Development of New Dual-Purpose Environmental Strategies for Effective Antibiotic Degradation Using Red Mud-Based Fenton Oxidation Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Red Mud
2.2. Material Synthesis
3. Results and Discussion
3.1. Catalyst Characterization
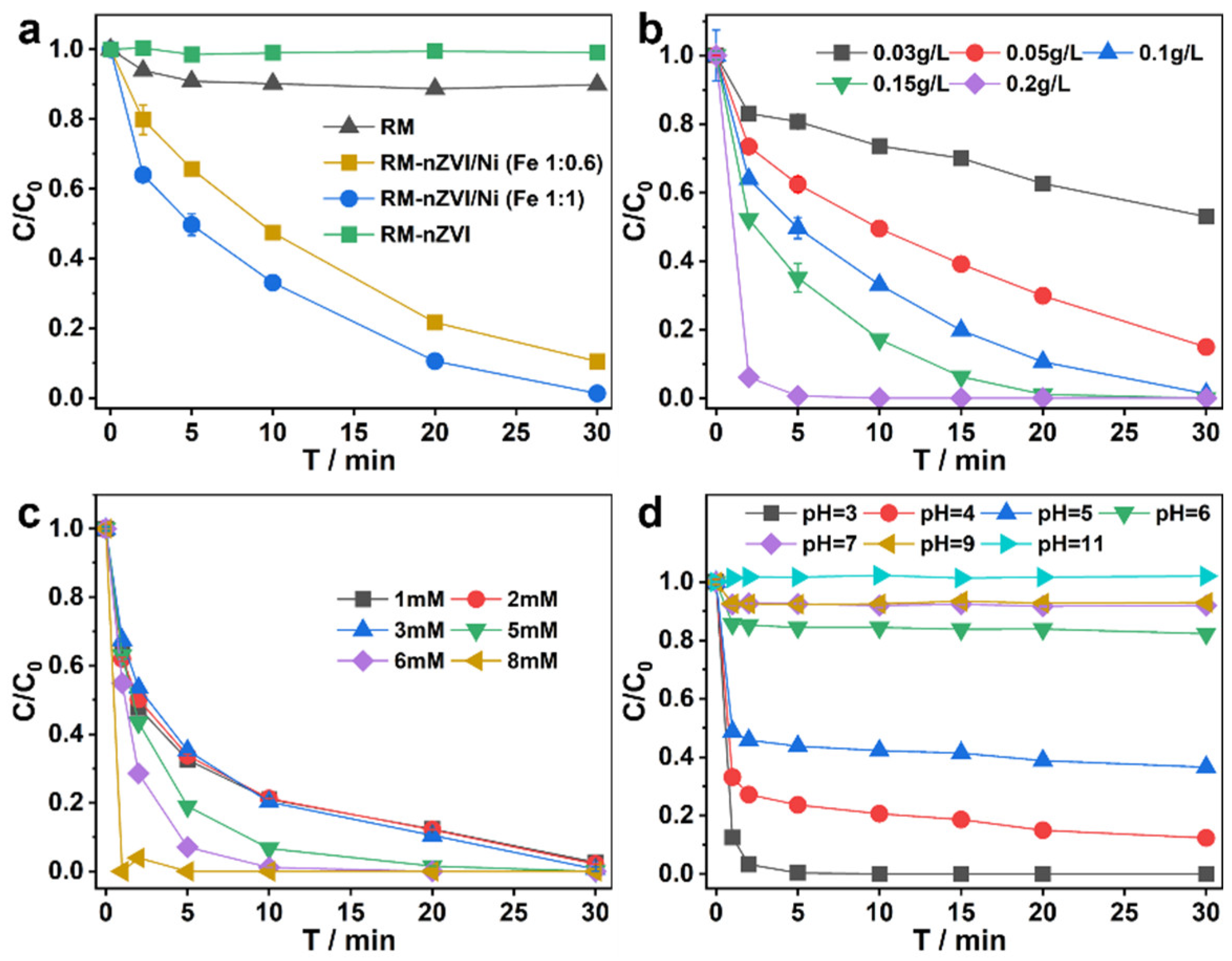
3.2. Assessment of the Performance of the RM-nZVI/Ni/H2O2 System
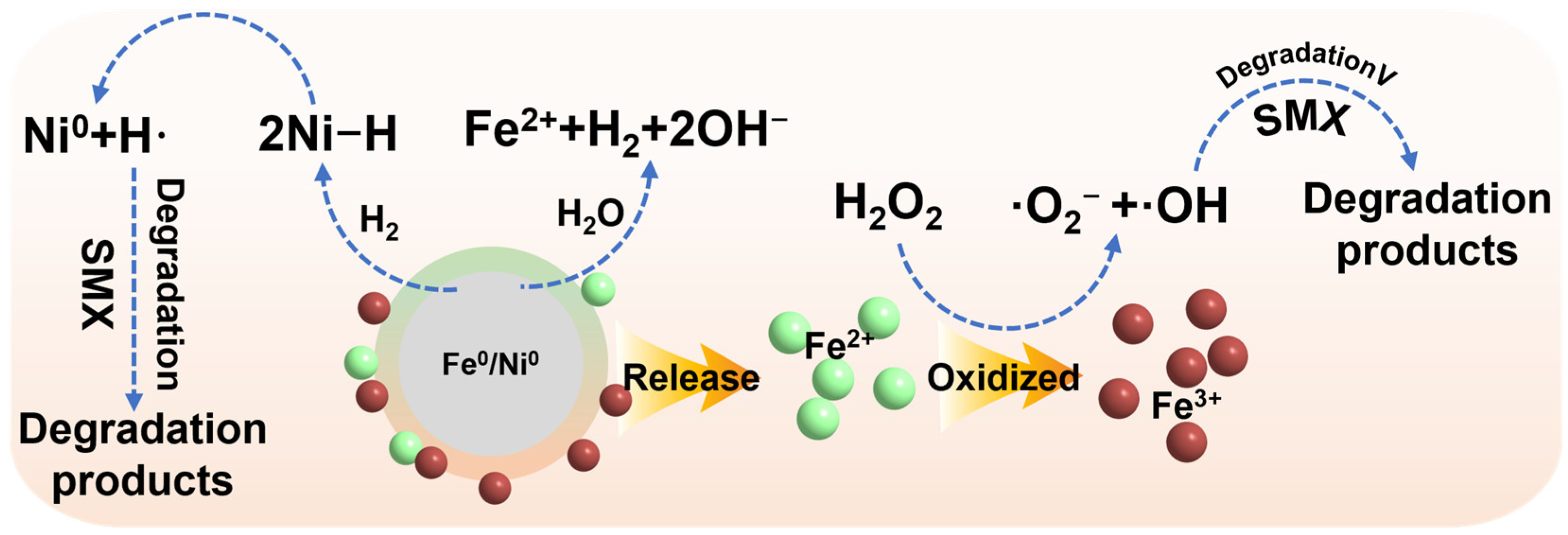
3.3. Removal Mechanism of SMX in the Reaction System
3.3.1. Identification of Key Reactive Oxygen Species (ROS) in the RM-nZVI/Ni/H2O2 System
3.3.2. Changes in Metal Oxidation States in the RM-nZVI/Ni/H2O2 System
3.4. Evaluation of the Practical Application Effect of the RM-nZVI/Ni/H2O2 System
3.4.1. Impact of Inorganic Anions
3.4.2. Practical Evaluation
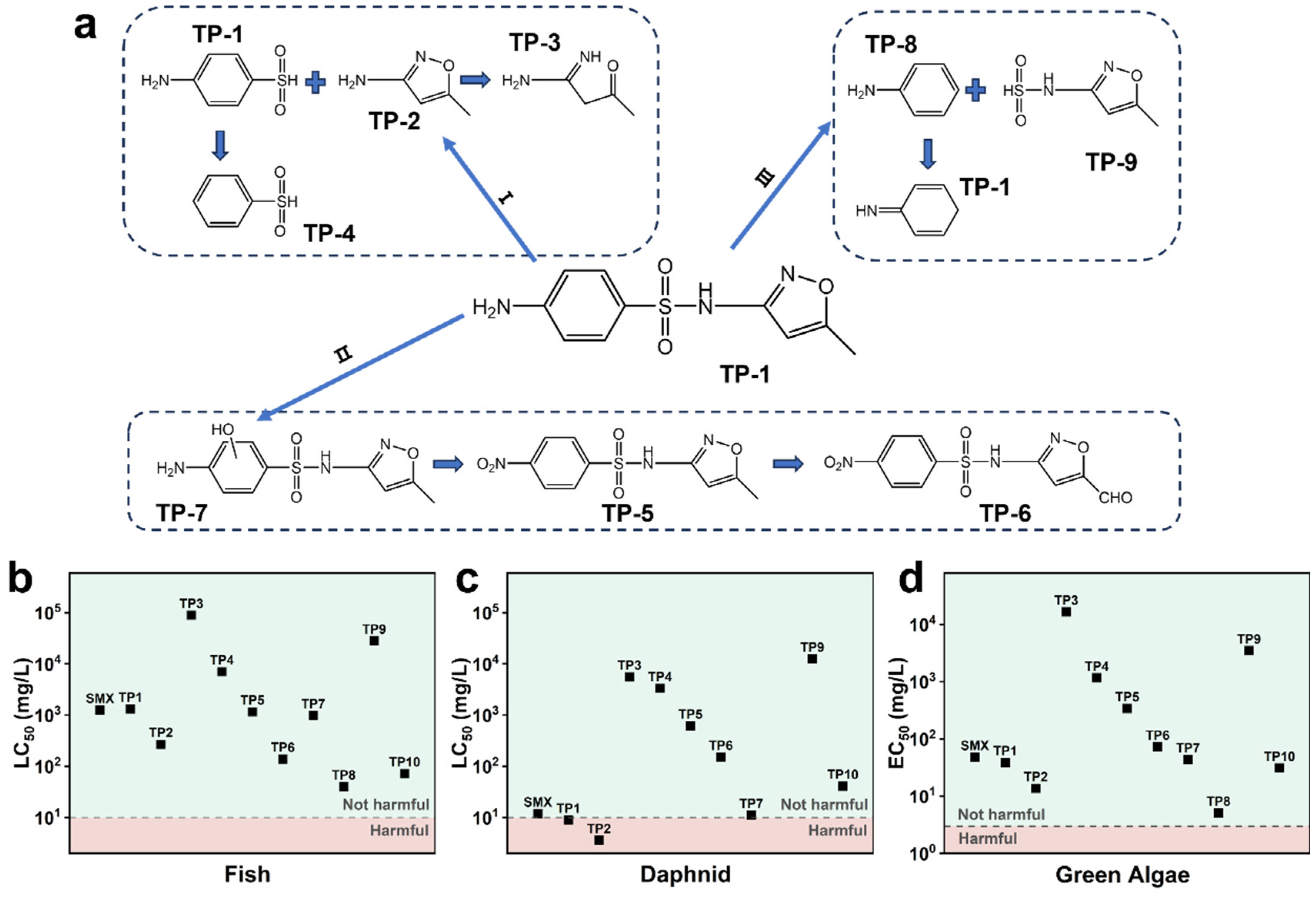
3.5. Degradation Pathway and Ecotoxicity Assessment
3.6. Cost Estimation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Jiang, L.; Zhai, W.; Wang, J.; Li, G.; Zhou, Z.; Li, B.; Zhuo, H. Antibiotics and antibiotic resistance genes in the water sources of the Wuhan stretch of the Yangtze River: Occurrence, distribution, and ecological risks. Environ. Res. 2023, 239, 117295. [Google Scholar] [CrossRef]
- Kamanmalek, S.; Rice-Boayue, J. Development of a national antibiotic multimetric index for identifying watersheds vulnerable to antibiotic pollution. Environ. Pollut. 2023, 339, 122670. [Google Scholar] [CrossRef]
- Li, Y.W.; Wu, X.L.; Mo, C.H.; Tai, Y.P.; Huang, X.P.; Xiang, L. Investigation of Sulfonamide, Tetracycline, and Quinolone Antibiotics in Vegetable Farmland Soil in the Pearl River Delta Area, Southern China. J. Agric. Food. Chem. 2011, 59, 7268–7276. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, T.; Cheng, F.; Yang, H.; Guo, Z.; Zhao, S.; Zhang, Y.-N.; Qu, J. Comprehensive analysis and risk assessment of Antibiotic contaminants, antibiotic-resistant bacteria, and resistance genes: Patterns, drivers, and implications in the Songliao Basin. Environ. Pollut. 2024, 361, 124852. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Wu, Y.; Li, S.; Hu, J.; Sun, W.; Ni, J. Profiles, drivers, and prioritization of antibiotics in China’s major rivers. J. Hazard. Mater. 2024, 477, 135399. [Google Scholar] [CrossRef]
- Li, S.; Zhu, L. Copper regulates degradation of typical antibiotics by microalgal-fungal consortium in simulated swine wastewater: Insights into metabolic routes and dissolved organic matters. Water Res. 2023, 245, 120654. [Google Scholar] [CrossRef]
- Li, N.; Sheng, G.P.; Lu, Y.Z.; Zeng, R.J.; Yu, H.Q. Removal of antibiotic resistance genes from wastewater treatment plant effluent by coagulation. Water Res. 2017, 111, 204–212. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhang, J.; Zhao, X.D.; Fu, H.F.; Chu, C.; Wang, P.; Wang, C.C. Visible-Light-Triggered Release of Sulfonamides in MOF/Ag-Based Nanoparticle Composites: Performance, Mechanism, and DFT Calculations. ACS Appl. Nano Mater. 2019, 2, 418–428. [Google Scholar] [CrossRef]
- Francisco-Márquez, M.; Soriano-Correa, C.; Sainz-Díaz, C.I. Adsorption of Sulfonamides on Phyllosilicate Surfaces by Molecular Modeling Calculations. J. Phys. Chem. C 2017, 121, 2905–2914. [Google Scholar] [CrossRef]
- Qin, C.; Yang, Y.; Wu, X.; Chen, L.; Liu, Z.; Tang, L.; Lyu, L.; Huang, D.; Wang, D.; Zhang, C.; et al. Twistedly hydrophobic basis with suitable aromatic metrics in covalent organic networks govern micropollutant decontamination. Nat. Commun. 2023, 14, 6740. [Google Scholar] [CrossRef]
- Tang, M.; Wan, J.; Wang, Y.; Ye, G.; Yan, Z.; Ma, Y.; Sun, J. Overlooked role of void-nanoconfined effect in emerging pollutant degradation: Modulating the electronic structure of active sites to accelerate catalytic oxidation. Water Res. 2024, 249, 120950. [Google Scholar] [CrossRef]
- Xue, W.; Shi, X.; Guo, J.; Wen, S.; Lin, W.; He, Q.; Gao, Y.; Wang, R.; Xu, Y. Affecting factors and mechanism of removing antibiotics and antibiotic resistance genes by nano zero-valent iron (nZVI) and modified nZVI: A critical review. Water Res. 2024, 253, 121309. [Google Scholar] [CrossRef]
- Chen, A.Q.; Wang, H.R.; Zhan, X.P.; Gong, K.L.; Xie, W.W.; Liang, W.Y.; Zhang, W.; Peng, C. Applications and synergistic degradation mechanisms of nZVI-modified biochar for the remediation of organic polluted soil and water: A review. Sci. Total Environ. 2024, 911, 168548. [Google Scholar] [CrossRef]
- McKone, J.R.; Sadtler, B.F.; Werlang, C.A.; Lewis, N.S.; Gray, H.B. Ni–Mo Nanopowders for Efficient Electrochemical Hydrogen Evolution. ACS Catal. 2013, 3, 166–169. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Z.; Gao, S.; Su, J.; Lun, Z.; Xia, G.; Chen, J.; Zhang, R.; Chen, Q. Tuning Electronic Structures of Nonprecious Ternary Alloys Encapsulated in Graphene Layers for Optimizing Overall Water Splitting Activity. ACS Catal. 2017, 7, 469–479. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Bimetallic Sites for Catalysis: From Binuclear Metal Sites to Bimetallic Nanoclusters and Nanoparticles. Chem. Rev. 2023, 123, 4855–4933. [Google Scholar] [CrossRef]
- Li, R.; Zhang, T.; Liu, Y.; Lv, G.; Xie, L. Calcification–carbonation method for red mud processing. J. Hazard. Mater. 2016, 316, 94–101. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Bandopadhyay, A. Innovative methodologies for the utilisation of wastes from metallurgical and allied industries. Resour. Conserv. Recycl. 2006, 48, 301–314. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X. Applications of red mud as an environmental remediation material: A review. J. Hazard. Mater. 2021, 408, 124420. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Zhang, J.; Dai, T.; Yi, H.; Chen, F.; Zhou, M.; Hou, H. Multiple environmental risk assessments of heavy metals and optimization of sludge dewatering: Red mud–reed straw biochar combined with Fe2+ activated H2O2. J. Environ. Manag. 2022, 316, 115210. [Google Scholar] [CrossRef]
- Lu, C.; Gu, J.; Wei, G.; Ba, J.; Zhang, L.; Li, Z.; Pei, R.; Li, J.; Wei, J. Three-dimensional electro-Fenton degradation of ciprofloxacin catalyzed by CuO doped red mud particle electrodes: Influencing factors, possible degradation pathways and energy consumption. J. Environ. Chem. Eng. 2022, 10, 107737. [Google Scholar] [CrossRef]
- Ma, S.; Cheng, F.; Meng, J.; Ge, H.; Lu, P.; Song, T. Ni-enhanced red mud oxygen carrier for chemical looping steam methane reforming. Fuel Process. Technol. 2022, 230, 107204. [Google Scholar] [CrossRef]
- Subagyo, R.; Tehubijuluw, H.; Utomo, W.P.; Rizqi, H.D.; Kusumawati, Y.; Bahruji, H.; Prasetyoko, D. Converting red mud wastes into mesoporous ZSM-5 decorated with TiO2 as an eco-friendly and efficient adsorbent-photocatalyst for dyes removal. Arab. J. Chem. 2022, 15, 103754. [Google Scholar] [CrossRef]
- Kamel, M.S.; Abd-Alla, M.H.; Abdul-Raouf, U.M. Characterization of anodic biofilm bacterial communities and performance evaluation of a mediator-free microbial fuel cell. Environ. Eng. Res. 2020, 25, 862–870. [Google Scholar] [CrossRef]
- Xu, K.; Lin, Q.; Fan, X.; Zheng, J.; Liu, Y.; Ma, Y.; He, J. Enhanced degradation of sulfamethoxazole by activation of peroxodisulfate with red mud modified biochar: Synergistic effect between adsorption and nonradical activation. Chem. Eng. J. 2023, 460, 141578. [Google Scholar] [CrossRef]
- Qi, L.; Sun, Z.; Tang, Q.; Wang, J.; Huang, T.; Sun, C.; Gao, F.; Tang, C.; Dong, L. Getting insight into the effect of CuO on red mud for the selective catalytic reduction of NO by NH3. J. Hazard. Mater. 2020, 396, 122459. [Google Scholar] [CrossRef]
- Li, Y.; Wei, G.; Shao, L.; Li, Z.; Yu, F.; Liu, J.; Yang, X.; Lu, Q.; Li, A.; Huang, Y.; et al. Green synthesis of red mud based ZnOFe2O3 composite used for photo-Fenton reaction under visible light. J. Clean. Prod. 2019, 207, 717–727. [Google Scholar] [CrossRef]
- Shi, W.; Ren, H.; Huang, X.; Li, M.; Tang, Y.; Guo, F. Low cost red mud modified graphitic carbon nitride for the removal of organic pollutants in wastewater by the synergistic effect of adsorption and photocatalysis. Sep. Purif. Technol. 2020, 237, 116477. [Google Scholar] [CrossRef]
- Ahmed, M.H.M.; Batalha, N.; Qiu, T.; Hasan, M.D.M.; Atanda, L.; Amiralian, N.; Wang, L.; Peng, H.; Konarova, M. Red-mud based porous nanocatalysts for valorisation of municipal solid waste. J. Hazard. Mater. 2020, 396, 122711. [Google Scholar] [CrossRef]
- Shi, W.; Ren, H.; Li, M.; Shu, K.; Xu, Y.; Yan, C.; Tang, Y. Tetracycline removal from aqueous solution by visible-light-driven photocatalytic degradation with low cost red mud wastes. Chem. Eng. J. 2020, 382, 122876. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, G.; Huang, B.; Cai, N.; Guo, P.; Chen, L. Preparation and application of a novel biochar-supported red mud catalyst: Active sites and catalytic mechanism. J. Hazard. Mater. 2021, 408, 124802. [Google Scholar] [CrossRef]
- Seyedi, M.S.; Sohrabi, M.R.; Motiee, F.; Mortazavinik, S. Synthesis and characterization of activated carbon@zerovalent iron–nickel nanoadsorbent for highly efficient removal of Reactive Orange 16 from aqueous sample: Experimental design, kinetic, isotherm and thermodynamic studies. Res. Chem. Intermed. 2020, 46, 1645–1662. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, F.; Wu, Y.; Naidu, R.; Chen, Z. Comparison of degradation mechanisms of microcystin-LR using nanoscale zero-valent iron (nZVI) and bimetallic Fe/Ni and Fe/Pd nanoparticles. Chem. Eng. J. 2016, 285, 459–466. [Google Scholar] [CrossRef]
- Li, Q.; Wei, G.; Duan, G.; Zhang, L.; Li, Z.; Yan, F. Valorization of ball-milled waste red mud into heterogeneous catalyst as effective peroxymonosulfate activator for tetracycline hydrochloride degradation. J. Environ. Manag. 2022, 324, 116301. [Google Scholar] [CrossRef]
- Jian, C.; Chen, P.; Cheng, Z.; Liu, L.; Yan, C.; Qiu, F. Hydrogenated red mud biochar as visible-light-driven peroxymonosulfate (PMS) activators for efficient degradation of antibiotic: Resource utilization, mechanism insights and toxicity assessment. Environ. Res. 2025, 273, 121233. [Google Scholar] [CrossRef]
- Ma, D.; Wang, J.; Feng, K.; Liu, B.; Xie, G.; Xing, D. A green strategy from waste red mud to Fe0-based biochar for sulfadiazine treatment by peroxydisulfate activation. Chem. Eng. J. 2022, 446, 136944. [Google Scholar] [CrossRef]
- Wang, J.; Shen, M.; Wang, H.; Du, Y.; Zhou, X.; Liao, Z.; Wang, H.; Chen, Z. Red mud modified sludge biochar for the activation of peroxymonosulfate: Singlet oxygen dominated mechanism and toxicity prediction. Sci. Total Environ. 2020, 740, 140388. [Google Scholar] [CrossRef]
- Gu, J.; Wei, G.; He, Y.; Zhang, Y.; Xiong, D.; Zhang, L.; Zhou, Y.; He, S. Red mud-based Fe2O3/Cu-Al LDH prepared through mechanochemical synthesis as effective peroxymonosulfate activator for lomefloxacin hydrochloride degradation via radical-nonradical cooperation mechanisms. J. Water Process Eng. 2024, 68, 106517. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, L.; Ji, Y.; Xue, R.; Duan, G.; Wei, G.; Li, B. Novel red mud-based FeS2 composite used as an effective heterogeneous catalyst for the degradation of levofloxacin: Preparation, application and degradation mechanism. Mater. Res. Bull. 2025, 182, 113143. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, L.; He, Y.; Gu, J.; Zhou, Y.; Wei, G.; Li, B.; Wei, L. Efficient degradation of ciprofloxacin by peroxymonosulfate activated using red mud-based ZIF-67 composite as a heterogeneous catalyst. Sep. Purif. Technol. 2024, 337, 126407. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, J.; Liu, Y.; Yang, B.; Hong, M.; Yu, S.; Qiu, G.; Fang, Y. Heterogeneous Fenton degradation of ciprofloxacin by RM-Co PBA prepared with red mud as iron source and carrier. J. Environ. Manag. 2025, 375, 124356. [Google Scholar] [CrossRef]
- Thi Mai, N.; Van Thanh, D.; Nhat Huy, N.; Danh Bich, D.; Thi Minh Hang, T.; Huu Hao, N.; Manh Khai, N. Red mud supported on rice husk biochar as sono-photo-Fenton catalysts for degradation of ciprofloxacin in water. Sep. Purif. Technol. 2025, 354, 129039. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Xu, D.; Dai, P. Co3O4 decoration on iron-contained biochar composite fabricated by co-pyrolysis of red mud and spent coffee ground: A synergistic hybrid for Rhodamine B degradation via peroxymonosulfate activation. J. Environ. Chem. Eng. 2023, 11, 110706. [Google Scholar] [CrossRef]
- Tang, J.; Cheng, Z.; Xu, Q.; Sun, J.; Li, F.; Qiu, F. Bagasse modification of red mud for efficient photocatalytic degradation of real dye wastewater: An utilization attempt for massive waste. Environ. Res. 2025, 267, 120608. [Google Scholar] [CrossRef]
- Chen, H.; Long, Q.; Shu, J.; Wei, F.; Zhang, Y. Efficient degradation of m-cresol by MnO-doped red mud catalyst activating peroxymonosulfate process: Performance and mechanism. J. Saudi Chem. Soc. 2024, 28, 101857. [Google Scholar] [CrossRef]
- Sun, X.; He, K.; Chen, Z.; Yuan, H.; Guo, F.; Shi, W. Construction of visible-light-response photocatalysis-self-Fenton system for the efficient degradation of amoxicillin based on industrial waste red mud/CdS S-scheme heterojunction. Sep. Purif. Technol. 2023, 324, 124600. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Gu, H. Synthesis of submicron ferrous oxalate from red mud with high Fenton catalytic performance on degradation of methylene blue. Environ. Sci. Pollut. Res. 2023, 30, 85210–85222. [Google Scholar] [CrossRef]
- Sun, X.; Ni, X.; Wang, X.; Xu, D. Preparation of zero-valent iron-based composite catalyst with red mud and scrap tire as starting materials for Fenton-like degradation of methyl blue. Surf. Interfaces 2022, 31, 102053. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.L. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Jiang, D.; Hu, X.; Wang, R.; Yin, D. Oxidation of nanoscale zero-valent iron under sufficient and limited dissolved oxygen: Influences on aggregation behaviors. Chemosphere 2015, 122, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Wan, S.; Kuang, P.; Cheng, B.; Yu, L.; Yu, J. Crystalline/amorphous Ni/NixSy supported on hierarchical porous nickel foam for high-current-density hydrogen evolution. Appl. Catal. B Environ. 2024, 340, 123195. [Google Scholar] [CrossRef]
- Shen, W.; Lin, F.; Jiang, X.; Li, H.; Ai, Z.; Zhang, L. Efficient removal of bromate with core-shell Fe@Fe2O3 nanowires. Chem. Eng. J. 2017, 308, 880–888. [Google Scholar] [CrossRef]
- Ni, Y.Q.; Zhou, C.X.; Xing, M.Y.; Zhou, Y. Oxidation of emerging organic contaminants by in-situ H2O2 fenton system. Green Energy Environ. 2024, 9, 417–434. [Google Scholar] [CrossRef]
- Hou, J.; Li, Y.; Ci, H.; Miao, L.; You, G.; Wu, J.; Xu, Y. Influence of aggregation and sedimentation behavior of bare and modified zero-valent-iron nanoparticles on the Cr(VI) removal under various groundwater chemistry conditions. Chemosphere 2022, 296, 133905. [Google Scholar] [CrossRef]
- Ryu, A.; Jeong, S.-W.; Jang, A.; Choi, H. Reduction of highly concentrated nitrate using nanoscale zero-valent iron: Effects of aggregation and catalyst on reactivity. Appl. Catal. B Environ. 2011, 105, 128–135. [Google Scholar] [CrossRef]
- Abbasi, A.; Qi, L.; Chen, G. Transport of nanoscale zero-valent iron in the presence of rhamnolipid. Sci. Total Environ. 2024, 927, 172279. [Google Scholar] [CrossRef]
- Ioannidi, A.; Oulego, P.; Collado, S.; Petala, A.; Arniella, V.; Frontistis, Z.; Angelopoulos, G.N.; Diaz, M.; Mantzavinos, D. Persulfate activation by modified red mud for the oxidation of antibiotic sulfamethoxazole in water. J. Environ. Manag. 2020, 270, 110820. [Google Scholar] [CrossRef]
- Jiang, Y.; Mao, K.; Ran, J.; Su, J.; Huang, G.; Zheng, X.; Zhang, K.; Guan, H.; Yang, C.; Zhang, H. Carbon nitride-type polymers compounded with FeOCl to enhance the catalytic removal of antibiotics over a wide pH range: Performance and mechanism. J. Water Process Eng. 2023, 53, 103601. [Google Scholar] [CrossRef]
- Li, X.; Cui, K.; Guo, Z.; Yang, T.; Cao, Y.; Xiang, Y.; Chen, H.; Xi, M. Heterogeneous Fenton-like degradation of tetracyclines using porous magnetic chitosan microspheres as an efficient catalyst compared with two preparation methods. Chem. Eng. J. 2020, 379, 122324. [Google Scholar] [CrossRef]
- Wang, J.; Shen, M.; Gong, Q.; Wang, X.; Cai, J.; Wang, S.; Chen, Z. One-step preparation of ZVI-sludge derived biochar without external source of iron and its application on persulfate activation. Sci. Total Environ. 2020, 714, 136728. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Zhou, G.; He, C.; Li, W.; Wang, Y.; Liu, X.; Lai, B.; Mu, Y. Enhanced contaminant degradation by FeS under oxic conditions with the coexistence of cobalt and nickel. Appl. Catal. B Environ. 2024, 341, 123350. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Dong, H.; Pang, Z.; Zhao, M.; Huang, D.; Dong, J.; Li, L. Highly efficient activation of peracetic acid via zero-valent iron-copper bimetallic nanoparticles (nZVIC) for the oxidation of sulfamethazine in aqueous solution under neutral condition. Appl. Catal. B Environ. 2024, 340, 123183. [Google Scholar] [CrossRef]
- Chen, L.S.; Ni, R.; Yuan, T.J.; Gao, Y.; Kong, W.J.; Zhang, P.; Yue, Q.Y.; Gao, B.Y. Effects of green synthesis, magnetization, and regeneration on ciprofloxacin removal by bimetallic nZVI/Cu composites and insights of degradation mechanism. J. Hazard. Mater. 2020, 382, 121008. [Google Scholar] [CrossRef]
- Cai, M.Q.; Sun, P.Z.; Zhang, L.Q.; Huang, C.H. UV/Peracetic Acid for Degradation of Pharmaceuticals and Reactive Species Evaluation. Environ. Sci. Technol. 2017, 51, 14217–14224. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Zhao, X.; Jing, G.; Zhou, Z. Activation of peracetic acid with zero-valent iron for tetracycline abatement: The role of Fe(II) complexation with tetracycline. J. Hazard. Mater. 2022, 424, 127653. [Google Scholar] [CrossRef]
- Selvakumar, K.; Oh, T.H.; Wang, Y.; Sadhasivam, T.; Sadhasivam, S.; Swaminathan, M. Fabrication of single tungsten/copper/cobalt atom oxide anchored BiVO4-rGO for boosting photodegradation of norfloxacin and rhodamine B. J. Clean. Prod. 2023, 423, 138693. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Yea, Y.; Saravanakumar, K.; Lee, E.; Yoon, Y.; Park, C.M. Adsorptive and photocatalytic performance of cobalt-doped ZnTiO3/Ti3C2Tx MXene nanohybrids towards tetracycline: Kinetics and mechanistic insight. J. Hazard. Mater. 2023, 443, 130165. [Google Scholar] [CrossRef]
- Li, Q.; Wei, G.; Zhang, L.; Li, Z.; Li, J. Activation of peroxymonosulfate by a waste red mud-supported Co3O4 quantum dots under visible light for the degradation of levofloxacin. Chem. Eng. J. 2023, 452, 139382. [Google Scholar] [CrossRef]
- Yao, B.; Liu, M.; Tang, T.; Hu, X.; Yang, C.; Chen, Y. Enhancement of anaerobic digestion of ciprofloxacin wastewater by nano zero-valent iron immobilized onto biochar. Bioresour. Technol. 2023, 385, 129462. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Y.; Duan, X.; Liu, Y.; Wang, S. Density Functional Theory Calculations for Insight into the Heterocatalyst Reactivity and Mechanism in Persulfate-Based Advanced Oxidation Reactions. ACS Catal. 2021, 11, 11129–11159. [Google Scholar] [CrossRef]
- Yubo Zhiye Industrial Research Institute. China Report Hall[DB/OL] [2024-05-25]. Available online: http://www.chinabgao.com/ (accessed on 22 December 2023).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Rev. A.03; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Realization of conceptual density functional theory and information-theoretic approach in multiwfn program. In Conceptual Density Functional Theory: Towards a New Chemical Reactivity Theory; Wiley: Hoboken, NJ, USA, 2022; pp. 631–647. [Google Scholar] [CrossRef]



| Pollutant | Concentration (mg/L) | Catalyst (g/L) | Oxidant | Concentration (mM) | pH | Time | Major ROS | Ref. |
|---|---|---|---|---|---|---|---|---|
| TC-HCl | 20 | 0.2 | PMS | 1.0 | 3–11 | 30 min | SO4·−, 1O2 | [35] |
| TC-HCl | 40 | 0.3 | PMS/vis | 3.0 | 3–9 | 60 min | SO4·− | [36] |
| TC-HCl | 20 | 0.2 | PMS | 1.0 | 3–11 | 60 min | SO4·−, ·OH, 1O2, ·O2− | [36] |
| SMX | 5.0 | 0.6 | PDS | 2.0 | 3–9 | 120 min | 1O2 | [26] |
| SDZ | 20 | 0.2 | PDS | 2 | 3–9 | 20 min | SO4·−, ·O2− | [37] |
| Orange II | 40 | 0.1 | H2O2/vis | 0.02 | 3–11 | 4 h | ·OH | [28] |
| SMX | 5.0 | 1.5 | PMS | 0.15 | 5–9 | 50 min | 1O2 | [38] |
| LOM-HCl | 20 | 0.66 | PMS | 3.0 | 6.2 | 30 min | 1O2 | [39] |
| LVF | 10 | 0.2 | PMS | 1.0 | 3–9 | 60 min | 1O2 | [40] |
| CIP | 20 | 0.1 | PMS | 1.0 | 3–7 | 30 min | SO4·−, ·OH | [41] |
| CIP | 20 | 0.5 | H2O2 | 0.05 | 3–11 | 60 min | ·OH, 1O2, ·O2− | [42] |
| CIP | 20 | 1.0 | H2O2/vis/50 °C | 0.02 | 3 | 180 min | ·OH | [43] |
| Rh B | 10 | 0.2 | PMS | 0.09 | 3–7 | 60 min | SO4·−, ·OH, 1O2 | [44] |
| Rh B | 20 | 2.0 | H2O2/vis | 0.0485 | 3–8.6 | 110 min | ·OH, 1O2, ·O2− | [45] |
| M-cresol | 50 | 2.0 | PMS | 10 | 3–8 | 90 min | 1O2 | [46] |
| AMX | 20 | 0.5 | H2O2/vis | 1 | 3–7 | 120 min | ·OH, ·O2− | [47] |
| MB | 50 | 0.1 | H2O2/vis | 0.02 | 3–9 | 15 min | ·OH | [48] |
| 50 | 0.1 | H2O2 | 0.02 | 3–9 | 30 min | ·OH | ||
| MB | 40 | 0.1 | H2O2 | 0.3 | 2–4 | 15 min | ·OH, ·O2− | [49] |
| Raw Material | Market Price [72] (USD/ton) | Converted Price (USD/ton) |
|---|---|---|
| FeSO4·7H2O | 35.83 | 28.94 |
| N2 | 62.84 | 1.38 |
| RM | 0 | 0 |
| Ni(NO3)2·6H2O | 124.03 | 20.67 |
| KBH4 | 12,127.56 | 2650.15 |
| Cost calculation (USD/ton) | 2701.14 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Su, J.; Zhou, B.; Li, F.; Mao, K.; Umair, M.; Huang, G.; Zhang, H. Development of New Dual-Purpose Environmental Strategies for Effective Antibiotic Degradation Using Red Mud-Based Fenton Oxidation Catalysts. Molecules 2025, 30, 1298. https://doi.org/10.3390/molecules30061298
Zhao Y, Su J, Zhou B, Li F, Mao K, Umair M, Huang G, Zhang H. Development of New Dual-Purpose Environmental Strategies for Effective Antibiotic Degradation Using Red Mud-Based Fenton Oxidation Catalysts. Molecules. 2025; 30(6):1298. https://doi.org/10.3390/molecules30061298
Chicago/Turabian StyleZhao, Yirong, Junxia Su, Bingqi Zhou, Fujie Li, Kang Mao, Muhammad Umair, Guopei Huang, and Hua Zhang. 2025. "Development of New Dual-Purpose Environmental Strategies for Effective Antibiotic Degradation Using Red Mud-Based Fenton Oxidation Catalysts" Molecules 30, no. 6: 1298. https://doi.org/10.3390/molecules30061298
APA StyleZhao, Y., Su, J., Zhou, B., Li, F., Mao, K., Umair, M., Huang, G., & Zhang, H. (2025). Development of New Dual-Purpose Environmental Strategies for Effective Antibiotic Degradation Using Red Mud-Based Fenton Oxidation Catalysts. Molecules, 30(6), 1298. https://doi.org/10.3390/molecules30061298






.jpg)


