Tailoring Catalysts for CO2 Hydrogenation: Synthesis and Characterization of NH2–MIL–125 Frameworks
Abstract
1. Introduction
2. Results and Discussion
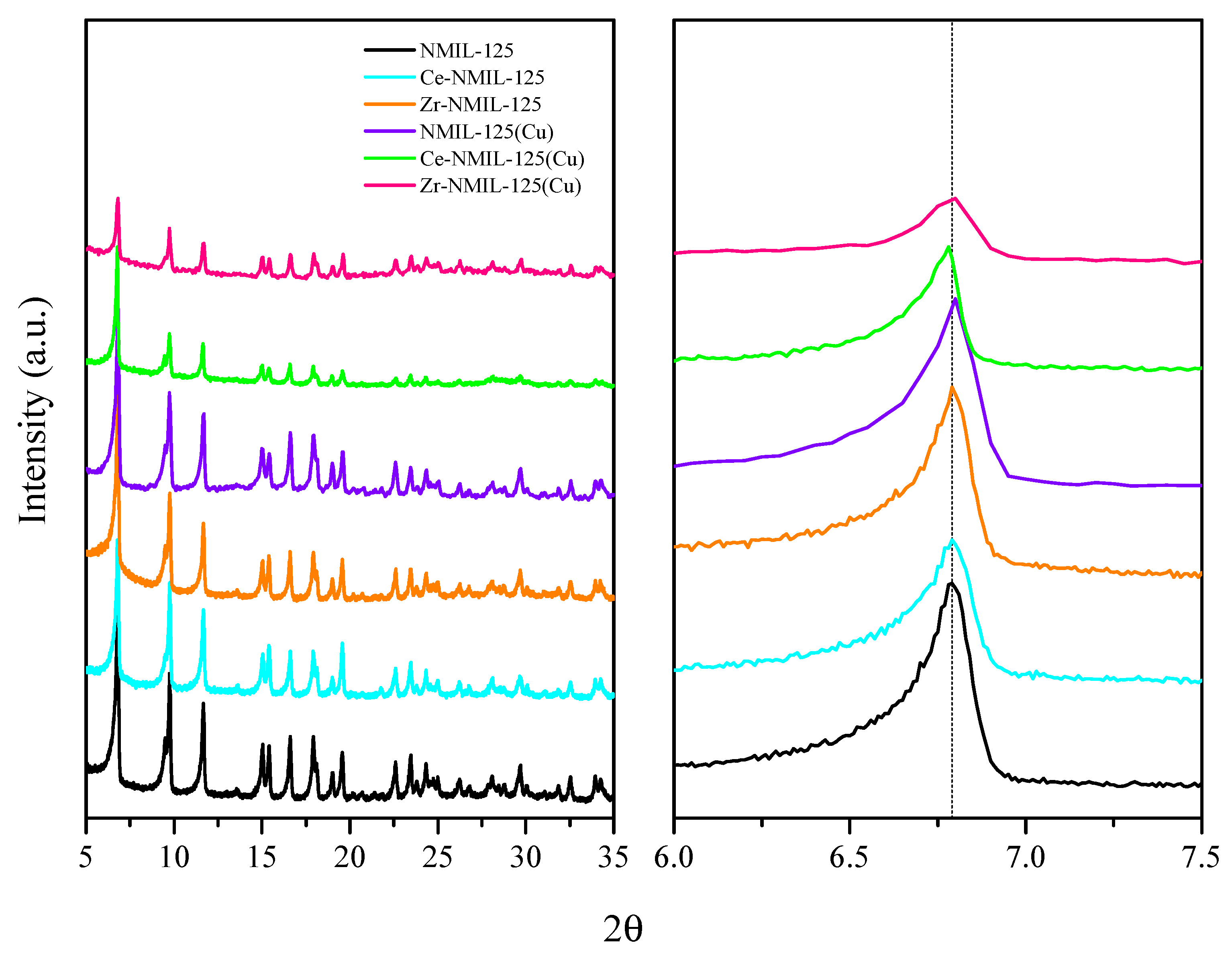
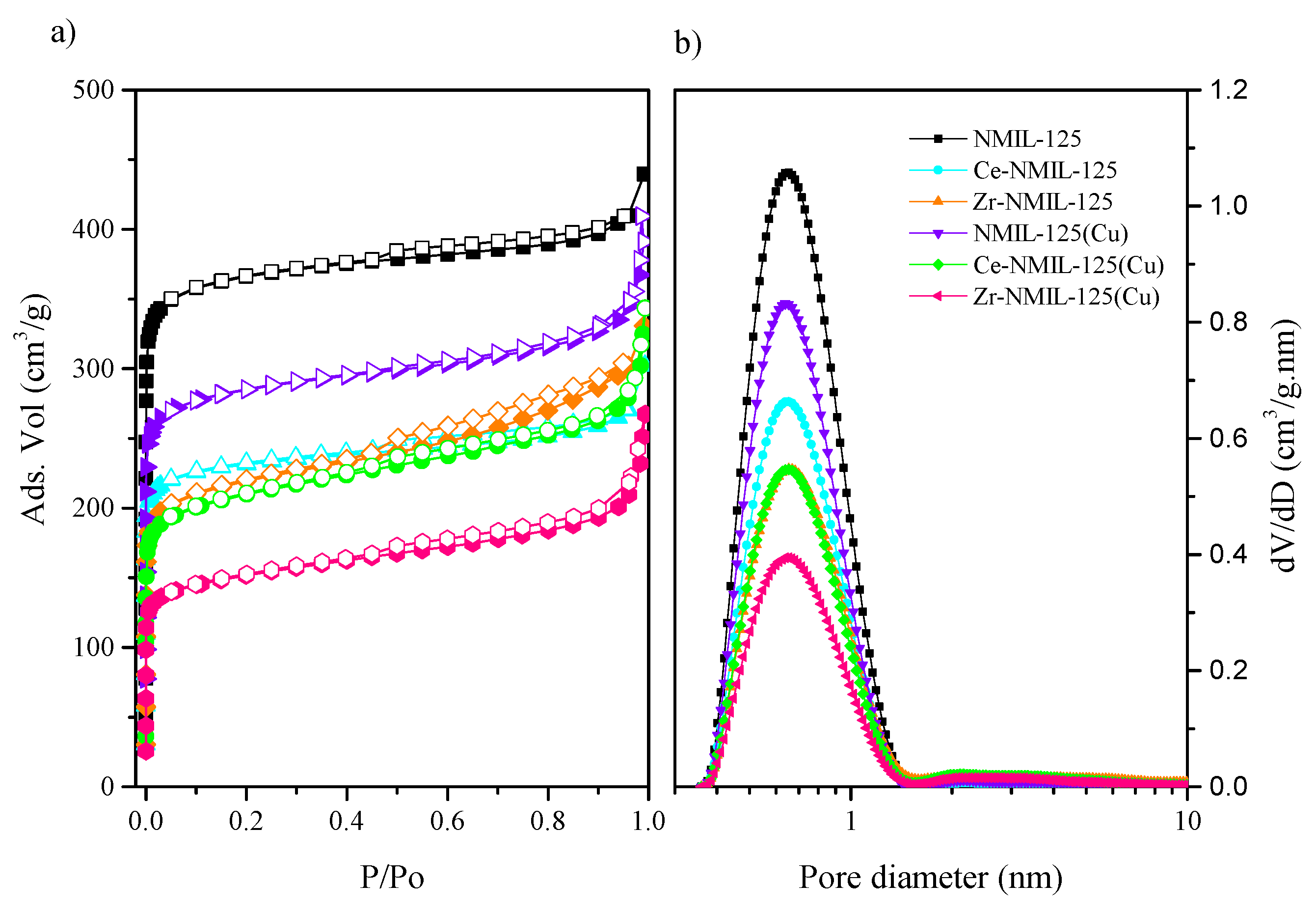
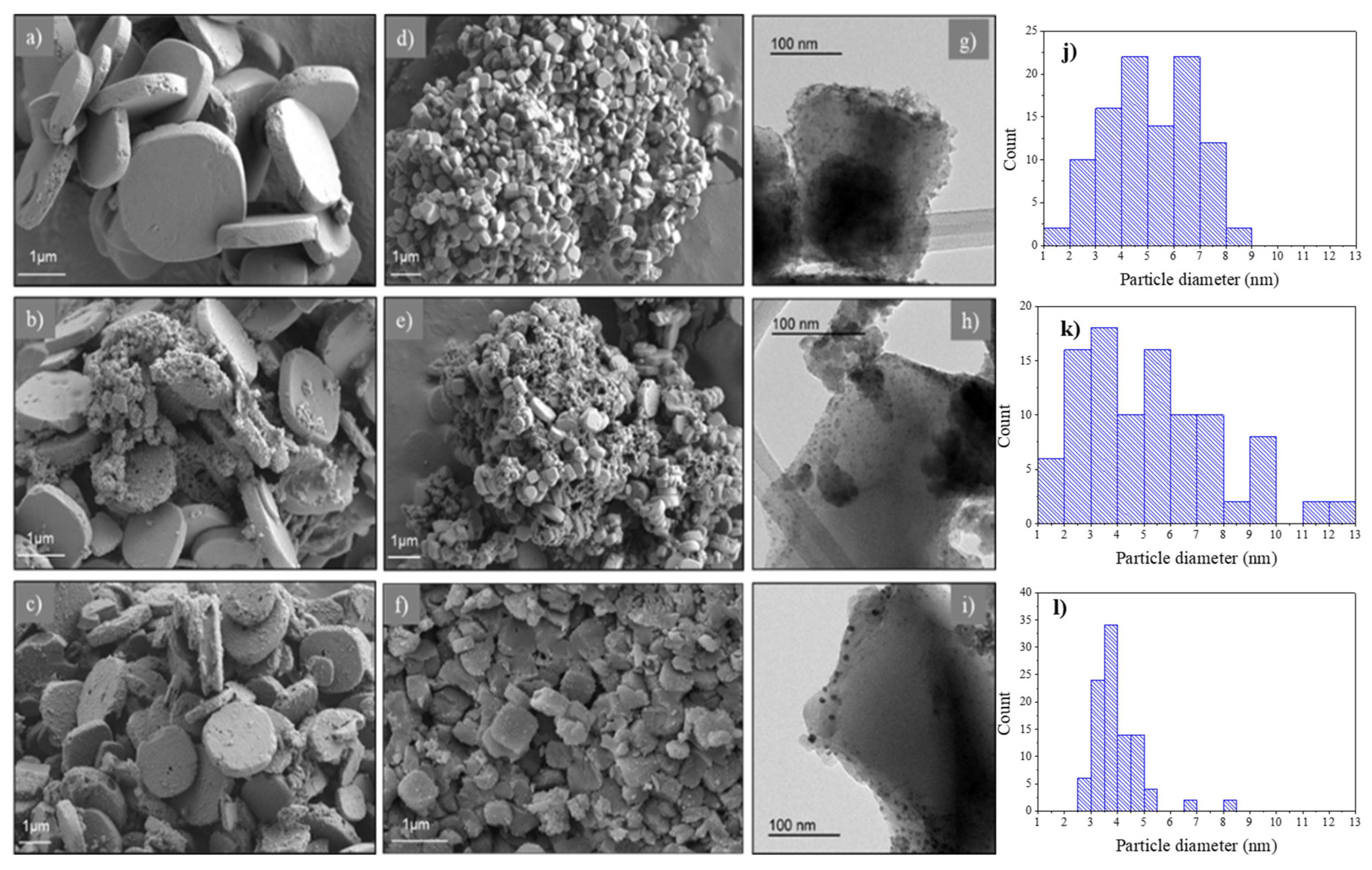
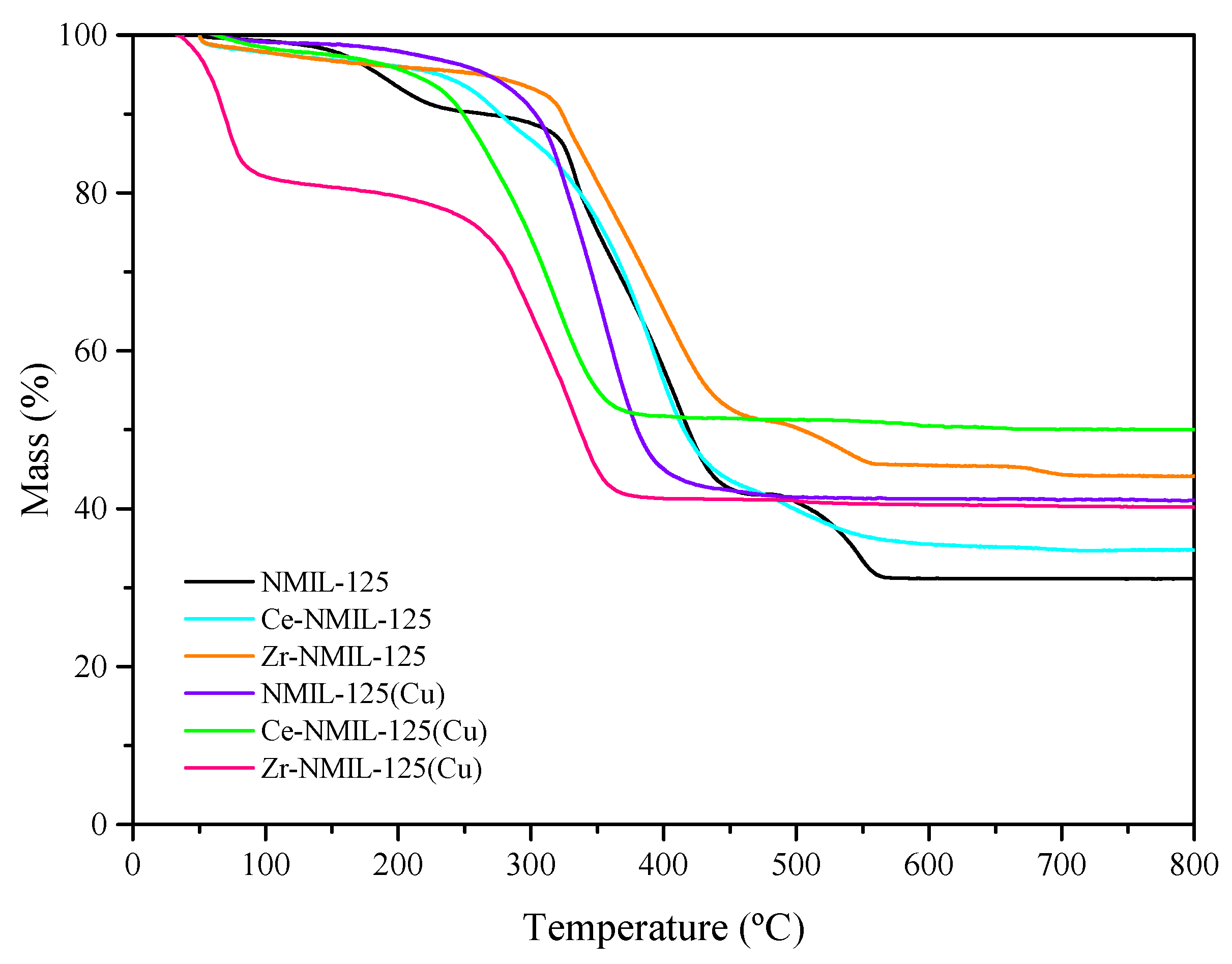
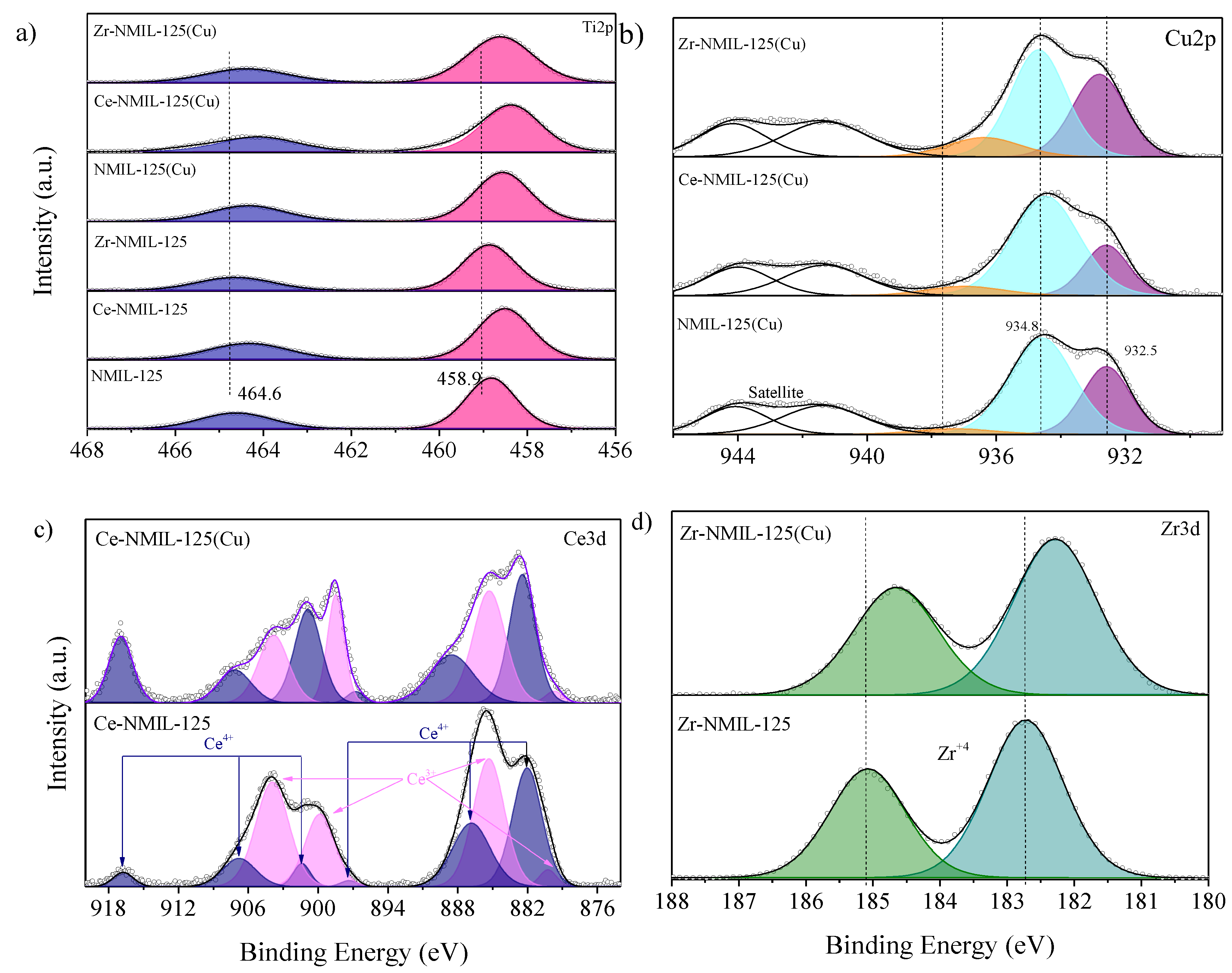
2.1. Characterization of Catalysts
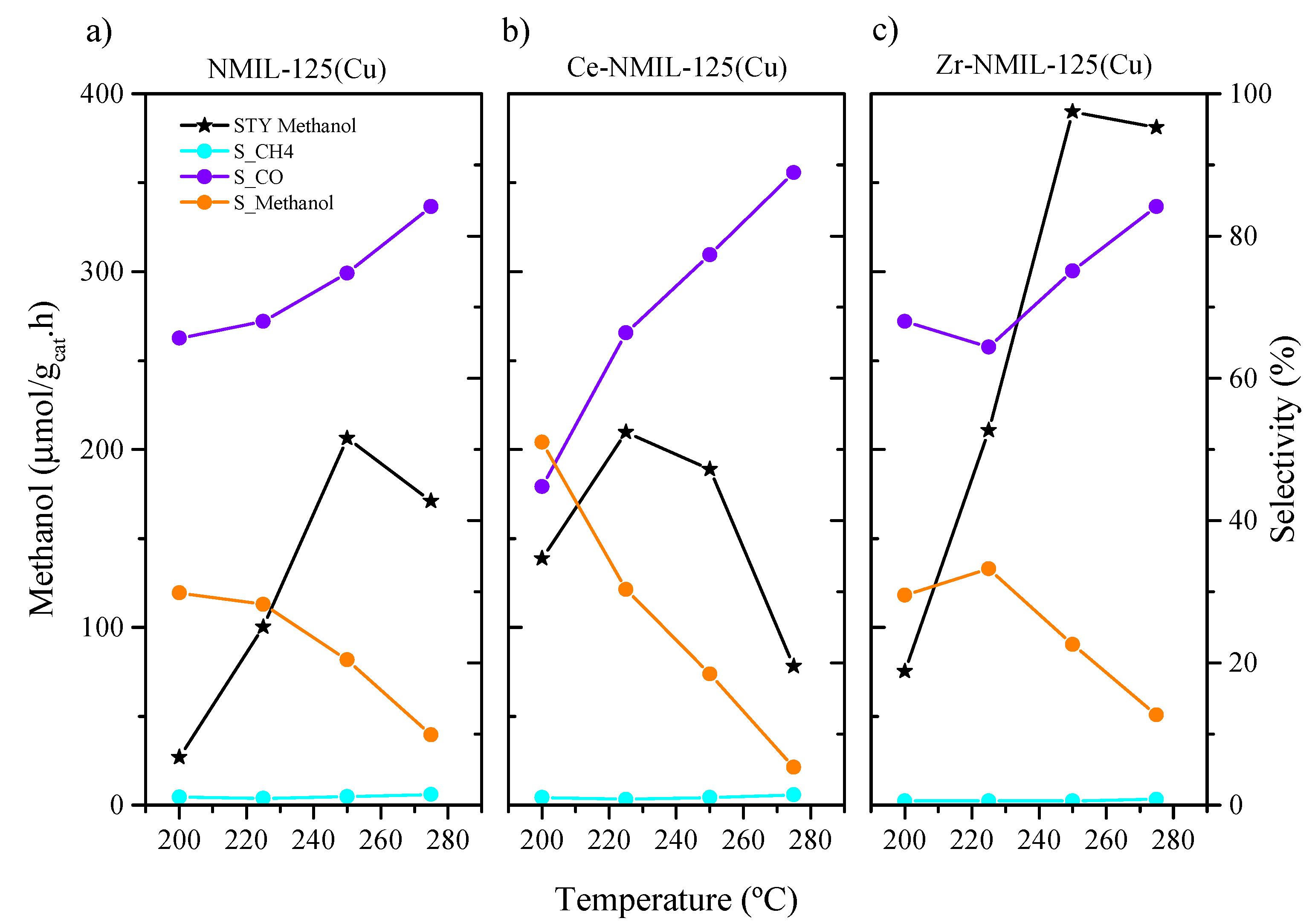
2.2. CO2 Hydrogenation to Methanol Reaction
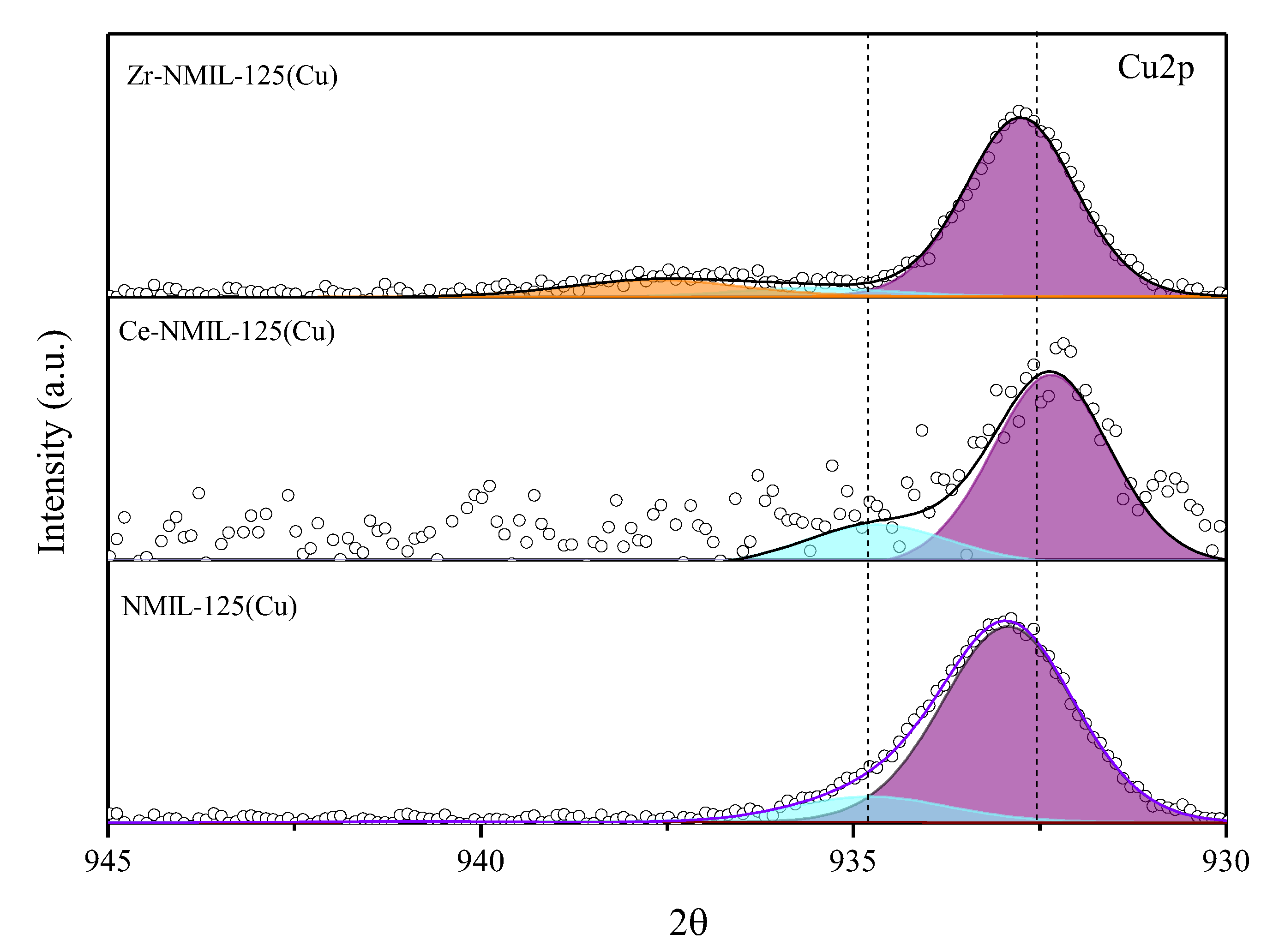
2.3. Characterization After Catalytic Tests
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Materials
3.3. Characterization
3.4. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Browning, S.; Lawrence, T.; Joshi, C.; Seay, J. Analysis of green pesticide production by valorization of husks from Croton megalocarpus tree nuts. Environ. Prog. Sustain. Energy 2020, 39, e13312. [Google Scholar] [CrossRef]
- De Oliveira Silva, R.R.; Calvo, P.V.C.; Merfels, C.A.; Lima, M.V.R.; Santana, H.S.; Converti, A.; Palma, M.S.A. Synthesis of Lobeglitazone inter-mediates seeking for continuous drug production in flow capillary microreactor. J. Ind. Eng. Chem. 2022, 116, 239–249. [Google Scholar] [CrossRef]
- Curry, N.; VanEvery, K.; Snyder, T.; Susnjar, J.; Bjorklund, S. Performance testing of suspension plasma sprayed thermal barrier coatings produced with varied suspension parameters. Coatings 2015, 5, 338–356. [Google Scholar] [CrossRef]
- Dang, S.; Yang, H.; Gao, P.; Wang, H.; Li, X.; Wei, W.; Sun, Y. A review of research progress on het-erogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today 2019, 330, 61–75. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper Nanocrystals Encapsulated in Zr-based Metal-Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar] [CrossRef]
- Martin, O.; Martín, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferré, D.; Pérez-Ramírez, J. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew. Chemie—Int. Ed. 2016, 55, 6261–6265. [Google Scholar] [CrossRef]
- Bavykina, A.; Yarulina, I.; Al Abdulghani, A.J.; Gevers, L.; Hedhili, M.N.; Miao, X.; Galilea, A.R.; Pustovarenko, A.; Dikhtiarenko, A.; Cadiau, A.; et al. Turning a Methanation Co Catalyst into an In-Co Methanol Producer. ACS Catal. 2019, 9, 6910–6918. [Google Scholar] [CrossRef]
- Sun, K.; Fan, Z.; Ye, J.; Yan, J.; Ge, Q.; Li, Y.; He, W.; Yang, W.; Liu, C.J. Hydrogenation of CO2 to methanol over In2O3 catalyst. J. CO2 Util. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Ojelade, O.A.; Zaman, S.F.; Daous, M.A.; Al-Zahrani, A.A.; Malik, A.S.; Driss, H.; Shterk, G.; Gascon, J. Optimizing Pd:Zn molar ratio in PdZn/CeO2 for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 584, 117185. [Google Scholar] [CrossRef]
- Song, J.; Liu, S.; Yang, C.; Wang, G.; Tian, H.; Jian Zhao, Z.; Mu, R.; Gong, J. The role of Al doping in Pd/ZnO catalyst for CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2020, 263, 118367. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Capel Berdiell, I.; Airi, A.; Ho Chung, S.; Mancuso, J.L.; Redekop, E.A.; Fabris, C.; Figueroa Quintero, L.; Navarro de Miguel, J.C.; Narciso, J.; et al. Transitioning from Methanol to Olefins (MTO) toward a Tandem CO2 Hydrogenation Process: On the Role and Fate of Heteroatoms (Mg, Si) in MAPO-18 Zeotypes. JACS Au 2024, 4, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Abou-Hamad, E.; Gong, X.; Sho-inkhorova, T.B.; Dokania, A.; Gascón, J.; Chowdhur, A. Mapping the Methanol-to-Gasoline Process Over Zeolite Beta. Angew. Chem. Int. Ed. Engl. 2023, 62, e202303124. [Google Scholar] [CrossRef]
- Mahnaz, F.; Dunlap, V.; Helmer, R.; Shirish Borkar, S.; Navar, R.; Yang, X.; Shetty, M. Selective Valorization of CO2 towards Valuable Hydrocar-bons through Methanol-mediated Tandem. ChemCatChem 2023, 15, e202300402. [Google Scholar] [CrossRef]
- Filosa, C.; Gong, X.; Bavykina, A.; Chow-dhury, A.D.; Gallo, J.M.R.; Gascon, J. Enabling the Methanol Economy: Opportunities and Challenges for Heterogeneous Catalysis in the Production of Liquid Fuels via Methanol. Acc. Chem. Res. 2023, 56, 3492–3503. [Google Scholar] [CrossRef]
- Frei, E.; Gaur, A.; Lichtenberg, H.; Zwiener, L.; Scherzer, M.; Girgsdies, F.; Lunkenbein, T.; Schlögl, R. Cu−Zn Alloy Formation as Unfavored State for Efficient Methanol Catalysts. ChemCatChem 2020, 12, 4029–4033. [Google Scholar] [CrossRef]
- Yang, X.; Kattel, S.; Senanayake, S.D.; Boscoboinik, J.A.; Nie, X.; Graciani, J.; Rodriguez, J.A.; Liu, P.; Stacchiola, D.J.; Chen, J.G. Low pressure CO2 hydrogenation to methanol over gold nano-particles activated on a CeOx/TiO2 Interface. J. Am. Chem. Soc. 2015, 137, 10104–10107. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Pettinari, C.; Tombesi, A. Metal–organic frameworks for chemical conversion of carbon dioxide. MRS Energy Sustain. 2020, 7, 31. [Google Scholar] [CrossRef]
- Jin, C.; Shi, S.; Liu, S.; Xia, S.; Lou, Y.; Wang, S.; Wang, H.; Chen, C. Post-Synthetic Ligand Exchange by Mechanochemistry: Toward Green, Efficient, and Large-Scale Preparation of Functional Metal–Organic Frameworks. Chem. Mater. 2023, 35, 4489–4497. [Google Scholar] [CrossRef]
- Ye, R.; Ma, L.; Mao, J.; Wang, X.; Hong, X.; Gallo, A.; Ma, Y.; Luo, W.; Wang, B.; Zhang, R.; et al. A Ce-CuZn catalyst with abundant Cu/Zn-OV-Ce active sites for CO2 hydrogenation to methanol. Nat. Commun. 2024, 15, 2159. [Google Scholar] [CrossRef]
- Hou, S.-L.; Dong, J.; Zhao, X.-Y.; Li, X.-S.; Ren, F.-Y.; Zhao, J.; Zhao, B. Thermocatalytic Conversion of CO2 to Valuable Products Activated by Noble-Metal-Free Metal-Organic Frameworks. Angew. Chem. 2023, 62, e202305213. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Quintero, L.; Villalgordo-Hernández, D.; Delgado-Marín, J.J.; Narciso, J.; Kumar Velisoju, V.; Castaño, P.; Gascón, J. Post-Synthetic Surface Modification of Metal Organic Frameworks and Their Potential Applications. Small Methods 2023, 7, 2201413. [Google Scholar] [CrossRef] [PubMed]
- Villalgordo-Hernández, D.; Antonio Diaz-Perez, M.; Balloi, V.; Anabel Lara-Angulo, M.; Narciso, J.; Serrano-Ruiz, J.C.; Ramos-Fernandez, E.V. Post-synthetic ligand exchange as a route to improve the affinity of ZIF-67 towards CO2. Chem. Eng. J. 2023, 476, 146846. [Google Scholar] [CrossRef]
- Chen, X.; Lyu, Y.; Wang, Z.; Qiao, X.; Gates, B.C.; Yang, D. Tuning Zr12O22 Node Defects as Catalytic Sites in the Metal-Organic Framework hcp UiO-66. ACS Catal. 2020, 10, 2906–2914. [Google Scholar] [CrossRef]
- Li, Z.; Peters, A.W.; Bernales, V.; Ortuño, M.A.; Schweitzer, N.M.; Destefano, M.R.; Gallington, L.C.; Platero-Prats, A.E.; Chapman, K.W.; Cramer, C.J.; et al. Metal-Organic Framework Supported Cobalt Catalysts for the Oxidative Dehydrogenation of Propane at Low Temperature. ACS Cent. Sci. 2017, 3, 31–38. [Google Scholar] [CrossRef]
- Otake, K.I.; Ye, J.; Mandal, M.; Islamoglu, T.; Buru, C.T.; Hupp, J.T.; Delferro, M.; Truhlar, D.G.; Cramer, C.J.; Farha, O.K. Enhanced Activity of Heterogeneous Pd(II) Catalysts on Acid-Functionalized Metal-Organic Frameworks. ACS Catal. 2019, 9, 5383–5390. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Primo, A.; García, H. Selective Gas-Phase Hydrogenation of CO2 to Methanol Catalysed by Metal-Organic Frameworks. Angew. Chem. Int. Ed. Engl. 2024, 63, e202311241. [Google Scholar] [CrossRef]
- Niu, J.; Liu, H.; Jin, Y.; Fan, B.; Qi, W.; Ran, J. Comprehensive review of Cu-based CO2 hydrogenation to CH3OH: Insights from experimental work and theoretical analysis. Int. J. Hydrogen Energy 2022, 47, 9183–9200. [Google Scholar] [CrossRef]
- An, B.; Zhang, J.; Cheng, K.; Ji, P.; Wang, C.; Lin, W. Confinement of Ultrasmall Cu/ZnOx Nanopar-ticles in Metal-Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO2. J. Am. Chem. Soc. 2017, 139, 3834–3840. [Google Scholar] [CrossRef]
- Velisoju, V.K.; Cerrillo, J.L.; Ahmad, R.; Mohamed, H.O.; Attada, Y.; Cheng, Q.; Yao, X.; Zheng, L.; Shekhah, O.; Telalovic, S.; et al. Copper nanoparticles encapsulated in zeolitic imidazolate framework-8 as a stable and selective CO2 hydrogenation catalyst. Nat. Commun. 2024, 15, 2045. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Liang, Y.; Zhang, L.; Li, H.; Zhu, N.-X.; Zhong Tang, B.; Zhao, D.; Liu, B. CO2-Based Stable Porous Metal–Organic Frameworks for CO2 Utilization. J. Am. Chem. Soc. 2024, 146, 14835–14843. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, S.; Li, Y.; She, J.; Tian, X.; Ding, M. Highly dispersed Cu on hollow spherical CeO2: An efficient and stable catalyst for the RWGS reaction. Appl. Catal. B 2025, 366, 125003. [Google Scholar] [CrossRef]
- Stawowy, A.; Ciesielski, M.; Maniecki, R.; Matus, T.; Łużny, K.; Trawczynski, R.; Silvestre-Albero, J.; Łamacz, J. CO2 Hydrogenation to Methanol over Ce and Zr Containing UiO-66 and Cu/UiO-66. Catalysts 2020, 10, 39. [Google Scholar] [CrossRef]
- Kim, S.N.; Kim, J.; Kim, H.Y.; Cho, H.Y.; Ahn, W.S. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93. [Google Scholar] [CrossRef]
- Muelas-Ramos, V.; Peñas-Garzón, M.; Rodriguez, J.J.; Bedia, J.; Belver, C. Solar photocatalytic degradation of emerging contaminants using NH2-MIL-125 grafted by heterocycles. Sep. Purif. Technol. 2022, 297, 121442. [Google Scholar] [CrossRef]
- Guo, F.; Yang, M.; Li, R.X.; He, Z.Z.; Wang, Y.; Sun, W.Y. Nanosheet-Engineered NH2-MIL-125 with Highly Active Facets for Enhanced Solar CO2 Reduction. ACS Catal. 2022, 12, 9486–9493. [Google Scholar] [CrossRef]
- Bakiro, M.; Ahmed, S.H.; Alzamly, A. Cycloaddition of CO2 to propylene oxide using BiN-bO4/NH2-MIL-125(Ti) composites as visible-light photocatalysts. J. Environ. Chem. Eng. 2020, 8, 104461. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An Amine-Functionalized Titanium Metal Organic Framework Photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chemie Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Solís, R.R.; Gómez-Avilés, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Microwave-assisted synthesis of NH2-MIL-125(Ti) for the solar photocatalytic degradation of aqueous emerging pollutants in batch and continuous tests. J. Environ. Chem. Eng. 2021, 9, 106230. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Hu, S.; Liu, M.; Li, K.; Song, C.; Zhang, G.; Guo, X. Surfactant-assisted synthesis of hierarchical NH2-MIL-125 for the removal of organic dyes. RSC Adv. 2017, 7, 581–587. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Lei, H.; Chen, J.; Wang, S.; Chen, C. Combining shell confinement and outside decoration to boost the selectivity of Pt nanocatalysts in hollow-flower like Zr-MOFs double shell micro-reactor for hydrogenation cinnamaldehyde to unsaturated alcohol. Appl. Surf. Sci. 2022, 599, 153899. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Li, K.; Zuo, Y.; Zhang, A.; Song, C.; Zhang, G.; Guo, X. Solvothermal synthesis of NH2-MIL-125(Ti) from circular plate to octahedron. CrystEngComm 2014, 16, 9645–9650. [Google Scholar] [CrossRef]
- Hu, J.; Ding, J.; Zhong, Q. In situ fabrication of amorphous TiO2/NH2-MIL-125(Ti) for enhanced photocatalytic CO2 into CH4 with H2O under visible-light irradiation. J. Colloid Interface Sci. 2020, 560, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, J.; Ye, J.; Cui, Y.; Koh, K.; Kovarik, L.; Camaioni, D.M.; Fulton, J.L.; Truhlar, D.G.; Neurock, M.; et al. Copper-zirconia interfaces in UiO-66 en-able selective catalytic hydrogenation of CO2 to methanol. Nat. Commun. 2020, 11, 5849. [Google Scholar] [CrossRef]
- Zhang, K.; Chu, F.; Hu, Y.; Huang, X.; Zhao, G.; Wang, G. Ce-doped MIL-125-NH2 coupled Ce4+/Ce3+ and Ti4+/Ti3+ redox mediators for thermo-enhanced photocatalytic oxidative desulfurization. Chin. Chem. Lett. 2023, 34, 4–8. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, Y.; Wang, Z.; Chen, M.; Sun, L.; Liu, X. Titanium incorporated with UiO-66(Zr)-type Metal-Organic Framework (MOF) for photo-catalytic application. RSC Adv. 2016, 6, 3671–3679. [Google Scholar] [CrossRef]
- Bibi, R.; Wei, L.; Shen, Q.; Tian, W.; Oder-inde, O.; Li, N.; Zhou, J. Effect of Amino Functionality on the Uptake of Cationic Dye by Titanium-Based Metal Organic Frameworks. J. Chem. Eng. Data 2017, 62, 1615–1622. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Wu, Z.; Jiang, L.; Li, H. Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photo-catalytic activity for Cr(VI) reduction. J. Hazard. Mater. 2015, 286, 187–194. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakani-shi, S.; Hashimoto, K. Nitrogen-doped carbon nano-materials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 2–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, X.; Jiang, X.; Chen, H.; Long, Z. Ce-based UiO-66 metal-organic frameworks as a new redox catalyst for atomic spectrometric determination of Se(VI) and colorimetric sensing of Hg(II). Microchem. J. 2019, 149, 103967. [Google Scholar] [CrossRef]
- Vazirov, R.A.; Sokovnin, S.Y.; Ilves, V.G.; Bazhukova, I.N.; Pizurova, N.; Kuznetsov, M.V. Physi-cochemical characterization and antioxidant prop-erties of cerium oxide nanoparticles. J. Phys. Conf. Ser. 2018, 1115, 032094. [Google Scholar] [CrossRef]
- Kang, M.; Huang, S.; Wang, M.; Oderinde, O.; Wang, M.; Zhang, Z. Defective zirconium/titanium bimetallic metal–organic framework as a highly selective and sensitive electrochemical aptasensor for deoxynivalenol determination in foodstuffs. Microchim. Acta 2023, 190, 358. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wu, C.K.; Lou, Y.; Burda, C.; Ko-berstein, J.T.; Zhu, Y.; O’Brien, S. Copper oxide nano-crystals. J. Am. Chem. Soc. 2005, 127, 9506–9511. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Taylor, J.M.; Mitsuka, Y.; Ogiwara, N.; Yamamoto, T.; Toriyama, T.; Matsumu-ra, S.; Kitagawa, H. Charge transfer dependence on CO2 hydrogenation activity to methanol in Cu na-noparticles covered with metal-organic framework systems. Chem. Sci. 2019, 10, 3289–3294. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiao, S.; Wang, H.; Wang, W.; Cai, Y.; Li, G.; Qiao, M.; Zhu, J.; Li, H.; Zhang, D.; et al. MOFs Conferred with Transient Metal Centers for Enhanced Photocatalytic Activity. Angew. Chemie—Int. Ed. 2020, 59, 17182–17186. [Google Scholar] [CrossRef]
- Liu, H.-X.; Li, S.-Q.; Wang, W.-W.; Yu, W.-Z.; Zhang, W.-J.; Ma, C.; Jia, C.-J. Partially sintered copper–ceria as excellent catalyst for the high-temperature reverse water gas shift reaction. Nat. Commun. 2022, 13, 867. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; Cheng, L.; Wang, C.; Mei, X. Engineering the Self-Assembly Induced Emission of Copper Nanoclusters as 3D Nanomaterials with Mesoporous Sphere Structures by the Crosslinking of Ce3+. ACS Omega 2018, 3, 14755–14765. [Google Scholar] [CrossRef]
- Villalgordo-Hernández, D.; Grau-Atienza, A.; García-Marín, A.A.; Ramos-Fernández, E.V.; Narciso, J. Manufacture of Carbon Materials with High Nitrogen Content. Materials 2022, 15, 2415. [Google Scholar] [CrossRef]
- Narciso, J.; Ramos-Fernandez, E.V.; Delgado-Marin, J.J.; Affolter, C.W.; Olsbye, U.; Redekop, E.A. New route for the synthesis of Co-MOF from metal substrates. Microporous Mesoporous Mater. 2021, 324, 111310. [Google Scholar] [CrossRef]

| at% | Ratio | Formula Units | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Ti | Zr | Ce | Cu | Ti/M | Ti | Zr | Ce |
| NMIL–125 | 100 | –– | –– | –– | –– | 8 | –– | –– |
| Ce–NMIL–125 | 85.7 | –– | 14.3 | –– | 6.0 | 6.86 | –– | 1.14 |
| Zr–NMIL–125 | 81.0 | 19.0 | –– | –– | 4.3 | 6.48 | 1.52 | –– |
| NMIL–125(Cu) | 72 | –– | –– | 28.0 | –– | –– | –– | –– |
| Ce–NMIL–125(Cu) | 60.3 | –– | 10.2 | 29.5 | 5.9 | –– | –– | –– |
| Zr–NMIL–125(Cu) | 60.4 | 12.2 | –– | 27.4 | 4.9 | –– | –– | –– |
| Sample | Specific Surface Area BET (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|
| NMIL–125 | 1452 | 0.57 | 0.11 | 0.65 |
| Ce–NMIL–125 | 914 | 0.36 | 0.11 | 0.65 |
| Zr–NMIL–125 | 847 | 0.34 | 0.17 | 0.66 |
| NMIL–125(Cu) | 1122 | 0.44 | 0.19 | 0.64 |
| Ce–NMIL–125(Cu) | 805 | 0.32 | 0.21 | 0.66 |
| Zr–NMIL–125(Cu) | 581 | 0.23 | 0.18 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa-Quintero, L.; Cordero-Lanzac, T.; Ramos-Fernandez, E.V.; Olsbye, U.; Narciso, J. Tailoring Catalysts for CO2 Hydrogenation: Synthesis and Characterization of NH2–MIL–125 Frameworks. Molecules 2025, 30, 1458. https://doi.org/10.3390/molecules30071458
Figueroa-Quintero L, Cordero-Lanzac T, Ramos-Fernandez EV, Olsbye U, Narciso J. Tailoring Catalysts for CO2 Hydrogenation: Synthesis and Characterization of NH2–MIL–125 Frameworks. Molecules. 2025; 30(7):1458. https://doi.org/10.3390/molecules30071458
Chicago/Turabian StyleFigueroa-Quintero, Leidy, Tomás Cordero-Lanzac, Enrique V. Ramos-Fernandez, Unni Olsbye, and Javier Narciso. 2025. "Tailoring Catalysts for CO2 Hydrogenation: Synthesis and Characterization of NH2–MIL–125 Frameworks" Molecules 30, no. 7: 1458. https://doi.org/10.3390/molecules30071458
APA StyleFigueroa-Quintero, L., Cordero-Lanzac, T., Ramos-Fernandez, E. V., Olsbye, U., & Narciso, J. (2025). Tailoring Catalysts for CO2 Hydrogenation: Synthesis and Characterization of NH2–MIL–125 Frameworks. Molecules, 30(7), 1458. https://doi.org/10.3390/molecules30071458









