The Role of Orthobunyavirus Glycoprotein Gc in the Viral Life Cycle: From Viral Entry to Egress
Abstract
1. OB Disease and Epidemiology
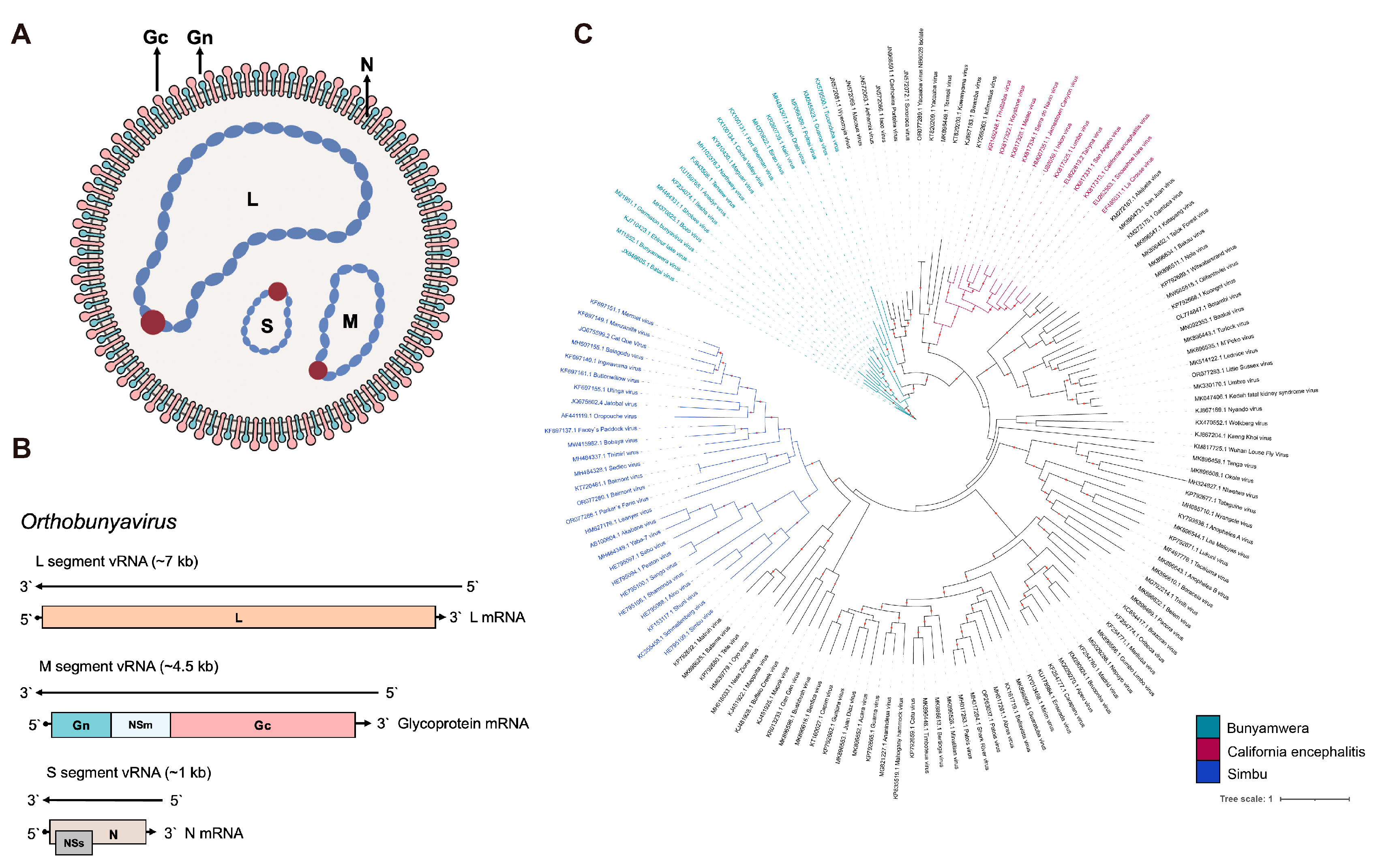
2. OBV Molecular Properties
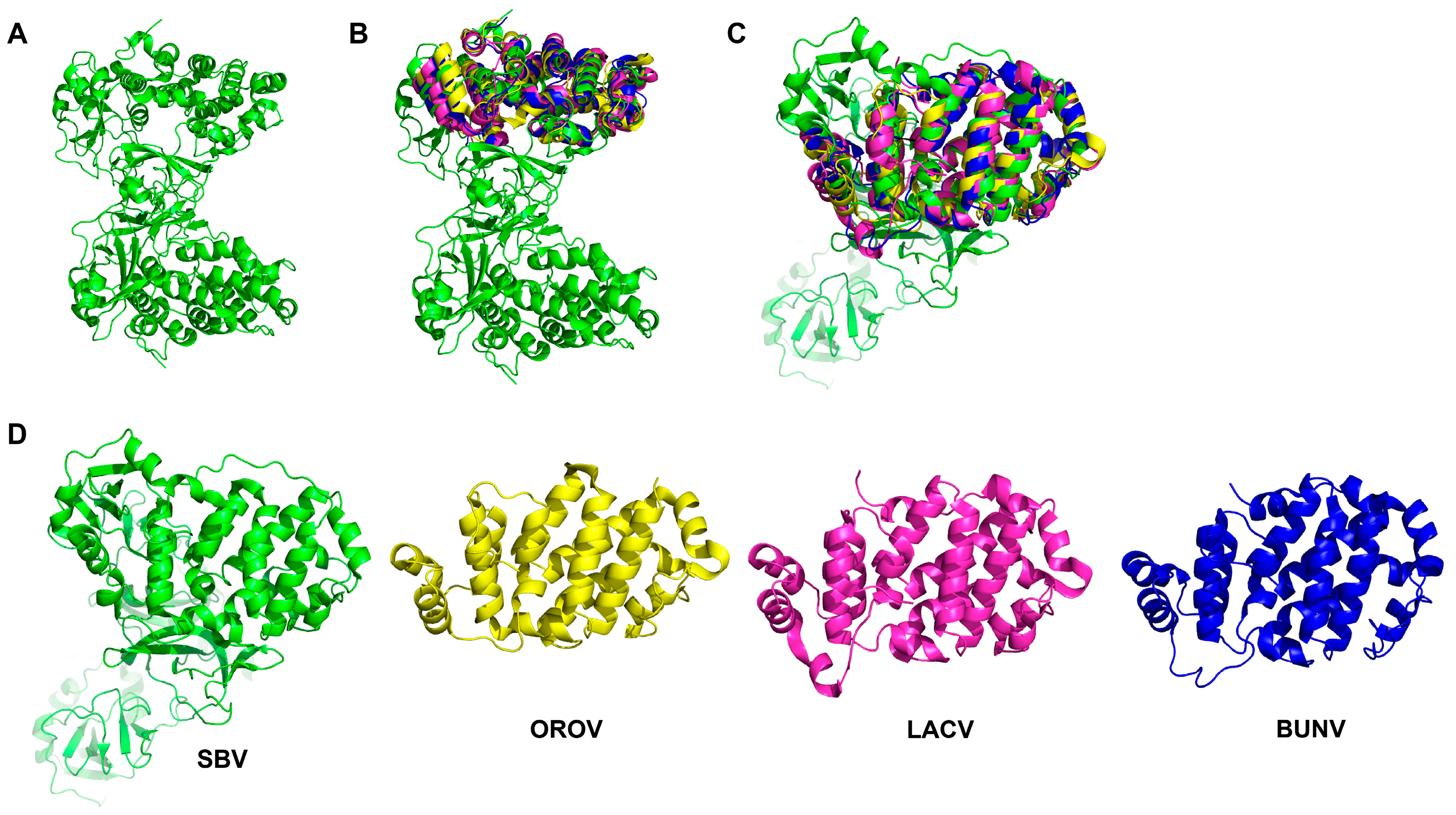
3. OBV Gc Protein
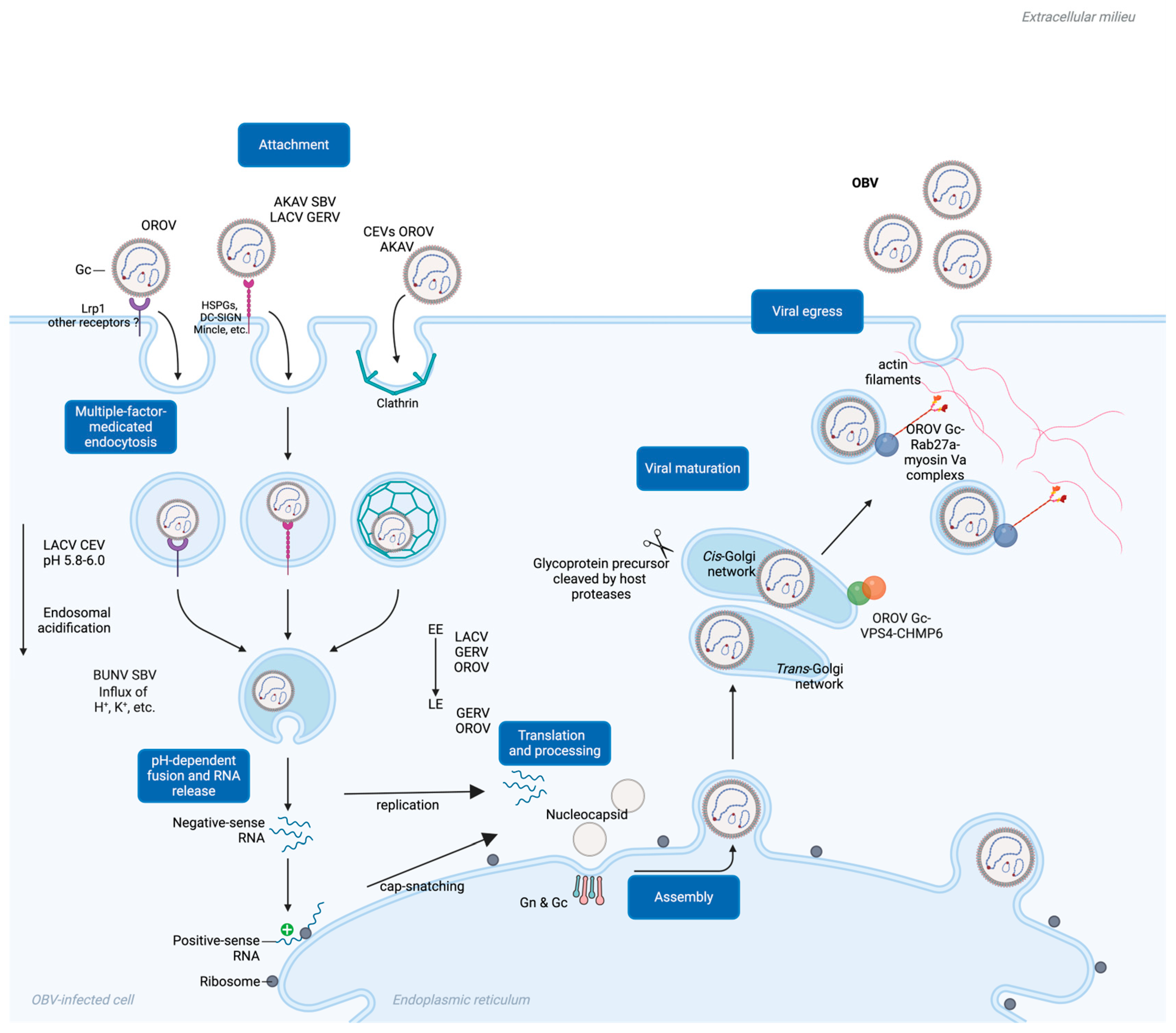
4. Gc-Mediated Viral Entry
5. Gc-Mediated Membrane Fusion and Viral Penetration
6. Gc Pre-Maturation Under Endosome Acidification Facilitates Entry
7. Gc Maturation in the Golgi Apparatus and Its Role in OBV Egress
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuhn, J.H.; Brown, K.; Adkins, S.; de la Torre, J.C.; Digiaro, M.; Ergunay, K.; Firth, A.E.; Hughes, H.R.; Junglen, S.; Lambert, A.J.; et al. Promotion of order Bunyavirales to class Bunyaviricetes to accommodate a rapidly increasing number of related polyploviricotine viruses. J. Virol. 2024, 98, e0106924. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Edridge, A.W.D.; van der Hoek, L. Emerging orthobunyaviruses associated with CNS disease. PLoS Negl. Trop. Dis. 2020, 14, e0008856. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Cheng, R.; Xu, X.; Xu, Z.; Wang, J.; Fu, S.; Xu, H.; Zhang, S.; He, Y.; Li, F.; et al. Isolation and identification of Tete virus group (Peribunyaviridae: Orthobunyavirus) from Culicoides biting midges collected in Lichuan County, China. Front Cell Infect Microbiol 2023, 13, 1193184. [Google Scholar] [CrossRef] [PubMed]
- Jost, H.; Bialonski, A.; Schmetz, C.; Gunther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation and phylogenetic analysis of Batai virus, Germany. Am. J. Trop. Med. Hyg. 2011, 84, 241–243. [Google Scholar] [CrossRef]
- Yanase, T.; Kato, T.; Yamakawa, M.; Takayoshi, K.; Nakamura, K.; Kokuba, T.; Tsuda, T. Genetic characterization of Batai virus indicates a genomic reassortment between orthobunyaviruses in nature. Arch. Virol. 2006, 151, 2253–2260. [Google Scholar] [CrossRef]
- Geevarghese, G.; Prasanna, N.Y.; Jacob, P.G.; Hanumaiah; Bhat, H.R. Isolation of Batai virus from sentinel domestic pig from Kolar district in Karnataka State, India. Acta Virol. 1994, 38, 239–240. [Google Scholar]
- Omoga, D.C.A.; Tchouassi, D.P.; Venter, M.; Ogola, E.O.; Eibner, G.J.; Kopp, A.; Slothouwer, I.; Torto, B.; Junglen, S.; Sang, R. Circulation of Ngari Virus in Livestock, Kenya. mSphere 2022, 7, e0041622. [Google Scholar] [CrossRef]
- Eiden, M.; Vina-Rodriguez, A.; El Mamy, B.O.; Isselmou, K.; Ziegler, U.; Hoper, D.; Jackel, S.; Balkema-Buschmann, A.; Unger, H.; Doumbia, B.; et al. Ngari virus in goats during Rift Valley fever outbreak, Mauritania, 2010. Emerg. Infect. Dis. 2014, 20, 2174–2176. [Google Scholar] [CrossRef]
- Groseth, A.; Weisend, C.; Ebihara, H. Complete genome sequencing of mosquito and human isolates of Ngari virus. J. Virol. 2012, 86, 13846–13847. [Google Scholar] [CrossRef]
- Gerrard, S.R.; Li, L.; Barrett, A.D.; Nichol, S.T. Ngari virus is a Bunyamwera virus reassortant that can be associated with large outbreaks of hemorrhagic fever in Africa. J. Virol. 2004, 78, 8922–8926. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.; Mugas, M.L.; Aguilar, J.J.; Marioni, J.; Contigiani, M.S.; Nunez Montoya, S.C.; Konigheim, B.S. First report of antiviral activity of nordihydroguaiaretic acid against Fort Sherman virus (Orthobunyavirus). Antiviral Res. 2021, 187, 104976. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, E.F.; Carneiro, I.O.; Ribas, J.R.L.; Fischer, C.; Marklewitz, M.; Junglen, S.; Netto, E.M.; Franke, C.R.; Drexler, J.F. Identification of animal hosts of Fort Sherman virus, a New World zoonotic orthobunyavirus. Transbound. Emerg. Dis. 2020, 67, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Kokernot, R.H.; Smithburn, K.C.; Paterson, H.E.; McIntosh, B.M. Isolation of Germiston virus, a hitherto unknown agent, from culicine mosquitoes, and a report of infection in two laboratory workers. Am. J. Trop. Med. Hyg. 1960, 9, 62–69. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Drexler, J.F. Re-emergence of Oropouche virus in Brazil and Latin America. Lancet Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Nielsen-Saines, K.; Brasil, P. Oropouche virus and potential birth defects. Lancet Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Desai, A.N.; Otter, A.; Koopmans, M.; Granata, G.; Grobusch, M.P.; Tunali, V.; Astorri, R.; Jokelainen, P.; Greub, G.; Ergonul, O.; et al. Oropouche virus: A re-emerging arbovirus of clinical significance. Int. J. Infect. Dis. 2024, 149, 107251. [Google Scholar] [CrossRef]
- LeDuc, J.W.; Hoch, A.L.; Pinheiro, F.P.; da Rosa, A.P. Epidemic Oropouche virus disease in northern Brazil. Bull. Pan Am. Health Organ. 1981, 15, 97–103. [Google Scholar]
- De Regge, N. Akabane, Aino and Schmallenberg virus-where do we stand and what do we know about the role of domestic ruminant hosts and Culicoides vectors in virus transmission and overwintering? Curr. Opin. Virol. 2017, 27, 15–30. [Google Scholar] [CrossRef]
- Uchinuno, Y.; Noda, Y.; Ishibashi, K.; Nagasue, S.; Shirakawa, H.; Nagano, M.; Ohe, R. Isolation of Aino virus from an aborted bovine fetus. J. Vet. Med. Sci. 1998, 60, 1139–1140. [Google Scholar] [CrossRef][Green Version]
- Oem, J.K.; Yoon, H.J.; Kim, H.R.; Roh, I.S.; Lee, K.H.; Lee, O.S.; Bae, Y.C. Genetic and pathogenic characterization of Akabane viruses isolated from cattle with encephalomyelitis in Korea. Vet. Microbiol. 2012, 158, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, P.D. Akabane virus infection. Rev. Sci. Tech. 2015, 34, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Tauro, L.B.; Rivarola, M.E.; Lucca, E.; Marino, B.; Mazzini, R.; Cardoso, J.F.; Barrandeguy, M.E.; Teixeira Nunes, M.R.; Contigiani, M.S. First isolation of Bunyamwera virus (Bunyaviridae family) from horses with neurological disease and an abortion in Argentina. Vet. J. 2015, 206, 111–114. [Google Scholar] [CrossRef]
- Zoller, D.; Saurich, J.; Metzger, J.; Jung, K.; Lepenies, B.; Becker, S.C. Innate Immune Response Against Batai Virus, Bunyamwera Virus, and Their Reassortants. Viruses 2024, 16, 1833. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.R.; Kenney, J.L.; Calvert, A.E. Cache Valley virus: An emerging arbovirus of public and veterinary health importance. J. Med. Entomol. 2023, 60, 1230–1241. [Google Scholar] [CrossRef]
- Wilson, M.R.; Suan, D.; Duggins, A.; Schubert, R.D.; Khan, L.M.; Sample, H.A.; Zorn, K.C.; Rodrigues Hoffman, A.; Blick, A.; Shingde, M.; et al. A novel cause of chronic viral meningoencephalitis: Cache Valley virus. Ann. Neurol. 2017, 82, 105–114. [Google Scholar] [CrossRef]
- Cho, J.J.; Wong, J.K.; Henkel, J.; DeJesus, R.O.; Nazario-Lopez, B. Acute Seroconversion of Eastern Equine Encephalitis Coinfection With California Serogroup Encephalitis Virus. Front. Neurol. 2019, 10, 242. [Google Scholar] [CrossRef]
- Coleman, K.J.; Chauhan, L.; Piquet, A.L.; Tyler, K.L.; Pastula, D.M. An Overview of Jamestown Canyon Virus Disease. Neurohospitalist 2021, 11, 277–278. [Google Scholar] [CrossRef]
- Lwande, O.W.; Bucht, G.; Ahlm, C.; Ahlm, K.; Naslund, J.; Evander, M. Mosquito-borne Inkoo virus in northern Sweden—Isolation and whole genome sequencing. Virol. J. 2017, 14, 61. [Google Scholar] [CrossRef]
- Matthews, E.; Chauhan, L.; Piquet, A.L.; Tyler, K.L.; Pastula, D.M. An Overview of La Crosse Virus Disease. Neurohospitalist 2022, 12, 587–588. [Google Scholar] [CrossRef]
- Ouellette, C.P. La Crosse virus encephalitis in children. Curr. Opin. Infect. Dis. 2024, 37, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wu, Z.; Feng, S.; Lu, K.; Zhu, W.; Sun, H.; Niu, G. Oropouche virus: A neglected global arboviral threat. Virus Res. 2024, 341, 199318. [Google Scholar] [CrossRef] [PubMed]
- Wernike, K.; Hoffmann, B.; Breard, E.; Botner, A.; Ponsart, C.; Zientara, S.; Lohse, L.; Pozzi, N.; Viarouge, C.; Sarradin, P.; et al. Schmallenberg virus experimental infection of sheep. Vet. Microbiol. 2013, 166, 461–466. [Google Scholar] [CrossRef]
- Wernike, K.; Elbers, A.; Beer, M. Schmallenberg virus infection. Rev. Sci. Tech. 2015, 34, 363–373. [Google Scholar] [CrossRef]
- Wernike, K.; Beer, M. Schmallenberg Virus: A Novel Virus of Veterinary Importance. Adv. Virus. Res. 2017, 99, 39–60. [Google Scholar] [CrossRef]
- Golender, N.; Varsano, J.S.; Nissimyan, T.; Tiomkin, E. Identification of Novel Reassortant Shuni Virus Strain in Clinical Cases of Israeli Ruminants, 2020-2021. Trop. Med. Infect. Dis. 2022, 7, 297. [Google Scholar] [CrossRef]
- Motlou, T.P.; Venter, M. Shuni Virus in Cases of Neurologic Disease in Humans, South Africa. Emerg. Infect. Dis. 2021, 27, 565–569. [Google Scholar] [CrossRef]
- Guarido, M.M.; Motlou, T.; Riddin, M.A.; MacIntyre, C.; Manyana, S.C.; Johnson, T.; Schrama, M.; Gorsich, E.E.; Brooke, B.D.; Almeida, A.P.G.; et al. Potential Mosquito Vectors for Shuni Virus, South Africa, 2014-2018. Emerg. Infect. Dis. 2021, 27, 3142–3146. [Google Scholar] [CrossRef]
- Hubalek, Z.; Sebesta, O.; Pesko, J.; Betasova, L.; Blazejova, H.; Venclikova, K.; Rudolf, I. Isolation of Tahyna Virus (California Encephalitis Group) From Anopheles hyrcanus (Diptera, Culicidae), a Mosquito Species New to, and Expanding in, Central Europe. J. Med. Entomol. 2014, 51, 1264–1267. [Google Scholar] [CrossRef]
- May, L.P.; Watts, S.L.; Maruniak, J.E. Molecular survey for mosquito-transmitted viruses: Detection of Tensaw virus in north central Florida mosquito populations. J. Am. Mosq. Control Assoc. 2014, 30, 61–64. [Google Scholar] [CrossRef]
- Calisher, C.H.; Lazuick, J.S.; Lieb, S.; Monath, T.P.; Castro, K.G. Human infections with Tensaw virus in south Florida: Evidence that Tensaw virus subtypes stimulate the production of antibodies reactive with closely related Bunyamwera serogroup viruses. Am. J. Trop. Med. Hyg. 1988, 39, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Coleman, P.H. Tensaw virus, a new member of the Bunyamwera arbovirus group from the Southern United States. Am. J. Trop. Med. Hyg. 1969, 18, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Danforth, M.E.; Snyder, R.E.; Feiszli, T.; Bullick, T.; Messenger, S.; Hanson, C.; Padgett, K.; Coffey, L.L.; Barker, C.M.; Reisen, W.K.; et al. Epidemiologic and environmental characterization of the Re-emergence of St. Louis Encephalitis Virus in California, 2015-2020. PLoS Negl. Trop. Dis. 2022, 16, e0010664. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.D.; Yuill, T.M. Snowshoe hare virus: Discovery, distribution, vector and host associations, and medical significance. J. Med. Entomol. 2023, 60, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Scheuch, M.; Hoper, D.; Jungblut, R.; Holsteg, M.; Schirrmeier, H.; Eschbaumer, M.; Goller, K.V.; Wernike, K.; Fischer, M.; et al. Novel orthobunyavirus in Cattle, Europe, 2011. Emerg. Infect. Dis. 2012, 18, 469–472. [Google Scholar] [CrossRef]
- Konig, P.; Wernike, K.; Hechinger, S.; Tauscher, K.; Breithaupt, A.; Beer, M. Fetal infection with Schmallenberg virus—An experimental pathogenesis study in pregnant cows. Transbound. Emerg. Dis. 2019, 66, 454–462. [Google Scholar] [CrossRef]
- Wernike, K.; Beer, M. Re-circulation of Schmallenberg virus, Germany, 2019. Transbound. Emerg. Dis. 2020, 67, 2290–2295. [Google Scholar] [CrossRef]
- Wernike, K.; Fischer, L.; Twietmeyer, S.; Beer, M. Extensive Schmallenberg virus circulation in Germany, 2023. Vet. Res. 2024, 55, 134. [Google Scholar] [CrossRef]
- Tang, H.B.; Ren, P.; Qin, S.; Lin, J.; Bai, A.; Qin, S.; Chen, F.; Liu, J.; Wu, J. Isolation, genetic analysis of the first Akabane virus from goat in China. J. Vet. Med. Sci. 2019, 81, 1445–1449. [Google Scholar] [CrossRef]
- Della-Porta, A.J.; O’Halloran, M.L.; Parsonson, I.M.; Snowdon, W.A.; Murray, M.D.; Hartley, W.J.; Haughey, K.J. Akabane disease: Isolation of the virus from naturally infected ovine foetuses. Aust. Vet. J. 1977, 53, 51–52. [Google Scholar] [CrossRef]
- Oya, A.; Okuno, T.; Ogata, T.; Kobayashii; Matsuyama, T. Akabane, a new arbor virus isolated in Japan. Jpn. J. Med. Sci. Biol. 1961, 14, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Takenaka-Uema, A.; Matsugo, H.; Ohira, K.; Sekine, W.; Murakami, S.; Horimoto, T. Different organ and tissue tropism between Akabane virus genogroups in a mouse model. Virus Res. 2022, 314, 198752. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Hoffmann, A.; Dorniak, P.; Filant, J.; Dunlap, K.A.; Bazer, F.W.; de la Concha-Bermejillo, A.; Welsh, C.J.; Varner, P.; Edwards, J.F. Ovine fetal immune response to Cache Valley virus infection. J. Virol. 2013, 87, 5586–5592. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, G.; Cutler, S. Evolution of the Koch postulates: Towards a 21st-century understanding of microbial infection. Clin. Microbiol. Infect. 2016, 22, 583–584. [Google Scholar] [CrossRef]
- Edridge, A.W.D.; Deijs, M.; Namazzi, R.; Cristella, C.; Jebbink, M.F.; Maurer, I.; Kootstra, N.A.; Buluma, L.R.; van Woensel, J.B.M.; de Jong, M.D.; et al. Novel Orthobunyavirus Identified in the Cerebrospinal Fluid of a Ugandan Child With Severe Encephalopathy. Clin. Infect. Dis. 2019, 68, 139–142. [Google Scholar] [CrossRef]
- Huang, B.; Firth, C.; Watterson, D.; Allcock, R.; Colmant, A.M.; Hobson-Peters, J.; Kirkland, P.; Hewitson, G.; McMahon, J.; Hall-Mendelin, S.; et al. Genetic Characterization of Archived Bunyaviruses and their Potential for Emergence in Australia. Emerg. Infect. Dis. 2016, 22, 833–840. [Google Scholar] [CrossRef]
- Tilston-Lunel, N.L.; Shi, X.; Elliott, R.M.; Acrani, G.O. The Potential for Reassortment between Oropouche and Schmallenberg Orthobunyaviruses. Viruses 2017, 9, 220. [Google Scholar] [CrossRef]
- de Souza, E.A. ICTV Virus Taxonomy Profile: Peribunyaviridae 2024. J. Gen. Virol. 2024, in press. [Google Scholar] [CrossRef]
- Guu, T.S.; Zheng, W.; Tao, Y.J. Bunyavirus: Structure and replication. Adv. Exp. Med. Biol. 2012, 726, 245–266. [Google Scholar] [CrossRef]
- Bermudez-Mendez, E.; Katrukha, E.A.; Spruit, C.M.; Kortekaas, J.; Wichgers Schreur, P.J. Visualizing the ribonucleoprotein content of single bunyavirus virions reveals more efficient genome packaging in the arthropod host. Commun. Biol. 2021, 4, 345. [Google Scholar] [CrossRef]
- Hellert, J.; Aebischer, A.; Wernike, K.; Haouz, A.; Brocchi, E.; Reiche, S.; Guardado-Calvo, P.; Beer, M.; Rey, F.A. Orthobunyavirus spike architecture and recognition by neutralizing antibodies. Nat. Commun. 2019, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Hover, S.; Charlton, F.W.; Hellert, J.; Swanson, J.J.; Mankouri, J.; Barr, J.N.; Fontana, J. Organisation of the orthobunyavirus tripodal spike and the structural changes induced by low pH and K(+) during entry. Nat. Commun. 2023, 14, 5885. [Google Scholar] [CrossRef] [PubMed]
- Hellert, J.; Aebischer, A.; Haouz, A.; Guardado-Calvo, P.; Reiche, S.; Beer, M.; Rey, F.A. Structure, function, and evolution of the Orthobunyavirus membrane fusion glycoprotein. Cell Rep. 2023, 42, 112142. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Li, S.; Li, M.; Harlos, K.; Bowden, T.A.; Huiskonen, J.T. Shielding and activation of a viral membrane fusion protein. Nat. Commun. 2018, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Behrens, A.J.; Harlos, K.; Huiskonen, J.T.; Elliott, R.M.; Crispin, M.; Brennan, B.; Bowden, T.A. Structure of a phleboviral envelope glycoprotein reveals a consolidated model of membrane fusion. Proc. Natl. Acad. Sci. USA 2016, 113, 7154–7159. [Google Scholar] [CrossRef]
- Thannickal, S.A.; Spector, S.N.; Stapleford, K.A. The La Crosse virus class II fusion glycoprotein ij loop contributes to infectivity and replication in vitro and in vivo. J. Virol. 2023, 97, e0081923. [Google Scholar] [CrossRef]
- Hollidge, B.S.; Salzano, M.V.; Ibrahim, J.M.; Fraser, J.W.; Wagner, V.; Leitner, N.E.; Weiss, S.R.; Weber, F.; Gonzalez-Scarano, F.; Soldan, S.S. Targeted Mutations in the Fusion Peptide Region of La Crosse Virus Attenuate Neuroinvasion and Confer Protection against Encephalitis. Viruses 2022, 14, 1464. [Google Scholar] [CrossRef]
- Soldan, S.S.; Hollidge, B.S.; Wagner, V.; Weber, F.; Gonzalez-Scarano, F. La Crosse virus (LACV) Gc fusion peptide mutants have impaired growth and fusion phenotypes, but remain neurotoxic. Virology 2010, 404, 139–147. [Google Scholar] [CrossRef]
- Plassmeyer, M.L.; Soldan, S.S.; Stachelek, K.M.; Roth, S.M.; Martin-Garcia, J.; Gonzalez-Scarano, F. Mutagenesis of the La Crosse Virus glycoprotein supports a role for Gc (1066–1087) as the fusion peptide. Virology 2007, 358, 273–282. [Google Scholar] [CrossRef]
- Guardado-Calvo, P.; Atkovska, K.; Jeffers, S.A.; Grau, N.; Backovic, M.; Perez-Vargas, J.; de Boer, S.M.; Tortorici, M.A.; Pehau-Arnaudet, G.; Lepault, J.; et al. A glycerophospholipid-specific pocket in the RVFV class II fusion protein drives target membrane insertion. Science 2017, 358, 663–667. [Google Scholar] [CrossRef]
- Shi, X.; Goli, J.; Clark, G.; Brauburger, K.; Elliott, R.M. Functional analysis of the Bunyamwera orthobunyavirus Gc glycoprotein. J. Gen. Virol. 2009, 90, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Kohl, A.; Li, P.; Elliott, R.M. Role of the cytoplasmic tail domains of Bunyamwera orthobunyavirus glycoproteins Gn and Gc in virus assembly and morphogenesis. J. Virol. 2007, 81, 10151–10160. [Google Scholar] [CrossRef]
- Wang, N.; Shi, X.; Jiang, L.; Zhang, S.; Wang, D.; Tong, P.; Guo, D.; Fu, L.; Cui, Y.; Liu, X.; et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013, 23, 986–993. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Z.; Hou, Y.; Deng, X.; Xu, W.; Zheng, T.; Wu, P.; Xie, S.; Bian, W.; Zhang, C.; et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021, 31, 126–140. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Spandidos, D.A.; Karteris, E. Not only ACE2-the quest for additional host cell mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 21. [Google Scholar] [CrossRef]
- Wei, C.; Wan, L.; Yan, Q.; Wang, X.; Zhang, J.; Yang, X.; Zhang, Y.; Fan, C.; Li, D.; Deng, Y.; et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat. Metab. 2020, 2, 1391–1400. [Google Scholar] [CrossRef]
- Ganaie, S.S.; Leung, D.W.; Hartman, A.L.; Amarasinghe, G.K. Host entry factors of Rift Valley Fever Virus infection. Adv. Virus Res. 2023, 117, 121–136. [Google Scholar] [CrossRef]
- Ganaie, S.S.; Schwarz, M.M.; McMillen, C.M.; Price, D.A.; Feng, A.X.; Albe, J.R.; Wang, W.; Miersch, S.; Orvedahl, A.; Cole, A.R.; et al. Lrp1 is a host entry factor for Rift Valley fever virus. Cell 2021, 184, 5163–5178 e5124. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.; Wang, Q.; Li, J.; Lv, S.; Han, S.; Zhang, L.; Ding, H.; Wang, C.Y.; Xiao, G.; et al. CCR2 is a host entry receptor for severe fever with thrombocytopenia syndrome virus. Sci. Adv. 2023, 9, eadg6856. [Google Scholar] [CrossRef]
- Schwarz, M.M.; Price, D.A.; Ganaie, S.S.; Feng, A.; Mishra, N.; Hoehl, R.M.; Fatma, F.; Stubbs, S.H.; Whelan, S.P.J.; Cui, X.; et al. Oropouche orthobunyavirus infection is mediated by the cellular host factor Lrp1. Proc. Natl. Acad. Sci. USA 2022, 119, e2204706119. [Google Scholar] [CrossRef]
- Watashi, K. HBV Slow Maturation Process Leads to Infection. Trends Microbiol. 2016, 24, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Somiya, M.; Kuroda, S. Elucidation of the early infection machinery of hepatitis B virus by using bio-nanocapsule. World J. Gastroenterol. 2016, 22, 8489–8496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, C.Z.; Swaroop, M.; Xu, M.; Wang, L.; Lee, J.; Wang, A.Q.; Pradhan, M.; Hagen, N.; Chen, L.; et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020, 6, 80. [Google Scholar] [CrossRef]
- Ye, N.; Wang, B.; Feng, W.; Tang, D.; Zeng, Z. PRRS virus receptors and an alternative pathway for viral invasion. Virus Res. 2022, 320, 198885. [Google Scholar] [CrossRef]
- Chen, Y.; Maguire, T.; Hileman, R.E.; Fromm, J.R.; Esko, J.D.; Linhardt, R.J.; Marks, R.M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 1997, 3, 866–871. [Google Scholar] [CrossRef]
- Thamamongood, T.; Aebischer, A.; Wagner, V.; Chang, M.W.; Elling, R.; Benner, C.; Garcia-Sastre, A.; Kochs, G.; Beer, M.; Schwemmle, M. A Genome-Wide CRISPR-Cas9 Screen Reveals the Requirement of Host Cell Sulfation for Schmallenberg Virus Infection. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Murakami, S.; Takenaka-Uema, A.; Kobayashi, T.; Kato, K.; Shimojima, M.; Palmarini, M.; Horimoto, T. Heparan Sulfate Proteoglycan Is an Important Attachment Factor for Cell Entry of Akabane and Schmallenberg Viruses. J. Virol. 2017, 91, e00503-17. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, M.; Takenouchi, A.; Shimoda, H.; Kimura, N.; Maeda, K. Distinct usage of three C-type lectins by Japanese encephalitis virus: DC-SIGN, DC-SIGNR, and LSECtin. Arch. Virol. 2014, 159, 2023–2031. [Google Scholar] [CrossRef]
- Wang, P.; Hu, K.; Luo, S.; Zhang, M.; Deng, X.; Li, C.; Jin, W.; Hu, B.; He, S.; Li, M.; et al. DC-SIGN as an attachment factor mediates Japanese encephalitis virus infection of human dendritic cells via interaction with a single high-mannose residue of viral E glycoprotein. Virology 2016, 488, 108–119. [Google Scholar] [CrossRef]
- Mason, C.P.; Tarr, A.W. Human lectins and their roles in viral infections. Molecules 2015, 20, 2229–2271. [Google Scholar] [CrossRef]
- Monteiro, J.T.; Schon, K.; Ebbecke, T.; Goethe, R.; Ruland, J.; Baumgartner, W.; Becker, S.C.; Lepenies, B. The CARD9-Associated C-Type Lectin, Mincle, Recognizes La Crosse Virus (LACV) but Plays a Limited Role in Early Antiviral Responses against LACV. Viruses 2019, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.; Li, X.; Zhang, X.; Liu, W.; Kuhl, A.; Kaup, F.; Soldan, S.S.; Gonzalez-Scarano, F.; Weber, F.; He, Y.; et al. Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines. J. Virol. 2013, 87, 4384–4394. [Google Scholar] [CrossRef] [PubMed]
- Windhaber, S.; Xin, Q.; Lozach, P.Y. Orthobunyaviruses: From Virus Binding to Penetration into Mammalian Host Cells. Viruses 2021, 13, 872. [Google Scholar] [CrossRef] [PubMed]
- Bangphoomi, N.; Takenaka-Uema, A.; Sugi, T.; Kato, K.; Akashi, H.; Horimoto, T. Akabane virus utilizes alternative endocytic pathways to entry into mammalian cell lines. J. Vet. Med. Sci. 2014, 76, 1471–1478. [Google Scholar] [CrossRef]
- Santos, R.I.; Rodrigues, A.H.; Silva, M.L.; Mortara, R.A.; Rossi, M.A.; Jamur, M.C.; Oliver, C.; Arruda, E. Oropouche virus entry into HeLa cells involves clathrin and requires endosomal acidification. Virus Res. 2008, 138, 139–143. [Google Scholar] [CrossRef]
- Boulant, S.; Stanifer, M.; Lozach, P.Y. Dynamics of virus-receptor interactions in virus binding, signaling, and endocytosis. Viruses 2015, 7, 2794–2815. [Google Scholar] [CrossRef]
- Hollidge, B.S.; Nedelsky, N.B.; Salzano, M.V.; Fraser, J.W.; Gonzalez-Scarano, F.; Soldan, S.S. Orthobunyavirus entry into neurons and other mammalian cells occurs via clathrin-mediated endocytosis and requires trafficking into early endosomes. J. Virol. 2012, 86, 7988–8001. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, J.S.; Yoon, Y.S.; Go, Y.Y.; Lee, H.W.; Kwon, O.S.; Park, S.; Park, M.S.; Kim, M. Salinomycin Inhibits Influenza Virus Infection by Disrupting Endosomal Acidification and Viral Matrix Protein 2 Function. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Soliman, M.; Seo, J.Y.; Kim, D.S.; Kim, J.Y.; Park, J.G.; Alfajaro, M.M.; Baek, Y.B.; Cho, E.H.; Kwon, J.; Choi, J.S.; et al. Activation of PI3K, Akt, and ERK during early rotavirus infection leads to V-ATPase-dependent endosomal acidification required for uncoating. PLoS Pathog. 2018, 14, e1006820. [Google Scholar] [CrossRef]
- Hacker, J.K.; Hardy, J.L. Adsorptive endocytosis of California encephalitis virus into mosquito and mammalian cells: A role for G1. Virology 1997, 235, 40–47. [Google Scholar] [CrossRef]
- Jacoby, D.R.; Cooke, C.; Prabakaran, I.; Boland, J.; Nathanson, N.; Gonzalez-Scarano, F. Expression of the La Crosse M segment proteins in a recombinant vaccinia expression system mediates pH-dependent cellular fusion. Virology 1993, 193, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Windhaber, S.; Xin, Q.; Uckeley, Z.M.; Koch, J.; Obr, M.; Garnier, C.; Luengo-Guyonnot, C.; Duboeuf, M.; Schur, F.K.M.; Lozach, P.Y. The Orthobunyavirus Germiston Enters Host Cells from Late Endosomes. J. Virol. 2022, 96, e0214621. [Google Scholar] [CrossRef] [PubMed]
- Shtanko, O.; Nikitina, R.A.; Altuntas, C.Z.; Chepurnov, A.A.; Davey, R.A. Crimean-Congo hemorrhagic fever virus entry into host cells occurs through the multivesicular body and requires ESCRT regulators. PLoS Pathog. 2014, 10, e1004390. [Google Scholar] [CrossRef]
- Hover, S.; Foster, B.; Fontana, J.; Kohl, A.; Goldstein, S.A.N.; Barr, J.N.; Mankouri, J. Bunyavirus requirement for endosomal K+ reveals new roles of cellular ion channels during infection. PLoS Pathog. 2018, 14, e1006845. [Google Scholar] [CrossRef]
- Charlton, F.W.; Hover, S.; Fuller, J.; Hewson, R.; Fontana, J.; Barr, J.N.; Mankouri, J. Cellular cholesterol abundance regulates potassium accumulation within endosomes and is an important determinant in bunyavirus entry. J. Biol. Chem. 2019, 294, 7335–7347. [Google Scholar] [CrossRef]
- Sandler, Z.J.; Firpo, M.R.; Omoba, O.S.; Vu, M.N.; Menachery, V.D.; Mounce, B.C. Novel Ionophores Active against La Crosse Virus Identified through Rapid Antiviral Screening. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Hulswit, R.J.G.; Paesen, G.C.; Bowden, T.A.; Shi, X. Recent Advances in Bunyavirus Glycoprotein Research: Precursor Processing, Receptor Binding and Structure. Viruses 2021, 13, 353. [Google Scholar] [CrossRef]
- Vincent, M.J.; Sanchez, A.J.; Erickson, B.R.; Basak, A.; Chretien, M.; Seidah, N.G.; Nichol, S.T. Crimean-Congo hemorrhagic fever virus glycoprotein proteolytic processing by subtilase SKI-1. J. Virol. 2003, 77, 8640–8649. [Google Scholar] [CrossRef]
- Shi, X.; Kohl, A.; Leonard, V.H.; Li, P.; McLees, A.; Elliott, R.M. Requirement of the N-terminal region of orthobunyavirus nonstructural protein NSm for virus assembly and morphogenesis. J. Virol. 2006, 80, 8089–8099. [Google Scholar] [CrossRef]
- Barbosa, N.S.; Concha, J.O.; daSilva, L.L.P.; Crump, C.M.; Graham, S.C. Oropouche Virus Glycoprotein Topology and Cellular Requirements for Glycoprotein Secretion. J. Virol. 2023, 97, e0133122. [Google Scholar] [CrossRef]
- Concha, J.O.; Gutierrez, K.; Barbosa, N.; Rodrigues, R.L.; de Carvalho, A.N.; Tavares, L.A.; Rudd, J.S.; Costa, C.S.; Andrade, B.Y.G.; Espreafico, E.M.; et al. Rab27a GTPase and its effector Myosin Va are host factors required for efficient Oropouche virus cell egress. PLoS Pathog. 2024, 20, e1012504. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, Y.; Wang, P.; Xiao, X. Mosquito Defense Strategies against Viral Infection. Trends Parasitol. 2016, 32, 177–186. [Google Scholar] [CrossRef]
- Andreolla, A.P.; Borges, A.A.; Nagashima, S.; Vaz de Paula, C.B.; de Noronha, L.; Zanchin, N.I.T.; Bordignon, J.; Duarte Dos Santos, C.N. Development of monoclonal antibodies against oropouche virus and its applicability to immunohistochemical diagnosis. Virol. J. 2024, 21, 81. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Wei, F.; Yu, R.; Xu, S.; Lin, X.; Wu, S. Identification of a broadly neutralizing epitope within Gc protein of Akabane virus using newly prepared neutralizing monoclonal antibodies. Vet. Microbiol. 2024, 295, 110123. [Google Scholar] [CrossRef]
- Dagnaw, M.; Solomon, A.; Dagnew, B. Serological prevalence of the Schmallenberg virus in domestic and wild hosts worldwide: A systematic review and meta-analysis. Front. Vet. Sci. 2024, 11, 1371495. [Google Scholar] [CrossRef]
- Stubbs, S.H.; Cornejo Pontelli, M.; Mishra, N.; Zhou, C.; de Paula Souza, J.; Mendes Viana, R.M.; Lipkin, W.I.; Knipe, D.M.; Arruda, E.; Whelan, S.P.J. Vesicular Stomatitis Virus Chimeras Expressing the Oropouche Virus Glycoproteins Elicit Protective Immune Responses in Mice. mBio 2021, 12, e0046321. [Google Scholar] [CrossRef]
- Roman-Sosa, G.; Karger, A.; Kraatz, F.; Aebischer, A.; Wernike, K.; Maksimov, P.; Lillig, C.H.; Reimann, I.; Brocchi, E.; Keller, M.; et al. The amino terminal subdomain of glycoprotein Gc of Schmallenberg virus: Disulfide bonding and structural determinants of neutralization. J. Gen Virol. 2017, 98, 1259–1273. [Google Scholar] [CrossRef]
- Wernike, K.; Aebischer, A.; Audonnet, J.C.; Beer, M. Vaccine development against Schmallenberg virus: From classical inactivated to modified-live to scaffold particle vaccines. One Health Outlook 2022, 4, 13. [Google Scholar] [CrossRef]
- Wernike, K.; Mundt, A.; Link, E.K.; Aebischer, A.; Schlotthauer, F.; Sutter, G.; Fux, R.; Beer, M. N-terminal domain of Schmallenberg virus envelope protein Gc delivered by recombinant equine herpesvirus type 1 and modified vaccinia virus Ankara: Immunogenicity and protective efficacy in cattle. Vaccine 2018, 36, 5116–5123. [Google Scholar] [CrossRef]
- Bian, T.; Wang, B.; Fu, G.; Hao, M.; Chen, Y.; Fang, T.; Liu, S.; Yu, C.; Li, J.; Chen, W. Single-dose of a replication-competent adenovirus-vectored vaccine provides sterilizing protection against Rift Valley fever virus challenge. Front. Immunol. 2022, 13, 907675. [Google Scholar] [CrossRef]
- Bian, T.; Hao, M.; Zhao, X.; Zhao, C.; Luo, G.; Zhang, Z.; Fu, G.; Yang, L.; Chen, Y.; Wang, Y.; et al. A Rift Valley fever mRNA vaccine elicits strong immune responses in mice and rhesus macaques. NPJ Vaccines 2023, 8, 164. [Google Scholar] [CrossRef] [PubMed]
| Species | Virus Name | Host | Vector | Clinical Signs | References |
|---|---|---|---|---|---|
| Orthobunyavirus ainoense | Aino virus (AINOV) | cows | biting midge, mosquito | Hydranencephaly | [19,20] |
| Orthobunyavirus akabaneense | Akabane virus (AKAV) | cows, sheep, goats, pigs | biting midge, mosquito | Abortion and neonatal hydranencephaly | [21,22] |
| Orthobunyavirus bataiense | Batai virus (BATV) | humans, cows, sheep, goats, rodents, birds | mosquito | Meningoencephalitis | [5] |
| Orthobunyavirus bunyamweraense | Bunyamwera virus (BUNV) | humans, horses | mosquito | CNS disease and abortion | [23,24] |
| Orthobunyavirus bunyamweraense | Germiston virus (GERV) | humans | mosquito | Mental confusion | [14] |
| Orthobunyavirus cacheense | Cache Valley virus (CVV) | humans, sheep | mosquito | Encephalitis and meningitis (acute and chronic) | [25,26] |
| Orthobunyavirus encephalitidis | California encephalitis virus (CEV) | humans, horses | mosquito | Encephalitis | [27] |
| Orthobunyavirus jamestownense | Jamestown Canyon virus (JCV) | humans | mosquito, horse fly | Meningitis and meningoencephalitis | [28] |
| Orthobunyavirus jamestownense | Inkoo virus (INKV) | humans | mosquito | Encephalitis and meningitis | [29] |
| Orthobunyavirus lacrosseense | La Crosse virus (LACV) | humans, dogs | mosquito | Encephalitis and meningitis | [30,31] |
| Orthobunyavirus oropoucheense | Oropouche virus (OROV) | humans | biting midge, mosquito | Encephalitis and meningitis | [17,32] |
| Orthobunyavirus schmallenbergense | Schmallenberg virus (SBV) | cows, sheep, goats | biting midge | Abortion and neonatal hydranencephaly | [33,34,35] |
| Orthobunyavirus shuniense | Shuni virus (SHUV) | humans, cows, horses, sheep, goats | biting midge, mosquito | Encephalitis and meningitis | [36,37,38] |
| Orthobunyavirus tahynaense | Ťahyňa virus (TAHV) | humans | mosquito | Encephalitis and meningitis | [39] |
| Orthobunyavirus tensawense | Tensaw virus (TENV) | humans, foxes | mosquito | Encephalitis and rabies-like symptoms | [40,41,42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Zhao, D.; Li, C.; Deng, M.; Li, G.; Chen, S.; Zhao, M.; Qin, L.; Zhang, K. The Role of Orthobunyavirus Glycoprotein Gc in the Viral Life Cycle: From Viral Entry to Egress. Molecules 2025, 30, 503. https://doi.org/10.3390/molecules30030503
Gao H, Zhao D, Li C, Deng M, Li G, Chen S, Zhao M, Qin L, Zhang K. The Role of Orthobunyavirus Glycoprotein Gc in the Viral Life Cycle: From Viral Entry to Egress. Molecules. 2025; 30(3):503. https://doi.org/10.3390/molecules30030503
Chicago/Turabian StyleGao, Han, Dengshuai Zhao, Canyuan Li, Menghua Deng, Gan Li, Shengfeng Chen, Mengmeng Zhao, Limei Qin, and Keshan Zhang. 2025. "The Role of Orthobunyavirus Glycoprotein Gc in the Viral Life Cycle: From Viral Entry to Egress" Molecules 30, no. 3: 503. https://doi.org/10.3390/molecules30030503
APA StyleGao, H., Zhao, D., Li, C., Deng, M., Li, G., Chen, S., Zhao, M., Qin, L., & Zhang, K. (2025). The Role of Orthobunyavirus Glycoprotein Gc in the Viral Life Cycle: From Viral Entry to Egress. Molecules, 30(3), 503. https://doi.org/10.3390/molecules30030503





