Sustainable Supercritical Carbon Dioxide Extraction of Value-Added Lignan from Sesame Meal: Achieving Green Neuroprotection and Waste Valorization by Optimizing Temperature, Solvent, and Pressure
Abstract
1. Introduction
2. Results
2.1. Investigation of Parameters for Supercritical Carbon Dioxide Extraction
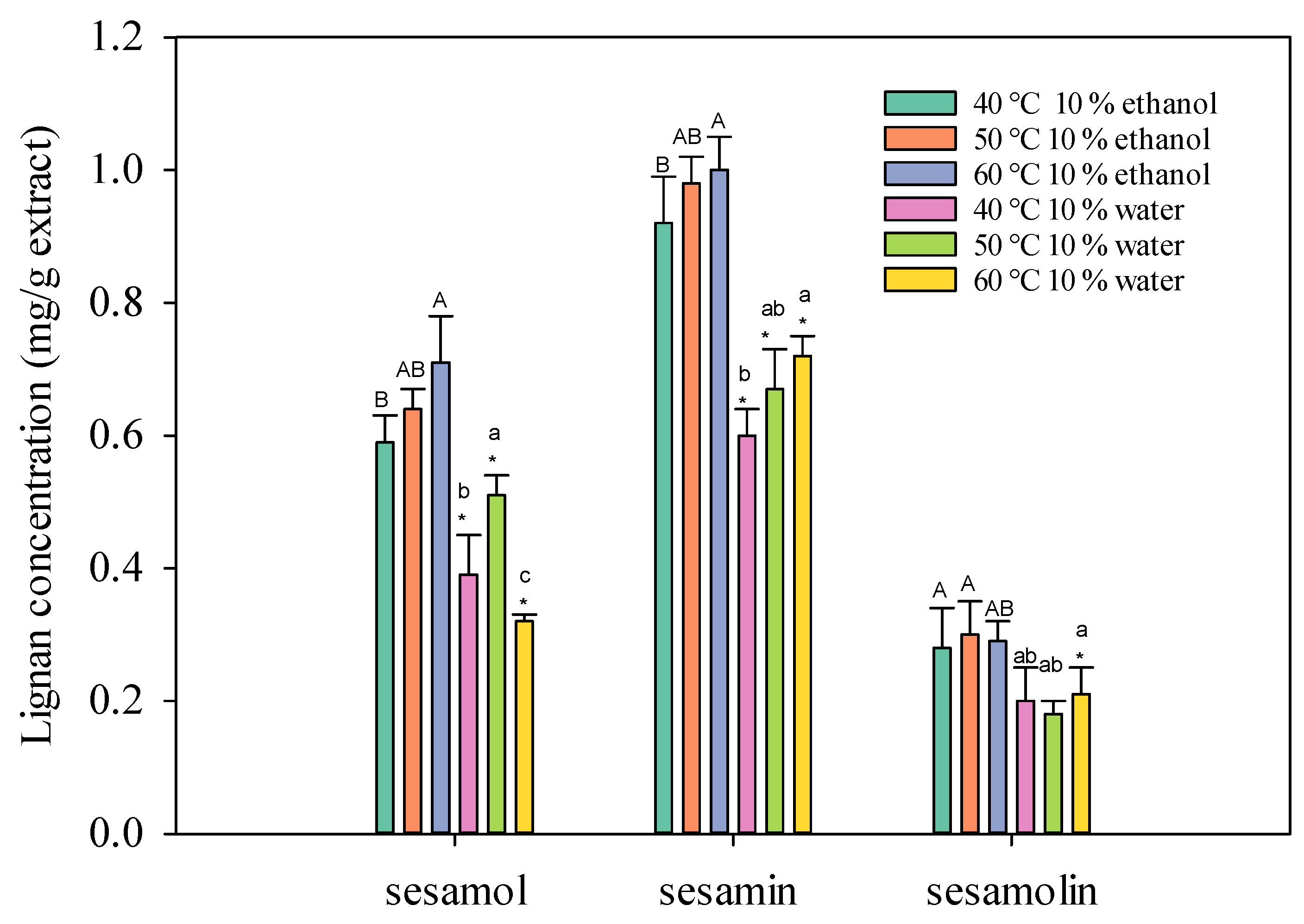
2.1.1. Effect of Supercritical Carbon Dioxide Extraction Temperature on Sesame Lignans
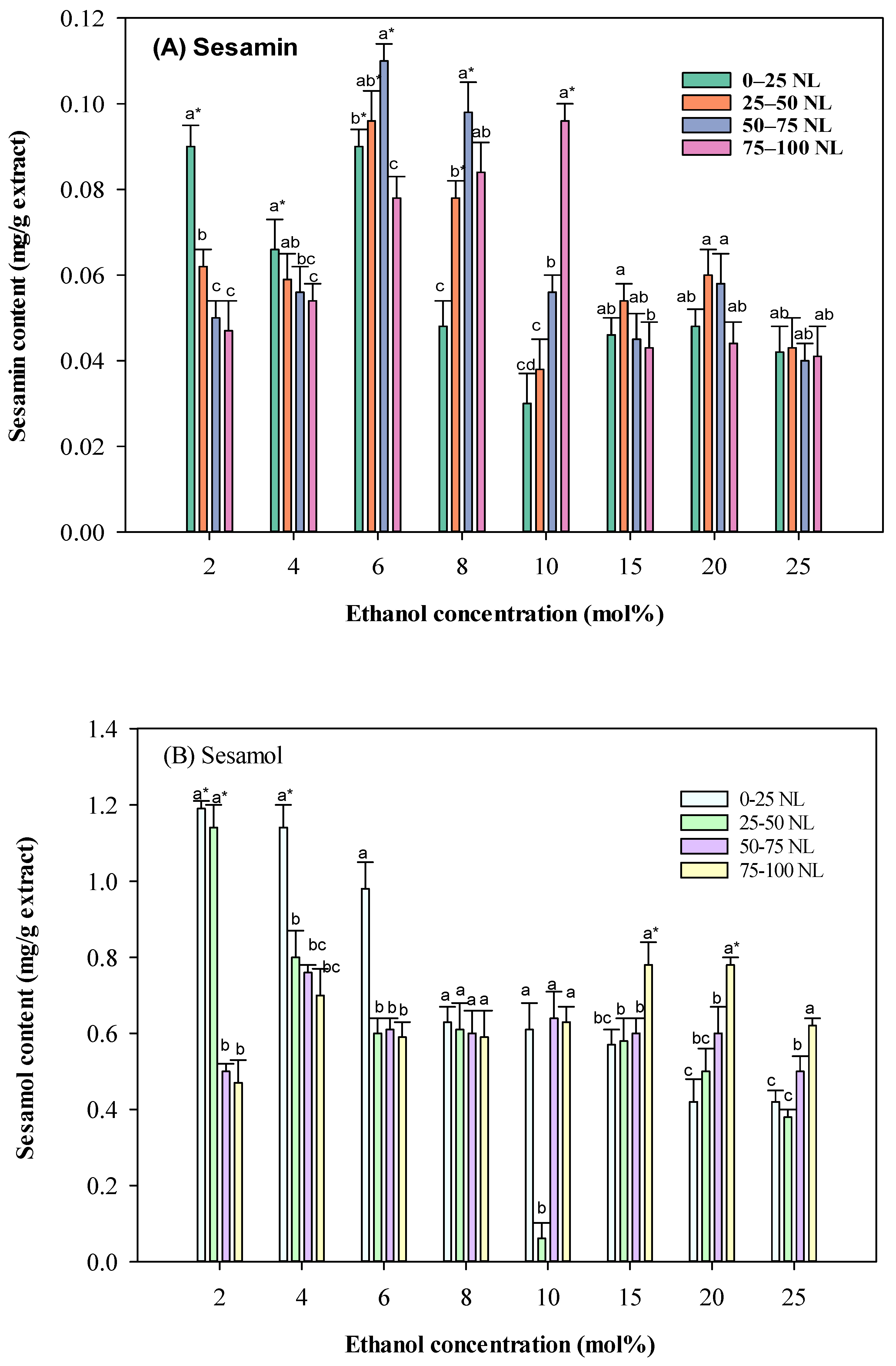
2.1.2. Effects of Different Co-Solvent Concentrations and Carbon Dioxide Consumption on the Supercritical Fluid Extraction of Sesame Lignans
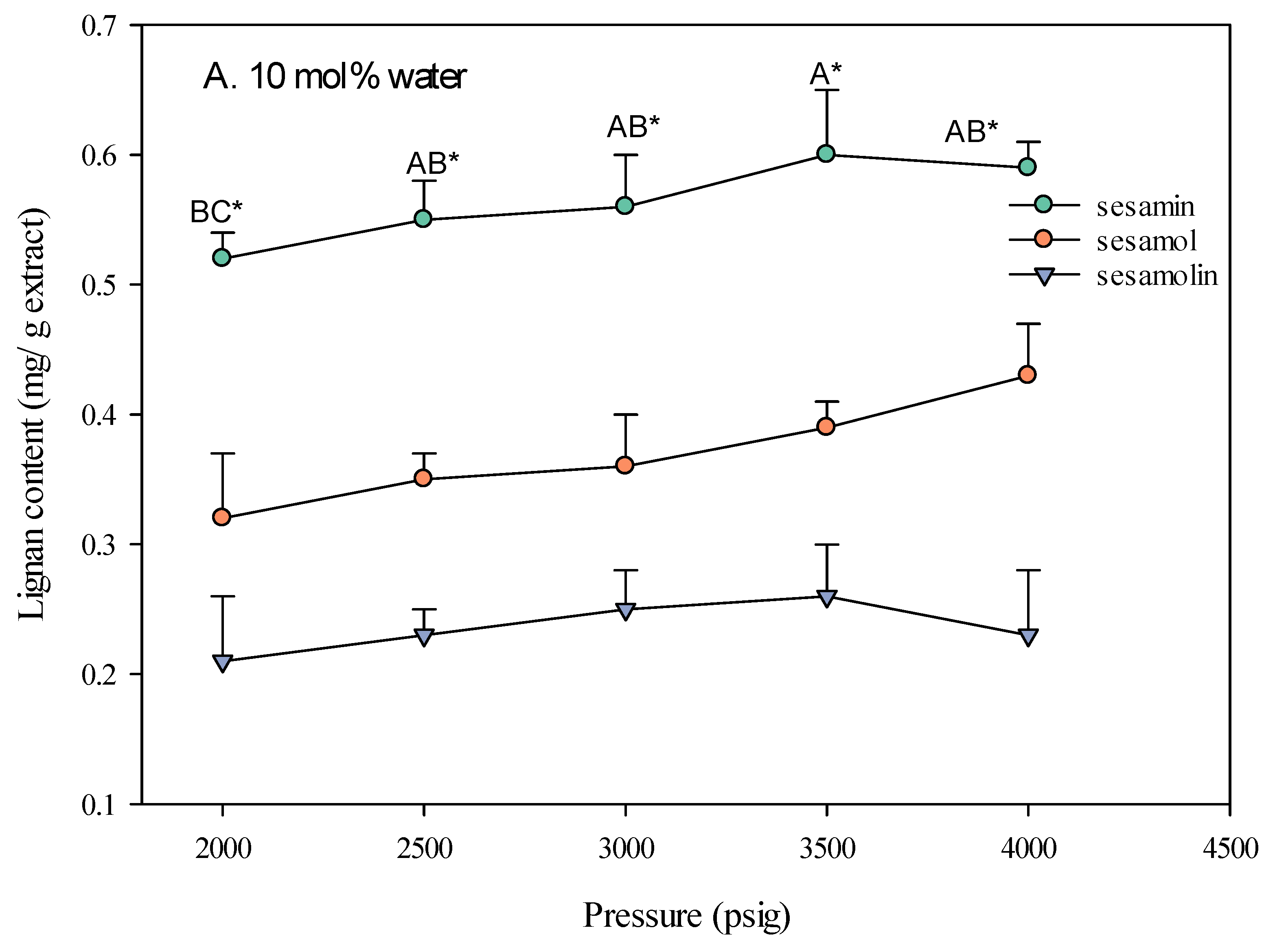
2.1.3. The Influence of Pressure on SC-CO2 Extraction of Sesame Lignans Yield
2.2. HPLC Results
2.3. Evaluation of the Bioactivity (LDH Release) of Supercritical Fluid Extraction of Sesame Lignans
2.4. Sustainability Impacts of the Results
3. Materials and Methods
3.1. Materials and Chemicals
3.1.1. Reagents and Chemicals
3.1.2. Sesamolin Isolation and Structural Characterization
3.2. Supercritical Carbon Dioxide Extraction of Sesame Lignans
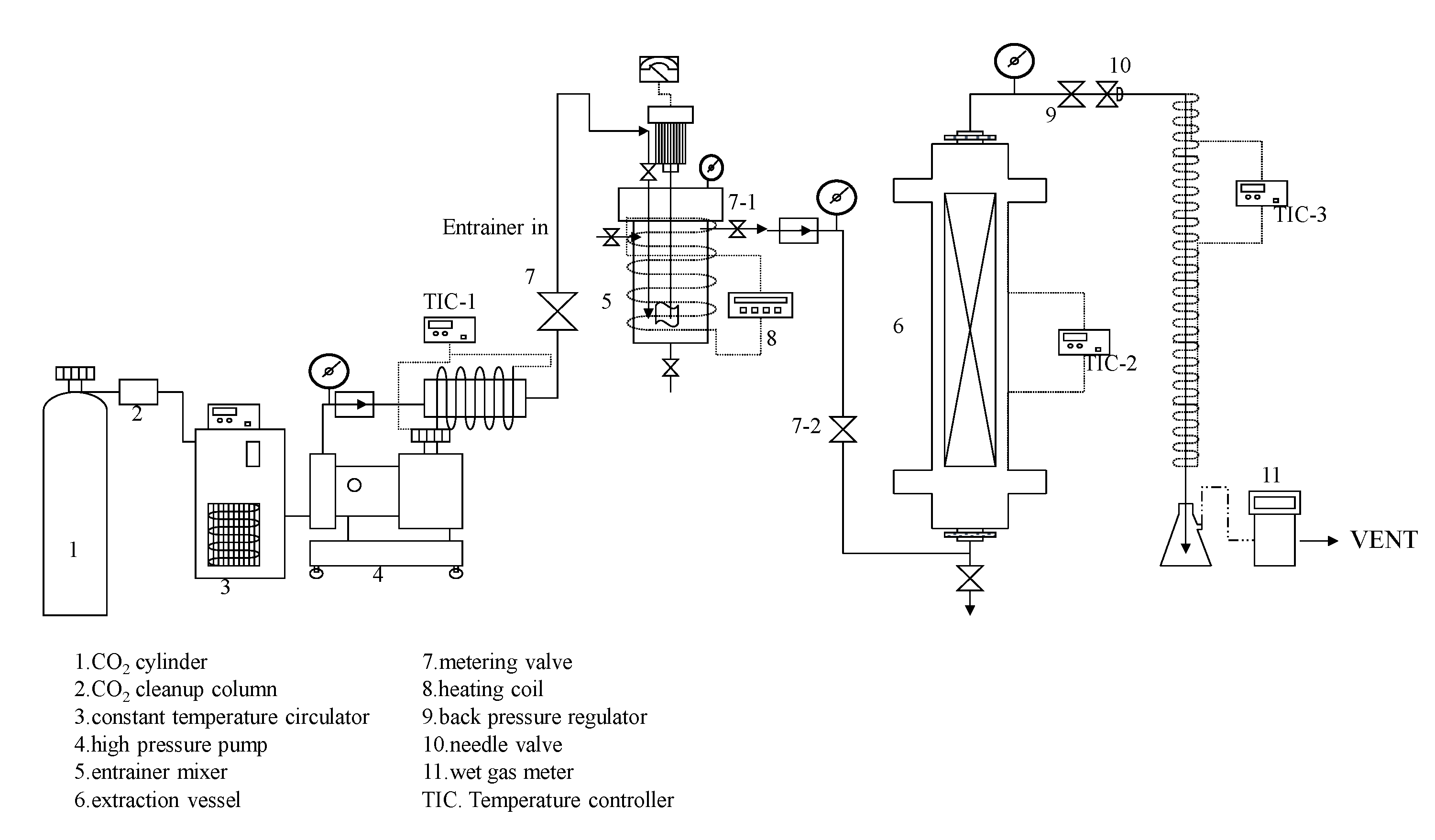
3.2.1. Supercritical Carbon Dioxide Extraction System
3.2.2. Supercritical CO2 Extraction with Co-Solvent Method
3.3. High-Performance Liquid Chromatography (HPLC) of Lignans
3.4. Ischemic Stroke In Vitro Model and Treatments
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nair, A.B.; Dalal, P.; Kadian, V.; Kumar, S.; Garg, M.; Rao, R.; Almuqbil, R.M.; Alnaim, A.S.; Aldhubiab, B.; Alqattan, F. Formulation strategies for enhancing pharmaceutical and nutraceutical potential of sesamol: A natural phenolic bioactive. Plants 2023, 12, 1168. [Google Scholar] [CrossRef] [PubMed]
- Doungwichitrkul, T.; Damsud, T.; Phuwapraisirisan, P. α-Glucosidase inhibitors from cold-pressed black sesame (Sesamum indicum) meal: Characterization of new furofuran lignans, kinetic study, and in vitro gastrointestinal digestion. J. Agric. Food Chem. 2024, 72, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.J.; Zu, H.D.; Lee, J.; Kang, M.C.; Park, J.; Song, K.M.; Lee, N.H. Development of an ultrasonic system for industrial extraction of unheated sesame oil cake. Food Chem. 2021, 354, 129582. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Sheng, J.; Wei, Y.; Sun, Z.; Xiao, X.; Zhang, L. Sesamol augments paclitaxel-induced apoptosis in human cervical cancer cell lines. Nutr. Cancer 2022, 74, 3692–3700. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Ahmed, H.I.; Zaky, H.S.; Badr, A.M. Sesame oil mitigates memory impairment, oxidative stress, and neurodegeneration in a rat model of Alzheimer’s disease. A pivotal role of NF-ΚB/P38MAPK/BDNF/PPAR-γ pathways. J. Ethnopharmacol. 2021, 267, 113468. [Google Scholar] [CrossRef]
- Mehta, S.; Kadian, V.; Dalal, S.; Dalal, P.; Kumar, S.; Garg, M.; Rao, R. A fresh look on bergenin: Vision of its novel drug delivery systems and pharmacological activities. Future Pharmacol. 2022, 2, 64–91. [Google Scholar] [CrossRef]
- Gupta, B.; Dalal, P.; Rao, R. Cyclodextrin decorated nanosponges of sesamol: Antioxidant, anti-tyrosinase and photostability assessment. Food Biosci. 2021, 42, 101098. [Google Scholar] [CrossRef]
- Jan, K.C.; Wang, T.Y.; Sun Hwang, L.; Gavahian, M. Biotransformation of sesaminol triglycoside by intestinal microflora of swine supplemented with probiotic or antibiotic diet. Qual. Assur. Saf. Crops Foods 2022, 14, 19–29. [Google Scholar] [CrossRef]
- Chaosuan, N.; Phimolsiripol, Y.; Gavahian, M. Sustainable electrical-based technologies for extraction and modification of pectin from agri-food waste. Innov. Food Sci. Emerg. Technol. 2024, 96, 103779. [Google Scholar] [CrossRef]
- Makoter, T.Ž.; Kotnik, P.; Makoter, T.; Knez, Ž.; Marevci, M.K. Optimization of the supercritical extraction and decarboxylation process of industrial hemp. J. CO2 Util. 2025, 91, 103007. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Verma, D.K.; Thakur, M.; Tripathy, S.; Patel, A.R.; Shah, N.; Utama, G.L.; Srivastav, P.P.; Benavente-Valdés, J.R.; Chávez-González, M.L.; et al. Supercritical fluid extraction (SCFE) as green extraction technology for high-value metabolites of algae, its potential trends in food and human health. Food Res. Int. 2021, 150, 110746. [Google Scholar] [CrossRef] [PubMed]
- Dashtian, K.; Kamalabadi, M.; Ghoorchian, A.; Ganjali, M.R.; Rahimi-Nasrabadi, M. Integrated supercritical fluid extraction of essential oils. J. Chromatogr. A 2024, 1733, 465240. [Google Scholar] [CrossRef]
- Qamar, S.; Torres, Y.J.M.; Parekh, H.S.; Falconer, J.R. Effects of ethanol on the supercritical carbon dioxide extraction of cannabinoids from near equimolar (THC and CBD Balanced) cannabis flower. Separations 2021, 8, 154. [Google Scholar] [CrossRef]
- Buelvas-Puello, L.M.; Franco-Arnedo, G.; Martínez-Correa, H.A.; Ballesteros-Vivas, D.; Sánchez-Camargo, A.D.P.; Miranda-Lasprilla, D.; Narváez-Cuenca, C.E.; Parada-Alfonso, F. Supercritical fluid extraction of phenolic compounds from mango (Mangifera indica L.) seed kernels and their application as an antioxidant in an edible oil. Molecules 2021, 26, 7516. [Google Scholar] [CrossRef]
- Villacís-Chiriboga, J.; Vera, E.; Van Camp, J.; Ruales, J.; Elst, K. Valorization of byproducts from tropical fruits: A review, Part 2: Applications, economic, and environmental aspects of biorefinery via supercritical fluid extraction. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2305–2331. [Google Scholar] [CrossRef]
- Atwi-Ghaddar, S.; Zerwette, L.; Destandau, E.; Lesellier, E. Supercritical fluid extraction (SFE) of polar compounds from camellia sinensis leaves: Use of ethanol/water as a green polarity modifier. Molecules 2023, 28, 5485. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A critical comparison of the advanced extraction techniques applied to obtain health-promoting compounds from seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Wu, D.; Ge, D.; Dai, Y.; Chen, Y.; Fu, Q.; Jin, Y. Extraction and isolation of diphenylheptanes and flavonoids from Alpinia officinarum Hance using supercritical fluid extraction followed by supercritical fluid chromatography. J. Sep. Sci. 2023, 46, e2300156. [Google Scholar] [CrossRef]
- Cao, P.H.; Zhang, C.X.; Ma, Y.X.; Yu, Y.M.; Liu, H.M.; Wang, X.D.; Zheng, Y.Z. Extraction of protein from sesame meal: Impact of deep eutectic solvents on protein structure and functionality. LWT 2023, 187, 115366. [Google Scholar] [CrossRef]
- Fraguela-Meissimilly, H.; Bastías-Monte, J.M.; Vergara, C.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Flores, M.; Toledo-Merma, P.; Alcázar-Alay, S.; Gallón-Bedoya, M. New trends in supercritical fluid technology and pressurized liquids for the extraction and recovery of bioactive compounds from agro-industrial and marine food waste. Molecules 2023, 28, 4421. [Google Scholar] [CrossRef] [PubMed]
- Radzali, S.A.; Markom, M.; Md Saleh, N. Parameter effects and optimisation in supercritical fluid extraction of phenolic compounds from Labisia pumila. Separations 2022, 9, 385. [Google Scholar] [CrossRef]
- Srisongkram, T.; Weerapreeyakul, N. Route of intracellular uptake and cytotoxicity of sesamol, sesamin, and sesamolin in human melanoma SK-MEL-2 cells. Biomed. Pharmacother. 2022, 146, 112528. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Chuang, C.H.; Rejano, C.J.F.; Tayo, L.L.; Hsieh, C.Y.; Huang, S.K.H.; Tsai, P.W. Sesamin: A promising therapeutic agent for ameliorating symptoms of diabetes. Molecules 2023, 28, 7255. [Google Scholar] [CrossRef]
- Mostashari, P.; Mousavi Khaneghah, A. Sesame seeds: A nutrient-rich superfood. Foods 2024, 13, 1153. [Google Scholar] [CrossRef]
- Ramazani, E.; Ebrahimpour, F.; Emami, S.A.; Shakeri, A.; Javadi, B.; Sahebkar, A.; Tayarani-Najaran, Z. Neuroprotective effects of Sesamum indicum, sesamin and sesamolin against 6-OHDA-induced apoptosis in PC12 cells. Recent. Adv. Food Nutr. Agric. 2023, 14, 126–133. [Google Scholar] [CrossRef]
- Ghaderi, M.A.; Emami, S.A.; Beirak Olia, M.D.; Javadi, B. The Role of Sesamin in Targeting Neurodegenerative Disorders: A Systematic Review. Mini. Rev. Med. Chem. 2023, 23, 756–770. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R.; Dehpour, A.R.; Azhdarzadeh, M.; Dinarvand, M. Application of nanostructured lipid carriers: The prolonged protective effects for sesamol in in vitro and in vivo models of ischemic stroke via activation of PI3K signalling pathway. Daru 2017, 25, 25. [Google Scholar] [CrossRef]
- Gavahian, M. Opinion on the prospects of emerging food processing technologies to achieve sustainability in the industry by reduced energy consumption, waste reduction and valorisation, and improved food nutrition. Int. J. Food Sci. Tech. 2024, 59, 8135–8140. [Google Scholar] [CrossRef]
- Montiel, I.; Cuervo-Cazurra, A.; Park, J.; Antolín-López, R.; Husted, B.W. Implementing the United Nations’ sustainable development goals in international business. J. Int. Bus. Stud. 2021, 52, 999–1030. [Google Scholar] [CrossRef]
- Jan, K.C.; Ku, K.L.; Chu, Y.H.; Hwang, L.S.; Ho, C.T. Intestinal distribution and excretion of sesaminol and its tetrahydrofuranoid metabolites in rats. J. Agric. Food Chem. 2011, 59, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Grougnet, R.; Magiatis, P.; Mitaku, S.; Terzis, A.; Tillequin, F.; Skaltsounis, A.L. New lignans from the perisperm of Sesamum indicum. J. Agric. Food Chem. 2006, 54, 7570–7574. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jung, Y.J.; Yoon, H.J.; Kwon, H.J.; Hong, S.P. Simultaneous quantification method for eleutheroside B, eleutheroside E, chiisanoside, and sesamin using reverse-phase high-performance liquid chromatography coupled with ultraviolet detection and integrated pulsed amperometric detection. Heliyon 2023, 9, e12684. [Google Scholar] [CrossRef]


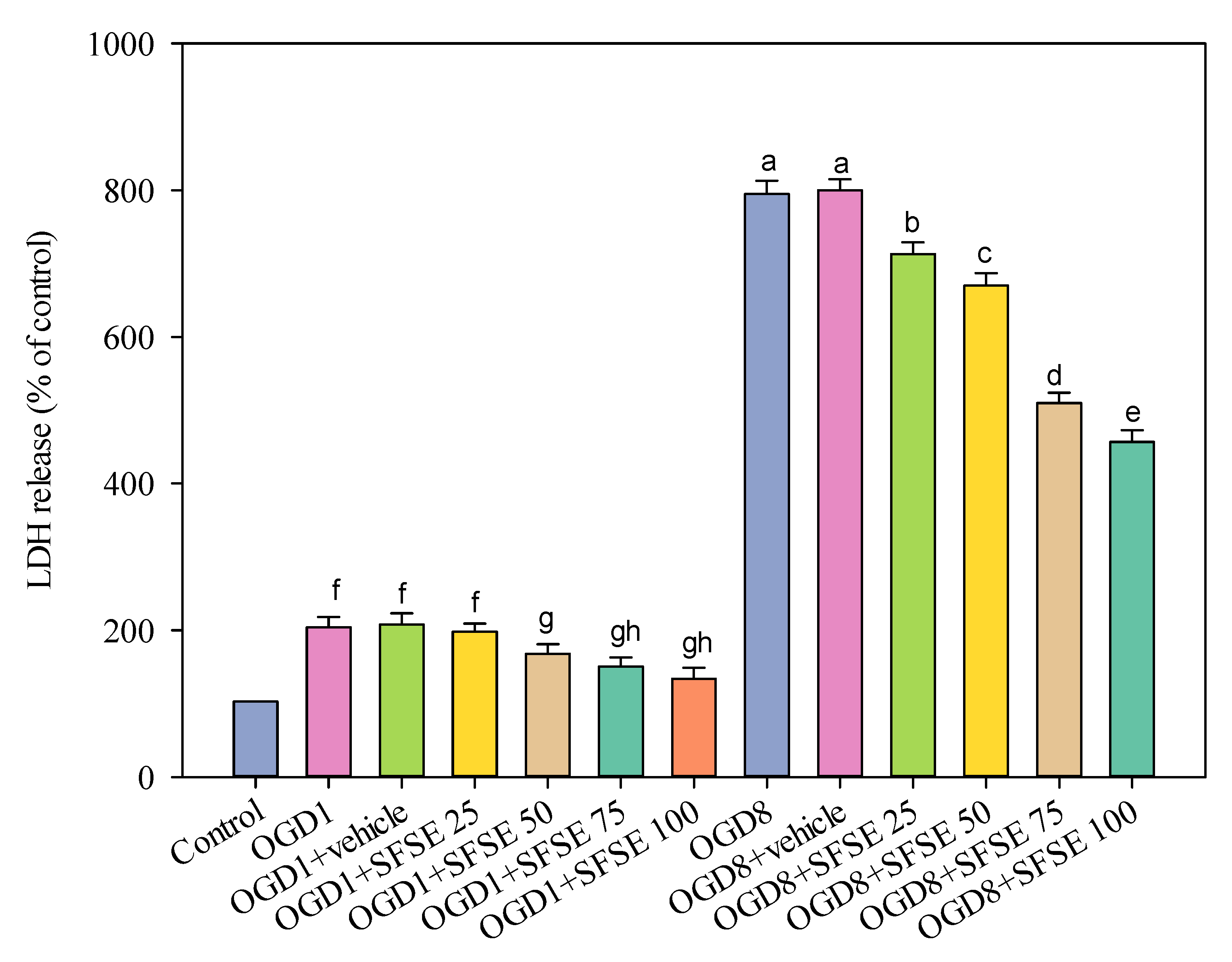
| Time (min) | Mobile Phase Ratio (%) | |
|---|---|---|
| A [Pure Water] | B [Methanol: Pure Water (8:2)] | |
| 0 | 90 | 10 |
| 6.0 | 90 | 10 |
| 7.0 | 85 | 15 |
| 10.0 | 85 | 15 |
| 25.0 | 50 | 50 |
| 26 | 25 | 75 |
| 35 | 25 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jan, K.-C.; Gavahian, M. Sustainable Supercritical Carbon Dioxide Extraction of Value-Added Lignan from Sesame Meal: Achieving Green Neuroprotection and Waste Valorization by Optimizing Temperature, Solvent, and Pressure. Molecules 2025, 30, 539. https://doi.org/10.3390/molecules30030539
Jan K-C, Gavahian M. Sustainable Supercritical Carbon Dioxide Extraction of Value-Added Lignan from Sesame Meal: Achieving Green Neuroprotection and Waste Valorization by Optimizing Temperature, Solvent, and Pressure. Molecules. 2025; 30(3):539. https://doi.org/10.3390/molecules30030539
Chicago/Turabian StyleJan, Kuo-Ching, and Mohsen Gavahian. 2025. "Sustainable Supercritical Carbon Dioxide Extraction of Value-Added Lignan from Sesame Meal: Achieving Green Neuroprotection and Waste Valorization by Optimizing Temperature, Solvent, and Pressure" Molecules 30, no. 3: 539. https://doi.org/10.3390/molecules30030539
APA StyleJan, K.-C., & Gavahian, M. (2025). Sustainable Supercritical Carbon Dioxide Extraction of Value-Added Lignan from Sesame Meal: Achieving Green Neuroprotection and Waste Valorization by Optimizing Temperature, Solvent, and Pressure. Molecules, 30(3), 539. https://doi.org/10.3390/molecules30030539








