From the Soil to the Wine—Elements’ Migration in Monovarietal Bulgarian Wines
Abstract
1. Introduction
2. Results
2.1. Elements’ Content in Soil, Leave, Must and Wine
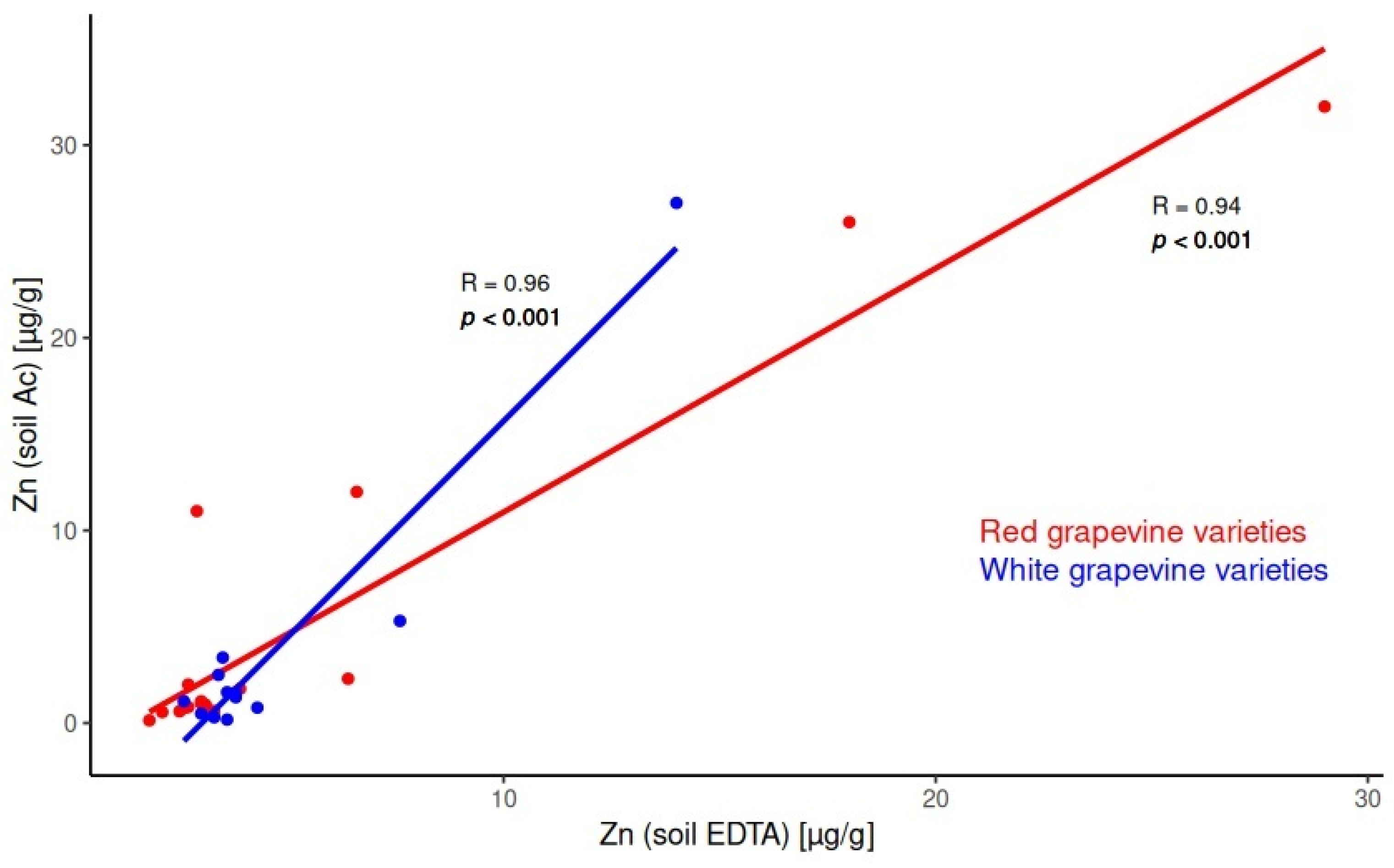
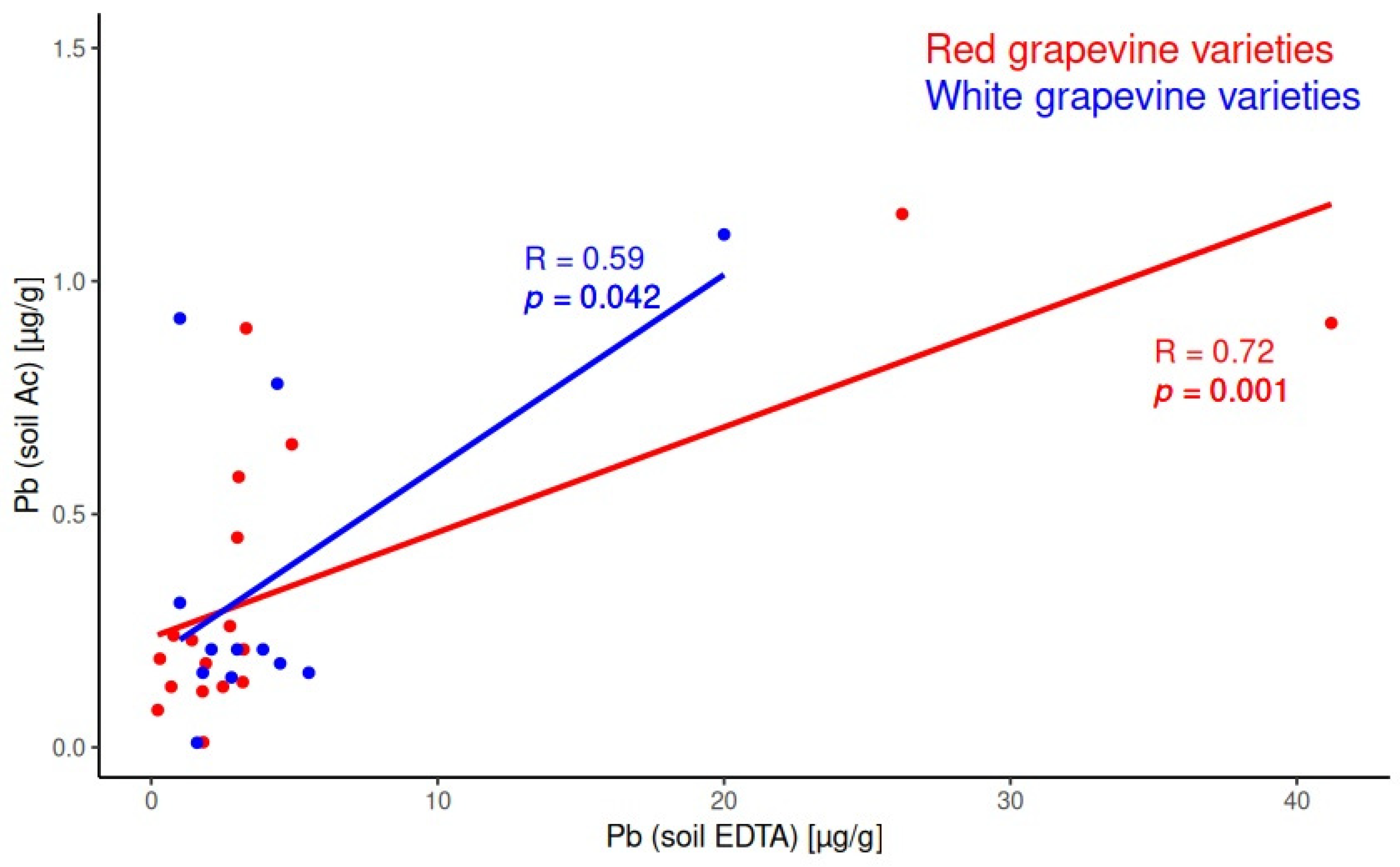
2.2. Correlations in the System Soil/Soil
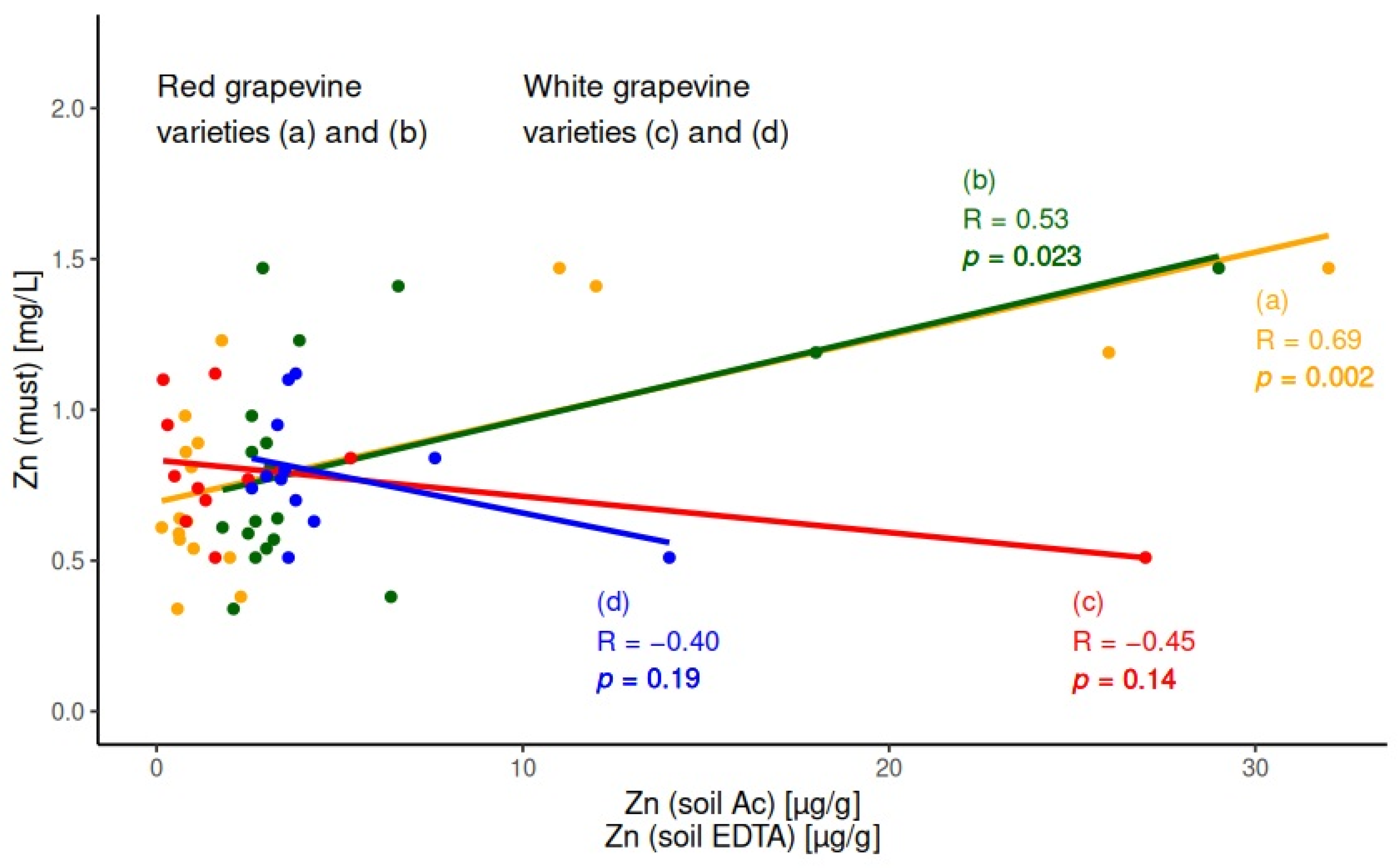
2.3. Correlations in the System Soil/Must
2.4. Elements’ Decreasing Level in the System Must—Wine
3. Discussion
3.1. Soil Characterization Based on Soil Extracts
3.2. Chemical Elements Migration in the System Soil/Plant
3.3. Chemical Elements in the System Must/Wine
4. Materials and Methods
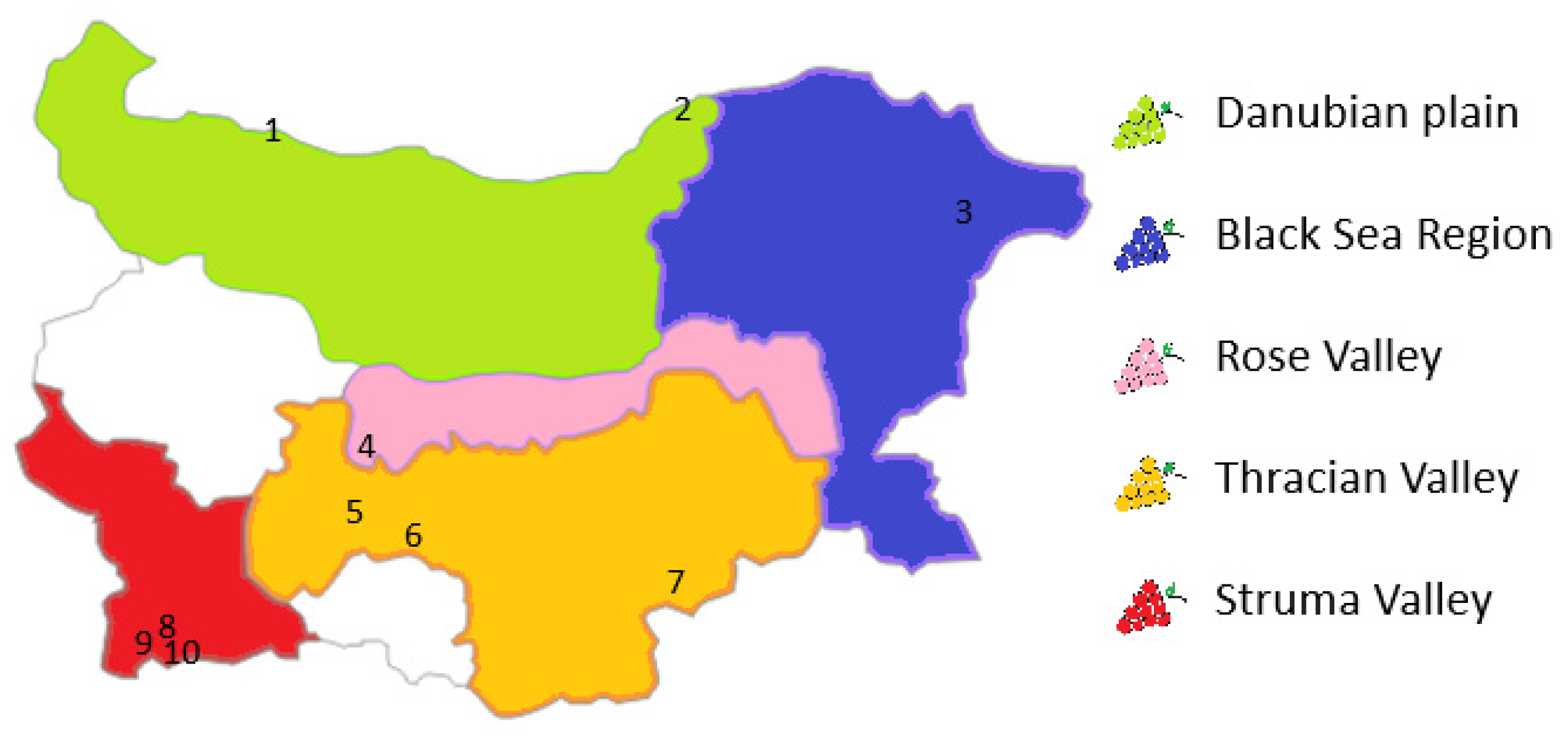
4.1. Sample Collection
4.1.1. Sampling Points
4.1.2. Soils
4.1.3. Leaves
4.1.4. Must
4.1.5. Row Wine
4.2. Sample Pretreatment
4.2.1. Soil Samples
4.2.2. Leaf Samples
4.2.3. Must Samples
4.2.4. Wine Samples
4.3. Instrumental Measurement
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, R.S. Wines: Types of Table Wines. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 556–561. [Google Scholar]
- Rocha, S.; Pinto, E.; Almeida, A.; Fernandes, E. Multi-Elemental Analysis as a Tool for Characterization and Differentiation of Portuguese Wines According to Their Protected Geographical Indication. Food Control 2019, 103, 27–35. [Google Scholar] [CrossRef]
- Gnilomedova, N.V.; Anikina, N.S.; Kolesnov, A.Y. A Review of Methodological Approaches to Authenticating the Geographical Origin of Wines. Food Process. Tech. Technol. 2023, 53, 231–246. [Google Scholar] [CrossRef]
- Pohl, P. What do metals tell us about wine? TrAC 2007, 26, 941–949. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology—The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley & Sons: Chichester, UK, 2006; pp. 301–332. [Google Scholar]
- Blesic, M.; Drmac, M.; Batinic, K.; Spaho, N.; Smajic Murtic, M.; Zele, M. Levels of selected metals in wines from different Herzegovinian viticultural localities. Croat. J. Food Sci. Technol. 2017, 9, 1–10. [Google Scholar] [CrossRef]
- Nicolini, G.; Larcher, R.; Pangrazzi, P.; Bontempo, L. Changes in the contents of micro- and trace elements in wine due to winemaking treatments. Vitis 2004, 43, 41–45. [Google Scholar] [CrossRef]
- Tariba, B. Metals in Wine—Impact on Wine Quality and Health Outcomes. Biol. Trace Elem. Res. 2011, 144, 143–156. [Google Scholar] [CrossRef]
- Deng, Z.-H.; Zhang, A.; Yang, Z.-W.; Zhong, Y.-L.; Mu, J.; Wang, F.; Liu, Y.-X.; Zhang, J.-J.; Fang, Y.-L. A Human Health Risk Assessment of Trace Elements Present in Chinese Wine. Molecules 2019, 24, 248. [Google Scholar] [CrossRef] [PubMed]
- Parr, W.V.; Maltman, A.J.; Easton, S.; Ballester, J. Minerality in Wine: Towards the Reality behind the Myths. Beverages 2018, 4, 77. [Google Scholar] [CrossRef]
- Compendium of International Methods of Wine and Must Analysis, International Organization of Vine and Wine. 2024. Available online: https://www.oiv.int/sites/default/files/publication/2024-06/Compendium%20MA%20Wines%202024%20EN%20complet_0.pdf (accessed on 11 November 2024).
- Temerdashev, Z.A.; Abakumov, A.G.; Kaunova, A.A.; Sheludko, O.N.; Tsyupko, T.G. Assessment of quality and regional origin of wines. J. Anal. Chem. 2023, 78, 1724–1740. [Google Scholar] [CrossRef]
- Petrini, R.; Sansone, L.; Slejko, F.F.; Buccianti, A.; Marcuzzo, P.; Tomasi, D. The 87Sr/86Sr strontium isotopic systematics applied to Glera vineyards: A tracer for the geographical origin of the Prosecco. Food Chem. 2015, 170, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, H.-C.; Wang, Y.; Gao, X.-T.; Li, H.-Q.; Tian, M.-B.; Shi, N.; Li, M.-Y.; Yang, X.-L.; He, F.; et al. Region, vintage, and grape maturity co-shaped the ionomic signatures of the Cabernet Sauvignon wines. Food. Res. Inter. 2023, 163, 112165. [Google Scholar] [CrossRef]
- Luykx, D.M.A.M.; van Ruth, S.M. An overview of analytical methods for determining the geographical origin of food products. Food Chem. 2008, 107, 897–911. [Google Scholar] [CrossRef]
- Georgiou, C.A.; Danezis, G.P. Advanced Mass Spectrometry for Food Safety and Quality. Compr. Anal. Chem. 2015, 68, 131–243. [Google Scholar] [CrossRef]
- Coelho, I.; Epova, E.; Barre, J.; Castanheira, I.; Donald, O.F.X. Mineral and Isotopic Characterization of Wines from the Douro Demarcated Region. Curr. Dev. Nutr. 2022, 6 (Suppl. 1), 478. [Google Scholar] [CrossRef]
- Karadjova, I.; Izgi, B.; Gucer, S. Fractionation and speciation of Cu, Zn and Fe in wine samples by atomic absorption spectrometry. Spectrochim. Acta B At. Spectrosc. 2002, 57, 581–590. [Google Scholar] [CrossRef]
- Krist, J.; Veber, M.; Slekovec, M. The application of ETAAS to the determination of Cr, Pb and Cd in samples taken during different stages of the winemaking process. Anal. Bioanal. Chem. 2002, 373, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Mafakheri, N.; Shamsipur, M.; Babajani, N. Development of a dispersive liquid–liquid microextraction procedure based on a natural deep eutectic solvent for ligand-less preconcentration and determination of heavy metals from water and food samples. Microchem. J. 2024, 199, 110010. [Google Scholar] [CrossRef]
- Tashev, K.; Karadjova, I.; Boev, I.; Stafilov, T. On CV-AAS determination and speciation of mercury in wine. J. Agric. Food Environ. Sci. 2023, 77, 39–48. [Google Scholar] [CrossRef]
- Lemos, A.; Lujan, C.E.; Oviedo, M.; Acha, C.; Wuilloud, R. Magnetic ionic liquid effervescence assisted liquid-liquid microextraction and atomic fluorescence spectrometry for sensitive determination of mercury species in water and wine samples. J. Food Comp. Anal. 2024, 136, 106710. [Google Scholar] [CrossRef]
- Blotevogel, S.; Schreck, E.; Laplanche, C.; Besson, P.; Saurin, N.; Audry, S.; Viers, J.; Oliva, P. Soil chemistry and meteorological conditions influence the elemental profiles of West European wines. Food Chem. 2019, 298, 125033. [Google Scholar] [CrossRef] [PubMed]
- Bora, F.D.; Bunea, C.I.; Rusu, T.; Pop, N. Vertical distribution and analysis of micro-, macroelements and heavy metals in the system soil-grapevine-wine in vineyard from North-West Romania. Chem. Cent. J. 2015, 9, 19. [Google Scholar] [CrossRef][Green Version]
- Hao, X.; Gao, F.; Wu, H.; Song, Y.; Zhang, L.; Li, H.; Wang, H. From Soil to Grape and Wine: Geographical Variations in Elemental Profiles in Different Chinese Regions. Foods 2021, 10, 3108. [Google Scholar] [CrossRef] [PubMed]
- Kment, P.; Mihaljevič, M.; Ettler, V.; Šebek, O.; Strnad, L.; Rohlová, L. Differentiation of Czech wines using multielement composition–A comparison with vineyard soil. Food Chem. 2005, 91, 157–165. [Google Scholar] [CrossRef]
- Palma-Lopez, J.; Sanchez-Rodríguez, A.R.; del Campillo, M.C.; Leon-Gutierrez, J.M.; Ramírez-Perez, P. Influence of soil properties on grape and must quality in the Montilla–Moriles protected designation of origin (southern Spain). Catena 2024, 241, 108041. [Google Scholar] [CrossRef]
- Maltman, A. Minerality in Wine: A Geological Perspective. J. Wine Res. 2013, 24, 169–181. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Vasconcelos, M.T.S.D. Lead contamination in Portuguese red wines from the Douro region: From the vineyard to the final product. J. Agric. Food Chem. 2003, 51, 3012–3023. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: London, UK, 2012; pp. 645–651. [Google Scholar]
- Jiménez-Ballesta, R.; Bravo, S.; Amorós, J.A.; Pérez-de-los-Reyes, C.; García-Pradas, J.; Sanchez, M.; García-Navarro, F.J. Soil and Leaf Mineral Element Contents in Mediterranean Vineyards: Bioaccumulation and Potential Soil Pollution. Water Air Soil Pollut. 2022, 233, 20. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Temerdashev, Z.; Abakumov, A.; Khalafyan, A.; Bolshov, M.; Lukyanov, A.; Vasilyev, A.; Gipich, E. The Influence of the Soil Profile on the Formation of the Elemental Image of Grapes and Wine of the Cabernet Sauvignon Variety. Molecules 2024, 29, 2251. [Google Scholar] [CrossRef]
- Wang, X.; Shao, X.; Zhang, Z.; Zhong, X.; Ji, X.; Shi, X.; Liu, C.; Wang, Z.; Li, F.; Wang, F. Multi-nutrient fertilization-based analysis of fruit quality and mineral element composition during fruit development in Merlot wine grapevine. J. Integr. Agric. 2024; in press. [Google Scholar] [CrossRef]
- Hirzel, D.R.; Steenwerth, K.; Parikh, S.J.; Oberholster, A. Impact of winery wastewater irrigation on soil, grape and wine composition. Agric. Water Manag. 2017, 180 Pt A, 178–189. [Google Scholar] [CrossRef]
- Garinie, T.; Nusillard, W.; Lelièvre, Y.; Taranu, Z.E.; Goubault, M.; Thiéry, D.; Moreau, J.; Louâpre, P. Adverse effects of the Bordeaux mixture copper-based fungicide on the non-target vineyard pest Lobesia botrana. Pest Manag. Sci. 2024, 80, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- de la Guardia, M.; Garrigues, S. Handbook of Mineral Elements in Food, 1st ed.; John Wiley & Sons, Ltd.: Chicheter, UK, 2015. [Google Scholar]
- Santos, S.; Costa, C.A.E.; Duarte, A.C.; Scherer, H.W.; Schneider, R.J.; Esteves, V.I.; Santos, E.B.H. Influence of different organic amendments on the potential availability of metals from soil: A study on metal fractionation and extraction kinetics by EDTA. Chemosphere 2010, 78, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Milićević, T.; Urošević, M.A.; Relić, D.; Vuković, G.; Škrivanj, S.; Popović, A. Bioavailability of potentially toxic elements in soil–grapevine (leaf, skin, pulp and seed) system and environmental and health risk assessment. Sci. Total Environ. 2018, 626, 528–545. [Google Scholar] [CrossRef]
- Mpelasoka, B.S.; Schachtman, D.P.; Treeby, M.T.; Thomas, M.R. A review of potassium nutrition in grapevines with special emphasis on berry accumulation. Aust. J. Grape Wine Res. 2003, 9, 154–168. [Google Scholar] [CrossRef]
- Ribeiro-Filho, N.; Linforth, R.; Powell, C.D.; Fisk, I.D. Influence of essential inorganic elements on flavour formation during yeast fermentation. Food Chem. 2021, 361, 130025. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, E.; Bakardzhiyski, I.; Dimitrova, E. Investigation of Elemental Composition in White Wine Treated with Varying Doses of Bentonite. Beverages 2024, 10, 114. [Google Scholar] [CrossRef]
- Ettler, V. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 2016, 64, 56–74. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Ferreira, I.M.P.L.V.O. Assessment of metal(loid)s phytoavailability in intensive agricultural soils by the application of single extractions to rhizosphere soil. Ecotoxicol. Environ. Saf. 2015, 113, 418–424. [Google Scholar] [CrossRef]
- Ortiz-Villajos, J.A.A.; Navaro, F.J.G.; Perez de los Reyes, C.; Gallego, J.A.C.; Mrtin-Consuegra, S.B.; Ballesta, R.J.; Moreno, R.G. Geochemical influence of soil on leaf and grape (Vitis vinifera L. ‘Cencibel’) composition in La Mancha region (Spain). Vitis 2012, 51, 111–118. [Google Scholar] [CrossRef]
- Li, H.; Santos, F.; Butler, K.; Herndon, E. A Critical Review on the Multiple Roles of Manganese in Stabilizing and Destabilizing Soil Organic Matter. Environ. Sci. Technol. 2021, 55, 12136–12152. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marshner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar]
- McCain, D.C.; Markley, J.L. More manganese accumulates in maple sun leaves than in shade leaves. Plant Physiol. 1989, 90, 1417–1421. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A Central Regulator of Plant Growth and Development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 309–323. [Google Scholar] [CrossRef]
- Tang, R.-J.; Luan, S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Vršič, S.; Gumzej, M.; Lešnik, M.; Perko, A.; Pulko, B. Patterns of Copper Bioaccumulation and Translocation in Grapevine Grafts Depending on Rootstocks. Agriculture 2023, 13, 1768. [Google Scholar] [CrossRef]
- Lai, H.Y.; Juang, K.W.; Chen, B.C. Copper concentrations in grapevines and vineyard soils in central Taiwan. Soil Sci. Plant Nutr. 2010, 56, 601–606. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Cambrolle, J.; García, J.L.; Ocete, R.; Figueroa, M.E.; Cantos, M. Growth and photosynthetic responses to copper in wild grapevine. Chemosphere 2013, 93, 294–301. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pandey, S.D.; Misra, V. Stability Constants of Metal–Humic Acid Complexes and Its Role in Environmental Detoxification. Ecotoxicol. Environ. Saf. 2000, 47, 195–200. [Google Scholar] [CrossRef]
- Shimizu, H.; Akamatsu, F.; Kamada, A.; Koyama, K.; Iwashita, K.; Goto-Yamamoto, N. Variation in the mineral composition of wine produced using different winemaking techniques. J. Biosci. Bioeng. 2020, 30, 166–172. [Google Scholar] [CrossRef]
- Chobanova, D. Enology (Exercise Guide), 1st ed.; UFT Academic Publishing House: Plovdiv, Bulgaria, 2007. (In Bulgarian) [Google Scholar]
- Chobanova, D. Enology Part 1. Wine Composition, 1st ed.; UFT Academic Publishing House: Plovdiv, Bulgaria, 2012. (In Bulgarian) [Google Scholar]
- Aguilar, M.V.; Martinez, M.C.; Masoud, T.A. Arsenic content in some Spanish wines. Influence of the wine-making technique on arsenic content in musts and wines. Z. Lebensm. Unters. Forsch. 1987, 185, 185–191. [Google Scholar] [CrossRef]
- OIV Compendium of International Methods of Analysis: Reducing Substances. OIV-MA-AS311-01A. Available online: http://www.oiv.int (accessed on 10 January 2024).
- Žemberyová, M.; Barteková, J.; Závadská, M.; Šišoláková, M. Determination of bioavailable fractions of Zn, Cu, Ni, Pb and Cd in soils and sludges by atomic absorption spectrometry. Talanta 2007, 71, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Quevauviller, P. Operationally defined extraction procedures for soil and sediment analysis I. Standardization. TrAC 1998, 17, 289–298. [Google Scholar] [CrossRef]
- Voyslavov, T.; Mladenova, E.; Balkanska, R. New approach for determination of the botanical origin of monofloral bee honey, Ccombining mineral content, physicochemical parameters, and self-organizing maps. Molecules 2021, 26, 7219. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.A. Descriptive Methods in Regression and Correlation. In Introductory Statistics, 10th ed.; Lynch, D., Ed.; Pearson: Essex, UK, 2016; pp. 640–678. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 7 January 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
| Red Wines | White Wines | |||
|---|---|---|---|---|
| Element | Min% | Max% | Min% | Max% |
| Al | 4.92 | 94.71 | 7.72 | 86.65 |
| B | 2.68 | 33.34 | 9.77 | 34.24 |
| Ba | 23.36 | 96.01 | 10.99 | 94.45 |
| Ca | 4.96 | 67.49 | 16.58 | 59.61 |
| Cu | 54.45 | 99.62 | 7.60 | 97.65 |
| Fe | 11.35 | 84.27 | 17.10 | 64.92 |
| K | 5.76 | 59.16 | 28.59 | 67.04 |
| Mg | 9.47 | 34.38 | 8.81 | 44.36 |
| Mn | 8.28 | 60.54 | 15.76 | 73.53 |
| Na | 41.23 | 86.13 | 6.13 | 83.99 |
| P | 10.19 | 39.50 | 14.13 | 53.14 |
| Sr | 17.20 | 71.88 | 24.71 | 62.23 |
| Zn | 9.44 | 72.03 | 20.26 | 53.24 |
| Red Wines | White Wines | |||
|---|---|---|---|---|
| Element | Min% | Max% | Min% | Max% |
| As | 56.52 | 94.32 | 10.35 | 93.03 |
| Cd | 22.22 | 94.92 | 20.00 | 75.00 |
| Co | 8.78 | 91.20 | 13.79 | 72.41 |
| Cr | 10.34 | 57.14 | 9.38 | 34.88 |
| Li | 14.29 | 64.52 | 24.00 | 45.45 |
| Ni | 44.25 | 90.08 | 32.91 | 90.67 |
| Pb | 23.08 | 44.44 | 23.08 | 44.44 |
| Grapevine Variety | Sub-Regions * | Region | Sample Number |
|---|---|---|---|
| Chardonnay 1 | Oryahovo (1) | Danubian plain | 1 |
| Chardonnay 2 | 2 | ||
| Chardonnay 3 | 3 | ||
| Chardonnay 4 | 4 | ||
| Sauvignon Blanc | 5 | ||
| Viognier | 6 | ||
| Egiodola | 7 | ||
| Cabernet Franc | 8 | ||
| Cabernet Sauvignon | 9 | ||
| Marselan | 10 | ||
| Merlot | 11 | ||
| Pinot Noir 1 | 12 | ||
| Pinot Noir 2 | 13 | ||
| Syrah | 14 | ||
| Muscat Ottonel | Pirgovo (2) | 15 | |
| Chardonnay | Suvorovo (3) | Black Sea Region | 16 |
| Sauvignon Blanc | 17 | ||
| Cabernet Sauvignon | Starosel (4) | Rose Valley | 18 |
| Chardonnay | Topoli dol (5) | Thracian Valley | 19 |
| Tamyanka | 20 | ||
| Cabernet Sauvignon | 21 | ||
| Syrah | 22 | ||
| Sauvignon Blanc | Brestnik (6) | 23 | |
| Cabernet Franc | 24 | ||
| Syrah | 25 | ||
| Cabernet Sauvignon | Chernodab (7) | 26 | |
| Cabernet Sauvignon | Levunovo (8) | Struma Valley | 27 |
| Merlot | 28 | ||
| Melnik | General Todorov (9) | 29 | |
| Shiroka Melnishka Loza | Vranya (10) | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladenova, E.; Voyslavov, T.; Bakardzhiyski, I.; Karadjova, I. From the Soil to the Wine—Elements’ Migration in Monovarietal Bulgarian Wines. Molecules 2025, 30, 475. https://doi.org/10.3390/molecules30030475
Mladenova E, Voyslavov T, Bakardzhiyski I, Karadjova I. From the Soil to the Wine—Elements’ Migration in Monovarietal Bulgarian Wines. Molecules. 2025; 30(3):475. https://doi.org/10.3390/molecules30030475
Chicago/Turabian StyleMladenova, Elisaveta, Tsvetomil Voyslavov, Ivan Bakardzhiyski, and Irina Karadjova. 2025. "From the Soil to the Wine—Elements’ Migration in Monovarietal Bulgarian Wines" Molecules 30, no. 3: 475. https://doi.org/10.3390/molecules30030475
APA StyleMladenova, E., Voyslavov, T., Bakardzhiyski, I., & Karadjova, I. (2025). From the Soil to the Wine—Elements’ Migration in Monovarietal Bulgarian Wines. Molecules, 30(3), 475. https://doi.org/10.3390/molecules30030475








