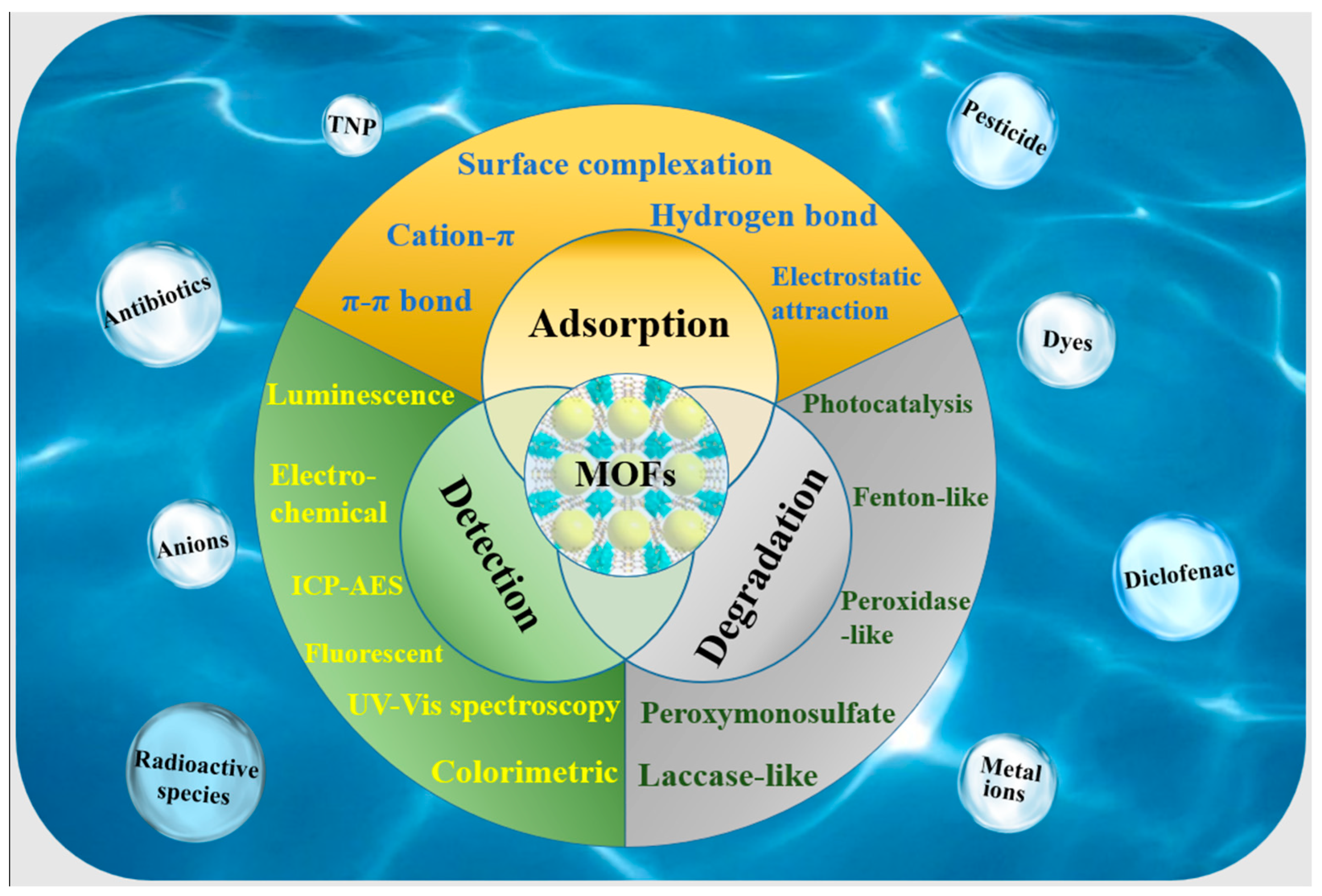
The Application of Multifunctional Metal–Organic Frameworks for the Detection, Adsorption, and Degradation of Contaminants in an Aquatic Environment
Abstract
1. Introduction
2. Simultaneous Detection and Adsorption-Based Removal Contaminants
| Catalyst | Contaminant | Detection | Adsorption Capacity | Time for Equilibrium | Year | Ref. | |
|---|---|---|---|---|---|---|---|
| Method | LOD | ||||||
| Zr-MOF | TC and OTC | Fluorescent | 6.14 and 14.59 nM | 262.46 and 267.92 mg/g | 30–360 min | 2024 | [6] |
| Znq2@ZIF-8 | TC | Fluorescent | 0.13 μmol/L | 377.02 mg/g | 30–360 min | 2024 | [8] |
| apt-NiCoFe-MOF-74 | TC, OTC, CTC, and DOX | Fluorescent | 1.3, 1.4, 5.5, and 3.0 nM | 97.4, 105.2, 115.6, and 114.7 mg/g | 10 min | 2024 | [9] |
| Colorimetric | 6, 10, 49, and 2.8 nM | ||||||
| Zr-MOF (BMAT3H5) | TC | Fluorescent | 28 ± 0.012 nM | 317.6 mg/g. | 5 min | 2025 | [10] |
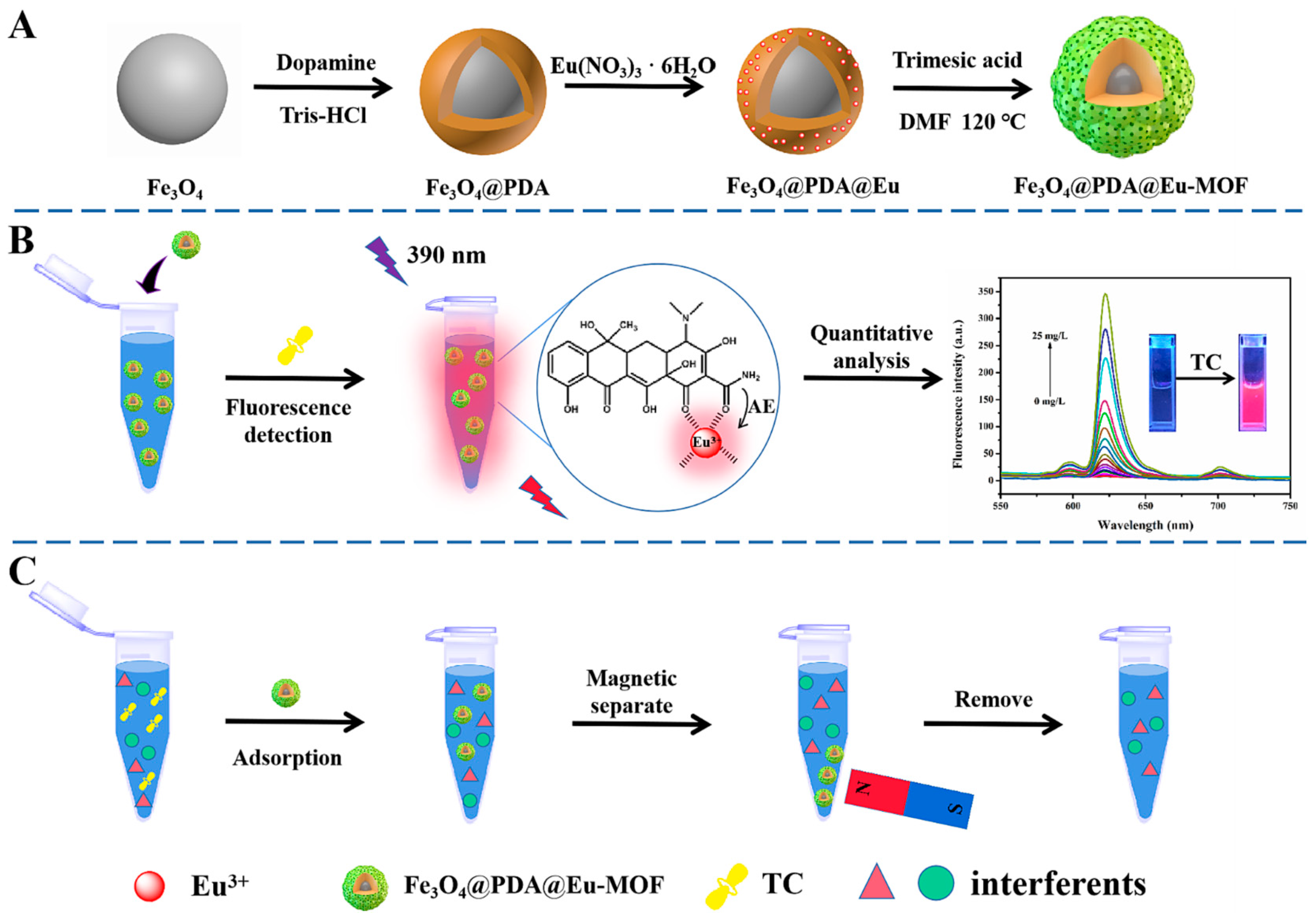
| Fe3O4@PDA@Eu-MOF | TC | Fluorescent | 2 μg/L | 144.9 mg/g | 80 min | 2023 | [11] |
| Zn-MOF | OTC | Fluorescent | 26.9 nM | 5.8 mg/g | 4 h | 2023 | [51] |
| Eu-CuBi2O4@ZIF-8 | TC | Fluorescent | 17 nM | 377.07 mg/g | 2 h | 2022 | [52] |
| Eu-MOF | TC | Fluorescent | 3 nM | 387.14 mg/g | 500 min | 2021 | [53] |
| Eu/Zr-MOF | TC | Fluorescent | 0.92 ng/mL | 289 mg/g | 150 min | 2021 | [54] |
| Cd-MOF | chloramphenicol (CHL) | Fluorescent | 91 ppb | - | 50 h | 2020 | [55] |
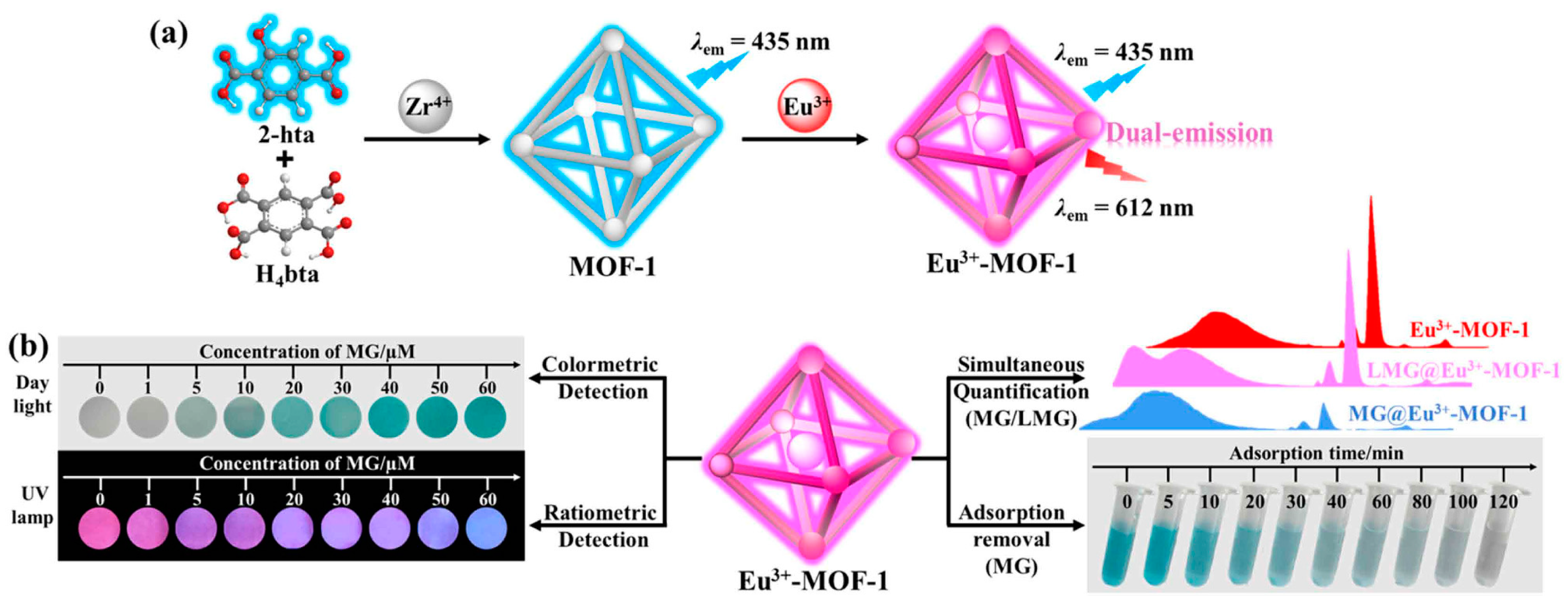
| Eu3+-MOF | Malachite green (MG) | Fluorescent | 34.20 nM | 97.64% based on 20 mg Eu3+-MOF in 10 mL MG (10 mg/L) under pH 7 at 35 °C in 120 min. | 120 min | 2024 | [15] |
| Leuco-malachite green (LMG) | 1.98 nM | - | - | ||||
| Zr-Sti | 2,4,6-trinitrophenyl phenol (TNP) | Fluorescent | 0.68 μM (156 ppb) | ca. 78 mg/g | - | 2023 | [56] |
| Cd-MOF@ macroporous melamine foam | TNP | Luminescence | 0.38 μM | 456.16 mg/g | 30 min | 2022 | [57] |
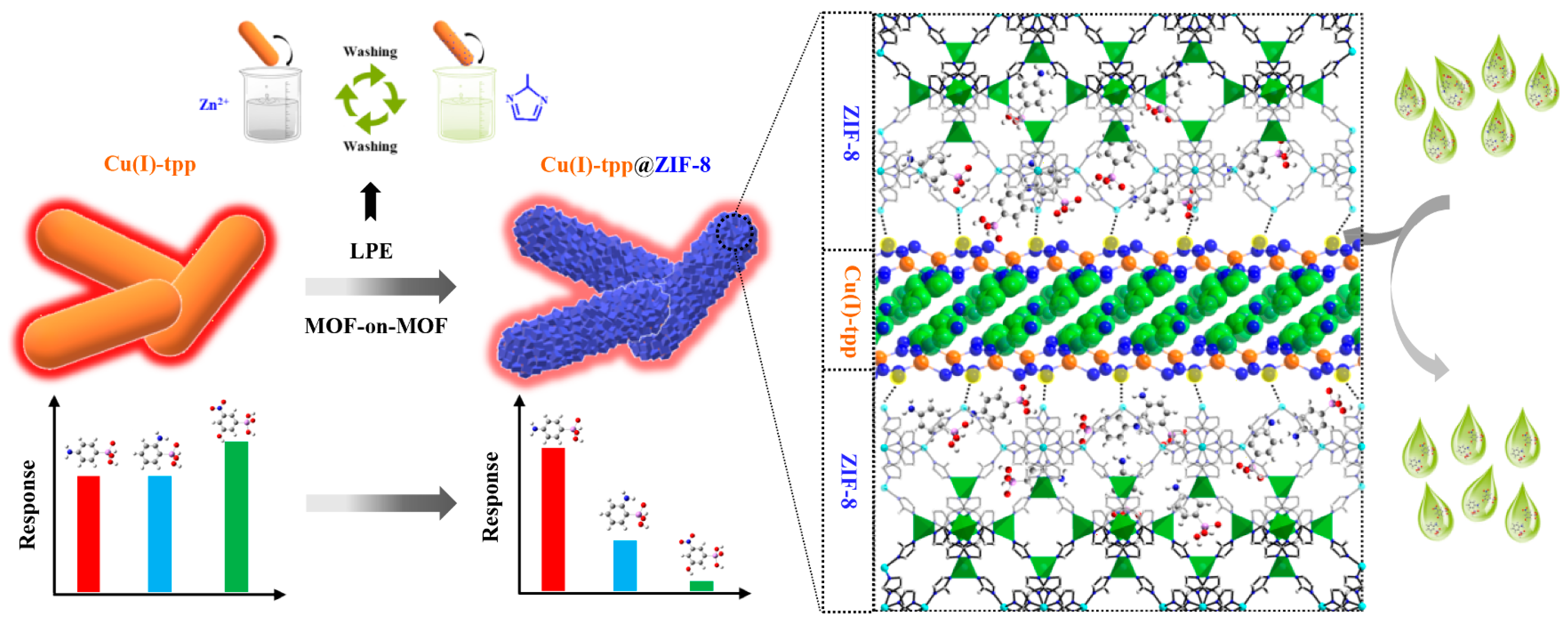
| Cu+-tpp@ZIF-8 | P-arsanilic acid | Fluorescent | 0.4 µg/L | 303.0 mg/g | 240 min | 2022 | [58] |
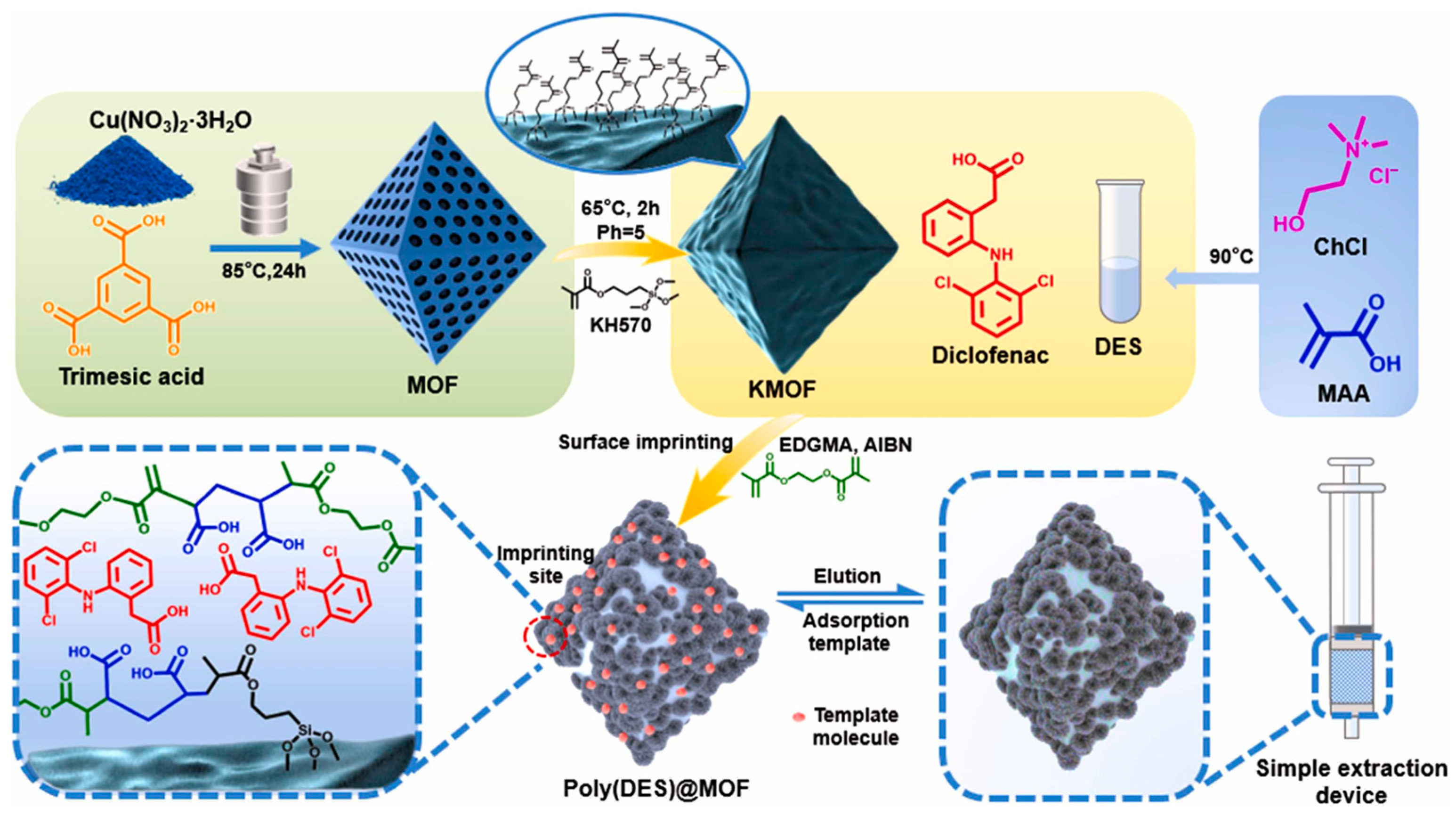
| Poly(DES)@MOF | Diclofenac | UV spectrophotometer | 0.84 μg/mL | 19.39 mg/g | 2 h | 2024 | [59] |
| ZIF-8-on-Zn2@SA | Pesticide: thiophanate-methyl [1.2-α-(3-methoxycarbonyl-2-thioureido)benzene] | Fluorescent | 0.14 μM | 161.8 mg/g | ~280 min | 2022 | [37] |
| kgd-M1@ACPs | Pesticide: 2,6-dichloro-4-nitroaniline | Fluorescent | 0.09 µM | 83.3 mg/g | 240 min | 2022 | [60] |
| Zn2@ZIF-8@SA | Pesticide: quinclorac, 2,6-dichloro-4-nitroaniline, and thiabendazole | Fluorescent | 0.08, 0.09, and 0.37 μM | 142.1 mg/g | 20 h | 2022 | [61] |
| UiO-66-NH2@AuNCs/ZIF-8 | Hg2+ | Fluorescent | 0.42 ppb | 129.9 mg/g | 30 min | 2024 | [62] |
| Sm-MOF | Hg2+ | Fluorescent | 0.87 μM | 0.97 μmol/4 mg | 2 h | 2021 | [63] |
| Zn2(BDC)2(TzTz)2 | Hg2+ | Colorimetric | - | 1428 mg/g | 30 s–30 min | 2021 | [64] |
| Thioketone Al-MOF nanorods | Hg2+ | Colorimetric | 0.8 ppb | 1110 mg/g | - | 2020 | [65] |
| Zr-Sti | Cr2O72− | Fluorescent | 0.73 μM (159 ppb) | 43.38 mg/g | - | 2023 | [56] |
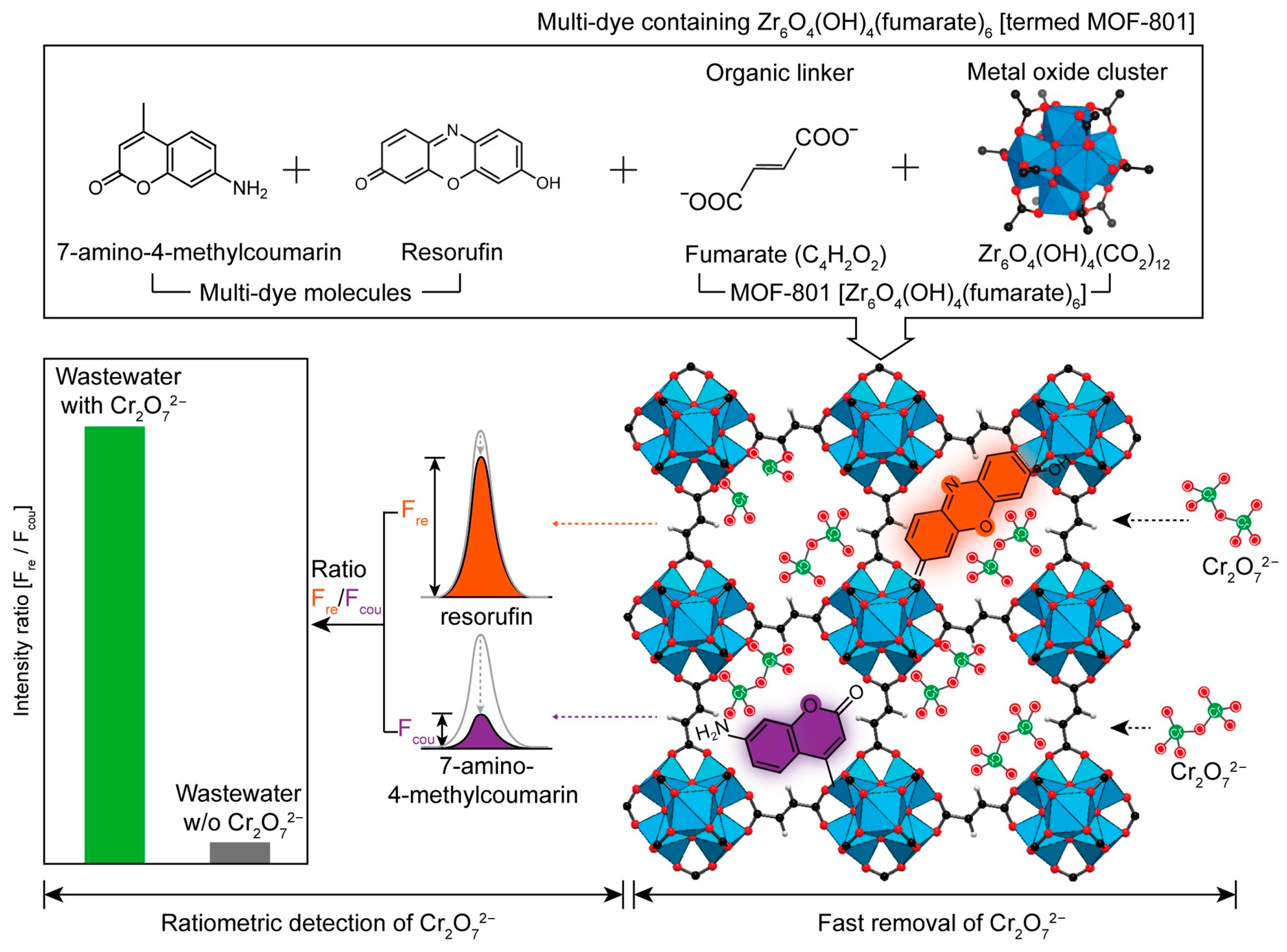
| Dyes⊂MOF-801 | Cr2O72− | Fluorescent | 0.03 mM | 83 mg/g | 3 min | 2019 | [66] |
| HSB-W15-NS | Fe3+ | Fluorescent | 0.837 μM | 250.81 mg/g | 5 min | 2024 | [67] |
| Zn-MOF | Fe2+ | UV-Vis spectroscopy | 0.129 μM | 208.7 mg/g | 30 min | 2023 | [68] |
| Pb2+ | 0.113 μM | 192.6 mg/g | |||||
| V5+ | 0.246 μM | 203.6 mg/g | |||||
| MIL-101(Fe) | Fe3+ | Fluorescent | 1.8 μM | 3.5 mM/g | 180 min | 2019 | [69] |
| Cu2+ | 1.6 μM | 0.9 mM/g | 180 min | ||||
| Pb2+ | 5.2 μM | 1.1 mM/g | 180 min | ||||
| Fe3O4/MOF/L-cysteine | Cd2+ | ICP-AES | 10.6 ng/mL | 248.24 mg/g | 10 min | 2018 | [70] |
| Fluorescent | 0.94 ng/mL | ||||||
| NH2-MIL-88(Fe) | As5+ | Fluorescent | 4.2 ppb | 125 mg/g | 24 h | 2017 | [71] |
| Tb-BTC | phosphorus | Fluorescent | 2.97 μM | 222.2 mg/g | 30 min | 2023 | [72] |
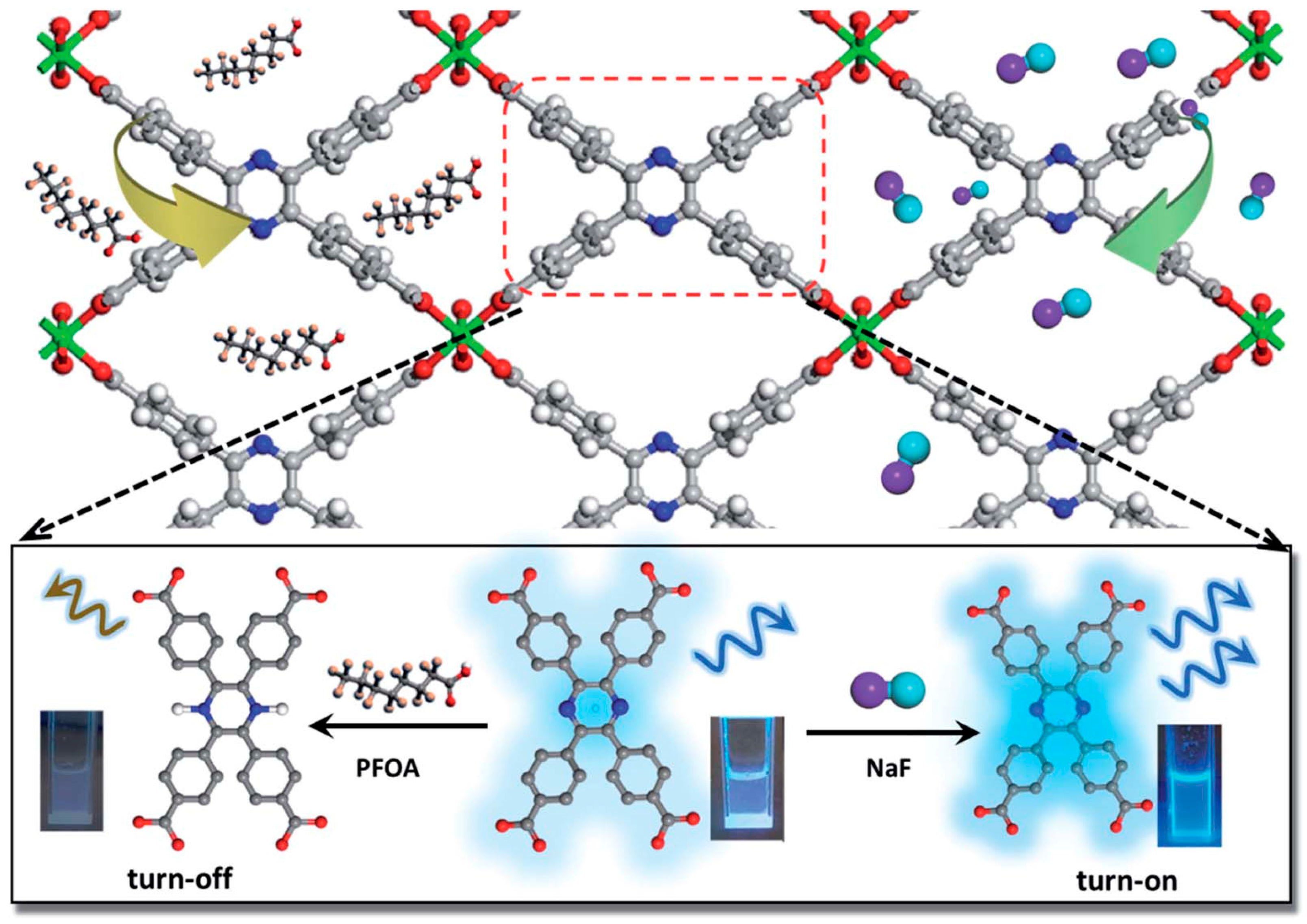
| In(tcpp) | F− | Fluorescent | 1.3 μg/L | 36.7 mg/g | 30 min | 2021 | [73] |
| Perfluorooctanoic acid | 1.9 μg/L | 980.0 mg/g | 30 min | ||||
| Ag@UiO-66-(COOH)2 | I− | Fluorescent | 0.58 ppm | 235.5 mg/g | 60 min | 2022 | [74] |
2.1. Organics
2.1.1. Antibiotic Treatment
2.1.2. Treatment of Other Organic Contaminants
2.2. Inorganics
2.2.1. Heavy Metal Ion Treatment
2.2.2. Inorganic Anion Treatment
3. Simultaneous Detection and Degradation of Contaminants
3.1. Antibiotics
3.2. Phenolic Compounds
3.3. Other Contaminants
| Catalyst | Contaminant | Detection | Adsorption Capacity | Degradation | Year | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Method | LOD | Method | Rate | |||||
| CaO2@Cu-MOF | TC | Fluorescent | 11.8 fg/mL | - | Fenton-like | 95% per 60 min | 2024 | [21] |
| In2S3@PCN-224 | TC | Fluorescent | 55 nM | 60% | Photocatalysis | 20% | 2023 | [36] |
| Cu2O@NMOF-Lac | 2,4-dichlorophenol | UV colorimetric | 0.29 µM | - | Peroxidase-like | 82% | 2023 | [100] |
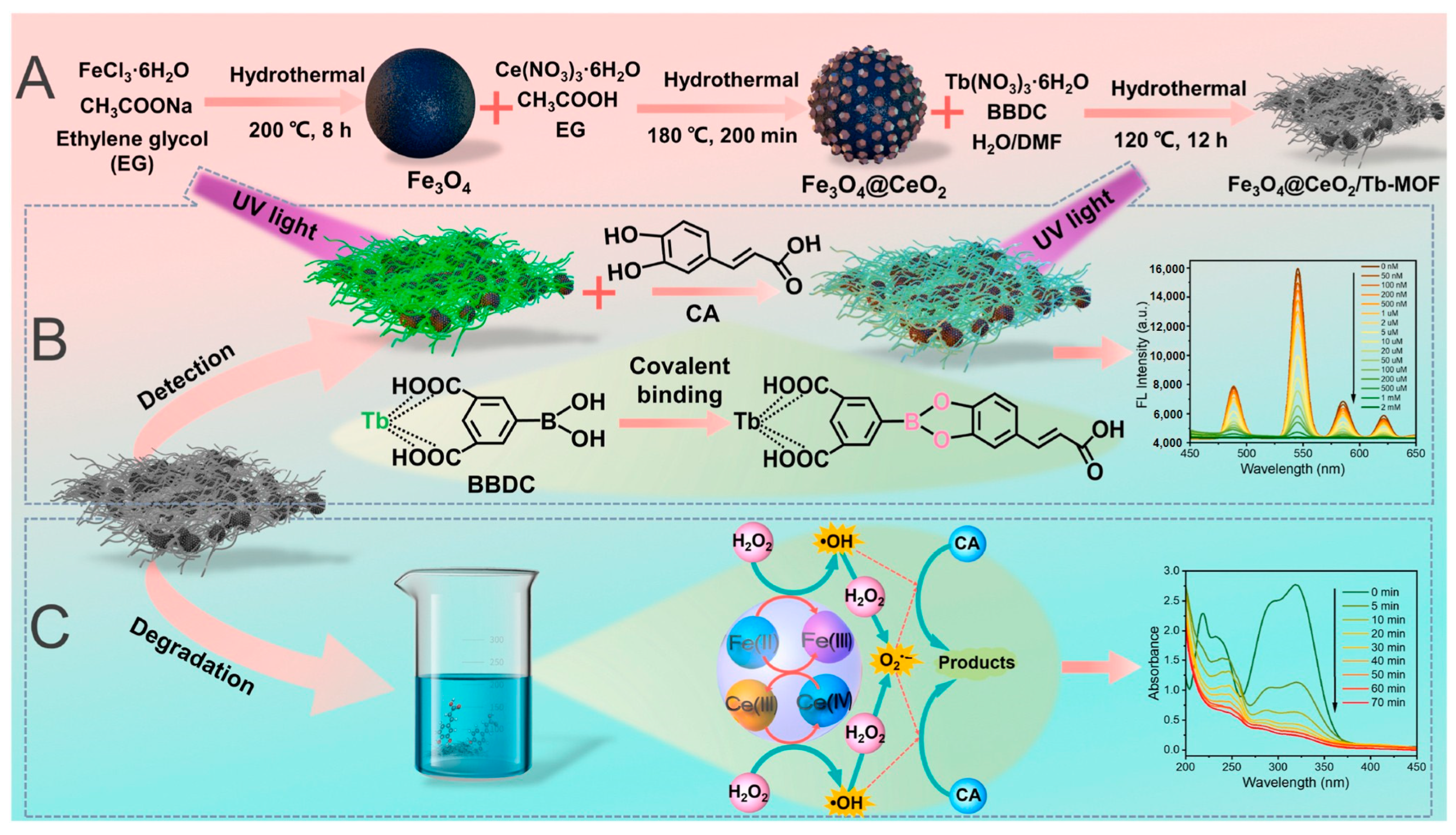
| Fe3O4@CeO2/Tb-MOF | Caffeic acid | Fluorescent | 18.9 nM | - | Peroxidase-like | 95% | 2024 | [101] |
| ZnO/NH2-MIL-125 (Ti) | Ibuprofen | Fluorescent | 0.15 μM | ~25% | Photocatalytic | 88.9% | 2024 | [111] |
| Tb-OBBA-Hemin | 17β-estradiol | Fluorescent | 50 pM | - | Peroxidase-like | 88% | 2020 | [102] |
4. One Stone, Two Birds
4.1. Different Functions for Different Contaminants
| Catalyst | Removal | Detection | Degradation | Other Function | Year | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Contaminant | Adsorption Capacity and Removal Rate | Contaminant | Method and LOD | Contaminant | Method and Rate | ||||
| Zn-MOF@MCHS | Cu2+ | 523.56 mg/g, 99% | 2,4,6-trinitrophenol (TNP), and Cu2+ | Fluorescent, 0.301 and 0.368 µM | - | - | - | 2025 | [116] |
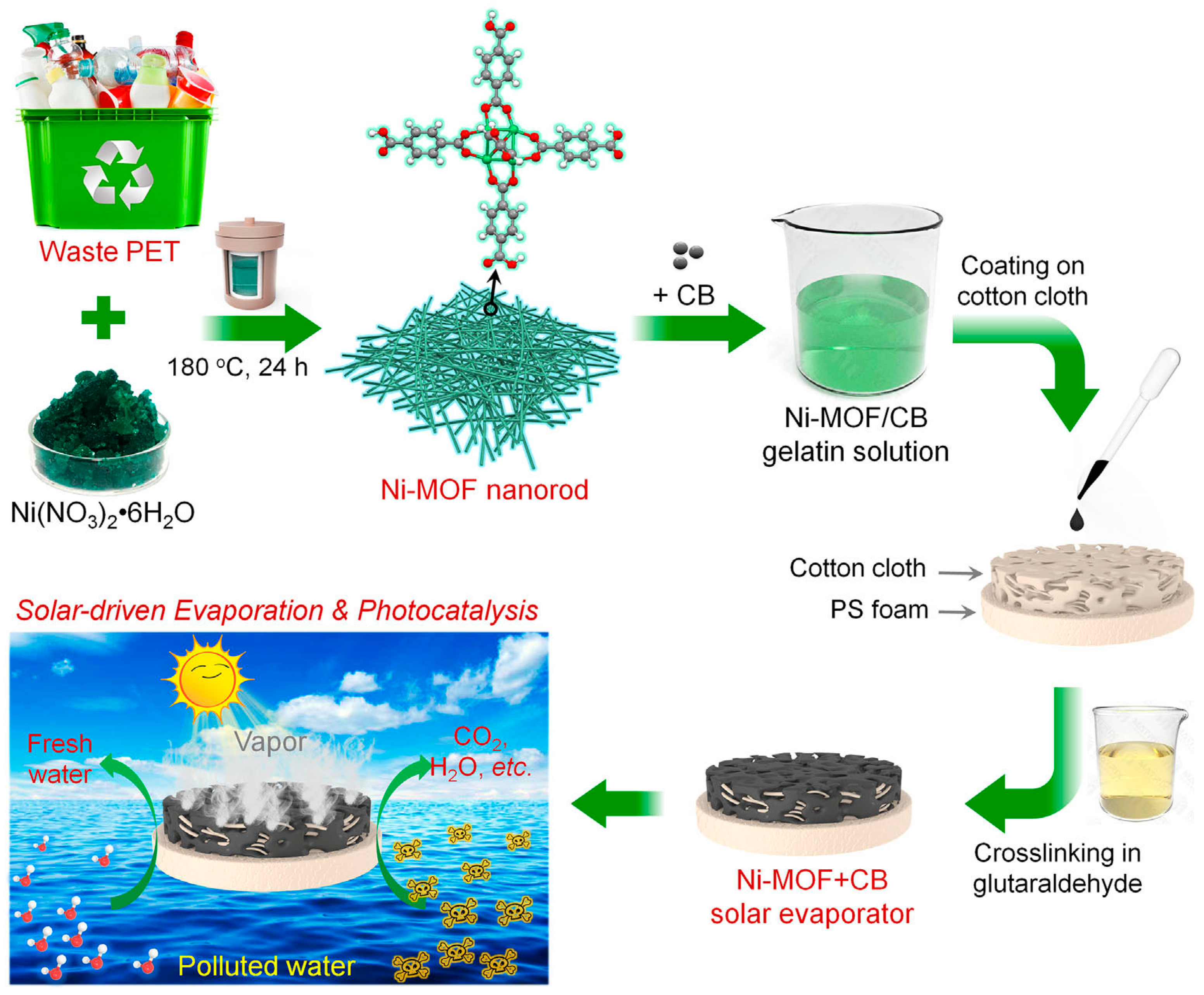
| Ni-MOF | - | - | - | - | TC | Peroxymonosulfate, 91% | Interfacial solar evaporation (2.25 kg/m2/h) | 2023 | [40] |
| Co-MOF@CNT | Methylene blue and Methyl orange | 98% and 72% | Cr6+ | Fluorescent, 0.125 μM | - | - | - | 2022 | [118] |
| TMU-57 | Hg2+ | 570 mg/g | 2,4,6-trinitrophenol | Fluorescent, 2 ppb | - | - | - | 2022 | [119] |
| MOFL-TpBD | Pb2+ | 21.74 mg/g | 2,4,6-trinitrophenol | 0.32 μg/L | - | - | - | 2021 | [120] |
| Eu-CMOF | MnO4− | 1.1 g/g | Nitrofurantoin and Nitrofurazone | Fluorescent, 1.33 and 2.80 μM | - | - | - | 2022 | [117] |
| CA-Cu | - | - | Dopamine | UV colorimetric, 2.23 μM | chlorophenol and diphenol | Laccase-like, 80% and 50% | - | 2022 | [121] |
| Zr-BBI | - | - | - | - | Reducing Cr2O72− to Cr3+ | Photocatalysis, k = 0.073 min−1 | pH sensor (pH 4.6–7.12) | 2021 | [122] |
4.2. One Function for Different Contaminants
4.2.1. Removal of Various Contaminants
| Catalyst | Removal Species (Adsorption Capacity, Time for Equilibrium) | Year | Ref. |
|---|---|---|---|
| MTV-MOF | Hg2+, Pb2+, and Tl+; Pyronin Y, Auramine O, brilliant green, and methylene blue, 0–48 h | 2019 | [16] |
| Zr-MOF | Methylene blue (169 mg/g) Lead ions (100 mg/g) Cadmium ions (37 mg/g) | 2021 | [125] |
| CaFu MOF | Imidacloprid (467.23 mg/g, 150 min) Cd2+ ions (781.2 mg/g, 40 min) | 2021 | [126] |
| Cu-MOFs/CMFP | Methylene blue, malachite green, and rhodamine B; Pb2+ and Cd2+ | 2021 | [132] |
| ZnO-NP@Zn-MOF-74 | Cu (106.27 mg/g, 2 h) Tetracycline (137.17 mg/g, 2 h) | 2021 | [17] |
| ZIF8/MWCNT | Phosphate (188.5 mg/g, 12 h) Acetaminophen (0.51 mol/g, 12 h) Triclosan (0.35 mol/g, 12 h) | 2021 | [128] |
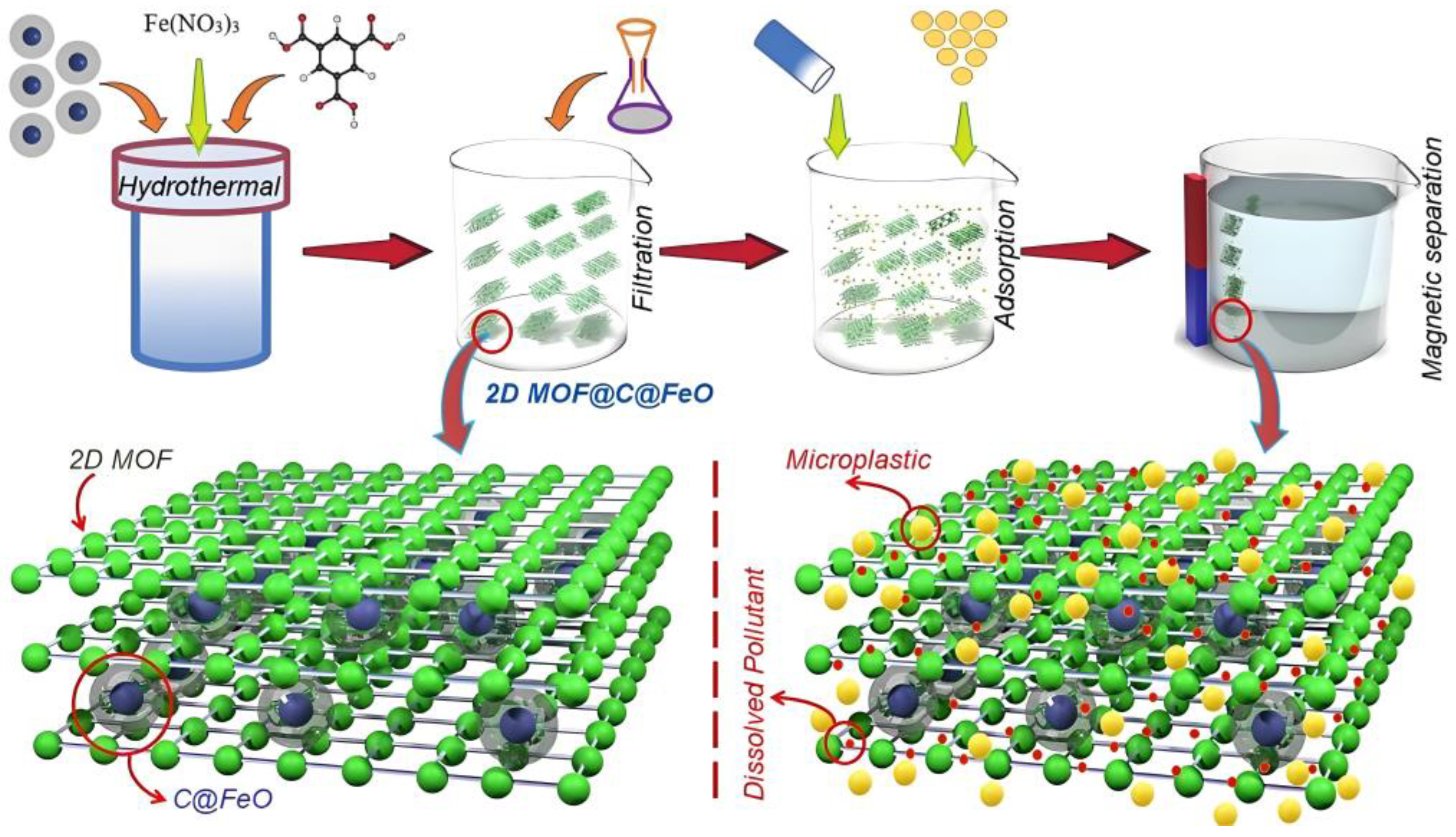
| 2D-MOF@C@FeO | Microplastic (100% removal, 60 min) Methylene blue (100% removal, 60 min) | 2023 | [129] |
| MOF-5/COF | Auramine O (17.95 mg/g, 10 min) RhodamineB (16.18 mg/g, 10 min) | 2020 | [30] |
| apt-NiCoFe-MOF-74 | TC (97.4 mg/g) | 2024 | [9] |
| OTC (105.2 mg/g, 10 min) | |||
| CTC (115.6 mg/g, 10 min) | |||
| DOX (114.7 mg/g, 10 min) | |||
| Eu3+-MOF | Malachite green (97.64%, 120 min), | 2024 | [15] |
| Leuco-malachite green | |||
| Zr-MOF | Pb2+ (715.2 mg/g, 30 min) Hg2+ (862.7 mg/g, 30 min) Cd2+ (450.5 mg/g, 30 min) | 2024 | [130] |
| NH2-MIL-101-Fe, and MOF-808-EDTA | Hg2+(272.7 mg/g) As3+ (151.29 mg/g) Mn2+ (125.9 mg/g) | 2024 | [77] |
| CeO2@UiO-66 | As3+, As5+, Cd2+, Cr3+, Cr6+, Cu2+, Pb2+, and Hg2+ | 2020 | [131] |
4.2.2. Detection of Different Contaminants
| Catalyst | Detection Method | Detection Species (LOD) | Year | Ref. |
|---|---|---|---|---|
| Cu/Co-MOF | Electrochemical | Malathion (0.015 pM) Acetamiprid (0.018 pM) | 2021 | [38] |
| Ce(III, IV)-MOF | Electroluminescence | Malathion (0.038 pM) Chlorpyrifos (0.045 pM) | 2022 | [39] |
| Ce-MOF/CNTs | Electrochemical | Catechol (3.5 μM) Hydroquinone (5.3 μM) | 2021 | [50] |
| CoNi-MOF/GO | Electrochemical | Catechol (0.03 μM) Hydroquinone (0.05 μM) | 2023 | [137] |
| CP-rGO-CoZn-MOF | Electrochemical | Cd2+ (0.565 nM) Pb2+ (0.588 nM) | 2022 | [138] |
| Yb-MOF | Electrochemical | Cd2+ (3.0 ppb) Pb2+ (1.6 ppb) | 2021 | [139] |
| Cu-MOF | Electrochemical | Tl+ (0.11 ppb) Hg2+ (0.17 ppb) | 2020 | [140] |
5. Conclusions, Outlooks, and Recommendations
5.1. Conclusions
5.2. Future Outlooks
5.3. Future Recommendation
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.S.; Zhu, S.; Chen, S.B. Metal-Organic Frameworks (MOFs) for Oxo-Anion Removal in Wastewater Treatment: Advancements and Applications. Chem. Eng. J. 2024, 500, 157396. [Google Scholar] [CrossRef]
- Manzoor, M.H.; Naz, N.; Naqvi, S.M.G.; Ashraf, S.; Ashiq, M.Z.; Verpoort, F. Wastewater treatment using Metal-Organic Frameworks (MOFs). Appl. Mater. Today 2024, 40, 102358. [Google Scholar] [CrossRef]
- Han, J.; Zhang, H.; Fan, Y.; Zhou, L.; Zhang, Z.; Li, P.; Li, Z.; Du, Y.; Meng, Q. Progressive Insights into Metal-Organic Frameworks and Metal-Organic Framework-Membrane Composite Systems for Wastewater Management. Molecules 2024, 29, 1615. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wei, K.; Li, Y.; Zhang, L.; Xu, S.; Shi, X.; Tang, B. Efficient synthesis of multifunctional α-Fe2O3 QDs/TS-1 as an artificial nanozyme for simultaneous colorimetric detection and photodegradation of tetracyclines. Chem. Eng. J. 2024, 498, 155225. [Google Scholar] [CrossRef]
- Meng, F.; Ling, Y.; Li, Y.; Liu, D.; Wei, K.; Sun, L.; Sang, Z. Synthesis of visible-light-driven photocatalyst of TiO2 modified waste red mud and its application in tetracycline hydrochloride removal. Surf. Interfaces 2022, 35, 102482. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, T.; Wang, X.; Lu, G.; Tong, K.; Wang, Q.; Li, B. Fabrication of dual-functional Zr-based MOF incorporating amino and sulfoxide derivatives for simultaneous removal and detection of tetracycline antibiotics. Sep. Purif. Technol. 2024, 339, 126676. [Google Scholar] [CrossRef]
- Alessandretti, I.; Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Dettmer, A. Removal of diclofenac from wastewater: A comprehensive review of detection, characteristics and tertiary treatment techniques. J. Environ. Chem. Eng. 2021, 9, 106743. [Google Scholar] [CrossRef]
- Yang, L.; Xu, T.; Zou, H.; Zhang, S.; Huang, J.; Cai, L.; Zou, D.; Huang, J.; Yao, M. Self-assembled fluorescent Zn-MOF with high specific surface area based on the coordination interaction for sensitive detection and selective removal of tetracycline antibiotic in water. Opt. Mater. 2024, 157, 116126. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Sun, H.; Yang, Y.; Zhang, D.; Li, X.; Han, L.; Wang, G.; Zhang, Y. Sensitive dual-signal detection and effective removal of tetracycline antibiotics in honey based on a hollow triple-metal organic framework nanozymes. Food Chem. 2024, 442, 138383. [Google Scholar] [CrossRef]
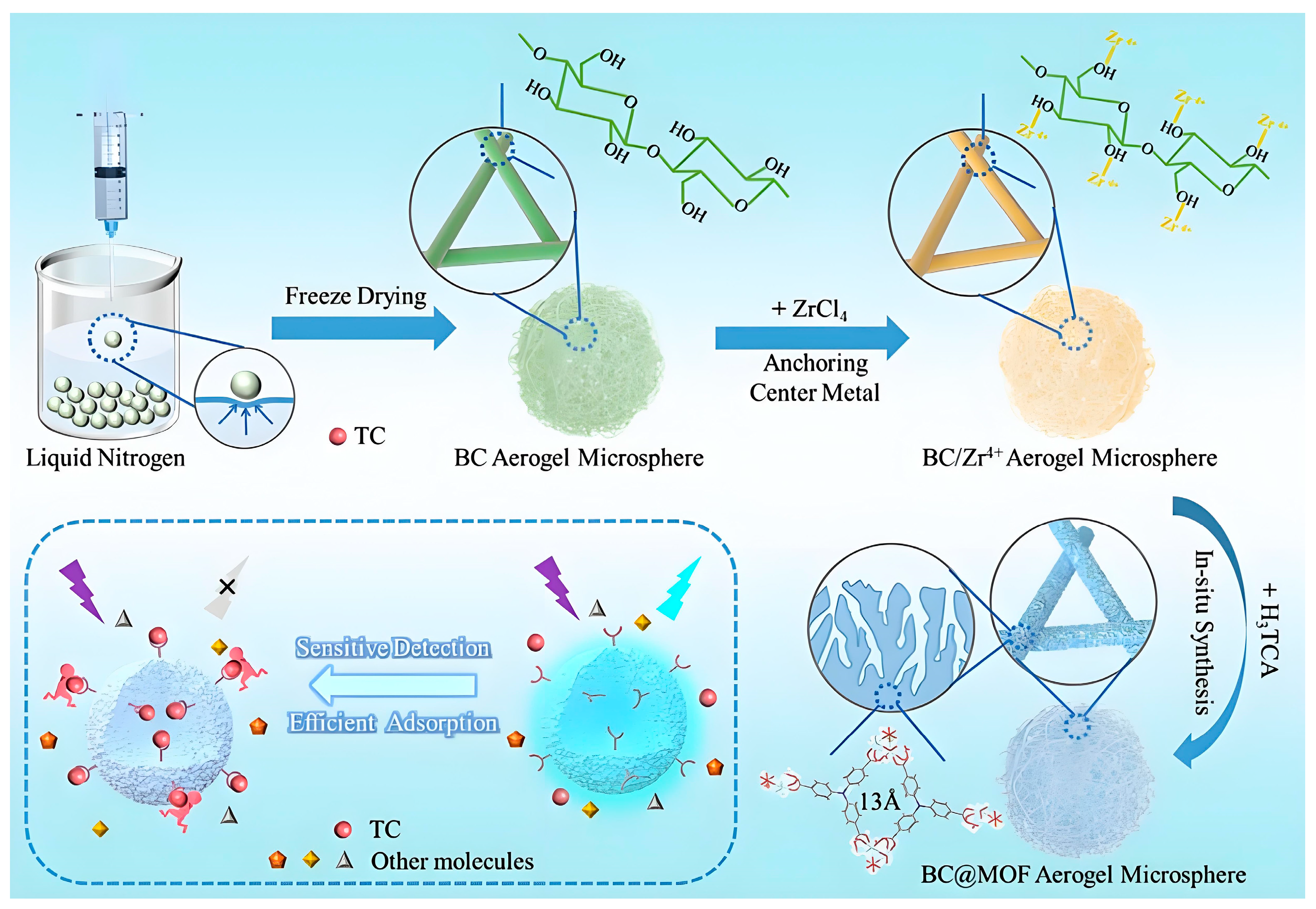
- Yan, Z.; Meng, L.; Jiang, S.; Deng, Y.; Xi, J.; Zhang, L.; Li, P.; Xiao, H.; Wu, W. Bifunctional Nanocellulose@MOF composite aerogel for selective fluorescent detection and efficient removal of tetracycline. Carbohydr. Polym. 2025, 347, 122697. [Google Scholar] [CrossRef]
- Li, J.; Yao, R.; Deng, B.; Li, Z.; Tuo, K.; Fan, C.; Liu, G.; Pu, S. A facile construction of bifunctional core-shell-shell structured magnetic metal-organic frameworks for detection and removal of tetracycline. Chem. Eng. J. 2023, 464, 142626. [Google Scholar] [CrossRef]
- Yi, M.; Xia, Q.; Tan, J.; Shang, J.; Cheng, X. Catalytic-separation technology for highly efficient removal of emerging pollutants, desalination, and antimicrobials: A new strategy for complex wastewater treatment. Chem. Eng. J. 2024, 493, 152568. [Google Scholar] [CrossRef]
- Lu, X.; Shen, L.; Chen, C.; Yu, W.; Wang, B.; Kong, N.; Zeng, Q.; Chen, S.; Huang, X.; Wang, Y.; et al. Advance of self-cleaning separation membranes for oil-containing wastewater treatment. Environ. Funct. Mater. 2024, 3, 72–93. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Fang, K.; Gong, H.; Jin, Z.; He, Q.; Wang, Q. Up-concentration processes of organics for municipal wastewater treatment: New trends in separation. Sci. Total Environ. 2021, 787, 147690. [Google Scholar] [CrossRef]
- Xia, Y.-F.; Yuan, H.-Q.; Qiao, C.; Li, W.; Wang, R.; Chen, P.; Li, Y.-X.; Bao, G.-M. Multifunctional Eu3+-MOF for simultaneous quantification of malachite green and leuco-malachite green and efficient adsorption of malachite green. J. Hazard. Mater. 2024, 465, 133386. [Google Scholar] [CrossRef] [PubMed]
- Mon, M.; Bruno, R.; Tiburcio, E.; Viciano-Chumillas, M.; Kalinke, L.H.G.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Multivariate Metal-Organic Frameworks for the Simultaneous Capture of Organic and Inorganic Contaminants from Water. J. Am. Chem. Soc. 2019, 141, 13601–13609. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, F.; Yang, R.; Sun, L.; Li, Y.; Xu, J. Preparation of novel ZnO-NP@Zn-MOF-74 composites for simultaneous removal of copper and tetracycline from aqueous solution. Sep. Purif. Technol. 2021, 274, 118949. [Google Scholar] [CrossRef]
- Miyah, Y.; El Messaoudi, N.; Benjelloun, M.; Georgin, J.; Franco, D.S.P.; Acikbas, Y.; Kusuma, H.S.; Sillanpää, M. MOF-derived magnetic nanocomposites as potential formulations for the efficient removal of organic pollutants from water via adsorption and advanced oxidation processes: A review. Mater. Today Sustain. 2024, 28, 100985. [Google Scholar] [CrossRef]
- Maru, K.; Kalla, S.; Jangir, R. Efficient Dye Extraction from Wastewater Using Indium-MOF-Immobilized Polyvinylidene Fluoride Membranes with Selective Filtration for Enhanced Remediation. Langmuir 2024, 40, 8144–8161. [Google Scholar] [CrossRef]
- Li, Y.; Pang, J.; Bu, X.-H. Multi-functional metal-organic frameworks for detection and removal of water pollutions. Chem. Commun. 2022, 58, 7890–7908. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, C.; Yu, X.; Lai, G.; Li, X. Sense and Shoot: Multifunctional CaO2-loaded Cu-MOF nanosheets for integrated fluorescence detection and fenton-like degradation of tetracycline. Chem. Eng. J. 2024, 481, 148635. [Google Scholar] [CrossRef]
- Khan, M.S.; Li, Y.; Li, D.-S.; Qiu, J.; Xu, X.; Yang, H.Y. A review of metal-organic framework (MOF) materials as an effective photocatalyst for degradation of organic pollutants. Nanoscale Adv. 2023, 5, 6318–6348. [Google Scholar] [CrossRef]
- Dong, F.; Pang, Z.; Yang, S.; Lin, Q.; Song, S.; Li, C.; Ma, X.; Nie, S. Improving Wastewater Treatment by Triboelectric-Photo/Electric Coupling Effect. ACS Nano 2022, 16, 3449–3475. [Google Scholar] [CrossRef]
- Houska, J.; Stocco, L.; Hofstetter, T.B.; Gunten, U.v. Hydrogen Peroxide Formation during Ozonation of Olefins and Phenol: Mechanistic Insights from Oxygen Isotope Signatures. Environ. Sci. Technol. 2023, 57, 18950–18959. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Cheng, S.; Han, Y.; Pu, L.; Du, E.; Peng, M.; Li, A.; Li, W. Chlorination of Biopterin in Water: Deciphering the Kinetics, Disinfection Byproducts, and Toxicity. Environ. Sci. Technol. 2024, 58, 20137–20146. [Google Scholar] [CrossRef]
- Scandura, G.; Eid, S.; Alnajjar, A.A.; Paul, T.; Karanikolos, G.N.; Shetty, D.; Omer, K.; Alqerem, R.; Juma, A.; Wang, H.; et al. Photo-responsive metal-organic frameworks—Design strategies and emerging applications in photocatalysis and adsorption. Mater. Adv. 2023, 4, 1258–1285. [Google Scholar] [CrossRef]
- Kubiak, A.; Jaruga, M.; Lusina, A.; Nazim, T.; Sobańska, K.; Pietrzyk, P.; Cegłowski, M. Real-world application of molecularly imprinted TiO2-graphite photocatalysts: Efficient pharmaceutical removal under energy-optimized LED system. J. Water Process Eng. 2025, 69, 106894. [Google Scholar] [CrossRef]
- Kallawar, G.; Thakare, N.; Bonde, S.; Barai, D.; Bhanvase, B.A.; Sonawane, A.; Sonawane, S.H.; Manickam, S. Exploring sonochemical synthesis for photocatalyst nanocomposites in water and wastewater treatment: An in-depth review. J. Clean. Prod. 2024, 485, 144279. [Google Scholar] [CrossRef]
- Bui, T.K.; Nguyen, T.L.; Van Pham, V. Review of g-C3N4-based photocatalysts for Amoxicillin photocatalytic degradation. J. Water Process Eng. 2024, 67, 106257. [Google Scholar] [CrossRef]
- Firoozi, M.; Rafiee, Z.; Dashtian, K. New MOF/COF Hybrid as a Robust Adsorbent for Simultaneous Removal of Auramine O and Rhodamine B Dyes. ACS Omega 2020, 5, 9420–9428. [Google Scholar] [CrossRef]
- Wang, C.-C.; Yi, X.-H.; Wang, P. Powerful combination of MOFs and C3N4 for enhanced photocatalytic performance. Appl. Catal. B 2019, 247, 24–48. [Google Scholar] [CrossRef]
- Patra, S.; Schaming, D.; Picot, P.; Pignié, M.-C.; Brubach, J.-B.; Sicard, L.; Le Caër, S.; Thill, A. Inorganic nanotubes with permanent wall polarization as dual photo-reactors for wastewater treatment with simultaneous fuel production. Environ. Sci. Nano 2021, 8, 2523–2541. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Pang, H. A Review of MOFs and Their Composites-Based Photocatalysts: Synthesis and Applications. Adv. Funct. Mater. 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Li, C.; Huang, N.-Y.; Yang, Y.; Xu, Q.; Liao, G. Emerging metal-organic framework-based photocatalysts for solar-driven fuel production. Coord. Chem. Rev. 2025, 524, 216292. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal-Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef]
- Chen, F.-Z.; Li, Y.-J.; Zhou, M.; Gong, X.-X.; Gao, Y.; Cheng, G.; Ren, S.-B.; Han, D.-M. Smart multifunctional direct Z-scheme In2S3@PCN-224 heterojunction for simultaneous detection and photodegradation towards antibiotic pollutants. Appl. Catal. B 2023, 328, 122517. [Google Scholar] [CrossRef]
- Jia, W.; Fan, R.; Zhang, J.; Zhu, K.; Gai, S.; Yin, Y.; Yang, Y. Smart MOF-on-MOF Hydrogel as a Simple Rod-shaped Core for Visual Detection and Effective Removal of Pesticides. Small 2022, 18, 2201510. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Z.; Yi, J.; Ma, D.; Xia, F.; Tian, D.; Zhou, C. A dual-signal electroluminescence aptasensor based on hollow Cu/Co-MOF-luminol and g-C3N4 for simultaneous detection of acetamiprid and malathion. Sens. Actuators B 2021, 331, 129412. [Google Scholar] [CrossRef]
- Ma, D.; Liu, J.; Liu, H.; Yi, J.; Xia, F.; Tian, D.; Zhou, C. Multiplexed electrochemical aptasensor based on mixed valence Ce(III, IV)-MOF for simultaneous determination of malathion and chlorpyrifos. Anal. Chim. Acta 2022, 1230, 340364. [Google Scholar] [CrossRef]
- Fan, Z.; He, P.; Bai, H.; Liu, J.; Liu, H.; Liu, L.; Niu, R.; Gong, J. Green recycling of waste poly(ethylene terephthalate) into Ni-MOF nanorod for simultaneous interfacial solar evaporation and photocatalytic degradation of organic pollutants. EcoMat 2024, 6, 12422. [Google Scholar] [CrossRef]
- Arya, K.; Kumar, A.; Kataria, R. Recent advances in MOF-based composites for the detection and adsorptive removal of Pb(II) ions in aqueous phase. Mater. Today Sustain. 2025, 29, 101057. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Wang, M.; Wang, X.; Cui, H.; Wei, J.; Li, X. The role of metal-organic frameworks in removing emerging contaminants in wastewater. J. Clean. Prod. 2023, 429, 139526. [Google Scholar] [CrossRef]
- Alsehli, B.R. Toward sustainable environmental cleanup: Metal-organic frameworks in adsorption—A review. Desalin. Water Treat. 2023, 316, 44–70. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, T.; Wang, B.; Wang, W. The Application of Metal–Organic Frameworks in Water Treatment and Their Large-Scale Preparation: A Review. Materials 2024, 17, 1972. [Google Scholar] [CrossRef]
- Singh, S.; Sivaram, N.; Nath, B.; Khan, N.A.; Singh, J.; Ramamurthy, P.C. Metal organic frameworks for wastewater treatment, renewable energy and circular economy contributions. npj Clean Water 2024, 7, 124. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, X.; Zhang, Q.; Han, W.; Liu, X.; Zhang, H.; Liu, H.; Wu, Z.; Shi, G. Unveiling the adsorption behavior and mass transfer mechanism of Rb+ and Cs+ adsorption on FeMn-MOF. Chem. Eng. J. 2024, 502, 157999. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Z.; Wang, X.; Liu, X.; Kapteijn, F. Water Adsorption in MOFs: Structures and Applications. Adv. Funct. Mater. 2024, 34, 2304788. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.-A.; Yang, F.; Liu, Q.-K.; Ma, J.-P.; Dong, Y.-B. Cd(II)-MOF: Adsorption, Separation, and Guest-Dependent Luminescence for Monohalobenzenes. Inorg. Chem. 2014, 53, 9087–9094. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Y.; Chen, Z.; Chen, J.; Hu, Y.; Zhu, J.-J. Electrochemical sensor based on Ce-MOF/carbon nanotube composite for the simultaneous discrimination of hydroquinone and catechol. J. Hazard. Mater. 2021, 416, 125895. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, L.; Zhang, S.; Su, P.; Li, X.; Hao, X.; Wang, S.; Sun, M. Dual-functional fluorescent metal-organic framework based beads for visual detection and removal of oxytetracycline in real aqueous solution. Appl. Surf. Sci. 2023, 625, 157202. [Google Scholar] [CrossRef]
- Wu, N.; Guo, H.; Wang, M.; Cao, Y.; Sun, L.; Yang, F.; Zhang, T.; Peng, L.; Liu, Y.; Yang, W. A novel core-shell coordination assembled hybrid via postsynthetic metal exchange for simultaneous detection and removal of tetracycline. Anal. Chim. Acta 2022, 1190, 339247. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Wang, H.; Zhangsun, H.; Sun, X.; Bu, T.; Liu, Y.; Wang, W.; Xu, Z.; Wang, L. Europium-based metal-organic framework containing characteristic metal chains: A novel turn-on fluorescence sensor for simultaneous high-performance detection and removal of tetracycline. Sens. Actuators B 2021, 334, 129610. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Huang, Z.; Qian, C.; Tian, Y.; Duan, Y. An Eu-doped Zr-metal-organic framework for simultaneous detection and removal of antibiotic tetracycline. J. Environ. Chem. Eng. 2021, 9, 106012. [Google Scholar] [CrossRef]
- Wu, R.; Bi, C.; Zhang, D.; Fan, C.; Wang, L.; Zhu, B.; Liu, W.; Li, N.; Zhang, X.; Fan, Y. Highly selective, sensitive and stable three-dimensional luminescent metal-organic framework for detecting and removing of the antibiotic in aqueous solution. Microchem. J. 2020, 159, 105349. [Google Scholar] [CrossRef]
- Yin, R.; Bu, Y.; Zhu, H.; Su, P.; Ye, E.; Li, Z.; Jun Loh, X.; Yuan, C.; Wang, S. Simultaneous detection and removal of 2,4,6-trinitrophenyl phenol and dichromate by metal-organic framework. Spectrochim. Acta Part A 2023, 297, 122735. [Google Scholar] [CrossRef]
- Lei, M.; Ge, F.; Ren, S.; Gao, X.; Zheng, H. A water-stable Cd-MOF and corresponding MOF@melamine foam composite for detection and removal of antibiotics, explosives, and anions. Sep. Purif. Technol. 2022, 286, 120433. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, J.; Fan, R.; Cao, Y.; Lu, H.; Wang, B.; Zheng, X.; Yin, Y.; Wang, P.; Yang, Y. Selective adsorption and detection of p-arsanilic acid on MOF-on-MOF heterostructure induced by nitrogen-rich self-assembly template. Chem. Eng. J. 2022, 427, 131483. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Li, H.; Han, M.; Feng, Z.; Du, K.; Li, X. Approach for concurrent detection and removal of diclofenac in wastewater: Integration of MOF with Poly(deep eutectic solvent) imprinting method. J. Environ. Chem. Eng. 2024, 12, 114107. [Google Scholar] [CrossRef]
- Jia, W.; Fan, R.; Zhang, J.; Geng, Z.; Li, P.; Sun, J.; Gai, S.; Zhu, K.; Jiang, X.; Yang, Y. Portable metal-organic framework alginate beads for high-sensitivity fluorescence detection and effective removal of residual pesticides in fruits and vegetables. Food Chem. 2022, 377, 132054. [Google Scholar] [CrossRef]
- Jia, W.; Fan, R.; Zhang, J.; Zhu, K.; Gai, S.; Nai, H.; Guo, H.; Wu, J.; Yang, Y. Home-made multifunctional auxiliary device for in-situ imaging detection and removal of quinclorac residues through MOF decorated gel refills. Chem. Eng. J. 2022, 450, 138303. [Google Scholar] [CrossRef]
- Bi, X.; Liu, X.; Luo, L.; Liu, S.; He, Y.; Zhang, L.; Li, L.; You, T. Isolation of Sensing Units and Adsorption Groups Based on MOF-on-MOF Hierarchical Structure for Both Highly Sensitive Detection and Removal of Hg2+. Inorg. Chem. 2024, 63, 2224–2233. [Google Scholar] [CrossRef]
- Wu, N.; Guo, H.; Xue, R.; Wang, M.; Cao, Y.; Wang, X.; Xu, M.; Yang, W. A free nitrogen-containing Sm-MOF for selective detection and facile removal of mercury(II). Colloids Surf. A 2021, 618, 126484. [Google Scholar] [CrossRef]
- Safaei, S.; Kazemian, H.; Junk, P.C. Dual functional MOF as a selective fluorescent naked-eye detector and effective sorbent for mercury ion. J. Solid State Chem. 2021, 300, 122267. [Google Scholar] [CrossRef]
- Radwan, A.; El-Sewify, I.M.; Shahat, A.; Azzazy, H.M.E.; Khalil, M.M.H.; El-Shahat, M.F. Multiuse Al-MOF Chemosensors for Visual Detection and Removal of Mercury Ions in Water and Skin-Whitening Cosmetics. ACS Sustain. Chem. Eng. 2020, 8, 15097–15107. [Google Scholar] [CrossRef]
- Yoo, J.; Ryu, U.; Kwon, W.; Choi, K.M. A multi-dye containing MOF for the ratiometric detection and simultaneous removal of Cr2O72− in the presence of interfering ions. Sens. Actuators B 2019, 283, 426–433. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, S.; Zhang, S.; Wang, L.; Wu, X.-T.; Zhu, Q.-L.; Wen, Y. Two-dimensional layered MOF nanosheets with multiple binding sites for selective detection and removal of Fe(III) ions. Sep. Purif. Technol. 2024, 336, 126294. [Google Scholar] [CrossRef]
- Arya, K.; Kumar, A.; Mehra, S.; Divya; Kumar, A.; Kumar Mehta, S.; Kataria, R. Exploration and removal of multiple metal ions using mixed-linker-architected Zn-MOF in aqueous media. Sep. Purif. Technol. 2023, 307, 122551. [Google Scholar] [CrossRef]
- Lv, S.-W.; Liu, J.-M.; Li, C.-Y.; Zhao, N.; Wang, Z.-H.; Wang, S. A novel and universal metal-organic frameworks sensing platform for selective detection and efficient removal of heavy metal ions. Chem. Eng. J. 2019, 375, 122111. [Google Scholar] [CrossRef]
- Fan, L.; Deng, M.; Lin, C.; Xu, C.; Liu, Y.; Shi, Z.; Wang, Y.; Xu, Z.; Li, L.; He, M. A multifunctional composite Fe3O4/MOF/l-cysteine for removal, magnetic solid phase extraction and fluorescence sensing of Cd(ii). RSC Adv. 2018, 8, 10561–10572. [Google Scholar] [CrossRef]
- Xie, D.; Ma, Y.; Gu, Y.; Zhou, H.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Bifunctional NH2-MIL-88(Fe) metal-organic framework nanooctahedra for highly sensitive detection and efficient removal of arsenate in aqueous media. J. Mater. Chem. A 2017, 5, 23794–23804. [Google Scholar] [CrossRef]
- Ding, S.; Wang, X. Terbium-based metal organic framework and its immobilized nanofibrous membrane for selective detection and efficient removal of phosphate. Chem. Eng. J. 2023, 464, 142751. [Google Scholar] [CrossRef]
- Yin, H.-Q.; Tan, K.; Jensen, S.; Teat, S.J.; Ullah, S.; Hei, X.; Velasco, E.; Oyekan, K.; Meyer, N.; Wang, X.-Y.; et al. A switchable sensor and scavenger: Detection and removal of fluorinated chemical species by a luminescent metal-organic framework. Chem. Sci. 2021, 12, 14189–14197. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Shao, L.; Ren, Y.; Jiang, J.; Wang, H.; Tang, H.; Deng, H.; Xia, T. Highly Sensitive Adsorption and Detection of Iodide in Aqueous Solution by a Post-Synthesized Zirconium-Organic Framework. Molecules 2022, 27, 8547. [Google Scholar] [CrossRef]
- Barathe, P.; Kaur, K.; Reddy, S.; Shriram, V.; Kumar, V. Antibiotic pollution and associated antimicrobial resistance in the environment. J. Hazard. Mater. Lett. 2024, 5, 100105. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Z.; Wan, D.; Wang, X.; Zhang, X.; Zhu, Y.; Guo, J. Pollution characteristics of heavy metals, antibiotic and antibiotic resistance genes in the crested ibis and their habitat across different lifestyle and geography. Environ. Res. 2024, 261, 119701. [Google Scholar] [CrossRef]
- Saeed, F.; Anil Reddy, K.; Sridhar, S. Efficient removal of environmentally hazardous heavy metal ions from water by NH2-MIL-101-Fe and MOF-808-EDTA based polyether sulfone adsorbents. Chem. Eng. J. 2024, 497, 154688. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Nasr Esfahani, P.; Davar, F.; Aminabhavi, T.M.; Vasseghian, Y. Adsorptive removal of malachite green using novel GO@ZnO-NiFe2O4-αAl2O3 nanocomposites. Chem. Eng. J. 2023, 471, 144485. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Abbas, A.; Lam, S.-M.; Park, S.; Chon, K.; Kim, E.-S.; Cho, K.H. Machine learning approaches to predict the photocatalytic performance of bismuth ferrite-based materials in the removal of malachite green. J. Hazard. Mater. 2023, 442, 130031. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]
- Khan, I.; Shah, T.; Tariq, M.R.; Ahmad, M.; Zhang, B. Understanding the toxicity of trinitrophenol and promising decontamination strategies for its neutralization: Challenges and future perspectives. J. Environ. Chem. Eng. 2024, 12, 112720. [Google Scholar] [CrossRef]
- Umapathi, R.; Raju, C.V.; Safarkhani, M.; Haribabu, J.; Lee, H.U.; Rani, G.M.; Huh, Y.S. Versatility of MXene based materials for the electrochemical detection of phenolic contaminants. Coord. Chem. Rev. 2025, 525, 216305. [Google Scholar] [CrossRef]
- Wumaer, M.; Huo, T.; Gong, H.; Yalikun, N. Synthesis of coal tar pitch derived porous carbon and its application for electrochemical detection of phenol isomers. Microchem. J. 2025, 209, 112691. [Google Scholar] [CrossRef]
- Hu, X.-J.; Li, Y.-L.; Liu, H.-X.; Ying, S.-M.; Yin, Q.; Liu, T.-F. Removal of diclofenac sodium from water using a polyacrylonitrile mixed-matrix membrane embedded with MOF-808. RSC Adv. 2024, 14, 12142–12146. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, T.; Zhao, F.; Huang, G.; Bi, J. A hybrid linker-MOF fibrous composite for efficient diclofenac removal and self-cleaning. Sep. Purif. Technol. 2024, 337, 126260. [Google Scholar] [CrossRef]
- Chen, Z.; He, G.; You, T.; Zhang, T.; Liu, B.; Wang, Y. Complex pollution of Fluoroquinolone antibiotics and metal oxides/metal ions in water: A review on occurrence, formation mechanisms, removal and ecotoxicity. J. Environ. Chem. Eng. 2024, 12, 112191. [Google Scholar] [CrossRef]
- Yan, C.; Qu, Z.; Wang, J.; Cao, L.; Han, Q. Microalgal bioremediation of heavy metal pollution in water: Recent advances, challenges, and prospects. Chemosphere 2022, 286, 131870. [Google Scholar] [CrossRef]
- Cheng, W.; Yin, H.; Dong, F.; Li, X.; Zhang, D.; Lu, C. Analysis and probabilistic health risk assessment of vertical heavy metal pollution in the water environment of reservoir in the west coast new area of Qingdao, China. Environ. Pollut. 2024, 362, 125021. [Google Scholar] [CrossRef]
- Kalisinska, E.; Lanocha-Arendarczyk, N.; Kosik-Bogacka, D.; Budis, H.; Pilarczyk, B.; Tomza-Marciniak, A.; Podlasinska, J.; Cieslik, L.; Popiolek, M.; Pirog, A.; et al. Muscle mercury and selenium in fishes and semiaquatic mammals from a selenium-deficient area. Ecotoxicol. Environ. Saf. 2017, 136, 24–30. [Google Scholar] [CrossRef]
- Bi, C.-Y.; He, Y.-C.; Zhang, H.-Y.; Dong, Y.-H.; Su, S.-J.; Shen, Z.-S.; Wang, L.; Jing, Z. Two novel three-dimensional Pb(II)-based coordination polymers for the detection of Fe3+ and Cr2O72− in water. J. Mol. Struct. 2025, 1326, 141158. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Fan, L.; Zhang, J.; Sun, C.; Li, W.; Chang, Z. A multifunctional metal–organic complex fluorescent probe for highly sensitive detection of lysine, CrO42−/Cr2O72−, Fe3+ and nitro-aromatic compounds. Inorg. Chim. Acta 2025, 574, 122415. [Google Scholar] [CrossRef]
- Li, D.; Liu, A.; Xing, Y.; Li, Z.; Luo, Y.; Zhao, S.; Dong, L.; Xie, T.; Guo, K.; Li, J. A smart chemosensor with different response mechanisms to multi-analytes: Chromogenic and fluorogenic recognition of Cu2+, Fe3+, and Zn2+. Dyes Pigments 2023, 213, 111180. [Google Scholar] [CrossRef]
- Yuna, Z. Review of the Natural, Modified, and Synthetic Zeolites for Heavy Metals Removal from Wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Xiao, H.; Jiang, M.; Su, R.; Luo, Y.; Jiang, Y.; Hu, R. Fertilization intensities at the buffer zones of ponds regulate nitrogen and phosphorus pollution in an agricultural watershed. Water Res. 2024, 250, 121033. [Google Scholar] [CrossRef]
- Xiong, X.; Li, Y.; Zhang, C. Enhanced phosphorus removal from anoxic water using oxygen-carrying iron-rich biochar: Combined roles of adsorption and keystone taxa. Water Res. 2024, 266, 122433. [Google Scholar] [CrossRef]
- Talpur, S.A.; Rashad, M.; Ahmed, A.; Rosatelli, G.; Baloch, M.Y.J.; Khan, A.H.A.; Talpur, H.A.; Iqbal, J. Pollution indicators and human health risk assessment of fluoride contaminated drinking groundwater in southern Pakistan. HydroResearch 2025, 8, 167–177. [Google Scholar] [CrossRef]
- Kunarbekova, M.; Busquets, R.; Sailaukhanuly, Y.; Mikhalovsky, S.V.; Toshtay, K.; Kudaibergenov, K.; Azat, S. Carbon adsorbents for the uptake of radioactive iodine from contaminated water effluents: A systematic review. J. Water Process Eng. 2024, 67, 106174. [Google Scholar] [CrossRef]
- Lv, W.; Song, Y.; Mo, Z. Synthesis of metal-organic framework-luminescent guest (MOF@LG) composites and their applications in environmental health sensing: A mini review. Talanta 2025, 283, 127105. [Google Scholar] [CrossRef]
- Yuan, L.; Chai, J.; Wang, S.; Li, T.; Yan, X.; Wang, J.; Yin, H. Biomimetic Laccase-Cu2O@MOF for synergetic degradation and colorimetric detection of phenolic compounds in wastewater. Environ. Technol. Innov. 2023, 30, 103085. [Google Scholar] [CrossRef]
- Liao, X.; Li, B.; Wang, L.; Chen, Y. Boric acid functionalized Fe3O4@CeO2/Tb-MOF as a luminescent nanozyme for fluorescence detection and degradation of caffeic acid. Biosens. Bioelectron. 2024, 264, 116637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y. Luminescence-Sensing Tb-MOF Nanozyme for the Detection and Degradation of Estrogen Endocrine Disruptors. ACS Appl. Mater. Interfaces 2020, 12, 8351–8358. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Guo, X.; Li, F. Antibiotic resistance genes in bioaerosols: Emerging, non-ignorable and pernicious pollutants. J. Clean. Prod. 2022, 348, 131094. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Z.; Gudda, F.O.; Tang, L.; Wang, H.; Czech, B.; Oleszczuk, P.; Gao, Y. Hydroxyl groups and vacancy defects modified Mo2C MXene as peroxymonosulfate activator for antibiotics degradation. J. Clean. Prod. 2025, 486, 144540. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Mishra, S.R.; Gadore, V.; Yadav, G.; Roy, S.; Bhattacharjee, B.; Bhuyan, A.; Hazarika, B.; Darabdhara, J.; Kumari, K. Phenolic compounds in water: From toxicity and source to sustainable solutions—An integrated review of removal methods, advanced technologies, cost analysis, and future prospects. J. Environ. Chem. Eng. 2024, 12, 112964. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Tabassum, S. Based on nanocomposites for degradation of phenolic compounds from aqueous environments by advanced oxidation processes: A review. J. Water Process Eng. 2024, 61, 105286. [Google Scholar] [CrossRef]
- Diaz, N.O.; Rodríguez, C.A.; Durán-Álvarez, J.C.; Talreja, N.; Quispe-Fuentes, I.; Martínez-Avelar, C.; Bizarro, M.; Valdés, H.; Mera, A.C. A theoretical and experimental approach for photocatalytic degradation of caffeic acid using BiOBr microspheres. Mater. Sci. Eng. B 2021, 273, 115432. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Lv, L.; Wang, J. Degradation of caffeic acid by dielectric barrier discharge plasma combined with Ce doped CoOOH catalyst. J. Hazard. Mater. 2021, 402, 123772. [Google Scholar] [CrossRef]
- Tan, C.; Huang, Y.; Lin, X.; Li, P.; Su, L.; Wang, Q. Insights into ibuprofen degradation in boron-doped CuO/PMS systems: Role of Cu, kinetics, mechanisms and degradation pathways. J. Water Process Eng. 2025, 69, 106711. [Google Scholar] [CrossRef]
- Fidelis, M.Z.; dos Santos, A.S.G.G.; de Paula, E.T.; Lenzi, G.G.; Soares, O.S.G.P.; Andreo, O.A.B. Nb2O5/MWCNT nanocomposites for the degradation of ibuprofen via photocatalysis and catalytic ozonation. Catal. Commun. 2024, 187, 106853. [Google Scholar] [CrossRef]
- Garg, R.; Sabouni, R.; Ghommem, M. Dual detection and degradation of anti-inflammatory drugs in aqueous environments using amino-functionalized MOF/metal oxide based ‘turn-on’ sensors. J. Water Process Eng. 2024, 66, 105947. [Google Scholar] [CrossRef]
- Cosma, D.-V.; Roşu, M.-C.; Socaci, C.; Rostas, A.M.; Urda, A.; Radu, T.; Turza, A.; Dan, M.; Costescu, R.; Gustavsen, K.R.; et al. Adsorption-catalysis synergy in the visible-light-driven removal of 17β-estradiol by (Au)TiO2 nanotubes-graphene composites. J. Environ. Chem. Eng. 2024, 12, 112885. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, J.; Lu, Y.; Feng, Z.; Zhang, S.; Sun, T. Prepared Sandwich structure WS2/ag@MIP composite for ultrasensitive SERS detection of trace 17β-estradiol in food. Food Chem. 2024, 460, 140731. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Han, J. Treatment techniques for removal of polybrominated diphenyl ethers (PBDEs) from real wastewater: Limitations, challenges, and future research directions. J. Water Process Eng. 2024, 63, 105463. [Google Scholar] [CrossRef]
- Ardiati, F.C.; Anita, S.H.; Nurhayat, O.D.; Chempaka, R.M.; Yanto, D.H.Y.; Watanabe, T.; Wilén, B.-M. Evaluation of batch and fed-batch rotating drum biological contactor using immobilized Trametes hirsuta EDN082 for non-sterile real textile wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 113241. [Google Scholar] [CrossRef]
- Sharma, I.; Kumar, A.; Arya, K.; Mehra, S.; Kumar, A.; Kumar Mehta, S.; Kataria, R. Dual-functional luminescent Zn-MOF@MCHS nanocomposite for TNP detection and copper(II) adsorptive removal. Sep. Purif. Technol. 2025, 355, 129538. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Zhang, L.; Yang, Y.; Zhang, K.; Cai, Y.; Xu, Y.; Gai, Y.; Xiong, K. Viologen-Based Cationic Metal-Organic Framework for Antibiotics Detection and MnO4– Removal in Water. Cryst. Growth Des. 2022, 22, 3991–3997. [Google Scholar] [CrossRef]
- Qasem, K.M.A.; Khan, S.; Chinnam, S.; Saleh, H.A.M.; Mantasha, I.; Zeeshan, M.; Manea, Y.K.; Shahid, M. Sustainable fabrication of Co-MOF@CNT nano-composite for efficient adsorption and removal of organic dyes and selective sensing of Cr(VI) in aqueous phase. Mater. Chem. Phys. 2022, 291, 126748. [Google Scholar] [CrossRef]
- Miao, Q.; Hakimifar, A.; Akbar Razavi, S.A.; Abbasi, H.; Tehrani, A.A.; Chen, J.-Q.; Hu, M.-L.; Morsali, A.; Retailleau, P. Multi-functionalization strategy for environmental monitoring: A metal-organic framework for high capacity Mercury(II) removal and exceptionally sensitive detection of nitroaromatics. J. Clean. Prod. 2022, 376, 134301. [Google Scholar] [CrossRef]
- Li, W.-T.; Hu, Z.-J.; Meng, J.; Zhang, X.; Gao, W.; Chen, M.-L.; Wang, J.-H. Zn-based metal organic framework-covalent organic framework composites for trace lead extraction and fluorescence detection of TNP. J. Hazard. Mater. 2021, 411, 125021. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; He, Z. Preparation of amorphous MOF based biomimetic nanozyme with high laccase- and catecholase-like activity for the degradation and detection of phenolic compounds. Chem. Eng. J. 2022, 434, 134677. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Shi, Z.; Lu, T.; Wang, Q.; Li, B. Multifunctional Zr-MOF Based on Bisimidazole Tetracarboxylic Acid for pH Sensing and Photoreduction of Cr(VI). ACS Appl. Mater. Interfaces 2021, 13, 54217–54226. [Google Scholar] [CrossRef]
- Zayed, A.M.; Ragab, A.H.; Al-Mhyawi, S.R.; Gumaah, N.F.; Masoud, M.A.; El Maghrabi, A.H.; El-Rabiee, M.M.; Abdelaziz, M.H.; Metwally, B.S. Innovative chrysotile/polyamide nanotextile filter for sustainable treatment of aqueous solutions and real wastewater: Fabrication, characterization, and performance evaluation. J. Water Process Eng. 2025, 69, 106727. [Google Scholar] [CrossRef]
- Luo, Q.; Li, J.; Zhang, Z.; Liu, G.; Chen, J.; Chen, Y.; Zhao, Z. Formation and adaptation of algal-bacterial granular sludge in real Chinese liquor brewing wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 112288. [Google Scholar] [CrossRef]
- Nimbalkar, M.N.; Bhat, B.R. Simultaneous adsorption of methylene blue and heavy metals from water using Zr-MOF having free carboxylic group. J. Environ. Chem. Eng. 2021, 9, 106216. [Google Scholar] [CrossRef]
- Singh, S.; Kaushal, S.; Kaur, J.; Kaur, G.; Mittal, S.K.; Singh, P.P. CaFu MOF as an efficient adsorbent for simultaneous removal of imidacloprid pesticide and cadmium ions from wastewater. Chemosphere 2021, 272, 129648. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, J.; Fan, L.; Li, Y.; He, Y.; Wang, Y.; Li, L. Controllable Preparation of Cu-MOF-Coated Carboxyl Filter Paper for Simultaneous Removal of Organic Dye and Metal Ions. Ind. Eng. Chem. Res. 2021, 60, 7311–7319. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Z.; Shang, Y.; Qi, Z.; Zhao, W.; Peng, Y. Proportional modulation of zinc-based MOF/carbon nanotube hybrids for simultaneous removal of phosphate and emerging organic contaminants with high efficiency. Chem. Eng. J. 2021, 417, 128063. [Google Scholar] [CrossRef]
- Haris, M.; Khan, M.W.; Zavabeti, A.; Mahmood, N.; Eshtiaghi, N. Self-assembly of C@FeO nanopillars on 2D-MOF for simultaneous removal of microplastic and dissolved contaminants from water. Chem. Eng. J. 2023, 455, 140390. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, J.; Wang, L.; Li, F.; Bai, H.; Wang, S.; Yang, X. Multi-ligand strategy for enhanced removal of heavy metal ions by thiol-functionalized defective Zr-MOFs. J. Hazard. Mater. 2024, 479, 135723. [Google Scholar] [CrossRef]
- Boix, G.; Troyano, J.; Garzón-Tovar, L.; Camur, C.; Bermejo, N.; Yazdi, A.; Piella, J.; Bastus, N.G.; Puntes, V.F.; Imaz, I.; et al. MOF-Beads Containing Inorganic Nanoparticles for the Simultaneous Removal of Multiple Heavy Metals from Water. ACS Appl. Mater. Interfaces 2020, 12, 10554–10562. [Google Scholar] [CrossRef]
- Yu, J.; Wei, Z.; Li, Q.; Wan, F.; Chao, Z.; Zhang, X.; Lin, L.; Meng, H.; Tian, L. Advanced Cancer Starvation Therapy by Simultaneous Deprivation of Lactate and Glucose Using a MOF Nanoplatform. Adv. Sci. 2021, 8, 2101467. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, J.; Wang, L.; Zhu, Z.; Han, B.; Chen, L.; Liu, W. Pollution characteristics, environmental issues, and green development of neonicotinoid insecticides in China: Insights from Imidacloprid. Environ. Pollut. 2025, 365, 125394. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, N.; Ashraf, S.; Nayana, R.U.K.; Anju, P.B.; Showkat, M.; Perveen, K.; Bukhari, N.A.; Sayyed, R.Z.; Mastinu, A. Evaluation of suitability and biodegradability of the organophosphate insecticides to mitigate insecticide pollution in onion farming. Heliyon 2024, 10, e32580. [Google Scholar] [CrossRef]
- Achache, M.; Seddik, N.B.; Bouchta, D.; Draoui, K.; Choukairi, M. NiO nanoparticles modified carbon paste electrode for the voltammetric simultaneous detection of catechol and hydroquinone as environmental pollutants. Microchem. J. 2025, 208, 112578. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, A.; Sui, J. One-step synthesis of AuNPs decorated PEDOT nano-horns for simultaneously sensing hydroquinone and catechol. Int. J. Electrochem. Sci. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, N.; Li, L.; Liu, T.; Zhang, Y.; Tang, J.; Guo, J.; Su, S. Synthesis of Graphene Oxide-Coupled CoNi Bimetallic MOF Nanocomposites for the Simultaneous Analysis of Catechol and Hydroquinone. Sensors 2023, 23, 6957. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, X.; Huo, D.; Liu, H.; Yang, M.; Hou, C. Simultaneous detection of Cd2+ and Pb2+ in food based on sensing electrode prepared by conductive carbon paper, rGO and CoZn·MOF (CP-rGO-CoZn·MOF). Anal. Chim. Acta 2022, 1220, 339812. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Nga, D.T.N.; Thu, V.T.; Piro, B.; Truong, T.N.P.; Yen, P.T.H.; Le, G.H.; Hung, L.Q.; Vu, T.A.; Ha, V.T.T. Novel nanoscale Yb-MOF used as highly efficient electrode for simultaneous detection of heavy metal ions. J. Mater. Sci. 2021, 56, 8172–8185. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Safapour Moghaddam, B.; Nodehi, M. Cu-Based MOF for Simultaneous Determination of Trace Tl (I) and Hg (II) by Stripping Voltammetry. J. Electrochem. Soc. 2020, 167, 167522. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, J.; Wu, J.; Jiang, Z.; Zhang, X.; Meng, F. The Application of Multifunctional Metal–Organic Frameworks for the Detection, Adsorption, and Degradation of Contaminants in an Aquatic Environment. Molecules 2025, 30, 1336. https://doi.org/10.3390/molecules30061336
Liu Y, Yang J, Wu J, Jiang Z, Zhang X, Meng F. The Application of Multifunctional Metal–Organic Frameworks for the Detection, Adsorption, and Degradation of Contaminants in an Aquatic Environment. Molecules. 2025; 30(6):1336. https://doi.org/10.3390/molecules30061336
Chicago/Turabian StyleLiu, Yachen, Jinbin Yang, Junlin Wu, Zehao Jiang, Xinyu Zhang, and Fanjun Meng. 2025. "The Application of Multifunctional Metal–Organic Frameworks for the Detection, Adsorption, and Degradation of Contaminants in an Aquatic Environment" Molecules 30, no. 6: 1336. https://doi.org/10.3390/molecules30061336
APA StyleLiu, Y., Yang, J., Wu, J., Jiang, Z., Zhang, X., & Meng, F. (2025). The Application of Multifunctional Metal–Organic Frameworks for the Detection, Adsorption, and Degradation of Contaminants in an Aquatic Environment. Molecules, 30(6), 1336. https://doi.org/10.3390/molecules30061336





