Profile of Free and Conjugated Phenolic Compounds of Okra Pods Subjected to High-Humidity Hot-Air Impingement Blanching (HHAIB)
Abstract
1. Introduction
2. Results and Discussion
2.1. Profile and Content of Phenolic Acids in Okra Pods Subjected to HHAIB by Micro-HPLC-QTRAP/MS/MS
2.2. Profile and Content of Flavonoids in Okra Pods Subjected to HHAIB by Micro-HPLC-QTRAP/MS/MS
3. Materials and Methods
3.1. Materials
3.2. Chemicals
3.3. High-Humidity Hot Air Impingement Blanching (HHAIB)
3.4. Profile and Content of Phenolic Acids and Flavonoids in Okra Pods
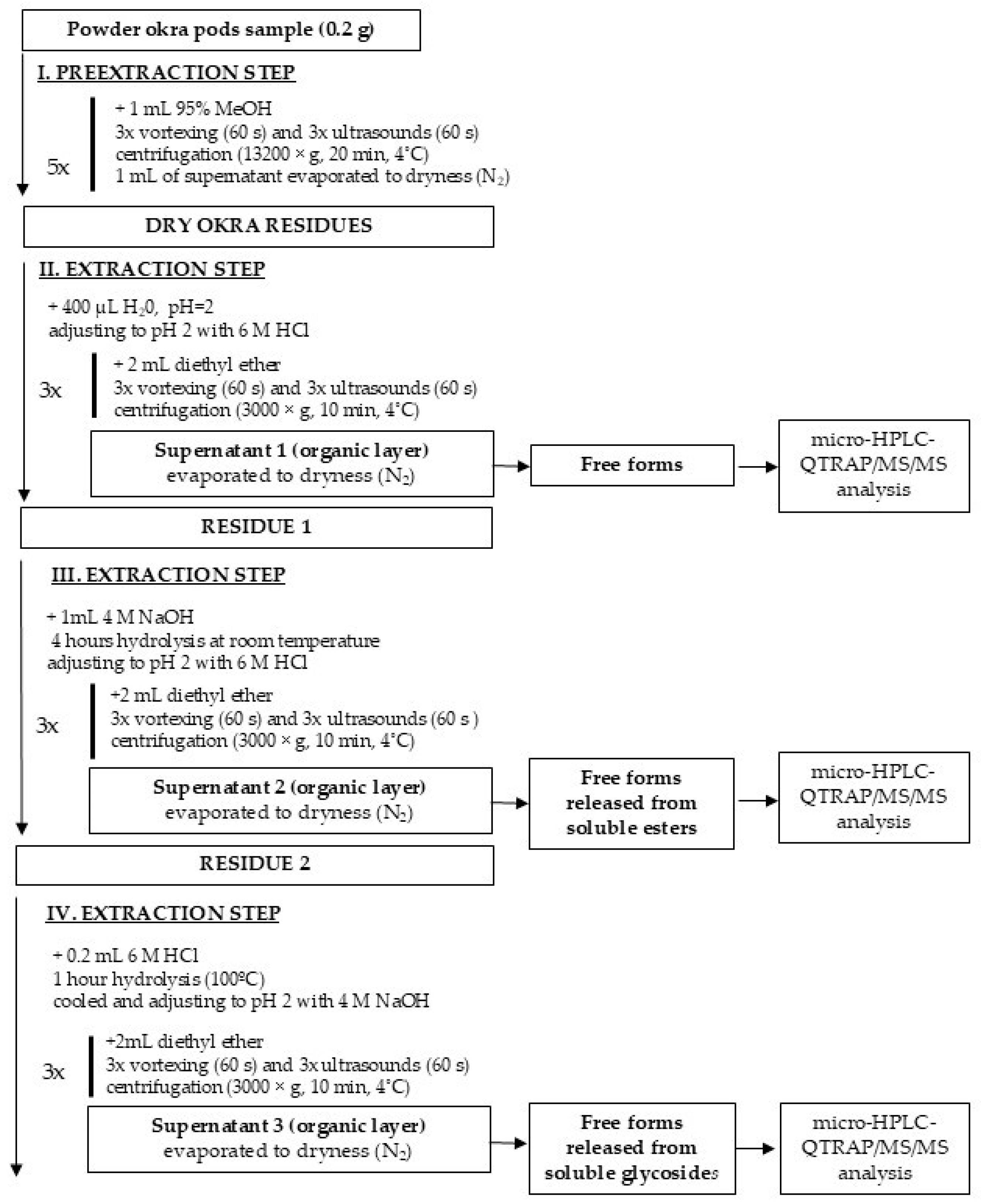
3.4.1. Extraction of Free, Ester, and Glycoside-Bound Forms of Phenolic Acids and Flavonoids
3.4.2. Chromatographic Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olivera, D.F.; Mugridge, A.; Chaves, A.R.; Mascheroni, R.H.; Viña, S.Z. Quality attributes of okra (Abelmoschus esculentus L. Moench) pods as affected by cultivar and fruit size. J. Food Res. 2021, 1, 224. [Google Scholar] [CrossRef]
- Arapitsas, P. Identification and quantification of polyphenolic compounds from okra seeds and skin. Food Chem. 2008, 110, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Adelakun, O.E.; Oyelade, O.J.; Ade-Omowaye, B.I.O.; Adeyemi, I.A.; Van de Venter, M. Chemical composition and the antioxidative properties of Nigerian okra seed (Abelmoschus esculentus moench) flour. Food Chem. Toxicol. 2009, 47, 1123–1126. [Google Scholar] [CrossRef]
- Ames, J.M.; Macleod, G. Volatile components of okra. Phytochemistry 1990, 29, 1201–1207. [Google Scholar] [CrossRef]
- Camciuc, M.; Bessiere, J.M.; Vilarem, G.; Gaset, A. Volatile components in okra seed coat. Phytochemistry 1998, 48, 311–315. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.M.; Paganda, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2021, 2, 270–278. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, A.; Barros, L.; Ferreira, I.C.F.R. Chemical composition, nutritional value and antioxidant properties of Mediterranean okra genotypes in relation to harvest stage. Food Chem. 2018, 242, 466–474. [Google Scholar] [CrossRef]
- Romdhane, M.H.; Chahdoura, H.; Barros, L.; Dias, M.I.; Corrêa, R.C.G.; Morales, P.; Ciudad-Mulero, M.; Flamini, G.; Majdoub, H.; Ferreira, I.C.F.R. Chemical composition, nutritional value, and biological evaluation of Tunisian okra pods (Abelmoschus esculentus L. Moench). Molecules 2020, 25, 4739. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, B.; Eaves, D.H.; Shikany, J.M.; Pace, R.D. Phenolic compound profile of selected vegetables frequently consumed by African Americans in the southeast United States. Food Chem. 2007, 103, 1395–1402. [Google Scholar] [CrossRef]
- Dehnad, D.; Jafari, S.M.; Afrasiabi, M. Influence of drying on functional properties of food biopolymers: From traditional to novel dehydration techniques. Trends Food Sci. Technol. 2016, 57, 116–131. [Google Scholar] [CrossRef]
- Afolabi, T.J.; Agarry, S.E. Thin layer drying kinetics and modelling of okra (Abelmoschus esculentus (L.) moench) slices under natural and forced convective air drying. Food Sci. Qual. Manag. 2014, 28, 35–49. [Google Scholar]
- Huang, J.; Zhang, M. Effect of three drying methods on the drying characteristics and quality of okra. Dry. Technol. 2015, 34, 900–911. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.H.; Mujumdar, A.S.; Fang, X.M.; Zhang, Q.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. Effects of high-humidity hot air impingement blanching (HHAIB) pretreatment on the change of antioxidant capacity, the degradation kinetics of red pigment, ascorbic acid in dehydrated red peppers during storage. Food Chem. 2018, 259, 65–72. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Q.; Zhao, B. Comparison of drying methods on drying efficiency and physicochemical quality of okra (Abelmoschus esculentus) cultivated in China. J. Food Process Eng. 2019, 42, e13163. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Monteiro, F.; Passos, C.P.; Silva, A.M.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Blanching impact on pigments, glucosinolates, and phenolics of dehydrated broccoli by-products. Food Res. Int. 2020, 132, 109055. [Google Scholar] [CrossRef]
- Deng, L.Z.; Mujumdar, A.; Yang, X.H.; Wang, J.; Zhang, Q.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. High humidity hot air impingement blanching (HHAIB) enhances drying rate and softens texture of apricot via cell wall pectin polysaccharides degradation and ultrastructure modification. Food Chem. 2018, 261, 292–300. [Google Scholar] [CrossRef]
- Deng, L.Z.; Pan, Z.; Mujumdar, A.; Zhao, J.H.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. High-humidity hot air impingement blanching (HHAIB) enhances drying quality of apricots by inactivating the enzymes, reducing drying time and altering cellular structure. Food Control 2019, 96, 104–111. [Google Scholar] [CrossRef]
- Wang, H.; Fang, X.M.; Sutar, P.P.; Meng, J.S.; Wang, J.; Yu, X.L.; Xiao, H.W. Effects of vacuum-steam pulsed blanching on drying kinetics, colour, phytochemical contents, antioxidant capacity of carrot and the mechanism of carrot quality changes revealed by texture, microstructure and ultrastructure. Food Chem. 2021, 338, 12799. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, V.; Ferreira, C.D.; Hoffmann, J.F.; Chaves, F.C.; Vanier, N.L.; de Oliveira, M.; Elias, M.C. Cooking quality properties and free and bound phenolics content of brown, black, and red rice grains stored at different temperatures for six months. Food Chem. 2018, 242, 427–434. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.M.; Mujumdar, A.S.; Qian, J.Y.; Zhang, Q.; Yang, X.H.; Liu, Y.H.; Gao, Z.J.; Xiao, H.W. Effect of high-humidity hot air impingement blanching (HHAIB) on drying and quality of red pepper (Capsicum annuum L.). Food Chem. 2017, 220, 145–152. [Google Scholar] [CrossRef]
- Mounir, S.; Ghandour, A.; Tellez-Perez, C.; Aly, A.A.; Mujumdar, A.S.; Allaf, K. Phytochemicals, chlorophyll pigments, antioxidant activity, relative expansion ratio, and microstructure of dried okra pods: Swell-drying by instant controlled pressure drop versus conventional shade drying. Dry. Technol. 2020, 39, 2145–2159. [Google Scholar] [CrossRef]
- Gong, X.; Huang, X.; Yang, T.; Wen, J.; Zhou, W.; Li, J. Effect of drying methods on physicochemical properties and antioxidant activities of okra pods. J. Food Process. Preserv. 2019, 43, e14277. [Google Scholar] [CrossRef]
- Zielinska, S.; Staniszewska, I.; Cybulska, J.; Zdunek, A.; Szymanska-Chargot, M.; Zielinska, D.; Liu, Z.; Xiao, H.; Pan, Z.; Zielinska, M. The effect of high humidity hot air impingement blanching on the changes in cell wall polysaccharides and phytochemicals of okra pods. J. Sci. Food Agric. 2022, 102, 5965–5973. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; Damin, F.M.; Caldeirao, L.; Filho, M.D.J.; da Silva, L.C.; Constant, L.D.S.; Filho, J.T.; Wagner, R.; Godoy, H.T. Study of new sources of six chlorogenic acids and caffeic acid. J. Food Compos. Anal. 2019, 82, 103244. [Google Scholar] [CrossRef]
- D’Urso, G.; Napolitano, A.; Cannavacciuolo, C.; Masullo, M.; Piacente, S. Okra fruit: LC-ESI/LTQOrbitrap/MS/MS n based deep insight on polar lipids and specialized metabolites with evaluation of antioxidant and anti-hyperglycemic activity. Food Funct. 2020, 11, 7856–7865. [Google Scholar] [CrossRef]
- Mokgalabone, T.T.; Mpai, S.; Nyakudya, T.T.; Ndhlala, A.R. Amino acids, UPLC-MS phenolic metabolites and multivariate approach for elucidating the effect of two growing conditions on growth and yield attributes in okra pods and leaves. Food Chem. 2025, 467, 142220. [Google Scholar] [CrossRef]
- Shui, G.; Peng, L.L. An improved method for the analysis of major antioxidants of Hibiscus esculentus Linn. J. Chromatogr. A 2004, 1048, 17–24. [Google Scholar] [CrossRef]
- Khomsug, P.; Thongjaroenbuangam, W.; Pakdeenarong, N.; Suttajit, M.; Chantiratikul, P. Antioxidative activities and phenolic content of extracts from okra (Abelmoschus esculentus L.). Res. J. Biol. Sci. 2010, 5, 310–313. [Google Scholar] [CrossRef]
- Amirabbasi, S.; Elhamirad, A.H.; Saeediasl, M.R.; Armin, M.; Ziaolhagh, S.H.R. Optimization of polyphenolic compounds extraction methods from okra stem. J. Food Meas. Charact. 2021, 15, 717–734. [Google Scholar] [CrossRef]
- Wu, D.T.; Nie, X.R.; Shen, D.D.; Li, H.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; Qin, W. Phenolic compounds, antioxidant activities, and inhibitory effects on digestive enzymes of different cultivars of okra (Abelmoschus esculentus). Molecules 2020, 25, 1276. [Google Scholar] [CrossRef] [PubMed]
- AOAC: Official Method of Analysis. Association of Official Analytical Chemists (No. 934.06); AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Płatosz, N.; Sawicki, T.; Wiczkowski, W. Profile of Phenolic Acids and Flavonoids of Red Beet and Its Fermentation Products. Does Long-Term Consumption of Fermented Beetroot Juice Affect Phenolics Profile in Human Blood Plasma and Urine? Pol. J. Food Nutr. Sci. 2020, 70, 55–65. [Google Scholar] [CrossRef]
| No | Compounds | Rt (min) | [M-H]- (m/z) | MS/MS (m/z) |
|---|---|---|---|---|
| phenolic acids | ||||
| 1 | protocatechuic acid | 1.91 | 153 | 109/91 |
| 2 | chlorogenic acid | 2.21 | 353 | 191/161 |
| 3 | vanillic acid | 2.27 | 167 | 123/108/91 |
| 4 | p-hydroxybenzoic acid | 2.3 | 137 | 119/93/65 |
| 5 | 3,4-dihydroxyhydrocinnamic acid | 2.31 | 181 | 137/75/59 |
| 6 | cynarin | 2.36 | 515 | 353/191/179 |
| 7 | gentisic acid | 2.41 | 153 | 135/109 |
| 8 | caffeic acid | 2.41 | 179 | 161/135/107 |
| 9 | homovanillic acid | 2.44 | 181 | 135/122 |
| 10 | syringic acid | 2.45 | 197 | 182/153/123 |
| 11 | p-coumaric acid | 2.51 | 163 | 145/119/93 |
| 12 | ferulic acid | 2.52 | 193 | 178/134/ |
| 13 | sinapic acid | 2.55 | 223 | 208/179/164 |
| 14 | salicylic acid | 2.79 | 137 | 119/93/65 |
| flavonoids | ||||
| 15 | vitexin | 1.92 | 431 | 323/311/283 |
| 16 | epigallocatechin | 2.01 | 305 | 165/125 |
| 17 | epicatechin | 2.33 | 289 | 245/203/109 |
| 18 | rutin | 2.34 | 609 | 463/301 |
| 19 | verbascoside | 2.46 | 623 | 461/161 |
| 20 | myricetin | 2.57 | 317 | 151/137 |
| 21 | luteolin | 2.71 | 285 | 151/133 |
| 22 | quercetin | 2.72 | 301 | 179/151 |
| 23 | orientin | 2.82 | 447 | 357/339/296 |
| 24 | apigenin | 3.00 | 269 | 225/151/117 |
| Phenolic Compounds | Contents [μg/g dm] | ||||||
|---|---|---|---|---|---|---|---|
| C | HHAIB 15 s | HHAIB 30 s | HHAIB 60 s | HHAIB 90 s | HHAIB 120 s | ||
| Phenolic acids | |||||||
| 1 | protocatechuic acid | 134.70 ± 4.50 cd | 168.89 ± 15.05 ab | 110.30 ± 9.44 d | 137.45 ± 10.03 cd | 192.94 ± 15.97 a | 141.62 ± 1.28 bc |
| 2 | chlorogenic acid | 0.24 ± 0.00 a | 0.01 ± 0.00 d | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.01 ± 0.00 d | 0.04 ± 0.00 b |
| 3 | vanillic acid | 185.88 ± 5.55 a | 60.16 ± 3.29 b | 50.07 ± 3.22 c | 36.30 ± 1.66 d | 57.88 ± 3.77 bc | 37.71 ± 1.78 d |
| 4 | p-hydroxybenzoic acid | 137.57 ± 5.81 a | 37.17 ± 3.63 b | 29.23 ± 1.96 bc | 23.36 ± 1.27 c | 35.77 ± 2.7 b | 29.19 ± 2.40 bc |
| 5 | 3.4-dihydroxyhydrocinnamic acid | 0.24 ± 0.01 a | 0.12 ± 0.01 d | 0.20 ± 0.02 b | 0.17 ± 0.01 bc | 0.18 ± 0.01 bc | 0.15 ± 0.02 cd |
| 6 | cynarin | 39.92 ± 2.60 a | 0.87 ± 0.07 b | nd | nd | nd | nd |
| 7 | gentisic acid | 64.76 ± 2.81 bc | 73.15 ± 3.99 ab | 74.48 ± 4.92 a | 35.87 ± 0.35 d | 60.62 ± 2.79 c | 62.84 ± 4.11 c |
| 8 | caffeic acid | 104.22 ± 5.89 c | 164.55 ± 3.20 b | 178.45 ± 14.94 b | 189.50 ± 13.76 b | 367.16 ± 34.26 a | 107.38 ± 6.76 c |
| 9 | homovanillic acid | 366.41 ± 28.27 a | 300.44 ± 29.62 b | 297.15 ± 24.47 b | 244.11 ± 12.2 bc | 231.45 ± 14.98 c | 229.73 ± 18.28 c |
| 10 | syringic acid | 32.69 ± 1.37 b | 21.68 ± 1.60 c | 39.42 ± 2.41 a | 16.72 ± 1.59 c | 39.70 ± 1.08 a | 31.68 ± 2.81 b |
| 11 | p-coumaric acid | 265.44 ± 8.12 a | 224.56 ± 22.95 ab | 184.89 ± 14.73 bc | 174.44 ± 9.77 c | 199.79 ± 12.31 bc | 193.77 ± 19.42 bc |
| 12 | ferulic acid | 298.68 ± 6.38 a | 209.09 ± 14.82 b | 203.02 ± 15.73 b | 226.46 ± 20.44 b | 217.67 ± 21.81 b | 225.41 ± 5.90 b |
| 13 | sinapic acid | 189.40 ± 14.08 b | 228.82 ± 11.88 a | 220.83 ± 9.69 ab | 189.77 ± 18.10 b | 233.28 ± 13.82 a | 222.57 ± 13.03 ab |
| 14 | salicylic acid | 101.98 ± 6.21 a | 27.27 ± 1.94 b | 21.43 ± 1.33 bc | 17.46 ± 0.94 c | 27.07 ± 1.60 b | 21.76 ± 1.63 bc |
| Flavonoids | |||||||
| 15 | vitexin | 0.49 ± 0.04 a | 0.09 ± 0.01 c | nd | 0.14 ± 0.02 bc | 0.17 ± 0.03 b | 0.13 ± 0.01 bc |
| 16 | (−)epigallocatechin | 36.27 ± 2.27 a | 23.20 ± 2.75 b | 14.21 ± 0.44 c | 13.55 ± 0.53 c | 8.69 ± 0.75 d | 8.45 ± 0.72 d |
| 17 | epicatechin | 43.74 ± 3.02 a | 36.14 ± 2.26 b | 30.79 ± 1.13 b | 31.71 ± 0.98 b | 34.00 ± 1.71 b | 30.76 ± 2.02 b |
| 18 | rutin | 4.06 ± 0.35 d | 11.64 ± 1.21 b | 19.63 ± 0.99 a | 10.24 ± 1.10 b | 7.25 ± 0.36 c | 5.18 ± 0.14 cd |
| 19 | verbascoside | 10.31 ± 0.10 d | 152.09 ± 7.06 a | 120.32 ± 15.99 b | 72.75 ± 3.90 c | 88.23 ± 8.09 c | 83.35 ± 4.75 c |
| 20 | myricetin | 36.98 ± 2.79 b | 45.59 ± 1.70 a | 25.31 ± 1.57 d | 31.02 ± 1.31 c | 21.51 ± 1.39 d | 32.02 ± 2.49 bc |
| 21 | luteolin | 0.02 ± 0.01 a | 0.03 ± 0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.01 a | 0.03 ± 0.01 a | nd |
| 22 | quercetin | 37.43 ± 1.25 c | 64.31 ± 4.02 a | 42.91 ± 1.12 bc | 27.98 ± 0.69 d | 38.33 ± 2.06 c | 44.67 ± 0.57 b |
| 23 | orientin | 4.64 ± 0.33 b | 4.9 ± 0.34 ab | 3.61 ± 0.16 c | 5.54 ± 0.36 ab | 5.83 ± 0.59 a | 2.71 ± 0.10 c |
| 24 | apigenin | 0.02 ± 0.01 b | 0.07 ± 0.01 a | 0.06 ± 0.01 a | 0.06 ± 0.01 a | 0.07 ± 0.01 a | 0.06 ± 0.01 a |
| Phenolic Compounds | Contents [μg/g dm] | ||||||
|---|---|---|---|---|---|---|---|
| C | HHAIB 15 s | HHAIB 30 s | HHAIB 60 s | HHAIB 90 s | HHAIB 120 s | ||
| (a) | |||||||
| Phenolic acids | |||||||
| 1 | protocatechuic acid | 5.95 ± 0.58 a | 1.49 ± 0.09 b | 1.03 ± 0.02 b | 0.99 ± 0.02 b | 1.01 ± 0.13 b | 1.14 ± 0.03 b |
| 2 | chlorogenic acid | 0.24 ± 0.00 a | 0.01 ± 0.00 b | 0.03 ± 0.0 b | 0.03 ± 0.00 b | 0.01 ± 0.0 b | 0.04 ± 0.00 b |
| 3 | vanillic acid | 146.06 ± 2.91 a | 12.51 ± 1.02 cd | 16.37 ± 1.05 b | 11.17 ± 0.39 d | 12.08 ± 0.56 c | 9.82 ± 0.41 e |
| 4 | p-hydroxybenzoic acid | 86.35 ± 4.46 a | 12.55 ± 1.56 b | 8.97 ± 0.47 d | 10.24 ± 0.38 c | 11.60 ± 1.08bc | 10.11 ± 0.81 c |
| 5 | 3,4-dihydroxyhydrocinnamic acid | 0.09 ± 0.00 | nd | nd | nd | nd | nd |
| 6 | cynarin | 17.25 ± 1.26 | nd | nd | nd | nd | nd |
| 7 | gentisic acid | 15.15 ± 0.40 d | 34.26 ± 1.68 b | 44.95 ± 4.36 a | 15.28 ± 0.06 d | 24.25 ± 0.95 c | 36.86 ± 2.46 b |
| 8 | caffeic acid | 11.54 ± 0.46 b | 5.14 ± 0.26e | 8.80 ± 0.47 d | 10.00 ± 0.30 c | 19.21 ± 1.77 a | 18.20 ± 1.74 a |
| 9 | homovanillic acid | 138.07 ± 9.98 a | 108.10 ± 9.35 b | 122.08 ± 5.89 b | 94.18 ± 5.37 c | 83.74 ± 8.34 cd | 73.48 ± 7.52 d |
| 10 | syringic acid | 3.66 ± 0.20 c | 5.77 ± 0.53 b | 6.26 ± 0.39 b | 3.93 ± 0.49 c | 9.50 ± 0.25 a | 2.50 ± 0.37 d |
| 11 | p-coumaric acid | 35.48 ± 2.71 a | 29.13 ± 2.12 b | 18.82 ± 1.88 d | 16.36 ± 0.88 d | 21.74 ± 1.67 c | 18.21 ± 1.60 d |
| 12 | ferulic acid | 122.05 ± 0.92 a | 48.66 ± 2.07 c | 54.79 ± 1.60 b | 51.47 ± 5.72 bc | 53.76 ± 4.65 bc | 56.83 ± 2.73 b |
| 13 | sinapic acid | 110.32 ± 8.06 d | 169.71 ± 7.04 a | 149.28 ± 7.51b | 138.42 ± 13.26 bc | 145.38 ± 9.03b | 119.51 ± 7.14 cd |
| 14 | salicylic acid | 63.57 ± 5.20 a | 8.31 ± 0.75 b | 6.72 ± 0.35 c | 7.68 ± 0.28 b | 8.70 ± 0.81 b | 7.58 ± 0.61 c |
| Flavonoids | |||||||
| 15 | vitexin | 0.38 ± 0.03 a | 0.09 ± 0.01 b | nd | 0.14 ± 0.02 b | 0.17 ± 0.03 b | 0.13 ± 0.01 b |
| 16 | (−)epigallocatechin | 36.27 ± 2.27 a | 23.20 ± 2.75 b | 14.21 ± 0.44 c | 13.55 ± 0.53 c | 8.69 ± 0.75 d | 8.45 ± 0.72d |
| 17 | epicatechin | 22.28 ± 1.40 a | 18.33 ± 1.92 a | 14.85 ± 0.88 b | 18.80 ± 0.47 a | 19.99 ± 2.09 a | 19.68 ± 1.42 a |
| 18 | rutin | 2.85 ± 0.25 f | 11.10 ± 1.17 b | 17.86 ± 0.89 a | 9.11 ± 0.94 c | 5.67 ± 0.26 d | 4.45 ± 0.10 e |
| 19 | verbascoside | 10.31 ± 0.10 f | 152.09 ± 7.06 b | 120.32 ± 15.99 a | 72.75 ± 3.90 e | 88.23 ± 8.09 d | 83.35 ± 4.75 c |
| 20 | myricetin | 7.76 ± 0.56 c | 36.97 ± 1.18 a | 15.24 ± 0.78 b | 15.46 ± 1.02 b | 15.38 ± 1.18 b | 16.99 ± 1.49 b |
| 21 | luteolin | 0.01 ± 0.01 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | nd |
| 22 | quercetin | 34.01 ± 1.10 c | 63.67 ± 3.97 a | 41.10 ± 1.10 b | 26.53 ± 0.58 d | 34.58 ± 1.79 c | 39.73 ± 0.35 b |
| 23 | orientin | 4.64 ± 0.33 a | 3.00 ± 0.26 c | 2.22 ± 0.11 e | 3.73 ± 0.26 b | 3.41 ± 0.38 b | 2.71 ± 0.02 d |
| 24 | apigenin | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.01 a |
| Total phenolic acids and flavonoids | 874.32 ± 43.20 a | 744.13 ± 44.81 b | 663.93 ± 44.20 bc | 519.87 ± 34.89 d | 567.14 ± 43.83 cd | 529.80 ± 34.29 d | |
| (b) | |||||||
| Phenolic acids | |||||||
| 1 | protocatechuic acid | 16.58 ± 0.87 a | 16.40 ± 1.24 a | 10.35 ± 0.82 c | 12.23 ± 1.35 b | 13.62 ± 1.02 b | 11.61 ± 1.36 b |
| 2 | chlorogenic acid | nd | nd | nd | nd | nd | nd |
| 3 | vanillic acid | 19.40 ± 0.93 c | 28.99 ± 1.70 a | 26.45 ± 1.55 ab | 20.20 ± 0.83 c | 25.11 ± 1.23 b | 12.31 ± 0.89 d |
| 4 | p-hydroxybenzoic acid | 10.34 ± 0.64 b | 10.89 ± 0.99 b | 11.08 ± 0.62 b | 12.38 ± 0.83 ab | 13.55 ± 0.71 a | 13.05 ± 0.92 a |
| 5 | 3,4-dihydroxyhydrocinnamic acid | 0.15 ± 0.01 c | 0.12 ± 0.01 d | 0.20 ± 0.02 a | 0.17 ± 0.01 ab | 0.18 ± 0.01 ab | 0.15 ± 0.02 bcd |
| 6 | cynarin | 22.67 ± 1.34 a | 0.87 ± 0.07 b | nd | nd | nd | nd |
| 7 | gentisic acid | 32.75 ± 0.81 a | 20.19 ± 0.60 c | 20.49 ± 0.34 c | 20.59 ± 0.29 c | 23.00 ± 0.78 b | 21.10 ± 1.18 bc |
| 8 | caffeic acid | 84.61 ± 4.87 d | 154.11 ± 2.92 c | 164.47 ± 14.09 bc | 179.50 ± 13.46 b | 347.95 ± 32.49 a | 89.18 ± 5.02 d |
| 9 | homovanillic acid | 203.81 ± 17.07 a | 192.34 ± 20.27 a | 175.07 ± 18.58 a | 149.93 ± 6.83 b | 147.71 ± 6.64 b | 156.25 ± 10.76 b |
| 10 | syringic acid | 14.99 ± 0.54 c | 10.81 ± 0.75 d | 19.79 ± 1.01 b | 10.62 ± 0.96 d | 20.13 ± 0.37 b | 24.13 ± 2.35 a |
| 11 | p-coumaric acid | 223.81 ± 4.99 a | 192.79 ± 20.55 b | 159.91 ± 12.34 bc | 154.58 ± 8.58 c | 174.53 ± 10.28 bc | 169.63 ± 17.23 bc |
| 12 | ferulic acid | 170.43 ± 4.84 a | 157.04 ± 12.45 a | 145.92 ± 13.86 a | 173.38 ± 14.58 a | 161.03 ± 16.86 a | 165.85 ± 2.92 a |
| 13 | sinapic acid | 60.29 ± 5.28 c | 51.70 ± 4.61 d | 65.23 ± 1.58 c | 50.02 ± 4.81 d | 82.70 ± 4.42 b | 95.71 ± 5.61 a |
| 14 | salicylic acid | 7.75 ± 0.48 c | 7.80 ± 0.34 c | 8.31 ± 0.47 c | 9.29 ± 0.62 bc | 10.41 ± 0.11 a | 9.78 ± 0.69 ab |
| Flavonoids | |||||||
| 15 | vitexin | 0.11 ± 0.01 | nd | nd | nd | nd | nd |
| 16 | (−)epigallocatechin | nd | nd | nd | nd | nd | nd |
| 17 | epicatechin | 12.75 ± 1.13 a | 9.21 ± 0.21 b | 6.75 ± 0.28 d | 9.63 ± 0.16 b | 9.95 ± 0.74 c | 7.67 ± 0.34 c |
| 18 | rutin | 1.21 ± 0.10 b | 0.54 ± 0.04 d | 1.77 ± 0.10 a | 1.13 ± 0.16 b | 1.58 ± 0.10 a | 0.73 ± 0.04 c |
| 19 | verbascoside | nd | nd | nd | nd | nd | nd |
| 20 | myricetin | 25.33 ± 1.88 a | 8.62 ± 0.52 d | 10.07 ± 0.79 c | 15.56 ± 0.59 b | 5.84 ± 0.18 e | 14.78 ± 0.98 b |
| 21 | luteolin | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | nd |
| 22 | quercetin | 2.44 ± 0.10 c | 0.51 ± 0.04 f | 1.75 ± 0.02 d | 1.44 ± 0.11 e | 3.33 ± 0.25 b | 4.39 ± 0.17 a |
| 23 | orientin | nd | 1.90 ± 0.08 b | 1.39 ± 0.05 c | 1.81 ± 0.10 b | 2.42 ± 0.21 a | nd |
| 24 | apigenin | nd | nd | nd | nd | nd | nd |
| Total phenolic acids and flavonoids | 909.43 ± 45.89 bc | 864.84 ± 67.39 c | 829.01 ± 66.52 c | 822.47 ± 54.27 c | 1043.05 ± 76.40 a | 796.32 ± 50.48 c | |
| (c) | |||||||
| Phenolic acids | |||||||
| 1 | protocatechuic acid | 112.17 ± 3.05 d | 151.00 ± 13.72 b | 98.92 ± 8.60 d | 124.23 ± 8.66 c | 178.31 ± 14.82 a | 128.87 ± 8.89 c |
| 2 | chlorogenic acid | nd | nd | nd | nd | nd | nd |
| 3 | vanillic acid | 20.42 ± 1.71 a | 18.66 ± 0.57 a | 7.25 ± 0.62 c | 4.93 ± 0.44 d | 20.69 ± 1.98 a | 15.58 ± 0.48 b |
| 4 | p-hydroxybenzoic acid | 40.88 ± 0.71 a | 13.73 ± 1.08 b | 9.18 ± 0.87 c | 0.74 ± 0.06 e | 10.62 ± 0.91c | 6.03 ± 0.67 d |
| 5 | 3.4-dihydroxyhydrocinnamic acid | nd | nd | nd | nd | nd | nd |
| 6 | cynarin | nd | nd | nd | nd | nd | nd |
| 7 | gentisic acid | 16.86 ± 1.60 a | 18.70 ± 1.71 a | 9.04 ± 0.22 c | nd | 13.37 ± 1.06 b | 4.88 ± 0.47 d |
| 8 | caffeic acid | 8.07 ± 0.56 a | 5.30 ± 0.02b | 5.18 ± 0.38 b | nd | nd | nd |
| 9 | homovanillic acid | 24.53 ± 1.22 | nd | nd | nd | nd | nd |
| 10 | syringic acid | 14.04 ± 0.63 a | 5.10 ± 0.32 c | 13.37 ± 1.01 a | 2.17 ± 0.14 d | 10.07 ± 0.46 b | 5.05 ± 0.09 c |
| 11 | p-coumaric acid | 6.15 ± 0.42 a | 2.64 ± 0.28 c | 6.16 ± 0.51 a | 3.50 ± 0.31 b | 3.52 ± 0.36 b | 5.93 ± 0.59 a |
| 12 | ferulic acid | 6.20 ± 0.62 a | 3.39 ± 0.30 b | 2.31 ± 0.27 d | 1.61 ± 0.14 e | 2.88 ± 0.30 bc | 2.73 ± 0.25 c |
| 13 | sinapic acid | 18.79 ± 0.74 a | 7.41 ± 0.23 b | 6.32 ± 0.60 c | 1.33 ± 0.03 e | 5.20 ± 0.37 d | 7.35 ± 0.28 b |
| 14 | salicylic acid | 30.66 ± 0.53 a | 11.16 ± 0.85 b | 6.40 ± 0.51 d | 0.49 ± 0.04 f | 7.96 ± 0.68 c | 4.40 ± 0.33 e |
| Flavonoids | |||||||
| 15 | vitexin | nd | nd | nd | nd | nd | nd |
| 16 | (−)epigallocatechin | nd | nd | nd | nd | nd | nd |
| 17 | epicatechin | 8.71 ± 0.49 ab | 8.60 ± 0.13 b | 9.19 ± 0.27 a | 3.28 ± 0.35 d | 4.06 ± 0.22 c | 3.41 ± 0.26 d |
| 18 | rutin | nd | nd | nd | nd | nd | nd |
| 19 | verbascoside | nd | nd | nd | nd | nd | nd |
| 20 | myricetin | 3.89 ± 0.35 a | nd | nd | nd | 0.29 ± 0.03 b | 0.25 ± 0.02 b |
| 21 | luteolin | nd | nd | nd | nd | nd | nd |
| 22 | quercetin | 0.98 ± 0.05 a | 0.13 ± 0.01 d | 0.06 ± 0.00 e | 0.01 ± 0.00 f | 0.42 ± 0.02 c | 0.55 ± 0.05 b |
| 23 | orientin | nd | nd | nd | nd | nd | nd |
| 24 | apigenin | nd | 0.05 ± 0.00 a | 0.04 ± 0.00 a | 0.03 ± 0.00 a | 0.05 ± 0.00 a | 0.03 ± 0.00 a |
| Total phenolic acids and flavonoids | 312.35 ± 12.68 a | 245.87 ± 19.22 b | 173.42 ± 0.27 cd | 142.32 ± 10.17 d | 257.44 ± 21.21 b | 185.06 ± 12.38 c | |
| Phenolic Compounds | C | HHAIB 15 s | HHAIB 30 s | HHAIB 60 s | HHAIB 90 s | HHAIB 120 s | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Free | Conj. | Free | Conj. | Free | Conj. | Free | Conj. | Free | Conj. | Free | Conj. | |
| Phenolic acids | ||||||||||||
| protocatechuic acid | 5 | 95 | 1 | 99 | 1 | 99 | 1 | 99 | 1 | 99 | 1 | 99 |
| chlorogenic acid | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| vanillic acid | 78 | 22 | 21 | 79 | 33 | 67 | 30 | 70 | 21 | 79 | 26 | 74 |
| p-hydroxybenzoic acid | 63 | 37 | 34 | 66 | 31 | 69 | 44 | 56 | 33 | 67 | 35 | 65 |
| 3.4-dihydroxyhydrocinnamic acid | 37 | 63 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 |
| cynarin | 43 | 57 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| gentisic acid | 23 | 73 | 47 | 53 | 60 | 40 | 43 | 57 | 40 | 60 | 59 | 41 |
| caffeic acid | 11 | 89 | 3 | 97 | 5 | 95 | 5 | 95 | 5 | 95 | 17 | 83 |
| homovanillic acid | 38 | 62 | 36 | 64 | 41 | 59 | 39 | 61 | 36 | 64 | 32 | 68 |
| syringic acid | 11 | 89 | 27 | 73 | 16 | 84 | 23 | 77 | 24 | 76 | 8 | 92 |
| p-coumaric acid | 13 | 87 | 13 | 87 | 10 | 90 | 10 | 90 | 11 | 89 | 10 | 90 |
| ferulic acid | 41 | 59 | 23 | 77 | 27 | 73 | 23 | 77 | 25 | 75 | 25 | 75 |
| sinapic acid | 58 | 42 | 74 | 26 | 68 | 32 | 73 | 27 | 62 | 28 | 54 | 46 |
| salicylic acid | 62 | 38 | 30 | 70 | 31 | 69 | 44 | 56 | 32 | 68 | 35 | 65 |
| Flavonoids | ||||||||||||
| vitexin | 76 | 24 | 100 | 0 | nd | nd | 100 | 0 | 100 | 0 | 100 | 0 |
| rutin | 70 | 30 | 95 | 5 | 91 | 9 | 89 | 11 | 78 | 22 | 86 | 14 |
| (−)epigallocatechin | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| myricetin | 21 | 79 | 81 | 29 | 60 | 40 | 50 | 50 | 71 | 29 | 53 | 47 |
| verbascoside | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| epicatechin | 51 | 49 | 51 | 49 | 48 | 52 | 59 | 41 | 59 | 41 | 64 | 26 |
| quercetin | 91 | 9 | 99 | 1 | 96 | 4 | 95 | 5 | 90 | 10 | 89 | 11 |
| luteolin | 50 | 50 | 67 | 33 | 50 | 50 | 67 | 33 | 67 | 33 | nd | nd |
| orientin | 100 | 0 | 61 | 39 | 62 | 38 | 67 | 33 | 58 | 42 | 100 | 0 |
| apigenin | 100 | 0 | 28 | 72 | 33 | 67 | 50 | 50 | 28 | 72 | 50 | 50 |
| Phenolic Compounds | Contents [μg/g dm] | |||||
|---|---|---|---|---|---|---|
| C | HHAIB 15 s | HHAIB 30 s | HHAIB 60 s | HHAIB 90 s | HHAIB 120 s | |
| Phenolic acids (free form) | 755.78 ± 38.79 a | 435.64 ± 26.47 bc | 438.10 ± 23.99 b | 359.75 ± 27.15 cd | 390.98 ± 29.24 bcd | 354.28 ± 25.42 d |
| Phenolic acids (ester bound) | 867.58 ± 42.67 b | 844.05 ± 66.50 b | 807.27 ± 65.28 b | 792.89 ± 53.15 b | 1019.92 ± 74.92 a | 768.75 ± 48.95 b |
| Phenolic acids (glycoside bound) | 298.77 ± 11.79 a | 237.09 ± 19.08 b | 164.13 ± 13.59 cd | 139.00 ± 9.82 d | 252.62 ± 20.94 b | 180.82 ± 12.05 c |
| Total content of phenolic acids | 1922.13 ± 93.25 a | 1516.78 ± 112.05 bc | 1409.50 ± 102.86 bc | 1291.64 ± 90.12 c | 1663.52 ± 125.10 ab | 1303.85 ± 86.42 c |
| Flavonoids (free form) | 118.54 ± 6.06 d | 308.49 ± 18.34 a | 225.83 ± 20.21 b | 160.12 ± 7.74 cd | 176.16 ± 14.59 c | 175.52 ± 8.87 c |
| Flavonoids (ester bound) | 41.85 ± 3.22 a | 20.79 ± 0.89 c | 21.74 ± 1.24 c | 29.58 ± 1.12 b | 23.13 ± 1.48 c | 27.57 ± 1.53 bc |
| Flavonoids (glycoside bound) | 13.58 ± 0.89 a | 8.78 ± 0.14 b | 9.29 ± 0.27 b | 3.32 ± 0.35 d | 4.82 ± 0.35 c | 4.24 ± 0.33 cd |
| Total content of flavonoids | 173.97 ± 10.17 bc | 338.06 ± 19.37 a | 256.86 ± 21.72 b | 193.02 ± 9.21 c | 204.11 ± 16.42 c | 207.33 ± 10.73 c |
| Total content of phenolic acids and flavonoids | 2096.10 ± 103.42 a | 1854.84 ± 131.42 ab | 1666.36 ± 124.58 bc | 1484.66 ± 99.33 c | 1867.63 ± 141.52 ab | 1511.18 ± 97.15 c |
| Apparatus | LC-200, Eksigent, Vanghan, ON, Canada |
|---|---|
| Detector | Mass spectrometer QTRAP 5500, AB Sciex, Vaughan, ON, Canada |
| Column | HALO C18 column (50 mm × 0.5 mm, 2.7 µm), Eksigent, Vaughan, ON, Canada |
| Mobile phase | Solvent A (water/formic acid; 99.05/0.95; v/v) Solvent B (acetonitrile/formic acid, 99.05/0.95, v/v) |
| Gradient program | 5% B (0–0.5 min) 5% to 90% B (0.5–2 min) 90% B (2–2.5 min) 90% to 5% B (2.5–2.7 min) 5% B (3 min) |
| Optical ESI-MS/MS conditions | Negative ionization, curtain gas: 20 L/min; collision gas: 9 L/min; ion spray voltage: −5300 V; temperature: 350 °C; 1 ion source gas: 35 L/min; 2 ion source gas: 30 L/min; declastering potential: 100 V; entrance potential: 10 V; collision energy: 40 eV; collision cell exit potential: 20 V |
| Injection volume | 5 µL |
| Flow rate | 15 µL/min |
| Column temperature | 45 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, D.; Płatosz, N.; Górski, K.; Zielińska, M.; Xiao, H.-W.; Zieliński, H. Profile of Free and Conjugated Phenolic Compounds of Okra Pods Subjected to High-Humidity Hot-Air Impingement Blanching (HHAIB). Molecules 2025, 30, 4665. https://doi.org/10.3390/molecules30244665
Zielińska D, Płatosz N, Górski K, Zielińska M, Xiao H-W, Zieliński H. Profile of Free and Conjugated Phenolic Compounds of Okra Pods Subjected to High-Humidity Hot-Air Impingement Blanching (HHAIB). Molecules. 2025; 30(24):4665. https://doi.org/10.3390/molecules30244665
Chicago/Turabian StyleZielińska, Danuta, Natalia Płatosz, Kacper Górski, Magdalena Zielińska, Hong-Wei Xiao, and Henryk Zieliński. 2025. "Profile of Free and Conjugated Phenolic Compounds of Okra Pods Subjected to High-Humidity Hot-Air Impingement Blanching (HHAIB)" Molecules 30, no. 24: 4665. https://doi.org/10.3390/molecules30244665
APA StyleZielińska, D., Płatosz, N., Górski, K., Zielińska, M., Xiao, H.-W., & Zieliński, H. (2025). Profile of Free and Conjugated Phenolic Compounds of Okra Pods Subjected to High-Humidity Hot-Air Impingement Blanching (HHAIB). Molecules, 30(24), 4665. https://doi.org/10.3390/molecules30244665







