Extract from Rosa spp. as a Factor Influencing the Growth Rate of Coagulase-Negative Staphylococcus Strains
Abstract
1. Introduction
2. Results and Discussion
2.1. Growth Inhibition Zone of Test Bacteria Depending on the Extract Concentration
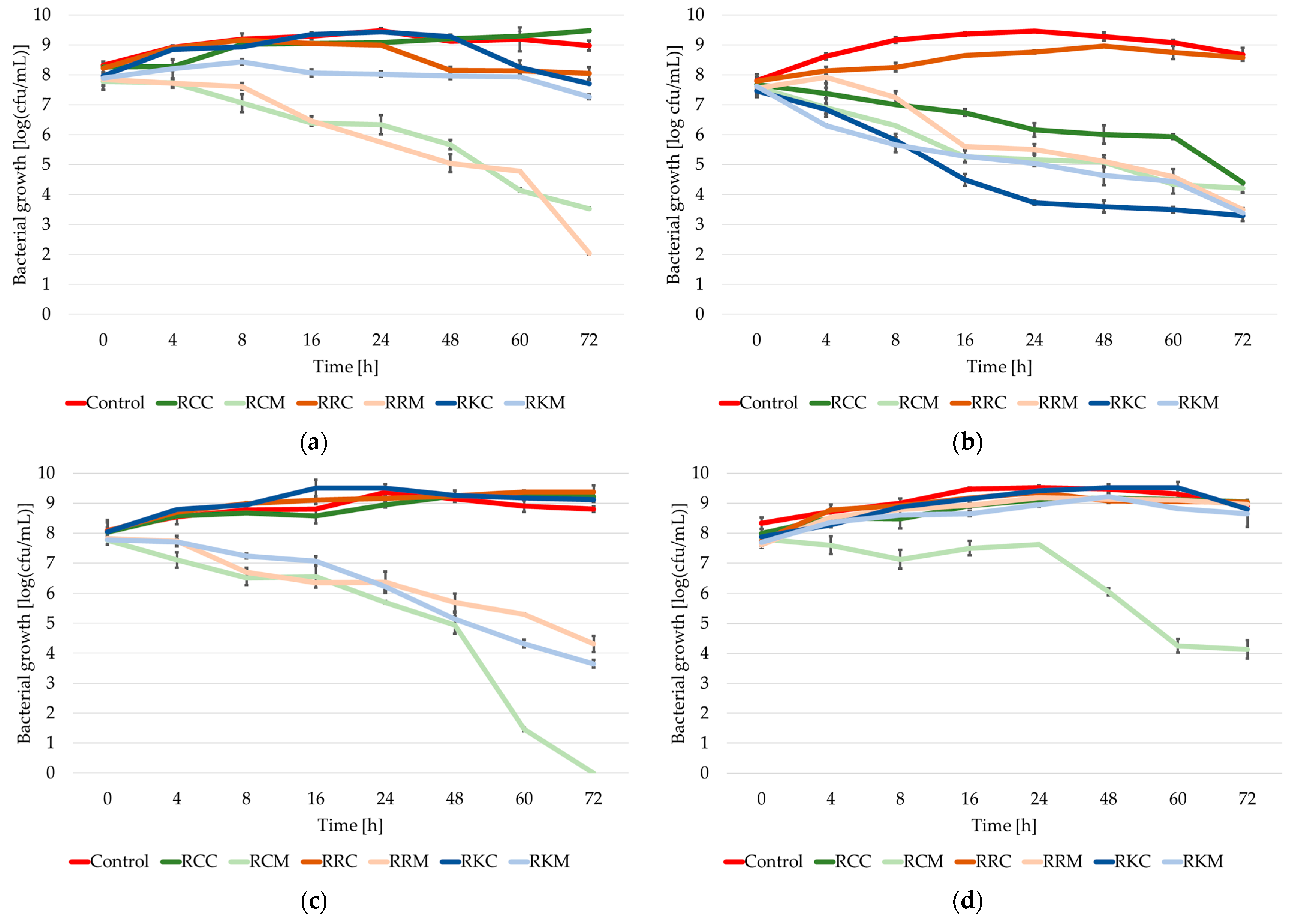
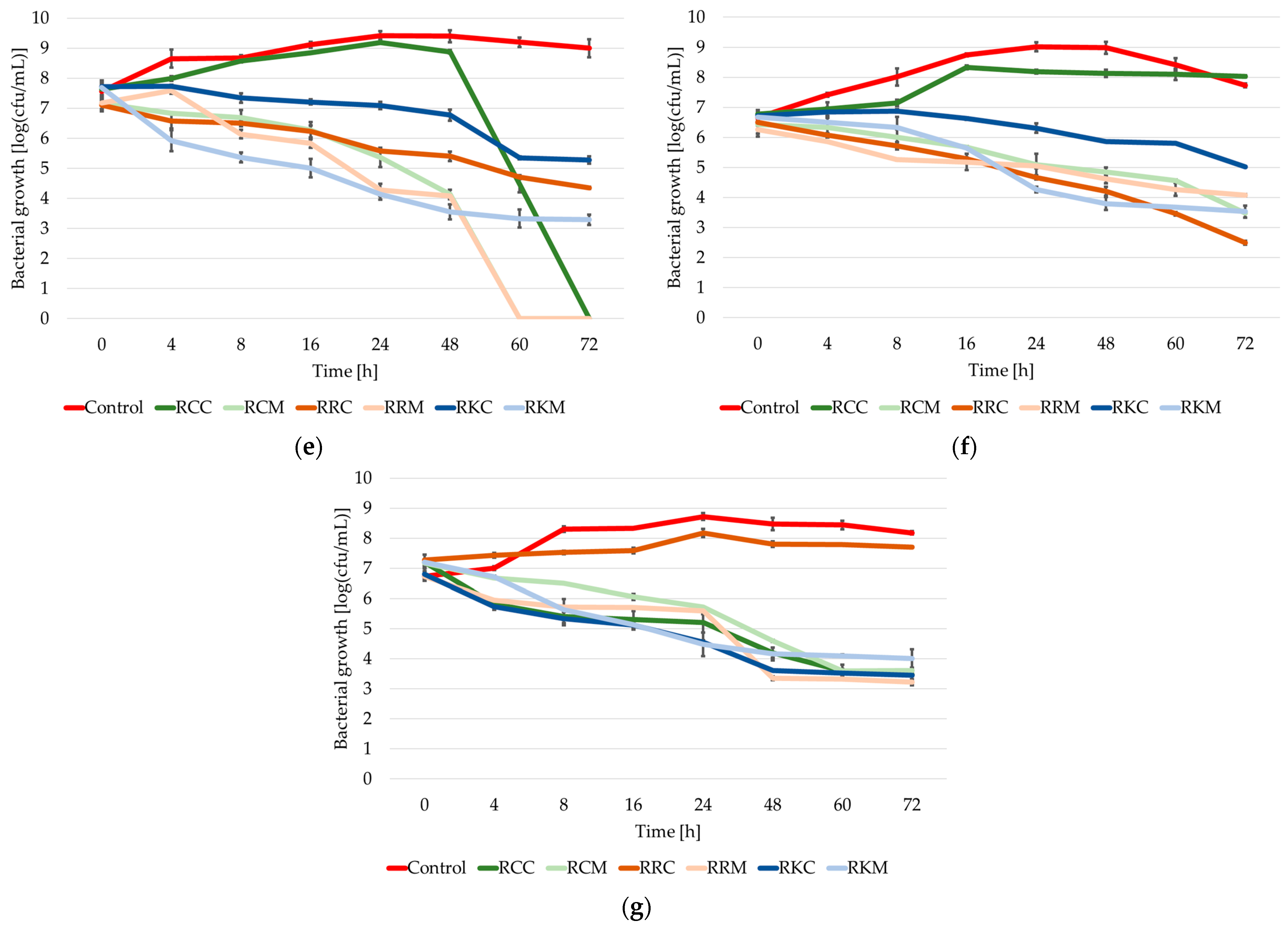
2.2. Growth Dynamics of the Tested Strains of the Staphylococcus Genus in Cultures with the Addition of Extracts from Rosa spp.
2.3. The Growth/Death Rate of Staphylococcus spp. Bacteria in Cultures with the Addition of Extracts
2.4. Scanning Electron Microscopy—Assessment of Changes in Staphylococcus spp. Cell Morphology
3. Materials and Methods
3.1. Biological Material
3.2. Studied Material
3.3. Antimicrobial Properties of Extracts
3.4. Growth of Staphylococcus Bacteria in an Environment with Added Rosa spp. Extracts
3.5. Scanning Electron Microscopy
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIC | Minimal inhibitory concentration |
| SEM | Scanning electron microscopy |
| RCC | Extract from the whole pseudo-fruit of Rosa canina |
| RCM | Rosa canina flesh extract |
| RRC | Extract from the whole pseudo-fruit of Rosa rugosa |
| RRM | Rosa rugosa flesh extract |
| RKC | Extract from the whole pseudo-fruit of Rosa pomifera ‘Karpatia’ |
| RKM | Rosa pomifera ‘Karpatia’ flesh extract |
References
- Anal, A.K.; Perpetuini, G.; Petchkongkaew, A.; Tan, R.; Avallone, S.; Tofalo, R.; Van Nguyen, H.; Chu-Ky, S.; Ho, P.H.; Phan, T.T.; et al. Food safety risks in traditional fermented food from South-East Asia. Food Control 2020, 109, 106922. [Google Scholar] [CrossRef]
- Heo, S.; Lee, J.H.; Jeong, D.W. Food-derived coagulase-negative Staphylococcus as starter cultures for fermented foods. Food Sci. Biotechnol. 2020, 29, 1023–1035. [Google Scholar] [CrossRef]
- Amadoro, C.; Rossi, F.; Poltronieri, P.; Marino, L.; Colavita, G. Diversity and safety aspects of coagulase-negative staphylococci in Ventricina del Vastese Italian Dry Fermented Sausage. Appl. Sci. 2022, 12, 13042. [Google Scholar] [CrossRef]
- Banaszkiewicz, S.; Wałecka-Zacharska, E.; Schubert, J.; Tabi’s, A.; Król, J.; Stefaniak, T.; Węsierska, E.; Bania, J. Staphylococcal enterotoxin genes in coagulase-negative staphylococci—Stability, expression, and genomic context. Int. J. Mol. Sci. 2022, 23, 2560. [Google Scholar] [CrossRef] [PubMed]
- Chajęcka-Wierzchowska, W.; Gajewska, J.; Wiśniewski, P.; Zadernowska, A. Enterotoxigenic potential of coagulase-negative staphylococci from ready-to-eat food. Pathogens 2020, 9, 734. [Google Scholar] [CrossRef]
- Mantzourani, I.; Daoutidou, M.; Dasenaki, M.; Nikolaou, A.; Alexopoulos, A.; Terpou, A.; Thomaidis, N.; Plessas, S. Plant extract and essential oil application against food-borne pathogens in raw pork meat. Foods 2022, 11, 861. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. In vitro antioxidant activities of the methanol extracts of five Allium species from Turkey. Food Chem. 2005, 92, 89–92. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Gao, H.; Cheng, N.; Zhou, J.; Wang, B.; Deng, J.; Cao, W. Antioxidant activities and phenolic compounds of date plum persimmon (Diospyros lotus L.) fruits. Int. J. Food Sci. Technol. 2014, 51, 950–956. [Google Scholar] [CrossRef]
- Baranowska, M.; Bartoszek, A. Antioxidant and antimicrobial properties of bioactive phytochemicals from cranberry. Post. Hig. Med. Dosw. 2016, 70, 1460–1468. [Google Scholar] [CrossRef]
- Yamanaka, A.; Kimizuka, R.; Kato, T.; Okuda, K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol. Immunol. 2004, 19, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Z.; Meng, H.; Yu, S. The antibiotic activity and mechanisms of sugar beet (Beta vulgaris) molasses polyphenols against selected food-borne pathogens. LWT Food Sci. Technol. 2017, 82, 354–360. [Google Scholar] [CrossRef]
- Fei, P.; Ali, M.A.; Gong, S.; Sun, Q.; Bi, X.; Liu, S.; Guo, L. Antimicrobial activity and mechanism of action of olive oil polyphenols extract against Cronobacter sakazakii. Food Control 2018, 94, 289–294. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E. Mutual influence of polyphenols and Lactobacillus spp. bacteria in food: A review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial effect of different herbal plant extracts against different microbial population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 24, 1639. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi, J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Atef, N.M.; Shanab, S.M.; Negm, S.I.; Abbas, Y.A. Evaluation of antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Bull. Natl. Res. Cent. 2019, 43, 144. [Google Scholar] [CrossRef]
- Wei, L.; Li, J.; Yang, Y.; Zhu, M.; Zhao, M.; Yang, J.; Yang, Z.; Zhou, L.; Zhou, S.; Gong, J.; et al. Characterization and potential bioactivity of polyphenols of Rosa rugosa. Food Biosci. 2022, 50, 102108. [Google Scholar] [CrossRef]
- Nowak, R.; Gawlik-Dziki, U. Polyphenols of Rosa L. leaves extracts and their radical scavenging activity. Z. Naturforsch C J. Biosci. 2007, 62, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Faur, C.A.; Zăhan, M.; Bunea, C.I.; Hârșan, E.; Bora, F.D.; Bunea, A. Antiproliferative and biochemical evaluation of rose extracts: Impact on tumor and normal skin cells. Front. Plant Sci. 2024, 30, 1477243. [Google Scholar] [CrossRef]
- Chroho, M.; Bouymajane, A.; Oulad El Majdoub, Y.; Cacciola, F.; Mondello, L.; Aazza, M.; Zair, T.; Bouissane, L. Phenolic composition, antioxidant and antibacterial activities of extract from flowers of Rosa damascena from Morocco. Separations 2022, 9, 247. [Google Scholar] [CrossRef]
- Klewicka, E.; Piekarska-Radzik, L.; Milala, J.; Klewicki, R.; Sójka, M.; Rosół, N.; Otlewska, A.; Matysiak, B. Antagonistic activity of lactic acid bacteria and Rosa rugosa Thunb. pseudo-fruit extracts against Staphylococcus spp. strains. Appl. Sci. 2022, 12, 4005. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E.; Milala, J.; Klewicki, R.; Rosół, N.; Matysiak, B.; Sójka, M.; Markowski, J. Impact of polyphenols from Rosa rugosa Thunb. pseudofruits pomace on growth of Lactobacillus bacteria. ZNTJ 2019, 26, 73–87. [Google Scholar] [CrossRef]
- Jafari-Sales, A.; Jafari, B.; Khaneshpour, H.; Pashazadeh, M. Antibacterial effect of methanolic extract of Rosa damascena on standard bacteria Staphylococcus aureus, Bacillus cereus, Escherichia coli and Pseudomonas aeruginosa in Vitro. Int. J. Nat. Sci. 2020, 4, 40–46. [Google Scholar]
- Jung, I.-G.; Jeong, J.-Y.; Yum, S.-H.; Hwang, Y.-J. Inhibitory effects of selected medicinal plants on bacterial growth of methicillin-resistant Staphylococcus aureus. Molecules 2022, 27, 7780. [Google Scholar] [CrossRef]
- Safdar, Y.; Malik, T. Antibacterial activity of the rose extract. Open Access J. Complement. Altern. 2020, 4, 194–201. [Google Scholar] [CrossRef]
- Cendrowski, A.; Kraśniewska, K.; Przybył, J.L.; Zielińska, A.; Kalisz, S. Antibacterial and antioxidant activity of extracts from Rose fruits (Rosa rugosa). Molecules 2020, 25, 1365. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Jaiswal, S. Modelling the effects of natural antimicrobials as food preservatives. In Handbook of Natural Antimicrobials for Food Safety and Quality; Taylor, M., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2014; pp. 259–284. [Google Scholar]
- Bshabshe, A.A.; Joseph, M.R.P.; Awad El-Gied, A.A.; Fadul, A.N.; Chandramoorthy, H.C.; Hamid, M.E. Clinical relevance and antimicrobial profiling of methicillin-resistant Staphylococcus aureus (MRSA) on routine antibiotics and ethanol extract of mango kernel (Mangifera indica L.). BioMed Res. Int. 2020, 2020, 4150678. [Google Scholar] [CrossRef] [PubMed]
- Beressa, T.B.; Deyno, S.; Alele, P.E. Antifungal activity of the essential oil of Echinops kebericho Mesfin: An In Vitro Study. Evid. Based Complement. Alternat Med. 2020, 12, 3101324. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wan, Q.; Li, J.; Li, Q.; Hu, K.; Ao, X.; Chen, S.; He, L.; Hu, X.; Hu, B.; et al. Rose bud extract as a natural antimicrobial agent against Staphylococcus aureus: Mechanisms and application in maintaining pork safety. LWT Food Sci. Technol. 2023, 176, 114527. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, D.; Lv, C.; Qin, K.; Zhou, Q.; Pu, N.; Song, S.; Wang, X. Antimicrobial and anti-biofilm activity of Polygonum chinense L. aqueous extract against Staphylococcus aureus. Sci. Rep. 2022, 12, 21988. [Google Scholar] [CrossRef]
- Gong, S.; Fei, P.; Sun, Q.; Guo, L.; Jiang, L.; Duo, K.; Bi, X.; Yun, X. Action mode of cranberry anthocyanin on physiological and morphological properties of Staphylococcus aureus and its application in cooked meat. Food Microbiol. 2021, 94, 103632. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Bi, X.; Duo, K.; Sun, Q.; Yun, X.; Zhang, Y.; Fei, P.; Han, J. Antimicrobial activity and mechanism of action of the amaranthus tricolor crude extract against Staphylococcus aureus and potential application in cooked meat. Foods 2020, 9, 359. [Google Scholar] [CrossRef]
- Kitsiou, M.; Purk, L.; Ioannou, C.; Wantock, T.; Sandison, G.; Harle, T.; Gutierrez-Merino, J.; Klymenko, O.V.; Velliou, E. On the evaluation of the antimicrobial effect of grape seed extract and cold atmospheric plasma on the dynamics of Listeria monocytogenes in novel multiphase 3D viscoelastic models. Int. J. Food Microbiol. 2023, 406, 110395. [Google Scholar] [CrossRef]
- Gavriil, A.; Zilelidou, E.; Papadopoulos, A.E.; Siderakou, D.; Kasiotis, K.M.; Haroutounian, S.A.; Gardeli, C.; Giannenas, I.; Skandamis, P.N. Evaluation of antimicrobial activities of plant aqueous extracts against Salmonella Typhimurium and their application to improve safety of pork meat. Sci. Rep. 2021, 11, 21971. [Google Scholar] [CrossRef] [PubMed]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial activities of ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef]
- Milala, J.; Piekarska-Radzik, L.; Sójka, M.; Klewicki, R.; Matysiak, B.; Klewicka, E. Rosa spp. extracts as a factor that limits the growth of Staphylococcus spp. bacteria, a food contaminant. Molecules 2021, 26, 4590. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Määttä-Riihinen, K.; Oksman-Caldentey, K.-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.N.; Coelho-Fernandes, S.; Teixeira, J.A.; Cadavez, V.; Gonzales-Barron, U. Dynamic modelling to describe the effect of plant extracts and customised starter culture on Staphylococcus aureus survival in goat’s raw milk soft cheese. Foods 2023, 12, 2683. [Google Scholar] [CrossRef] [PubMed]
- Buldain, D.; Gortari Castillo, L.; Marchetti, M.L.; Julca Lozano, K.; Bandoni, A.; Mestorino, N. Modeling the growth and death of Staphylococcus aureus against Melaleuca armillaris essential oil at different pH conditions. Antibiotics 2021, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zhang, N.; Santos, R.M. Mineral characterization using Scanning Electron Microscopy (SEM): A Review of the fundamentals, advancements, and research directions. Appl. Sci. 2023, 13, 12600. [Google Scholar] [CrossRef]
- Morshdy, A.E.M.A.; Abdallah, K.M.E.; Abdallah, H.E.; Algahtani, F.D.; Elabbasy, M.T.; Atique, S.; Ahmad, K.; Al-Najjar, M.A.A.; Abdallah, H.M.; Mahmoud, A.F.A. Potential of natural phenolic compounds as antimicrobial agents against multidrug-resistant Staphylococcus aureus in chicken meat. Molecules 2023, 28, 6742. [Google Scholar] [CrossRef]
- Chen, X.; Chen, T.; Liu, J.; Wei, Y.; Zhou, W. Physicochemical stability and antibacterial mechanism of theabrownins prepared from tea polyphenols catalyzed by polyphenol oxidase and peroxidase. Food Sci. Biotechnol. 2023, 33, 47–61. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E.; Olewska, A. Analysis of phenotypic and genotypic antibiotic resistance of food isolates of Staphylococcus spp. Acta Sci. Pol. Technol. Aliment. 2022, 22, 411–428. [Google Scholar] [CrossRef]

| Zone of Inhibition [mm] | |||||||
|---|---|---|---|---|---|---|---|
| Rosa canina—Pseudo-Fruit | |||||||
| Extract Concentration [mg/mL] | ATCC 25923 | DSMZ 3270 | A5 | M5 | M6 | KR6 | KR2A |
| 500 | 20.50 ± 0.58 A | 15.50 ± 0.50 A | 24.00 ± 0.82 A | 20.33 ± 0.58 A | 18.30 ± 0.50 A | 18.50 ± 0.50 A | 18.00 ± 0.00 A |
| 400 | 20.00 ± 0.00 AB | 14.67 ± 0.58 A | 21.00 ± 1.00 A | 19.67 ± 0.58 A | 17.67 ± 0.58 A | 18.00 ± 0.00 AB | 16.67 ± 0.58 B |
| 300 | 19.33 ± 0.58 BC | 13.00 ± 0.00 B | 20.00 ± 1.73 AB | 18.00 ± 0.00 B | 17.00 ± 0.00 A | 16.67 ± 0.58 BC | 15.33 ± 0.58 BC |
| 200 | 18.33 ± 0.58 C | 12.00 ± 0.00 B | 20.00 ± 1.73 AB | 16.00 ± 0.00 C | 16.00 ± 0.00 B | 15.33 ± 0.58 CD | 15.00 ± 0.00 C |
| 100 | 16.33 ± 0.58 D | no | 19.67 ± 2.31 AB | 14.33 ± 0.58 D | 15.00 ± 0.00 C | 15.00 ± 1.00 D | 12.67 ± 0.58 C |
| 50 | 15.00 ± 0.00 E | no | 18.33 ± 1.15 ABC | 11.33 ±0.58 E | 12.00 ± 0.00 D | 14.00 ± 0.00 D | 12.33 ± 0.58 D |
| 25 | 13.67 ± 0.58 F | no | 16.37 ± 0.58 BC | no | no | no | no |
| 12.5 | 12.00 ± 0.00 G | no | 16.33 ± 0.58 BC | no | no | no | no |
| 6.25 | no | no | 16.67 ± 0.58 BC | no | no | no | no |
| 3.13 | no | no | 14.33 ± 1.53 C | no | no | no | no |
| 1.56 | no | no | no | no | no | no | no |
| Rosa canina flesh (rose flesh) | |||||||
| 500 | 14.33 ± 1.89 A | 12.33 ± 0.58 A | 15.33 ± 1.50 A | 17.50 ± 0.50 A | 11.80 ± 0.50 A | 17.33 ± 0.58 A | 14.50 ± 1.29 A |
| 400 | 13.67 ± 0.58 A | 11.00 ± 0.00 B | 14.00 ± 0.00 A | 14.67 ± 0.58 B | 11.00 ± 0.00 A | 14.33 ± 1.15 B | 12.67 ± 0.58 AB |
| 300 | 12.33 ± 0.58 A | no | 13.00 ± 0.00 B | 14.33 ± 0.58 B | no | 14.33 ± 0.58 B | 12.00 ± 0.00 B |
| 200 | no | no | no | 11.00 ± 0.00 C | no | 12.67 ± 0.58 C | no |
| 100 | no | no | no | no | no | no | no |
| 50 | no | no | no | no | no | no | no |
| 25 | no | no | no | no | no | no | no |
| 12.5 | no | no | no | no | no | no | no |
| 6.25 | no | no | no | no | no | no | no |
| 3.13 | no | no | no | no | no | no | no |
| 1.56 | no | no | no | no | no | no | no |
| Rosa rugosa—pseudo-fruit | |||||||
| 500 | 23.80 ± 0.96 A | 22.50 ± 2.38 A | 28.50 ± 1.29 A | 24.00 ± 0.82 A | 20.00 ± 0.82 A | 20.33 ± 0.96 A | 21.33 ± 0.96 A |
| 400 | 22.67 ± 0.58 A | 20.67 ± 0.58 A | 26.33 ± 0.58 A | 21.67 ± 0.58 B | 19.67 ± 0.58 A | 20.33 ± 0.58 AB | 21.00 ± 0.00 A |
| 300 | 21.00 ± 0.00 B | 19.67 ± 0.58 A | 24.67 ± 0.58 B | 20.67 ± 0.58 BC | 18.67 ± 0.58 A | 20.00 ± 0.00 AB | 20.00 ± 0.00 A |
| 200 | 20.00 ± 0.00 BC | 17.67 ± 0.58 B | 23.67 ± 0.58 BC | 20.33 ± 0.58 CD | 15.67 ± 0.58 B | 19.33 ± 0.58 B | 19.67 ± 0.58 A |
| 100 | 19.67 ±0.58 BC | 16.33 ± 0.58 BC | 22.67 ± 0.58 C | 19.33 ± 0.58 D | 14.67 ± 0.58 B | 18.00 ± 0.00 C | 19.33 ± 0.58 A |
| 50 | 19.00 ± 0.00 C | 15.67 ± 0.58 C | 21.00 ± 0.00 D | 16.67 ± 0.58 E | no | 17.00 ± 0.00 C | 16.33 ± 0.58 B |
| 25 | 16.67 ± 0.58 D | 13.67 ± 0.58 D | 20.00 ± 0.00 DE | 15.00 ± 0.00 F | no | 15.00 ± 0.00 D | 16.00 ± 0.00 B |
| 12.5 | 15.33 ± 0.58 D | no | 20.00 ± 0.00 DE | 13.00 ± 0.00 G | no | 12.67 ± 0.58 E | 15.33 ± 0.58 B |
| 6.25 | 13.33 ± 0.58 E | no | 19.00 ± 0.00 E | no | no | no | 13.00 ± 0.00 C |
| 3.13 | no | no | no | no | no | no | no |
| 1.56 | no | no | no | no | no | no | no |
| Rosa rugosa—flesh (rose flesh) | |||||||
| 500 | 13.00 ± 0.00 A | 14.00 ± 1.15 A | 13.50 ± 0.50 A | 23.00 ± 0.00 A | 11.50 ± 0.50 A | 18.33 ± 1.53 A | 14.50 ± 0.50 A |
| 400 | no | 12.00 ± 0.00 A | 12.67 ± 0.58 A | 21.00 ± 0.00 B | no | 17.67 ± 1.53 A | 12.00 ± 0.00 B |
| 300 | no | no | no | 19.00 ± 0.00 C | no | 15.00 ± 1.00 B | no |
| 200 | no | no | no | 18.00 ± 0.00 D | no | 14.00 ± 0.00 BC | no |
| 100 | no | no | no | 16.00 ± 0.00 E | no | 13.00 ± 0.00 BC | no |
| 50 | no | no | no | 15.00 ± 0.00 F | no | 12.00 ± 0.00 C | no |
| 25 | no | no | no | 14.00 ± 0.00 G | no | no | no |
| 12.5 | no | no | no | 12.00 ± 0.00 H | no | no | no |
| 6.25 | no | no | no | no | no | no | no |
| 3.13 | no | no | no | no | no | no | no |
| 1.56 | no | no | no | no | no | no | no |
| Rosa pomifera‘Karpatia’—pseudo-fruit | |||||||
| 500 | 20.00 ± 1.83 A | 18.80 ± 0.96 A | 22.80 ± 0.96 A | 23.00 ± 0.00 A | 18.50 ± 1.29 A | 20.70 ± 0.58 A | 19.00 ± 1.83 A |
| 400 | 19.67 ± 0.58 A | 16.33 ± 0.58 B | 20.33 ± 0.58 B | 22.00 ± 0.00 B | 17.67 ± 0.58 A | 18.67 ± 0.58 B | 17.33 ± 0.58 B |
| 300 | 18.00 ± 0.00 B | 14.00 ± 0.00 C | 19.67 ± 0.58 BC | 20.67 ± 0.58 C | 15.33 ± 0.58 AB | 17.67 ± 0.58 B | 16.00 ± 0.00 C |
| 200 | 17.00 ± 0.00 B | no | 19.00 ± 0.00 BCD | 19.00 ± 0.00 D | 14.33 ± 0.58 BC | 15.67 ± 0.58 C | 14.67 ± 0.58 D |
| 100 | 15.67 ± 0.58 C | no | 18.67 ± 0.58 CD | 17.67 ± 0.58 E | 13.33 ± 0.58 C | 15.00 ± 0.00 CD | 13.67 ± 0.58 D |
| 50 | 14.67 ± 0.58 C | no | 18.00 ± 0.00 CD | 14.67 ± 0.58 F | no | 14.33 ± 0.58 CD | no |
| 25 | 13.00 ± 0.00 D | no | 17.67 ± 0.58 DE | 13.00 ± 0.00 G | no | 14.00 ± 0.00 D | no |
| 12.5 | no | no | 17.00 ± 0.00 DE | 12.00 ± 0.00 H | no | no | no |
| 6.25 | no | no | 14.67 ± 0.58 E | no | no | no | no |
| 3.13 | no | no | 13.67 ± 0.58 F | no | no | no | no |
| 1.56 | no | no | no | no | no | no | no |
| Rosa pomifera ‘Karpatia’—flesh (rose flesh) | |||||||
| 500 | 13.00 ± 0.00 A | 13.00 ± 0.00 A | 18.50 ± 1.29 A | 19.80 ± 0.96 A | 13.00 ± 0.82 A | 16.50 ± 173 A | 13.00 ± 0.00 A |
| 400 | no | 12.00 ± 0.00 B | no | 19.33 ± 0.58 A | no | 14.67 ± 0.58 A | no |
| 300 | no | no | no | 18.00 ± 0.58 B | no | 13.67 ± 0.58 A | no |
| 200 | no | no | no | 17.67 ± 0.58 B | no | no | no |
| 100 | no | no | no | 16.00 ± 0.00 C | no | no | no |
| 50 | no | no | no | 15.00 ± 0.58 CD | no | no | no |
| 25 | no | no | no | 14.67 ± 0.58 D | no | no | no |
| 12.5 | no | no | no | 13.00 ± 0.00 E | no | no | no |
| 6.25 | no | no | no | no | no | no | no |
| 3.13 | no | no | no | no | no | no | no |
| 1.56 | no | no | no | no | no | no | no |
| Staphylococcus Strain | R2 Coefficient for Linear Trend Line | |||||
|---|---|---|---|---|---|---|
| RCC | RCM | RRC | RRM | RKC | RKM | |
| ATCC 25923 | 0.4337 | X | 0.3547 | X | 0.4358 | X |
| DSMZ 3270 | 0.7339 | X | 0.4332 | X | X | X |
| A5 | 0.2521 | X | 0.2949 | X | 0.2305 | X |
| M5 | 0.6079 | 0.7941 | 0.4050 | 0.4730 | 0.5224 | 0.3619 |
| M6 | 0.4699 | X | 0.5981 | X | 0.5870 | X |
| KR6 | 0.4167 | 0.6740 | 0.3585 | 0.5351 | 0.3743 | X |
| KR2A | 0.4757 | X | 0.2756 | X | 0.5480 | X |
| R2 for Logarithmic Trend Line | ||||||
| RCC | RCM | RRC | RRM | RKC | RKM | |
| ATCC 25923 | 0.7786 | X | 0.7381 | X | 0.7419 | X |
| DSMZ 3270 | 0.8812 | X | 0.7330 | X | X | X |
| A5 | 0.6204 | X | 0.6126 | X | 0.6213 | X |
| M5 | 0.8633 | 0.9232 | 0.7795 | 0.7859 | 0.8425 | 0.6913 |
| M6 | 0.7697 | X | 0.8096 | X | 0.8033 | X |
| KR6 | 0.6865 | 0.8356 | 0.7372 | 0.7792 | 0.6515 | X |
| KR2A | 0.7489 | X | 0.6916 | X | 0.7758 | X |
| Staphylococcus Strain | ∆μ [h−1] | |||
|---|---|---|---|---|
| Culture Without Extract Addition | ||||
| 0–24 h | 24–72 h | |||
| ATCC 25923 | 0.006 | 0.001 * | ||
| DSMZ 3270 | 0.009 | 0.002 * | ||
| A5 | 0.007 | 0.001 * | ||
| M5 | 0.006 | 0.001 * | ||
| M6 | 0.010 | 0.001 * | ||
| KR6 | 0.015 | 0.003 * | ||
| KR2A | 0.012 | 0.001 * | ||
| Staphylococcus Strain | ∆µ [h−1] | |||
| Culture with Addition of Rosa canina Extract | ||||
| Flesh (Rose Flesh) | Whole Pseudo-Fruit | |||
| 0–24 h | 24–72 h | 0–24 h | 24–72 h | |
| ATCC 25923 | 0.008 * | 0.009 * | 0.004 | 0.001 |
| DSMZ 3270 | 0.014 * | 0.004 * | 0.009 * | 0.006 * |
| A5 | 0.011 * | 0.021 * | 0.005 | 0.001 |
| M5 | 0.001 * | 0.010 * | 0.006 | 0.000 |
| M6 | 0.010 * | 0.021 * | 0.008 | 0.021 * |
| KR6 | 0.009 * | 0.007 * | 0.009 | 0.000 |
| KR2A | 0.008 * | 0.008 * | 0.012 * | 0.007 * |
| Staphylococcus Strain | ∆µ [h−1] | |||
| Culture with Addition of Rosa rugosa Extract | ||||
| Flesh (Rose Flesh) | Whole Pseudo-Fruit | |||
| 0–24 h | 24–72 h | 0–24 h | 24–72 h | |
| ATCC 25923 | 0.011 * | 0.013 * | 0.004 | 0.002 * |
| DSMZ 3270 | 0.011 * | 0.008 * | 0.005 | 0.000 |
| A5 | 0.008 * | 0.007 * | 0.006 | 0.001 |
| M5 | 0.009 | 0.001 * | 0.009 | 0.001 * |
| M6 | 0.017 * | 0.021 * | 0.009 * | 0.005 * |
| KR6 | 0.008 * | 0.004 * | 0.012 * | 0.010 * |
| KR2A | 0.007 * | 0.009 * | 0.005 | 0.001 * |
| Staphylococcus Strain | ∆µ [h−1] | |||
| Culture with Addition of Rosa pomifera ‘Karpatia’ Extract | ||||
| Flesh (Rose Flesh) | Whole Pseudo-Fruit | |||
| 0–24 h | 24–72 h | 0–24 h | 24–72 h | |
| ATCC 25923 | 0.001 | 0.002 * | 0.008 | 0.004 * |
| DSMZ 3270 | 0.014 * | 0.007 * | 0.021 * | 0.002 * |
| A5 | 0.008 * | 0.009 * | 0.008 | 0.001 * |
| M5 | 0.007 | 0.001 * | 0.008 | 0.001 * |
| M6 | 0.019 * | 0.005 * | 0.003 * | 0.005 * |
| KR6 | 0.015 * | 0.004 * | 0.003 * | 0.004 * |
| KR2A | 0.016 * | 0.002 * | 0.14 * | 0.05 * |
| Staphylococcus Strain | Extract Concentration [mg/mL] | |||||
|---|---|---|---|---|---|---|
| Rosa canina | Rosa rugosa | Rosa pomifera ‘Karpatia’ | ||||
| RCC | RCM | RRC | RRM | RKC | RKM | |
| ATCC 25923 | 3.125 | 75 | 0.781 | 125 | 6.25 | 125 |
| DSMZ 3270 | 50 | 125 | 6.25 | 100 | 75 | 100 |
| A5 | 0.781 | 75 | 1.563 | 100 | 0.781 | 25 |
| M5 | 12.5 | 75 | 1.563 | 3.125 | 3.125 | 12.5 |
| M6 | 12.5 | 125 | 25 | 125 | 25 | 100 |
| KR6 | 6.25 | 50 | 3.125 | 12.5 | 6.25 | 75 |
| KR2A | 6.25 | 75 | 1.563 | 100 | 25 | 125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekarska-Radzik, L.; Milala, J.; Klewicki, R.; Sójka, M.; Żyżelewicz, D.; Matysiak, B.; Klewicka, E. Extract from Rosa spp. as a Factor Influencing the Growth Rate of Coagulase-Negative Staphylococcus Strains. Molecules 2025, 30, 1443. https://doi.org/10.3390/molecules30071443
Piekarska-Radzik L, Milala J, Klewicki R, Sójka M, Żyżelewicz D, Matysiak B, Klewicka E. Extract from Rosa spp. as a Factor Influencing the Growth Rate of Coagulase-Negative Staphylococcus Strains. Molecules. 2025; 30(7):1443. https://doi.org/10.3390/molecules30071443
Chicago/Turabian StylePiekarska-Radzik, Lidia, Joanna Milala, Robert Klewicki, Michał Sójka, Dorota Żyżelewicz, Bożena Matysiak, and Elżbieta Klewicka. 2025. "Extract from Rosa spp. as a Factor Influencing the Growth Rate of Coagulase-Negative Staphylococcus Strains" Molecules 30, no. 7: 1443. https://doi.org/10.3390/molecules30071443
APA StylePiekarska-Radzik, L., Milala, J., Klewicki, R., Sójka, M., Żyżelewicz, D., Matysiak, B., & Klewicka, E. (2025). Extract from Rosa spp. as a Factor Influencing the Growth Rate of Coagulase-Negative Staphylococcus Strains. Molecules, 30(7), 1443. https://doi.org/10.3390/molecules30071443








