Abstract
Citri grandis Exocarpium (Chinese name Huajuhong, HJH) is a traditional Chinese medicinal herb widely used in traditional medicines and foods in China due to its efficacy in treating coughs and excessive phlegm. This study employed HS-SPME-GC-MS to analyze the volatile compounds in HJH samples from different regions, with the aim of distinguishing samples from Huazhou from those of other origins and exploring their potential relationship with ecological factors. A multidimensional strategy was utilized to analyze the relationships between volatile oils, climatic factors, and soil elements, examining how volatile compounds responded to ecological factors. From 47 batches of HJH samples across various regions, eight significantly different volatile compounds were identified, serving as chemical markers for HJH from Huazhou. The findings elucidate the impact of ecological factors on the volatile compounds of HJH, highlighting environmental factors relating to the authenticity of HJH from Huazhou. The results indicate that the authenticity of HJH is shaped by the unique climatic and soil environments of Huazhou.
1. Introduction
According to the Chinese Pharmacopoeia (ChP 2020), Citrus grandis ‘Tomentosa’ (Huazhouyou) and Citrus grandis (L.) Osbeck are legitimate sources of the traditional Chinese medicine material Huajuhong (HJH), which is an important medicinal herb used to treat cough and excessive phlegm [1]. The aroma of HJH is fragrant, and it has a bitter and slightly acrid taste. Given that pomelo is widely distributed in southern China, regions such as Zhejiang, Hunan, Hubei, Jiangxi, Guangxi, and Guangdong are recognized as production regions of HJH. Among these, Huazhou is designated as the authentic production region, while the other locations are classified as non-authentic production regions. HJH cultivated in Huazhou is regarded as the highest-quality authentic herbal medicine [2], commanding a higher market value. This has led to unscrupulous traders using HJH from other regions instead of HJH from Huazhou [3], which adversely affects the safety and efficacy of its clinical application. Therefore, it is imperative to establish reliable methods for distinguishing between authentic and non-authentic HJH to address market discrepancies. Moreover, elucidating the scientific basis of HJH’s authenticity could facilitate the sustainable development of its industrial production.
The principal bioactive compounds of HJH include flavonoids, volatile oils, and other related substances. To date, flavonoid compounds contained in HJH have been analyzed and reported in numerous studies [4,5,6], while volatile compounds have received relatively limited attention. Previous studies have found that volatile substances possess antitussive and expectorant effects [7,8,9]. Notably potent compounds include D-limonene [10], β-pinene [11], γ-terpinene [12], and terpinene-4-ol [13], which exhibit significant antibacterial, anti-inflammatory, antioxidant, and expectorant activities. The volatile compounds of Citri grandis primarily consist of terpenes and their derivatives, such as limonene, pinene, and terpinen-4-ol [14]. The variety and abundance of these volatile compounds are crucial in the medicinal efficacy of Citri grandis [14]. However, reports on the variation of volatile compounds of HJH from different regions remains limited. Nevertheless, volatile compounds in herbal medicines have demonstrated good performance in distinguishing materials from different regions. For instance, the volatile compounds of mugwort leaves [15] and the volatile constituents of citrus peels can be utilized to differentiate products originating from various species or different geographical regions [16,17]. Volatile compounds possess significant efficacy [18] and as plant secondary metabolites, their composition and abundance are closely correlated with their geographical origin [19]. Therefore, comparative analysis of products from different production regions holds substantial significance.
Environmental factors are one of the key determinants of authenticity, primarily in relation to the geographical origin of herbal medicine, as crucial prerequisites for the accumulation of active components [20]. The growth of medicinal plants is dependent on their environmental conditions, leading to differences in the material composition of medicinal herbs from various production regions [21,22,23,24,25,26]. Differences in climate characteristics and soil physicochemical properties result in variations in the effective component content of medicinal plants [25,26]. For instance, ecological factors in the production region are the key factors affecting variations of the active components in Salvia miltiorrhiza [27], with appropriate low temperatures promoting the accumulation of ginsenosides in cells [28,29,30]. The mineral elements in the soil of different production regions also contribute to the accumulation of herbal medicine constituents. For instance, the synthesis and accumulation of monoterpene components in the fruit of Citrus reticulata ‘Chachi’ are influenced by soil nutrients and salinity [31]. Soil factors affect the accumulation of active components in herbal medicine [32].
For individual herbal medicines, the genetic mechanisms generally remain consistent in standard agricultural practices. The primary factors that contribute to the authenticity of herbal medicines are predominantly the results of changes in key environmental factors, which subsequently alter the broader ecological context [33]. For example, under drought stress, Scutellaria baicalensis can stimulate the expression of key enzyme genes involved in biosynthetic pathways of active components in its roots and leaves [34]. Environmental factors significantly influence the accumulation of effective components in herbal medicines, and the environmental factors influencing HJH vary across different production regions. Ancient Chinese medical books noted that HJH from Huazhou demonstrated enhanced cough-suppressing and expectorant effects, attributed to the rich presence of Chloriti Lapis [35] components in the soil where it was cultivated, yet there has been a paucity of research regarding the impact of these factors on its quality. Accordingly, elucidating the authenticity of HJH from an environmental perspective might further enhance the scientific understanding of its authenticity effects.
This study utilized immature pomelo fruits sourced from various regions in China as research materials for sample preparation. It employed headspace solid-phase microextraction gas chromatography–mass spectrometry (HS-SPME-GC-MS) technology for the relative quantitative analysis of volatile substances in samples from different regions. Additionally, ICP-OES was utilized to determine the total concentrations of Al, B, Ca, Cu, Fe, K, Mg, Mn, Na, P, and S in the soil, while climatic factor information was derived according to the geographic coordinates of the sampling points. A chemometric approach was applied to establish a discrimination model for HJH from different production regions, identifying material with variability in quality. By integrating soil and climatic factor data for correlation analysis, this study aimed to elucidate and clarify the influence of these factors on the primary active components in HJH, highlighting the most significant influencing variables. The findings clarify the relationship between ecological factors and the secondary metabolites in HJH, thereby explaining the environmental basis of the authenticity of HJH from Huazhou.
2. Results
2.1. GC MS Analysis
Volatile compounds were extracted from HJH samples sourced from various origins using solid-phase microextraction (SPME) and subsequently analyzed via gas chromatography–mass spectrometry (GC-MS). The reproducibility of the method was assessed by performing six replicate measurements on a specified sample. The relative standard deviation (RSD) values ranged from 2% to 12%, indicating the method’s good reproducibility (generally close to or lower than 10%) [17]. The acquired results showed that the conditions for extraction and GC-MS analysis were reliable and robust. A total of 33 volatile compounds were identified, primarily consisting of terpene compounds, aligning with findings reported by Fan et al. [14] that identified D-limonene, γ-terpinene, and β-pinene as the predominant constituents of HJH essential oil. Notably, m-cymene was identified as a unique component in COREs; detailed compound information is available in Table 1. Figure 1A,B illustrate the total ion chromatograms (TICs) of essential oils from COREs compared with those from Non-COREs, revealing that COREs contained a greater diversity of compounds. Further characterization of these components revealed 12 monoterpenes, 19 sesquiterpenes, as well as two ester compounds (results depicted in Table 1). Monoterpenes, such as D-limonene, are classified as compounds composed of two isoprene units, typically containing ten carbon atoms, with the fundamental carbon skeleton formed by head-to-tail sequence connection of isoprene units. Oxygenated derivatives of monoterpenes, such as compounds 11, 13, and 14, were also present. In contrast, sesquiterpenes consist of three isoprene units and contain fifteen carbon atoms, with γ-cadinene illustrated as an example of this class, exhibiting a similar head-to-tail connection of isoprene units.
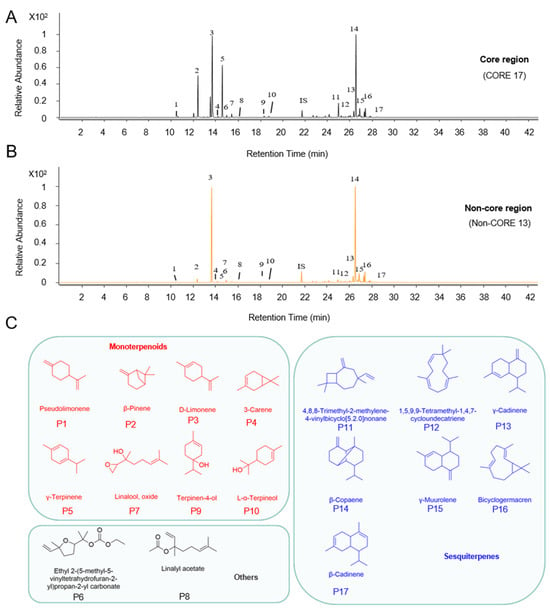
Figure 1.
GC-MS typical total ion chromatograms (TICs) of volatile compounds in the pomelo. Comparative analysis of essential oils in the fruit of pumelo in different planting areas: (A) TICs of volatile compounds in the HJH from the core region (CORE 17); (B) TICs of volatile compounds in the HJH from the non-core region (Non-CORE 13). Numbers represent the common peak order in both COREs and Non-COREs; (C) Classification of volatile oil from HJH. Numbers represent the peak order. The peak number represents the common peak in (A,B).
From the mass spectrometric data of 47 samples from different origins, 17 common peaks were selected (Figure 1C), encompassing the principal compounds in HJH essential oil. The relative peak areas of these compounds were compiled into a dataset, including the specific data detailed in Table 2; the average sum of the relative peak areas accounted for 88.37%. Studies have shown [36,37] that solid-phase microextraction (SPME) can also be used for quantitative analysis in a non-equilibrium state. Therefore, as long as the samples are extracted under the same conditions, the differences in volatile compounds of HJH samples from different regions can be compared. Research indicates that the compounds represented by common peaks, as well as the peaks with higher relative abundances, demonstrate faster identification rates, enhanced stability, and improved quantitative repeatability [38]. Accordingly, the analysis excluded certain minor or trace volatile compounds.

Table 2.
Identification of prevalent volatile compounds in HJH.

Table 1.
Detailed compound information.
Table 1.
Detailed compound information.
| No. | RT/min | Compound | Formula | CAS No. | RI | Reference | Sim Score |
|---|---|---|---|---|---|---|---|
| 1 | 10.56 | α-Pinene | C10H16 | 80-56-8 | 930 | [39] | 94.73 |
| 2 | 11.97 | Pseudolimonene | C10H16 | 499-97-8 | 975 | [40] | 95.35 |
| 3 | 12.36 | β-Pinene | C10H16 | 127-91-3 | 988 | [41] | 96.21 |
| 4 | 12.89 | α-Phellandrene | C10H16 | 99-83-2 | 1004 | [42] | 91.13 |
| 5 | 13.48 | m-Cymene | C10H14 | 535-77-3 | 1023 | [43] | 97.13 |
| 6 | 13.65 | D-Limonene | C10H16 | 5989-27-5 | 1029 | [44] | 98.35 |
| 7 | 14.15 | 3-Carene | C10H16 | 13466-78-9 | 1045 | [40] | 95.09 |
| 8 | 14.55 | β-Terpinene | C10H16 | 99-85-4 | 1058 | [45] | 97.75 |
| 9 | 14.93 | Ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl) propan-2-yl carbonate | C13H22O4 | - | 1048 | [46] | 95.8 |
| 10 | 15.38 | α-Terpinene | C10H16 | 99-86-5 | 1084 | [45] | 95.51 |
| 11 | 15.43 | Linalool oxide | C10H18O2 | 1365-19-1 | 1086 | [47] | 92.93 |
| 12 | 15.82 | Linalyl acetate | C12H20O2 | 115-95-7 | 1093 | [48] | 80.05 |
| 13 | 18.29 | Terpinen-4-ol | C10H18O | 562-74-3 | 1178 | [42] | 93.14 |
| 14 | 18.72 | α-Terpineol | C10H18O | 10482-56-1 | 1191 | [49] | 94.51 |
| 15 | 22.68 | δ-EIemene | C15H24 | 20307-84-0 | 1410 | [39] | 95.33 |
| 16 | 23 | Cadina-3,5-diene | C15H24 | 267665-20-3 | 1416 | [46] | 94.22 |
| 17 | 23.23 | Humulene | C15H24 | 6753-98-6 | 1421 | [50] | 85.33 |
| 18 | 23.78 | Copaene | C15H24 | 3856-25-5 | 1433 | [41] | 96.18 |
| 19 | 24.12 | Guaia-10(14),11-diene | C15H24 | - | 1440 | - | 95.09 |
| 20 | 24.95 | 2-methylene-4,8,8-trimethyl-4-vinyl-bicyclo[5.2.0]nonane | C15H24 | - | 1458 | - | 97.36 |
| 21 | 25.43 | Valencene | C15H24 | 4630-07-3 | 1469 | [51] | 94.78 |
| 22 | 25.86 | 1,5,9,9-Tetramethyl-1,4,7-cycloundecatriene | C15H24 | - | 1478 | [52] | 94.77 |
| 23 | 26.02 | Bicyclosesquiphellandrene | C15H24 | 54324-03-7 | 1481 | [53] | 94.8 |
| 24 | 26.32 | γ-Cadinene | C15H24 | 483-74-9 | 1488 | [50] | 96.69 |
| 25 | 26.52 | β-Copaene | C15H24 | 18252-44-3 | 1492 | [42] | 97.35 |
| 26 | 26.76 | γ-Muurolene | C15H24 | 30021-74-0 | 1497 | [48] | 95.3 |
| 27 | 26.86 | Bicyclogermacren | C15H24 | 67650-90-2 | 1499 | [54] | 95.28 |
| 28 | 27.01 | δ-Cadinene | C15H24 | 483-76-1 | 1502 | [50] | 92.8 |
| 29 | 27.37 | β-Cadinene | C15H24 | 523-47-7 | 1510 | [55] | 94.82 |
| 30 | 27.46 | Calamenene | C15H24 | 72937-55-4 | 1512 | [46] | 93.19 |
| 31 | 27.73 | 4-Isopropyl-1,6-dimethyl-1,2,3,4,4a,7-hexahydronaphthalene | C15H24 | 16728-99-7 | 1518 | [56] | 86.44 |
| 32 | 27.83 | α-Amorphene | C15H24 | 483-75-0 | 1520 | [57] | 94.92 |
| 33 | 28.4 | Germacrene B | C15H24 | 15423-57-1 | 1532 | [39] | 91.84 |
| IS | 21.69 | n-Tridecane | C13H28 | 629-50-5 | 1300 | - | 96.59 |
‘’RT’’, retention time (min). “-” indicates a compound without a CAS number. “RI”, retention index.
2.2. Discrimination Between COREs and Non-COREs Based on Multivariate Analysis
This study employed principal component analysis (PCA) to investigate potential differences between COREs and Non-COREs. Upon determining the projections for each principal component, each sample was assigned a score based on these components. A PCA score plot was generated using the scores PC1 and PC2 from the two principal components, as illustrated in Figure 2A. In the presented study, four principal components accounting for 82.4% (R2X) of the total variance were considered significant (PC1 described 56.5% of the sample variability, and PC2 described 11.2%). The results indicated that PC1 had the highest cumulative contribution rate to the total variance, and the predictive ability of the model (Q2) was 0.508, suggesting that the model required further optimization (Q2 ≥ 0.50). The score plot enables observation of the degree of clustering and dispersion among samples; closer distribution points indicate greater similarity, while greater separation suggests significant differences. In the plot, the COREs and Non-COREs are located within distinct regions, with a noticeable separation between them, indicating significant differences in volatile compounds between the samples from the two groups. Comparing the score plot and the loading plot, differential intra-group or inter-group compounds could be rapidly identified. The significance of the differentiation was validated through statistical analyses, including t-tests and ANOVA.
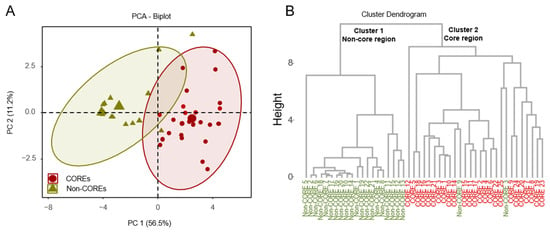
Figure 2.
Comparative analysis of essential oils in pomelo fruit collected from different regions: (A) Principal component analysis (PCA) of Euclidean distance for the essential oil contents in core and the non-core region samples; (B) Dendrograms of the hierarchical cluster analysis (HCA) result. Red points mean COREs. Olive green points mean Non-COREs. Red fond means COREs, and olive green fond means Non-COREs.
Additionally, hierarchical clustering was performed using the Ward method, generating a dendrogram as illustrated in Figure 2B. The results indicate that the 47 samples could be broadly categorized into two groups: COREs and Non-COREs, highlighting a clear distinction in volatile compounds between HJH from Huazhou and samples from other regions.
To achieve more precise results, the mass spectrometry data of COREs and Non-COREs were evaluated based on supervised OPLS-DA. The OPLS-DA results for the volatile oil components of HJH indicated a clear separation between the authentic and non-authentic regional samples (see Figure 3). The scores for the first principal component (horizontal axis) positioned the COREs predominantly on the negative half of the axis, while the Non-COREs were situated on the positive half of the axis, suggesting significant differences in volatile oil composition based on geographical origin. The model identified one predictive component and two orthogonal components. The fitting index for the independent variables (R2X) was 0.717, indicating that the three principal components accounted for 71.7% of the variance in the X variable, with the predictive component contributing 47.2% and the orthogonal components contributing 24.5%. The fitting index for the dependent variable (R2Y) was 0.903, signifying that the predictive component accounted for 90.3% of the variance in the Y variable. In addition, the model’s predictive index (Q²) was 0.823, demonstrating 82.3% prediction accuracy for different origins of HJH. Both R² and Q2 exceeded 0.5, indicating a good model fit. In the permutation test results, the R2Y and Q2Y values of the actual and simulated models, after random permutation, yielded scatter plots where the model R2Y and Q2Y (scatter) values were both lower than the true values (indicated by the horizontal line). Additionally, the p-values for the R²Y fitting index and the Q2 predictive index were both equal to 0.05, suggesting that the model fitted well without overfitting. The variable contribution within the OPLS-DA model was assessed based on the Variable Importance for the Projection (VIP) score, defined as the weighted sum of the PLS weights. Variables with a VIP score greater than 1 were considered significant in the model. Moreover, the Log2FC for each compound was calculated based on its relative abundance. Components with both VIP and Log2FC values greater than 1 were selected as potential quality markers, resulting in a total of eight components (α-terpineol (P10), γ-cadinene (P13), β-copaene (P14), γ-muurolene (P15), bicyclogermacren (P16), β-cadinene (17), β-pinene (2), and terpinen-4-ol (P9)). These eight compounds exhibited significant differences in content between COREs and Non-COREs (p < 0.001), indicating their potential as distinguishing biomarkers.
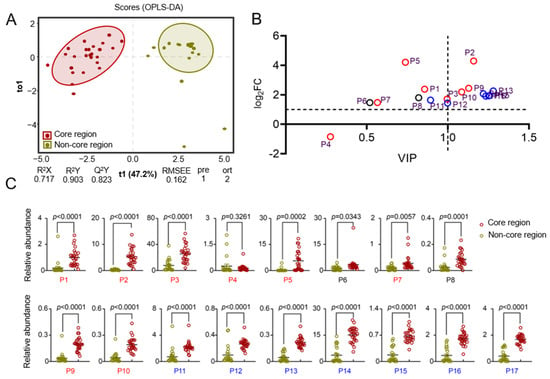
Figure 3.
Screening of volatile compound quality markers across samples from different regions: (A) Score plot of orthogonal partial least squares discrimination analysis (OPLS-DA) models for the classification of pomelo; (B) Quadrant plot constructed using the Variable Importance in Projection (VIP) values obtained from OPLS-DA as the x-axis, while the y-axis represents the Log2FC values of the relative abundance of volatile compounds. Compounds with VIP values greater than 1 and Log2FC values greater than 1 were selected as potential quality markers; (C) Results of t-tests on the relative abundance of compounds across different regions. P1, pseudolimonene; P2, β-pinene; P3, D-limonene; P4, 3-carene; P5, γ-terpinene; P6, ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl)propan-2-yl carbonate; P7, linalool oxide; P8, linalyl acetate; P9, terpinen-4-ol; P10, α-terpineol; P11, 2-methylene-4,8,8-trimethyl-4-vinyl-bicyclo[5.2.0] nonane; P12, 1,5,9,9-tetramethyl-1,4,7-cycloundecatriene; P13, γ-cadinene; P14, β-copaene; P15, γ-muurolene; P16, bicyclogermacren; P17, β-cadinene. Red represents monoterpenes, blue represents sesquiterpenes, and black represents other types of compounds in Figure 3B.
2.3. Environmental Factors Analysis
The synthesis and accumulation of plant secondary metabolites are influenced not just by species, organ, and growth developmental stages but also by ecological factors [58]. The concentration of secondary metabolites in plants is correlated with climatic and soil conditions in their respective regions. To investigate the impact of different cultivation areas on the accumulation of volatile compounds in HJH, it is essential to ascertain whether ecological factors differ across various production regions. The results indicate that the climatic conditions in the core and non-core regions are significantly distinct (Figure 4A). For the first principal component (PC1), the climatic factors of the core region predominantly clustered on the positive side, while those of the non-core region were mainly positioned on the negative side. This suggests that geographic factors were the primary contributors to the observed climatic differences, with an explanatory power of 67.3% for PC1. Moreover, PCA dimensionality reduction analysis revealed that soil factors from different regions could generally be grouped into two categories (Figure 4C). For the first principal component (PC1), the soil factors of the core region were primarily located on the negative side of the horizontal axis, whereas those from the non-core region clustered on the positive side. Similarly to climatic factors, regional differences emerged as a significant influence on soil element variation, accounting for 36.8% of the explanatory power. These results underscore the evident differences in climatic and soil factors between the core and non-core regions, with the variability in climatic factors being more pronounced than that in soil factors, suggesting that they may be crucial determinants of volatile oil content in HJH.
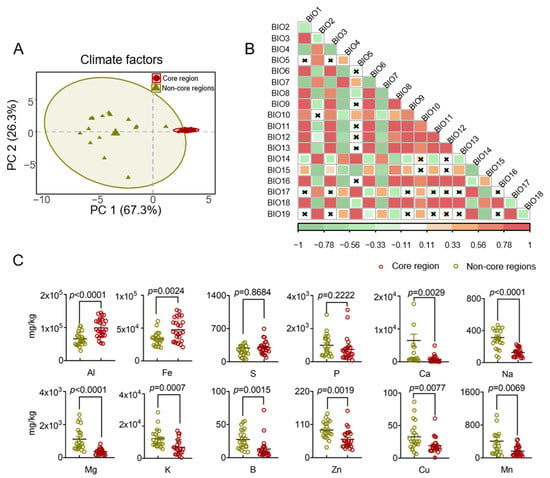
Figure 4.
Comparative analysis of environmental factors in different pomelo-collecting regions: (A) Principal component analysis of Euclidean distance for climatic factors in core and non-core region samples; (B) Auto-correlation of climatic factors by Pearson analysis; (C) Soil physical and chemical properties in rhizosphere soil samples from the core region differed from those in samples from the non-core region. Significance between core and non-core regions indicated by t-test. Red means core regions, and olive green means non-core regions.
To address the issue of multicollinearity among climate factor variables, the selection of variables (temperature, temperature change, precipitation, precipitation change, and soil) was carried out based on distinct climate factor categories. Environmental factors with similar characteristics and low contribution rates were excluded. In this study, four principal components accounting for 98.3% (R2X) of the total variance were considered significant (PC1 described 67.3% of the sample variability, and PC2 described 26.3%). The predictive ability of the model (Q2) was 0.956, which indicated that it was a good model (Q2 ≥ 0.50). Both domains (core region and non-core region) were clearly separated from each other. Through the integration of PCA and Pearson correlation analysis, environmental factors with absolute loadings exceeding 0.80 in the PCA were identified. In cases where two or more correlation coefficients ∣r∣ ≥ 0.8 were observed among the same environmental factors, the factor with the highest contribution rate was chosen to differentiate between the two regions [59]. The soil factors were selected to include differences between the two groups and contain statistically significant elements. The screening results are presented in Table 3.

Table 3.
Environmental factors.
2.4. Correlation Analysis of Climatic Factors and Soil Factors with Volatile Compounds
Plants produce a variety of secondary metabolites to adapt to diverse environmental stresses during their growth. These secondary metabolites often possess multiple biological activities, providing humans with healthy food and medicinal products [60]. In this study, a correlation analysis was conducted to examine the relationship between the volatile compounds content from HJH of different origins characterized by various climatic factors and soil mineral elements (Figure 5A). Specifically, BIO15, BIO18, and BIO6 exhibited a positive correlation with the volatile oil content of HJH, while BIO7 showed a negative correlation. Furthermore, BIO18 was negatively correlated with sodium (Na), manganese (Mn), and magnesium (Mg) in the soil, whereas BIO15 had a negative correlation with zinc (Zn) content. Additionally, BIO6 demonstrated negative correlations with Zn, Na, Mn, and Mg. Conversely, BIO7 was positively correlated with the concentrations of Zn, Na, Mn, Mg, and aluminum (Al) in the soil. The soil boron (B) content was negatively correlated with BIO6 and BIO18 but positively correlated with BIO7. B was positively correlated with Na, Mg, and potassium (K), while calcium (Ca) was positively associated with Zn, Na, Mn, Mg, and copper (Cu). Copper (Cu) showed a positive correlation with Na, and Al was positively correlated with iron (Fe). Notably, Al was also positively correlated with the volatile oil component P9. The volatile oil components α-terpineol (P10), γ-cadinene (P13), β-copaene (P14), γ-muurolene (P15), bicyclogermacren (P16), β-cadinene (P17), β-pinene (P2), and terpinen-4-ol (P9) exhibited positive correlations with the climatic factors BIO15, BIO18, and BIO6, while demonstrating negative correlations with BIO7 and the soil elements Zn, Na, Mg, and Ca.
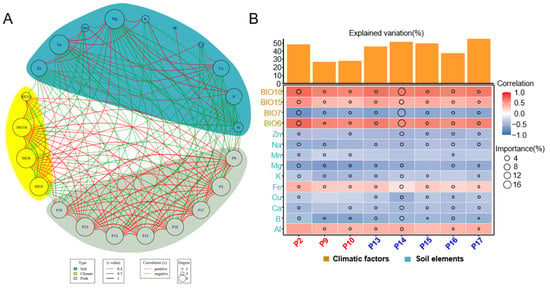
Figure 5.
Correlation analysis of soil and climate elements with volatile compounds in pomelos sourced from core and non-core regions: (A) A network was constructed by correlation of compounds (grey), climatic factors (yellow), and soil elements (green). Node size corresponds to the degree of each node. The thickness and color of the edges denote strength and significance, respectively. Red and green lines indicate positive and negative correlations; (B) Contributions of soil physical–chemical properties and climatic factors to the components with varying levels among different regions in plants. P 10, α-terpineol; P13, γ-cadinene; P14, β-copaene; P15, γ-muurolene; P16, bicyclogermacren; P17 β-cadinene; P2, β-pinene; P9, terpinen-4-ol.
The analysis was conducted through multiple linear regression and variance decomposition (Figure 5B). The results revealed that the selected climatic and soil factors collectively explained 48.62% of the variance in β-pinene (P2), 26.58% in terpinen-4-ol (P9), 28.13% in α-terpineol (P10), 45.59% in γ-cadinene (P13), 51.55% in β-copaene (P14), 49.63% in γ-muurolene (P15), 37.55% in bicyclogermacren (P16), and 55.64% in β-cadinene (P17) (Figure 5B). Notably, the climatic factors—Precipitation in the Warmest Quarter (BIO18), Precipitation Seasonality (BIO15), Precipitation in the Driest Quarter (BIO7), and Precipitation in the Wettest Quarter (BIO6), as well as the concentrations of B, Mg, Ca, Zn, and Fe in the soil, were identified as key determinants for distinguishing material with potential variability in quality across different regions. The influence of climatic factors, particularly concerning the variability of β-copaene (P14), was notably pronounced, showing a positive correlation with BIO18, BIO15, BIO6, and soil Fe, while displaying a negative correlation with soil concentrations of Ca, B, and Zn. In comparison to climatic factors, the mineral element in the soil had less influence on the differences in the content of potential quality differentiators in HJH, and the concentrations of Fe and Al in the soil exhibited a positive correlation with the levels of material with potential variability in quality, whereas other elements generally correlated negatively.
A random forest analysis was employed to further identify the factors associated with the volatile components in traditional medicines, with the results presented in Figure 6A. BIO7, BIO15, and BIO18 were identified as the primary factors influencing monoterpenes and sesquiterpenes. The main influencers of monoterpene content included BIO6, BIO7, BIO15, and BIO18, while the key factors impacting sesquiterpene content included BIO6, BIO7, BIO18, Na, Cu, and Ca. Additionally, considering the geological characteristics of the core region, factors such as Al and Fe (elements that are primarily found in Chloriti Lapis, which is rich in the soil of Huazhou ) were included in the subsequent SEM validation model. In the SEM model constructed in this study, a p-value less than 0.05 indicated that the model exhibited a good fit to the data and was suitable for data validation. The outcome showed that BIO6 had a positive correlation with the abundance of volatile compounds, whereas Cu content in soil was negatively correlated with the abundance of volatile compounds. The results indicate that the unique climate in the core region and soil elements together affect the volatile compounds in medicinal materials.
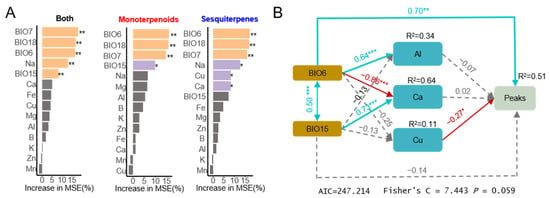
Figure 6.
The major factors influencing volatile compounds evaluated by Random Forest and SEM analysis: (A) The main factors influencing the relative abundance of compounds were analyzed and evaluated based on a random forest model. Both means the sum of the relative abundances of monoterpene compounds and sesquiterpene compounds. The monoterpenes part represents the average relative abundance of monoterpene compounds included in the model. The sesquiterpenes part represents the average relative abundance of sesquiterpene compounds included in the model; (B) The direct and indirect relationships between climatic factors, soil elements, and the total relative abundance of compounds, assessed based on structural equation modeling (SEM). The arrows represent the direction of hypothesized causation. The red line represents adverse effects, the blue line represents positive effects, and the gray line represents no significant effect. * p < 0.05, ** p < 0.01 and *** p < 0.001. In this part, “peaks” means the sum result of the relative peak areas of the eight selected compounds.
3. Discussion
This study employed HS-SPME-GC-MS technology for the relative quantification of volatile compounds in the COREs and Non-COREs. Subsequently, OPLS-DA was conducted to identify variable-quality material in the essential oils from different regions. Permutation tests indicated a good fit for the model, with no evidence of overfitting, suggesting that the identification of materials with potential variability in quality was statistically significant. The volatile compounds in HJH are known to have expectorant and antitussive effects [7,8,9]. Based on their chemical structures, these compounds can be categorized into three main types: monoterpenoids, sesquiterpenoids, and a small amount of other compounds. The monoterpenoid compounds include pseudolimonene, β-pinene, D-limonene, 3-carene, γ-terpinene, linalool oxide, terpinen-4-ol, and α-terpineol. The sesquiterpenoid compounds mainly comprise γ-cadinene, β-copaene, γ-muurolene, bicyclogermacrane, β-cadinene, 2-methylene-4,8,8-trimethyl-4-vinyl-bicyclo[5.2.0]nonane, and 1,5,9,9-tetramethyl-1,4,7-cycloundecatriene. Other compounds include ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl) propan-2-yl carbonate and bicyclo[5.2.0]nonane, as well as linalyl acetate. Currently, there are few studies on the biological activities of Pseudolimonene; however, as a monoterpenoid, it may exhibit potential antimicrobial and antioxidant properties [61]. β-Pinene has demonstrated antimicrobial and antifungal activities, as well as anti-inflammatory and analgesic effects [11]. D-Limonene, the most abundant component in Citrus reticulata volatile oils, exhibits significant antioxidant, antimicrobial, anti-inflammatory properties, along with notable expectorant and antitussive effects [10,61]. 3-Carene shows both antimicrobial and antioxidant activities [62], while γ-terpinene is effective in scavenging free radicals and offers analgesic and expectorant effects [12]. Both terpinen-4-ol and α-terpineol exhibit antimicrobial, anti-inflammatory, and antioxidant activities [13]. Among the sesquiterpenoids, γ-cadinene demonstrates antimicrobial and antioxidant effects. Studies have shown its ability to inhibit the growth of various bacteria and fungi [61]. β-Copaene, γ-muurolene, bicyclogermacrane, and β-cadinene all exhibit antimicrobial and antioxidant activities as well. Additionally, linalool oxide and linalyl acetate not only have anti-inflammatory and antioxidant effects but also inhibit the growth of various bacteria and fungi [63]. While there has been limited research on the biological activities of ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl) propan-2-yl carbonate, 2-methylene-4,8,8-trimethyl-4-vinyl-bicyclo[5.2.0]nonane, and 1,5,9,9-tetramethyl-1,4,7-cycloundecatriene, their chemical structures suggest that they may possess potential antimicrobial and antioxidant properties [61]. These volatile compounds play an important role in the pharmacological activity of traditional Chinese medicine, with D-limonene [10], β-pinene [11], γ-terpinene [12], and terpinen-4-ol [13] being among the most potent and abundant compounds in HJH volatile oils. In conclusion, volatile compounds play a significant role in the pharmacology of traditional Chinese medicine, and drying treatments do not affect their biological activities. This provides a scientific foundation for the development and application of traditional Chinese medicine.
GC-MS data analysis revealed differences in the relative abundance of volatile compounds between the COREs and Non-COREs. Eight potential chemical markers were identified, including α-terpineol (P10), γ-cadinene (P13), β-copaene (P14), γ-muurolene (P15), bicyclogermacrane (P16), β-cadinene (P17), β-pinene (P2), and terpinen-4-ol (P9). Among these, β-pinene, γ-cadinene, terpinen-4-ol, and α-terpineol are known to have significant antitussive and expectorant effects [11,12,13,64,65]. The other four compounds, which have structures similar to those of the aforementioned compounds, may also promote similar effects. Notably, all eight compounds were found to be present in higher concentrations in the COREs, which could be one of the reasons for the superior therapeutic efficacy of authentic medicinal materials.
Furthermore, ICP-OES was utilized to determine the concentrations of Al, B, Ca, Cu, Fe, K, Mg, Mn, Na, P, and S in the soil. Climate factor information was obtained based on the latitude and longitude of sampling points. Significant climate and soil factors distinguishing authentic from non-authentic regions were identified through PCA dimensionality reduction and Pearson correlation tests. Ultimately, correlation analyses between the abundance of volatile compounds in HJH and the soil elements and climatic factors revealed that climatic factors exerted the greatest influence on the volatile compounds, followed by soil elements.
Currently, the recognized functions of volatile compounds in plants generally include disease resistance, pest resistance, and tolerance to environmental stressors [66]. The volatile compounds in HJH are predominantly terpenoids, which play significant defensive roles in plant growth [67,68]. This research indicates that the volatile compounds from authentic HJH-producing regions exhibit greater diversity compared with those from non-authentic regions, with some compounds present in higher abundance. Furthermore, the formation of authentic HJH is closely linked to climatic factors, characterized by relatively high average temperatures, minimal annual temperature fluctuations, and significant yet variable precipitation, indicating instability and frequent abnormal weather patterns. These climatic features relate to Huazhou’s geographical location; situated at latitudes of 21°29′–22°13′ N and longitudes of 110°20′–110°45′ E, it falls within a subtropical climate zone with mild temperatures and abundant rainfall influenced by maritime conditions, leading to frequent summer and autumn typhoons and heavy rainfall. Meanwhile, studies suggest that the biosynthesis of secondary metabolites in plants is related to environmental temperature [23,69,70,71]. Investigations into the volatile compound content of HJH have demonstrated that increasing temperatures correlate with higher abundance of volatile substances, potentially due to the higher temperatures promoting the biosynthesis of volatile compounds [72]. However, reports also indicate that secondary metabolites may decrease under extreme temperature [22]. Temperature is a critical ecological factor influencing community distribution, not only providing the necessary heat for the growth of medicinal plants but also altering the climatic environment of these plants, thereby affecting the production and allocation of secondary metabolites through various mechanisms [73]. Research has found that temperature is a primary factor influencing the release rates of monoterpenes and other volatile compounds, with release rates increasing with temperature within a certain range, probably due to changes in vapor pressure [74,75,76,77]. Additionally, variations in precipitation patterns affect biomass accumulation and allocation to reproductive organs, leading to changes in reproductive strategies that enhance plants’ adaptability to their environments [78]. The above indicates that climatic factors are one of the key factors influencing the authenticity of HJH.
The soil environment is another one of the key factors influencing plant growth [79], particularly for medicinal herbs. The quality of authentic herbal medicines is strongly dependent on environmental conditions, and the environmental conditions are largely determined by the soil. For instance, soil nutrients have been shown to significantly enhance quality accumulation in herbal medicines such as Citrus reticulata ‘Chachi’ [31], Chrysanthemum morifolium Ramat. [80], and Cinnamomum migao endemic [81]. This enhancement may stem from the influence of various factors, including climate and soil nutrients, on the absorption and accumulation of elements by plants [81]. Soil serves as the fundamental substrate for medicinal plants, and soil nutrients are critical determinants of growth rate and the abundance of active ingredients [82,83]. It is recorded in ancient Chinese medical books that the soil in Huazhou is rich in the mineral component Chloriti Lapis (Chinese name Mengshi), a traditional medicinal mineral primarily utilized for its cough-suppressing and phlegm-relieving properties [84], which aligns with the medicinal efficacy of HJH. The primary elements present in Chloriti Lapis include Al, Fe, Si, Mg, Ca, K, and Na, which together account for approximately 90.70% [35]. According to Zhonghua Bencao, Chloriti Lapis is notably abundant in Fe and Al, consistent with the high concentrations of these elements observed in the soil of Huazhou. In this study, the concentrations of Fe and Al in soil samples from authentic regions were significantly higher than those from non-authentic regions, and a strong positive correlation (r = 0.64) was found between the concentrations of Fe and Al (As shown in Figure 7). Experimental results indicated that the levels of Fe and Al in the soil were positively correlated with the volatile compound content in the HJH samples. This correlation suggests that the unique soil environment of Huazhou contributes to the distinctive quality of the essential oils derived from HJH in this region. Furthermore, among climatic factors, temperature and precipitation have substantial effects on the weathering, leaching, and deposition of substances in the soil [85]. The results of this study indicate that the concentrations of Al and Fe in the soil from authentic regions are higher than those in non-authentic regions. Conversely, levels of other elements such as B, Ca, Cu, K, Mg, Mn, Na, and P are significantly lower in the authentic regions. A positive correlation was found between the volatile compound content in HJH samples and the soil concentrations of Al and Fe, while a negative correlation was observed with the concentrations of B, Ca, Cu, K, Mg, Mn, Na, and P. This phenomenon may be attributed to the subtropical climate of Huazhou, characterized by intense weathering and leaching processes that lead to substantial loss of easily soluble mineral elements, while sparingly soluble elements like Al and Fe remain in the soil, contributing to the reddish color of the soil. This characteristic is consistent with the predominantly reddish hue observed during sampling. The climatic conditions in Huazhou result in low levels of soluble inorganic nutrients, significantly reducing the availability of elements that can be effectively utilized by plants. This situation may induce stress in plants, consequently leading to a measurable increase in the abundance of volatile compounds in HJH. The relative increase in the concentrations of Fe and Al in the soil may represent an external manifestation of soil element loss. In summary, the relatively high concentrations of Fe and Al elements in the soils of the Huazhou region are likely to have been influenced by both geological and climatic factors. The unique soil characteristic may be one of the key determinants shaping the quality of the volatile oils in HJH.
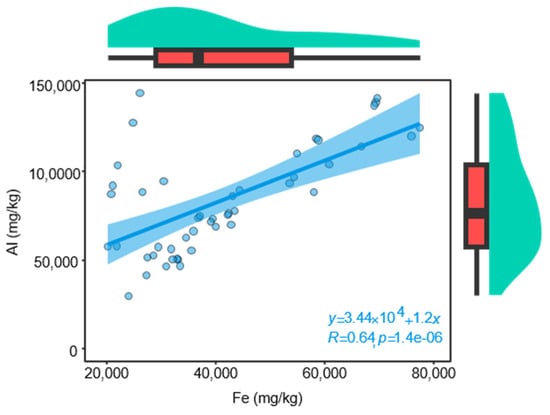
Figure 7.
Pearson Correlation analysis of Fe and Al elements in soil. Green shows the data’s kernel density, red represents the data distribution in the box plot, and the blue area indicates the 95% confidence interval.
The climatic factors and soil elements of medicinal plant habitats exert varying degrees of influence on the quality of herbal medicines. A deficiency in mineral elements within the soil can affect plant growth and the formation of secondary metabolites. According to the resource availability hypothesis, when a plant’s potential growth rate decreases, the production of secondary metabolites for defense increases [86]. The growth-differentiation balance hypothesis assumes that under conditions of abundant resources, plants prioritize growth; however, under poor resource conditions, growth and differentiation are both reduced. At moderate resource levels—such as mild drought, or habitats with warm or cold temperatures—differentiation becomes the primary focus, accompanied by greater accumulation of secondary metabolites [86]. It has been reported that the soil environment shapes unique rhizosphere microbial communities, which in turn contribute to the authenticity of herbal medicines [31]. In this study, the soil element content was found to influence the levels of volatile compounds in HJH. The total concentrations of elements such as Al, B, Ca, Cu, Fe, K, Mg, Mn, Na, P, and S were measured, but without separately assessing the plant-available valence states of these elements. Research indicates that plants can utilize soil elements not only directly but also indirectly through microorganisms [31,87]. This might be one of the complex factors relating to the authenticity of herbal medicines, warranting further investigation into how plants absorb and utilize soil elements. It should be noted that this study focused solely on the total concentration of elements in the soil without addressing their bioavailability, which has a significant effect on absorption in plants. However, the bioavailability of elements in soil involves multiple influencing factors, including soil physicochemical properties, soil fertility, and soil microecology [22,31]. Our group will continue to work on this topic.
4. Materials and Methods
4.1. Subsection
Field sampling was carried out between May and July 2023. In this study, to ensure consistency and comparability of the experiments, all immature pomelo fruit samples were harvested at a standardized time exactly two months after flowering. This standardized harvesting schedule aimed to eliminate potential variability caused by different harvesting times, thereby ensuring the accuracy and reliability of the experimental results. A total of 47 batches of fruit samples and soil samples (10–20cm) from around fruit trees were selected from sites in seven provinces of China (Guangdong, Guangxi, Hunan, Sichuan, Guizhou, Jiangxi, and Zhejiang). The latitude and longitude information at the sampling points was recorded using an Element Latitude Camera. Sampling sites were categorized based on their geographical region, into authentic producing areas (specifically Huazhou in Guangdong Province) and non-authentic producing areas (other regions), designated as core regions and non-core regions, respectively. Corresponding samples were recorded as COREs and Non-COREs.
In total, 47 batch samples of immature pomelo fruits from various regions were collected (22 Non-COREs and 25 COREs). These samples were authenticated by Professor Wen-bo Liao at Sun Yat-sen University in China and were stored at the School of Life Sciences, Sun Yat-sen University. Sampling information is shown in Table 4.

Table 4.
Sampling information.
Currently, dried immature young fruits are widely used as HJH for medicine [88]. The sample processing method and duration were established based on the temperature typically used for drying immature fruits in the local region of Huazhou. All samples underwent drying using a heat pump dryer at a temperature set to 70 °C, humidity 5%, for a period of 7 days.
Additionally, 47 batches of soil samples were air-dried, crushed, sieved, and stored in the laboratory for future use according to the requirements of HJ/T 166.
4.2. HS-SPME-GC-MS
The method described by Zheng et al. [17] was modified slightly. Prior to the HS-SPME analysis, the samples were powdered in a mill and passed through a 60-mesh sieve. Then, 20mg of the sample powder was introduced into a 20 mL headspace, adding 5 μL n-tridecane (0.3024 mg/mL dissolved in methanol) serving as an internal standard.
According to the established method [17], GC-MS analysis was carried out using a Trace GC Ultra gas chromatograph coupled with a triple quadrupole mass spectrometer (Agilent Technologies Inc., Palo Alto, CA, USA). Injections were performed in split mode (1:50), and volatile compounds were chromatographed on a HP-5MS column (30 m × 0.25 mm × 0. 25 µm) provided by Agilent Technologies Inc. (Palo Alto, CA, USA), with helium as the carrier gas at a constant flow of 1.0 mL/min. The oven temperature was initially set at 40 °C for 3 min, followed by a gradual increase to 200 °C at a rate of 5 °C/min. This was subsequently increased to 250 °C at a rate of 10 °C/min, and the temperature was then maintained at 250 °C for 3 min. The inlet and ion source temperatures were both set at 230 °C, and MS was scanned at 70 eV in electronic ionization mode. Mass spectra were acquired using the full-scan monitoring mode, covering a range of m/z 29–448, on an Agilent MassHunter Workstation (NIST17.L, National Institute of Standards and Technology, Gaithersburg, MD, USA). The extraction parameters were set as follows: fiber type 80 μm DVB/C-WR/PDMS-gray, sampling temperature at 50 °C, and a sampling duration of 30 min. Subsequently, the volatile compounds were desorbed in the vaporization chamber at 220 °C for 10 bmin.
For the qualitative analysis, 1 μL of the n-alkane standard mixture was injected under the same analytical conditions as the samples, and the retention times of each n-alkane wre recorded. The retention indices (RIs) of the components were calculated using the Kovats retention index formula. The standard mass spectral database NIST17.L was utilized for retrieval and matching, selecting data with similar retention indices (RI) and a match value exceeding 800.
4.3. Climatic Factors
Data for annual mean temperature (BIO1), mean diurnal temperature range (BIO 2), isothermality (BIO 3), temperature seasonality (BIO4), maximum temperature of warmest month (BIO5), minimum temperature of coldest month (BIO6), annual temperature range (BIO7), mean temperature of wettest quarter (BIO8), mean temperature of driest quarter (BIO9), mean temperature of warmest quarter (BIO10), mean temperature of coldest quarter (BIO11), annual precipitation (BIO12), precipitation in the wettest month (BIO13), precipitation in the driest month (BIO14), precipitation seasonality (BIO15), Precipitation in the wettest quarter (BIO16), precipitation in the driest quarter (BIO17), precipitation in the warmest quarter (BIO18), and precipitation in the coldest quarter (BIO19) were obtained from the WorldClim global climate and weather database (http://www.worldclim.org) for the various sampling points. WorldClim is a global database offering high-spatial-resolution weather and climate data, extensively used in the ecological research of medicinal plants [89,90,91].
4.4. Soil Elemental Analysis
The soil samples were initially pretreated using a Super Microwave Platform (Ultra WAVE, Milestone, Italy) and were then analyzed for their concentrations of Al, B, Ca, Cu, Fe, K, Mg, Mn, Na, P, S, and Zn via inductively coupled plasma optical emission spectrometry (ICP-OES, ICAP6500 Duo, Thermo Fisher Scientific, Waltham, MA, USA).
In this process, 50 mg of soil was precisely weighed and placed in a polytetrafluoroethylene digestion tube and 4 mL of concentrated nitric acid was added and allowed to pre-digest overnight. On the subsequent day, microwave digestion was conducted. After completion of digestion, the solution was cooled to room temperature, diluted to 10 mL with high-purity water, and designated as the stock solution. Subsequently, the stock solution was diluted 100-fold with 5% diluted nitric acid for analysis.
As in previous studies [38], the operational parameters of the Super Microwave Platform(Ultra WAVE, Milestone, Italy) included pre-pressurizing to 50 bar in advance. Subsequently, the temperature was ramped from room temperature to 90 °C over 10 min, followed by a 5 min equilibrium; then, the temperature was raised from 90 °C to 160 °C over 15 min, with a 5 min equilibrium, then increased from 160 °C to 240 °Cover an additional 15 min, followed by a 30 min equilibrium.
The operational parameters of the Inductively Coupled Plasma Optical Emission Spectrometer(Thermo Fisher Scientific, Waltham, MA, USA) were as follows: RF power set at 1150 W; auxiliary gas flow rate maintained at 0.5 L/min; nebulizer flow rate at 0.5 L/min; and cooling gas flow rate at 12 L/min. Each sample was measured three times to ensure repeatability, and the average value was subsequently calculated.
4.5. Data Analysis and Statistics
Qualitative and Quantitative Analysis: Qualitative analysis was performed using the Agilent MassHunter Workstation(Agilent Technologies Inc., Palo Alto, CA, USA) to automatically deconvolute the total ion chromatogram (TIC) of volatile compounds in HJH. The mass spectrometry results were cross-referenced against the 2017 NIST 17.L standard spectral library, applying a filter for substances with a score exceeding 80. The standard mass spectral database NIST17.L was utilized for retrieval and matching, selecting data with similar retention indices (RIs) and match values exceeding 800. For relative quantitative analysis, the ratio of the peak area of each compound to the peak area of the internal standard (n-tridecane) was calculated, which served as an indicator for the abundance of volatile components. This allowed the comparison of compositional variations among HJH samples sourced from different production regions.
The data obtained were primarily processed using R 4.4.1 packages for principal component analysis (PCA), hierarchical cluster analysis (HCA), orthogonal partial least squares discriminant analysis (OPLS-DA), Pearson correlation, multiple linear regression, variance decomposition analysis, random forest, and structural equation modeling (SEM). The Variable Importance in Projection (VIP) and Log2FC values were also calculated and predicted. Additionally, statistical significance was assessed using GraphPad Prism 8.0.1, where the selection criteria for volatile compounds included p < 0.05, VIP > 1, and Log2FC > 1. Differences in soil element data among soil element groups were analyzed and visualized using GraphPad Prism 8.0.1.
5. Conclusions
The volatile compounds of HJH are primarily monoterpenes or sesquiterpenes. Differences in the volatile compounds between authentic and non-authentic producing regions were observed, with eight materials of variable quality identified, all of which were significantly higher in samples from authentic regions. This variation might be one of the key factors contributing to confirming the authenticity of HJH. The differential components of volatile compounds across regions included three monoterpenes—β-pinene, α-terpineol, and terpinen-4-ol, the latter two being oxygenated monoterpenoid alcohols—and five sesquiterpenes, namely γ-cadinene, β-copaene, γ-muurolene, bicyclogermacren, and β-cadinene. Modern pharmacological research has shown that β-pinene, γ-cadinene, terpinen-4-ol, and α-terpineol have significant antitussive and expectorant effects [11,12,13,64,65]. The other four compounds, having similar structures, might also possess comparable therapeutic properties. Therefore, HJH from authentic regions might have higher medicinal value, though further validation is required. Additionally, the unique geological and climatic conditions of Huazhou, including climatic factors and soil elements, have been shown to influence the volatile compounds in HJH. In conclusion, the authenticity of HJH is shaped by the distinctive climate and soil composition of Huazhou.
Author Contributions
Conceptualization, S.H., P.L. and W.S.; methodology, S.H.; formal analysis, S.H.; investigation, S.H.; resources, W.P. and W.S.; data curation, S.H. and A.Z.; writing—original draft preparation, S.H. and H.W.; writing—review and editing, P.L., W.P. and W.S.; visualization, S.H., H.W. and A.Z.; project administration, W.S.; funding acquisition, W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “the Open Competition Program of Ten Major Directions of Agricultural Science and Technology Innovation for the 14th Five Year Plan of Guangdong Province (No. 2022SDZG07)”, “Guangdong Province Special Project for Rural Revitalization Strategy (No. 2023SDZG07, No. 2024KJ22)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We extend our sincere appreciation to the Huazhou Municipal People’s Government for their invaluable sampling support. Special acknowledgment is due to the proprietor of the sample collection orchard for graciously permitting us to gather experimental samples. We are deeply grateful to Zhizhong Huang and Junpeng Li from the special class of Citri grandis Exocarpium of Huazhou Municipal People’s Government for their pivotal role in facilitating negotiations and sample acquisition. A special note of gratitude is extended to Zhencai Hu of Huazhou for his generous assistance in transportation. We would like to acknowledge the invaluable support of Xin Cai from the State Key Laboratory of Biocontrol at Sun Yat-sen University for her unwavering assistance throughout the GC-MS analysis. We also thank Sujun Yan from the Instrument Analysis and Research Center for her invaluable guidance in exploring experimental conditions using GC-MS. Lastly, we wish to express our appreciation to Minsi Liang and Zhisheng Lu from the Instrument Analysis and Research Center at Sun Yat-sen University for their significant contributions to the ICP-OES analysis super-microwave digestion. We are very grateful to Zhaorong Lun from Sun Yat-sen University for the suggestions he has made to improve our manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zheng, W.K.; Zhang, W.; Liu, D.H.; Yin, M.Q.; Wang, X.; Wang, S.C.; Shen, S.Q.; Liu, S.J.; Huang, Y.; Li, X.X.; et al. Evolution-guided multiomics provide insights into the strengthening of bioactive flavone biosynthesis in medicinal pummelo. Plant Biotechnol. J. 2023, 21, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Sahu, S.K.; Huang, L.Y.; Fan, Y.N.; Lin, J.H.; Su, J.M.; Bai, M.; Chen, Y.W.; Wang, S.J.; Ye, P.; et al. The draft genome and multi-omics analyses reveal new insights into geo-herbalism properties of ‘Tomentosa’. Plant Sci. 2022, 325, 111489. [Google Scholar] [CrossRef]
- Su, C.; Wong, K.-L.; But, P.P.-H.; Su, W.-W.; Shaw, P.-C. Molecular Authentication of the Chinese Herb Huajuhong and Related Medicinal Material by DNA Sequencing and ISSR Markers. J. Food Drug Anal. 2010, 18, 161–170. [Google Scholar] [CrossRef]
- Li, P.L.; Liu, M.H.; Hu, J.H.; Su, W.W. Systematic chemical profiling of Citrus grandis ‘Tomentosa’ by ultra-fast liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 90, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.Y.; Zhu, C.Y.; Qiu, D.Y.; Mao, G.L.; Mueller-Roeber, B.; Zeng, J.W. Integrated transcriptomic and metabolomic analyses reveal key genes controlling flavonoid biosynthesis in ‘Tomentosa’ fruits. Plant Physiol. Bioch. 2023, 196, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, C.; Tu, H.; Zhou, J.; Liu, X.; Cheng, Y.; Luo, J.; Deng, X.; Zhang, H.; Xu, J. Characterization and Metabolic Diversity of Flavonoids in Citrus Species. Sci. Rep. 2017, 7, 10549. [Google Scholar] [CrossRef]
- Jiang, K.; Song, Q.; Wang, L.; Xie, T.; Wu, X.; Wang, P.; Yin, G.; Ye, W.; Wang, T. Antitussive, expectorant and anti-inflammatory activities of different extracts from Exocarpium Citri grandis. J. Ethnopharmacol. 2014, 156, 97–101. [Google Scholar] [CrossRef]
- Valussi, M.; Antonelli, M.; Donelli, D.; Firenzuoli, F. Appropriate use of essential oils and their components in the management of upper respiratory tract symptoms in patients with COVID-19. J. Herb. Med. 2021, 28, 100451. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, A.; Ziaian, B.; Hessami, K.; Kashkooe, A.; Pasalar, M. An Evidence-Based Review of Antitussive Herbs Containing Essential Oils in Traditional Persian Medicine. Curr. Drug Discov. Technol. 2021, 18, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Santana, H.S.R.; de Carvalho, F.O.; dos Santos, D.M.; da Silva, E.A.P.; Silva, É.R.; Shanmugam, S.; Heimfarth, L.; Nunes, P.S.; e Silva, A.M.d.O.; de Souza Araújo, A.A. Inhaled D-Limonene minimizes acute lung injury and reduces oxidative stress induced by smoke in rats. Phytomedicine Plus 2022, 2, 100308. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; LD Jayaweera, S.; A. Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Baschieri, A.; Amorati, R.; Valgimigli, L. Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chem. 2021, 345, 128468. [Google Scholar] [CrossRef] [PubMed]
- Deen, J.I.; Zawad, A.S.; Uddin, M.; Chowdhury, M.A.H.; Al Araby, S.Q.; Rahman, M.A. Terpinen-4-ol, a volatile terpene molecule, extensively electrifies the biological systems against the oxidative stress-linked pathogenesis. Adv. Redox Res. 2023, 9, 100082. [Google Scholar] [CrossRef]
- Fan, R.Y.; Qiu, D.Y.; Mao, G.L.; Zeng, J.W. Combined analysis of GC-MS, RNA-seq and ATAC-seq elucidates the essential oils variation and terpenes biosynthesis in Citrus grandis ‘Tomentosa’. Ind. Crop Prod. 2024, 209, 117996. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, L.P.; Teng, Z.Q.; Zhan, Z.L.; Nan, T.G.; Zhou, A.X.; Guo, L.P. Comparison of volatile constituents in two types of mugwort leaves (produced in Qichun and Nanyang) using the headspace GC-MS. J. Acupunct. Tuina Sci. 2016, 14, 164–169. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Zeng, X.; Peng, W.; Wu, Z.; Su, W.W. Study on the Discrimination between Citri Reticulatae Pericarpium Varieties Based on HS-SPME-GC-MS Combined with Multivariate Statistical Analyses. Molecules 2018, 23, 1235. [Google Scholar] [CrossRef]
- Fan, R.Y.; Zhu, C.Y.; Qiu, D.Y.; Zeng, J.W. Comparison of the bioactive chemical components and antioxidant activities in three tissues of six varieties of Citrus grandis ‘Tomentosa’ fruits. Int. J. Food Prop. 2019, 22, 1848–1862. [Google Scholar] [CrossRef]
- Gouinguené, S.; Turlings, T. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 2002, 129, 1296–1307. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Z.; Li, X. Moderate warming will expand the suitable habitat of Ophiocordyceps sinensis and expand the area of O. sinensis with high adenosine content. Sci. Total Environ. 2021, 787, 147605. [Google Scholar] [CrossRef]
- El-Guezzane, C.; El-Moudden, H.; Harhar, H.; Chahboun, N.; Zarrouk, A. A comparative study of the antioxidant activity of two Moroccan prickly pear cultivars collected in different regions. Chem. Data Collect. 2020, 31, 100637. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Chen, C.H.; Yuan, J.H.; Xue, J.; Chen, H.J.; Liu, X.H.; Cai, Z.C.; Wu, N.; Yang, W.; Cheng, J.M. A study for quality evaluation of Lysimachiae herba from different origins based on fingerprint-activity relationship modeling and multi-component content determination. J. Ethnopharmacol. 2024, 325, 118019. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, B.; Jia, X.; Chen, G. Differential Expression of Calycosin-7-O-β-D-glucoside Biosynthesis Genes and Accumulation of Related Metabolites in Different Organs of Astragalus membranaceus Bge.var. mongholicus (Bge.) Hsiao Under Drought Stress. Appl. Biochem. Biotechnol. 2021, 194, 3182–3195. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, H.; Li, P.; Jin, J.; Li, Z. The bacterial consortia promote plant growth and secondary metabolite accumulation in Astragalus mongholicus under drought stress. BMC Plant Biol. 2022, 22, 475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Peng, H.S.; Shen, Y.; Zhao, R.; Huang, L.Q. The profiling of bioactive ingredients of differently aged Salvia miltiorrhiza roots. Microsc. Res. Techniq. 2013, 76, 947–954. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, Y.; Han, M.; Yang, L.M. Changes in the physiological characteristics of Panax ginseng embryogenic calli and molecular mechanism of ginsenoside biosynthesis under cold stress. Planta 2021, 253, 79. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.L.; Liu, J.; Quan, X.L.; Quan, L.H.; Wu, S.Q. Different chilling stresses stimulated the accumulation of different types of ginsenosides in Panax ginseng cells. Acta Physiol. Plant. 2016, 38, 210. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Zhang, J.; Li, C.; Xu, Y.H.; Zheng, H.; Zhou, J.H.; Zha, L.P.; Jiang, C.; Jin, Y.; et al. Ginsenosides regulate adventitious root formation in via a CLE45-WOX11 regulatory module. J. Exp. Bot. 2020, 71, 6396–6407. [Google Scholar] [CrossRef]
- Su, J.M.; Wang, Y.Y.; Bai, M.; Peng, T.H.; Li, H.S.; Xu, H.J.; Guo, G.F.; Bai, H.Y.; Rong, N.; Sahu, S.K.; et al. Soil conditions and the plant microbiome boost the accumulation of monoterpenes in the fruit of Citrus reticulata ‘Chachi ’. Microbiome 2023, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ma, S.S.; Yan, H.F.; Wang, L.B.; Li, J.L. Geo-herbalism research of Polygalae Radix based on element profiles and chemometrics. Spectrosc. Lett. 2017, 50, 352–357. [Google Scholar] [CrossRef]
- Cheng, L.; Han, M.; Yang, L.M.; Yang, L.; Sun, Z.; Zhang, T. Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis Georgi under drought stress. Ind. Crop. Prod. 2018, 122, 473–482. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Y.J.; Wu, C.; Chen, S.Q.; Wang, Z.Y.; Yang, Z.C.; Qin, S.S.; Huang, L.Q. Water Deficit Affected Flavonoid Accumulation by Regulating Hormone Metabolism in Georgi Roots. PLoS ONE 2012, 7, e42946. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Wu, D.K.; Lin, R.C.; Liu, X.H.; Hu, Q.P.; Kong, Q.Y. ICP-MS analytical studies on inorganic elements of Mineral Chinese Medicine Chloriti Lapis. Chin. J. Pharm. Anal. 2010, 30, 2067–2074. [Google Scholar]
- Ai, J. Headspace solid phase microextraction. Dynamics and quantitative analysis before reaching a partition equilibrium. J. Anal. Chem. 1997, 69, 3260–3266. [Google Scholar] [CrossRef]
- Ai, J. Solid phase microextraction for quantitative analysis in nonequilibrium situations. J Anal. Chem. 1997, 69, 1230–1236. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, P.; Pan, Z.; Xu, H.; Luo, Y.; Wang, X. Discrimination of oolong tea (Camellia sinensis) varieties based on feature extraction and selection from aromatic profiles analysed by HS-SPME/GC–MS. J. Food Chem. 2013, 141, 259–265. [Google Scholar] [CrossRef]
- Siani, A.C.; Garrido, I.S.; Monteiro, S.S.; Carvalho, E.S.; Ramos, M.F.S. Protium icicariba as a source of volatile essences. Biochem. Syst. Ecol. 2004, 32, 477–489. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, W.; Hu, S.; Wu, H.; Dong, L.; Su, W. Dynamic change and activity analysis of volatile oil in the flowers of Citri grandis Exocarpium. Acta Sci. Nat. Univ. Sunyatseni 2024, 63, 88–95. [Google Scholar]
- Jalali-Heravi, M.; Zekavat, B.; Sereshti, H. Characterization of essential oil components of Iranian geranium oil using gas chromatography–mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A 2006, 1114, 154–163. [Google Scholar] [CrossRef]
- Asuming, W.A.; Beauchamp, P.S.; Descalzo, J.T.; Dev, B.C.; Dev, V.; Frost, S.; Ma, C.W.J.B.S. Essential oil composition of four Lomatium Raf. species and their chemotaxonomy. Biochem. Syst. Ecol. 2005, 33, 17–26. [Google Scholar] [CrossRef]
- Romanenko, E.; Tkachev, A. Identification by GC—MS of cymene isomers and 3, 7, 7-trimethylcyclohepta-1, 3, 5-triene in essential oils. Chem. Nat. Compd. 2006, 42, 699–701. [Google Scholar] [CrossRef]
- Harzallah-Skhiri, F.; Jannet, H.B.; Hammami, S.; Mighri, Z. Variation of volatile compounds in two Prosopis farcta (Banks et Sol.) Eig.(Fabales, Fabaceae= Leguminosae) populations. Flavour Fragr. J. 2006, 21, 484–487. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef] [PubMed]
- Andriamaharavo, N.R. Retention Data; NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014.
- Pripdeevech, P.; Chumpolsri, W.; Suttiarporn, P.; Wongpornchai, S. The chemical composition and antioxidant activities of basil from Thailand using retention indices and comprehensive two-dimensional gas chromatography. J. Serbian Chem. Soc. 2010, 75, 1503–1513. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Venskutonis, P.R.; Viškelis, P.; Dambrauskienė, E. Influence of nitrogen fertilizers on the yield and composition of thyme (Thymus vulgaris). J. Agric. Food Chem. 2003, 51, 7751–7758. [Google Scholar] [CrossRef] [PubMed]
- Skaltsa, H.D.; Mavrommati, A.; Constantinidis, T. A chemotaxonomic investigation of volatile constituents in Stachys subsect. Swainsonianeae (Labiatae). Phytochemistry 2001, 57, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Couladis, M.; Chinou, I.; Tzakou, O.; Petrakis, P. Composition and antimicrobial activity of the essential oil of Hypericum rumeliacum subsp. apollinis (Boiss. & Heldr.). Phytother. Res. 2003, 17, 152–154. [Google Scholar]
- Siani, A.; Ramos, M.d.S.; Menezes-de-Lima Jr, O.; Ribeiro-dos-Santos, R.; Fernadez-Ferreira, E.; Soares, R.; Rosas, E.; Susunaga, G.; Guimarães, A.; Zoghbi, M.d.G. Evaluation of anti-inflammatory-related activity of essential oils from the leaves and resin of species of Protium. J. Ethnopharmacol. 1999, 66, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Liang, Y.Z.; Zhou, T.; Zhang, L.X.; Hu, C.D. Comparative analysis of volatile constituents between recipe jingfangsan and its single herbs by GC-MS combined with alternative moving window factor analysis method. J. Sep. Sci. 2009, 32, 258–266. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) NE Brown, grown in Colombia, and evaluation of its in vitro antioxidant activity. J. Chromatogr. A 2004, 1025, 93–103. [Google Scholar] [CrossRef]
- Radulović, N.; Đorđević, N.; Marković, M.; Palić, R. Volatile constituents of Glechoma hirsute Waldst. & Kit. and G. hederacea L. (Lamiaceae). Bull. Chem. Soc. Ethiop. 2010, 24, 67–76. [Google Scholar]
- Schmidt, J.M.; Noletto, J.A.; Vogler, B.; Setzer, W.N. Abaco bush medicine: Chemical composition of the essential oils of four aromatic medicinal plants from Abaco Island, Bahamas. J. Herbs Spices Med. Plants 2007, 12, 43–65. [Google Scholar] [CrossRef]
- Marilena, I.; Davorin, K.; Josip, F. Differentiation of F1 hybrids P. nigra JF Arnold× P. sylvestris L., P. nigra JF Arnold× P. densiflora Siebold et Zucc., P. nigra JF Arnold× P. thunbergiana Franco and their parental species by needle volatile composition. Biochem. Syst. Ecol. 2005, 33, 427–439. [Google Scholar]
- Zarai, Z.; Kadri, A.; Ben Chobba, I.; Ben Mansour, R.; Bekir, A.; Mejdoub, H.; Gharsallah, N. The in-vitro evaluation of antibacterial, antifungal and cytotoxic properties of Marrubium vulgare L. essential oil grown in Tunisia. Lipids Health Dis. 2011, 10, 1–8. [Google Scholar] [CrossRef]
- Zraik, M.; Booth, T.; Piercey-Normore, M.D. Relationship between lichen species composition, secondary metabolites and soil pH, organic matter, and grain characteristics in Manitoba. Botany 2018, 96, 267–279. [Google Scholar] [CrossRef]
- Sun, X.; Pei, J.; Zhao, L.; Ahmad, B.; Huang, L.F. Fighting climate change: Soil bacteria communities and topography play a role in plant colonization of desert areas. Environ. Microbiol. 2021, 23, 6876–6894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, K.; Wang, Q.; Yang, X.; Meng, D. A multidimensional strategy for characterization, distinction, and quality control of two Clinopodium medicinal plants. J. Ethnopharmacol. 2024, 327, 118019. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, Z.; Sun, Y.; Zhang, Y.; Wang, S.; Zhang, Q.; Cai, T.; Xiang, W.; Zeng, C.; Tang, J. D-Limonene: Promising and Sustainable Natural Bioactive Compound. Appl. Sci. 2024, 14, 4605. [Google Scholar] [CrossRef]
- Kang, G.-Q.; Duan, W.-G.; Lin, G.-S.; Yu, Y.-P.; Wang, X.-Y.; Lu, S.-Z.J.M. Synthesis of bioactive compounds from 3-carene (II): Synthesis, antifungal activity and 3D-QSAR study of (Z)-and (E)-3-caren-5-one oxime sulfonates. Molecules 2019, 24, 477. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L.J.P. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wu, X.; Wang, Q.; Qi, P.; Zhang, Y.; Wang, L.; Sun, C. Characterization of γ-Cadinene Enzymes in Ganoderma lucidum and Ganoderma sinensis from Basidiomycetes Provides Insight into the Identification of Terpenoid Synthases. ACS Omega 2022, 7, 7229–7239. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- He, J.P.; Yao, L.; Pecoraro, L.; Liu, C.X.; Wang, J.; Huang, L.Q.; Gao, W.Y. Cold stress regulates accumulation of flavonoids and terpenoids in plants by phytohormone, transcription process, functional enzyme, and epigenetics. Crit. Rev. Biotechnol. 2023, 43, 680–697. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Jeong, B.R. Night Temperature Affects the Growth, Metabolism, and Photosynthetic Gene Expression in Astragalus membranaceus and Codonopsis lanceolata Plug Seedlings. Plants 2019, 8, 407. [Google Scholar] [CrossRef]
- Kanno, K.; Mae, T.; Makino, A. High night temperature stimulates photosynthesis, biomass production and growth during the vegetative stage of rice plants. Soil Sci. Plant Nutr. 2009, 55, 124–131. [Google Scholar] [CrossRef]
- Wang, B.; Guo, C.; Wan, Y.; Li, J.; Li, Y.E. Air warming and CO2 enrichment increase N use efficiency and decrease N surplus in a Chinese double rice cropping system. Sci. Total Environ. 2019, 706, 136063. [Google Scholar] [CrossRef]
- Naghiloo, S.; Movafeghi, A.; Delazar, A.; Nazemiyeh, H.; Dadpour, M.R. Ontogenetic Variation of Total Phenolics and Antioxidant Activity in Roots, Leaves and Flowers of Astragalus compactus Lam. (Fabaceae). Bioimpacts 2012, 2, 105. [Google Scholar]
- Zhang, W.J.; Bjorn, L.O. The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants. Fitoterapia 2009, 80, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Bufler, U.; Wegmann, K. Diurnal variation of monoterpene concentrations in open-top chambers and in the welzheim forest air, F.R.G. Atmos. Environ. Part A Gen. Top. 1991, 25, 251–256. [Google Scholar] [CrossRef]
- Dement, W.A.; Tyson, B.J.; Mooney, H.A. Mechanism of monoterpene volatilization in Salvia mellifera. Phytochemistry 1975, 12, 2555–2557. [Google Scholar] [CrossRef]
- Tingey, D.T.; Manning, M.; Grothaus, L.C.; Burns, W.F. Influence of Light and Temperature on Monoterpene Emission Rates from Slash Pine. Plant Physiol. 1980, 65, 797–801. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Loreto, F. Water stress, temperature, and light effects on isoprene emission and photosynthesis of Kudzu leaves. In Proceedings of the IEEE Topical Meeting on Electrical Performance of Electronic Packaging, Monterey, CA, USA, 20–22 October 1993. [Google Scholar]
- Zhang, G.G.; Kang, Y.M.; Han, G.D.; Sakurai, K. Effect of climate change over the past half century on the distribution, extent and NPP of ecosystems of Inner Mongolia. Glob. Change Biol. 2011, 17, 377–389. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Kaur, T.; Devi, R.; Saxena, A.K. Biodiversity, and biotechnological contribution of beneficial soil microbiomes for nutrient cycling, plant growth improvement and nutrient uptake. Biocatal. Agric. Biotechnol. 2021, 33, 102009. [Google Scholar] [CrossRef]
- Yao, M.; Bai, X.; Wen, F.; Liu, K.; Yang, J.; Chen, H.; Yang, X. Accurate origin identification of Chinese white Chrysanthemi flos by analysis of C, N, O, H stable isotope ratios and mineral elements combined with chemometrics. J. Food Compos. Anal. 2023, 124, 105703. [Google Scholar] [CrossRef]
- Chen, J.Z.; Huang, X.L.; Tong, B.L.; Wang, D.; Liu, J.M.; Liao, X.F.; Sun, Q.W. Effects of rhizosphere fungi on the chemical composition of fruits of the medicinal plant Cinnamomum migao endemic to southwestern China. BMC Microbiol. 2021, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Biswas, P.K.; Al Hasan, S.M.; Rahman, M.M.; Lee, S.H.; Kim, K.H.; Rahman, S.M.; Islam, M.R. The occurrences of heavy metals in farmland soils and their propagation into paddy plants. Environ. Monit. Assess. 2018, 190, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fang, H.; Zhao, Y.; Zheng, Y.; Jiang, J.; Gu, X. Responses of soil nutrients and rhizosphere microbial communities of a medicinal plant Pinelliaternatato vermicompost. 3 Biotech 2023, 13, 353. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Huang, H.C.; Hao, S.B.; Tang, W.C.; Gao, L.; Gao, H.; Zheng, Z.Y.; Zhang, Z.L. Characteristics of the Lapis chloriti analyzed by the terahertz time-domain technology. In Proceedings of the International Conference on Information Optics and Photonics, Beijing, China, 8–11 July 2018. [Google Scholar]
- Tripathi, A.; Pandey, V.; Ranjan, M.R. Climate Change and Its Impact on Soil Properties. In Climate Change and the Microbiome; Springer: Cham, Switzerland, 2021; pp. 139–153. [Google Scholar]
- Su, W.; Zhang, H.; Li, X.; Ou, X. Relationship between accumulation of secondary metabolism in medicinal plant and environmental condition. Chin. Tradit. Herb. Drugs 2005, 36, 1415–1418. [Google Scholar]
- Melton, E.D.; Swanner, E.D.; Behrens, S.; Schmidt, C.; Kappler, A. The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat. Rev. Microbiol. 2014, 12, 797–808. [Google Scholar] [CrossRef]
- Li, P.; Peng, W.; Wu, H.; Wang, Y.; Li, P.; Zhang, M.; Su, W. Suggestions for the quality standard in Chinese pharmacopoeia of Citri grandis Exocarpium and the establishment of its grade standard. Acta Sci. Nat. Univ. Sunyatseni 2019, 58, 1–13. [Google Scholar]
- Zhang, B.Y.; Chen, B.R.; Zhou, X.Y.; Zou, H.; Duan, D.T.; Zhang, X.Y.; Zhang, X.X. Distribution and protection of Thesium chinense Turcz. under climate and land use change. Sci. Rep. 2024, 14, 6475. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Zuo, Z.T.; Xu, F.R.; Wang, Y.Z. Study of the suitable climate factors and geographical origins traceability of Panax notoginseng based on correlation analysis and spectral images combined with machine learning. Front. Plant Sci. 2023, 13, 1009727. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Akkemik, U.; Dogan, O.; Yilmaz, H.; Sevgi, O.; Sevgi, E. The missing part of the past, current, and future distribution model of Quercus ilex L.: The eastern edge. Iforest—Biogeosciences For. 2024, 17, 90–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).