Innovative Sorbents for the Removal of Micropollutants from Water
Abstract
1. Introduction
2. Traditional Modified Sorbents
2.1. Activated Carbon
2.2. Composites with Clays
2.3. Biochar Modified with Transition Metals
3. New or Innovated Types of Sorbents
3.1. Carbon-Based Sorbents
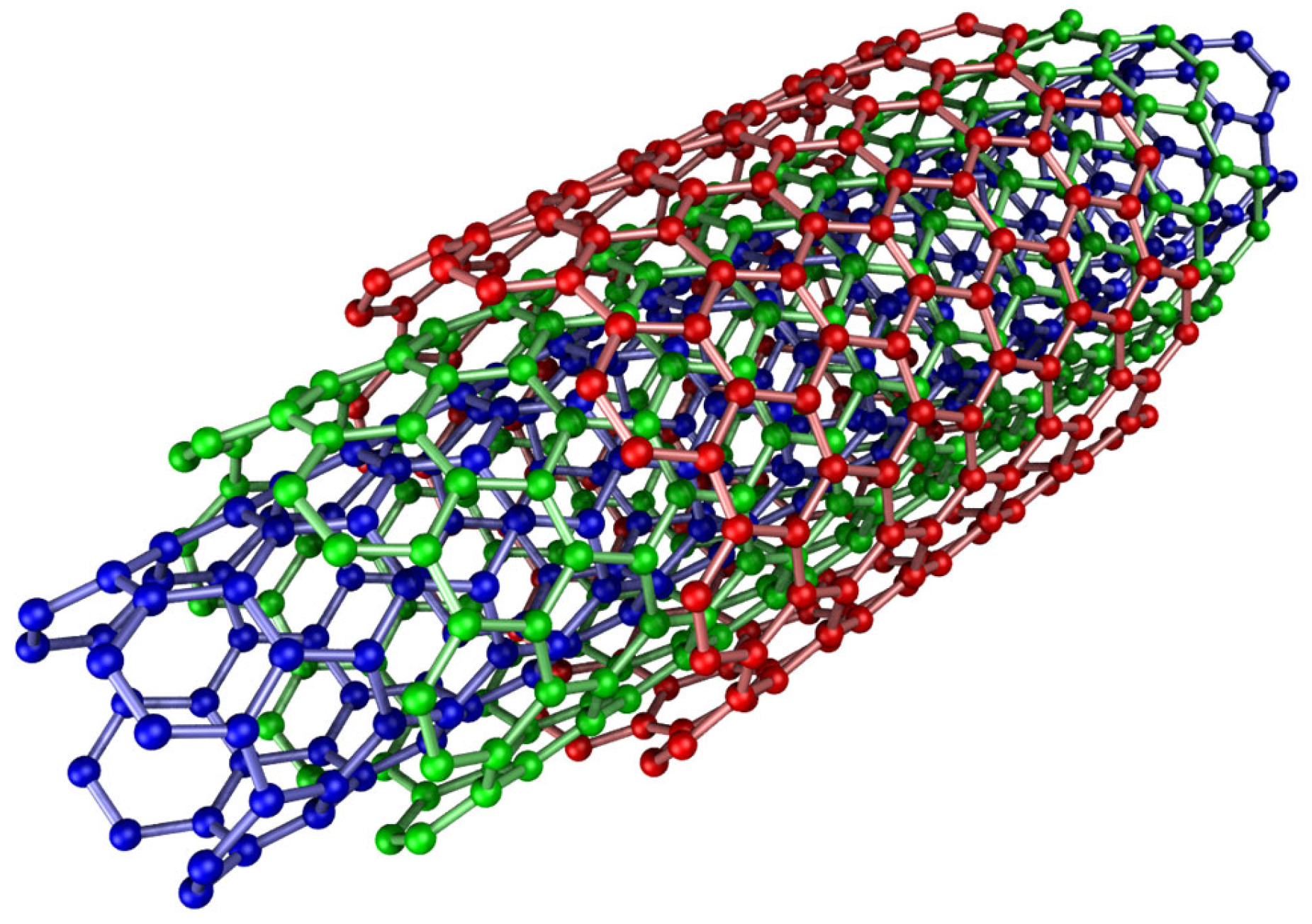
3.1.1. Carbon and Metal-Based Nanotubes
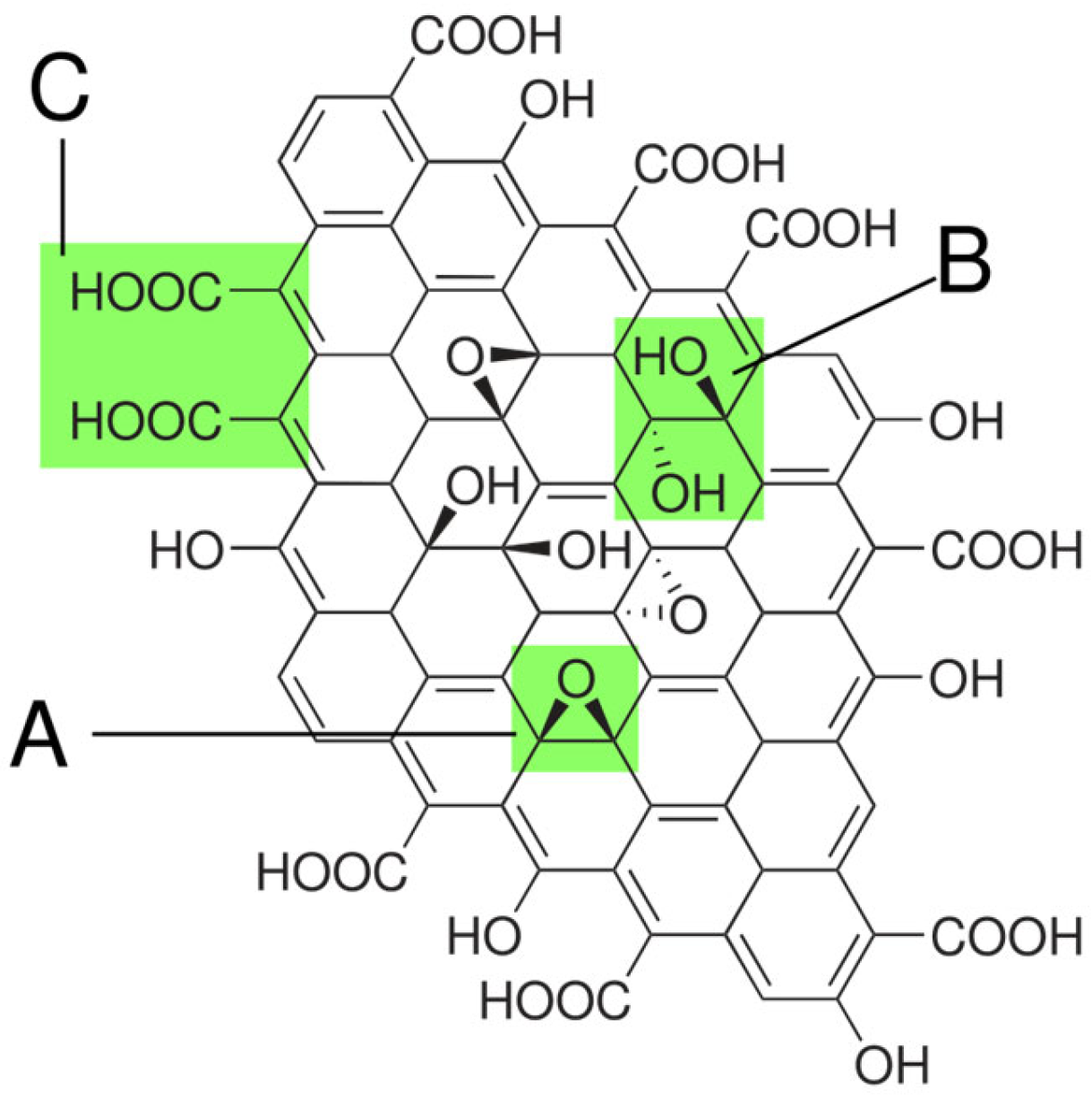
3.1.2. Graphene/Graphene Oxide
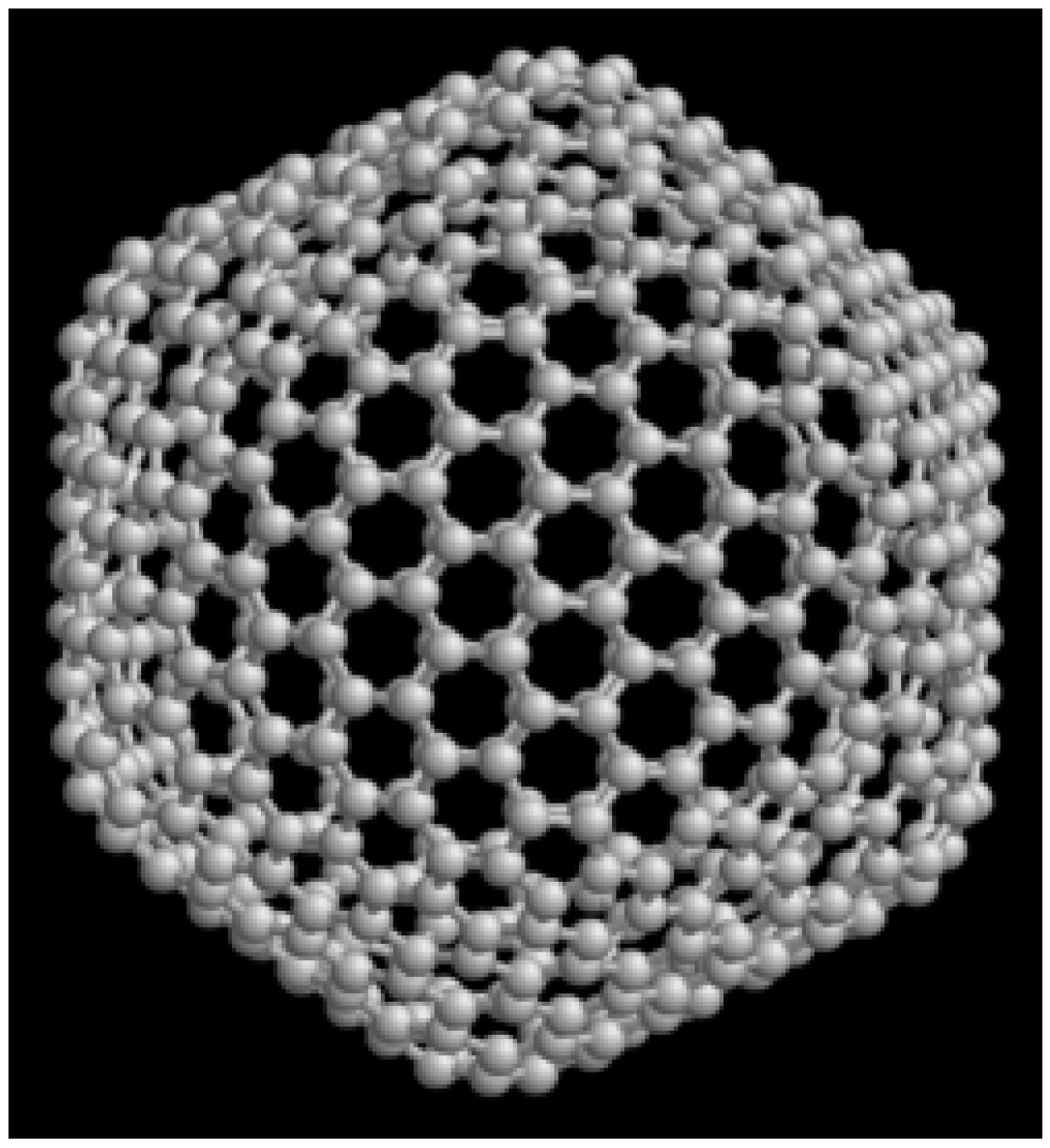
3.1.3. Fullerenes
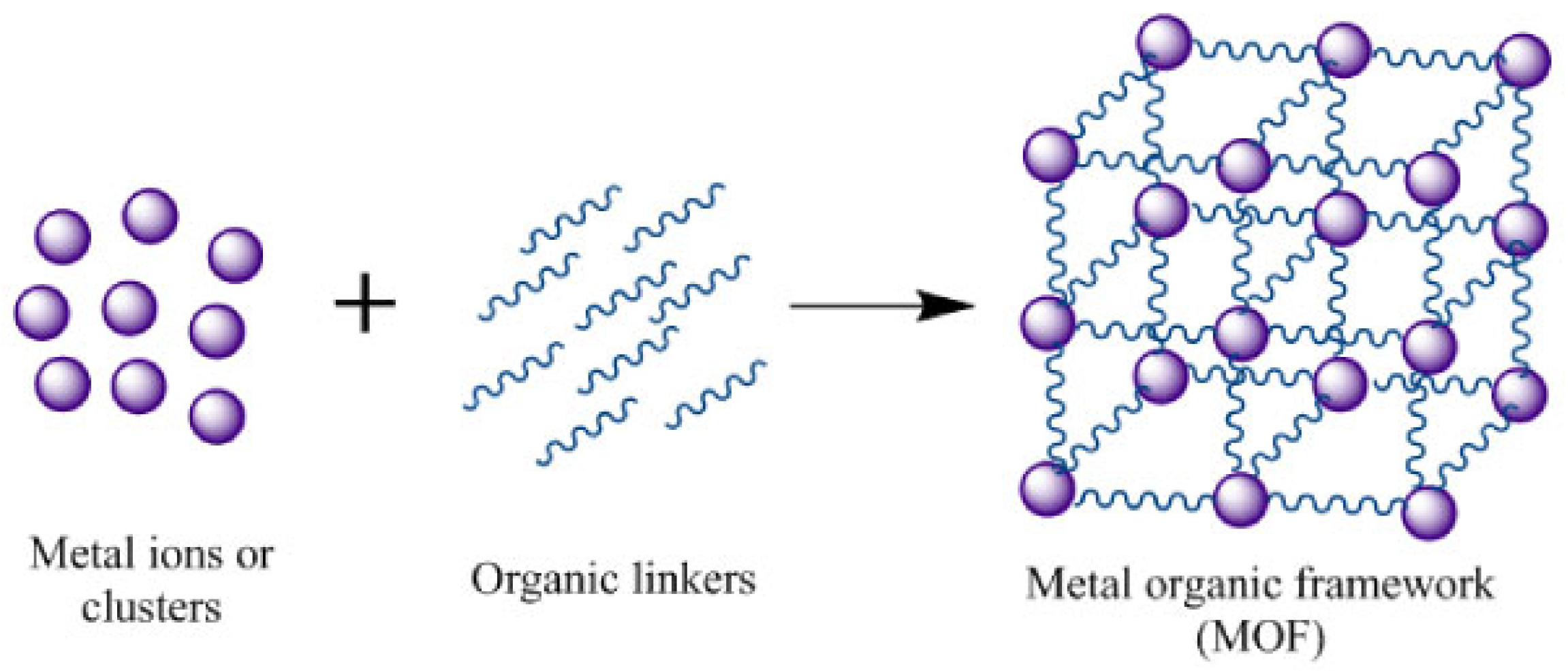
3.2. MOF—Metal Organic Framework
3.3. Aerogels as Innovative Sorbents
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ampC | Antibiotic Resistance Gene of β-Lactam |
| BDC | 1,4-benzenedicarboxylate |
| BDMHDA | benzyldimethylhexadecylammonium |
| CNTs | Carbon nanotubes |
| DDAB | Didodecyldimethylammonium bromide |
| DMF | dimethylformamide |
| ecfX | Antibiotic Resistance Gene |
| ermB | Antibiotic Resistance Gene of Macrolide |
| GO | Graphene oxide |
| HDTMA | hexadecyltrimethylammonium bromide |
| HPVP | poly-4-vinylpyridine-co-styrene |
| MOF | Metal organic framework |
| NZVI | Nano-zero valent iron |
| ODTMA | octadecyltrimethylammonium |
| PET | Polyethyleneglycol terephthalate |
| PFAS | polyfluoroalkyl substances |
| PFOA | perfluorooctanoic acid |
| POE | Portulaca oleracea L. extract |
| POPs | Persistent Organic Pollutants |
| rGO | Reduced graphene oxide |
| SA | Silica Aerogel |
| Sul2 | Antibiotic Resistance Gene of Sulfanilamide |
| tetA | Antibiotic Resistance Gene of Tetracycline |
| WWTPs | Wastewater treatment plants |
References
- Khan, N.A.; Khan, S.U.; Ahmed, S.; Farooqi, I.H.; Yousefi, M.; Mohammadi, A.A.; Changani, F. Recent Trends in Disposal and Treatment Technologies of Emerging-Pollutants—A Critical Review. Trends Anal. Chem. 2020, 122, 115744. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A Review on Effective Removal of Emerging Contaminants from Aquatic Systems: Current Trends and Scope for Further Research. J. Hazard Mater. 2021, 409, 124413. [Google Scholar] [CrossRef] [PubMed]
- Di Marcantonio, C.; Chiavola, A.; Dossi, S.; Cecchini, G.; Leoni, S.; Frugis, A.; Spizzirri, M.; Boni, M.R. Occurrence, Seasonal Variations and Removal of Organic Micropollutants in 76 Wastewater Treatment Plants. Process Saf. Environ. Prot. 2020, 141, 61–72. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef]
- Speth, T. PFAS Treatment in drinking water and wastewater. US EPA Office of Research and Development. In Proceedings of the PFAS Science Webinars for EPA Region 1 and State & Tribal Partners, Web Conference, 16 September 2020. [Google Scholar]
- Solcova, O.; Dlaskova, M.; Kastanek, F. Challenges and Advances in Tertiary Waste Water Treatment for Municipal Treatment Plants. Processes 2024, 12, 2084. [Google Scholar] [CrossRef]
- Adewuyi, A. Chemically Modified Biosorbents and Their Role in the Removal of Emerging Pharmaceutical Waste in the Water System. Water 2020, 12, 1551. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An Overview of the Modification Methods of Activated Carbon for Its Water Treatment Applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Giwa, A.S.; Ndungutse, J.M.; Li, Y.; Mabi, A.; Liu, X.; Vakili, M.; Memon, A.G.; Ai, L.; Chenfeng, Z.; Sheng, M. Modification of Biochar with Fe 3 O 4 and Humic Acid-Salt for Removal of Mercury from Aqueous Solutions: A Review. Environ. Pollut. Bioavailab. 2022, 34, 352–364. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagrán, D. Green Synthesis of Magnetic MOF@GO and MOF@CNT Hybrid Nanocomposites with High Adsorption Capacity towards Organic Pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef]
- Koga, H.; Kitaoka, T. Activated Carbon Water Purification Filter Prepared by Wet Molding with a DualPolyelectrolyte Retention System. Sen’i Gakkaishi 2011, 67, 81–85. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Sreńscek-Nazzal, J. An Innovative and Environmentally Friendly Bioorganic Synthesis of Activated Carbon Based on Olive Stones and Its Potential Application for CO2 Capture. Sustain. Mater. Technol. 2023, 38, e00717. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.M.; Ribeiro Carrott, M.M.L.; Singh, R.; Singh, L.P.; Chaudhary, M. An Innovative Approach to Develop Microporous Activated Carbons in Oxidising Atmosphere. J. Clean. Prod. 2017, 156, 549–555. [Google Scholar] [CrossRef]
- Pam, A.A. Innovative Activated Carbon Based on Deep Eutectic Solvents (DES) and H3PO4. C 2019, 5, 43. [Google Scholar] [CrossRef]
- Tian, H.; Pan, J.; Zhu, D.; Guo, Z.; Yang, C.; Xue, Y.; Li, S.; Wang, Y. Innovative One-Step Preparation of Activated Carbon from Low-Rank Coals Activated with Oxidized Pellets. J. Clean. Prod. 2021, 313, 127877. [Google Scholar] [CrossRef]
- Koo-amornpattana, W.; Phadungbut, P.; Kunthakudee, N.; Jonglertjunya, W.; Ratchahat, S.; Hunsom, M. Innovative Metal Oxides (CaO, SrO, MgO) Impregnated Waste-Derived Activated Carbon for Biohydrogen Purification. Sci. Rep. 2023, 13, 4705. [Google Scholar] [CrossRef]
- Ajayi, O.; Bowaje, M.; Ojo, A.; Ogunnaiya, B.; Idowu, E.; Oni, S.; Ajayi, O.; Dosunmu, B. A Review on Natural Clay Application for Removal of Pharmaceutical Residue in Wastewater. Prog. Chem. Biochem. Res. 2023, 6, 71–87. [Google Scholar]
- Mahouachi, L.; Rastogi, T.; Palm, W.-U.; Ghorbel-Abid, I.; Ben Hassen Chehimi, D.; Kümmerer, K. Natural Clay as a Sorbent to Remove Pharmaceutical Micropollutants from Wastewater. Chemosphere 2020, 258, 127213. [Google Scholar] [CrossRef]
- Viegas, R.M.A.; Melo, M.L.; Brandão Lima, L.C.; Garcia, R.R.P.; Filho, E.C.S.; Osajima, J.A.; Chiavone-Filho, O. Carbamazepine Adsorption with a Series of Organoclays: Removal and Toxicity Analyses. Appl. Water Sci. 2024, 14, 133. [Google Scholar] [CrossRef]
- Lelario, F.; Gardi, I.; Mishael, Y.; Dolev, N.; Undabeytia, T.; Nir, S.; Scrano, L.; Bufo, S.A. Pairing Micropollutants and Clay-Composite Sorbents for Efficient Water Treatment: Filtration and Modeling at a Pilot Scale. Appl. Clay Sci. 2017, 137, 225–232. [Google Scholar] [CrossRef]
- Khan, S.; Ajmal, S.; Hussain, T.; Rahman, M.U. Clay-Based Materials for Enhanced Water Treatment: Adsorption Mechanisms, Challenges, and Future Directions. J. Umm Al Qura Univ. Appl. Sci. 2023, 9, 1–16. [Google Scholar] [CrossRef]
- Kovalchuk, I. Clay-Based Sorbents for Environmental Protection from Inorganic Pollutants. Environ. Sci. Proc. 2023, 25, 34. [Google Scholar] [CrossRef]
- de Farias, M.B.; Spaolonzi, M.P.; da Silva, T.L.; da Silva, M.G.C.; Vieira, M.G.A. Natural and Synthetic Clay-Based Materials Applied for the Removal of Emerging Pollutants from Aqueous Medium. In Advanced Materials for Sustainable Environmental Remediation: Terrestrial and Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2022; pp. 359–392. [Google Scholar] [CrossRef]
- Munir, M.; Nazar, M.F.; Zafar, M.N.; Zubair, M.; Ashfaq, M.; Hosseini-Bandegharaei, A.; Khan, S.U.-D.; Ahmad, A. Effective Adsorptive Removal of Methylene Blue from Water by Didodecyldimethylammonium Bromide-Modified Brown Clay. ACS Omega 2020, 5, 16711–16721. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Mu, B.; Zhang, T.; Dong, C.; Zhu, Y.; Zong, L.; Wang, A. Synthesis of Biochar/Clay Mineral Nanocomposites Using Oil Shale Semi-Coke Waste for Removal of Organic Pollutants. Biochar 2023, 5, 7. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A Critical Review of Clay-Based Composites with Enhanced Adsorption Performance for Metal and Organic Pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Atugoda, T.; Ashiq, A.; Keerthanan, S.; Wijekoon, P.; Ramanayaka, S.; Vithanage, M. Biochar Amalgamation with Clay: Enhanced Performance for Environmental Remediation. Adv. Chem. Pollut. Environ. Manag. Prot. 2021, 7, 1–37. [Google Scholar] [CrossRef]
- da Silva Neto, L.D.; de Sá, Í.M.G.L.; Gabriel, R.; dos Santos Lins, P.V.; Freire, J.T.; Meili, L. Application of Clay-Biochar Composites as Adsorbents for Water Treatment. In Clay Composites; Springer: Singapore, 2023; pp. 113–142. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.C.; Zhao, Z.; Liu, D.; Ren, J.; Luo, Y. Synthesis and Characterization of Clay-Biochars Produced with Facile Low-Temperature One-Step in the Presence of Air for Adsorbing Methylene Blue from Aqueous Solution. Front. Environ. Sci. 2023, 11, 1137284. [Google Scholar] [CrossRef]
- Rallet, D.; Paltahe, A.; Tsamo, C.; Loura, B. Synthesis of Clay-Biochar Composite for Glyphosate Removal from Aqueous Solution. Heliyon 2022, 8, e09112. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of Pollutants from Wastewater by Biochar: A Review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Qin, Y.; Li, G.; Gao, Y.; Zhang, L.; Ok, Y.S.; An, T. Persistent Free Radicals in Carbon-Based Materials on Transformation of Refractory Organic Contaminants (ROCs) in Water: A Critical Review. Water Res. 2018, 137, 130–143. [Google Scholar] [CrossRef]
- Li, X.; Cheng, H. Mn-Modified Biochars for Efficient Adsorption and Degradation of Cephalexin: Insight into the Enhanced Redox Reactivity. Water Res. 2023, 243, 120368. [Google Scholar] [CrossRef]
- Xu, Z.; Xiang, Y.; Zhou, H.; Yang, J.; He, Y.; Zhu, Z.; Zhou, Y. Manganese Ferrite Modified Biochar from Vinasse for Enhanced Adsorption of Levofloxacin: Effects and Mechanisms. Environ. Pollut. 2021, 272, 115968. [Google Scholar] [CrossRef]
- Niu, Z.; Feng, W.; Huang, H.; Wang, B.; Chen, L.; Miao, Y.; Su, S. Green Synthesis of a Novel Mn–Zn Ferrite/Biochar Composite from Waste Batteries and Pine Sawdust for Pb2+ Removal. Chemosphere 2020, 252, 126529. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Li, Y.; Zeng, W.; Yang, G.; Zeng, J.; Nie, J.; Zhou, Y. Synergistic Adsorption and Oxidation of Trivalent Antimony from Groundwater Using Biochar Supported Magnesium Ferrite: Performances and Mechanisms. Environ. Pollut. 2023, 323, 121318. [Google Scholar] [CrossRef]
- Gul, E.; Alrawashdeh, K.A.B.; Masek, O.; Skreiberg, Ø.; Corona, A.; Zampilli, M.; Wang, L.; Samaras, P.; Yang, Q.; Zhou, H.; et al. Production and Use of Biochar from Lignin and Lignin-Rich Residues (Such as Digestate and Olive Stones) for Wastewater Treatment. J. Anal. Appl. Pyrolysis 2021, 158, 105263. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic Biochar for Environmental Remediation: A Review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Kumar, A.; Lai, C.W.; Naushad, M.; Shehnaz; Iqbal, J.; Stadler, F.J. Activated Carbon as Superadsorbent and Sustainable Material for Diverse Applications. Adsorpt. Sci. Technol. 2022, 2022, 4184809. [Google Scholar] [CrossRef]
- Aslam, M.M.-A.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Cukierman, A.L.; Nunell, G.V.; Bonelli, P.R. Removal of Emerging Pollutants from Water through Adsorption onto Carbon-Based Materials. In Emerging and Nanomaterial Contaminants in Wastewater: Advanced Treatment Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–213. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Hoang, T.V.; Malwade, K.; Kanel, S.R.; Harper, W.F.; Struckhoff, G. Application of Carbon Nanotubes for Removal of Emerging Contaminants of Concern in Engineered Water and Wastewater Treatment Systems. Nanotechnol. Environ. Eng. 2019, 4, 12. [Google Scholar] [CrossRef]
- Taleb, A.; Naif Al-sharif, M.; Ali Al-mutair, M.; Almasoudi, S.; Madkhali, O.; Muzibur Rahman, M. Modification and Application of Carbon Nanotubes for the Removal of Emerging Contaminants from Wastewater: A Review. In Carbon Nanotubes—Recent Advances, New Perspectives and Potential Applications; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Multi-Walled Carbon Nanotube. Available online: https://commons.wikimedia.org/wiki/File:Multi-walled_Carbon_Nanotube.png (accessed on 19 December 2024).
- Synthesis of Carbon Nanotube. Available online: https://en.wikipedia.org/wiki/Synthesis_of_carbon_nanotubes (accessed on 19 December 2024).
- Timesnano. Available online: http://www.timesnano.com/en/article.php?prt=1,21 (accessed on 19 December 2024).
- Spaolonzi, M.P.; Duarte, E.D.V.; Oliveira, M.G.; Costa, H.P.S.; Ribeiro, M.C.B.; Silva, T.L.; Silva, M.G.C.; Vieira, M.G.A. Green-Functionalized Carbon Nanotubes as Adsorbents for the Removal of Emerging Contaminants from Aqueous Media. J. Clean. Prod. 2022, 373, 133961. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption Mechanisms of Organic Chemicals on Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Orona-Návar, C.; García-Morales, R.; Rubio-Govea, R.; Mahlknecht, J.; Hernandez-Aranda, R.I.; Ramírez, J.G.; Nigam, K.D.P.; Ornelas-Soto, N. Adsorptive Removal of Emerging Pollutants from Groundwater by Using Modified Titanate Nanotubes. J. Environ. Chem. Eng. 2018, 6, 5332–5340. [Google Scholar] [CrossRef]
- Cao, Y.; Li, X. Adsorption of Graphene for the Removal of Inorganic Pollutants in Water Purification: A Review. Adsorption 2014, 20, 713–727. [Google Scholar] [CrossRef]
- Li, X.; Tao, Y.; Li, F.; Huang, M. Efficient Preparation and Characterization of Functional Graphene with Versatile Applicability. J. Harbin Inst. Technol. 2016, 23, 1–29. [Google Scholar]
- Jia, Y.; Guo, L.; Lu, W.; Guo, Y.; Lin, J.; Zhu, K.; Chen, L.; Huang, Q.; Huang, J.; Li, Z.; et al. Fabrication and Characterization of Graphene Derived from SiC. Sci. China Phys. Mech. Astron. 2013, 56, 2386–2394. [Google Scholar] [CrossRef]
- Munuera, J.; Britnell, L.; Santoro, C.; Cuéllar-Franca, R.; Casiraghi, C. A Review on Sustainable Production of Graphene and Related Life Cycle Assessment. 2D Mater. 2021, 9, 012002. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, C.; Chen, Y.; Nie, Z. Research Progress on the Preparation and Applications of Laser-Induced Graphene Technology. Nanomaterials 2022, 12, 2336. [Google Scholar] [CrossRef]
- Graphen. Available online: https://cs.wikipedia.org/wiki/Grafen (accessed on 19 December 2024).
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene as a New Sorbent in Analytical Chemistry. Trends Anal. Chem. 2013, 51, 33–43. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (RGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Graphite Oxide. Available online: https://en.wikipedia.org/wiki/Graphite_oxide#/media/File:Graphite_oxide.svg (accessed on 19 December 2024).
- Anegbe, B.; Ifijen, I.H.; Maliki, M.; Uwidia, I.E.; Aigbodion, A.I. Graphene Oxide Synthesis and Applications in Emerging Contaminant Removal: A Comprehensive Review. Environ. Sci. Eur. 2024, 36, 15. [Google Scholar] [CrossRef]
- Li, G.; Du, R.; Cao, Z.; Li, C.; Xue, J.; Ma, X.; Wang, S. Research Progress in Graphene-Based Adsorbents for Wastewater Treatment: Preparation, Adsorption Properties and Mechanisms for Inorganic and Organic Pollutants. C 2024, 10, 78. [Google Scholar] [CrossRef]
- Lü, M.; Li, J.; Yang, X.; Zhang, C.; Yang, J.; Hu, H.; Wang, X. Applications of Graphene-Based Materials in Environmental Protection and Detection. Chin. Sci. Bull. 2013, 58, 2698–2710. [Google Scholar] [CrossRef]
- MSE Suppliers. Is Graphene Hydrophilic or Hydrophobic? Available online: https://www.msesupplies.com/blogs/news/is-graphene-hydrophilic-or-hydrophobic (accessed on 7 January 2025).
- Kulakova, I.I.; Lisichkin, G.V. Prospects for Using Graphene Nanomaterials: Sorbents, Membranes, and Gas Sensors. Russ. J. Appl. Chem. 2021, 94, 1177–1188. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Han, L.; Wang, J.; Zhu, L.; Zeng, H. Graphene-Based Materials for Adsorptive Removal of Pollutants from Water and Underlying Interaction Mechanism. Adv. Colloid Interface Sci. 2021, 289, 102360. [Google Scholar] [CrossRef]
- Machado, A.B.; Schmitt, P.; Maraschin, T.G.; Osorio, D.M.M.; Basso, N.R.D.S.; Berlese, D.B. Adsorption Capacity of Pollutants from Water by Graphene and Graphene-Based Materials: A Bibliographic Review. Contrib. Cienc. Sociales 2024, 17, e4707. [Google Scholar] [CrossRef]
- Baig, N.; Ihsanullah; Sajid, M.; Saleh, T.A. Graphene-Based Adsorbents for the Removal of Toxic Organic Pollutants: A Review. J. Environ. Manag. 2019, 244, 370–382. [Google Scholar] [CrossRef]
- Rosli, F.A.; Ahmad, H.; Jumbri, K.; Abdullah, A.H.; Kamaruzaman, S.; Fathihah Abdullah, N.A. Efficient Removal of Pharmaceuticals from Water Using Graphene Nanoplatelets as Adsorbent. R. Soc. Open Sci. 2021, 8, 201076. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Graphene Oxide and Its Application as an Adsorbent for Wastewater Treatment. J. Chem. Technol. Biotechnol. 2013, 89, 196–205. [Google Scholar] [CrossRef]
- Nanografi. Available online: https://nanografi.com/about-us-references/ (accessed on 6 January 2025).
- Bytesnikova, Z.; Richtera, L.; Smerkova, K.; Adam, V. Graphene Oxide as a Tool for Antibiotic-Resistant Gene Removal: A Review. Environ. Sci. Pollut. Res. 2019, 26, 20148–20163. [Google Scholar] [CrossRef]
- Yu, W.; Zhan, S.; Shen, Z.; Zhou, Q.; Yang, D. Efficient Removal Mechanism for Antibiotic Resistance Genes from Aquatic Environments by Graphene Oxide Nanosheet. Chem. Eng. J. 2017, 313, 836–846. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.S.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.P.; et al. Removal of Antibiotics, Antibiotic-Resistant Bacteria and Their Associated Genes by Graphene-Based TiO2 Composite Photocatalysts under Solar Radiation in Urban Wastewaters. Appl. Catal. B Environ. 2018, 224, 810–824. [Google Scholar] [CrossRef]
- Pant, A.; Jain, R.; Ahammad, S.Z.; Ali, S.W. Removal of Antibiotic Resistance Genes from Wastewater Using Diethylaminoethyl Cellulose as a Promising Adsorbent. J. Water Process Eng. 2023, 55, 104109. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Ham, S.; Qiao, R. Graphene Oxide and Its Derivatives as Adsorbents for PFOA Molecules. J. Phys. Chem. B 2023, 127, 9620–9629. [Google Scholar] [CrossRef]
- Tunioli, F.; Marforio, T.D.; Favaretto, L.; Mantovani, S.; Pintus, A.; Bianchi, A.; Kovtun, A.; Agnes, M.; Palermo, V.; Calvaresi, M.; et al. Chemical Tailoring of Β-Cyclodextrin-Graphene Oxide for Enhanced Per- and Polyfluoroalkyl Substances (PFAS) Adsorption from Drinking Water. Chem. A Eur. J. 2023, 29, e202301854. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of Pollutants by Porous Carbon, Carbon Nanotubes and Fullerene- An Overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Fullerene. Available online: https://cs.wikipedia.org/wiki/Fullereny (accessed on 7 January 2025).
- Elessawy, N.A.; El-Sayed, E.M.; Ali, S.; Elkady, M.F.; Elnouby, M.; Hamad, H.A. One-Pot Green Synthesis of Magnetic Fullerene Nanocomposite for Adsorption Characteristics. J. Water Process Eng. 2020, 34, 101047. [Google Scholar] [CrossRef]
- Alomar, M.; Khan, A.A. Porphyrin like Porous Fullerene Functionalized with Ga as an Effective Adsorbent for the Removal of Methylene Blue from Wastewater Effluent. Surf. Interfaces 2024, 52, 104883. [Google Scholar] [CrossRef]
- Kausar, A. Fullerene in Water Remediation Nanocomposite Membranes—Cutting Edge Advancements. Charact. Appl. Nanomater. 2024, 7, 4945. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Carbon Nanomaterials for the Treatment of Heavy Metal-Contaminated Water and Environmental Remediation. Nanoscale Res. Lett. 2019, 14, 341. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Iii, T.G.; et al. Tuning the Structure and Function of Metal–Organic Frameworks via Linker Design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Ossila. MOF Ligands. Available online: https://www.ossila.com/collections/mof-ligands (accessed on 6 January 2025).
- Kumar, M.; Kulkarni, N.V. Metal-Organic Frameworks (MOFs). Available online: https://www.amrita.edu/news/metal-organic-frameworks-mofs/ (accessed on 6 January 2025).
- Kaye, S.S.; Dailly, A.; Yaghi, O.M.; Long, J.R. Impact of Preparation and Handling on the Hydrogen Storage Properties of Zn4O(1,4-Benzenedicarboxylate)3 (MOF-5). J. Am. Chem. Soc. 2007, 129, 14176–14177. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, M.; Wang, X.; Cui, H.; Wei, J.; Li, X. The Role of Metal-Organic Frameworks in Removing Emerging Contaminants in Wastewater. J. Clean. Prod. 2023, 429, 139526. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.-L.; Feng, D.; Xie, L.-H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Highly Stable Zr(IV)-Based Metal–Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of Water Stable Metal–Organic Frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Ramezanalizadeh, H.; Manteghi, F. Synthesis of a Novel MOF/CuWO4 Heterostructure for Efficient Photocatalytic Degradation and Removal of Water Pollutants. J. Clean. Prod. 2018, 172, 2655–2666. [Google Scholar] [CrossRef]
- Beydaghdari, M.; Saboor, F.H.; Babapoor, A.; Asgari, M. Recent Progress in Adsorptive Removal of Water Pollutants by Metal-Organic Frameworks. Chemnanomat 2022, 8, e202100400. [Google Scholar] [CrossRef]
- Darabdhara, J.; Ahmaruzzaman, M. Recent Developments in MOF and MOF Based Composite as Potential Adsorbents for Removal of Aqueous Environmental Contaminants. Chemosphere 2022, 304, 135261. [Google Scholar] [CrossRef]
- Zadehahmadi, F.; Eden, N.T.; Mahdavi, H.; Konstas, K.; Mardel, J.I.; Shaibani, M.; Banerjee, P.C.; Hill, M.R. Removal of Metals from Water Using MOF-Based Composite Adsorbents. Environ. Sci. Water Res. Technol. 2023, 9, 1305–1330. [Google Scholar] [CrossRef]
- Yan, C.; Jin, J.; Wang, J.; Zhang, F.; Tian, Y.; Liu, C.; Zhang, F.; Cao, L.; Zhou, Y.; Han, Q. Metal–Organic Frameworks (MOFs) for the Efficient Removal of Contaminants from Water: Underlying Mechanisms, Recent Advances, Challenges, and Future Prospects. Coord. Chem. Rev. 2022, 468, 214595. [Google Scholar] [CrossRef]
- Wei, Z.; Su, Q.; Lin, Q.; Wang, X.; Long, S.; Zhang, G.; Yang, J. Multifunctional Oxidized Poly (Arylene Sulfide Sulfone)/UiO-66 Nanofibrous Membrane with Efficient Adsorption/Separation Ability in Harsh Environment. Chem. Eng. J. 2022, 430, 133021. [Google Scholar] [CrossRef]
- Martell Mendoza, M.; Alberto Méndez Cuesta, C.; Angel Zavala Sánchez, M.; Cuauhtemoc Pérez Montiel, E.; Mata Berbudez, A.; Pérez González, C. Metal Organic Frameworks Used as Antibiotic Removal Agents in Water. In Wastewater Treatment—Past and Future Perspectives; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Z.; Yu, G.; Wu, H.; Chen, H.; Zhou, L.; Zhang, Y.; Su, Y.; Tan, S.; Yang, L.; et al. A Review of Metal Organic Framework (MOFs)-Based Materials for Antibiotics Removal via Adsorption and Photocatalysis. Chemosphere 2021, 272, 129501. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Ge, F.; Ren, S.; Gao, X.; Zheng, H. A Water-Stable Cd-MOF and Corresponding MOF@melamine Foam Composite for Detection and Removal of Antibiotics, Explosives, and Anions. Sep. Purif. Technol. 2022, 286, 120433. [Google Scholar] [CrossRef]
- Xu, Z.; Wen, Y.; Tian, L.; Li, G. Efficient and Selective Adsorption of Nitroaromatic Explosives by Zr-MOF. Inorg. Chem. Commun. 2017, 77, 11–13. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, C.; Xia, W.; Liang, Y.; Zeng, Y.; Zhao, X.; Xiong, W.; Cheng, M.; Wang, Z. Recent Advances in Metal–Organic Framework-Based Materials for Removal of Fluoride in Water: Performance, Mechanism, and Potential Practical Application. Chem. Eng. J. 2022, 446, 137299. [Google Scholar] [CrossRef]
- Lal, S.; Singh, P.; Singhal, A.; Kumar, S.; Singh Gahlot, A.P.; Gandhi, N.; Kumari, P. Advances in Metal–Organic Frameworks for Water Remediation Applications. RSC Adv. 2024, 14, 3413–3446. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Abedpour, H.; Moghaddas, J.S.; Borhani, M.N.; Borhani, T.N. Separation of Toxic Contaminants from Water by Silica Aerogel-Based Adsorbents: A Comprehensive Review. J. Water Process Eng. 2023, 53, 103676. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica Aerogel; Synthesis, Properties and Characterization. J. Mater. Process Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Štandeker, S.; Novak, Z.; Knez, Ž. Adsorption of Toxic Organic Compounds from Water with Hydrophobic Silica Aerogels. J. Colloid Interface Sci. 2007, 310, 362–368. [Google Scholar] [CrossRef]
- Franco, P.; Cardea, S.; Tabernero, A.; De Marco, I. Porous Aerogels and Adsorption of Pollutants from Water and Air: A Review. Molecules 2021, 26, 4440. [Google Scholar] [CrossRef] [PubMed]
- Sekwele, K.G.; Tichapondwa, S.M.; Mhike, W. Cellulose, Graphene and Graphene-Cellulose Composite Aerogels and Their Application in Water Treatment: A Review. Discov. Mater. 2024, 4, 23. [Google Scholar] [CrossRef]
- Aylaz, G.; Okan, M.; Duman, M.; Aydin, H.M. Study on Cost-Efficient Carbon Aerogel to Remove Antibiotics from Water Resources. ACS Omega 2020, 5, 16635–16644. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Kamaruddin, M.A.; HPS, A.K.; Yahya, E.B.; Muhammad, S.; Rizal, S.; Ahmad, M.I.; Surya, I.; Abdullah, C.K. Recent Advances in Nanocellulose Aerogels for Efficient Heavy Metal and Dye Removal. Gels 2023, 9, 416. [Google Scholar] [CrossRef]
- Boccia, A.C.; Neagu, M.; Pulvirenti, A. Bio-Based Aerogels for the Removal of Heavy Metal Ions and Oils from Water: Novel Solutions for Environmental Remediation. Gels 2023, 10, 32. [Google Scholar] [CrossRef]
- Lv, T.; Wu, F.; Zhang, Z.; Liu, Z.; Zhao, Y.; Yu, L.; Zhang, J.; Yu, C.; Zhao, C.; Xing, G. TiVCT X MXene/Graphene Nanosheet-Based Aerogels for Removal of Organic Contaminants from Wastewater. ACS Appl. Nano Mater. 2024, 7, 7312–7326. [Google Scholar] [CrossRef]
- Niu, X.; Si, J.; Chen, B.; Wang, Q.; Zeng, S.; Cui, Z. Preparation of Bioaerogel from Iron-Rich Microalgae for the Removal of Water Pollutants. Processes 2024, 12, 1313. [Google Scholar] [CrossRef]
- Ganesamoorthy, R.; Vadivel, V.K.; Kumar, R.; Kushwaha, O.S.; Mamane, H. Aerogels for Water Treatment: A Review. J. Clean. Prod. 2021, 329, 129713. [Google Scholar] [CrossRef]
- Garg, S.; Singh, S.; Shehata, N.; Sharma, H.; Samuel, J.; A Khan, N.; Ramamurthy, P.C.; Singh, J.; Mubashir, M.; Bokhari, A.; et al. Aerogels in Wastewater Treatment: A Review. J. Taiwan Inst. Chem. Eng. 2023, 166, 105299. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Lopes, D.; Girão, A.V.; Silva, R.F.; Malfait, W.J.; Durães, L. Carbon Nanostructures—Silica Aerogel Composites for Adsorption of Organic Pollutants. Toxics 2023, 11, 232. [Google Scholar] [CrossRef]
- Sharma, S.K.; Ranjani, P.; Mamane, H.; Kumar, R. Preparation of Graphene Oxide-Doped Silica Aerogel Using Supercritical Method for Efficient Removal of Emerging Pollutants from Wastewater. Sci. Rep. 2023, 13, 16448. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Torres, R.B.; Vareda, J.P.; Lopes, D.; Ferreira, M.; Valente, V.; Girão, A.V.; Valente, A.J.M.; Durães, L. Amine Modification of Silica Aerogels/Xerogels for Removal of Relevant Environmental Pollutants. Molecules 2019, 24, 3701. [Google Scholar] [CrossRef] [PubMed]
- Gorgolis, G.; Kotsidi, M.; Paterakis, G.; Koutroumanis, N.; Tsakonas, C.; Galiotis, C. Graphene Aerogels as Efficient Adsorbers of Water Pollutants and Their Effect of Drying Methods. Sci. Rep. 2024, 14, 8029. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solcova, O.; Dlaskova, M.; Kastanek, F. Innovative Sorbents for the Removal of Micropollutants from Water. Molecules 2025, 30, 1444. https://doi.org/10.3390/molecules30071444
Solcova O, Dlaskova M, Kastanek F. Innovative Sorbents for the Removal of Micropollutants from Water. Molecules. 2025; 30(7):1444. https://doi.org/10.3390/molecules30071444
Chicago/Turabian StyleSolcova, Olga, Martina Dlaskova, and Frantisek Kastanek. 2025. "Innovative Sorbents for the Removal of Micropollutants from Water" Molecules 30, no. 7: 1444. https://doi.org/10.3390/molecules30071444
APA StyleSolcova, O., Dlaskova, M., & Kastanek, F. (2025). Innovative Sorbents for the Removal of Micropollutants from Water. Molecules, 30(7), 1444. https://doi.org/10.3390/molecules30071444







