Luminescent Manganese(II) Iminophosphorane Derivatives
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation of NPh=PPh3 and of Its Zinc(II) Bromide Derivative
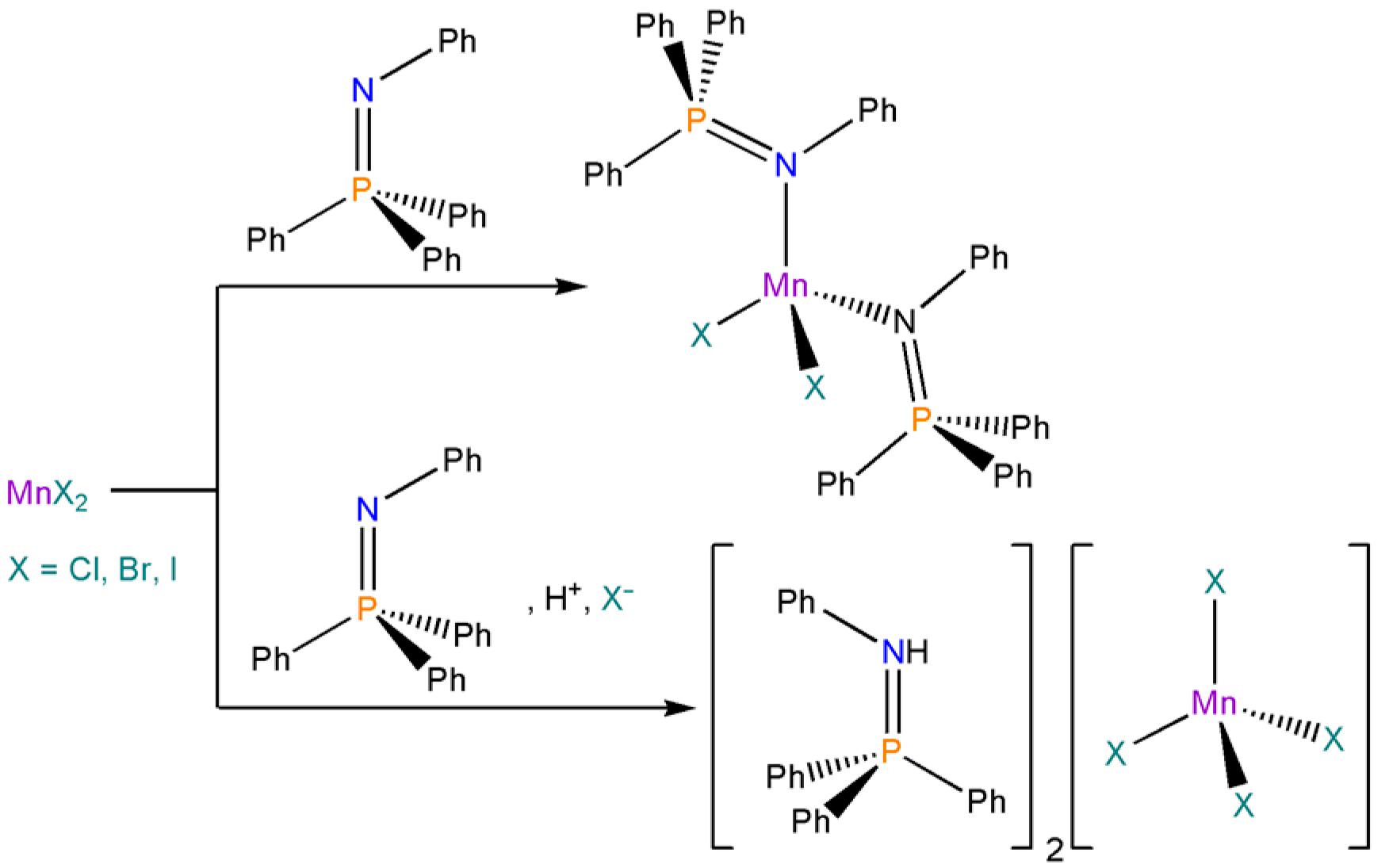
2.2. Synthesis and Luminescence of [MnX2(NPh=PPh3)2]
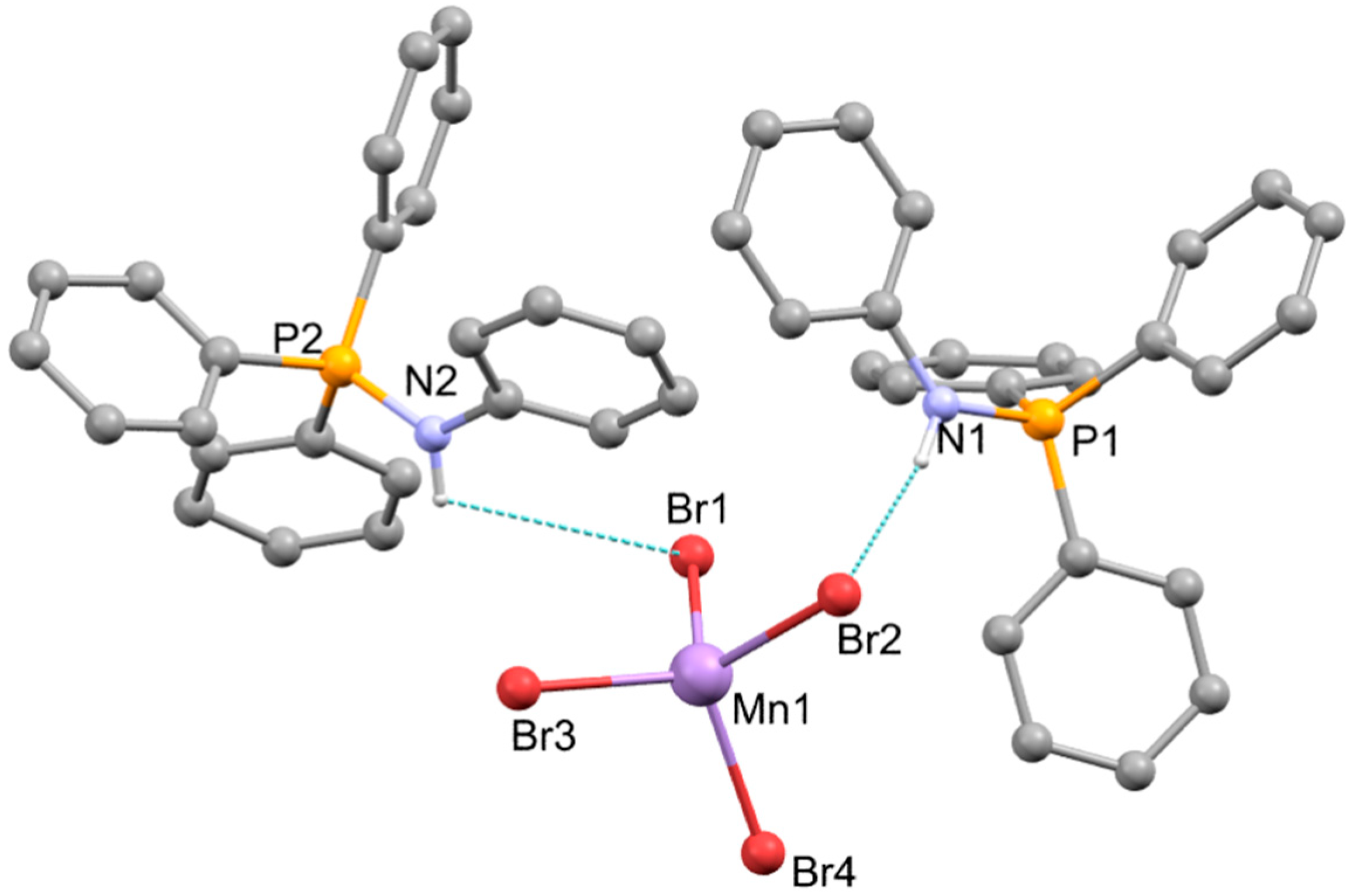
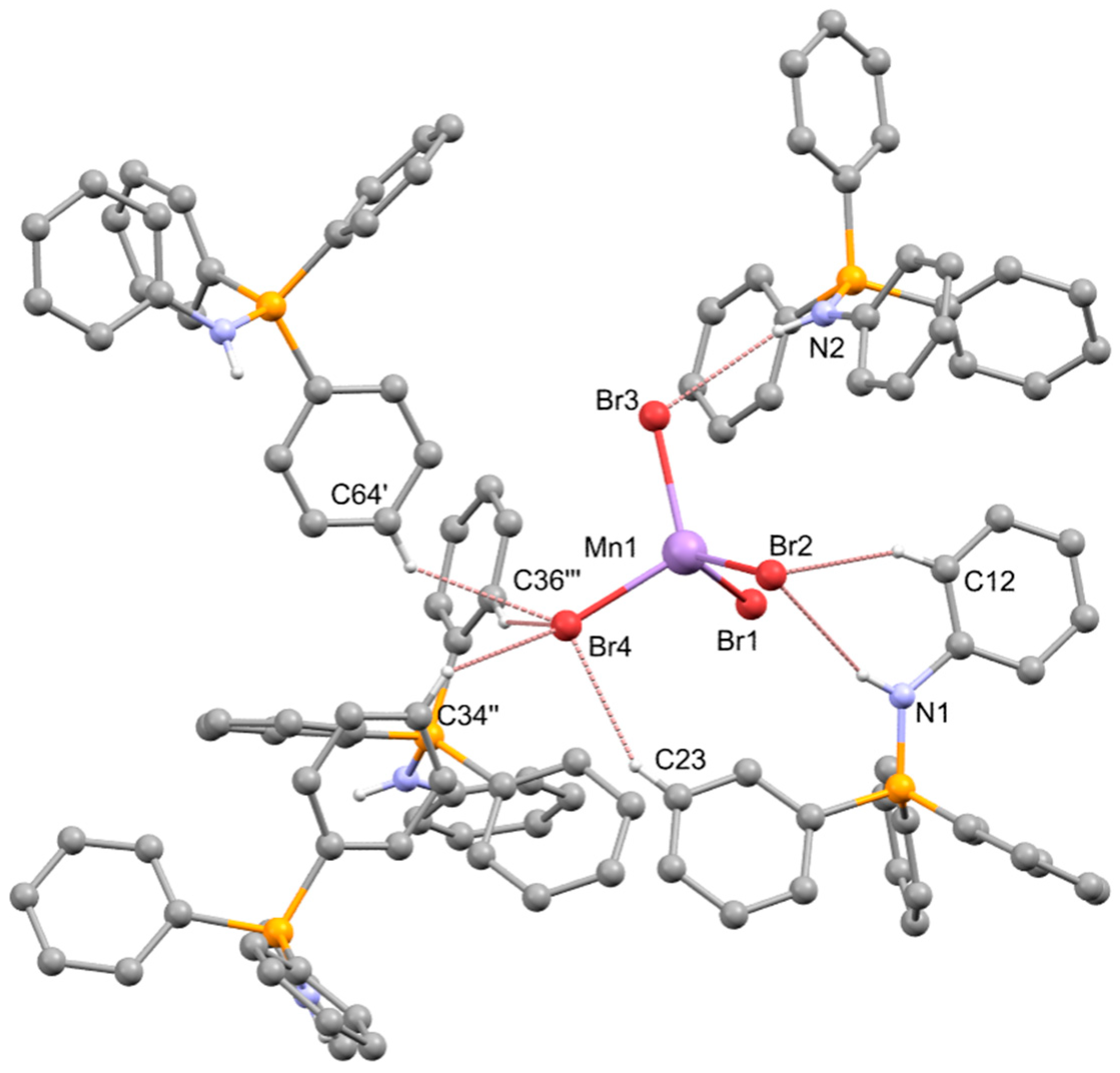
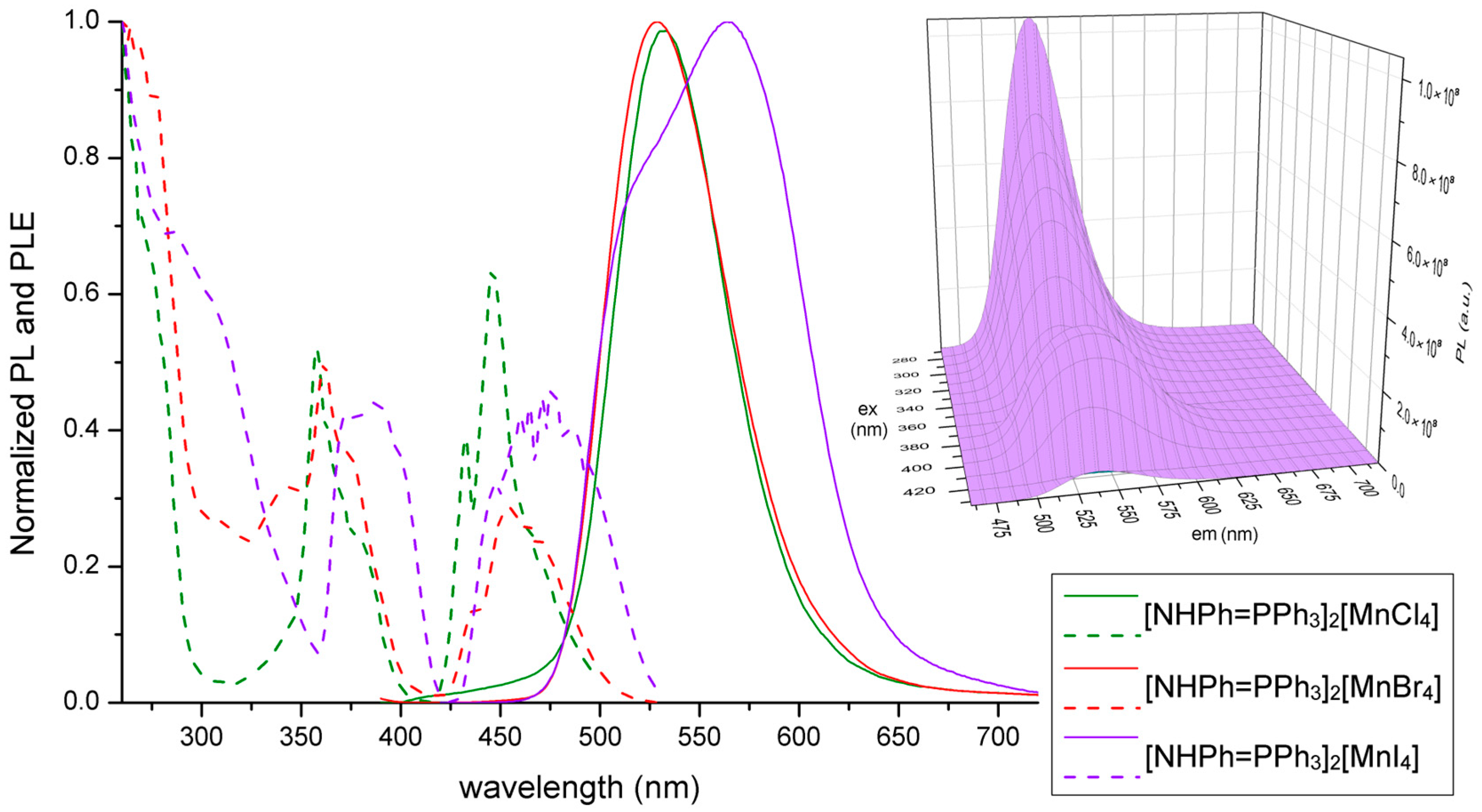
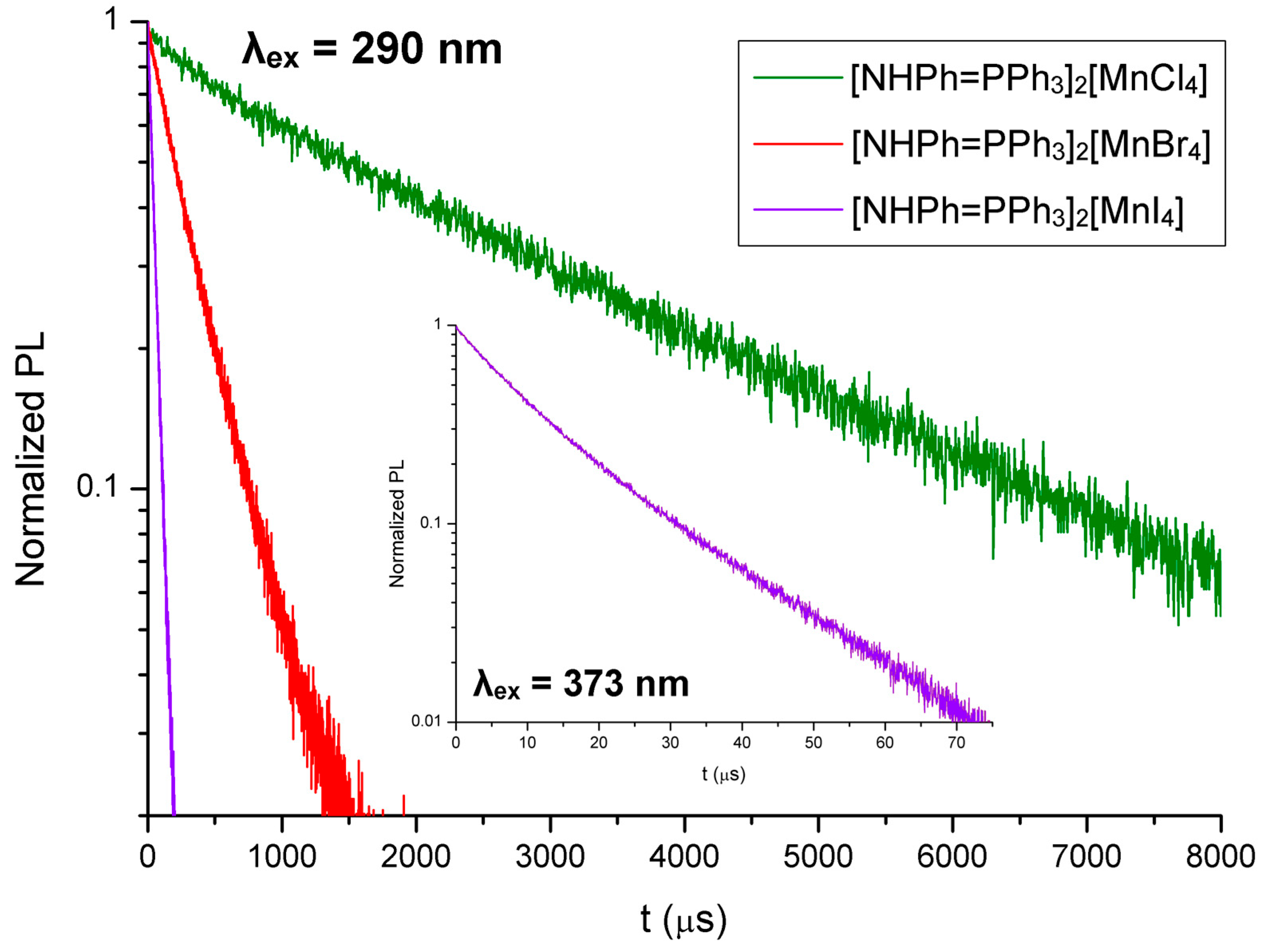
2.3. Synthesis of [NHPh=PPh3]2[MnX4], X-ray Structure Determination of the Bromo-Derivative and Luminescence Measurements
3. Experimental Section
3.1. Materials and Methods
3.2. Characterisations
3.3. Synthesis of [ZnBr2(NPh=PPh3)2]
3.4. Synthesis of [MnX2(NPh=PPh3)2] (X = Cl, Br, I)
3.5. Synthesis of [NHPh=PPh3)]2[MnX4] (X = Cl, Br)
3.6. Synthesis of [NHPh=PPh3)]2[MnI4]
3.7. Absorption and Luminescence Measurements
3.8. Single Crystal X-ray Structure Determination and Powder X-ray Measurements
3.9. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, Y.; She, P.; Huang, X.; Huang, W.; Zhao, Q. Luminescent manganese(II) complexes: Synthesis, properties and optoelectronic applications. Coord. Chem. Rev. 2020, 416, 213331. [Google Scholar] [CrossRef]
- Tao, P.; Liu, S.-J.; Wong, W.-J. Phosphorescent manganese(II) complexes and their emerging applications. Adv. Opt. Mater. 2020, 8, 2000985. [Google Scholar] [CrossRef]
- Liang, D.; Xiao, H.; Cai, W.; Lu, S.; Zhao, S.; Zang, Z.; Xie, L. Mn2+-Based Luminescent Metal Halides: Syntheses, Properties, and Applications. Adv. Opt. Mater. 2023, 11, 2202997. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, W.; Li, L.; Huang, P.; Xu, J.; Zhang, W.; Shao, Z.; Chen, X. Unlocking the Potential of Organic-Inorganic Hybrid Manganese Halides for Advanced Optoelectronic Applications. Adv. Mater. 2024, 36, 2408777. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, W.-Q.; Fu, D.-W.; Ye, H.-Y.; Liu, C.-M.; Chen, Z.-N.; Xiong, R.-G. The first organic-inorganic hybrid luminescent multiferroic: (pyrrolidinium)MnBr3. Adv. Mater. 2015, 27, 3942–3946. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, W.-Q.; Fu, D.-W.; Ye, H.-Y.; Chen, Z.-N.; Xiong, R.-G. Highly efficient red-light emission in an organic-inorganic hybrid ferroelectric: (pyrrolidinium)MnCl3. J. Am. Chem. Soc. 2015, 137, 4928–4931. [Google Scholar] [CrossRef]
- Ye, H.-Y.; Zhou, Q.; Niu, X.; Liao, W.-Q.; Fu, D.-W.; Zhang, Y.; You, Y.-M.; Wang, J.; Chen, Z.-N.; Xiong, R.-G. High-temperature ferroelectricity and photoluminescence in a hybrid organic-inorganic compound: (3-Pyrrolinium)MnCl3. J. Am. Chem. Soc. 2015, 137, 13148–13154. [Google Scholar] [CrossRef]
- Jiang, C.; Zhong, N.; Luo, C.; Lin, H.; Zhang, Y.; Peng, H.; Duan, C.-G. (Diisopropylammonium)2MnBr4: A multifunctional ferroelectric with efficient green-emission and excellent gas sensing properties. Chem. Commun. 2017, 53, 5954–5957. [Google Scholar] [CrossRef]
- You, Y.-M.; Liao, W.-Q.; Zhao, D.; Ye, H.-Y.; Zhang, Y.; Zhou, Q.; Niu, X.; Wang, J.; Li, P.-F.; Fu, D.-W.; et al. An organic-inorganic perovskite ferroelectric with large piezoelectric response. Science 2017, 357, 306–309. [Google Scholar] [CrossRef]
- Mei, Y.; Yu, H.; Wei, Z.; Mei, G.; Cai, H. Two coordinated geometries of Mn2+ ions in one single molecule: Organic–inorganic hybrids constructed with tris(2-aminoethyl)amine and manganese halide and fluorescent properties. Polyhedron 2017, 127, 458–463. [Google Scholar] [CrossRef]
- Chen, S.; Gao, J.; Chang, J.; Zhang, Y.; Feng, L. Organic-inorganic manganese (II) halide hybrids based paper sensor for the fluorometric determination of pesticide ferbam. Sens. Actuators B 2019, 297, 126701. [Google Scholar] [CrossRef]
- Mao, L.; Guo, P.; Wang, S.; Cheetham, A.K.; Seshadri, R. Design principles for enhancing photoluminescence quantum yield in hybrid manganese bromides. J. Am. Chem. Soc. 2020, 142, 13582–13589. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ge, C.; Yang, Z.; Song, Y.; Wang, A.; Kang, Y.; Li, B.; Dong, Q. Guanidinetemplated manganese halides single crystals toward efficient mechanoluminescence and photoluminescence by supramolecular interactions modulation. Adv. Opt. Mater. 2021, 9, 2100862. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Zhou, J.; Ming, H.; Wang, C.-H.; Jing, X.; Ye, S.; Zhang, Q. Structural design enables highly-efficient green emission with preferable blue light excitation from zero-dimensional manganese(II) hybrids. Chem. Eng. J. 2021, 421, 129886. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Zhou, Y.; Li, M.; Wang, W.; Ming, H.; Jing, X.; Ye, S. DipoleOrientation-dependent Förster resonance energy transfer from aromatic head groups to MnBr42− blocks in organic-inorganic hybrids. J. Phys. Chem. Lett. 2021, 12, 8692–8698. [Google Scholar] [CrossRef]
- Jana, A.; Sree, V.G.; Ba, Q.; Cho, S.C.; Lee, S.U.; Cho, S.; Jo, Y.; Meena, A.; Kim, H.; Im, H. Efficient organic manganese(II) bromide green-light-emitting diodes enabled by manipulating the hole and electron transport layer. J. Mater. Chem. C 2021, 9, 11314–11323. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Kou, T.; Zhou, Y.; Liang, Y.; Wu, Z.; Huang, J.; Chang, T.; Peng, C.; Wei, Q.; et al. Lead-free MnII-based red-emitting hybrid halide (CH6N3)2MnCl4 toward high performance warm WLEDs. J. Mater. Chem. C 2021, 9, 4895–4902. [Google Scholar] [CrossRef]
- He, J.; Zhao, H.; Hu, X.; Fangg, Z.; Wang, J.; Zhang, R.; Zheng, G.; Zhou, B.; Long, F. Synthesis, structure, and photoelectric properties of novel 0-dimensional organicinorganic hybrid perovskite (2-5-py)2MnBr4. J. Phys. Chem. C 2021, 125, 22898–22906. [Google Scholar] [CrossRef]
- Yan, S.; Tian, W.; Chen, H.; Tang, K.; Lin, T.; Zhong, G.; Qiu, L.; Pan, X.; Wang, W. Synthesis of 0D Manganese-Based Organic–Inorganic Hybrid Perovskite and Its Application in Lead-Free Red Light-Emitting Diode. Adv. Funct. Mater. 2021, 31, 2100855. [Google Scholar] [CrossRef]
- Berezin, A.S.; Davydova, M.P.; Samsonenko, D.G.; Sukhikh, T.S.; Artem’ev, A.V. A family of brightly emissive homo- and mixed-halomanganates(II): The effect of halide on optical and magnetic properties. J. Lumin. 2021, 236, 118069. [Google Scholar] [CrossRef]
- Liu, H.-L.; Ru, H.-Y.; Sun, M.-E.; Wang, Z.-Y.; Zang, S.-Q. Organic−Inorganic Manganese Bromide Hybrids with Water-Triggered Luminescence for Rewritable Paper. Adv. Opt. Mater. 2022, 10, 2101700. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, Y.-H.; Tang, Y.-Z.; Fan, X.-W.; Peng, X.-L.; Han, R.-R.; Li, Y.-K.; Wang, F.-X. Two Manganese(II)-Based Hybrid Multifunctional Phase Transition Materials with Strong Photoluminescence, High Quantum Yield, and Switchable Dielectric Properties: (C6NH16)2MnBr4 and (C7NH18)2MnBr4. Inorg. Chem. 2022, 61, 10454–10460. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Wang, L.; Peng, Z.; Wei, Z. Two manganese chloride organic–inorganic hybrid compounds showing strong luminescence and switchable phase transition and dielectric anomaly. New J. Chem. 2022, 46, 20005–20009. [Google Scholar] [CrossRef]
- Davydova, M.P.; Meng, L.; Rakhmanova, M.I.; Bagryanskaya, I.Y.; Sulyaeva, V.S.; Meng, H.; Artem’ev, A.V. Highly Emissive Chiral Mn(II) Bromide Hybrids for UV-Pumped Circularly Polarized LEDs and Scintillator Image Applications. Adv. Opt. Mater. 2023, 11, 2202811. [Google Scholar] [CrossRef]
- Wei, X.; He, J.; Zhu, Y.; Qin, Z.; Zheng, G.; Zhang, R.; Mo, S.; Wang, J.; Yao, D.; Lin, B.; et al. Synthesis, structure, and photoelectric properties of a novel zero-dimensional organic-inorganic hybrid perovskite (C6H9N2)2MnI4. Opt. Mater. 2023, 136, 113360. [Google Scholar] [CrossRef]
- Wu, L.-K.; Zou, Q.-H.; Yao, H.-Q.; Ye, H.-Y.; Li, J.-R. Zero-dimensional organic–inorganic hybrid manganese bromide with coexistence of dielectric–thermal double switches and efficient photoluminescence. Dalton Trans. 2023, 52, 11558–11564. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Zhang, B.; Li, F.; Meng, H.; Liu, S.; Zhao, Q. Chiral hybrid manganese(II) halide clusters with circularly polarized luminescence for X-ray imaging. J. Mater. Chem. C 2023, 11, 3206–3212. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Z.; Wang, C.; Li, Y.; Kurosawa, S.; Ren, G.; OuYang, X.; Wu, Y. Red-Emitting Organic–Inorganic Hybrid Manganese(II)Halides for X-Ray Imaging. Adv. Sensor Res. 2023, 2, 2200083. [Google Scholar] [CrossRef]
- Dong, G.; Hu, B.; Chen, C.; Yu, H.; Han, Q.; Wu, W. Two Organic–Inorganic Hybrid Manganese Bromides with Highly Efficient Emission toward White LEDs. Inorg. Chem. 2024, 63, 20830–20839. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, R.; Tang, Z.; Bai, Y.; Zhang, S.; Dai, Y. Efficient green emission in organic-inorganic hybrid (C13H22N)2MnCl4 metal halides with large Mn–Mn spacing distance. J. Lumin. 2024, 267, 120403. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, X.; Cao, C.; Xu, H.; Liu, X.; Li, X.; Wang, H.; Huang, K.; Yang, W.; Wang, D.; et al. Manganese-manganese distance modulation in zero-dimensional hybrid manganese halides using pyridine quaternary ammonium salts as templates. Mater. Today Chem. 2024, 42, 102343. [Google Scholar] [CrossRef]
- Yu, X.; Zhong, S.; Guo, Z.; Guan, J.; Tang, H.; He, X.; Chen, Y.; Pan, S. Switchable circularly polarized luminescent Mn-based hybrid metal halides. J. Mater. Chem. C 2025, 13, 2190–2197. [Google Scholar] [CrossRef]
- Goodgame, D.M.L.; Cotton, F.A. Phosphine oxide complexes. Part V. Tetrahedral complexes of manganese(II) containing triphenylphosphine oxide, and triphenylarsine oxide as ligands. J. Chem. Soc. 1961, 3735–3741. [Google Scholar] [CrossRef]
- Chandra, B.P.; Kaza, B.R. Mechanoluminescence, electroluminescence and high-pressure photoluminescence of Mn(Ph3PO)2Br2. J. Lumin. 1982, 27, 101–107. [Google Scholar] [CrossRef]
- Cotton, F.A.; Daniels, L.M.; Huang, P. Correlation of structure and triboluminescence for tetrahedral manganese(II) compounds. Inorg. Chem. 2001, 40, 3576–3578. [Google Scholar] [CrossRef]
- Tang, Y.-Y.; Wang, Z.-X.; Li, P.-F.; You, Y.-M.; Stroppa, A.; Xiong, R.-G. Brilliant triboluminescence in a potential organic–inorganic hybrid ferroelectric: (Ph3PO)2MnBr2. Inorg. Chem. Front. 2017, 4, 154–159. [Google Scholar] [CrossRef]
- Huang, X.; Qin, Y.; She, P.; Meng, H.; Liu, S.; Zhao, Q. Functionalized triphenylphosphine oxide-based manganese(II) complexes for luminescent printing. Dalton Trans. 2021, 50, 8831–8836. [Google Scholar] [CrossRef]
- Berezin, A. Birefringence and polarized luminescence of a manganese(II) chloride–triphenylphosphine oxide compound: Application in LEDs and photolithography. Mater. Chem. Front. 2023, 7, 2475–2483. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Zheng, F.-K.; Liu, Z.-F.; Wang, S.-H.; Wu, A.-Q.; Guo, G.-C. Intense photo- and tribo-luminescence of three tetrahedral manganese(II) dihalides with chelating bidentate phosphine oxide ligand. Dalton Trans. 2015, 44, 3289–3294. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Zhang, Y.-Q.; Yang, M.; Chen, Z.-N. Achievement of ligand-field induced thermochromic luminescence via two-step single-crystal to single-crystal transformations. Chem. Commun. 2018, 54, 13961–13964. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Xu, L.-J.; Yang, M.; Chen, Z.-N. Luminescent vapochromism due to a change of the ligand field in a one-dimensional manganese(II) coordination polymer. Inorg. Chem. 2018, 57, 9175–9181. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J.; Enrichi, F.; Vomiero, A.; Busato, M.; Huang, W. Green-emitting manganese(II) complexes with phosphoramide and phenylphosphonic diamide ligands. Inorg. Chem. Commun. 2018, 92, 145–150. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J.; Trave, E.; Dallan, D.; Favaretto, S. Orange-emitting manganese(II) complexes with chelating phosphine oxides. Inorg. Chem. Commun. 2018, 90, 105–107. [Google Scholar] [CrossRef]
- Berezin, A.S.; Samsonenko, D.G.; Brel, V.K.; Artem’ev, A.V. “Two-in-one” organic–inorganic hybrid MnII complexes exhibiting dual-emissive phosphorescence. Dalton Trans. 2018, 47, 7306–7315. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J. Dibromomanganese(II) complexes with hexamethylphosphoramide and phenylphosphonic bis(amide) ligands. J. Coord. Chem. 2019, 72, 309–327. [Google Scholar] [CrossRef]
- Berezin, A.S.; Davydova, M.P.; Bagryanskaya, I.Y.; Artyushin, O.I.; Brel, V.K.; Artem’ev, A.V. A red-emitting Mn(II)-based coordination polymer build on 1,2,4,5- tetrakis(diphenylphosphinyl)benzene, Inorg. Chem. Commun. 2019, 107, 107473. [Google Scholar] [CrossRef]
- Qin, Y.; Tao, P.; Gao, L.; She, P.; Liu, S.; Li, X.; Li, F.; Wang, H.; Zhao, Q.; Miao, Y.; et al. Designing Highly Efficient Phosphorescent Neutral Tetrahedral Manganese(II) Complexes for Organic Light-Emitting Diodes. Adv. Opt. Mater. 2019, 7, 1801160. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Davydova, M.P.; Berezin, A.S.; Brel, V.K.; Morgalyuk, V.P.; Bagryanskaya, I.Y.; Samsonenko, D.G. Luminescence of the Mn2+ ion in non-Oh and Td coordination environments: The missing case of square pyramid. Dalton Trans. 2019, 48, 16448–16456. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J.; Gobbo, A.; Ferraro, V.; Pietrobon, L.; Antoniutti, S. Tetrahedral photoluminescent manganese(II) halide complexes with 1,3-dimethyl-2-phenyl-1,3-diazaphospholidine-2-oxide as a ligand. New J. Chem. 2020, 44, 571–579. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J.; Gobbo, A.; Ferraro, V.; Pietrobon, L. Light harvesting indolyl-substituted phosphoramide ligand for the enhancement of Mn(II) luminescence. Dalton Trans. 2020, 49, 7525–7534. [Google Scholar] [CrossRef]
- Davydova, M.P.; Bauer, I.A.; Brel, V.K.; Rakhmanova, M.I.; Bagryanskaya, I.Y.; Artem’ev, A.V. Manganese(II) thiocyanate complexes with bis(phosphine oxide) ligands: Synthesis and excitation wavelength-dependent multicolor luminescence. Eur. J. Inorg. Chem. 2020, 2020, 695–703. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Davydova, M.P.; Berezin, A.S.; Sukhikh, T.S.; Samsonenko, D.G. Photo- and triboluminescent robust 1D polymers made of Mn(II) halides and meta-carborane based bis(phosphine oxide). Inorg. Chem. Front. 2021, 8, 2261–2270. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Davydova, M.P.; Rakhmanova, M.I.; Bagryanskaya, I.Y.; Pishchur, D.P. A family of Mn(II) complexes exhibiting strong photo- and triboluminescence as well as polymorphic luminescence. Inorg. Chem. Front. 2021, 8, 3767–3774. [Google Scholar] [CrossRef]
- Meng, H.; Zhu, W.; Li, F.; Huang, X.; Qin, Y.; Liu, S.; Yang, Y.; Huang, W.; Zhao, Q. Highly emissive and stable five-coordinated manganese(II) complex for X-ray imaging. Laser Photonics Rev. 2021, 15, 2100309. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J.; Di Vera, A.; Palù, A.; Ferraro, V. Manganese(II) bromo- and iodo-complexes with phosphoramidate and phosphonate ligands: Synthesis, characterization and photoluminescence. New J. Chem. 2021, 45, 12871–12878. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Ferraro, V.; Castro, J. Synthesis and photoluminescence of manganese(II) naphtylphosphonic diamide complexes. Dalton Trans. 2021, 50, 3132–3136. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J.; Ferraro, V. Dual emission from Mn(II) complexes with carbazolyl-substituted phosphoramides. Inorg. Chim. Acta 2022, 536, 120896. [Google Scholar] [CrossRef]
- Ferraro, V.; Castro, J.; Agostinis, L.; Bortoluzzi, M. Dual-emitting Mn(II) and Zn(II) halide complexes with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide as ligand. Inorg. Chim. Acta 2023, 545, 121285. [Google Scholar] [CrossRef]
- Tan, G.-H.; Chen, Y.-N.; Chuang, Y.-T.; Lin, H.-C.; Hsieh, C.-A.; Chen, Y.-S.; Lee, T.-Y.; Miao, W.-C.; Kuo, H.-C.; Chen, L.-Y.; et al. Highly Luminescent Earth-Benign Organometallic Manganese Halide Crystals with Ultrahigh Thermal Stability of Emission from 4 to 623 K. Small 2023, 19, 2205981. [Google Scholar] [CrossRef]
- Davydova, M.P.; Meng, L.; Rakhmanova, M.I.; Jia, Z.; Berezin, A.S.; Bagryanskaya, I.Y.; Lin, Q.; Meng, H.; Artem’ev, A.V. Strong Magnetically-Responsive Circularly Polarized Phosphorescence and X-Ray Scintillation in Ultrarobust Mn(II)–Organic Helical Chains. Adv. Mater. 2023, 35, 2303611. [Google Scholar] [CrossRef]
- Zhou, Z.; Meng, H.; Li, F.; Jiang, T.; Yang, Y.; Liu, S.; Zhao, Q. Highly Luminescent Nonclassical Binuclear Manganese(II) Complex Scintillators for Efficient X-ray Imaging. Inorg. Chem. 2023, 62, 5279–5736. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Castro, J.; Bortoluzzi, M. Luminescent Behavior of Zn(II) and Mn(II) Halide Derivatives of 4-Phenyldinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepine 4-Oxide and Single-Crystal X-ray Structure Determination of the Ligand. Molecules 2024, 29, 239. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.W.; Mucklejohn, S.A. The chemistry of phosphinimines. Phosphorus Sulfur Silicon Relat. Elem. 1981, 9, 235–266. [Google Scholar] [CrossRef]
- Appel, R.; Schaaff, R. Triphenylphosphinimin-Komplexe Von Übergangselementen. Z. Naturforsch. B 1961, 16, 405. [Google Scholar] [CrossRef]
- Hieber, W.; Winter, E.; Schubert, E. Reaktionen des Vanadinhexacarbonyls mit verschiedenartigen Basen und die Säurefunktion von Vanadincarbonylwasserstoff-Verbindungen, II. Chem. Ber. 1962, 95, 3070–3076. [Google Scholar] [CrossRef]
- Seidel, W. Complexes of Iminophosphoranes with Metal Salts. Angew. Chem. Int. Ed. 1965, 4, 785. [Google Scholar] [CrossRef]
- Bock, H.; Dieck, H.T. Triphenylphosphin-imin, -oxid und -äthylen als Liganden in Molybdän[0]- und Wolfram[0]-Carbonyl-Komplexen. Z. Naturforsch. B 1966, 21, 739–746. [Google Scholar] [CrossRef]
- Fukui, M.; Itoh, K.; Ishii, Y. Iminophosphorane Complexes of Palladium(II). Bull. Chem. Soc. Jpn. 1975, 48, 2044–2046. [Google Scholar] [CrossRef]
- Abel, E.W.; Mucklejohn, S.A.; Cameron, T.S.; Cordes, R.E. The Dimeric Phosphinimine Complex [CdI2(HN:PPh3)2]2 Containing N-H···I Hydrogen Bonds. Z. Naturforsch. B 1978, 33, 339–340. [Google Scholar] [CrossRef]
- Dilworth, J.R.; de Liefde Meijer, H.J.; Teuben, J.H. Cyclopentadienyl titanium complexes with aryldiazenido- or phosphiniminato-ligands. J. Organomet. Chem. 1978, 159, 47–52. [Google Scholar] [CrossRef]
- Abel, E.W.; Mucklejohn, S.A. Some triphenylphosphinimine complexes of zinc, cadmium, mercury, rhodium and palladium. Inorg. Chim. Acta 1979, 37, 107–111. [Google Scholar] [CrossRef]
- Fenske, D.; Böhm, E.; Dehnicke, K.; Strähle, J. N-Trimethylsilyl-iminotriphenylphosphoran-Kupfer(II)-chlorid, [Me3SiNPPh3 · CuCl2]2 Synthese und Kristallstruktur. Z. Naturforsch. B 1988, 43, 1–4. [Google Scholar] [CrossRef]
- Maurer, A.; Fenske, D.; Beck, J.; Strähle, J.; Böhm, E.; Dehnicke, K. Kupfer(I)-chlorid-Addukte von Phosphoraniminen Die Kristallstrukturen von Ph3PNPh·CuCl und von 2,3-Bis(triphenylphosphoranylidenamino)maIeinsäure-N-methylimid- Kupfer(I)-chlorid. Z. Naturforsch. B 1988, 43, 5–11. [Google Scholar] [CrossRef]
- Imhoff, P.; Elsevier, C.J.; Stam, C.H. Iminophosphorane complexes of rhodium(I) and X-ray crystal structure of [Rh(COD)Cl(Et3P=N-p-tolyl)]. Inorg. Chim. Acta 1990, 175, 209–216. [Google Scholar] [CrossRef]
- Meyer Zu Köcker, R.; Frenzen, G.; Neumüller, B.; Dehnicke, K.; Magull, J. Synthese und Kristallstrukturen der Phosphanimin-Komplexe MCl2(Me3SiNPMe3)2 mit M = Zn und Co, und CoCl2(HNPMe3)2. Z. Anorg. Allg. Chem. 1994, 620, 431–437. [Google Scholar] [CrossRef]
- Miekisch, T.; Mai, H.J.; Meyer zu Köcker, R.; Dehnicke, K.; Magull, J.; Goesmann, H. Silylierte Phosphanimin-Komplexe von Chrom(II), Palladium(II) und Kupfer(II). Die Kristallstrukturen von [CrCl2(Me3SiNPMe3)2], [PdCl2(Me3SiNPEt3)2] und [CuCl2(Me3SiNPMe3)]2. Z. Anorg. Allg. Chem. 1996, 622, 583–588. [Google Scholar] [CrossRef]
- Braun, T.P.; Gutsch, P.A.; Zimmer, H. Complexes of N-Aryltriphenylphosphinimines with Mercury(II) Halides. Z. Naturforsch. B 1999, 54, 858–862. [Google Scholar] [CrossRef]
- Wheaton, C.A.; Ireland, B.J.; Hayes, P.G. Synthesis and Structural Diversity of ZnCl2 and Zn(C6F5)2 Complexes of a Phosphinimine Ligand. Z. Anorg. Allg. Chem. 2011, 637, 2111–2119. [Google Scholar] [CrossRef]
- Venderbosch, B.; Oudsen, J.-P.H.; van der Vlugt, J.I.; Korstanje, T.J.; Tromp, M. Cationic Copper Iminophosphorane Complexes as CuAAC Catalysts: A Mechanistic Study. Organometallics 2020, 39, 3480–3489. [Google Scholar] [CrossRef]
- Miller, J.S.; Visscher, M.O.; Caulton, K.G. Spectra and Structure of Mo2(CO)6(μ-Ph3PNH)3. A New Bonding Mode for Phosphinimines. Inorg. Chem. 1974, 13, 1632–1639. [Google Scholar] [CrossRef]
- Appel, R.; Volz, P. 1,2-Bis-triphenylphosphoranylidenamino-äthan als zweizähniger Ligand in Übergangsmetallkomplexen. Z. Anorg. Allg. Chem. 1975, 413, 45–50. [Google Scholar] [CrossRef]
- Imhoff, P.; van Asselt, R.; Elsevier, C.J.; Zoutberg, M.C.; Stam, C.H. Reactions of bis(iminophosphoranyl)methanes with chloro-bridged rhodium or iridium dimers giving complexes in which the ligand is coordinated either as a σ-N,σ-N′ or as a σ-N, σ-C chelate. X-ray crystal structure of the σ-N, σ-N′ Rh(I) complex Rh{(4-CH3-C6H4-N=PPh2)2CH2}(COD)]PF6. Inorg. Chim. Acta 1991, 184, 73–87. [Google Scholar] [CrossRef]
- Avis, M.W.; Vrieze, K.; Kooijman, H.; Veldman, N.; Spek, A.L.; Elsevier, C.J. Selective Formation of Four-Membered Metallacyclic Pt-N-P-C Compounds from Reactions of Bis((N-arylimino)phosphoranyl)methanes with Halide-Bridged Platinum(II) Phosphine Dimers. X-ray Crystal Structures of [PtCl(PMe2Ph){CH(PPh2=NC6H4-4-CH3)(PPh2NHC6H4-4-CH3)}]+Cl− and [PtCl(PEt3){CH(PPh2=NC6H4-4-CH3)(PPh2NHC6H4-4-CH3)}]+[PtCl3(PEt3)]−. Inorg. Chem. 1995, 34, 4092–4105. [Google Scholar] [CrossRef]
- Reetz, M.T.; Bohres, E.; Goddard, R. Chiral diiminophosphoranes: A new class of ligands for enantioselective transition metal catalysis. Chem. Commun. 1998, 935–936. [Google Scholar] [CrossRef]
- Al-Benna, S.; Sarsfield, M.J.; Thornton-Pett, M.; Ormsby, D.L.; Maddox, P.J.; Brès, P.; Bochmann, M. Sterically hindered iminophosphorane complexes of vanadium, iron, cobalt and nickel: A synthetic, structural and catalytic study. J. Chem. Soc. Dalton Trans. 2000, 4247–4257. [Google Scholar] [CrossRef]
- Steiner, A.; Zacchini, S.; Richards, P.I. From neutral iminophosphoranes to multianionic phosphazenates. The coordination chemistry of imino–aza-P(V) ligands. Coord. Chem. Rev. 2002, 227, 193–216. [Google Scholar] [CrossRef]
- Jain, A.; Karmakar, H.; Roesky, P.W.; Panda, T.K. Role of Bis(phosphinimino)methanides as Universal Ligands in the Coordination Sphere of Metals across the Periodic Table. Chem. Rev. 2023, 123, 13323–13373. [Google Scholar] [CrossRef]
- Evans, D.J.; Hill, M.S.; Hitchcock, P.B. Tuning low-coordinate metal environments: High spin d5–d7 complexes supported by bis(phosphinimino)methyl ligation. Dalton Trans. 2003, 570–574. [Google Scholar] [CrossRef]
- Hill, M.S.; Hitchcock, P.B. Three-coordinate bis(phosphinimino)methanide derivatives of ‘open shell’ [M(II)] (M = Mn, Fe, Co) transition metals. J. Organomet. Chem. 2004, 689, 3163–3167. [Google Scholar] [CrossRef]
- García-Álvarez, J.; García-Garrido, S.E.; Cadierno, V. Iminophosphorane–phosphines: Versatile ligands for homogeneous catalysis. J. Organomet. Chem. 2014, 751, 792–808. [Google Scholar] [CrossRef]
- Frik, M.; Jiménez, J.; Vasilevski, V.; Carreira, M.; de Almeida, A.; Gascón, E.; Benoit, F.; Sanaú, M.; Casini, A.; Contel, M. Luminescent iminophosphorane gold, palladium and platinum complexes as potential anticancer agents. Inorg. Chem. Front. 2014, 1, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.-J.; Wocadlo, S.; Massa, W.; Weller, F.; Dehnicke, K.; Maichle-Mössmer, C.; Strähle, J. Phosphanimin-Komplexe von Mangan(II)halogeniden. Die Kristallstrukturen von [MnCl2(Me3SiNPEt3)]2, [MnI2(Me3SiNPEt3)2] und [MnI2(Me2Si(NPEt3)2)]. Z. Naturforsch. B 1995, 50, 1215–1221. [Google Scholar] [CrossRef]
- Foster, R.S.; Jakobi, H.; Harrity, J.P.A. A General and Regioselective Synthesis of 5-Trifluoromethyl-pyrazoles. Org. Lett. 2012, 14, 4858–4861. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, T.; Chen, Q. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon 2020, 165, 461–467. [Google Scholar] [CrossRef]
- Yang, H.; Bard, A.J. The application of fast scan cyclic voltammetry. Mechanistic study of the initial stage of electropolymerization of aniline in aqueous solutions. J. Electroanal. Chem. 1992, 339, 423–449. [Google Scholar] [CrossRef]
- Sakai, Y.; Sagara, Y.; Nomura, H.; Nakamura, N.; Suzuki, Y.; Miyazaki, H.; Adachi, C. Zinc complexes exhibiting highly efficient thermally activated delayed fluorescence and their application to organic light-emitting diodes. Chem. Commun. 2015, 51, 3181–3184. [Google Scholar] [CrossRef]
- Berezin, A.S.; Vinogradova, K.A.; Krivopalov, V.P.; Nikolaenkova, E.B.; Plyusnin, V.F.; Kupryakov, A.S.; Pervukhina, N.V.; Naumov, D.Y.; Bushuev, M.B. Excitation-Wavelength-Dependent Emission and Delayed Fluorescence in a Proton-Transfer System. Chem. Eur. J. 2018, 24, 12790–12795. [Google Scholar] [CrossRef]
- Xiong, J.; Li, K.; Teng, T.; Chang, X.; Wei, Y.; Wu, C.; Yang, C. Dinuclear ZnII Complexes Exhibiting Thermally Activated Delayed Fluorescence and Luminescence Polymorphism. Chem. Eur. J. 2020, 26, 6887–6893. [Google Scholar] [CrossRef]
- Ferraro, V.; Baggio, F.; Castro, J.; Bortoluzzi, M. Green Phosphorescent Zn(II) Halide Complexes with N,N,N′,N′-tetramethyl-P-indol-1-ylphosphonic Diamide as Ligand. Eur. J. Inorg. Chem. 2022, 2022, e202200119. [Google Scholar] [CrossRef]
- Yersin, H. Highly Efficient OLEDs: Materials Based on Thermally Activated Delayed Fluorescence; Wiley-VCH: Weinheim, Germany, 2018; pp. 4–46. [Google Scholar]
- Bertini, I.; Luchinat, C. The hyperfine shift. Coord. Chem. Rev. 1996, 150, 29–75. [Google Scholar] [CrossRef]
- Liu, X.; Dronskowski, R.; Glaum, R.; Tchougréeff, A.L. Experimental and Quantum-Chemical Investigations of the UV/Vis Absorption Spectrum of Manganese Carbodiimide, MnNCN. Z. Anorg. Allg. Chem. 2010, 636, 343–348. [Google Scholar] [CrossRef]
- Wrighton, M.; Ginley, D. Excited state decay of tetrahalomanganese(II) complexes. Chem. Phys. 1974, 4, 295–299. [Google Scholar] [CrossRef]
- Gusev, A.; Kiskin, M.; Braga, E.; Zamnius, E.; Lisichnikova, A.; Linert, W. New highly efficient phosphorescent manganese halides as green and blue emitters. Dalton Trans. 2025, 54, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Cirera, J.; Alemany, P.; Alvarez, S. Mapping the Stereochemistry and Symmetry of Tetracoordinate Transition-Metal Complexes. Chem. Eur. J. 2004, 10, 190–207. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Coordination polymers and molecular structures among complexes of mercury(II) halides with selected 1-benzoylthioureas. Polyhedron 2015, 90, 47–57. [Google Scholar] [CrossRef]
- Böhm, E.; Dehnicke, K.; Beck, J.; Hiller, W.; Strähle, J.; Maurer, A.; Fenske, D. Die Kristallstrukturen von Ph3PNPh, [Ph3PN(H)Ph][AuI2] und von 2,3-Bis(triphenylphosphoranimino)maleinsäure-N-methylimid. Z. Naturforsch. B 1988, 43, 138–144. [Google Scholar] [CrossRef]
- Jiang, C.; Stephan, D.W. Phosphinimine–borane combinations in frustrated Lewis pair chemistry. Dalton Trans. 2013, 42, 630–637. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996; pp. 67–68, 215–216. [Google Scholar]
- Lindsay, R.O.; Allen, C.F.H. Phenyl azide. Org. Synth. 1942, 22, 96–97. [Google Scholar] [CrossRef]
- Deucher, N.C.; Silva de Souza, R., Jr.; Borges, E.M. Teaching Precipitation Titration Methods: A Statistical Comparison of Mohr, Fajans, and Volhard Techniques. J. Chem. Educ. 2025, 102, 364–371. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Pratical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- APEX4 v.2022.1-1, SAINT v.8.40B, XPREP v.2014/2, SADABS-2016/2, Bruker AXS Inc.: Madison, WI, USA, 2022.
- McArdle, P. Oscail, a program package for small-molecule single-crystal crystallography with crystal morphology prediction and molecular modelling. J. Appl. Crystallogr. 2017, 50, 320–326. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. checkCIF validation ALERTS: What they mean and how to respond. Acta Crystallogr. Sect. E 2020, 76, 1–11. [Google Scholar] [CrossRef]
- Grimme, S.; Brandenburg, J.G.; Bannwarth, C.; Hansen, A. Consistent structures and interactions by density functional theory with small atomic orbital basis sets. J. Chem. Phys. 2015, 143, 054107. [Google Scholar] [CrossRef]
- Kruse, H.; Grimme, S. A geometrical correction for the inter- and intra-molecular basis set superposition error in Hartree-Fock and density functional theory calculations for large systems. J. Chem. Phys. 2012, 136, 154101. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software update: The ORCA program system -Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1616. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
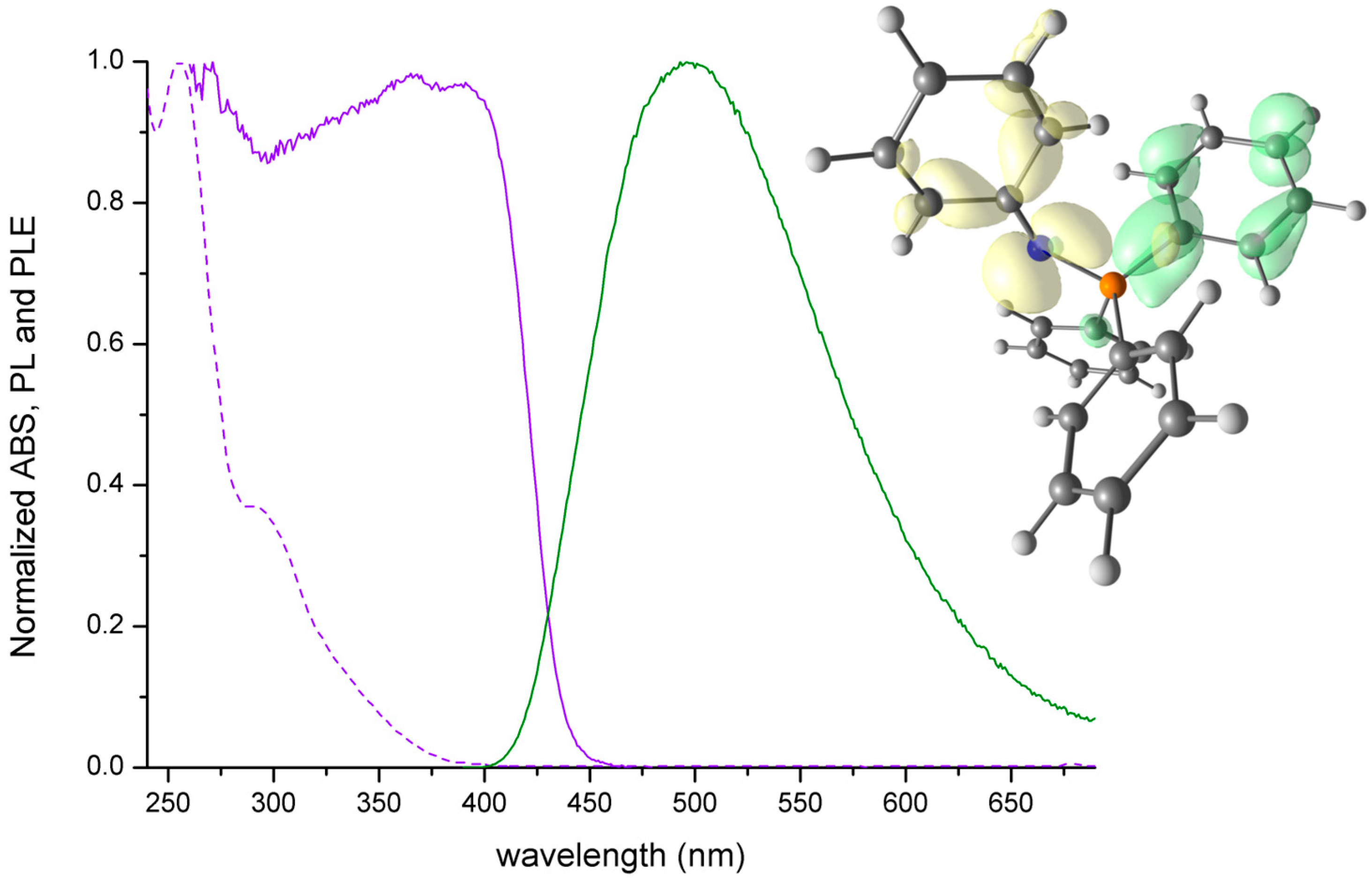
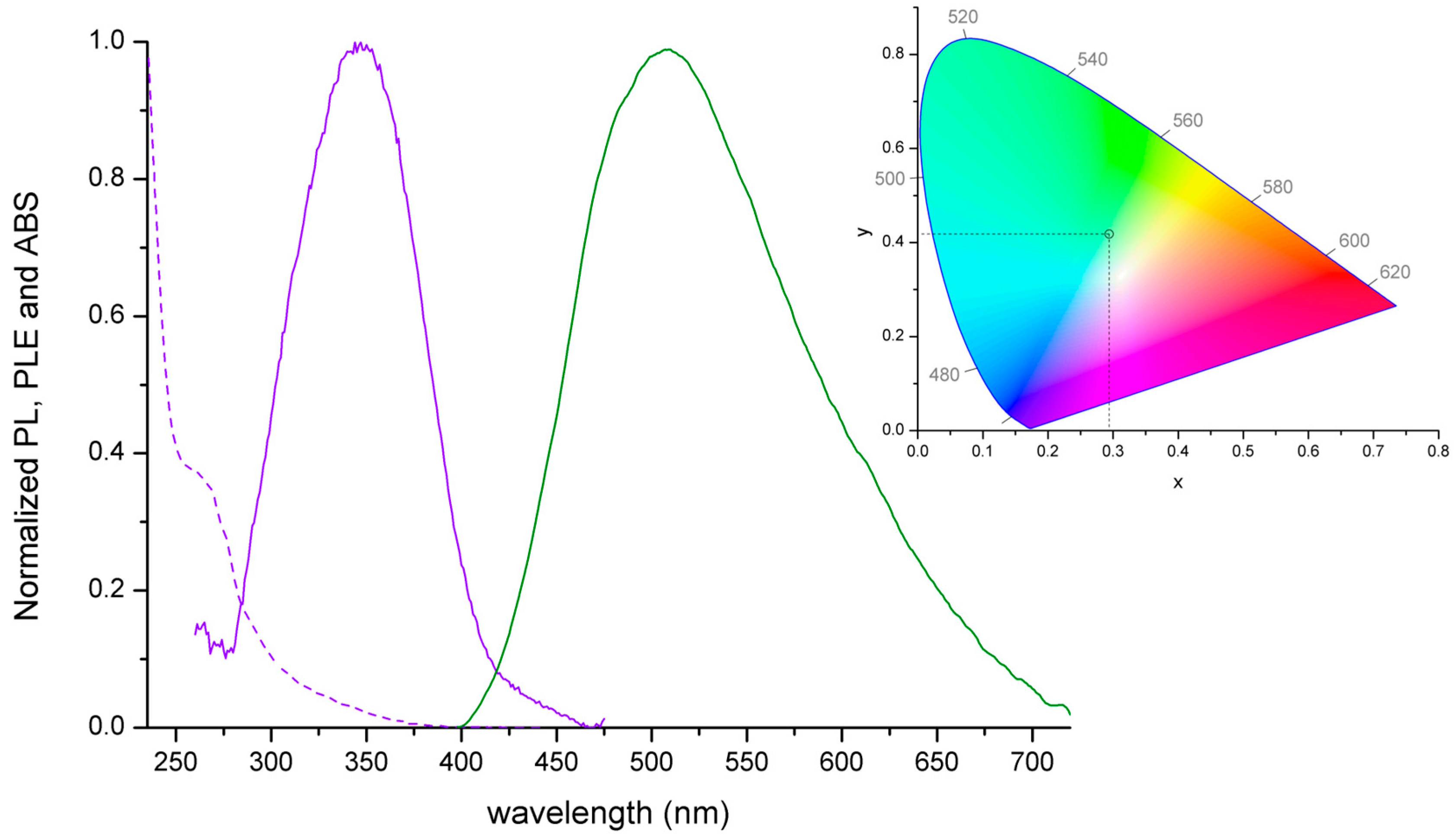
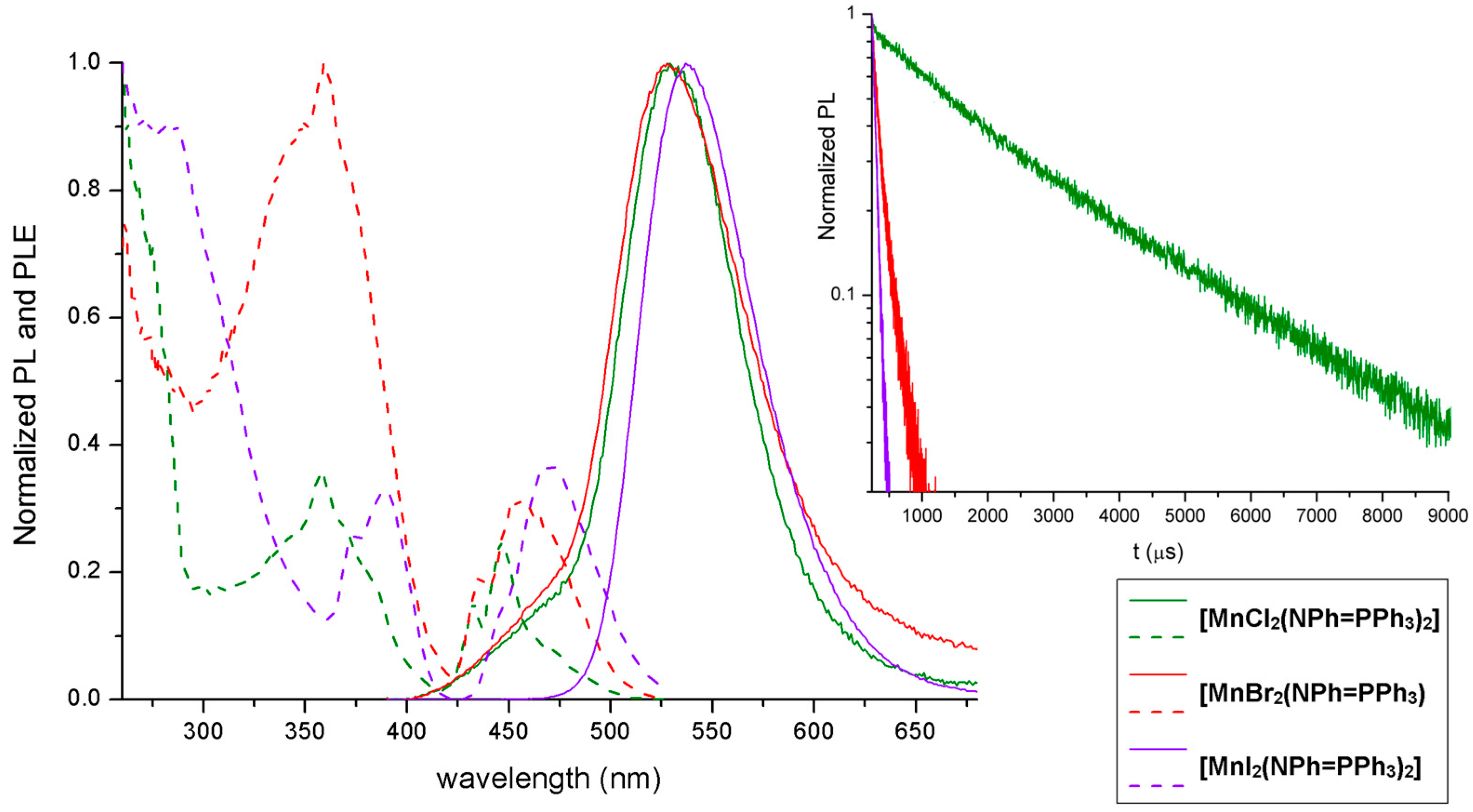
| Compound | Photoluminescence Data |
|---|---|
| NPh=PPh3 | UV-VIS (nm): <400, 342 sh, 298 sh, 256. PL (λex = 300 nm, nm): 496 (FWHM = 4800 cm−1). PLE (λem = 510 nm, nm): <450. τ (λex = 373 nm, λem = 415 nm, ns, TCSPC): 2. |
| [ZnBr2(NPh=PPh3)2] | UV-VIS (nm): <400, 335 sh, 262 sh. PL (λex = 350 nm, nm): 510 (FWHM = 5300 cm−1). PLE (λem = 510 nm, nm): <470, max 347. τ (λex = 373 nm, λem = 510 nm, ns, TCSPC): 222 (av.). τ (λex = 290 nm, λem = 510 nm, μs, MCS): 303 (av.). Φ: 3%. |
| [MnCl2(NPh=PPh3)2] | UV-VIS (nm): <350, 295 sh, 276 sh, 269, 264. PL (λex = 358 nm, nm): 460 sh, 530 (FWHM = 2400 cm−1). PLE (λem = 560 nm, nm): 470, 446, 432, 420–300 (358 max), <295. τ (λex = 373 nm, λem = 410 nm, ns, TCPSC): 2. τ (λex = 290 nm, λem = 535 nm, μs, MCS): 2478. |
| [MnBr2(NPh=PPh3)2] | UV-VIS (nm): <350, 295 sh, 276 sh, 268, 261. PL (λex = 358 nm, nm): 528 (FWHM = 2700 cm−1). PLE (λem = 560 nm, nm): 470 sh, 454, 435, 420–300 (359 max), <295. τ (λex = 373 nm, λem = 410 nm, ns, TCSPC): 2. τ (λex = 290 nm, λem = 535 nm, μs, MCS): 151. Φ: 3%. |
| [MnI2(NPh=PPh3)2] | UV-VIS (nm): <400, 364, 292, 276, 268. PL (λex = 358 nm, nm): 538 (FWHM = 2200 cm−1). PLE (λem = 560 nm, nm): 472, 447 sh, 390, 372, <360. τ (λex = 290 nm, λem = 535 nm, μs, MCS): 55. Φ: 7%. |
| [NHPh=PPh3]2[MnCl4] | UV-VIS (nm): 299 sh, 276, 269, 262. PL (λex = 370 nm, nm): 532 (FWHM = 2300 cm−1). PLE (λem = 560 nm, nm): 467 sh, 448, 432, 382 sh, 371 sh, 358, <295. τ (λex = 290 nm, λem = 530 nm, μs, MCS): 2832. |
| [NHPh=PPh3]2[MnBr4] | UV-VIS (nm): 299 sh, 276, 269, 262. PL (λex = 370 nm, nm): 529 (FWHM = 2500 cm−1). PLE (λem = 560 nm, nm): 471 sh, 454, 436, 375 sh, 362, 343, 312, <295. τ (λex = 290 nm, λem = 535 nm, μs, MCS): 313. Φ: 22%. |
| [NHPh=PPh3]2[MnI4] | UV-VIS (nm): 365, 299 sh, 276, 269, 262, <255. PL (λex = 370 nm, nm): 565, 525 sh. PL (λex = 280 nm, nm): 537 (FWHM = 2200 cm−1). PLE (λem = 560 nm, nm): 471, 446 sh, 390, 373, <355. τ (λex = 290 nm, λem = 520 nm, μs, MCS): 49. τ (λex = 373 nm, λem = 565 nm, μs, MCS): 10 (55%), 49 a (45%). Φ: 23%. |
| Mn(1)-Br(1) | 2.5055(10) | Mn(1)-Br(3) | 2.4916(11) |
| Mn(1)-Br(2) | 2.5263(11) | Mn(1)-Br(4) | 2.4748(11) |
| P(1)-N(1) | 1.633(5) | P(2)-N(2) | 1.631(5) |
| P(1)-C(31) | 1.785(5) | P(2)-C(61) | 1.773(6) |
| P(1)-C(21) | 1.786(6) | P(2)-C(71) | 1.783(7) |
| P(1)-C(41) | 1.795(6) | P(2)-C(81) | 1.798(7) |
| N(1)-C(11) | 1.423(7) | N(2)-C(51) | 1.421(8) |
| Br(1)-Mn(1)-Br(2) | 106.94(4) | Br(3)-Mn(1)-Br(1) | 109.01(4) |
| Br(4)-Mn(1)-Br(1) | 108.17(4) | Br(3)-Mn(1)-Br(2) | 113.74(4) |
| Br(4)-Mn(1)-Br(2) | 108.77(4) | Br(4)-Mn(1)-Br(3) | 110.03(4) |
| N(1)-P(1)-C(31) | 111.2(2) | N(2)-P(2)-C(61) | 104.9(3) |
| N(1)-P(1)-C(21) | 106.5(2) | N(2)-P(2)-C(71) | 112.2(3) |
| N(1)-P(1)-C(41) | 111.8(3) | N(2)-P(2)-C(81) | 111.6(3) |
| C(11)-N(1)-P(1) | 128.6(4) | C(51)-N(2)-P(2) | 128.5(4) |
| D-H…A | (D-H) | d(H…A) | d(D…A) | <(DHA) |
|---|---|---|---|---|
| N(1)-H(1)…Br(2) | 0.86 | 2.76 | 3.586(5) | 161.9 |
| C(12)-H(12)…Br(2) | 0.93 | 2.96 | 3.757(7) | 144.5 |
| N(2)-H(2)…Br(3) | 0.86 | 2.68 | 3.520(5) | 164.8 |
| C(23)-H(23)…Br(4) | 0.93 | 2.89 | 3.720(7) | 149.6 |
| C(64i)-H(64i)…Br(4) | 0.93 | 2.97 | 3.658(8) | 132.3 |
| C(34ii)-H(34ii)…Br(4) | 0.93 | 2.96 | 3.627(7) | 130.0 |
| C(36iii)-H(36iii)…Br(4) | 0.93 | 2.91 | 3.769(6) | 154.1 |
| Empirical formula | C48 H42 Br4 Mn N2 P2 | Index ranges | −18 ≤ h ≤ 21 |
| Moiety formula | (C24 H21 N P)2(Mn Br4) | −18 ≤ k ≤ 18 | |
| Formula weight | 1083.35 | −47 ≤ l ≤ 44 | |
| Temperature | 296(2) K | Reflections collected | 95,854 |
| Wavelength | 0.71073 Å | Independent reflections | 9780 [Rint = 0.0581 Rσ = 0.0382] |
| Crystal system | Orthorhombic | Reflections observed (>2σ) | 6622 |
| Space group | Pbca | Data completeness | 0.999 |
| Unit cell dimensions | a = 17.7894(8) Å | Absorption correction | Semi-empirical from equivalents |
| b = 15.3223(6) Å | Max. and min. transmission | 0.8620 and 0.6668 | |
| c = 39.1289(17) Å | Refinement method | Full-matrix least-squares on F2 | |
| Volume | 10,665.5(8) Å3 | Data/restraints/parameters | 9780/0/514 |
| Z | 8 | Goodness-of-fit on F2 | 1.048 |
| Density (calculated) | 1.349 Mg/m3 | Final R indices [I > 2σ(I)] | R1 = 0.0548 |
| Absorption coefficient | 3.335 mm−1 | wR2 = 0.1363 | |
| F(000) | 4312 | R indices (all data) | R1 = 0.0862 |
| Crystal size | 0.141 mm × 0.092 mm × 0.048 mm | wR2 = 0.1508 | |
| Theta range for data collection | 2.082 to 25.368° | Largest diff. peak and hole | 0.516 and −0.521 e.Å−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccolo, D.; Castro, J.; Rosa-Gastaldo, D.; Bortoluzzi, M. Luminescent Manganese(II) Iminophosphorane Derivatives. Molecules 2025, 30, 1319. https://doi.org/10.3390/molecules30061319
Piccolo D, Castro J, Rosa-Gastaldo D, Bortoluzzi M. Luminescent Manganese(II) Iminophosphorane Derivatives. Molecules. 2025; 30(6):1319. https://doi.org/10.3390/molecules30061319
Chicago/Turabian StylePiccolo, Domenico, Jesús Castro, Daniele Rosa-Gastaldo, and Marco Bortoluzzi. 2025. "Luminescent Manganese(II) Iminophosphorane Derivatives" Molecules 30, no. 6: 1319. https://doi.org/10.3390/molecules30061319
APA StylePiccolo, D., Castro, J., Rosa-Gastaldo, D., & Bortoluzzi, M. (2025). Luminescent Manganese(II) Iminophosphorane Derivatives. Molecules, 30(6), 1319. https://doi.org/10.3390/molecules30061319






