1. Introduction
In 1981, two manuscripts were submitted for publication, in which the synthesis of disilenes, hitherto unknown molecules with silicon-silicon double bonds, were described. West et al. reported in their paper [
1] the dimerization of silylenes, whereas Masamune et al. [
2] prepared disilene by photofragmentation of a cyclotrisilane, another hitherto unknown molecule. That hexakis(2,6-dimethylphenyl)cyclotrisilane was “stable to oxygen, moisture, and heat to its melting point” indicated unexpected thermodynamic stability. When the cyclotrisilane was irradiated with ultraviolet light for 5 min, it was nearly quantitatively converted into disilene and silylene [
2]. This suggests that large aryl substituents increase the kinetic stabilization of the three-membered ring against oxidation and fragmentation, at least in the electronic ground state. When the aryl substituents were replaced by alkyl substituents [
3], the cyclotrisilanes were found to be rather sensitive to exposure to oxygen, but the light-induced fragmentation took several hours. These experimental results did not explain why the cyclotrisilanes were that stable; after all, according to chemical knowledge, both the smallest cyclic silanes and unsaturated silanes were regarded as nonexistent compounds or compounds of low stability [
4]. To explain the lower stability of the silicon species when compared with the carbon analogs, the overlap of the silicon valence orbitals was claimed to be much poorer. In the case of disilenes, the side-on overlap of the p
π atomic orbitals (AO) was considered to be too small; in the case of the small ring systems, the small overlap of sp
3 AOs was claimed to be the cause of low stability and of high reactivity. The high stability caused by suitable substituents was surprising and demanded a correction of the explanation.
In this article, we study the dissociation and recombination reactions of the 3-rings and concentrate on the description of spin reorganization and charge polarization using a wave function method designed for studying such processes, which are more difficult to describe than the formation of covalent single bonds. Since chemical reactions are dominated by local processes—Levine simply says “
Chemistry is local” [
5]—it is necessary to transform the state function into a form that reveals the local processes during breaking and forming covalent bonds. Most important are the coupling of fragment high-spin states and the preparation of reactants for bonding.
Bonding is the stabilizing process in a system composed of several subsystems [
6]; in molecular systems, the subsystems are either atoms or stable atom groups, where “stable” means that the atom group does not spontaneously disintegrate into smaller atom groups or atoms. Stable atom groups, also termed molecular species or molecular compounds, can be radicals or molecules; molecules are molecular species that do not react violently with other species of their own kind [
7]. In this paper, we will call any subsystem of a molecular system a fragment. An important criterion for any kind of bonding is the total energy: if the total energy of a system decreases during some process, the system is stabilized and thus bonded. For chemists, the following structural criterion must be fulfilled for chemical or covalent bonding in a system: at least one atom group with a significant short interatomic distance, which did not exist in the system before the stabilization process started, must be created. This criterion together with the claimed role of the Lewis electron pair and the valence concept is the basis of the traditional chemical explanation of covalent bonding. According to Lewis, the formation of the atom groups is caused by electron pairs shared between the atoms, meaning that each atom in the atom group contributes one electron to the electron pair (Lewis’ rule of two). The valence of an atom is equal to the number of unpaired electrons it has and to the number of electron pairs the atom can share with other atoms. In chemistry parlance, one speaks of a covalent single AB bond if the AB atom group is the result of the formation of one shared electron pair between atoms A and B. If not all of the
N unpaired valence electrons are used to form single bonds to different atoms, two or more electron pairs can stabilize an AB group and one speaks then of the existence of double bonds, triple bonds, etc., in the AB group. Pairs of valence electrons that are located at one atom but are not shared electron pairs are called lone pairs. A serious deficiency of Lewis’ electron pair model is that it does not say when electrons will pair.
As soon as the fermionic character of electrons was recognized, it became clear that a shared electron pair is an electron distribution in a singlet spin state, called a low-spin state. That means the two electrons must be in spin projection states with different quantum numbers; in chemical parlance, one electron has
spin, the other has
spin or, simply said, they are singlet coupled. If the spins of both electrons are in the same spin projection state, either both are
or both are
electrons, the electron distribution is in a high-spin triplet state, the electrons are unpaired. Singlet coupled valence electrons are always paired and cannot immediately participate in the formation of shared electron pairs, whereas triplet coupled valence electrons are unpaired and therefore immediately ready for bonding. The difference in the dimerization of methylene and of silylene demonstrates this nicely. The ground state of methylene is a triplet state; the two valence electrons are triplet coupled lone pair electrons, which are ready for the formation of a double bond. The ground state of silylene, however, is a low spin singlet state; the lone pair electrons are nor ready for bonding but must be prepared by exciting the lone pair from the low-spin state to the high-spin state [
8].
However, pairing of unpaired electrons is not sufficient for covalent bonding. Since the first quantum theoretical studies of bonding in the hydrogen molecule, it is known that charge shifts during bonding must be accounted for [
9]. And the more valence electrons the atoms connected by covalent bonds have, the more inter- and intra-fragment interactions can occur and must be properly treated. These interactions may cause dramatic changes in the distribution of charges and spins and, accordingly, in the structure of the molecules involved; multi-configurational self-consistent field (MCSCF) wave functions, especially the complete active space self-consistent field (CASSCF) variant, allow the sound description of reactions in such molecular systems. But local effects remain hidden if the wave functions are built with delocalized molecular orbitals (MO), and they can be revealed when valence bond (VB) methods based on localized orbitals are used. The orthogonal valence bond (OVB) analysis of CASSCF wave functions [
8] was developed to reveal the hidden local features by transforming delocalized CASSCF MOs into localized fragment MOs (FMO).
To find out which processes are constitutive for the creating or breaking of covalent bonds, comparative studies of reactions with homologous reactants are helpful. The insertion of methylene [
10] and silylene [
11,
12,
13] into the hydrogen molecule were the first reactions that stimulated a re-investigation with the OVB method, followed by the dimerization of methylene and silylene to ethene and disilene, respectively [
8,
14], as well as the formation of silaethene from methylene and silylene [
15]. The insertion reaction into hydrogen is a prototype reaction for insertions of all carbene analogs into
-single bonds. In these reactions the bonding electron pair of the
bond and the singlet coupled lone pair of the carbene analogs must simultaneously be excited from low- to high-spin states, and, since all reactions are studied in systems with singlet ground states, these spin rearrangements in the reactants must occur simultaneously. Depending on the system’s symmetry, polarization of the charge distribution can enhance the decoupling, and make symmetry-forbidden reactions symmetry-allowed, as found for the dimerization of carbene analogs [
14].
In this paper, the elimination of methylene and silylene from cyclic 3-rings and the reverse addition of methylene and silylene to the bonds in ethene, disilene, and silaethene are studied in both high and low symmetry. In high symmetry, elimination of carbene analogs is costly; in low symmetry, elimination proceeds without any energy barriers. This was found for all 3-rings, and explains why 3-rings with large, bulky substituents, which prohibit a deformation to symmetry, are rather stable. Comparison of the reactions in the cyclopropane and cyclotrisilane systems show intrinsic similarities and differences of the two small ring systems. The reactions of silacyclopropane and disilacyclopropane show the differences in the electron structure between a molecular system with a C-C double bond and silylene compared with the system with an Si-Si double bond and methylene.
2. The Role of the Pauli Exclusion Principle
That electrons are fermions is fundamental for the electron distribution in many-electron systems. Fermions are quantum particles with a half-integer quantum number
s. To each quantum number
s, there are
different projections of the spin onto an external axis, and thus
different spin states with spin projection quantum numbers running from
to
in steps of one. Electrons are fermions with spin quantum number
, having two spin projections, called spin-up and spin-down; in chemistry, electrons in the spin-up state are called
-electrons and electrons in the spin-down state are called
-electrons. Electrons in the same spin projection state are identical electrons. The most simple way to describe an
N-particle systems is by looking at the probability to find one particle in a certain one-particle state
, a second in a one-particle state
, and so on. This probability
is the square modulus of the, in general, complex, quantum amplitude
or simply
. Quantum amplitudes of many-fermion systems are totally antisymmetric,
. This implies that a fermion-wave function is zero whenever two individual states are identical or, in other words, a system of fermions can never be in a state in which two individual states are identical. This is the Pauli exclusion principle (PEP). If the individual states are characterized by quantum numbers, the PEP can be expressed as follows: no two individual states can agree in all quantum numbers; if the individual states are position states, the PEP says that two identical fermions can never be at the same position in space. Considering that the square of the modulus of the quantum amplitude can be interpreted as a probability, one can also say that, for
with
, the probability to find two identical electrons at
and
goes to zero. That two identical electrons avoid coming close in space resembles a repulsive interaction, but one that is much more effective than any other physical interaction like, e.g., the Coulomb interaction. Identical electrons avoid each other because of the PEP and the Coulomb repulsion; non-identical electrons experience only the Coulomb repulsion. This has an important effect on the spatial extension of orbitals: if two electrons occupy the same orbital, they must be non-identical; thus, they can come arbitrarily near and experience the strong Coulomb repulsion. To diminish the repulsion, the spatial region where two non-identical electrons are located must increase, which means the orbital must expand. The same is true if two non-identical electrons occupy two different orbitals that occupy regions in space that are very close, for example, the AOs representing the subshells of an electron shell in a many-electron atom. But if the AOs are occupied by identical electrons, the Coulomb repulsion is strongly reduced due to the PEP and the AOs contract. Given incompletely filled subshells of the valence shell, the degenerate AOs can be occupied with non-identical or with identical electrons. The total spin quantum number
S is proportional to the difference of the number of
-electrons,
, and
-electrons,
, with
.
ranges from zero (for even
N) or 1/2 (for odd
N) to
. Spin states corresponding to small total spin quantum numbers are low-spin states, those corresponding to large spin quantum numbers are high-spin states. The number
is called the multiplicity of the state. If the degenerate AOs are occupied with identical electrons, the system is in the state with the highest spin. In the carbon and the silicon atom, only two electrons occupy the three AOs of the p-subshell. If the two electrons are identical, the state of the total spin is a high-spin state and the electrons must occupy different AOs; if the electrons are non-identical, the state of the total spin is a low-spin state, and the electrons can either occupy the same AO or different AOs. In both cases, the low-spin state has a higher total energy than the high-spin state. This is expressed in one of Hund’s rules, which states that for a given electron configuration (distribution of electrons in AOs), the state with the highest multiplicity has the lowest energy (rule of maximum multiplicity). Hund’s rules were originally formulated for many-electron atoms, but they apply also to molecular states [
16].
When atoms from different periods in the periodic table are studied, one must also consider the different extensions of the AOs in the valence shell. The silicon atom has a larger extension than the carbon atom, and the same is true for the valence AOs; consequently, the Coulomb repulsion of two non-identical electrons in a single silicon valence AO is smaller than in a carbon valence AO. Hund’s rule of maximum multiplicity can be used to predict the ground states of molecules with partially occupied degenerate MOs such as the O2 molecule with the configuration, or linear methylene or linear silylene, both with the configuration. All three molecules have triplet (high-spin) ground states. If the triatomic molecules bend, the degeneracy is lifted and the MOs develop into atom-centered lone pair MOs: one becomes an sp-hybrid with a large s-contribution and a second MO that is essentially a p AO; the energy of the sp-hybrid is lower than that of the p AO. Now, the different extensions decide on the orbital occupation; in methylene, the extensions of both lone pair MOs are much smaller than the extensions of the respective MOs in silylene, single occupation of both orbitals with identical electrons is preferable to a double occupation of one AO, and the ground state of bent methylene is indeed a high-spin triplet. The large extension of the MOs in silylene on the other hand allows a double occupation of the energetically lower sp-hybrid and the ground state of silylene is a low-spin singlet.
The PEP also governs the electron and spin distribution in larger molecules. The existence of two identical electrons in triplet methylene immediately allows the formation of two covalent bonds when two ground state methylenes combine to form singlet ethene; the two lone pair electrons at each carbon atom remain also in the ethene molecule in a local high-spin arrangement. In the reaction of two singlet silylenes to singlet disilene, the two non-identical lone pair electrons, which occupy the energetically low lone pair sp-hybrid, are not able to form covalent bonds between the silicon atoms. They must be transformed into a pair of identical electrons by a spin flip, by which the singlet coupled spins are decoupled. This means that the lone pair electrons in the methylene and silylene fragments in ethene, disilene, or silaethene are identical electrons. Speaking of lone pair electrons in fragments assumes that the lone pair MOs of the isolated fragments retain their characteristics also in the molecule and that it makes sense to say that in the molecule, the lone pair electrons occupy FMOs that resemble the lone pair MOs of the isolated fragments. And only then can one say that identical electrons located in lone-pair FMOs constitute a local high-spin state; similarly, non-identical electrons in lone pair FMOs constitute local low-spin states. One must not forget, however, that these local spin states are not fragment states, which are always mixed states [
17]; the justification for speaking of local spin states is the possibility to construct spin eigenfunctions of the molecular system from spin eigenfunctions of the fragments [
18].
3. The OVB Analysis of CASSCF Wave Functions
The configuration state functions (CSFs) of an
M electron system are antisymmetrized products of a spatial wave function, in most cases, a product of spatial MOs, and an eigenfunction of the spin operator
[
18]. The most simple CSF of a system with
electrons is a closed-shell CSF. It consists of the
N lowest MOs, all occupied with two electrons, combined with a singlet spin eigenfunction. In general, the MOs are calculated with the self-consistent field (SCF) method. A wave function that consists of only one closed-shell CSF cannot properly describe the change in the electron distribution of a molecular system during chemical reactions, but a linear combination of CSFs, called a multi-configurational (MC) wave function, can do it. For chemical reactions, only valence electrons are relevant, which are the electrons in the valence shell, that is, the outermost electron shell of an atom; the sum of all atomic valence electrons is the number of valence electrons of a molecule. Valence AOs are all AOs describing the valence shell of an atom; valence MOs are all MOs that are linear combinations of valence AOs. The CSFs of an MC wave function are constructed by taking a certain number of electrons, called the active electrons, out of doubly occupied MOs of the closed shell CSF and placing them into some unoccupied MOs. Active MOs and active valence electrons are defined as those valence MOs and valence electrons, respectively, that are regarded as important for the description of a chemical reaction. The coefficients of the CSFs in MC wave functions are termed configuration interaction (CI) coefficients. In the multi-configurational SCF (MCSCF) method all active MOs and the CI coefficients are simultaneously optimized. An important class of MCSCF wave functions is the so called complete active space SCF (CASSCF) wave functions made with the maximum number of CSFs that can be constructed with
n active electrons and
m active MOs. The symbol for such a CASSCF wave function is CAS(
n,
m). The number of possible CSFs, given by the Robinson–Weyl formula [
18], depends not only on
n and
m but also on the spin quantum number
S of the wave function, as follows:
The specification of the active MOs and active electrons is part of the modeling of a chemical reaction. CASSCF wave functions are designed to give optimal descriptions of reactions; therefore, they are also called fully optimized reaction space (FORS) wave functions.
Because of the variational optimization of CASSCF wave functions, all information about the interaction between reactants in a chemical reaction is stored in the orthogonal CASSCF MOs. And because the MOs are orthogonal, the CSFs made with them are also orthogonal. This allows interpretion of the square of the CI coefficient of a CSF as the weight of the CSF in the wave function. If CSFs of an MC wave function are constructed with non-orthogonal orbitals, the CSFs are also non-orthogonal and the square of the CI coefficient of a CSF cannot be directly interpreted as the weight of the CSF.
As mentioned above, the spin functions of composite molecular systems can be constructed from spin functions of the subsystems, that is, the fragments. The antisymmetrized product of FMOs and such a spin function gives a CSF that can be interpreted as the antisymmetrized product of subsystem CSFs or fragment CSFs (pFCSFs). If the FMOs are obtained from the delocalized CASSCF MOs by an orthogonal transformation [
8,
19], the FMOs are also orthogonal as are the pFCSFs. The FMOs store the same interaction information as do the original MOs, but now it is possible to trace the interactions in the molecular system to specific interactions between the fragments. In other words, the wave function of the composite system can be represented by
CSFs with delocalized MOs or by the same number of pFCSFs. This is the basis of the OVB analysis of CASSCF wave functions [
8,
14,
15,
20].
The charge distribution of electrons in an isolated neutral fragment is described by a neutral pFCSF. If the molecular system is composed of two fragments, the following descriptions of the fragments are possible: the system consists of a pair of neutral fragments, or of a cation/anion fragment pair, or of a dication/dianion fragment pair and so on. Accordingly, the pFCSFs are products of neutral FCSFs, or of one FCSF describing a cationic charge distribution and a second one describing an anion, and so on. is, in general, much larger than the number of neutral pFCSFs, but it is equal to the sum of all neutral and all ionic pFCSFs that are products of the FCSFs describing the states of the cation/anion fragment pairs. A single ionic pFCSF describes the charge distribution in a cation/anion pair, which can be regarded as the result of a charge transfer between neutral fragments; accordingly, ionic pFCSFs are frequently called charge-transfer CSFs. However, only wave functions of the molecular system that consist of a single charge-transfer pFCSF describe a physical electron transfer; in an MC wave function, charge-transfer pFCSFs describe charge shifts (charge polarization) in the composite system.
FMOs that are calculated for one isolated fragment with an SCF method are orthogonal to each other, but they are not, in general, orthogonal to FMOs of another fragment. pFCSFs made with such FMOs are not orthogonal to each other, except when the fragments are spatially far from each other, that is, when the system is dissociated. Because of the non-orthogonality of the FMOs, an electron occupying an FMO in the first fragment has a finite probability to be found in an FMO of the second fragment, and this interference also describes a charge shift. This means that neutral pFCSFs constructed with non-orthogonal FMOs also contribute to the description of charge shifts, which are hidden by the mathematical form of the pFCSFs. This makes a unique and sound physical interpretation of such pFCSFs impossible. When orthogonal FMOs are used, the electron distributions in the fragments are always either neutral or ionic, and the charge shifts are described by linear combinations of neutral and ionic pFCSFs. As mentioned in the Introduction, the dimerization of silylene is only possible when the singlet coupled lone pair electrons in each fragment are decoupled to a triplet high spin state, and these local triplet states are coupled to a global singlet state. Local spin-rearrangements in neutral fragments are invisible when wave functions with delocalized MOs are used; they are, however, revealed by an OVB analysis. In a recent paper by one of the authors [
20], the charge and spin rearrangements during the dissociation of the C
2 molecule were investigated.
In the following sections, we will discuss only properties of pFCSF, so we replace this acronym by CSF.
4. The Description of Chemical Reactions
4.1. Potential Energy Surface, Minimum Energy Path, Potential Energy Curve
A fundamental theoretical tool for the study of processes in molecular systems is the potential energy surface (PES), which is a consequence of the Born–Oppenheimer approximation. A lucid discussion of this topic was given by Sutcliffe and Woolley [
21]. Separating the translational and rotational contributions from the Hamiltonian of an
N-atomic molecular system yields a system of moving electrons and nuclei; the latter can vibrate, and permutations of identical nuclei are also possible. In a last step, one can assume that the nuclei do not change their relative positions, which are given by the
n general coordinates
, and the
n-tuples are elements of the nuclear configuration space
X. Any point
, also called the system point, represents a certain geometry of the molecular system; changing
P means changing the geometry of the molecular system, as happens during chemical reactions. The system at point
P is described by the clamped-nuclei Hamiltonian
; the eigenstates
and the eigenvalues
also depend on
P. The energy is the so called electronic energy of the system; the energy
as a function of
P is the PES, which is a hypersurface in the
-dimensional space
. the eigenstates and eigenvalues can be indexed
at any point
P in order of increasing energy
E. The eigenstates of a clamped-nuclei Hamiltonian are called adiabatic, as are the wave functions describing an eigenstate and the corresponding PES. Because of the way adiabatic PESs are constructed, they can have local minima connected by saddle points, troughs separated by ridges and so on; adiabatic PESs are not smooth. The interpretation of processes in the molecular system is, therefore, more difficult than when the PESs are smooth. A non-smooth adiabatic PES indicates often large changes in the molecular system accompanied by dramatic changes in the electronic structure. Atchity and Ruedenberg, therefore, classified states as adiabatic if “…in certain regions of coordinate space drastic changes occur in the electronic structures…” [
22,
23]. Chemists are used to representing the equilibrium structures of molecular systems by conventional structural formulas, which store essential chemical information like the connectivity and the type and number of bonds between connected atoms, and represent important parts of the characteristics of the electronic structure of the system. In an adiabatic reaction, A → B, a system A with its essential characteristics is smoothly transformed into a system B having different characteristics.
A curve (path, trajectory) in X is a subset of X that can be indexed (parametrized) by a single continuous parameter ; if the arc length of the curve is used as a parameter, one speaks of the natural parametrization of the curve. In chemistry, the arc length is called the reaction coordinate. It may happen that, for a certain curve in X, one of the n coordinates makes the largest contribution to the arc length, or is regarded as describing the most important aspect of the structural change in the system considered. For example, when during a chemical reaction two fragments approach each other, the structure of the reactants will change as will the relative orientation of the fragments, but the distance R between the reactants is often considered to be most important. Then, in chemical parlance, the coordinate R is often called the reaction coordinate, although it is in fact only an approximate reaction coordinate, describing only one aspect of the change in the molecular structure during the reaction. The reaction coordinate used in this paper is always an approximate coordinate R that measures the distance between the reactants. If a reaction occurs without any symmetry restrictions for the system’s geometry, then, for a certain , all the internal coordinates will be different; if there are symmetry restrictions, some internal coordinates will have either equal values or they will differ by their sign. Then, the curve will lie in a subset of X, whereas, without symmetry restrictions, the curve is allowed to meander through a much larger subset of the space X.
An adiabatic PES that describes a simple chemical reaction typically has two trough-shaped channels, the entrance and the exit channel, which are, in general, connected by a saddle point. The curve at the bottom of the troughs passing the saddle point is called the energy profile or the potential energy curve (PEC); it is a curve in and can be regarded as the graph of the energy function over as domain, called the minimum energy path (MEP). A PEC lies always on a PES. PECs are often used to make important aspects of a PES more obvious, such as reaction barriers, energy differences, etc. If and represent the structures A and B of the molecular system, the MEP between and has a length , which is, in general, assumed to be finite. For the graphical representation of the energy profile, the MEP is always rectified, meaning that the curve is replaced by a line segment having the same length as the MEP. The reaction coordinate is then an element of an interval with . By this, the energy profile becomes the graph of the function , ; the graph is a subset of the Cartesian product . A PEC must be a curve without kinks or jumps; the PEC of a reaction going over a saddle point always has a local maximum and frequently two local minima. In practice, the true reaction coordinate is mostly replaced by an approximate reaction coordinate R, and the true reaction profile is replaced by an approximate potential energy curve . Because of the high dimension, , of the configurational space X, it is impossible to imagine all geometry changes along a path , but the n coordinate curves , , can help to do this. If the approximate reaction coordinate R is used, the curves are used. Too often the change in the coordinates along a path is not appropriately considered, although only they can describe structural changes; changes in the energy alone, as described by a PEC, cannot do this. Only a PEC together with all the coordinate curves can give a hint of the physical processes behind a chemical reaction.
4.2. Diabatic and Adiabatic States
Two reactants C and D without unpaired electrons cannot form a product with covalent bonds between them; only when the reactants are prepared for bonding, C* and D*, can they form the product C*D*. Both the combination reaction C + D → C*D* and the dissociation reaction C*D* → C + D are adiabatic reactions, in which the electron structure of the molecular system changes dramatically. On the other hand, in the two reactions, C + D → CD and C*D* → C* + D*, the electronic characteristics of the initial structures are not changed. In the recombination reaction, the fragments are compressed to the structure of the product, but they are still not prepared for bonding; in the dissociation reaction, the fragments are still prepared for bonding, but the distance between them is large. According to Atchity and Ruedenberg, the corresponding wave functions, and the reactions are called diabatic if “…[the] electronic structures maintain their essential characteristics over the entirety of such regions” [
22,
23].
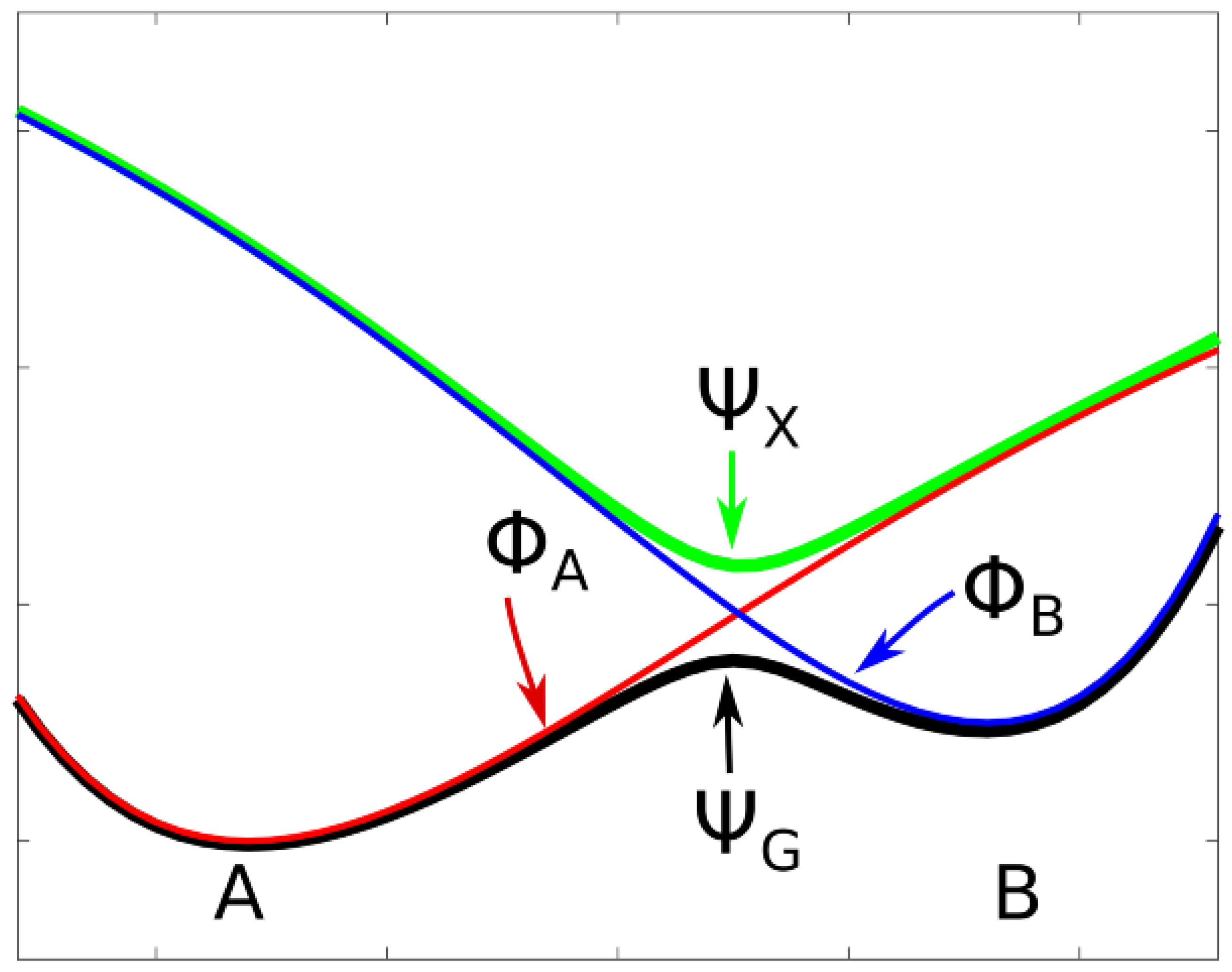
Adiabatic states are well defined as eigenstates of the Hamiltonian of the system; the adiabatic PECs are made with the corresponding eigenvalues. The PEC of an adiabatic state with wave function
that describes the ground state of a molecular system having two stable structures A and B will show two local minima connected by a local maximum (see
Figure 1). There will be an excited adiabatic state with wave function
, which has the same symmetry as
. The PEC of this excited state has a local minimum where the ground state PEC has a local maximum; one speaks of an avoided crossing as a consequence of the non-crossing rule for states with the same spin or spatial symmetry (see, for example, Cohen-Tannaoudji [
17]). The diabatic states,
and
, are not eigenstates of a Hamiltonian. They must be constructed, for example, by linear combinations of adiabatic states, but this can be done in different ways as described by Shu et al. [
24]. Each of the diabatic PECs has a single local minimum at one of the two stable structures; there, the shapes of the diabatic PECs are nearly identical with the shape of the PEC of the adiabatic ground state. And the PECs of the diabatic states cross where the adiabatic PECs show the avoided crossing. In
Figure 1, the two diabatic states and the two adiabatic states describe a reaction following a single MEP. Thermal reactions are nearly always adiabatic reactions; most photochemical reactions or electron transfer reactions and, in general, all fast reactions are diabatic reactions—they connect system structures in the ground state with system structures in excited states. A PEC that consists of the parts of the diabatic PECs below the crossing point is a curve with a cusp. It never describes a real chemical reaction along the MEP.
In our studies, we frequently describe forward and reverse reactions following different, non-crossing MEPs; the reaction valleys are then two skew troughs that do not coalesce. In this case, the PECs resemble those of diabatic states, but they are indeed adiabatic PECs because the energies are the lowest eigenvalues of the Hamiltonian. As long as one does not study the potential energy surface in detail, one can assume that the two skew troughs are separated by a ridge of unknown height. But chemical reactions never occur in such a way that the system point strictly follows an MEP; the vibrations of the atoms result in trajectories that oscillate about the MEP, similarly to the motions of a skater in a half-pipe. If the system energy is higher than the energy at the bottom of the trough, and if the oscillations of the system point are so strong that its energy is larger than the height of the ridge, the system point can jump from one trough to the other.
If the MEPs are rectified and parametrized over the same interval I by the approximate reaction coordinates R, and if we assume that the PEC of the forward reaction is a monotonically increasing curve and that of the backward reaction is monotonically decreasing, the two PECs will intersect at the crossing point , resembling crossing diabatic PECs. Only when all the coordinate curves are considered does it become clear that the crossing curves are PECs, belonging to reactions that follow different MEPs, for which no non-crossing rule holds.
Central to the treatment of diabatic states by Ruedenberg and Atchity [
22,
23] is the observation that diabatic states fulfill the criterion of electronic uniformity, meaning that in certain regions of the nuclear configuration space, the electronic structure does not drastically change, whereas the electronic structure in adiabatic states does. The electronic uniformity criterion can be equivalently replaced by the configurational uniformity criterion. This criterion is based on the representation of a wave function in terms of CSFs made with some kind of MOs. If only a few CSFs have non-neglectable weight, and if the weight of these CSFs remains large along a path in configuration space, one speaks of configurational uniformity and, since the CSFs represent a certain electronic structure, of electronic uniformity. Now, it is found that diabatic CSFs can fulfill the configurational uniformity criterion only with proper MOs, called diabatic MOs. Different strategies have been proposed for constructing diabatic MOs [
24], but it was found for the (H
2)
2 system [
25] that the diabatic MOs are localized on the two H
2 fragments. We found that the CSF made with FMOs localized on the fragments fulfills the configurational uniformity criterion, and, since it is easy to attribute a certain charge and spin distribution to each CSF (see next section), also the criterion of electronic uniformity. This leads us to the conclusion that the reactions along different MEPs show the properties of diabatic reactions.
In this paper, we study ground state reactions, and many of them indeed follow different MPEs. The PECs describing these reactions and shown in the figures consist of the branches of two PECs that lie below the crossing point . The region with will be called the dissociated system, the fragments are mostly in low-spin states, and we will say that the wave function has low-spin characteristics; the region with is the bonded system, the fragments are in high-spin states, and therefore, the wave function has high-spin characteristics. The PEC of a reaction that follows a single MEP is a smooth curve without singular points like cusps or jump discontinuities. In some systems, jumps are caused by slow geometry optimization on a PES that is obviously not quadratic, indicating a vanishing ridge between the troughs; in other systems, there is indeed a sudden change in the structure of a fragment.
In this paper, an OVB analysis is made only for the wave functions corresponding to the PECs shown in the figures. These curves can be either smooth curves following a single MEP or they can consist of pieces of PECs over different MEPs. In this case, the energy curves may have cusps or even jumps. Coordinate curves have jump discontinuities where the energy curves either cross or have jumps. Nevertheless, all curves in this paper are represented as if they were graphs of continuous functions. In the
Supplementary Materials, the continuous representation is compared with the correct discontinuous one.
5. The OVB Analysis of the CAS(4,4) Wave Functions
All the molecular systems studied in this paper are in singlet ground states; the dissociated system consists of two fragments: an ethene analog and a carbene analog; the bonded system consists of a single fragment, the 3-ring. The chemical reactions are modeled with four active FMOs and four active electrons. In the bonded system, the four delocalized active MOs are the bonding and antibonding MOs describing the two
bonds between the two fragments; the four active electrons are in the two bonding MOs. In the dissociated system, the four active MOs are the bonding and the antibonding
MOs in the ethene analog, and the s-type and the p-type lone pair AOs in the carbene analog. The four active electrons are the electron pair occupying the
MO and two electrons that can occupy the two lone pair MOs. The delocalized active MOs in the dissociated system are already strongly localized at the two fragments, and the delocalized MOs will be localized onto the MOs of these fragments. This choice of active orbitals assumes that the
MO in the double bond of the ethene analog is not involved in the reaction, and a CAS(6,6) wave function is not necessary to describe the reactions; two reactions were treated with both CAS(4,4) and CAS(6,6) wave functions to check whether this assumption is reliable (see the
Supplementary Materials).
For an OVB analysis of a system with geometry
, the delocalized CASSCF MOs are localized on the FMOs calculated for the non-interacting fragments at geometry
. The four orthogonal FMOs of the CAS(4,4) wave function are, in the order they occur in the CSFs, the
and
FMOs of the ethene analog, and the
s and
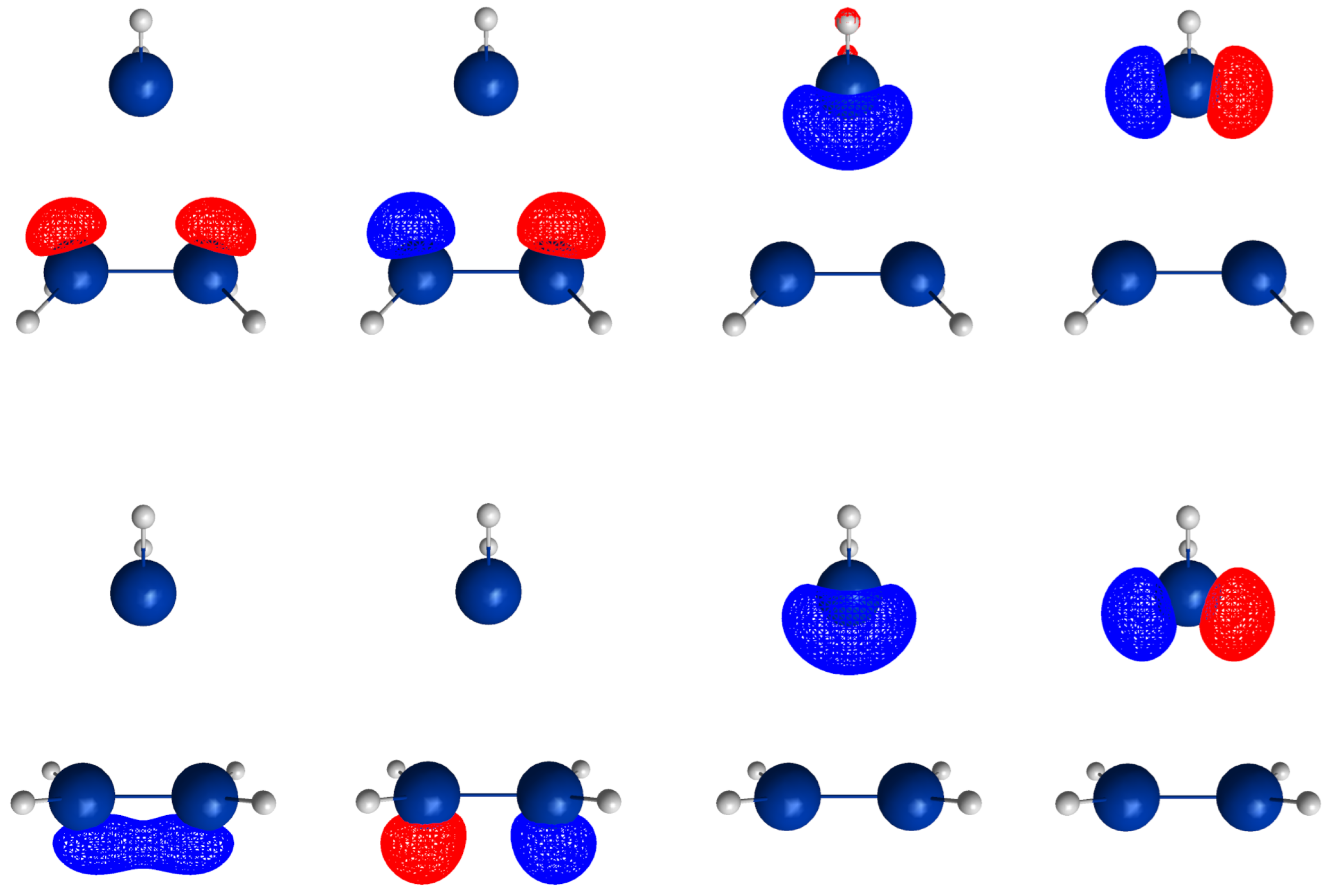
p lone pair FMOs of the carbene analog. A CAS(4,4) wave function for a singlet state is a linear combination of at most 20 CSFs. The electron and spin distribution and the labels characterizing it can be seen in
Figure 2. The CSFs NB, TT, SS, and QX describe a unique distribution; all other labels describe two distributions, which differ by switching the two fragments (in
Figure 2, only one electron distribution is shown). In
Table 1, the occupation types of all 20 CSFs are listed together with the character strings describing the orbital occupation. Note that the graphical representations of the CSFs do not contain any energetic information.
- (1)
NB is the single No-Bond CSF; the two low-energy FMOs are occupied by electron pairs. Both fragments are in singlet states; therefore, no unpaired electrons are available for bond making. This CSF describes spin distributions in the fragments for which, during the reaction, the PEP will be most important. In each fragment, the lowest FMO is doubly occupied by an spin pair. Therefore, there are two inter-fragment spin pairs and , which will strongly resist the approach of the fragments (no-bond). This CSF dominates the dissociated system. All other CSFs can be described as excitations or charge transfers with respect to the NB CSF.
- (2)
X labels the two CSFs with a single excitation in one fragment without spin flip.
- (3)
C labels the two CSFs with a single electron transfer from one fragment to the other.
- (4)
DX labels the two CSFs with a double excitation of a singlet coupled electron pair in one fragment.
- (5)
DC labels the two CSFs describing the transfer of an electron pair from one fragment to the other.
- (6)
CX1 labels the two CSFs describing a single charge transfer and a single local excitation without spin flip from one doubly occupied MO.
- (7)
CX2 labels the two CSFs describing a local double excitation in one fragment and a single charge transfer from the other.
- (8)
TT labels the single CSF describing two fragments in local triplet states coupled to a singlet. In both fragments, two unpaired electrons are ready for bond making. There are no inter-fragment pairs of identical electrons, and the PEP is not effective. This CSF is important for the description of the bonded system. With respect to NB, this CSF describes in each fragment a single excitation with spin flip.
- (9)
SS labels the single CSF describing two fragments in local excited singlet states. In CSF NB, there are two inter-fragment pairs of identical electrons and the PEP effectively resists the approach of the fragments. The electron configuration described by SS is never important.
- (10)
TX labels the two CSFs describing triple excitations, that is, a double excitation in one and a single excitation without spin flip in the other fragment.
- (11)
TXC labels the two CSFs describing a triple excitation plus a single charge transfer.
- (12)
QX labels the single CSF describing a quadruple excitation, that is, a double excitation in each fragment. As in NB, there are two inter-fragment spin pairs and , which will strongly resist the approach of the fragments. This CSF is never important.
The CSFs NB, X, DX, TT, SS, TX, and QX describe neutral electron structures, and in each fragment there are the same number of active electrons; all CSFs containing a C in the label are ionic.
Covalent bonding needs essentially mutual charge shifts from one bonding partner towards the other, as was shown by Ruedenberg and coworkers [
26,
27,
28,
29,
30]. This charge shift is a consequence of the constructive interference of fragment wave functions. When orthogonal FMOs are used, ionic CSFs are necessary to describe the charge shift.
All reactions are studied in either the high or in the low symmetry group. In , the low-energy FMOs and s belong to an irreducible representation (IRREP) and the high-energy FMOs and p belong to IRREP , so the eight CSFs X, C, TX, and TXC do not belong to the totally symmetric IRREP but to IRREP . The totally symmetric wave function in is, therefore, a linear combination of only twelve CSFs. The bonded system with two covalent bonds between the fragments is described by a superposition of the neutral CSF TT and some ionic CSFs. CSFs CX1 describe charge shifts between the low-energy FMOs; CSFs CX2 describe charge shifts between the high-energy FMOs. The DX CSFs are necessary to describe left-right correlation in the ethene analogs or angular correlation in the carbene analogs.
In , all active orbitals belong to IRREP , and therefore, the totally symmetric singlet ground state wave function is a linear combination of all 20 CSFs. The eight CSFs that did not contribute in describe asymmetric local charge deformations and charge shifts, only C and X are found to be important.
The important data obtained in an OVB analysis are the diagonal elements of the CI matrix, that is, the energy expectation values calculated with the CSFs and the eigenvector of the CI matrix describing the investigated system state. The squares of the CI coefficients are the weights of the CSFs in the wave function. In the figures, the weight and energy curves of large CSFs only are shown, which are those CSFs for which the weight was larger than 0.1 (10 percent) somewhere along the reaction coordinate. To show how much the large CSFs contribute to the molecular wave function, the sum of the weights of the large and of the small CSFs is shown for all reactions.
6. Results
Figure 3 shows a sketch of the molecular geometry of the 3-rings. The three heavy atoms X, Y, and Z lie in the x–z plane; atoms X and Y lie on the x-axis. The x-coordinate of atom X is always larger than that of atom Y; the normal distance from atom Z to the x-axis is the approximate reaction coordinate
R. Of all the internal coordinates, only the three out-of-plane angles
,
, and
are indicated. In
symmetry, atoms X and Y are symmetry equivalent. A total of 7 of the 21 internal coordinates are unique, the four bond lengths r(X–Z), r(X–Y), r(X–H), and r(Z–H), three angles, and the three bond angles HXH and HZH, and the out-of-plane angle
. The out-of-plane angle
always has a value of 90 degrees. In low
symmetry, all three atoms are non-equivalent. The 12 internal coordinates are unique: the six bond lengths r(X–Z), r(Y–Z), r(X–Y), r(X–H), r(Y–H), and r(Z–H), and the six bond angles, HXH, HYH, HZH, and the out-of-plane angles
,
, and
.
The out-of-plane angles and have positive values if the hydrogen atoms lie below the x–y plane. The positive values of angle are counted counter-clockwise from 0 degrees to 180 degrees in the first and second quadrants; the negative values are counted clockwise from 0 degrees to degrees in the fourth and third quadrants. That means if the hydrogen atoms of the ZH2 moiety are closer to atom X than to atom Y, the values are in the first or the fourth quadrant. If the ZH2 moiety is parallel to the x–y plane, is either 0 degrees or 180 degrees (equal to −180 degrees); 90 degrees means that ZH2 is parallel to the y–z plane with atom Z closer to the X-Y moiety than the hydrogen atoms.
The following reactions are investigated; all systems are in their respective totally symmetric singlet ground states.
R1v c-C3H6 ⇌ C2H4 + CH2 in .
R1s c-C3H6 ⇌ C2H4 + CH2 in .
R2v c-Si3H6 ⇌ Si2H4 + SiH2 in .
R2s c-Si3H6 ⇌ Si2H4 + SiH2 in .
R3v c-CSi2H6 ⇌ Si2H4 + CH2 in .
R3s c-CSi2H6 ⇌ Si2H4 + CH2 in .
R4v c-SiC2H6 ⇌ C2H4 + SiH2 in .
R4s c-SiC2H6 ⇌ C2H4 + SiH2 in .
R5s c-CSi2H6 ⇌ CSiH4 + SiH2 in .
R6s c-SiC2H6 ⇌ CSiH4 + CH2 in .
With the CAS(4,4) wave function, only the bonding pairs of the two bonds in the 3-ring are correlated; the bond of the double bond is never correlated. Therefore, the equilibrium geometries of cyclopropane and cyclotrisilane do not have the correct but only symmetry. In reactions R5s and R6s, the system always has symmetry; the C-Si MO in silaethene is always doubly occupied, but for the other C-Si bond a correlated description is used. Therefore, the two C-Si bonds in c-SiC2H6 and in c-SiC2H6 are not equivalent.
The geometry data of the ground and excited states calculated for the isolated fragments can be found in the
Supplementary Materials.
6.1. The Reactions c-C3H6 ⇌ C2H4 + CH2
All three atoms X, Y, and Z from the sketch in
Figure 3 are carbon atoms. We investigated the elimination of CH
2 from the 3-ring as well as the recombination of CH
2 and ethene (addition reaction); both reactions were performed in
(
R1v) and
symmetry (
R1s). The approximate reaction coordinate
R for both reactions varies between
Å (“molecule”) and
Å (“dissociated system”). At the equilibrium geometry of c-C
3H
6, the approximate reaction coordinate is
Å.
During the addition of methylene to the
bond in ethene, the geometry around the C atoms in ethene changes from planar (sp
2 hybridization) to pyramidal (sp
3 hybridization), and this also affects the C-C
bond. One could assume that adding the C-C
MO to the set of active MOs will change the electron structure considerably and will strongly influence the shape of the curves describing the total energy and the geometry parameters. The results of the CAS(6,6) optimization can be found in the
Supplementary Materials; the agreement with the CAS(4,4) results shows, however, that a CAS(4,4) ansatz is indeed sufficient for the description of this reaction.
6.1.1. R1v: The Elimination and Recombination Reactions in
The PECs for both reactions (see
Figure 4) have a prominent cusp at the crossing point (see comments on curve representation in
Section 4.2) of two different energy profiles (see the discussion in
Section 4), from which only the branches with energies below the crossing point are depicted. These branches belong to different energy profiles with different MEPs.
This becomes obvious when all the internal coordinates describing the structure of the molecular system are considered (see
Figure 5). For
Å and
Å, the PECs of the elimination and the recombination reactions are identical; the crossing points are at
Å and
Å, respectively. This means that the jump between the different reaction channels occurs in the elimination reaction at a larger separation of the fragments than in the recombination reaction.
As the geometry parameters of the fragments show, the dissociated system consists of ethene and methylene in their lowest singlet states. In the bonded system, the fragment geometries resemble those of the fragments in their lowest triplet states; this justifies speaking of the low-spin and the high-spin part of the reaction. In the case of ethene, we do not mean the fully relaxed triplet geometry, which has symmetry, but the geometry of the triplet state. Similarly, we speak of the low-spin and high-spin characteristics, respectively, of the wave function. During the recombination reaction, the electronic structure of the dissociated system has low-spin characteristic up to Å, and the total energy increases monotonically; at Å the energy is below that at the crossing point, and the fragment geometries are those of the high-spin states in the bonded system. The elimination reaction starts at a geometry close to the relaxed 3-ring geometry of the bonded system; the energy increases monotonically and the fragments remain in high-spin structures. At Å, the characteristics of the electronic structure change from high-spin to low-spin; the total energy is lower than at the crossing point, and the fragments have adopted low-spin geometries. The different crossing points of the energy curves of the recombination and elimination reactions show that for each reaction, the system stays in the entrance channel and follows the MEP to geometries where the energy is higher than the energy of the entrance channel of the reverse reaction, and only then does the system jump to the lower lying channel. Every jump of the system to structures with lower total energy is accompanied by sudden changes in the fragment geometries that indicate characteristic changes in the electron structures of the fragments; frequently the changes can be traced to spin-rearrangements, such as spin flips.
In the recombination reactions, both fragments must change from low-spin to high-spin states to create unpaired electrons in the fragments, making them ready for bonding; in elimination reactions, the spin flip must change the high-spin states of the fragments to low-spin if the ground state of the dissociated system is a low-spin state. This is supported by the geometry data. In the recombination reaction, the methylene fragment has, in the dissociated system, the characteristic geometry of the lowest
state (1-
): the CH bond length is 1.098 Å and the HCH angle is 102.2 degrees; the geometry parameters of the ethene fragment are very close to the equilibrium values of the free fragment: the C=C bond length is 1.331 Å, the CH bond lengths are 1.073 Å and the HCH bond angles are 116.4 degrees. During the approach of the fragments, the geometry parameters change very little: at
Å, the CH bond length in methylene is about 1.103 Å and the bond angle is 101.7 degrees; the C=C length in ethene is 1.332 Å, the CH bond lengths are 1.073 Å, and the HCH bond angles are 116.3 degrees. There is a slight deviation from planarity; the out-of-plane angles are −3.5 degrees with the CH bonds bent towards the methylene fragment. At
Å, the system already has the high-spin geometry: the C=C bond length in ethene (1.427 Å) has increased by 0.1 Å, the CH bonds in ethene have reduced to 1.071 Å, and the HCH bond angles (116.7 degrees) are nearly unchanged, indicating that the CH moieties in ethene are nearly unchanged; only the out-of-plane angles have increased by about 18 degrees, but they are still much smaller than in the 3-ring. The CH bond length in methylene (1.063 Å) has decreased by 0.04 Å, and the bond angle (139.9 degrees) has increased by roughly 40 degrees. The geometry parameters of methylene are markedly different from either the 1-
state or the
state; indeed, these values are typical for the second
state (2-
) of methylene (see
Supplementary Materials). The geometry parameters of the methylene fragment indicate that at
Å, the fragments are still in low-spin states; the change in the 1-
methylene state to the
ground state proceeds via an intermediate excited singlet state, in which the lone pair changes from the s-type AO to the p-type AO and only at about 1.9 Å follows the spin flip to the
state with singly occupied lone pair FMOs. As long as the methylene is in a singlet state, also the ethene fragment must be in some excited singlet state, as the increase in the C=C bond length and the deviation from planarity show. Further decrease in the distance between the two fragments causes a linear change in all the geometry parameters, except the C=C bond length, towards the values of the equilibrium structure of the 3-ring with CH bond lengths of about 1.074 Å, HCH bond angles of about 114 degrees, out-of-plane angles of about 30 degrees, and with the length of the former C=C bond now about 1.48 Å.
In the elimination reaction, the characteristics of the bonded system change without any sudden geometry changes. At
Å, the methylene fragment has already adopted the 2-
geometry, but, with increasing reaction coordinate, the geometry parameters of methylene do not show the expected change to the 1-
geometry. Instead, the CH bond length decreases and the HCH angle increases further. At
Å, the CH bond length is only 1.058 Å, and the HCH bond angle is about 155 degrees. The change in the geometry parameters of ethene is rather slow (see
Figure 5). At
Å, the system is in a dissociated state and all the geometry parameters have the expected values.
The unexpected change in the methylene geometry during both reactions needs further consideration. The two lowest states of bent methylene, the 1- and the 2- state, correlate with one state and one component of the lowest state of linear methylene (CH bond length 1.056 Å). Both states are made with the two degenerate MOs, traditionally labeled and , which are centered at the carbon atom. The is proportional to ; the two components are proportional to and .
In symmetry, that is, when methylene bends, one MO becomes the s-type lone pair AO of symmetry and the other MO becomes the p-type AO of symmetry. The former state and the component of the state have symmetry; the second component has symmetry. To obtain a totally symmetric ground state of the molecular system, the state must couple with an excited singlet state of ethylene of the same symmetry, which is described by the CSF SS but never plays a role in the composition of the total wave functions. The two states of methylene are dominated by doubly occupied lone pair AOs, the 1- state is dominated by s2, and the 2- state is dominated by p2. During the approach of methylene and ethene, the lone pair in the s-type AO point directly to the doubly occupied MO of ethene, which is resisted by the PEP. An angular change in the lone pair density from the s-type to the p-type AO will certainly minimize the so called Pauli repulsion but increase the total energy considerably. A spin-flip from the local 2- to the state yields an energetic stabilization and prepares the methylene fragment for bonding. The OVB analysis of the CASSCF wave functions can shed more light on the bonding processes.
A final note: The geometry parameters of methylene change much more strongly than the corresponding parameters of ethene: the maximal variation of the CH length in methylene is 0.046 Å, in ethene it is only 0.003 Å; the maximal variation of the HCH angle in methylene is 39.4 degrees, in ethene it is 2.9 degrees. The variations differ roughly by a factor of 10.
In the dissociated system, the neutral low-spin NB CSF has the largest weight in the wave function, the DX CSF
has the second largest weight, which describes the angular correlation of the lone pair electrons in the 1-
state of methylene (see
Figure 6). In the bonded system, the neutral CSF TT has the highest weight. The contributions of the two CX1 CSFs
and
, and of one CX2 CSF, namely,
, are important between
Å and
Å. These ionic CSFs describe the charge shift during covalent bonding. CSF DX
, which corresponds to the intermediate 2-
, has the second highest weight only between
Å and
Å, where it supports the change from 1-
to
. At the equilibrium geometry, the sum of the ionic CSFs is larger than the weight of CSF TT. The only differences between the elimination and recombination reaction are found between
Å and
Å. Since the crossing point of the energy profile for the elimination reaction is at
Å, the weight of CSF
becomes even larger than that of CSF TT.
The CSF energy curves show the effect of the contraction of the lowest FMOs; in the dissociated system, the lowest FMO in each fragment is doubly occupied, and the Coulomb repulsion of non-identical electrons causes an extension of at least these two FMOs; in the bonded system, the FMOs of each fragment are singly occupied by identical electrons and therefore all FMOs contract. When during the recombination reaction, the extended FMOs contract, the energy of CSF NB increases because of the increase in the Coulomb repulsion in the less extended FMOs, and the energy of all other CSFs, not only of TT, decreases (see
Figure 6).
Since the descriptions of both reactions are nearly identical, the sums of the weights of the large CSFs and the respective complementary contributions of the small CSFs are also nearly identical. The complementary contributions show that the small CSFs, which are mostly ionic CSFs, become important when the new covalent bonds are formed; the various ionic CSFs describe all possible deviations of the electron distribution during the reaction (see
Figure 6).
It is important to remember that the curves of the CSF energies and weights are also composed of the contributions of two reactions following different MEPs, even when the continuous curves suggest a reaction following a single MEP.
6.1.2. R1s: The Elimination and Recombination Reactions in
There is no structural and energetic difference between the elimination and recombination reactions—both reactions follow the same trough and the total energy decreases monotonously during the recombination reaction. In the dissociated system, the methylene molecule lies roughly parallel to ethene; indeed,
is negative, which means that the H atoms of the methylene fragment are closer to the ethene molecule than the carbon atom. This “parallel” structure remains during the approach in the recombination reaction up to
Å, where the methylene fragment rotates to
degrees, and the full
symmetry is reached. Between
Å and
Å, most of the geometry parameters change significantly. The CH bond length in methylene decreases from 1.098 Å to 1.066 Å and the HCH bond angle increases from 102 degrees to about 126 degrees; at
Å, the geometry of methylene resembles the geometry in the
state (see
Figure 7). During the approach of the fragments, atom Z is closer to atom Y than to atom X; accordingly, the lengths of the two C-C single bonds are different for
Å—they are equal only when the molecular structure has adopted
symmetry. Because of the asymmetric approach of methylene and ethene, the geometry parameters of ethene at the two carbon atoms are significantly different. Note that in
symmetry, the 2-
state of methylene is not involved in both reactions, as the curves of the geometry parameters show.
When the system’s symmetry is reduced to
, all active MOs have
symmetry, and therefore, all 20 CSFs are totally symmetric, but only eight CSFs are large (see
Figure 8). In the dissociated system, only CSF NB and the DX CSF
, describing angular correlation in methylene, are important; they have the same weights as in the reaction in
. The neutral TT does not contribute significantly before
Å; the weight increases slowly and reaches its maximum at
Å, when the molecular system again has
symmetry. Then, the weights of the ionic CSFs become, as expected, larger than the weight of the neutral TT CSF. In contrast to the reaction in
, the C CSF
, with a doubly occupied s-type lone pair FMO and the two singly occupied FMOs
and the p-type FMO, contributes very early, its weight being already significant at about
Å. At large distances, the overlap of the two FMOs contributes to the stabilization of the dissociated system with methylene lying “parallel” to the ethene; at short
R values, the CSF describes a charge shift from the doubly occupied
FMO to the empty p lone pair FMO, and this is only possible when the p FMO points towards the
FMO, so that they can overlap when
R decreases. Indeed, the empty p lone pair FMO does not point to the
FMO, but only to one of the p AOs spanning the
MO. The second C CSF
, which describes a charge shift from the doubly occupied s lone pair of methylene to the empty
in ethene, is much less important. Both C CSFs are not totally symmetric in
and therefore cannot contribute to the wave function as soon as the molecular system has adopted
symmetry. The charge shift during covalent bonding is thus described by different CSFs: in
, it is mainly the C CSFs; in
, it is only the CX1 and CX2 CSFs. The CSF energies in
Figure 8 show that the different composition of the wave function also influences the extension of the FMOs. Since there is no sudden replacement of CSF NB by CSF TT, change in the FMO extensions is not as sudden as it is in the
reaction, and the drop in the energies is much more moderate.
Figure 8 shows that in
symmetry, the sum of the weights of the large CSFs decreases between
Å and
Å. Then, after the system has adopted
symmetry, the contribution of the large CSFs is larger again, and then slowly decreases, whereas the polarizing small CSFs become more important.
6.2. The Reactions c-Si3H6 → Si2H4 + SiH2
The second homonuclear 3-ring of this study is cyclotrisilane.
All three atoms X, Y, and Z in the sketch in
Figure 3 are silicon atoms. We investigated the elimination of SiH
2 from the 3-ring as well as the recombination of SiH
2 and disilene in both
(
R2v) and
symmetry (
R2s). For all reactions, the approximate reaction coordinate
R is in the range of
Å to
Å; at the equilibrium geometry of cyclotrisilane, the
R value is 2.088 Å. We also checked whether, in
symmetry, a CAS(4,4) wave function is sufficient for the description of the reactions studied. The results obtained with a CAS(6,6) wave function can be found in the
Supplementary Materials; they agree very well with those obtained with the CAS(4,4) wave functions—the only difference worth mentioning is that the CAS(6,6) wave function yields planar disilene at
Å, whereas with CAS(4,4), disilene is slightly cis-bent. But this has no influence on the bonding process. The trans-bent equilibrium structure of disilene has
symmetry; with CAS(4,4), the out-of-plane angle is 43 degrees, and with CAS(6,6), the angle is 37 degrees. This, again, shows that for these reactions, a CAS(4,4) wave function is sufficient. And this strengthened our opinion that this is the case for all other reactions.
In
symmetry, the elimination and recombination reactions follow different MEPs; in
symmetry, the recombination reaction does not lead to the 3-ring but to the open chain trisilylene diradical. It turns out that this recombination reaction is different from all others investigated in this study. The differences between the reactions of cyclopropane and cyclotrisilane, in
symmetry, are caused by the low-spin ground state of silylene, and by the flexible, non-planar structure of disilene. Disilene has a trans-bent ground state structure, which it can adopt only when the reactions occur in
symmetry; in
symmetry, the ground state structure is cis-bent. In the dissociated system, both trans-bent and cis-bent disilene have pyramidal structures at both silicon atoms. In the cis-bent structure, all four hydrogen atoms point off the silylene fragment, as they do in the 3-ring; in the trans-bent structure, the hydrogen atoms at one silicon atom point off the silylene fragment and the hydrogen atom at the other silicon atom point towards it. We speak of correct pyramidality if the pyramidality is the same as in the 3-ring, otherwise of wrong pyramidality. In the case of correct pyramidality, the out-of-plane angle
is positive; in the case of wrong pyramidality, it is negative. In
symmetry, two different structures of the dissociated system are possible: in the first, the silicon atom of silylene points to the silicon atom in disilene with the correct pyramidality and the hydrogen atoms point to the silicon atom with the wrong pyramidality; this structure is designated with S1. In the second case, the silylene fragment lies the other way around; this structure is designated with S2 (see
Figure 9). This classification is used for all systems with non-planar ethene analogs.
6.2.1. R2v: The Elimination and Recombination Reactions in
For
Å, the description of the dissociated system is identical in both the elimination and the recombination reactions; the same is true for the description of the bonded system for
Å (see
Figure 10).
In the elimination reaction, the PEC (see
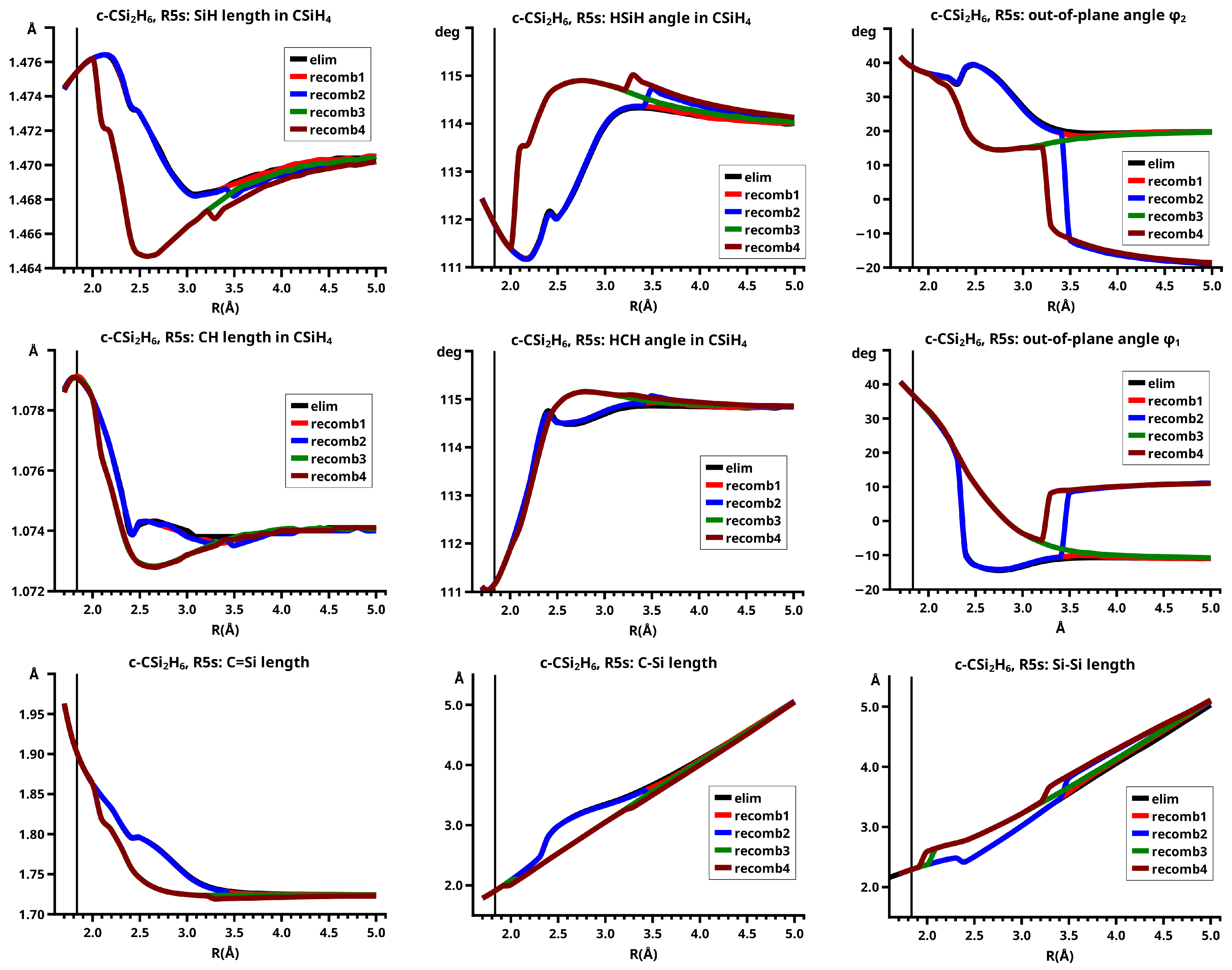
Figure 10) increases monotonically from the equilibrium structure up to the crossing point
Å, where the silylene fragment still has the typical triplet geometry, with an SiH length of about 1.48 Å and an HSiH angle of about 113 degrees. The SiSi length of
Å in the disilene fragment is typical for an Si-Si single bond. In cis-bent disilene, both silicon atoms have correct pyramidality with an out-of-plane angle of about 40 degrees. Between
Å and
Å, the system jumps from the state with the high-spin characteristics to the state with low-spin characteristics: silylene has long SiH bonds (about 1.52 Å) and a small HSiH bond angle (about 95 degrees); the disilene fragment has a short Si-Si bond length of about 2.17 Å, the SiH bond length is about 1.471 Å, and the HSiH angle is about 115 degrees. The fragment is still cis-bent with an out-of-plane angle of only −20 deg, but now the pyramidality is wrong. This is the result of an inversion of the pyramid, which is also called “umbrella inversion”. When the separation of the fragments increases, the geometry of silylene remains nearly constant—the geometry of the disilene fragment changes slowly, at
Å, disilene is only slightly cis-bent with an out-of-plane angle of about −10 degrees, and the HSiH angle is 115 degrees, which is equal to the HSiH angle in planar disilene (see
Figure 11).
The recombination reaction is remarkably different from that of cyclopropane. Starting from the structure obtained in the elimination reaction, the silylene fragment remains nearly unchanged up to the crossing point Å. The same holds for the SiH length and the HSiH angle in disilene; only the Si=Si length and the out-of-plane angle change. At the crossing point, the Si=Si length has increased by 0.05 Å and the pyramidality has increased, the out-of-plane angle has a value of −29 degrees, and the energy has reached its highest value. Then, the system again jumps from the MEP describing the low-spin part of the reaction to the MEP corresponding to the high-spin part. The geometry of silylene has changed to that of triplet silylene; the Si-Si bond length in disilene has increased by 0.16 Å to 2.38 Å, which is longer than that of the Si-Si single bonds in silanes, the pyramidality is still wrong and more pronounced than before the jump, the out-of-plane angle is −42 degrees, and the SiH length and the HSiH angle in disilene are 1.48 Å and 111 degrees, respectively. The energy at Å is lower than that at but it is about 23 millihartree higher than the energy of the elimination reaction for the same R value. The change from low-spin to high-spin characteristics explains the moderate decrease in energy; the high energy value, compared with the elimination reaction, is caused by the wrong pyramidality. To reach the equilibrium structure of cyclotrisilane, an “umbrella inversion” is necessary; this happens between Å and Å and causes the jump discontinuity in the CSF energy curves. For R values smaller than Å, the geometry of the system and its energy are identical with those in the elimination reaction.
Pyramidal inversion in XY3 molecules, like ammonia, can be described by a single MEP, with the out-of-plane angle changing continuously from negative to positive values. Reduction in the pyramidality means a decrease in the sp3 hybridization of atom X, and this causes an increase in the YXY angles and a decrease in the XY length; in the planar structure atom, Y is sp2 hybridized, the YXY bond angle has its maximum, and the XY bond length has its minimum value; then, the pyramidality increases again, the hybridization of atom X changes from sp2 to sp3, and the geometry parameters change accordingly. Such a continuous change is, however, not observed for the inversion in the disilene fragment. Instead, one finds rather sudden geometry changes in the out-of-plane angle, the HSiH angle, and the SiH length, indicating jumps between different MEPs. At both singular points of the PEC, one observes discontinuous changes in the disilene fragment. At the crossing point ( Å), the wave function changes its characteristics from low-spin to high-spin, with corresponding changes in the system structure without a reversion of the pyramidality of disilene; at the second singular point ( Å), the pyramidality is reversed without a change in the high-spin characteristics of the wave function.
The elimination reaction is described by six large OVB CSFs. In the bonded system, the neutral CSF TT has the largest weight, whereas the weight of NB at
Å is only 0.1. The four ionic CSFs CX1 and CX2, which describe the charge polarization during the covalent bonding, have nearly identical energies and weights—the sum of their weights is about twice as large as that of TT. In the dissociated system, NB has a weight of about 0.9, the CSF
describing the angular correlation in silylene has too small a value to be shown in
Figure 12.
The change in the wave function from high-spin to low-spin characteristics at the crossing point is accompanied by a strong change in the shape of the active FMOs (see
Figure 13). For
Å, the lobes of the
orbitals point towards the silylene fragment; at
Å, where the disilene fragment has wrong pyramidality, the lobes of the
orbitals point in the opposite direction. The recombination reaction is dominated by the same CSFs as for the elimination reaction. When the system approaches
Å, the disilene fragment is strongly but wrongly pyramidalized, with the lobes of the
orbitals pointing off the silylen fragment (see
Figure 14). When the wave function changes from low-spin to high-spin characteristics, there is a spin flip, but the geometry with the wrong pyramidality is retained, and the wave function, dominated by TT, is therefore constructed with FMOs, where the lobes of the
orbitals point off the silylene fragment. The shape of the
FMOs and the local high-spin configuration reduces the Coulomb repulsion strongly; the CSF energies are therefore very low, and only the energy of CSF NB increases. The shape of the
FMOs also prohibits the stabilization of the system by forming new bonds, and therefore the total energy is higher than in the elimination reaction. After the umbrella inversion, new covalent bonds can be formed. This decreases the total energy, but at the same time, the Coulomb repulsion increases and this causes higher CSF energies. This explains the upward jumps in the CSF energy curves at the second singular point.
Note the difference between the reactions of c-Si3H6 and c-C3H6 caused by the different ground state structures of the ethene analogs and the different ground state multiplicities of the carbene analogs.
6.2.2. R2s: The Elimination and Recombination Reactions in
Atom X in the sketch in
Figure 3 is Si
1 in the graphs, Y is Si
2, and Z is Si
7.
The fully relaxed dissociated cyclotrisilane system consists of trans-bent disilene and silylene. Because disilene has a trans-bent ground state structure, the energy of the dissociated system in lies below the dissociated system in , where the disilene fragment has a less favorable cis-bent structure.
In the beginning, the elimination reaction follows the same MEP as that for the reaction in
, but between
Å and
Å, the system’s symmetry changes from
to
. At
Å, the disilene fragment has already changed from cis-bent to trans-bent, and the pyramidality of atom X has changed from correct to wrong. The silylene fragment, which stands initially normal to the disilene fragment, has folded down, the angle
having changed from 90 degrees to about 30 degrees. As the reaction proceeds, the disilene fragment adopts the ground state geometry with a short Si=Si bond length, and the silylene geometry develops into the silylene 1-
structure, lying parallel to the disilene fragment, see
Figure 15; the dissociated system has an S1 structure.
The recombination reaction is an exception to all other recombination reactions in
as it does not lead to c-Si
3H
6 but to the open chain trisilylene (SiH
2)
3. Starting from the S1 structure, the PECs of the elimination and the recombination reactions are identical between
Å and
Å, as are the curves of the geometry parameters, see
Figure 16.
However, for
Å, all physical quantities calculated for the two reactions are different and they change in a non-uniform way, except for the distance between atoms Z and Y, which is the silicon atom in the disilene fragment closest to the silicon atom in silylene. Between these two silicon atoms, the new covalent single bond is formed; in the equilibrium structure, it will have the same bond length as the former Si=Si double bond, which also becomes an Si-Si single bond. Note that the two Si-Si single bonds, X-Y and Y-Z, are not equivalently described because the CAS(4,4) wave function allows for the new bond between atoms Y and Z a correlated description only but not for the bond between X and Y, which is the former
of the double bond in disilene. Accordingly, the two terminal SiH
2 moieties in trisilylene are also not equivalently described—only a CAS(6,6) wave function for the three Si-Si bonds in the 3-ring could do this. In the relaxed trisilylene, there is no covalent bond between the two terminal SiH
2 moieties with atoms Z and X; the distance between these two atoms is about 80 percent larger than the lengths of the two Si-Si single bonds. The following description of the bonding process leading to the open chain molecule is only rudimentary. In trisilylene, the terminal SiH
2 moieties have the pyramidal structure of silyl groups; both carry a single electron, but the pyramidality of the terminal SiH
2 moieties is such that the lobes of the singly occupied FMOs do not point to each other. Therefore, the creation of an Si-Si bond is impossible. The formation of the new Si-Si single bond needs an optimum overlap of the FMOs between Z and Y. For this, the silylene must shift towards atom Y. As long as silylene lies parallel to disilene, the empty p FMO can only overlap with the doubly occupied
FMO; the spatial extension of the doubly occupied s FMO and the empty
FMO does not allow the necessary overlap. With decreasing
R, the silylene fragment folds up and then the lone pair electrons of silylene can contribute to bonding (see
Figure 17). The recombination follows a single MEP—there are no discontinuous changes in the geometry parameters.
When the recombination reaction starts from structure S2, there is an umbrella inversion at Å, but after that, the reaction proceeds as in the reaction starting from S1.
In the initial phase of the elimination reaction, when the system has
symmetry, the neutral CSF TT has the largest weight, and CSF NB has zero weight; polarization is described by the two CSFs CX1
and
, and the two CX2 CSFs
and
. When the symmetry is reduced to
, the two C CSFs
and
replace the CX1 and CX2 CSFs, and NB gains weight. In the dissociated system, the NB CSF dominates; its weight is 0.85, while the sum of the weight of all other 19 CSFs is only 0.15 (see
Figure 18).
Between Å and Å, the description of the dissociated system is identical in both the recombination and the elimination reactions. Going from Å to Å, the weight of CSF NB decreases and the C CSF becomes slowly important; the weight of the second C CSF increases more slowly, and both CSFs become equally important around Å. In the wave function of the bonded system, CSF TT gives the largest contribution. At the equilibrium structure, the weight of TT is 0.38, and CSF NB and the two C CSFs have equal weight of about 0.1.
A comment on the singly ionic CSFs and , which are very important in all reactions in symmetry, not just in the currently considered recombination reaction. CSF describes a cation/anion fragment pair, which is the result of the transfer of an electron from the doubly occupied FMO of disilene to the empty p FMO of silylene. At large distances, this CSF contributes to the stabilization of the parallel structure of the fragments; with decreasing distance between the fragments, it describes a polarization of the electrons of disilene, which prepares the fragments for bonding. The second C CSF describes the anion/cation fragment pair that is the result of the transfer of an electron from the doubly occupied s FMO to the empty FMO; it is not obvious that this CSF helps to stabilize the dissociated system at large distances, but at medium distances, both CSFs polarize mutually the charges in both fragments. The distance-dependent mix of the ionic CSFs and the two neutral CSFs NB and TT describe distance-dependent charge and spin distributions, which cause the oscillation of the geometry parameters, especially for the disilene moiety at silicon atom 1, which has the weakest interaction with the silylene fragment in the early stage of the recombination reaction.
6.3. c-CSi2H6 → Si2H4 + CH2
In c-CSi
2H
6 the atoms X and Y from the sketch in
Figure 3 are equal to Si
1 and Si
2, respectively; atom Z is the carbon atom C.
The creation of disilacyclopropane c-CSi2H6 by addition of methylene to disilene and the elimination of methylene from the 3-ring is studied in high (R3v) and low symmetry (R3s). The approximate reaction coordinate R is in the range of Å to Å. At the equilibrium of c-CSi2H6 the approximate reaction coordinate is Å.
The PECs (
Figure 19) show, first, that in
symmetry, the elimination and recombination reactions of the c-CSi
2H
6 system follow different MEPs; second, in contrast to the elimination reactions for cyclopropane and cyclotrisilane, the c-CSi
2H
6 system does not jump during the elimination reaction to the lower reaction channel; therefore, the dissociated system consists of methylene and disilene both in their lowest triplet states. The reaction has the properties of a diabatic reaction. The PEC for the recombination reaction resembles that for the cyclotrisilane system. The reactions in
symmetry are different from those of cyclotrisilane. First, the elimination reaction also leads to triplet fragments, as the shapes of the PECs and of the geometry parameters suggest; second, the recombination reactions starting from both structures S1 and S2 lead to disilacyclopropane, so three different reactions in
were investigated.
6.3.1. R3v: The Elimination and Recombination Reactions of c-CSi2H6 in
The methylene elimination follows a single MEP, as the PEC (
Figure 19) and all curves of the geometry parameters show (see
Figure 20); there is no jump to the lower lying entrance valley of the recombination reaction. In the dissociated system, both fragments are in their respective triplet states. During the recombination reaction between
Å and
Å, both fragments are in low-spin states, and both silicon atoms of disilene have wrong pyramidality, disilene being cis-bent; the methylene fragment is in its 1-
state. The total energy increases and reaches its highest value at
Å, then the fragments change to high-spin states; the geometry parameters of methylene are the best indicators for the change in multiplicity. In disilene, the Si-Si distance increases from 2.20 Å to 2.37 Å. The Si-H length increases and the pyramidality at the silicon atoms increases as well, but it is still wrong. With decreasing
R, the energy decreases also, but is still higher than in the elimination reaction. In this phase of the reaction, only the disilene parameters change but not the methylene parameters. At
Å, umbrella inversion allows the formation of the new bonds and the total energy is lowered to that of the elimination reaction. The change from low-spin to high-spin at the crossing point at
Å means a reduction in the Coulomb repulsion for the disilene fragment, and, thus, a lowering of the total energy for
Å. This is similar to the reaction in the c-Si
3H
6 system. In methylene, the change means the replacement of an excited low-spin state by the high-spin ground state, and causes a lowering of the total energy. In this aspect, the reaction in the c-CSi
2H
6 system is different from the reaction in the c-Si
3H
6 system. However, because of the wrong pyramidality, the
lobes point off the methylene fragment, so that no new bonds can be formed. This is only possible after the umbrella inversion.
The weight of CSF TT supports the description of the elimination reaction. In the dissociated system, TT has weight 1.0, and no other CSF contributes to the wave function; in the bonded system, the CX1 CSF and the CX2 CSF describe the charge shift in the covalent C-Si bonds. Around the equilibrium geometry, the doubly ionic CSF enforces the charge shift towards the carbon atom; this is in agreement with chemical explanations based on the different electronegativities of silicon and carbon. Altogether, the wave function describing the elimination reaction is a linear combination of only four large CSFs, but, as the sum of the weights shows, the CSFs with weights smaller than 0.1 contribute together about 30 percent at the equilibrium geometry, most of which are ionic CSFs describing the polarization of the electron densities in the fragments. This means that during the elimination reaction, the system’s wave function does not change its characteristics; the elimination reaction has the character of a diabatic reaction.
The dissociated system in the recombination reaction is dominated by the CSF NB and the CSF
, which describe the left-right correlation of the
bond; the
CSF for the angular correlation of the lone pair electrons in methylene has too small a weight to be shown. Between
Å and
Å, the sum of their weights is larger than 0.94. When the wave function acquires high-spin characteristics at about
Å, TT is the dominating CSF; the necessary charge shifts during covalent bonding are described by CSFs CX1
, CX2
, and DC
. Before the umbrella inversion, only
contributes to the wave function, after the umbrella inversion,
also contributes and then both CSFs are equally important, as the weights show (see
Figure 21).
6.3.2. R3s: The Elimination and Recombination Reactions of c-CSi2H6 in
The elimination reaction in
symmetry also follows a single MEP; in the dissociated system, both fragments are in triplet states. Up to
Å, the MEP is the same as for the reaction in
, but then the methylene fragment folds down to
degrees, and the methylene fragment shifts along the Si=Si direction so that the Si-C distances have different lengths, Z-X is longer than Z-Y. The Si=Si distance increases continuously, the out-of-plane angles increase slightly from 20 to 50 degrees, and the disilene fragment remains cis-bent; the pyramidality of the SiH
2 moiety at atom 1 becomes more pronounced than that of the second SiH
2 moiety at atom 2. Note that the energy of the dissociated system is equal for the reactions in
and in
symmetry (see
Figure 19).
The recombination reactions, labeled recomb1 and recomb2, respectively, can start from structure S1 or S2, and both recombination reactions yield the 3-ring. In reaction recomb1, the system’s energy is nearly constant (
Figure 19) between
Å and
Å; the geometry parameters of both fragments are either nearly constant or change only moderately (see
Figure 22,
Figure 23 and
Figure 24). In this phase of the reaction, methylene is in the 1-
state and disilene is in its ground state with trans-bent geometry. At about
Å, the wave function has changed from low-spin to high-spin characteristics, methylene has changed from singlet to triplet geometry, the short Si=Si double bond is now a long Si-Si single bond, and the geometries of the two SiH
2 moieties in disilene have changed very differently. In the SiH
2 moiety at silicon atom 2, to which the first new Si-C bond is formed, both the SiH bond lengths and the HSiH bond angle change rather smoothly from the values in disilene to the values in the 3-ring, the SiH lengths decrease, and the HSiH angle increases. Neither geometry parameter changes suddenly at
Å. This is different from the other SiH
2 moiety, where both parameters show jumps at
Å. Subsequently, the SiH bond lengths increase and the HSiH angles decrease in both moieties, their geometries developing towards the pyramidal structure of a silyl group. At
Å, the symmetry changes from
to
and the two SiH
2 moieties become equivalent; this happens with a jump in the total energy.
In structure S2, the carbon atom points to the silicon atom with the wrong pyramidality; the distance of the carbon atom to both silicon atoms is equal. In reaction recomb2, the change from low-spin to high-spin occurs at Å. At this point, the pyramidality of both silicon atoms changes, as the out-of-plane angles show, and the methylene fragment shifts relative to the disilene fragment, so that the two C-Si distances are now different, as occurs when the reaction starts at structure S1. From now on, reaction recomb2 proceeds as reaction recomb1.
The wave function that describes the elimination reaction is composed of five CSFs: the dominant neutral TT CSF and four ionic CSFs. In the part of the reaction, that is up to Å, the same four CSFs TT, CX1, CX2, DC dominate the wave function, as in the reaction; when the system changes to symmetry, the C CSF is the most important ionic CSF, CX2, and DC disappear, and CX1 has weights smaller than 0.1. For Å, the wave function is described solely by CSF TT; at Å, its weight is 1.00.
In the recombination reactions, the wave function is composed of six CSFs; CSF NB dominates the dissociated system, at
Å, its weight is 0.86. The remaining 14 percent stem from “small” CSFs, but not from any other “large” CSF. At
Å, NB still has a weight of 0.72; the weight of the second large CSF, the ionic CSF C
, is only 0.07. At
Å, the weight of NB has dropped to 0.17, and the largest CSF is TT with a weight of 0.57. The weights of the ionic CSFs C, CX1, and CX2 increase as expected; at
Å, the system has changed to
symmetry, and then the reaction proceeds as in the
elimination reaction (see
Figure 25).
6.4. c-SiC2H6 → C2H4 + SiH2
The creation of silacyclopropane c-SiC
2H
6 by addition of silylene to ethene and the elimination of silylene from the 3-ring is studied in high
(
R4v) and low
symmetry (
R4s). The approximate reaction coordinate
R is in the range of
Å to
Å. At the equilibrium of c-SiC
2H
6, the value of the approximate reaction coordinate is
Å. In c-SiC
2H
6, the atoms X and Y from the sketch in
Figure 3 are equal to C; atom Z is the silicon atom.
Both the elimination and the recombination reactions give the same PEC, which has a prominent cusp at
Å (
Figure 26). The two branches describe the reactions in the bonded and the dissociated system, respectively; at the crossing point, the system jumps immediately between the two troughs—the system never follows the MEPs beyond the crossing point. Therefore, there is only a single curve for each geometry parameter, and there is only a single set of CSF energy and weight curves.
6.4.1. R4v: The Elimination and Recombination Reactions of c-SiC2H6 in
The PEC (
Figure 26) and all curves of the geometry parameters (
Figure 27) show that the silylene elimination and the recombination follow the same two MEPs; the crossing point for both reactions is at
Å. When the recombination starts at the dissociated geometry obtained in the elimination reaction, the reaction yields effectively the same geometries and energies (
Figure 28). In the bonded system, both fragments are in high-spin states, while in the dissociated system, both fragments are in their respective singlet ground states, and the ethene fragment is planar.
The dissociated system is described by the neutral CSFs NB and DX , which describe the angular correlation in the silylene fragment. The contribution of the small CSFs is only 7 percent. In the bonded system, the neutral CSF TT has the largest weight. The CX1 CSF , the CX2 CSFs and the C CSF describe the charge shifts in the covalent C-Si bonds. The DC CSF has a weight larger than 0.1 only for R smaller than the equilibrium value; it is thus irrelevant. The wave function is thus a linear combination of seven large CSFs. Two dominate the dissociated system, four the bonded system, and one is irrelevant.
6.4.2. R4s: The Elimination and Recombination Reactions of c-SiC2H6 in
The elimination reaction follows the
MEP up to
Å, where the total energy is about 0.038 millihartrees above the energy in the entrance channel of the recombination reaction. At this geometry, the system symmetry changes from
to
and the wave function changes from high-spin to low-spin characteristics. The geometry parameters change very differently. The least changes are observed for the C-Si bond lengths, while there is a slight shift of the silylene fragment parallel to the ethene fragment, so that atom Z is closer to carbon atom 2 than to carbon atom 1. The geometry parameters of the CH
2 moiety at atom 2 change pronouncedly more smoothly than those of the other CH
2 moiety. The most abrupt changes show the SiH
2 moiety. Because of the planarity of ethene, there is only one starting geometry for the recombination reaction, and, thus, a single recombination reaction. Up to
Å, the total energy is nearly constant during the approach of the fragments, and the wave function retains the low-spin characteristics. The geometry parameters are rather constant up to
Å, then all parameters start changing, but up to
Å, the parameter curves are identical with those for the elimination reaction. Between
Å and
Å, the changes increase, the largest differences being found for the parameters describing the different CH
2 moieties of the ethene fragment. At
Å, the total energy is higher than that of the elimination reaction, but at
Å, the symmetry changes to
and the recombination follows the
MEP (see
Figure 29,
Figure 30 and
Figure 31).
As long as the system has
symmetry in the elimination reaction, the wave function is a linear combination of the neutral CSF TT, the CX1 CSF
, the two CX2 CSFs
and
, and the DC CSF
. The CX1 and CX2 CSFs describe the charge shifts in the covalent Si-C single bonds, and CSF CSF
describes in the dissociated system the angular correlation in the silylene
ground state; in the bonded system, it helps to describe the deformation of the electron distribution in the silylene fragment. The doubly ionic CSF
has a weight larger than 0.1 only at small
R values; indeed, this CSF is rather unimportant for the description of the reaction. When the symmetry changes to
, the wave function changes the characteristics from high-spin to low spin, and CSF TT is replaced by CSF NB and the C CSF
. With increasing
R, the weight of NB increases and that of
decreases; at
Å, only NB and the correlating CSF
survive, and all other CSFs contribute not more than 4 percent. In the recombination reaction, up to
Å, the wave function is dominated by the CSF TT contribution; at
Å, the weight of the C CSF
increases, and at
Å, also that of the C CSF
. At
Å, the system symmetry becomes
, the C CSFs no longer contribute to the wave functions, and the wave function has the same composition as it has in the elimination reaction. See
Figure 32.
6.5. c-CSi2H6 → CSiH4 + SiH2
The elimination of silylene from disilirane and the reverse addition of silylene to silaethene, reaction
R5s, proceeds only in
symmetry; the approximate reaction coordinate
R is in the range of
Å to
Å, and at the equilibrium of c-CSi
2H
6, the value of the approximate reaction coordinate is
Å. In the molecular system, the atoms X and Y from the sketch in
Figure 3 are carbon and silicon, respectively; atom Z is silicon.
This is the first of two reactions with silaethene as an ethene analog. It has a heteroatomic double bond and a non-zero dipole moment and is not planar but trans-bent, while the pyramidality at the silicon atom is much larger than at the carbon atom. For each of the two trans-bent structures of silaethene, there are two possible orientations of the carbene analog, giving four possible structures as starting structures of the recombination reactions; this is different from the reactions studied above. In structure S1, the silicon atom of silylene is close to the silicon atom in silaethene having correct pyramidality; in structure S2, the pyramidality is wrong. When the orientation of silylene in structure S1 is reverted, structure S3 is obtained; reverting the orientation of silylene in structure S2 yields structure S4. In all four possible structures, the silylene fragment lies “parallel” to the double bond (see
Figure 33).
The elimination reaction follows a single MEP, the total energy increases monotonously, and the dissociated system has structure S1. In this structure, the dipole moments of silylene and silaethene are antiparallel, so one can assume that this structure is also electrostatically stabilized when the fragments approach each other. The PECs of the elimination reaction and the recombination reaction recomb1, starting from structure S1, are essentially identical, and the maximum difference between the energies is 30 microhartrees; in
Figure 34, left, the PECs cannot be distinguished. Also the corresponding geometry parameter curves are identical. Therefore, only the recombination reactions are discussed.
At Å, atom Z has roughly the same distance from atoms X and Y; at Å, the Z-Y distance, i.e., the Si-Si distance, is shorter than the Z-X distance (Si-C) by 0.33 Å; the interaction between the two silicon atoms is apparently larger than between the silylene silicon atom and the carbon atom. Around Å, the silaethene fragment becomes cis-bent; the silylene fragment changes from singlet to triplet geometry, and the “parallel” relative position is changed to a “vertical” one. The Z-Y distance is shorter than the Z-X distance, so the new Si-Si bond is created before the C-Si bond. In the silaethene fragment, the SiH and CH lengths increase and the HSiH and HCH angles decrease, and the “planar” fragment adopts the non-planar structure in the 3-ring. In the 3-ring, the Si-Si distance is longer than the Si-C distance.
In reaction recomb2, starting from structure S2, the PEC lies slightly above that of reaction recomb1 up to
Å; the energy differences are not larger than 1.4 millihartrees. All the geometry parameters, except for the out-of-plane angles are equal to those of recomb1. At
Å, the pyramids in silaethene are inverted, and the energy drops only by 3 millihartrees; for
Å, reactions recomb1 and recomb2 are equal, as are all the geometry parameters (see
Figure 35 and
Figure 36).
Whereas in reactions recomb1 and recomb2, “parallel” means that
varies in a small interval
degrees around
degrees, in reactions recomb3 and recomb4,
has values
or
. This is the origin of the jump in the
curve for recomb3 and recomb4 in
Figure 36. In recomb3 and recomb4, silylene remains “parallel” up to
Å. At this point, the characteristics of the wave function change from low-spin to high-spin. The silicon atom in silylene is close to the carbon atom in silaethene, which has wrong pyramidality. Because of the parallel orientation of the dipole moments of the fragments, one might assume that this structure is electrostatically destabilized, but the energy difference of 100 microhartrees between structures S1 and S3 shows that the dipole–dipole interaction is of minor importance for the stabilization at large distances. More important is the different interaction of the silicon atom in silylene with the atoms in silaethene. In structure S3, the Z-X (Si-C) distance is about 0.06 Å shorter than the Z-Y (Si-Si) distance, and the difference becomes even larger with decreasing
R values. During the approach of the two fragments, angle
at the carbon atom changes from negative to positive values; angle
is already positive, as it must be in the 3-ring. Comparison of the total energies shows that the interaction between the two silicon atoms in recomb1 is much larger than that between silicon and carbon in recomb3; between
Å and
Å, the energy of recomb3 is approximately 10 millihartrees higher than the energy of recomb1. At about
Å, the wave function changes its characteristics from low-spin to high-spin, the CH
2 and the SiH
2 moieties in silaethene change to the typical values in the 3-ring, the silylene fragment changes the orientation, and
jumps to a value of about 45 degrees. Only at
Å, when the 3-ring is formed, does the the silylene fragment change to an upright position. Structure S4, the starting structure of reaction recomb4, can be regarded as the result of an umbrella inversion at both atoms in the double bond (double umbrella inversion) in structure S3. The only difference between recomb3 and recomb4 are the different out-of-plane angles of silaethene up to
Å. The total energy of reaction recomb4 lies slightly above that of reaction recomb3, the largest difference is 2.3 millihartrees. At
Å, the molecular system changes by a double umbrella inversion to that of reaction recomb3. The difference between recomb3 and recomb4 is minimal, as is the difference between recomb1 and recomb2. The large difference between recomb1 and recomb3, and between recomb2 and recomb4, respectively, is caused by the much larger interaction of the silicon atom in silylene with the silicon atom than with the carbon atom in silaethene.
The wave functions describing all five reactions are linear combinations of seven CSFs. In the dissociated system, the wave functions are spanned by only the neutral CSFs NB and X
, which describes the polarized
density in silaethene. The sum of their weights is larger than 90 percent. When the fragments approach, the weights of both CSFs decrease, and the weight of CSF TT increases as do the weights of the C CSFs
and
. The first one describes a charge shift from the bonding
FMO to the empty p lone pair FMO in silylene, the second one a shift from the doubly occupied s lone pair FMO to the antibonding
FMO. If one considers that, in the dissociated system, carbon AOs dominate the
FMO and silicon AOs, the
FMO, CSF
describes mainly a charge shift from the
electron density, located at the carbon atom, to the empty lone pair FMO in the silylene fragment. CSF
describes a charge shift from the doubly occupied s lone pair FMO in the silylene fragment to the empty silicon AOs in the
FMO. So, the first CSF is relevant for the description of the new C-Si bond, and the second CSF for the description of the new Si-Si bond. In reactions recomb1 and recomb2, both CSFs contribute between
Å and
Å. At
Å, the weight of
is about 60 percent larger than that of
; then, the contribution of both CSFs is suddenly only half of that before. As the sums of the weights show, several small CSFs must replace the two ionic CSFs. In reactions recomb3 and recomb4, only C CSF
contributes up to
Å; CSF
starts to contribute when the weight of
has already reached its maximum. This is when the pyramidality of the CH
2 moiety in silaethene is inverted, and the wave function changes its characteristics from low-spin to high-spin (see
Figure 37 and
Figure 38).
All five reactions obviously follow a single MEP—there are no significant jumps in the energy and geometry curves. The CSF energies also vary smoothly, even where the characteristics of the wave functions change.
6.6. c-SiC2H6 → CSiH4 + CH2
Reaction
R6s, the elimination of methylene from silirane and the addition of methylene to silaethene, proceeds only in
symmetry; the approximate reaction coordinate
R is in the range of
Å to
Å, and at the equilibrium of c-SiC
2H
6, the value of the approximate reaction coordinate is
Å. In c-CSi
2H
6, the atoms X and Y from the sketch in
Figure 3 are carbon and silicon; atom Z is carbon.
The four starting structures for the recombination reaction (see
Figure 33) are the same as for reaction
R5s.
Structure S1 is the result of the elimination reaction and the starting structure for reaction recomb1; both reactions are essentially identical, with the maximal difference between the PECs being 27 microhartrees. Structure S1 consists of methylene in the 1- state and trans-bent silaethene in the singlet ground state. The SiH2 moiety already has correct pyramidality, and angle has 10 degrees. The CH2 moiety has wrong pyramidality with degrees—the pyramid is very flat. The methylene fragment is “parallel” to silaethene, with the carbon atom close to the silicon atom in silaethene.
Between Å and Å, all the geometry parameters of the fragments are essentially constant—only the Z-X (C-C) and Z-Y (C-Si) bond lengths change linearly, C-Si up to Å, C-C only up to Å, with C-C always being slightly longer than C-Si. At Å, the methylene fragment has an HCH angle of 102 degrees and a CH length of 1.098 Å; at Å, the wave function changes its characteristics from low-spin to high-spin, and the interaction between the fragments increases. Between Å and Å, the HCH angle in methylene widens rapidly to 115 deg, which is neither close to the angle in the state, nor in the state or in the state; then, it increases further and reaches a maximum value at Å. The CH length decreases from 1.098 Å to 1.08 Å and reaches a minimum of 1.072 Å, again at Å. The value of neither geometry parameter can be compared to those of methylene in either of the pure states. The out-of-plane angle increases from about 0 degrees at Å to 40 degrees at Å and then nearly linearly to 75 degrees in the 3-ring. The geometry parameters of silaethene change very differently. The out-of-plane angle of the CH2 moiety, , is essentially constant between Å and Å; then, it changes rapidly to 25 degrees in the 3-ring. The CH bond length and the HCH bond angle are also constant until the flat pyramid inverts at Å. The continuous inversion of the pyramid via a flat intermediate structure is accompanied by a change from sp3 hybridization to sp2 and then again to sp3 hybridization. The out-of-plane angle of the SiH2 moiety increases from 20 degrees to 40 degrees, and then decreases to 25 degrees in the 3-ring; both the SiH and the CH bond lengths have a minimum at Å, and both the HSiH and the HCH angles have a maximum at the same position. Again, these changes correspond with the planarization of both pyramids, and, thus, with a change in hybridization from sp3 towards sp2. Between Å and Å, the length of the Si=C bond increases from 1.73 Å to 1.81 Å. This value is kept up to Å. Then it decreases and has a minimal value of 1.79 Å at Å, and then increases again. Although several geometry parameters change considerably between Å and Å, the total energy decreases nearly linearly.
The change in the initial structure from S1 to S2 results in a very small difference between reactions recomb1 and recomb2. Up to Å, all the geometry parameters change slowly but are significantly stronger than in recomb1. Most prominent are the different signs of angles and . Nevertheless, the differences in the total energy are not larger than 1.4 millihartrees before the double umbrella inversion at Å; after the umbrella inversion, both reactions have identical energies.
The different orientation of the methylene fragment in structure S3 influences the total energy of reactions recomb3 and recomb1 only between
Å and
Å (see
Figure 39). The only geometry parameter that is essentially different from the corresponding recomb1 parameter is
. All other parameters have similar shapes; the positions where the largest changes in the parameter values occur are where the wave function characteristics change—this is in recomb3 at about
Å and in recomb1 at about
Å. For recomb1 and recomb3, the curves of the HCH angle in silaethene are very similar; in contrast to the recomb1 curve for the CH length, the recomb3 curve has a minimum at
Å. The parameter curves with the largest differences between recomb1 and recomb3 are the HSiH curves. As expected, the Z-X lengths are smaller and the Z-Y lengths larger than in the case of recomb1.
The major differences between reactions recomb3 and recomb4 are again due to the different pyramidality of silaethene in structure S4. Nevertheless, the PECs for recomb3 and recomb4 are identical, and they are nearly constant between
Å and
Å, where the energy drops to the recomb1 and recomb2 values and then decreases linearly between
Å and
Å. All significant changes in the geometry parameters occur at about
Å, where the characteristics of the wave function change (see
Figure 40 and
Figure 41).
The wave functions describing all five reactions are linear combinations of the three neutral CSFs NB, TT, and the X CSF
, and of the C CSF
, the CX1 CSF
, and the CX2 CSF
. The dissociated system is dominated by CSF NB and the local excitation
; the sum of their weights is 93 percent for all reactions. Between
Å and
Å, the C CSF starts to contribute, and at the same time the weight of NB declines. The weight of NB drops suddenly to about 0.2 and the weight of CSF TT increases to about 0.4 at those
R values, where the fragment start to interact. In recomb1 and recomb2, it is between
Å and
Å, while in recomb3 and recomb4, it is between
Å and
Å. Up to
Å, CSFs NB, TT, and C are the only dominating large CSFs; in this interval, the weight of NB goes to zero, the weight of TT approaches 0.3, and the weight of CSF C is below 0.05. Now, CSFs CX1 and CX2 start to contribute. The graphs with the sum of all the large CSFs and their complement show that a large part of the fragment interaction is represented by small CSFs; as soon as the interaction starts, the sum of the weights of the large CSFs decreases linearly to about 0.6 at the equilibrium of the 3-ring. So, even when only three large CSFs dominate the wave function of the interacting fragments, the large number of small CSFs makes it impossible to attribute one of them to the incomprehensible geometric features. An example is the methylene structure with a CH bond length of 1.072 Å and an HCH bond angle of 115 deg (see
Figure 42 and
Figure 43).
7. Discussion
Although the MCSCF reaction energies do not contain the large contributions of dynamic correlation, comparison of the shifted PECs in
Figure 44 nevertheless provides a reasonable estimate of the stability of the 3-rings. In
, c-CSi
2H
6 is most stable with respect to elimination of a carbene analog, followed by c-C
3H
6, c-Si
3H
6, and c-SiC
2H
6, which is least stable. But one must not forget that the energy at the crossing point is not an adiabatic reaction barrier—it is the energy of the two isoenergetic structures at the bottom of the troughs of the diabatic elimination and recombination reactions. As the PECs show, the resistance against the elimination of methylene is much higher than against the elimination of silylene. The conventional thermodynamical measure of the stability of the 3-rings is the reaction energy of the elimination reaction with all reactants in their respective ground states. In our calculations, the molecular system is always in the singlet ground state; therefore, some fragments can be in excited states, and the energies must be corrected (see the
Supplementary Materials for the details). According to the corrected
values, c-SiC
2H
6 (412.7 kJ/mol) is most stable against elimination of CH
2 in
symmetry, followed by c-C
3H
6 (300.9 kJ/mol); the elimination of SiH
2 costs less energy, c-Si
3H
6 (231.4 kJ/mol) and c-SiC
2H
6 (103.2 kJ/mol). That c-CSi
2H
6 is more stable than c-C
3H
6 is surprising, that c-Si
3H
6 is less stable than c-C
3H
6 was expected.
The difference between the crossing point energy and the energy of the stable 3-ring is more important when the elimination reaction is forced to follow the MEP, for example, because of large substituents of the 3-rings that prohibit a deformation of the system towards geometries. Because the elimination of CH2 from c-SiC2H6 is a diabatic reaction, there is no crossing point and therefore no estimate of a possible reaction barrier. The largest “barrier” is thus found for the methylene elimination from c-C3H6 (492.4 kJ/mol); for the silylene elimination, we find 311.2 kJ/mol for c-Si3H6, and 273.4 kJ/mol for c-SiC2H6. Also for the recombination reaction, we obtain estimates of the barrier, and now the order is different. The largest barrier is found for the addition of methylene to ethene, c-C3H6 (188.3 kJ/mol), then for the addition of silylene to ethene, c-SiC2H6 (170.2 kJ/mol), then follows the addition of methylene to disilene, c-CSi2H6 (101.9 kJ/mol), and finally, the addition of silylene to disilene, c-Si3H6 (79.8 kJ/mol).
The PECs for the different system symmetries show that resistance against elimination of a methylene analog is highest in symmetry. Since most stable 3-rings have large ligands, folding down of the carbene analogs is thus hampered and the elimination proceeds along an MEP that is close to the MEP in . Also the elimination of carbene analogs from c-CSi2H6 and c-SiC2H6 in reaction R5s and R6s will proceed along an MEP that is close to the MEPs in when the 3-rings have large substituents, and there will be reaction barriers considerably above the reaction energies. These results are relevant for all reactions, where steric factors force the elimination of the carbene analog to proceed in high symmetry. Without such steric hindrances, the reactions can proceed in symmetry and the high barriers disappear.
With respect to the stability of c-Si3H6, one can say that there are no intrinsic reasons for a thermodynamic instability; that cyclotrisilanes could not be made for a long time reflects only kinetic reasons.
To find out why a molecule is stable or unstable, it is not sufficient just to look just at the energetics of a reaction; a reliable description of a reaction must consider both the physical interactions in a molecular system and all consequences of the fermionic character of electrons. Strangely enough, although it is well known that the latter is of utmost importance for the description of many-electron systems, the role of charge and therefore the Coulomb interaction dominates too many discussions and explanations of chemical reactions and molecular structure. One can only speculate about the reasons for this. One possible reason could be that MO-based wave functions use eigenfunctions of an energy operator, the Fock operator; or it is may simply be the fact that arguments regarding the energy or charge of electrons are more comprehensible than those regarding the spin. It is, nevertheless, sheer ignorance of what Lennard-Jones said seventy years ago about the importance of the Pauli exclusion principle for molecular structure and of the reactions leading to it: “…a property which holds for all electronic systems, whether they are atoms, molecules or solids:
Electrons of like spins tend to avoid each other. This effect is most powerful, much more powerful than that of electrostatic forces. It does more to determine the shapes and properties of molecules than any other single factor. It is the exclusion principle which plays the dominant role in chemistry” [
31]. And Lévy-Leblond and Balibar stated that the exclusion principle, “plays the role of a fictitious, although highly effective, mutual repulsion being exerted within the system, irrespective of any other actual forces or interactions […] that might be present” [
32]. The fictitious short-range repulsion, also called “Pauli repulsion”, was addressed by Daudel et al., who simply stated: “This shows that the ‘Pauli repulsion’ between electrons possessing the same spin is very significant. In fact, this
repulsion is the main origin of bond angles" [
33]. Because the “Pauli repulsion” applies to identical spins and because it is short-range, it mainly concerns electrons with a high-spin arrangement localized on an atom. Any spin flip in an electron distribution that increases the number of identical spins has immediate consequences not only for the angular structure of the atom as bond lengths are also affected. In a composite system, any change in the spin arrangement in one subsystem affects the spin arrangement in another, spatially close subsystem. Monitoring the geometry changes during a chemical reaction often allows to conjecture as to which spin arrangement was changed in which subsystem. The full information about the change in the molecular structure plus possible information about the local spin and charge arrangements is stored in the MEP, that is, the set of the
n internal coordinates
as a function of the approximate reaction coordinates. If, for whatever reason, some internal coordinates are not optimized, in technical parlance, if they are frozen, one obtains an approximate MEP, which can be simply wrong. If a change in the spin arrangement causes a strong change in some internal coordinates, and if these coordinates are frozen, the reaction is erroneously described—instead of a correct diabatic PEC one gets, for example, a pseudo-adiabatic one. But even if all the geometry parameters are fully optimized, and the information delivered by them is not properly interpreted, one can miss important information, such as the existence of different MEPs and jumps, but also the absence of jumps between the corresponding reaction valleys. Reactions in different valleys are described by wave functions with different essential characteristics, representing different local charge and spin distributions, which are hardly seen when wave functions based on delocalized MOs are used, but which can easily be seen when localized MOs, for example, FMOs, are used.
In symmetry, the elimination reaction and recombination reaction occur in (at least) two different reaction valleys with corresponding MEPs; one valley describes the stretching of two single bonds in the 3-ring, the second describes the compression of the ethene and the carbene analogs. The wave functions describing these reactions have different essential characteristics. A reaction following a third MEP is the recombination reaction to c-CSi2H6, where after the crossing point, when the wave function already describes fragments with unpaired electrons, the total energy shows another jump. This jump is caused by the double umbrella inversion, which does not change the spin distribution but the shape of the FMOs. Only this change in the orbital shape makes the formation of the new covalent bonds possible. Wave functions made with such different FMOs have different essential characteristics again.
The metaphoric description of the change in the MEP during a reaction as a jump between the troughs of the reaction valleys has its origin in the neglect of nuclear motions in the Born–Oppenheimer approximation; indeed, the elementary processes should be described by an electron-phonon model, which can explain the coupling of the electronic states in the two different regions in the configuration space, and how the system can overcome the barrier between the trough. Note that even an electron-phonon model would be rather approximate, because it allows only vibrations that retain the symmetry.
All reactions in this study that occur in
are orbital symmetry forbidden according to the Woodward–Hoffmann rules. In the dissociated system, each reactant has a doubly occupied
HOMO and an empty
LUMO. For each symmetry, there is one positive and one negative linear combination with bonding and antibonding character, respectively. In the dissociated system, the positive and the negative linear combinations of the
HOMOs are doubly occupied, while the two linear combinations of the
LUMOs are unoccupied. In the 3-ring, the two
bonds between the fragments are described by the doubly occupied bonding
and the bonding
MOs; the antibonding
and
MOs are unoccupied. This implies that the occupied bonding
orbitals in the 3-ring and the dissociated system are correlated, as are the unoccupied antibonding
orbitals; but the occupied bonding
orbital in the 3-ring correlates with the unoccupied bonding
linear combination in the dissociated system, and the unoccupied antibonding
orbital in the 3-ring correlates with the occupied antibonding
linear combination in the dissociated system. This shows that the orbital symmetry of the occupied and the unoccupied orbitals is not conserved, and, therefore, the reaction is forbidden. If, however, the system adopts the lower
point group symmetry, all occupied and all unoccupied orbitals belong to the same
IRREP, the orbital symmetry is conserved, and the reaction is orbital symmetry allowed. With the help of the Woodward–Hoffmann rules, it is possible to understand in which point group symmetry of the system a certain reaction is allowed and in which it is forbidden, but it does not give a physical explanation of the local processes involved in the making or breaking of covalent bonds. This can only be done when the so called static correlation is accounted for by using proper multi-configurational wave functions from methods like configuration interaction (CI), MCSCF, or coupled cluster (CC), which are based on delocalized MOs. Such MOs lack, in general, the ability to describe local effects and processes. VB wave functions are constructed with atom-centered orbitals and are able to do this. See the excellent book by LucjanPiela for a discussion of the benefits of different methods for the interpretation of reactions using multi-configurational wave functions [
34]. There are some very old studies on the methylene addition to ethene. Zurawski and Kutzelnigg [
35] studied this reaction at the SCF level in
symmetry. They used a small double-
basis and calculated the total energy at the CEPA level of theory with larger basis sets. Their geometry data agree qualitatively with ours. In 1993, Reuter et al. [
36] studied the addition of both singlet and triplet methylene to ethene in
symmetry. They used a large CI wave function with 18 active electrons distributed among 54 MOs; the basis set was a split valence basis with polarization functions. The PECs were calculated for selected distances between the reactants, and the geometry parameters were optimized at the CI level. The authors discuss the change in the parameters of methylene and ethene, showing good agreement with ours. In 1983, Anwari and Gordon [
37] calculated the addition of silylene to ethene in
and
symmetry. They used small basis sets for the geometry optimizations at the SCF level. Not surprisingly, the geometry data for the
reaction are rather different; the energy at the so-called avoided crossing is about half as large as our energy at the crossing point. For the
reaction, they also find for large separation of silylene and ethene a parallel structure, and they observe large changes in the relative orientation of the reactants when the bonding starts. In 1997, Lennartz et al. [
38] studied photochemical reactions of silylene with ethene and silaethene. Unfortunately, the scarce geometry data do not allow a comparison of their reactions with our results. In the few studies of reactions in
, the authors mention avoided crossings; that these points are indeed crossing points of PECs for different MEPs could not be seen, the small number of points of the approximate reaction coordinates prevent this being seen. In none of these studies, the changes in the geometry parameters of the fragments were related to changes in the fragment multiplicities; the use of delocalized MOs did not allow this. The same was true in the study of the insertion of silylene into the hydrogen molecule by one of the present authors [
13]. The assumption that a key step in the reaction is the simultaneous change in the multiplicities in both fragments was confirmed with an OVB analysis of the CASSCF wave function much later [
8,
14].
Our strategy for calculating the PECs allowed finding out whether a reaction and the reverse reaction follow the same MEP or different MEPs. In the latter case, large changes in the system structure indicate jumps between the MEPs, probably stimulated by internal vibrations. The investigated model systems have the smallest possible substituents, replacement of the hydrogen atoms by large, bulky substituents, or embedding of the 3-ring into a larger structure. The structural constraints may prohibit large changes in the geometry of the 3-ring, and some jumps can be prohibited, or at least retarded but they can also be enhanced. The different stability of the cyclotrisilanes reported by Masamune supports this view. See also, for example, reference [
39], where we showed that deformation of the molecular structure can strengthen bonds. As we already mentioned, large substituents could force the system to follow in reactions
R5s and
R6s an MEP that resembles that of reactions in high
symmetry. As our OVB analysis shows, jumps between different MEPs are accompanied by local changes in charge and spin distribution, but such an analysis is not always necessary. Frequently, conspicuous changes in the molecular geometry, which any proper electronic structure method can correctly describe, already indicate changes in the spin distribution and thus of the bonding.
The question that also an OVB analysis cannot answer as yet is: What causes the reactants to change their electron distribution so that they have unpaired electrons, or in other words, what causes the simultaneous spin flip in the reactants by which the geometries change from typical low-spin to typical high-spin geometries? Any attempt to explain this by referring to energies fails, be it orbital energies of delocalized orbitals, as used in Woodward–Hoffmann-type studies, or lowering the total energy as a driving force for the stabilization of the system. The total energy is the sum of many different contributions, caused by various interactions or just properties of a many fermion system; it is the most important scalar physical quantity that measures the result of the interactions, but it is not the cause of a system change. After all, in many reactions, there is first an energy increase, before, after some changes in the structure, the system develops into a thermodynamically stabilized system. Metaphorically speaking, the system had to know in advance, that after surmounting barriers, it is on the way to the desired stable molecular structure. Such types of argumentation have the odor of quantum teleology. So, what triggers a system, when the reactants have reached a certain distance, to simultaneously change the spin distribution that equips the reactants with the unpaired electrons needed for covalent bonding? We know that the PEP plays the dominant role for the molecular structure, as with quantum theoretical methods one can monitor the structural changes during a reaction; the OVB results show that, at certain distances between the reactants, the reactants undergo simultaneous changes in their spin distribution, and this is connected with the fermionic character of the electrons. Currently, no mathematical description exists for the physical causes of such changes. In our opinion, it must reflect the significance of the fermionic character of electrons in general, and the role of the PEP in particular, which are indisputable for any correct description of processes in molecular systems. Or, to quote again Lévy-Leblond and Balibar: “The fermionic nature of electrons plays an absolutely essential role and the Pauli exclusion principle reigns supreme, ‘without which things would never be what they are’.” [
32].
8. Method
The main deficiencies of the Hartree–Fock method are the inability to correctly describe dissociation, and the large difference between the exact non-relativistic energy of a system and the Hartree–Fock energy (in the complete basis set limit); the energy difference is called the correlation energy. The Hartree-Fock wave function is a single CSF made with delocalized MOs, which are eigenfunctions of the Hartree–Fock operator; the highest occupied MO (HOMO) must be non-degenerate. If, for certain structures of the system, the HOMO is degenerate or nearly degenerate, two or more CSFs of similar energy can be made and, therefore, only a linear combination of these CSFs gives in the lowest (zeroth) order of approximation, a correct wave function. When the MOs are eigenfunctions of the Hartree–Fock operator, only one component of the (nearly) degenerate HOMO is variationally optimized; the other components are not optimized virtual MOs. MCSCF is the method in which all HOMO components are simultaneously optimized for a linear combination of CSFs; this wave function is then the best zeroth-order wave function. What such a wave function describes, and a Hartree–Fock wave function cannot describe, is called static or non-dynamic correlation; the energy improvement is the static correlation energy. The correlation energy is thus separated in a static and a dynamic correlation contribution; the latter is essential for high-precision total energy calculation. For a correct description of systems with degenerate states or of chemical reactions, the static correlation must be correctly accounted for, and for this, the linear combination must include all CSFs that can be made for a certain number of active electrons distributed among a certain number of active MOs and for a given spin multiplicity of the electronic state. Such a wave function is called a CASSCF or FORS wave function. With such wave functions, it is possible to describe systems composed of subsystems with different spin multiplicities, but this information is hidden as long as delocalized MOs are used to construct CSFs. But such wave functions can be analyzed using the OVB method, revealing the hidden information. Electronic structure methods like the approximate Kohn–Sham DFT, which uses a one-electron density constructed in the same way as in the Hartree–Fock method, can never answer a question of the kind: How important is the coupling of fragment triplet states during a dissociation reaction [
40]?
For all calculations, we used CAS(4,4) wave functions with all non-valence MOs kept frozen. The comparative calculations with CAS(6,6) wave functions were only carried out for two systems. The geometry optimizations were performed with delocalized MOs, while for the subsequent OVB analysis, localized FMOs were used. All calculations were performed with a local version of GAMESS [
41]. The 6-311G(2d) basis set was used throughout. For the calculation of the PECs, the approximate reaction coordinate
R, was incremented in steps of 0.1 Å; all other geometry parameters were optimized. In
and
symmetry, 11 and 6 degrees of freedom must be optimized, respectively. The elimination reactions started with MOs optimized at the equilibrium geometry of the 3-rings. As active MOs, the two bonding and the corresponding antibonding MOs were chosen that describe the two
bonds that are broken during the elimination reaction; the active MOs for the recombination reactions were the
and
MOs of the ethene analogs and the s-type and p-type lone pair AOs of the carbene analogs calculated for the molecular system with the fragments separated by
Å. For the calculation of the PECs, the following strategy was used: take the system structure optimized for a fixed
R value; increment the
R value; use the new geometry as a starting point for the next constrained optimization. Similarly, the MOs optimized for the structure at
R were used as initial MOs for the energy calculation at the incremented geometry. With this strategy, the electron structure along the approximate reaction coordinate was conserved.
For each optimized structure, the optimized CASSCF MOs were localized on the fragments using an orthogonal Procrustes transformation [
19]. In brief: Assuming that the electron structure of the reactants changes slowly during a reaction, the fragment MOs of the non-interacting reactants at the optimized structure at
R must be contained in the delocalized MOs of the molecular system. So, the geometry of the fragments in the optimized geometry of the molecular system is taken and calculated for each fragment simple fragment MOs by a cheap SCF method (RHF or UHF), and the fragment MOs of the different fragments are not orthogonal to each other. The overlap matrix
of these fragment MOs contains all the information about the non-interacting but deformed fragments in the interacting molecular system. The optimized CASSCF MOs contain all the information on the electron structure of the interacting system. By an orthogonal transformation of the MOs, one can localize the MOs on the fragments, and the localized FMO form an orthogonal set. The overlap between the non-orthogonal and the orthogonal FMOs forms the matrix
S. The orthogonal transformation is obtained by demanding that the norm of the matrix difference
is as small as possible. This procedure is the Procrustes transformation. As a result, the doubly occupied non-active MOs are transformed into doubly occupied FMOs; active MOs are transformed into active FMOs, which resemble either AOs or hybrid AOs of the carbene analogs, or the
and
orbitals of the ethene analogs. CSFs constructed with these FMOs are dubbed OVB CSFs. Finally, the CI matrix is set up with the OVB CSFs and diagonalized. The diagonal elements of the CI matrix are the CSF energies; the lowest eigenvalue of the CI matrix must be identical with the CAS(4,4) energy obtained with delocalized MOs; the squared CI coefficients are the weights for the OVB CSFs.