Magnetic Solid-Phase Extraction Based on C18 Nanoparticles for the Determination of Pesticides in Aquaculture Water Samples
Abstract
1. Introduction
2. Results and Discussion
2.1. Preliminary Experiments
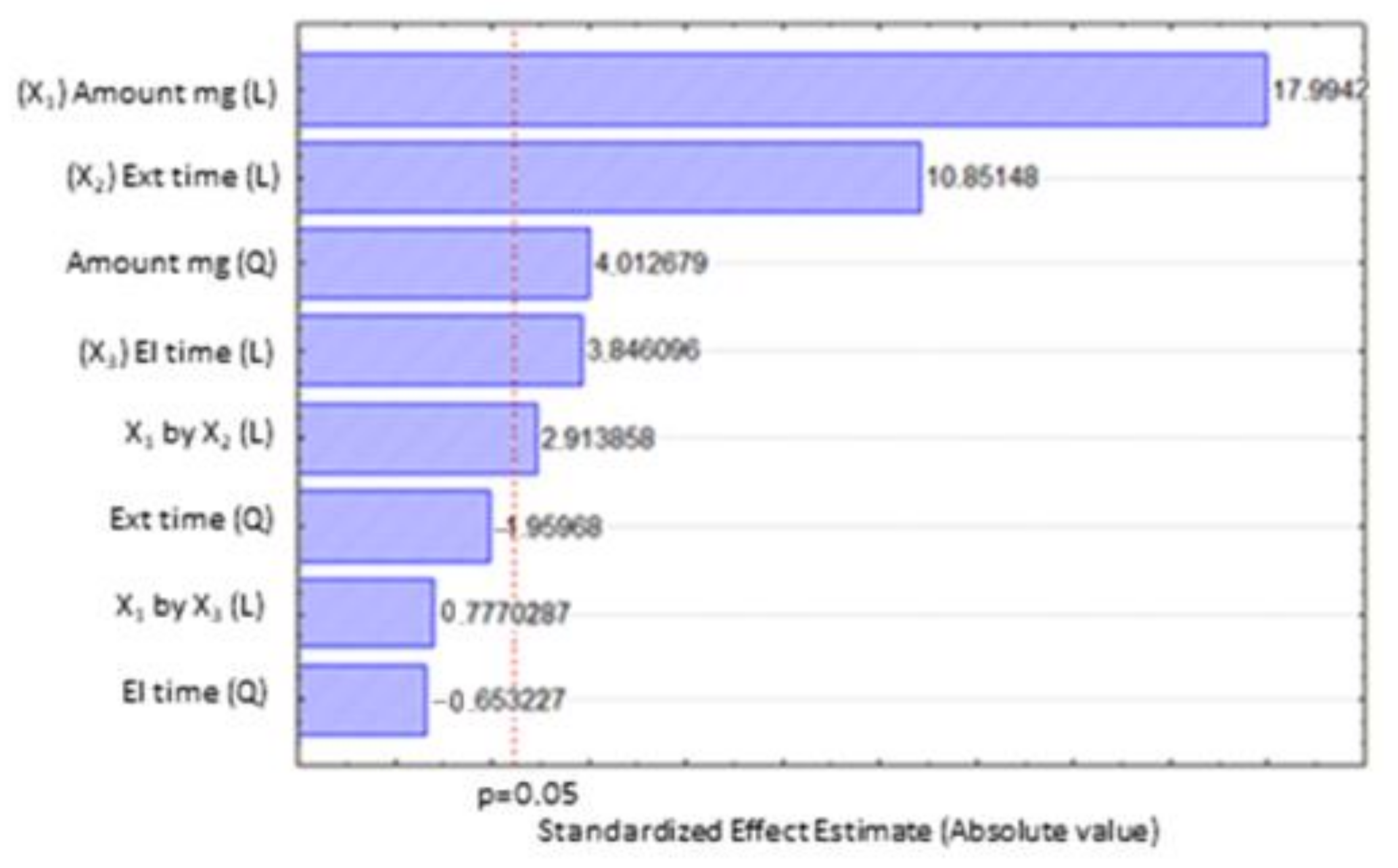
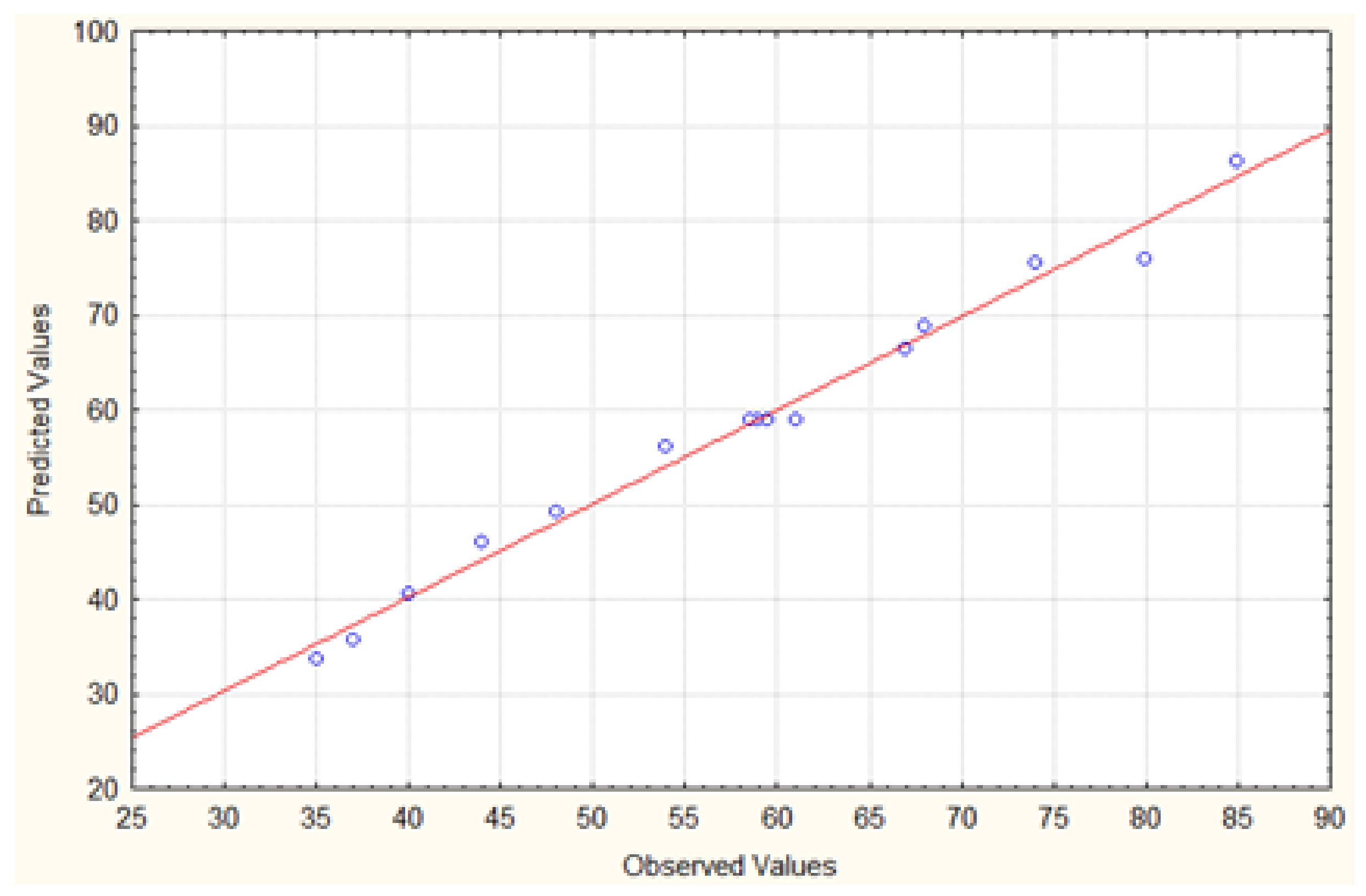
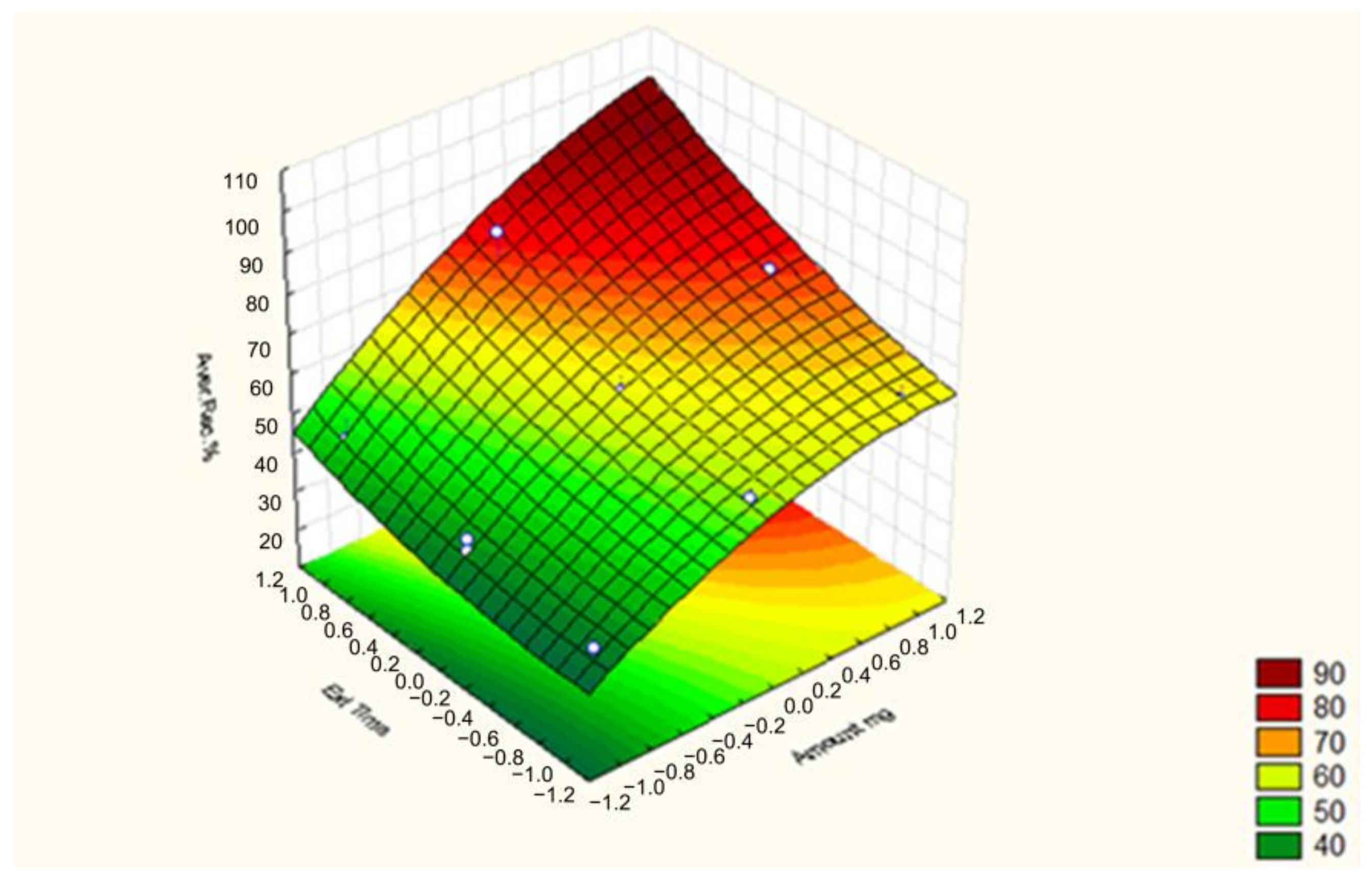
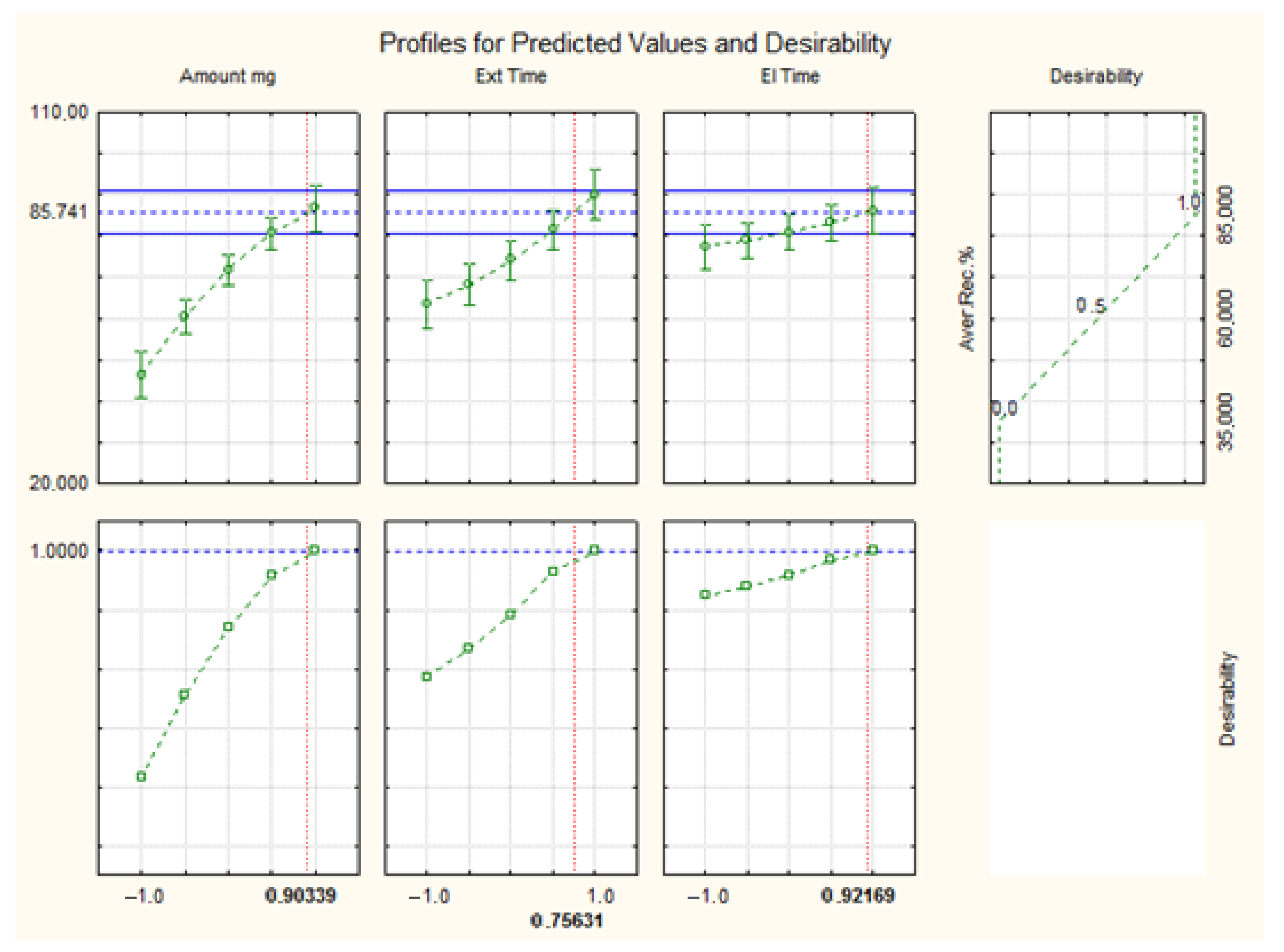
2.2. Box–Behnken Design (BBD)
2.3. Analytical Performance and Method Validation
2.4. Reusability of Fe3O4@SiO2@C18
2.5. Analysis of Target Compounds in Real Water Samples
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Instrumental Analysis
3.3. Preparation of C18 Functionalized Magnetic Nanoparticles
3.4. Characterization of MNPs
3.5. Magnetic Solid-Phase Extraction (MSPE) Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSPE | Magnetic Solid-phase extraction |
| GC–MS | Gas Chromatography–Mass Spectrometry |
| MNPs | Magnetic Nanoparticles |
| BBD | Box–Behnken design |
References
- Food and Agriculture Organization of the United Nations, Economic and Social Department. The State of Food Insecurity in the World, 2004: Monitoring Progress Towards the World Food Summit and Millennium Development Goals; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; ISBN 925105178X. [Google Scholar]
- Ismail, N.A.H.; Wee, S.Y.; Aris, A.Z. Multi-Class of Endocrine Disrupting Compounds in Aquaculture Ecosystems and Health Impacts in Exposed Biota. Chemosphere 2017, 188, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, X.; Chen, J.; Li, X.; Jia, G.; Zou, Y.; Zhang, Y.; Cui, Y. Occurrence, Distribution and Ecological Risks of Antibiotics and Pesticides in Coastal Waters around Liaodong Peninsula, China. Sci. Total Environ. 2019, 656, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Masci, M.; Orban, E.; Nevigato, T. Organochlorine Pesticide Residues: An Extensive Monitoring of Italian Fishery and Aquaculture. Chemosphere 2014, 94, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.; Martínez Bueno, M.J.; Agüera, A.; Fernández-Alba, A.R. Environmental and Human Health Risk Assessment of Organic Micro-Pollutants Occurring in a Spanish Marine Fish Farm. Environ. Pollut. 2010, 158, 1809–1816. [Google Scholar] [CrossRef]
- Barbieri, M.V.; Postigo, C.; Guillem-Argiles, N.; Monllor-Alcaraz, L.S.; Simionato, J.I.; Stella, E.; Barceló, D.; López de Alda, M. Analysis of 52 Pesticides in Fresh Fish Muscle by QuEChERS Extraction Followed by LC-MS/MS Determination. Sci. Total Environ. 2019, 653, 958–967. [Google Scholar] [CrossRef]
- Aslam, S.N.; Venzi, M.S.; Venkatraman, V.; Mikkelsen, Ø. Chemical Assessment of Marine Sediments in Vicinity of Norwegian Fish Farms—A Pilot Study. Sci. Total Environ. 2020, 732, 139130. [Google Scholar] [CrossRef]
- Arisekar, U.; Shakila, R.J.; Jeyasekaran, G.; Shalin, R.; Kumar, P.; Malani, A.H.; Rani, V. Accumulation of Organochlorine Pesticide Residues in Fish, Water, and Sediments in the Thamirabarani River System of Southern Peninsular India. Environ. Nanotechnol. Monit. Manag. 2018, 11, 100194. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Meseguer, J.; Esteban, M.A. Risks of Using Antifouling Biocides in Aquaculture. Int. J. Mol. Sci. 2012, 13, 1541–1560. [Google Scholar] [CrossRef]
- European Commission, E. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013. Off. J. Eur. Union 2013, 1–17. [Google Scholar]
- Köck-Schulmeyer, M.; Postigo, C.; Farré, M.; Barceló, D.; López de Alda, M. Medium to Highly Polar Pesticides in Seawater: Analysis and Fate in Coastal Areas of Catalonia (NE Spain). Chemosphere 2019, 215, 515–523. [Google Scholar] [CrossRef]
- Ccanccapa, A.; Masiá, A.; Navarro-Ortega, A.; Picó, Y.; Barceló, D. Pesticides in the Ebro River Basin: Occurrence and Risk Assessment. Environ. Pollut. 2016, 211, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Ziarrusta, H.; Olivares, M.; Delgado, A.; Posada-Ureta, O.; Zuloaga, O.; Etxebarria, N. Multiscreening Determination of Organic Pollutants in Molluscs Using Matrix Solid Phase Dispersion. J. Chromatogr. A 2015, 1391, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qu, B.; Liu, H.; Ding, J.; Ren, N. Analysis of Organochlorine Pesticides in Surface Water of the Songhua River Using Magnetoliposomes as Adsorbents Coupled with GC-MS/MS Detection. Sci. Total Environ. 2018, 618, 70–79. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, X.; Hu, S.; Bai, X. Applications of Liquid-Phase Microextraction Techniques in Natural Product Analysis: A Review. J. Chromatogr. A 2014, 1368, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bonansea, R.I.; Amé, M.V.; Wunderlin, D.A. Determination of Priority Pesticides in Water Samples Combining SPE and SPME Coupled to GC–MS. A Case Study: Suquía River Basin (Argentina). Chemosphere 2013, 90, 1860–1869. [Google Scholar] [CrossRef]
- Ccanccapa-Cartagena, A.; Masiá, A.; Picó, Y. Simultaneous Determination of Pyrethroids and Pyrethrins by Dispersive Liquid-Liquid Microextraction and Liquid Chromatography Triple Quadrupole Mass Spectrometry in Environmental Samples. Anal. Bioanal. Chem. 2017, 409, 4787–4799. [Google Scholar] [CrossRef]
- Latif, U.; Yaqub, S.; Dickert, F.L. Sensitive Coatings Based on Molecular-Imprinted Polymers for Triazine Pesticides’ Detection. Sensors 2024, 24, 5934. [Google Scholar] [CrossRef]
- Maddah, B.; Shamsi, J. Extraction and Preconcentration of Trace Amounts of Diazinon and Fenitrothion from Environmental Water by Magnetite Octadecylsilane Nanoparticles. J. Chromatogr. A 2012, 1256, 40–45. [Google Scholar] [CrossRef]
- Cloutier, P.L.; Fortin, F.; Groleau, P.E.; Brousseau, P.; Fournier, M.; Desrosiers, M. QuEChERS Extraction for Multi-Residue Analysis of PCBs, PAHs, PBDEs and PCDD/Fs in Biological Samples. Talanta 2017, 165, 332–338. [Google Scholar] [CrossRef]
- Hurtado-Sánchez, M.C.; Romero-González, R.; Rodríguez-Cáceres, M.I.; Durán-Merás, I.; Frenich, A.G. Rapid and Sensitive On-Line Solid Phase Extraction-Ultra High Performance Liquid Chromatography-Electrospray-Tandem Mass Spectrometry Analysis of Pesticides in Surface Waters. J. Chromatogr. A 2013, 1305, 193–202. [Google Scholar] [CrossRef]
- Mondal, R.; Mukherjee, A.; Biswas, S.; Kole, R.K. GC-MS/MS Determination and Ecological Risk Assessment of Pesticides in Aquatic System: A Case Study in Hooghly River Basin in West Bengal, India. Chemosphere 2018, 206, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-Phase Extraction of Organic Compounds: A Critical Review. Part Ii. TrAC Trends Anal. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Rashidi Nodeh, H.; Wan Ibrahim, W.A.; Kamboh, M.A.; Sanagi, M.M. New Magnetic Graphene-Based Inorganic–Organic Sol-Gel Hybrid Nanocomposite for Simultaneous Analysis of Polar and Non-Polar Organophosphorus Pesticides from Water Samples Using Solid-Phase Extraction. Chemosphere 2017, 166, 21–30. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; García-Jares, C.; Dagnac, T. Environmental Applications of Solid-Phase Microextraction. TrAC Trends Anal. Chem. 2019, 112, 1–12. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, L.; Chen, Z.; Hong, L. Analysis of Phthalate Acid Esters in Environmental Water by Magnetic Graphene Solid Phase Extraction Coupled with Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2014, 1329, 24–29. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern Trends in Solid Phase Extraction: New Sorbent Media. TrAC Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Zhao, G.; Song, S.; Wang, C.; Wu, Q.; Wang, Z. Determination of Triazine Herbicides in Environmental Water Samples by High-Performance Liquid Chromatography Using Graphene-Coated Magnetic Nanoparticles as Adsorbent. Anal. Chim. Acta 2011, 708, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qi, P.; Wang, J.; Wang, Z.; Di, S.; Xu, H.; Zhao, H.; Wang, Q.; Wang, X.; Wang, X. Development, Validation, Comparison, and Implementation of a Highly Efficient and Effective Method Using Magnetic Solid-Phase Extraction with Hydrophilic-Lipophilic-Balanced Materials for LC-MS/MS Analysis of Pesticides in Seawater. Sci. Total Environ. 2020, 708, 135221. [Google Scholar] [CrossRef]
- Heidari, H.; Razmi, H. Multi-Response Optimization of Magnetic Solid Phase Extraction Based on Carbon Coated Fe3O4 Nanoparticles Using Desirability Function Approach for the Determination of the Organophosphorus Pesticides in Aquatic Samples by HPLC-UV. Talanta 2012, 99, 13–21. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Ahmadi, F.; Pawliszyn, J. A Critical Review of Solid Phase Microextraction for Analysis of Water Samples. TrAC Trends Anal. Chem. 2016, 85, 133–143. [Google Scholar] [CrossRef]
- Wierucka, M.; Biziuk, M. Application of Magnetic Nanoparticles for Magnetic Solid-Phase Extraction in Preparing Biological, Environmental and Food Samples. TrAC Trends Anal. Chem. 2014, 59, 50–58. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Yadeghari, A.; Khoshmaram, L. Magnetic Solid Phase Extraction Using Fe3O4@SiO2@C8nanoparticles Performed in a Narrow-Bore Tube Followed by Dispersive Liquid-Liquid Microextraction for Extraction and Preconcentration of Nine Pesticides. New J. Chem. 2018, 42, 6215–6224. [Google Scholar] [CrossRef]
- Synaridou, M.-E.S.; Sakkas, V.A.; Stalikas, C.D.; Albanis, T.A. Evaluation of Magnetic Nanoparticles to Serve as Solid-Phase Extraction Sorbents for the Determination of Endocrine Disruptors in Milk Samples by Gas Chromatography Mass Spectrometry. J. Chromatogr. A 2014, 1348, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Boruah, P.K.; Sharma, B.; Hussain, N.; Das, M.R. Magnetically Recoverable Fe3O4/Graphene Nanocomposite towards Efficient Removal of Triazine Pesticides from Aqueous Solution: Investigation of the Adsorption Phenomenon and Specific Ion Effect. Chemosphere 2017, 168, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Aydin, M.E.; Beduk, F.; Ulvi, A. Removal of Antibiotics from Aqueous Solution by Using Magnetic Fe3O4/Red Mud-Nanoparticles. Sci. Total Environ. 2019, 670, 539–546. [Google Scholar] [CrossRef]
- Kalaboka, M.; Sakkas, V. Magnetic Solid-Phase Extraction Based on Silica and Graphene Materials for Sensitive Analysis of Emerging Contaminants in Wastewater with the Aid of UHPLC-Orbitrap-MS. Molecules 2023, 28, 2277. [Google Scholar] [CrossRef]
- Wang, P.; Luo, M.; Liu, D.; Zhan, J.; Liu, X.; Wang, F.; Zhou, Z.; Wang, P. Application of a Magnetic Graphene Nanocomposite for Organophosphorus Pesticide Extraction in Environmental Water Samples. J. Chromatogr. A 2018, 1535, 9–16. [Google Scholar] [CrossRef]
- Ebrahimzadeh, P.; Maleki, B.; Ghani, M.; Peiman, S. High-Performance Fe₃O₄@SiO₂@Mel@DABCO Catalyst for Synthesis of Chromene Derivatives and Solid Phase Microextraction of Fipronil and Prometryn in Food Samples Followed by HPLC-UV Determination. Chem. Methodol. 2024, 8, 833–855. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Z.; Meng, X.; Sun, S.; Lv, Z.; Liang, Q.; Jiang, T.; Feng, J. Construction of Fe3O4/XSiO2/YSiO2 Nanoparticles for Pesticide Removal from Water: Improved Dispersion Stability and Adsorption Capacity. Langmuir 2023, 39, 8749–8759. [Google Scholar] [CrossRef]
- Behpour, M.; Shadi, M.; Nojavan, S. Preparation of an Efficient Magnetic Nano-Sorbent Based on Modified Cellulose and Carboxylated Carbon Nano-Tubes for Extraction of Pesticides from Food and Agricultural Water Samples before GC-FID Analysis. Food Chem. 2023, 407, 135067. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Khoshmaram, L.; Nabil, A.A.A. Determination of Pyrethroid Pesticides Residues in Vegetable Oils Using Liquid-Liquid Extraction and Dispersive Liquid-Liquid Microextraction Followed by Gas Chromatography-Flame Ionization Detection. J. Food Compos. Anal. 2014, 34, 128–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Kuang, M.; Zhang, L.; Yang, P.; Lu, H. An Accessible Protocol for Solid-Phase Extraction of N-Linked Glycopeptides through Reductive Amination by Amine-Functionalized Magnetic Nanoparticles. Anal. Chem. 2013, 85, 5535–5541. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Yamini, Y.; Moradi, M.; Esrafili, A. Polythiophene-Coated Fe3O4 Superparamagnetic Nanocomposite: Synthesis and Application as a New Sorbent for Solid-Phase Extraction. Anal. Chim. Acta 2013, 770, 68–74. [Google Scholar] [CrossRef]
- Smith, G.; Wermuth, U.D.; Healy, P.C. Hydrogen Bonding in Proton-Transfer Compounds of 5-Sulfosalicylic Acid with Ortho-Substituted Monocyclic Heteroaromatic Lewis Bases. J. Chem. Crystallogr. 2006, 36, 841–849. [Google Scholar] [CrossRef]
- Morel, M.; Martínez, F.; Mosquera, E. Synthesis and Characterization of Magnetite Nanoparticles from Mineral Magnetite. J. Magn. Magn. Mater. 2013, 343, 76–81. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, H.; Zhang, Y.; Liu, J.; Shi, Y.; Zhang, X.; Cai, Y. Biocompatible Phosphatidylcholine Bilayer Coated on Magnetic Nanoparticles and Their Application in the Extraction of Several Polycyclic Aromatic Hydrocarbons from Environmental Water and Milk Samples. J. Chromatogr. A 2012, 1238, 38–45. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Jian, G.-Q.; He, X.-W.; Chen, L.-X.; Zhang, Y.-K. Preparation and Application of Core-Shell Structural Carbon Nanotubes-Molecularly Imprinted Composite Material for Determination of Nafcillin in Egg Samples. Chin. J. Anal. Chem. 2013, 41, 161–166. [Google Scholar] [CrossRef]
- Bekele, H.; Megersa, N. Green Analytical Method Based on Salt Assisted Graphene Oxide Dispersive Solid Phase Extraction of Symmetrical Triazine Herbicides in Environmental Water Samples for Liquid Chromatographic Determination. Int. J. Environ. Anal Chem. 2024, 104, 7029–7045. [Google Scholar] [CrossRef]
- Gabardo, R.P.; Toyama, N.P.; do Amaral, B.; Boroski, M.; Toci, A.T.; Benassi, S.F.; Peralta-Zamora, P.G.; Cordeiro, G.A.; Liz, M.V. de Determination of Atrazine and Main Metabolites in Natural Waters Based on a Simple Method of QuEChERS and Liquid Chromatography Coupled to a Diode-Array Detector. Microchem. J. 2021, 168, 106392. [Google Scholar] [CrossRef]
- Prukjareonchook, A.; Alahmad, W.; Kulsing, C.; Chaisuwan, T.; Dubas, L. Selective Solid-Phase Extraction of Atrazine from Agricultural Environmental Water Samples Using High Permeability Nanoporous Carbon Derived from Melamine-Based Polybenzoxazine Followed by HPLC-UV. Int. J. Environ. Anal. Chem. 2024, 104, 2041–2055. [Google Scholar] [CrossRef]
- Gil García, M.D.; Dahane, S.; Arrabal-Campos, F.M.; SocíasViciana, M.M.; García, M.A.; Fernández, I.; Martínez Galera, M. MCM-41 as Novel Solid Phase Sorbent for the Pre-Concentration of Pesticides in Environmental Waters and Determination by Microflow Liquid Chromatography-Quadrupole Linear Ion Trap Mass Spectrometry. Microchem. J. 2017, 134, 181–190. [Google Scholar] [CrossRef]
- Arnnok, P.; Patdhanagul, N.; Burakham, R. Dispersive Solid-Phase Extraction Using Polyaniline-Modified Zeolite NaY as a New Sorbent for Multiresidue Analysis of Pesticides in Food and Environmental Samples. Talanta 2017, 164, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, G.G.; De Queiroz, M.E.L.R.; Victor, R.P.D.; Noronha, L.M.; Neves, A.A.; De Oliveira, A.F.; Heleno, F.F. DLLME-GC/ECD Method for the Residual Analysis of Parathion-Methyl and Its Application in the Study of the UV-Photodegradation Process. Article J. Braz. Chem. Soc. 2017, 28, 2045–2053. [Google Scholar] [CrossRef]
- Nasiri, M.; Ahmadzadeh, H.; Amiri, A. Organophosphorus Pesticides Extraction with Polyvinyl Alcohol Coated Magnetic Graphene Oxide Particles and Analysis by Gas Chromatography-Mass Spectrometry: Application to Apple Juice and Environmental Water. Talanta 2021, 227, 122078. [Google Scholar] [CrossRef] [PubMed]
- Song, N.E.; Jung, Y.S.; Choi, J.Y.; Koo, M.; Choi, H.K.; Seo, D.H.; Lim, T.G.; Nam, T.G. Development and Application of a Multi-Residue Method to Determine Pesticides in Agricultural Water Using QuEChERS Extraction and LC-MS/MS Analysis. Separations 2020, 7, 52. [Google Scholar] [CrossRef]
| Compound | R2 | Linearity (ng L−1) | LOD (ng L−1) | LOQ (ng L−1) | RSDR Inter (%) | Concentration Level | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOQ | 5 LOQ | 10 LOQ | |||||||||
| R% | RSDr Intra (%) | R% | RSDr Intra (%) | R% | RSDr Intra (%) | ||||||
| Ethoxyquine | 0.9993 | 30–500 | 9.0 | 30 | 17 | 63 | 15 | 65 | 9.6 | 63 | 3.3 |
| Atrazine | 0.9995 | 27–500 | 8.1 | 27 | 12 | 85 | 11 | 83 | 8.2 | 88 | 3.9 |
| Chlorothalonil | 0.9901 | 87–500 | 26 | 87 | 14 | 62 | 14 | 63 | 9.0 | 60 | 3.6 |
| Chlorpyriphos-methyl | 0.9999 | 6.4–500 | 1.9 | 6.4 | 9.6 | 91 | 8.3 | 90 | 6.1 | 92 | 2.4 |
| Methyl-parathion | 0.9978 | 52–500 | 16 | 52 | 8.8 | 98 | 8.4 | 99 | 6.3 | 99 | 2.9 |
| Chlorpyriphos | 0.9901 | 69–500 | 21 | 69 | 9.9 | 96 | 8.2 | 95 | 6.6 | 97 | 2.3 |
| Resmethrin | 0.9909 | 31–500 | 9.5 | 31 | 17 | 67 | 15 | 67 | 8.9 | 64 | 4.1 |
| λ-cyhalothrin | 0.9931 | 90–500 | 27 | 90 | 17 | 60 | 15 | 60 | 9.1 | 61 | 4.0 |
| Permethrin | 0.9916 | 206–500 | 62 | 206 | 14 | 75 | 12 | 76 | 8.4 | 83 | 3.1 |
| Irgarol | 0.9996 | 99–500 | 30 | 99 | 12 | 84 | 10 | 84 | 7.9 | 85 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapsi, M.; Sakkas, V.; Boti, V.; Albanis, T. Magnetic Solid-Phase Extraction Based on C18 Nanoparticles for the Determination of Pesticides in Aquaculture Water Samples. Molecules 2025, 30, 2076. https://doi.org/10.3390/molecules30092076
Kapsi M, Sakkas V, Boti V, Albanis T. Magnetic Solid-Phase Extraction Based on C18 Nanoparticles for the Determination of Pesticides in Aquaculture Water Samples. Molecules. 2025; 30(9):2076. https://doi.org/10.3390/molecules30092076
Chicago/Turabian StyleKapsi, Margarita, Vasileios Sakkas, Vasiliki Boti, and Triantafyllos Albanis. 2025. "Magnetic Solid-Phase Extraction Based on C18 Nanoparticles for the Determination of Pesticides in Aquaculture Water Samples" Molecules 30, no. 9: 2076. https://doi.org/10.3390/molecules30092076
APA StyleKapsi, M., Sakkas, V., Boti, V., & Albanis, T. (2025). Magnetic Solid-Phase Extraction Based on C18 Nanoparticles for the Determination of Pesticides in Aquaculture Water Samples. Molecules, 30(9), 2076. https://doi.org/10.3390/molecules30092076









