Optimization of Natural Deep Eutectic Solvent-Assisted Extraction of Rosmarinic Acid from Thunbergia laurifolia Lindl. and Evaluation of Antioxidant Activity
Abstract
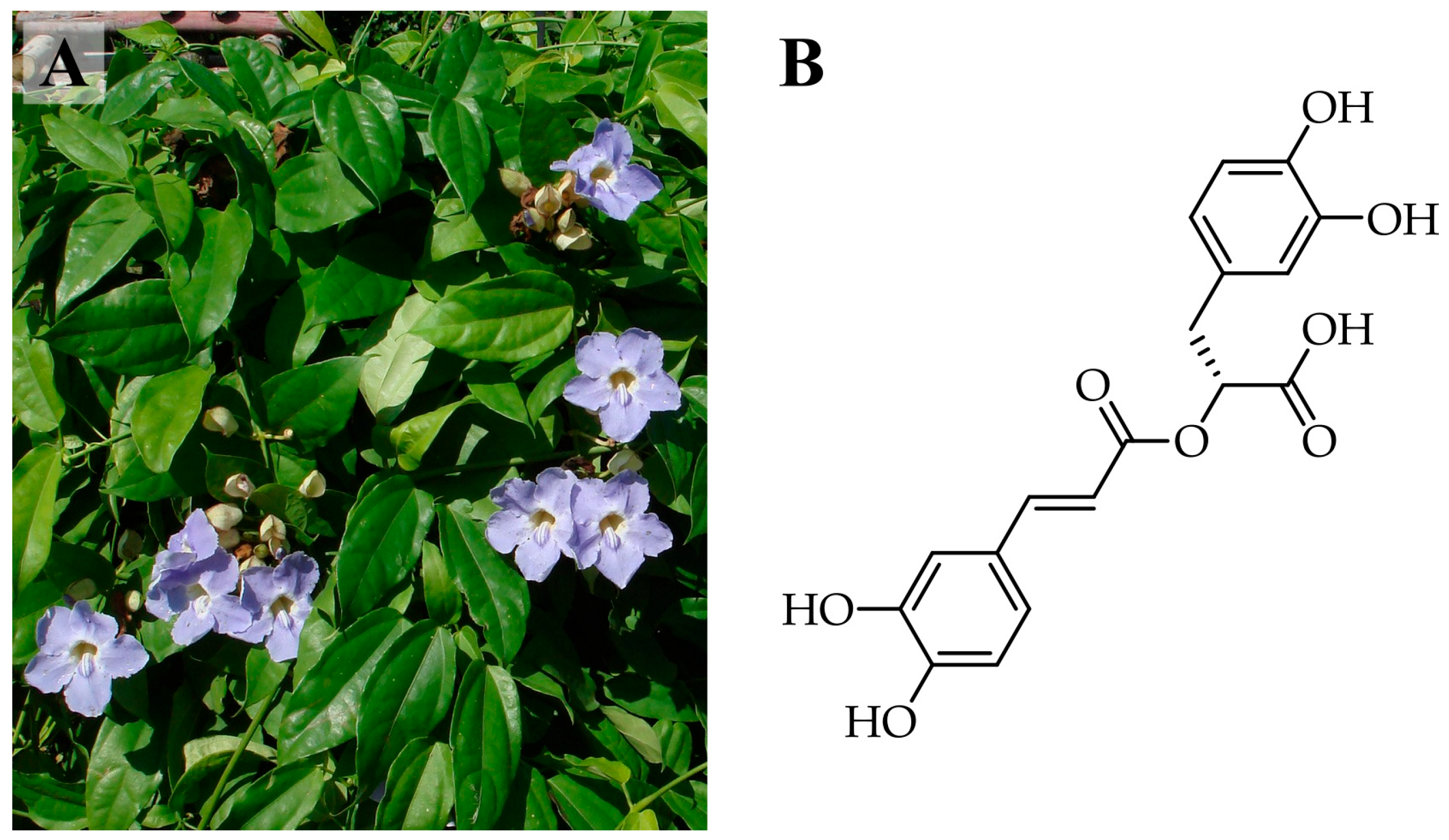
1. Introduction
2. Results and Discussion
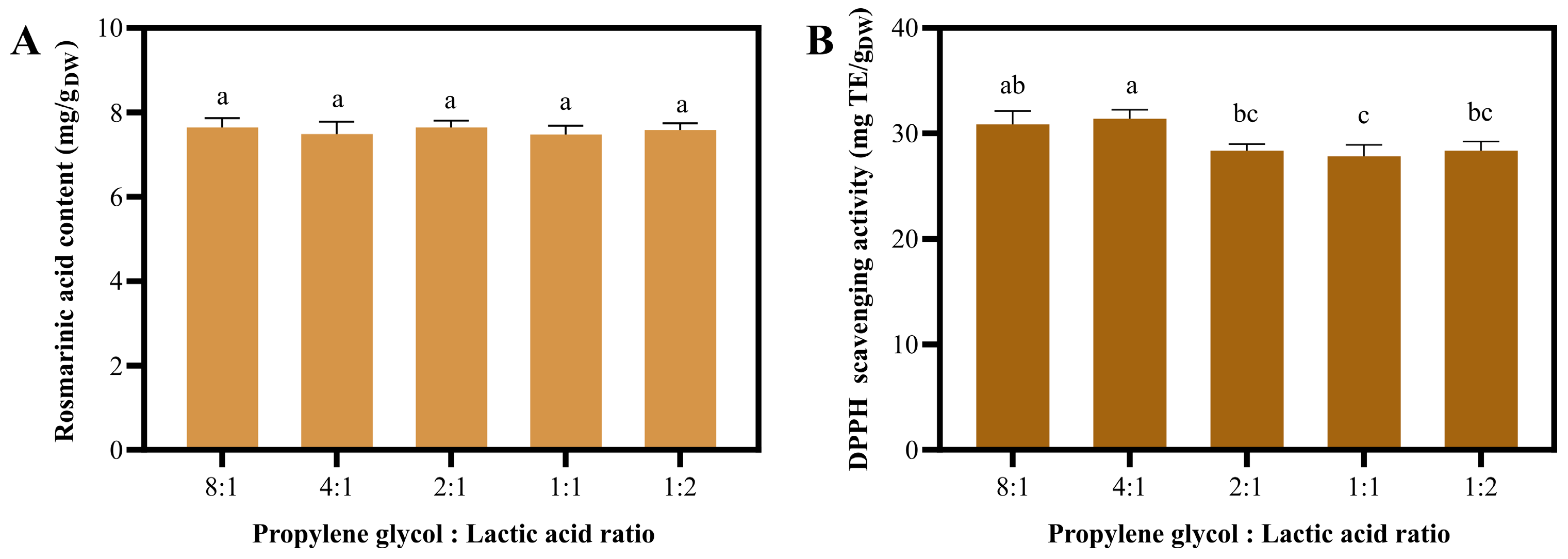
2.1. The Suitable NaDES Combination for Ultrasonic-Assisted Extraction of T. laurifolia Was Determined by Rosmarinic Acid Content and DPPH Scavenging Activity
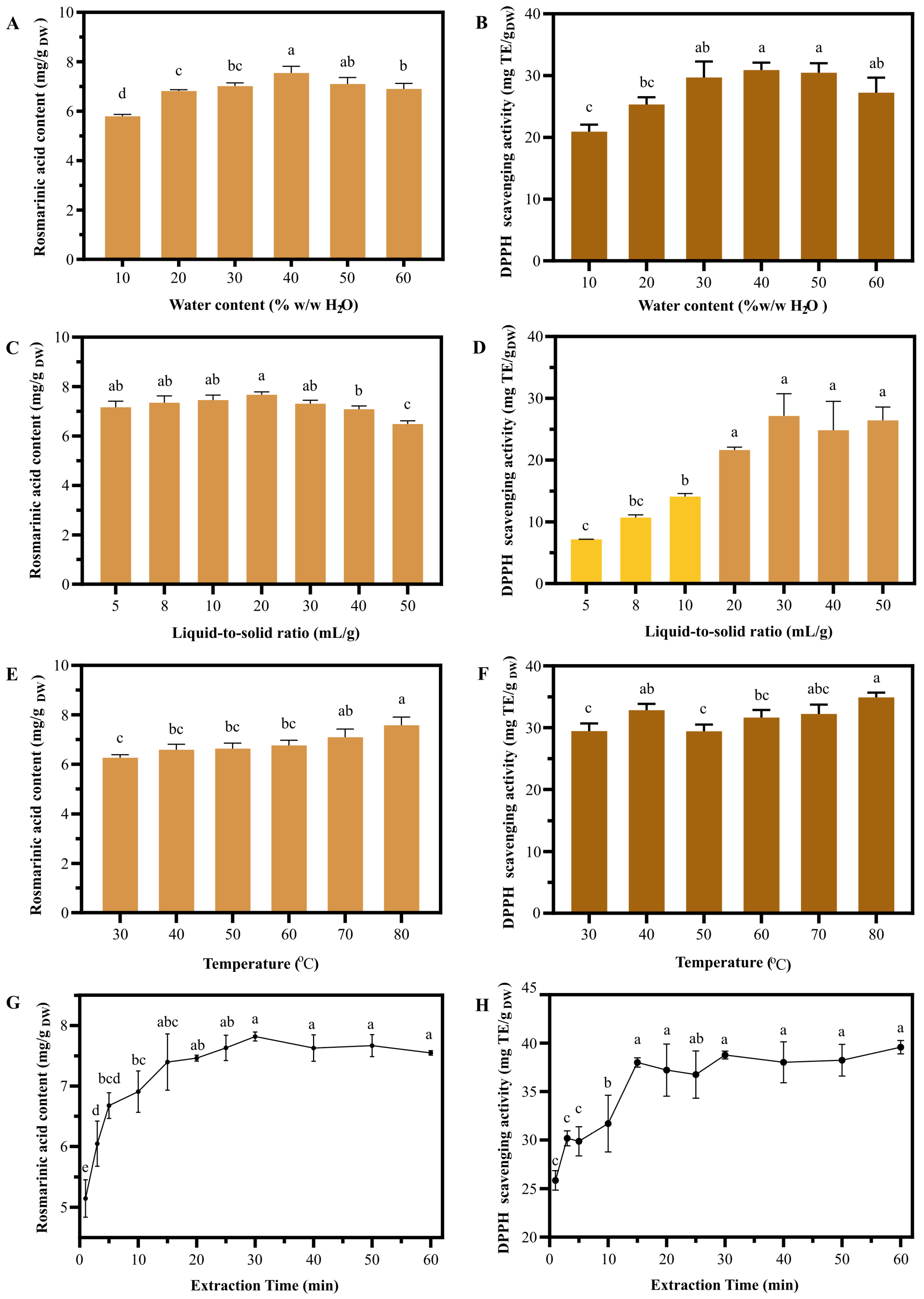
2.2. Optimal Extraction Parameters Were Successively Obtained from One-Factor Experiments
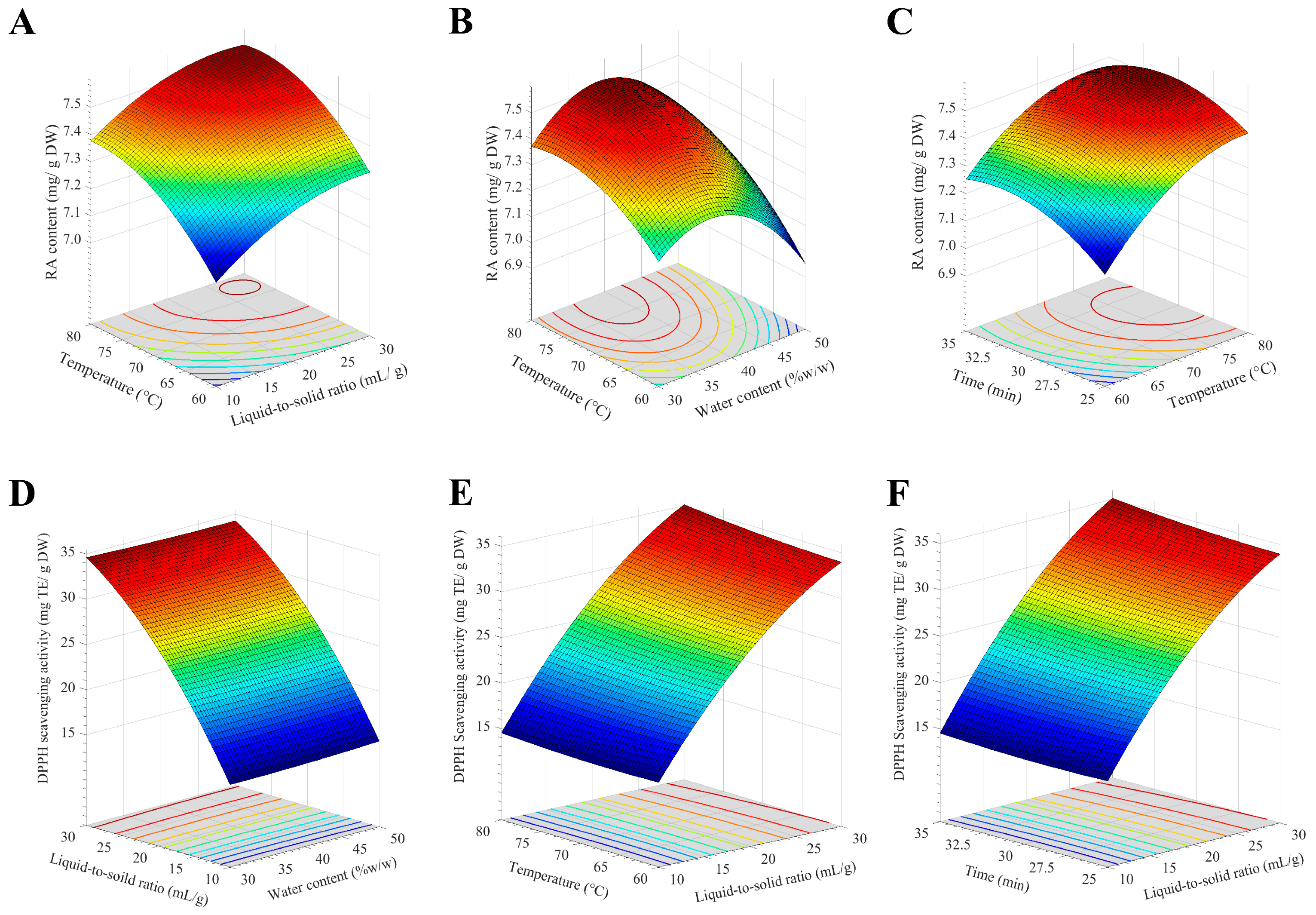
2.3. The Optimized Extraction Conditions Were Achieved by Response Surface Methodology with Box–Behnken Design
− 5.0639 × 10−4 W × L + 3.9121 × 10−4 W × T − 9.3343 × 10−4 W × M
− 1.2810 × 10−4 L × T + 1.2070 × 10−4 L × M − 5.1194 × 10−4 T × M
− 1.8449 × 10−3 W2 − 5.8046 × 10−4 L2 − 8.1489 × 10−4 T2
− 2.8930 × 10−3 M2
(mg TE/g DW) − 1.9003 × 10−3 W × L − 2.2598 × 10−3 W × T − 2.4652 × 10−3 W × M
+ 2.1571 × 10−3 L × T + 1.7633 × 10−2 L × M + 1.1642 × 10−3 T × M
+ 1.0129 × 10−3 W2 − 2.4110 × 10−2 L2 + 2.2541 × 10−3 T2
+ 2.5349 × 10−2 M2
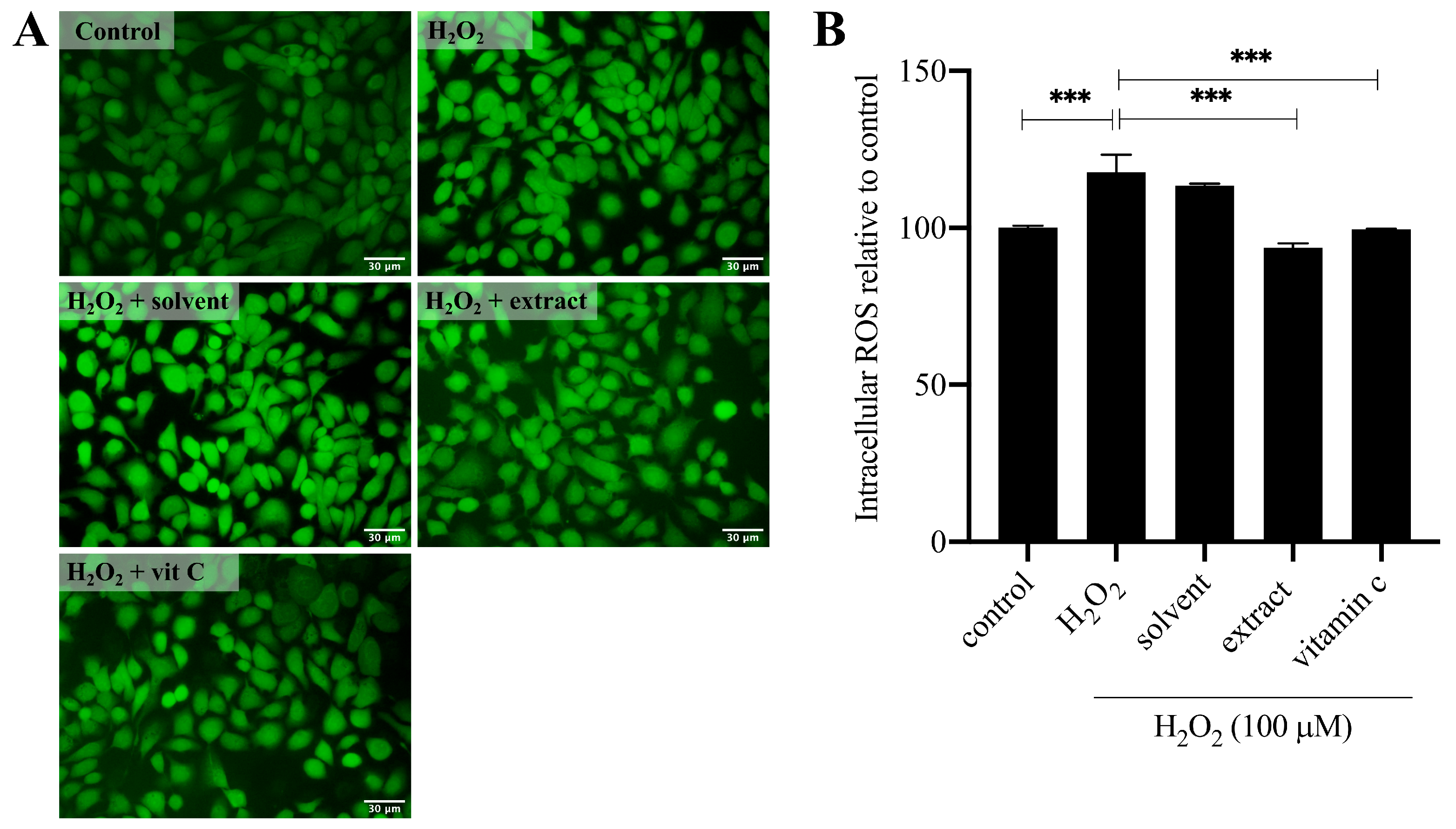
2.4. NaDES-Assisted T. laurifolia Extract Suppresses H2O2-Induced Intracellular ROS Production
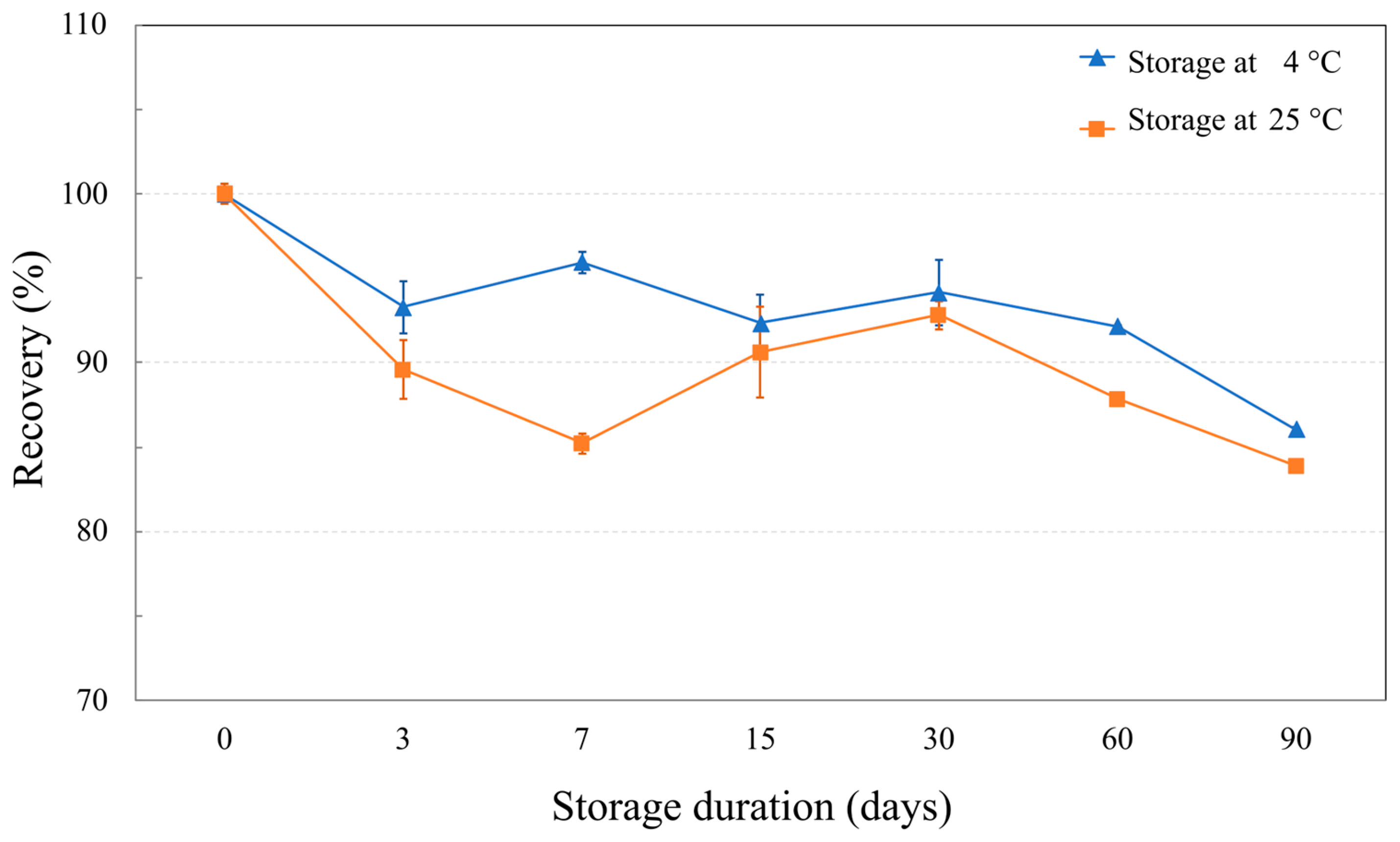
2.5. NaDES-Assisted T. laurifolia Extract Was Stable Under Stress and Various Storage Conditions
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. Preparing and Screening of NaDES
3.4. Determination of Rosmarinic Acid Content Using HPLC
3.5. Evaluation of Antioxidant Activity of T. laurifolia Extracts
3.6. One Factor Experiments
3.7. Multi-Factor Experimental Designs and Response Surface Methodology (RSM)
3.8. Reactive Oxygen Species Scavenging Assay on H2O2-Induced Keratinocytes
3.8.1. Cell Culture
3.8.2. Cell Viability Assay
3.8.3. Measurement of Intracellular ROS
3.9. Chemical Stability Study of NaDES-Assisted T. laurifolia Extract
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cit | Citric acid |
| ChCl | Choline chloride |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FBS | Fetal bovine serum |
| Glu | Glucose |
| Gly | Glycerol |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| La | Lactic acid |
| NaDES | natural deep eutectic solvent |
| PD | Propandiol |
| PG | Propylene glycol |
| RA | Rosmarinic acid |
| Sorb | Sorbitol |
| VitC | Ascorbic acid |
References
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Sanchez Viera, M.; Marrot, L.; Chuberre, B.; Dreno, B. The impact of airborne pollution on skin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496–1505. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M. The dark side of beauty: An in-depth analysis of the health hazards and toxicological impact of synthetic cosmetics and personal care products. Front. Public Health 2024, 12, 1439027. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Goyal, N.; Jerold, F. Biocosmetics: Technological advances and future outlook. Environ. Sci. Pollut. Res. 2023, 30, 25148–25169. [Google Scholar] [CrossRef] [PubMed]
- Thunbergia laurifolia Lindl. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:56339-1/general-information (accessed on 22 October 2025).
- Chan, E.W.C.; Eng, S.Y.; Tan, Y.P.; Wong, Z.C. Phytochemistry and Pharmacological Properties of Thunbergia laurifolia: A Review. Pharmacogn. J. 2011, 3, 1–6. [Google Scholar] [CrossRef]
- Taguchi, R.; Hatayama, K.; Takahashi, T.; Hayashi, T.; Sato, Y.; Sato, D.; Ohta, K.; Nakano, H.; Seki, C.; Endo, Y.; et al. Structure–activity relations of rosmarinic acid derivatives for the amyloid β aggregation inhibition and antioxidant properties. Eur. J. Med. Chem. 2017, 138, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Suwanchaikasem, P.; Chaichantipayuth, C.; Sukrong, S. Antioxidant-guided Isolation of Rosmarinic Acid, a Major Constituent from Thunbergia laurifolia, and Its Use as a Bioactive Marker for Standardization. Chiang Mai J. Sci. 2014, 41, 117–127. [Google Scholar]
- Popova, M.; Bankova, V. Contemporary methods for the extraction and isolation of natural products. BMC Chem. 2023, 17, 68. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jayakody, J.T.M.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Villa, C.; Caviglia, D.; Robustelli della Cuna, F.S.; Zuccari, G.; Russo, E. NaDES Application in Cosmetic and Pharmaceutical Fields: An Overview. Gels 2024, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, D.; Russo, E.; Schito, A.M.; Robustelli della Cuna, F.S.; Grignani, E.; Lionetti, N.; Villa, C. NaDES-Based Extracts by Microwave Activation from Laurus nobilis L. Leaves: Sustainable Multifunctional Ingredients for Potential Cosmetic and Pharmaceutical Applications. Molecules 2025, 30, 3006. [Google Scholar] [CrossRef] [PubMed]
- Srimawong, C.; Torkaew, P.; Putalun, W. Polyphenol-enriched extraction from Thunbergia laurifolia using natural deep eutectic solvents for enhanced antioxidant and anti-inflammatory activities. RSC Adv. 2025, 15, 22086–22096. [Google Scholar] [CrossRef]
- 2006/257/EC: Commission Decision of 9 February 2006 Amending Decision 96/335/EC Establishing an Inventory and a Common Nomenclature of Ingredients Employed in Cosmetic Products (Text with EEA Relevance). Available online: http://data.europa.eu/eli/dec/2006/257/oj (accessed on 22 October 2025).
- GRAS Substances (SCOGS) Database. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database (accessed on 23 October 2025).
- COSMILE Europe. Available online: https://cosmileeurope.eu/ (accessed on 22 October 2025).
- Kim, S.B.; Bisson, J.; Friesen, J.B.; Pauli, G.F.; Simmler, C. Selective Chlorophyll Removal Method to “Degreen” Botanical Extracts. J. Nat. Prod. 2020, 83, 1846–1858. [Google Scholar] [CrossRef]
- Kovács, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Rojsanga, P.; Raksaskulwong, G.; Ruaysaptawee, K.; Chooluck, K. Preliminary findings of the effect of infusion variables on marker contents and antioxidant activity of Thunbergia laurifolia tea. Pharm. Sci. Asia 2018, 45, 243–251. [Google Scholar] [CrossRef]
- Choi, D.-I.; Park, J.-H.; Choi, J.-Y.; Piao, M.; Suh, M.-S.; Lee, J.-B.; Yun, S.-J.; Lee, S.-C. Keratinocytes-Derived Reactive Oxygen Species Play an Active Role to Induce Type 2 Inflammation of the Skin: A Pathogenic Role of Reactive Oxygen Species at the Early Phase of Atopic Dermatitis. Ann. Dermatol. 2021, 33, 26–36. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products. Cosmetics 2018, 5, 19. [Google Scholar] [CrossRef]
- Naharro-Rodriguez, J.; Bacci, S.; Hernandez-Bule, M.L.; Perez-Gonzalez, A.; Fernandez-Guarino, M. Decoding Skin Aging: A Review of Mechanisms, Markers, and Modern Therapies. Cosmetics 2025, 12, 144. [Google Scholar] [CrossRef]
- Woottisin, N.; Kongkiatpaiboon, S.; Sukprasert, S.; Sathirakul, K. Development and Validation of Stability Indicating HPLC Method for Determination of Caffeic Acid, Vitexin and Rosmarinic Acid in Thunbergia laurifolia Leaf Extract. Pharmacogn. J. 2020, 12, 611–618. [Google Scholar] [CrossRef]
- Bodalska, A.; Kowalczyk, A.; Fecka, I. Stability of Rosmarinic Acid and Flavonoid Glycosides in Liquid Forms of Herbal Medicinal Products—A Preliminary Study. Pharmaceuticals 2021, 14, 1139. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Hanczné Lakatos, E.; Kapcsándi, V.; Székelyhidi, R.; Ajtony, Z. Investigation of the long-term stability of various tinctures belonging to the lamiaceae family by HPLC and spectrophotometry method. Chem. Pap. 2021, 75, 5781–5791. [Google Scholar] [CrossRef]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation Study of Carnosic Acid, Carnosol, Rosmarinic Acid, and Rosemary Extract (Rosmarinus officinalis L.) Assessed Using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef]
- Santana, A.P.R.; Mora-Vargas, J.A.; Guimarães, T.G.S.; Amaral, C.D.B.; Oliveira, A.; Gonzalez, M.H. Sustainable synthesis of natural deep eutectic solvents (NADES) by different methods. J. Mol. Liq. 2019, 293, 111452. [Google Scholar] [CrossRef]
- Thongphichai, W.; Hasriadi, H.; Wasana, P.W.D.; Jayashan, S.S.; Sritularak, B.; Towiwat, P.; Sukrong, S. Anti-inflammatory activity of Curcuma wanenlueanga Saensouk, Thomudtha & Boonma rhizomes and the search for its bioactive markers by harmonizing bioassay-guided isolation and network pharmacology. BMC Complement. Med. Ther. 2025, 25, 143. [Google Scholar] [CrossRef]
- Li, S.; Wang, G.; Zhao, J.; Ou, P.; Yao, Q.; Wang, W. Ultrasound-Assisted Extraction of Phenolic Compounds from Celtuce (Lactuca sativa var. augustana) Leaves Using Natural Deep Eutectic Solvents (NADES): Process Optimization and Extraction Mechanism Research. Molecules 2024, 29, 2385. [Google Scholar] [CrossRef]
- Vo, T.P.; Pham, T.V.; Weina, K.; Tran, T.N.H.; Vo, L.T.V.; Nguyen, P.T.; Bui, T.L.H.; Phan, T.H.; Nguyen, D.Q. Green extraction of phenolics and flavonoids from black mulberry fruit using natural deep eutectic solvents: Optimization and surface morphology. BMC Chem. 2023, 17, 119. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual [Internet]; Markossian, S., Grossman, A., Baskir, H., Eds.; Eli Lilly & Company: Indianapolis, IN, USA; The National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013. [Google Scholar]

| No. | Name of NaDES | Component 1 | Component 2 |
|---|---|---|---|
| 1 | ChCl:La | Choline chloride | Lactic acid |
| 2 | PD:La | Propanediol | Lactic acid |
| 3 | Gly:La | Glycerol | Lactic acid |
| 4 | PG:La | Propylene glycol | Lactic acid |
| 5 | ChCl:Glu | Choline chloride | Glucose |
| 6 | PD:Glu | Propanediol | Glucose |
| 7 | Gly:Glu | Glycerol | Glucose |
| 8 | PG:Glu | Propylene glycol | Glucose |
| 9 | ChCl:Sorb | Choline chloride | Sorbitol |
| 10 | PD:Sorb | Propanediol | Sorbitol |
| 11 | Gly:Sorb | Glycerol | Sorbitol |
| 12 | PG:Sorb | Propylene glycol | Sorbitol |
| 13 | Cit:Sorb | Citric acid | Sorbitol |
| 14 | La:Sorb | Lactic acid | Sorbitol |
| 15 | EtOH95 | 95% (v/v) Aqueous ethanol | |
| 16 | EtOH70 | 70% (v/v) Aqueous ethanol |
| Factors | Units | Symbols | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Water content | % w/w | W | 30 | 40 | 50 |
| Liquid-to-solid ratio | mL/g | L | 10 | 20 | 30 |
| Extraction temperature | °C | T | 60 | 70 | 80 |
| Extraction time | min | M | 25 | 30 | 35 |
| Stress Type | Reagent | Condition | %Recovery |
|---|---|---|---|
| Control | - | - | 100.00 ± 0.39 |
| Acidic | 37% HCl | 60 °C, 60 min | 81.77 ± 0.44 *** |
| Basic | 5N NaOH | 60 °C, 60 min | 100.19 ± 3.63 |
| Oxidative | 30% H2O2 | 60 °C, 60 min | 101.61 ± 3.81 |
| Photolytic | - | Fluorescent lamp 4000 Lux, ambient (25 °C), 72 h | 95.72 ± 2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kriengsaksri, K.; Thongphichai, W.; Uttarawichien, T.; Khoochonthara, J.; Towiwat, P.; Sukrong, S. Optimization of Natural Deep Eutectic Solvent-Assisted Extraction of Rosmarinic Acid from Thunbergia laurifolia Lindl. and Evaluation of Antioxidant Activity. Molecules 2025, 30, 4795. https://doi.org/10.3390/molecules30244795
Kriengsaksri K, Thongphichai W, Uttarawichien T, Khoochonthara J, Towiwat P, Sukrong S. Optimization of Natural Deep Eutectic Solvent-Assisted Extraction of Rosmarinic Acid from Thunbergia laurifolia Lindl. and Evaluation of Antioxidant Activity. Molecules. 2025; 30(24):4795. https://doi.org/10.3390/molecules30244795
Chicago/Turabian StyleKriengsaksri, Krittima, Wisuwat Thongphichai, Tamonwan Uttarawichien, Jasadakorn Khoochonthara, Pasarapa Towiwat, and Suchada Sukrong. 2025. "Optimization of Natural Deep Eutectic Solvent-Assisted Extraction of Rosmarinic Acid from Thunbergia laurifolia Lindl. and Evaluation of Antioxidant Activity" Molecules 30, no. 24: 4795. https://doi.org/10.3390/molecules30244795
APA StyleKriengsaksri, K., Thongphichai, W., Uttarawichien, T., Khoochonthara, J., Towiwat, P., & Sukrong, S. (2025). Optimization of Natural Deep Eutectic Solvent-Assisted Extraction of Rosmarinic Acid from Thunbergia laurifolia Lindl. and Evaluation of Antioxidant Activity. Molecules, 30(24), 4795. https://doi.org/10.3390/molecules30244795







