Three-Dimensional Electrosorption for Pharmaceutical Wastewater Management and Sustainable Biochar Regeneration
Abstract
1. Introduction
2. Results and Discussion
2.1. BC Characterization
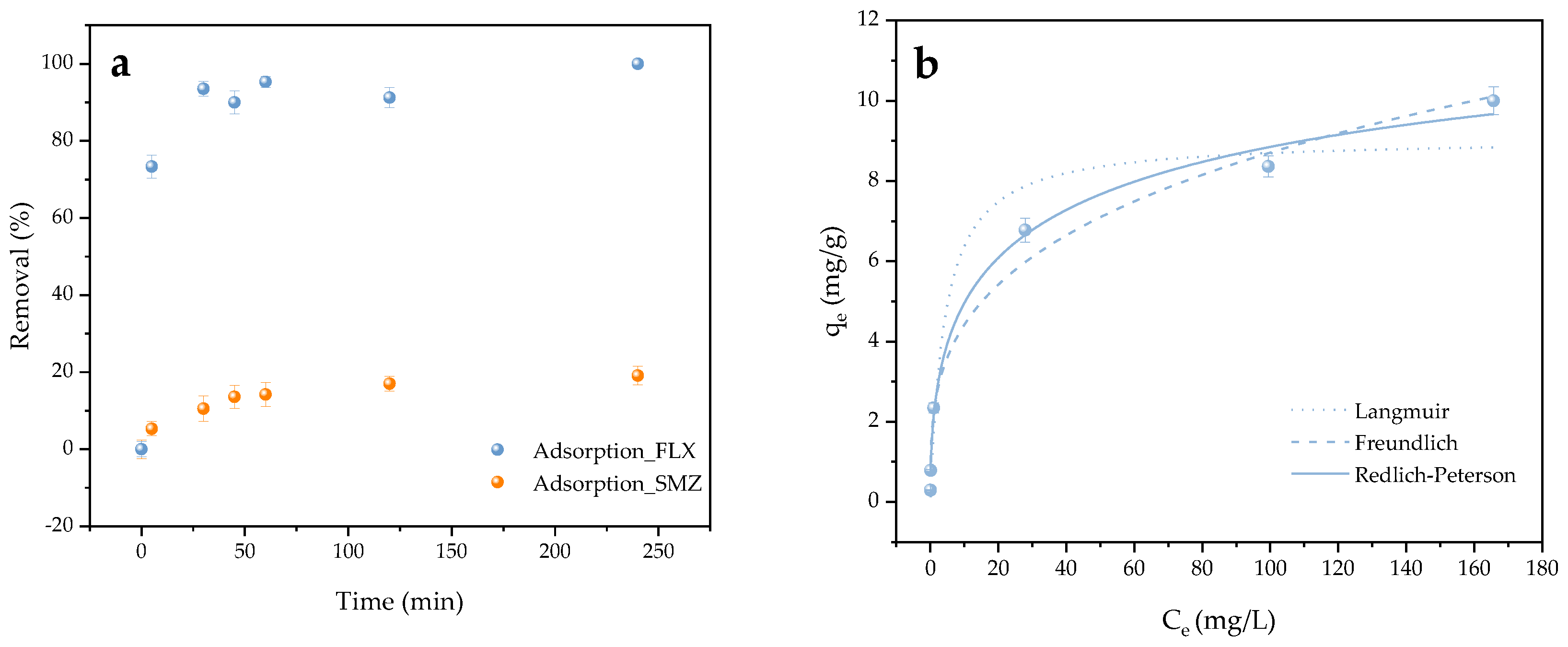
2.2. Cationic and Anionic Adsorption
2.3. Enhanced Sulfonamide Adsorption
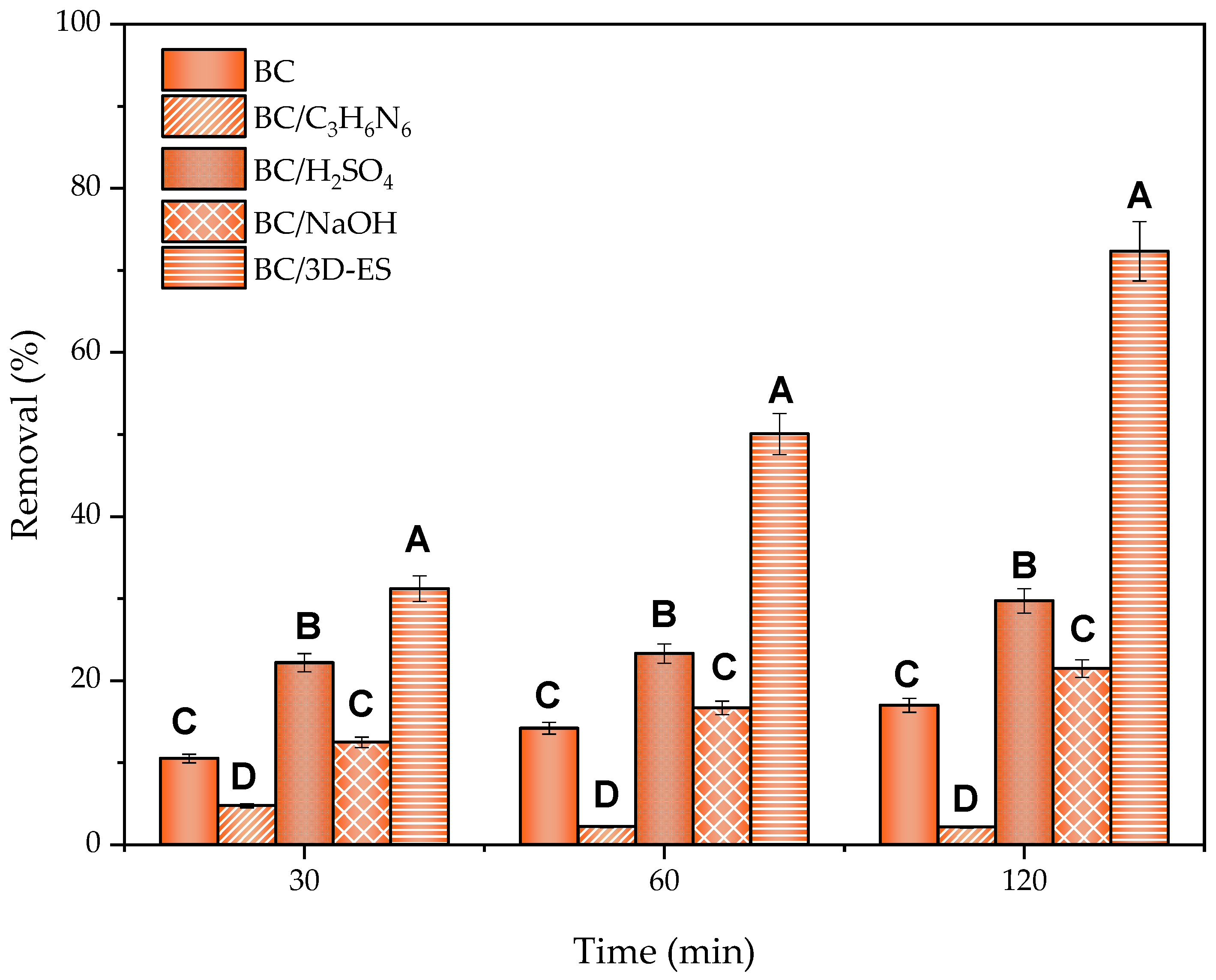
2.4. The 3D Electrosorption Method
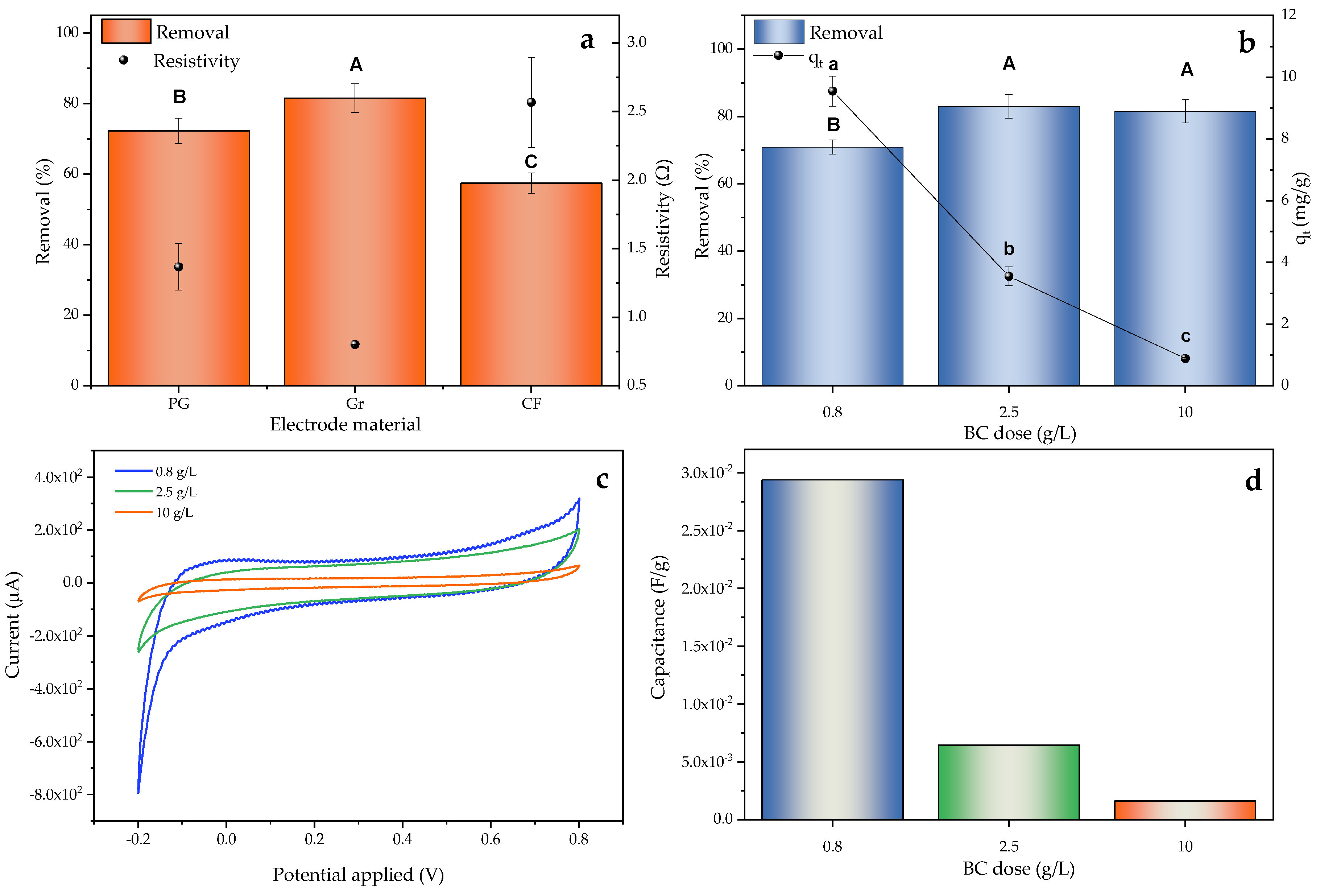
2.4.1. Electrode and Dose Effect
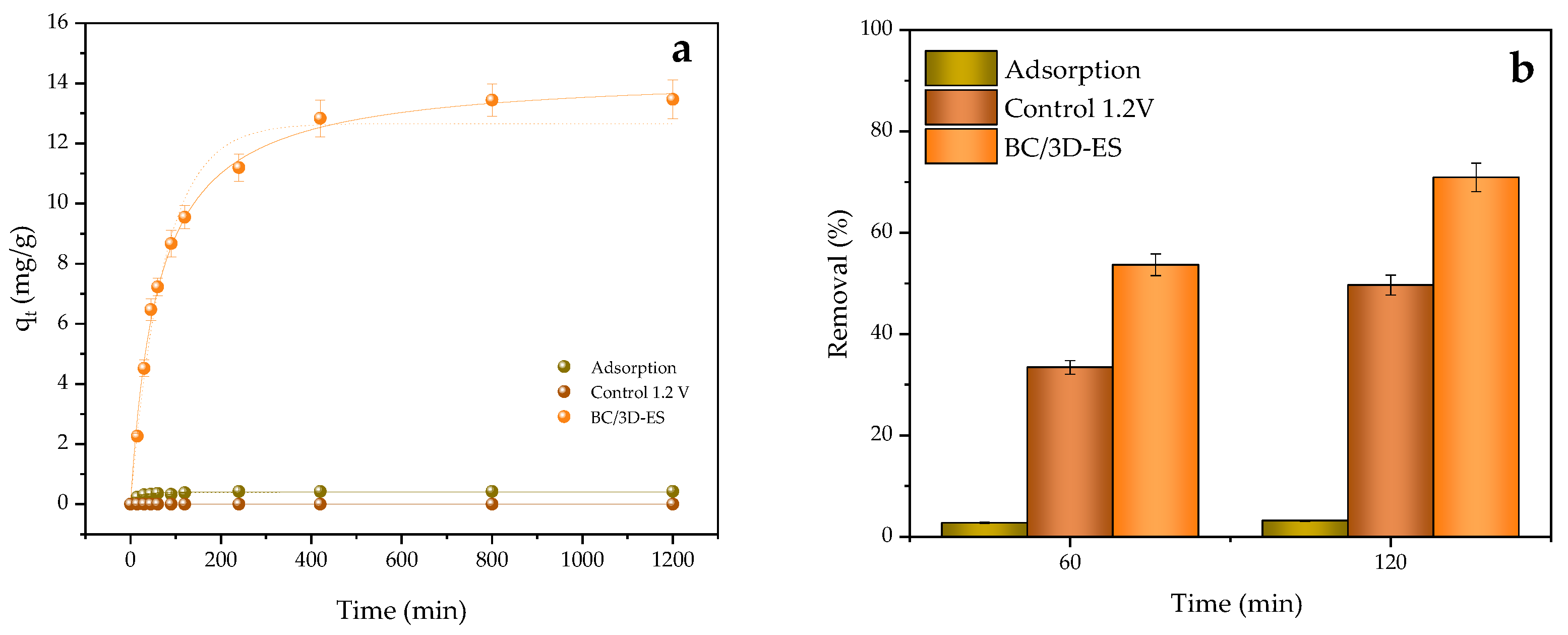
2.4.2. Kinetic Study
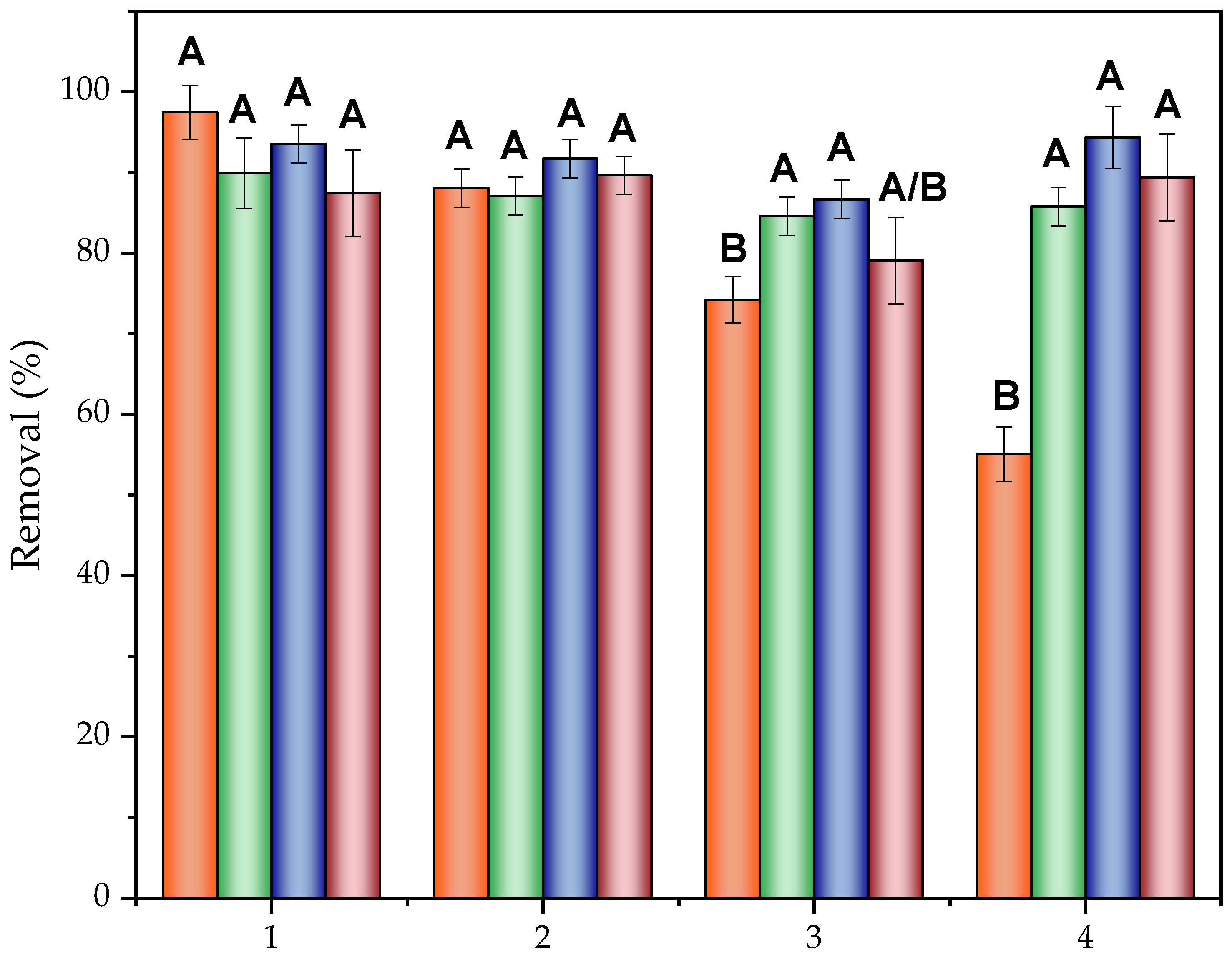
2.4.3. Synergistic Electrosorption Effect
2.5. Regeneration and Reusability
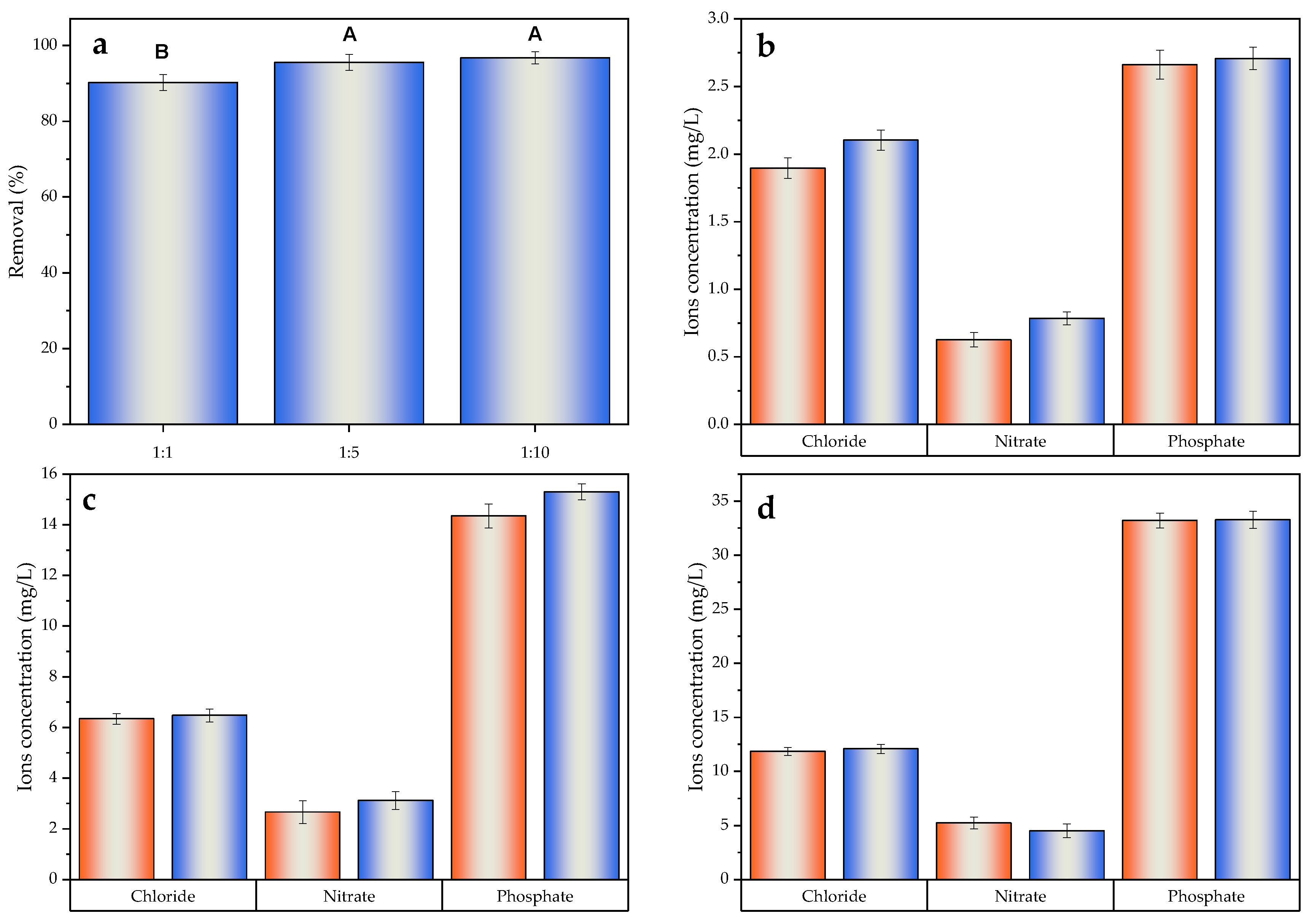
2.6. Ionic Strength
3. Materials and Methods
3.1. Reagents and Materials
3.2. Biochar Characterization
3.3. Experimental Set-Up
3.3.1. Adsorption Assays
3.3.2. Biochar Property Modification
- Incorporation of functional groups with C3H3N6.An autoclave hydrothermal treatment was performed by mixing 5 g of BC in a 60 mL 0.5 M solution of C3H6N6 [136]. The treatment was maintained at 220 °C for 12 h and the recovered material was washed 5 times. The modified BC, denoted as BC/C3H6N6, was dried overnight at 60 °C. Once the treatment was completed, adsorption tests of BC/C3H6N6 with SMZ were performed, keeping the conditions specified in Section 3.3.1, but reducing the contact time to 120 min.
- Acid–base modifications with sulfuric acid and sodium hydroxide.Acid and basic treatments were applied to the BC by using 2 M solutions of H2SO4 or NaOH. In both cases, the material was placed in contact with each solution by agitation for 5 h at 60 °C. The material was then recovered and washed with water until a neutral pH was obtained in the wash water (pH ≈ 7). Finally, the BC was left to dry overnight at 60 °C. As in the previous case, once the modified materials were obtained, which were named BC/H2SO4 and BC/NaOH, the corresponding adsorption tests were performed with SMZ.
- The 3D electrosorption (BC/3D-ES).Continuing with the methodology specified in Section 3.3.1, a 3D SMZ electrosorption test was carried out by incorporating an anode and a cathode of graphite paper obtained from Mersen to the system, with dimensions of 15 × 25 × 0.5 mm. The electrodes were immersed in the solution until a working area of 2.3 cm2 was reached, and a potential difference of 1.2 V was applied on them with a Hanmatek HM305 power supply. This potential was reported in previous works as the maximum applicable voltage without triggering secondary reactions such as water electrolysis, which could reduce the process efficiency [121]. To ensure conductivity throughout the solution, a 10 mM concentration of Na2SO4 was added as an electrolyte. This treatment will hereafter be referred to as BC/3D-ES, and also remained active for 120 min.Under these same conditions, the effect of using different carbonaceous materials in the electrodes (PG, CF and Gr provided by Mersen, Carbon-Lorraine and AliExpress, respectively) and different doses of BC (10 g/L, 2.5 g/L and 0.8 g/L) were evaluated. The choice of electrodes was made based on the material’s low cost, while the adsorbent dosage was adjusted to the minimum amount necessary to maintain high FLX adsorption following the behavior of adsorption isotherms.To determine the electrochemical properties of the material, a Metrohm Autolab potentiostat (Metrohm AG, Herisau, Switzerland) was used to perform the measurements and the Nova 2.1.7 software was used for data visualization. The cyclic voltammetry (CV) technique was used in a 0.5 M Na2SO4 solution, since it was the working electrolyte, with a potential window between −0.2 and 0.8 V, a scan rate of 0.1 V/s and a 0.002 V step. Likewise, electrochemical impedance spectroscopy (EIS) was applied in a 0.5 M H2SO4 solution in the frequency range from 106 to 10−2 Hz, with a sinusoidal perturbation of 0.02 Vs. These conditions made it possible to obtain CV curves and spectroscopies free of noise and with clear signals.
3.4. Reusability and Regeneration
3.4.1. Desorption of Adsorbed Pollutant
3.4.2. Electro-Regeneration
3.5. Coexistence of Ions
3.6. Analytical and Statistic Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, M.-K.; Truong, Q.-M.; Thai, V.-A.; Pham, M.-T.; Chang, S.W.; Nguyen, D.D. Microplastics and Pharmaceuticals from Water and Wastewater: Occurrence, Impacts, and Membrane Bioreactor-based Removal. Sep. Purif. Technol. 2025, 361, 131489. [Google Scholar] [CrossRef]
- Tang, J.; Li, Z.; Xiao, X.; Liu, B.; Huang, W.; Xie, Q.; Lan, C.; Luo, S.; Tang, L. Recent Advancements in Antibiotics Removal by Bio-electrochemical Systems (BESs): From Mechanisms to Application of Emerging Combined Systems. Water Res. 2025, 268, 122683. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Tripathi, G.; Mishra, V.; Ilyas, T.; Firdaus, S.; Ahmad, S.; Farooqui, A.; Yadav, N.; Rustagi, S.; Shreaz, S.; et al. Antibiotic Contamination in Wastewater Treatment Plant Effluents: Current Research and Future Perspectives. Environ. Nanotechnol. Monit. Manag. 2025, 23, 101047. [Google Scholar] [CrossRef]
- Khan, A.H.; Aziz, H.A.; Palaniandy, P.; Naushad, M.; Cevik, E.; Zahmatkesh, S. Pharmaceutical Residues in the Ecosystem: Antibiotic Resistance, Health Impacts, and Removal Techniques. Chemosphere 2023, 339, 139647. [Google Scholar] [CrossRef] [PubMed]
- Miglione, A.; Raucci, A.; Cristiano, F.; Mancini, M.; Gioia, V.; Frugis, A.; Cinti, S. Paper-based 2D Configuration for the Electrochemical and Facile Detection of Paracetamol in Wastewaters. Electrochim. Acta 2024, 488, 144255. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Ali, H. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Anoob, F.; Arachchi, S.; Azamathulla, H.M.; Al-Mahbashi, N.; Rathnayake, U. Nanoadsorbents as an Effective Wastewater Treatment Candidate for Pharmaceutical Contaminants; Towards Sustainable Policy Development. Case Stud. Chem. Environ. Eng. 2024, 9, 100639. [Google Scholar] [CrossRef]
- Tominaga, F.K.; Boiani, N.F.; Silva, T.T.; Garcia, V.S.G.; Borrely, S.I. Acute and Chronic Ecotoxicological Effects of Pharmaceuticals and Their Mixtures in Daphnia Similis. Chemosphere 2022, 309, 136671. [Google Scholar] [CrossRef]
- Duarte, I.A.; Reis-Santos, P.; Novais, S.C.; Rato, L.D.; Lemos, M.F.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Cabral, H.N.; Fonseca, V.F. Depressed, Hypertense and Sore: Long-term Effects of Fluoxetine, Propranolol and Diclofenac Exposure in a Top Predator Fish. Sci. Total Environ. 2020, 712, 136564. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Y.; Liu, Q.; Chang, C.-C.; Hou, W. Electrosorption of Methylene Blue from Aqueous Solution on Graphene-Titanium Electrode: Adsorption Kinetics Studies. Int. J. Chem. React. Eng. 2019, 17, 20180025. [Google Scholar] [CrossRef]
- Castillo-Zacarías, C.; Barocio, M.E.; Hidalgo-Vázquez, E.; Sosa-Hernández, J.E.; Parra-Arroyo, L.; López-Pacheco, I.Y.; Barceló, D.; Iqbal, H.N.; Parra-Saldívar, R. Antidepressant Drugs as Emerging Contaminants: Occurrence in Urban and Non-Urban Waters and Analytical Methods for Their Detection. Sci. Total Environ. 2021, 757, 143722. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.J.; Paíga, P.; Silva, A.; Llaguno, C.P.; Carvalho, M.; Vázquez, F.M.; Delerue-Matos, C. Antibiotics and Antidepressants Occurrence in Surface Waters and Sediments Collected in the North of Portugal. Chemosphere 2020, 239, 124729. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gautam, L.; Hall, S.W. The Detection of Drugs of Abuse and Pharmaceuticals in Drinking Water Using Solid-Phase Extraction and Liquid Chromatography-Mass Spectrometry. Chemosphere 2019, 223, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Morales, D.; Masís-Mora, M.; Montiel-Mora, J.R.; Cambronero-Heinrichs, J.C.; Briceño-Guevara, S.; Rojas-Sánchez, C.E.; Méndez-Rivera, M.; Arias-Mora, V.; Tormo-Budowski, R.; Brenes-Alfaro, L.; et al. Occurrence of Pharmaceuticals, Hazard Assessment and Ecotoxicological Evaluation of Wastewater Treatment Plants in Costa Rica. Sci. Total Environ. 2020, 746, 141200. [Google Scholar] [CrossRef]
- Duan, W.; Cui, H.; Jia, X.; Huang, X. Occurrence and Ecotoxicity of Sulfonamides in the Aquatic Environment: A Review. Sci. Total Environ. 2022, 820, 153178. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Rehman, M.Y.A.; Lv, M.; Yue, L.; Xu, N.; Malik, R.N. First Insight into the Occurrence, Spatial Distribution, Sources, and Risks Assessment of Antibiotics in Groundwater from Major Urban-Rural Settings of Pakistan. Sci. Total Environ. 2021, 791, 148298. [Google Scholar] [CrossRef]
- Qin, L.-T.; Pang, X.-R.; Zeng, H.-H.; Liang, Y.-P.; Mo, L.-Y.; Wang, D.-Q.; Dai, J.-F. Ecological and Human Health Risk of Sulfonamides in Surface Water and Groundwater of Huixian Karst Wetland in Guilin, China. Sci. Total Environ. 2020, 708, 134552. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Errayess, S.A.; Amine, A. Voltammetric Determination of Sulfonamides Using Paste Electrodes Based on Various Carbon Nanomaterials. Microchim. Acta 2016, 183, 2169–2176. [Google Scholar] [CrossRef]
- Grenni, P.; Patrolecco, L.; Rauseo, J.; Spataro, F.; Di Lenola, M.; Aimola, G.; Zacchini, M.; Pietrini, F.; Di Baccio, D.; Stanton, I.C.; et al. Sulfamethoxazole Persistence in a River Water Ecosystem and Its Effects on the Natural Microbial Community and Lemna Minor Plant. Microchem. J. 2019, 149, 103999. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Zhang, Q.; Song, K.; Zhou, X.; Tang, Z.; Zhou, X. Occurrence and Ecological Risk Assessment of Selected Antibiotics in the Freshwater Lakes Along the Middle and Lower Reaches of Yangtze River Basin. J. Environ. Manag. 2019, 249, 109396. [Google Scholar] [CrossRef]
- Faleye, A.; Adegoke, A.; Ramluckan, K.; Bux, F.; Stenström, T.A. Antibiotic Residue in the Aquatic Environment: Status in Africa. Open Chem. 2018, 16, 890–903. [Google Scholar] [CrossRef]
- Chen, H.; Jing, L.; Teng, Y.; Wang, J. Characterization of Antibiotics in A Large-scale River System of China: Occurrence Pattern, Spatiotemporal Distribution and Environmental Risks. Sci. Total Environ. 2018, 618, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Santos, L. Antibiotics in the Aquatic Environments: A Review of the European Scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Garoma, T.; Umamaheshwar, S.K.; Mumper, A. Removal of Sulfadiazine, Sulfamethizole, Sulfamethoxazole, and Sulfathiazole from Aqueous Solution by Ozonation. Chemosphere 2010, 79, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Gorito, A.M.; Pesqueira, J.F.; Moreira, N.F.; Ribeiro, A.R.; Pereira, M.F.R.; Nunes, O.C.; Almeida, C.M.R.; Silva, A.M. Ozone-based Water Treatment (O3, O3/UV, O3/H2O2) for Removal of Organic Micropollutants, Bacteria Inactivation and Regrowth Prevention. J. Environ. Chem. Eng. 2021, 9, 105315. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Tian, M.; Ahmad, N.; Jia, K.; Luo, Z.; Qiao, B.; Cheng, J.; Zhao, C. Porphyrinic Based Hydrogen-bonded Organic Framework/TiO2 Nanocomposites for Efficient Photocatalytic Degradation of Sulfadiazine. J. Environ. Sci. 2025, 152, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fan, Y.; Shang, S.; Li, P.; Li, X.-Y. Fabrication of A Permeable SnO2-Sb Reactive Anodic Filter for High-Efficiency Electrochemical Oxidation of Antibiotics in Wastewater. Environ. Int. 2021, 157, 106827. [Google Scholar] [CrossRef]
- Martínez-Costa, J.; Rivera-Utrilla, J.; Leyva-Ramos, R.; Sánchez-Polo, M.; Velo-Gala, I.; Mota, A. Individual and Simultaneous Degradation of the Antibiotics Sulfamethoxazole and Trimethoprim in Aqueous Solutions by Fenton, Fenton-like and Photo-Fenton Processes Using Solar and UV Radiations. J. Photochem. Photobiol. A Chem. 2018, 360, 95–108. [Google Scholar] [CrossRef]
- Hamadeen, H.M.; Elkhatib, E.A. New Nanostructured Activated Biochar for Effective Removal of Antibiotic Ciprofloxacin from Wastewater: Adsorption Dynamics and Mechanisms. Environ. Res. 2022, 210, 112929. [Google Scholar] [CrossRef]
- Kong, Z.; Zhang, H.; Zhou, T.; Xie, L.; Wang, B.; Jiang, X. Biomass-Derived Functional Materials: Preparation, Functionalization, and Applications in Adsorption and Catalytic Separation of Carbon Dioxide and Other Atmospheric Pollutants. Sep. Purif. Technol. 2025, 354, 129099. [Google Scholar] [CrossRef]
- Sarkar, D.; Panicker, T.F.; Mishra, R.K.; Kini, M.S. A Comprehensive Review of Production and Characterization of Biochar for Removal of Organic Pollutants from Water and Wastewater. Water-Energy Nexus 2024, 7, 243–265. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Goh, H.H.; Gikas, P.; Chong, K.-K.; Chew, K.W. Challenges and Opportunities for Biochar to Promote Circular Economy and Carbon Neutrality. J. Environ. Manag. 2023, 332, 117429. [Google Scholar] [CrossRef]
- Usman, I.M.T.; Ho, Y.-C.; Baloo, L.; Lam, M.-K.; Sujarwo, W. A Comprehensive Review on the Advances of Bioproducts from Biomass Towards Meeting Net Zero Carbon Emissions (NZCE). Bioresour. Technol. 2022, 366, 128167. [Google Scholar] [CrossRef]
- Kung, C.-C.; Mu, J.E. Prospect of China’s Renewable Energy Development from Pyrolysis and Biochar Applications under Climate Change. Renew. Sustain. Energy Rev. 2019, 114, 109343. [Google Scholar] [CrossRef]
- Olugbenga, O.S.; Adeleye, P.G.; Oladipupo, S.B.; Adeleye, A.T.; John, K.I. Biomass-derived Biochar in Wastewater Treatment- A Circular Economy Approach. Waste Manag. Bull. 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Afshar, M.; Mofatteh, S. Biochar for A Sustainable Future: Environmentally Friendly Production and Diverse Applications. Results Eng. 2024, 23, 102433. [Google Scholar] [CrossRef]
- Georgieva, V.G.; Gonsalvesh, L.; Tavlieva, M.P. Thermodynamics and Kinetics of the Removal of Nickel (II) Ions from Aqueous Solutions by Biochar Adsorbent Made from Agro-waste Walnut Shells. J. Mol. Liq. 2020, 312, 112788. [Google Scholar] [CrossRef]
- Aghababaei, A.; Ncibi, M.C.; Sillanpää, M. Optimized Removal of Oxytetracycline and Cadmium from Contaminated Waters Using Chemically-activated and Pyrolyzed Biochars from Forest and Wood-Processing Residues. Bioresour. Technol. 2017, 239, 28–36. [Google Scholar] [CrossRef]
- Birer, A.M.; Gözmen, B.; Sönmez, Ö.; Kalderis, D. Evaluation of Sewage Sludge Biochar and Modified Derivatives as Novel SPE Adsorbents for Monitoring of Bisphenol A. Chemosphere 2021, 268, 128866. [Google Scholar] [CrossRef]
- Liu, H.; Xu, G.; Li, G. Preparation of Porous Biochar Based on Pharmaceutical Sludge Activated by NaOH and Its Application in the Adsorption of Tetracycline. J. Colloid Interface Sci. 2021, 587, 271–278. [Google Scholar] [CrossRef]
- Ayala, J.; Fernández, B. Industrial Waste Materials as Adsorbents for the Removal of as and Other Toxic Elements from an Abandoned Mine Spoil Heap Leachate: A Case Study in Asturias. J. Hazard. Mater. 2020, 384, 121446. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Q.; Feng, W.; Ni, S.; Li, G. Coadsorption of Tetracycline and Copper(II) by KOH-Modified Biomass and Biochar Derived from Corn Straw in Aqueous Solution. Water 2025, 17, 284. [Google Scholar] [CrossRef]
- Naghipour, D.; Amouei, A.; Ghasemi, K.T.; Taghavi, K. Removal of Cefixime from Aqueous Solutions by the Biosorbent Prepared from Pine Cones: Kinetic and Isotherm Studies. Desalination Water Treat. 2020, 201, 219–227. [Google Scholar] [CrossRef]
- Alawa, B.; Singh, S.; Chakma, S.; Kishor, R.; Lundborg, C.S.; Diwan, V. Development of Novel Biochar Adsorbent Using Agricultural Waste Biomass for Enhanced Removal of Ciprofloxacin from Water: Insights into the Isotherm, Kinetics, and Thermodynamic Analysis. Chemosphere 2025, 375, 144252. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Zhuang, S.; Wang, J. Biochar: A Potential and Green Adsorbent for Antibiotics Removal from Aqueous Solution. Rev. Environ. Sci. Bio/Technol. 2024, 23, 1065–1103. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the Preparation and Application of Modified Biochar for Improved Contaminant Removal from Water and Wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Philip, L. Sustainability Assessment of Acid-modified Biochar as Adsorbent for the Removal of Pharmaceuticals and Personal Care Products from Secondary Treated Wastewater. J. Environ. Chem. Eng. 2022, 10, 107592. [Google Scholar] [CrossRef]
- González-Hourcade, M.; Dos Reis, G.S.; Grimm, A.; Dinh, V.M.; Lima, E.C.; Larsson, S.H.; Gentili, F.G. Microalgae Biomass as a Sustainable Precursor to Produce Nitrogen-Doped Biochar for Efficient Removal of Emerging Pollutants from Aqueous Media. J. Clean. Prod. 2022, 348, 131280. [Google Scholar] [CrossRef]
- Paunovic, O.; Pap, S.; Maletic, S.; Taggart, M.A.; Boskovic, N.; Sekulic, M.T. Ionisable Emerging Pharmaceutical Adsorption Onto Microwave Functionalised Biochar Derived from Novel Lignocellulosic Waste Biomass. J. Colloid Interface Sci. 2019, 547, 350–360. [Google Scholar] [CrossRef]
- Mahdi, Z.; El Hanandeh, A.; Yu, Q.J. Electro-Assisted Adsorption of Heavy Metals from Aqueous Solutions by Biochar. Water Sci. Technol. 2020, 81, 801–812. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, M.Á. Advancing in Wastewater Treatment Using Sustainable Electrosorbents. Curr. Opin. Electrochem. 2024, 44, 101450. [Google Scholar] [CrossRef]
- Ji, F.; Wang, L.; Yang, J.; Wu, X.; Li, M.; Jiang, S.; Lin, S.; Chen, Z. Highly Compact, Free-Standing Porous Electrodes from Polymer-derived Nanoporous Carbons for Efficient Electrochemical Capacitive Deionization. J. Mater. Chem. A 2019, 7, 1768–1778. [Google Scholar] [CrossRef]
- Martinez, J.; Colán, M.; Catillón, R.; Huamán, J.; Paria, R.; Sánchez, L.; Rodríguez, J.M. Desalination Using the Capacitive Deionization Technology with Graphite/AC Electrodes: Effect of the Flow Rate and Electrode Thickness. Membranes 2022, 12, 717. [Google Scholar] [CrossRef]
- Cuong, D.V.; Wu, P.-C.; Liu, N.-L.; Hou, C.-H. Hierarchical Porous Carbon Derived from Activated Biochar as an Eco-friendly Electrode for the Electrosorption of Inorganic Ions. Sep. Purif. Technol. 2020, 242, 116813. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Y.; Fang, C.; Shi, J.; Han, H.; Li, M.; Zhao, J. Electrochemically Enhanced Adsorption of Organic Dyes from Aqueous Using A Freestanding Metal–organic Frameworks/cellulose-derived Porous Monolithic Carbon Foam. Bioresour. Technol. 2022, 347, 126424. [Google Scholar] [CrossRef]
- Acuña-Bedoya, J.D.; Alvarez-Pugliese, C.E.; Castilla-Acevedo, S.F.; Bravo-Suárez, J.J.; Marriaga-Cabrales, N. Degradation of Diclofenac Aqueous Solutions in A 3D Electrolytic Reactor Using Carbon-based Materials as Pseudo Third Electrodes in Fluidized Bed, Anodic and Cathodic Configurations. J. Environ. Chem. Eng. 2022, 10, 108075. [Google Scholar] [CrossRef]
- Alighardashi, A.; Aghta, R.S.; Ebrahimzadeh, H. Improvement of Carbamazepine Degradation by A Three-Dimensional Electrochemical (3-EC) Process. Int. J. Environ. Res. 2018, 12, 451–458. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Wu, D.; Fu, R. Feasibility Study of Using Carbon Aerogel as Particle Electrodes for Decoloration of RBRX Dye Solution in A Three-dimensional Electrode Reactor. Chem. Eng. J. 2008, 138, 47–54. [Google Scholar] [CrossRef]
- González-Prieto, Ó.; Torres, L.O.; Torres, A.V. Comparison of Waste Biomass from Pine, Eucalyptus, and Acacia and the Biochar Elaborated Using Pyrolysis in A Simple Double Chamber Biomass Reactor. Appl. Sci. 2024, 14, 1851. [Google Scholar] [CrossRef]
- Wang, G.; Yong, X.; Luo, L.; Yan, S.; Wong, J.W.; Zhou, J. Structure-performance Correlation of High Surface Area and Hierarchical Porous Biochars as Chloramphenicol Adsorbents. Sep. Purif. Technol. 2022, 296, 121374. [Google Scholar] [CrossRef]
- Sing, K.S.W. International Union of Pure and Applied Chemistry Physical Chemistry Division Commission on Colloid and Surface Chemistry Including Catalysis* Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity Reporting Physisorption Data for Gas/Solid Systems-with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, D.; Pan, Z.; Yao, Y.; Li, J.; Qiu, Y. Pore Structure and Its Impact on CH4 Adsorption Capacity and Flow Capability of Bituminous and Subbituminous Coals from Northeast China. Fuel 2013, 103, 258–268. [Google Scholar] [CrossRef]
- Yue, J.; Wang, Z.; Chen, J.; Zheng, M.; Wang, Q.; Lou, X. Investigation of Pore Structure Characteristics and Adsorption Characteristics of Coals with Different Destruction Types. Adsorpt. Sci. Technol. 2019, 37, 623–648. [Google Scholar] [CrossRef]
- Yargicoglu, E.N.; Sadasivam, B.Y.; Reddy, K.R.; Spokas, K. Physical and Chemical Characterization of Waste Wood Derived Biochars. Waste Manag. 2015, 36, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Gámiz, B.; Hall, K.; Spokas, K.A.; Cox, L. Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption. Agronomy 2019, 9, 588. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Qin, Y.; Yin, X.; Huang, A. Released Volatile Organic Compounds in Southern Yellow Pine Before and After Heat Treatment. Int. J. Environ. Res. Public Health 2018, 15, 2579. [Google Scholar] [CrossRef]
- Singh, B.; Fang, Y.; Johnston, C. A Fourier-Transform Infrared Study of Biochar Aging in Soils. Soil Sci. Soc. Am. J. 2016, 80, 613–622. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared Spectrum Characteristics and Quantification of OH Groups in Coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption Behavior and Mechanism of Hydrophilic Organic Chemicals to Virgin and Aged Microplastics in Freshwater and Seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Y.; Huang, M.; Fu, S.; Hao, Y.; Hu, S.; Lai, D.; Zhao, L. Adsorption Behaviors and Mechanisms of Antibiotic Norfloxacin on Degradable and Nondegradable Microplastics. Sci. Total Environ. 2022, 807, 151042. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar Surface Functional Groups as Affected by Biomass Feedstock, Biochar Composition and Pyrolysis Temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Esteves, B.; Marques, A.V.; Domingos, I.; Pereira, H. Chemical Changes of Heat Treated Pine and Eucalypt Wood Monitored by FTIR. Maderas-Cienc. Tecnol. 2013, 15, 245–258. [Google Scholar] [CrossRef]
- Askeland, M.; Clarke, B.; Paz-Ferreiro, J. Comparative Characterization of Biochars Produced At Three Selected Pyrolysis Temperatures from Common Woody and Herbaceous Waste Streams. PeerJ 2019, 7, E6784. [Google Scholar] [CrossRef]
- Rodrigues, D.L.C.; Fuhr, A.C.F.P.; Guido, J.A.; De Azevedo, C.F.; Rangel, A.M.; Dotto, G.L.; Alomar, S.Y.; Machado, F.M. Olive Biomass-derived Magnetic Activated Biochar for Ciprofloxacin Removal: Integrated Kinetic, Isotherm, Thermodynamic, and Spectroscopic Analysis. Sep. Purif. Technol. 2025, 360, 131014. [Google Scholar] [CrossRef]
- He, Y.; Liu, Z.; Chen, J.; Deng, Y. Performance and Mechanism of Sulfadiazine and Norfloxacin Adsorption from Aqueous Solution by Magnetic Coconut Shell Biochar. Environ. Sci. Pollut. Res. 2024, 31, 48561–48575. [Google Scholar] [CrossRef] [PubMed]
- Pellenz, L.; De Oliveira, C.R.S.; Júnior, A.H.d.S.; Da Silva, L.J.S.; De Souza, A.A.U.; Souza, S.M.d.A.G.U.d.; Borba, F.H.; Da Silva, A. A Comprehensive Guide for Characterization of Adsorbent Materials. Sep. Purif. Technol. 2023, 305, 122435. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kavitha, D. IR, XRD and SEM Studies on the Mechanism of Adsorption of Dyes and Phenols by Coir Pith Carbon from Aqueous Phase. Microchem. J. 2006, 82, 43–48. [Google Scholar] [CrossRef]
- Gupta, G.K.; Gupta, P.K.; Mondal, M.K. Experimental Process Parameters Optimization and In-depth Product Characterizations for Teak Sawdust Pyrolysis. Waste Manag. 2019, 87, 499–511. [Google Scholar] [CrossRef]
- He, P.; Liu, Y.; Shao, L.; Zhang, H.; Lü, F. Particle Size Dependence of the Physicochemical Properties of Biochar. Chemosphere 2018, 212, 385–392. [Google Scholar] [CrossRef]
- Yadav, S.K.; Bag, R. Effect of Bamboo Biochar on Strength and Water Retention Properties of Low Plastic Clay and Silty Sand. Sci. Rep. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Faye, M.C.A.; Zhang, Y. Characterization of Biochar Derived from Rice Husks and Its Potential in Chlorobenzene Degradation. Carbon 2018, 130, 730–740. [Google Scholar] [CrossRef]
- Chang, Z.; Tian, L.; Zhang, J.; Zhou, D. Comparative Study on the Relative Significance of Low-/high-condensation Aromatic Moieties in Biochar to Organic Contaminant Sorption. Ecotoxicol. Environ. Saf. 2022, 238, 113598. [Google Scholar] [CrossRef]
- Nan, H.; Yin, J.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X. Pyrolysis Temperature-dependent Carbon Retention and Stability of Biochar with Participation of Calcium: Implications to Carbon Sequestration. Environ. Pollut. 2021, 287, 117566. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Burachevskaya, M.; Minkina, T.; Bauer, T.; Lobzenko, I.; Fedorenko, A.; Mazarji, M.; Sushkova, S.; Mandzhieva, S.; Nazarenko, A.; Butova, V.; et al. Fabrication of Biochar Derived from Different Types of Feedstocks as an Efficient Adsorbent for Soil Heavy Metal Removal. Sci. Rep. 2023, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Piersa, P.; Szufa, S.; Czerwińska, J.; Ünyay, H.; Adrian, Ł.; Wielgosinski, G.; Obraniak, A.; Lewandowska, W.; Marczak-Grzesik, M.; Dzikuć, M.; et al. Pine Wood and Sewage Sludge Torrefaction Process for Production Renewable Solid Biofuels and Biochar as Carbon Carrier for Fertilizers. Energies 2021, 14, 8176. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Méndez, A.; Gascó, G.; Lado, M.; Paz-González, A. the Effects of Feedstock, Pyrolysis Temperature, and Residence Time on the Properties and Uses of Biochar from Broom and Gorse Wastes. Appl. Sci. 2024, 14, 4283. [Google Scholar] [CrossRef]
- Boraah, N.; Chakma, S.; Kaushal, P. Optimum Features of Wood-based Biochars: A Characterization Study. J. Environ. Chem. Eng. 2023, 11, 109976. [Google Scholar] [CrossRef]
- Essandoh, M.; Kunwar, B.; Pittman, C.U., Jr.; Mohan, D.; Mlsna, T. Sorptive Removal of Salicylic Acid and Ibuprofen from Aqueous Solutions Using Pine Wood Fast Pyrolysis Biochar. Chem. Eng. J. 2015, 265, 219–227. [Google Scholar] [CrossRef]
- Brooks, B.W.; Foran, C.M.; Richards, S.M.; Weston, J.; Turner, P.K.; Stanley, J.K.; Solomon, K.R.; Slattery, M.; La Point, T.W. Aquatic Ecotoxicology of Fluoxetine. Toxicol. Lett. 2003, 142, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photochemical Fate of Sulfa Drugs in the Aquatic Environment: Sulfa Drugs Containing Five-Membered Heterocyclic Groups. Environ. Sci. Technol. 2004, 38, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of Ionizable and Ionic Organic Compounds to Biochar, Activated Carbon and Other Carbonaceous Materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Do, T.T.; Dao, U.P.; Bui, H.T.; Nguyen, T.T. Effect of Electrostatic Interaction Between Fluoxetine and Lipid Membranes on the Partitioning of Fluoxetine Investigated Using Second Derivative Spectrophotometry and FTIR. Chem. Phys. Lipids 2017, 207, 10–23. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Moreira, M.M.; Paíga, P.; Dias, D.; Bernardo, M.; Carvalho, M.; Lapa, N.; Fonseca, I.; Morais, S.; Figueiredo, S.; et al. Evaluation of the Adsorption Potential of Biochars Prepared from Forest and Agri-food Wastes for the Removal of Fluoxetine. Bioresour. Technol. 2019, 292, 121973. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Ledesma, B.; Laginhas, C.; Titirici, M.-M. Surface Interactions During the Removal of Emerging Contaminants by Hydrochar-Based Adsorbents. Molecules 2020, 25, 2264. [Google Scholar] [CrossRef]
- Lago, A.; Silva, B.; Tavares, T. Biowaste Valorization on Pharmaceuticals and Pesticides Abatement in Aqueous Environments. Sustain. Mater. Technol. 2024, 39, E00792. [Google Scholar] [CrossRef]
- Nkana, G.R.N.; Chabot, B.; Nguyen-Tri, P. Adsorption of Fluoxetine in Aqueous Environments: Development of Macroporous Cryogels Based on Sodium Poly(acrylate) and Carboxymethyl Chitosan. React. Funct. Polym. 2024, 205, 106069. [Google Scholar] [CrossRef]
- Ji, L.; Wan, Y.; Zheng, S.; Zhu, D. Adsorption of Tetracycline and Sulfamethoxazole on Crop Residue-Derived Ashes: Implication for the Relative Importance of Black Carbon to Soil Sorption. Environ. Sci. Technol. 2011, 45, 5580–5586. [Google Scholar] [CrossRef]
- Hou, J.; Bao, W.; Zhang, J.; Yu, J.; Chen, L.; Di, G.; Zhou, Q.; Li, X. Characteristics and Mechanisms of Sulfamethoxazole Adsorption Onto Modified Biochars with Hierarchical Pore Structures: Batch, Predictions Using Artificial Neural Network and Fixed Bed Column Studies. J. Water Process. Eng. 2023, 54, 103975. [Google Scholar] [CrossRef]
- Jaria, G.; Calisto, V.; Gil, M.V.; Otero, M.; Esteves, V.I. Removal of Fluoxetine from Water by Adsorbent Materials Produced from Paper Mill Sludge. J. Colloid Interface Sci. 2015, 448, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Martins, M.; Rosca, M.; Rocha, V.; Lago, A.; Neves, I.C.; Tavares, T. Waste-based Biosorbents as Cost-effective Alternatives to Commercial Adsorbents for the Retention of Fluoxetine from Water. Sep. Purif. Technol. 2020, 235, 116139. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of Magnetic Biochar from Pine Sawdust Via Oxidative Hydrolysis of FeCl2 for the Removal Sulfamethoxazole from Aqueous Solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef]
- Cabral, L.L.; Bottini, R.C.R.; Gonçalves, A.J.; Junior, M.M.; Rizzo-Domingues, R.C.P.; Lenzi, M.K.; Nagalli, A.; Passig, F.H.; Dos Santos, P.M.; De Carvalho, K.Q. Food Dye Adsorption in Single and Ternary Systems by the Novel Passion Fruit Peel Biochar Adsorbent. Food Chem. 2025, 464, 141592. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z.; Skic, K.; Tomczyk, A. Chemically Engineered Biochar—Effect of Concentration and Type of Modifier on Sorption and Structural Properties of Biochar from Wood Waste. Fuel 2019, 256, 115893. [Google Scholar] [CrossRef]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar Pyrolytically Produced from Municipal Solid Wastes for Aqueous As(V) Removal: Adsorption Property and Its Improvement with KOH Activation. Bioresour. Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, H.; Shi, S.; Zhang, G.; Ni, J. Electrolytic Treatment of C.I. Acid Orange 7 in Aqueous Solution Using A Three-dimensional Electrode Reactor. Dye. Pigment. 2008, 77, 158–164. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Liu, W.-F.; Qin, L.; Liu, X.-G. the Use of Carbon-based Particle Electrodes in Three-dimensional Electrode Reactors for Wastewater Treatment. Xinxing Tan Cailiao/New Carbon Mater. 2024, 39, 973–991. [Google Scholar] [CrossRef]
- Correia-Sá, L.; Soares, C.; Freitas, O.M.; Moreira, M.M.; Nouws, H.P.A.; Correia, M.; Paíga, P.; Rodrigues, A.J.; Oliveira, C.M.; Figueiredo, S.A.; et al. A Three-Dimensional Electrochemical Process for the Removal of Carbamazepine. Appl. Sci. 2021, 11, 6432. [Google Scholar] [CrossRef]
- Duan, X.; Ren, D.; Wang, S.; Zhang, M.; Sun, Y.; Sun, S.; Huo, Z.; Zhang, N. Carbon Materials in Electrocatalytic Oxidation Systems for the Treatment of Organic Pollutants in Wastewater: A Review. Carbon Resour. Convers. 2023, 6, 262–273. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of Cadmium by Biochar Derived from Municipal Sewage Sludge: Impact Factors and Adsorption Mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Chen, Y.-D.; Yang, Z.-K.; Nagarajan, D.; Chang, J.-S.; Ren, N.-Q. High-efficiency Removal of Lead from Wastewater by Biochar Derived from Anaerobic Digestion Sludge. Bioresour. Technol. 2017, 246, 142–149. [Google Scholar] [CrossRef]
- Fan, S.S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X.D. Removal of Methylene Blue from Aqueous Solution by Sewage Sludge-derived Biochar: Adsorption Kinetics, Equilibrium, Thermodynamics and Mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Chu, M.; Tian, W.; Zhao, J.; Zhang, D.; Zou, M.; Lu, Z.; Jiang, J. Dual-activated Biochar with A Multichannel Structure Enhanced Electrosorption Capacity of Capacitive Deionization for Sulfate Removal from Mining Wastewater. Desalination 2023, 556, 116588. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Ellis, N.; Gyenge, E. Electrosorption on Activated Biochar: Effect of Thermo-chemical Activation Treatment on the Electric Double Layer Capacitance. J. Appl. Electrochem. 2014, 44, 141–157. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, R.; Zhao, H.; Li, K.; Guo, Z.; Chang, Y.; Shen, M. KOH Activated Walnut Shell Biochar Electrode of Capacitive Deionization and Its Desalination Performance. J. Electrochem. Soc. 2024, 171, 113501. [Google Scholar] [CrossRef]
- Ding, J.; Liang, J.; Wang, Q.; Tan, X.; Xie, W.; Chen, C.; Li, C.; Li, D.; Li, J.; Chen, X. Enhanced Tetracycline Adsorption Using KOH-Modified Biochar Derived from Waste Activated Sludge in Aqueous Solutions. Toxics 2024, 12, 691. [Google Scholar] [CrossRef]
- Ma, X.; Cao, Y.; Deng, J.; Shao, J.; Feng, X.; Li, W.; Li, S.; Zhang, R. Synergistic Enhancement of N, S Co-modified Biochar for Removal of Tetracycline Hydrochloride from Aqueous Solution: Tunable Micro-mesoporosity and Chemisorption Sites. Chem. Eng. J. 2024, 492, 152189. [Google Scholar] [CrossRef]
- Shao, S.; Zhang, M.; Lv, Z.; Sun, Y.; Li, P.; Zhou, R. Research on Efficient Removal of Ciprofloxacin Through Sequential Rice Straw Biochar Modification Via Alkali Activation and Manganese Oxides. Environ. Technol. Innov. 2024, 34, 103611. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Cheng, J.; Hu, C.; Yang, Z.; Wu, C. Three-dimensional Particle Electrode System Treatment of Organic Wastewater: A General Review Based on Patents. J. Clean. Prod. 2021, 308, 127324. [Google Scholar] [CrossRef]
- Puga, A.; Meijide, J.; Pazos, M.; Rosales, E.; Sanromán, M. Electric Field as A Useful Tool to Improve the Poor Adsorption Affinity of Pollutants on Carbonaceous Aerogel Pellets. J. Mol. Liq. 2022, 366, 120269. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Abdelfatah, A.M.; Hosny, M.; Fawzy, M. Novel Biogenic Synthesis of A Ag@Biochar Nanocomposite as an Antimicrobial Agent and Photocatalyst for Methylene Blue Degradation. ACS Omega 2022, 7, 8046–8059. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-García, V.; Rosales, E.; Puga, A.; Pazos, M.; Sanromán, M. Synthesis and Use of Efficient Adsorbents under the Principles of Circular Economy: Waste Valorisation and Electroadvanced Oxidation Process Regeneration. Sep. Purif. Technol. 2020, 242, 116796. [Google Scholar] [CrossRef]
- Lu, K.-H.; Chen, C.-Y.; Lee, M.-R. Trace Determination of Sulfonamides Residues in Meat with A Combination of Solid-phase Microextraction and Liquid Chromatography–mass Spectrometry. Talanta 2007, 72, 1082–1087. [Google Scholar] [CrossRef]
- Puga, A.; Rosales, E.; Sanromán, M.A.; Pazos, M. Environmental Application of Monolithic Carbonaceous Aerogels for the Removal of Emerging Pollutants. Chemosphere 2020, 248, 125995. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Shan, R.; Li, M.; Gu, J.; Yuan, H.; Chen, Y. Efficient Adsorption of A Sulfonamide Antibiotic in Aqueous Solutions with N-doped Magnetic Biochar: Performance, Mechanism, and Reusability. ACS Omega 2023, 8, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Ma, C.; Zhou, J.; Wang, H.; Yang, H. Enhancing Sulfonamide Removal from Water Using Wood Biochar: Adsorption Mechanisms and Regeneration Potential. J. Taiwan Inst. Chem. Eng. 2025, 167, 105910. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Ye, X.; Chen, J.; Huang, J. Advanced Oxidation Processes for Activated Carbon Regeneration: From Fundamental to Application. ACS ES&T Water 2024, 4, 3119–3130. [Google Scholar] [CrossRef]
- Sun, M.; Liu, Y.; Xiang, W.; Zhai, L.-F. Electricity-induced Catalytic Oxidation of RhB by O2 At A Graphite Anode. Electrochim. Acta 2015, 158, 314–320. [Google Scholar] [CrossRef]
- Borràs, N.; Arias, C.; Oliver, R.; Brillas, E. Anodic Oxidation, Electro-Fenton and Photoelectro-Fenton Degradation of Cyanazine Using A Boron-doped Diamond Anode and an Oxygen-diffusion Cathode. J. Electroanal. Chem. 2013, 689, 158–167. [Google Scholar] [CrossRef]
- Pariente, M.; Segura, Y.; Álvarez-Torrellas, S.; Casas, J.; De Pedro, Z.; Diaz, E.; García, J.; López-Muñoz, M.; Marugán, J.; Mohedano, A.; et al. Critical Review of Technologies for the On-site Treatment of Hospital Wastewater: From Conventional to Combined Advanced Processes. J. Environ. Manag. 2022, 320, 115769. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Han, Z.; Gan, Y.; Zhen, H.; Li, Z.; Luo, J.; Jiang, K.; Ji, C.; Yang, W.; Lu, X.; et al. Titanium (IV) Hydroxide-modified Biochar Electrode for Efficient and Selective Fluorine Removal by Flow-electrode Capacitive Deionization. Sep. Purif. Technol. 2025, 361, 131329. [Google Scholar] [CrossRef]
- Maged, A.; Dissanayake, P.D.; Yang, X.; Pathirannahalage, C.; Bhatnagar, A.; Ok, Y.S. New Mechanistic Insight into Rapid Adsorption of Pharmaceuticals from Water Utilizing Activated Biochar. Environ. Res. 2021, 202, 111693. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Huang, H.; Gao, S. Adsorption of Triclosan Onto Different Aged Polypropylene Microplastics: Critical Effect of Cations. Sci. Total Environ. 2020, 717, 137033. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, S.; Chakraborty, S.; Saikia, M.D. Surface Modified Pineapple Crown Leaf for Adsorption of Cr(VI) and Cr(III) Ions from Aqueous Solution. J. Environ. Chem. Eng. 2018, 6, 2492–2501. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, Y.; Qian, S.; Deng, Z.; Liu, Y.; Ma, J.; Zhang, Z. Ball Milling Boosted Hydrothermal N-doped Sludge-derived Biochar Towards Efficiently Adsorptive Removal of Sulfamethoxazole from Waters: Behavior, Mechanism and DFT Study. Sep. Purif. Technol. 2024, 338, 126453. [Google Scholar] [CrossRef]
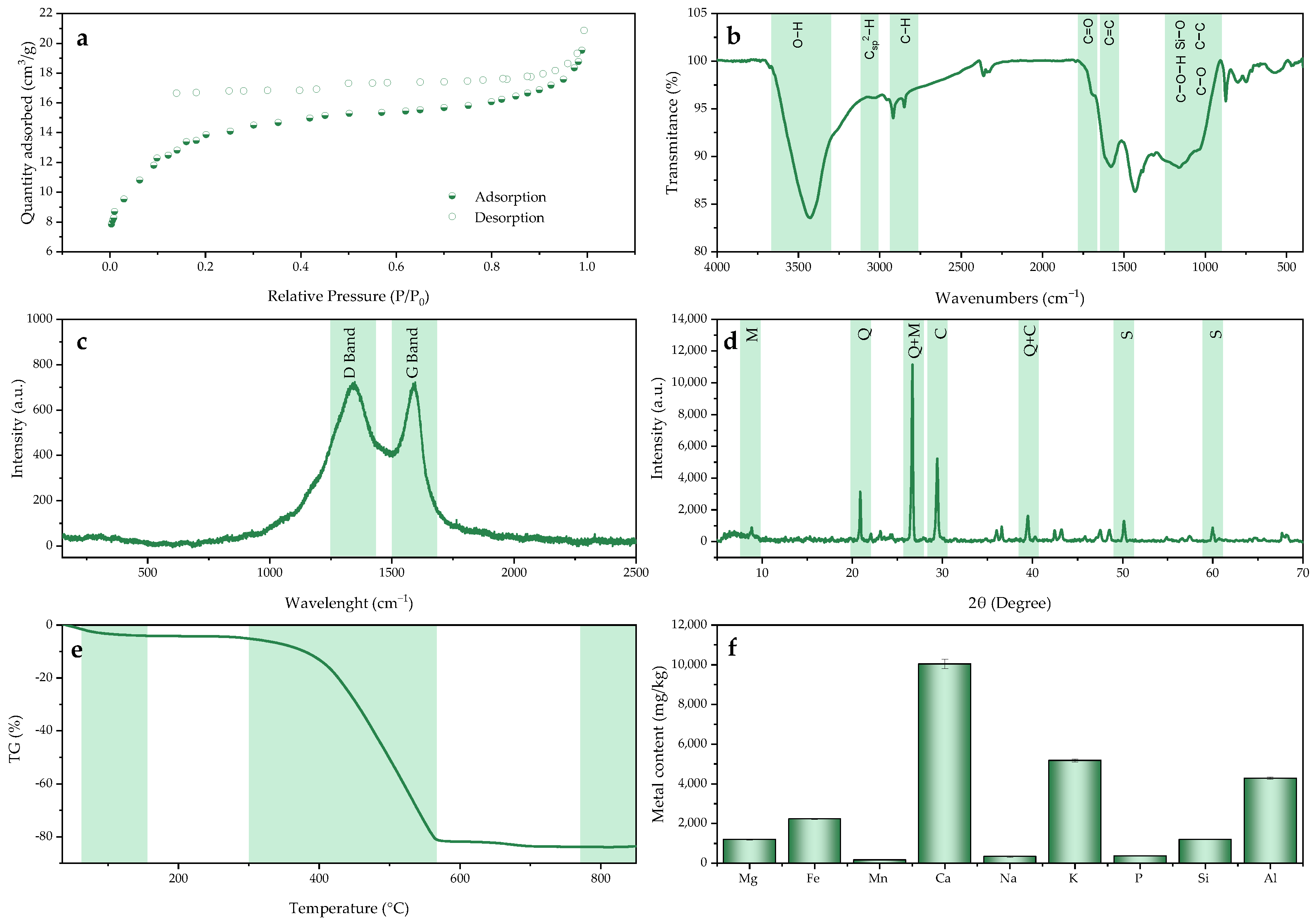
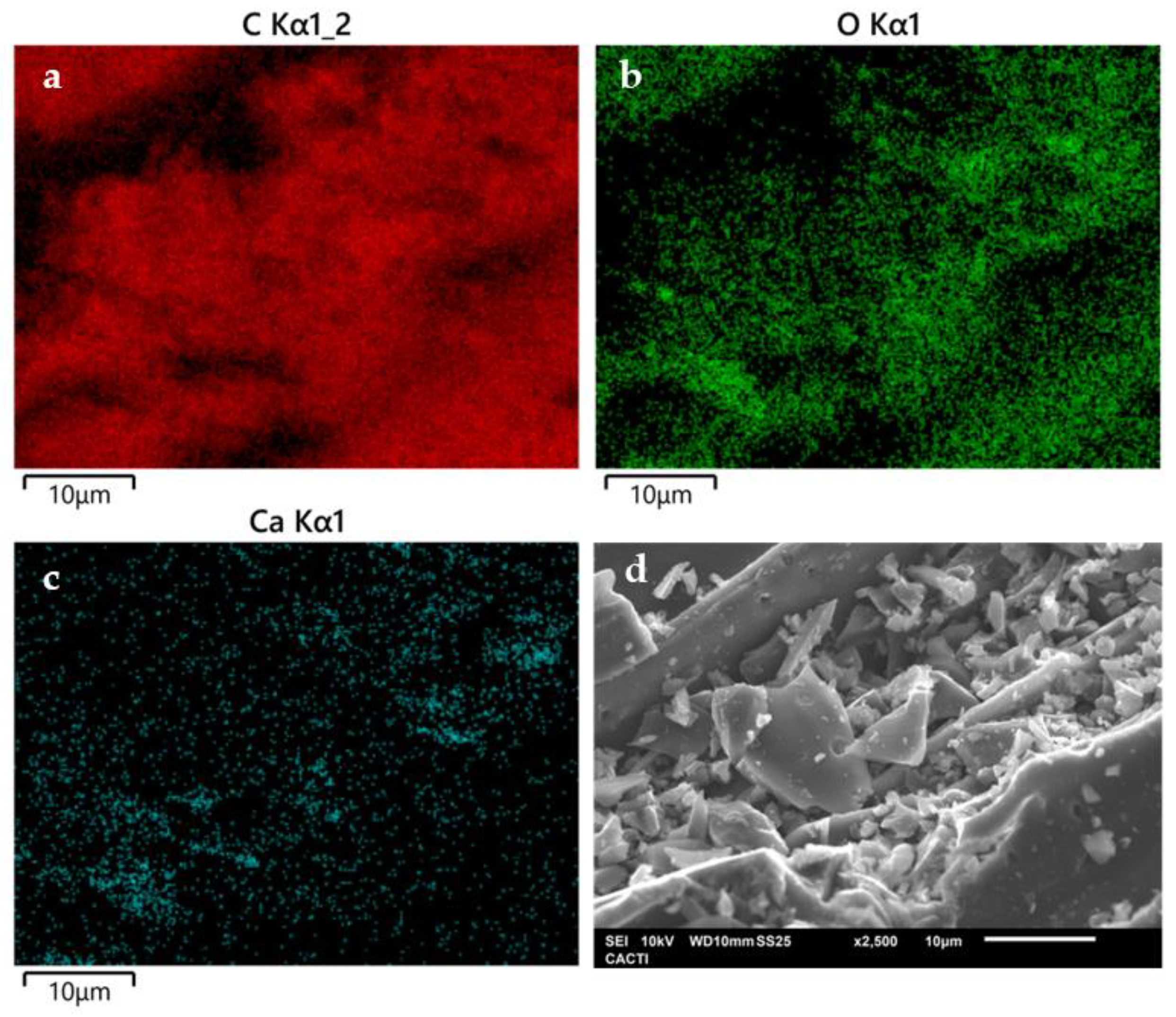
| BET parameters | |
| SBET (m2/g) | 49.64 |
| SEBET (m2/g) | 32.76 |
| SMP (m2/g) | 16.89 |
| VMP (cm3/g) | 0.0068 |
| Elemental analysis parameters | |
| Carbon (Wt %) | 71.95 |
| Oxygen (Wt %) | 14.71 |
| Hydrogen (Wt %) | 2.71 |
| Nitrogen (Wt %) | 0.53 |
| Ash (Wt %) | 10.10 |
| Electrochemical parameters | |
| Zero-point charge (pHzpc) | 7.67 |
| Compound | pKa Acid | pKa Basic | Log Kow | Ref. |
|---|---|---|---|---|
| FLX | - | 10.06 | 1.57 | [91] |
| SMZ | 2.1 | 5.3 | 0.54 | [92] |
| Adsorbent Material | Zeta Potential (mV) |
|---|---|
| BC | −20.43 |
| BC/C3H6N6 | −21.10 |
| BC/H2SO4 | −38.10 |
| BC/NaOH | −22.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernárdez-Rodas, N.; Rosales, E.; Pazos, M.; González-Prieto, Ó.; Torres, L.O.; Sanromán, M.Á. Three-Dimensional Electrosorption for Pharmaceutical Wastewater Management and Sustainable Biochar Regeneration. Molecules 2025, 30, 1435. https://doi.org/10.3390/molecules30071435
Bernárdez-Rodas N, Rosales E, Pazos M, González-Prieto Ó, Torres LO, Sanromán MÁ. Three-Dimensional Electrosorption for Pharmaceutical Wastewater Management and Sustainable Biochar Regeneration. Molecules. 2025; 30(7):1435. https://doi.org/10.3390/molecules30071435
Chicago/Turabian StyleBernárdez-Rodas, Nuria, Emilio Rosales, Marta Pazos, Óscar González-Prieto, Luis Ortiz Torres, and M. Ángeles Sanromán. 2025. "Three-Dimensional Electrosorption for Pharmaceutical Wastewater Management and Sustainable Biochar Regeneration" Molecules 30, no. 7: 1435. https://doi.org/10.3390/molecules30071435
APA StyleBernárdez-Rodas, N., Rosales, E., Pazos, M., González-Prieto, Ó., Torres, L. O., & Sanromán, M. Á. (2025). Three-Dimensional Electrosorption for Pharmaceutical Wastewater Management and Sustainable Biochar Regeneration. Molecules, 30(7), 1435. https://doi.org/10.3390/molecules30071435









