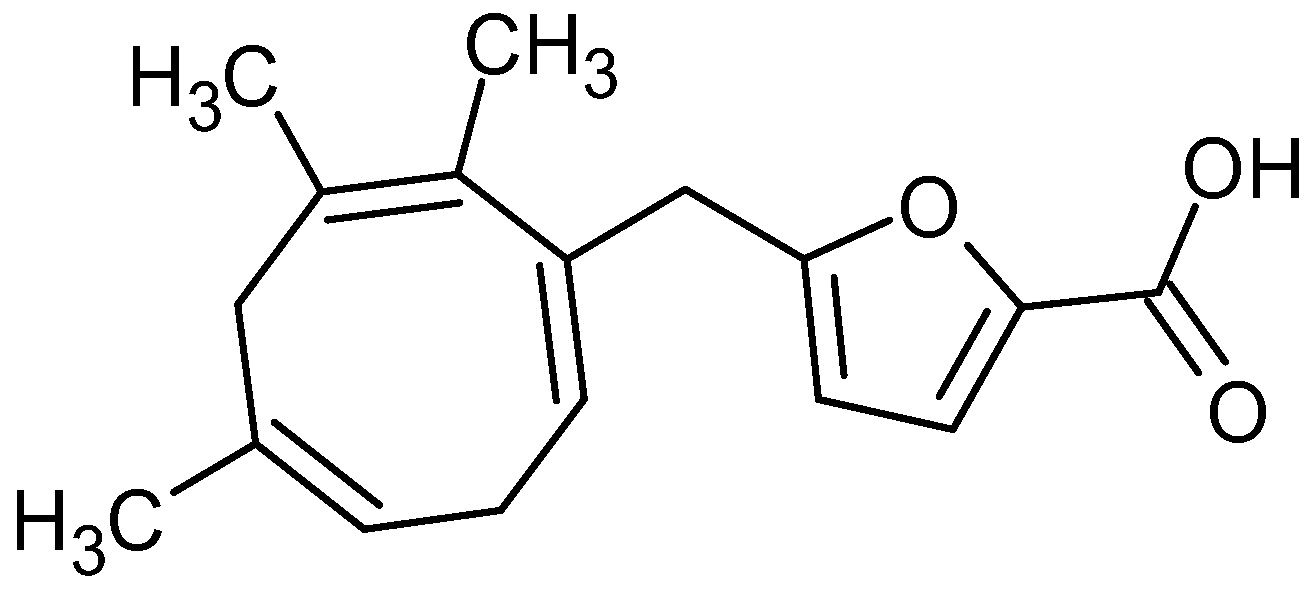
Novel Trinorditerpene from Dysoxylum parasiticum (Osbeck) Kosterm: Leaf Extract with Cytotoxic, Antioxidant and α-Glucosidase Inhibitory Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Cytotoxic Activity
2.2. Antioxidant Activity
2.3. α-Glucosidase Enzyme Inhibition
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation
3.3. Plant Materials
3.4. Extraction
3.5. Isolation and Purification
3.6. Cell Culture and Cytotoxic Activity
3.7. DPPH Free Radical Scavenging Activity
3.8. α-Glucosidase Inhibitory Activity
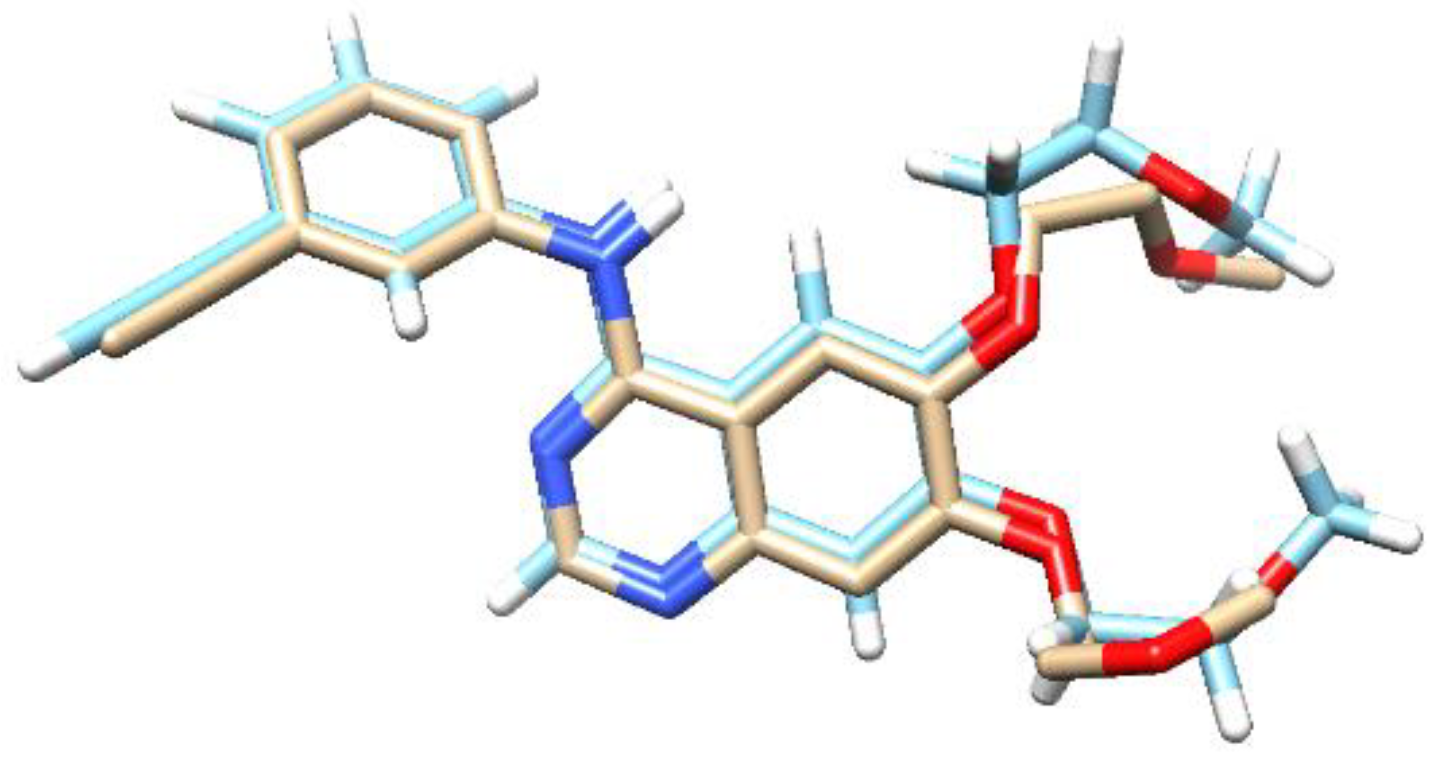
3.9. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, H.S. Oxidative Stress and ROS Link Diabetes and Cancer. J. Mol. Pathol. 2024, 5, 96–119. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Bray, F. The Evolving Scale and Profile of Cancer Worldwide: Much Ado About Everything. Cancer Epidemiol. Biomark. Prev. 2016, 25, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.K. The Diabetes-Cancer Link. Diabetes Spectr. 2014, 27, 276–280. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Bhutani, J.; OKeefe, J.H. Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2015, 2, e000327. [Google Scholar] [CrossRef] [PubMed]
- Nakhaee, A.; Sanjari, M. Evaluation of effect of acarbose consumption on weight losing in non-diabetic overweight or obese patients in Kerman. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 391–394. [Google Scholar]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Busselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G. Chemoprevention of Human Prostate Cancer by Oral Administration of Green Tea Catechins in Volunteers with High-Grade Prostate Intraepithelial Neoplasia: A Preliminary Report from a One-Year Proof-of-Principle Study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef]
- Bushman, J.L. Green tea and cancer in humans: A review of the literature. Nutr. Cancer 1998, 31, 151–159. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Hossain, M.; Islam, M.; Jahan, I.; Hasan, M.K. Aphanamixis polystachya: Pharmacological benefits, health benefits and other potential benefits. Phytomed. Plus 2023, 3, 100448. [Google Scholar] [CrossRef]
- Naini, A.A.; Mayanti, T.; Nurlelasari; Harneti, D.; Maharani, R.; Safari, A.; Hidayat, A.T.; Farabi, K.; Lesmana, R.; Supratman, U.; et al. Cytotoxic sesquiterpenoids from Dysoxylum parasiticum (Osbeck) Kosterm. stem bark. Phytochem. Lett. 2022, 47, 102–106. [Google Scholar] [CrossRef]
- Subarnas, A.; Diantini, A.; Abdulah, R.; Zuhrotun, A.; Yamazaki, C.; Nakazawa, M.; Koyama, H. Antiproliferative activity of primates-consumed plants against MCF-7 human breast cancer cell lines. E3 J. Med. Res. 2012, 1, 038–43. [Google Scholar]
- Sofian, F.F.; Subarnas, A.; Hakozaki, M.; Uesugi, S.; Koseki, T.; Shiono, Y. Bidysoxyphenols A–C, dimeric sesquiterpene phenols from the leaves of Dysoxylum parasiticum (Osbeck) Kosterm. Fitoterapia 2022, 158, 105157. [Google Scholar] [CrossRef]
- Sofian, F.F.; Subarnas, A.; Hakozaki, M.; Uesugi, S.; Koseki, T.; Shiono, Y. Tridysoxyphenols A and B, two new trimeric sesquiterpene phenols from Dysoxylum parasiticum leaves. Phytochem. Lett. 2022, 50, 134–140. [Google Scholar] [CrossRef]
- Naini, A.; Mayanti, T.; Harneti, D.; Nurlelasari, D.; Maharani, R.; Farabi, K.; Herlina, T.; Supratman, U.; Fajriah, S.; Kuncoro, H.; et al. Sesquiterpenoids and sesquiterpenoid dimers from the stem bark of Dysoxylum parasiticum (osbeck) kosterm. Phytochemistry 2023, 205, 113477. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Cheng, J.-C.; Hwang, T.-L.; Shen, D.Y.; Wu, T.S. Trinorditerpenes from the roots of Flueggea virosa. Bioorg Med. Chem. Lett. 2014, 24, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, H.; Li, H.; Zhang, W.D. Podocarpane trinorditerpenes from Celastrus angulatus and their biological activities. Fitoterapia 2018, 130, 156–162. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Osman, A.-M.M.; Bayoumi, H.M.; Al-Harthi, S.E.; Damanhouri, Z.A.; ElSal, M.F. Modulation of doxorubicin cytotoxicity by resveratrol in a human breast cancer cell line. Cancer Cell Int. 2012, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, E.P.; Bini, R.D.; Endo, K.M.; de Oliveira, V.A., Jr.; de Almeida, I.V.; Dias, G.S.; dos Santos, I.A.; de Oliveira, P.N.; Vicentini, V.E.P.; Cotica, L.F. Doxorubicin-Loaded Magnetic Nanoparticles: Enhancement of Doxorubicin’s Effect on Breast Cancer Cells (MCF-7). Magnetochemistry 2022, 8, 114. [Google Scholar] [CrossRef]
- Lafi, Z.; Alshaer, W.; Gharaibeh, L.; Alqudah, D.A.; Alquaissi, B.; Bashaireh, B.; Ibrahim, A.A. Synergistic combination of doxorubicin with hydralazine, and disulfiram against MCF-7 breast cancer cell line. PLoS ONE 2023, 18, e0291981. [Google Scholar] [CrossRef]
- Voon, K.J.; Sivasothy, Y.; Sundralingam, U.; Lalmahomed, A.; Goh, A.P.T. Cytotoxic Labdane Diterpenes, Norlabdane Diterpenes and Bis-Labdanic Diterpenes from the Zingiberaceae: A Systematic Review. Pharmaceuticals 2022, 15, 1517. [Google Scholar] [CrossRef]
- Hernández, Á.P.; Chamorro, P.; Rodríguez, M.L.; del Corral, J.M.M.; García, P.A.; Francesch, A.; San Feliciano, A.; Castro, M.A. New Antineoplastic Naphthohydroquinones Attached to Labdane and Rearranged Diterpene Skeletons. Molecules 2021, 26, 474. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J. Why the Epidermal Growth Factor Receptor? The Rationale for Cancer Therapy. Oncologist 2002, 7, 2–8. [Google Scholar] [CrossRef]
- Yuanita, E.; Pranowo, H.D.; Mustofa, M.; Swasono, R.T.; Syahri, J.; Jumina, J. Synthesis, Characterization and Molecular Docking of Chloro-substituted Hydroxyxanthone Derivatives. Chem. J. Mold. 2019, 14, 68–76. [Google Scholar] [CrossRef]
- Ismail, N.S.M.; Ali, E.M.H.; Ibrahim, D.A.; Serya, R.A.T.; Abou El Ella, D.A. Pyrazolo [3,4-d]pyrimidine based scaffold derivatives targeting kinases as anticancer agents. Future J. Pharm. Sci. 2016, 2, 20–30. [Google Scholar] [CrossRef]
- Cosconati, S.; Forli, S.; Perryman, A.L.; Harris, R.; Goodsell, D.S.; Olson, A.J. Virtual screening with AutoDock: Theory and practice. Expert Opin. Drug Discov. 2010, 5, 597–607. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa Has More Phenolic Phytochemicals and a Higher Antioxidant Capacity than Teas and Red Wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef]
- Vo, Q.V.; Tam, N.M.; Hieu, L.T.; Bay, M.V.; Thong, N.M.; Huyen, T.L.; Hoa, N.T.; Mechler, A. The antioxidant activity of natural diterpenes: Theoretical insights. RSC Adv. 2020, 10, 14937–14943. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Capanoglu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, A. Structural Diversity and Bioactivity of Labdane and Clerodane Diterpenoids. J. Synth. Org. Chem. Jpn. 2004, 62, 872–881. [Google Scholar] [CrossRef]
- Ngo, T.C.; Dao, D.Q.; Mai, T.V.-T.; Nguyen, T.L.A.; Huynh, L.K. On The Radical Scavenging and DNA Repairing Activities by Natural Oxygenated Diterpenoids: Theoretical Insights. J. Chem. Inf. Model. 2022, 62, 2365–2377. [Google Scholar] [CrossRef]
- Stobiecka, A. A DFT Study on the Radical-Scavenging Properties of Ferruginol-Type Diterpenes. Food Biophys. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Yin, X.Y.; Gao, M.K.; Wang, H.T.; Chen, Q.; Kong, B.H. Probing the interaction between selected furan derivatives and porcine myofibrillar proteins by spectroscopic and molecular docking approaches. Food Chem. 2022, 397, 133776. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xu, B.; Niu, Y.; Xu, F.R.; Liang, L.; Wang, C.; Yu, J.P.; Yan, G.; Wang, W.; Jin, H.W.; et al. Synthesis, Bioactivity, Docking and Molecular Dynamics Studies of Furan-Based Peptides as 20S Proteasome Inhibitors. ChemMedChem 2015, 10, 498–510. [Google Scholar] [CrossRef]
- Doorandishan, M.; Pirhadi, S.; Gholami, M.; Jassbi, A.R. In silico studies of bis-spiro- and Furano-Labdane diterpenoids from Rydingia persica Scheen (Otostegia persica) as α-glucosidase enzyme inhibitor. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Ghosh, S.; Rangan, L. Molecular Docking Inhibition Kinetics of α-glucosidase Activity by Labdane Diterpenes Isolated from Tora Seeds (Alpinia nigra, B.L. Burtt.). Appl. Biochem. Biotechnol. 2015, 175, 1477–1489. [Google Scholar] [CrossRef]
- Gafar, M.K.; Salim, F.; Anouar, E.H.; Shah, S.A.A.; Azman, I.I.N.; Ahmad, R. Diastereotopic labdane diterpenoids from rhizomes of Hedychium coronarium with α-glucosidase activity and their molecular docking study. Phytochem. Lett. 2023, 53, 47–55. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Dhanawansa, R.; Faridmoayer, A.; van der Merwe, G.; Li, X.Y.; Scaman, C.H. Overexpression, purification, and partial characterization of Saccharomyces cerevisiae processing alpha glucosidase I. Glycobiology 2002, 12, 229–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Alhadramy, M.S. Diabetes and oral therapies. J. Taibah Univ. Med. Sci. 2016, 11, 317–329. [Google Scholar] [CrossRef]
- Artanti, N.; Handayani, S.; Devi, A.F.; Dewijanti, I.D.; Mulyani, H.; Dewi, R.T.; Udin, L.Z.; Hanafi, M.; Musdalifah, D.; Lelono, R.A.A.; et al. In vitro bioactivities analysis of Indonesian mistletoes and selaginella herbal tea infusions. In Proceedings of the 4th International Symposium on Applied Chemistry, Banten, Indonesia, 1–2 November 2018. [Google Scholar]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Wang, M.-H.; Rhee, H.-I. A novel α-glucosidase inhibitor from pine bark. Carbohydr. Res. 2004, 339, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Mustika, C.R.; Astuti, E.; Mardjan, M.I.D. Molecular Docking Synthesis In Vitro Antiplasmodium Assay of Monoketone Curcumin Analogous from 2-Chlorobenzaldehyde Indones. J. Chem. 2024, 24, 638–651. [Google Scholar]




| Compound | Concentration (µg/mL) | % Inhibition | IC50 (µg/mL) | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Average | |||
| Erlotinib | 40 | 99.73 | 98.83 | 98.64 | 99.1 ± 0.5 | 6.6 ± 0.4 |
| 20 | 87.21 | 86.26 | 87.56 | 87.0 ± 0.6 | ||
| 10 | 63.19 | 61.02 | 62.12 | 63.0 ± 0.9 | ||
| 5 | 33.74 | 32.83 | 33.63 | 33.0 ± 0.4 | ||
| Parasitic acid | 100 | 92.6 | 93.1 | 92.8 | 92.8 ± 0.3 | 29.0 ± 0.8 |
| 50 | 70.8 | 71.0 | 70.2 | 70.7 ± 0.4 | ||
| 25 | 52.0 | 50.3 | 51.9 | 51.4 ± 1.0 | ||
| 12.5 | 32.7 | 31.2 | 31.5 | 31.8 ± 0.8 | ||
| Compound | Binding Energy (kcal mol−1) | Hydrogen Bond |
|---|---|---|
| Erlotinib | −8.27 | Lys705, Met769, Cys773 |
| Parasitic acid | −8.18 | Met769 |
| Compound | Concentration (µg/mL) | %Reduction Activity | IC50 (µg/mL) |
|---|---|---|---|
| Quercetin | 1 | 6.91 | 6.1 ± 0.1 |
| 2.5 | 17.30 | ||
| 5 | 56.82 | ||
| 10 | 94.66 | ||
| Parasitic acid | 5 | 26.58 | 10.91 ± 0.04 |
| 10 | 53.51 | ||
| 25 | 92.93 |
| Compound | Concentration (µg/mL) | %Inhibition | IC50 (µg/mL) |
|---|---|---|---|
| Quercetin | 1 | 46.84 | 1.29 ± 0.06 |
| 2.5 | 58.89 | ||
| 5 | 73.87 | ||
| 10 | 86.16 | ||
| Parasitic acid | 5 | 0.39 | 36 ± 1 |
| 10 | 2.32 | ||
| 20 | 15.12 | ||
| 25 | 28.18 |
| Compound | Binding Energy (k cal mol−1) | Hydrogen Bond |
|---|---|---|
| α-glucopyranose | −6.99 | Asp69, His112, Asp215, Arg213, Glu277, His351, Asp352, Agr442 |
| Quercetin | −7.16 | Arg213, Asp215, Glu277, Gln279, Glu411 |
| Parasitic acid | −5.28 | Tyr72, Asp215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahon, P.J.; Hanafi, M.; Artanti, N.; Wibawa, I.P.A.H.; Hermawan, F.; Minarti; Lotulung, P.D.; Butardo, V.M., Jr. Novel Trinorditerpene from Dysoxylum parasiticum (Osbeck) Kosterm: Leaf Extract with Cytotoxic, Antioxidant and α-Glucosidase Inhibitory Activities. Molecules 2025, 30, 4747. https://doi.org/10.3390/molecules30244747
Mahon PJ, Hanafi M, Artanti N, Wibawa IPAH, Hermawan F, Minarti, Lotulung PD, Butardo VM Jr. Novel Trinorditerpene from Dysoxylum parasiticum (Osbeck) Kosterm: Leaf Extract with Cytotoxic, Antioxidant and α-Glucosidase Inhibitory Activities. Molecules. 2025; 30(24):4747. https://doi.org/10.3390/molecules30244747
Chicago/Turabian StyleMahon, Peter J., Muhammad Hanafi, Nina Artanti, I Putu Agus Hendra Wibawa, Faris Hermawan, Minarti, Puspa Dewi Lotulung, and Vito M. Butardo, Jr. 2025. "Novel Trinorditerpene from Dysoxylum parasiticum (Osbeck) Kosterm: Leaf Extract with Cytotoxic, Antioxidant and α-Glucosidase Inhibitory Activities" Molecules 30, no. 24: 4747. https://doi.org/10.3390/molecules30244747
APA StyleMahon, P. J., Hanafi, M., Artanti, N., Wibawa, I. P. A. H., Hermawan, F., Minarti, Lotulung, P. D., & Butardo, V. M., Jr. (2025). Novel Trinorditerpene from Dysoxylum parasiticum (Osbeck) Kosterm: Leaf Extract with Cytotoxic, Antioxidant and α-Glucosidase Inhibitory Activities. Molecules, 30(24), 4747. https://doi.org/10.3390/molecules30244747








