Protein O-Fucosyltransferases: Biological Functions and Molecular Mechanisms in Mammals
Abstract
1. Introduction
2. GDP-Fucose Synthesis and Transport
2.1. De Novo Pathway
2.2. Salvage Pathway
2.3. GDP-Fucose Transport and Cellular GDP-Fucose Pool
3. Fucosyltransferases (FUTs)
3.1. Golgi-Localized FUTs
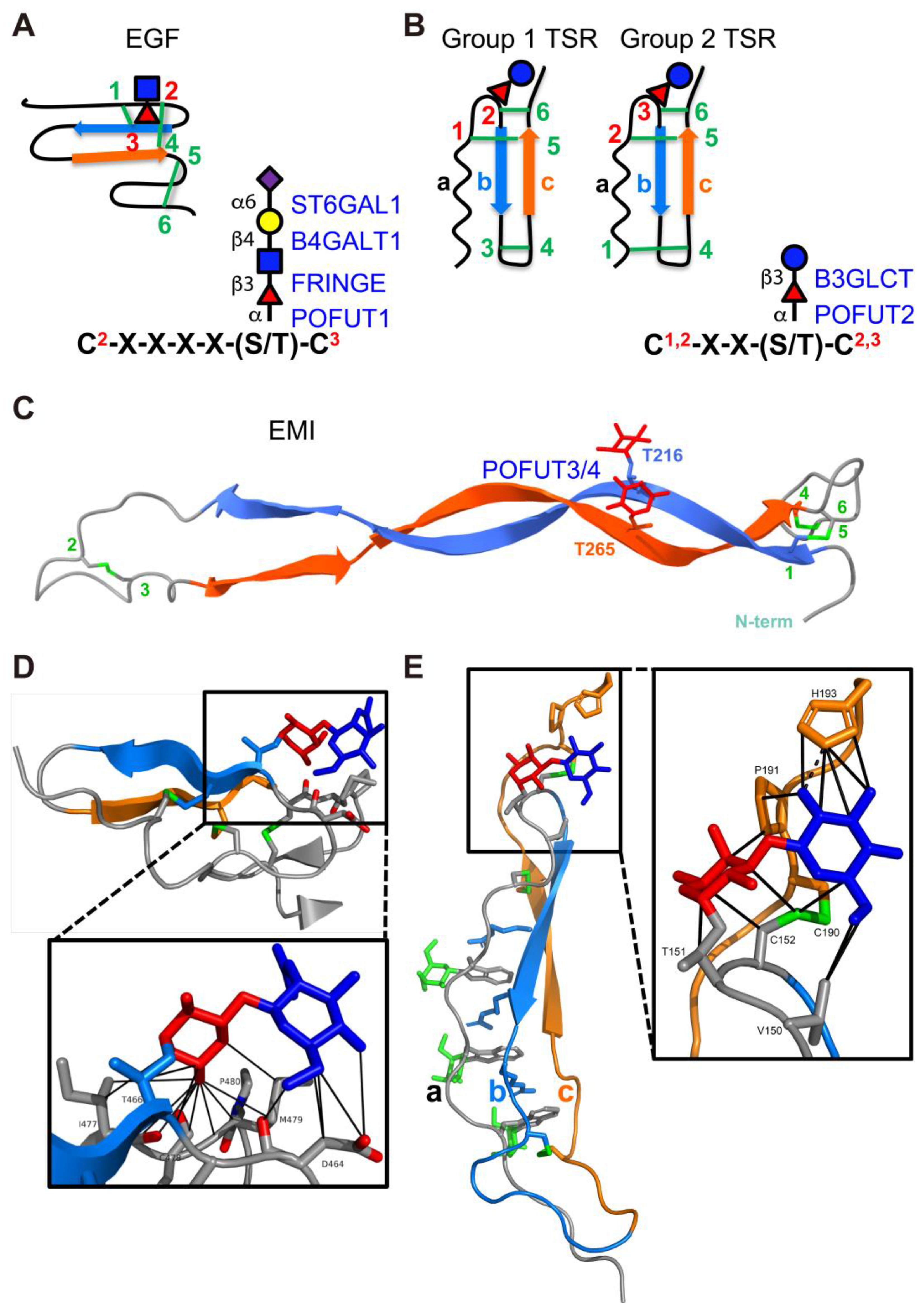
3.2. ER-Localized POFUTs
3.2.1. POFUT1 (FUT12)
| UniProt ID | Gene Name | Protein Name | Consensus/Total | Subcellular Location | Known Human Pathology (If Any) |
|---|---|---|---|---|---|
| H0YMF1 | ACAN | Aggrecan core protein | 1/2 | ECM | - |
| Q14246 | ADGRE1 | Adhesion G protein-coupled receptor E1 | 4/6 | CM | - |
| Q9UHX3 | ADGRE2 | Adhesion G protein-coupled receptor E2 | 2/5 | CM | Vibratory urticaria (VBU) [80] |
| Q9BY15 | ADGRE3 | Adhesion G protein-coupled receptor E3 | 1/2 | ECM, CM | - |
| Q86SQ3 | ADGRE4P | Putative adhesion G protein-coupled receptor E4P | 1/2 | ECM, CM | - |
| P48960 | ADGRE5 | Adhesion G protein-coupled receptor E5 | 2/5 | ECM, CM | - |
| O00468 | AGRN | Agrin | 2/4 [81] | ECM | Myasthenic syndrome, congenital, 8 (CMS8) [82,83,84] |
| Q6UW56 | ATRAID | All-trans retinoic acid-induced differentiation factor | 1/1 | CM, N | - |
| Q96GW7 | BCAN | Brevican core protein | 1/1 | ECM, CM | - |
| Q9NPY3 | CD93 | Complement component C1q receptor | 1/5 | CM | - |
| Q9NYQ6 | CELSR1 | Cadherin EGF LAG seven-pass G-type receptor 1 | 2/8 | CM | Neural tube defects (NTD) [85]; Lymphatic malformation 9 (LMPHM9) [86,87] |
| Q9HCU4 | CELSR2 | Cadherin EGF LAG seven-pass G-type receptor 2 | 2/7 | CM | - |
| Q9NYQ7 | CELSR3 | Cadherin EGF LAG seven-pass G-type receptor 3 | 2/8 | CM | - |
| P0CG37 | CFC1 | Cryptic protein | 1/1 | ECM, CM | Heterotaxy, visceral, 2, autosomal (HTX2) [88,89] |
| P0CG36 | CFC1B | Cryptic family protein 1B | 1/1 | ECM | - |
| Q8WYK1 | CNTNAP5 | Contactin-associated protein-like 5 | 1/2 | CM | - |
| P82279 | CRB1 | Protein crumbs homolog 1 | 8/19 | ECM, CM | Retinitis pigmentosa 12 (RP12) [90,91]; Leber congenital amaurosis 8 (LCA8) [92]; Pigmented paravenous chorioretinal atrophy (PPCRA) [93] |
| Q5IJ48 | CRB2 | Protein crumbs homolog 2 | 9/15 | ECM, CM, CYT | Focal segmental glomerulosclerosis 9 (FSGS9) [94]; Retinitis pigmentosa (RP) [95]; Ventriculomegaly with cystic kidney disease (VMCKD) [96] |
| P13385 | CRIPTO | Protein Cripto | 1/1 [97,98] | ECM, CM | - |
| P51864 | CRIPTO3 | Putative protein CRIPTO3 | 1/1 | CM | - |
| O60494 | CUBN | Cubilin | 4/7 | CM | Imerslund-Grasbeck syndrome 1 (IGS1) [99,100,101]; Proteinuria, chronic benign (PROCHOB) [102] |
| P80370 | DLK1 | Protein delta homolog 1 | 3/6 [103] | CM, CYT | - |
| Q6UY11 | DLK2 | Protein delta homolog 2 | 1/6 | CM | - |
| O00548 | DLL1 | Delta-like protein 1 | 4/8 [56,58] | CM | Neurodevelopmental disorder with non-specific brain abnormalities and with or without seizures (NEDBAS) [104] |
| Q9NYJ7 | DLL3 | Delta-like protein 3 | 2/6 [105] | CM | Spondylocostal dysostosis 1, autosomal recessive (SCDO1) [106] |
| Q9NR61 | DLL4 | Delta-like protein 4 | 5/8 | CM | Adams-Oliver syndrome 6 (AOS6) [107] |
| Q8NFT8 | DNER | Delta and Notch-like epidermal growth factor-related receptor | 6/10 | CM | - |
| O43854 | EDIL3 | EGF-like repeat and discoidin I-like domain-containing protein 3 | 1/3 [108] | ECM | - |
| O95967 | EFEMP2 | EGF-containing fibulin-like extracellular matrix protein 2 | 1/6 | ECM | Cutis laxa, autosomal recessive, 1B (ARCL1B) [109] |
| P01133 | EGF | Pro-epidermal growth factor | 1/9 | CM | Hypomagnesemia 4 (HOMG4) [110] |
| Q9UHF1 | EGFL7 | Epidermal growth factor-like protein 7 | 1/2 | ECM | - |
| Q63HQ2 | EGFLAM | Pikachurin | 2/3 | ECM | - |
| Q5T1H1 | EYS | Protein eyes shut homolog | 11/27 | ECM, CYT | Retinitis pigmentosa 25 (RP25) [111,112] |
| P08709 | F7 | Coagulation factor VII | 1/2 [50] | ECM | Factor VII deficiency (FA7D) [113,114,115] |
| P00740 | F9 | Coagulation factor IX | 1/2 [52,116] | ECM | Hemophilia B (HEMB) [117,118]; Thrombophilia, X-linked, due to factor IX defect (THPH8) [119]; Warfarin sensitivity, X-linked (WARFS) [120] |
| P00748 | F12 | Coagulation factor XII | 1/2 [51] | ECM | Factor XII deficiency (FA12D) [121,122]; Angioedema, hereditary, 3 (HAE3) [123,124] |
| Q14517 | FAT1 | Protocadherin Fat 1 | 2/5 | CM, N | - |
| Q9NYQ8 | FAT2 | Protocadherin Fat 2 | 1/2 | CM | Spinocerebellar ataxia 45 (SCA45) [125] |
| Q8TDW7 | FAT3 | Protocadherin Fat 3 | 3/4 | CM | - |
| Q6V0I7 | FAT4 | Protocadherin Fat 4 | 5/6 | CM | Van Maldergem syndrome 2 (VMLDS2) [126]; Hennekam lymphangiectasia-lymphedema syndrome 2 (HKLLS2) [127] |
| P23142 | FBLN1 | Fibulin-1 | 3/9 | ECM | Complex type of synpolydactyly [128]; associated with human breast cancer [129] |
| P98095 | FBLN2 | Fibulin-2 | 2/10 | ECM | - |
| Q9UBX5 | FBLN5 | Fibulin-5 | 1/6 | ECM | Charcot-Marie-Tooth disease, demyelinating, 1H (CMT1H) [130]; Cutis laxa, autosomal dominant, 2 (ADCL2) [131]; Cutis laxa, autosomal recessive, 1A (ARCL1A) [132]; Macular degeneration, age-related, 3 (ARMD3) [133] |
| Q53RD9 | FBLN7 | Fibulin-7 | 2/3 | ECM | - |
| P35555 | FBN1 | Fibrillin-1 | 1/47 [134] | ECM | Marfan syndrome (MFS) [135,136]; Ectopia lentis 1, isolated, autosomal dominant (ECTOL1) [137]; Weill–Marchesani syndrome 2 (WMS2) [138]; Overlap connective tissue disease (OCTD) [139]; Stiff skin syndrome (SSKS) [140]; Geleophysic dysplasia 2 (GPHYSD2) [141]; Acromicric dysplasia (ACMICD) [141]; Marfanoid-progeroid-lipodystrophy syndrome (MFLS) [142] |
| P35556 | FBN2 | Fibrillin-2 | 2/47 | ECM | Contractural arachnodactyly, congenital (CCA) [143]; Macular degeneration, early-onset (EOMD) [144] |
| Q75N90 | FBN3 | Fibrillin-3 | 1/44 | ECM | - |
| Q14520 | HABP2 | Hyaluronan-binding protein 2 | 1/3 | ECM | Thyroid cancer, non-medullary, 5 (NMTC5) [145,146] |
| Q04756 | HGFAC | Hepatocyte growth factor activator | 2/2 | ECM | - |
| P98160 | HSPG2 | Basement membrane-specific heparan sulfate proteoglycan core protein | 3/4 | ECM | Schwartz-Jampel syndrome (SJS1) [147]; Dyssegmental dysplasia Silverman-Handmaker type (DDSH) [148] |
| P78504 | JAG1 | Jagged-1 | 11/16 [56,149] | CM | Alagille syndrome 1 (ALGS1) [150]; Tetralogy of Fallot (TOF) [151]; Deafness, congenital heart defects, and posterior embryotoxon (DCHE) [152]; Charcot-Marie-Tooth disease, axonal, 2HH (CMT2HH) [153] |
| Q9Y219 | JAG2 | Jagged-2 | 9/16 | CM | Muscular dystrophy, limb-girdle, autosomal recessive 27 (LGMDR27) [154] |
| Q07954 | LRP1 | Prolow-density lipoprotein receptor-related protein 1 | 5/22 | CM, CYT | Keratosis pilaris atrophicans (KPA) [155]; Developmental dysplasia of the hip 3 (DDH3) [156] |
| Q9NZR2 | LRP1B | Low-density lipoprotein receptor-related protein 1B | 4/22 | CM | - |
| Q14767 | LTBP2 | Latent-transforming growth factor beta-binding protein 2 | 1/20 | ECM | Glaucoma 3, primary congenital, D (GLC3D) [157]; Microspherophakia and/or megalocornea, with ectopia lentis and with or without secondary glaucoma (MSPKA) [158]; Weill–Marchesani syndrome 3 (WMS3) [159] |
| Q5VYJ5 | MALRD1 | MAM and LDL-receptor class A domain-containing protein 1 | 1/1 | CYT | - |
| O75095 | MEGF6 | Multiple epidermal growth factor-like domains protein 6 | 5/27 | ECM | - |
| Q7Z7M0 | MEGF8 | Multiple epidermal growth factor-like domains protein 8 | 2/5 | CM | Carpenter syndrome 2 (CRPT2) [160] |
| Q96KG7 | MEGF10 | Multiple epidermal growth factor-like domains protein 10 | 5/15 | CM | Congenital myopathy 10A, severe variant (CMYP10A) [161]; Congenital myopathy 10B, mild variant (CMYP10B) [162] |
| A6BM72 | MEGF11 | Multiple epidermal growth factor-like domains protein 11 | 8/14 | CM | - |
| Q13201 | MMRN1 | Multimerin-1 [45,163] | 1/1 | ECM | Factor V Quebec [164] |
| Q9UK23 | NAGPA | N-acetylglucosamine-1-phosphodiester alpha-N-acetylglucosaminidase | 1/2 | GA | Stuttering [165] |
| A0A087WY62 | NBPF26 | NBPF member 26 | 5/6 | CYT | - |
| O14594 | NCAN | Neurocan core protein | 2/2 | ECM | - |
| Q92832 | NELL1 | Protein kinase C-binding protein NELL1 | 1/5 | ECM, CYT | - |
| Q14112 | NID2 | Nidogen-2 | 1/5 | ECM | - |
| P46531 | NOTCH1 | Neurogenic locus notch homolog protein 1 | 21/36 [67,70] | CM | Aortic valve disease 1 (AOVD1) [166]; Adams-Oliver syndrome 5 (AOS5) [167] |
| Q04721 | NOTCH2 | Neurogenic locus notch homolog protein 2 | 20/36 [66,168] | CM | Alagille syndrome 2 (ALGS2) [169]; Hajdu-Cheney syndrome (HJCYS) [170] |
| Q7Z3S9 | NOTCH2NLA | Notch homolog 2 N-terminal-like protein A | 5/6 | ECM, CYT | Microcephaly, macrocephaly [171] |
| P0DPK3 | NOTCH2NLB | Notch homolog 2 N-terminal-like protein B | 4/6 | ECM | Microcephaly, macrocephaly [171] |
| P0DPK4 | NOTCH2NLC | Notch homolog 2 N-terminal-like protein C | 5/6 | ECM | Neuronal intranuclear inclusion disease (NIID) [172,173,174]; Oculopharyngodistal myopathy 3 (OPDM3) [175] |
| Q9UM47 | NOTCH3 | Neurogenic locus notch homolog protein 3 | 14/34 [176] | CM | Cerebral arteriopathy, autosomal dominant, with subcortical infarcts and leukoencephalopathy, 1 (CADASIL1) [177,178]; Myofibromatosis, infantile 2 (IMF2) [179]; Lateral meningocele syndrome (LMNS) [180] |
| Q99466 | NOTCH4 | Neurogenic locus notch homolog protein 4 | 18/29 | CM | - |
| Q96CW9 | NTNG2 | Netrin-G2 | 1/1 | CM | Neurodevelopmental disorder with behavioral abnormalities, absent speech, and hypotonia (NEDBASH) [181,182] |
| Q6UXH9 | PAMR1 | Inactive serine protease PAMR1 | 1/1 [183] | ECM | - |
| Q5VY43 | PEAR1 | Platelet endothelial aggregation receptor 1 | 3/9 | CM | Cardiovascular disease [184] |
| P00750 | PLAT | Tissue-type plasminogen activator | 1/1 [49] | ECM | Increased activity results in excessive bleeding; Defective release results in thrombosis or embolism [185] |
| P00749 | PLAU | Urokinase-type plasminogen activator | 1/1 [47,48] | ECM | Quebec platelet disorder (QPD) [186] |
| P04070 | PROC | Vitamin K-dependent protein C | 1/2 | ECM | Thrombophilia due to protein C deficiency, autosomal dominant (THPH3) [187]; Thrombophilia due to protein C deficiency, autosomal recessive (THPH4) [188] |
| P22891 | PROZ | Vitamin K-dependent protein Z | 1/2 | ECM | - |
| P78509 | RELN | Reelin | 2/8 | ECM | Lissencephaly 2 (LIS2) [189]; Epilepsy, familial temporal lobe, 7 (ETL7) [190] |
| Q96GP6 | SCARF2 | Scavenger receptor class F member 2 | 1/7 | CM | Van den Ende-Gupta syndrome (VDEGS) [191] |
| O75093 | SLIT1 | Slit homolog 1 protein | 2/9 | ECM | - |
| O94813 | SLIT2 | Slit homolog 2 protein | 3/7 | ECM | - |
| O75094 | SLIT3 | Slit homolog 3 protein | 3/9 | ECM | - |
| Q8TER0 | SNED1 | Sushi, nidogen and EGF-like domain-containing protein 1 | 10/15 | ECM | - |
| Q9NY15 | STAB1 | Stabilin-1 | 3/16 | CM | Hyperferritinemia (HRFT) [192] |
| Q8WWQ8 | STAB2 | Stabilin-2 | 6/17 | CM, CYT | - |
| Q6UWL2 | SUSD1 | Sushi domain-containing protein 1 | 2/3 | CM | - |
| Q4LDE5 | SVEP1 | Sushi, von Willebrand factor type A, EGF and pentraxin domain-containing protein 1 | 4/9 | ECM, CYT | Coronary artery disease [193]; artherosclerosis [194] |
| Q9UKZ4 | TENM1 | Teneurin-1 | 1/8 | CM | - |
| Q9NT68 | TENM2 | Teneurin-2 | 2/8 | CM | - |
| Q9P273 | TENM3 | Teneurin-3 | 1/7 | CM | Microphthalmia/Coloboma 9 (MCOPCB9) [195]; Microphthalmia, syndromic, 15 (MCOPS15) [196] |
| Q6N022 | TENM4 | Teneurin-4 | 2/8 | CM | Tremor, hereditary essential 5 (ETM5) [197] |
| P49746 | THBS3 | Thrombospondin-3 | 1/3 | ECM | - |
| P35590 | TIE1 | Tyrosine-protein kinase receptor Tie-1 | 1/3 | CM | Lymphatic malformation 11 (LMPHM11) [198] |
| P07911 | UMOD | Uromodulin | 3/4 | ECM, CM | Tubulointerstitial kidney disease, autosomal dominant, 1 (ADTKD1) [199,200] |
| Q5DID0 | UMODL1 | Uromodulin-like 1 | 1/3 | CM, CYT | - |
| Q6EMK4 | VASN | Vasorin | 1/1 | ECM, CM | - |
| P13611 | VCAN | Versican core protein | 2/2 | ECM | Wagner vitreoretinopathy (WGVRP) [201,202] |
| Q5GFL6 | VWA2 | von Willebrand factor A domain-containing protein 2 | 2/2 [203] | ECM | - |
| Q8N2E2 | VWDE | von Willebrand factor D and EGF domain-containing protein | 3/7 | ECM | - |
| Q9Y5W5 | WIF1 | Wnt inhibitory factor 1 | 2/5 | ECM | - |
| Q0PNF2 | - | FEX1 | 3/20 | - | - |
3.2.2. POFUT2 (FUT13)
| UniProt ID | Gene Name | Protein Name | Consensus/Total | Subcellular Location | Known Human Pathology (If Any) |
|---|---|---|---|---|---|
| Q9UHI8 | ADAMTS1 | A disintegrin and metalloproteinase with thrombospondin motifs 1 | 3/3 | ECM | - |
| O95450 | ADAMTS2 | A disintegrin and metalloproteinase with thrombospondin motifs 2 | 3/4 | ECM | Ehlers-Danlos syndrome, dermatosparaxis type (EDSDERMS) [215] |
| O15072 | ADAMTS3 | A disintegrin and metalloproteinase with thrombospondin motifs 3 | 3/4 | ECM | Hennekam lymphangiectasia-lymphedema syndrome 3 (HKLLS3) [216] |
| O75173 | ADAMTS4 | A disintegrin and metalloproteinase with thrombospondin motifs 4 | 1/1 | ECM | - |
| Q9UNA0 | ADAMTS5 | A disintegrin and metalloproteinase with thrombospondin motifs 5 | 2/2 [217] | ECM | - |
| Q9UKP5 | ADAMTS6 | A disintegrin and metalloproteinase with thrombospondin motifs 6 | 3/5 [218] | ECM | - |
| Q9UKP4 | ADAMTS7 | A disintegrin and metalloproteinase with thrombospondin motifs 7 | 6/8 | ECM | - |
| Q9UP79 | ADAMTS8 | A disintegrin and metalloproteinase with thrombospondin motifs 8 | 2/2 | ECM | - |
| Q9P2N4 | ADAMTS9 | A disintegrin and metalloproteinase with thrombospondin motifs 9 | 12/15 [219] | ECM | - |
| Q9H324 | ADAMTS10 | A disintegrin and metalloproteinase with thrombospondin motifs 10 | 3/5 [219] | ECM | Weill–Marchesani syndrome 1 (WMS1) [220,221] |
| P58397 | ADAMTS12 | A disintegrin and metalloproteinase with thrombospondin motifs 12 | 7/8 | ECM | - |
| Q76LX8 | ADAMTS13 | A disintegrin and metalloproteinase with thrombospondin motifs 13 | 7/8 [206] | ECM | Thrombotic thrombocytopenic purpura, hereditary (TTP) [222] |
| Q8WXS8 | ADAMTS14 | A disintegrin and metalloproteinase with thrombospondin motifs 14 | 3/4 | ECM | - |
| Q8TE58 | ADAMTS15 | A disintegrin and metalloproteinase with thrombospondin motifs 15 | 3/3 | ECM | Arthrogryposis, distal, 12 (DA12) [223] |
| Q8TE57 | ADAMTS16 | A disintegrin and metalloproteinase with thrombospondin motifs 16 | 6/6 | ECM | - |
| Q8TE56 | ADAMTS17 | A disintegrin and metalloproteinase with thrombospondin motifs 17 | 4/5 [224] | ECM | Weill–Marchesani syndrome 4 (WMS4) [225] |
| Q8TE60 | ADAMTS18 | A disintegrin and metalloproteinase with thrombospondin motifs 18 | 4/5 | ECM | Microcornea, myopic chorioretinal atrophy, and telecanthus (MMCAT) [226] |
| Q8TE59 | ADAMTS19 | A disintegrin and metalloproteinase with thrombospondin motifs 19 | 4/5 | ECM | Cardiac valvular dysplasia 2 (CVDP2) [227,228] |
| P59510 | ADAMTS20 | A disintegrin and metalloproteinase with thrombospondin motifs 20 | 12/15 [229] | ECM | - |
| Q8N6G6 | ADAMTSL1 | ADAMTS-like protein 1 | 9/9 [207] | ECM | - |
| Q86TH1 | ADAMTSL2 | ADAMTS-like protein 2 | 6/7 [230] | ECM | Geleophysic dysplasia 1 (GPHYSD1) [231,232] |
| P82987 | ADAMTSL3 | ADAMTS-like protein 3 | 9/10 | ECM | - |
| Q6UY14 | ADAMTSL4 | ADAMTS-like protein 4 | 2/6 | ECM | Ectopia lentis 2, isolated, autosomal recessive (ECTOL2) [233]; Ectopia lentis et pupillae (ECTOLP) [234] |
| Q6ZMM2 | ADAMTSL5 | ADAMTS-like protein 5 | 1/1 | ECM | - |
| O14514 | ADGRB1 | Adhesion G protein-coupled receptor B1 | 4/5 [235] | CM | - |
| O60241 | ADGRB2 | Adhesion G protein-coupled receptor B2 | 4/4 | CM | - |
| O60242 | ADGRB3 | Adhesion G protein-coupled receptor B3 | 4/4 | CM | - |
| P13671 | C6 | Complement component C6 | 1/3 | ECM | Complement component 6 deficiency (C6D) [236] |
| O00622 | CCN1 | CCN family member 1 | 1/1 [237] | ECM | - |
| P29279 | CCN2 | CCN family member 2 | 1/1 [219] | ECM | - |
| P48745 | CCN3 | CCN family member 3 | 1/1 | ECM, CYT | - |
| O95388 | CCN4 | CCN family member 4 | 1/1 | ECM | - |
| O76076 | CCN5 | CCN family member 5 | 1/1 | ECM | - |
| O95389 | CCN6 | Cellular communication network factor 6 | 1/1 | ECM | Progressive pseudorheumatoid dysplasia (PPRD) [238] |
| P27918 | CFP | Properdin | 4/7 [239] | ECM | Properdin deficiency (PFD) [240] |
| Q8IUL8 | CILP2 | Cartilage intermediate layer protein 2 | 1/1 | ECM | - |
| Q96RW7 | HMCN1 | Hemicentin-1 | 6/6 | ECM, CYT | Macular degeneration, age-related, 1 (ARMD1) [241] |
| B1AKI9 | ISM1 | Isthmin-1 | 1/1 | ECM | - |
| O95428 | PAPLN | Papilin | 4/5 | ECM | - |
| Q13591 | SEMA5A | Semaphorin-5A | 2/7 | CM | - |
| Q9P283 | SEMA5B | Semaphorin-5B | 2/5 | CM | - |
| Q9HCB6 | SPON1 | Spondin-1 | 5/6 [242] | ECM | - |
| A2VEC9 | SSPOP | SCO-spondin | 16/25 [243] | ECM | - |
| P07996 | THBS1 | Thrombospondin-1 | 3/3 [204] | ECM | - |
| P35442 | THBS2 | Thrombospondin-2 | 3/3 [209] | ECM | Intervertebral disc disease (IDD) [244] |
| Q9NS62 | THSD1 | Thrombospondin type-1 domain-containing protein 1 | 1/1 | ECM | Lymphatic malformation 13 (LMPHM13) [245,246]; Aneurysm, intracranial berry, 12 (ANIB12) [247,248] |
| Q6ZMP0 | THSD4 | Thrombospondin type-1 domain-containing protein 4 | 3/6 | ECM | Aortic aneurysm, familial thoracic 12 (AAT12) [249] |
| Q9UPZ6 | THSD7A | Thrombospondin type-1 domain-containing protein 7A | 7/19 | ECM, CM | Pathogenic autoantigen in membranous nephropathy (MN) [250,251] |
| Q9C0I4 | THSD7B | Thrombospondin type-1 domain-containing protein 7B | 6/18 | CM | - |
3.2.3. POFUT3 (FUT10) and POFUT4 (FUT11)
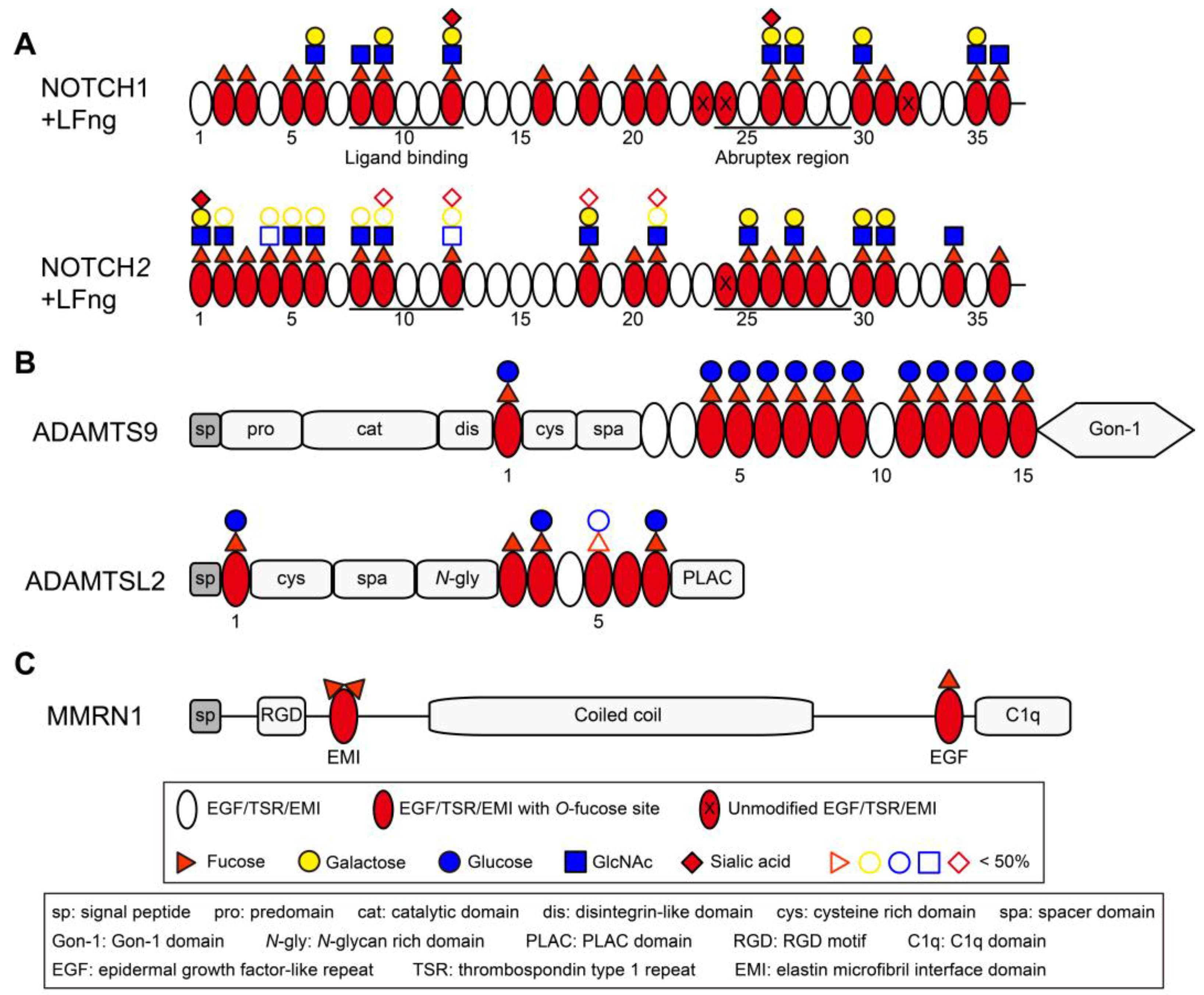
4. O-Fucosylation: Physiological and Pathological Significance
4.1. Regulation of Lymphoid and Myeloid Cell Development by Notch O-Fucosylation
4.2. Cancer with Altered O-Fucosylation
4.3. Peters-Plus Syndrome (PTRPLS)
4.4. Spondylocostal Dysostosis 3 (SCDO3)
4.5. Other Biological Processes Where O-Fucosylation May Play a Role

5. Molecular Mechanisms of How O-Fucose Regulates Protein Functions
5.1. O-Fucosylation Forms Direct Contacts with Binding Partners
5.2. O-Fucosylation Generates Intramolecular Interactions That Stabilize Protein Domains and Facilitate Secretion
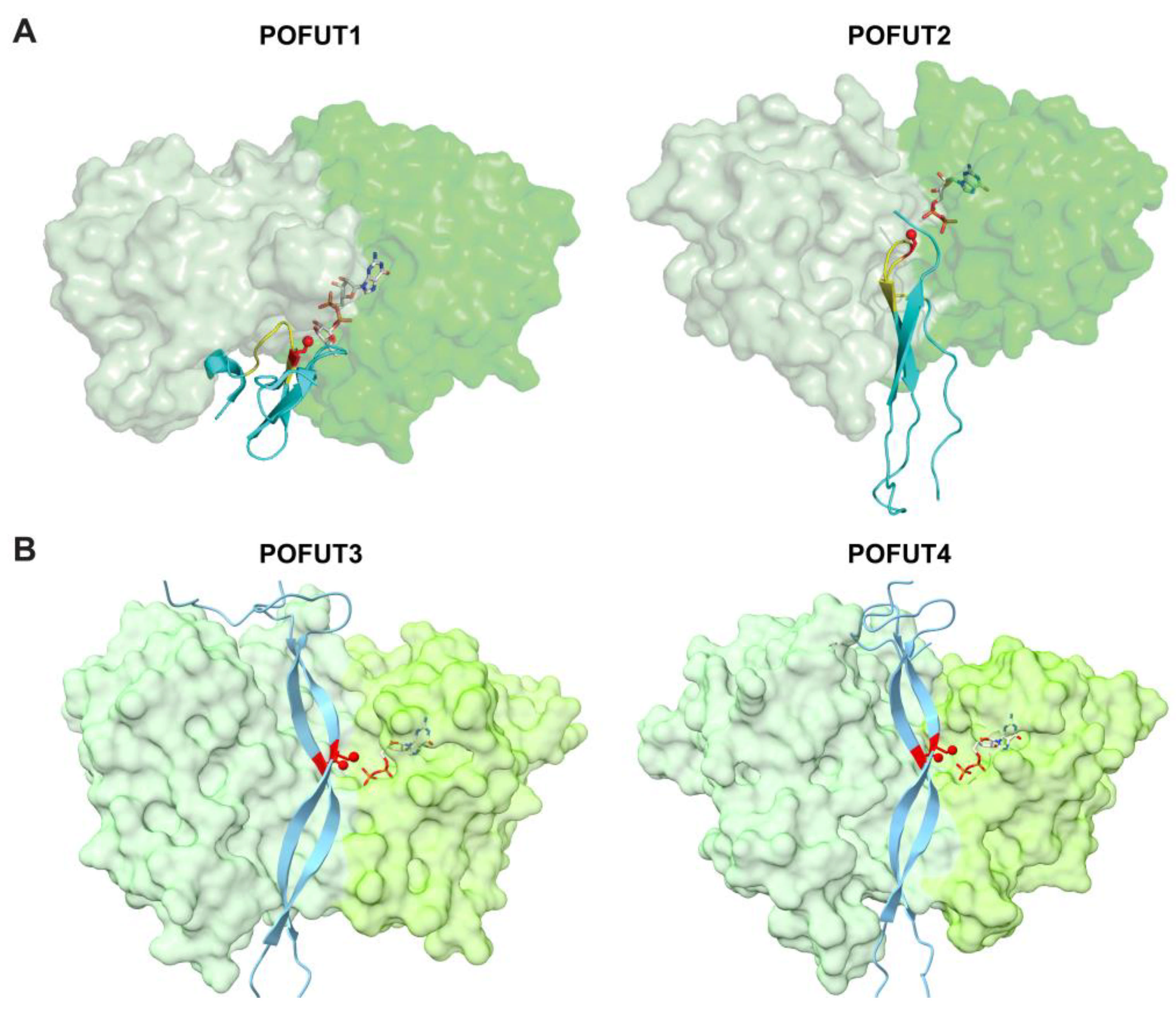
6. Inhibitors/Modulators of O-Fucosylation
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ginsburg, V. Formation of guanosine diphosphate L-fucose from guanosine diphosphate D-mannose. J. Biol. Chem. 1960, 235, 2196–2201. [Google Scholar] [PubMed]
- Tonetti, M.; Sturla, L.; Bisso, A.; Benatti, U.; De Flora, A. Synthesis of GDP-L-fucose by the human FX protein. J. Biol. Chem. 1996, 271, 27274–27279. [Google Scholar] [PubMed]
- Coffey, J.W.; Miller, O.N.; Sellinger, O.Z. The metabolism of L-fucose in the rat. J. Biol. Chem. 1964, 239, 4011–4017. [Google Scholar] [PubMed]
- Kaufman, R.L.; Ginsburg, V. The metabolism of L-fucose by HeLa cells. Exp. Cell Res. 1968, 50, 127–132. [Google Scholar]
- Ishihara, H.; Heath, E.C. The metabolism of L-fucose. IV. The biosynthesis of guanosine diphosphate L-fucose in porcine liver. J. Biol. Chem. 1968, 243, 1110–1115. [Google Scholar]
- Ishihara, H.; Massaro, D.J.; Heath, E.C. The metabolism of L-fucose. 3. The enzymatic synthesis of beta-L-fucose 1-phosphate. J. Biol. Chem. 1968, 243, 1103–1109. [Google Scholar]
- Wiese, T.J.; Dunlap, J.A.; Yorek, M.A. L-fucose is accumulated via a specific transport system in eukaryotic cells. J. Biol. Chem. 1994, 269, 22705–22711. [Google Scholar]
- Ng, B.G.; Sosicka, P.; Xia, Z.; Freeze, H.H. GLUT1 is a highly efficient L-fucose transporter. J. Biol. Chem. 2023, 299, 102738. [Google Scholar]
- Lühn, K.; Laskowska, A.; Pielage, J.; Klämbt, C.; Ipe, U.; Vestweber, D.; Wild, M.K. Identification and molecular cloning of a functional GDP-fucose transporter in Drosophila melanogaster. Exp. Cell Res. 2004, 301, 242–250. [Google Scholar]
- Lühn, K.; Wild, M.K.; Eckhardt, M.; Gerardy-Schahn, R.; Vestweber, D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat. Genet. 2001, 28, 69–72. [Google Scholar]
- Lu, L.; Varshney, S.; Yuan, Y.; Wei, H.X.; Tanwar, A.; Sundaram, S.; Nauman, M.; Haltiwanger, R.S.; Stanley, P. In vivo evidence for GDP-fucose transport in the absence of transporter SLC35C1 and putative transporter SLC35C2. J. Biol. Chem. 2023, 299, 105406. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hou, X.; Shi, S.; Körner, C.; Stanley, P. SLC35C2 promotes Notch1 fucosylation and is required for optimal Notch signaling in mammalian cells. J. Biol. Chem. 2010, 285, 36245–36254. [Google Scholar] [CrossRef] [PubMed]
- Ashikov, A.; Routier, F.; Fuhlrott, J.; Helmus, Y.; Wild, M.; Gerardy-Schahn, R.; Bakker, H. The human solute carrier gene SLC35B4 encodes a bifunctional nucleotide sugar transporter with specificity for UDP-xylose and UDP-N-acetylglucosamine. J. Biol. Chem. 2005, 280, 27230–27235. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D.; Atkinson, P.H. Fucosyl-glycoprotein and precursor polls in HeLa cells. Biochemistry 1975, 14, 3107–3114. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Atkinson, P.H. Equilibration of fucosyl glycoprotein pools in HeLa cells. Biochemistry 1977, 16, 944–953. [Google Scholar] [CrossRef]
- Feichtinger, R.G.; Hullen, A.; Koller, A.; Kotzot, D.; Grote, V.; Rapp, E.; Hofbauer, P.; Brugger, K.; Thiel, C.; Mayr, J.A.; et al. A spoonful of L-fucose-an efficient therapy for GFUS-CDG, a new glycosylation disorder. EMBO Mol. Med. 2021, 13, e14332. [Google Scholar] [CrossRef]
- Sosicka, P.; Ng, B.G.; Pepi, L.E.; Shajahan, A.; Wong, M.; Scott, D.A.; Matsumoto, K.; Xia, Z.J.; Lebrilla, C.B.; Haltiwanger, R.S.; et al. Origin of cytoplasmic GDP-fucose determines its contribution to glycosylation reactions. J. Cell Biol. 2022, 221, e202205038. [Google Scholar] [CrossRef]
- Skurska, E.; Szulc, B.; Maszczak-Seneczko, D.; Wiktor, M.; Wiertelak, W.; Makowiecka, A.; Olczak, M. Incorporation of fucose into glycans independent of the GDP-fucose transporter SLC35C1 preferentially utilizes salvaged over de novo GDP-fucose. J. Biol. Chem. 2022, 298, 102206. [Google Scholar] [CrossRef]
- Bisso, A.; Sturla, L.; Zanardi, D.; De Flora, A.; Tonetti, M. Structural and enzymatic characterization of human recombinant GDP-D-mannose-4,6-dehydratase. FEBS Lett. 1999, 456, 370–374. [Google Scholar] [CrossRef]
- Sullivan, F.X.; Kumar, R.; Kriz, R.; Stahl, M.; Xu, G.Y.; Rouse, J.; Chang, X.J.; Boodhoo, A.; Potvin, B.; Cumming, D.A. Molecular cloning of human GDP-mannose 4,6-dehydratase and reconstitution of GDP-fucose biosynthesis in vitro. J. Biol. Chem. 1998, 273, 8193–8202. [Google Scholar] [CrossRef]
- Richards, W.L.; Kilker, R.D.; Serif, G.S. Metabolite control of L-fucose utilization. J. Biol. Chem. 1978, 253, 8359–8361. [Google Scholar] [PubMed]
- Becker, D.J.; Lowe, J.B. Fucose: Biosynthesis and biological function in mammals. Glycobiology 2003, 13, 41R–53R. [Google Scholar] [PubMed]
- Ma, B.; Simala-Grant, J.L.; Taylor, D.E. Fucosylation in prokaryotes and eukaryotes. Glycobiology 2006, 16, 158R–184R. [Google Scholar]
- Schneider, M.; Al-Shareffi, E.; Haltiwanger, R.S. Biological functions of fucose in mammals. Glycobiology 2017, 27, 601–618. [Google Scholar] [PubMed]
- Flynn, R.A.; Pedram, K.; Malaker, S.A.; Batista, P.J.; Smith, B.A.H.; Johnson, A.G.; George, B.M.; Majzoub, K.; Villalta, P.W.; Carette, J.E.; et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell 2021, 184, 3109–3124.e22. [Google Scholar] [CrossRef]
- Oriol, R.; Mollicone, R. Fucosyltransferases 1, 2. GDP-Fucose Galactoside α2-Fucosyltransferases. FUT1 or H Blood Group, FUT2 or ABH Secretor Status and Sec1 (FUT1, FUT2, Sec1). In Handbook of Glycosyltransferases and Related Genes; Springer: Tokyo, Japan, 2014; pp. 515–530. [Google Scholar]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Lowe, J.B. The blood group-specific human glycosyltransferases. Bailliere’s Clin. Haematol. 1993, 6, 465–492. [Google Scholar]
- Scharberg, E.A.; Olsen, C.; Bugert, P. The H blood group system. Immunohematology 2016, 32, 112–118. [Google Scholar]
- Kannagi, R. Fucosyltransferase 5. GDP-Fucose Lactosamine α3/4-Fucosyltransferase (FUT5). In Handbook of Glycosyltransferases and Related Genes; Springer: Tokyo, Japan, 2014; pp. 549–558. [Google Scholar]
- Kannagi, R. Fucosyltransferase 6. GDP-Fucose Lactosamine α3-Fucosyltransferase (FUT6). In Handbook of Glycosyltransferases and Related Genes; Springer: Tokyo, Japan, 2014; pp. 559–571. [Google Scholar]
- Kudo, T.; Narimatsu, H. Fucosyltransferase 3. GDP-Fucose Lactosamine α1,3/4-Fucosyltransferase. Lea and Leb Histo-Blood Groups (FUT3, Lewis Enzyme). In Handbook of Glycosyltransferases and Related Genes; Springer: Tokyo, Japan, 2014; pp. 531–539. [Google Scholar]
- Kudo, T.; Narimatsu, H. Fucosyltransferase 4. GDP-Fucose Lactosamine α1,3-Fucosyltransferase. Myeloid Specific (FUT4). In Handbook of Glycosyltransferases and Related Genes; Springer: Tokyo, Japan, 2014; pp. 541–547. [Google Scholar]
- Kudo, T.; Narimatsu, H. Fucosyltransferase 7. GDP-Fucose Lactosamine α1,3-Fucosyltransferase. Sialyl-Lex Specific (FUT7). In Handbook of Glycosyltransferases and Related Genes; Springer: Tokyo, Japan, 2014; pp. 573–580. [Google Scholar]
- Kudo, T.; Narimatsu, H. Fucosyltransferase 9. GDP-Fucose Lactosamine α1,3-Fucosyltransferase. Lex Specific (FUT9). In Handbook of Glycosyltransferases and Related Genes; Springer: Tokyo, Japan, 2014; pp. 597–603. [Google Scholar]
- Stowell, C.P.; Stowell, S.R. Biologic roles of the ABH and Lewis histo-blood group antigens Part I: Infection and immunity. Vox Sang. 2019, 114, 426–442. [Google Scholar] [CrossRef]
- Ihara, H.; Tsukamoto, H.; Gu, J.; Miyoshi, E.; Taniguchi, N.; Ikeda, Y. Fucosyltransferase 8. GDP-Fucose N-Glycan Core α6-Fucosyltransferase (FUT8). In Handbook of Glycosyltransferases and Related Genes; Springer: Tokyo, Japan, 2014; pp. 581–596. [Google Scholar]
- Boruah, B.M.; Kadirvelraj, R.; Liu, L.; Ramiah, A.; Li, C.; Zong, G.; Bosman, G.P.; Yang, J.Y.; Wang, L.X.; Boons, G.J.; et al. Characterizing human α-1,6-fucosyltransferase (FUT8) substrate specificity and structural similarities with related fucosyltransferases. J. Biol. Chem. 2020, 295, 17027–17045. [Google Scholar] [CrossRef]
- Bastian, K.; Scott, E.; Elliott, D.J.; Munkley, J. FUT8 Alpha-(1,6)-Fucosyltransferase in Cancer. Int. J. Mol. Sci. 2021, 22, 455. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, C.; Grau, S.; Jäger, C.; Sondermann, P.; Brünker, P.; Waldhauer, I.; Hennig, M.; Ruf, A.; Rufer, A.C.; Stihle, M.; et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA 2011, 108, 12669–12674. [Google Scholar] [PubMed]
- Luo, Y.; Haltiwanger, R.S. O-fucosylation of notch occurs in the endoplasmic reticulum. J. Biol. Chem. 2005, 280, 11289–11294. [Google Scholar] [PubMed]
- Luo, Y.; Koles, K.; Vorndam, W.; Haltiwanger, R.S.; Panin, V.M. Protein O-fucosyltransferase 2 adds O-fucose to thrombospondin type 1 repeats. J. Biol. Chem. 2006, 281, 9393–9399. [Google Scholar]
- Kakuda, S.; Haltiwanger, R.S. Fucosyltransferases 12, 13: Protein O-Fucosyltransferases 1 and 2 (POFUT1, POFUT2). In Handbook of Glycosyltransferases and Related Genes; Springer: Tokyo, Japan, 2014; pp. 623–633. [Google Scholar]
- Holdener, B.C.; Haltiwanger, R.S. Protein O-fucosylation: Structure and function. Curr. Opin. Struct. Biol. 2019, 56, 78–86. [Google Scholar]
- Hao, H.; Yuan, Y.; Ito, A.; Eberand, B.M.; Tjondro, H.; Cielesh, M.; Norris, N.; Moreno, C.L.; Maxwell, J.W.C.; Neely, G.G.; et al. FUT10 and FUT11 are protein O-fucosyltransferases that modify protein EMI domains. Nat. Chem. Biol. 2025. [Google Scholar] [CrossRef]
- Luo, Y.; Nita-Lazar, A.; Haltiwanger, R.S. Two distinct pathways for O-fucosylation of epidermal growth factor-like or thrombospondin type 1 repeats. J. Biol. Chem. 2006, 281, 9385–9392. [Google Scholar]
- Kentzer, E.J.; Buko, A.; Menon, G.; Sarin, V.K. Carbohydrate composition and presence of a fucose-protein linkage in recombinant human pro-urokinase. Biochem. Biophys. Res. Commun. 1990, 171, 401–406. [Google Scholar]
- Buko, A.M.; Kentzer, E.J.; Petros, A.; Menon, G.; Zuiderweg, E.R.; Sarin, V.K. Characterization of a posttranslational fucosylation in the growth factor domain of urinary plasminogen activator. Proc. Natl. Acad. Sci. USA 1991, 88, 3992–3996. [Google Scholar]
- Harris, R.J.; Leonard, C.K.; Guzzetta, A.W.; Spellman, M.W. Tissue plasminogen activator has an O-linked fucose attached to threonine-61 in the epidermal growth factor domain. Biochemistry 1991, 30, 2311–2314. [Google Scholar]
- Bjoern, S.; Foster, D.C.; Thim, L.; Wiberg, F.C.; Christensen, M.; Komiyama, Y.; Pedersen, A.H.; Kisiel, W. Human plasma and recombinant factor VII. Characterization of O-glycosylations at serine residues 52 and 60 and effects of site-directed mutagenesis of serine 52 to alanine. J. Biol. Chem. 1991, 266, 11051–11057. [Google Scholar] [PubMed]
- Harris, R.J.; Ling, V.T.; Spellman, M.W. O-linked fucose is present in the first epidermal growth factor domain of factor XII but not protein C. J. Biol. Chem. 1992, 267, 5102–5107. [Google Scholar] [PubMed]
- Harris, R.J.; van Halbeek, H.; Glushka, J.; Basa, L.J.; Ling, V.T.; Smith, K.J.; Spellman, M.W. Identification and structural analysis of the tetrasaccharide NeuAc alpha(2-->6)Gal beta(1-->4)GlcNAc beta(1-->3)Fuc alpha 1-->O-linked to serine 61 of human factor IX. Biochemistry 1993, 32, 6539–6547. [Google Scholar] [PubMed]
- Wang, Y.; Lee, G.F.; Kelley, R.F.; Spellman, M.W. Identification of a GDP-L-fucose:polypeptide fucosyltransferase and enzymatic addition of O-linked fucose to EGF domains. Glycobiology 1996, 6, 837–842. [Google Scholar]
- Wang, Y.; Spellman, M.W. Purification and characterization of a GDP-fucose:polypeptide fucosyltransferase from Chinese hamster ovary cells. J. Biol. Chem. 1998, 273, 8112–8118. [Google Scholar]
- Wang, Y.; Shao, L.; Shi, S.; Harris, R.J.; Spellman, M.W.; Stanley, P.; Haltiwanger, R.S. Modification of epidermal growth factor-like repeats with O-fucose. Molecular cloning and expression of a novel GDP-fucose protein O-fucosyltransferase. J. Biol. Chem. 2001, 276, 40338–40345. [Google Scholar]
- Panin, V.M.; Shao, L.; Lei, L.; Moloney, D.J.; Irvine, K.D.; Haltiwanger, R.S. Notch ligands are substrates for protein O-fucosyltransferase-1 and Fringe. J. Biol. Chem. 2002, 277, 29945–29952. [Google Scholar]
- Shao, L.; Moloney, D.J.; Haltiwanger, R. Fringe modifies O-fucose on mouse Notch1 at epidermal growth factor-like repeats within the ligand-binding site and the Abruptex region. J. Biol. Chem. 2003, 278, 7775–7782. [Google Scholar]
- Müller, J.; Rana, N.A.; Serth, K.; Kakuda, S.; Haltiwanger, R.S.; Gossler, A. O-fucosylation of the notch ligand mDLL1 by POFUT1 is dispensable for ligand function. PLoS ONE 2014, 9, e88571. [Google Scholar]
- Luther, K.B.; Haltiwanger, R.S. 1.07—O-Fucosylation of Proteins. In Comprehensive Glycoscience, 2nd ed.; Barchi, J.J., Ed.; Elsevier: Oxford, UK, 2021; pp. 182–203. [Google Scholar]
- Li, M.; Cheng, R.; Liang, J.; Yan, H.; Zhang, H.; Yang, L.; Li, C.; Jiao, Q.; Lu, Z.; He, J.; et al. Mutations in POFUT1, encoding protein O-fucosyltransferase 1, cause generalized Dowling-Degos disease. Am. J. Hum. Genet. 2013, 92, 895–903. [Google Scholar]
- Shi, S.; Stanley, P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 5234–5239. [Google Scholar] [PubMed]
- Okamura, Y.; Saga, Y. Pofut1 is required for the proper localization of the Notch receptor during mouse development. Mech. Dev. 2008, 125, 663–673. [Google Scholar] [PubMed]
- Varshney, S.; Stanley, P. Multiple roles for O-glycans in Notch signalling. FEBS Lett. 2018, 592, 3819–3834. [Google Scholar] [PubMed]
- Artavanis-Tsakonas, S.; Muskavitch, M.A. Notch: The past, the present, and the future. Curr. Top. Dev. Biol. 2010, 92, 1–29. [Google Scholar]
- Stanley, P.; Tanwar, A. Regulation of myeloid and lymphoid cell development by O-glycans on Notch. Front. Mol. Biosci. 2022, 9, 979724. [Google Scholar]
- Kakuda, S.; LoPilato, R.K.; Ito, A.; Haltiwanger, R.S. Canonical Notch ligands and Fringes have distinct effects on NOTCH1 and NOTCH2. J. Biol. Chem. 2020, 295, 14710–14722. [Google Scholar]
- Kakuda, S.; Haltiwanger, R.S. Deciphering the Fringe-Mediated Notch Code: Identification of Activating and Inhibiting Sites Allowing Discrimination between Ligands. Dev. Cell 2017, 40, 193–201. [Google Scholar]
- Moloney, D.J.; Panin, V.M.; Johnston, S.H.; Chen, J.; Shao, L.; Wilson, R.; Wang, Y.; Stanley, P.; Irvine, K.D.; Haltiwanger, R.S.; et al. Fringe is a glycosyltransferase that modifies Notch. Nature 2000, 406, 369–375. [Google Scholar]
- Brückner, K.; Perez, L.; Clausen, H.; Cohen, S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 2000, 406, 411–415. [Google Scholar]
- Moloney, D.J.; Shair, L.H.; Lu, F.M.; Xia, J.; Locke, R.; Matta, K.L.; Haltiwanger, R.S. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J. Biol. Chem. 2000, 275, 9604–9611. [Google Scholar]
- Chen, J.; Moloney, D.J.; Stanley, P. Fringe modulation of Jagged1-induced Notch signaling requires the action of beta 4galactosyltransferase-1. Proc. Natl. Acad. Sci. USA 2001, 98, 13716–13721. [Google Scholar] [PubMed]
- Luca, V.C.; Jude, K.M.; Pierce, N.W.; Nachury, M.V.; Fischer, S.; Garcia, K.C. Structural biology. Structural basis for Notch1 engagement of Delta-like 4. Science 2015, 347, 847–853. [Google Scholar] [PubMed]
- Luca, V.C.; Kim, B.C.; Ge, C.; Kakuda, S.; Wu, D.; Roein-Peikar, M.; Haltiwanger, R.S.; Zhu, C.; Ha, T.; Garcia, K.C. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science 2017, 355, 1320–1324. [Google Scholar] [PubMed]
- Taylor, P.; Takeuchi, H.; Sheppard, D.; Chillakuri, C.; Lea, S.M.; Haltiwanger, R.S.; Handford, P.A. Fringe-mediated extension of O-linked fucose in the ligand-binding region of Notch1 increases binding to mammalian Notch ligands. Proc. Natl. Acad. Sci. USA 2014, 111, 7290–7295. [Google Scholar]
- Wang, W.; Okajima, T.; Takeuchi, H. Significant Roles of Notch O-Glycosylation in Cancer. Molecules 2022, 27, 1783. [Google Scholar] [CrossRef]
- Saiki, W.; Ma, C.; Okajima, T.; Takeuchi, H. Current Views on the Roles of O-Glycosylation in Controlling Notch-Ligand Interactions. Biomolecules 2021, 11, 309. [Google Scholar] [CrossRef]
- Pandey, A.; Niknejad, N.; Jafar-Nejad, H. Multifaceted regulation of Notch signaling by glycosylation. Glycobiology 2021, 31, 8–28. [Google Scholar]
- Matsumoto, K.; Luther, K.B.; Haltiwanger, R.S. Diseases related to Notch glycosylation. Mol. Aspects Med. 2021, 79, 100938. [Google Scholar]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar]
- Boyden, S.E.; Desai, A.; Cruse, G.; Young, M.L.; Bolan, H.C.; Scott, L.M.; Eisch, A.R.; Long, R.D.; Lee, C.C.; Satorius, C.L.; et al. Vibratory Urticaria Associated with a Missense Variant in ADGRE2. N. Engl. J. Med. 2016, 374, 656–663. [Google Scholar]
- Kim, M.L.; Chandrasekharan, K.; Glass, M.; Shi, S.; Stahl, M.C.; Kaspar, B.; Stanley, P.; Martin, P.T. O-fucosylation of muscle agrin determines its ability to cluster acetylcholine receptors. Mol. Cell. Neurosci. 2008, 39, 452–464. [Google Scholar]
- Huzé, C.; Bauché, S.; Richard, P.; Chevessier, F.; Goillot, E.; Gaudon, K.; Ben Ammar, A.; Chaboud, A.; Grosjean, I.; Lecuyer, H.A.; et al. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am. J. Hum. Genet. 2009, 85, 155–167. [Google Scholar] [PubMed]
- Maselli, R.A.; Fernandez, J.M.; Arredondo, J.; Navarro, C.; Ngo, M.; Beeson, D.; Cagney, O.; Williams, D.C.; Wollmann, R.L.; Yarov-Yarovoy, V.; et al. LG2 agrin mutation causing severe congenital myasthenic syndrome mimics functional characteristics of non-neural (z-) agrin. Hum. Genet. 2012, 131, 1123–1135. [Google Scholar] [PubMed]
- Nicole, S.; Chaouch, A.; Torbergsen, T.; Bauché, S.; de Bruyckere, E.; Fontenille, M.J.; Horn, M.A.; van Ghelue, M.; Løseth, S.; Issop, Y.; et al. Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain 2014, 137 Pt 9, 2429–2443. [Google Scholar] [PubMed]
- Robinson, A.; Escuin, S.; Doudney, K.; Vekemans, M.; Stevenson, R.E.; Greene, N.D.; Copp, A.J.; Stanier, P. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum. Mutat. 2012, 33, 440–447. [Google Scholar]
- Gonzalez-Garay, M.L.; Aldrich, M.B.; Rasmussen, J.C.; Guilliod, R.; Lapinski, P.E.; King, P.D.; Sevick-Muraca, E.M. A novel mutation in CELSR1 is associated with hereditary lymphedema. Vasc. Cell 2016, 8, 1. [Google Scholar]
- Maltese, P.E.; Michelini, S.; Ricci, M.; Maitz, S.; Fiorentino, A.; Serrani, R.; Lazzerotti, A.; Bruson, A.; Paolacci, S.; Benedetti, S.; et al. Increasing evidence of hereditary lymphedema caused by CELSR1 loss-of-function variants. Am. J. Med. Genet. A 2019, 179, 1718–1724. [Google Scholar]
- Bamford, R.N.; Roessler, E.; Burdine, R.D.; Saplakoğlu, U.; dela Cruz, J.; Splitt, M.; Goodship, J.A.; Towbin, J.; Bowers, P.; Ferrero, G.B.; et al. Loss-of-function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat. Genet. 2000, 26, 365–369. [Google Scholar]
- Goldmuntz, E.; Bamford, R.; Karkera, J.D.; dela Cruz, J.; Roessler, E.; Muenke, M. CFC1 mutations in patients with transposition of the great arteries and double-outlet right ventricle. Am. J. Hum. Genet. 2002, 70, 776–780. [Google Scholar]
- den Hollander, A.I.; Heckenlively, J.R.; van den Born, L.I.; de Kok, Y.J.; van der Velde-Visser, S.D.; Kellner, U.; Jurklies, B.; van Schooneveld, M.J.; Blankenagel, A.; Rohrschneider, K.; et al. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am. J. Hum. Genet. 2001, 69, 198–203. [Google Scholar]
- den Hollander, A.I.; ten Brink, J.B.; de Kok, Y.J.; van Soest, S.; van den Born, L.I.; van Driel, M.A.; van de Pol, D.J.; Payne, A.M.; Bhattacharya, S.S.; Kellner, U.; et al. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat. Genet. 1999, 23, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Lotery, A.J.; Jacobson, S.G.; Fishman, G.A.; Weleber, R.G.; Fulton, A.B.; Namperumalsamy, P.; Héon, E.; Levin, A.V.; Grover, S.; Rosenow, J.R.; et al. Mutations in the CRB1 gene cause Leber congenital amaurosis. Arch. Ophthalmol. 2001, 119, 415–420. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.J.; Clarke, S.; Davis, J.A.; Simpson, D.A.; Silvestri, G. Pigmented paravenous chorioretinal atrophy is associated with a mutation within the crumbs homolog 1 (CRB1) gene. Investig. Ophthalmol. Vis. Sci. 2005, 46, 322–328. [Google Scholar] [CrossRef]
- Ebarasi, L.; Ashraf, S.; Bierzynska, A.; Gee, H.Y.; McCarthy, H.J.; Lovric, S.; Sadowski, C.E.; Pabst, W.; Vega-Warner, V.; Fang, H.; et al. Defects of CRB2 cause steroid-resistant nephrotic syndrome. Am. J. Hum. Genet. 2015, 96, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, C.; Yang, D.; Sun, R.; Wang, M.; Sun, H.; Xu, M.; Zhou, L.; Chen, M.; Xie, P.; et al. CRB2 mutation causes autosomal recessive retinitis pigmentosa. Exp. Eye Res. 2019, 180, 164–173. [Google Scholar] [CrossRef]
- Slavotinek, A.; Kaylor, J.; Pierce, H.; Cahr, M.; DeWard, S.J.; Schneidman-Duhovny, D.; Alsadah, A.; Salem, F.; Schmajuk, G.; Mehta, L. CRB2 mutations produce a phenotype resembling congenital nephrosis, Finnish type, with cerebral ventriculomegaly and raised alpha-fetoprotein. Am. J. Hum. Genet. 2015, 96, 162–169. [Google Scholar] [CrossRef]
- Yan, Y.T.; Liu, J.J.; Luo, Y.; E, C.; Haltiwanger, R.S.; Abate-Shen, C.; Shen, M.M. Dual roles of Cripto as a ligand and coreceptor in the nodal signaling pathway. Mol. Cell. Biol. 2002, 22, 4439–4449. [Google Scholar] [CrossRef]
- Schiffer, S.G.; Foley, S.; Kaffashan, A.; Hronowski, X.; Zichittella, A.E.; Yeo, C.Y.; Miatkowski, K.; Adkins, H.B.; Damon, B.; Whitman, M.; et al. Fucosylation of Cripto is required for its ability to facilitate nodal signaling. J. Biol. Chem. 2001, 276, 37769–37778. [Google Scholar] [CrossRef]
- Aminoff, M.; Carter, J.E.; Chadwick, R.B.; Johnson, C.; Gräsbeck, R.; Abdelaal, M.A.; Broch, H.; Jenner, L.B.; Verroust, P.J.; Moestrup, S.K.; et al. Mutations in CUBN, encoding the intrinsic factor-vitamin B12 receptor, cubilin, cause hereditary megaloblastic anaemia 1. Nat. Genet. 1999, 21, 309–313. [Google Scholar] [CrossRef]
- Kristiansen, M.; Aminoff, M.; Jacobsen, C.; de La Chapelle, A.; Krahe, R.; Verroust, P.J.; Moestrup, S.K. Cubilin P1297L mutation associated with hereditary megaloblastic anemia 1 causes impaired recognition of intrinsic factor-vitamin B(12) by cubilin. Blood 2000, 96, 405–409. [Google Scholar] [CrossRef]
- Storm, T.; Zeitz, C.; Cases, O.; Amsellem, S.; Verroust, P.J.; Madsen, M.; Benoist, J.F.; Passemard, S.; Lebon, S.; Jønsson, I.M.; et al. Detailed investigations of proximal tubular function in Imerslund-Gräsbeck syndrome. BMC Med. Genet. 2013, 14, 111. [Google Scholar]
- Bedin, M.; Boyer, O.; Servais, A.; Li, Y.; Villoing-Gaudé, L.; Tête, M.J.; Cambier, A.; Hogan, J.; Baudouin, V.; Krid, S.; et al. Human C-terminal CUBN variants associate with chronic proteinuria and normal renal function. J. Clin. Investig. 2020, 130, 335–344. [Google Scholar]
- Krogh, T.N.; Bachmann, E.; Teisner, B.; Skjødt, K.; Højrup, P. Glycosylation analysis and protein structure determination of murine fetal antigen 1 (mFA1)--the circulating gene product of the delta-like protein (dlk), preadipocyte factor 1 (Pref-1) and stromal-cell-derived protein 1 (SCP-1) cDNAs. Eur. J. Biochem. 1997, 244, 334–342. [Google Scholar] [PubMed]
- Fischer-Zirnsak, B.; Segebrecht, L.; Schubach, M.; Charles, P.; Alderman, E.; Brown, K.; Cadieux-Dion, M.; Cartwright, T.; Chen, Y.; Costin, C.; et al. Haploinsufficiency of the Notch Ligand DLL1 Causes Variable Neurodevelopmental Disorders. Am. J. Hum. Genet. 2019, 105, 631–639. [Google Scholar] [PubMed]
- Serth, K.; Schuster-Gossler, K.; Kremmer, E.; Hansen, B.; Marohn-Köhn, B.; Gossler, A. O-fucosylation of DLL3 is required for its function during somitogenesis. PLoS ONE 2015, 10, e0123776. [Google Scholar]
- Bulman, M.P.; Kusumi, K.; Frayling, T.M.; McKeown, C.; Garrett, C.; Lander, E.S.; Krumlauf, R.; Hattersley, A.T.; Ellard, S.; Turnpenny, P.D. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat. Genet. 2000, 24, 438–441. [Google Scholar]
- Meester, J.A.; Southgate, L.; Stittrich, A.B.; Venselaar, H.; Beekmans, S.J.; den Hollander, N.; Bijlsma, E.K.; Helderman-van den Enden, A.; Verheij, J.B.; Glusman, G.; et al. Heterozygous Loss-of-Function Mutations in DLL4 Cause Adams-Oliver Syndrome. Am. J. Hum. Genet. 2015, 97, 475–482. [Google Scholar]
- Schürpf, T.; Chen, Q.; Liu, J.H.; Wang, R.; Springer, T.A.; Wang, J.H. The RGD finger of Del-1 is a unique structural feature critical for integrin binding. FASEB J. 2012, 26, 3412–3420. [Google Scholar]
- Hucthagowder, V.; Sausgruber, N.; Kim, K.H.; Angle, B.; Marmorstein, L.Y.; Urban, Z. Fibulin-4: A novel gene for an autosomal recessive cutis laxa syndrome. Am. J. Hum. Genet. 2006, 78, 1075–1080. [Google Scholar]
- Groenestege, W.M.; Thébault, S.; van der Wijst, J.; van den Berg, D.; Janssen, R.; Tejpar, S.; van den Heuvel, L.P.; van Cutsem, E.; Hoenderop, J.G.; Knoers, N.V.; et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J. Clin. Investig. 2007, 117, 2260–2267. [Google Scholar]
- Abd El-Aziz, M.M.; Barragan, I.; O’Driscoll, C.A.; Goodstadt, L.; Prigmore, E.; Borrego, S.; Mena, M.; Pieras, J.I.; El-Ashry, M.F.; Safieh, L.A.; et al. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat. Genet. 2008, 40, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Collin, R.W.; Littink, K.W.; Klevering, B.J.; van den Born, L.I.; Koenekoop, R.K.; Zonneveld, M.N.; Blokland, E.A.; Strom, T.M.; Hoyng, C.B.; den Hollander, A.I.; et al. Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am. J. Hum. Genet. 2008, 83, 594–603. [Google Scholar] [PubMed]
- Hao, X.; Cheng, X.; Ye, J.; Wang, Y.; Yang, L.; Wang, M.; Jin, Y. Severe coagulation factor VII deficiency caused by a novel homozygous mutation (p. Trp284Gly) in loop 140s. Blood Coagul. Fibrinolysis 2016, 27, 461–463. [Google Scholar] [PubMed]
- O’Brien, D.P.; Gale, K.M.; Anderson, J.S.; McVey, J.H.; Miller, G.J.; Meade, T.W.; Tuddenham, E.G. Purification and characterization of factor VII 304-Gln: A variant molecule with reduced activity isolated from a clinically unaffected male. Blood 1991, 78, 132–140. [Google Scholar]
- Wulff, K.; Herrmann, F.H. Twenty two novel mutations of the factor VII gene in factor VII deficiency. Hum. Mutat. 2000, 15, 489–496. [Google Scholar]
- Nishimura, H.; Takao, T.; Hase, S.; Shimonishi, Y.; Iwanaga, S. Human factor IX has a tetrasaccharide O-glycosidically linked to serine 61 through the fucose residue. J. Biol. Chem. 1992, 267, 17520–17525. [Google Scholar]
- Bertina, R.M.; van der Linden, I.K.; Mannucci, P.M.; Reinalda-Poot, H.H.; Cupers, R.; Poort, S.R.; Reitsma, P.H. Mutations in hemophilia Bm occur at the Arg180-Val activation site or in the catalytic domain of factor IX. J. Biol. Chem. 1990, 265, 10876–10883. [Google Scholar]
- Ludwig, M.; Sabharwal, A.K.; Brackmann, H.H.; Olek, K.; Smith, K.J.; Birktoft, J.J.; Bajaj, S.P. Hemophilia B caused by five different nondeletion mutations in the protease domain of factor IX. Blood 1992, 79, 1225–1232. [Google Scholar]
- Simioni, P.; Tormene, D.; Tognin, G.; Gavasso, S.; Bulato, C.; Iacobelli, N.P.; Finn, J.D.; Spiezia, L.; Radu, C.; Arruda, V.R. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N. Engl. J. Med. 2009, 361, 1671–1675. [Google Scholar]
- Chu, K.; Wu, S.M.; Stanley, T.; Stafford, D.W.; High, K.A. A mutation in the propeptide of Factor IX leads to warfarin sensitivity by a novel mechanism. J. Clin. Investig. 1996, 98, 1619–1625. [Google Scholar]
- Bernardi, F.; Marchetti, G.; Patracchini, P.; del Senno, L.; Tripodi, M.; Fantoni, A.; Bartolai, S.; Vannini, F.; Felloni, L.; Rossi, L.; et al. Factor XII gene alteration in Hageman trait detected by TaqI restriction enzyme. Blood 1987, 69, 1421–1424. [Google Scholar] [PubMed]
- Schloesser, M.; Hofferbert, S.; Bartz, U.; Lutze, G.; Lämmle, B.; Engel, W. The novel acceptor splice site mutation 11396(G-->A) in the factor XII gene causes a truncated transcript in cross-reacting material negative patients. Hum. Mol. Genet. 1995, 4, 1235–1237. [Google Scholar] [PubMed]
- Cichon, S.; Martin, L.; Hennies, H.C.; Müller, F.; Van Driessche, K.; Karpushova, A.; Stevens, W.; Colombo, R.; Renné, T.; Drouet, C.; et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am. J. Hum. Genet. 2006, 79, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Dewald, G.; Bork, K. Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem. Biophys. Res. Commun. 2006, 343, 1286–1289. [Google Scholar]
- Nibbeling, E.A.R.; Duarri, A.; Verschuuren-Bemelmans, C.C.; Fokkens, M.R.; Karjalainen, J.M.; Smeets, C.; de Boer-Bergsma, J.J.; van der Vries, G.; Dooijes, D.; Bampi, G.B.; et al. Exome sequencing and network analysis identifies shared mechanisms underlying spinocerebellar ataxia. Brain 2017, 140, 2860–2878. [Google Scholar] [CrossRef]
- Cappello, S.; Gray, M.J.; Badouel, C.; Lange, S.; Einsiedler, M.; Srour, M.; Chitayat, D.; Hamdan, F.F.; Jenkins, Z.A.; Morgan, T.; et al. Mutations in genes encoding the cadherin receptor-ligand pair DCHS1 and FAT4 disrupt cerebral cortical development. Nat. Genet. 2013, 45, 1300–1308. [Google Scholar] [CrossRef]
- Alders, M.; Al-Gazali, L.; Cordeiro, I.; Dallapiccola, B.; Garavelli, L.; Tuysuz, B.; Salehi, F.; Haagmans, M.A.; Mook, O.R.; Majoie, C.B.; et al. Hennekam syndrome can be caused by FAT4 mutations and be allelic to Van Maldergem syndrome. Hum. Genet. 2014, 133, 1161–1167. [Google Scholar]
- Debeer, P.; Schoenmakers, E.F.; Twal, W.O.; Argraves, W.S.; De Smet, L.; Fryns, J.P.; Van De Ven, W.J. The fibulin-1 gene (FBLN1) is disrupted in a t(12;22) associated with a complex type of synpolydactyly. J. Med. Genet. 2002, 39, 98–104. [Google Scholar] [CrossRef]
- Greene, L.M.; Twal, W.O.; Duffy, M.J.; McDermott, E.W.; Hill, A.D.; O’Higgins, N.J.; McCann, A.H.; Dervan, P.A.; Argraves, W.S.; Gallagher, W.M. Elevated expression and altered processing of fibulin-1 protein in human breast cancer. Br. J. Cancer 2003, 88, 871–878. [Google Scholar]
- Auer-Grumbach, M.; Weger, M.; Fink-Puches, R.; Papić, L.; Fröhlich, E.; Auer-Grumbach, P.; El Shabrawi-Caelen, L.; Schabhüttl, M.; Windpassinger, C.; Senderek, J.; et al. Fibulin-5 mutations link inherited neuropathies, age-related macular degeneration and hyperelastic skin. Brain 2011, 134 Pt 6, 1839–1852. [Google Scholar]
- Markova, D.; Zou, Y.; Ringpfeil, F.; Sasaki, T.; Kostka, G.; Timpl, R.; Uitto, J.; Chu, M.L. Genetic heterogeneity of cutis laxa: A heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am. J. Hum. Genet. 2003, 72, 998–1004. [Google Scholar] [PubMed]
- Loeys, B.; Van Maldergem, L.; Mortier, G.; Coucke, P.; Gerniers, S.; Naeyaert, J.M.; De Paepe, A. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum. Mol. Genet. 2002, 11, 2113–2118. [Google Scholar] [PubMed]
- Stone, E.M.; Braun, T.A.; Russell, S.R.; Kuehn, M.H.; Lotery, A.J.; Moore, P.A.; Eastman, C.G.; Casavant, T.L.; Sheffield, V.C. Missense variations in the fibulin 5 gene and age-related macular degeneration. N. Engl. J. Med. 2004, 351, 346–353. [Google Scholar]
- Neupane, S.; Williamson, D.B.; Roth, R.A.; Halabi, C.M.; Haltiwanger, R.S.; Holdener, B.C. Poglut2/3 double knockout in mice results in neonatal lethality with reduced levels of fibrillin in lung tissues. J. Biol. Chem. 2024, 300, 107445. [Google Scholar]
- Dietz, H.C.; Cutting, G.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.G.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991, 352, 337–339. [Google Scholar] [PubMed]
- Dietz, H.C.; Saraiva, J.M.; Pyeritz, R.E.; Cutting, G.R.; Francomano, C.A. Clustering of fibrillin (FBN1) missense mutations in Marfan syndrome patients at cysteine residues in EGF-like domains. Hum. Mutat. 1992, 1, 366–374. [Google Scholar]
- Lönnqvist, L.; Child, A.; Kainulainen, K.; Davidson, R.; Puhakka, L.; Peltonen, L. A novel mutation of the fibrillin gene causing ectopia lentis. Genomics 1994, 19, 573–576. [Google Scholar]
- Faivre, L.; Gorlin, R.J.; Wirtz, M.K.; Godfrey, M.; Dagoneau, N.; Samples, J.R.; Le Merrer, M.; Collod-Beroud, G.; Boileau, C.; Munnich, A.; et al. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J. Med. Genet. 2003, 40, 34–36. [Google Scholar]
- Glesby, M.J.; Pyeritz, R.E. Association of mitral valve prolapse and systemic abnormalities of connective tissue. A phenotypic continuum. JAMA 1989, 262, 523–528. [Google Scholar]
- Loeys, B.L.; Gerber, E.E.; Riegert-Johnson, D.; Iqbal, S.; Whiteman, P.; McConnell, V.; Chillakuri, C.R.; Macaya, D.; Coucke, P.J.; De Paepe, A.; et al. Mutations in fibrillin-1 cause congenital scleroderma: Stiff skin syndrome. Sci. Transl. Med. 2010, 2, 23ra20. [Google Scholar]
- Le Goff, C.; Mahaut, C.; Wang, L.W.; Allali, S.; Abhyankar, A.; Jensen, S.; Zylberberg, L.; Collod-Beroud, G.; Bonnet, D.; Alanay, Y.; et al. Mutations in the TGFβ binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am. J. Hum. Genet. 2011, 89, 7–14. [Google Scholar] [PubMed]
- Graul-Neumann, L.M.; Kienitz, T.; Robinson, P.N.; Baasanjav, S.; Karow, B.; Gillessen-Kaesbach, G.; Fahsold, R.; Schmidt, H.; Hoffmann, K.; Passarge, E. Marfan syndrome with neonatal progeroid syndrome-like lipodystrophy associated with a novel frameshift mutation at the 3’ terminus of the FBN1-gene. Am. J. Med. Genet. A 2010, 152, 2749–2755. [Google Scholar]
- Putnam, E.A.; Zhang, H.; Ramirez, F.; Milewicz, D.M. Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat. Genet. 1995, 11, 456–458. [Google Scholar] [PubMed]
- Ratnapriya, R.; Zhan, X.; Fariss, R.N.; Branham, K.E.; Zipprer, D.; Chakarova, C.F.; Sergeev, Y.V.; Campos, M.M.; Othman, M.; Friedman, J.S.; et al. Rare and common variants in extracellular matrix gene Fibrillin 2 (FBN2) are associated with macular degeneration. Hum. Mol. Genet. 2014, 23, 5827–5837. [Google Scholar]
- Gara, S.K.; Jia, L.; Merino, M.J.; Agarwal, S.K.; Zhang, L.; Cam, M.; Patel, D.; Kebebew, E. Germline HABP2 Mutation Causing Familial Nonmedullary Thyroid Cancer. N. Engl. J. Med. 2015, 373, 448–455. [Google Scholar]
- Willeit, J.; Kiechl, S.; Weimer, T.; Mair, A.; Santer, P.; Wiedermann, C.J.; Roemisch, J. Marburg I polymorphism of factor VII--activating protease: A prominent risk predictor of carotid stenosis. Circulation 2003, 107, 667–670. [Google Scholar]
- Nicole, S.; Davoine, C.S.; Topaloglu, H.; Cattolico, L.; Barral, D.; Beighton, P.; Hamida, C.B.; Hammouda, H.; Cruaud, C.; White, P.S.; et al. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz-Jampel syndrome (chondrodystrophic myotonia). Nat. Genet. 2000, 26, 480–483. [Google Scholar]
- Arikawa-Hirasawa, E.; Wilcox, W.R.; Le, A.H.; Silverman, N.; Govindraj, P.; Hassell, J.R.; Yamada, Y. Dyssegmental dysplasia, Silverman-Handmaker type, is caused by functional null mutations of the perlecan gene. Nat. Genet. 2001, 27, 431–434. [Google Scholar]
- Thakurdas, S.M.; Lopez, M.F.; Kakuda, S.; Fernandez-Valdivia, R.; Zarrin-Khameh, N.; Haltiwanger, R.S.; Jafar-Nejad, H. Jagged1 heterozygosity in mice results in a congenital cholangiopathy which is reversed by concomitant deletion of one copy of Poglut1 (Rumi). Hepatology 2016, 63, 550–565. [Google Scholar]
- Li, L.; Krantz, I.D.; Deng, Y.; Genin, A.; Banta, A.B.; Collins, C.C.; Qi, M.; Trask, B.J.; Kuo, W.L.; Cochran, J.; et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat. Genet. 1997, 16, 243–251. [Google Scholar]
- Eldadah, Z.A.; Hamosh, A.; Biery, N.J.; Montgomery, R.A.; Duke, M.; Elkins, R.; Dietz, H.C. Familial Tetralogy of Fallot caused by mutation in the jagged1 gene. Hum. Mol. Genet. 2001, 10, 163–169. [Google Scholar] [PubMed]
- Le Caignec, C.; Lefevre, M.; Schott, J.J.; Chaventre, A.; Gayet, M.; Calais, C.; Moisan, J.P. Familial deafness, congenital heart defects, and posterior embryotoxon caused by cysteine substitution in the first epidermal-growth-factor-like domain of jagged 1. Am. J. Hum. Genet. 2002, 71, 180–186. [Google Scholar] [PubMed]
- Sullivan, J.M.; Motley, W.W.; Johnson, J.O.; Aisenberg, W.H.; Marshall, K.L.; Barwick, K.E.; Kong, L.; Huh, J.S.; Saavedra-Rivera, P.C.; McEntagart, M.M.; et al. Dominant mutations of the Notch ligand Jagged1 cause peripheral neuropathy. J. Clin. Investig. 2020, 130, 1506–1512. [Google Scholar] [PubMed]
- Coppens, S.; Barnard, A.M.; Puusepp, S.; Pajusalu, S.; Õunap, K.; Vargas-Franco, D.; Bruels, C.C.; Donkervoort, S.; Pais, L.; Chao, K.R.; et al. A form of muscular dystrophy associated with pathogenic variants in JAG2. Am. J. Hum. Genet. 2021, 108, 840–856. [Google Scholar]
- Klar, J.; Schuster, J.; Khan, T.N.; Jameel, M.; Mäbert, K.; Forsberg, L.; Baig, S.A.; Baig, S.M.; Dahl, N. Whole exome sequencing identifies LRP1 as a pathogenic gene in autosomal recessive keratosis pilaris atrophicans. J. Med. Genet. 2015, 52, 599–606. [Google Scholar]
- Yan, W.; Zheng, L.; Xu, X.; Hao, Z.; Zhang, Y.; Lu, J.; Sun, Z.; Dai, J.; Shi, D.; Guo, B.; et al. Heterozygous LRP1 deficiency causes developmental dysplasia of the hip by impairing triradiate chondrocytes differentiation due to inhibition of autophagy. Proc. Natl. Acad. Sci. USA 2022, 119, e2203557119. [Google Scholar]
- Ali, M.; McKibbin, M.; Booth, A.; Parry, D.A.; Jain, P.; Riazuddin, S.A.; Hejtmancik, J.F.; Khan, S.N.; Firasat, S.; Shires, M.; et al. Null mutations in LTBP2 cause primary congenital glaucoma. Am. J. Hum. Genet. 2009, 84, 664–671. [Google Scholar]
- Kumar, A.; Duvvari, M.R.; Prabhakaran, V.C.; Shetty, J.S.; Murthy, G.J.; Blanton, S.H. A homozygous mutation in LTBP2 causes isolated microspherophakia. Hum. Genet. 2010, 128, 365–371. [Google Scholar]
- Haji-Seyed-Javadi, R.; Jelodari-Mamaghani, S.; Paylakhi, S.H.; Yazdani, S.; Nilforushan, N.; Fan, J.B.; Klotzle, B.; Mahmoudi, M.J.; Ebrahimian, M.J.; Chelich, N.; et al. LTBP2 mutations cause Weill-Marchesani and Weill-Marchesani-like syndrome and affect disruptions in the extracellular matrix. Hum. Mutat. 2012, 33, 1182–1187. [Google Scholar]
- Twigg, S.R.; Lloyd, D.; Jenkins, D.; Elçioglu, N.E.; Cooper, C.D.; Al-Sannaa, N.; Annagür, A.; Gillessen-Kaesbach, G.; Hüning, I.; Knight, S.J.; et al. Mutations in multidomain protein MEGF8 identify a Carpenter syndrome subtype associated with defective lateralization. Am. J. Hum. Genet. 2012, 91, 897–905. [Google Scholar]
- Logan, C.V.; Lucke, B.; Pottinger, C.; Abdelhamed, Z.A.; Parry, D.A.; Szymanska, K.; Diggle, C.P.; van Riesen, A.; Morgan, J.E.; Markham, G.; et al. Mutations in MEGF10, a regulator of satellite cell myogenesis, cause early onset myopathy, areflexia, respiratory distress and dysphagia (EMARDD). Nat. Genet. 2011, 43, 1189–1192. [Google Scholar] [PubMed]
- Boyden, S.E.; Mahoney, L.J.; Kawahara, G.; Myers, J.A.; Mitsuhashi, S.; Estrella, E.A.; Duncan, A.R.; Dey, F.; DeChene, E.T.; Blasko-Goehringer, J.M.; et al. Mutations in the satellite cell gene MEGF10 cause a recessive congenital myopathy with minicores. Neurogenetics 2012, 13, 115–124. [Google Scholar] [PubMed]
- Houlahan, C.B.; Kong, Y.; Johnston, B.; Cielesh, M.; Chau, T.H.; Fenwick, J.; Coleman, P.R.; Hao, H.; Haltiwanger, R.S.; Thaysen-Andersen, M.; et al. Analysis of the Healthy Platelet Proteome Identifies a New Form of Domain-Specific O-Fucosylation. Mol. Cell. Proteom. 2024, 23, 100717. [Google Scholar]
- Hayward, C.P.; Rivard, G.E.; Kane, W.H.; Drouin, J.; Zheng, S.; Moore, J.C.; Kelton, J.G. An autosomal dominant, qualitative platelet disorder associated with multimerin deficiency, abnormalities in platelet factor V, thrombospondin, von Willebrand factor, and fibrinogen and an epinephrine aggregation defect. Blood 1996, 87, 4967–4978. [Google Scholar]
- Kang, C.; Riazuddin, S.; Mundorff, J.; Krasnewich, D.; Friedman, P.; Mullikin, J.C.; Drayna, D. Mutations in the lysosomal enzyme-targeting pathway and persistent stuttering. N. Engl. J. Med. 2010, 362, 677–685. [Google Scholar]
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005, 437, 270–274. [Google Scholar]
- Stittrich, A.B.; Lehman, A.; Bodian, D.L.; Ashworth, J.; Zong, Z.; Li, H.; Lam, P.; Khromykh, A.; Iyer, R.K.; Vockley, J.G.; et al. Mutations in NOTCH1 cause Adams-Oliver syndrome. Am. J. Hum. Genet. 2014, 95, 275–284. [Google Scholar]
- Shimizu, K.; Chiba, S.; Saito, T.; Kumano, K.; Takahashi, T.; Hirai, H. Manic fringe and lunatic fringe modify different sites of the Notch2 extracellular region, resulting in different signaling modulation. J. Biol. Chem. 2001, 276, 25753–25758. [Google Scholar]
- McDaniell, R.; Warthen, D.M.; Sanchez-Lara, P.A.; Pai, A.; Krantz, I.D.; Piccoli, D.A.; Spinner, N.B. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am. J. Hum. Genet. 2006, 79, 169–173. [Google Scholar]
- Simpson, M.A.; Irving, M.D.; Asilmaz, E.; Gray, M.J.; Dafou, D.; Elmslie, F.V.; Mansour, S.; Holder, S.E.; Brain, C.E.; Burton, B.K.; et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat. Genet. 2011, 43, 303–305. [Google Scholar]
- Fiddes, I.T.; Lodewijk, G.A.; Mooring, M.; Bosworth, C.M.; Ewing, A.D.; Mantalas, G.L.; Novak, A.M.; van den Bout, A.; Bishara, A.; Rosenkrantz, J.L.; et al. Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell 2018, 173, 1356–1369.e22. [Google Scholar] [PubMed]
- Tian, Y.; Wang, J.L.; Huang, W.; Zeng, S.; Jiao, B.; Liu, Z.; Chen, Z.; Li, Y.; Wang, Y.; Min, H.X.; et al. Expansion of Human-Specific GGC Repeat in Neuronal Intranuclear Inclusion Disease-Related Disorders. Am. J. Hum. Genet. 2019, 105, 166–176. [Google Scholar] [PubMed]
- Ishiura, H.; Shibata, S.; Yoshimura, J.; Suzuki, Y.; Qu, W.; Doi, K.; Almansour, M.A.; Kikuchi, J.K.; Taira, M.; Mitsui, J.; et al. Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nat. Genet. 2019, 51, 1222–1232. [Google Scholar] [PubMed]
- Sone, J.; Mitsuhashi, S.; Fujita, A.; Mizuguchi, T.; Hamanaka, K.; Mori, K.; Koike, H.; Hashiguchi, A.; Takashima, H.; Sugiyama, H.; et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat. Genet. 2019, 51, 1215–1221. [Google Scholar]
- Ogasawara, M.; Iida, A.; Kumutpongpanich, T.; Ozaki, A.; Oya, Y.; Konishi, H.; Nakamura, A.; Abe, R.; Takai, H.; Hanajima, R.; et al. CGG expansion in NOTCH2NLC is associated with oculopharyngodistal myopathy with neurological manifestations. Acta Neuropathol. Commun. 2020, 8, 204. [Google Scholar]
- Arboleda-Velasquez, J.F.; Rampal, R.; Fung, E.; Darland, D.C.; Liu, M.; Martinez, M.C.; Donahue, C.P.; Navarro-Gonzalez, M.F.; Libby, P.; D’Amore, P.A.; et al. CADASIL mutations impair Notch3 glycosylation by Fringe. Hum. Mol. Genet. 2005, 14, 1631–1639. [Google Scholar]
- Dichgans, M.; Filippi, M.; Brüning, R.; Iannucci, G.; Berchtenbreiter, C.; Minicucci, L.; Uttner, I.; Crispin, A.; Ludwig, H.; Gasser, T.; et al. Quantitative MRI in CADASIL: Correlation with disability and cognitive performance. Neurology 1999, 52, 1361–1367. [Google Scholar]
- Joutel, A.; Vahedi, K.; Corpechot, C.; Troesch, A.; Chabriat, H.; Vayssière, C.; Cruaud, C.; Maciazek, J.; Weissenbach, J.; Bousser, M.G.; et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet 1997, 350, 1511–1515. [Google Scholar]
- Martignetti, J.A.; Tian, L.; Li, D.; Ramirez, M.C.; Camacho-Vanegas, O.; Camacho, S.C.; Guo, Y.; Zand, D.J.; Bernstein, A.M.; Masur, S.K.; et al. Mutations in PDGFRB cause autosomal-dominant infantile myofibromatosis. Am. J. Hum. Genet. 2013, 92, 1001–1007. [Google Scholar]
- Gripp, K.W.; Robbins, K.M.; Sobreira, N.L.; Witmer, P.D.; Bird, L.M.; Avela, K.; Makitie, O.; Alves, D.; Hogue, J.S.; Zackai, E.H.; et al. Truncating mutations in the last exon of NOTCH3 cause lateral meningocele syndrome. Am. J. Med. Genet. A 2015, 167, 271–281. [Google Scholar]
- Abu-Libdeh, B.; Ashhab, M.; Shahrour, M.; Daana, M.; Dudin, A.; Elpeleg, O.; Edvardson, S.; Harel, T. Homozygous frameshift variant in NTNG2, encoding a synaptic cell adhesion molecule, in individuals with developmental delay, hypotonia, and autistic features. Neurogenetics 2019, 20, 209–213. [Google Scholar]
- Dias, C.M.; Punetha, J.; Zheng, C.; Mazaheri, N.; Rad, A.; Efthymiou, S.; Petersen, A.; Dehghani, M.; Pehlivan, D.; Partlow, J.N.; et al. Homozygous Missense Variants in NTNG2, Encoding a Presynaptic Netrin-G2 Adhesion Protein, Lead to a Distinct Neurodevelopmental Disorder. Am. J. Hum. Genet. 2019, 105, 1048–1056. [Google Scholar] [PubMed]
- Pennarubia, F.; Germot, A.; Pinault, E.; Maftah, A.; Legardinier, S. The single EGF-like domain of mouse PAMR1 is modified by O-Glucose, O-Fucose and O-GlcNAc. Glycobiology 2021, 31, 55–68. [Google Scholar] [PubMed]
- Elenbaas, J.S.; Pudupakkam, U.; Ashworth, K.J.; Kang, C.J.; Patel, V.; Santana, K.; Jung, I.-H.; Lee, P.C.; Burks, K.H.; Amrute, J.M.; et al. SVEP1 is an endogenous ligand for the orphan receptor PEAR1. Nat. Commun. 2023, 14, 850. [Google Scholar] [PubMed]
- Byeon, I.J.; Llinás, M. Solution structure of the tissue-type plasminogen activator kringle 2 domain complexed to 6-aminohexanoic acid an antifibrinolytic drug. J. Mol. Biol. 1991, 222, 1035–1051. [Google Scholar]
- Paterson, A.D.; Rommens, J.M.; Bharaj, B.; Blavignac, J.; Wong, I.; Diamandis, M.; Waye, J.S.; Rivard, G.E.; Hayward, C.P. Persons with Quebec platelet disorder have a tandem duplication of PLAU, the urokinase plasminogen activator gene. Blood 2010, 115, 1264–1266. [Google Scholar]
- Romeo, G.; Hassan, H.J.; Staempfli, S.; Roncuzzi, L.; Cianetti, L.; Leonardi, A.; Vicente, V.; Mannucci, P.M.; Bertina, R.; Peschle, C.; et al. Hereditary thrombophilia: Identification of nonsense and missense mutations in the protein C gene. Proc. Natl. Acad. Sci. USA 1987, 84, 2829–2832. [Google Scholar]
- Grundy, C.B.; Chisholm, M.; Kakkar, V.V.; Cooper, D.N. A novel homozygous missense mutation in the protein C (PROC) gene causing recurrent venous thrombosis. Hum. Genet. 1992, 89, 683–684. [Google Scholar]
- Hong, S.E.; Shugart, Y.Y.; Huang, D.T.; Shahwan, S.A.; Grant, P.E.; Hourihane, J.O.; Martin, N.D.; Walsh, C.A. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat. Genet. 2000, 26, 93–96. [Google Scholar]
- Dazzo, E.; Fanciulli, M.; Serioli, E.; Minervini, G.; Pulitano, P.; Binelli, S.; Di Bonaventura, C.; Luisi, C.; Pasini, E.; Striano, S.; et al. Heterozygous reelin mutations cause autosomal-dominant lateral temporal epilepsy. Am. J. Hum. Genet. 2015, 96, 992–1000. [Google Scholar]
- Anastasio, N.; Ben-Omran, T.; Teebi, A.; Ha, K.C.; Lalonde, E.; Ali, R.; Almureikhi, M.; Der Kaloustian, V.M.; Liu, J.; Rosenblatt, D.S.; et al. Mutations in SCARF2 are responsible for Van Den Ende-Gupta syndrome. Am. J. Hum. Genet. 2010, 87, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Monfrini, E.; Pelucchi, S.; Hollmén, M.; Viitala, M.; Mariani, R.; Bertola, F.; Majore, S.; Di Fonzo, A.; Piperno, A. A form of inherited hyperferritinemia associated with bi-allelic pathogenic variants of STAB1. Am. J. Hum. Genet. 2023, 110, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Stitziel, N.O.; Stirrups, K.E.; Masca, N.G.; Erdmann, J.; Ferrario, P.G.; König, I.R.; Weeke, P.E.; Webb, T.R.; Auer, P.L.; Schick, U.M.; et al. Coding Variation in ANGPTL4, LPL, and SVEP1 and the Risk of Coronary Disease. N. Engl. J. Med. 2016, 374, 1134–1144. [Google Scholar] [PubMed]
- Jung, I.H.; Elenbaas, J.S.; Alisio, A.; Santana, K.; Young, E.P.; Kang, C.J.; Kachroo, P.; Lavine, K.J.; Razani, B.; Mecham, R.P.; et al. SVEP1 is a human coronary artery disease locus that promotes atherosclerosis. Sci. Transl. Med. 2021, 13, eabe0357. [Google Scholar] [CrossRef]
- Aldahmesh, M.A.; Mohammed, J.Y.; Al-Hazzaa, S.; Alkuraya, F.S. Homozygous null mutation in ODZ3 causes microphthalmia in humans. Genet. Med. 2012, 14, 900–904. [Google Scholar] [CrossRef]
- Chassaing, N.; Ragge, N.; Plaisancié, J.; Patat, O.; Geneviève, D.; Rivier, F.; Malrieu-Eliaou, C.; Hamel, C.; Kaplan, J.; Calvas, P. Confirmation of TENM3 involvement in autosomal recessive colobomatous microphthalmia. Am. J. Med. Genet. A 2016, 170, 1895–1898. [Google Scholar] [CrossRef]
- Hor, H.; Francescatto, L.; Bartesaghi, L.; Ortega-Cubero, S.; Kousi, M.; Lorenzo-Betancor, O.; Jiménez-Jiménez, F.J.; Gironell, A.; Clarimón, J.; Drechsel, O.; et al. Missense mutations in TENM4, a regulator of axon guidance and central myelination, cause essential tremor. Hum. Mol. Genet. 2015, 24, 5677–5686. [Google Scholar] [CrossRef]
- Michelini, S.; Ricci, M.; Veselenyiova, D.; Kenanoglu, S.; Kurti, D.; Baglivo, M.; Fiorentino, A.; Basha, S.H.; Priya, S.; Serrani, R.; et al. TIE1 as a Candidate Gene for Lymphatic Malformations with or without Lymphedema. Int. J. Mol. Sci. 2020, 21, 6780. [Google Scholar] [CrossRef]
- Hart, T.C.; Gorry, M.C.; Hart, P.S.; Woodard, A.S.; Shihabi, Z.; Sandhu, J.; Shirts, B.; Xu, L.; Zhu, H.; Barmada, M.M.; et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J. Med. Genet. 2002, 39, 882–892. [Google Scholar] [CrossRef]
- Rampoldi, L.; Caridi, G.; Santon, D.; Boaretto, F.; Bernascone, I.; Lamorte, G.; Tardanico, R.; Dagnino, M.; Colussi, G.; Scolari, F.; et al. Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Hum. Mol. Genet. 2003, 12, 3369–3384. [Google Scholar] [CrossRef]
- Miyamoto, T.; Inoue, H.; Sakamoto, Y.; Kudo, E.; Naito, T.; Mikawa, T.; Mikawa, Y.; Isashiki, Y.; Osabe, D.; Shinohara, S.; et al. Identification of a novel splice site mutation of the CSPG2 gene in a Japanese family with Wagner syndrome. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2726–2735. [Google Scholar]
- Kloeckener-Gruissem, B.; Neidhardt, J.; Magyar, I.; Plauchu, H.; Zech, J.C.; Morlé, L.; Palmer-Smith, S.M.; Macdonald, M.J.; Nas, V.; Fry, A.E.; et al. Novel VCAN mutations and evidence for unbalanced alternative splicing in the pathogenesis of Wagner syndrome. Eur. J. Hum. Genet. 2013, 21, 352–356. [Google Scholar] [PubMed]
- Gebauer, J.M.; Müller, S.; Hanisch, F.G.; Paulsson, M.; Wagener, R. O-glucosylation and O-fucosylation occur together in close proximity on the first epidermal growth factor repeat of AMACO (VWA2 protein). J. Biol. Chem. 2008, 283, 17846–17854. [Google Scholar] [PubMed]
- Hofsteenge, J.; Huwiler, K.G.; Macek, B.; Hess, D.; Lawler, J.; Mosher, D.F.; Peter-Katalinic, J. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J. Biol. Chem. 2001, 276, 6485–6498. [Google Scholar]
- Valero-González, J.; Leonhard-Melief, C.; Lira-Navarrete, E.; Jiménez-Osés, G.; Hernández-Ruiz, C.; Pallarés, M.C.; Yruela, I.; Vasudevan, D.; Lostao, A.; Corzana, F.; et al. A proactive role of water molecules in acceptor recognition by protein O-fucosyltransferase 2. Nat. Chem. Biol. 2016, 12, 240–246. [Google Scholar] [CrossRef]
- Ricketts, L.M.; Dlugosz, M.; Luther, K.B.; Haltiwanger, R.S.; Majerus, E.M. O-fucosylation is required for ADAMTS13 secretion. J. Biol. Chem. 2007, 282, 17014–17023. [Google Scholar]
- Wang, L.W.; Dlugosz, M.; Somerville, R.P.; Raed, M.; Haltiwanger, R.S.; Apte, S.S. O-fucosylation of thrombospondin type 1 repeats in ADAMTS-like-1/punctin-1 regulates secretion: Implications for the ADAMTS superfamily. J. Biol. Chem. 2007, 282, 17024–17031. [Google Scholar] [CrossRef]
- Adams, J.C.; Tucker, R.P. The thrombospondin type 1 repeat (TSR) superfamily: Diverse proteins with related roles in neuronal development. Dev. Dyn. 2000, 218, 280–299. [Google Scholar] [CrossRef]
- Leonhard-Melief, C.; Haltiwanger, R.S. O-fucosylation of thrombospondin type 1 repeats. Methods Enzymol. 2010, 480, 401–416. [Google Scholar]
- Shcherbakova, A.; Preller, M.; Taft, M.H.; Pujols, J.; Ventura, S.; Tiemann, B.; Buettner, F.F.; Bakker, H. C-mannosylation supports folding and enhances stability of thrombospondin repeats. eLife 2019, 8, e52978. [Google Scholar] [CrossRef]
- Furmanek, A.; Hofsteenge, J. Protein C-mannosylation: Facts and questions. Acta Biochim. Pol. 2000, 47, 781–789. [Google Scholar] [PubMed]
- Du, J.; Takeuchi, H.; Leonhard-Melief, C.; Shroyer, K.R.; Dlugosz, M.; Haltiwanger, R.S.; Holdener, B.C. O-fucosylation of thrombospondin type 1 repeats restricts epithelial to mesenchymal transition (EMT) and maintains epiblast pluripotency during mouse gastrulation. Dev. Biol. 2010, 346, 25–38. [Google Scholar] [PubMed]
- Benz, B.A.; Nandadasa, S.; Takeuchi, M.; Grady, R.C.; Takeuchi, H.; LoPilato, R.K.; Kakuda, S.; Somerville, R.P.T.; Apte, S.S.; Haltiwanger, R.S.; et al. Genetic and biochemical evidence that gastrulation defects in Pofut2 mutants result from defects in ADAMTS9 secretion. Dev. Biol. 2016, 416, 111–122. [Google Scholar] [PubMed]
- Lesnik Oberstein, S.A.; Kriek, M.; White, S.J.; Kalf, M.E.; Szuhai, K.; den Dunnen, J.T.; Breuning, M.H.; Hennekam, R.C. Peters Plus syndrome is caused by mutations in B3GALTL, a putative glycosyltransferase. Am. J. Hum. Genet. 2006, 79, 562–566. [Google Scholar]
- Colige, A.; Sieron, A.L.; Li, S.W.; Schwarze, U.; Petty, E.; Wertelecki, W.; Wilcox, W.; Krakow, D.; Cohn, D.H.; Reardon, W.; et al. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am. J. Hum. Genet. 1999, 65, 308–317. [Google Scholar]
- Brouillard, P.; Dupont, L.; Helaers, R.; Coulie, R.; Tiller, G.E.; Peeden, J.; Colige, A.; Vikkula, M. Loss of ADAMTS3 activity causes Hennekam lymphangiectasia-lymphedema syndrome 3. Hum. Mol. Genet. 2017, 26, 4095–4104. [Google Scholar]
- Wang, L.W.; Leonhard-Melief, C.; Haltiwanger, R.S.; Apte, S.S. Post-translational modification of thrombospondin type-1 repeats in ADAMTS-like 1/punctin-1 by C-mannosylation of tryptophan. J. Biol. Chem. 2009, 284, 30004–30015. [Google Scholar]
- Neupane, S.; Berardinelli, S.J.; Cameron, D.C.; Grady, R.C.; Komatsu, D.E.; Percival, C.J.; Takeuchi, M.; Ito, A.; Liu, T.W.; Nairn, A.V.; et al. O-fucosylation of thrombospondin type 1 repeats is essential for ECM remodeling and signaling during bone development. Matrix Biol. 2022, 107, 77–96. [Google Scholar]
- Dubail, J.; Vasudevan, D.; Wang, L.W.; Earp, S.E.; Jenkins, M.W.; Haltiwanger, R.S.; Apte, S.S. Impaired ADAMTS9 secretion: A potential mechanism for eye defects in Peters Plus Syndrome. Sci. Rep. 2016, 6, 33974. [Google Scholar]
- Dagoneau, N.; Benoist-Lasselin, C.; Huber, C.; Faivre, L.; Mégarbané, A.; Alswaid, A.; Dollfus, H.; Alembik, Y.; Munnich, A.; Legeai-Mallet, L.; et al. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am. J. Hum. Genet. 2004, 75, 801–806. [Google Scholar]
- Kutz, W.E.; Wang, L.W.; Dagoneau, N.; Odrcic, K.J.; Cormier-Daire, V.; Traboulsi, E.I.; Apte, S.S. Functional analysis of an ADAMTS10 signal peptide mutation in Weill-Marchesani syndrome demonstrates a long-range effect on secretion of the full-length enzyme. Hum. Mutat. 2008, 29, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.G.; Nichols, W.C.; Lian, E.C.; Foroud, T.; McClintick, J.N.; McGee, B.M.; Yang, A.Y.; Siemieniak, D.R.; Stark, K.R.; Gruppo, R.; et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 2001, 413, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Boschann, F.; Cogulu, M.; Pehlivan, D.; Balachandran, S.; Vallecillo-Garcia, P.; Grochowski, C.M.; Hansmeier, N.R.; Coban Akdemir, Z.H.; Prada-Medina, C.A.; Aykut, A.; et al. Biallelic variants in ADAMTS15 cause a novel form of distal arthrogryposis. Genet. Med. 2022, 24, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Hubmacher, D.; Schneider, M.; Berardinelli, S.J.; Takeuchi, H.; Willard, B.; Reinhardt, D.P.; Haltiwanger, R.S.; Apte, S.S. Unusual life cycle and impact on microfibril assembly of ADAMTS17, a secreted metalloprotease mutated in genetic eye disease. Sci. Rep. 2017, 7, 41871. [Google Scholar] [CrossRef]
- Morales, J.; Al-Sharif, L.; Khalil, D.S.; Shinwari, J.M.; Bavi, P.; Al-Mahrouqi, R.A.; Al-Rajhi, A.; Alkuraya, F.S.; Meyer, B.F.; Al Tassan, N. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am. J. Hum. Genet. 2009, 85, 558–568. [Google Scholar] [CrossRef]
- Aldahmesh, M.A.; Alshammari, M.J.; Khan, A.O.; Mohamed, J.Y.; Alhabib, F.A.; Alkuraya, F.S. The syndrome of microcornea, myopic chorioretinal atrophy, and telecanthus (MMCAT) is caused by mutations in ADAMTS18. Hum. Mutat. 2013, 34, 1195–1199. [Google Scholar] [CrossRef]
- Massadeh, S.; Alhashem, A.; van de Laar, I.; Alhabshan, F.; Ordonez, N.; Alawbathani, S.; Khan, S.; Kabbani, M.S.; Chaikhouni, F.; Sheereen, A.; et al. ADAMTS19-associated heart valve defects: Novel genetic variants consolidating a recognizable cardiac phenotype. Clin. Genet. 2020, 98, 56–63. [Google Scholar] [CrossRef]
- Wünnemann, F.; Ta-Shma, A.; Preuss, C.; Leclerc, S.; van Vliet, P.P.; Oneglia, A.; Thibeault, M.; Nordquist, E.; Lincoln, J.; Scharfenberg, F.; et al. Loss of ADAMTS19 causes progressive non-syndromic heart valve disease. Nat. Genet. 2020, 52, 40–47. [Google Scholar] [CrossRef]
- Holdener, B.C.; Percival, C.J.; Grady, R.C.; Cameron, D.C.; Berardinelli, S.J.; Zhang, A.; Neupane, S.; Takeuchi, M.; Jimenez-Vega, J.C.; Uddin, S.M.Z.; et al. ADAMTS9 and ADAMTS20 are differentially affected by loss of B3GLCT in mouse model of Peters plus syndrome. Hum. Mol. Genet. 2019, 28, 4053–4066. [Google Scholar] [CrossRef]
- Zhang, A.; Berardinelli, S.J.; Leonhard-Melief, C.; Vasudevan, D.; Liu, T.W.; Taibi, A.; Giannone, S.; Apte, S.S.; Holdener, B.C.; Haltiwanger, R.S. O-Fucosylation of ADAMTSL2 is required for secretion and is impacted by geleophysic dysplasia-causing mutations. J. Biol. Chem. 2020, 295, 15742–15753. [Google Scholar] [CrossRef]
- Allali, S.; Le Goff, C.; Pressac-Diebold, I.; Pfennig, G.; Mahaut, C.; Dagoneau, N.; Alanay, Y.; Brady, A.F.; Crow, Y.J.; Devriendt, K.; et al. Molecular screening of ADAMTSL2 gene in 33 patients reveals the genetic heterogeneity of geleophysic dysplasia. J. Med. Genet. 2011, 48, 417–421. [Google Scholar] [PubMed]
- Le Goff, C.; Morice-Picard, F.; Dagoneau, N.; Wang, L.W.; Perrot, C.; Crow, Y.J.; Bauer, F.; Flori, E.; Prost-Squarcioni, C.; Krakow, D.; et al. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation. Nat. Genet. 2008, 40, 1119–1123. [Google Scholar] [PubMed]
- Ahram, D.; Sato, T.S.; Kohilan, A.; Tayeh, M.; Chen, S.; Leal, S.; Al-Salem, M.; El-Shanti, H. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am. J. Hum. Genet. 2009, 84, 274–278. [Google Scholar] [PubMed]
- Christensen, A.E.; Fiskerstrand, T.; Knappskog, P.M.; Boman, H.; Rødahl, E. A novel ADAMTSL4 mutation in autosomal recessive ectopia lentis et pupillae. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6369–6373. [Google Scholar]
- Wang, J.; Miao, Y.; Wicklein, R.; Sun, Z.; Wang, J.; Jude, K.M.; Fernandes, R.A.; Merrill, S.A.; Wernig, M.; Garcia, K.C.; et al. RTN4/NoGo-receptor binding to BAI adhesion-GPCRs regulates neuronal development. Cell 2021, 184, 5869–5885.e25. [Google Scholar]
- Ikinciogullari, A.; Tekin, M.; Dogu, F.; Reisli, I.; Tanir, G.; Yi, Z.; Garrison, N.; Brilliant, M.H.; Babacan, E. Meningococccal meningitis and complement component 6 deficiency associated with oculocutaneous albinism. Eur. J. Pediatr. 2005, 164, 177–179. [Google Scholar] [CrossRef]
- Niwa, Y.; Suzuki, T.; Dohmae, N.; Simizu, S. O-Fucosylation of CCN1 is required for its secretion. FEBS Lett. 2015, 589, 3287–3293. [Google Scholar]
- Hurvitz, J.R.; Suwairi, W.M.; Van Hul, W.; El-Shanti, H.; Superti-Furga, A.; Roudier, J.; Holderbaum, D.; Pauli, R.M.; Herd, J.K.; Van Hul, E.V.; et al. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat. Genet. 1999, 23, 94–98. [Google Scholar] [CrossRef]
- Hess, D.; Keusch, J.J.; Oberstein, S.A.; Hennekam, R.C.; Hofsteenge, J. Peters Plus syndrome is a new congenital disorder of glycosylation and involves defective Omicron-glycosylation of thrombospondin type 1 repeats. J. Biol. Chem. 2008, 283, 7354–7360. [Google Scholar]
- Fredrikson, G.N.; Westberg, J.; Kuijper, E.J.; Tijssen, C.C.; Sjöholm, A.G.; Uhlén, M.; Truedsson, L. Molecular characterization of properdin deficiency type III: Dysfunction produced by a single point mutation in exon 9 of the structural gene causing a tyrosine to aspartic acid interchange. J. Immunol. 1996, 157, 3666–3671. [Google Scholar]
- Schultz, D.W.; Klein, M.L.; Humpert, A.J.; Luzier, C.W.; Persun, V.; Schain, M.; Mahan, A.; Runckel, C.; Cassera, M.; Vittal, V.; et al. Analysis of the ARMD1 locus: Evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum. Mol. Genet. 2003, 12, 3315–3323. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Peredo, A.; Klein, D.; Macek, B.; Hess, D.; Peter-Katalinic, J.; Hofsteenge, J. C-mannosylation and o-fucosylation of thrombospondin type 1 repeats. Mol. Cell. Proteom. 2002, 1, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Goto, J.; Berardinelli, S.J.; Ito, A.; Haltiwanger, R.S.; Holdener, B.C. Hydrocephalus in mouse B3glct mutants is likely caused by defects in multiple B3GLCT substrates in ependymal cells and subcommissural organ. Glycobiology 2021, 31, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Chiba, K.; Karasugi, T.; Nakajima, M.; Kawaguchi, Y.; Mikami, Y.; Furuichi, T.; Mio, F.; Miyake, A.; Miyamoto, T.; et al. A functional polymorphism in THBS2 that affects alternative splicing and MMP binding is associated with lumbar-disc herniation. Am. J. Hum. Genet. 2008, 82, 1122–1129. [Google Scholar] [CrossRef]
- Al Rawi, W.N.; Ibrahim, F.H.; El Nakeib, O.A.S.; Al Zidgali, F.M. Manifestations of thrombospondin type-1 domain-containing protein 1 gene mutation in an extremely premature infant with nonimmune hydrops fetalis. Am. J. Med. Genet. A 2021, 185, 1598–1601. [Google Scholar] [CrossRef]
- Shamseldin, H.E.; Tulbah, M.; Kurdi, W.; Nemer, M.; Alsahan, N.; Al Mardawi, E.; Khalifa, O.; Hashem, A.; Kurdi, A.; Babay, Z.; et al. Identification of embryonic lethal genes in humans by autozygosity mapping and exome sequencing in consanguineous families. Genome Biol. 2015, 16, 116. [Google Scholar] [CrossRef]
- Rui, Y.N.; Xu, Z.; Fang, X.; Menezes, M.R.; Balzeau, J.; Niu, A.; Hagan, J.P.; Kim, D.H. The Intracranial Aneurysm Gene THSD1 Connects Endosome Dynamics to Nascent Focal Adhesion Assembly. Cell Physiol. Biochem. 2017, 43, 2200–2211. [Google Scholar] [CrossRef]
- Santiago-Sim, T.; Fang, X.; Hennessy, M.L.; Nalbach, S.V.; DePalma, S.R.; Lee, M.S.; Greenway, S.C.; McDonough, B.; Hergenroeder, G.W.; Patek, K.J.; et al. THSD1 (Thrombospondin Type 1 Domain Containing Protein 1) Mutation in the Pathogenesis of Intracranial Aneurysm and Subarachnoid Hemorrhage. Stroke 2016, 47, 3005–3013. [Google Scholar] [CrossRef]
- Elbitar, S.; Renard, M.; Arnaud, P.; Hanna, N.; Jacob, M.P.; Guo, D.C.; Tsutsui, K.; Gross, M.S.; Kessler, K.; Tosolini, L.; et al. Pathogenic variants in THSD4, encoding the ADAMTS-like 6 protein, predispose to inherited thoracic aortic aneurysm. Genet. Med. 2021, 23, 111–122. [Google Scholar] [CrossRef]
- Seifert, L.; Hoxha, E.; Eichhoff, A.M.; Zahner, G.; Dehde, S.; Reinhard, L.; Koch-Nolte, F.; Stahl, R.A.K.; Tomas, N.M. The Most N-Terminal Region of THSD7A Is the Predominant Target for Autoimmunity in THSD7A-Associated Membranous Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 1536–1548. [Google Scholar] [CrossRef]
- Tomas, N.M.; Beck, L.H., Jr.; Meyer-Schwesinger, C.; Seitz-Polski, B.; Ma, H.; Zahner, G.; Dolla, G.; Hoxha, E.; Helmchen, U.; Dabert-Gay, A.S.; et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 2014, 371, 2277–2287. [Google Scholar] [PubMed]
- Roos, C.; Kolmer, M.; Mattila, P.; Renkonen, R. Composition of Drosophila melanogaster proteome involved in fucosylated glycan metabolism. J. Biol. Chem. 2002, 277, 3168–3175. [Google Scholar] [PubMed]
- Fabini, G.; Freilinger, A.; Altmann, F.; Wilson, I.B. Identification of core alpha 1,3-fucosylated glycans and cloning of the requisite fucosyltransferase cDNA from Drosophila melanogaster: Potential basis of the neural anti-horseadish peroxidase epitope. J. Biol. Chem. 2001, 276, 28058–28067. [Google Scholar] [PubMed]
- Rendic, D.; Linder, A.; Paschinger, K.; Borth, N.; Wilson, I.B.; Fabini, G. Modulation of neural carbohydrate epitope expression in Drosophila melanogaster cells. J. Biol. Chem. 2006, 281, 3343–3353. [Google Scholar]
- Baboval, T.; Smith, F.I. Comparison of human and mouse Fuc-TX and Fuc-TXI genes, and expression studies in the mouse. Mamm. Genome 2002, 13, 538–541. [Google Scholar]
- Patnaik, S. Characterization of Fut10 and Fut11, Putative Alpha-1–3/4 Fucosyltransferase Genes Important for Vertebrate Development. Nat. Preced. 2007. [Google Scholar] [CrossRef]
- Rendić, D.; Klaudiny, J.; Stemmer, U.; Schmidt, J.; Paschinger, K.; Wilson, I.B. Towards abolition of immunogenic structures in insect cells: Characterization of a honey-bee (Apis mellifera) multi-gene family reveals both an allergy-related core alpha1,3-fucosyltransferase and the first insect Lewis-histo-blood-group-related antigen-synthesizing enzyme. Biochem. J. 2007, 402, 105–115. [Google Scholar]
- Mollicone, R.; Moore, S.E.; Bovin, N.; Garcia-Rosasco, M.; Candelier, J.J.; Martinez-Duncker, I.; Oriol, R. Activity, splice variants, conserved peptide motifs, and phylogeny of two new alpha1,3-fucosyltransferase families (FUT10 and FUT11). J. Biol. Chem. 2009, 284, 4723–4738. [Google Scholar]
- Kumar, A.; Torii, T.; Ishino, Y.; Muraoka, D.; Yoshimura, T.; Togayachi, A.; Narimatsu, H.; Ikenaka, K.; Hitoshi, S. The Lewis X-related α1,3-fucosyltransferase, Fut10, is required for the maintenance of stem cell populations. J. Biol. Chem. 2013, 288, 28859–28868. [Google Scholar] [CrossRef]
- Capuano, A.; Bucciotti, F.; Farwell, K.D.; Tippin Davis, B.; Mroske, C.; Hulick, P.J.; Weissman, S.M.; Gao, Q.; Spessotto, P.; Colombatti, A.; et al. Diagnostic Exome Sequencing Identifies a Novel Gene, EMILIN1, Associated with Autosomal-Dominant Hereditary Connective Tissue Disease. Hum. Mutat. 2016, 37, 84–97. [Google Scholar]
- Iacomino, M.; Doliana, R.; Marchese, M.; Capuano, A.; Striano, P.; Spessotto, P.; Bosisio, G.; Iodice, R.; Manganelli, F.; Lanteri, P.; et al. Distal motor neuropathy associated with novel EMILIN1 mutation. Neurobiol. Dis. 2020, 137, 104757. [Google Scholar]
- Boutboul, S.; Black, G.C.; Moore, J.E.; Sinton, J.; Menasche, M.; Munier, F.L.; Laroche, L.; Abitbol, M.; Schorderet, D.F. A subset of patients with epithelial basement membrane corneal dystrophy have mutations in TGFBI/BIGH3. Hum. Mutat. 2006, 27, 553–557. [Google Scholar] [PubMed]
- Chakravarthi, S.V.; Kannabiran, C.; Sridhar, M.S.; Vemuganti, G.K. TGFBI gene mutations causing lattice and granular corneal dystrophies in Indian patients. Investig. Ophthalmol. Vis. Sci. 2005, 46, 121–125. [Google Scholar]
- Fujiki, K.; Hotta, Y.; Nakayasu, K.; Yokoyama, T.; Takano, T.; Yamaguchi, T.; Kanai, A. A new L527R mutation of the betaIGH3 gene in patients with lattice corneal dystrophy with deep stromal opacities. Hum. Genet. 1998, 103, 286–289. [Google Scholar]
- Rozzo, C.; Fossarello, M.; Galleri, G.; Sole, G.; Serru, A.; Orzalesi, N.; Serra, A.; Pirastu, M. A common beta ig-h3 gene mutation (delta f540) in a large cohort of Sardinian Reis Bücklers corneal dystrophy patients. Mutations in brief no. 180. Online. Hum. Mutat. 1998, 12, 215–216. [Google Scholar]
- Yamamoto, S.; Okada, M.; Tsujikawa, M.; Shimomura, Y.; Nishida, K.; Inoue, Y.; Watanabe, H.; Maeda, N.; Kurahashi, H.; Kinoshita, S.; et al. A kerato-epithelin (betaig-h3) mutation in lattice corneal dystrophy type IIIA. Am. J. Hum. Genet. 1998, 62, 719–722. [Google Scholar]
- Dupuy, F.; Germot, A.; Julien, R.; Maftah, A. Structure/function study of Lewis alpha3- and alpha3/4-fucosyltransferases: The alpha1,4 fucosylation requires an aromatic residue in the acceptor-binding domain. Glycobiology 2004, 14, 347–356. [Google Scholar]
- Dupuy, F.; Germot, A.; Marenda, M.; Oriol, R.; Blancher, A.; Julien, R.; Maftah, A. Alpha1,4-fucosyltransferase activity: A significant function in the primate lineage has appeared twice independently. Mol. Biol. Evol. 2002, 19, 815–824. [Google Scholar]
- Dupuy, F.; Petit, J.M.; Mollicone, R.; Oriol, R.; Julien, R.; Maftah, A. A single amino acid in the hypervariable stem domain of vertebrate alpha1,3/1,4-fucosyltransferases determines the type 1/type 2 transfer. Characterization of acceptor substrate specificity of the lewis enzyme by site-directed mutagenesis. J. Biol. Chem. 1999, 274, 12257–12262. [Google Scholar]
- Loriol, C.; Dupuy, F.; Rampal, R.; Dlugosz, M.A.; Haltiwanger, R.S.; Maftah, A.; Germot, A. Molecular evolution of protein O-fucosyltransferase genes and splice variants. Glycobiology 2006, 16, 736–747. [Google Scholar]
- Callebaut, I.; Mignotte, V.; Souchet, M.; Mornon, J.P. EMI domains are widespread and reveal the probable orthologs of the Caenorhabditis elegans CED-1 protein. Biochem. Biophys. Res. Commun. 2003, 300, 619–623. [Google Scholar] [PubMed]
- Doliana, R.; Bot, S.; Bonaldo, P.; Colombatti, A. EMI, a novel cysteine-rich domain of EMILINs and other extracellular proteins, interacts with the gC1q domains and participates in multimerization. FEBS Lett. 2000, 484, 164–168. [Google Scholar] [PubMed]
- Colombatti, A.; Spessotto, P.; Doliana, R.; Mongiat, M.; Bressan, G.M.; Esposito, G. The EMILIN/Multimerin family. Front. Immunol. 2011, 2, 93. [Google Scholar]
- Cao, W.; Zeng, Z.; Lan, J.; Yang, Y.; Lu, M.; Lei, S. Knockdown of FUT11 inhibits the progression of gastric cancer via the PI3K/AKT pathway. Heliyon 2023, 9, e17600. [Google Scholar]
- Cao, W.; Zeng, Z.; Pan, R.; Wu, H.; Zhang, X.; Chen, H.; Nie, Y.; Yu, Z.; Lei, S. Hypoxia-Related Gene FUT11 Promotes Pancreatic Cancer Progression by Maintaining the Stability of PDK1. Front. Oncol. 2021, 11, 675991. [Google Scholar]
- Huang, Y.; Yang, X.; Wei, M.; Yang, X.; Yuan, Z.; Huang, J.; Wei, J.; Tian, L. FUT11 expression in gastric cancer: Its prognostic significance and role in immune regulation. Discov. Oncol. 2024, 15, 250. [Google Scholar]
- Ruan, W.; Yang, Y.; Yu, Q.; Huang, T.; Wang, Y.; Hua, L.; Zeng, Z.; Pan, R. FUT11 is a target gene of HIF1α that promotes the progression of hepatocellular carcinoma. Cell Biol. Int. 2021, 45, 2275–2286. [Google Scholar]
- Wang, P.; Liu, X.; Yu, J.; Meng, Z.; Lv, Z.; Shang, C.; Geng, Q.; Wang, D.; Xue, D.; Li, L. Fucosyltransferases Regulated by Fusobacterium Nucleatum and Act as Novel Biomarkers in Colon Adenocarcinoma. J. Inflamm. Res. 2023, 16, 747–768. [Google Scholar]
- Zhang, P.; Tang, W.; Jiang, Y.; Lyu, F.; Liu, Z.; Xiao, Y.; Wang, D. Dry and wet experiments reveal the significant role of FUT11 in clear cell renal cell carcinoma. Int. Immunopharmacol. 2022, 113 Pt B, 109447. [Google Scholar]
- Zodro, E.; Jaroszewski, M.; Ida, A.; Wrzesiński, T.; Kwias, Z.; Bluyssen, H.; Wesoly, J. FUT11 as a potential biomarker of clear cell renal cell carcinoma progression based on meta-analysis of gene expression data. Tumour Biol. 2014, 35, 2607–2617. [Google Scholar]
- Iwasaki, H.; Akashi, K. Hematopoietic developmental pathways: On cellular basis. Oncogene 2007, 26, 6687–6696. [Google Scholar] [PubMed]
- Stier, S.; Cheng, T.; Dombkowski, D.; Carlesso, N.; Scadden, D.T. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 2002, 99, 2369–2378. [Google Scholar] [PubMed]
- Ohishi, K.; Varnum-Finney, B.; Bernstein, I.D. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(-) cord blood cells. J. Clin. Investig. 2002, 110, 1165–1174. [Google Scholar]
- Yao, D.; Huang, Y.; Huang, X.; Wang, W.; Yan, Q.; Wei, L.; Xin, W.; Gerson, S.; Stanley, P.; Lowe, J.B.; et al. Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions. Blood 2011, 117, 5652–5662. [Google Scholar] [PubMed]
- Yu, V.W.; Saez, B.; Cook, C.; Lotinun, S.; Pardo-Saganta, A.; Wang, Y.H.; Lymperi, S.; Ferraro, F.; Raaijmakers, M.H.; Wu, J.Y.; et al. Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. J. Exp. Med. 2015, 212, 759–774. [Google Scholar]
- Bhandoola, A.; Sambandam, A. From stem cell to T cell: One route or many? Nat. Rev. Immunol. 2006, 6, 117–126. [Google Scholar]
- Maillard, I.; Fang, T.; Pear, W.S. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu. Rev. Immunol. 2005, 23, 945–974. [Google Scholar]
- Tanigaki, K.; Honjo, T. Regulation of lymphocyte development by Notch signaling. Nat. Immunol. 2007, 8, 451–456. [Google Scholar]
- Koch, U.; Lacombe, T.A.; Holland, D.; Bowman, J.L.; Cohen, B.L.; Egan, S.E.; Guidos, C.J. Subversion of the T/B lineage decision in the thymus by lunatic fringe-mediated inhibition of Notch-1. Immunity 2001, 15, 225–236. [Google Scholar]
- Feyerabend, T.B.; Terszowski, G.; Tietz, A.; Blum, C.; Luche, H.; Gossler, A.; Gale, N.W.; Radtke, F.; Fehling, H.J.; Rodewald, H.R. Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity 2009, 30, 67–79. [Google Scholar]
- Schneider, M.; Kumar, V.; Nordstrøm, L.U.; Feng, L.; Takeuchi, H.; Hao, H.; Luca, V.C.; Garcia, K.C.; Stanley, P.; Wu, P.; et al. Inhibition of Delta-induced Notch signaling using fucose analogs. Nat. Chem. Biol. 2018, 14, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Visan, I.; Tan, J.B.; Yuan, J.S.; Harper, J.A.; Koch, U.; Guidos, C.J. Regulation of T lymphopoiesis by Notch1 and Lunatic fringe-mediated competition for intrathymic niches. Nat. Immunol. 2006, 7, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Yahata, T.; Negishi, N.; Nakano, Y.; Habu, S.; Hozumi, K.; Ando, K. Expression of Delta-like 1 in the splenic non-hematopoietic cells is essential for marginal zone B cell development. Immunol. Lett. 2008, 121, 33–37. [Google Scholar] [PubMed]
- Saito, T.; Chiba, S.; Ichikawa, M.; Kunisato, A.; Asai, T.; Shimizu, K.; Yamaguchi, T.; Yamamoto, G.; Seo, S.; Kumano, K.; et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity 2003, 18, 675–685. [Google Scholar] [CrossRef]
- Tan, J.B.; Xu, K.; Cretegny, K.; Visan, I.; Yuan, J.S.; Egan, S.E.; Guidos, C.J. Lunatic and manic fringe cooperatively enhance marginal zone B cell precursor competition for delta-like 1 in splenic endothelial niches. Immunity 2009, 30, 254–263. [Google Scholar]
- Aster, J.C.; Pear, W.S.; Blacklow, S.C. The Varied Roles of Notch in Cancer. Annu. Rev. Pathol. 2017, 12, 245–275. [Google Scholar] [CrossRef]
- Postma, C.; Hermsen, M.A.; Coffa, J.; Baak, J.P.; Mueller, J.D.; Mueller, E.; Bethke, B.; Schouten, J.P.; Stolte, M.; Meijer, G.A. Chromosomal instability in flat adenomas and carcinomas of the colon. J. Pathol. 2005, 205, 514–521. [Google Scholar]
- Chabanais, J.; Labrousse, F.; Chaunavel, A.; Germot, A.; Maftah, A. POFUT1 as a Promising Novel Biomarker of Colorectal Cancer. Cancers 2018, 10, 411. [Google Scholar] [CrossRef]
- Deschuyter, M.; Pennarubia, F.; Pinault, E.; Legardinier, S.; Maftah, A. Functional Characterization of POFUT1 Variants Associated with Colorectal Cancer. Cancers 2020, 12, 1430. [Google Scholar] [CrossRef]
- Ma, L.; Dong, P.; Liu, L.; Gao, Q.; Duan, M.; Zhang, S.; Chen, S.; Xue, R.; Wang, X. Overexpression of protein O-fucosyltransferase 1 accelerates hepatocellular carcinoma progression via the Notch signaling pathway. Biochem. Biophys. Res. Commun. 2016, 473, 503–510. [Google Scholar] [CrossRef]
- Sawey, E.T.; Chanrion, M.; Cai, C.; Wu, G.; Zhang, J.; Zender, L.; Zhao, A.; Busuttil, R.W.; Yee, H.; Stein, L.; et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell 2011, 19, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, J.; Ma, X.; Wang, M.; Zhou, L. POFUT1 acts as a tumor promoter in glioblastoma by enhancing the activation of Notch signaling. J. Bioenerg. Biomembr. 2021, 53, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Kroes, R.A.; Dawson, G.; Moskal, J.R. Focused microarray analysis of glyco-gene expression in human glioblastomas. J. Neurochem. 2007, 103 (Suppl. 1), 14–24. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, Z.; Huang, B.; Zhang, J.; Ge, Y.; Fan, Q.; Wang, Z. Bioinformatics insight into glycosyltransferase gene expression in gastric cancer: POFUT1 is a potential biomarker. Biochem. Biophys. Res. Commun. 2017, 483, 171–177. [Google Scholar] [CrossRef]
- Yokota, S.; Ogawara, K.; Kimura, R.; Shimizu, F.; Baba, T.; Minakawa, Y.; Higo, M.; Kasamatsu, A.; Endo-Sakamoto, Y.; Shiiba, M.; et al. Protein O-fucosyltransferase 1: A potential diagnostic marker and therapeutic target for human oral cancer. Int. J. Oncol. 2013, 43, 1864–1870. [Google Scholar] [CrossRef]
- Milde-Langosch, K.; Karn, T.; Schmidt, M.; zu Eulenburg, C.; Oliveira-Ferrer, L.; Wirtz, R.M.; Schumacher, U.; Witzel, I.; Schutze, D.; Muller, V. Prognostic relevance of glycosylation-associated genes in breast cancer. Breast Cancer Res. Treat. 2014, 145, 295–305. [Google Scholar] [CrossRef]
- Wahby, S.; Jarczyk, J.; Fierek, A.; Heinkele, J.; Weis, C.A.; Eckstein, M.; Martini, T.; Porubsky, S.; Hafner, M.; Erben, P. POFUT1 mRNA expression as an independent prognostic parameter in muscle-invasive bladder cancer. Transl. Oncol. 2021, 14, 100900. [Google Scholar] [CrossRef]
- Rampias, T.; Vgenopoulou, P.; Avgeris, M.; Polyzos, A.; Stravodimos, K.; Valavanis, C.; Scorilas, A.; Klinakis, A. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat. Med. 2014, 20, 1199–1205. [Google Scholar] [CrossRef]
- Larose, H.; Prokoph, N.; Matthews, J.D.; Schlederer, M.; Högler, S.; Alsulami, A.F.; Ducray, S.P.; Nuglozeh, E.; Fazaludeen, F.M.S.; Elmouna, A.; et al. Whole Exome Sequencing reveals NOTCH1 mutations in anaplastic large cell lymphoma and points to Notch both as a key pathway and a potential therapeutic target. Haematologica 2021, 106, 1693–1704. [Google Scholar] [CrossRef]
- Pennarubia, F.; Ito, A.; Takeuchi, M.; Haltiwanger, R.S. Cancer-associated Notch receptor variants lead to O-fucosylation defects that deregulate Notch signaling. J. Biol. Chem. 2022, 298, 102616. [Google Scholar] [CrossRef]
- Weh, E.; Reis, L.M.; Tyler, R.C.; Bick, D.; Rhead, W.J.; Wallace, S.; McGregor, T.L.; Dills, S.K.; Chao, M.C.; Murray, J.C.; et al. Novel B3GALTL mutations in classic Peters plus syndrome and lack of mutations in a large cohort of patients with similar phenotypes. Clin. Genet. 2014, 86, 142–148. [Google Scholar] [PubMed]
- Aliferis, K.; Marsal, C.; Pelletier, V.; Doray, B.; Weiss, M.M.; Tops, C.M.; Speeg-Schatz, C.; Lesnik, S.A.; Dollfus, H. A novel nonsense B3GALTL mutation confirms Peters plus syndrome in a patient with multiple malformations and Peters anomaly. Ophthalmic Genet. 2010, 31, 205–208. [Google Scholar] [PubMed]
- Kozma, K.; Keusch, J.J.; Hegemann, B.; Luther, K.B.; Klein, D.; Hess, D.; Haltiwanger, R.S.; Hofsteenge, J. Identification and characterization of abeta1,3-glucosyltransferase that synthesizes the Glc-beta1,3-Fuc disaccharide on thrombospondin type 1 repeats. J. Biol. Chem. 2006, 281, 36742–36751. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sato, M.; Kiyohara, K.; Sogabe, M.; Shikanai, T.; Kikuchi, N.; Togayachi, A.; Ishida, H.; Ito, H.; Kameyama, A.; et al. Molecular cloning and characterization of a novel human beta1,3-glucosyltransferase, which is localized at the endoplasmic reticulum and glucosylates O-linked fucosylglycan on thrombospondin type 1 repeat domain. Glycobiology 2006, 16, 1194–1206. [Google Scholar] [CrossRef]
- Zhang, A.; Venkat, A.; Taujale, R.; Mull, J.L.; Ito, A.; Kannan, N.; Haltiwanger, R.S. Peters plus syndrome mutations affect the function and stability of human beta1,3-glucosyltransferase. J. Biol. Chem. 2021, 297, 100843. [Google Scholar]
- Mead, T.J.; Apte, S.S. ADAMTS proteins in human disorders. Matrix Biol. 2018, 71–72, 225–239. [Google Scholar]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015, 16, 113. [Google Scholar]
- Vasudevan, D.; Takeuchi, H.; Johar, S.S.; Majerus, E.; Haltiwanger, R.S. Peters plus syndrome mutations disrupt a noncanonical ER quality-control mechanism. Curr. Biol. 2015, 25, 286–295. [Google Scholar]
- Sparrow, D.B.; Chapman, G.; Wouters, M.A.; Whittock, N.V.; Ellard, S.; Fatkin, D.; Turnpenny, P.D.; Kusumi, K.; Sillence, D.; Dunwoodie, S.L. Mutation of the LUNATIC FRINGE gene in humans causes spondylocostal dysostosis with a severe vertebral phenotype. Am. J. Hum. Genet. 2006, 78, 28–37. [Google Scholar] [CrossRef]
- Wengryn, P.; Fenrich, F.; Silveira, K.D.C.; Oborn, C.; Mizumoto, S.; Beke, A.; Soltys, C.L.; Yamada, S.; Kannu, P. Integrative analysis of Lunatic Fringe variants associated with spondylocostal dysostosis type-III. FASEB J. 2024, 38, e23753. [Google Scholar] [CrossRef]
- Wang, L.; Mizumoto, S.; Zhang, R.; Zhang, Y.; Liu, Y.; Cheng, W.; Li, X.; Dan, M.; Zhang, C.; Gao, X.; et al. Identification of a novel LFNG variant in a Chinese fetus with spondylocostal dysostosis and a systematic review. J. Hum. Genet. 2024, 69, 321–327. [Google Scholar] [PubMed]
- Nóbrega, A.; Maia-Fernandes, A.C.; Andrade, R.P. Altered Cogs of the Clock: Insights into the Embryonic Etiology of Spondylocostal Dysostosis. J. Dev. Biol. 2021, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Wahi, K.; Bochter, M.S.; Cole, S.E. The many roles of Notch signaling during vertebrate somitogenesis. Semin. Cell Dev. Biol. 2016, 49, 68–75. [Google Scholar] [PubMed]
- Evrard, Y.A.; Lun, Y.; Aulehla, A.; Gan, L.; Johnson, R.L. lunatic fringe is an essential mediator of somite segmentation and patterning. Nature 1998, 394, 377–381. [Google Scholar]
- Zhang, N.; Gridley, T. Defects in somite formation in lunatic fringe-deficient mice. Nature 1998, 394, 374–377. [Google Scholar]
- Moran, J.L.; Levorse, J.M.; Vogt, T.F. Limbs move beyond the radical fringe. Nature 1999, 399, 742–743. [Google Scholar]
- Zhang, N.; Norton, C.R.; Gridley, T. Segmentation defects of Notch pathway mutants and absence of a synergistic phenotype in lunatic fringe/radical fringe double mutant mice. Genesis 2002, 33, 21–28. [Google Scholar]
- Moran, J.L.; Shifley, E.T.; Levorse, J.M.; Mani, S.; Ostmann, K.; Perez-Balaguer, A.; Walker, D.M.; Vogt, T.F.; Cole, S.E. Manic fringe is not required for embryonic development, and fringe family members do not exhibit redundant functions in the axial skeleton, limb, or hindbrain. Dev. Dyn. 2009, 238, 1803–1812. [Google Scholar]
- Harvey, B.M.; Rana, N.A.; Moss, H.; Leonardi, J.; Jafar-Nejad, H.; Haltiwanger, R.S. Mapping Sites of O-Glycosylation and Fringe Elongation on Drosophila Notch. J. Biol. Chem. 2016, 291, 16348–16360. [Google Scholar]
- Li, Z.; Han, K.; Pak, J.E.; Satkunarajah, M.; Zhou, D.; Rini, J.M. Recognition of EGF-like domains by the Notch-modifying O-fucosyltransferase POFUT1. Nat. Chem. Biol. 2017, 13, 757–763. [Google Scholar]
- Berardinelli, S.J.; Eletsky, A.; Valero-Gonzalez, J.; Ito, A.; Manjunath, R.; Hurtado-Guerrero, R.; Prestegard, J.H.; Woods, R.J.; Haltiwanger, R.S. O-fucosylation stabilizes the TSR3 motif in thrombospondin-1 by interacting with nearby amino acids and protecting a disulfide bond. J. Biol. Chem. 2022, 298, 102047. [Google Scholar] [CrossRef]
- Moremen, K.W.; Haltiwanger, R.S. Emerging structural insights into glycosyltransferase-mediated synthesis of glycans. Nat. Chem. Biol. 2019, 15, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Yu, H.; Hao, H.; Takeuchi, M.; Ito, A.; Li, H.; Haltiwanger, R.S. O-Glycosylation modulates the stability of epidermal growth factor-like repeats and thereby regulates Notch trafficking. J. Biol. Chem. 2017, 292, 15964–15973. [Google Scholar] [CrossRef] [PubMed]
- Okajima, T.; Xu, A.; Lei, L.; Irvine, K.D. Chaperone activity of protein O-fucosyltransferase 1 promotes notch receptor folding. Science 2005, 307, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Ajima, R.; Suzuki, E.; Saga, Y. Pofut1 point-mutations that disrupt O-fucosyltransferase activity destabilize the protein and abolish Notch1 signaling during mouse somitogenesis. PLoS ONE 2017, 12, e0187248. [Google Scholar] [CrossRef]
- McMillan, B.J.; Zimmerman, B.; Egan, E.D.; Lofgren, M.; Xu, X.; Hesser, A.; Blacklow, S.C. Structure of human POFUT1, its requirement in ligand-independent oncogenic Notch signaling, and functional effects of Dowling-Degos mutations. Glycobiology 2017, 27, 777–786. [Google Scholar] [CrossRef]
- Stahl, M.; Uemura, K.; Ge, C.; Shi, S.; Tashima, Y.; Stanley, P. Roles of Pofut1 and O-fucose in mammalian Notch signaling. J. Biol. Chem. 2008, 283, 13638–13651. [Google Scholar] [CrossRef]
- Zandberg, W.F.; Kumarasamy, J.; Pinto, B.M.; Vocadlo, D.J. Metabolic inhibition of sialyl-Lewis X biosynthesis by 5-thiofucose remodels the cell surface and impairs selectin-mediated cell adhesion. J. Biol. Chem. 2012, 287, 40021–40030. [Google Scholar] [CrossRef]
- Okeley, N.M.; Alley, S.C.; Anderson, M.E.; Boursalian, T.E.; Burke, P.J.; Emmerton, K.M.; Jeffrey, S.C.; Klussman, K.; Law, C.L.; Sussman, D.; et al. Development of orally active inhibitors of protein and cellular fucosylation. Proc. Natl. Acad. Sci. USA 2013, 110, 5404–5409. [Google Scholar] [CrossRef]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef]
- Allen, J.G.; Mujacic, M.; Frohn, M.J.; Pickrell, A.J.; Kodama, P.; Bagal, D.; San Miguel, T.; Sickmier, E.A.; Osgood, S.; Swietlow, A.; et al. Facile Modulation of Antibody Fucosylation with Small Molecule Fucostatin Inhibitors and Cocrystal Structure with GDP-Mannose 4,6-Dehydratase. ACS Chem. Biol. 2016, 11, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Nakano, M.; Yamaguchi, Y.; Nakajima, K.; Oka, R.; Sato, K.; Ren, C.T.; Hsu, T.L.; Wong, C.H.; Taniguchi, N. An Alkynyl-Fucose Halts Hepatoma Cell Migration and Invasion by Inhibiting GDP-Fucose-Synthesizing Enzyme FX, TSTA3. Cell Chem. Biol. 2017, 24, 1467–1478.e5. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Funayama, S.; Shogomori, H.; Nakano, M.; Nakajima, K.; Oka, R.; Kitazume, S.; Yamaguchi, Y.; Sano, M.; Korekane, H.; et al. High-Sensitivity and Low-Toxicity Fucose Probe for Glycan Imaging and Biomarker Discovery. Cell Chem. Biol. 2016, 23, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Gilormini, P.A.; Thota, V.N.; Fers-Lidou, A.; Ashmus, R.A.; Nodwell, M.; Brockerman, J.; Kuo, C.W.; Wang, Y.; Gray, T.E.; Nitin; et al. A metabolic inhibitor blocks cellular fucosylation and enables production of afucosylated antibodies. Proc. Natl. Acad. Sci. USA 2024, 121, e2314026121. [Google Scholar] [CrossRef]
- Al-Shareffi, E.; Chaubard, J.L.; Leonhard-Melief, C.; Wang, S.K.; Wong, C.H.; Haltiwanger, R.S. 6-alkynyl fucose is a bioorthogonal analog for O-fucosylation of epidermal growth factor-like repeats and thrombospondin type-1 repeats by protein O-fucosyltransferases 1 and 2. Glycobiology 2013, 23, 188–198. [Google Scholar] [CrossRef]
- Ma, C.; Takeuchi, H.; Hao, H.; Yonekawa, C.; Nakajima, K.; Nagae, M.; Okajima, T.; Haltiwanger, R.S.; Kizuka, Y. Differential Labeling of Glycoproteins with Alkynyl Fucose Analogs. Int. J. Mol. Sci. 2020, 21, 6007. [Google Scholar] [CrossRef]
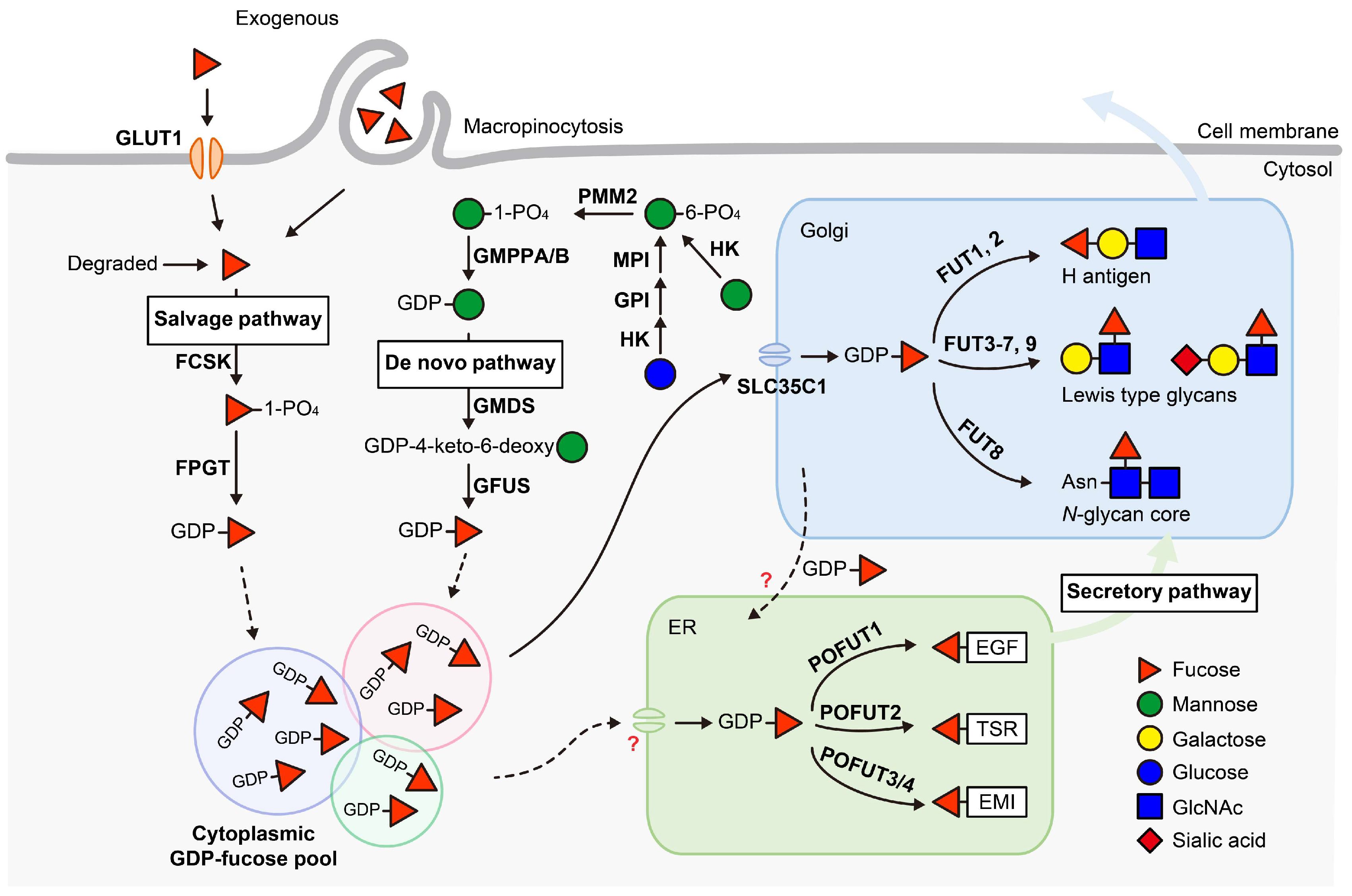
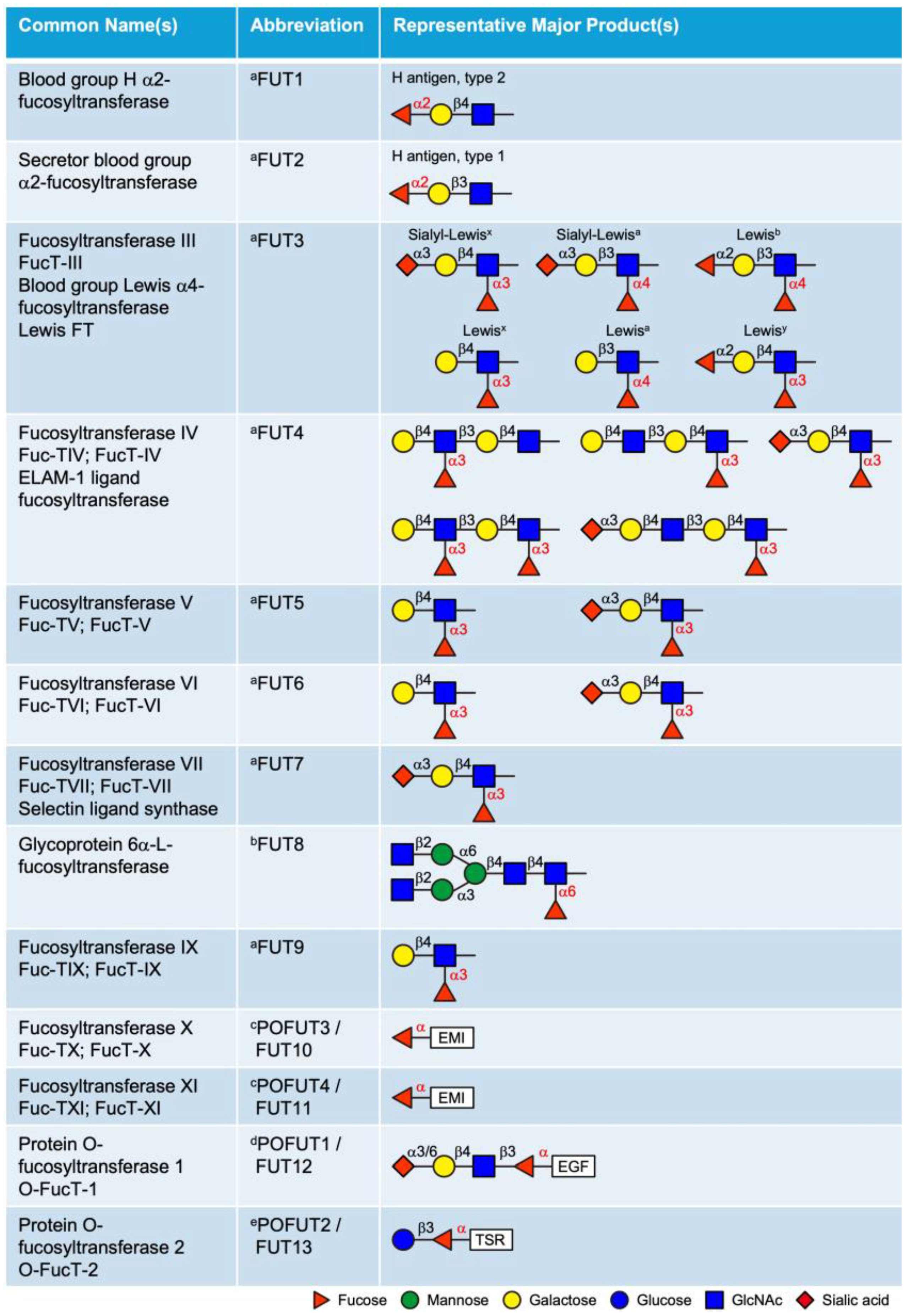
| UniProt ID | Gene Name | Protein Name | Subcellular Location | Known Human Pathology (If Any) |
|---|---|---|---|---|
| Q96A83 | COL26A1 | Collagen alpha-1(XXVI) chain | ECM | - |
| Q9UHF1 | EGFL7 | Epidermal growth factor-like protein 7 | ECM | - |
| Q99944 | EGFL8 | Epidermal growth factor-like protein 8 | ECM | - |
| Q96A84 | EMID1 | EMI domain-containing protein 1 [45] | ECM | - |
| Q9Y6C2 | EMILIN1 | EMILIN-1 | ECM | Neuronopathy, distal hereditary motor, autosomal dominant 10 (HMND10) [260,261] |
| Q9BXX0 | EMILIN2 | EMILIN-2 | ECM | - |
| Q9NT22 | EMILIN3 | EMILIN-3 | ECM | - |
| O75095 | MEGF6 | Multiple epidermal growth factor-like domains protein 6 | ECM | - |
| Q96KG7 | MEGF10 | Multiple epidermal growth factor-like domains protein 10 | CM | Congenital myopathy 10A, severe variant (CMYP10A) [161]; Congenital myopathy 10B, mild variant (CMYP10B) [162] |
| A6BM72 | MEGF11 | Multiple epidermal growth factor-like domains protein 11 | CM | - |
| Q13201 | MMRN1 | Multimerin-1 [45,163] | ECM | Factor V Quebec [164] |
| Q9H8L6 | MMRN2 | Multimerin-2 [45] | ECM | - |
| Q5VY43 | PEAR1 | Platelet endothelial aggregation receptor 1 | CM | Cardiovascular disease [184] |
| Q15063 | POSTN | Periostin | ECM | - |
| A2VEC9 | SSPOP | SCO-spondin | ECM | - |
| Q15582 | TGFBI | Transforming growth factor-beta-induced protein ig-h3 | ECM | Corneal dystrophy, epithelial basement membrane (EBMD) [262]; Corneal dystrophy, Groenouw type 1 (CDGG1) [263]; Corneal dystrophy, lattice type 1 (CDL1) [264]; Corneal dystrophy, Thiel-Behnke type (CDTB); Corneal dystrophy, Reis-Bucklers type (CDRB) [265]; Corneal dystrophy, lattice type 3A (CDL3A) [266]; Corneal dystrophy, Avellino type (CDA) |
| Q5DID0 | UMODL1 | Uromodulin-like 1 | CM, CYT | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, H.; Eberand, B.M.; Larance, M.; Haltiwanger, R.S. Protein O-Fucosyltransferases: Biological Functions and Molecular Mechanisms in Mammals. Molecules 2025, 30, 1470. https://doi.org/10.3390/molecules30071470
Hao H, Eberand BM, Larance M, Haltiwanger RS. Protein O-Fucosyltransferases: Biological Functions and Molecular Mechanisms in Mammals. Molecules. 2025; 30(7):1470. https://doi.org/10.3390/molecules30071470
Chicago/Turabian StyleHao, Huilin, Benjamin M. Eberand, Mark Larance, and Robert S. Haltiwanger. 2025. "Protein O-Fucosyltransferases: Biological Functions and Molecular Mechanisms in Mammals" Molecules 30, no. 7: 1470. https://doi.org/10.3390/molecules30071470
APA StyleHao, H., Eberand, B. M., Larance, M., & Haltiwanger, R. S. (2025). Protein O-Fucosyltransferases: Biological Functions and Molecular Mechanisms in Mammals. Molecules, 30(7), 1470. https://doi.org/10.3390/molecules30071470






