Current Progress of Hederagenin and Its Derivatives for Disease Therapy (2017–Present)
Abstract
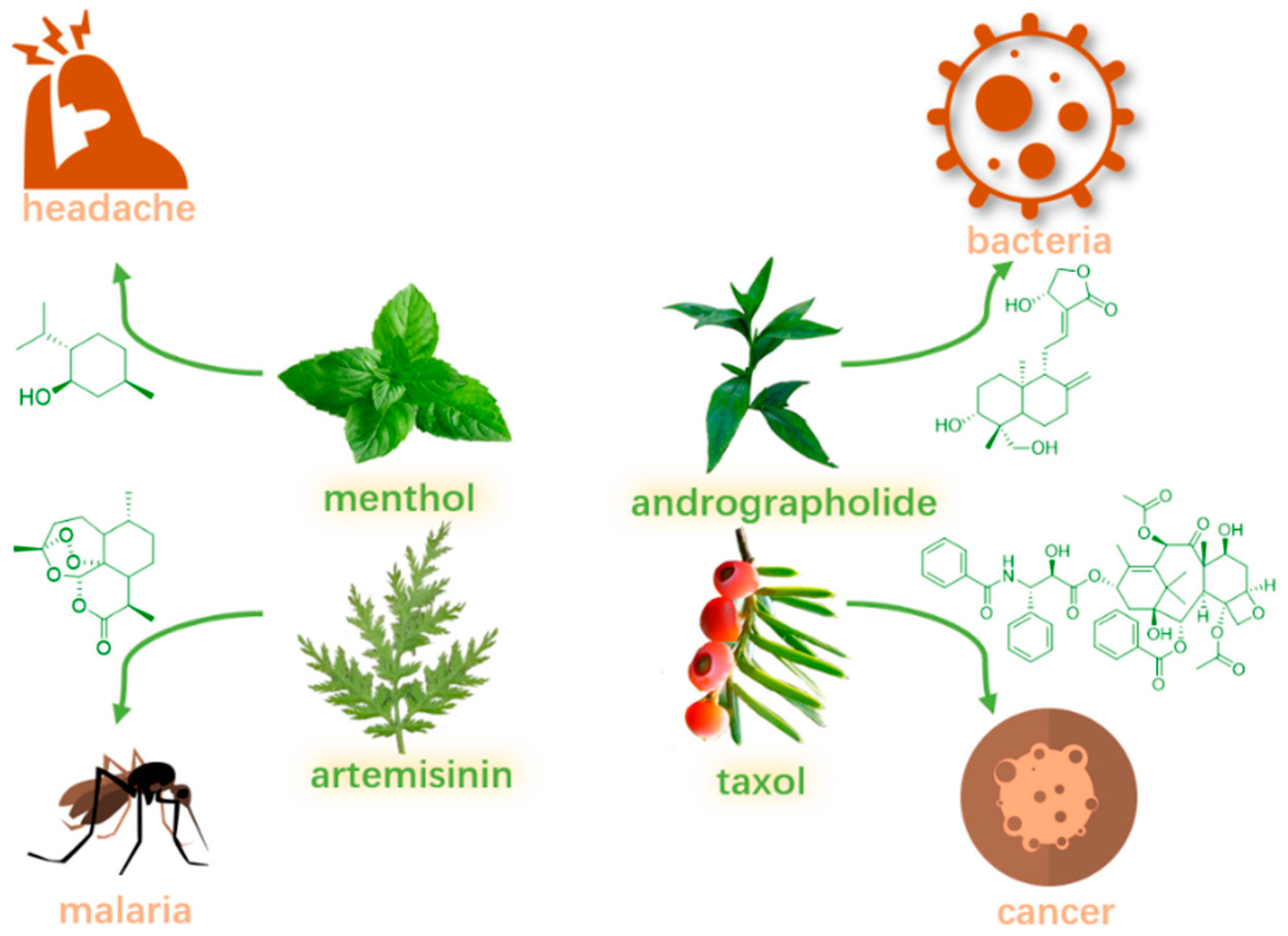
1. Introduction
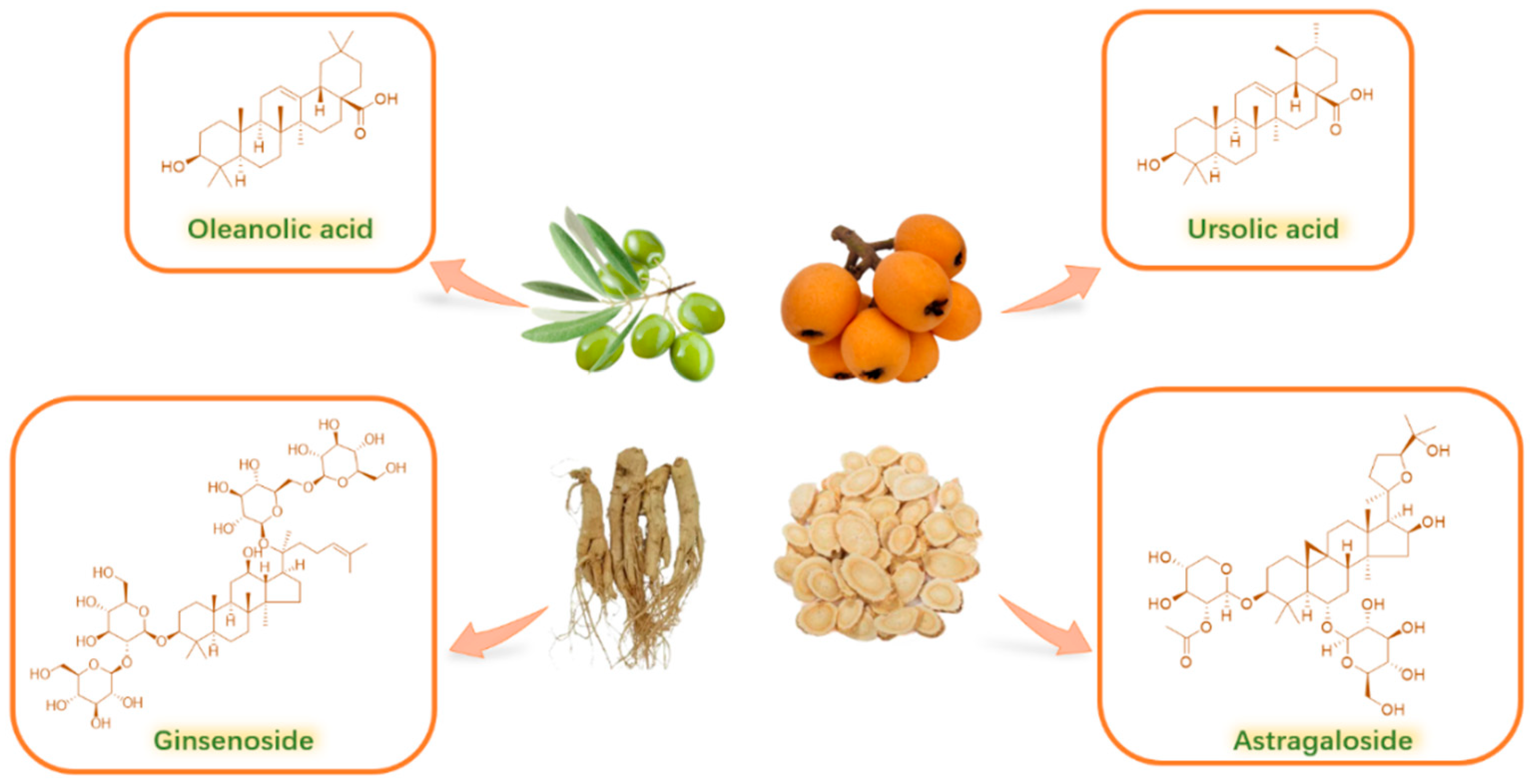
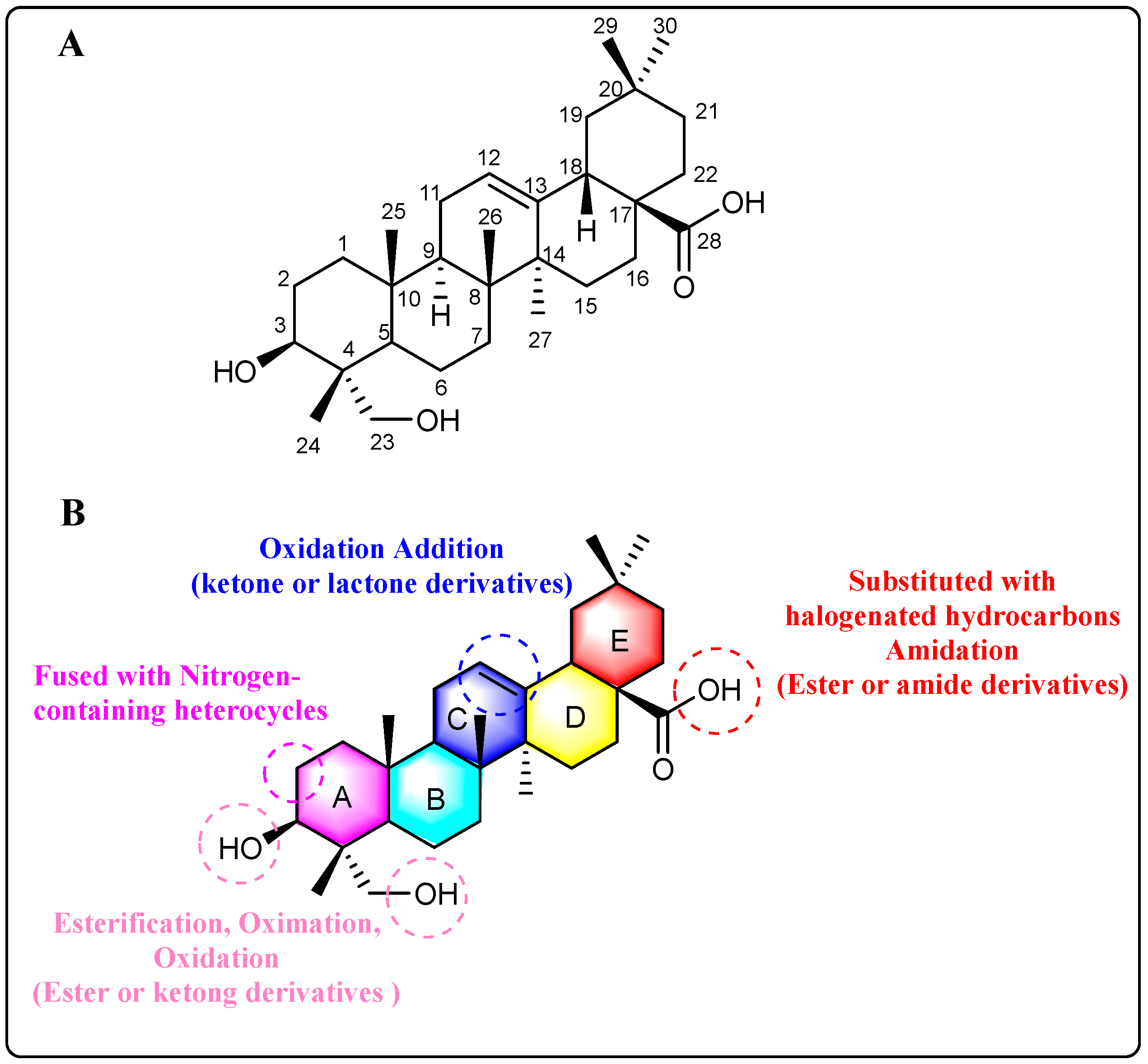
2. Properties and Sources
3. Sites of Modification
4. Derivatives and Bioactivities
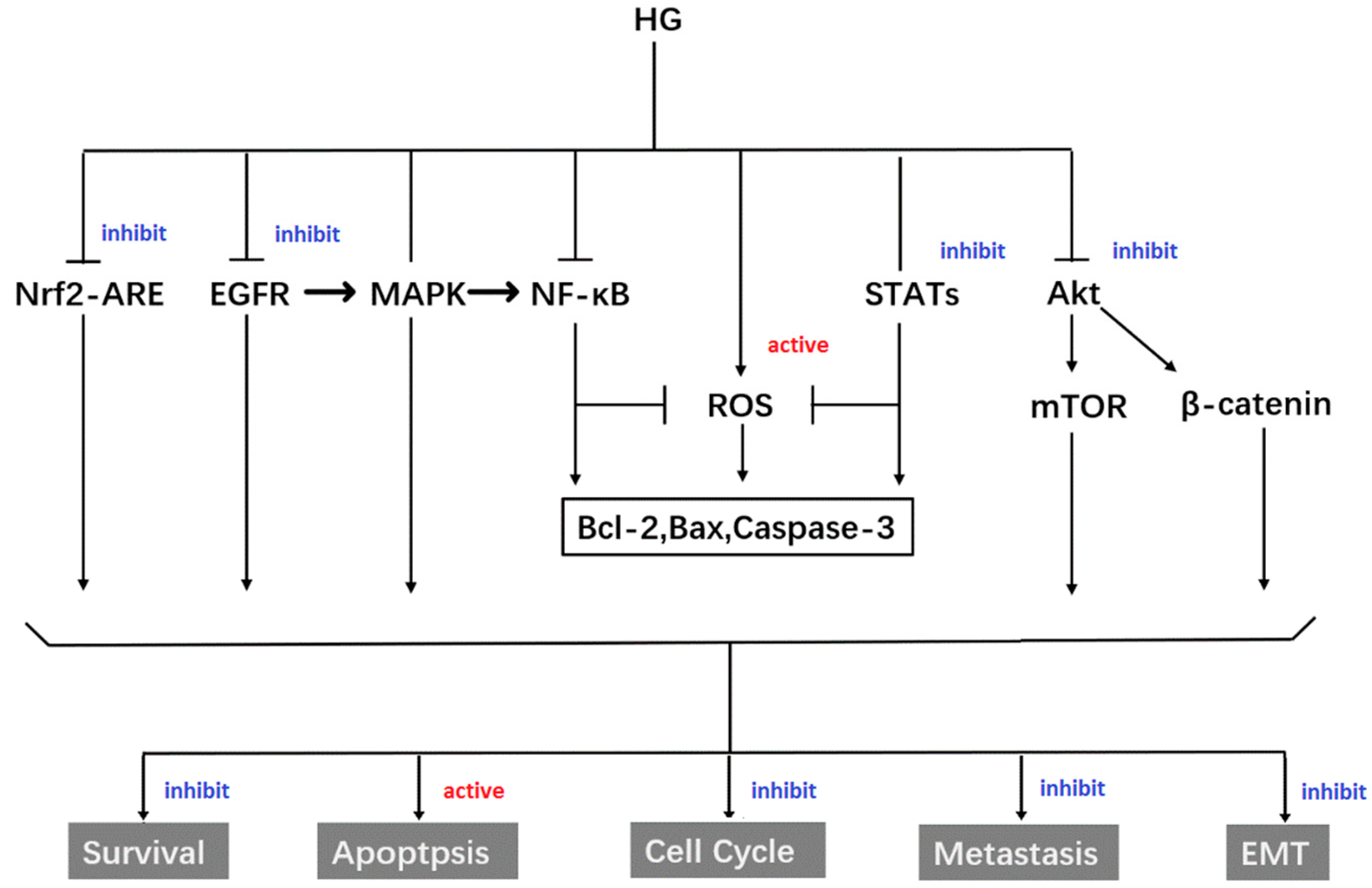
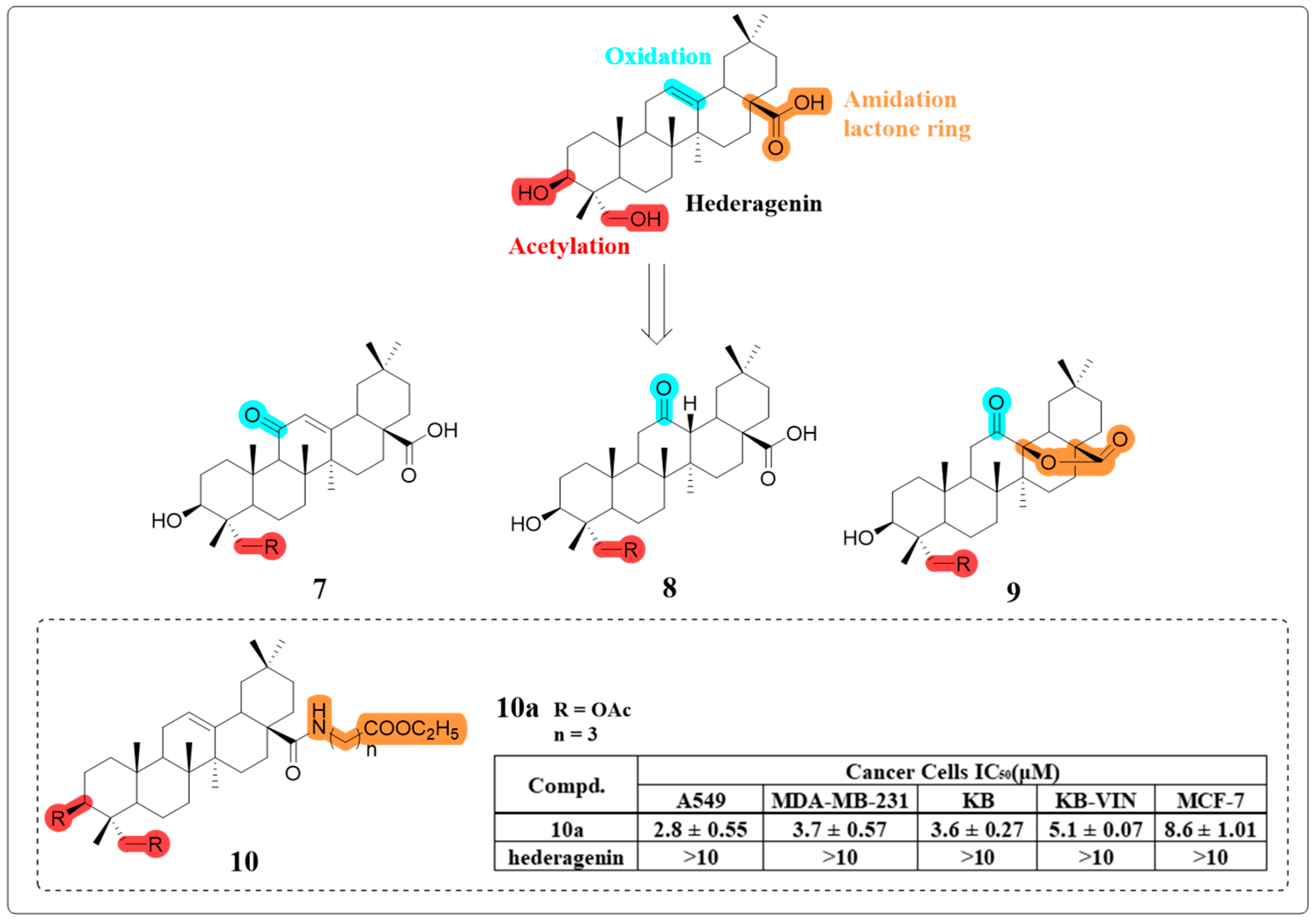
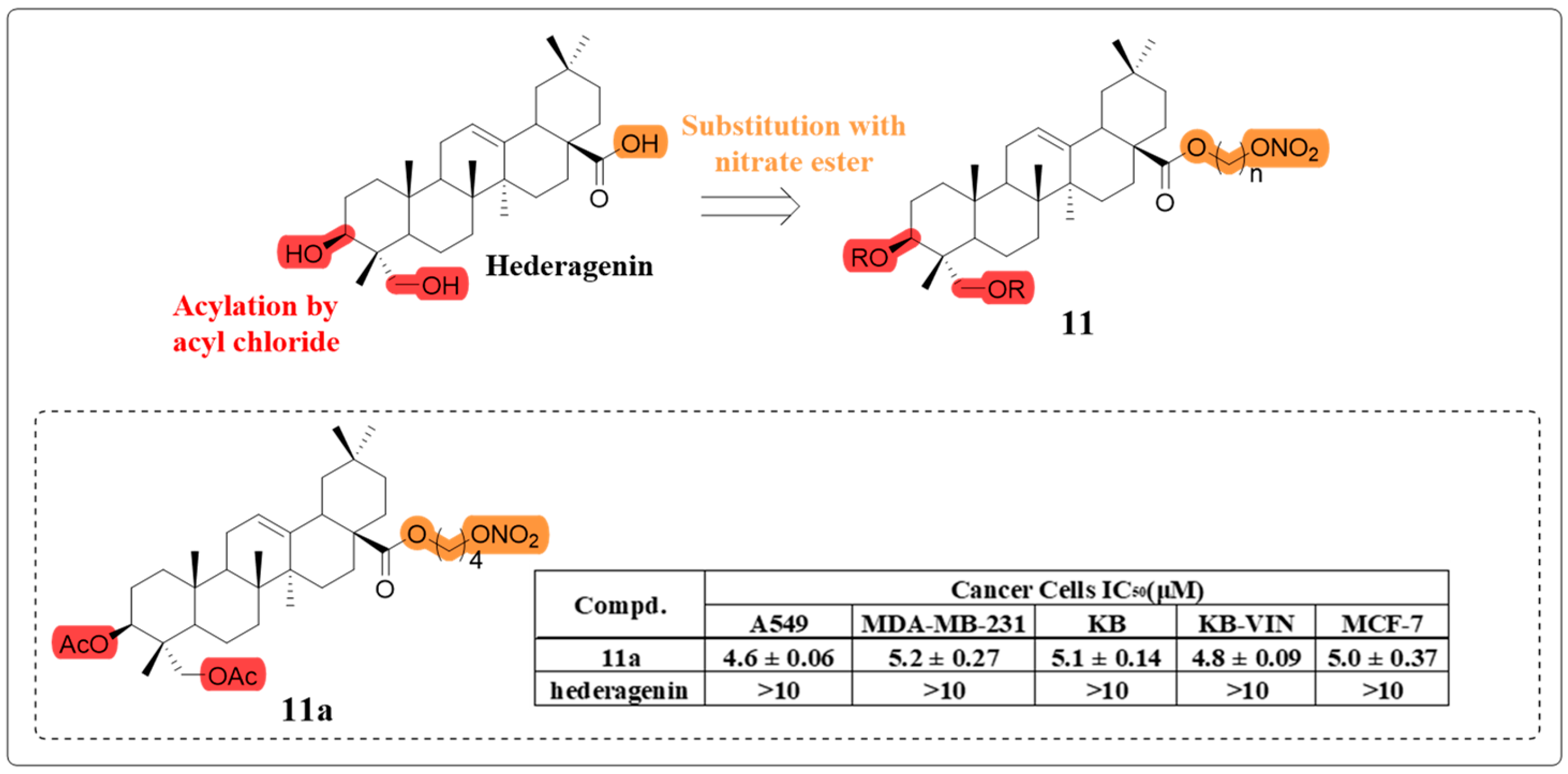
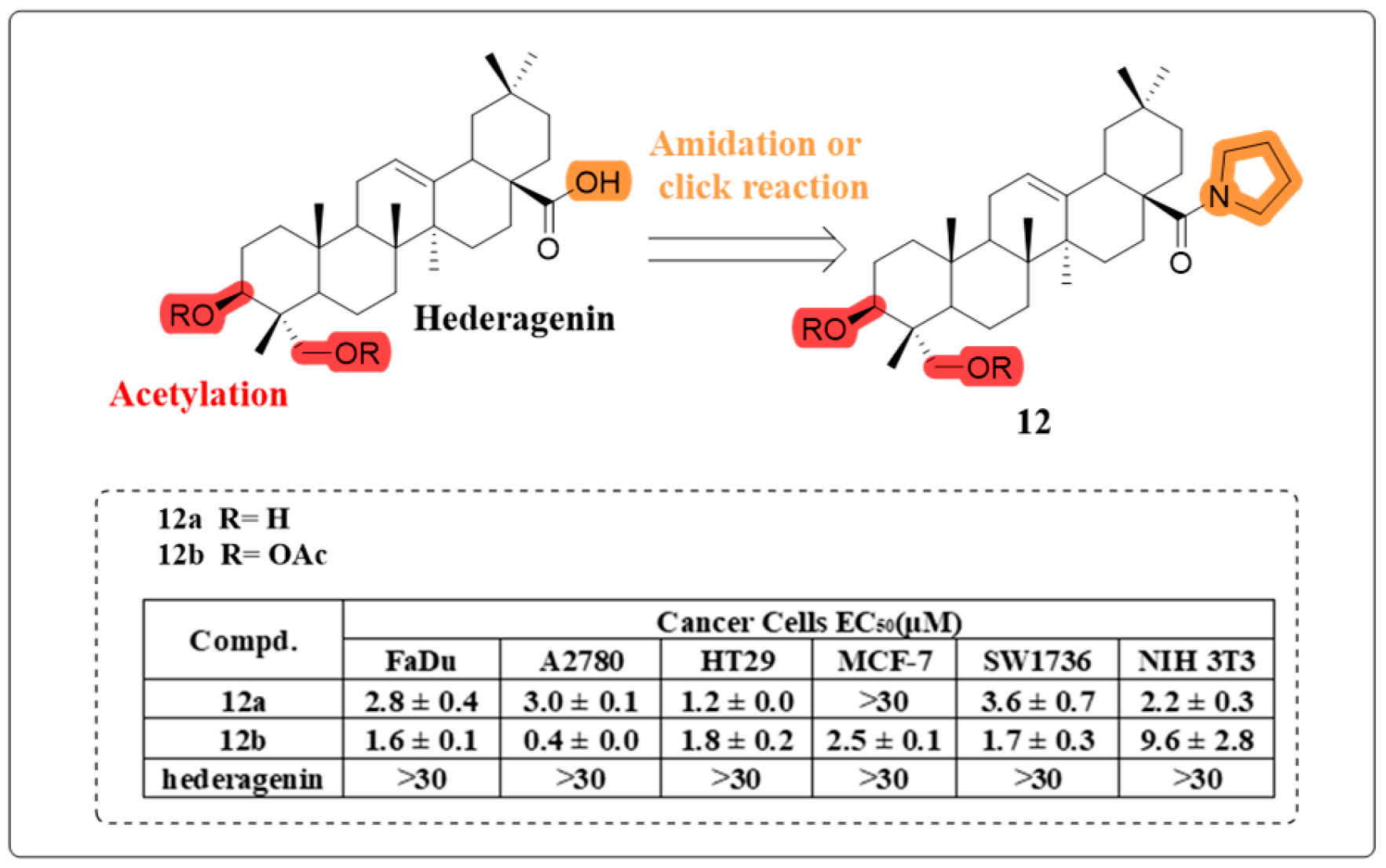
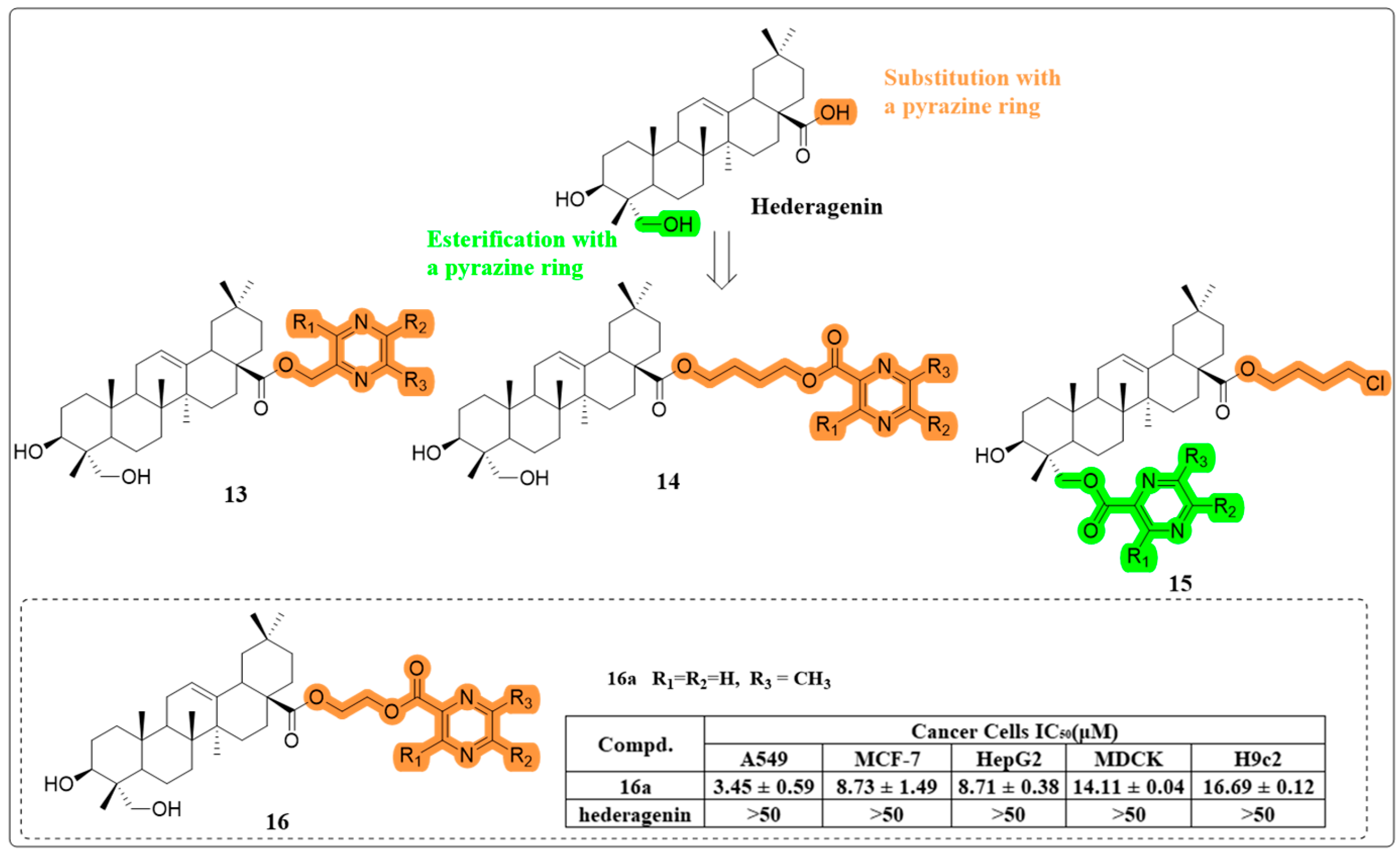
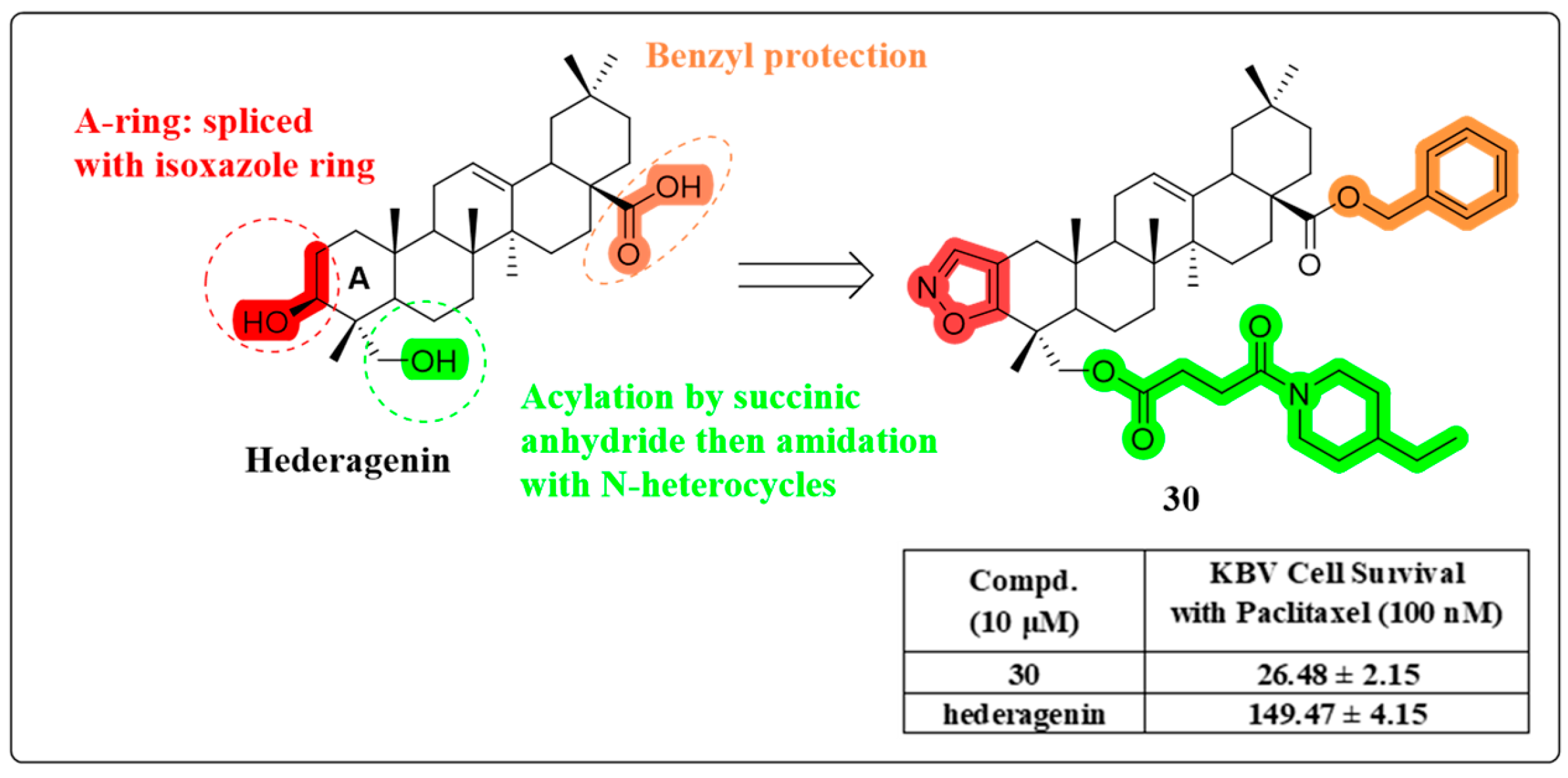
4.1. Anti-Cancer Activity
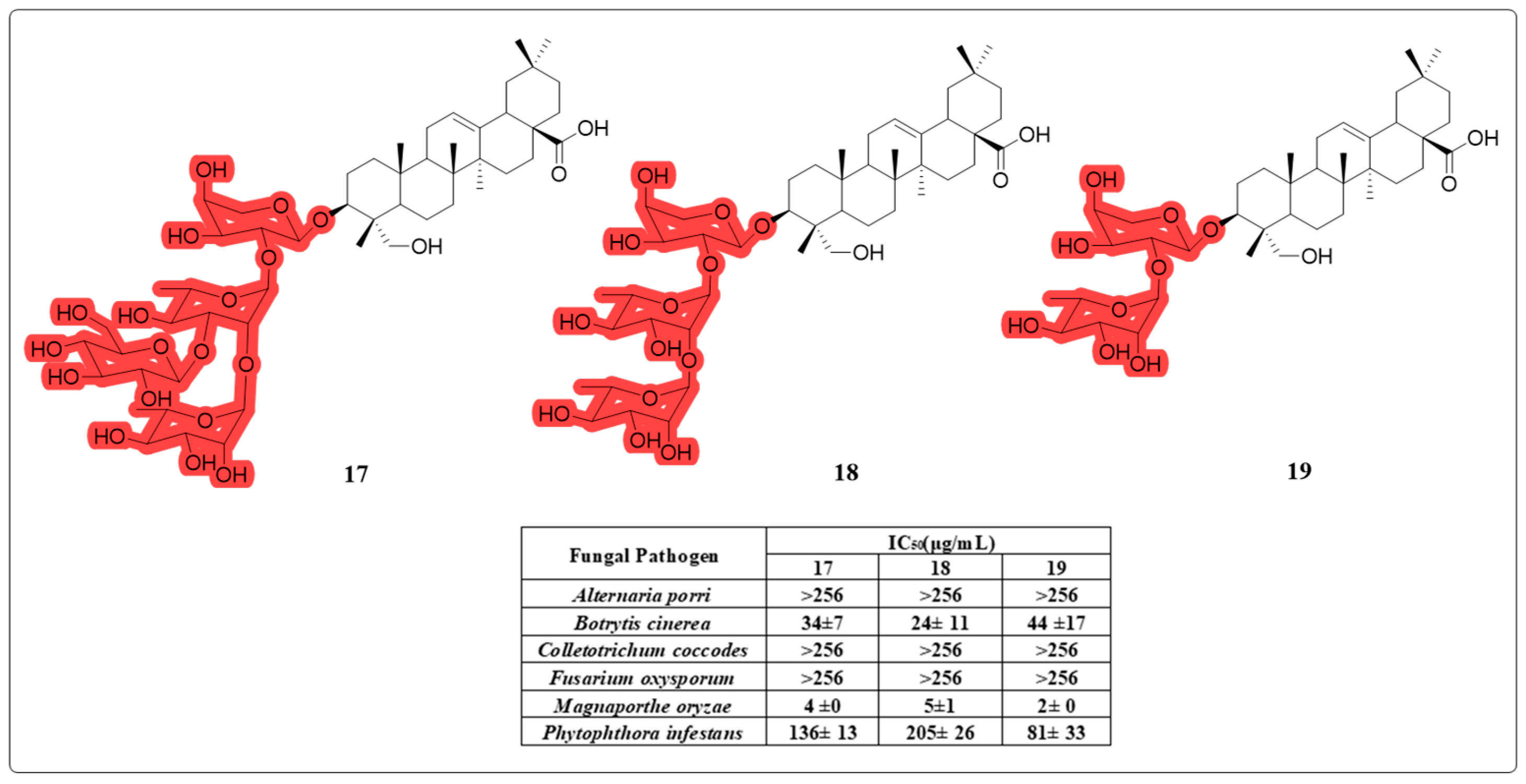
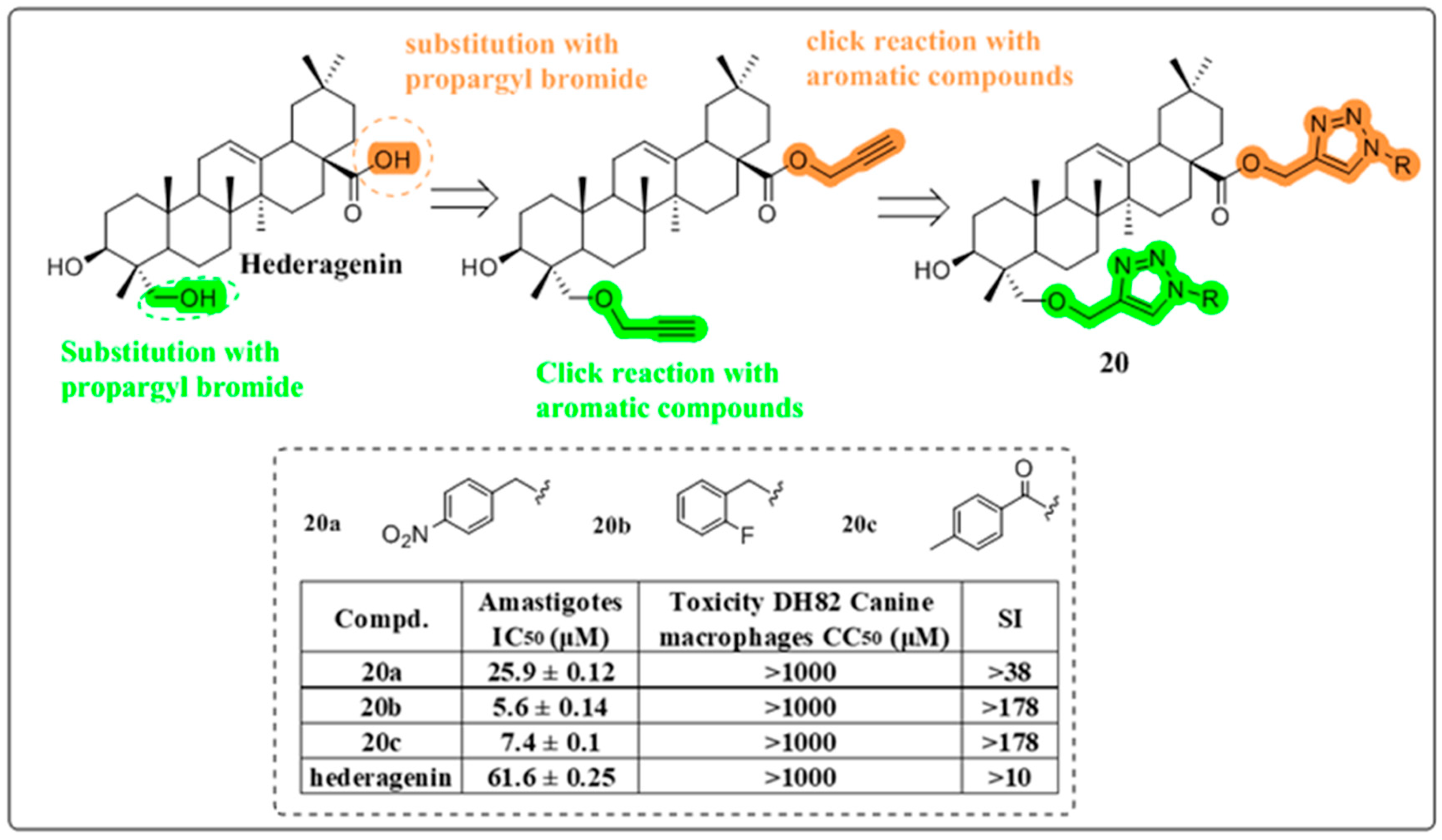
4.2. Anti-Fungal and Anti-Leishmania Activity
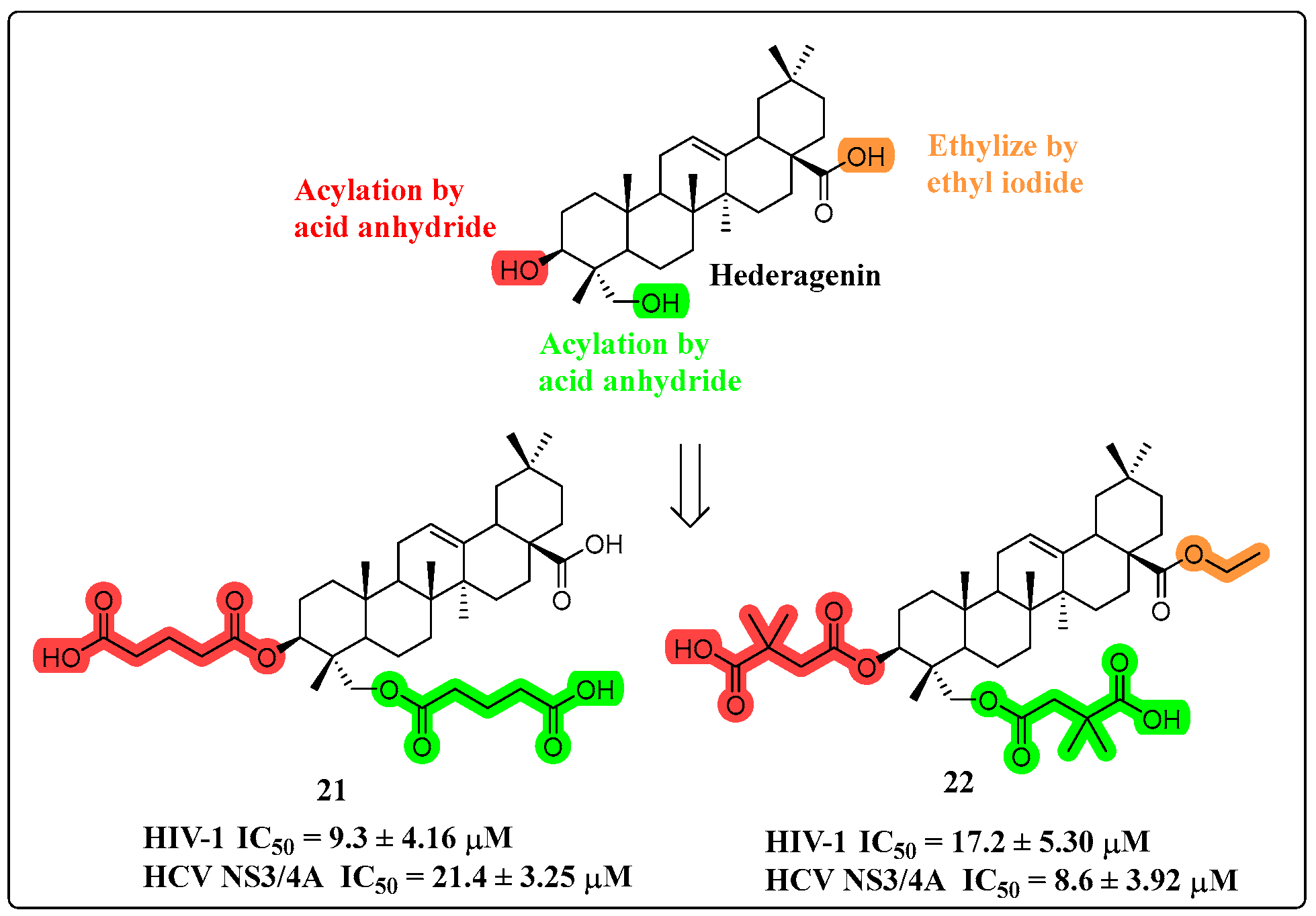
4.3. Anti-Viral Activity
4.4. Anti-Fibrosis
4.5. Neuro-Related Activity
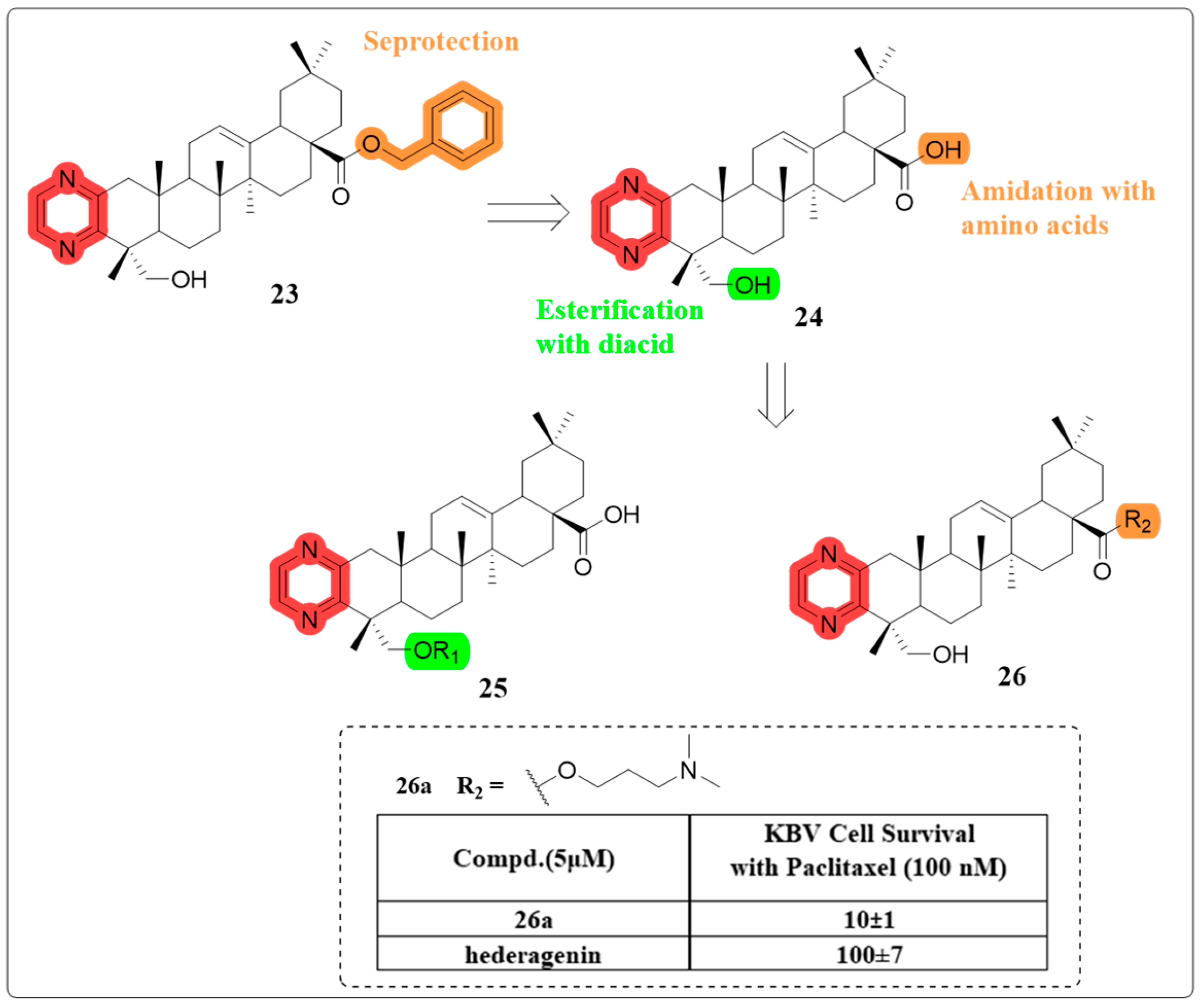
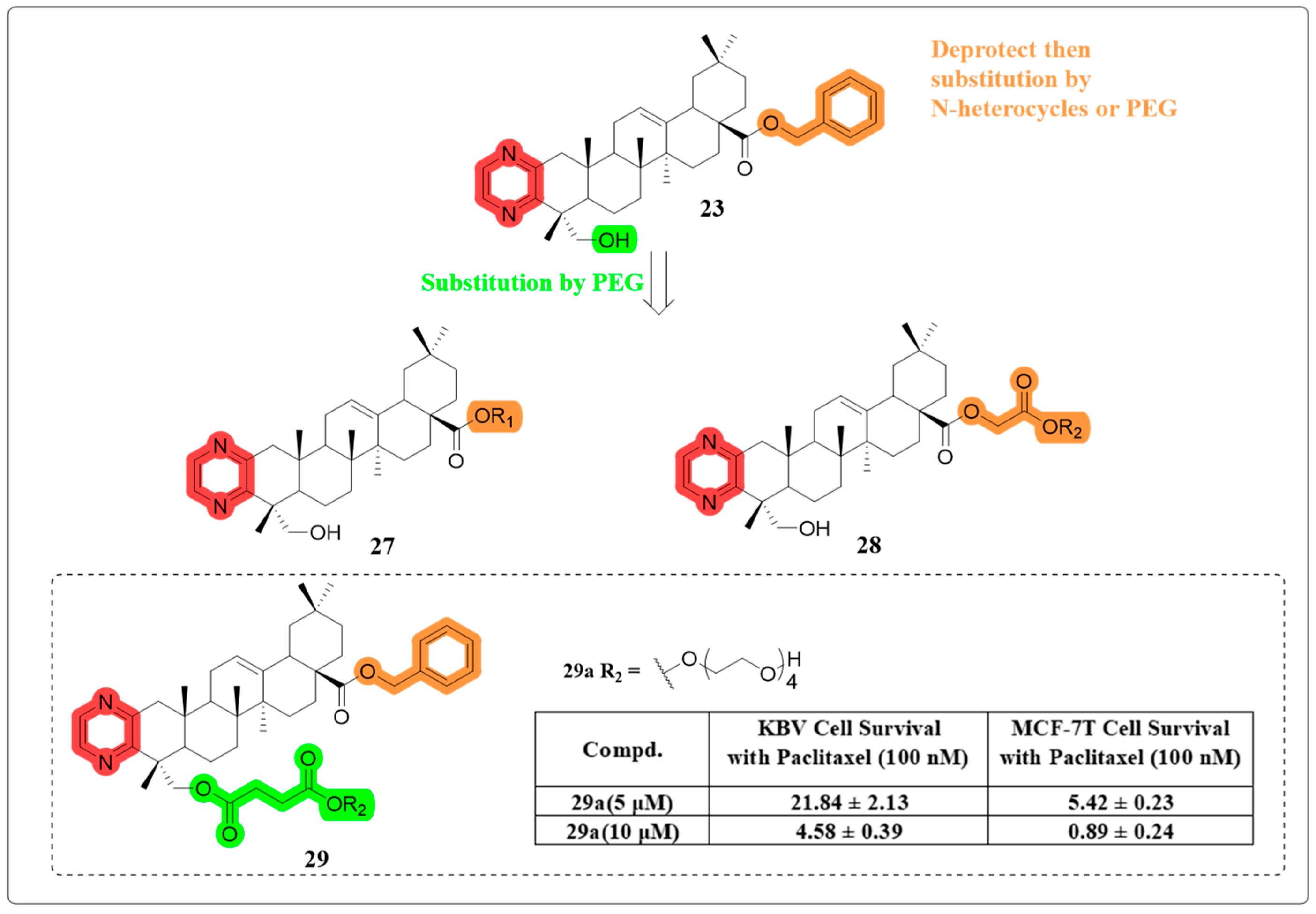
4.6. Multidrug Resistance Reverse
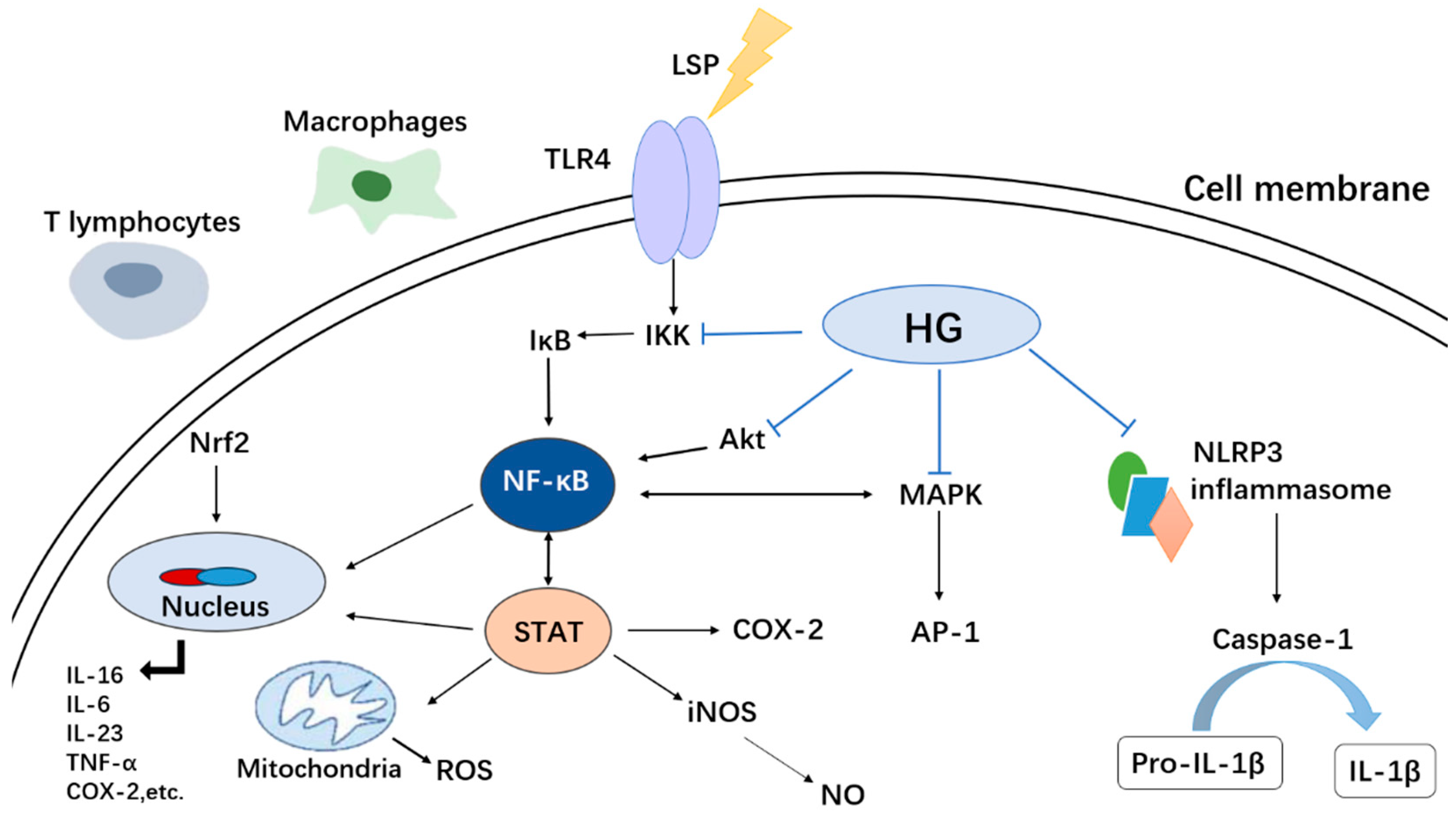
4.7. Anti-Inflammatory Activity
4.8. Osteoclast Inhibition Activity
4.9. Renal Disease
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, G.; Lou, H.X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2018, 38, 1255–1294. [Google Scholar] [CrossRef] [PubMed]
- Naaz, F.; Haider, M.R.; Shafi, S.; Yar, M.S. Anti-tubulin agents of natural origin: Targeting taxol, vinca, and colchicine binding domains. Eur. J. Med. Chem. 2019, 171, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xu, W.; Yan, Y.S.; Chen, M.J.; Li, H.; Chen, L.X. Cembrane diterpenoids: Chemistry and pharmacological activities. Phytochemistry 2023, 212, 113703. [Google Scholar] [CrossRef] [PubMed]
- Farco, J.A.; Grundmann, O. Menthol—Pharmacology of an Important Naturally Medicinal “Cool”. Mini-Rev. Med. Chem. 2013, 13, 124–131. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Goncalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.H.; Li, C.; Wang, X.L.; He, X.J. Triterpenes isolated from acorns of Quercus serrata var. brevipetiolata exert anti-inflammatory activity. Ind. Crops Prod. 2016, 91, 302–309. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef]
- Mabhida, S.E.; Dludla, P.V.; Johnson, R.; Ndlovu, M.; Louw, J.; Opoku, A.R.; Mosa, R.A. Protective effect of triterpenes against diabetes-induced beta-cell damage: An overview of in vitro and in vivo studies. Pharmacol. Res. 2018, 137, 179–192. [Google Scholar] [CrossRef]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef]
- Sanchez-Quesada, C.; Lopez-Biedma, A.; Warleta, F.; Campos, M.; Beltran, G.; Gaforio, J.J. Bioactive Properties of the Main Triterpenes Found in Olives, Virgin Olive Oil, and Leaves of Olea europaea. J. Agric. Food. Chem. 2013, 61, 12173–12182. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Imura, K.; Yamaguchi, T.; Akagi, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Hepatoprotective triterpenes from traditional Tibetan medicine Potentilla anserina. Phytochemistry 2014, 102, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Theoduloz, C.; Schmeda-Hitschmann, G.; Razmilic, I.; Yanez, T.; Rodriguez, J.A. Gastroprotective and ulcer-healing activity of oleanolic acid derivatives: In vitro-in vivo relationships. Life Sci. 2006, 79, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, Q.; Xuan, C.X.; Wang, X.; Luo, H.Q.; Liu, J.E.; Wang, L.L.; Li, T.T.; Chen, Y.P.; Liu, J. 3-Acetyl-oleanolic acid ameliorates non-alcoholic fatty liver disease in high fat diet-treated rats by activating AMPK-related pathways. Acta Pharmacol. Sin. 2018, 39, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.K.; Pan, Q.Q.; Ji, K.; Tian, Z.; Zhou, H.Y.; Li, S.H.; Luo, C.C.; Li, J. Review on the protective mechanism of astragaloside IV against cardiovascular diseases. Front. Pharmacol. 2023, 14, 1187910. [Google Scholar] [CrossRef]
- Yin, M.C.; Chan, K.C. Nonenzymatic antioxidative and antiglycative effects of oleanolic acid and ursolic acid. J. Agric. Food. Chem. 2007, 55, 7177–7181. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Liu, Q.P.; An, P.; Jia, M.; Luan, X.; Tang, J.Y.; Zhang, H. Ginsenoside Rd: A promising natural neuroprotective agent. Phytomedicine 2022, 95, 153883. [Google Scholar] [CrossRef]
- Woldemichael, G.M.; Wink, M. Identification and biological activities of triterpenoid saponins from Chenopodium quinoa. J. Agric. Food. Chem. 2001, 49, 2327–2332. [Google Scholar] [CrossRef]
- Lee, C.W.; Park, S.M.; Zhao, R.; Lee, C.; Chun, W.; Son, Y.; Kim, S.H.; Jung, J.Y.; Jegal, K.H.; Cho, I.J.; et al. Hederagenin, a major component of Clematis mandshurica Ruprecht root, attenuates inflammatory responses in RAW 264.7 cells and in mice. Int. Immunopharmacol. 2015, 29, 528–537. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, D.; Barbosa, L.C.A.; Demuner, A.J.; de Almeida, R.M.; Fujiwara, R.T.; Ferreira, S.R. Highly potent anti-leishmanial derivatives of hederagenin, a triperpenoid from Sapindus saponaria L. Eur. J. Med. Chem. 2016, 124, 153–159. [Google Scholar] [CrossRef]
- Luo, J.-G.; Liu, J.; Kong, L.-Y. New pentacyclic triterpenes from Gypsophila oldhamiana and their biological evaluation as glycogen phosphorylase inhibitors. Chem. Biodivers. 2008, 5, 751–757. [Google Scholar] [CrossRef]
- Jin, Z.-L.; Gao, N.; Zhou, D.; Chi, M.-G.; Yang, X.-M.; Xu, J.-P. The extracts of Fructus Akebiae, a preparation containing 90% of the active ingredient hederagenin: Serotonin, norepinephrine and dopamine reuptake inhibitor. Pharmacol. Biochem. Behav. 2012, 100, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Huang, T.; Xue, M.; Chen, J.X.; Feng, L.L.; Du, R.F.; Feng, Y. Current knowledge and development of hederagenin as a promising medicinal agent: A comprehensive review. RSC Adv. 2018, 8, 24188–24202. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Li, G.L.; Chen, L.Y.; Zhang, C.; Wan, X.X.; Xu, J.P. Quantitative determination of hederagenin in rat plasma and cerebrospinal fluid by ultra fast liquid chromatography-tandem mass spectrometry method. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2011, 879, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, H.; Ding, L.; Yang, Z.L. Determination of asperosaponin VI and its active metabolite hederagenin in rat tissues by LC-MS/MS: Application to a tissue distribution study. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2014, 959, 22–26. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y.; Yang, K.; Wu, C.; Tang, R.; Li, H.; Chen, L. Synthesis of novel andrographolide beckmann rearrangement derivatives and evaluation of their HK2-related anti-inflammatory activities. Eur. J. Med. Chem. 2019, 173, 282–293. [Google Scholar] [CrossRef]
- Oh, S.R.; Jung, K.Y.; Son, K.H.; Park, S.H.; Lee, I.S.; Ahn, K.S.; Lee, H.K. In vitro anticomplementary activity of hederagenin saponins isolated from roots of Dipsacus asper. Arch. Pharmacal Res. 1999, 22, 317–319. [Google Scholar] [CrossRef]
- Zhou, D.; Jin, H.; Lin, H.-B.; Yang, X.-M.; Cheng, Y.-F.; Deng, F.-J.; Xu, J.-P. Antidepressant effect of the extracts from Fructus Akebiae. Pharmacol. Biochem. Behav. 2010, 94, 488–495. [Google Scholar] [CrossRef]
- Liang, B.-F.; Huang, F.; Wang, H.-T.; Wang, G.-H.; Yuan, X.; Zhang, M.-Z.; Guo, H.-B.; Cheng, Y.-F.; Xu, J.-P. Involvement of norepinephrine and serotonin system in antidepressant-like effects of hederagenin in the rat model of unpredictable chronic mild stress-induced depression. Pharm. Biol. 2015, 53, 368–377. [Google Scholar] [CrossRef]
- Wu, A.-G.; Zeng, W.; Wong, V.K.-W.; Zhu, Y.-Z.; Lo, A.C.Y.; Liu, L.; Law, B.Y.-K. Hederagenin and α-hederin promote degradation of proteins in neurodegenerative diseases and improve motor deficits in MPTP-mice. Pharmacol. Res. 2017, 115, 25–44. [Google Scholar] [CrossRef]
- Wu, A.-G.; Wong, V.K.-W.; Zeng, W.; Liu, L.; Law, B.Y.-K. Identification of novel autophagic Radix Polygalae fraction by cell membrane chromatography and UHPLC-(Q) TOF-MS for degradation of neurodegenerative disease proteins. Sci. Rep. 2015, 5, 17199. [Google Scholar] [CrossRef]
- Lu, S.-H.; Guan, J.-H.; Huang, Y.-L.; Pan, Y.-W.; Yang, W.; Lan, H.; Huang, S.; Hu, J.; Zhao, G.-P. Experimental Study of Antiatherosclerosis Effects with Hederagenin in Rats. Evid. Based Complement. Alternat. Med. 2015, 2015, 456354. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, D.H.; Choi, J.W.; Park, J.H.; Han, Y.N. A potent anti-diabetic agent from Kalopanax pictus. Arch. Pharmacal Res. 1998, 21, 24–29. [Google Scholar] [CrossRef]
- Liu, B.-X.-Z.; Zhou, J.-Y.; Li, Y.; Zou, X.; Wu, J.; Gu, J.-F.; Yuan, J.-R.; Zhao, B.-J.; Feng, L.; Jia, X.-B.; et al. Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway. BMC Complement. Altern. Med. 2014, 14, 412. [Google Scholar] [CrossRef]
- Gao, Y.; He, C.; Bi, W.; Wu, G.; Altman, E. Bioassay Guided Fractionation Identified Hederagenin as a Major Cytotoxic Agent from Cyclocarya paliurus Leaves. Planta Med. 2016, 82, 171–179. [Google Scholar] [CrossRef]
- Wu, X.; Gao, H.; Hou, Y.; Yu, J.; Sun, W.; Wang, Y.; Chen, X.; Feng, Y.; Xu, Q.-m.; Chen, X. Dihydronortanshinone, a natural product, alleviates LPS-induced inflammatory response through NF-κB, mitochondrial ROS, and MAPK pathways. Toxicol. Appl. Pharmacol. 2018, 355, 1–8. [Google Scholar] [CrossRef]
- Ziech, D.; Anestopoulos, I.; Hanafi, R.; Voulgaridou, G.P.; Franco, R.; Georgakilas, A.G.; Pappa, A.; Panayiotidis, M.I. Pleiotrophic effects of natural products in ROS-induced carcinogenesis: The role of plant-derived natural products in oral cancer chemoprevention. Cancer Lett. 2012, 327, 16–25. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, W.; Zhao, L.-F.; Liang, J.-J.; Liu, Y.; Zhang, X.; Feng, K.; Liu, H.-M. Design, synthesis and biological mechanisms research on 1,2,3-triazole derivatives of Jiyuan Oridonin A. Biorg. Med. Chem. 2018, 26, 4761–4773. [Google Scholar] [CrossRef]
- Kim, E.H.; Baek, S.; Shin, D.; Lee, J.; Roh, J.L. Hederagenin Induces Apoptosis in Cisplatin-Resistant Head and Neck Cancer Cells by Inhibiting the Nrf2-ARE Antioxidant Pathway. Oxidative Med. Cell. Longev. 2017, 2017, 5498908. [Google Scholar] [CrossRef]
- Wang, K.; Liu, X.D.; Liu, Q.M.; Ho, I.H.; Wei, X.L.; Yin, T.; Zhan, Y.J.; Zhang, W.J.; Zhang, W.B.; Chen, B.N.; et al. Hederagenin potentiated cisplatin- and paclitaxel-mediated cytotoxicity by impairing autophagy in lung cancer cells. Cell Death Dis. 2020, 11, 611. [Google Scholar] [CrossRef]
- Diwanji, N.; Bergmann, A. An unexpected friend—ROS in apoptosis-induced compensatory proliferation: Implications for regeneration and cancer. Semin. Cell Dev. Biol. 2018, 80, 74–82. [Google Scholar] [CrossRef]
- Chen, H.; Guan, X.; Liu, Q.; Yang, L.; Guo, J.; Gao, F.; Qi, Y.; Wu, X.; Zhang, F.; Tian, X. Co-assembled Nanocarriers of De Novo Thiol-Activated Hydrogen Sulfide Donors with an RGDFF Pentapeptide for Targeted Therapy of Non-Small-Cell Lung Cancer. ACS Appl. Mater. Interfaces 2022, 14, 53475–53490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tan, X.N.; Peng, L.; Gao, W.H.; Zeng, P.H. Hederagenin Induces Apoptosis of Human Hepatoma HepG2 Cells via the Mitochondrial Pathway. Comb. Chem. High. Throughput Screen. 2024, 27, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.X.; Masra, N.; Zhou, L.; Yu, C.; Jin, W.; Ni, H.B. Hederagenin suppresses glioma cell biological activities via Nur77 in vitro study. Food Sci. Nutr. 2023, 11, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Wang, W.; Sun, D.; Liang, J.; Zhu, M.; Li, H.; Chen, L. PROTAC technology as a novel tool to identify the target of lathyrane diterpenoids. Acta Pharm. Sin. B 2022, 12, 4262–4265. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Han, Y.; Shang, Y.C.; Wang, X.Y.; Sun, J.W. Proteomics identifies differentially expressed proteins in glioblastoma U87 cells treated with hederagenin. Proteome Sci. 2023, 21, 7. [Google Scholar] [CrossRef]
- Su, F.; Sui, X.; Xu, J.B.; Liu, Q.L.; Li, J.F.; Liu, W.H.; Xu, Y.; Zhang, Z.Q.; Tao, F.F. Hederagenin suppresses ovarian cancer via targeting mitochondrial fission through dynamin-related protein 1. Eur. J. Pharmacol. 2024, 963, 176188. [Google Scholar] [CrossRef]
- Hu, S.; Chu, Y.; Zhou, X.; Wang, X. Recent advances of ferroptosis in tumor: From biological function to clinical application. Biomed. Pharmacother. 2023, 166, 115419. [Google Scholar] [CrossRef]
- Lu, J.Y.; Guo, Q.X.; Zhao, H.; Liu, H. Hederagenin promotes lung cancer cell death by activating CHAC1-dependent ferroptosis pathway. Biochem. Biophys. Res. Commun. 2024, 718, 150085. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, L.; Wu, J.; Zhou, X.J.; Li, X.X.; Sun, X.; Zhu, L.; Xia, T.S.; Ding, Q. A hederagenin saponin isolated from Clematis ganpiniana induces apoptosis in breast cancer cells via the mitochondrial pathway. Oncol. Lett. 2018, 15, 1737–1743. [Google Scholar] [CrossRef]
- Liu, X.X.; Yang, Y.T.; Wang, X.; Wang, K.Y.; Liu, J.Q.; Lei, L.; Luo, X.M.; Zhai, R.; Fu, F.H.; Wang, H.B.; et al. Design, synthesis and biological evaluation of novel alpha-hederagenin derivatives with anticancer activity. Eur. J. Med. Chem. 2017, 141, 427–439. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, H.; Wang, M.; Han, L.; Liu, Y.; Zhu, Y.; Yang, S. Synthesis, cytotoxicity and haemolytic activity of Pulsatilla saponin A, D derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 2550–2554. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, H.Q.; Tong, X.H.; Hsu, P.L.; Han, L.; Morris-Natschke, S.L.; Yang, S.L.; Liu, W.; Lee, K.H. Cytotoxicity, Hemolytic Toxicity, and Mechanism of Action of Pulsatilla Saponin D and Its Synthetic Derivatives. J. Nat. Prod. 2018, 81, 465–474. [Google Scholar] [CrossRef]
- Fu, X.; Lu, H.; Gao, M.; Li, P.; He, Y.; He, Y.; Luo, X.; Rao, X.; Liu, W. Nitric oxide in the cardio-cerebrovascular system: Source, regulation and application. Nitric Oxide-Biol. Chem. 2024, 152, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, K.Y.; Ling, Y.; Goto, M.; Duan, H.Q.; Tong, X.H.; Liu, Y.L.; Cheng, Y.Y.; Morris-Natschke, S.L.; Yang, P.C.; et al. Discovery of an Oleanolic Acid/Hederagenin-Nitric Oxide Donor Hybrid as an EGFR Tyrosine Kinase Inhibitor for Non-Small-Cell Lung Cancer. J. Nat. Prod. 2019, 82, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, D.; Barbosa, L.C.A.; Demuner, A.J.; Martins, J.P.A.; Fischer, L.; Csuk, R. Hederagenin amide derivatives as potential antiproliferative agents. Eur. J. Med. Chem. 2019, 168, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Zhang, X.H.; Han, Y.T.; Wu, G.R.; Cai, D.S.; Xue, N.N.; Guo, W.B.; Yang, Y.Q.; Chen, M.; Zhang, X.Y.; et al. Design, Synthesis, and Cytotoxic Analysis of Novel Hederagenin-Pyrazine Derivatives Based on Partial Least Squares Discriminant Analysis. Int. J. Mol. Sci. 2018, 19, 2994. [Google Scholar] [CrossRef]
- Anderson, R.; Feldman, C. Pneumolysin as a potential therapeutic target in severe pneumococcal disease. J. Infect. 2017, 74, 527–544. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, Y.; Xu, X.Z.; Hou, Y.F.; Nie, J.; Deng, X.M.; Qiu, J.Z.; Lv, Q.H. Inhibitory effect of hederagenin on Streptococcus pneumoniae pneumolysin in vitro. Microb. Infect. 2022, 24, 104888. [Google Scholar] [CrossRef]
- Xu, M.; Xu, J.; Hao, M.; Zhang, K.; Lv, M.; Xu, H. Evaluation of andrographolide-based analogs derived from Andrographis paniculata against Mythimna separata Walker and Tetranychus cinnabarinus Boisduval. Bioorg. Chem. 2019, 86, 28–33. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, B.; Xu, H. Synthesis of andrographolide-related esters as insecticidal and acaricidal agents. Bioorg. Med. Chem. Lett. 2018, 28, 360–364. [Google Scholar] [CrossRef]
- Kim, B.; Han, J.W.; Ngo, M.T.; Quang Le, D.; Kim, J.-C.; Kim, H.; Choi, G.J. Identification of novel compounds, oleanane- and ursane-type triterpene glycosides, from Trevesia palmata: Their biocontrol activity against phytopathogenic fungi. Sci. Rep. 2018, 8, 14522. [Google Scholar] [CrossRef] [PubMed]
- Coffeng, L.E.; de Vlas, S.J.; Singh, R.P.; James, A.; Bindroo, J.; Sharma, N.K.; Ali, A.; Singh, C.; Sharma, S.; Coleman, M. Effect of indoor residual spraying on sandfly abundance and incidence of visceral leishmaniasis in India, 2016–2022: An interrupted time-series analysis and modelling study. Lancet. Infect. Dis. 2024, 24, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, D.; Barbosa, L.C.A.; Demuner, A.J.; Nain-Perez, A.; Ferreira, S.R.; Fujiwara, R.T.; de Almeida, R.M.; Heller, L.; Csuk, R. Leishmanicidal and cytotoxic activity of hederagenin-bistriazolyl derivatives. Eur. J. Med. Chem. 2017, 140, 624–635. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, C.M.; Chen, D.Y.; Hattori, M. Anti-HIV-1 protease triterpenoids from Stauntonia obovatifoliola Hayata subsp intermedia. Phytochemistry 2008, 69, 1875–1879. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, Y.; Hao, Y.; Yang, J.; Pan, B.; Yang, X.; Zhou, Y.; Wang, X. Synthesis and Evaluation of Acylated Derivatives of Hederagenin as Inhibitors of HIV-1 and HCV NS3/4A Proteases. Nat. Prod. Commun. 2022, 17, 1934578X2210750. [Google Scholar] [CrossRef]
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 515–546. [Google Scholar] [CrossRef]
- Ma, W.J.; Huang, Q.S.; Xiong, G.F.; Deng, L.J.; He, Y. The protective effect of Hederagenin on pulmonary fibrosis by regulating the Ras/JNK/NFAT4 axis in rats. Biosci. Biotechnol. Biochem. 2020, 84, 1131–1138. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Jiang, H.; Pan, J.; Huang, X.R.; Wang, Y.C.; Huang, H.F.; To, K.F.; Nikolic-Paterson, D.J.; Lan, H.Y.; Chen, J.H. Macrophage-to-Myofibroblast Transition Contributes to Interstitial Fibrosis in Chronic Renal Allograft Injury. J. Am. Soc. Nephrol. 2017, 28, 2053–2067. [Google Scholar] [CrossRef]
- Jia, J.; Xu, L.H.; Deng, C.; Zhong, X.; Xie, K.H.; Han, R.Y.; Su, H.W.; Tan, R.Z.; Wang, L. Hederagenin ameliorates renal fibrosis in chronic kidney disease through blocking ISG15 regulated JAK/STAT signaling. Int. Immunopharmacol. 2023, 118, 110122. [Google Scholar] [CrossRef]
- Lin, R.H.; Liu, L.L.; Silva, M.; Fang, J.K.; Zhou, Z.W.; Wang, H.T.; Xu, J.P.; Li, T.J.; Zheng, W.H. Hederagenin Protects PC12 Cells Against Corticosterone-Induced Injury by the Activation of the PI3K/AKT Pathway. Front. Pharmacol. 2021, 12, 712876. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Yang, L.-E.; Yang, Z. Hederagenin Modulates M1 Microglial Inflammatory Responses and Neurite Outgrowth. Nat. Prod. Commun. 2020, 15, 1934578X2094625. [Google Scholar] [CrossRef]
- Kargiotis, O.; Safouris, A.; Psychogios, K.; Saposnik, G.; Yaghi, S.; Merkler, A.; Kamel, H.; Filippatos, G.; Tsivgoulis, G. Heart failure and stroke: The underrepresentation of the heart failure with preserved ejection fraction subtype in randomized clinical trials of therapeutic anticoagulation. J. Neurol. Sci. 2024, 466, 123231. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Song, L.L.; Cao, X.; Li, W.; Zhao, Y.Y.; Chen, J.; Li, J.; Chen, Y.Z.; Yu, W.K.; Xu, Y. Hederagenin Attenuates Cerebral Ischaemia/Reperfusion Injury by Regulating MLK3 Signalling. Front. Pharmacol. 2020, 11, 1173. [Google Scholar] [CrossRef]
- Chen, T.; Xiao, Z.; Liu, X.; Wang, T.; Wang, Y.; Ye, F.; Su, J.; Yao, X.; Xiong, L.; Yang, D.-H. Natural products for combating multidrug resistance in cancer. Pharmacol. Res. 2024, 202, 107099. [Google Scholar] [CrossRef]
- Xia, Y.-Z.; Ni, K.; Guo, C.; Zhang, C.; Geng, Y.-D.; Wang, Z.-D.; Yang, L.; Kong, L.-Y. Alopecurone B reverses doxorubicin-resistant human osteosarcoma cell line by inhibiting P-glycoprotein and NF-kappa B signaling. Phytomedicine 2015, 22, 344–351. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Wang, C.C.N.; Wang, J.-Y.; Lee, T.-E.; Cheng, Y.-Y.; Morris-Natschke, S.L.; Lee, K.-H.; Hung, C.-C. Tenulin and isotenulin inhibit P-glycoprotein function and overcome multidrug resistance in cancer cells. Phytomedicine 2019, 53, 252–262. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lu, Y.-J.; Yang, C.-C.; Tuo, W.-C.; Lo, Y.-Q.; Huang, Y.-H.; Hsieh, C.-H.; Hung, T.-H.; Wu, C.-P. Hernandezine, a Bisbenzylisoquinoline Alkaloid with Selective Inhibitory Activity against Multidrug-Resistance-Linked ATP-Binding Cassette Drug Transporter ABCB1. J. Nat. Prod. 2016, 79, 2135–2142. [Google Scholar] [CrossRef]
- Yang, Y.T.; Guan, D.K.; Lei, L.; Lu, J.; Liu, J.Q.; Yang, G.Q.; Yan, C.H.; Zhai, R.; Tian, J.W.; Bi, Y.; et al. H6, a novel hederagenin derivative, reverses multidrug resistance in vitro and in vivo. Toxicol. Appl. Pharmacol. 2018, 341, 98–105. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Q.W.; Liu, X.X.; Yang, Y.T.; Wang, B.H.; Zhai, R.; Qi, J.G.; Tian, J.W.; Wang, H.B.; Bi, Y. Synthesis and biological evaluation of novel H6 analogues as drug resistance reversal agents. Eur. J. Med. Chem. 2019, 161, 364–377. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.; Huang, W.; Ma, M.; Chen, X.; Zeng, W.; Liang, K.; Wang, H.; Bi, Y.; Li, X. Design, synthesis, and biological evaluation of hederagenin derivatives with improved aqueous solubility and tumor resistance reversal activity. Eur. J. Med. Chem. 2021, 211, 113107. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Xu, S.; Qiao, H.; Cheng, H.; Wang, L.; Liu, S.; Tian, Q.; Wang, R.; Wang, H.; et al. Design, synthesis, and tumor drug resistance reversal activity of novel hederagenin derivatives modified by nitrogen-containing heterocycles. Eur. J. Med. Chem. 2022, 232, 114207. [Google Scholar] [CrossRef] [PubMed]
- Duryee, M.J.; Aripova, N.; Hunter, C.D.; Ruskamp, R.J.; Tessin, M.R.; Works, D.R.; Mikuls, T.R.; Thiele, G.M. A novel reactive aldehyde species inhibitor prevents the deleterious effects of ethanol in an animal model of alcoholic liver disease. Int. Immunopharmacol. 2022, 113, 109400. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-J.; Song, D.H.; Yoo, H.S.; Chung, K.-H.; Lee, K.J.; An, J.H. Hederagenin Supplementation Alleviates the Pro-Inflammatory and Apoptotic Response to Alcohol in Rats. Nutrients 2017, 9, 41. [Google Scholar] [CrossRef]
- Li, Y.; Dong, J.; Shang, Y.; Zhao, Q.; Li, P.; Wu, B. Anti-inflammatory effects of hederagenin on diabetic cardiomyopathy via inhibiting NF-kappa B and Smads signaling pathways in a type-2 diabetic mice model. RSC Adv. 2019, 9, 26238–26247. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.X.; Meng, Q.H.; Long, Y.Y.; Shi, X.F.; Wang, Y.L. Adipose tissue-derived mesenchymal stromal cells attenuate acute lung injury induced by trauma and haemorrhagic shock. Immunobiology 2023, 228, 152765. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, M. Suppression of NOD-like receptor protein 3 inflammasome activation and macrophage M1 polarization by hederagenin contributes to attenuation of sepsis-induced acute lung injury in rats. Bioengineered 2022, 13, 7262–7276. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, S.J.; Chen, P.; Dai, Y.; Yang, D.; Lin, Y.; Yi, L.S. Maresins as novel anti-inflammatory actors and putative therapeutic targets in sepsis. Pharmacol. Res. 2024, 202, 107113. [Google Scholar] [CrossRef]
- Yu, T.; Cheng, H.R.; Li, X.L.; Huang, W.T.; Li, H.X.; Gao, X.J.; Zhao, J.N.; Zhang, X.; Gu, X.X.; Bi, Y.; et al. Design and synthesis of hederagenin derivatives modulating STING/NF-?B signaling for the relief of acute liver injury in septic mice. Eur. J. Med. Chem. 2023, 245, 114911. [Google Scholar] [CrossRef]
- Pimenta-Lopes, C.; Sanchez-de-Diego, C.; Deber, A.; Egea-Cortes, A.; Valer, J.A.; Alcala, A.; Mendez-Lucas, A.; Esteve-Codina, A.; Rosa, J.L.; Ventura, F. Inhibition of C5AR1 impairs osteoclast mobilization and prevents bone loss. Mol. Ther. 2023, 31, 2507–2523. [Google Scholar] [CrossRef]
- Tian, K.; Su, Y.; Ding, J.; Wang, D.; Zhan, Y.; Li, Y.; Liang, J.; Lin, X.; Song, F.; Wang, Z.; et al. Hederagenin protects mice against ovariectomy-induced bone loss by inhibiting RANKL-induced osteoclastogenesis and bone resorption. Life Sci. 2020, 244, 117336. [Google Scholar] [CrossRef] [PubMed]
- Atak, M.; Yigit, E.; Huner Yigit, M.; Topal Suzan, Z.; Yilmaz Kutlu, E.; Karabulut, S. Synthetic and non-synthetic inhibition of ADAM10 and ADAM17 reduces inflammation and oxidative stress in LPS-induced acute kidney injury in male and female mice. Eur. J. Pharmacol. 2024, 983, 176964. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.H.; Liu, X.H.; Jia, J.; Zhong, X.; Han, R.Y.; Tan, R.Z.; Wang, L. Hederagenin ameliorates cisplatin-induced acute kidney injury via inhibiting long non-coding RNA A330074k22Rik/Axin2/13-catenin signalling pathway. Int. Immunopharmacol. 2022, 112, 109247. [Google Scholar] [CrossRef] [PubMed]
- ElKhooly, I.A.; El-Bassossy, H.M.; Mohammed, H.O.; Atwa, A.M.; Hassan, N.A. Vitamin B1 and calcitriol enhance glibenclamide suppression of diabetic nephropathy: Role of HMGB1/TLR4/NF-kappaB/TNF-alpha/Nrf2/alpha-SMA trajectories. Life Sci. 2024, 357, 123046. [Google Scholar] [CrossRef]
- Jia, J.; Tan, R.Z.; Xu, L.H.; Wang, H.L.; Li, J.C.; Su, H.W.; Zhong, X.; Liu, P.; Wang, L. Hederagenin improves renal fibrosis in diabetic nephropathy by regulating Smad3/NOX4/SLC7A11 signaling-mediated tubular cell ferroptosis. Int. Immunopharmacol. 2024, 135, 112303. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Jin, Y.; Liu, M.-K.; Zhang, S.-Y.; Chen, H.; Song, J. Current Progress of Hederagenin and Its Derivatives for Disease Therapy (2017–Present). Molecules 2025, 30, 1275. https://doi.org/10.3390/molecules30061275
Wang W, Jin Y, Liu M-K, Zhang S-Y, Chen H, Song J. Current Progress of Hederagenin and Its Derivatives for Disease Therapy (2017–Present). Molecules. 2025; 30(6):1275. https://doi.org/10.3390/molecules30061275
Chicago/Turabian StyleWang, Wang, Yan Jin, Meng-Ke Liu, Sai-Yang Zhang, Hong Chen, and Jian Song. 2025. "Current Progress of Hederagenin and Its Derivatives for Disease Therapy (2017–Present)" Molecules 30, no. 6: 1275. https://doi.org/10.3390/molecules30061275
APA StyleWang, W., Jin, Y., Liu, M.-K., Zhang, S.-Y., Chen, H., & Song, J. (2025). Current Progress of Hederagenin and Its Derivatives for Disease Therapy (2017–Present). Molecules, 30(6), 1275. https://doi.org/10.3390/molecules30061275






