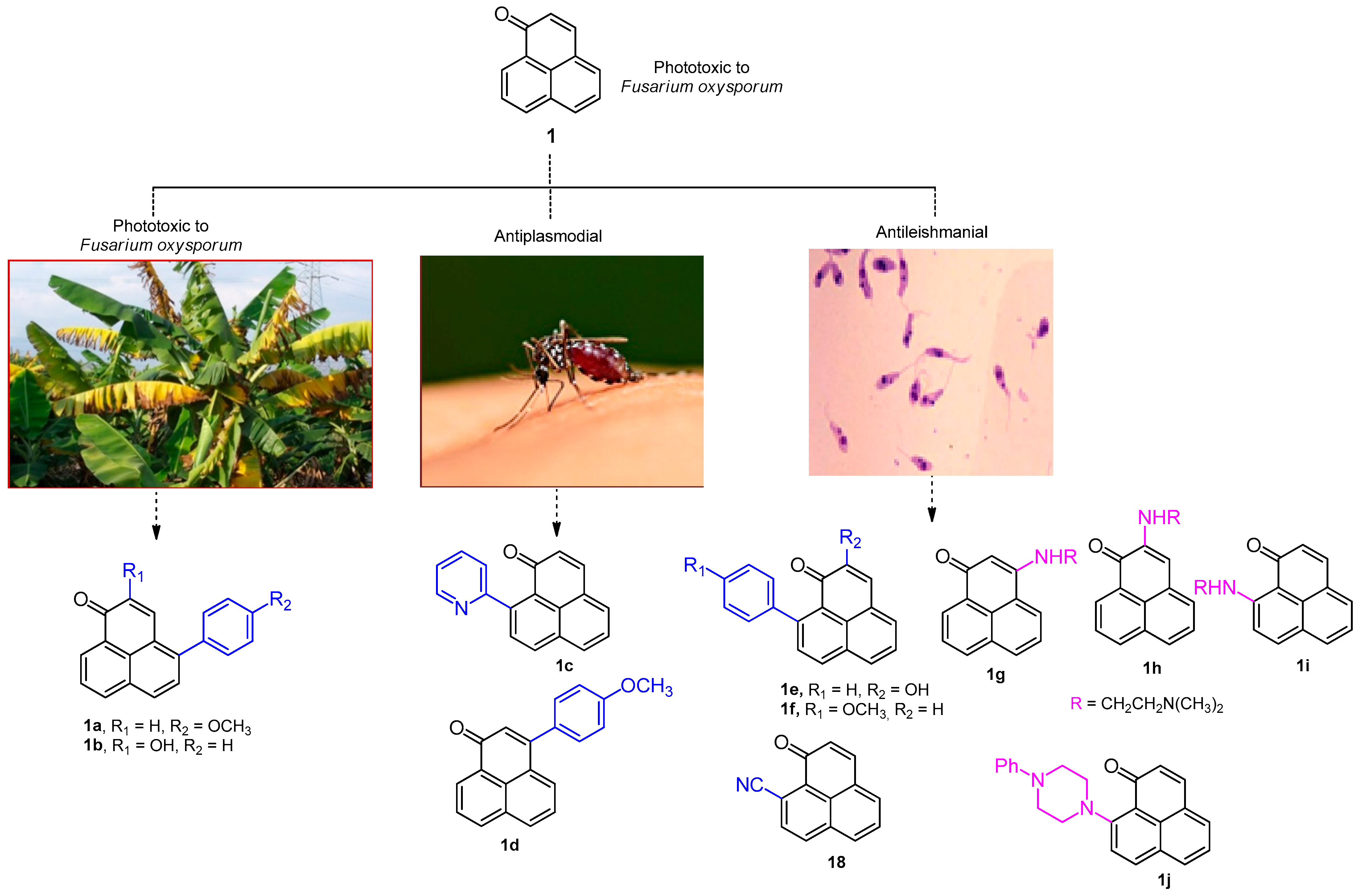
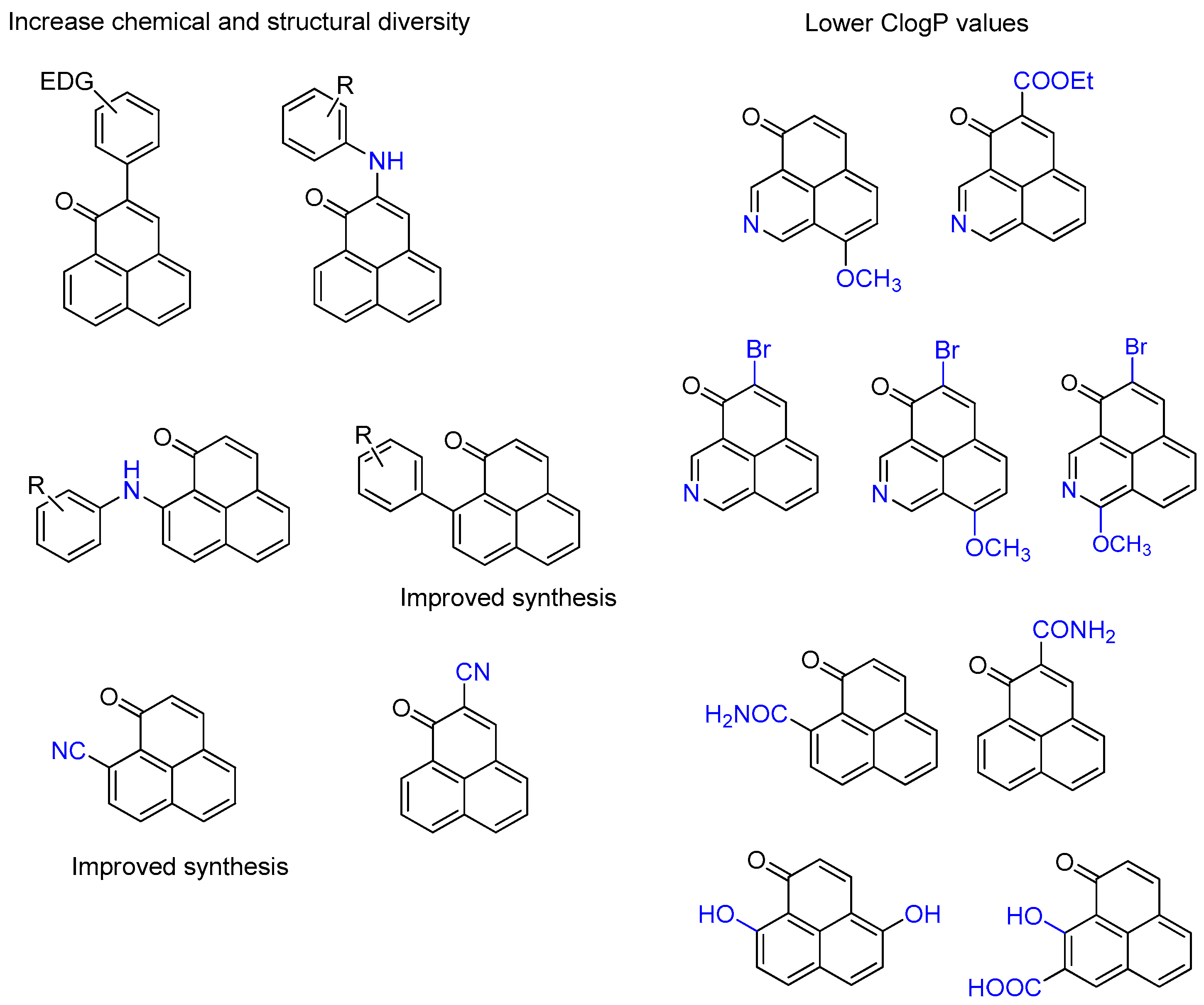
3.2. Synthesis
Compounds
2 [
28],
3 [29],
8 [
21] and
35 [
26] were prepared as described in the references.
3.2.1. Synthesis of 2-Bromo-1H-phenalen-1-one (2)
1H-Phenalen-1-one (1) (3.0 g, 16.6 mmol), neutral alumina (22.6 g), and NBS (5.0 g, 28.3 mmol) were mixed in a mortar until a uniformly yellow mixture was obtained. This mixture was then heated at 45 °C for 22 h. The crude product was filtered, washing with CH2Cl2, and the solvent was evaporated to dryness under reduced pressure using a rotary evaporator. The resulting mixture was washed with hot water and filtered while hot to afford compound 2 (4.0 g, 93%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ: 8.64 (1H, dd, J = 0.9, 7.4 Hz, H-9), 8.19 (1H, d, J = 8.0 Hz, H-7), 8.14 (1H, s, H-3), 8.02 (1H, d, J = 8.4, H-6), 7.74 (1H, dd, J = 8.0, 7.6 Hz, H-8), 7.67 (1H, d, J = 7.0 Hz, H-4), 7.56 (1H, dd, J = 8.0, 7.2 Hz, H-5). 13C NMR (100 MHz, CDCl3) δ: 178.7 (C=O), 143.1 (CH, C-3), 135.6 (CH), 132.5 (CH), 132.2 (CH), 132.2 (C), 131.5 (CH), 128.6 (C), 127.9 (C), 127.5 (CH), 126.9 (CH), 126.6 (C), 125.9 (C). UV-Vis (EtOH) λmax: 403, 362, 328 nm. Mp: 150–152 °C.
3.2.2. Synthesis of 2-Iodo-1H-phenalen-1-one (3)
A solution of iodine (6.0 g, 20 mmol) in CCl4:Py (20 mL, 1:1) was added dropwise to a solution of 1H-phenalen-1-one (1) (2.0 g, 11.1 mmol) in CCl4:Py (20 mL, 1:1) at 0 °C under an argon atmosphere. The mixture was stirred at room temperature for 48 h. It was then diluted with ether (100 mL) and washed successively with water (50 mL), 1 N HCl solution (2 × 50 mL), water (50 mL), and an aqueous 20% Na2S2O3 solution (50 mL). The organic layer was dried over Na2SO4, filtered, and the solvent was evaporated to dryness under reduced pressure. The crude product was purified by recrystallization from ether to afford compound 3 (1.56 g, 46%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ: 8.55 (1H, dd, J = 1.2, 7.6 Hz, H-9), 8.37 (1H, s, H-3), 8.12 (1H, d, J = 7.2 Hz, H-7), 7.96 (1H, d, J = 8.4 Hz, H-6), 7.66 (1H, dd, J = 7.6, 8.0 Hz, H-8), 7.57 (1H, d, J = 6.8 Hz, H-4), 7.50 (1H, dd, J = 8, 7.2 Hz, H-5). 13C NMR (100 MHz, CDCl3) δ: 179.5 (C=O), 150.5 (CH, C-3), 135.4 (CH), 132.6 (CH), 132.4 (CH), 132.0 (C), 131.2 (C), 128.7 (C), 127.4 (CH), 127.0 (C), 126.9 (C), 126.8 (CH), 106.0 (C, C-2). EIMS: m/z 306 (M+, 15), 305 (M-1, 100), 179 (M-I+, 22), 151 (47), 111 (26), 97 (37), 57 (59). HRMS (EI): calcd. for C13H7IO (M+) 305.9542, found 305.9540.
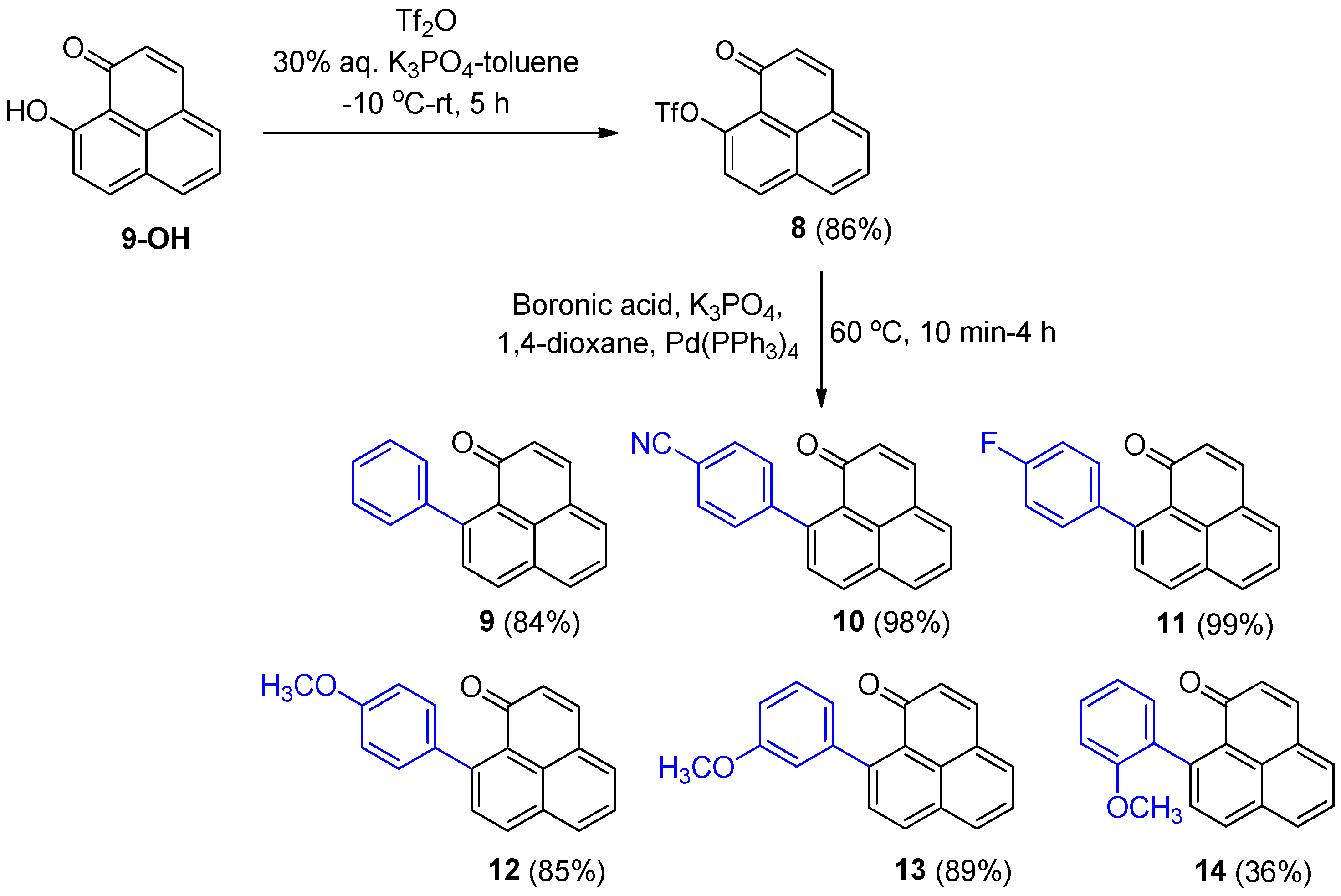
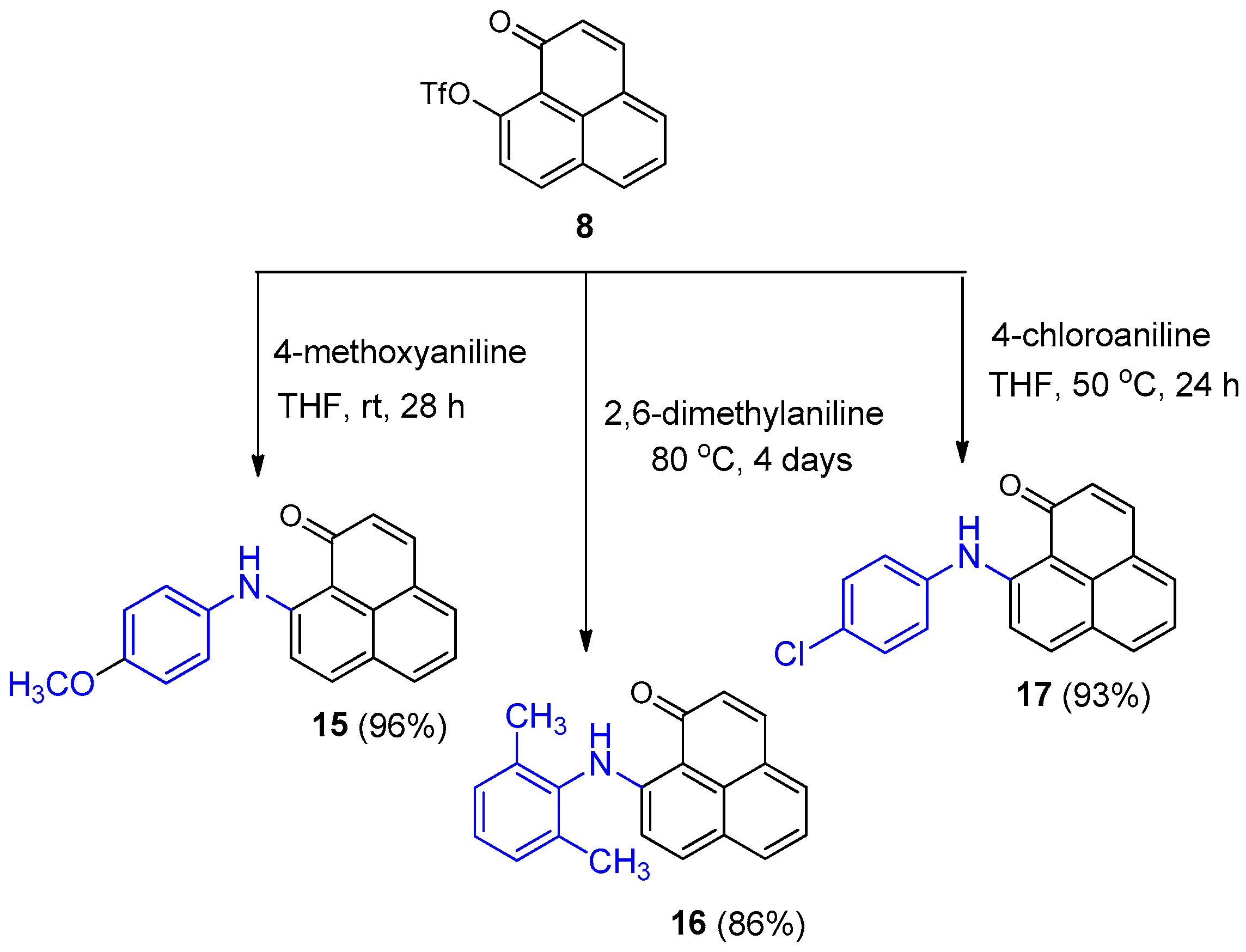
3.2.3. Synthesis of 1-Oxo-1H-phenalen-9-trifluoromethanesulfonate (8)
To a solution of 9-hydroxy-1H-phenalen-1-one (1) (400 mg, 2.04 mmol) in toluene (20 mL) was added 20 mL of a 30% K3PO4 solution (8.6 g, 40.8 mmol). The mixture was cooled to −10 °C, then triflic anhydride (0.42 mL, 2.45 mmol) was slowly added, and the reaction was stirred at room temperature for 5 h. The aqueous phase was extracted with toluene (3 × 15 mL), the combined organic layers were dried (MgSO4), filtered, and the solvent was removed under reduced pressure. The crude product was purified by silica gel column chromatography (hexane/EtOAc, 10–30%) to afford compound 8 (575 mg, 86%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ: 8.29 (1H, d, J = 8.8 Hz), 8.07 (1H, d, J = 8.2 Hz), 7.87 (1H, d, J = 7 Hz), 7.76 (1H, d, J = 9.8 Hz), 7.70 (1H, dd, J = 7.8, 7.5 Hz), 7.57 (1H, d, J = 8.8, Hz), 6.74 (1H, d, J = 9.8 Hz). EIMS: m/z 139 (100), 166 (31), 195 (35), 196 (16). 236 (32) 327 (73), 328 (M+, 14). HRMS (EI): calcd. for C14H7F3O4S (M+) 328.0017, found 327.0019. UV-Vis (EtOH) λmax: 307, 340, 355, 382 nm.
3.2.4. Synthesis of 2-Aryl-1H-phenalen-1-one Derivatives: General Procedure
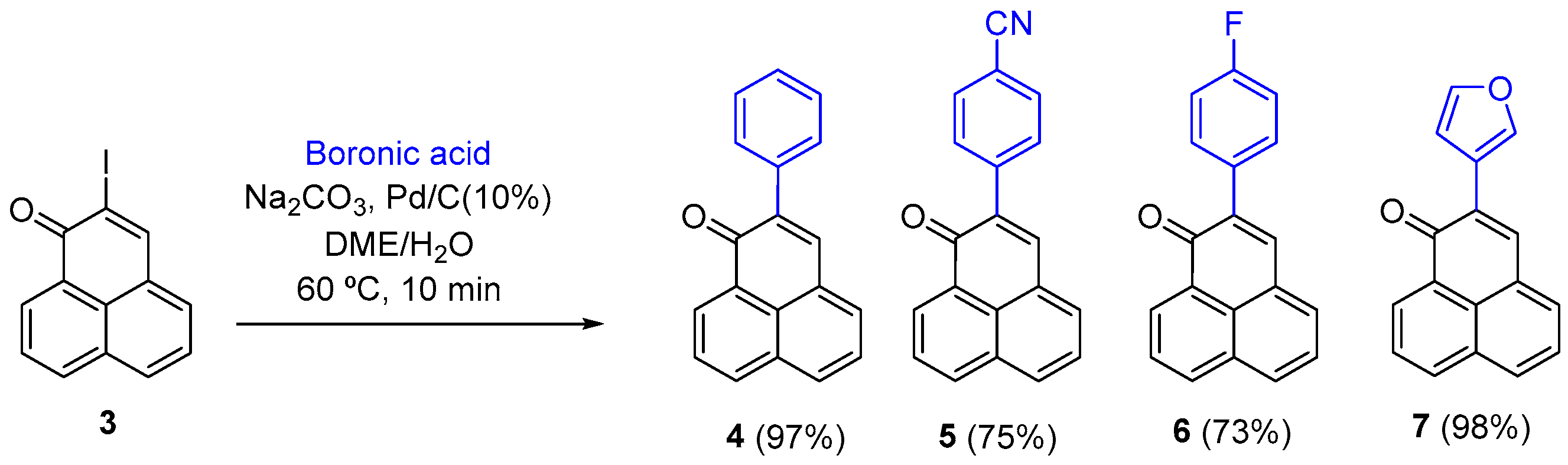
To a solution of 2-iodo-1H-phenalen-1-one (3) in a 1:1 DME/H2O mixture, Na2CO3, boronic acid, and 10% Pd/C were added. The reaction mixture was heated at 60 °C. After this time, EtOAc (10 mL) was added and the mixture was filtered over Celite®. H2O (20 mL) was then added, the mixture was extracted with EtOAc (3 × 8 mL), and dried (MgSO4). After filtration and concentration under vacuum, the crude product was purified by column chromatography or on a preparative silica gel plate.
From 2-iodo-1H-phenalen-1-one (3) (51.8 mg, 0.169 mmol), DME/H2O (1 mL), Na2CO3 (41.6 mg, 0.392 mmol), phenylboronic acid (46.1 mg, 0.378 mmol), and 10% Pd/C (15.6 mg), the mixture was heated for 10 min; column chromatography (CH2Cl2); product 4 (42.3 mg, 97%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ: 8.71 (1H, d, J = 7.4 Hz, H-9), 8.20 (1H, d, J = 8.0 Hz), 8.01 (1H, d, J = 8.3 Hz), 7.85 (1H, s, H-3), 7.85–7.78 (2H, m), 7.69 (2H, d, J = 8.0 Hz), 7.61 (1H, t, J = 7.6), 7.49–7.45 (2H, m), 7.42–7.38 (1H, m). 13C NMR (100 MHz, CDCl3) δ: 184.1 (C=O), 139.7 (CH), 139.4 (C), 136.7 (C), 134.7 (CH), 132.1 (C), 131.5 (CH), 131.1 (CH), 130.0 (C), 129.2 (2 × CH), 128.3 (2 × CH), 128.3 (C), 128.2 (CH), 127.4 (CH), 127.3 (C), 126.9 (CH, C-3). EIMS: m/z 301 (40), 280 (23), 279 (M+ + Na, 100). HRMS (ESI): calcd. for C19H12ONa (M+ + Na) 279.0787, found 279.0787. Mp: 147–149 °C.
Starting with 2-iodo-1H-phenalen-1-one (3) (70.0 mg, 0.228 mmol), DME/H2O (1.4 mL), Na2CO3 (56.0 mg, 0.524 mmol), (4-cyanophenyl)boronic acid (77.0 mg, 0.524 mmol), and 10% Pd/C (15.6 mg), the mixture was heated for 1 h and 20 min; column chromatography (hexane/EtOAc, 4:1 to 3:2) yielded product 5 (48.0 mg, 75%) as a yellow solid. 1H NMR (500 MHz, CDCl3) δ: 8.73 (1H, dd, J = 1.1, 7.4 Hz, H-9), 8.27 (1H, dd, J = 0.9, 8.0 Hz, H-7), 8.09 (1H, d, J = 8.2 Hz), 7.91 (1H, s, H-3), 7.88–7.85 (2H, m), 7.81 (2H, dd, J = 1.9, 6.0 Hz, H-2’, H-6’,), 7.74 (2H, dd, J = 2.0, 6.6 Hz, H-3’, H-5’), 7.66 (1H, dd, J = 7.6, 8.2 Hz, H-5). 13C NMR (125 MHz, CDCl3) δ: 183.4 (C=O), 141.4 (C, C-2), 140.9 (CH), 137.6 (C), 135.3 (CH), 132.5 (CH), 132.4 (CH), 132.2 (C), 132.1 (2 × CH), 131.5 (CH), 129.9 (2 × CH), 129.7 (C), 127.7 (CH), 127.6 (C), 127.4 (C), 127.1 (CH), 119.1 (CN), 111.7 (C, C-1’). EIMS: m/z 281 (M+, 56), 280 (10), 251 (11), 140 (11). HRMS (EI): calcd. for C20H11NO (M+) 281.0841, found 281.0834. UV-Vis (EtOH) λmax: 400, 363, 282, 249 nm. IR (film) νmax: 2852, 1731, 1634, 1115, 837 cm−1. Mp: 232–234 °C.
Starting with 2-iodo-1H-phenalen-1-one (3) (70.0 mg, 0.228 mmol), DME/H2O (1.4 mL), Na2CO3 (56.0 mg, 0.524 mmol), (4-fluorophenyl)boronic acid (87.0 mg, 0.524 mmol), and 10% Pd/C (15.6 mg), the mixture was heated for 10 min; column chromatography (hexane/AcOEt, 4:1) gave product 6 (46.0 mg, 73%) as a yellow solid. 1H NMR (500 MHz, CDCl3) δ: 8.70 (1H, dd, J = 1.1, 7.3 Hz, H-9), 8.23 (1H, dd, J = 0.8, 8.0 Hz, H-7), 8.04 (1H, d, J = 8.1 Hz), 7.83 (1H, s, H-3), 7.81 (2H, t, J = 7.6 Hz), 7.66 (1H, dd, J = 2.1, 5.5 Hz), 7.62 (1H, dd, J = 7.1, 8.2 Hz, H-8), 7.14 (1H, dd, J = 2.1, 5.5 Hz). 13C NMR (125 MHz, CDCl3) δ: 184.1 (C=O), 162.9 (d, JC-F = 245.7 Hz), 139.6 (CH), 138.4 (C), 134.9 (CH), 132.6 (d, JC-F = 3.5 Hz), 132.2 (C), 131.7 (CH), 131.6 (CH), 131.2 (CH), 131.0 (d, JCH-F = 8.0 Hz, 2 × CHF), 129.9 (C), 128.1 (C), 127.5 (CH), 127.3 (C), 127.0 (CH), 115.3 (d, JCH-F = 21.2 Hz, 2 × CHF). 19F NMR (470 MHz, CDCl3) δ: −113.8. EIMS: m/z 274 (M+, 68), 273 (100), 244 (17), 136 (11). HRMS (EI): calcd. for C19H11FO (M+) 274.0794, found 274.0786. UV-Vis (EtOH) λmax: 405, 361, 254 nm. IR (film) νmax: 2850, 1631, 1577, 1288, 1220, 833cm−1. Mp: 200–202 °C.
From 2-iodo-1H-phenalen-1-one (3) (50.0 mg, 0.163 mmol), DME/H2O (1 mL), Na2CO3 (34.6 mg, 0.326 mmol), 3-furylboronic acid (35.4 mg, 0.316 mmol), and 10% Pd/C (15.6 mg), the mixture was heated for 10 min; column chromatography (CH2Cl2) gave product 7 (39.5 mg, 98%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ: 8.68 (1H, d, J = 7.3 Hz, H-9), 8.56 (1H, s, H-2’), 8.18 (1H, d, J = 7.9 Hz, H-7), 7.97 (1H, d, J = 8.2 Hz), 7.87 (1H, s, H-3), 7.80–7.76 (2H, m), 7.58 (1H, t, J = 7.6 Hz, H-5), 7.50 (1H, s, H-5’), 6.84 (1H, s, H-4’). 13C NMR (100 MHz, CDCl3) δ: 183.8 (C=O), 143.8 (CH), 142.6 (CH), 135.9 (CH), 134.9 (CH), 132.0 (C), 131.3 (CH), 131.2 (CH), 131.0 (CH), 130.8 (C), 129.7 (C), 128.1 (C), 127.3 (CH), 126.9 (CH), 126.5 (s), 120.3 (C), 108.2 (CH). EIMS: m/z 270 (17), 269 (M+ + Na+,100). HRMS (ESI): calcd. for C17H10O2Na (M+ + Na+) 269.0578, found 269.0572. UV-Vis (EtOH) λmax: 407, 362, 246 nm. IR (film) νmax: 3393, 1754, 1636, 1573, 936 cm−1. Mp: 133–135 °C.
3.2.5. Synthesis of 9-Phenyl-1H-phenalen-1-one (9)
To a solution of 1-oxo-1H-phenalen-9-trifluoromethylsulfonate (8) (46.0 mg, 0.140 mmol), K3PO4 (44.0 mg, 0.210 mmol), and Pd(PPh3)4 (10.5 mg, 0.009 mmol) in 1,4-dioxane (1.2 mL), 95% phenylboronic acid (28 mg, 0.229 mmol) was added, and the mixture was heated for 3 h at 60 °C. The reaction crude was concentrated on a rotary evaporator, H2O (10 mL) was added, extracted with EtOAc (3 × 8 mL), dried (Na2SO4), filtered and the solvent was removed on a rotary evaporator. The crude was purified by preparative silica gel plate chromatography (hexane/EtOAc, 3:2) to obtain product 9 (30 mg, 84%) as a red oil. 1H NMR (500 MHz, CDCl3) δ: 8.16 (1H, d, J = 8.3 Hz, H-7), 8.03 (1H, dd, J = 0.8, 8.2 Hz), 7.77 (1H, dd, J = 0.7, 7.0 Hz), 7.69 (1H, d, J = 9.7 Hz), 7.61 (1H, dd, J = 7.1, 8.2 Hz), 7.59 (1H, d, J = 8.2 Hz), 7.49–7.37 (5H, m), 6.60 (1H, d, J = 9.7 Hz).
To a solution of 1-oxo-1H-phenalen-9-trifluoromethylsulfonate (8) (50.0 mg, 0.152 mmol), K3PO4 (48 mg, 0.228 mmol), and Pd(PPh3)4 (10 mg, 0.009 mmol) in 1,4-dioxane (1.3 mL), (4-cyanophenyl)boronic acid (36 mg, 0.243 mmol) was added, and the mixture was heated for 2 h and 30 min at 60 °C. The reaction crude was concentrated on a rotary evaporator, H2O (10 mL) was added, the mixture was extracted with EtOAc (3 × 5 mL), dried (Na2SO4), filtered, and the solvent was removed on a rotary evaporator. The crude was purified by silica gel column chromatography (hexane/EtOAc, 4:1 to 3:2) to give product 10 (42 mg, 98%) as an orange solid. 1H NMR (500 MHz, CDCl3) δ: 8.22 (1H, d, J = 8.2 Hz, H-7), 8.07 (1H, dd, J = 0.7, 8.2 Hz), 7.82 (1H, d, J = 6.5 Hz), 7.74–7.72 (3H, m), 7.56 (1H, dd, J = 7.1, 8.2 Hz, H-5), 7.51 (1H, d, J = 8.2 Hz), 7.45 (2H, dd, J = 8.4, 1.9 Hz, H-2’, H-6’), 6.58 (1H, d, J = 9.7 Hz, H-2). 13C NMR (125 MHz, CDCl3) δ: 185.6 (C=O), 148.2 (C, C-9), 145.3 (C), 141.1 (CH), 134.3 (CH), 132.3 (C), 132.2 (2 × CH, C-2’, C-6’), 132.1 (CH), 132.0 (CH), 130.6 (CH), 130.2 (CH), 128.8 (2 × CH, C-3’, C-5’), 128.6 (C), 128.3 (C), 127.1 (CH), 126.1 (C), 119.2 (C, CN), 111.0 (C, C-1’). EIMS: m/z 281 (M+, 40), 280 (100), 251 (7), 140 (8) HRMS (EI): calcd. for C20H11NO (M+) 281.0841, found 281.0833. UV-Vis (EtOH) λmax: 361, 257 nm. IR (film) νmax: 2222, 1623, 1546, 832 cm−1. Mp: 191–193 °C.
To a solution of 1-oxo-1H-phenalene-9-trifluoromethylsulfonate (8) (50.0 mg, 0.152 mmol), K3PO4 (48 mg, 0.228 mmol), and Pd(PPh3)4 (10 mg, 0.009 mmol) in 1,4-dioxane (1.3 mL), 95% (4-fluorophenyl)boronic acid (42 mg, 0.253 mmol) was added, and the mixture was heated for 1 h and 30 min at 60 °C. The reaction crude was concentrated on a rotary evaporator, H2O (8 mL) was added, the mixture was extracted with EtOAc (3 × 5 mL), dried (Na2SO4), filtered, and the solvent was removed on a rotary evaporator. The crude was purified by silica gel column chromatography (hexane/EtOAc, 4:1 to 3:2) to give product 11 (41.5 mg, 99%) as an orange solid. 1H NMR (500 MHz, CDCl3) δ: 8.16 (1H, d, J = 8.3 Hz, H-7), 8.03 (1H, dd, J = 0.95, 8.2 Hz), 7.76 (1H, dd, J = 0.8, 7.0 Hz), 7.68 (1H, d, J = 9.7 Hz, H-3), 7.61 (1H, dd, J = 7.1, 8.1 Hz, H-5), 7.55 (1H, d, J = 8.3, H-8), 7.35 (1H, d, J = 8.7 Hz, H-3’, H-5’), 7.34 (1H, d, J = 8.8 Hz), 7.16 (1H, d, J = 8.7, H-2’, H-6’), 7.14 (1H, d, J = 8.8 Hz), 6.59 (1H, d, J = 9.7 Hz, H-2). 13C NMR (125 MHz, CDCl3) δ: 185.9 (C=O), 163.3 (d, JC-F = 244.5 Hz), 146.6 (C), 140.6 (CH), 138.8 (d, JC-F = 3.6 Hz), 133.9 (CH), 131.9 (C), 131.9 (CH), 131.7 (2 × CH), 130.6 (CH), 129.8 (d, JCH-F = 8.0 Hz, 2 × CHF), 128.5 (C), 128.4 (C), 126.6 (CH), 126.2 (C), 115.4 (d, JCH-F = 21.7 Hz, 2 × CHF). 19F NMR (470 MHz, CDCl3) δ: −115.4. EIMS: m/z 274 (M+, 33), 273 (100), 136 (7), 113 (4). HRMS (EI): calcd. for C19H11FO (M+) 274.0794, found 274.0802. UV-Vis (EtOH) λmax: 398, 362, 252 nm. IR (film) νmax: 1624, 1552, 1350, 1211, 829 cm−1. Mp: 136–138 °C.
To a solution of 1-oxo-1H-phenalene-9-trifluoromethylsulfonate (8) (28.0 mg, 0.085 mmol), K3PO4 (27 mg, 0.128 mmol), and Pd(PPh3)4 (6 mg, 0.005 mmol) in 1,4-dioxane (0.7 mL) was added 95% (4-methoxyphenyl)boronic acid (33 mg, 0.217 mmol) and heated for 1 h and 30 min at 60 °C under an inert atmosphere. The reaction crude was concentrated on a rotary evaporator, H2O (10 mL) was added, the mixture was extracted with EtOAc (3 × 5 mL), dried (Na2SO4), filtered, and the solvent was removed on a rotary evaporator. The crude was purified by silica gel column chromatography (hexane/EtOAc, 10–30%) to give product 12 (20.7 mg, 85%) as an orange solid. 1H NMR (400 MHz, CDCl3) δ: 8.14 (1H, d, J = 8.3 Hz, H-7), 8.07 (1H, d, J = 8.2 Hz, H-6), 7.77 (1H, d, J = 7 Hz, H-4), 7.68 (1H, d, J = 9.7 Hz, H-3), 7.68 (2H, m, H-5, H-8), 7.34 (2H, d, J = 8.6, Hz, H-2’, H-6’), 7.0 (2H, d, J = 8.6 Hz, H-3’, H-5’), 6.60 (1H, d, J = 9.7 Hz, H-2), 3.88 (3H, s, OCH3). Mp: 175–178 °C.
To a solution of 1-oxo-1H-phenalene-9-trifluoromethylsulfonate (8) (80.0 mg, 0.244 mmol), K3PO4 (76 mg, 0.366 mmol), and Pd(PPh3)4 (17 mg, 0.015 mmol) in 1,4-dioxane (2 mL) was added (3-methoxyphenyl)boronic acid (59 mg, 0.390 mmol) and heated for 4 h at 60 °C. The reaction crude was concentrated on a rotary evaporator, H2O (8 mL) was added, the mixture was extracted with EtOAc (3 × 5 mL), dried (Na2SO4), filtered, and the solvent was removed on a rotary evaporator. The crude oil was purified by silica gel column chromatography (hexane/EtOAc, 19:1 to 17:3) to give product 13 (62 m, 89%) as an orange oil. 1H NMR (500 MHz, CDCl3) δ: 8.16 (1H, d, J = 8.3 Hz, H-7), 8.04 (1H, dd, J = 0.9, 8.2 Hz), 7.78 (1H, d, J = 6.7 Hz), 7.68 (1H, d, J = 9.7 Hz, H-3), 7.61 (1H, dd, J = 7.1, 8.2 Hz, H-5), 7.60 (1H, d, J = 8.3, H-8), 7.37 (1H, t, J = 7.8 Hz, H-5), 6.95 (2H, dd, J = 2.1, 7.9 Hz, H-4’, H-6’), 6.90 (1H, m, H-2’), 6.59 (1H, d, J = 9.7 Hz, H-2), 3.83 (3H, s, CH3). 13C NMR (125 MHz, CDCl3) δ: 185.8 (C=O), 159.8 (C, C-3’), 147.5 (C), 144.5 (C), 140.5 (CH), 138.8 (CH), 131.9 (C), 131.8 (CH), 131.6 (CH), 131.5 (CH), 130.6 (CH), 129.4 (CH), 128.7 (C), 128.5 (C), 126.5 (CH), 126.3 (C), 120.4 (CH), 113.7 (CH), 112.7 (CH), 56.4 (CH3, OCH3). EIMS: m/z 286 (M+, 74), 285 (100), 271 (28), 256 (19), 255 (95), 242 (30), 213 (19). HRMS (EI): calcd. for C20H14O2 (M+) 286.0994, found 286.1003. UV-Vis (EtOH) λmax: 362, 253 nm. IR (film) νmax: 1640, 1558, 1240, 850 cm−1.
To a solution of 1-oxo-1H-phenalene-9-trifluoromethylsulfonate (8) (20.0 mg, 0.058 mmol), K3PO4 (18 mg, 0.086 mmol), and Pd(PPh3)4 (4 mg, 0.003 mmol) in 1,4-dioxane (0.5 mL) was added (2-methoxyphenyl)boronic acid (14 mg, 0.093 mmol) and heated for 4 h at 60 °C. The reaction crude was concentrated on a rotary evaporator, H2O (8 mL) was added, the mixture was extracted with EtOAc (3 × 5 mL), dried (Na2SO4), filtered, and the solvent was removed on a rotary evaporator. The crude oil was purified by preparative silica gel plate chromatography (CH2Cl2) to give product 14 (6 mg, 36%) as a yellow solid. 1H NMR (500 MHz, CDCl3) δ: 8.18 (1H, d, J = 8.2 Hz, H-7), 8.03 (1H, dd, J = 0.9, 8.2 Hz), 7.75 (1H, dd, J = 0.9, 7.0 Hz), 7.67 (1H, d, J = 9.7 Hz, H-3), 7.60 (1H, dd, J = 7.1, 8.2 Hz, H-5), 7.58 (1H, d, J = 8.2 Hz, H-8), 7.39 (1H, ddd, J = 1.7, 7.4, 8.2 Hz), 7.16 (1H, dd, J = 1.8, 7.5 Hz), 7.07 (1H, ddd, J = 0.9, 7.3, 8.3 Hz), 6.99 (1H, dd, J = 0.6, 8.2 Hz), 6.57 (1H, d, J = 9.7 Hz, H-2), 3.70 (3H, s, CH3). 13C NMR (125 MHz, CDCl3) δ: 185.8 (C=O), 156.6 (C, C-2’), 143.9 (C), 140.4 (CH), 133.9 (CH), 132.4 (C), 132.0 (C), 131.8 (CH), 131.7 (CH), 131.2 (CH), 130.4 (CH), 128.9 (CH), 128.8 (CH), 128.7 (C), 128.4 (C), 127.1 (C), 126.3 (CH), 121.1 (CH), 110.9 (CH), 56.2 (CH3, OCH3). Mp: 123–125 °C.
3.2.6. Synthesis of 9-Arylamino-1H-phenalen-1-ones Derivatives
To a solution of 1-oxo-1H-phenalene-9-trifluromethylsulfonate (8) (25 mg, 0.076 mmol) in THF (0.65 mL) was added (4-methoxyphenyl)amine (21 mg, 0.173 mmol) and stirred at room temperature for 28 h. After that time, the THF was removed under high vacuum. The crude product was purified by silica gel column chromatography (hexane/EtOAc, 4:1, 7:3) to give product 15 (22 mg, 96%) as a red solid. 1H NMR (500 MHz, CDCl3) δ: 13.64 (1H, br s, NH), 7.88–7.85 (4H, m), 7.44 (1H, t, J = 7.6 Hz, H-5), 7.35 (1H, d, J = 9.3 Hz, H-8), 7.30 (2H, d, J = 8.5 Hz, H-3’, H-5’), 7.03 (1H, d, J = 9.4 Hz, H-2), 6.98 (2H, d, J = 8.6 Hz, H-2’, H-6’), 3.86 (3H, s, OCH3). Mp: 128–130 °C.
A solution of 1-oxo-1H-phenalene-9-trifluromethylsulfonate (8) (50 mg, 0.152 mmol) and 2,6-dimethylaniline (0.6 mL) was heated at 80 °C for 4 days. After that time, the amine was removed under high vacuum. The crude product was purified by silica gel column chromatography (hexane/EtOAc, 1:1 to 1:9) to obtain product 16 (39 mg, 86%) as a yellow solid. 1H NMR (500 MHz, CDCl3) δ: 13.38 (1H, br s, NH), 7.94 (4H, m), 7.51 (1H, t, J = 7.5 Hz, H-5), 7.26 (3H, m), 7.13 (1H, d, J = 9.4 Hz), 6.77 (1H, d, J = 9.2 Hz, H-2), 2.29 (6H, s, 2 × CH3). 13C NMR (125 MHz, CDCl3) δ: 185.1 (C=O), 155.5 (C, C-9), 138.9 (CH), 138.4 (CH), 136.2 (CH), 135.9 (C), 131.9 (CH), 131.5 (CH), 128.9 (CH), 128.7 (2 × CH), 128.5 (C), 127.6 (2 × C), 125.4 (C), 125.0 (C), 122.2 (CH), 115.7 (CH), 108.2 (C), 18.4 (2 × CH3). EIMS: m/z 299 (M+, 80), 298 (100), 141 (11). HRMS (EI): calcd. for C21H17NO (M+) 299.1310, found 299.1315. UV-Vis (EtOH) λmax: 468, 441, 355, 250 nm. IR (film) νmax: 3397, 1632, 1509, 1242, 852 cm−1. Mp: 205–207 °C.
To a solution of 1-oxo-1H-phenalene-9-trifluoromethylsulfonate (8) (50 mg, 0.152 mmol) in THF (1 mL) was added (4-chlorophenyl)amino (54.5 mg, 0.427 mmol) and heated at 50 °C for 24 h. After that time, THF was removed under high vacuum. The crude product was purified by silica gel column chromatography (hexane/EtOAc, 9:1 to 4:1) to give product 17 (43 mg, 93%) as an orange solid. 1H NMR (500 MHz, CDCl3) δ: 13.73 (1H, br s, NH), 7.92 (1H, d, J = 9.3 Hz, H-7), 7.91–7.86 (3H, m), 7.46 (1H, t, J = 7.6 Hz, H-5), 7.42 (1H, d, J = 9.2, H-3), 7.40 (2H, d, J = 8.6 Hz, H-2’, H-6’), 7.31 (2H, d, J = 8.6 Hz, H-3’, H-5’), 7.00 (1H, d, J = 9.4 Hz, H-2). 13C NMR (125 MHz, CDCl3) δ: 185.1 (C=O), 153.6 (C, C-9), 139.3 (CH), 138.2 (CH), 137.2 (C), 132.2 (CH), 131.6 (CH), 131.5 (CH), 131.8 (C), 129.8 (2 × CH, C-2’, C-6’), 128.8 (CH), 128.3 (C), 126.2 (2 × CH, C-3’, C-5’), 125.6 (C), 125.4 (C), 122.7 (CH), 115.5 (CH), 109.1 (C). EIMS: m/z 307 (34), 305 (M+, 100), 270 (18), 241 (12), 120 (23), HRMS (EI): calcd. for C19H12ClNO (M+) 305.0607, found 305.0611. UV-Vis (EtOH) λmax: 469, 358, 283, 244 nm. IR (film) νmax: 3092, 1630, 1581, 1510, 1139, 830cm−1. Mp: 133–135 °C.
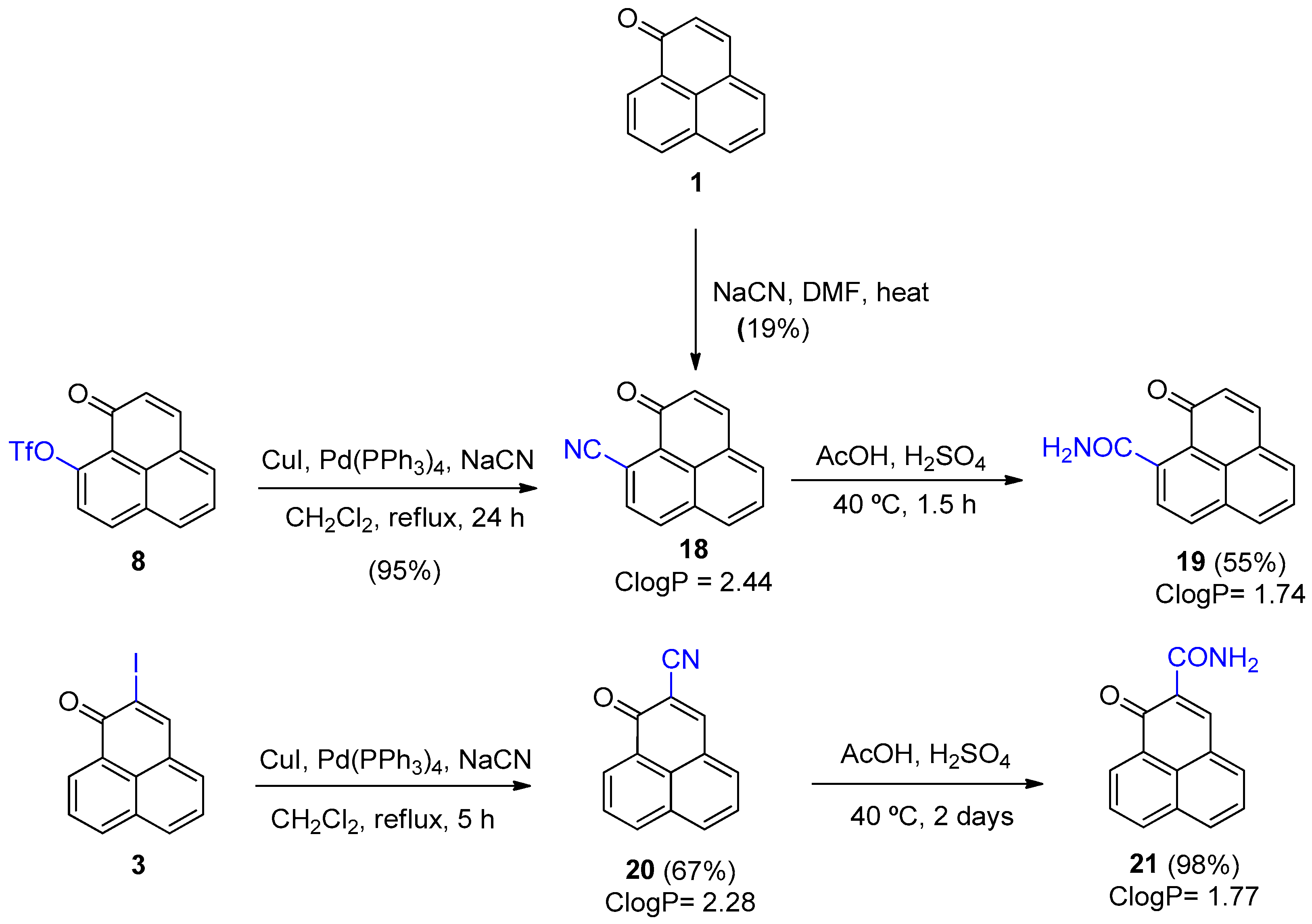
3.2.7. Synthesis of 1-Oxo-1H-phenalene-9-carbonitrile (18) and Its Derivative (19)
To a solution of 1-oxo-1H-phenalene-9-trifluoromethylsulfonate (8) (200 mg, 0.609 mmol), CuI (60 mg, 0.318 mmol), and Pd(PPh3)4 (106 mg, 0.092 mmol) in DCM (4 mL), NaCN (60 mg, 1.223 mmol) was added, and the mixture was heated under reflux for 24 h. After this time, the mixture was filtered over Celite®, a saturated solution of NH4Cl (10 mL) was added, the mixture was extracted with CH2Cl2 (3 × 5 mL), dried (Na2SO4), filtered, and the solvent was removed using a rotary evaporator. The crude product was purified by column chromatography on silica gel (hexane/CH2Cl2, 9:1 to 2:3) to give product 18 (118 mg, 95%) as an orange solid. 1H NMR (500 MHz, CDCl3) δ: 8.28 (1H, d, J = 8.3 Hz, H-7), 8.06 (1H, d, J = 8.4 Hz, H-6), 8.02 (1H, d, J = 8.03 Hz, H-3), 7.84 (1H, d, J = 6.6 Hz, H-4), 7.75 (1H, dd, J = 8.7, 10, H-5), 7.73 (1H, d, J = 8.3 Hz, H-8), 6.79 (1H, d, J = 9.8 Hz, H-2). Mp: 172–175 °C.
Concentrated H2SO4 (2 mL) was added to a solution of 1-oxo-1H-phenalene-9-carbonitrile (18) (30.0 mg, 0.146 mmol) in AcOH (2 mL) and heated for 1 h and 30 min at 40 °C. H2O (10 mL) was added to the reaction mixture, which was extracted with EtOAc (3 × 8 mL), washed with 1N NaOH solution until pH = 7–8, dried (Na2SO4), filtered, and the solvent was removed on a rotary evaporator. The crude oil was purified by preparative silica gel plate chromatography (hexane/CH2Cl2, 1:4) to give product 19 (18 mg, 55%) as a yellow solid. 1H NMR (500 MHz, CDCl3) δ: 8.25 (1H, d, J = 8.2 Hz, H-7), 8.04 (1H, d, J = 8.2 Hz, H-6), 7.81 (1H, d, J = 7.0 Hz, H-4), 7.74 (2H, d, J = 9.1 Hz, H-8, H-3), 7.65 (1H, dd, J = 7.1, 8.1 Hz, H-5), 6.71 (1H, d, J = 9.7, H-2), 5.84 (2H, s, NH2). 13C NMR (125 MHz, CDCl3) δ: 185.1 (C=O), 141.9 (C, CONH2), 139.9 (C, C-9), 135.2 (CH), 132.6 (CH), 132.5 (CH), 132.0 (2 × C), 129.0 (CH), 128.3 (C), 127.5 (2 × C), 126.5 (CH), 125.2 (C). EIMS: m/z 223 (M+, 100), 207 (80), 271 (28), 195 (35), 180 (30), 152 (49), 151 (68). HRMS (EI): calcd. for C14H9NO2 (M+) 223.0633, found 223.0638. UV-Vis (EtOH) λmax: 391, 359, 247 nm. IR (film) νmax: 3378, 3262, 1610, 1390, 1264, 847 cm−1. Mp: 242–244 °C.
3.2.8. Synthesis of 1-Oxo-1H-phenalene-2-carbonitrile (20) and Its Derivative (21)
To a solution of 2-iodo-1H-phenalen-1-one (3) (100.0 mg, 0.327), anhydrous CuI (30 mg, 0.163 mmol), and Pd(PPh3)4 (57.0 mg, 0.049 mmol) in anhydrous DCM (2 mL), finely ground NaCN (32 mg, 0.654 mmol) was added and the mixture was refluxed for 5 h under an inert atmosphere. After this time, the mixture was filtered over Celite®, a saturated NH4Cl solution (10 mL) was added, the mixture was extracted with CH2Cl2 (3 × 5 mL), dried (Na2SO4), filtered, and the solvent was removed on a rotary evaporator. The crude oil was purified by silica gel column chromatography (hexane/CH2Cl2, 9:1 to 7:3) to obtain product 20 (45.0 mg, 67%) as a yellow solid. 1H NMR (500 MHz, CDCl3) δ: 8.73 (1H, dd, J = 1.2, 7.4 Hz, H-9), 8.32 (1H, dd, J = 2.1, 7.0 Hz, H-7), 8.32 (1H, s, H-3), 8.27 (1H, dd, J = 0.8, 8.3 Hz, H-6), 7.96 (1H, dd, J = 0.8, 7.1 Hz, H-4), 7.87 (1H, t, J = 7.2 Hz, H-8), 7.71 (1H, dd, J = 7.1, 8.2 Hz, H-5). 13C NMR (125 MHz, CDCl3) δ: 179.8 (C=O), 150.1 (CH), 136.4 (CH), 135.6 (CH), 134.7 (CH), 132.4 (CH), 132.3 (C), 128.4 (C), 128.1 (CH), 127.8 (C), 127.4 (C), 125.7 (C), 115.4 (C), 114.9 (C). EIMS: m/z 206 (15), 205 (M+, 100), 177 (50), 88 (9). HRMS (EI): calcd. for C14H7NO (M+) 205.0528, found 205.0530. UV-Vis (EtOH) λmax: 397, 365, 253 nm. IR (film) νmax: 2226, 1640, 1582, 1357, 1257, 845 cm−1. Mp: 225–227 °C.
To a solution of 1-oxo-1H-phenalene-2-carbonitrile (20) (34.0 mg, 0.165 mmol) in AcOH (2 mL) was added H2SO4 (2 mL) and heated for 2 days at 40 °C. The crude product was poured onto an ice-water mixture, and the precipitate was filtered, washed with water and 5% Na2CO3 solution until the filtrate reached a pH = 6–7, to obtain product 21 (36.0 mg, 98%) as a yellow solid. 1H NMR (500 MHz, CDCl3) δ: 9.24 (1H, br s, NH), 8.97 (1H, s, H-3), 8.71 (1H, d, J = 7.4 Hz, H-9), 8.26 (1H, d, J = 7.9 Hz, H-7), 8.16 (1H, d, J = 8.3 Hz), 8.02 (1H, d, J = 7.0 Hz), 7.83 (1H, dd, J = 7.6, 7.8 Hz, H-5), 7.67 (1H, dd, J = 7.5, 7.8 Hz), 5.98 (1H, br s, NH). 13C NMR (125 MHz, CDCl3) δ: 184.7 (C=O), 165.7 (C), 148.6 (CH), 135.9 (CH), 135.5 (CH), 134.6 (CH), 132.1 (CH), 130.0 (C), 129.9 (C), 128.3 (C), 127.8 (C), 127.7 (CH), 127.3 (CH), 126.4 (C). EIMS: m/z 223 (M+, 100), 207 (52), 180 (61), 151 (50). HRMS (EI): calcd. for C14H9NO2 (M+) 223.0633, found 223.0633. UV-Vis (EtOH) λmax: 390, 252 nm. IR (film) νmax: 3347, 1695, 1574, 697cm−1. Mp: 186–188 °C.
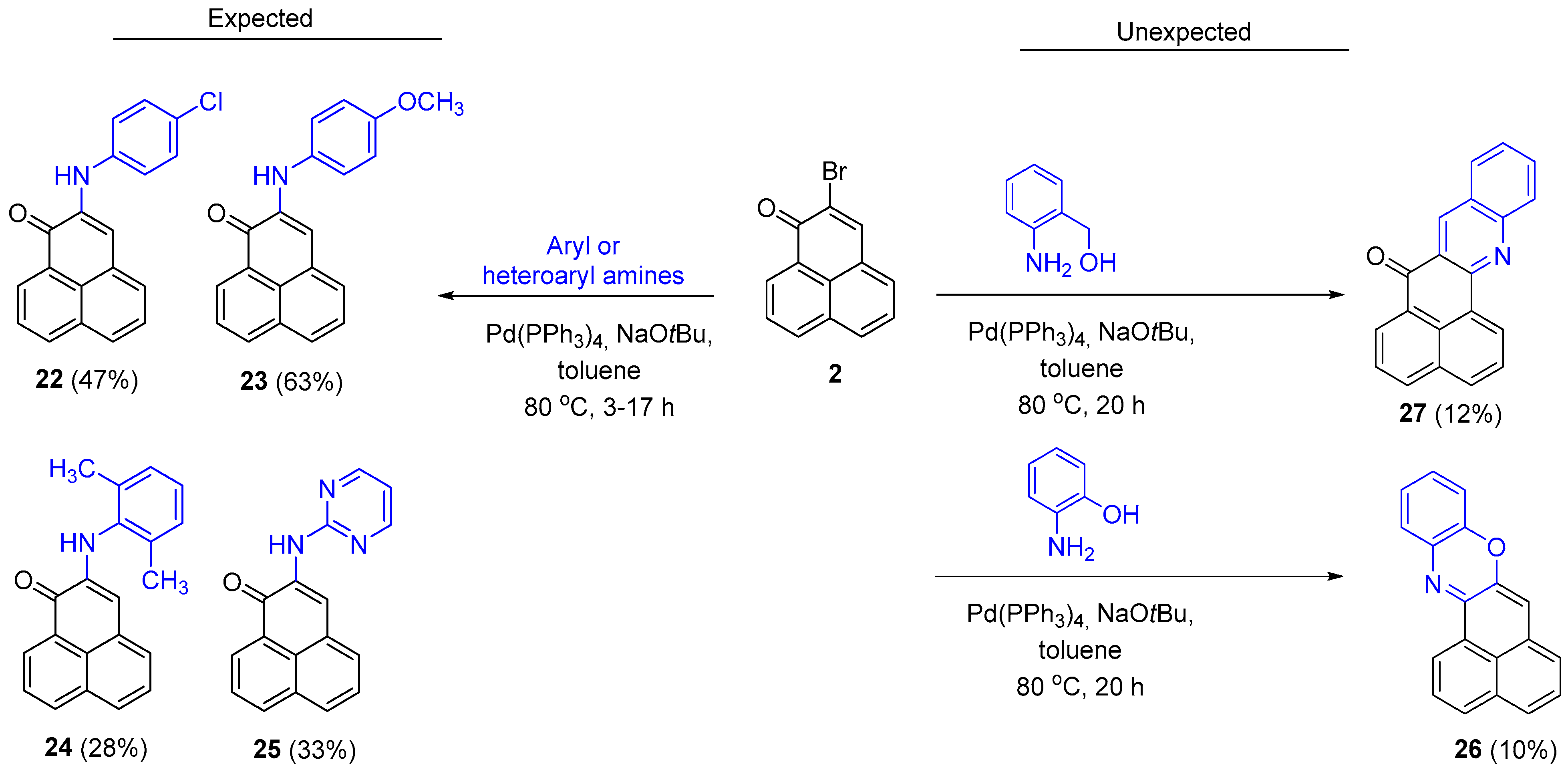
3.2.9. Synthesis of 2-Arylamino-1H-phenalen-1-one Derivatives: General Procedure
Arylamine was added to a solution of 2-bromo-1H-phenalen-1-one (2), NaOtBu, Pd(PPh3)4 in anhydrous toluene. The reaction mixture was heated to 80 °C with stirring for 2 h–4 days in the dark under an argon atmosphere. After this time, CH2Cl2 (10 mL) was added and the mixture was filtered over Celite®. The mixture was then concentrated under vacuum to dryness on a rotary evaporator, and the crude product was purified by column chromatography or preparative plate chromatography on silica gel.
From 2-bromo-1H-phenalen-1-one (2) (101.2 mg, 0.391 mmol), NaOtBu (53.8 mg, 0.560 mmol), Pd(PPh3)4 (46 mg, 0.04 mmol), anhydrous toluene (1.5 mL) and 4-chloroaniline (63.7 mg, 0.5 mmol); it was heated at 80 °C for 17 h; column chromatography (hexane/EtOAc, 95:5); product 22 (56.4 mg, 47%) was obtained as a dark red solid. 1H NMR (400 MHz, CDCl3) δ: 8.75 (1H, dd, J = 0.8, 7.2 Hz, H-9), 8.24 (1H, d, J = 8 Hz, H-7), 7.85 (1H, d, J = 7.7 Hz), 7.80 (1H, dd, J = 7.6, 8.0 Hz), 7.60–7.53 (2H, m), 7.36 (2H, d, J =8.8 Hz, H-3’, H-5’), 7.28–7.23 (4H, m, H-3, H-2’, H-6’, NH). 13C HMR (100 MHz, CDCl3) δ: 180.28 (C=O), 139.56 (C, C-1’), 137.51 (C, C-2), 136.21 (CH), 132.06 (C), 131.11 (CH), 129.64 (2 × CH, C-3’, C-5’), 129.08 (C), 128.85 (CH), 128.45 (CH), 127.92 (C), 127.46 (C), 127.37 (CH), 126.87 (CH), 124.16 (C), 121.64 (2 × CH, C-2’, C-6’), 109.80 (C, C-3). EIMS: m/z 307 (34), 306 (23), 305 (M+, 100), 135 (25), 68 (20). HRMS (EI): calcd. for C19H12ClNO (M+) 305.0607, found 305.0601. Mp: 182–183 °C.
From 2-bromo-1H-phenalen-1-one (2) (105.8 mg, 0.408 mmol), NaOtBu (51.9 mg, 0.540 mmol), Pd(PPh3)4 (45.8 mg, 0.039 mmol), anhydrous toluene (1.5 mL) and 4-methoxyaniline (61.3 mg, 0.498 mmol); it was heated at 80 °C for 17 h; column chromatography (toluene/EOAc, 95:5); product 23 (77.0 mg, 63%) was obtained as a dark red solid. 1H NMR (400 MHz, CDCl3) δ: 8.72 (1H, dd, J = 1.0, 7.4 Hz, H-9), 8.19 (1H, d, J = 8 Hz, H-7), 7.76–7.73 (2H, m), 7.49–7.48 (2H, m), 7.24 (2H, d, J = 8.8 Hz, H-3’, H-5’), 7.05 (1H, s, H-3), 7.0 (1H, br s, NH), 6.97 (2H, dd, J =1.9, 8.8 Hz, H-2’, H-6’), 3.83 (3H, s, OCH3). 13C NMR (100 MHz, CDCl3) δ: 180.6 (C=O), 155.9 (C, C-4’), 139.2 (C), 135.9 (CH), 133.7 (C), 132.0 (C), 130.8 (CH), 129.6 (C), 128.1 (CH), 128.0 (C), 127.7 (CH), 127.3 (CH), 126.7 (CH), 123.9 (C), 123.6 (2 × CH, C-3’, C-5’), 114.8 (2 × CH, C-2’, C-6’), 107.7 (CH, C-3), 55.69 (CH3, OCH3). EIMS: m/z 302 (22), 301 (M+, 100), 286 (44). HRMS (EI): calcd. for C20H15NO2 (M+) 301.1103, found 301.1092. Mp: 137–139 °C.
From 2-bromo-1H-phenalen-1-one (2) (104.9 mg, 0.405 mmol), NaOtBu (53.3 mg, 0.555 mmol), Pd(PPh3)4 (47.5 mg, 0.041 mmol), anhydrous toluene (1.5 mL) and 2,6-dimethylaniline (58 μL, 0.463 mmol); it was heated at 80 °C for 4 days; column chromatography (toluene/EOAc, 95:5); product 24 (32.3 mg, 28%) was obtained as a red oil. 1H NMR (400 MHz, CDCl3) δ: 8.78 (1H, dd, J = 0.7, 7.2 Hz, H-9), 8.21 (1H, d, J = 8 Hz, H-7), 7.79 (1H, t, J = 7.6 Hz, H-5), 7.76 (1H, d, J = 7.5 Hz, H-6), 7.47 (1H, dd, J = 7.3, 8.0 Hz, H-8), 7.41 (1H, d, J = 7.0 Hz, H-4), 7.19 (3H, br s, H-3’, H-4’, H-5’), 6.61 (1H, br s, NH), 6.12 (1H, s, H-3), 2.28 (6H, s, CH3). 13C NMR (100 MHz, CDCl3) δ: 180.5 (C=O), 139.8 (C, C-1’), 136.9 (C, C-2), 136.4 (CH), 135.9 (CH), 132.1 (C), 130.7 (CH), 129.9 (C), 128.7 (CH, 2 × C, C-3’, C-5’), 128.3 (C), 127.9 (CH), 127.5 (CH), 127.3 (CH), 126.7 (C, CH, 2 × C), 123.9 (C), 107.5 (CH, C-3), 18.4 (CH3, 2 × C). EIMS: m/z 299 (M+, 100), 282 (44), 141 (29). HRMS (EI): calcd. for C21H17NO (M+) 299.1310, found 299.1310.
From 2-bromo-1H-phenalen-1-one (2) (50.7 mg, 0.196 mmol), NaOtBu (28.1 mg, 0.292 mmol), Pd(PPh3)4 (26.2 mg, 0.023 mmol), anhydrous toluene (0.75 mL) and pyrimidin-2-amine (25.6 mg, 0.269 mmol); it was heated at 80 °C for 3 h; column chromatography (hexane/EtOAc, 3:2); product 25 (17.2 mg, 33%) was obtained as a red solid. 1H NMR (400 MHz, CDCl3) δ: 8.86 (1H, s, H-3), 8.73 (1H, dd, J = 1.0, 7.4 Hz, H-9), 8.58 (1H, br s, NH), 8.53 (2H, d, J = 4.8 Hz, H-4’, H-6’), 8.21 (1H, dd, J = 0.6, 8.0 Hz, H-7), 7.88 (1H, d, J = 8.2 Hz), 7.79–7.74 (2H, m), 7.57 (1H, dd, J = 7.3, 8.0 Hz), 6.79 (1H, t, J = 4.8 Hz, H-5’). 13C NMR (100 MHz, CDCl3) δ: 179.7 (C=O), 159.9 (CH, C-2’), 158.0 (2 × CH, C-4’, C-6’), 135.9 (CH), 134.4 (C, C-2’), 132.0 (C), 131.1 (CH), 130.7 (CH), 129.4 (CH), 128.9 (C), 127.8 (C), 127.3 (CH), 126.8 (CH), 124.7 (C), 119.1 (CH), 113.3 (CH, C-3). EIMS: m/z 274 (18), 273 (M+, 100), 272 (54), 244 (14). HRMS (EI): calcd. for C17H11N3O (M+) 273.0902, found 273.0893. UV-Vis (EtOH) λmax: 457, 365, 282 nm. IR (film) νmax: 3349, 1622, 1564, 1519, 1395, 842 cm−1. Mp: 181–183 °C.
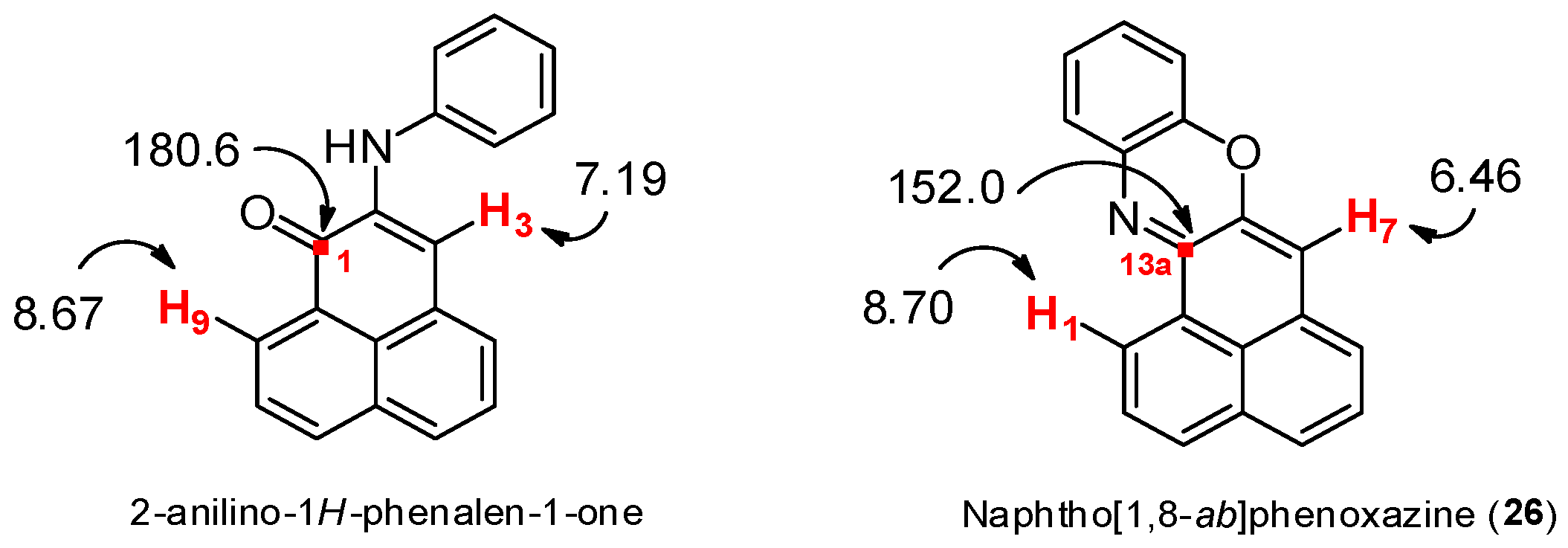
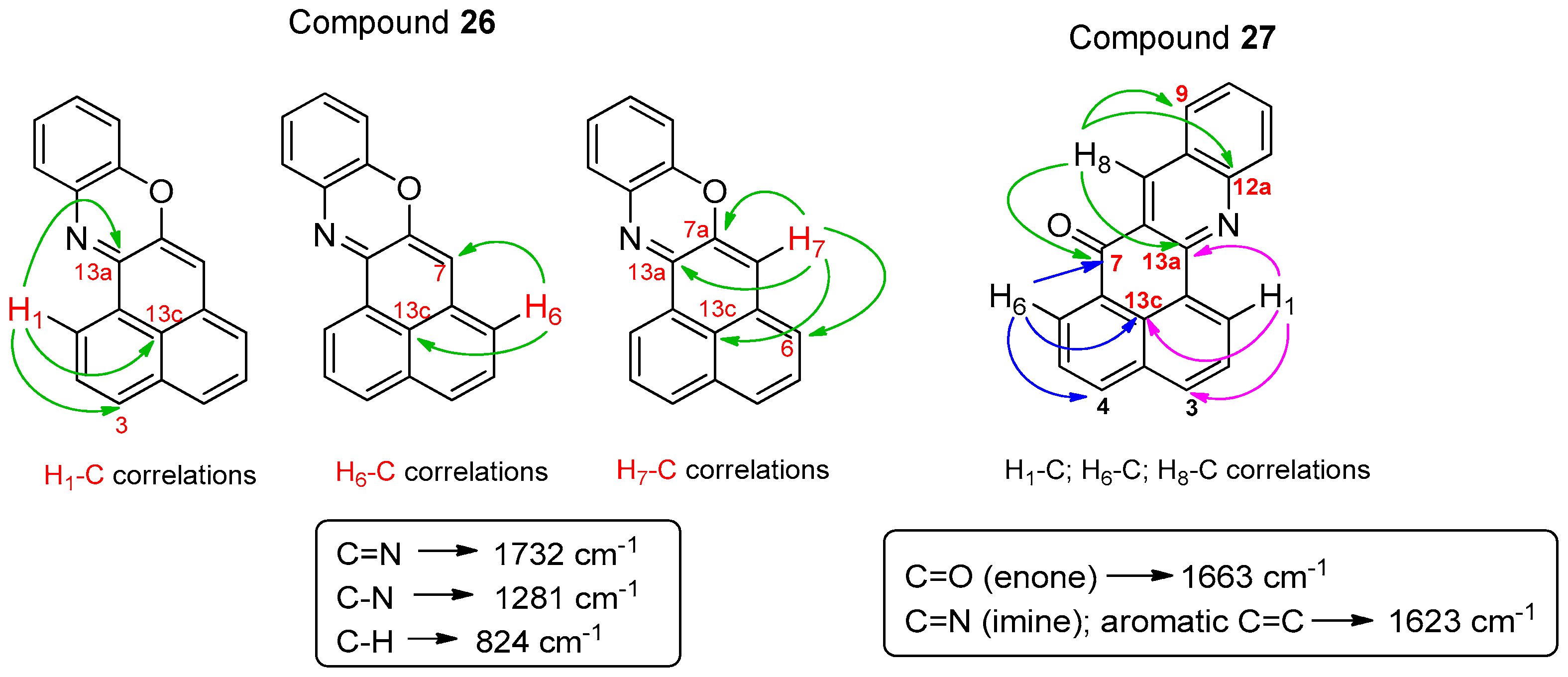
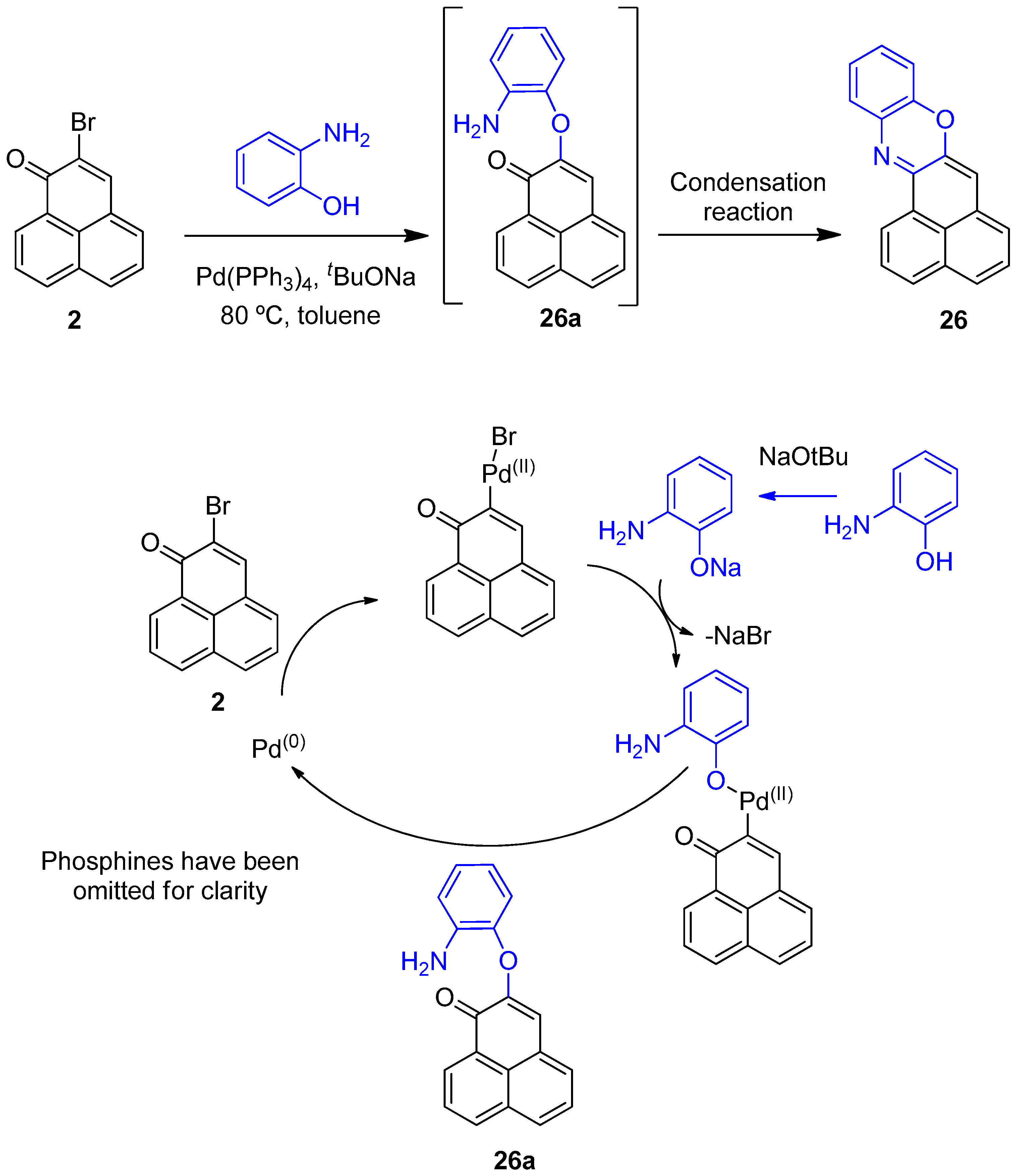
From 2-bromo-1H-phenalen-1-one (2) (105.2 mg, 0.406 mmol), NaOtBu (107.6 mg, 1.12 mmol), Pd(PPh3)4 (88 mg, 0.078 mmol), anhydrous toluene (1.5 mL) and 2-aminophenol (56.1 mg, 0.514 mmol); it was heated at 80 °C for 4 days; column chromatography (toluene/EtOAc, 9.5:0.5); product 26 (11.3 mg, 10%) was obtained as a red solid. 1H NMR (500 MHz, CDCl3) δ: 8.71 (1H, d, J = 7.5 Hz, H-1), 7.92 (1H, d, J = 8.0 Hz, H-3), 7.59–7.62 (2H, m, H-2, H-4), 7.38 (1H, dd, J = 1.2, 7.7 Hz), 7.35 (1H, t, J = 7.3 Hz, H-5), 7.24 (1H, m, H-6), 7.12 (1H, m), 7.03 (1H, m), 6.87 (1H, dd, J = 0.9, 7.9 Hz), 6.46 (1H, s, H-7). 13C NMR (125 MHz, CDCl3) δ: 152.7 (C, C-13a), 146.1 (C), 145.1 (C, C-7a), 135.0 (C), 133.3 (C, C-3a), 132.3 (CH, C-3), 131.4 (C, C-6a), 130.4 (C, C-13b), 128.8 (CH), 128.2 (CH), 127.1 (2 × CH), 127.0 (C, C-13c), 126.9 (CH), 125.5 (CH, C-1), 125.2 (CH, C-6), 124.1 (CH), 114.8 (CH), 108.5 (CH, C-7). EIMS: m/z 270 (M++1, 21) 269 (M+, 100), 240 (13), 134 (18). HRMS (EI): calcd. for C19H11NO (M+) 269.0841, found 269.0842. UV-Vis (EtOH) λmax: 524, 491, 269, 237 nm. IR (film) νmax: 1732, 1281, 824 cm−1. Mp: 168–170 °C.
From 2-bromo-1H-phenalen-1-one (2) (51.5 mg, 0.168 mmol), NaOtBu (23.0 mg, 0.239 mmol), Pd(PPh3)4 (19.6 mg, 0.017 mmol), anhydrous toluene (0.75 mL) and (2-aminophenyl)methanol (24.2 mg, 0.196 mmol); it was heated at 80 °C for 20 h; preparative plate chromatography (toluene/EtOAc, 9.8:0.2); product 27 (6.0 mg, 12%) was obtained as a yellow solid. 1H NMR (500 MHz, CDCl3) δ: 9.36 (1H, dd, J = 1.1, 7.3 Hz, H-1), 9.28 (1H, s, H-8), 8.79 (1H, d, J = 1.3, 7.3 Hz, H-6), 8.29 (1H, dd, J = 1.1, 8.1 Hz, H-4), 8.25 (1H, dd, J = 0.7, 8.5 Hz, H-12), 8.14 (1H, dd, J = 0.9, 8.1 Hz, H-3), 8.06 (1H, m, J = 7.0 Hz, H-9), 7.87 (1H, ddd, J = 1.5, 6.8, 8.4 Hz, H-11), 7.83 (1H, dd, J = 7.6, 7.9 Hz, H-2), 7.81 (1H, dd, J = 7.4, 7.9 Hz, H-5), 7.61 (1H, ddd, J = 1.1, 6.8, 8.0 Hz, H-10). 13C NMR (125 MHz, CDCl3) δ: 184.2 (C, C-7), 152.4 (C, C-13a), 150.4 (C, C-12a), 137.8 (CH, C-8), 135.6 (CH, C-4), 133.2 (C, C-6a), 132.4 (CH, C-11), 131.7 (CH, C-3), 129.9 (CH), 129.8 (CH), 129.7 (CH, C-9), 129.6 (C, C-13c), 128.2 (2 × C, C-3a, C-13b), 127.6 (C, 8a), 127.3 (CH, C-1), 127.2 (CH, C-2), 127.2 (CH, C-10), 126.5 (CH, C-5), 125.1 (C, C-7a). EIMS: m/z 311 (15), 305 (30), 304 (M+ + Na, 100), 301 (50). HRMS (ESI): calcd. for C20H11NO (M+ + Na) 304.0738, found 304.0743. Mp: 221–224 °C.
3.2.10. Synthesis of 9-Hydroxy-1H-phenalen-1-one Derivatives
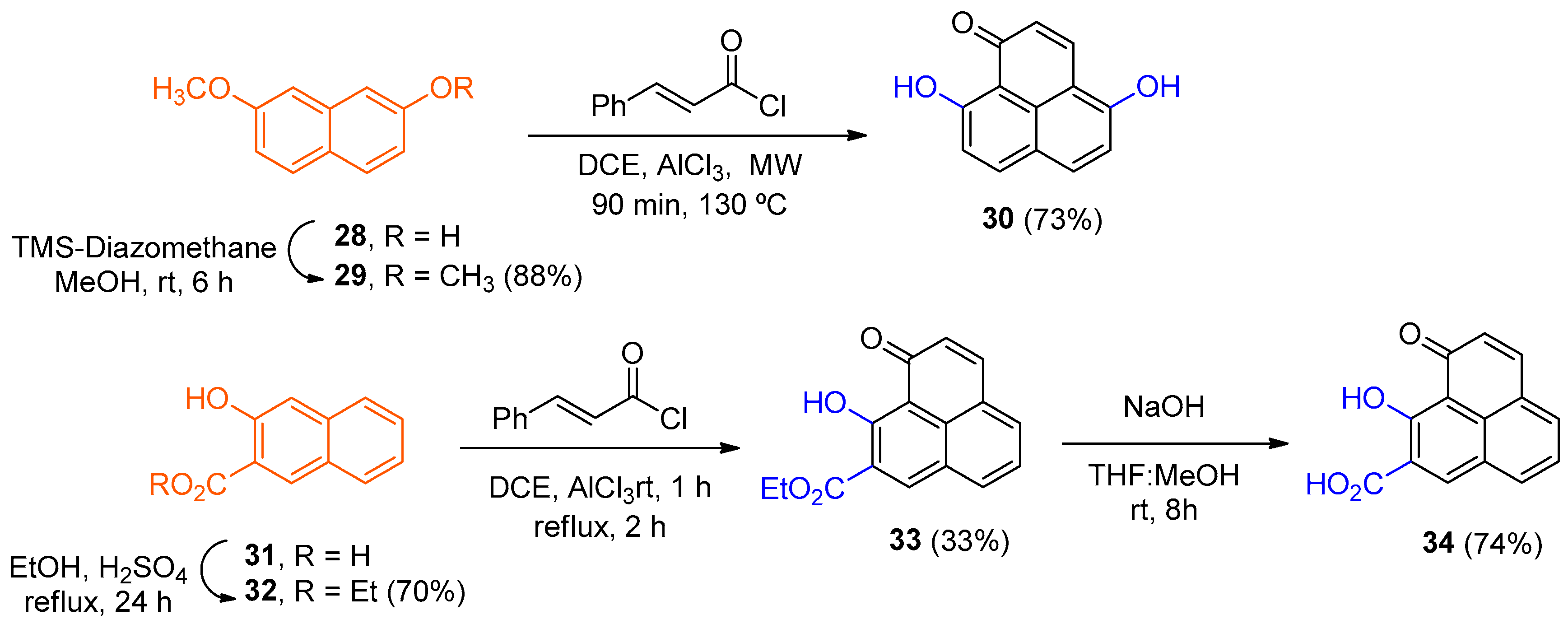
Diazomethyl(trimethyl)silane (2.03 mL, 4.018 mmol) was slowly added to a solution of 7-methoxynaphthalene-2-ol (28) (100.0 mg, 0.574 mmol) in MeOH (2 mL) and stirred for 6 h at room temperature. The resulting precipitate was gravity filtered to give product 29 (95.2 mg, 88%) as a white solid. 1H NMR (400 MHz, CDCl3) δ: 7.68 (2H, d, J = 8.8 Hz, H-4, H-5), 7.09 (2H, s, H-1, H-8), 7.03 (2H, d, J = 2.0, 8.8 Hz, H-3, H-6), 3.92 (6H, s, OCH3). EIMS: m/z 189 (21), 145 (51), 188 (M+, 100). HRMS (EI): calcd. for C12H12O2 (M+) 188.0737, found 188.0754. Mp: 135–137 °C.
To a solution of 2,7-dimethoxynaphthalene (29) (30.0 mg, 0.159 mmol) and (2E)-3-phenylacryloyl chloride (27.0 g, 159 mmol) in DCE (1 mL) was added AlCl3 (48 mg, 0.318 mmol) portionwise, and the mixture was irradiated with MW for 60 min at 130 °C. Additional DCE (1 mL) and AlCl3 (25 mg, 0.159 mmol) were then added, and the mixture was irradiated with MW for 30 min at 130 °C. The reaction crude was concentrated in a rotary evaporator, cooled in an ice bath, and a cold 1N HCl solution (1 mL) was added. The aqueous phase was extracted with EtOAc (3 × 15 mL). The organic phase was washed with a 1% NaHCO3 solution (3 × 15 mL) and with H2O (10 mL). On the other hand, the aqueous phase was acidified to pH = 2–3 and extracted with EtOAc (3 × 15 mL). The organic phase was dried (MgSO4), filtered, and the solvent was removed in a rotary evaporator. The crude was purified by silica gel column chromatography (hexane/EtOAc, 50–70%, EtOAc, acetone) to obtain product 30 (24.5 mg, 73%) as a yellow solid. 1H NMR (400 MHz, (CD3)2CO) δ: 10.57 (1H, br s, OH), 8.62 (1H, d, J = 9.6 Hz, H-3), 8.19 (1H, d, J = 9.2 Hz), 8.09 (1H, d, J = 8.4 Hz), 7.25 (1H, d, J = 8.4 Hz), 7.08 (1H, d, J = 9.6 Hz, H-2), 6.99 (1H, d, J = 9.2 Hz), 3.20 (br s, OH). EIMS: m/z 212 (M+, 100), 105 (89), 51 (15). HRMS (EI): calcd. for C13H8O3 (M+) 212.0473, found 212.0468. Mp: 240 °C.
Concentrated H2SO4 (0.1 mL) was added to a solution of 3-hydroxy-2-naphthoic acid (31) (1 g, 5.3 mmol) in EtOH (10 mL). The reaction mixture was heated under reflux for 24 h. The solvent was then removed, dissolved in a hexane/EtOAc mixture (10 mL, 2:1), and washed with H2O, saturated NaHCO3 solution, and saturated NaCl solution. The mixture was then dried (MgSO4), filtered, and concentrated under vacuum to give 32 (800 mg, 70%) as a white solid. 1H NMR (400 MHz, CDCl3) δ: 11.92 (1H, br s, OH), 10.87(1H, s, H-1), 9.09 (1H, d, J = 8.6 Hz), 8.58 (1H, s, H-4), 7.76 (2H, dt, J = 0.4, 6.5 Hz), 7.67–7.64 (1H, cd, J = 1.1, 5.5 Hz), 7.41–7.38 (1H, cd, J = 0.8, 5.5 Hz), 4.49 (2H, c, J = 7.1 Hz, CH2), 1.48 (3H, t, J = 7.1 Hz, CH3). EIMS: m/z 216 (M+, 45), 171 (20), 169 (100), 142 (45), 114 (19). HRMS (EI): calcd. for C13H12O3 (M+) 216.0786, found 216. 0782. Mp: 85–88 °C.
To a solution of ethyl 3-hydroxynaphthalene-2-carboxylate (32) (101 mg, 0.463 mmol) and (2E)-3-phenylacryloyl chloride (77.0 g, 463 mmol) in DCE (2 mL) was added AlCl3 (123 mg, 0.926 mmol) slowly, and the mixture was stirred at room temperature for 1 h. After this time, the mixture was heated under reflux for 2 h. The reaction mixture was then cooled and DCE (1 mL) and AlCl3 (62 mg, 0.463 mmol) were added, followed by reflux for 4 h. The crude product was concentrated on a rotary evaporator, cooled in an ice bath, and cold 1N HCl (1 mL) was added. The mixture was then lyophilized, the solid dissolved, and filtered with hot EtOAc. The crude product was purified by silica gel column chromatography (hexane/EtOAc, 10–80%) to yield 3 products: ethyl 4-hydroxy-3-oxo-1-phenyl-2,3-dihydro-1H-phenalene-5-carboxylate (7.1 mg, 4%), Ethyl 9-hydroxy-1-oxo-3-phenyl-1H-phenalene-8-carboxylate (2.1 mg, 1.3%), and product 33 (33 mg, 33%) as orange oils. Ethyl 4-hydroxy-3-oxo-1-phenyl-2,3-dihydro-1H-phenalene-5-carboxylate: 1H NMR (500 MHz, CDCl3) δ: 13.98 (1H, s, OH), 8.65 (1H, s, H-6), 7.77 (1H, d, J = 7.9 Hz), 7.36–7.27 (5H, m), 7.16 (2H, d, J = 7.2 Hz), 4.66 (1H, t, J = 7.2 Hz, H-1), 4.48 (2H, c, J = 7.1, Hz, H-1’), 3.28 (2H, d, J = 6.8 Hz, H-2), 1.46 (3H, t, J = 7.1 Hz, H-2’). 13C NMR (125 MHz, CDCl3) δ: 202.8 (C), 165.1 (C), 161.6 (C), 142.5 (C), 141.6 (CH), 134.11 (C), 133.3 (C), 130.0 (CH), 129.1 (2 × CH), 128.5 (CH), 127.9 (2 × CH), 127.5 (CH), 126.1 (C), 124.9 (CH), 121.4 (C), 111.2 (C), 61.7 (CH2), 44.91 (CH), 44.5 (CH2), 14.5 (CH3). EIMS: m/z 346 (M+, 100), 301 (27), 300 (43), 224 (25), 215 (19), 196 (14), 139 (14). HRMS (EI): calcd. for C22H18O4 (M+) 346.1205, found 346.1203. UV-Vis (EtOH) λmax: 378, 328, 234 nm. IR (film) νmax: 2983, 1729, 1631, 1207, cm−1. Ethyl 9-hydroxy-1-oxo-3-phenyl-1H-phenalene-8-carboxylate: 1H NMR (400 MHz, CDCl3) δ: 8.82 (1H, s, H-7), 8.15 (2H, d, J = 7.9 Hz, H-6, H-4), 7.60 (1H, t, J = 7.8 Hz, H-5), 7.56–7.51 (5H, m, J = 7.24 Hz, phenyl), 4.51 (2H, c, J = 7.12 Hz, H-1’), 1.48 (3H, t, J = 7.12 Hz, H-2’). 13C NMR (125 MHz, CDCl3) δ: 179 0, 176.8, 164.9, 153.9, 145.7, 138.1, 135.0, 134.4, 129.8 (2 × C), 129.0, 128.7 (3 × C), 125.6, 125.2, 124.5, 124.4, 123.7, 111.6, 61.7 (C-1’), 14.6 (C-2’). EIMS: m/z 344 (M+, 60), 299 (33), 272 (100), 270 (20), 215 (20), 213 (21). HRMS (EI): calcd. for C22H16O4 (M+) 344.1049, found 344.1047. UV-Vis (EtOH) λmax: 450, 425, 356, 256 nm. IR (film) νmax: 2921, 1719, 1625, 1200, 1110, 1025 cm−1. Compound 33: 1H NMR (500 MHz, CDCl3) δ: 8.72 (1H, s, H-7), 8.09 (1H, d, J = 9.2 Hz, H-3), 8.09–8.08 (2H, m), 7.62 (1H, t, J = 7.6 Hz, H-5), 7.20 (1H, d, J = 9.2 Hz, H-2), 4.48 (2H, c, J = 7.2 Hz, H-1’), 1.46 (3H, t, J = 7.1 Hz, H-2’). 13C NMR (125 MHz, CDCl3) δ: 178.8 (C), 177.7 (C), 164.8 (C), 145.5 (CH), 140.9 (CH), 135.0 (CH), 134 8 (H), 127.9 (C), 125.5 (2 × C), 124.6 (CH), 124.0 (C), 123.5 (CH), 111.7 (C), 61.6 (CH2), 14.5 (CH3). EIMS: m/z 268 (M+, 64), 223 (52), 222 (71), 196 (100), 194 (29), 139 (36), 138 (15). HRMS (EI): calcd. for C16H12O4 (M+) 268.0736, found 268.0742. UV-Vis (EtOH) λmax: 446, 421, 354, 252 nm. IR (film) νmax: 3333, 1630, 1001, 845 cm−1.
To a solution of the 33 (2.31 g, 7.77 mmol) in MeOH (1mL) and H2O (0.2 mL) was added NaOH (4 mg, 0.1 mmol). The mixture was stirred at rt for 8 h. The mixture was concentrated and the crude was diluted with H2O (5 mL) and acidified with an aq solution of 2M HCl (1 mL). This aqueous mixture was extracted with EtOAc (2 × 5 mL) and the combined organic extracts were washed with brine (10 mL), dried with MgSO4, and concentrated under reduce pressure to provide the product 34 (13 mg, 74%) as a yellow solid. 1H NMR (500 MHz, CDCl3) δ: 14.87 (1H, s, OH), 13.5 (1H, br s, OH), 9.24 (1H, d, J = 1.8 Hz, H-7), 8.37 (1H, d, J = 9.1 Hz, H-3), 8.32 (2H, d, J = 8.0 Hz, H-6, H-4), 7.79 (1H, t, J = 7.7 Hz, H-5), 7.47 (1H, d, J = 9.1 Hz, H-2). 13C NMR (125 MHz, CDCl3) δ: 185.7 (C, COOH), 172.8 (C, C-1), 165.3 (C, C-9), 149.9 (CH), 142.5 (CH), 137.7 (CH), 137.1 (CH), 127.8 (C), 125.9 (C), 125.5 (CH), 124.1 (C), 122.8 (C), 121.2 (CH), 111.1 (C). EIMS: m/z 240 (M+, 49), 222 (22), 196 (100), 194 (25), 168 (25), 83 (13). HRMS (EI): calcd. for C14H8O4 (M+) 240.0423, found 240.0417. UV-Vis (EtOH) λmax: 443, 351, 236 nm. IR (film) νmax: 3452, 2401, 1738, 1628, 1575, 1405 cm−1. Mp: 242–244 °C.
3.2.11. Synthesis of 4H-Benzo[de]isoquinolin-4-ones
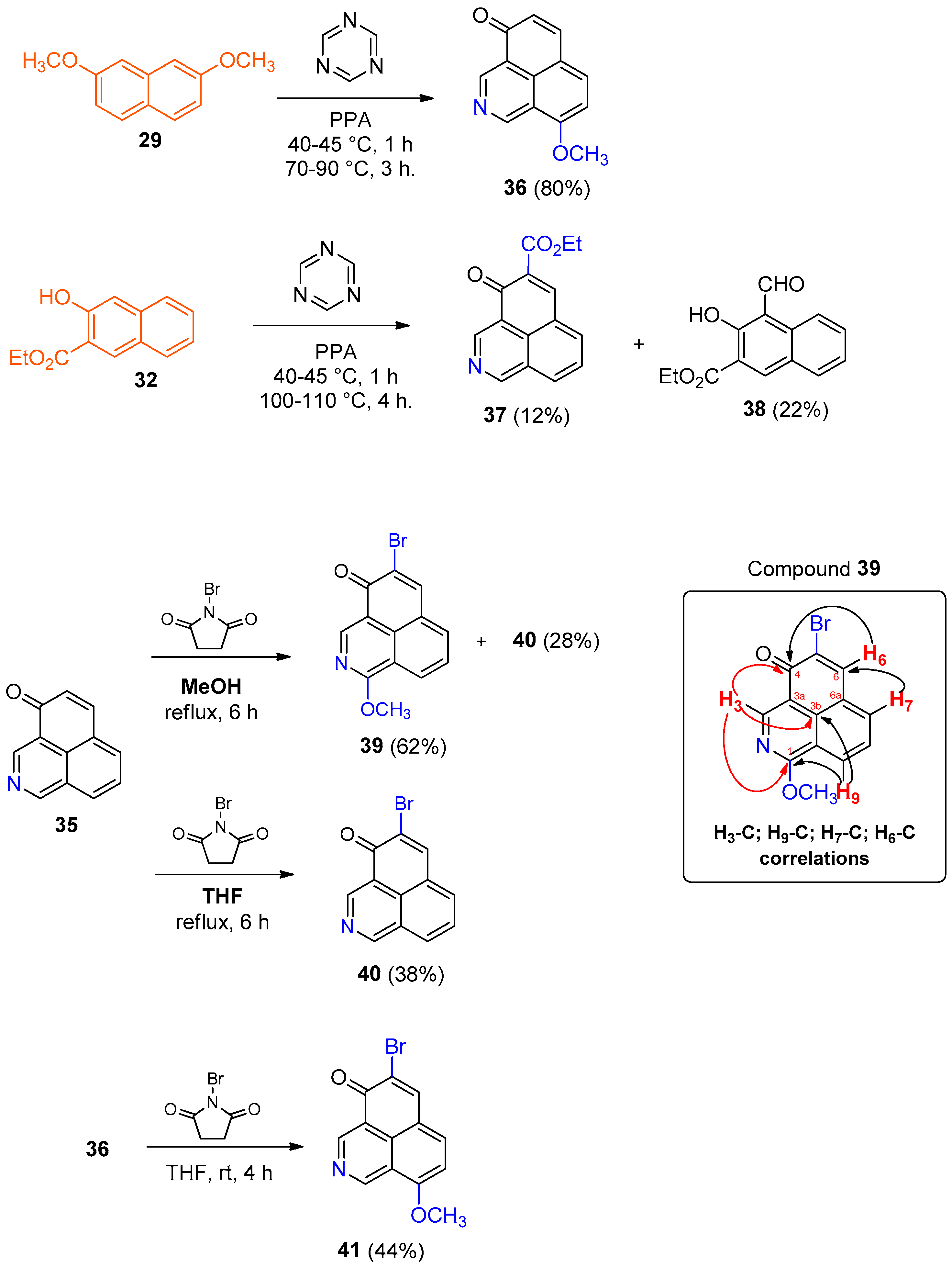
A mixture of 2-methoxynaphthalene (316 mg, 1.98 mmol) and triazine (180 mg, 2.22 mmol) in PPA (6–8 g) was stirred at 40–45 °C for 1 h and then at 100–110 °C for 3 h. The reaction mixture was poured into cold H2O (60 mL) with vigorous stirring. The resulting mixture was extracted with EtOAc (3 × 50 mL), dried (MgSO4), filtered, and the solvent was removed under vacuum. The residue was purified by column chromatography on silica gel (CH2Cl2) to give 2-methoxy-1,6-naphthalenedicarbaldehyde (38 mg, 9%) as a pale yellow solid. The aqueous phase was treated with ammonia until pH = 8–9 and, after cooling, the oil was extracted with EtOAc (3 × 50 mL). The organic phase was dried (MgSO4), filtered and the solvent was removed under vacuum and the residue was purified by column chromatography on silica gel (CH2Cl2) to give product 35 (41 mg, 11%) as a yellow solid. Compound 35: 1H NMR (600 MHz, CDCl3) δ: 9.53 (1H, s), 9.52 (1H, s), 8.15 (1H, d, J = 8.4 Hz), 7.93 (1H, d, J = 7.2 Hz), 7.78 (1H, d, J = 9.6 Hz, H-5), 7.72 (1H, dd, J = 7.6, 7.7 Hz, H-8), 6.74 (1H, d, J = 10.2 Hz, H-6). 13C NMR (150 MHz, CDCl3) δ: 185.6 (C), 157.1 (CH), 147.4 (CH), 141.0 (CH), 134.0 (CH), 130.9 (CH), 130.6 (C), 130.4 (CH), 128.4 (CH), 127.4 (C), 127.0 (C), 122.6 (C). EIMS: m/z 181 (M+, 100), 153 (58), 126 (15), 76 (15), 636 (17). HRMS (EI): calcd. for C12H7NO (M+) 181.0528, found 181.0520. UV-Vis (EtOH) λmax: 383, 340, 324, 252, 245, 232 nm. IR (film) νmax: 1638, 1574, 515, 495, 470 cm−1. Mp: 170–172 °C. 2-Methoxy-1,6-naphthalenedicarbaldehyde: 1H NMR (400 MHz, CDCl3) δ: 10.81 (1H, s), 10.05 (1H, s), 9.30 (1H, d, J = 8.9 Hz, H-8), 8.20 (1H, d, J = 1.2 Hz, H-5), 8.14 (1H, d, J = 9.2 Hz), 8.0 (1H, dd, J = 1.6, 8.9 Hz, H-7), 7.35 (1H, d, J = 9.8 Hz), 4.04 (3H, s). EIMS: m/z 214 (M+, 100), 213 (47), 185 (16), 154 (18). HRMS (EI): calcd. for C13H10O3 (M+) 214.0630, found 214.0620. IR (film) νmax: 2873, 1660, 1179, 1057, 506, 490 cm−1. Mp: 156–159 °C.
A mixture of 2,7-dimethoxynaphthalene (29) (100 mg, 0.53 mmol) and triazine (47 mg, 0.58 mmol) in APP (3–4 g) was stirred at 40–45 °C for 1 h and then at 70–90 °C for 3 h. The reaction mixture was poured into cold H2O (20 mL) with vigorous stirring. The resulting mixture was extracted with EtOAc (3 × 25 mL), dried (MgSO4), filtered, and the solvent was removed under vacuum. The aqueous phase was treated with ammonia until pH = 8–9 and, after cooling, the oil was extracted with EtOAc (3 × 25 mL). The organic phase was dried (MgSO4), filtered and the solvent was removed under vacuum and the residue was purified by silica gel column chromatography (CH2Cl2), to give product 36 (90 mg, 80%) as a yellow solid. 1H NMR (500 MHz, CDCl3) δ: 9.75 (1H, s), 9.48 (1H, s), 7.75 (1H, d, J = 8.1 Hz), 7.62 (1H, d, J = 9.7 Hz, H-5), 6.87 (1H, d, J = 8.1 Hz), 6.56 (1H, d, J = 9.7 Hz, H-6), 4.09 (3H, s, CH3). 13C NMR (125 MHz, CDCl3) δ: 185.5 (C, C-4), 160.5 (C), 152.1 (CH), 148.1 (CH), 141.1 (CH), 136.2 (CH), 131.4 (C), 127.5 (CH), 122.3 (C), 122.3 (C), 119.8 (C), 119.7 (C), 106.1 (CH), 56.4 (CH3, OCH3). EIMS: m/z 212 (14), 211 (M+, 100), 168 (17), 140 (27). HRMS (EI): calcd. for C13H9NO2 (M+) 211.0633, found 211.0626. UV-Vis (EtOH) λmax: 431, 278, 256, 230 nm. IR (film) νmax: 3336, 1644, 1581, 1236, 837, 489 cm−1. Mp: 202–204 °C.
A mixture of ethyl 3-hydroxy-2-naphthoate (32) (800 mg, 3.7 mmol) and triazine (329 mg, 4.1 mmol) in APP (8–9 g) was stirred at 40–45 °C for 1 h and then at 100–110 °C for 4 h. The reaction mixture was poured into cold H2O (80 mL) with vigorous stirring. The resulting mixture was extracted with EtOAc (3 × 60 mL), dried (MgSO4), filtered, and the solvent was removed under vacuum. The residue was purified by silica gel column chromatography (hexane/EtOAc, 1:2) to give ethyl 4-formyl-3-methoxy-2-naphthoate (38) (196 mg, 22%) as a pale yellow solid. The aqueous phase was treated with ammonia until pH = 8–9 and, after cooling, the oil was extracted with EtOAc (3 × 60 mL). The organic phase was dried (MgSO4), filtered and the solvent was removed under vacuum. The residue was purified by silica gel column chromatography (hexane/EtOAc, 1:2) to give product 37 (100 mg, 12%) as an orange oil. Compound 37: 1H NMR (500 MHz, CDCl3) δ: 9.58 (1H, s), 9.57 (1H, s), 8.45 (1H, s, H-6), 8.26 (1H, d, J = 8.3 Hz), 8.10 (1H, d, J = 7.0 Hz), 7.79 (1H, d, J = 7.6, 7.8 Hz, H-8), 4.45 (1H, c, J = 7.1 Hz), 1.44 (3H, t, J = 7.1 Hz). 13C NMR (125 MHz, CDCl3) δ: 181.2 (C, C-4), 164.9 (C), 157.0 (CH), 148.2 (CH), 144.9 (CH), 136.2 (CH), 132.2 (CH), 132.5 (CH), 131.4 (C), 130.7 (CH), 128.4 (CH), 126.7 (C), 125.7 (C), 123.0 (C), 61.7 (CH2), 14.3 (CH3). EIMS: m/z 209 (42), 208 (54), 181 (M+, 100), 180 (24), 152 (32), 148 (76), 57 (30). HRMS (EI): calcd. for C15H11NO3 (M+) 253.0739, found 253.0729. UV-Vis (EtOH) λmax: 389, 341, 326, 244, 223 nm. IR (film) νmax: 3024, 1218, 748, 672, 476 cm−1. Compound 38: 1H NMR (500 MHz, CDCl3) δ: 11.91 (1H, br s, OH), 10.87 (1H, s, CHO), 9.10 (1H, d, J = 8.6 Hz), 8.58 (1H, s, H-1), 7.76 (1H, d, J = 8.6 Hz), 7.66 (1H, ddd, J = 1.4, 6.9, 8.5 Hz), 7.40 (1H, ddd, J = 1.0, 6.9, 8.0 Hz), 4.49 (2H, c, J = 7.1 Hz), 1.49 (3H, t, J = 7.1Hz). EIMS: m/z 244 (M+, 35), 216 (28), 170 (100), 142 (34). HRMS (EI): calcd. for C14H12O4 (M+) 244.0736, found 244.0726. Mp: 140–142 °C.
To a solution of 35 (5 mg, 0.028 mmol) in MeOH (1 mL) was added NBS (5 mg, 0.028 mmol), and the reaction mixture was heated under reflux for 6 h. The solvent was removed under vacuum, and the residue was purified by preparative silica gel plate chromatography (hexane/EtOAc, 3:2) to give the products 40 (2 mg, 28%) as an orange oil and 39 (5 mg, 62%) as an orange solid. 1H NMR (500 MHz, CDCl3) δ: 9.24 (1H, s, H-3), 8.39 (1H, dd, J = 0.7, 8.7 Hz, H-9), 8.21 (1H, s, H-6), 7.15 (1H, d, J = 7.0 Hz, H-7), 7.64 (1H, dd, J = 8.1 Hz, H-8), 4.27 (3H, s, CH3). 13C NMR (125 MHz, CDCl3) δ: 177.8 (C, C-4), 165.1 (C, C-1), 150.1 (CH, C-3), 142.1 (CH, C-6), 133.0 (CH, C-7), 131.8 (C, C-9b), 127.8 (CH, C-9), 127.4 (CH, C-8), 127.3 (C, C-5), 127.0 (C, C-6a), 118.2 (C, C-3a), 117.1 (C, C-9a), 55.0 (CH3). EIMS: m/z 290 (M+, 100), 287 (98), 285 (35). HRMS (EI): calcd. for C13H8NO2Br (M+) 290.9716, found 290.9713. Mp: 220–223 °C.
To a solution of 35 (6 mg, 0.033 mmol) in THF (0.7 mL) was added NBS (5 mg, 0.028 mmol) and the reaction mixture was heated under reflux for 6 h. The solvent was removed under vacuum and the residue was purified by preparative silica gel plate chromatography (hexane/EtOAc, 3:2) to give product 40 (3.3 mg, 38%) as an orange oil. 1H NMR (500 MHz, CDCl3) δ: 9.62 (1H, s), 9.58 (1H, s), 8.28 (1H, s, H-6), 8.21 (1H, dd, J = 0.7, 8.2 Hz), 7.94 (1H, d, J = 7.1 Hz), 7.75 (1H, dd, J = 8.3, 7.3 Hz, H-8). 13C NMR (125 MHz, CDCl3) δ: 178.7 (s, C-4), 157.5 (d), 149.0 (d), 142.4 (d), 133.9 (d), 131.3 (d), 129.8 (s), 128.7 (d), 127.5 (s), 127.3 (s), 127.0 (s), 122.0 (s). EIMS: m/z 260 (M+, 100), 258 (99), 180 (32), 152 (46), 125 (20). HRMS (EI): calcd. for C12H6NOBr (M+) 258.9633, found 260.9641. UV-Vis (EtOH) λmax: 399, 344, 328, 252, 237 nm. IR (film) νmax: 3019, 1214, 647, 466 cm−1.
To a solution of 36 (37 mg, 0.175 mmol) in THF (2 mL) was added NBS (31 mg, 0.175 mmol), and the reaction mixture was stirred at room temperature for 4 h. The solvent was removed under vacuum, and the residue was purified by silica gel column chromatography (CH2Cl2) to give product 41 (22 mg, 44%) as an orange solid. 1H NMR (500 MHz, CDCl3) δ: 9.83 (1H, s), 9.60 (1H, s), 8.12 (1H, s, H-6), 7.79 (1H, d, J = 8.1 Hz), 6.94 (1H, d, J = 8.1 Hz), 4.15 (3H, s, CH3). 13C NMR (125 MHz, CDCl3) δ: 178.5 (s, C-4), 161.0 (s), 152.5 (d), 149.6 (d), 142.4 (d), 136.4 (d), 130.6 (s), 123.6 (s), 121.6 (s), 119.9 (s), 119.6 (s), 106.5 (d), 56.5 (c, CH3). EIMS: m/z 290 (M+, 100), 289 (49), 288 (99), 287 (37), 261 (30), 259 (30). HRMS (EI): calcd. for C13H8NO2Br (M+) 290.9718, found 290.9715. UV-Vis (EtOH) λmax: 397, 351, 257, 224 nm. IR (film) νmax: 3016, 1214, 744, 668, 462 cm−1. Mp: 215–217 °C.