If the parameters in Equation (2) refer to pure gases, then one speaks of ideal selectivity.
2.1. Solubility of Pure CO2, CH4 and Their Mixtures
The solubility of gaseous species i-th in Equations (1) and (2) is expressed as follows:
where C is the gas concentration in a polymer (cm
3(STP) cm
−3) and p is the gas pressure (bar). The concentrations of pure carbon dioxide and methane in the polysulfone-based membrane from the Air Products PRISM PA1020 module were measured gravimetrically in the temperature range of 283–303 K. The pressure range was 0–10 and 0–5 bar in the case of methane and carbon dioxide, respectively. Additionally, the CO
2 isotherm at 293 K was also measured for pressures from 0 to 10 bar. Each isotherm was determined in two runs: sorption and desorption. In the first one, the pressure was increased step by step from 0 to the maximum, and, in the second, the pressure was decreased the same way from the maximum pressure to 0. The experimental methodology is described in
Section 3.2.
In
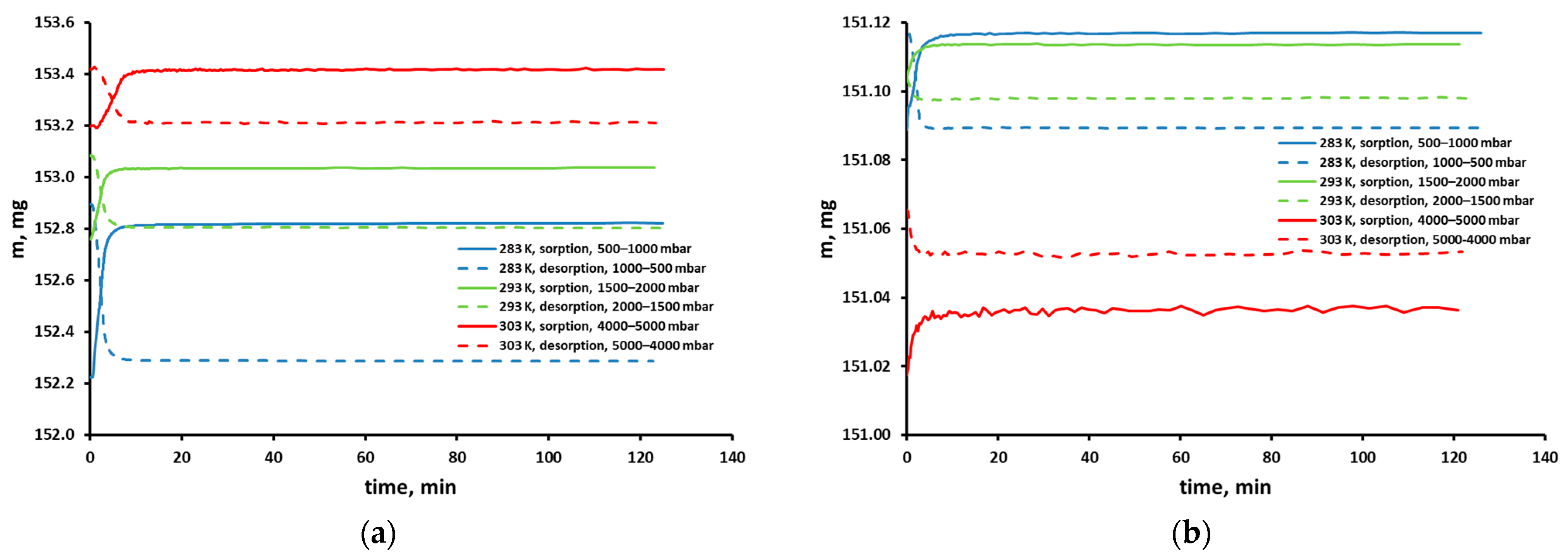
Figure 2, exemplary mass uptake curves are shown for different pressures and temperatures. Such uptake curves show the change in the sample mass over time due to gas sorption related to the change in its pressure and are registered with every isotherm point. As can be seen in these figures, the full mass uptake was reached after about 10 min. The sample weight did not change after this time, which means that sorption equilibrium was achieved. The changes in the sample weight are relatively small and amount to 200–600 µg and 10–25 µg for carbon dioxide and methane, respectively. However, they significantly exceed the measurement resolution of the microbalance (0.2 μg).
The fluctuations that are clearly visible in the case of the methane sorption curve shown in
Figure 2b (at 303 K and with an increase in pressure from 4000 to 5000 mbar) actually occur in all sorption curves. This microbalance is very sensitive. Its weighting resolution is ±0.2 μg. In turn, the temperature is stabilized with an accuracy of ±0.2 K and the pressure with an accuracy of ±0.02% of the measurement range. Therefore, the microbalance will record even a very small mass change associated with temperature and pressure fluctuations, once equilibrium has been reached at a given temperature and pressure.
The isotherms of CO
2 and CH
4 are shown in
Figure 3 and
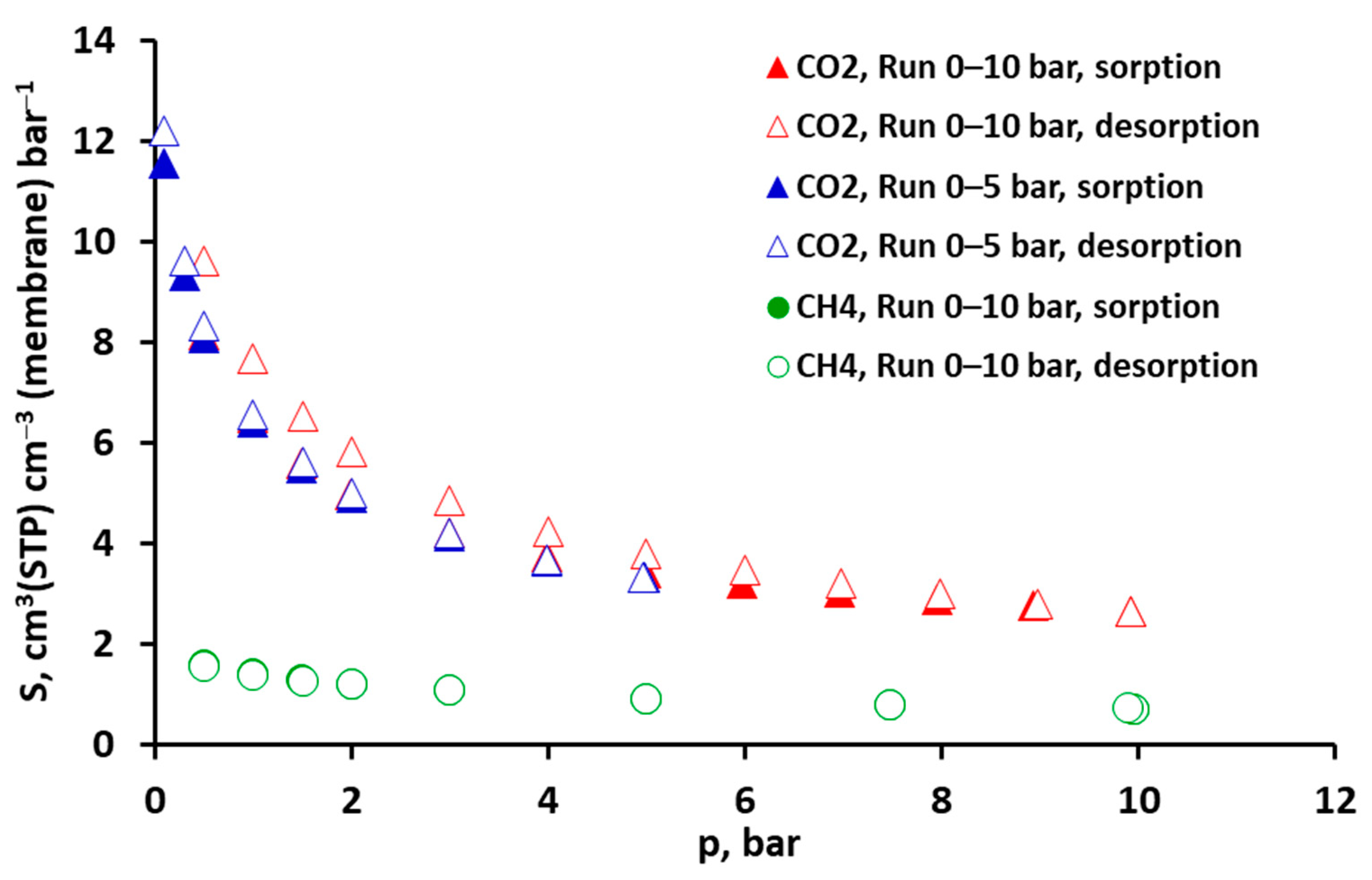
Figure 4, expressed as solubilities. As can be seen in these figures, the solubility of pure CO
2 is generally about two to six times higher than that of CH
4. The greatest differences occur for lower pressures and, additionally, the isotherm of carbon dioxide is strongly nonlinear. In the case of methane (
Figure 3) sorption and desorption, the points practically overlap in the entire pressure range. The same applies in principle to CO
2, when the isotherm was measured in the pressure range of 0–5 bar. This suggests that the process is fully reversible in these cases, i.e., penetrants do not cause any observable and lasting changes in the membrane material. The similar solubility of carbon dioxide and methane in other polysulfone-based materials is presented in [
23,
24,
25].
However, when the pressure range is extended to 10 bar in the case of CO
2, a hysteresis is observed, i.e., the desorption points are located above the sorption points. This phenomenon indicates that equilibrium was not reached during desorption or during both sorption and desorption. As can be seen in
Figure 2, equilibrium was established relatively quickly in all cases and the measurement time for a single isotherm point was sufficiently long. In this situation, it can be assumed that the appearance of hysteresis is related to the presence of another phenomenon that visibly accompanies the physical sorption/desorption.
In the case of the physical adsorption of gases or vapors in solids with a stable pore (voids) structure, hysteresis is a signal that condensation has occurred in the micropores. Typically, in such a situation, for the points on the lower hysteresis line (sorption points), equilibrium is reached, and the corresponding mass uptake curves look similar to those shown in
Figure 3. However, for points on the upper hysteresis line (desorption), equilibrium is not reached within the given measurement time because the desorption kinetics are much slower than the sorption kinetics.
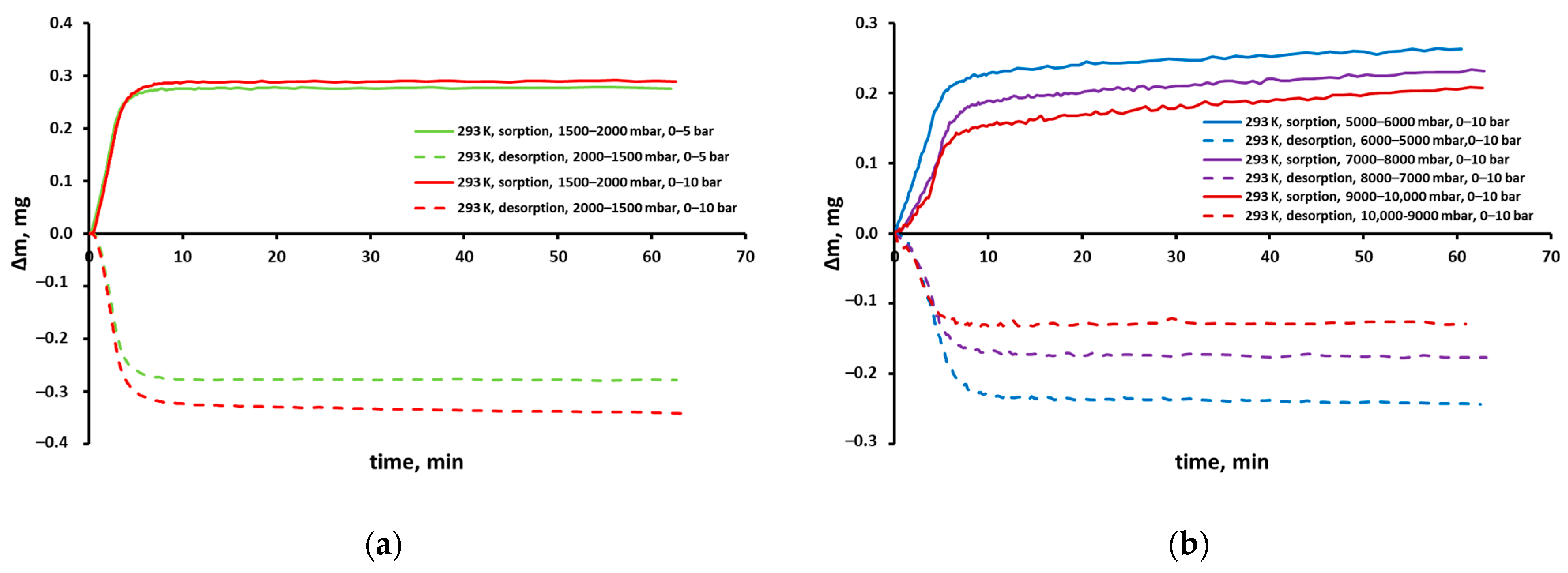
A similar picture could be drawn for the CO
2 isotherm in the polysulfone-based membrane determined in the pressure range of 0–10 bar, as is presented in
Figure 5a. The figure shows the sample weight gain/loss due to the CO
2 sorption/desorption between adjacent pressure points for several points of this isotherm. Therefore, when the pressure was changed from 1500 to 2000 mbar (solid red line) during the sorption stage, the sample weight increased by approximately 0.29 mg and then remained constant until the end of the measurement for that point. Virtually the same increase in the sample weight was recorded with such a pressure change when the CO
2 isotherm was determined in the independent measurement in the pressure range of 0–5 bar (solid green line). In turn, when the pressure was changed from 2000 to 1500 mbar (dashed red line) during the desorption stage, the sample weight decreased relatively quickly by about 0.32 mg, and then it decreased systematically until the end of the measurement for this isotherm point. It was different in the case of the isotherm determined in the pressure range of 0–5 bar when the sample weight remained constant until the end of the measurement after an initial decrease of about 0.27 mg (dashed green line).
It should be emphasized here that in the case of the isotherm determined between 0 and 5 bar, the change in the sample weight between adjacent pressure points is similar in absolute value. This is shown, for example, in
Figure 5a (green lines) and this means that equilibrium was also reached (i.e., the sorption/desorption was complete) for lower and higher pressures. However, the opposite is the case with the CO
2 isotherm determined between 0 and 10 bar. As shown earlier, the sample mass loss during the desorption outweighs its increase during the sorption, when the pressure was varied between 1500 and 2000 mbar. This would mean that the desorption at higher pressures may have been difficult and incomplete.
Indeed, as can be seen in
Figure 5b, at pressures higher than 5 bar, the mass loss during the desorption is lower than the mass gain during the sorption between corresponding adjacent pressure levels. The desorption is incomplete at these pressure levels, although the course of the mass uptake curves during the desorption (dashed lines in
Figure 5b) would suggest that an equilibrium state has been reached. This is also evidenced by the fact that the desorption points are located higher than the sorption points on the isotherm graph (
Figure 3). It should be noted, however, that in the case of the mass uptake curves recorded during the sorption (continuous lines in
Figure 5b), the equilibrium is not achieved at pressures higher than 5 bar. After an initial, relatively rapid increase, the mass of CO
2 in the polymer continues to increase monotonically until the end of the measurement for a given isotherm point. The latter observation suggests that there is a systematic increase in the sorption capacity of the tested polymer membrane sample, which means that it is most likely a swelling.
Both of these phenomena, the condensation of CO
2 in glassy polymers and their swelling upon exposure to this gas when its pressure is high, are widely described in the literature [
26,
27]. Both are unfavorable from the point of view of the separation of gas mixtures containing carbon dioxide, such as biogas, using membrane methods. Condensation of carbon dioxide in the fractional free volume (FFV) of the glassy polymer may result in hindered desorption of CO
2 from this region and, consequently, limited overall membrane permeability to this gas [
28]. In turn, the swelling of the membrane worsens its selectivity, facilitating the transport of mixture components being separated from carbon dioxide (e.g., methane in the case of biogas) [
12,
28,
29].
In the case considered in this paper, both of these phenomena already appear at a relatively low carbon dioxide pressure. However, this is not a problem from the point of view of the adsorptive–membrane process, which is related to the research presented in this paper. In this process, the partial pressure of CO
2 does not exceed 5 bar [
24,
30,
31]. Moreover, in real-world applications, membrane swelling should be avoided because the separation properties of the membrane would become unstable and unpredictable. Indeed, commercial membrane systems are dedicated to a specific process and specific process conditions. Only under such circumstances can the expected lifetime of the membrane system be guaranteed (e.g., 8–12 years). Therefore, in the further part of this work, the analysis was limited to the pressure range of 0–5 bar in the case of carbon dioxide, so as not to obscure the picture of the pure sorption–diffusion phenomena occurring in the tested membrane.
In the case of all the isotherms discussed above, including the one in which hysteresis occurred, the sample returned to its original state after regeneration, both in terms of its initial weight and the sorption capacity for CO
2 and CH
4. The sample regeneration method is presented in
Section 3.2. Both identified phenomena, CO
2 condensation in FFV (fractional free volume) and membrane swelling, are therefore reversible in the range of pressures and temperatures at which the research was carried out. This is consistent with the common view presented in the available literature regarding the influence of carbon dioxide on the condition of glassy membranes [
28,
32,
33,
34].
2.2. Solubility of Pure CO2, CH4 and Their Mixtures According to DMS Model
The sorption isotherms of carbon dioxide and methane in the polysulfone-based membrane sample were described using the Dual Mode Sorption (DMS) model. In this model, the simultaneous gas sorption in both fractions of membrane material is considered: Henry sorption in the matrix phase and Langmuir sorption in the microvoid region, called the fractional free volume (FFV). The model is presented in detail in
Section 3.3. The fitting was performed using the nonlinear least-squares method, separately for the experimental points expressed as C = f(p) and S = f(p). In the first case, hereinafter referred to as the model with minimized concentration squared differences, each isotherm was approximated by Equation (4). In the second case, hereinafter referred to as the model with minimized solubility squared differences, the DMS model coefficients were estimated using Equation (5). In this way, two sets of k
D, b and C’
H coefficients were obtained for each gas. Their temperature dependence is graphically presented in
Figures S1–S3 (points) in the
Supplementary Material. As can be observed from these figures, k
D, b and C’
H decrease with increasing temperature, according to Equation (8), Equation (9) and Equation (10), respectively. The coefficients of these equations were also determined using the least-squares method and are summarized in
Table 1.
It should be mentioned here that although the Dual Mode Sorption model is widely used and accepted for describing gas transport through polymer membranes, the issue of determining its coefficients is widely discussed in the literature [
11,
12,
13,
14,
35]. One of the problems indicated is that different sets of model coefficients can be obtained for the same experimental data set with good numerical fitting accuracy [
11,
36,
37,
38,
39]. It seems that an appropriate number of available experimental points determined in the appropriate range of the function argument (in this case pressure) does not guarantee obtaining DMS model parameter values that will well reflect the solubility in FFV and the polymer matrix. For gases such as CH
4 or N
2, solubility is low at low pressures. It changes nonlinearly with the pressure in this region, and at the same time, the number of experimental points that can be determined with sufficient accuracy is limited. This uncertainty in the determination of solubility is independent of whether it is determined indirectly, based on experimentally determined permeability and diffusivity, or directly, as in the present work. It is also reflected in the values of the b and C’
H coefficients, which describe the sorption in the Langmuir area. In the case of carbon dioxide sorption at higher pressures, swelling of the membrane, as discussed above, or plasticization [
32,
34,
37,
38] can occur. These phenomena directly affect the k
D value, which reports the amount of penetrant absorbed in the polymer matrix. In the present study, the physical significance of the obtained coefficients of the DMS model was evaluated based on several independent premises.
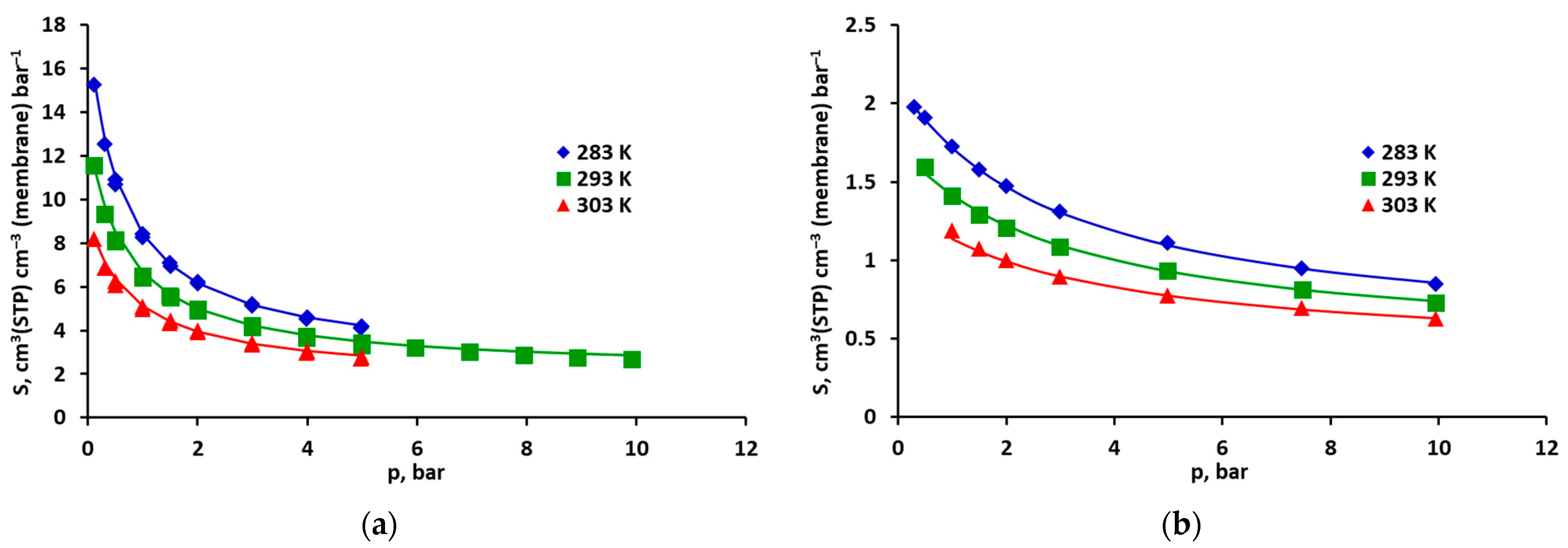
The sorption isotherms of CO
2 and CH
4 determined using the DMS model with the coefficients from
Table 1 are presented graphically (as lines), together with the experimental points, in
Figure 4a and
Figure 4b, respectively, for the case with minimized solubility squared differences and in
Figure S4 in the
Supplementary Material for the case with minimized concentration squared differences.
As can be seen in these figures, the qualitative agreement between the experimental isotherms and those determined by the DMS model is good for both gases and both methods of approximating the model coefficients. The quantitative agreement is also good, as indicated by the low values of the mean relative error (RE) and standard error of the estimate (SEE) given in
Table 1. These errors are defined by Equations (15) and (16), respectively. The latter was proposed to assess the quality of the DMS model fit in [
11]. As can be seen in
Figures S1–S3 in the
Supplementary Material, both approximation methods result in similar sets of k
D, b and C’
H coefficients for both gases.
Table 1 also lists the values of the C’
H1/−C’
H0 ratio, i.e., the theoretical intersection of the C’
H = f(T) line with the abscissa axis. This value refers to the temperature at which the fractional free volume in the polymer should theoretically disappear [
14]. The temperature is similar in each case, which can be considered as additional evidence of the good quality of the model fit to the experimental results, as well as the consistency of the obtained results. Thus, it can be stated that the conclusions and observations presented in the following sections regarding the solubility of the CH
4/CO
2 mixture components in the tested polysulfone-based membrane sample are independent of how the model coefficients are approximated.
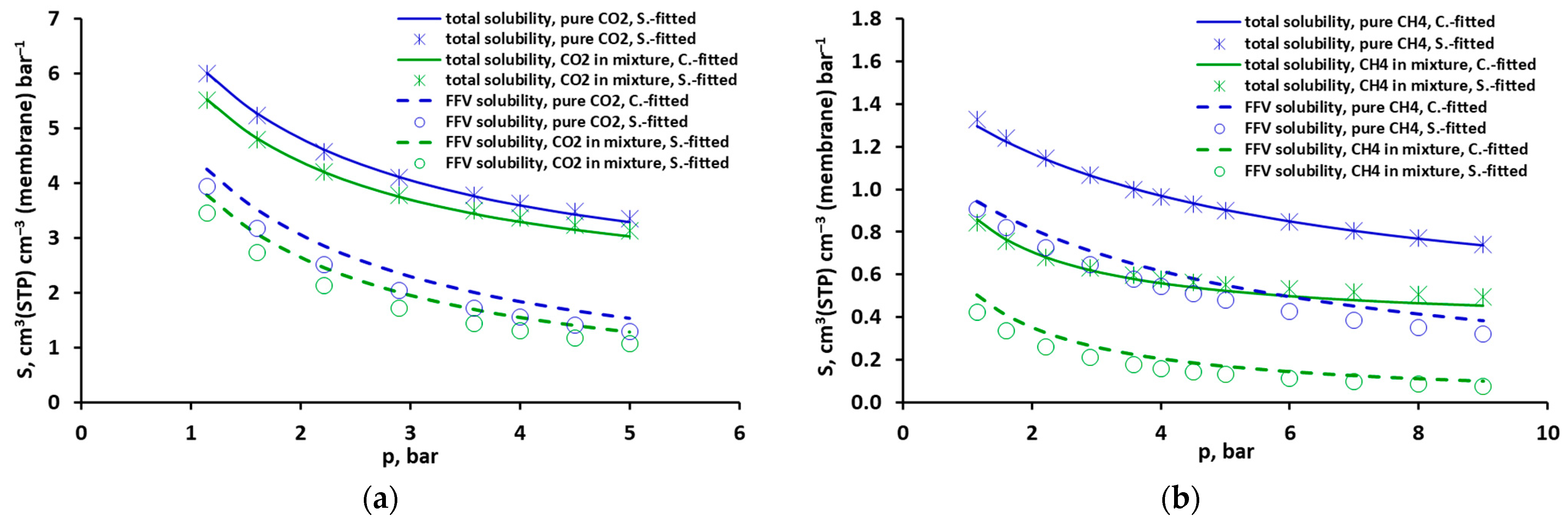
The solubility of carbon dioxide and methane, pure and mixed (CO
2: 50 vol.%/CH
4: 50 vol.%), as a function of gas partial pressure, determined from the DMS model for a temperature of 295 K is presented in
Figure 6. The same was performed for the mixture of CO
2: 40 vol.%/CH
4: 60 vol.% (cf.
Figure S5 in the
Supplementary Material). The mixture’s composition, temperature and pressure ranges correspond to the experimental conditions in which the permeation coefficients of both gases, pure and mixed, were determined [
3,
21]. As can be seen in
Figure 6 and
Figure S5 in the
Supplementary Material, the value of total solubility does not depend on how the coefficients of the DMS model are approximated, both for pure and mixed gases. However, the model with minimized concentration squared differences, on average, gives several percent higher FFV solubility values than the one with minimized solubility squared differences.
The total solubility of pure methane is about 4–5 times lower than that of pure carbon dioxide. For both gases, the fraction of solubility in the Langmuir area is significant and decreases with increases in pressure. In the pressure range of 1–5 bar, it decreases from 65% to 46% and from 73% to 43%, respectively, for CO
2 and CH
4 (in the case of minimized solubility squared differences). In the case of mixed gases, the presence of methane has only a slight effect on CO
2 solubility in the FFV of the studied polysulfone-based membrane, and consequently on its total solubility (
Figure 6a). As can be seen in
Figure 7, the ratio of pure and mixed carbon dioxide solubility is in the range of 0.89–0.93. On the other hand, the total solubility of methane significantly decreases in the presence of carbon dioxide due to a significant reduction in its solubility in the fractional free volume. In this case, the ratio of pure and mixed CH
4 solubility is in the range of 0.59–0.69 (
Figure 7). This clearly better affinity of carbon dioxide than methane during FFV adsorption is expressed by higher values of the parameter b, which are in the range of 1.22–1.61 bar
−1 and 0.31–0.33 bar
−1 for CO
2 and CH
4, respectively (the model with minimized solubility squared differences, cf.
Figure S3 in the
Supplementary Material). The heat of adsorption in FFV is also significantly higher for CO
2, as expressed by the −ΔH
L/R parameter. In the case of minimized solubility squared differences, it is equal to 1194.6 K
−1 for CO
2 and 305.5 K
−1 for CH
4 (cf.
Table 1).
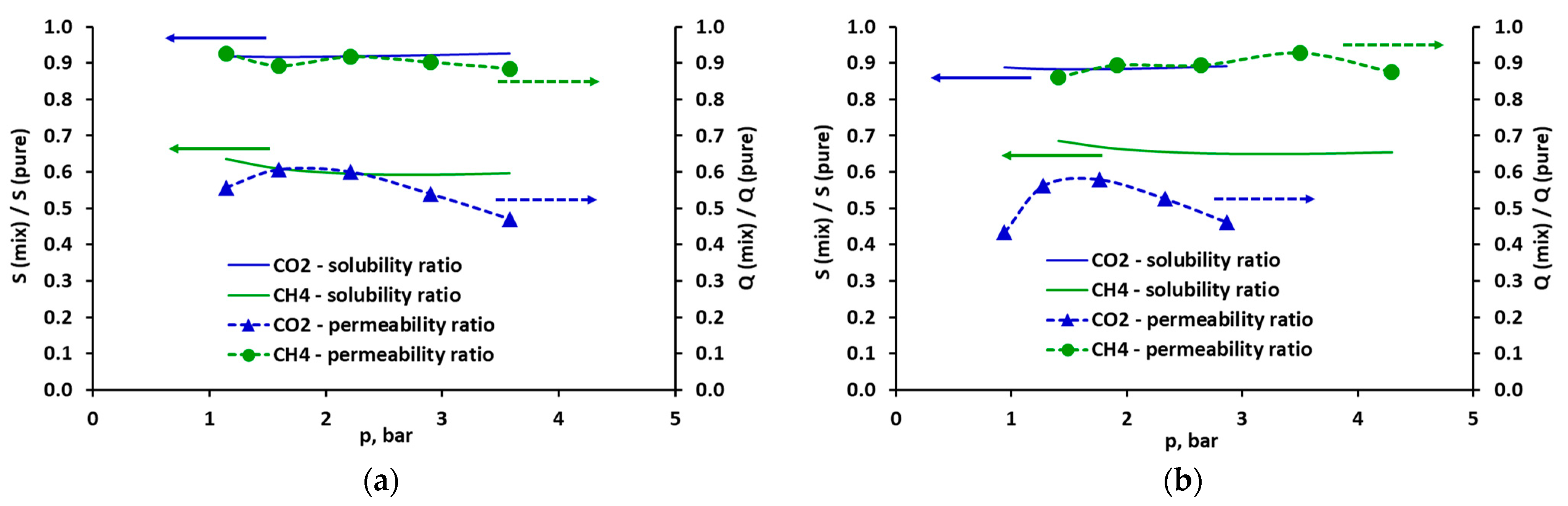
The comparison of the solubility of pure and mixed gas, as well as the permeability of pure and mixed CO
2 and CH
4, is presented in
Figure 7. The solubility was determined using the DMS model with minimized solubility squared differences. The similar plots obtained using the DMS model with minimized concentration squared differences are presented in
Figure S6 in the
Supplementary Material. The permeability coefficients determined experimentally in [
3,
21] are listed in
Tables S1 and S3 in the
Supplementary Material, and their corresponding ratios are marked in
Figure 7 and
Figure S6 in the
Supplementary Material by points connected by dotted lines. As can be seen in these figures, the better affinity of CO
2 towards the tested polysulfone membrane does not clearly imply the preference of this gas during the separation of the CO
2/CH
4 mixture. For both gases, the permeability coefficients of mixed gas are lower than in the case of pure gas. However, while the permeability of methane in the mixture is only slightly less than that of pure gas, the permeability of carbon dioxide is much less in the CO
2/CH
4 mixture than that of pure gas. The permeability ratio of mixed and pure CO
2 ranges from 0.43 to 0.61, while for methane it is 0.86 to 0.93 (
Figure 7). Moreover, the permeability coefficient of carbon dioxide in the CO
2/CH
4 mixture varies significantly with pressure, reaching a maximum value at a pressure of about 1.8 bar. The phenomenon of a decrease in CO
2 flux and unchanging CH
4 flux is often reported in the literature for the separation of CO
2/CH
4 mixtures in glassy polymer membranes. This phenomenon cannot be explained by the competitive sorption in FFV. Carbon dioxide is the dominant component in terms of its solubility in both the FFV and the polymer matrix, causing a significant reduction in the solubility of methane. This is also frequently reported [
28,
36,
39,
40], therefore the mentioned phenomenon is often explained by the fact that CO
2 present in the polymer matrix facilitates the diffusion of methane, pushing the polymer chains apart [
28,
36,
40].
2.3. Selectivity and Diffusivity of Pure CO2, CH4 and Their Mixtures
The simple relation between the permeability (or permeance), solubility and diffusivity provided by the solution–diffusion model (Equation (1)) allows one of these quantities to be determined, once the two others (usually including the permeability/permeance) have been directly measured [
32,
38,
39,
40,
41]. In the present study, the diffusion coefficients of methane and carbon dioxide, pure and mixed, were determined at 295 K based on the solubility and permeance of these gases in the polysulfone-based membrane sample. Their dependence on partial pressure is presented graphically in
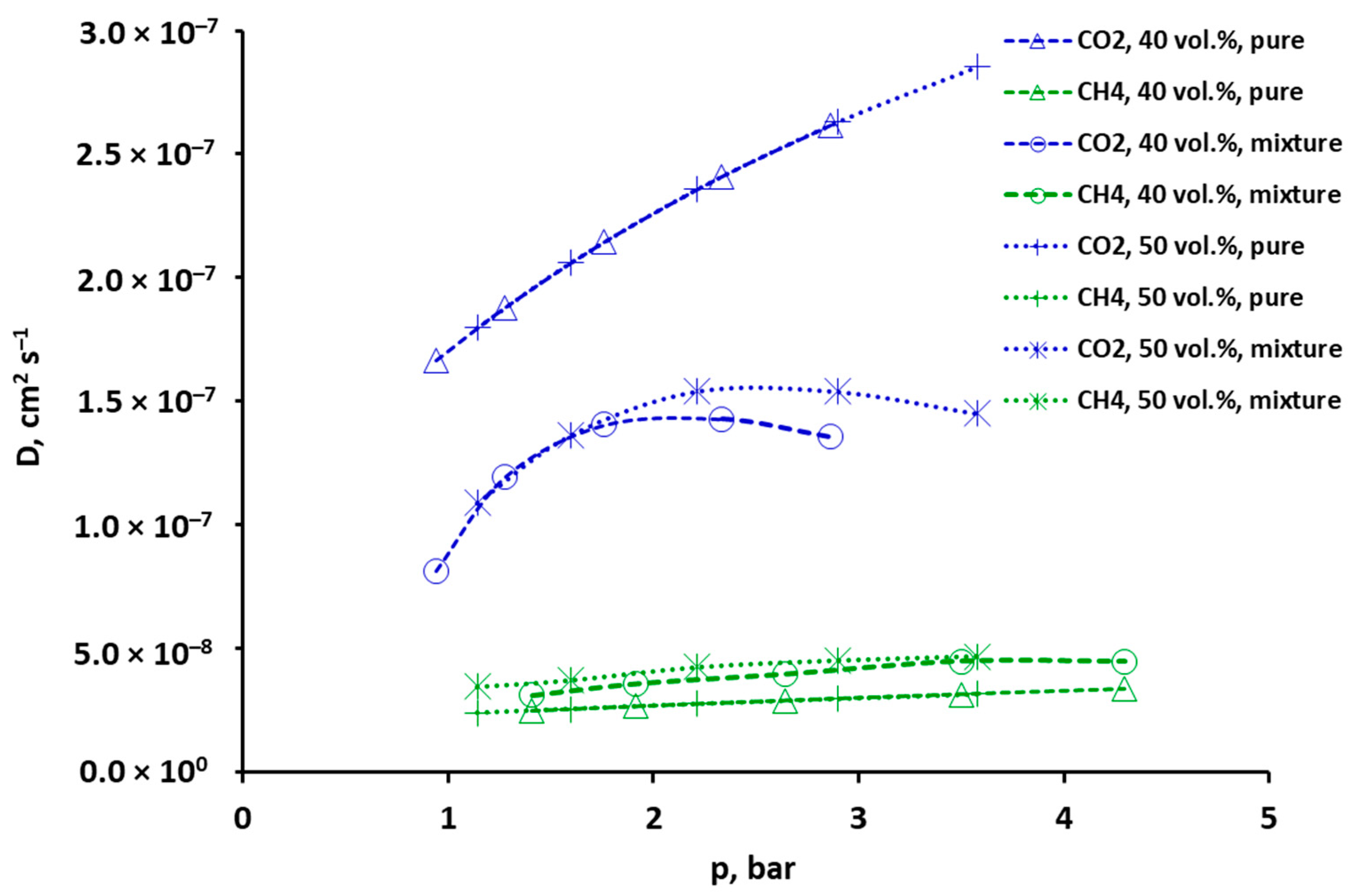
Figure 8 (as well as in
Figure S7 in the
Supplementary Material) and the values are given in
Tables S2 and S4 in the
Supplementary Material. The points in these figures correspond to the experimentally determined permeances, and the dotted lines are drawn to guide the eye. As can be noticed in
Figure 8 and
Figure S7 in the
Supplementary Material, the diffusion coefficients of pure CO
2 and pure and mixed methane increase monotonically and almost linearly with an increase in partial pressure. The diffusion of CH
4 in the mixture with CO
2 is slightly faster than that of the pure gas, with the points of both CO
2 concentrations (40 and 50 vol.%) practically aligning. In contrast, the diffusion of carbon dioxide is noticeably slower in the presence of methane. The diffusion coefficient of this gas initially increases with pressure to begin a slight decrease after reaching a maximum value. And near this maximum, the lines corresponding to both mixtures diverge.
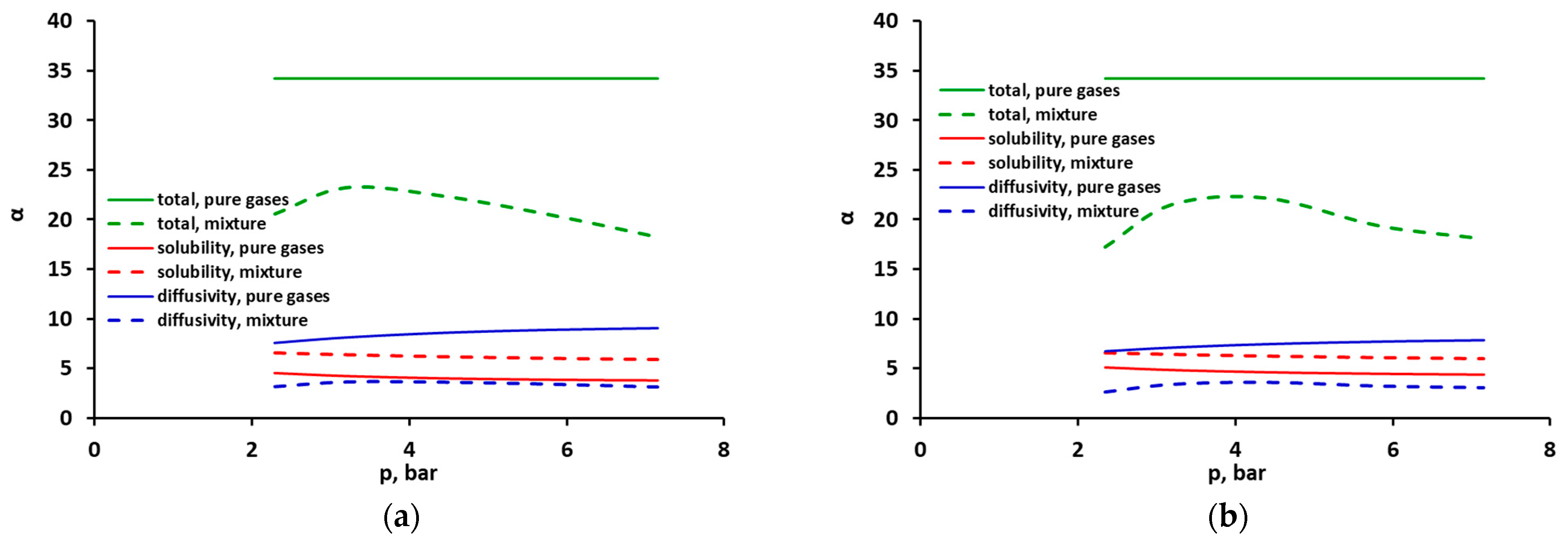
The interaction between carbon dioxide and methane in the glassy polymer is, of course, reflected in the selectivity (defined by Equation (2)), which is an important indicator that determines the efficiency of the separation process. As can be seen in
Figure 9 (and also in
Figure S8 in the
Supplementary Material), the overall permselectivity of CO
2 vs. CH
4 in the mixture in the tested polysulfone membrane is clearly lower than in the case of pure components (the ideal selectivity). It is true that there is an improvement in solubility selectivity in the mixture due to the dominance of CO
2 during adsorption in FFV. However, because of the decrease in the diffusion coefficient of this gas in the mixture, the diffusivity selectivity also decreases dramatically, which is a critical factor in the overall deterioration of the separation conditions for CO
2/CH
4 mixtures. The numerical values of the selectivity coefficients are presented in
Tables S1–S4 in the
Supplementary Material.
The experimentally determined diffusion coefficients of CO
2 and CH
4 are described using the partial immobilization model, presented in
Section 3.3. The linearized form of Equations (11) and (12), for pure and mixed gases, respectively, are shown graphically in
Figures S9–S12 in the
Supplementary Material. In this approach, the D
D coefficient, i.e., the diffusivity in the polymer matrix, is the slope of the straight line, and the ratio F of the diffusion coefficients in the Langmuir and Henry regions can be determined from the intercept (D
D∙K∙F). Both parameters are presented in
Table 2 for CO
2 and CH
4, pure and mixed, with both ways of approximating the DMS model coefficients. The average relative error in determining the diffusion coefficients of carbon dioxide and methane in the mixture was 11.8% and 5.6%, respectively, for fitting the DMS model coefficients with minimized solubility squared differences, and 19.2% and 14, 2% for the case with minimized concentration squared differences. Thus, in the former case, the accuracy of fitting the overall diffusion coefficient is clearly better, but the values of D
D and F are similar for both ways of approximating the DMS model coefficients.
As can be seen in
Table 2, for CH
4, in a mixture with carbon dioxide, the mobility in the FFV disappears (F = 0). On the other hand, the presence of methane induces the mobility of carbon dioxide in the fractional free volume (F increases from about 0 for pure CO
2 to about 0.29 or 0.35 for the mixture).
A comparison of the permeability of methane and carbon dioxide and their solubility in FFV and in the polymer matrix leads to the obvious conclusion that the former has a much lower affinity towards the tested glassy polymer. Thus, on the surface of the polymer voids, methane is likely to form a monolayer and fill the rest of the FFV while remaining in the gas phase. This would explain its mobility when it permeates alone. As a result, from the previous considerations, in the presence of CO2, methane can still occupy parts of the FFV surface, but it is probably no longer present there in the non-adsorbed phase, being displaced by carbon dioxide. In the case of the latter, the lack of mobility in the FFV in the pure state, with the strong affinity shown above towards the tested membrane, additionally indicates the possibility of CO2 condensation. On the other hand, the revealing of CO2 mobility in the FFV in the presence of methane may suggest that some carbon dioxide remains in the gas phase in this area.
When CH
4 permeates together with carbon dioxide, its diffusivity in the polymer matrix is increased (by about 6% in the case with minimized solubility squared differences). This observation would confirm the hypothesis, based on other premises, that CO
2 facilitates methane transport in the membrane matrix because it pushes the polymer chains apart [
11,
39,
40]. On the other hand, for carbon dioxide, the presence of methane means about a two-fold decrease in its diffusivity in the polymer matrix. It can be assumed that the presence of slower diffusing gas (methane) slows down the diffusion of faster diffusing gas (carbon dioxide), as was observed in [
42].
It should be noted here that the partial immobilization model does not predict phenomena that may accompany CO
2 permeation through polymer membranes, such as swelling or plasticization [
12,
13,
28,
38]. In particular, the D
D coefficient is a constant parameter in this model, and the dependence of the overall diffusion coefficient (D) on the concentration (in this case, the partial pressure) is captured by the product (Equation (11)) or sum of the products (Equation (12)) of the parameter b and the partial pressure. For gases that adsorb strongly (in this case, CH
4) or very strongly (in this case, CO
2) in the FFV, the overall value of the diffusion coefficient will theoretically tend toward D
D. Thus, in the case of changes in the diffusivity of CH
4/CO
2 mixture components compared to pure gases, it is only possible to indicate the observed trends without explicitly explaining them based on the theoretical description that is often used and also applied in this work.
As can be concluded from Equation (12), in the presence of strongly adsorbing CO
2, the total CH
4 diffusion coefficient is close to the value D
D and greater than in the case of pure gas. And this increase actually compensates for the decrease in methane solubility and keeps the CH
4 permeance in the mixture at a practically constant level compared to pure gas. It seems that when the diffusion of pure methane in the polymer matrix is hindered, it will tend to remain in the FFV (the coefficient F in Equation (11) is greater than zero for pure CH
4, cf.
Table 2). Carbon dioxide, by pushing methane out of the FFV, probably additionally forces its diffusion through the polymer matrix up to the D
D limit.