Spent Coffee Grounds as a Source of Chlorogenic Acid
Abstract
1. Introduction
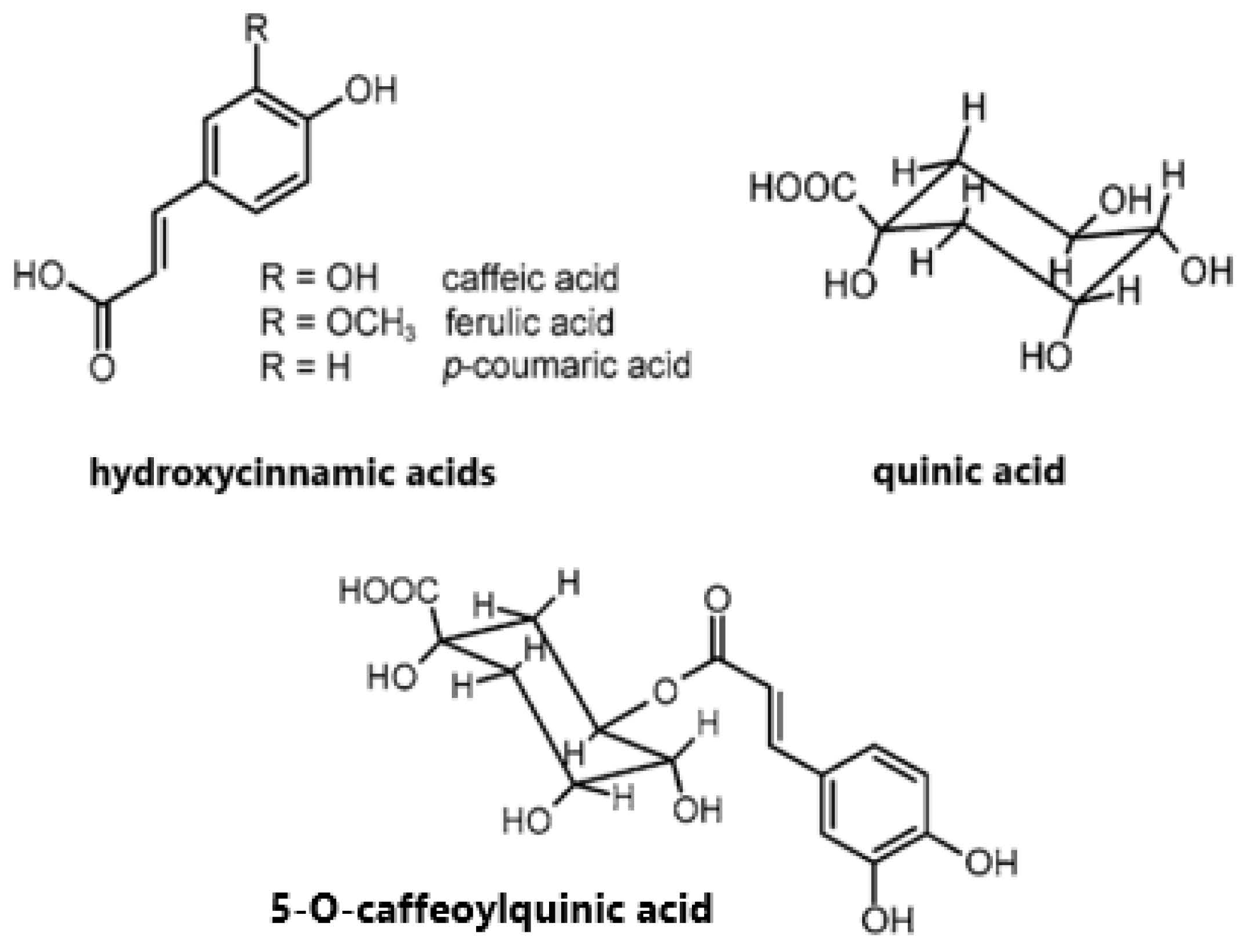
2. Chlorogenic Acids in Coffee
3. Extraction of Chlorogenic Acids from SCGs
3.1. Conventional Extraction Methods
3.2. Advanced Extraction Methods
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Pettinato, M.; Campardelli, R.; De Marco, I.; Perego, P. High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste. Appl. Sci. 2022, 12, 3642. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2024, 233, 352–370. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Brevik, E.C.; Bayoumi, Y.; Shalaby, T.A.; El-Mahrouk, M.E.; Taha, N.; Elbasiouny, H.; Elbehiry, F.; Amer, M.; Abdalla, N.; et al. An Overview of Agro-Waste Management in Light of the Water-Energy-Waste Nexus. Sustainability 2022, 14, 15717. [Google Scholar] [CrossRef]
- Frosi, I.; Montagna, I.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of Chlorogenic Acids from Agri-Food Wastes: Updates on Green Extraction Techniques. Molecules 2021, 26, 4515. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef]
- Pettinatoa, M.; Domínguezb, J.M.; Peregoa, P.; Bruno Fabiano, B. A Biorefinery from Spent Coffee Grounds: From High-added Value Compounds to Energy by Innovative Processes. Chem. Eng. Trans. 2023, 105, 331–336. [Google Scholar]
- Bevilacqua, E.; Cruzat, V.; Singh, I.; Rose’Meyer, R.B.; Panchal, S.K.; Brown, L. The Potential of Spent Coffee Grounds in Functional Food Development. Nutrients 2023, 16, 994. [Google Scholar] [CrossRef]
- Ahmed, H.; Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Toward Circular Economy: Potentials of Spent Coffee Grounds in Bioproducts and Chemical Production. Biomass 2023, 4, 286–312. [Google Scholar] [CrossRef]
- Cavanagh, Q.; Su-Ling Brooks, M.; Rupasinghe, H.P.V. Innovative technologies used to convert spent coffee grounds into new food ingredients: Opportunities, challenges, and prospects. Future Foods 2023, 8, 100255. [Google Scholar] [CrossRef]
- Arias, A.; Ioannidou, S.M.; Giannakis, N.; Feijoo, G.; Moreira, M.T.; Koutinas, A. Review of potential and prospective strategies for the valorization of coffee grounds within the framework of a sustainable and circular bioeconomy. Ind. Crops Prod. 2023, 205, 117504. [Google Scholar] [CrossRef]
- Pyrzynska, K. Useful Extracts from Coffee By-Products: A Brief Review. Separations 2024, 11, 334. [Google Scholar] [CrossRef]
- Yusufoğlu, B.; Kezer, G.; Wang, Y.; Ziora, Z.M.; Esatbeyoglu, T. Bio-recycling of spent coffee grounds: Recent advances and potential applications. Curr. Opin. Food Sci. 2024, 55, 1011111. [Google Scholar] [CrossRef]
- Solomakou, N.; Loukri, A.; Tsafrakidou, P.; Michaelidou, A.M.; Mourtzinos, I.; Goula, A.M. Recovery of phenolic compounds from spent coffee grounds through optimized extraction processes. Sustain. Chem. Pharm. 2022, 25, 100592. [Google Scholar] [CrossRef]
- Badr, A.N.; El-Attar, M.M.; Ali, H.S.; Elkhadragy, M.F.; Yehia, H.M.; Farouk, A. Spent Coffee Grounds Valorization as Bioactive Phenolic Source Acquired Antifungal, Anti-Mycotoxigenic, and Anti-Cytotoxic Activities. Toxins 2022, 14, 109. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef]
- Lee, Y.G.; Cho, E.J.; Maskey, S.; Nguyen, D.T.; Bae, H.J. Value-Added Products from Coffee Waste: A Review. Molecules 2023, 28, 3562. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Valorization of spent coffee grounds as the speciality material for dullness and ageing of skin treatments. Chem. Biol. Technol. Agric. 2021, 8, 55. [Google Scholar] [CrossRef]
- Bomfim, A.S.C.; Oliveira, D.M.; Voorwald, H.J.C.; Benini, K.C.; Dumont, M.J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. [Google Scholar] [CrossRef]
- Philipp-Dormston, W.G. Melasma: A Step-by-Step Approach Towards a Multimodal Combination Therapy. Clin. Cosmet. Investig. Dermatol. 2024, 22, 1203–1216. [Google Scholar] [CrossRef]
- Sugebo, B. A review on enhanced biofuel production from coffee by-products using different enhancement techniques. Mater. Renew. Sustain. Energy 2022, 11, 91–103. [Google Scholar] [CrossRef]
- Suasnabar, E.H.A.; Camarena Taxa, L.P.; Ordonez Galvez, J.J.; Benites Alfaro, E. Oil Extracted from Coffee Grounds to Obtain Biodiesel as Renewable Energy. Chem. Eng. Trans. 2023, 105, 517–522. [Google Scholar]
- Gu, J.; Lee, A.; Choe, C.; Lim, H. Comparative study of biofuel production based on spent coffee grounds transesterification and pyrolysis: Process simulation, techno-economic, and life cycle assessment. J. Clean. Prod. 2023, 428, 139308. [Google Scholar] [CrossRef]
- Solomakou, N.; Tsafrakidou, P.; Goula, A.M. Valorization of SCG through Extraction of Phenolic Compounds and Synthesis of New Biosorbent. Sustainability 2022, 14, 9358. [Google Scholar] [CrossRef]
- Bhosale, G.D.; Shobana, S.; Rajesh Banu, J.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Bhatia, S.K.; Atabani, A.E.; Mulone, V.; Yoon, J.J.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Biores. Technol. 2020, 314, 123800. [Google Scholar]
- Forcina, A.; Petrillo, A.; Travaglioni, M.; di Chiara, S.; De Felice, F. A comparative life cycle assessment of different spent coffee ground reuse strategies and a sensitivity analysis for verifying the environmental convenience based on the location of site. J. Clean. Prod. 2023, 385, 135727. [Google Scholar] [CrossRef]
- Johnson, K.; Liu, Y.; Lu, M. A Review of Recent Advances in Spent Coffee Grounds Upcycle Technologies and Practices. Front. Chem. Eng. 2022, 4, 838605. [Google Scholar] [CrossRef]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef]
- Leow, Y.; Yew, P.Y.M.; Chee, P.L.; Loh, X.J.; Kai, D. Recycling of spent coffee grounds for useful extracts and green composites. R. Soc. Chem. Adv. 2021, 11, 2682. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, Z.; Yu, T.; Yan, F. Spent coffee grounds: Present and future of environmentally friendly applications on industries—A review. Trends Food Sci. Technol. 2024, 14, 104312. [Google Scholar] [CrossRef]
- Garcia, C.V.; Kim, Y.T. Spent Coffee Grounds and Coffee Silverskin as Potential Materials for Packaging: A Review. J. Polym. Environ. 2021, 29, 2372–2384. [Google Scholar] [CrossRef]
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of Coffee Silver Skin as Potential Food-Safe Ingredient. Foods 2021, 10, 101367. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, A.; Squillante, J.; Velotto, S.; D’Auria, G.; Ferranti, P.; Mamone, G.; Errico, M.E.; Avolio, R.; Castaldo, R.; Cirillo, T.; et al. Valorization of coffee industry wastes: Comprehensive physicochemical characterization of coffee silverskin and multipurpose recycling applications. J. Clean. Prod. 2022, 370, 133520. [Google Scholar] [CrossRef]
- Hu, S.; Gil-Ramírez, A.; Martín-Trueba, M.; Benítez, V.; Aguilera, Y.; Martín-Cabrejas, M.A. Valorization of coffee pulp as a bioactive food ingredient by sustainable extraction methodologies. Curr. Res. Food Sci. 2023, 6, 100475. [Google Scholar] [CrossRef]
- Phuong, D.V.; Nguyen, L.T. Coffee pulp pretreatment methods: A comparative analysis of hydrolysis efficiency. Foods Raw Mater. 2024, 12, 133–141. [Google Scholar] [CrossRef]
- Campuzano, F.; Escobar, D.M.; Torres, A.M. Physicochemical characterization of coffee parchment of species Coffee arabica variety Castillo®. Coffee Sci. 2024, 19, e192182. [Google Scholar] [CrossRef]
- Soviguidi, D.R.J.; Pan, R.; Liu, Y.; Rao, L.; Zhang, W.; Yang, X. Chlorogenic Acid Metabolism: The Evolution and Roles in Plant Response to Abiotic Stress. Phyton Int. J. Exp. Bot. 2021, 91, 239–255. [Google Scholar]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzynska, K.; de Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Farah, A.; de Paula Lima, J. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Saygili, S.; Hegde, S.; Shi, X.-Z. Effects of Coffee on Gut Microbiota and Bowel Functions in Health and Diseases: A Literature Review. Nutrients 2024, 16, 3155. [Google Scholar] [CrossRef] [PubMed]
- Belayneh, A.; Molla, F. The Effect of Coffee on Pharmacokinetic Properties of Drugs: A Review. BioMed Res. Int. 2020, 2020, 7909703. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Choi, M.S.; Yoo, H.H.; Kim, D.H. The Intake of Coffee Increases the Absorption of Aspirin in Mice by Modifying Gut Microbiome. Pharmaceutics 2022, 14, 746. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 2023, 13, 1218015. [Google Scholar] [CrossRef]
- Arfian, N.; Wahyudi, D.A.P.; Zulfatina, I.B.; Citta, A.N.; Anggorowati, N.; Multazam, A.; Romi, M.M.; Sari, D.C.R. Chlorogenic Acid Attenuates Kidney Ischemic/Reperfusion Injury via Reducing Inflammation, Tubular Injury, and Myofibroblast Formation. BioMed Res. Int. 2019, 2019, 5423703. [Google Scholar] [CrossRef]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022, 186, 10650. [Google Scholar] [CrossRef]
- Neamțu, A.A.; Maghiar, T.A.; Turcuș, V.; Maghiar, P.B.; Căpraru, A.M.; Lazar, B.A.; Dehelean, C.A.; Pop, O.P.; Neamțu, C.; Totolici, B.D.; et al. A Comprehensive View on the Impact of Chlorogenic Acids on Colorectal Cancer. Curr. Issues Mol. Biol. 2024, 46, 6783–6804. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, Y.; Fu, S.; Li, Z.; Zhang, J.; Xu, Y.; Han, X.; Miao, J. Application of Chlorogenic acid as a substitute for antibiotics in Multidrug-resistant Escherichia coli-induced mastitis. Int. Immunopharmacol. 2023, 114, 109536. [Google Scholar] [CrossRef]
- Chen, C.; Wang, T.; Shen, J.L.; Liang, C.S.; Ling, F.; Li, P.F.; Wang, G.X. Evaluation of the antiviral activity of chlorogenic acid against white spot syndrome virus. Aquaculture 2024, 579, 740242. [Google Scholar] [CrossRef]
- Rai, S.P.; Ansari, A.H.; Singh, D.; Singh, S. Coffee, antioxidants, and brain inflammation. Prog. Brain Res. 2024, 289, 123–150. [Google Scholar] [PubMed]
- Porro, C.; Cianciulli, A.; Panaro, M.A. A cup of coffee for a brain long life. Neural Regen. Res. 2024, 19, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Di Pietrantonio, D.; Pace Palitti, V.; Cichelli, A.; Tacconelli, S. Protective Effect of Caffeine and Chlorogenic Acids of Coffee in Liver Disease. Foods 2024, 13, 2280. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of chlorogenic acid against Diabetes mellitus and its complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic Acids in Cardiovascular Disease: A Review of Dietary Consumption, Pharmacology, and Pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gryko, K.; Gołębiewska, E.; Świderski, G.; Lewandowska, H.; Pruszyński, M.; Zawadzka, M.; Kozłowski, M.; Sienkiewicz-Gromiuk, J.; Lewandowski, W. Fe(III) and Cu(II) Complexes of Chlorogenic Acid: Spectroscopic, Thermal, Anti-/Pro-Oxidant, and Cytotoxic Studies. Materials 2022, 15, 6832. [Google Scholar] [CrossRef]
- Pimpley, V.A.; Maity, S.; Murthy, P.S. Green coffee polyphenols in formulations of functional yoghurt and their quality attributes. Int. J. Dairy Technol. 2022, 75, 159–170. [Google Scholar] [CrossRef]
- Zain, M.Z.M.; Baba, A.S.; Shori, A.B. Effect of polyphenols enriched from green coffee bean on antioxidant activity and sensory evaluation of bread. J. King Saud Univ. Sci. 2018, 30, 278–282. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin–Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Inter. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.; Cimercati, C.; Costa, W.; Levate, M.L.; Pimenta, C.J. Effect of solvent, method, time and temperature of extraction on the recovery of phenolic compounds and antioxidants from spent coffee grounds. Int. J. Food Eng. 2022, 18, 325–336. [Google Scholar] [CrossRef]
- Ősz, B.E.; Jîtcă, G.; Ștefănescu, R.E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.E. Caffeine and Its Antioxidant Properties—It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 28, 13074. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.S.; Pal, K.; Asthana, N.; Bhattu, M.; Verma, M. Green synthesis by extraction of caffeine for cosmeceutical application: A review. J. Mol. Struct. 2024, 1305, 137733. [Google Scholar] [CrossRef]
- Sentkowska, A.; Ivanova-Petropulos, V.; Pyrzynska, K. What Can Be Done to Get More—Extraction of Phenolic Compounds from Plant Materials. Food Anal. Meth. 2024, 17, 594–610. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical Composition, Antioxidant and Enzyme Inhibitory Properties of Different Extracts Obtained from Spent Coffee Ground and Coffee Silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Campisi, B.; Peregrina, D.V.; Censi, R.; Khamitova, G.; Angeloni, S.; Caprioli, G.; Zannotti, M.; Ferraro, S.; Giovannetti, R.; et al. Optimization of the Extraction from Spent Coffee Grounds Using the Desirability Approach. Antioxidants 2020, 29, 370. [Google Scholar] [CrossRef]
- Vu, D.C.; Vu, Q.T.; Huynh, L.; Lin, C.H.; Alvarez, S.; Vo, X.T.; Nguyen, T.H.D. Evaluation of fatty acids, phenolics and bioactivities of spent coffee grounds prepared from Vietnamese coffee. Int. J. Food Prop. 2021, 24, 1548–1558. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, Identification and Quantification of Polyphenols from Spent Coffee Grounds by Chromatographic Methods and Chemometric Analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Bouhzam, I.; Cantero, R.; Margallo, M.; Aldaco, R.; Bala, A.; Fullana-i-Palmer, P.; Puig, R. Extraction of Bioactive Compounds from Spent Coffee Grounds Using Ethanol and Acetone Aqueous Solutions. Foods 2023, 12, 4400. [Google Scholar] [CrossRef] [PubMed]
- Ali Redha, A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef] [PubMed]
- García-Roldán, A.; Piriou, L.; Jauregi, P. Natural deep eutectic solvents as a green extraction of polyphenols from spent coffee ground with enhanced bioactivities. Front. Plant Sci. 2023, 13, 1072592. [Google Scholar] [CrossRef] [PubMed]
- Abi-Khattar, A.M.; Boussetta, N.; Rajha, H.N.; Abdel-Massih, R.M.; Louka, N.; Maroun, R.G.; Vorobiev, E.; Debs, E. Mechanical damage and thermal effect induced by ultrasonic treatment in olive leaf tissue. Impact on polyphenols recovery. Ultrason. Sonochem. 2022, 82, 105895. [Google Scholar] [CrossRef]
- Gupta, Y.; Barrett, B.; Vlachos, D.G. Understanding microwave-assisted extraction of phenolic compounds from diverse food waste feedstocks. Chem. Eng. Process. Process Intensif. 2024, 203, 109870. [Google Scholar] [CrossRef]
- Bouhzam, I.; Cantero, R.; Balcells, M.; Margallo, M.; Aldaco, R.; Bala, A.; Fullana-i-Palmer, P.; Puig, R. Environmental and Yield Comparison of Quick Extraction Methods for Caffeine and Chlorogenic Acid from Spent Coffee Grounds. Foods 2023, 12, 779. [Google Scholar] [CrossRef]
- Yoo, D.E.; Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep eutectic solvent-based valorization of spent coffee grounds. Food Chem. 2018, 255, 357–364. [Google Scholar] [CrossRef]
- Silva, C.N.d.; Silva, R.M.d.; Lemes, A.C.; Ribeiro, B.D. Recovery of Phenolic Compounds by Deep Eutectic Solvents in Orange By-Products and Spent Coffee Grounds. Sustainability 2024, 16, 7403. [Google Scholar] [CrossRef]
- Dewi, S.R.; Stevens, L.A.; Pearson, A.F.; Ferrari, R.; Irvine, D.J.; Binner, E.R. Investigating the role of solvent type and microwave selective heating on the extraction of phenolic compounds from cacao (Theobroma cacao L.) pod husk. Food Bioprod. Process. 2022, 134, 210–222. [Google Scholar] [CrossRef]
- Coelho, P.; Robalo, M.P.; Boyadzhieva, S.; Stateva, R.P. Microwave-Assisted Extraction of Phenolic Compounds from Spent Coffee Grounds. Process Optimization Applying Design of Experiments. Molecules 2021, 26, 7320. [Google Scholar] [CrossRef]
- Pettinato, M.; Alberto, A.; Perego, P. The role of heating step in microwave-assisted extraction of polyphenols from spent coffee grounds. Food Bioprod. Process. 2019, 114, 227–234. [Google Scholar] [CrossRef]
- Bhadange, Y.A.; Carpenter, J.; Saharan, V.K. A Comprehensive Review on Advanced Extraction Techniques for Retrieving Bioactive Components from Natural Sources. ACS Omega 2024, 9, 31274–31297. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič Višnjevec, A.; Barp, L.; Lucci, P.; Moret, S. Pressurized liquid extraction for the determination of bioactive compounds in plants with emphasis on phenolics. Trends Anal. Chem. 2024, 173, 117620. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Wang, C.Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food. Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Wu, C.T.; Agrawal, D.C.; Huang, W.Y.; Hsu, H.C.; Yang, S.J.; Huang, S.L.; Lin, Y.S. Functionality Analysis of Spent Coffee Ground Extracts Obtained by the Hydrothermal Method. J. Chem. 2019, 2019, 4671438. [Google Scholar] [CrossRef]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the Recovery of Phenolic Compounds from Spent Coffee Grounds (SCG) by Environmentally Friendly Extraction Techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Safavizadeh, V.; Yousefi, M.; Hosseini, S.M. A short review of supercritical fluid extraction of plant extracts. J. Food Meas. 2024, 18, 3651–3664. [Google Scholar] [CrossRef]
- Coelho, J.P.; Filipe, R.M.; Robalo, M.P.; Boyadzhieva, S.; Cholakov, G.S.; State, R.P. Supercritical CO2 extraction of spent coffee grounds. Influence of co-solvents and characterization of the extracts. J. Supercrit. Fluids 2020, 161, 104825. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Subcritical water and supercritical carbon dioxide: Efficient and selective eco-compatible solvents for coffee and coffee by-product valorization. Green Chem. 2020, 22, 8544–8571. [Google Scholar] [CrossRef]
- Andrade, K.S.; Gonçalvez, R.T.; Maraschin, M.; Ribeiro-do-Valle, R.M.; Martínez, J.; Ferreira, S.R. Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition. Talanta 2021, 88, 544–552. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Basile, G.; Nitride, C.; Pizzolongo, F.; Masi, P. The Use of Carbon Dioxide as a Green Approach to Recover Bioactive Compounds from Spent Coffee Grounds. Foods 2023, 12, 1958. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic-assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.D.S.; Vimercati, W.C.; Macedo, L.L.; Saraiva, S.H.; Teixeira, L.J.Q.; da Costa, J.M.G.; Pimenta, C.J. Encapsulation of phenolic and antioxidant compounds from spent coffee grounds using spray-drying and freeze-drying and characterization of dried powders. J. Food Sci. 2022, 87, 4056–4067. [Google Scholar] [CrossRef] [PubMed]
- Cosgun, G.; Gungor, K.K.; Balci-Torun, F.; Sahin, S.; Torun, M. Design of encapsulation method for chlorogenic acid and caffeine in coffee waste by-product. Phytochem. Anal. 2024, 35, 1720–1735. [Google Scholar] [CrossRef]
- Angeloni, S.; Nzekoue, F.K.; Navarini, L.; Sagratini, G.; Torregiani, E.; Vittori, S.; Caprioli, G. An analytical method for the simultaneous quantification of 30 bioactive compounds in spent coffee ground by HPLC-MS/MS. J. Mass Spectrom. 2020, 11, e4519. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Rodríguez Casas, A.; del Castillo, M.D. Interest of Coffee Melanoidins as Sustainable Healthier Food Ingredients. Front. Nutr. 2021, 8, 730343. [Google Scholar] [CrossRef]
- Viencz, T.; Acre, L.B.; Barros Rocha, R.; Alves, E.A.; Ramalho, A.R.; de Toledo Benassi, M. Caffeine, trigonelline, chlorogenic acids, melanoidins, and diterpenes contents of Coffea canephora coffees produced in the Amazon. J. Food Comp. Anal. 2023, 117, 105140. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the Antioxidant Activity of Melanoidins from Coffee Brews by Different Antioxidant Methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef]
- Binello, A.; Cravotto, G.; Menzio, J.; Tagliapietra, S. Polycyclic aromatic hydrocarbons in coffee samples: Enquiry into processes and analytical methods. Food Chem. 2021, 344, 128631. [Google Scholar] [CrossRef]
- Al Attiya, W.; Hassan, Z.U.; Al-Thani, R.; Jaoua, S. Prevalence of toxigenic fungi and mycotoxins in Arabic coffee (Coffea arabica): Protective role of traditional coffee roasting, brewing and bacterial volatiles. PLoS ONE 2021, 16, e0259302. [Google Scholar] [CrossRef]
- Yeoh, L.; Ng, K.S. Future Prospects of Spent Coffee Ground Valorisation Using a Biorefinery Approach. Resour. Conserv. Recycl. 2022, 179, 196123. [Google Scholar] [CrossRef]
- Almeida, F.S.; Dias, F.F.G.; Sato, A.C.K.; Leite, J.M.; de Moura Bell, N. Scaling up the Two-Stage Countercurrent Extraction of Oil and Protein from Green Coffee Beans: Impact of Proteolysis on Extractability, Protein Functionality, and Oil Recovery. Food Bioprocess Technol. 2022, 15, 1794–1809. [Google Scholar] [CrossRef]
- Shewa, W.A.; Hussain, A.; Chandra, R.; Lee, J.; Saha, S.; Lee, H.S. Valorization of food waste and economical treatment: Effect of inoculation methods. J. Clean. Prod. 2020, 261, 12117. [Google Scholar] [CrossRef]
- Peluso, M. Coffee By-Products: Economic Opportunities for Sustainability and Innovation in the Coffee Industry. Proceedings 2023, 89, 6. [Google Scholar] [CrossRef]
| Extraction Method | Extraction Conditions | CGAs Content mg/g | TPC (mg GAE/g) | Ref. |
|---|---|---|---|---|
| SLE | water, 1 g/20 mL, 80 °C, 30 min | - | 61.49 ± 1.36 | [68] |
| SLE | 0.7 g/4 mL, room temp., 1 min water 20% EtOH 40% EtOH 20% acetone 40% acetone | 0.78 0.74 0.35 0.83 0.69 | 3.83 3.98 3.93 4.40 4.37 | [71] |
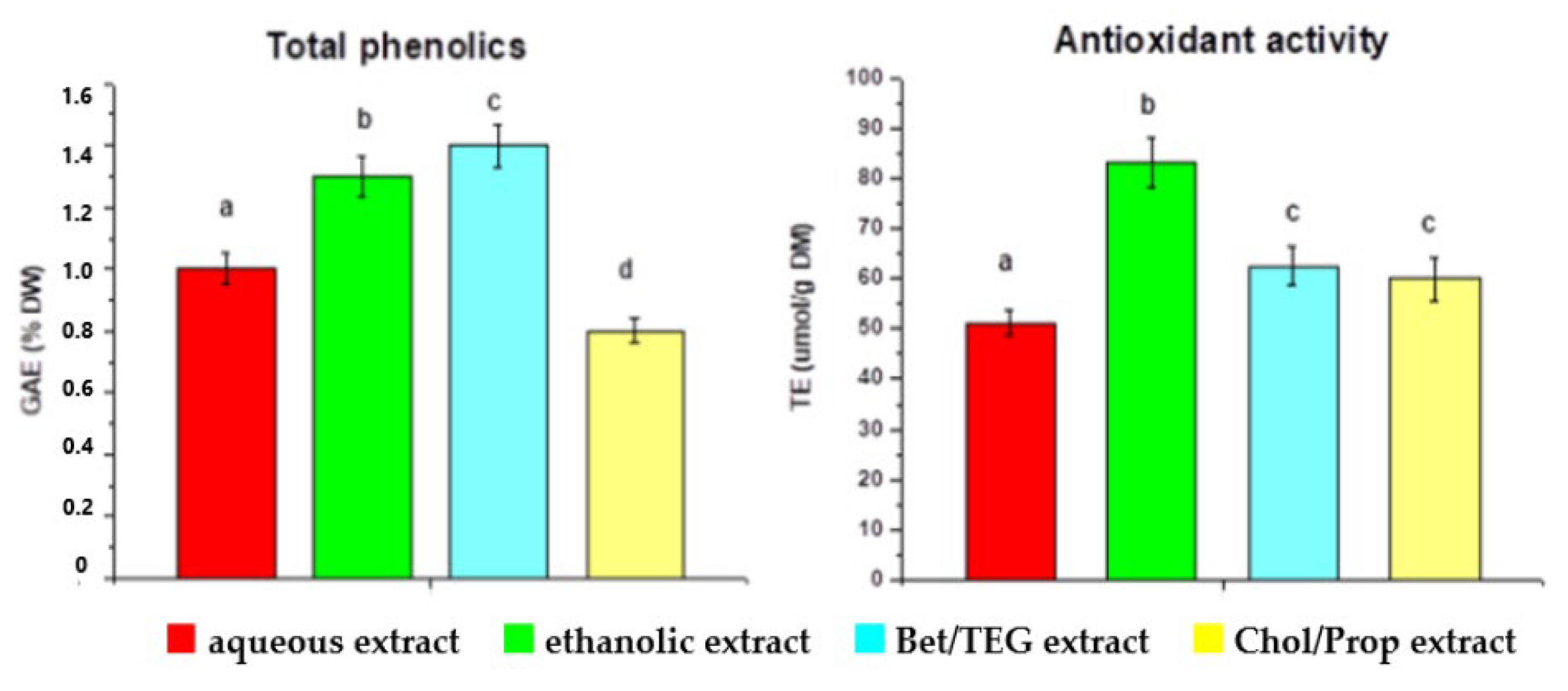
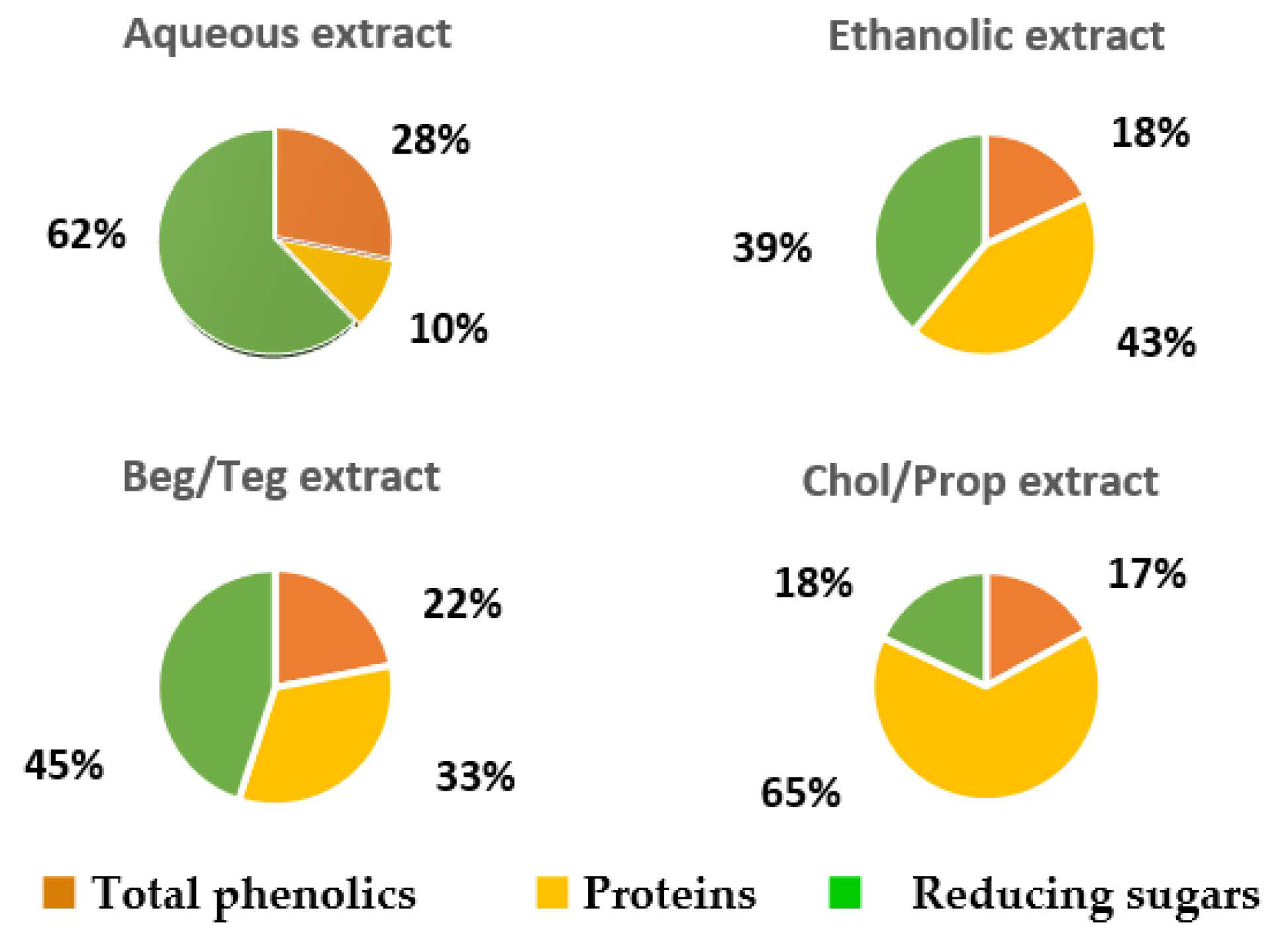
| SLE | NADES, 1 g/15 mL, 65 °C, 150 min Bet/TEG Chol/Prop 60% EtOH, 1 g/8 mL, 60 °C, 2 h water, 1 g/8 mL, 100 °C, 1 h | - | 1.42 1 0.78 1 1.26 1 0.97 1 | [73] |
| UAE | 10 g/50 mL, 20 °C, 120 min water 100% MeOH 50% MeOH 70% EtOH | 4.66 ± 0.25 9.79 ± 0.82 10.61 ± 0.90 8.62 ± 0.76 | 56.86 ± 0.16 63.25 ± 0.10 93.26 ± 0.14 93.55 ± 0.65 | [67] |
| UAE | water, 0.7 g/4 mL, 1 min, room temp. 50 °C | 1.15 1.02 | - | [76] |
| UAE | DES (1,6-hexanediol/choline chloride, 7:1), 100 mg/2.6 mL, 60 °C, 10 min | - | 17.0 ± 0.2 | [77] |
| MAE | 70% EtOH, 1 g/15 mL, 6 min, 75 °C | - | 117.7 ± 6.1 | [79] |
| SLE UAE HHPE | 80% MeOH, 50 °C, 30 min 80% MeOH, 15 min 80% MeOH, 15 min | 24.0 ± 0.3 2 85.0 ± 0.62 2 81.2 ± 1.1 2 | 6.40 ± 0.18 9.51 ± 0.06 9.42 ± 0.10 | [86] |
LE-CO2 SFE-CO2 | 10 mL/min flow rate, 30 MPa, 1 h 20 °C + 5% EtOH 60 °C + 5% EtOH | 1.41 ± 0.16 2 - 2.01 ± 0.06 2 | 692.75 ± 55.00 3 857.25 ± 37.00 3 419.50 ± 66.00 3 969.75 ± 35.00 3 | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrzynska, K. Spent Coffee Grounds as a Source of Chlorogenic Acid. Molecules 2025, 30, 613. https://doi.org/10.3390/molecules30030613
Pyrzynska K. Spent Coffee Grounds as a Source of Chlorogenic Acid. Molecules. 2025; 30(3):613. https://doi.org/10.3390/molecules30030613
Chicago/Turabian StylePyrzynska, Krystyna. 2025. "Spent Coffee Grounds as a Source of Chlorogenic Acid" Molecules 30, no. 3: 613. https://doi.org/10.3390/molecules30030613
APA StylePyrzynska, K. (2025). Spent Coffee Grounds as a Source of Chlorogenic Acid. Molecules, 30(3), 613. https://doi.org/10.3390/molecules30030613






