Iron Oxide Scale Formation Mechanism and Anti-Corrosion Technology from Induction Remelting of Boiler Coating in Waste Incineration Power Plant
Abstract
1. Introduction
2. Formation of Oxide Scale and Its Formation Mechanism
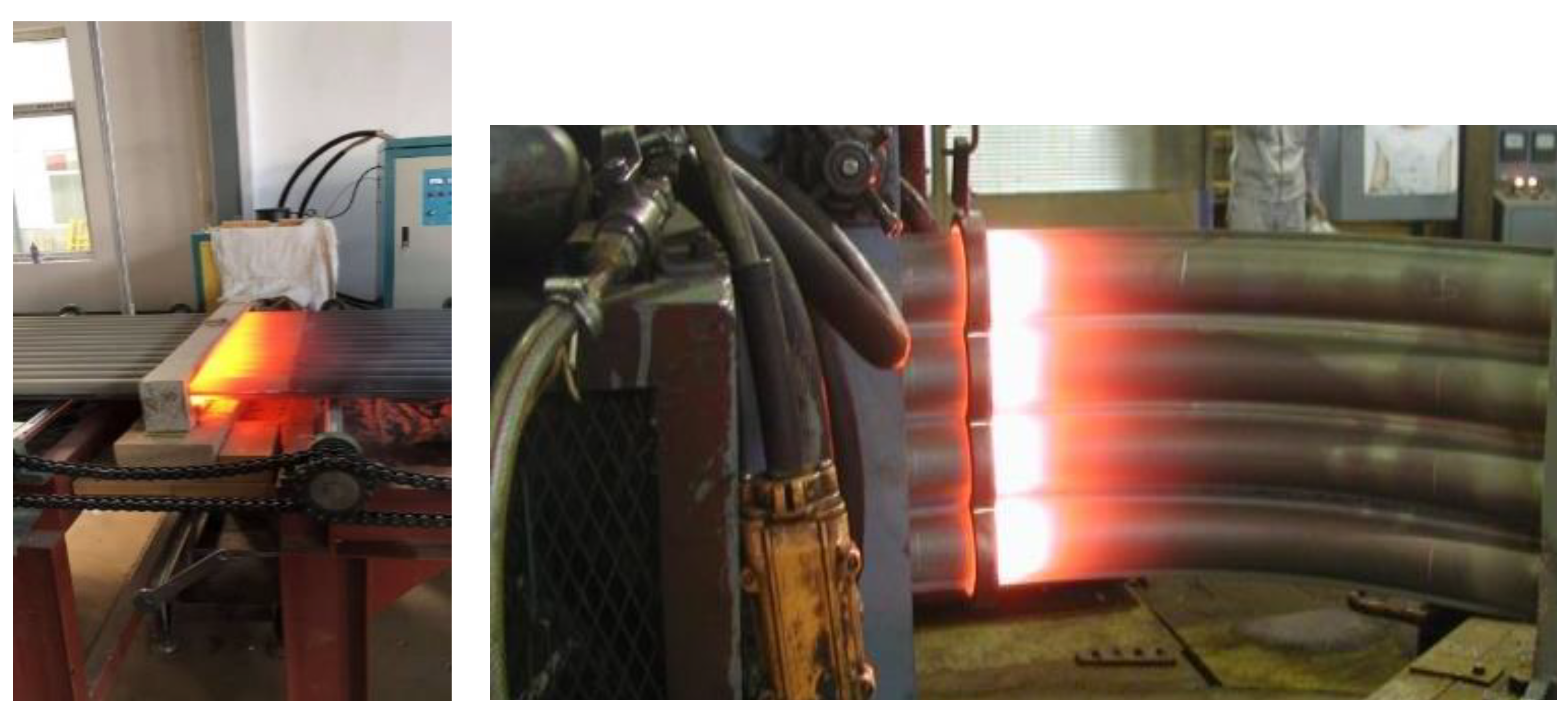
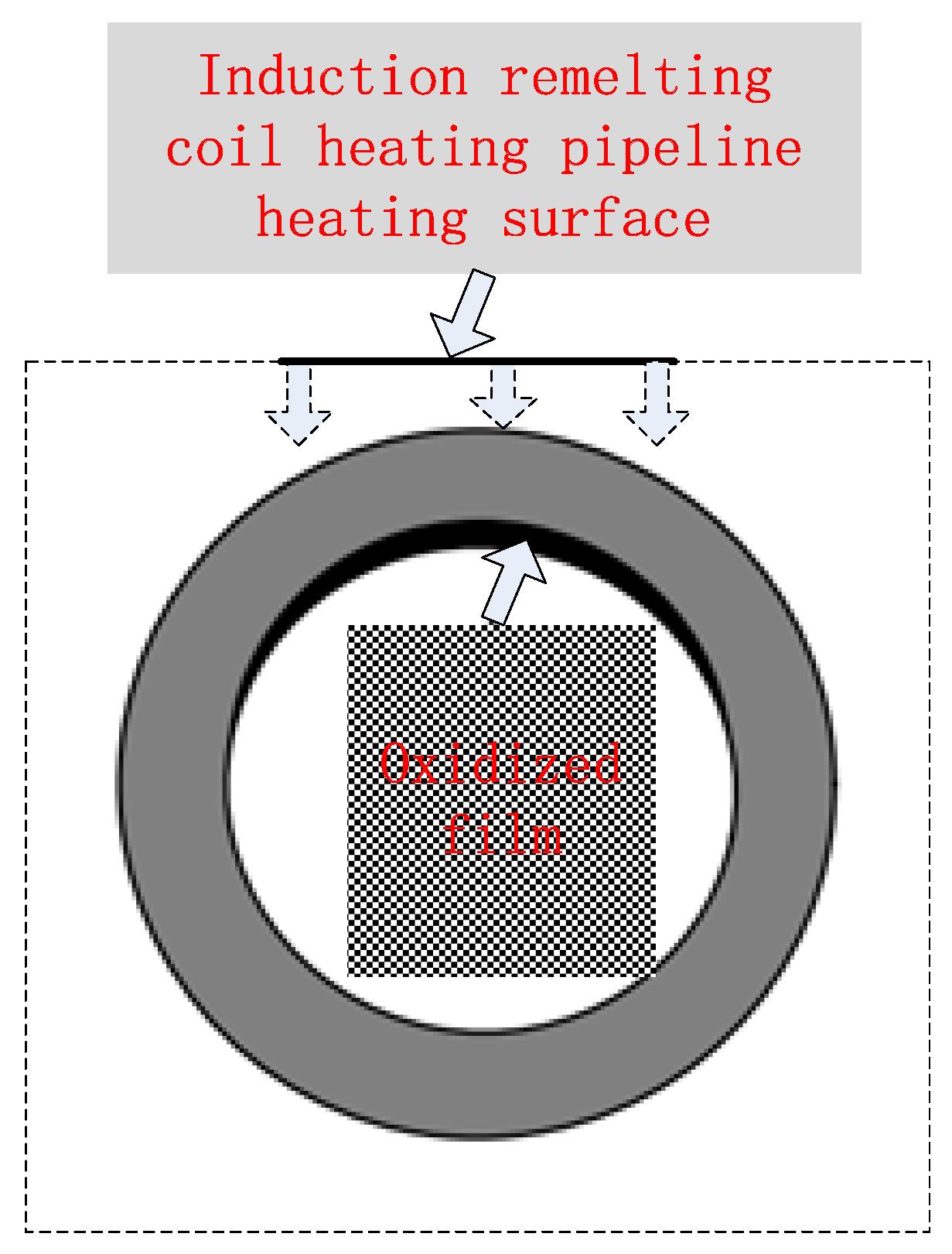
2.1. Formation of Iron Oxide Scale During Remelting
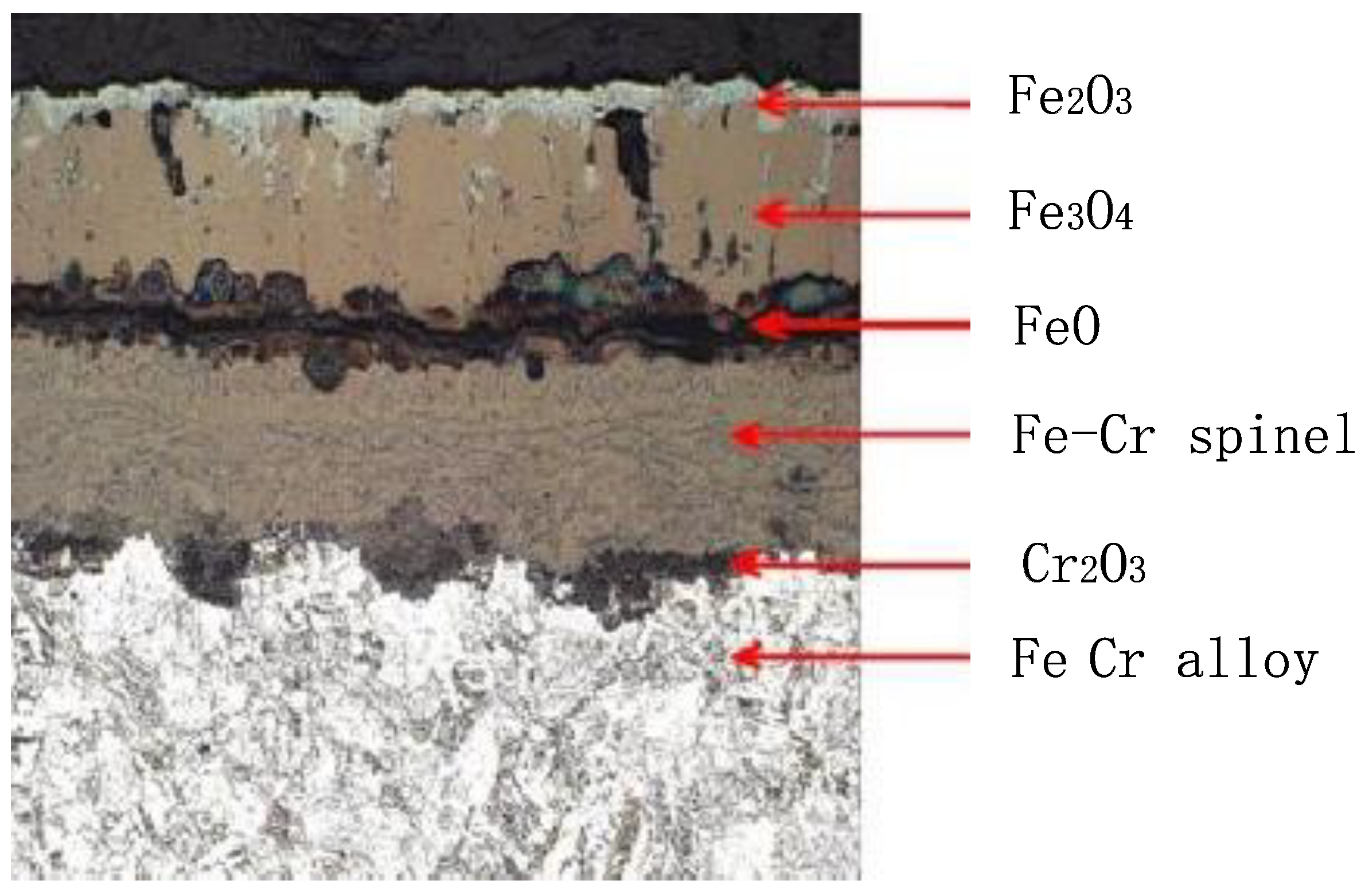
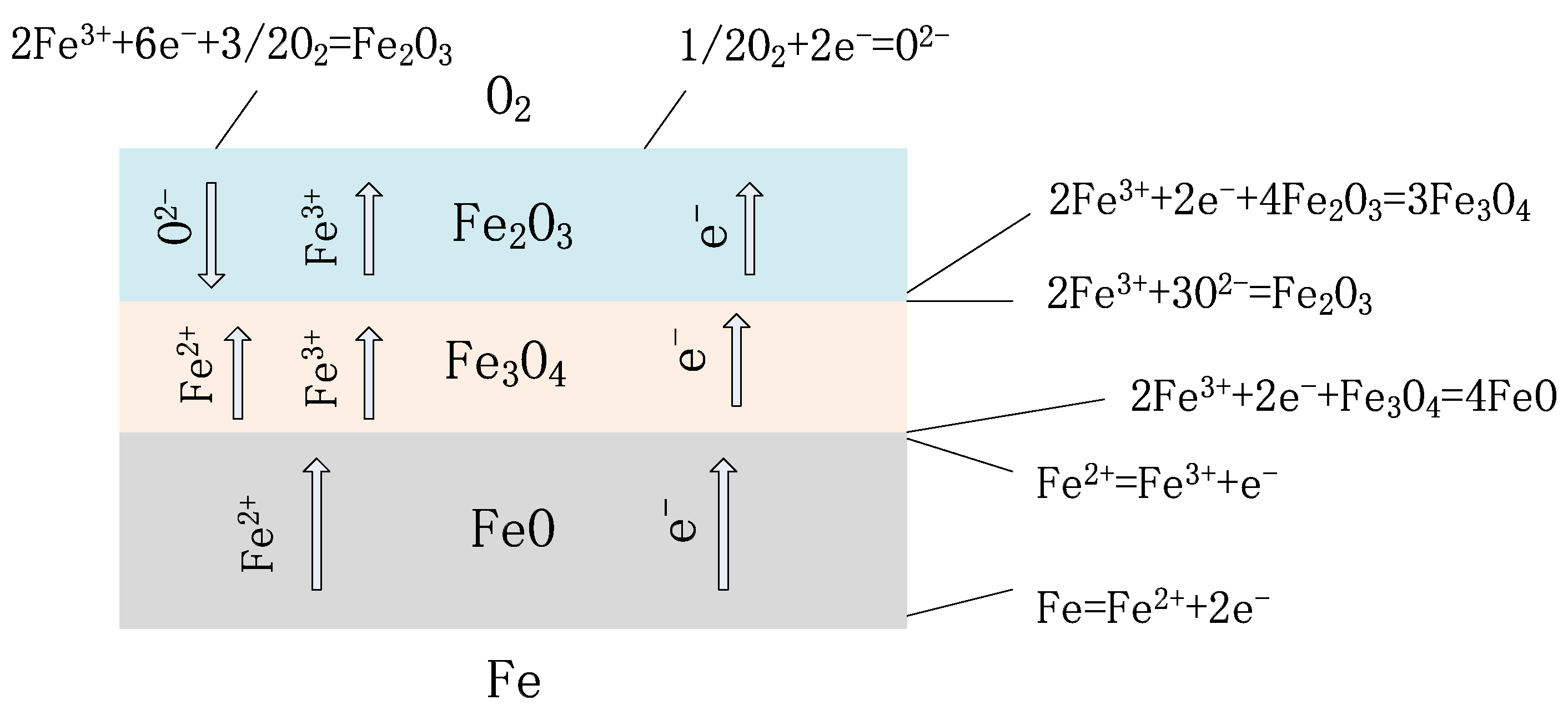
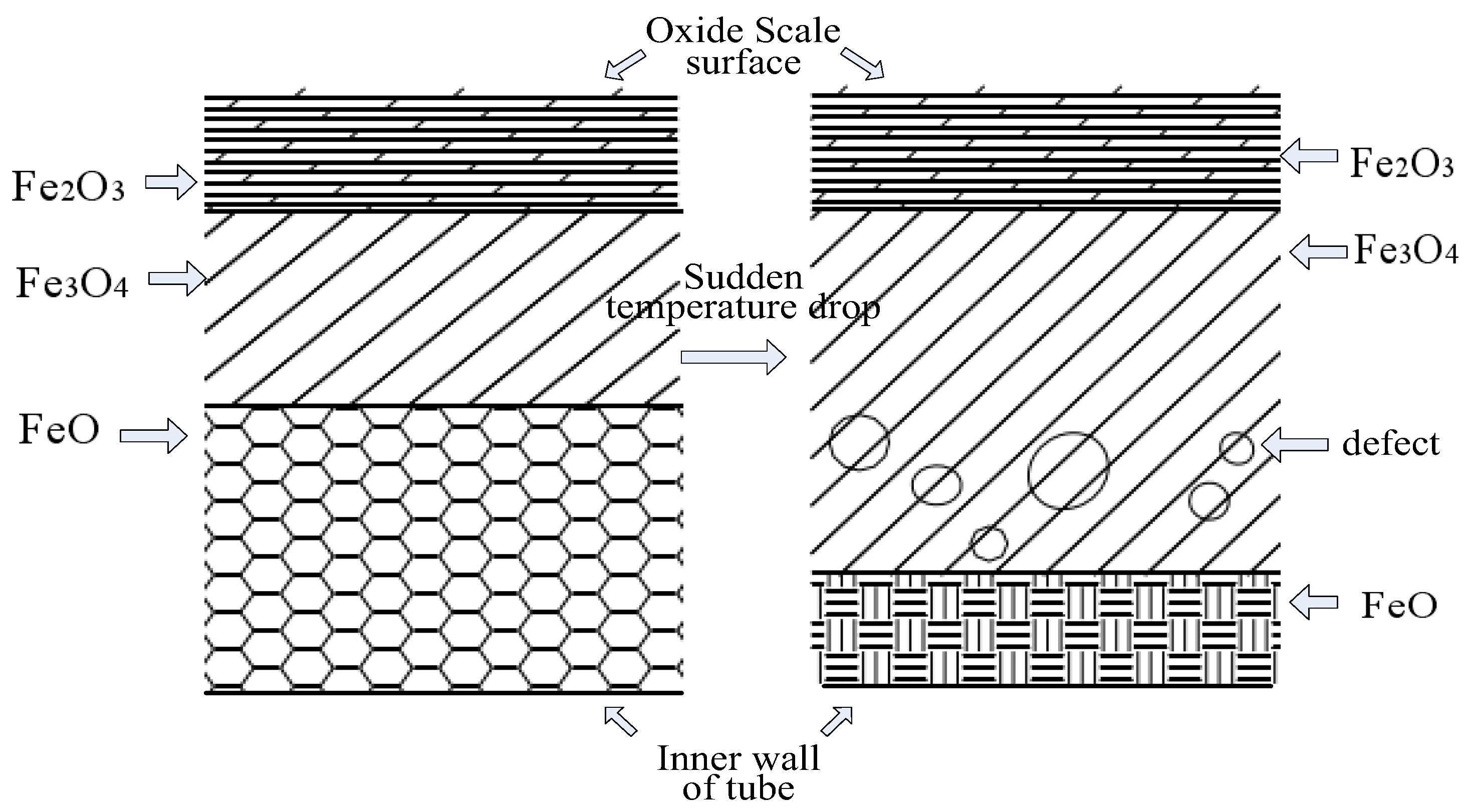
2.2. Formation Mechanism of Oxide Scale
2.3. Hazards of Oxide Scale Peel-Off
3. Influence of Oxide Scale on Mechanical Properties of Tube Inner Wall
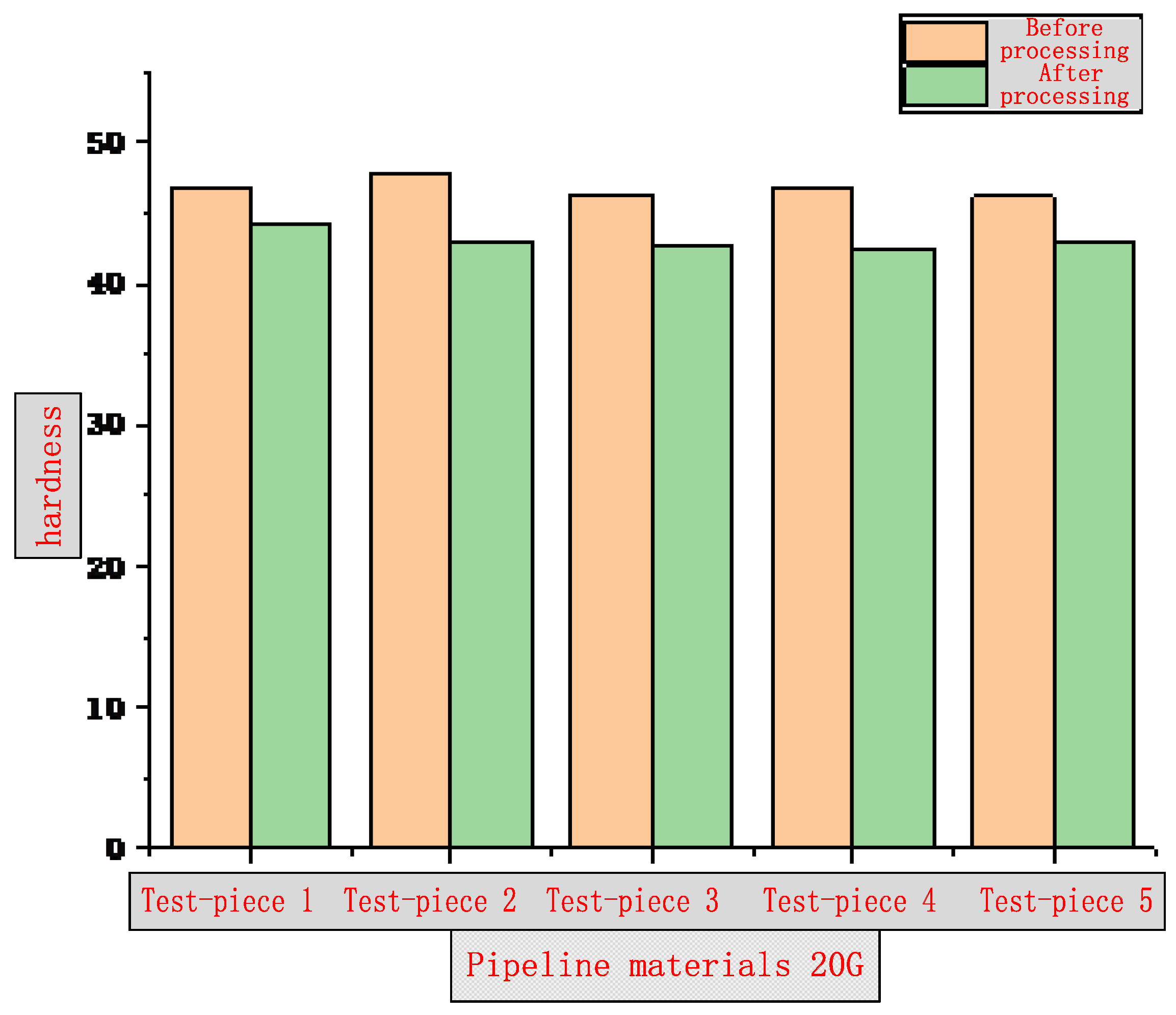
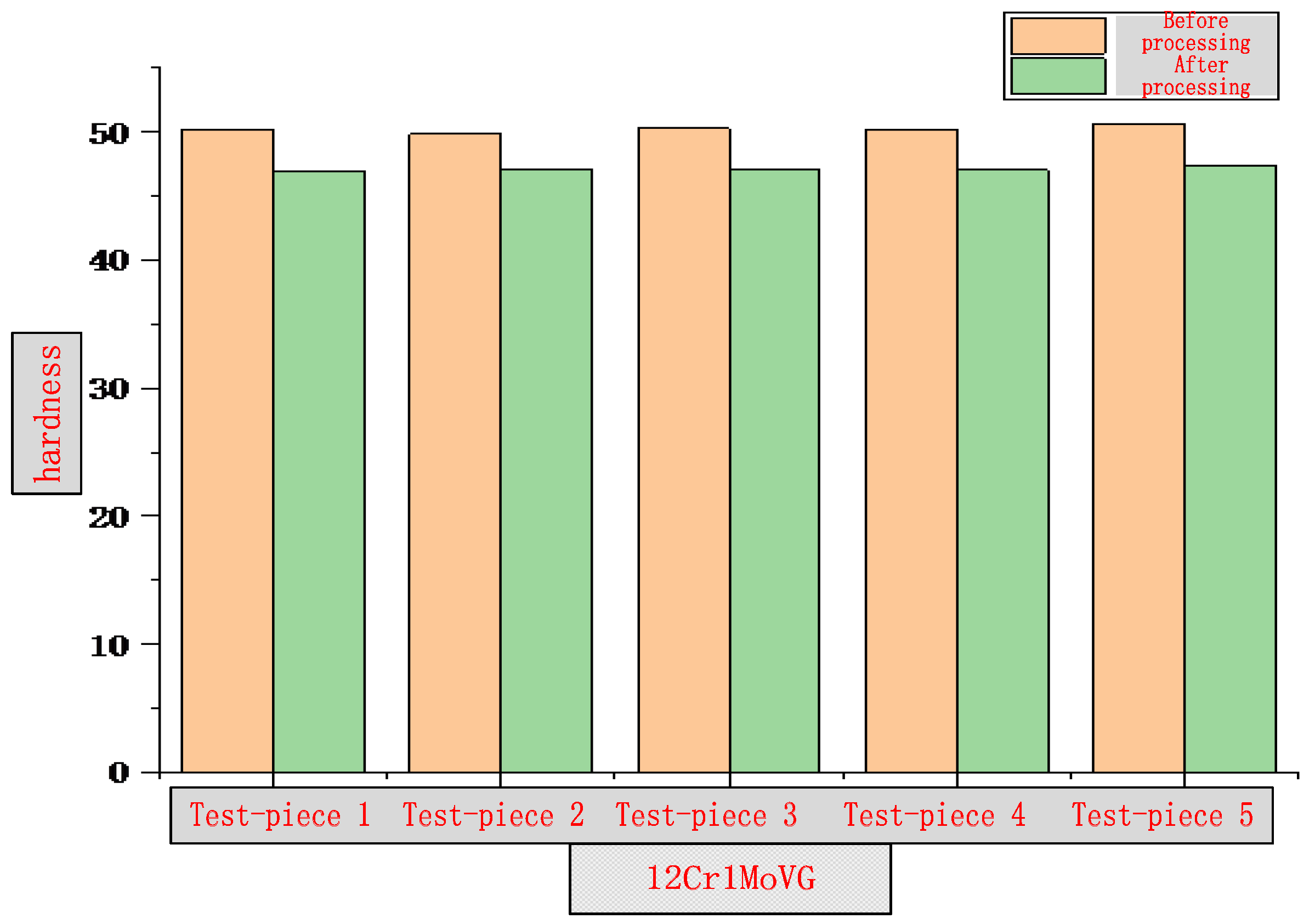
3.1. Surface Hardness
3.2. Decarburization Layer Depth Test
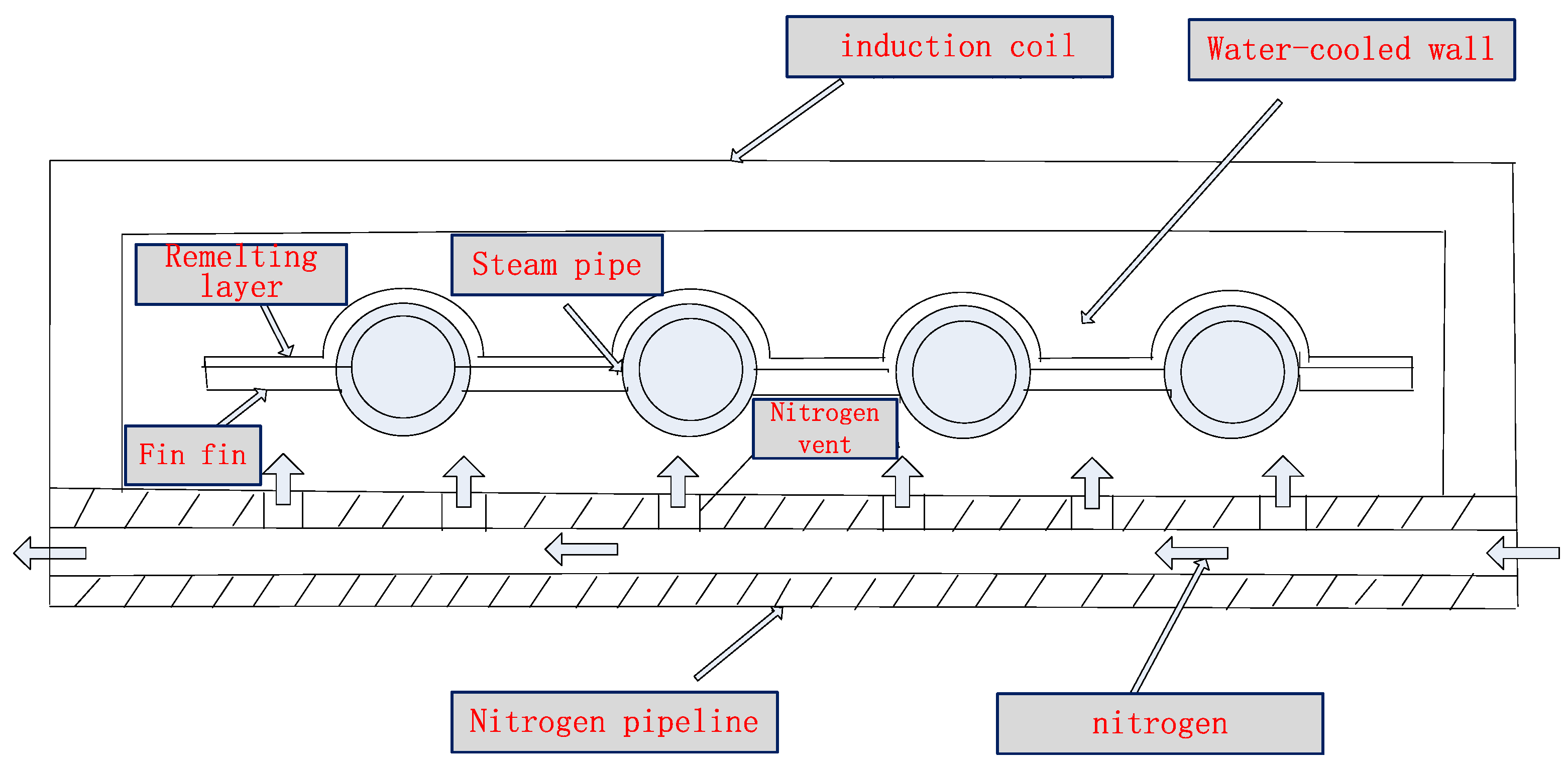
4. Technology of Inhibiting Tube Bundle Oxidization Depending on Coating Induction Remelting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Z.; Wu, X. Investigation on Internal Corrosion Failure of 15CrMo Reheater Tube. Phys. Test. Chem. Anal. Part A Phys. Test 2013, 49, 411–415. [Google Scholar]
- Wang, D.; Xu, X. Cause A nalysis of Scale Formation and Fall-off from High Temperature Heat Surfaces of 630MW Supercritical Boilers. Power Equip. 2008, 4, 310–312. [Google Scholar]
- Chen, Z. On site Anti-corrosion Welding Technology for Boiler Mode Water-cooled Wall. Weld. Technol. 2024, 53, 65–68. [Google Scholar]
- Du, J.; Wang, J.; Chen, K.; Yan, A. Online Separation and Accumulation Measurement of Oxide Scale in Supercritical Boiler Tubes. J. Eng. Therm. Energy Power 2020, 6, 103–108. [Google Scholar]
- Xu, K.; Zhang, Z.; Li, M.; Ye, X. Study on Mechanisms for Oxide Scale Formation and Exfoliation of T23 Tubes in Supercritical Boilers. Electr. Power Technol. Environ. Prot. 2014, 12, 54–57. [Google Scholar]
- Essuman, E.; Meier, G.H.; Żurek, J.; Hänsel, M.; Quadakkers, W.J. The Effect of Water Vapor on Selective Oxidation of Fe-Cr Alloy. Oxid. Met. 2008, 69, 143–162. [Google Scholar] [CrossRef]
- Sarver, J.M.; Tanzosh, J.M. An evaluation of the steam side oxidation of candidate use materials at 650 °C and 800 °C. In Proceedings of the 4th International Conference on Advances in Materials Technology for Fossil Power Plants, Hilton Head Island, SC, USA, 25–28 October 2004; pp. 1326–1340. [Google Scholar]
- Lin, J. Study of Forming Mechanism and Blockage Discipline of Oxide Skin in High-temperature Tube of Supercritical Boiler. Master’s Thesis, South China University of Technology, Guangzhou, China, May 2010. [Google Scholar]
- Holcomb, G.R. Superalloys for ultra-supercritical steam turbines-oxidation behavior. In Proceedings of the International Symposium on Superalloys, Champion, PA, USA, 14–18 September 2008; Volume 14, pp. 601–608. [Google Scholar]
- Gu, W.; Mu, S.; Song, G.; Gu, D. Analysis on Chlorine corrosion Issue from Waste incineration Boilers. Appl. Energy Technol. 2020, 269, 52–54. [Google Scholar]
- GB/T 230.1-2018; Metallic Materials—Rockwell Hardness Test—Part 1: Test Method. China National Standardization Administration: Beijing, China, 2018.
- GB/T224-2008; Determination of Depth of Decarburization of Steels. China National Standardization Administration: Beijing, China, 2008.
- GB/T 5310-2017; Seamless Steel Tubes and Pipes for High Pressure Boiler. China National Standardization Administration: Beijing, China, 2017.
- Jiang, X.; Liu, X. Research Progress and Direction Thinking on Corrosion of Key Heat Transfer Components in Waste Incineration Boilers. J. Chin. Soc. Corros. Prot. 2020, 40, 205–214. [Google Scholar]
- Qu, Z.; Tian, X. Research progress on high-temperature corrosion treatment of waste incineration power generation boilers. China Surf. Eng. 2020, 33, 50–60. [Google Scholar]








| Alloy Material | Temperature Range (°C) | Oxide Scale Thickness (μm) |
|---|---|---|
| 0–2%Cr | 500–700 | 520 |
| 9–12%Cr | 450–700 | 140 |
| 0–2%Cr | 470–1200 | 2307 |
| 0–2%Cr | 450–1200 | 603 |
| Temperature (°C) | 100 | 200 | 300 | 400 | 500 | 600 | 700 |
|---|---|---|---|---|---|---|---|
| T23 | 17.10 | 17.40 | 17.80 | 18.30 | 18.90 | 19.10 | 19.40 |
| Fe3O4 | - | - | - | - | 9.10 | - | - |
| Fe2O3 | - | - | - | - | 14.90 | - | - |
| FeO | - | - | - | - | 12.20 | - | - |
| Piping Material | Cr (%) | Power Generation Temperature (°C) | Detachment Time (h) | Thickness of Oxide Film (μm) |
|---|---|---|---|---|
| T91 | 8–9.5 | 600–605 | 11,000 | 330 |
| E911 | 8.5–9.5 | 600–605 | 11,000 | 460 |
| NF616 | 8.5–9.5 | 545 | 10,149 | 105 |
| NF616 | 8.5–9.5 | 600–605 | 11,000 | 325 |
| HCM12 | 11–13 | 545 | 10,149 | 60 |
| Esshetc 1250 | 14–16 | 600 | 12,463 | 40–80 |
| Esshetc 1250 | 14–16 | 660 | 12,719 | 100–148 |
| Esshetc 1250 | 14–16 | 633–677 | 6840 | 200–230 |
| Super 304H | 17–19 | 565 | 10,149 | 40–50 |
| NF709 | 19–22 | 565 | 10,149 | 20–25 |
| HR3C | 24–26 | 565 | 10,149 | 30 |
| AC66 | 26–28 | 600 | 12,463 | - |
| Position 1 (μm) | Position 2 (μm) | Position 3 (μm) | Position 4 (μm) | Position 5 (μm) | Average (μm) |
|---|---|---|---|---|---|
| GB/T 5310-2017 [13] 20 G Depth Requirement of Complete Decarburization Layer: Inner Surface < 400 μm | |||||
| 22.07 | 23.39 | 23.17 | 26.01 | 20.54 | 23.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Z.; Tian, X. Iron Oxide Scale Formation Mechanism and Anti-Corrosion Technology from Induction Remelting of Boiler Coating in Waste Incineration Power Plant. Molecules 2025, 30, 689. https://doi.org/10.3390/molecules30030689
Qu Z, Tian X. Iron Oxide Scale Formation Mechanism and Anti-Corrosion Technology from Induction Remelting of Boiler Coating in Waste Incineration Power Plant. Molecules. 2025; 30(3):689. https://doi.org/10.3390/molecules30030689
Chicago/Turabian StyleQu, Zuopeng, and Xinli Tian. 2025. "Iron Oxide Scale Formation Mechanism and Anti-Corrosion Technology from Induction Remelting of Boiler Coating in Waste Incineration Power Plant" Molecules 30, no. 3: 689. https://doi.org/10.3390/molecules30030689
APA StyleQu, Z., & Tian, X. (2025). Iron Oxide Scale Formation Mechanism and Anti-Corrosion Technology from Induction Remelting of Boiler Coating in Waste Incineration Power Plant. Molecules, 30(3), 689. https://doi.org/10.3390/molecules30030689






