Metabolite Profiling and Anti-Inflammatory Activities of Fritillaria cirrhosa D. Don Bulbs Derived from Tissue Culture
Abstract
1. Introduction
2. Results and Discussion
2.1. Tissue Culture of F. cirrhosa
2.2. Ion Chromatography Analysis
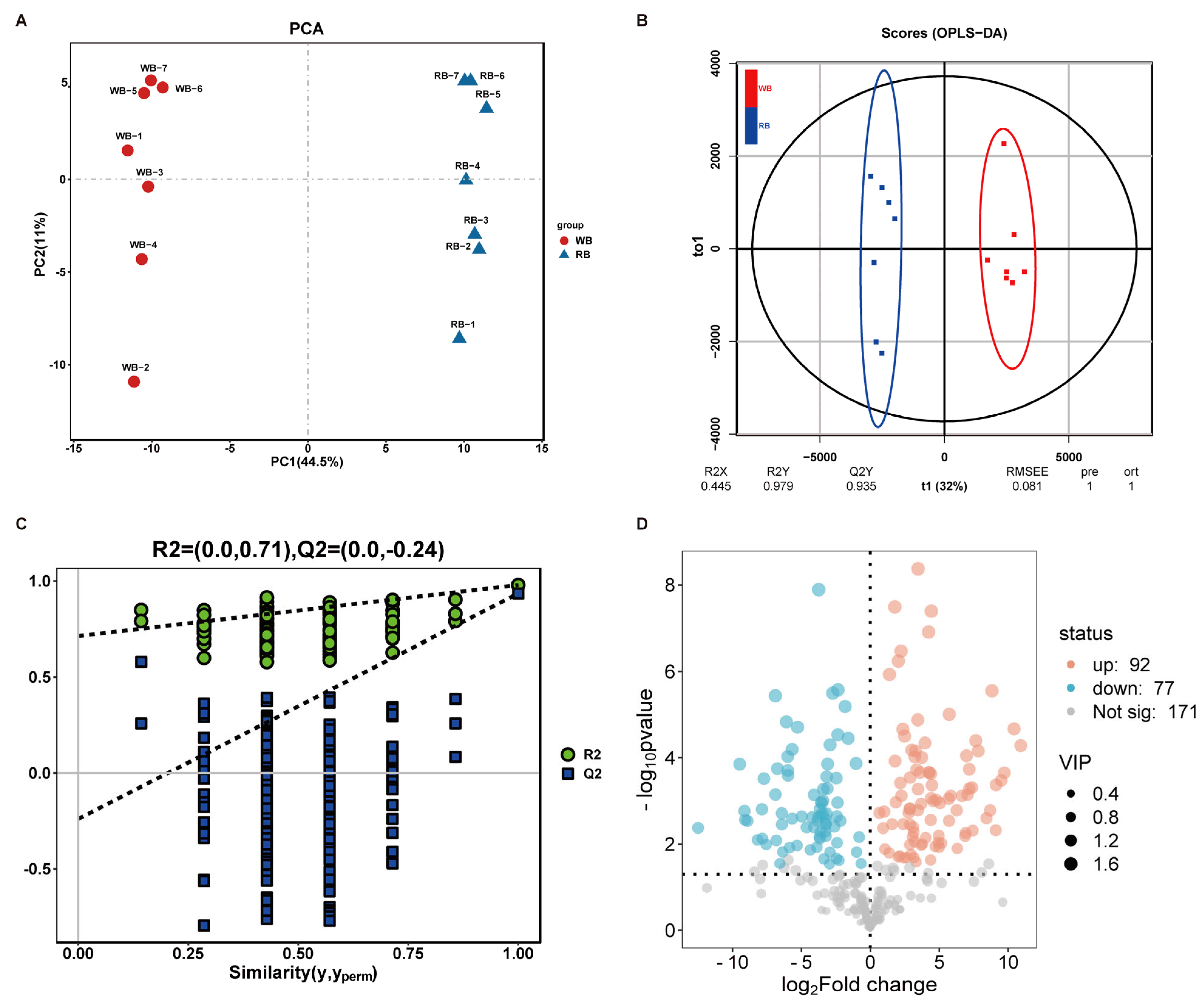
2.3. Chemotypic Differences in WB and RB Metabolites
2.4. WB and RB Attenuate the Expression of Inflammatory Factors
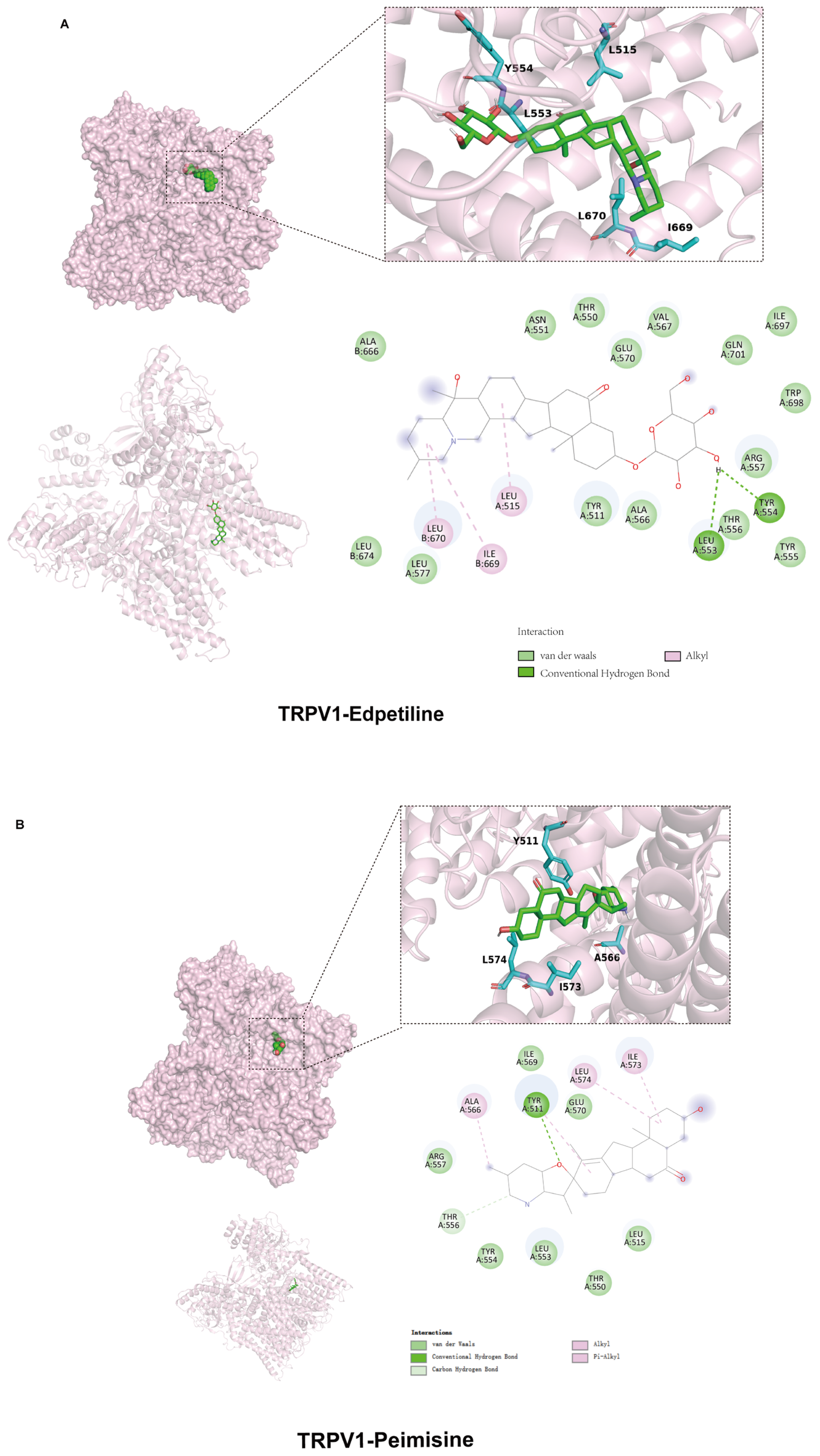
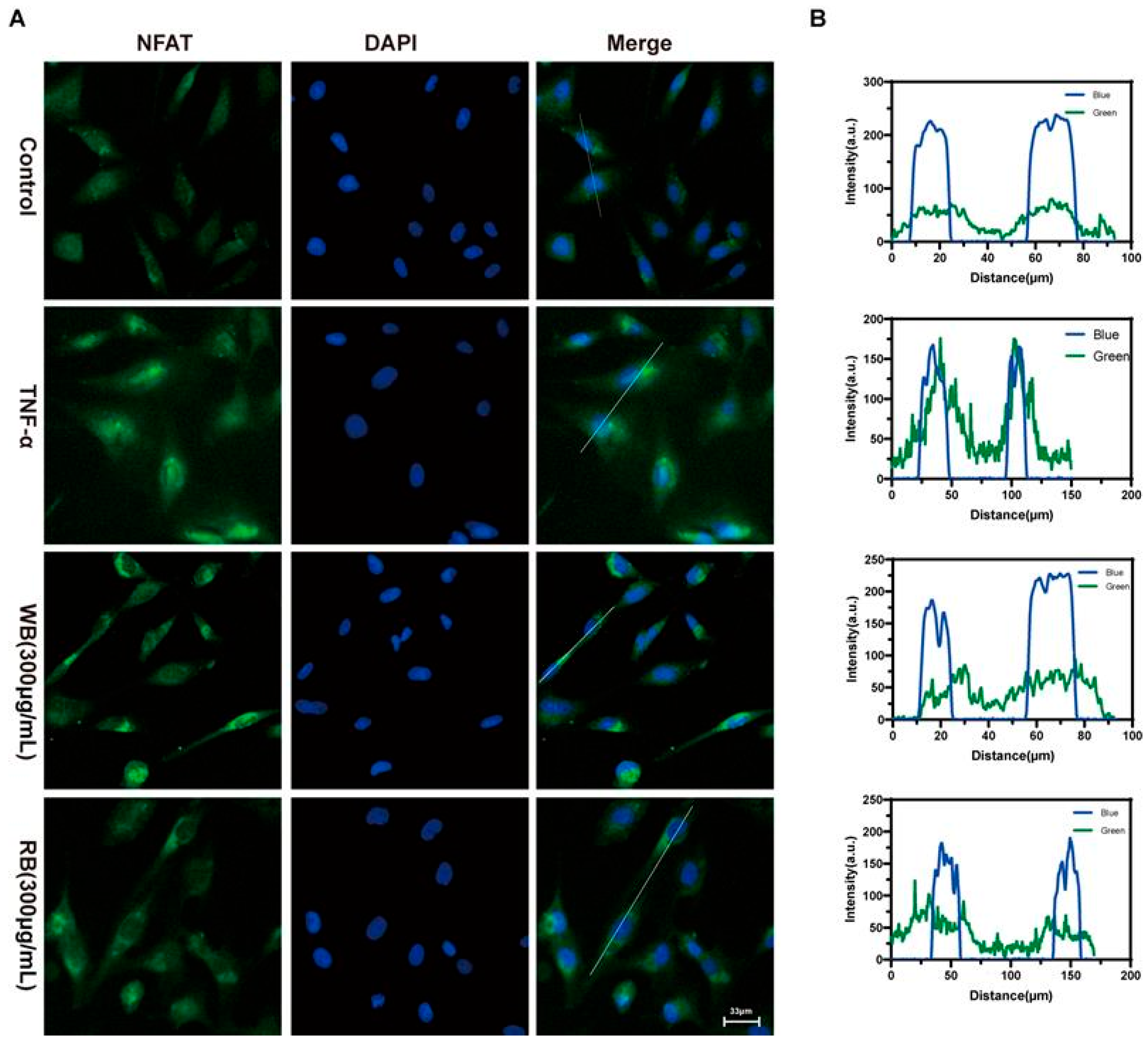
2.5. Inhibition of TRPV1 Signaling Pathway by WB and RB Suppresses Inflammatory Response in Beas-2B Cells
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and In Vitro Tissue Culture Growth Conditions
3.3. Preparation of Plant Extracts
3.4. UHPLC-Q-TOF/MS Analysis of Alkaloids
3.5. UHPLC-Q-TOF/MS Data Preprocessing and Multivariate Analysis
3.6. Cell Culture
3.7. Detection of Cell Viability with CCK8 (Cell Counting Kit-8 Assay) Kit
3.8. Molecular Docking
3.9. Immunofluorescence Assay for Detection of NFAT Entry into the Nucleus of Cells
3.10. Ca2+ Imaging
3.11. Western Blotting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSLP | Thymic Stromal Lymphopoietin |
| NFAT | Nuclear Factors of Activated T-cells |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| TNF-α | Tumor Necrosis Factor-α |
| BEAS-2B | Bronchial Epithelium transformed with Ad12-SV402B |
| PCA | Principal Component Analysis |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| CAP | Capsaicin |
| CPZ | Capsazepine |
| 6-BA | 6-Benzylaminopurine |
| NAA | Naphthaleneacetic Acid |
| CAN | Acetonitrile |
| DMEM | Dulbecco’s modified Eagle medium |
| CCK8 | Cell counting kit-8 assay |
| DMSO | Dimethyl Sulfoxide |
References
- Quan, Y.; Li, L.; Yin, Z.; Chen, S.; Yi, J.; Lang, J.; Zhang, L.; Yue, Q.; Zhao, J. Bulbus Fritillariae Cirrhosae as a Respiratory Medicine: Is There a Potential Drug in the Treatment of COVID-19? Front. Pharmacol. 2021, 12, 784335. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, J.; Wang, S.; Wang, X.; Ou, Y.; Wei, D.; Li, X. Antitussive, expectorant and anti-inflammatory alkaloids from Bulbus Fritillariae Cirrhosae. Fitoterapia 2011, 82, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, J.; Du, Q.; Li, H.; Wang, S. The total alkaloid fraction of bulbs of Fritillaria cirrhosa displays anti-inflammatory activity and attenuates acute lung injury. J. Ethnopharmacol. 2016, 193, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, S.; Feng, Y.; Zhang, L.; Li, Z.; Ma, J.; Luo, Y.; Xiao, W. Antitumor effects of Bulbus Fritillariae cirrhosae on Lewis lung carcinoma cells in vitro and in vivo. Ind. Crops Prod. 2014, 54, 92–101. [Google Scholar] [CrossRef]
- Chen, T.; Zhong, F.; Yao, C.; Chen, J.; Xiang, Y.; Dong, J.; Yan, Z.; Ma, Y. A systematic review on traditional uses, sources, phytochemistry, pharmacology, pharmacokinetics, and toxicity of Fritillariae cirrhosae bulbus. Evid. -Based Complement. Altern. Med. 2020, 2020, 1536534. [Google Scholar] [CrossRef]
- Li, X.; Song, J.; Wei, J.; Hu, Z.; Xie, C.; Luo, G. Natural Fostering in Fritillaria cirrhosa: Integrating herbal medicine production with biodiversity conservation. Acta Pharm. Sin. B 2012, 2, 77–82. [Google Scholar] [CrossRef]
- Carasso, V.; Hay, F.R.; Probert, R.J.; Mucciarelli, M. Temperature control of seed germination in Fritillaria tubiformis subsp. moggridgei (Liliaceae) a rare endemic of the South-west Alps. Seed Sci. Res. 2011, 21, 33–38. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Filová, A. Production of secondary metabolities in plant tissue cultures. Res. J. Agric. Sci. 2014, 46, 236–245. [Google Scholar]
- Zhu, Z.; Bao, Y.; Yang, Y.; Zhao, Q.; Li, R. Research Progress on Heat Stress Response Mechanism and Control Measures in Medicinal Plants. Int. J. Mol. Sci. 2024, 25, 8600. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.-L.; Li, J.; Li, J.-X.; Liu, S.-J.; Huang, L.-Q.; Gao, W.-Y. Production of Active Compounds in Medicinal Plants: From Plant Tissue Culture to Biosynthesis. Chin. Herb. Med. 2017, 9, 115–125. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E.; Cabi, E.; Kaya, Y. Production of secondary metabolites using tissue culture-based biotechnological applications. Front. Plant Sci. 2023, 14, 1132555. [Google Scholar] [CrossRef] [PubMed]
- Wawrosch, C.; Zotchev, S.B. Production of bioactive plant secondary metabolites through in vitro technologies—Status and outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Xie, H.-M.; Lee, M.-R.; Lin, C.-Y.; Yip, M.-K.; Agrawal, D.C.; Tsay, H.-S. In vitro propagation of bulblets and LC–MS/MS analysis of isosteroidal alkaloids in tissue culture derived materials of Chinese medicinal herb Fritillaria cirrhosa D. Don. Bot. Stud. 2020, 61, 1–9. [Google Scholar] [CrossRef]
- Kumar, P.; Ashrita; Acharya, V.; Warghat, A.R. Comparative transcriptome analysis infers bulb derived in vitro cultures as a promising source for sipeimine biosynthesis in Fritillaria cirrhosa D. Don (Liliaceae, syn. Fritillaria roylei Hook.)—High value Himalayan medicinal herb. Phytochemistry 2021, 183, 112631. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, C.; Su, H.; Wang, J.; Qi, Y.; Li, M. In vitro plant regeneration and bioactive metabolite production of endangered medicinal plant Fritillaria cirrhosa. Curr. Plant Biol. 2024, 39, 100363. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, H.; Ren, Q.; Hu, H.; Yang, T.; Li, X. Natural drug sources for respiratory diseases from Fritillaria: Chemical and biological analyses. Chin. Med. 2021, 16, 40. [Google Scholar] [CrossRef]
- Peng, M.; Li, J.; Zhou, J.; Zhang, B.; Liao, J.; Yang, D.; Wang, Y.; Yang, Y.; Li, R.; Tang, X. Total alkaloids of Fritillaria unibracteata var. wabuensis bulbus ameliorate chronic asthma via the TRPV1/Ca2+/NFAT pathway. Phytomedicine 2023, 118, 154946. [Google Scholar] [CrossRef]
- Paek, K.Y.; Murthy, H.N. High frequency of bulblet regeneration from bulb scale sections of Fritillaria thunbergii. Plant Cell Tissue Organ Cult. 2002, 68, 247–252. [Google Scholar] [CrossRef]
- Tao, Q.; Han, G.; Ma, B.; Jia, H.; Zhao, C.; Li, W.; Yan, Z. In Vitro Propagation and Artificial Seed Production of Fritillaria cirrhosa D. Don, an Endangered Medicinal Plant. Phyton-Int. J. Exp. Bot. 2024, 93, 1297–1310. [Google Scholar] [CrossRef]
- Kumar, P.; Partap, M.; Ashrita; Rana, D.; Kumar, P.; Warghat, A.R. Metabolite and expression profiling of steroidal alkaloids in wild tissues compared to bulb derived in vitro cultures of Fritillaria roylei—High value critically endangered Himalayan medicinal herb. Ind. Crops Prod. 2020, 145, 111945. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, R.; Zhang, Y.; Huang, K.; Wang, W.; Li, J. Transcriptome analysis reveals in vitro-cultured regeneration bulbs as a promising source for targeted Fritillaria cirrhosa steroidal alkaloid biosynthesis. 3 Biotech 2018, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kou, C.; Ren, Y.; Li, Q.; Wang, T.; Ma, R.; Sun, W.; Xue, Z.; Ma, P. Advances of Veratrum nigrum L. Steroid Alkaloids. Ind. Crops Prod. 2023, 191, 115946. [Google Scholar] [CrossRef]
- Jiang, Q.-W.; Chen, M.-W.; Cheng, K.-J.; Yu, P.-Z.; Wei, X.; Shi, Z. Therapeutic Potential of Steroidal Alkaloids in Cancer and Other Diseases. Med. Res. Rev. 2016, 36, 119–143. [Google Scholar] [CrossRef]
- Parnes, J.; Molfino, N.A.; Colice, G.; Martin, U.; Corren, J.; Menzies-Gow, A. Targeting TSLP in Asthma. J. Asthma Allergy 2022, 15, 749–765. [Google Scholar] [CrossRef]
- Nakajima, S.; Kabata, H.; Kabashima, K.; Asano, K. Anti-TSLP antibodies: Targeting a master regulator of type 2 immune responses. Allergol. Int. 2020, 69, 197–203. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Kurihara, M.; Kabata, H.; Irie, M.; Fukunaga, K. Current summary of clinical studies on anti-TSLP antibody, Tezepelumab, in asthma. Allergol. Int. 2023, 72, 24–30. [Google Scholar] [CrossRef]
- Mousa, A.M.; Almatroudi, A.; Alwashmi, A.S.; Abdulmonem, W.A.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Alsahli, M.A.; Alrumaihi, F.; Allemailem, K.S.; Abdellatif, A.A.H.; et al. Thyme oil alleviates Ova-induced bronchial asthma through modulating Th2 cytokines, IgE, TSLP and ROS. Biomed. Pharmacother. 2021, 140, 111726. [Google Scholar] [CrossRef]
- Al Sinani, S.S.S.; Eltayeb, E.A. The steroidal glycoalkaloids solamargine and solasonine in Solanum plants. South Afr. J. Bot. 2017, 112, 253–269. [Google Scholar] [CrossRef]
- Li, H.-J.; Jiang, Y.; Li, P. Chemistry, bioactivity and geographical diversity of steroidal alkaloids from the Liliaceae family. Nat. Prod. Rep. 2006, 23, 735–752. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, P.; Zhao, L.; Bai, L.; Su, Q.; Feng, Y.; Ma, W.; Zhu, J.; Yang, J.; Zhang, S. Colchicine Alleviates Interstitial Lung Disease in an Experimental Autoimmune Myositis Murine Model by Inhibiting the Formation of Neutrophil Extracellular Traps. Inflammation 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bertin, S.; Aoki-Nonaka, Y.; de Jong, P.R.; Nohara, L.L.; Xu, H.; Stanwood, S.R.; Srikanth, S.; Lee, J.; To, K.; Abramson, L.; et al. The ion channel TRPV1 regulates the activation and proinflammatory properties of CD4+ T cells. Nat. Immunol. 2014, 15, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; L’Herondelle, K.; Buscaglia, P.; Le Gall-Ianotto, C.; Philippe, R.; Legoux, N.; Mignen, O.; Buhé, V.; Leschiera, R.; Sakka, M.; et al. Major Role for TRPV1 and InsP3R in PAR2-Elicited Inflammatory Mediator Production in Differentiated Human Keratinocytes. J. Investig. Dermatol. 2018, 138, 1564–1572. [Google Scholar] [CrossRef]
- Baker, K.; Raemdonck, K.; Dekkak, B.; Snelgrove, R.J.; Ford, J.; Shala, F.; Belvisi, M.G.; Birrell, M.A. Role of the ion channel, transient receptor potential cation channel subfamily V member 1 (TRPV1), in allergic asthma. Respir. Res. 2016, 17, 67. [Google Scholar] [CrossRef]
- Cesare, P.; Moriondo, A.; Vellani, V.; McNaughton, P. Ion channels gated by heat. Proc. Natl. Acad. Sci. USA 1999, 96, 7658–7663. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, H.; Cao, X.; Yin, Y.; Zhang, B. Activation of TRPV1 mediates thymic stromal lymphopoietin release via the Ca2+/NFAT pathway in airway epithelial cells. FEBS Lett. 2014, 588, 3047–3054. [Google Scholar] [CrossRef]
- Zhou, L.; Hao, M.; Fan, X.; Lao, Z.; Li, M.; Shang, E. Effects of Houpo Mahuang Decoction on serum metabolism and TRPV1/Ca2+/TJs in asthma. J. Ethnopharmacol. 2023, 302, 115873. [Google Scholar] [CrossRef]
- Chinese Pharmacopeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]

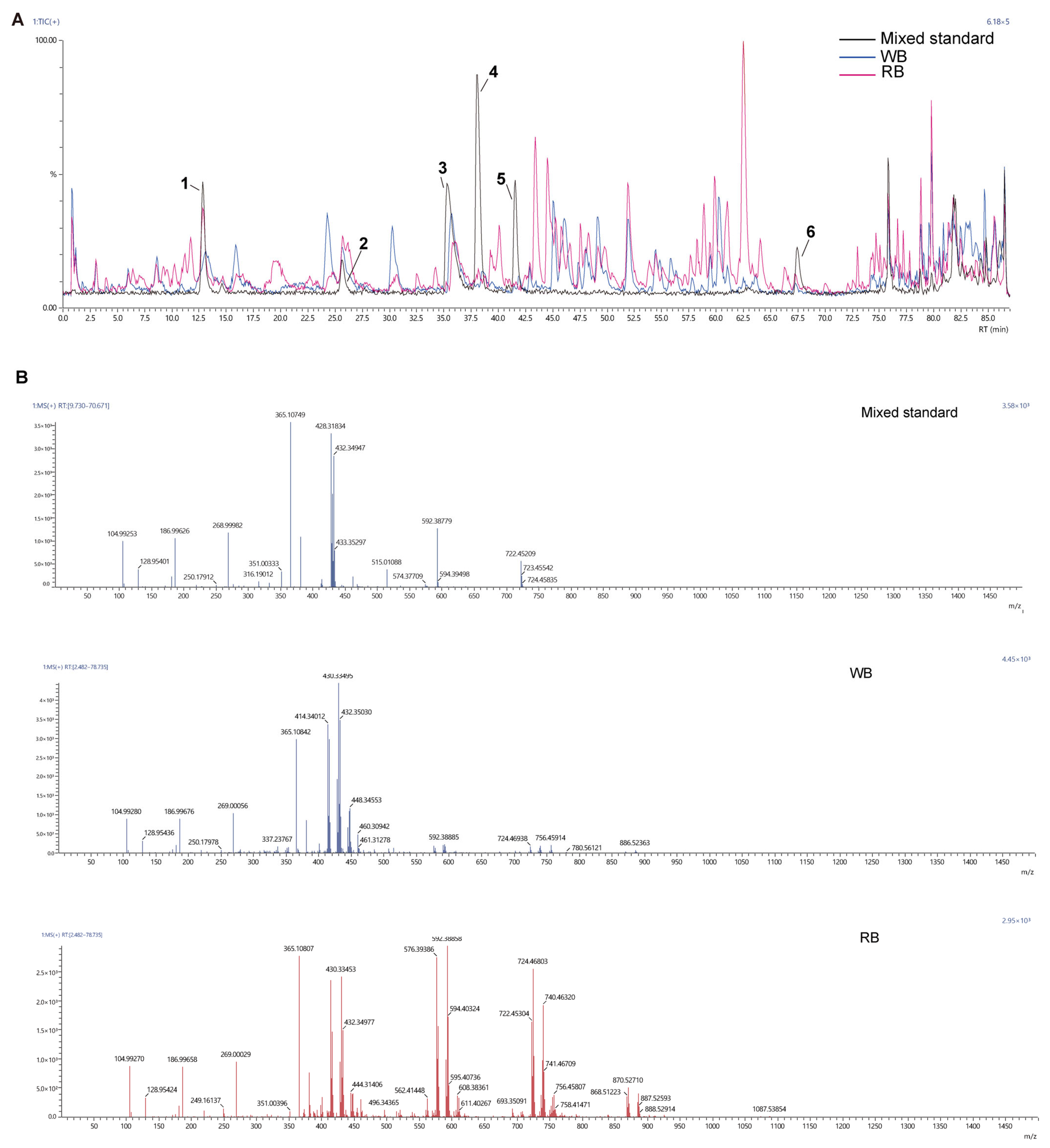


| NO. | Target Name | Target Formula | RT 1 in Mixed Standard | RT 1 in the WB | RT 1 in the RB | m/z in Mixed Standard | m/z in WB | m/z in RB | Response 2 in Mixed Standard | Response 2 in the WB | Response 2 in the RB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Edpetiline | C33H53NO8 | 12.9874 | 12.6933 | 12.5427 | 592.38779 | 592.38885 | 592.38885 | 1,170,550 | 527,306 | 992,544 |
| 2 | Sipeimine | C27H43NO3 | 26.0487 | 25.9012 | 25.9023 | 430.33157 | 430.33495 | 430.33453 | 502,110 | 757,323 | 868,954 |
| 3 | Peimisine | C27H41NO3 | 36.0988 | 36.0113 | 36.0851 | 428.31834 | 428.31674 | 428.31805 | 2,208,535 | 1,024,700 | 987,868 |
| 4 | Peimine | C27H45NO3 | 38.0533 | 38.3700 | 37.9571 | 432.34722 | 432.35030 | 432.34977 | 1,212,132 | 79,997 | 61,144 |
| 5 | Peiminine | C27H43NO3 | 41.6200 | 41.4829 | 41.7672 | 430.33157 | 430.33495 | 430.33453 | 1,492,489 | 100,065 | 76,702 |
| 6 | Khasianine | C39H63NO11 | 64.4504 | 67.4504 | 67.45404 | 722.45209 | 722.45195 | 722.45304 | 587,896 | 84,747 | 247,102 |
| Compound Name | Formula | Retention Time (min) | Fold Change | log2FC 1 | p-Value | VIP 2 | Type |
|---|---|---|---|---|---|---|---|
| Dehydroevodiamine | C19H15N3O | 83.46 | 0.0018 | −9.08 | 0.0027526 | 1.24 | down |
| Veratramine | C27H39NO2 | 75.473 | 120.2589 | 6.91 | 0.0055646 | 1.19 | up |
| Solasodine | C27H43NO2 | 51.608 | 52.7098 | 5.72 | 9.82 × 10−6 | 1.52 | up |
| Solanidine base + O-Hex-dHex | C39H63NO10 | 61.293 | 52.3457 | 5.71 | 0.0029032 | 1.25 | up |
| α-solanine | C45H73NO15 | 58.126 | 51.6251 | 5.69 | 0.0008903 | 1.33 | up |
| Edpetinosine | C33H55NO7 | 49.52 | 32.0000 | 5 | 0.011857 | 1.1 | up |
| Demissidine | C27H45NO | 75.732 | 30.4844 | 4.93 | 0.0092492 | 1.13 | up |
| Cycloposine | C33H51NO7 | 75.409 | 22.1618 | 4.47 | 0.0004324 | 1.36 | up |
| Edpetiline | C33H53NO8 | 12.789 | 13.6422 | 3.77 | 0.0100805 | 1.12 | up |
| Cyclopamine | C27H41NO2 | 79.683 | 13.2691 | 3.73 | 9.85 × 10−5 | 1.45 | up |
| β--Sitosterol | C29H50O | 85.805 | 12.9063 | 3.69 | 0.0011713 | 1.31 | up |
| Tomatidine | C27H45NO2 | 47.999 | 0.0921 | −3.44 | 0.0019842 | 1.28 | down |
| Diosgenin | C27H42O3 | 80.086 | 9.7136 | 3.28 | 6.99 × 10−5 | 1.47 | up |
| Sarsasapogenin | C27H44O3 | 79.977 | 5.1337 | 2.36 | 2.19 × 10−5 | 1.5 | up |
| 14-hydroxysprengerinin C | C44H70O17 | 74.336 | 4.5315 | 2.18 | 0.000384 | 1.36 | up |
| Timosaponin A1 | C33H54O8 | 75.292 | 2.9897 | 1.58 | 0.0034805 | 1.22 | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, J.; Zhang, E.; Yang, Y.; Lu, Q.; Zhu, Z.; Li, R. Metabolite Profiling and Anti-Inflammatory Activities of Fritillaria cirrhosa D. Don Bulbs Derived from Tissue Culture. Molecules 2025, 30, 623. https://doi.org/10.3390/molecules30030623
Wang Y, Liu J, Zhang E, Yang Y, Lu Q, Zhu Z, Li R. Metabolite Profiling and Anti-Inflammatory Activities of Fritillaria cirrhosa D. Don Bulbs Derived from Tissue Culture. Molecules. 2025; 30(3):623. https://doi.org/10.3390/molecules30030623
Chicago/Turabian StyleWang, Yu, Jiamin Liu, Enhao Zhang, Yixi Yang, Qiuxia Lu, Ziwei Zhu, and Rui Li. 2025. "Metabolite Profiling and Anti-Inflammatory Activities of Fritillaria cirrhosa D. Don Bulbs Derived from Tissue Culture" Molecules 30, no. 3: 623. https://doi.org/10.3390/molecules30030623
APA StyleWang, Y., Liu, J., Zhang, E., Yang, Y., Lu, Q., Zhu, Z., & Li, R. (2025). Metabolite Profiling and Anti-Inflammatory Activities of Fritillaria cirrhosa D. Don Bulbs Derived from Tissue Culture. Molecules, 30(3), 623. https://doi.org/10.3390/molecules30030623





