Synthesis of Magnetic Biosorbent from Bamboo Powders and Their Application for Methylene Blue Removal from Aqueous Solution: Kinetics, Isotherm, and Regeneration Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterizations of Raw BPs, Alkali-Treated BPs, and BPs/Fe3O4
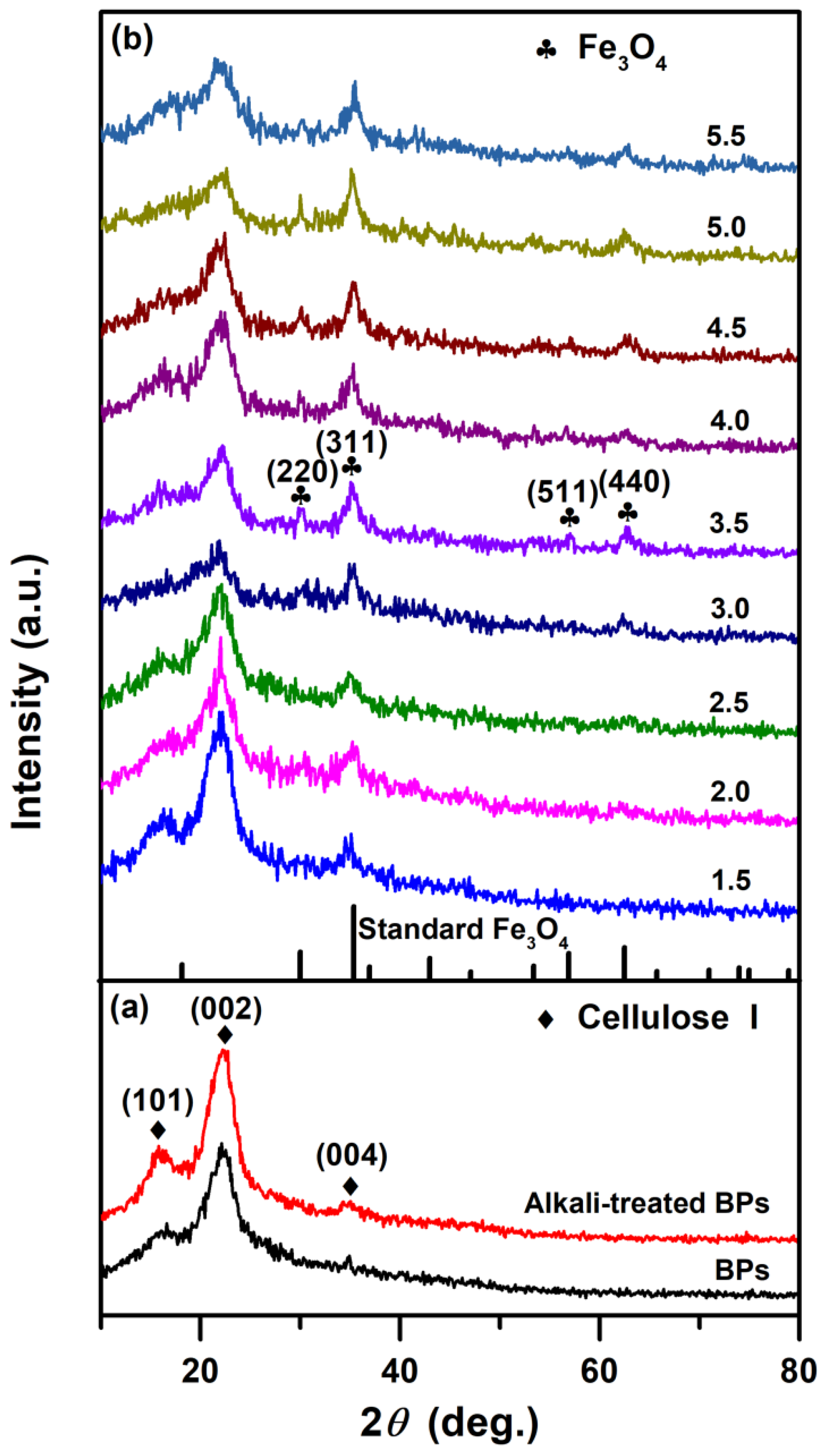
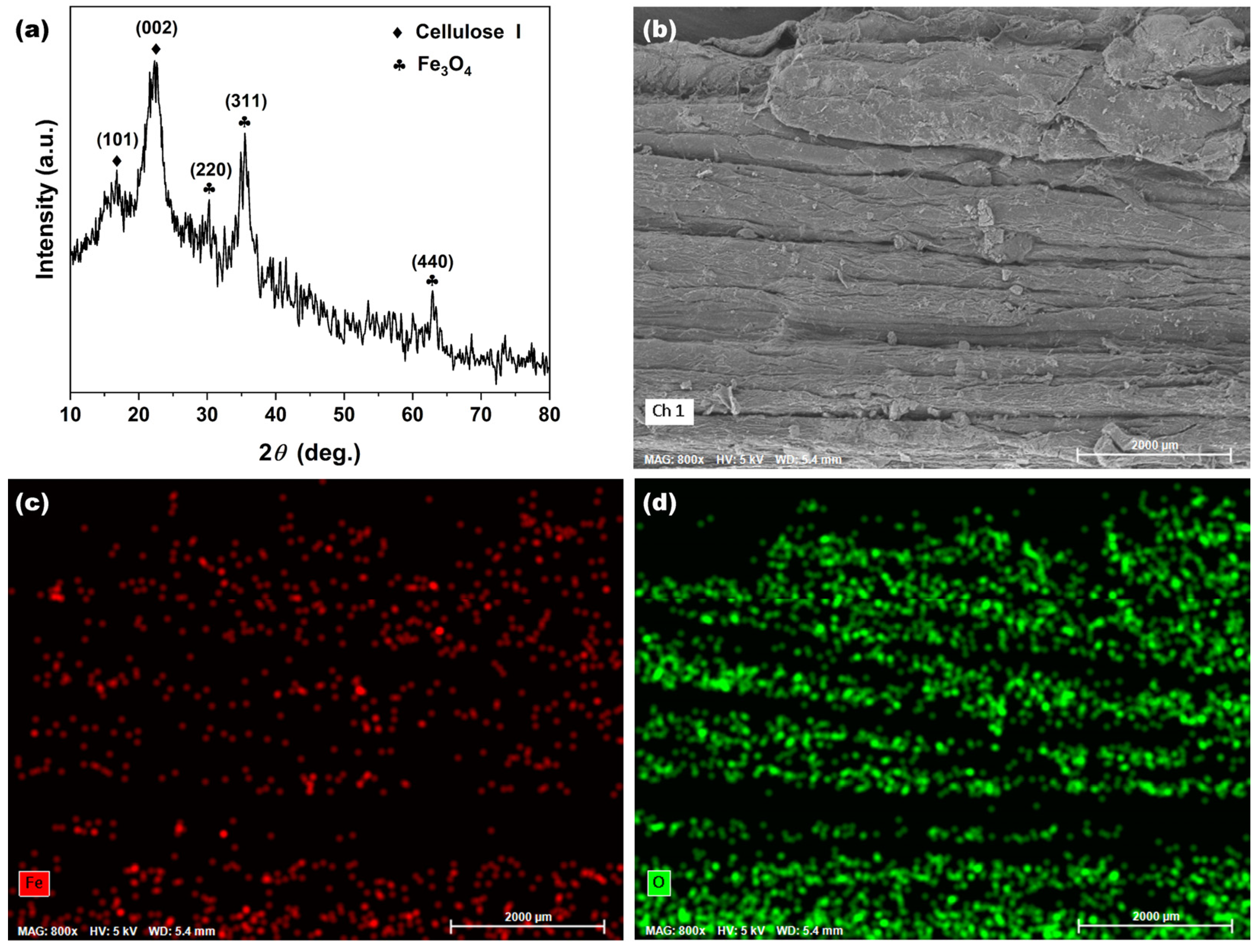
2.1.1. Phase Characterization
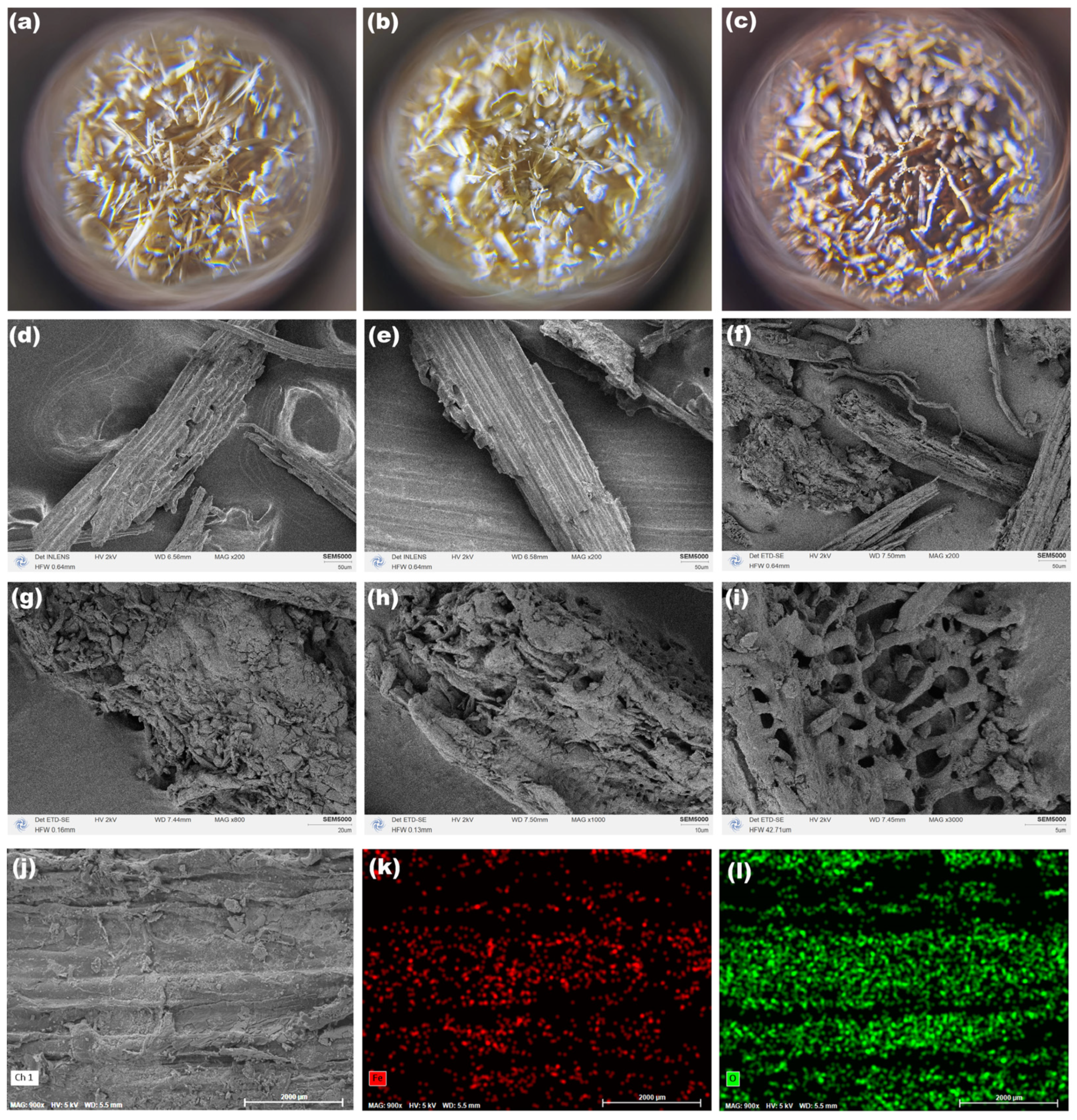
2.1.2. Morphology Characterization
2.2. Magnetic Response Behaviors of BPs/Fe3O4 Composites
2.3. Adsorption Results
2.3.1. Effect of Contact Time
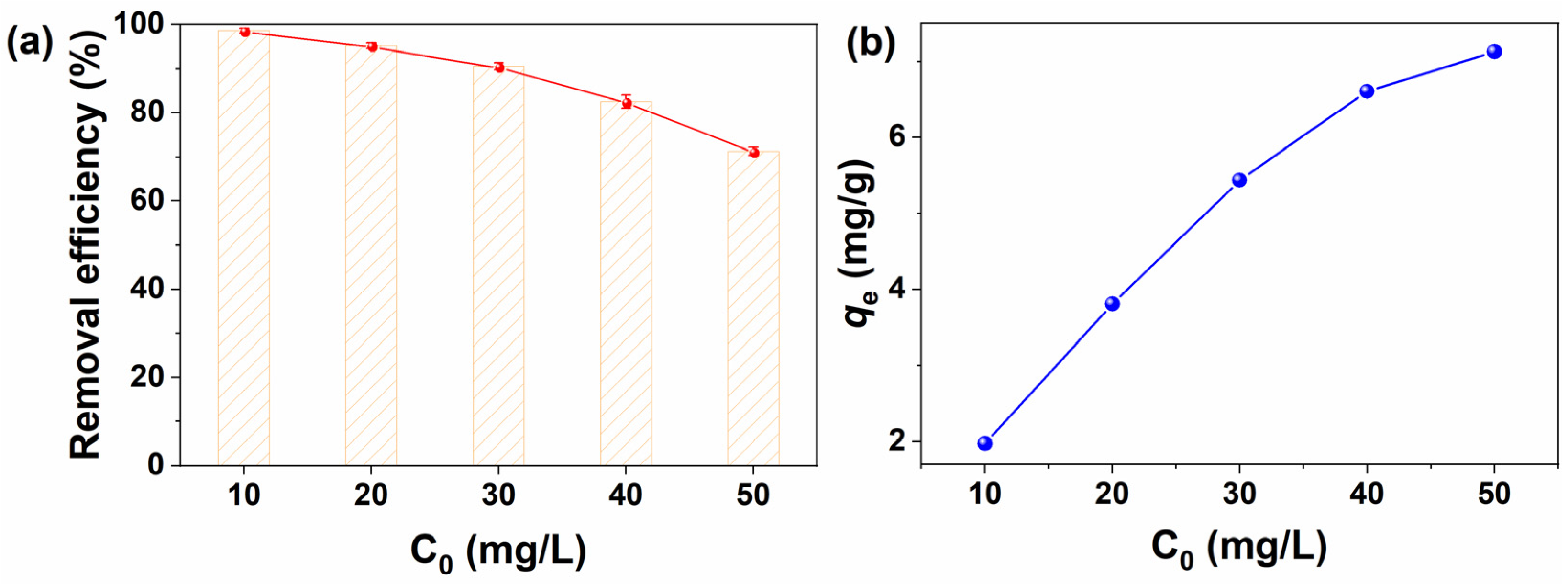
2.3.2. Effect of Initial MB Concentration
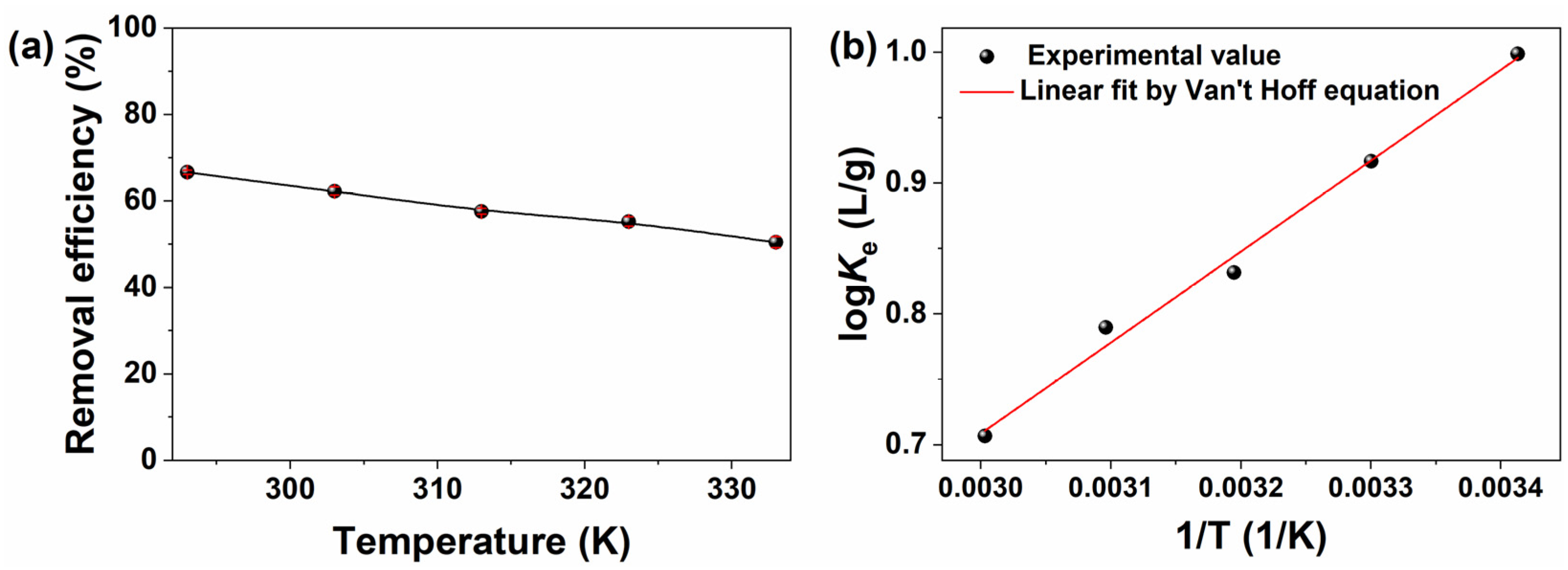
2.3.3. Effect of Temperature
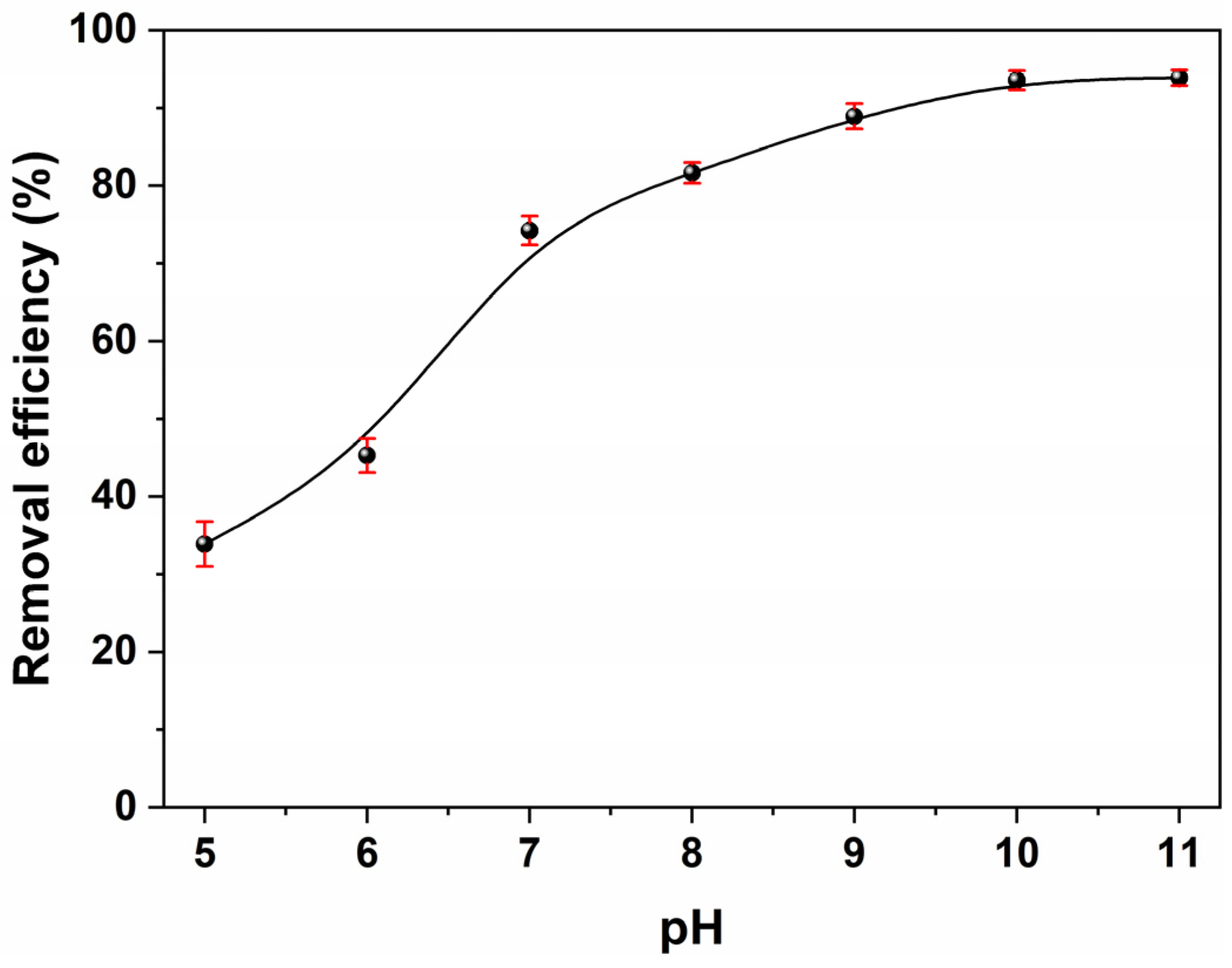
2.3.4. Effect of Solution pH
2.4. Adsorption Isotherms
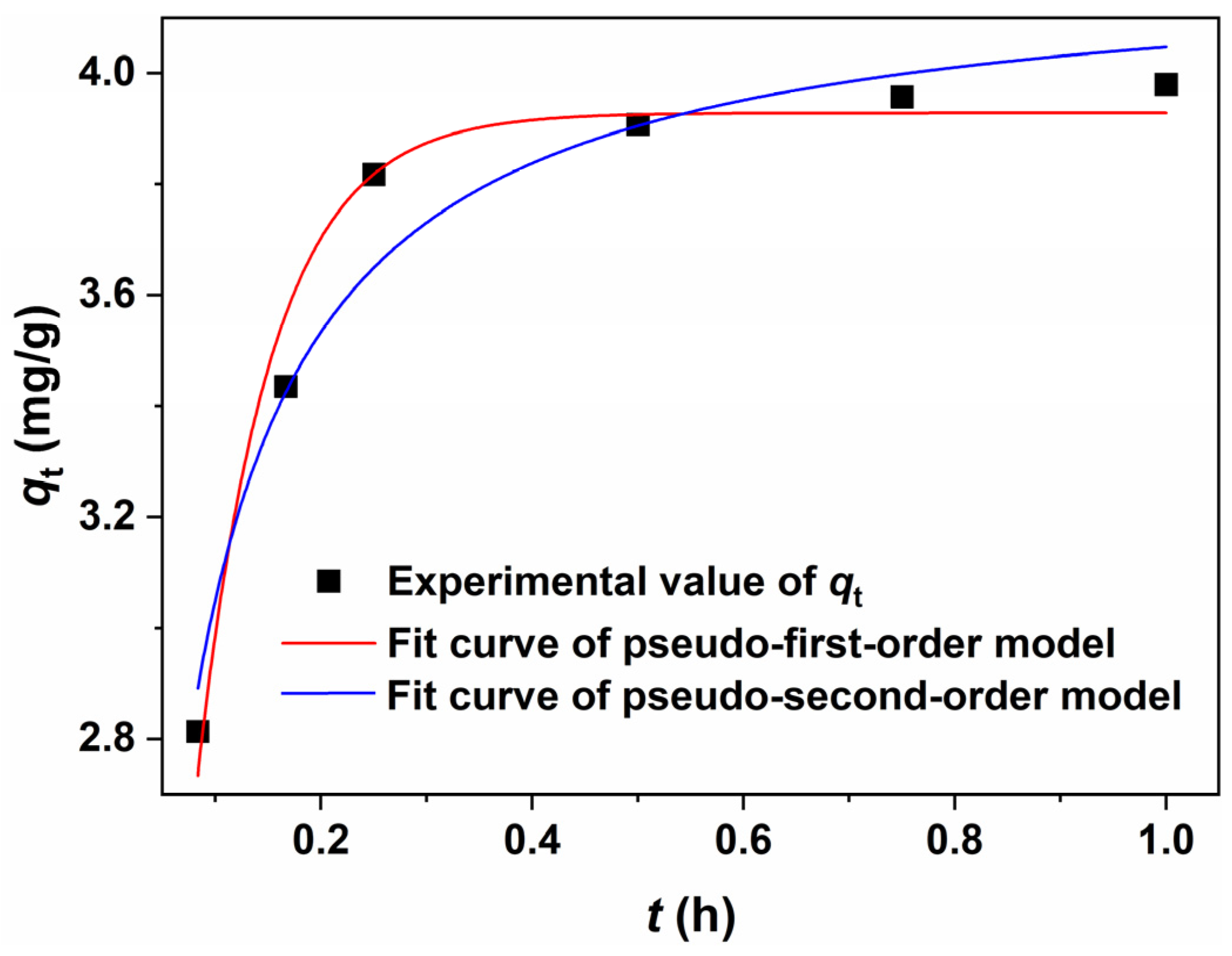
2.5. Adsorption Kinetics
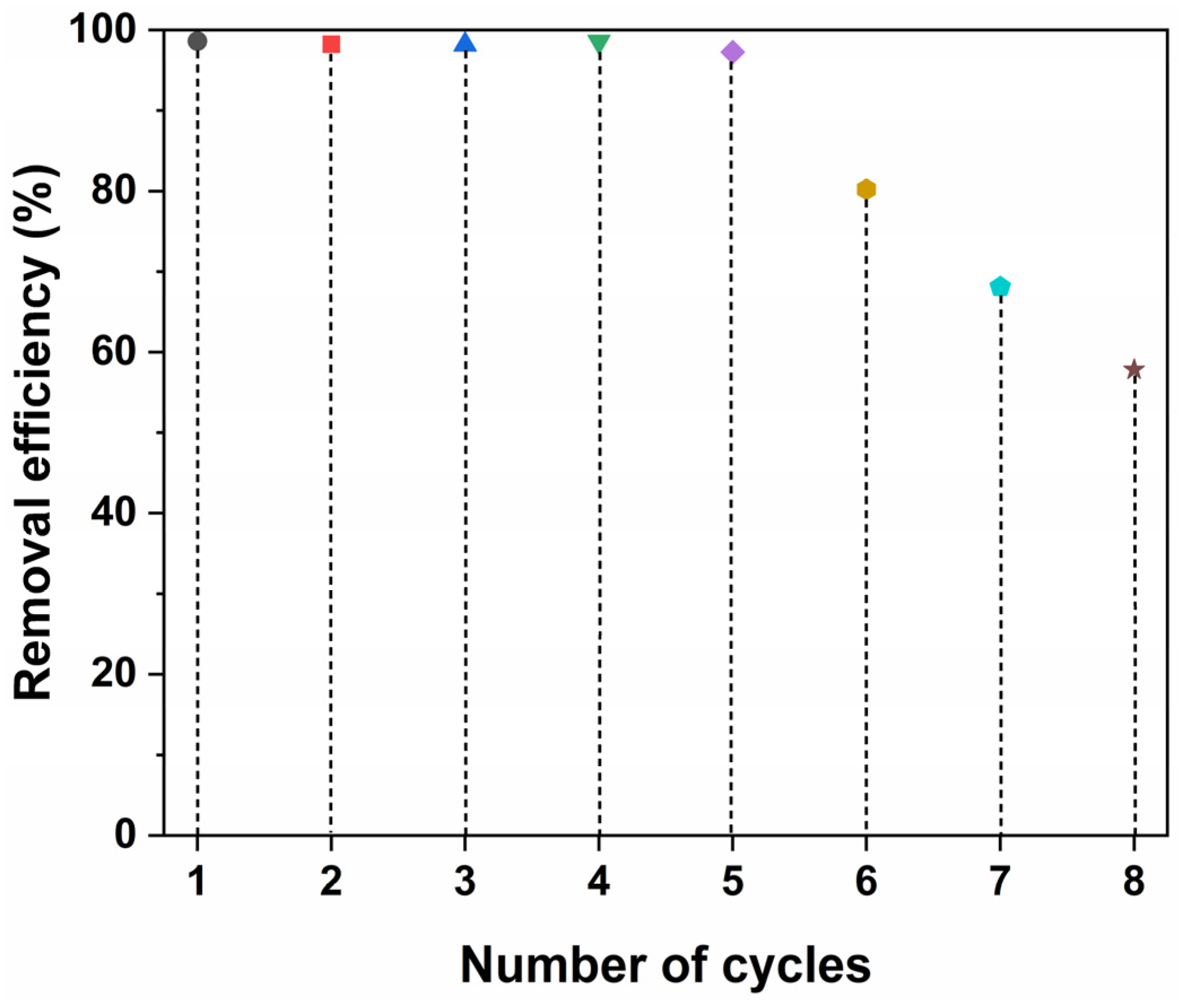
2.6. Regeneration
3. Experimental Procedure
3.1. Starting Materials
3.2. Synthesis
3.2.1. Pre-Treatment of BPs
3.2.2. Synthesis of BPs/Fe3O4 Composites
3.3. Characterization
3.4. Batch Adsorption Experiments
3.5. Data Analysis
3.6. Desorption and Reusability Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, S.; Chauhan, M.S. Treatment of Dye Wastewater from Textile Industry by Electrocoagulation and Fenton Oxidation: A Review. Water Qual. Manag. 2018, 79, 117–129. [Google Scholar] [CrossRef]
- Chiang, T.L.; Wang, Y.C.; Ding, W.H. Trace Determination of Rhodamine B and Rhodamine 6G Dyes in Aqueous Samples by Solid-phase Extraction and High-performance Liquid Chromatography Coupled with Fluorescence Detection. J. Chin. Chem. Soc. 2012, 59, 515–519. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Krafts, K.; Hempelmann, E.; Skórska-Stania, A. From methylene blue to chloroquine: A brief review of the development of an antimalarial therapy. Parasitol. Res. 2012, 111, 1–6. [Google Scholar] [CrossRef]
- Fransiska, D.; Jeo, W.S.; Moenadjat, Y.; Friska, D. Methylene Blue Effectiveness as Local Analgesic after Anorectal Surgery: A Literature Review. Adv. Med. 2017, 2017, 3968278. [Google Scholar] [CrossRef]
- Arasi, M.A.; Salem, A.; Salem, S. Extraction of nano-porous silica from hydrosodalite produced via modification of low grade kaolin for removal of methylene blue from wastewater. J. Chem. Technol. Biotechnol. 2020, 95, 1989–2000. [Google Scholar] [CrossRef]
- Orlando, R.M.; Dvoák, M.; Kubáň, P. Electroextraction of methylene blue from aqueous environmental samples using paper points coupled with hollow fiber membranes. Talanta 2024, 273, 125849. [Google Scholar] [CrossRef]
- Deba, S.A.H.; Wols, B.; Yntema, D.; Lammertink, R. Transport and surface reaction model of a photocatalytic membrane during the radical filtration of methylene blue. Chem. Eng. Sci. 2022, 254, 117617. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, M.; Piao, H.; Zuo, S.; Shi, X.; Quan, Q.; Zhu, R.; Huang, Q.; Xiao, C. ZnS/Ag2S decorated PES membrane with efficient near-infrared response and enhanced photocatalysis for pollutants photodegradation on high-turbidity water. Appl. Surf. Sci. 2023, 635, 157728. [Google Scholar] [CrossRef]
- Moharramzadeh, S.; Baghdadi, M. In situ sludge magnetic impregnation (ISSMI) as an efficient technology for enhancement of sludge sedimentation: Removal of methylene blue using nitric acid treated graphene oxide as a test process. J. Environ. Chem. Eng. 2016, 4, 2090–2102. [Google Scholar] [CrossRef]
- Kereszturi, É.; Sahin-Tóth, M. Intracellular Autoactivation of Human Cationic Trypsinogen Mutants Causes Reduced Trypsinogen Secretion and Acinar Cell Death. J. Biol. Chem. 2009, 284, 33392–33399. [Google Scholar] [CrossRef] [PubMed]
- Sawate, A.; Paul, N.; Sathe, B.; Katayama, T.; Furube, A.; Koinkar, P. Fabrication of MoO3/rGO/Au composite for increased photocatalytic degradation of methylene blue. Int. J. Mod. Phys. B 2024, 38, 2440010. [Google Scholar] [CrossRef]
- Tang, D.; Zhao, R.; Li, F.; Chen, T.; Tang, Y. Surface modification of bamboo-based activated carbon for methylene blue removal. Pap. Biomater. 2023, 8, 12–25. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, H.; Zhang, Y.; Li, C.; Zhang, Q.; Hu, G.; Li, Z. Carbon/Mn3O4/SrTiO3 microsphere photocatalyst for the efficient removal of high-concentration methylene blue from water. J. Chem. Technol. Biotechnol. 2023, 98, 1453–1464. [Google Scholar] [CrossRef]
- Mani, D.; Elango, D.; Priyadharsan, A.; Al-Humaid, L.; Al-Dahmash, N.D.; Ragupathy, S.; Jayanthi, P.; Ahn, Y. Groundnut shell chemically treated with KOH to prepare inexpensive activated carbon: Methylene blue adsorption and equilibrium isotherm studies. Environ. Res. 2023, 231, 116026. [Google Scholar] [CrossRef]
- Khan, T.A.; Nazir, M.; Khan, E.A. Adsorptive removal of rhodamine B from textile wastewater using water chestnut (Trapa natans L.) peel: Adsorption dynamics and kinetic studies. Toxicol. Environ. Chem. 2013, 95, 919–931. [Google Scholar] [CrossRef]
- Dahri, M.K.; Lim, L.B.L.; Mei, C.C. Cempedak durian as a potential biosorbent for the removal of Brilliant Green dye from aqueous solution: Equilibrium, thermodynamics and kinetics studies. Environ. Monit. Assess. 2015, 187, 546. [Google Scholar] [CrossRef]
- Wazir, A.H.; Waseem, I.; Qureshi, I.; Manan, A. Saccharum Arundinaceum Leaves as a Versatile Biosorbent for Removal of Methylene Blue Dye from Wastewater. Environ. Eng. Sci. 2020, 37, 737–745. [Google Scholar] [CrossRef]
- Khorramfar, S.; Mahmoodi, N.M.; Arami, M.; Gharanjig, K. Equilibrium and kinetic studies of the cationic dye removal capability of a novel biosorbent Tamarindus indica from textile wastewater. Color. Technol. 2010, 126, 261–268. [Google Scholar] [CrossRef]
- Venkata Rao, P.; Pydiraju, P.; Madhuri, V.; Vineeth, S.; Rahimuddin, S.; Vangalapati, M. Removal of indigo carmine dye from aqueous solution by adsorption on biomass of Grevillea Robusta leaves. Mater. Today Proc. 2020, 26, 3020–3023. [Google Scholar] [CrossRef]
- Mullick, A.; Neogi, S. Synthesis of potential biosorbent from used stevia leaves and its application for malachite green removal from aqueous solution: Kinetics, isotherm and regeneration studies. RSC Adv. 2016, 6, 65960–65975. [Google Scholar] [CrossRef]
- Gunturu, B.; Ramakrishnan, G.; Sahadevan, R. Equilibrium and Isotherm Studies on the Removal of Basic Textile Dye from Aqueous Solutions Using Kigelia africana Biosorbent. Appl. Mech. Mater. 2018, 877, 26–32. [Google Scholar] [CrossRef]
- Enache, A.C.; Cojocaru, C.; Samoila, P.; Ciornea, V.; Apolzan, R.; Predeanu, G.; Harabagiu, V. Adsorption of Brilliant Green Dye onto a Mercerized Biosorbent: Kinetic, Thermodynamic, and Molecular Docking Studies. Molecules 2023, 28, 4129. [Google Scholar] [CrossRef]
- Chen, X.; Lam, K.F.; Mak, S.F.; Ching, W.K.; Ng, T.N.; Yeung, K.L. Assessment of Sericin Biosorbent for Selective Dye Removal. Chin. J. Chem. Eng. 2012, 20, 426–432. [Google Scholar] [CrossRef]
- Jain, R.; Sikarwar, S. Removal of hazardous dye congored from waste material. J. Hazard. Mater. 2008, 152, 942–948. [Google Scholar] [CrossRef]
- Da Fontoura, J.T.; Rolim, G.S.; Mella, B.; Farenzena, M.; Gutterres, M. Defatted microalgal biomass as biosorbent for the removal of Acid Blue 161 dye from tannery effluent. J. Environ. Chem. Eng. 2017, 5, 5076–5084. [Google Scholar] [CrossRef]
- Hong, C.; Fang, J.; Jin, A.; Cai, J.; Guo, H.; Ren, J.; Shao, Q.; Zheng, B. Comparative Growth, Biomass Production and Fuel Properties Among Different Perennial Plants, Bamboo and Miscanthus. Bot. Rev. 2011, 77, 197–207. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon sequestration by Chinese bamboo forests and their ecological benefits: Assessment of potential, problems, and future challenges. Environ. Rev. 2011, 19, 418–428. [Google Scholar] [CrossRef]
- Wegst, U.G.K. Bamboo and Wood in Musical Instruments. Annu. Rev. Mater. Res. 2008, 38, 323–349. [Google Scholar] [CrossRef]
- Jia, Z.; Kou, K.; Qin, M.; Wu, H.; Puleo, F.; Liotta, L. Controllable and Large-Scale Synthesis of Carbon Nanostructures: A Review on Bamboo-Like Nanotubes. Catalysts 2017, 7, 256. [Google Scholar] [CrossRef]
- Peng, P.; She, D. Isolation, structural characterization, and potential applications of hemicelluloses from bamboo: A review. Carbohydr. Polym. 2014, 112, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.S.; Sasai, R. Arsenate removal from water using Fe3O4-loaded activated carbon prepared from waste biomass. Chem. Eng. J. 2010, 160, 57–62. [Google Scholar] [CrossRef]
- Zahoor, M.; Ullah, A.; Alam, S.; Muhammad, M.; Hendroko Setyobudi, R.; Zekker, I.; Sohail, A. Novel Magnetite Nanocomposites (Fe3O4/C) for Efficient Immobilization of Ciprofloxacin from Aqueous Solutions through Adsorption Pretreatment and Membrane Processes. Water 2022, 14, 724. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Yun, Y.; Lilliestråle, M.; Hedin, N. Iron Oxide Nanoparticles Embedded in Activated Carbons Prepared from Hydrothermally Treated Waste Biomass. ChemSusChem 2014, 7, 875–882. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Yang, F.; Wang, W. Study on the Preparation Process and Performance of a Conductive, Flexible, and Transparent Wood. J. Mater. Res. Technol. 2021, 15, 5396–5404. [Google Scholar] [CrossRef]
- Yu, H.; Gui, C.; Ji, Y.; Li, X.; Rao, F.; Huan, W.; Li, L. Changes in Chemical and Thermal Properties of Bamboo after Delignification Treatment. Polymers 2022, 14, 2573. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, L.; Zhou, Y. Synthesis of trifunctional inorganic/organic hybrid nanocomposites and their applications for recognition and elimination of heavy metal ions. Appl. Surf. Sci. 2022, 605, 154659. [Google Scholar] [CrossRef]
- Abdallah, R.; Taha, S. Biosorption of methylene blue from aqueous solution by nonviable Aspergillus fumigatus. Chem. Eng. J. 2012, 195–196, 69–76. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Nunell, G.V.; Bonelli, P.R.; Cukierman, A.L. Batch and dynamic biosorption of basic dyes from binary solutions by alkaline-treated cypress cone chips. Bioresour. Technol. 2012, 106, 55–62. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Ding, Z. Network-Polymer-Modified Superparamagnetic Magnetic Silica Nanoparticles for the Adsorption and Regeneration of Heavy Metal Ions. Molecules 2023, 28, 7385. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Morad, N.; Tan, K.A. Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel(II) from aqueous solution. J. Hazard. Mater. 2011, 186, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian Pirbazari, A.; Saberikhah, E.; Gholami Ahmad Gorabi, N. Fe3O4 nanoparticles loaded onto wheat straw: An efficient adsorbent for Basic Blue 9 adsorption from aqueous solution. Desalin. Water Treat. 2014, 57, 4110–4121. [Google Scholar] [CrossRef]
- Zang, Y.; Hang, N.; Sui, J.; Duan, S.; Zhao, W.; Tao, J.; Li, S. Magnetic Persimmon Leaf Composite: Preparation and Application in Magnetic Solid-Phase Extraction of Pesticides in Water Samples. Molecules 2024, 29, 45. [Google Scholar] [CrossRef]
- Nasrullah, A.; Khan, H.; Khan, A.S.; Man, Z.; Muhammad, N.; Khan, M.I.; Abd El-Salam, N.M. Potential Biosorbent Derived from Calligonum polygonoides for Removal of Methylene Blue Dye from Aqueous Solution. Sci. World J. 2015, 2015, 562693. [Google Scholar] [CrossRef]
- Hameed, B.H. Removal of cationic dye from aqueous solution using jackfruit peel as non-conventional low-cost adsorbent. J. Hazard. Mater. 2009, 162, 344–350. [Google Scholar] [CrossRef]
- Uddin, M.T.; Rukanuzzaman, M.; Khan, M.M.R.; Islam, A. Jackfruit (Artocarpus heterophyllus) leaf powder: An effective adsorbent for removal of methylene blue from aqueous solutions. Indian J. Chem. Techn. 2009, 16, 142–149. [Google Scholar] [CrossRef]
- Khattri, S.D.; Singh, M.K. Removal of malachite green from dye wastewater using neem sawdust by adsorption. J. Hazard. Mater. 2009, 167, 1089–1094. [Google Scholar] [CrossRef]
- Hameed, B.H.; Mahmoud, D.K.; Ahmad, A.L. Sorption of basic dye from aqueous solution by pomelo (Citrus grandis) peel in a batch system. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 78–84. [Google Scholar] [CrossRef]
- Nasuha, N.; Hameed, B.H. Adsorption of methylene blue from aqueous solution onto NaOH-modified rejected tea. Chem. Eng. J. 2011, 166, 783–786. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A.; Abou El-Reash, Y.G. Ion-imprinted modified chitosan resin for selective removal of Pd(II) ions. J. Colloid Interface Sci. 2016, 469, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Abdel Salam, M. Removal of heavy metal ions from aqueous solutions with multi-walled carbon nanotubes: Kinetic and thermodynamic studies. Int. J. Environ. Sci. Technol. 2012, 10, 677–688. [Google Scholar] [CrossRef]
- Yadav, S.; Srivastava, V.; Banerjee, S.; Gode, F.; Sharma, Y.C. Studies on the removal of nickel from aqueous solutions using modified riverbed sand. Environ. Sci. Pollut. Res. 2012, 20, 558–567. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Cengiz, S.; Cavas, L. Removal of methylene blue by invasive marine seaweed: Caulerpa racemosa var. cylindracea. Bioresour. Technol. 2008, 99, 2357–2363. [Google Scholar] [CrossRef]
- Garg, V.; Amita, M.; Kumar, R.; Gupta, R. Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: A timber industry waste. Dye. Pigment. 2004, 63, 243–250. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dye. Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Azizian, S.; Eris, S.; Wilson, L.D. Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem. Phys. 2018, 513, 99–104. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. J. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Tempkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys.-Chim. Sin. 1940, 12, 327–356. [Google Scholar]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Xu, S.; Xiao, H.; Jiang, X.; Liu, L.; Cao, M.; Wang, Z. Exploring of toxic Pb(II) removal by low-cost bio-adsorbent of camphor leaf forestry waste after camphor oil extraction. Environ. Sci. Pollut. Res. 2020, 27, 43625–43637. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.; Rodriguez, P.; Herrero, R.; Cremades, J.; Barbara, I.; Sastre de Vicente, M.E. Removal of Methylene Blue from aqueous solutions using as biosorbent Sargassum muticum: An invasive macroalga in Europe. J. Chem. Technol. Biotechnol. 2005, 80, 291–298. [Google Scholar] [CrossRef]
- Lim, L.B.L.; Priyantha, N.; Hei Ing, C.; Khairud Dahri, M.; Tennakoon, D.T.B.; Zehra, T.; Suklueng, M. Artocarpus odoratissimus skin as a potential low-cost biosorbent for the removal of methylene blue and methyl violet 2B. Desalin. Water Treat. 2015, 53, 964–975. [Google Scholar] [CrossRef]
- Saha, P. Assessment on the Removal of Methylene Blue Dye using Tamarind Fruit Shell as Biosorbent. Water Air Soil Pollut. 2010, 213, 287–299. [Google Scholar] [CrossRef]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium Isotherm Studies for the Sorption of Divalent Metal Ions onto Peat: Copper, Nickel and Lead Single Component Systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]






| Temperature (K) | Thermodynamic Parameters | ||
|---|---|---|---|
| △G0 (KJ/mol) | △H0 (KJ/mol) | △S0 (J/mol·K) | |
| 293 | –5.602 | –13.299 | –26.327 |
| 303 | –5.317 | ||
| 313 | –4.983 | ||
| 323 | –4.883 | ||
| 333 | –4.505 | ||
| Adsorption Isotherm | Langmuir | Freundlich | Temkin | Dubinin–Radushkevich | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | qm | KL | R2 | RSS | KF | n | R2 | RSS | KT | bT | R2 | RSS | qm | β | R2 | RSS |
| 7.46 | 1.28 | 0.9973 | 0.0047 | 3.70 | 3.52 | 0.9697 | 0.0050 | 1.00 | 2.15 | 0.9883 | 0.1558 | 5.86 | 4.2 × 10−8 | 0.8199 | 0.1489 | |
| Kinetic Model | Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | qe | k1 | R2 | RSS | qe | k2 | R2 | RSS |
| 195.98 | 0.0049 | 0.4608 | 1.3 × 10−5 | 4.1145 | 8.0584 | 0.9996 | 2.2 × 10−6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhou, Y.; Zhou, Y.; Wu, P.; Gao, L.; Ding, Z. Synthesis of Magnetic Biosorbent from Bamboo Powders and Their Application for Methylene Blue Removal from Aqueous Solution: Kinetics, Isotherm, and Regeneration Studies. Molecules 2025, 30, 1320. https://doi.org/10.3390/molecules30061320
Xu Y, Zhou Y, Zhou Y, Wu P, Gao L, Ding Z. Synthesis of Magnetic Biosorbent from Bamboo Powders and Their Application for Methylene Blue Removal from Aqueous Solution: Kinetics, Isotherm, and Regeneration Studies. Molecules. 2025; 30(6):1320. https://doi.org/10.3390/molecules30061320
Chicago/Turabian StyleXu, Yaohui, Yang Zhou, Yunxuan Zhou, Pingkeng Wu, Liangjuan Gao, and Zhao Ding. 2025. "Synthesis of Magnetic Biosorbent from Bamboo Powders and Their Application for Methylene Blue Removal from Aqueous Solution: Kinetics, Isotherm, and Regeneration Studies" Molecules 30, no. 6: 1320. https://doi.org/10.3390/molecules30061320
APA StyleXu, Y., Zhou, Y., Zhou, Y., Wu, P., Gao, L., & Ding, Z. (2025). Synthesis of Magnetic Biosorbent from Bamboo Powders and Their Application for Methylene Blue Removal from Aqueous Solution: Kinetics, Isotherm, and Regeneration Studies. Molecules, 30(6), 1320. https://doi.org/10.3390/molecules30061320









