New Difunctional Derivatives of Betulin: Preparation, Characterization and Antiproliferative Potential
Abstract
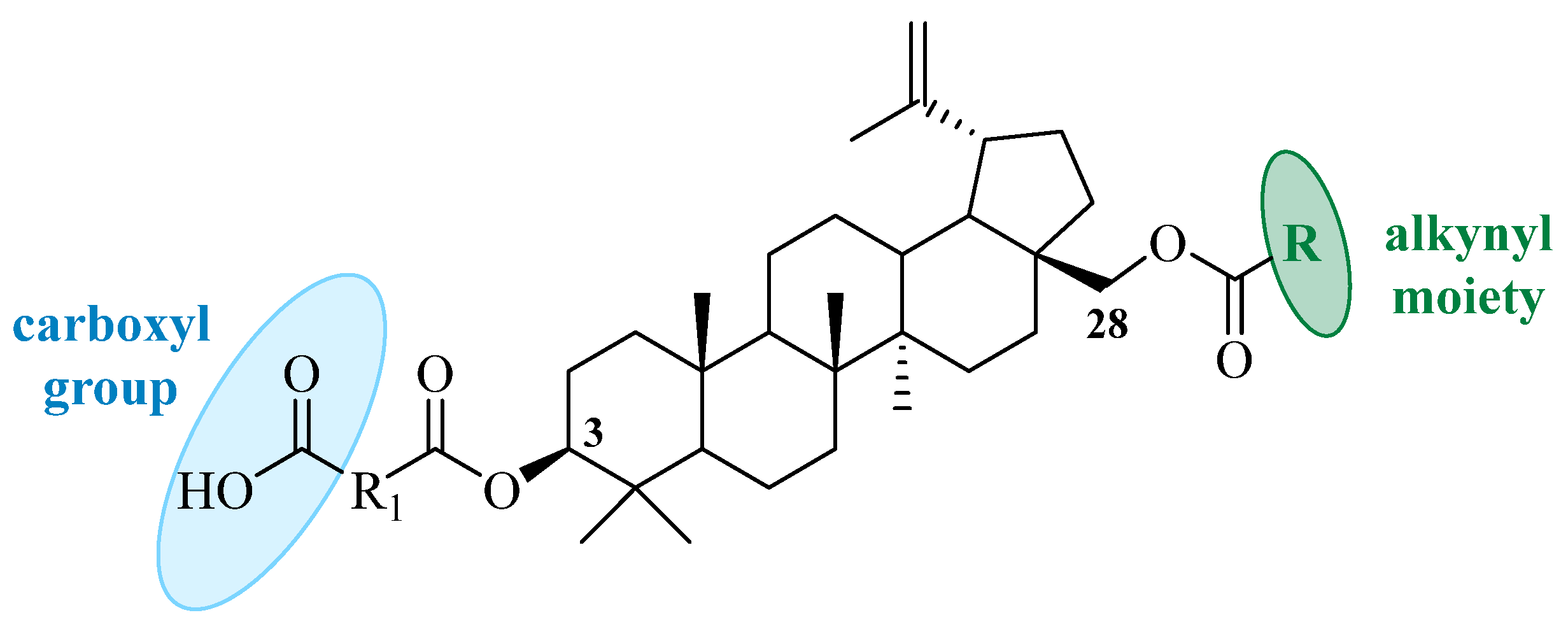
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antiproliferative Activity
2.3. Cell Cycle Study
2.4. Apoptosis Determination via Annexin V Staining
2.5. Caspase-3/7 Activity Study
2.6. Similarity to Drugs
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Procedure for the Synthesis of Betulin Derivatives
3.2.1. Procedure for the Preparation of 3-Carboxyacyl-28-O-Acetylbetulins 1-3
3.2.2. Procedure for the Preparation of 3-Carboxyacylbetulins 4-6
3.2.3. Procedure for Preparation of 3,28-Diacylbetulins 4a–f, 5a–5d, 6a, and 6b
3.3. In Vitro Studies
3.3.1. Biological Materials and Assays
3.3.2. Determination of Antiproliferative Activity
3.3.3. MTT Assay
3.3.4. SRB Assay
3.3.5. Cell Cycle Analysis
3.3.6. Apoptosis Studies Using Annexin V and Propidium Iodide Staining
3.3.7. Caspase-3/7 Activity Determination
3.3.8. Statistical Analysis
3.4. In Silico Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting Cancer Signaling Pathways by Natural Products: Exploring Promising Anti-Cancer Agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-Derived Anticancer Agents: A Green Anticancer Approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Adepoju, F.O.; Duru, K.C.; Li, E.; Kovaleva, E.G.; Tsurkan, M.V. Pharmacological Potential of Betulin as a Multitarget Compound. Biomolecules 2023, 13, 1105. [Google Scholar] [CrossRef]
- Drąg-Zalesińska, M.; Borska, S. Betulin and Its Derivatives—Precursors of New Drugs. World Sci. News 2019, 127, 123–138. [Google Scholar]
- Laiolo, J.; Graikioti, D.G.; Barbieri, C.L.; Joray, M.B.; Antoniou, A.I.; Vera, D.M.A.; Athanassopoulos, C.M.; Carpinella, M.C. Novel Betulin Derivatives as Multidrug Reversal Agents Targeting P-Glycoprotein. Sci. Rep. 2024, 14, 70. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Zou, Q.Y.; Ma, Y.H.; Morris-Natschke, S.L.; Li, X.Y.; Shi, L.C.; Ma, G.X.; Xu, X.D.; Yang, M.H.; Zhao, Z.J.; et al. Recent progress on triterpenoid derivatives and their anticancer potential. Phytochemistry 2025, 229, 114257. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Arora, S.; Singh, R. Isolation, characterization and biological activities of betulin from Acacia nilotica bark. Sci. Rep. 2022, 12, 9370. [Google Scholar] [CrossRef] [PubMed]
- Nistor, G.; Trandafirescu, C.; Prodea, A.; Milan, A.; Cristea, A.; Ghiulai, R.; Racoviceanu, R.; Mioc, A.; Mioc, M.; Ivan, V.; et al. Semisynthetic Derivatives of Pentacyclic Triterpenes Bearing Heterocyclic Moieties with Therapeutic Potential. Molecules 2022, 27, 6552. [Google Scholar] [CrossRef]
- Kuznetsova, S.A.; Shakhtshneider, T.P.; Mikhailenko, M.A.; Malyar, Y.N.; Kichkailo, A.S.; Drebushchak, V.A.; Kuznetsov, B.N. Preparation and Antitumor Activity of Betulin Dipropionate and Its Composites. Biointerface Res. Appl. Chem. 2021, 12, 6873–6894. [Google Scholar] [CrossRef]
- Prodea, A.; Milan, A.; Mioc, M.; Mioc, A.; Oprean, C.; Racoviceanu, R.; Negrea-Ghiulai, R.; Mardale, G.; Avram, Ș.; Balan-Porcărașu, M.; et al. Novel Betulin-1,2,4-Triazole Derivatives Promote In Vitro Dose-Dependent Anticancer Cytotoxicity. Processes 2024, 12, 24. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.H.; Wang, M.; Xu, G.B.; Liao, S.G. Prodrugs of Triterpenoids and Their Derivatives. Eur. J. Med. Chem. 2017, 5, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Pęcak, P.; Świtalska, M.; Chrobak, E.; Boryczka, G.; Bębenek, E. Betulin Acid Ester Derivatives Inhibit Cancer Cell Growth by Inducing Apoptosis through Caspase Cascade Activation: A Comprehensive In Vitro and In Silico Study. Int. J. Mol. Sci. 2023, 24, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, Q.; Morris-Natschke, S.L.; Chen, C.H.; Lee, K.H. Incorporation of privileged structures into bevirimat can improve activity against wild-type and bevirimat-resistant HIV-1. J. Med. Chem. 2016, 59, 9262–9268. [Google Scholar] [CrossRef]
- Shintiapina, A.B.; Shul’ts, E.E.; Petrenko, N.I.; Uzenkova, N.V.; Tolstikov, G.A.; Pronkina, N.V.; Kozhevnikov, V.S.; Pokrovskiĭ, A.G. Effect of nitrogen-containing derivatives of the plant triterpenes betulin and glycyrrhetic acid on the growth of MT-4, MOLT-4, CEM, and Hep G2 tumor cells. Russ. J. Bioorg. Chem. 2007, 33, 579–583. [Google Scholar] [CrossRef]
- Kvasnica, M.; Sarek, J.; Klinotova, E.; Dzubak, P.; Hajduch, M. Synthesis of Phthalates of Betulinic Acid and Betulin with Cytotoxic Activity. Bioorg. Med. Chem. 2005, 16, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Hosseininezhad, S.; Ramazani, A. Recent advances in the application of alkynes in multicomponent reactions. RSC Adv. 2024, 14, 278–352. [Google Scholar] [CrossRef]
- Talele, T.T. Acetylene Group, Friend or Foe in Medicinal Chemistry. J. Med. Chem. 2020, 63, 5625–5663. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Online. Database for Drug and Drug Target Info. Available online: https://go.drugbank.com/structures/search/small_molecule_drugs/structure (accessed on 9 October 2024).
- Jaroszewski, B.; Jelonek, K.; Kasperczyk, J. Drug Delivery Systems of Betulin and Its Derivatives: An Overview. Biomedicines 2024, 12, 1168. [Google Scholar] [CrossRef]
- Nazarov, M.A.; Polovnikova, A.A.; Tolmacheva, I.A. Synthesis of Betulin Conjugates and Its 1,2,3-Triazole Derivatives. Polym. Sci. Ser. D 2023, 16, 543–548. [Google Scholar] [CrossRef]
- Özdemir, Z.; Rybková, M.; Vlk, M.; Šaman, D.; Rárová, L.; Wimmer, Z. Synthesis and Pharmacological Effects of Diosgenin–Betulinic Acid Conjugates. Molecules 2020, 25, 3546. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Zhang, X.; Wang, J.; Li, K.; Liu, G.; Lu, K.; Zhang, X.; Xie, C.; Zheng, T.; et al. Synthesis and Biological Evaluation of Novel Allobetulon/Allobetulin–Nucleoside Conjugates as AntitumorAgents. Molecules 2022, 27, 4738. [Google Scholar] [CrossRef]
- Grymel, M.; Pastuch-Gawołek, G.; Lalik, A.; Zawojak, M.; Boczek, S.; Krawczyk, M.; Erfurt, K. Glycoconjugation of Betulin Derivatives Using Copper-Catalyzed 1,3-Dipolar Azido-Alkyne Cycloaddition Reaction and a Preliminary Assay of Cytotoxicity of the Obtained Compounds. Molecules 2020, 25, 6019. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Xu, X.; Liu, J.; Jia, Q.; Ke, C.; Zhang, H.; Xu, C.; Ou, E.; Tan, W.; Zhao, Y. Mitochondria-Targeted Triphenylphosphonium Conjugated C-3 Modified Betulin: Synthesis, Antitumor Properties and Mechanism of Action. Chem. Med. Chem. 2022, 16, e202100659. [Google Scholar] [CrossRef]
- Xiong, J.; Kashiwada, Y.; Chen, C.H.; Qian, K.; Morris-Natschke, S.L.; Lee, K.H.; Takaishi, Y. Conjugates of Betulin Derivatives with AZT as Potent Anti-HIV Agents. Bioorg. Med. Chem. 2010, 18, 6451–6469. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Sekiya, M.; Ikeshiro, Y.; Fujioka, T.; Kilgore, N.R.; Wild, C.T.; Allaway, G.P.; Lee, K.H. 3-O-Glutaryl-Dihydrobetulin and Related Monoacyl Derivatives as Potent Anti-HIV Agents. Bioorg. Med. Chem. Lett. 2004, 14, 5851–5853. [Google Scholar] [CrossRef]
- Gauthier, C.; Legault, J.; Lebrun, M.; Dufour, P.; Pichette, A. Glycosidation of lupane-type triterpenoids as potent in vitro cytotoxic agents. Bioorg. Med. Chem. 2006, 14, 6713–6725. [Google Scholar] [CrossRef] [PubMed]
- Marciniec, K.; Chrobak, E.; Dąbrowska, A.; Bębenek, E.; Kadela-Tomanek, M.; Pęcak, P.; Boryczka, S. Phosphate Derivatives of 3-Carboxyacylbetulin: Synthesis, In Vitro Anti-HIV and Molecular Docking Study. Biomolecules 2020, 10, 1148. [Google Scholar] [CrossRef]
- Talele, T.T. Natural Products Inspired Use of the Gem Dimethyl Group in Medicinal Chemistry. J. Med. Chem. 2018, 61, 2166–2210. [Google Scholar] [CrossRef]
- Ressmann, A.K.; Schneider, M.; Gaertner, P.; Weil, M.; Bica, K. Design and synthesis of basic ionic liquids for the esterification of triterpenic acids. Monatsh. Chem. 2017, 148, 139–148. [Google Scholar] [CrossRef]
- Chrobak, E.; Marciniec, K.; Dąbrowska, A.; Pęcak, P.; Bębenek, E.; Kadela-Tomanek, M.; Bak, A.; Jastrzębska, M.; Boryczka, S. New Phosphorus Analogs of Bevirimat: Synthesis, Evaluation of Anti-HIV-1 Activity and Molecular Docking Study. Int. J. Mol. Sci. 2019, 20, 5209. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Sczepek, R.; Siewert, B.; Nitsche, C. Cytotoxic betulin-derived hydroxypropargylamines trigger apoptosis. Bioorg. Med. Chem. 2013, 21, 425–435. [Google Scholar] [CrossRef]
- Vasilevsky, S.F.; Govdi, A.I.; Shults, E.E.; Shakirov, M.M.; Sorokina, I.V.; Tolstikova, T.G.; Baev, D.S.; Tolstikov, G.A.; Alabugin, I.V. Efficient synthesis of the first betulonic acid–acetylene hybrids and their hepatoprotective and anti-inflammatory activity. Bioorg. Med. Chem. 2009, 17, 5164–5169. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.B.; Giniyatullina, G.V.; Yamansarov, E.Y.; Tolstikov, G.A. Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorg. Med. Chem. Lett. 2010, 20, 4088–4090. [Google Scholar] [CrossRef]
- Chrobak, E.; Świtalska, M.; Wietrzyk, J.; Bębenek, E. Synthesis, Structure, and In Vitro Biological Evaluation of Semi-Synthetic Derivatives of Betulin. Appl. Sci. 2024, 14, 9970. [Google Scholar] [CrossRef]
- Csuk, R.; Barthel, A.; Sczepek, R.; Siewert, B.; Schwarz, S. Synthesis, encapsulation and antitumor activity of new betulin derivatives. Arch. Pharm. Chem. Life Sci. 2011, 1, 37–49. [Google Scholar] [CrossRef]
- Csuk, R.; Barthel, A.; Kluge, R.; Ströhl, D. Synthesis, cytotoxicity and liposome preparation of 28-acetylenic betulin derivatives. Bioorg. Med. Chem. 2010, 18, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y.H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhang, T.; Yuan, H.; Li, D.; Lou, H.; Fan, P. Mitochondria-targeted lupane triterpenoid derivatives and their selective apoptosis-inducing anticancer mechanisms. J. Med. Chem. 2017, 60, 6353–6363. [Google Scholar] [CrossRef]
- Zhuo, Z.J.; Xiao, M.J.; Lin, H.R.; Luo, J.; Wang, T. Novel betulin derivative induces anti-proliferative activity by G2/M phase cell cycle arrest and apoptosis in Huh7 cells. Oncol. Lett. 2018, 15, 2097–2104. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Han, Y.; Zhang, J.; Lin, Y.; Wang, H.; Wang, J.; Liu, J.; Bu, M. Design and Synthesis of Novel Betulin Derivatives Containing Thio-/Semicarbazone Moieties as Apoptotic Inducers through Mitochindria-Related Pathways. Molecules 2021, 26, 6356. [Google Scholar] [CrossRef] [PubMed]
- Agoni, C.; Olotu, F.A.; Ramharack, P.; Soliman, M.E. Druggability and drug-likeness concepts in drug design: Are biomodelling and predictive tools having their say? J. Mol. Model. 2020, 26, 120. [Google Scholar] [CrossRef]
- Protti, Í.F.; Rodrigues, D.R.; Fonseca, S.K.; Alves, R.J.; de Oliveira, R.B.; Maltarollo, V.G. Do Drug-likeness Rules Apply to Oral Prodrugs? Chem. Med. Chem. 2021, 16, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Czarnecka, M.; Świtalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis, characterization, and in vitro cancer cell growth inhibition evaluation of novel phosphatidylcholines with anisic and veratric acids. Molecules 2018, 23, 2022. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]








| Compound | IC50 [µM] | |||||||
|---|---|---|---|---|---|---|---|---|
| MV4-11 | A549 | MCF-7 | PC-3 | HCT116 | MiaPaca-1 | Hs249T | MCF-10A | |
| 1 | 2.90 ± 0.26 | 4.94 ± 0.48 | 7.02 ± 2.39 | 14.04 ± 7.02 | 6.38 ± 2.87 | 23.61 ± 3.83 | 11.64 ± 1.12 | 33.18 ± 1.91 |
| 2 | 4.40 ± 1.63 | 4.57 ± 1.79 | 16.64 ± 7.67 | 20.07 ± 2.12 | 10.93 ± 2.94 | 28.23 ± 4.40 | 27.57 ± 0.65 | 33.94 ± 4.24 |
| 3 | 3.93 ± 1.54 | 5.13 ± 0.85 | 17.27 ± 6.33 | 22.40 ± 2.56 | 13.16 ± 1.02 | 34.54 ± 5.47 | 21.88 ± 4.27 | 33.51 ± 1.88 |
| 4 | 4.77 ± 0.44 | 3.76 ± 2.05 | 12.14 ± 3.08 | 19.15 ± 3.76 | 11.11 ± 2.56 | 22.57 ± 6.50 | 24.45 ± 8.89 | 31.46 ± 1.71 |
| 4a | 0.38 ± 0.14 | 7.54 ± 1.25 | 16.48 ± 1.88 | 0.86 ± 0.25 | 7.85 ± 2.35 | 7.54 ± 0.47 | 0.49 ± 0.23 | 33.13 ± 5.18 |
| 4b | 3.19 ± 1.29 | 4.06 ± 1.05 | 11.73 ± 2.41 | 11.58 ± 4.51 | 7.82 ± 2.86 | 24.51 ± 2.56 | 10.68 ± 1.50 | 32.63 ± 2.41 |
| 4c | 3.23 ± 1.38 | 7.37 ± 2.30 | 16.59 ± 2.15 | 10.14 ± 2.46 | 8.45 ± 2.15 | 22.74 ± 5.53 | 9.68 ± 1.54 | 32.57 ± 2.15 |
| 4d | 5.15 ± 0.47 | 6.24 ± 1.25 | 20.59 ± 5.77 | 21.68 ± 4.52 | 16.85 ± 5.61 | 34.17 ± 2.96 | 18.72 ± 9.67 | 60.38 ± 15.91 |
| 4e | 3.16 ± 0.88 | 59.82 ± 22.16 | 25.70 ± 4.43 | 15.21 ± 5.91 | 23.19 ± 5.91 | 67.06 ± 14.48 | 19.79 ± 5.61 | 67.95 ± 34.86 |
| 4f | 46.56 ± 11.78 | Neg | 130.71 ± 8.83 | 95.79 ± 27.07 | Neg | Neg | Neg | Neg |
| 5 | 7.81 ± 2.80 | 9.11 ± 0.87 | 29.25 ± 3.85 | 26.97 ± 3.85 | 14.71 ± 4.55 | 30.65 ± 2.63 | 30.83 ± 1.40 | 26.80 ± 2.10 |
| 5a | 0.600 ± 0.175 | 10.72 ± 3.0 | 19.43 ± 3.1 | 0.85 ± 0.17 | 3.64 ± 0.6 | - | - | 12.7 ± 0.5 |
| 5b | 4.76 ± 0.92 | 4.15 ± 0.92 | 12.29 ± 2.92 | 21.05 ± 1.54 | 9.06 ± 2.92 | 28.42 ± 3.23 | 16.13 ± 4.76 | 33.80 ± 3.38 |
| 5c | 10.99 ± 1.10 | 6.59 ± 1.88 | 24.96 ± 4.87 | 22.92 ± 4.71 | 9.26 ± 0.47 | 32.03 ± 6.12 | 23.24 ± 3.77 | 32.03 ± 4.87 |
| 5d | 2.50 ± 0.48 | 4.62 ± 1.59 | 21.05 ± 9.89 | 16.59 ± 6.54 | 12.28 ± 4.15 | 29.19 ± 1.59 | 59.66 ± 38.60 | 34.13 ± 4.94 |
| 6 | 6.45 ± 2.03 | 8.66 ± 1.10 | 18.97 ± 6.45 | 26.16 ± 5.71 | 20.26 ± 5.53 | 38.13 ± 3.87 | 32.06 ± 1.66 | 31.87 ± 4.24 |
| 6a | 2.57 ± 1.01 | 12.94 ± 0.67 | 15.97 ± 1.01 | 0.41 ± 0.08 | 20.68 ± 9.08 | 18.49 ± 9.75 | 3.53 ± 0.67 | 49.42 ± 17.82 |
| 6b | 3.53 ± 0.64 | 3.21 ± 0.64 | 11.40 ± 4.17 | 13.16 ± 2.57 | 9.95 ± 0.64 | 27.45 ± 2.57 | 17.50 ± 6.26 | 30.66 ± 3.69 |
| A | 41.05 ± 8.46 | 27.84 ± 10.52 | 72.40 ± 6.60 | 80.65 ± 13.41 | 40.64 ± 13.61 | 126.86 ± 16.30 | 127.69 ± 14.23 | 123.98 ± 33.00 |
| B | 32.75 ± 12.87 | 14.00 ± 1.13 | 43.59 ± 15.81 | 51.50 ± 9.94 | 37.72 ± 7.45 | 144.33 ± 22.13 | 58.50 ± 15.58 | 98.48 ± 0.90 |
| C a | 0.021 ± 0.008 | 0.040 ± 0.012 | 0.150 ± 0.015 | 1.27 ± 0.2 | 0.118 ± 0.02 | - | - | 0.114 ± 0.013 |
| Compound | SI | ||||||
|---|---|---|---|---|---|---|---|
| MV4-11 | A549 | MCF-7 | PC-3 | HCT116 | MiaPaca-1 | Hs249T | |
| 1 | 11.44 | 6.71 | 4.73 | 2.36 | 5.20 | 1.41 | 2.85 |
| 2 | 7.70 | 7.43 | 2.04 | 1.69 | 3.10 | 1.20 | 1.23 |
| 3 | 8.82 | 6.53 | 1.94 | 1.50 | 2.55 | 0.97 | 1.53 |
| 4 | 6.60 | 8.36 | 2.60 | 1.64 | 2.83 | 1.39 | 1.29 |
| 4a | 98.92 | 4.40 | 2.01 | 38.57 | 4.22 | 4.40 | 67.0 |
| 4b | 10.20 | 8.04 | 2.78 | 2.82 | 4.17 | 1.33 | 3.06 |
| 4c | 10.10 | 4.42 | 1.96 | 3.21 | 3.85 | 1.43 | 3.37 |
| 4d | 11.73 | 9.68 | 2.93 | 2.78 | 3.58 | 1.77 | 3.23 |
| 4e | 21.50 | 1.14 | 2.60 | 4.47 | 2.93 | 1.01 | 3.40 |
| 5 | 3.43 | 2.94 | 0.92 | 1.01 | 1.82 | 0.87 | 0.87 |
| 5a | 21.17 | 1.18 | 0.65 | 14.94 | 3.49 | - | - |
| 5b | 7.10 | 8.15 | 2.75 | 1.61 | 3.73 | 1.19 | 2.10 |
| 5c | 2.91 | 4.86 | 1.28 | 1.40 | 3.46 | 1.00 | 1.38 |
| 5d | 13.63 | 7.38 | 1.62 | 2.06 | 2.78 | 1.17 | 0.57 |
| 6 | 4.94 | 3.68 | 1.68 | 1.22 | 1.57 | 0.84 | 0.99 |
| 6a | 19.22 | 3.82 | 3.09 | 121.0 | 2.39 | 2.67 | 14.0 |
| 6b | 8.68 | 9.55 | 2.69 | 2.33 | 3.08 | 1.12 | 1.75 |
| 28-O-acetylbetulin | 3.02 | 4.45 | 1.71 | 1.54 | 3.05 | 0.98 | 0.97 |
| betulin | 3.01 | 7.03 | 2.26 | 1.91 | 2.61 | 0.68 | 1.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrobak, E.; Świtalska, M.; Wietrzyk, J.; Bębenek, E. New Difunctional Derivatives of Betulin: Preparation, Characterization and Antiproliferative Potential. Molecules 2025, 30, 611. https://doi.org/10.3390/molecules30030611
Chrobak E, Świtalska M, Wietrzyk J, Bębenek E. New Difunctional Derivatives of Betulin: Preparation, Characterization and Antiproliferative Potential. Molecules. 2025; 30(3):611. https://doi.org/10.3390/molecules30030611
Chicago/Turabian StyleChrobak, Elwira, Marta Świtalska, Joanna Wietrzyk, and Ewa Bębenek. 2025. "New Difunctional Derivatives of Betulin: Preparation, Characterization and Antiproliferative Potential" Molecules 30, no. 3: 611. https://doi.org/10.3390/molecules30030611
APA StyleChrobak, E., Świtalska, M., Wietrzyk, J., & Bębenek, E. (2025). New Difunctional Derivatives of Betulin: Preparation, Characterization and Antiproliferative Potential. Molecules, 30(3), 611. https://doi.org/10.3390/molecules30030611






