Locust Bean Gum: A Natural Polysaccharide as an Eco-Friendly Corrosion Inhibitor for N80 Carbon Steel in CO2-Saturated Saline Solution, Useful for the Oil and Gas Industry
Abstract
1. Introduction
2. Results
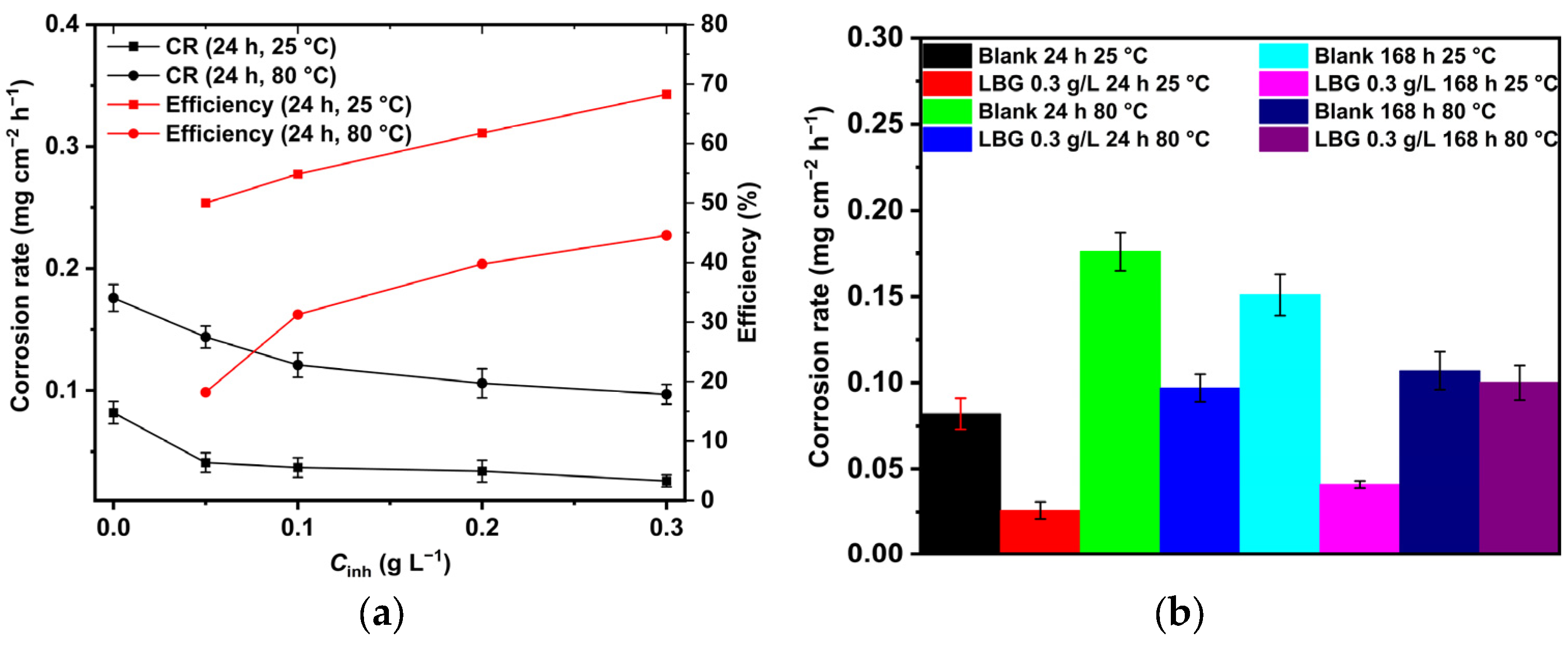
2.1. Weight Loss Measurements
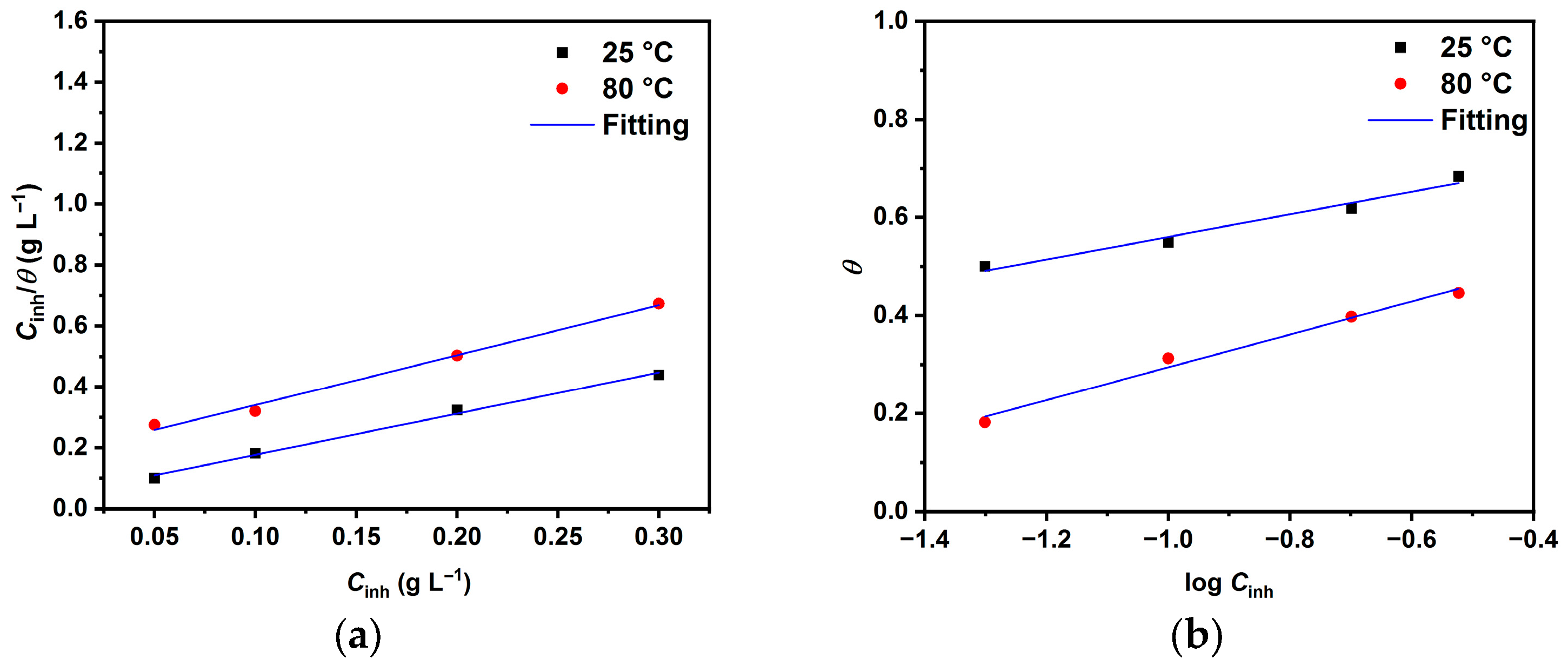
2.2. Adsorption Study and Activation Parameters
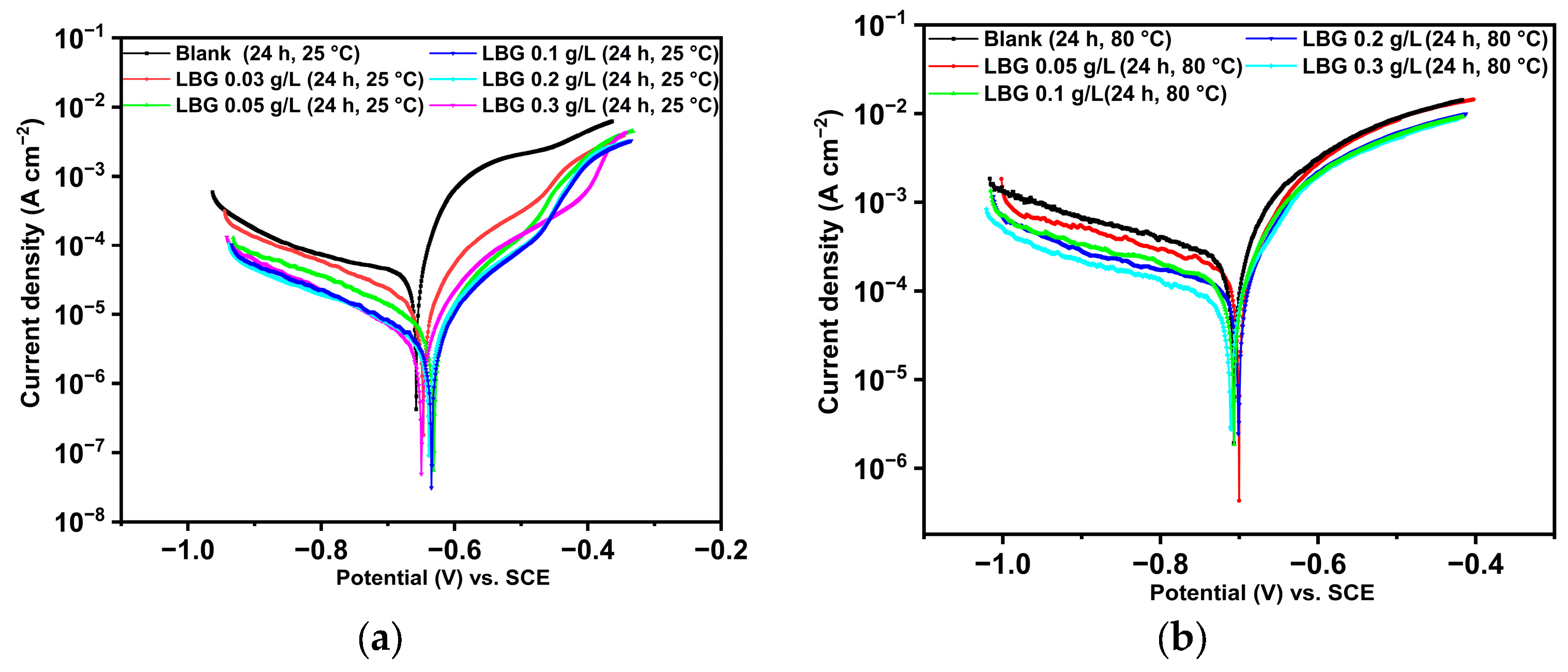
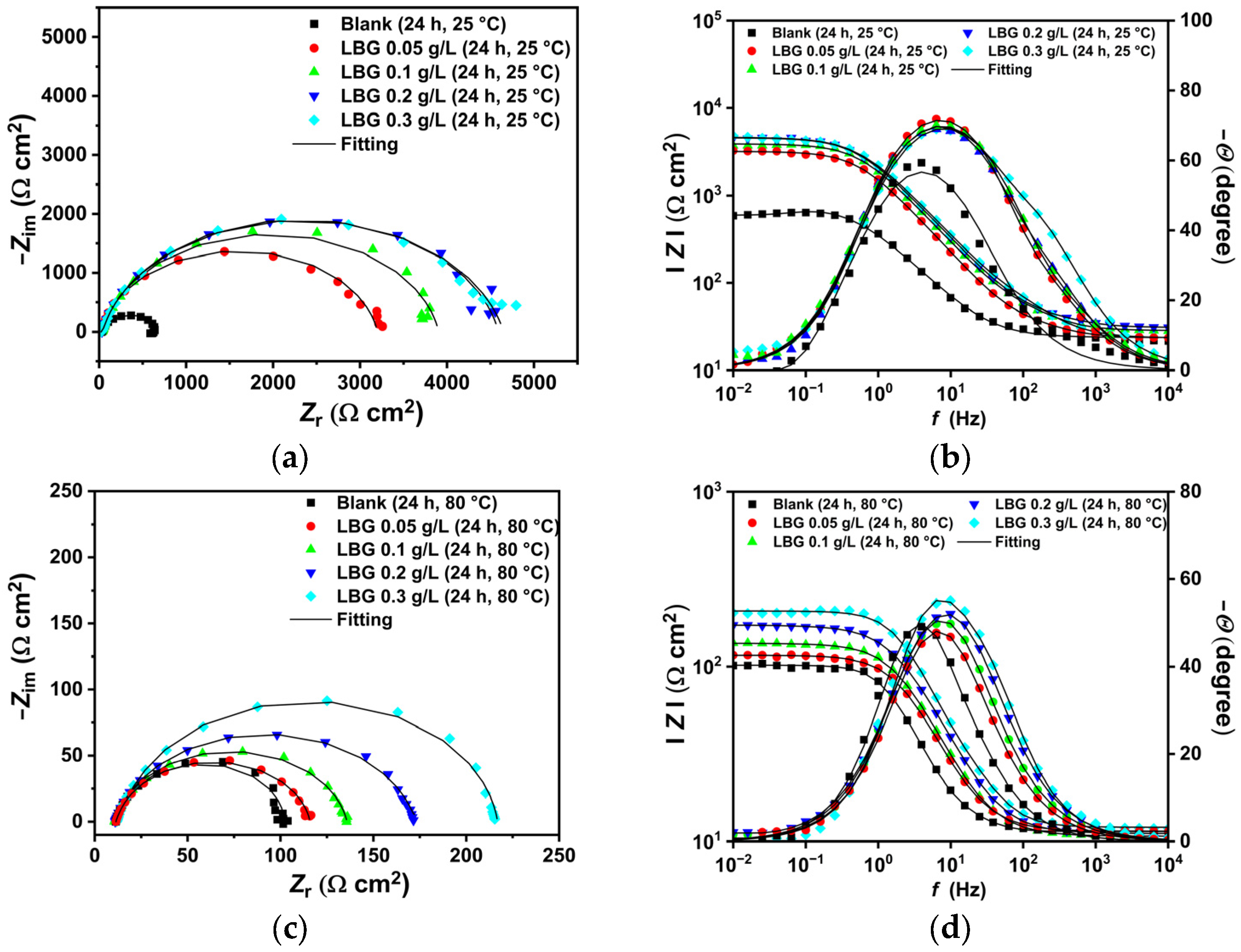
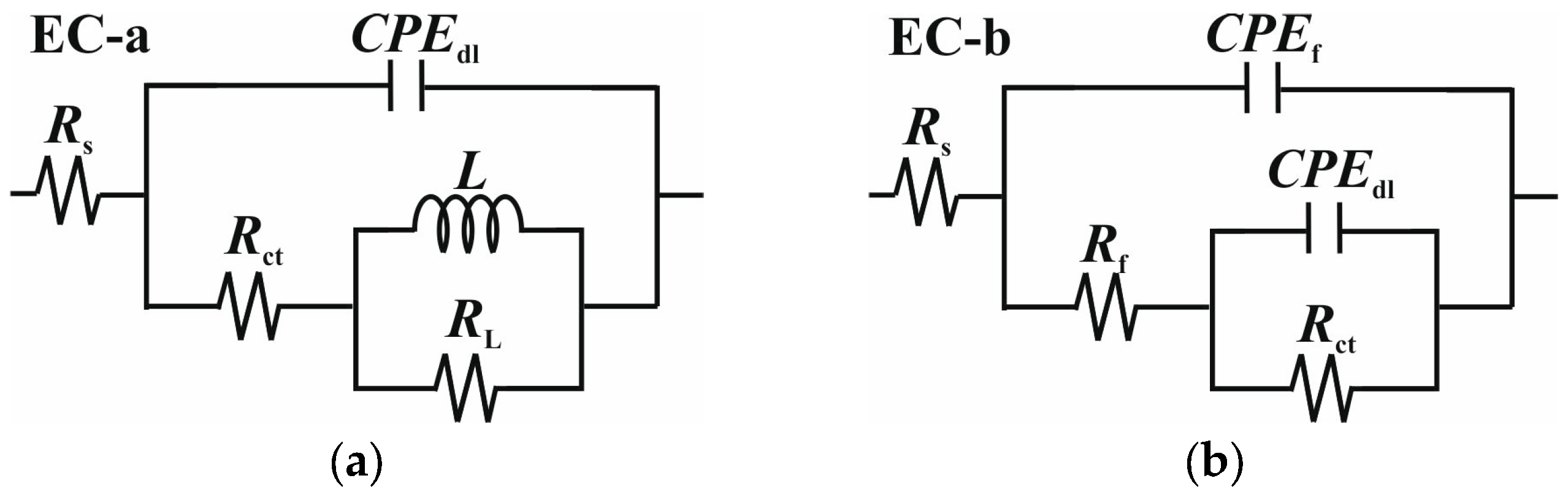
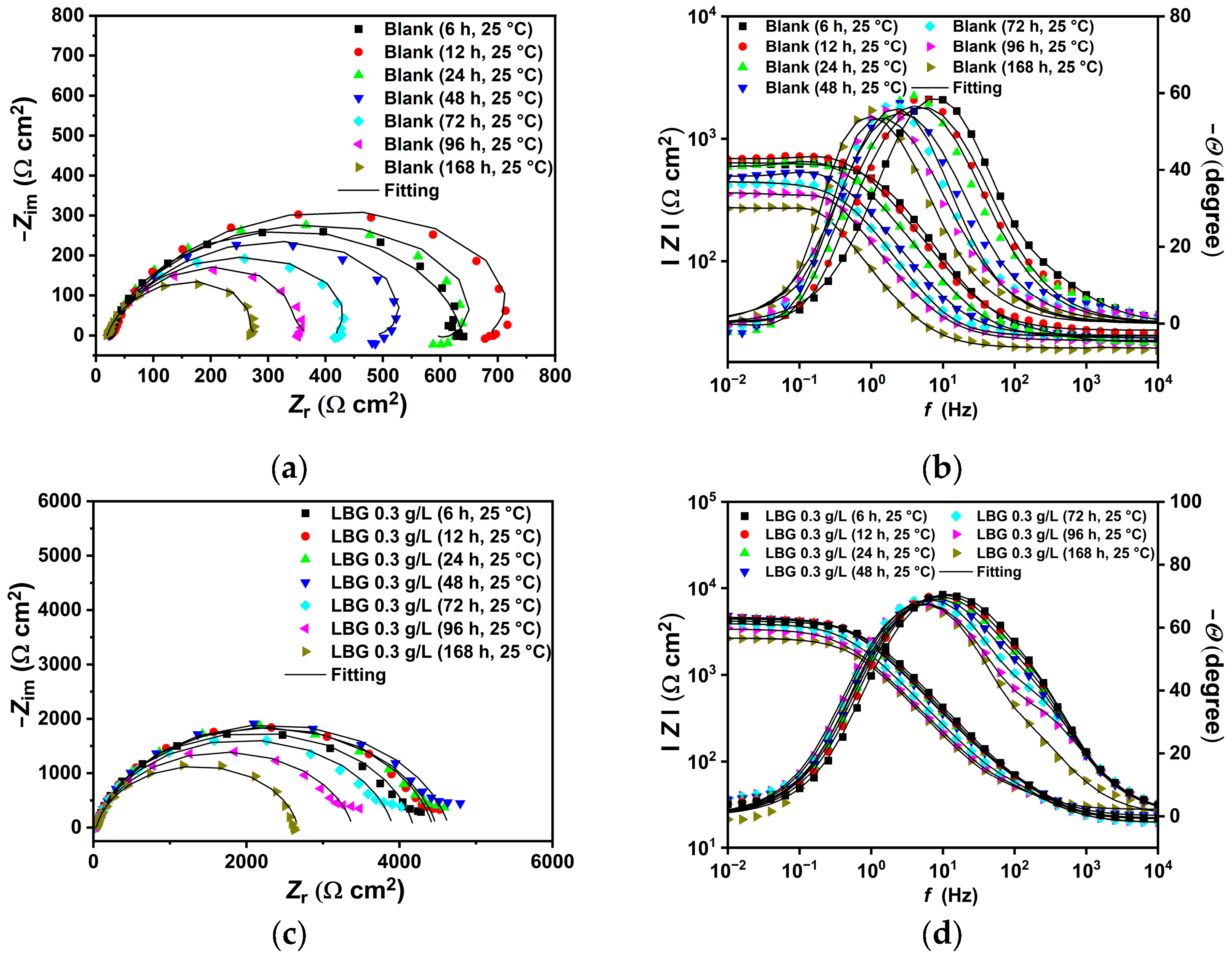
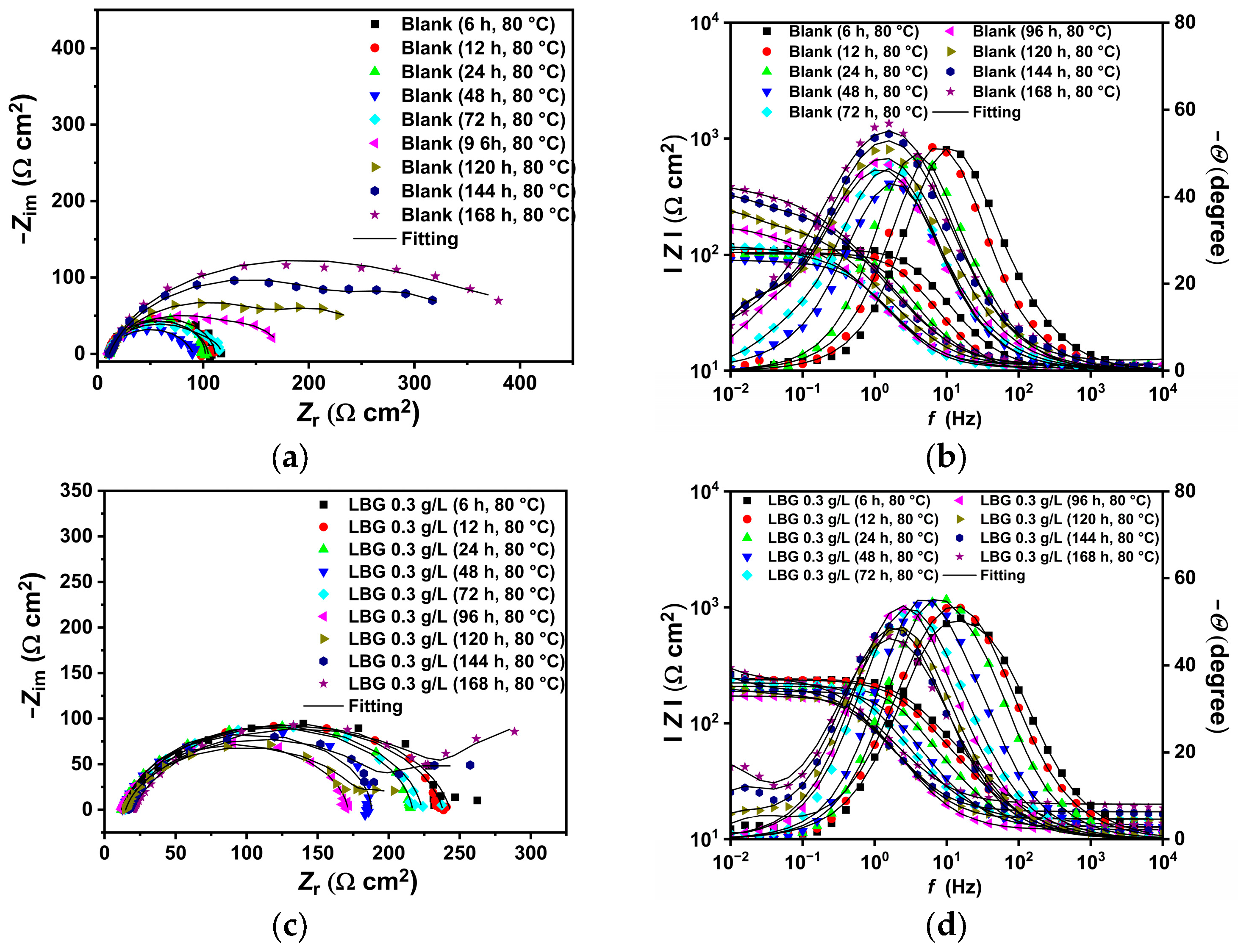
2.3. Electrochemical Measurements
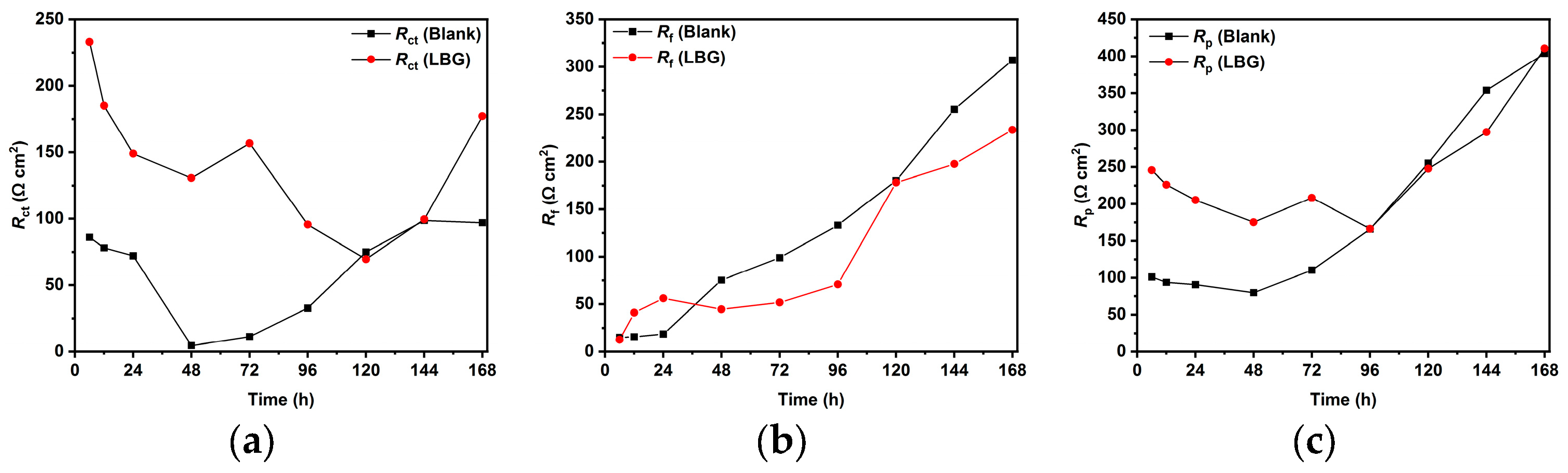
2.4. Effect of Time
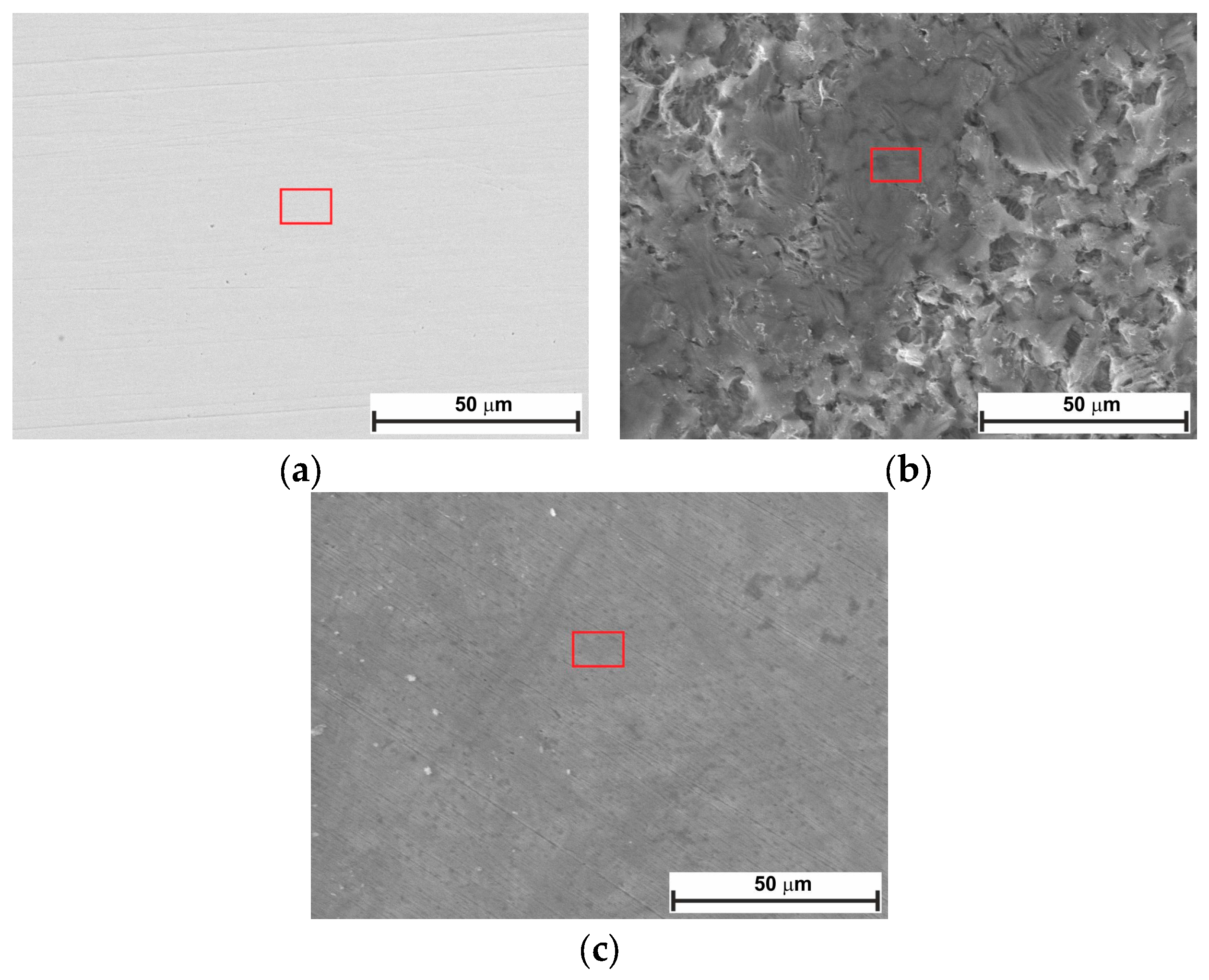
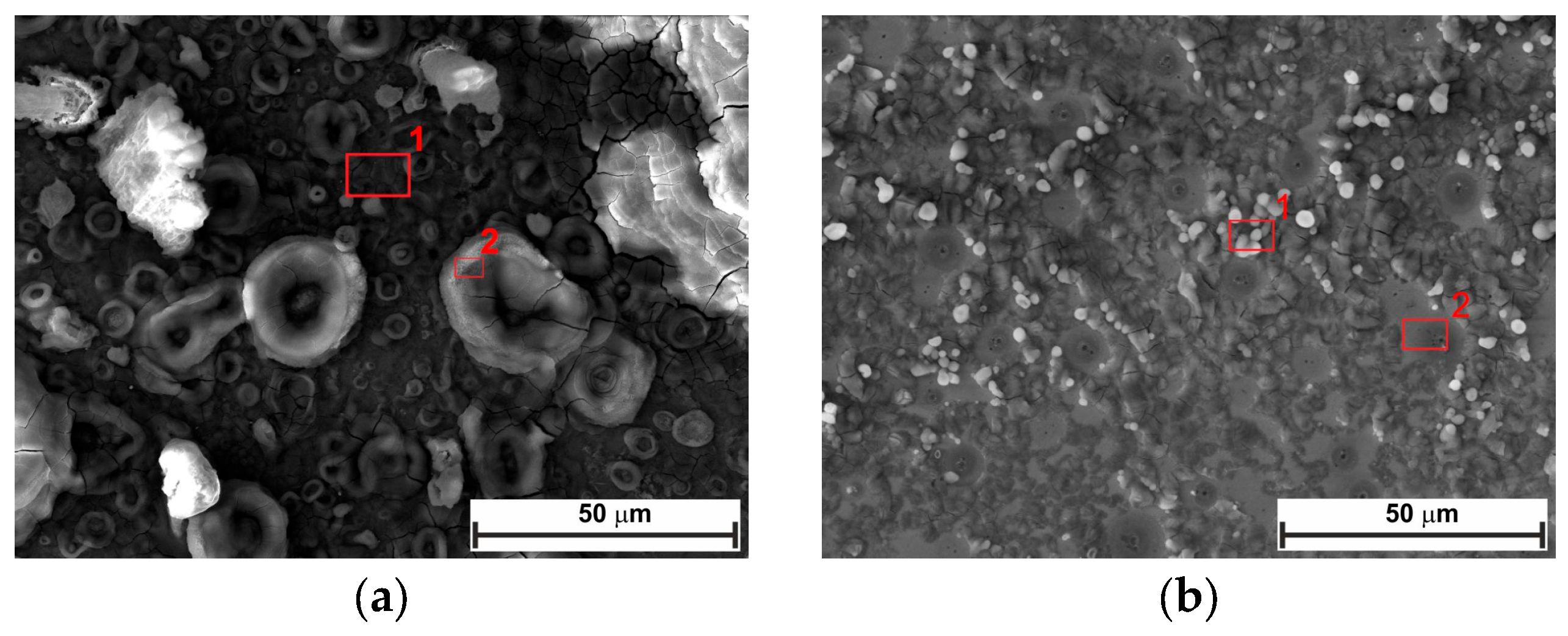
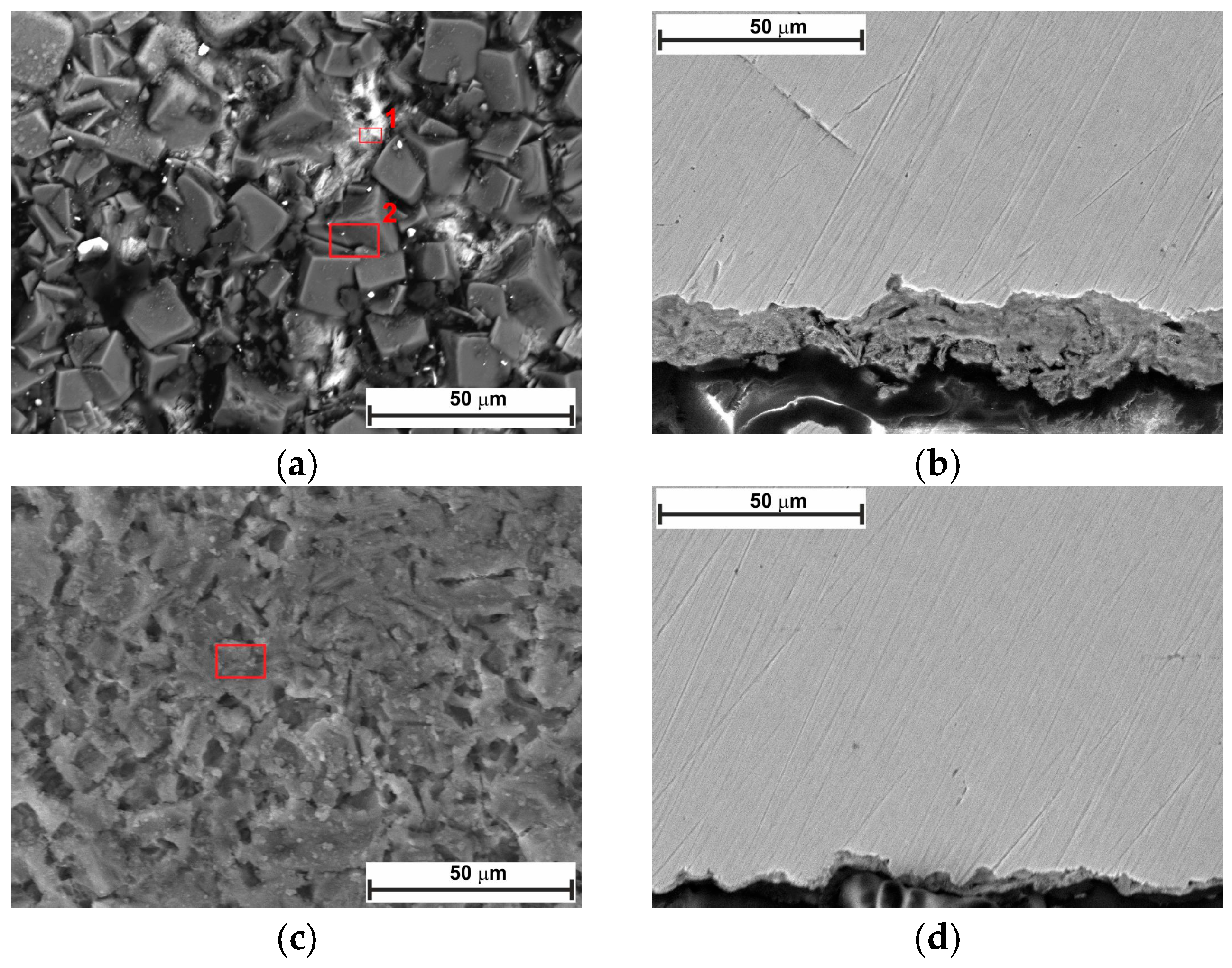
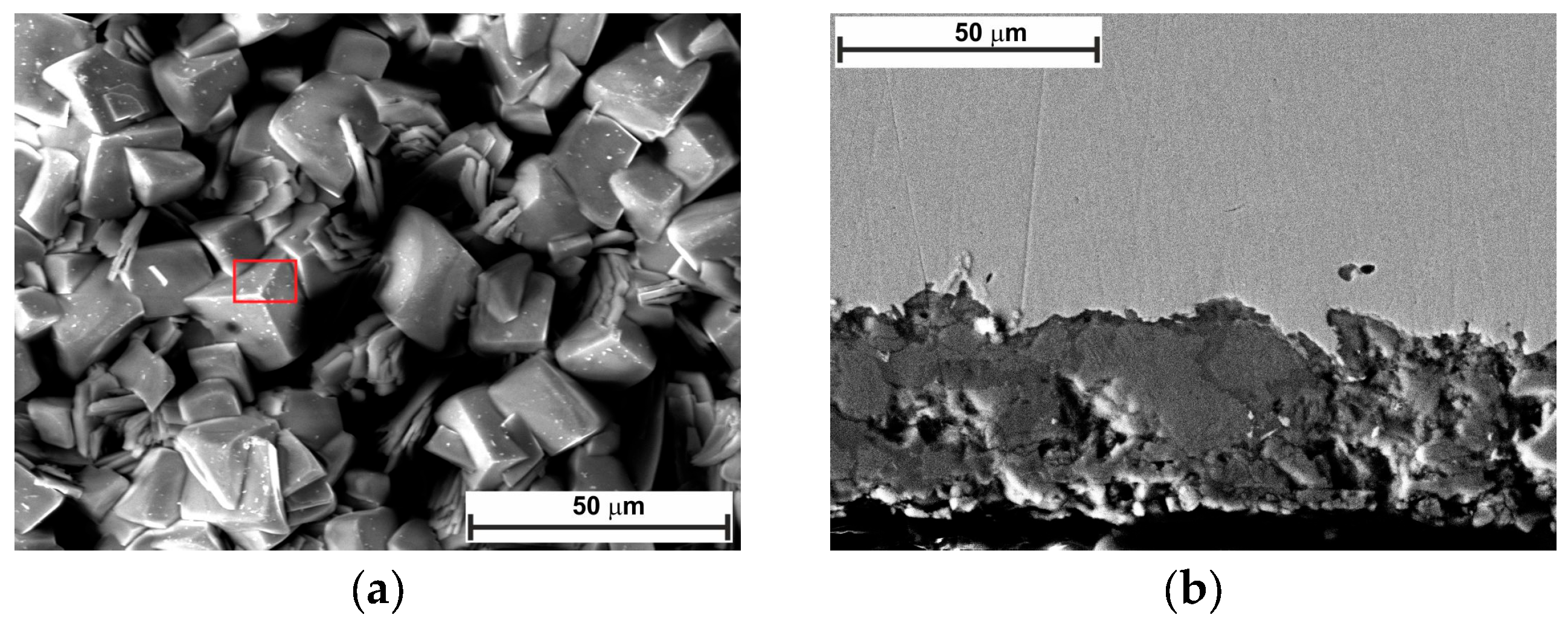
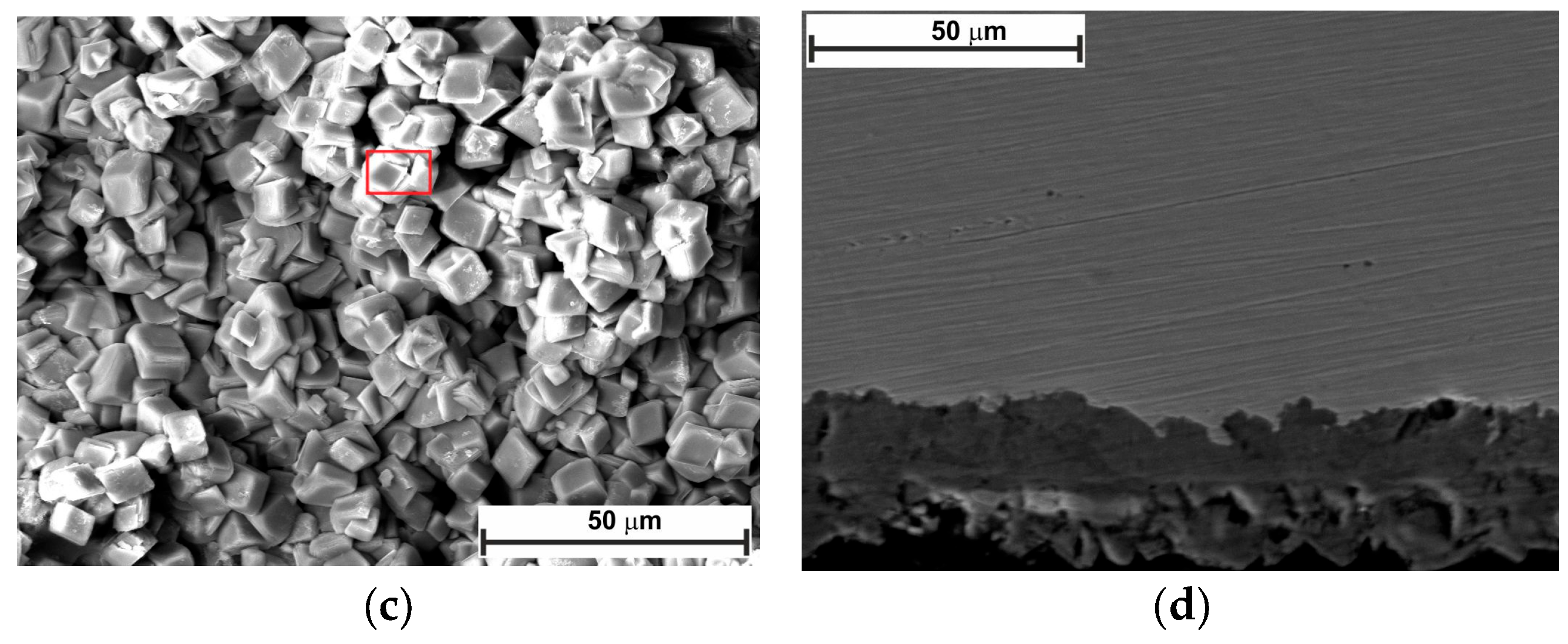
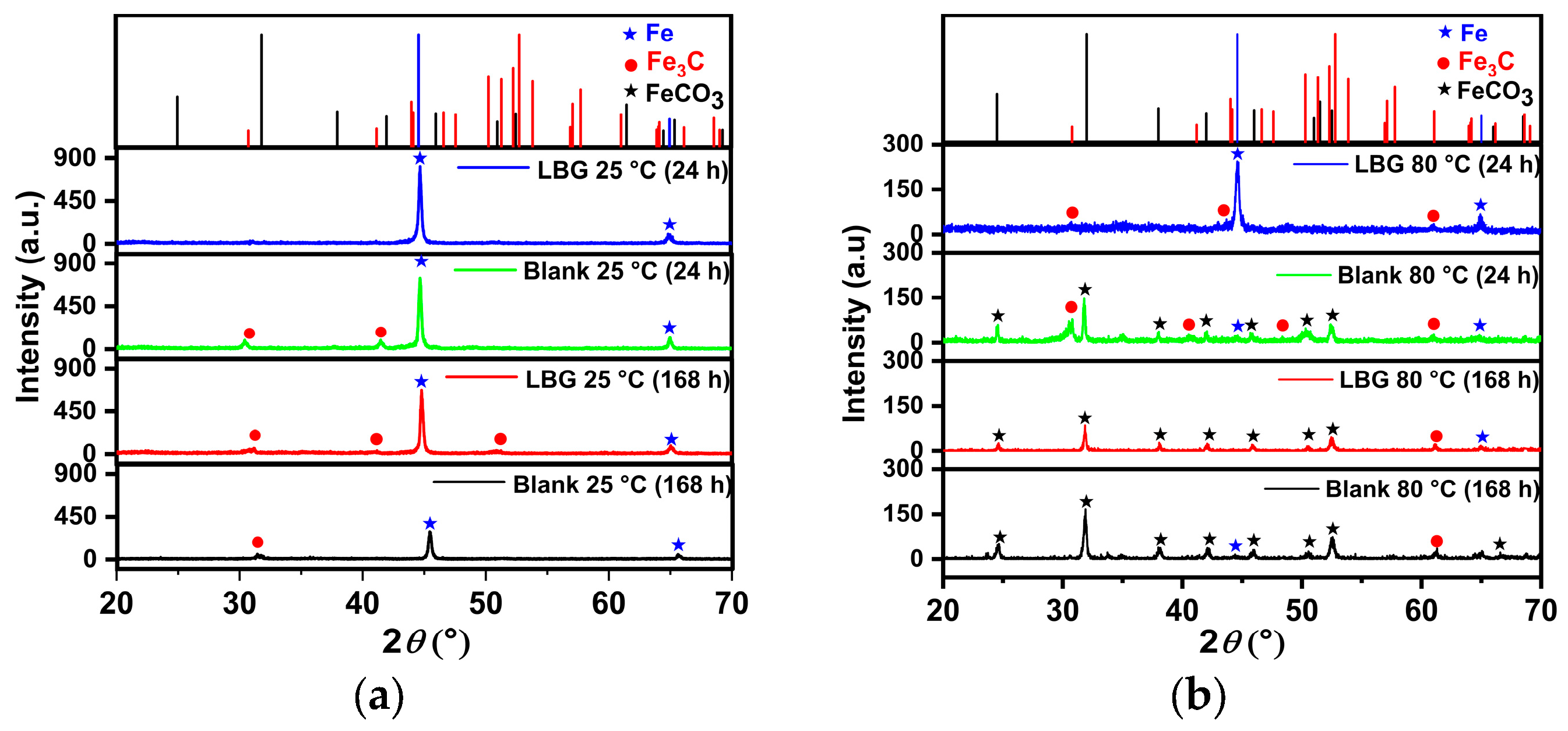
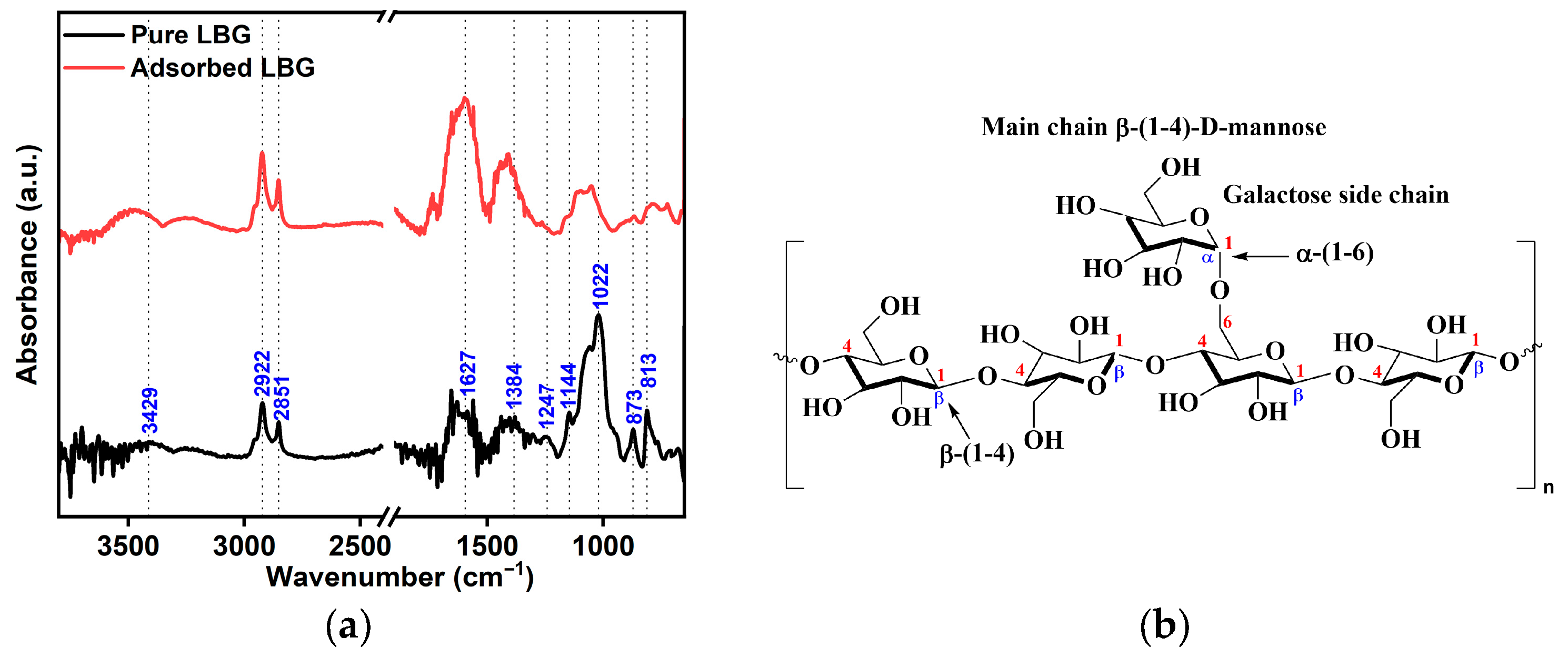
2.5. Surface Analysis
3. Discussion
3.1. Low Temperature (T = 25 °C)
3.2. High Temperature (T = 80 °C)
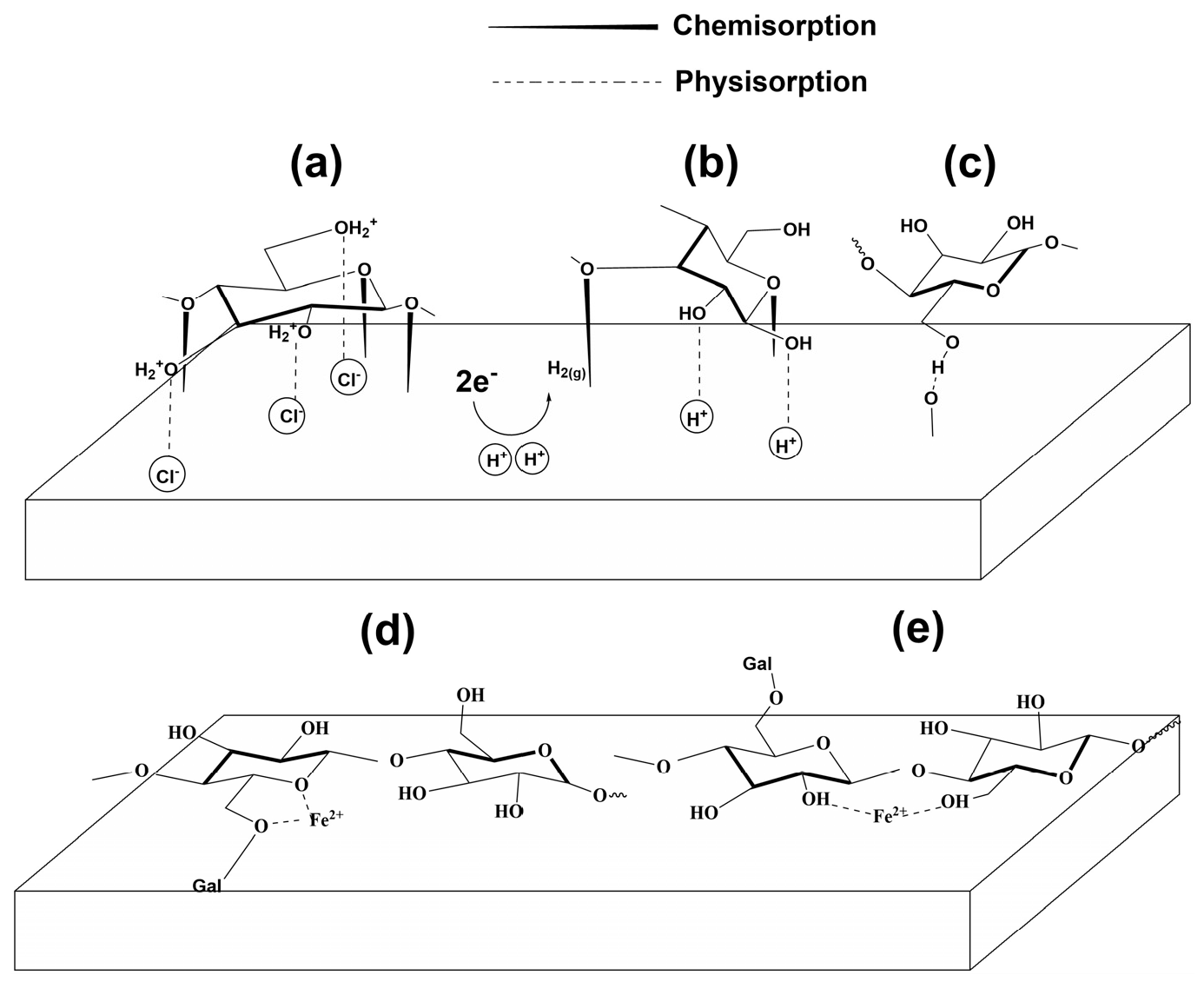
3.3. Mechanism of Inhibition
- Electrostatic adsorption
- Chemical adsorption
- Adsorption through hydrogen bonds
- Chelating Action
4. Experimental Procedure
4.1. Sample and Solution Preparation
4.2. Weight Loss Measurements
4.3. Electrochemical Measurements
4.4. Surface Analysis
5. Conclusions
- LBG demonstrated to be a strong and efficient corrosion inhibitor against CO2 corrosion, with its anticorrosive efficiency improving as concentration rose but declining with higher temperatures. EIS experiments showed that the highest inhibition efficiencies were 84.10% at 25 °C and 55.81% at 80 °C after 24 h of immersion, with 0.3 g L−1 of LBG.
- Long immersion experiments showed that the IE% at 25 °C stays steady throughout the experiment, with the IE reaching 83.97% after 168 h. However, at 80 °C and after 72 h (IE% = 47.04), LBG had no additional protective effect, due to the formation of a FeCO3 protective layer.
- PDP measurements indicate that LBG acts as a mixed-type inhibitor, reducing both cathodic and anodic reactions at both temperatures; however, at 80 °C, its suppression of the cathodic reaction was more pronounced.
- FTIR measurements show that LBG was strongly adsorbed onto the metal surface, with the adsorption process following the Temkin isotherm. The adsorption and activation data suggest that both physical and chemical mechanisms are involved in the process.
- SEM confirmed the efficacy of LBG as a corrosion inhibitor at both temperatures.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maleki, M.; Kazemzadeh, Y.; Dehghan Monfared, A.; Hasan-Zadeh, A.; Abbasi, S. Bio-enhanced oil recovery (BEOR) methods: All-important review of the occasions and challenges. Can. J. Chem. Eng. 2024, 102, 2364–2390. [Google Scholar] [CrossRef]
- Palumbo, G. Xanthan Gum as an Eco-Friendly Corrosion Inhibitor for N80 Carbon Steel Under High Pressure and High Temperature in Saline CO2-Saturated Solution. Materials 2025, 18, 4450. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, G.; Święch, D.; Górny, M. Guar Gum as an Eco-Friendly Corrosion Inhibitor for N80 Carbon Steel under Sweet Environment in Saline Solution: Electrochemical, Surface, and Spectroscopic Studies. Int. J. Mol. Sci. 2023, 24, 12269. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, G.; Górny, M.; Banaś, J. Corrosion Inhibition of Pipeline Carbon Steel (N80) in CO2-Saturated Chloride (0.5 M of KCl) Solution Using Gum Arabic as a Possible Environmentally Friendly Corrosion Inhibitor for Shale Gas Industry. J. Mater. Eng. Perform. 2019, 28, 6458–6470. [Google Scholar] [CrossRef]
- Okafor, P.C.; Liu, C.B.; Zhu, Y.J.; Zheng, Y.G. Corrosion and Corrosion Inhibition Behavior of N80 and P110 Carbon Steels in CO2-Saturated Simulated Formation Water by Rosin Amide Imidazoline. Ind. Eng. Chem. Res. 2011, 50, 7273–7281. [Google Scholar] [CrossRef]
- Zeng, D.; Dong, B.; Shi, S.; Pan, J.; Yu, H.; Li, J.; Ding, Y.; Cai, L. Effects of Temperature on Corrosion of N80 and 3Cr Steels in the Simulated CO2 Auxiliary Steam Drive Environment. Arab. J. Sci. Eng. 2018, 43, 3845–3854. [Google Scholar] [CrossRef]
- Peng, S.Y.; Jiang, Z.N.; Li, Y.R.; Dong, C.F.; Liu, H.F.; Zhang, G.A. A new exceptional imidazoline derivative corrosion inhibitor for carbon steel in supercritical CO2 environment. Corros. Sci. 2025, 245, 112663. [Google Scholar] [CrossRef]
- Solomon, M.M.; Onyeachu, I.B.; Njoku, D.I.; Nwanonenyi, S.C.; Oguzie, E.E. Adsorption and corrosion inhibition characteristics of 2-(chloromethyl)benzimidazole for C1018 carbon steel in a typical sweet corrosion environment: Effect of chloride ion concentration and temperature. Colloids Surf. A 2021, 610, 125638. [Google Scholar] [CrossRef]
- Hou, B.S.; Zhang, Q.H.; Li, Y.Y.; Zhu, G.Y.; Zhang, G.A. Influence of corrosion products on the inhibition effect of pyrimidine derivative for the corrosion of carbon steel under supercritical CO2 conditions. Corros. Sci. 2020, 166, 108442. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Umoren, S.A. Alternative corrosion inhibitor formulation for carbon steel in CO2-saturated brine solution under high turbulent flow condition for use in oil and gas transportation pipelines. Corros. Sci. 2019, 159, 108140. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Obot, I.B.; Sorour, A.A.; Abdul-Rashid, M.I. Green corrosion inhibitor for oilfield application I: Electrochemical assessment of 2-(2-pyridyl) benzimidazole for API X60 steel under sweet environment in NACE brine ID196. Corros. Sci. 2019, 150, 183–193. [Google Scholar] [CrossRef]
- Tang, J.; Hu, Y.; Han, Z.; Wang, H.; Zhu, Y.; Wang, Y.; Nie, Z.; Wang, Y. Experimental and Theoretical Study on the Synergistic Inhibition Effect of Pyridine Derivatives and Sulfur-Containing Compounds on the Corrosion of Carbon Steel in CO2-Saturated 3.5 wt.% NaCl Solution. Molecules 2018, 23, 3270. [Google Scholar] [CrossRef]
- Lin, H.; Chen, X.; Luo, Z.; Xu, J.; Lu, P.; Xie, T.; Tang, J.; Wang, H. Corrosion Inhibition Properties of Corrosion Inhibitors to under-Deposit Corrosion of X65 Steel in CO2 Corrosion Conditions. Molecules 2024, 29, 2611. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Guzman, N.B.; Canto, J.; Martinez-de-la-Escalera, L.M.; Neri, A.; Porcayo-Calderon, J. Inhibition of X52 Corrosion in CO2-Saturated Brine by a Dialkyl-Diamide from Coffee Bagasse Oil. Molecules 2023, 28, 763. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zou, C.; Wang, C.; Liang, H.; Lin, S.; Liao, Y.; Shi, L. β-cyclodextrin modified xanthan gum as an eco-friendly corrosion inhibitor for L80 steel in 1 M HCl. Cellulose 2021, 28, 11133–11152. [Google Scholar] [CrossRef]
- Abbout, S.; Zouarhi, M.; Chebabe, D.; Damej, M.; Berisha, A.; Hajjaji, N. Galactomannan as a new bio-sourced corrosion inhibitor for iron in acidic media. Heliyon 2020, 6, e03574. [Google Scholar] [CrossRef]
- Messali, M.; Lgaz, H.; Dassanayake, R.; Salghi, R.; Jodeh, S.; Abidi, N.; Hamed, O. Guar gum as efficient non-toxic inhibitor of carbon steel corrosion in phosphoric acid medium: Electrochemical, surface, DFT and MD simulations studies. J. Mol. Struct. 2017, 1145, 43–54. [Google Scholar] [CrossRef]
- Mobin, M.; Rizvi, M. Inhibitory effect of xanthan gum and synergistic surfactant additives for mild steel corrosion in 1 M HCl. Carbohydr. Polym. 2016, 136, 384–393. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Appl. Surf. Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Umoren, S.A. Inhibition of aluminium and mild steel corrosion in acidic medium using Gum Arabic. Cellulose 2008, 15, 751–761. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Kaya, T.; Kaya, E.; Umoren, S.A. Synergistic inhibition of St37 steel corrosion in 15% H2SO4 solution by chitosan and iodide ion additives. Cellulose 2016, 24, 931–950. [Google Scholar] [CrossRef]
- Roy, P.; Karfa, P.; Adhikari, U.; Sukul, D. Corrosion inhibition of mild steel in acidic medium by polyacrylamide grafted Guar gum with various grafting percentage: Effect of intramolecular synergism. Corros. Sci. 2014, 88, 246–253. [Google Scholar] [CrossRef]
- Azahar, S.S.; Raja, P.B.; Mohamad Ibrahim, M.N.; Awang, K.; Zakeyuddin, M.S.; Hamidon, T.S.; Hussin, M.H. Extraction of flavonoids from Butterfly blue pea (Clitoria ternatea) flower as carbon steel corrosion inhibitor in CO2 environment: Experimental and theoretical approaches. J. Mol. Liq. 2024, 396, 124056. [Google Scholar] [CrossRef]
- Ibrahim, T.H.; Gomes, E.E.; Obot, I.B.; Khamis, M.; Sabri, M.A. Mild steel green inhibition by Ficus carica leaves extract under practical field conditions. J. Adhes. Sci. Technol. 2017, 31, 2697–2718. [Google Scholar] [CrossRef]
- Ibrahim, T.; Gomes, E.; Obot, I.B.; Khamis, M.; Abou Zour, M. Corrosion inhibition of mild steel by Calotropis procera leaves extract in a CO2 saturated sodium chloride solution. J. Adhes. Sci. Technol. 2016, 30, 2523–2543. [Google Scholar] [CrossRef]
- Singh, A.; Lin, Y.; Ebenso, E.E.; Liu, W.; Pan, J.; Huang, B. Gingko biloba fruit extract as an eco-friendly corrosion inhibitor for J55 steel in CO2 saturated 3.5% NaCl solution. J. Ind. Eng. Chem. 2015, 24, 219–228. [Google Scholar] [CrossRef]
- Umoren, S.A.; AlAhmary, A.A.; Gasem, Z.M.; Solomon, M.M. Evaluation of chitosan and carboxymethyl cellulose as ecofriendly corrosion inhibitors for steel. Int. J. Biol. Macromol. 2018, 117, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose—Inhibition of cast iron corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-Y.; Li, S.-Y.; Cao, J.-J.; Hao, L.; Liao, B.-K.; Guo, X.-P. Waste licorice leaf extract as a sustainable corrosion inhibitor for copper in 0.5 M H2SO4: Intermolecular synergism effect and long-term protection performance. Ind. Crops Prod. 2025, 232, 121230. [Google Scholar] [CrossRef]
- Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Mazumder, M.A.J.; Singh, A. Chitosan Schiff base: An environmentally benign biological macromolecule as a new corrosion inhibitor for oil & gas industries. Int. J. Biol. Macromol. 2020, 144, 305–315. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Liang, G.; Lyu, X.; He, Y.; Feng, L.; Peng, H. Inhibition of carbon steel corrosion in CO2-saturated 3.5 wt.% NaCl solution by vanillin chitosan oligosaccharide and synergistic effect of KI additive. Petroleum 2024, 10, 705–718. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A. Inhibition effect of natural polysaccharide composite on hydrogen evolution and P110 steel corrosion in 3.5 wt% NaCl solution saturated with CO2: Combination of experimental and surface analysis. Int. J. Hydrogen Energy 2020, 45, 25398–25408. [Google Scholar] [CrossRef]
- Jalab, R.; Saad, M.; Benali, A.; Hussein, I.A.; Khaled, M. Biodegradable polysaccharide grafted polyacrylamide inhibitor for corrosion in CO2-saturated saline solution. Heliyon 2023, 9, e20304. [Google Scholar] [CrossRef]
- Petitjean, M.; Isasi, J.R. Locust Bean Gum, a Vegetable Hydrocolloid with Industrial and Biopharmaceutical Applications. Molecules 2022, 27, 8265. [Google Scholar] [CrossRef] [PubMed]
- Giridharaprasad, S.; Jadhav, H.B.; Shewale, S.; Annapure, U. Study on chemical modification of locust bean gum for enhanced functionality. J. Indian Chem. Soc. 2024, 101, 101360. [Google Scholar] [CrossRef]
- Büyüksağiş, A.; Baydır, A.T.; Dilek, M. Locust Bean Gum as Corrosion Inhibitors in NaCl Solution. Prot. Met. Phys. Chem. Surf. 2021, 57, 211–221. [Google Scholar] [CrossRef]
- Jano, A.; Lame, A.; Kokalari, E. Use of Extracted Green Inhibitors as a Friendly Choice in Corrosion Protection of Low Alloy Carbon Steel. J. Chem. Chem. Eng. 2012, 61, 497–503. [Google Scholar]
- Remita, E.; Tribollet, B.; Sutter, E.; Vivier, V.; Ropital, F.; Kittel, J. Hydrogen evolution in aqueous solutions containing dissolved CO2: Quantitative contribution of the buffering effect. Corros. Sci. 2008, 50, 1433–1440. [Google Scholar] [CrossRef]
- Abdulsada, J.A.; Chen, H.; Wei, B.; Zhang, D.; Fan, Y.; Ren, Y. A mini-review of water-alternating-CO2 injection process and derivations for enhanced oil recovery and CO2 storage in subsurface reservoirs. Petroleum 2025, 11, 410–421. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Allahkaram, S.R.; Kermani, M.B. The effects of temperature and pH on the characteristics of corrosion product in CO2 corrosion of grade X70 steel. Mater. Des. 2010, 31, 3559–3563. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Pang, X.; Zhou, M.; Liu, C.; Wei, L.; Gao, K. The behavior of pre-corrosion effect on the performance of imidazoline-based inhibitor in 3 wt.% NaCl solution saturated with CO2. Appl. Surf. Sci. 2015, 356, 63–72. [Google Scholar] [CrossRef]
- Souza, R.C.; Santos, B.A.F.; Gonçalves, M.C.; Mendes Júnior, E.P.; Simões, T.A.; Oliveira, J.R.; Vaz, G.L.; Caldeira, L.; Gomes, J.A.C.P.; Bueno, A.H. The role of temperature and H2S (thiosulfate) on the corrosion products of API X65 carbon steel exposed to sweet environment. J. Pet. Sci. Eng. 2019, 180, 78–88. [Google Scholar] [CrossRef]
- Waheed, A.; Baig, N.; Ullah, N.; Falath, W. Removal of hazardous dyes, toxic metal ions and organic pollutants from wastewater by using porous hyper-cross-linked polymeric materials: A review of recent advances. J. Environ. Manag. 2021, 287, 112360. [Google Scholar] [CrossRef] [PubMed]
- Umoren, S.A.; Li, Y.; Wang, F.H. Electrochemical study of corrosion inhibition and adsorption behaviour for pure iron by polyacrylamide in H2SO4: Synergistic effect of iodide ions. Corros. Sci. 2010, 52, 1777–1786. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Fadl-Allah, S.A. Testing validity of the Tafel extrapolation method for monitoring corrosion of cold rolled steel in HCl solutions—Experimental and theoretical studies. Corros. Sci. 2010, 52, 140–151. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfac. Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Lelek-Borkowska, U.; Palumbo, G.; Banaś, J. The Effect of the Methanol–Water Interaction on the Surface Layer on Titanium in CH3OH-H2O-LiClO4 Solutions. Electrochem 2020, 1, 87–103. [Google Scholar] [CrossRef]
- Wu, W.; Yin, H.; Zhang, H.; Kang, J.; Li, Y.; Dan, Y. Electrochemical Investigation of Corrosion of X80 Steel under Elastic and Plastic Tensile Stress in CO2 Environment. Metals 2018, 8, 949. [Google Scholar] [CrossRef]
- Liu, D.; Fu, C.Y.; Chen, Z.Y.; Guo, X.P. CO2 Corrosion Mechanism of Carbon Steel in the Presence of Acetate and Acetic Acid. Corros. Sci. Technol. 2007, 6, 227–232. [Google Scholar]
- Okafor, P.C.; Brown, B.; Nesic, S. CO2 corrosion of carbon steel in the presence of acetic acid at higher temperatures. J. Appl. Electrochem. 2009, 39, 873–877. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Drazic, D.; Despic, A.R. The electrode kinetics of the deposition and dissolution of iron. Electrochim. Acta 1961, 4, 325–361. [Google Scholar] [CrossRef]
- Gupta, K.K.; Haratian, S.; Gupta, S.; Mishin, O.V.; Ambat, R. The effect of cooling rate-induced microstructural changes on CO2 corrosion of low alloy steel. Corros. Sci. 2022, 209, 110769. [Google Scholar] [CrossRef]
- Islam, M.M.; Pojtanabuntoeng, T.; Gubner, R.; Kinsella, B. Electrochemical investigation into the dynamic mechanism of CO2 corrosion product film formation on the carbon steel under the water-condensation condition. Electrochim. Acta 2021, 390, 138880. [Google Scholar] [CrossRef]
- Belarbi, Z.; Dominguez Olivo, J.M.; Farelas, F.; Singer, M.; Young, D.; Nešić, S. Decanethiol as a Corrosion Inhibitor for Carbon Steels Exposed to Aqueous CO2. Corrosion 2019, 75, 1246–1254. [Google Scholar] [CrossRef]
- Mora-Mendoza, J.L.; Turgoose, S. Fe3C influence on the corrosion rate of mild steel in aqueous CO2 systems under turbulent flow conditions. Corros. Sci. 2002, 44, 1223–1246. [Google Scholar] [CrossRef]
- Farelas, F.; Galicia, M.; Brown, B.; Nesic, S.; Castaneda, H. Evolution of dissolution processes at the interface of carbon steel corroding in a CO2 environment studied by EIS. Corros. Sci. 2010, 52, 509–517. [Google Scholar] [CrossRef]
- Pessu, F.; Barker, R.; Neville, A. The Influence of pH on Localized Corrosion Behavior of X65 Carbon Steel in CO2-Saturated Brines. Corrosion 2015, 71, 1452–1466. [Google Scholar] [CrossRef]
- Pessu, F.; Barker, R.; Neville, A. Understanding Pitting Corrosion Behavior of X65 Carbon Steel in CO2-Saturated Environments: The Temperature Effect. Corrosion 2016, 72, 78–94. [Google Scholar] [CrossRef]
- Forero, A.B.; Núñez, M.M.G.; Bott, I.S. Analysis of the Corrosion Scales Formed on API 5L X70 and X80 Steel Pipe in the Presence of CO2. Mater. Res. 2014, 17, 461–471. [Google Scholar] [CrossRef]
- Palencsár, A.; Gulbrandsen, E.; Kosorú, K. Corrosion Inhibition under FeCO3-forming Conditions at Elevated Temperatures. In Proceedings of the SPE International Oilfield Corrosion Conference and Exhibition, Aberdeen, UK, 12–13 May 2014. [Google Scholar]
- Shaikhah, D.; Barker, R.; Taleb, W.; Lazareva, A.; Mohamed-Said, M.; Cowe, B.; Neville, A. Engineering of corrosion product-polymer hybrid layers for enhanced CO2 corrosion protection of carbon steel part one: Corrosion study and mechanical property investigation. Polymer 2022, 242, 124614. [Google Scholar] [CrossRef]
- Lazareva, A.; Owen, J.; Vargas, S.; Barker, R.; Neville, A. Investigation of the evolution of an iron carbonate layer and its effect on localized corrosion of X65 carbon steel in CO2 corrosion environments. Corros. Sci. 2021, 192, 109849. [Google Scholar] [CrossRef]
- Hua, Y.; Xu, S.; Wang, Y.; Taleb, W.; Sun, J.; Zhang, L.; Barker, R.; Neville, A. The formation of FeCO3 and Fe3O4 on carbon steel and their protective capabilities against CO2 corrosion at elevated temperature and pressure. Corros. Sci. 2019, 157, 392–405. [Google Scholar] [CrossRef]
- Karthik, G.; Sundaravadivelu, M. Studies on the inhibition of mild steel corrosion in hydrochloric acid solution by atenolol drug. Egypt. J. Pet. 2016, 25, 183–191. [Google Scholar] [CrossRef]
- Chen, L.-M.; Li, S.-J.; Mo, J.-L.; Chen, L.-Y.; Li, H.-Z.; Guo, X.-P.; Liao, B.-K. One Stone four birds: Expired butylphthalide drugs as a high-efficient corrosion inhibitor for mild steel in acidic media. J. Taiwan Inst. Chem. Eng. 2026, 180, 106472. [Google Scholar] [CrossRef]
- Busch, V.M.; Pepa, L.S.; Panizzolo, L.A.; Buera, M.d.P.; Ferreira, F. Effect of Ultrasound and Enzymatic Hydrolysis on the Physicochemical Properties of Neltuma ruscifolia Seed Gum and Other Galactomannan Gums. Food Bioprocess Technol. 2024, 17, 2380–2392. [Google Scholar] [CrossRef]
- Hadinugroho, W.; Martodihardjo, S.; Fudholi, A.; Riyanto, S. Esterification of citric acid with locust bean gum. Heliyon 2019, 5, e02337. [Google Scholar] [CrossRef] [PubMed]
- Thombare, N.; Mahto, A.; Singh, D.; Chowdhury, A.R.; Ansari, M.F. Comparative FTIR Characterization of Various Natural Gums: A Criterion for Their Identification. J. Polym. Environ. 2023, 31, 3372–3380. [Google Scholar] [CrossRef]
- Yuen, S.-N.; Choi, S.-M.; Phillips, D.L.; Ma, C.-Y. Raman and FTIR spectroscopic study of carboxymethylated non-starch polysaccharides. Food Chem. 2009, 114, 1091–1098. [Google Scholar] [CrossRef]
- Abdallah, M. Guar gum as corrosion inhibitor for carbon steel in sulfuric acid solutions. Port. Electrochim. Acta 2004, 22, 161–175. [Google Scholar] [CrossRef]
- Zhang, K.; Ge, F.; Tang, F.; Tan, L.; Qiu, Y.; Zhu, X. A structure-property study for konjac glucomannan and guar galactomannan: Selective carboxylation and scale inhibition. Carbohydr. Polym. 2023, 299, 120220. [Google Scholar] [CrossRef]
- Elkholy, A.E.; El-Taib Heakal, F.; Rashad, A.M.; Zakaria, K. Monte Carlo simulation for guar and xanthan gums as green scale inhibitors. J. Pet. Sci. Eng. 2018, 166, 263–273. [Google Scholar] [CrossRef]
- Lelek-Borkowska, U.; Wróbel, M.; Marzec, M.; Kustra, P.; Milenin, A. Mg–Ca Surgical Wires Degradation in Animal Serum. Metall. Mater. Trans. A 2024, 55, 2141–2152. [Google Scholar] [CrossRef]

| Temperature (°C) | R2 | a | Kads (g L−1) | (kJ mol−1) |
|---|---|---|---|---|
| Langmuir | ||||
| 25 | 0.995 | - | 24.39 | −25.46 |
| 80 | 0.996 | - | 5.68 | −23.23 |
| Temkin | ||||
| 25 | 0.973 | −5.01 | 2721 | −19.94 |
| 80 | 0.986 | −3.49 | 81 | −34.13 |
| Cinh (g L−1) | Ea (kJ mol−1) | Qads (kJ mol−1) |
|---|---|---|
| Blank | 12.16 | - |
| 0.05 | 20.00 | −23.95 |
| 0.1 | 18.86 | −15.67 |
| 0.2 | 18.10 | −12.10 |
| 0.3 | 20.96 | −15.48 |
| Cinh (g L−1) | −βc (V dec−1) | icorr (µA cm−2) | Ecorr (V) | IE (%) |
|---|---|---|---|---|
| 25 °C | ||||
| Blank | 0.466 ± 0.042 | 37.09 ± 4.38 | −0.657 ± 0.55 | - |
| 0.03 | 0.280 ± 0.038 | 16.52 ± 2.29 | −0.646 ± 0.94 | 55.46 |
| 0.05 | 0.253 ± 0.025 | 7.54 ± 1.25 | −0.630 ± 0.88 | 79.67 |
| 0.1 | 0.281 ± 0.035 | 6.05 ± 0.99 | −0.633 ± 0.23 | 83.69 |
| 0.2 | 0.296 ± 0.033 | 5.13 ± 1.01 | −0.637 ± 0.51 | 86.17 |
| 0.3 | 0.296 ± 0.033 | 4.69 ± 1.51 | −0.650 ± 0.31 | 87.35 |
| 80 °C | ||||
| Blank | 0.473 ± 0.041 | 171.15 ± 19.22 | −0.707 ± 0.11 | - |
| 0.05 | 0.415 ± 0.053 | 155.70 ± 21.09 | −0.700 ± 0.15 | 9.03 |
| 0.1 | 0.481 ± 0.044 | 126.43 ± 11.12 | −0.705 ± 0.09 | 26.13 |
| 0.2 | 0.520 ± 0.059 | 110.01 ± 5.09 | −0.699 ± 0.11 | 35.72 |
| 0.3 | 0.444 ± 0.031 | 90.17 ± 4.11 | −0.711 ± 0.10 | 47.32 |
| Cinh (g L−1) | Rs (Ω cm2) | CPEf | Rf (Ω cm2) | CPEdl | Rct (Ω cm2) | Cdl (µF cm−2) | L (H cm2) | RL (Ω cm2) | Rp (Ω cm2) | χ2 (×10−4) | IE (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yf (sn µΩ−1 cm−2) | nf | Ydl (sn µΩ−1 cm−2) | ndl | ||||||||||
| 25 °C | |||||||||||||
| Blank | 23.75 ± 3.41 | - | - | - | 510.51 ± 25.11 | 0.838 ± 0.06 | 574.72 ± 45.06 | 215.59 | 278.62 ± 85.06 | 150.33 ± 11.98 | 725.05 | 4.69 | - |
| 0.05 | 23.63 ± 2.10 | 80.39 ± 5.31 | 0.882 ± 0.05 | 57.65 ± 2.51 | 24.56 ± 2.40 | 0.982 ± 0.05 | 3119 ± 105.65 | 21.42 | - | - | 3176.65 | 3.77 | 77.18 |
| 0.1 | 28.26 ± 3.35 | 47.88 ± 3.95 | 0.867 ± 0.05 | 46.25 ± 2.05 | 32.15 ± 3.12 | 0.897 ± 0.05 | 3805 ± 88.56 | 14.37 | - | - | 3877.44 | 7.56 | 81.30 |
| 0.2 | 30.91 ± 3.21 | 46.44 ± 3.20 | 0.903 ± 0.09 | 72.44 ± 5.15 | 36.42 ± 3.41 | 0.889 ± 0.09 | 4514 ± 101.99 | 15.59 | - | - | 4560.25 | 3.63 | 84.10 |
| 0.3 | 21.73 ± 2.19 | 58.75 ± 4.44 | 0.862 ± 0.11 | 215.5 ± 13.43 | 13.81 ± 1.13 | 0.952 ± 0.06 | 4350 ± 115.15 | 9.17 | - | - | 4565.50 | 5.59 | 84.11 |
| 80 °C | |||||||||||||
| Blank | 11.38 ± 1.15 | 919.20 ± 25.66 | 0.907 ± 0.02 | 18.49 ± 0.41 | 621.40 ± 36.12 | 0.870 ± 0.06 | 72.13 ± 5.06 | 289.87 | - | - | 90.62 | 4.35 | - |
| 0.05 | 11.43 ± 1.01 | 769.20 ± 44.51 | 0.926 ± 0.05 | 22.43 ± 2.91 | 323.10 ± 35.23 | 0.860 ± 0.02 | 92.05 ± 3.65 | 127.32 | - | - | 114.48 | 1.19 | 20.83 |
| 0.1 | 10.75 ± 1.19 | 731.81 ± 46.11 | 0.907 ± 0.04 | 19.04 ± 2.11 | 219.81 ± 27.11 | 0.861 ± 0.02 | 106.11 ± 5.32 | 81.17 | - | - | 125.15 | 5.32 | 27.58 |
| 0.2 | 11.06 ± 1.26 | 686.84 ± 31.24 | 0.875 ± 0.05 | 15.01 ± 1.99 | 69.16 ± 5.88 | 0.88 ± 0.03 | 146.05 ± 9.11 | 25.74 | - | - | 161.51 | 0.66 | 45.25 |
| 0.3 | 11.98 ± 1.11 | 415.00 ± 28.06 | 0.931 ± 0.02 | 56.16 ± 9.01 | 189.10 ± 42.13 | 0.965 ± 0.06 | 148.90 ± 12.19 | 151.11 | - | - | 205.06 | 1.36 | 55.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, G.; Górny, M.; Święch, D.; Rai, A.; Youssif, M.M. Locust Bean Gum: A Natural Polysaccharide as an Eco-Friendly Corrosion Inhibitor for N80 Carbon Steel in CO2-Saturated Saline Solution, Useful for the Oil and Gas Industry. Molecules 2025, 30, 4534. https://doi.org/10.3390/molecules30234534
Palumbo G, Górny M, Święch D, Rai A, Youssif MM. Locust Bean Gum: A Natural Polysaccharide as an Eco-Friendly Corrosion Inhibitor for N80 Carbon Steel in CO2-Saturated Saline Solution, Useful for the Oil and Gas Industry. Molecules. 2025; 30(23):4534. https://doi.org/10.3390/molecules30234534
Chicago/Turabian StylePalumbo, Gaetano, Marcin Górny, Dominika Święch, Adarsh Rai, and Mahmoud M. Youssif. 2025. "Locust Bean Gum: A Natural Polysaccharide as an Eco-Friendly Corrosion Inhibitor for N80 Carbon Steel in CO2-Saturated Saline Solution, Useful for the Oil and Gas Industry" Molecules 30, no. 23: 4534. https://doi.org/10.3390/molecules30234534
APA StylePalumbo, G., Górny, M., Święch, D., Rai, A., & Youssif, M. M. (2025). Locust Bean Gum: A Natural Polysaccharide as an Eco-Friendly Corrosion Inhibitor for N80 Carbon Steel in CO2-Saturated Saline Solution, Useful for the Oil and Gas Industry. Molecules, 30(23), 4534. https://doi.org/10.3390/molecules30234534










