Therapeutic Potential of Myricitrin in a db/db Mouse Model of Type 2 Diabetes
Abstract
1. Introduction
2. Results
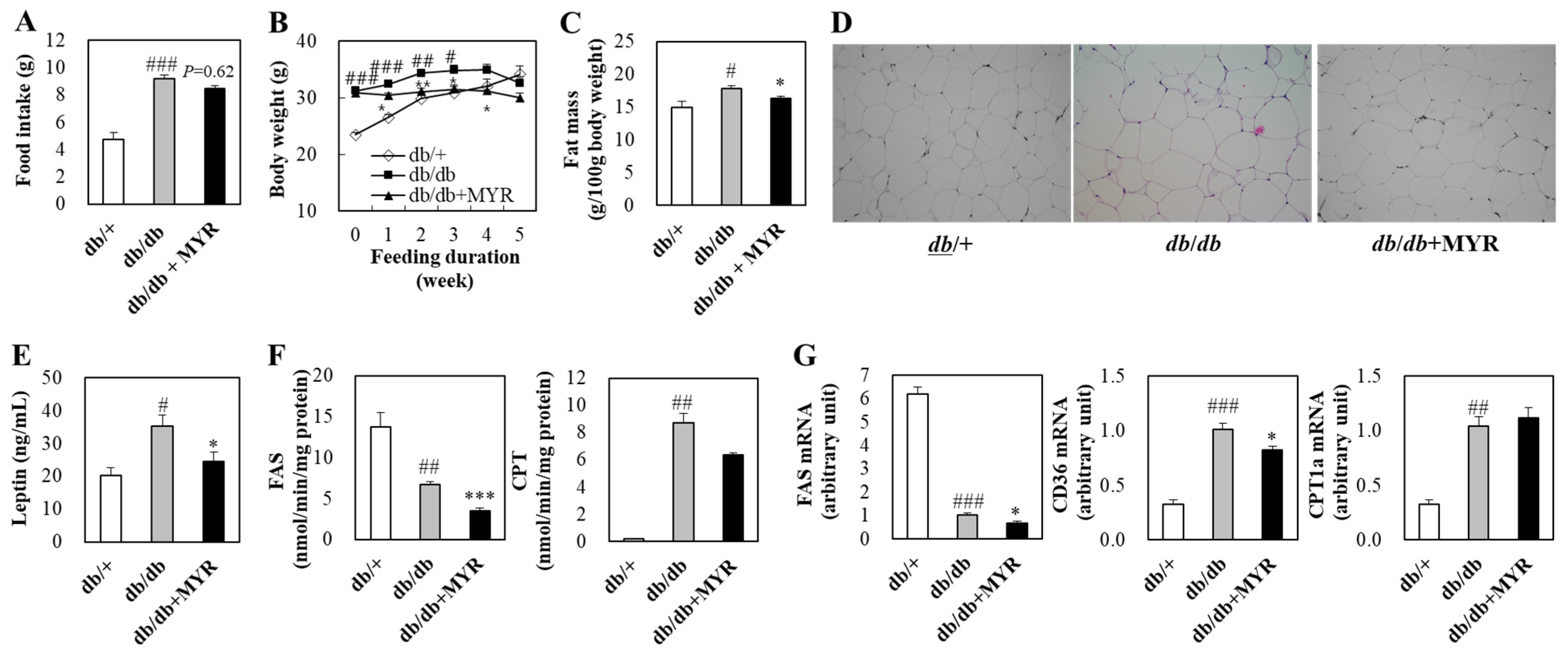
2.1. Effects of Myricitrin on Food Intake, Body Weight, and Fat Mass in db/db Mice
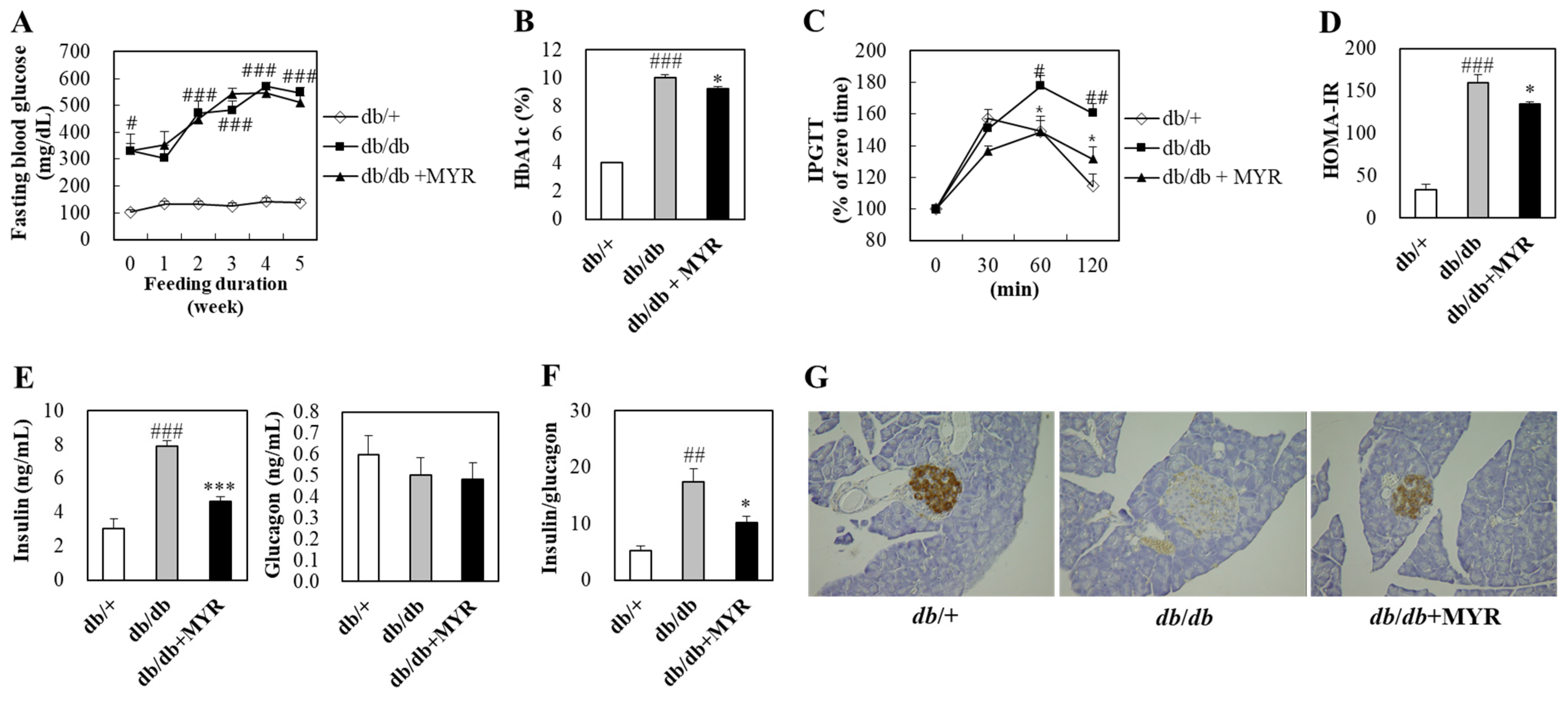
2.2. Effects of Myricitrin on Glucose Homeostasis and Insulin Regulation in db/db Mice
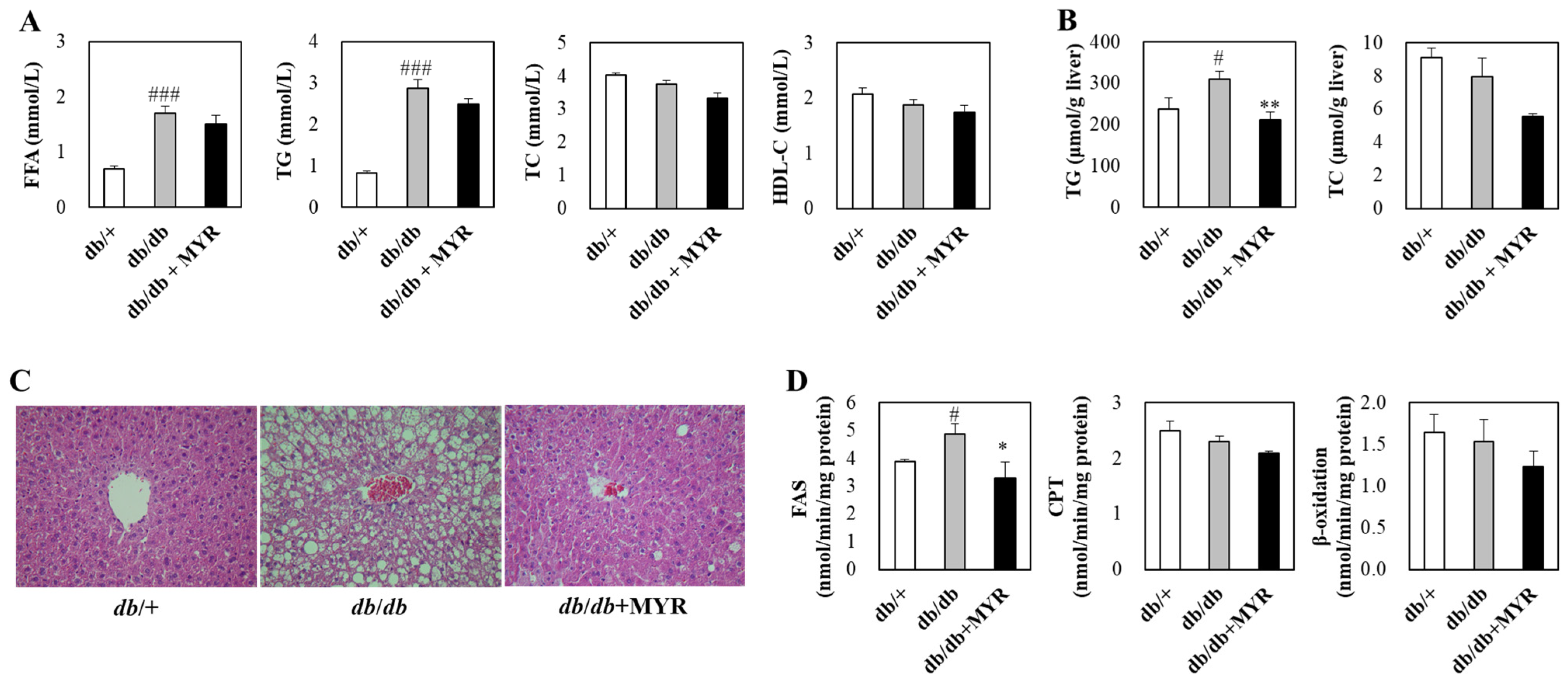
2.3. Effects of Myricitrin on Lipid Homeostasis and Liver Morphology in db/db Mice
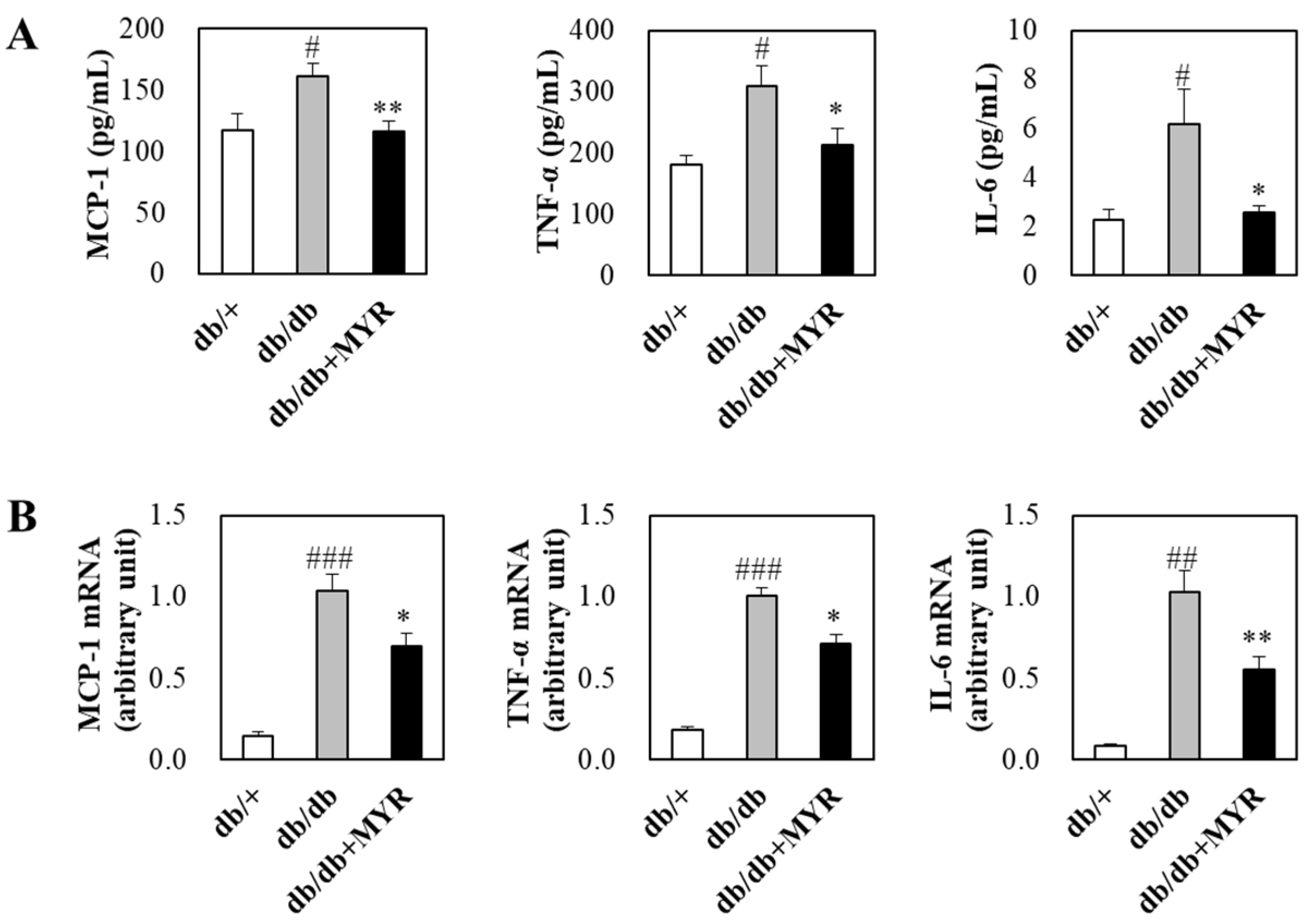
2.4. Effects of Myricitrin on Circulating and Adipose Inflammatory Markers in db/db Mice
3. Discussion
4. Materials and Methods
4.1. Animal and Diet
4.2. Blood Collection and Organ Harvesting
4.3. Fasting Blood Glucose, HbA1c, Plasma Insulin, Glucagon, Leptin, and Inflammatory Markers
4.4. Intraperitoneal Glucose Tolerance Test
4.5. Plasma and Hepatic Lipid Levels
4.6. Enzyme Activity in Liver and Adipose Tissues
4.7. RNA Extraction and Real-Time qPCR
4.8. Histopathological Analysis of Pancreatic, Liver, and Adipose Tissues
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| HDL | High-density lipoprotein |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| STZ | Streptozotocin |
| HFD | High-fat diet |
| FAS | Fatty acid synthase |
| CPT | Carnitine palmitoyltransferase |
| HbA1c | Hemoglobin A1c |
| IPGTT | Intraperitoneal glucose tolerance test |
| G6Pase | Glucose-6-phosphatase |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| GK | Glucokinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, D.J.; Krycer, J.R.; Kearney, A.L.; Hocking, S.L.; James, D.E. Muscle and adipose tissue insulin resistance: Malady without mechanism? J. Lipid Res. 2019, 60, 1720–1732. [Google Scholar] [PubMed]
- Santoro, A.; McGraw, T.E.; Kahn, B.B. Insulin action in adipocytes, adipose remodeling, and systemic effects. Cell Metab. 2021, 33, 748–757. [Google Scholar]
- Chait, A.; Eckel, R.H.; Vrablik, M.; Zambon, A. Lipid-lowering in diabetes: An update. Atherosclerosis 2024, 394, 117313. [Google Scholar]
- Nogueira, J.P.; Cusi, K. Role of insulin resistance in the development of nonalcoholic fatty liver disease in people with type 2 diabetes: From bench to patient care. Diabetes Spectr. 2024, 37, 20–28. [Google Scholar]
- Bae, J.C. No more NAFLD: The term is now MASLD. Endocrinol. Metab. 2024, 39, 92–94. [Google Scholar]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar]
- Huang, Q.; Gao, B.; Wang, L.; Hu, Y.Q.; Lu, W.G.; Yang, L.; Luo, Z.J.; Liu, J. Protective effects of myricitrin against osteoporosis via reducing reactive oxygen species and bone-resorbing cytokines. Toxicol. Appl. Pharmacol. 2014, 280, 550–560. [Google Scholar] [CrossRef]
- Sudarshan, K.; Aidhen, I.S. Convenient synthesis of 3-glycosylated isocoumarins. Eur. J. Org. Chem. 2017, 2017, 34–38. [Google Scholar]
- Hasegawa, T.; Tanaka, A.; Hosoda, A.; Takano, F.; Ohta, T. Antioxidant C-glycosyl flavones from the leaves of Sasa kurilensis var. gigantea. Phytochemistry 2008, 69, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Hiroe, K.; Kumazawa, T.; Jun-ichi, O. Total synthesis of two isoflavone C-glycosides: Genistein and orobol 8-C-beta-D-glucopyranosides. Carbohydr. Res. 2006, 341, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, G.B.; Du, Y.Y.; Tian, Y.; Liao, P.; Liu, X.S.; Ye, J.X.; Sun, X.B. Myricitrin protects cardiomyocytes from hypoxia/reoxygenation injury: Involvement of heat shock protein 90. Front. Pharmacol. 2017, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Domitrovic, R.; Rashed, K.; Cvijanovic, O.; Vladimir-Knezevic, S.; Skoda, M.; Visnic, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem. Biol. Interact. 2015, 230, 21–29. [Google Scholar]
- Gao, J.; Liu, C.; Zhang, H.; Sun, Z.; Wang, R. Myricitrin exhibits anti-atherosclerotic and anti-hyperlipidemic effects in diet-induced hypercholesterolemic rats. AMB Express 2019, 9, 204. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.R.; Kim, D.Y.; Woo, J.T.; Kwon, E.Y.; Han, Y.; Choi, M.S.; Jung, U.J. Supplementation of the flavonoid myricitrin attenuates the adverse metabolic effects of long-term consumption of a high-fat diet in mice. J. Med. Food 2019, 22, 1151–1158. [Google Scholar] [CrossRef]
- Omidi, M.; Ahangarpour, A.; Khorsandi, L.; Ramezani-AliAkbari, F. The antidiabetic and hepatoprotective effects of myricitrin on aged mice with D-galactose. Gastroenterol. Hepatol. Bed Bench 2020, 13, 247–253. [Google Scholar]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Solid lipid nanoparticles of myricitrin have antioxidant and antidiabetic effects on streptozotocin-nicotinamide-induced diabetic model and myotube cell of male mouse. Oxid. Med. Cell Longev. 2018, 2018, 7496936. [Google Scholar] [CrossRef]
- Dua, T.K.; Joardar, S.; Chakraborty, P.; Bhowmick, S.; Saha, A.; De Feo, V.; Dewanjee, S. Myricitrin, a glycosyloxyflavone in Myrica esculenta bark ameliorates diabetic nephropathy via improving glycemic status, reducing oxidative stress, and suppressing inflammation. Molecules 2021, 26, 258. [Google Scholar] [CrossRef]
- Belke, D.D.; Severson, D.L. Diabetes in mice with monogenic obesity: The db/db mouse and its use in the study of cardiac consequences. Methods Mol. Biol. 2012, 933, 47–57. [Google Scholar]
- Kim, K.E.; Jung, Y.; Min, S.; Nam, M.; Heo, R.W.; Jeon, B.T.; Song, D.H.; Yi, C.O.; Jeong, E.A.; Kim, H.; et al. Caloric restriction of db/db mice reverts hepatic steatosis and body weight with divergent hepatic metabolism. Sci. Rep. 2016, 6, 30111. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Formentini, G.; Calcaterra, F.; Lombardi, S.; Marini, F.; Zenari, L.; Saggiani, F.; Poli, M.; Perbellini, S.; Raffaelli, A.; et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: Prospective data from the Verona Diabetes Complications Study. Diabetes Care 2002, 25, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Al Rijjal, D.; Wheeler, M.B. A protocol for studying glucose homeostasis and islet function in mice. STAR Protoc. 2022, 3, 101171. [Google Scholar] [PubMed]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar]
- Esterson, Y.B.; Zhang, K.; Koppaka, S.; Kehlenbrink, S.; Kishore, P.; Raghavan, P.; Maginley, S.R.; Carey, M.; Hawkins, M. Insulin sensitizing and anti-inflammatory effects of thiazolidinediones are heightened in obese patients. J. Investig. Med. 2013, 61, 1152–1160. [Google Scholar]
- Schenk, S.; Harber, M.P.; Shrivastava, C.R.; Burant, C.F.; Horowitz, J.F. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J. Physiol. 2009, 587, 4949–4961. [Google Scholar]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar]
- Bo, T.; Gao, L.; Yao, Z.; Shao, S.; Wang, X.; Proud, C.G.; Zhao, J. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024, 36, 947–968. [Google Scholar]
- Ekberg, K.; Landau, B.R.; Wajngot, A.; Chandramouli, V.; Efendic, S.; Brunengraber, H.; Wahren, J. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 1999, 48, 292–298. [Google Scholar] [CrossRef]
- Sharabi, K.; Tavares, C.D.; Rines, A.K.; Puigserver, P. Molecular pathophysiology of hepatic glucose production. Mol. Aspects Med. 2015, 46, 21–33. [Google Scholar]
- Cherrington, A.D. The role of hepatic insulin receptors in the regulation of glucose production. J. Clin. Investig. 2005, 115, 1136–1139. [Google Scholar] [PubMed]
- Yabaluri, N.; Bashyam, M.D. Hormonal regulation of gluconeogenic gene transcription in the liver. J. Biosci. 2010, 35, 473–484. [Google Scholar] [PubMed]
- Altomonte, J.; Richter, A.; Harbaran, S.; Suriawinata, J.; Nakae, J.; Thung, S.N.; Meseck, M.; Accili, D.; Dong, H. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E718–E728. [Google Scholar] [PubMed]
- Cao, H.; Veer, E.; Ban, M.R.; Hanley, A.J.; Zinman, B.; Harris, S.B.; Young, T.K.; Pickering, J.G.; Hegele, R.A. Promoter polymorphism in PCK1 (phosphoenolpyruvate carboxykinase gene) associated with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2004, 89, 898–903. [Google Scholar]
- Liu, Y.; Nakagawa, Y.; Wang, Y.; Sakurai, R.; Tripathi, P.V.; Lutfy, K.; Friedman, T.C. Increased glucocorticoid receptor and 11{beta}-hydroxysteroid dehydrogenase type 1 expression in hepatocytes may contribute to the phenotype of type 2 diabetes in db/db mice. Diabetes 2005, 54, 32–40. [Google Scholar]
- Gómez-Valadés, A.G.; Méndez-Lucas, A.; Vidal-Alabró, A.; Blasco, F.X.; Chillon, M.; Bartrons, R.; Bermúdez, J.; Perales, J.C. Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice. Diabetes 2008, 57, 2199–2210. [Google Scholar]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef]
- Onyango, A.N. Excessive gluconeogenesis causes the hepatic insulin resistance paradox and its sequelae. Heliyon 2022, 8, e12294. [Google Scholar] [CrossRef]
- Berndt, J.; Kovacs, P.; Ruschke, K.; Klöting, N.; Fasshauer, M.; Schön, M.R.; Körner, A.; Stumvoll, M.; Blüher, M. Fatty acid synthase gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Diabetologia 2007, 50, 1472–1480. [Google Scholar]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012, 150, 1223–1234. [Google Scholar] [CrossRef]
- Okajima, Y.; Matsuzaka, T.; Miyazaki, S.; Motomura, K.; Ohno, H.; Sharma, R.; Shimura, T.; Istiqamah, N.; Han, S.I.; Mizunoe, Y.; et al. Morphological and functional adaptation of pancreatic islet blood vessels to insulin resistance is impaired in diabetic db/db mice. Biochim. Biophys. Acta-Mol. Basis Dis. 2022, 1868, 166339. [Google Scholar]
- Matsuoka, T.A.; Kaneto, H.; Miyatsuka, T.; Yamamoto, T.; Yamamoto, K.; Kato, K.; Shimomura, I.; Stein, R.; Matsuhisa, M. Regulation of MafA expression in pancreatic beta-cells in db/db mice with diabetes. Diabetes 2010, 59, 1709–1720. [Google Scholar] [PubMed]
- Karunakaran, U.; Park, K.-G. A systematic review of oxidative stress and safety of antioxidants in diabetes: Focus on islets and their defense. Diabetes Metab. J. 2013, 37, 106–112. [Google Scholar] [PubMed]
- Kuryłowicz, A.; Koźniewski, K. Anti-inflammatory strategies targeting metaflammation in type 2 diabetes. Molecules 2020, 25, 2224. [Google Scholar] [CrossRef]
- Ghorbani, A.; Rashidi, R.; Shafiee-Nick, R. Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomed. Pharmacother. 2019, 111, 947–957. [Google Scholar]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 607. [Google Scholar]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Coburn, C.T.; Knapp, F.F.J.; Febbraio, M.; Beets, A.L.; Silverstein, R.L.; Abumrad, N.A. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 2000, 275, 32523–32529. [Google Scholar]
- Yang, J.; Park, K.W.; Cho, S. Inhibition of the CD36 receptor reduces visceral fat accumulation and improves insulin resistance in obese mice carrying the BDNF-Val66Met variant. Biol. Chem. 2018, 293, 13338–13348. [Google Scholar]
- Kennedy, D.J.; Kuchibhotla, S.; Westfall, K.M.; Silverstein, R.L.; Morton, R.E.; Febbraio, M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc. Res. 2011, 89, 604–613. [Google Scholar]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, C.; He, J.; Liu, B.; Ji, W.; Wu, L.; He, L. ASK1-interacting protein 1 acts as a novel predictor of type 2 diabetes. Front. Endocrinol. 2022, 13, 896753. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Wang, D.; Wang, Y.; Gu, H.; Jv, J.; Yuan, L.; Wang, C.; Chen, L. Kaempferol alleviates adipose tissue inflammation and insulin resistance in db/db mice by inhibiting the STING/NLRP3 signaling pathway. Endocr. Connect. 2024, 13, e230379. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Tamura, Y.; Yano, M.; Kawao, N.; Okumoto, K.; Ueshima, S.; Kaji, H.; Matsuo, O. Enzamin ameliorates adipose tissue inflammation with impaired adipocytokine expression and insulin resistance in db/db mice. J. Nutr. Sci. 2013, 2, e37. [Google Scholar] [CrossRef]
- Bastard, J.P.; Maachi, M.; Van Nhieu, J.T.; Jardel, C.; Bruckert, E.; Grimaldi, A.; Robert, J.J.; Capeau, J.; Hainque, B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J. Clin. Endocrinol. Metab. 2002, 87, 2084–2089. [Google Scholar] [CrossRef]
- Sabio, G.; Das, M.; Mora, A.; Zhang, Z.; Jun, J.Y.; Ko, H.J.; Barrett, T.; Kim, J.K.; Davis, R.J. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008, 322, 1539–1543. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar]
- Orland, M.J.; Permutt, M.A. Quantitative analysis of pancreatic proinsulin mRNA in genetically diabetic (db/db) mice. Diabetes 1987, 36, 341–347. [Google Scholar] [CrossRef]
- Pretz, D.; Le Foll, C.; Rizwan, M.Z.; Lutz, T.A.; Tups, A. Hyperleptinemia as a contributing factor for the impairment of glucose intolerance in obesity. FASEB J. 2021, 35, e21216. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef] [PubMed]
- Baky, M.H.; Elshahed, M.; Wessjohann, L.; Farag, M.A. Interactions between dietary flavonoids and the gut microbiome: A comprehensive review. Br. J. Nutr. 2022, 128, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Shimabayashi, H.; Moriwaki, M. Enzymatic production of highly soluble myricitrin glycosides using β-galactosidase. Biosci. Biotechnol. Biochem. 2006, 70, 940–948. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, C.; Wang, W.; Shi, C.Y.; Xiong, W.; Wang, M.D.; Fang, J.G. Gastrointestinal stability of dihydromyricetin, myricetin, and myricitrin: An in vitro investigation. Int. J. Food Sci. Nutr. 2017, 68, 704–711. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90–121. [Google Scholar] [CrossRef]
- American Institute of Nutrition. Report of the American institute of nutrition ad hoc committee on standards for nutritional studies. J. Nutr. 1977, 107, 1340–1348. [Google Scholar] [CrossRef]
- Bieri, J.G. Second Report of ad hoc committee on standards for nutritional studies. J. Nutr. 1980, 110, 1717–1726. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, K.T.; Park, Y.B.; Jeon, S.M.; Choi, M.S. Dietary docosahexaenoic acid-rich diacylglycerols ameliorate hepatic stea- tosis and alter hepatic gene expressions in C57BL/6J-Lepob/ob mice. Mol. Nutr. Food Res. 2008, 52, 965–973. [Google Scholar] [CrossRef]
- Davidson, A.L.; Arion, W.J. Factors underlying significant under- estimations of glucokinase activity in crude liver extracts: Physiological implications of higher cellular activity. Arch. Biochem. Biophys. 1987, 253, 156–167. [Google Scholar] [PubMed]
- Alegre, M.; Ciudad, C.J.; Fillat, C.; Guinovart, J.J. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal. Biochem. 1988, 173, 185–189. [Google Scholar] [PubMed]
- Bentle, L.A.; Lardy, H.A. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J. Biol. Chem. 1976, 251, 2916–2921. [Google Scholar] [PubMed]
- Nepokroeff, C.M.; Lakshmanan, M.R.; Porter, J.W. Fatty acid synthase from rat liver. Methods Enzymol. 1975, 35, 37–44. [Google Scholar]
- Markwell, M.A.; McGroarty, E.J.; Bieber, L.L.; Tolbert, N.E. The subcellular distribution of carnitine acyltransferases in mamma-lian liver and kidney. A new peroxisomal enzyme. J. Biol. Chem. 1973, 248, 3426–3432. [Google Scholar]
- Lazarow, P.B. Assay of peroxisomal β-oxidation of fatty acids. Methods Enzymol. 1981, 72, 315–319. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.R.; Kim, Y.-J.; Kim, H.; Park, S.; Jung, U.J. Therapeutic Potential of Myricitrin in a db/db Mouse Model of Type 2 Diabetes. Molecules 2025, 30, 1460. https://doi.org/10.3390/molecules30071460
Kim SR, Kim Y-J, Kim H, Park S, Jung UJ. Therapeutic Potential of Myricitrin in a db/db Mouse Model of Type 2 Diabetes. Molecules. 2025; 30(7):1460. https://doi.org/10.3390/molecules30071460
Chicago/Turabian StyleKim, Sang Ryong, Young-Je Kim, HwiCheol Kim, Sojeong Park, and Un Ju Jung. 2025. "Therapeutic Potential of Myricitrin in a db/db Mouse Model of Type 2 Diabetes" Molecules 30, no. 7: 1460. https://doi.org/10.3390/molecules30071460
APA StyleKim, S. R., Kim, Y.-J., Kim, H., Park, S., & Jung, U. J. (2025). Therapeutic Potential of Myricitrin in a db/db Mouse Model of Type 2 Diabetes. Molecules, 30(7), 1460. https://doi.org/10.3390/molecules30071460






