Design, Synthesis and Anticancer Activity of 6-Substituted-1-(3,4,5-trimethoxyphenyl)-1H-indole Against Tubulin Polymerisation
Abstract
1. Introduction
2. Results and Discussion
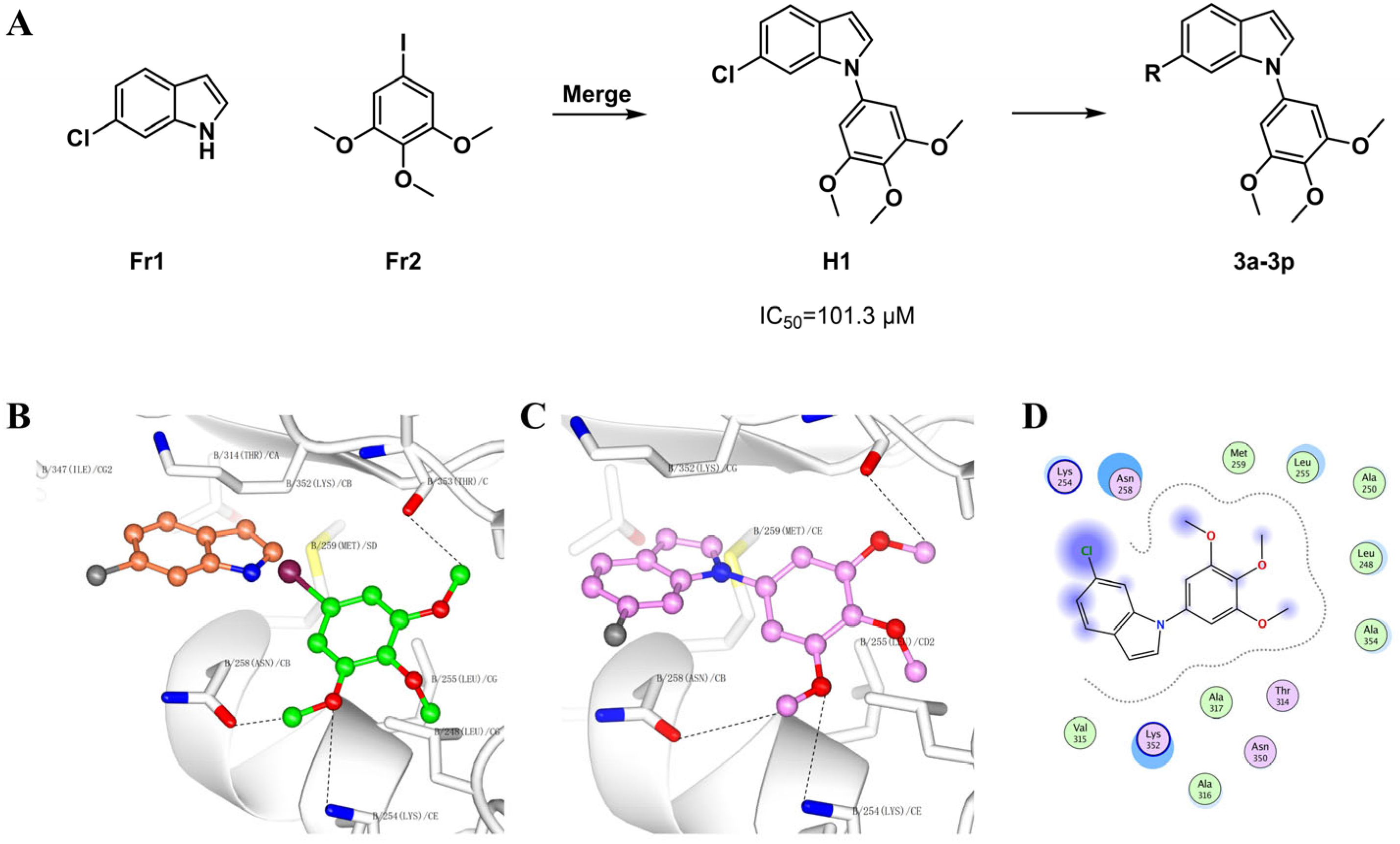
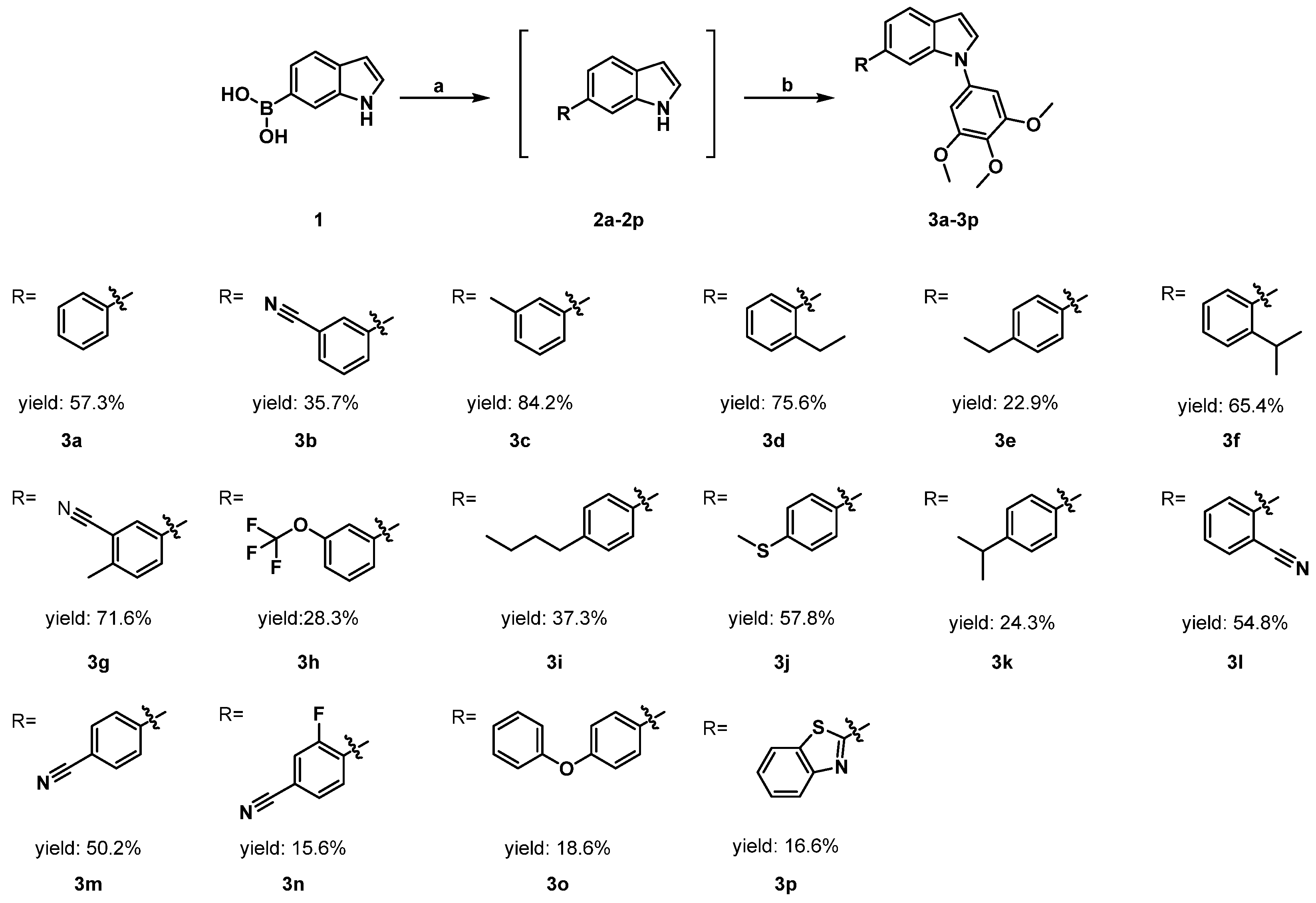
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Binding Affinity to Tubulin Protein
2.2.2. Antiproliferative Activity Against Cancer Cell Lines
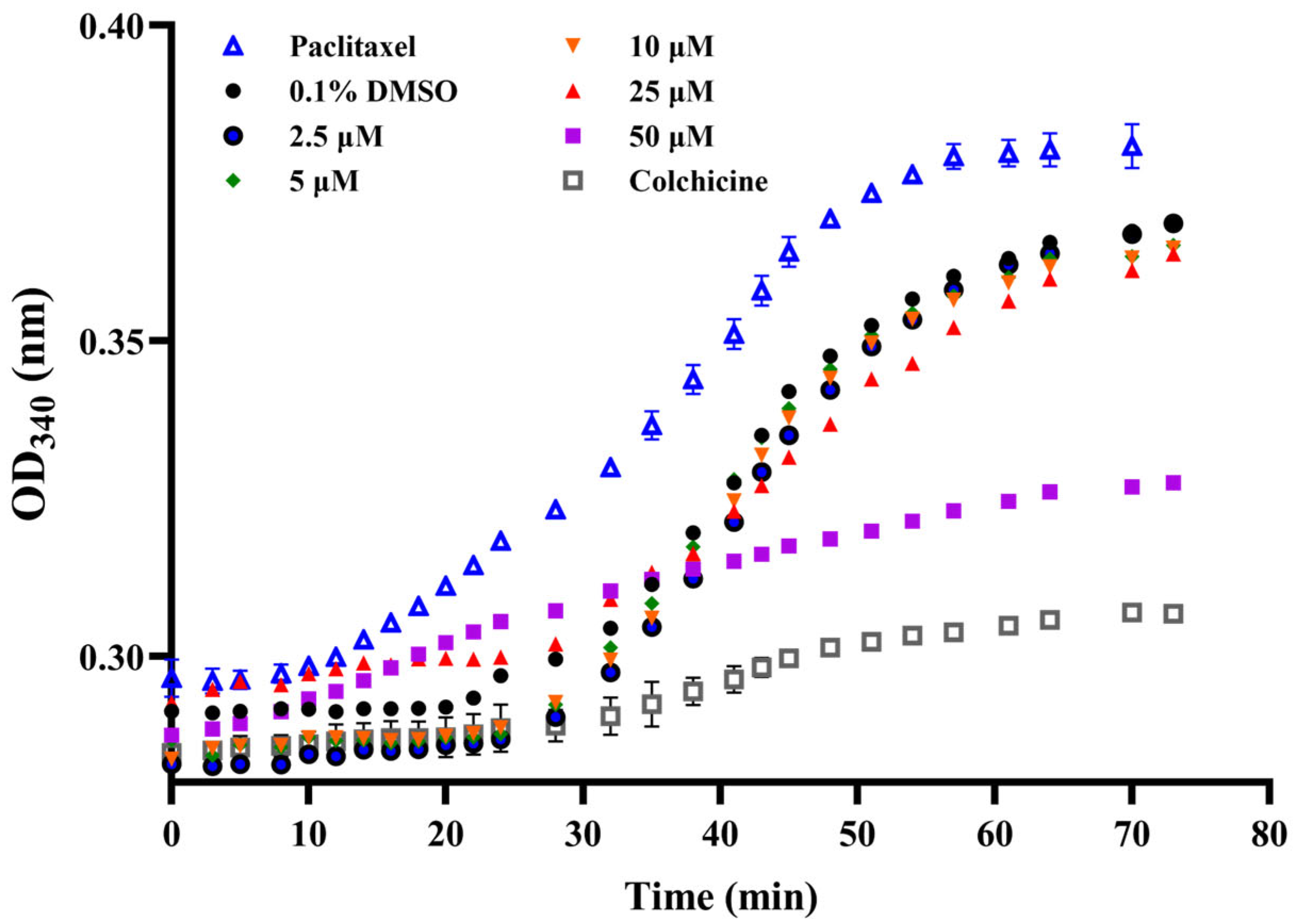
2.2.3. Inhibition of Tubulin Polymerization
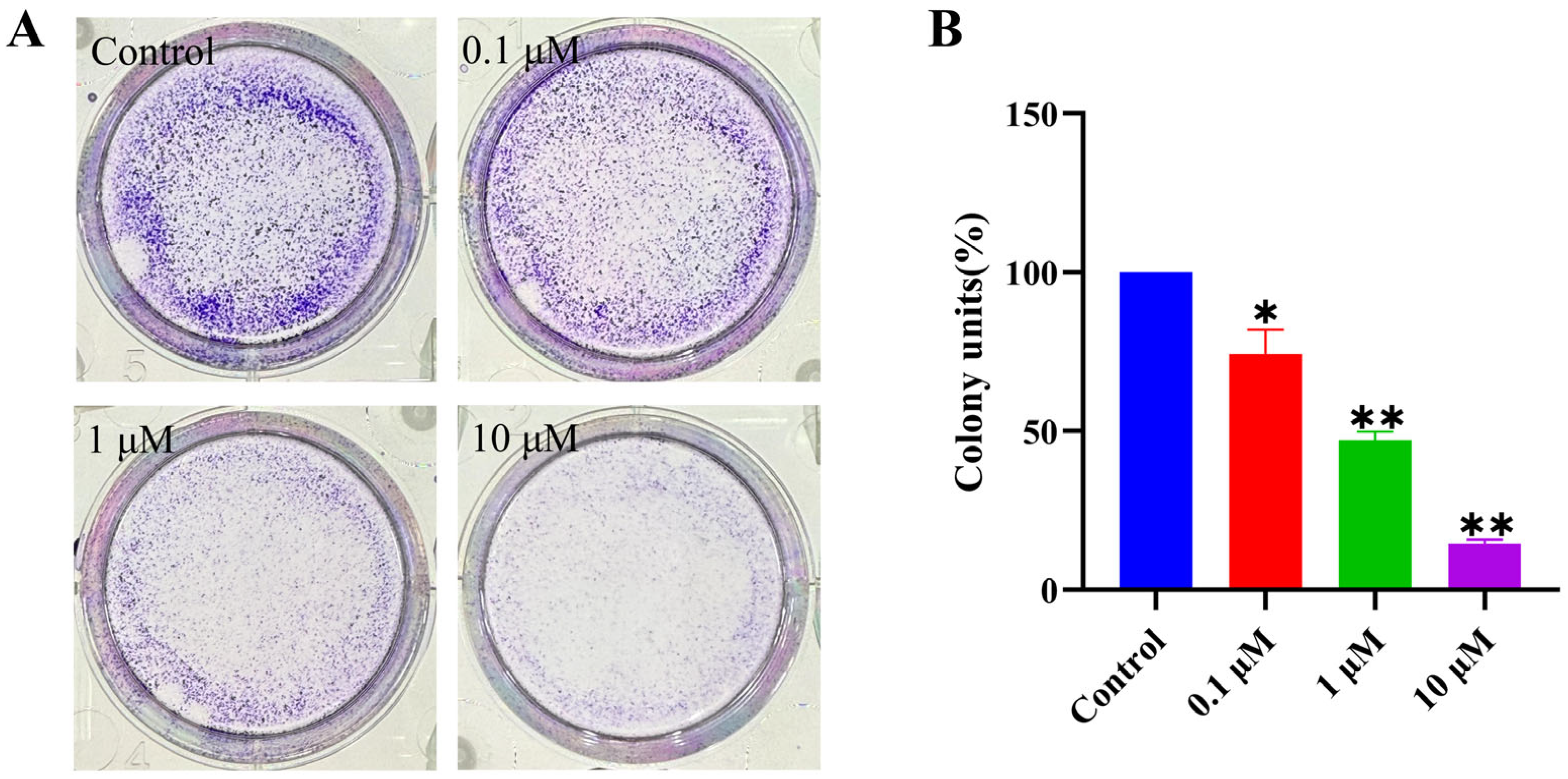
2.2.4. Inhibition of Cancer Cell Colony Formation
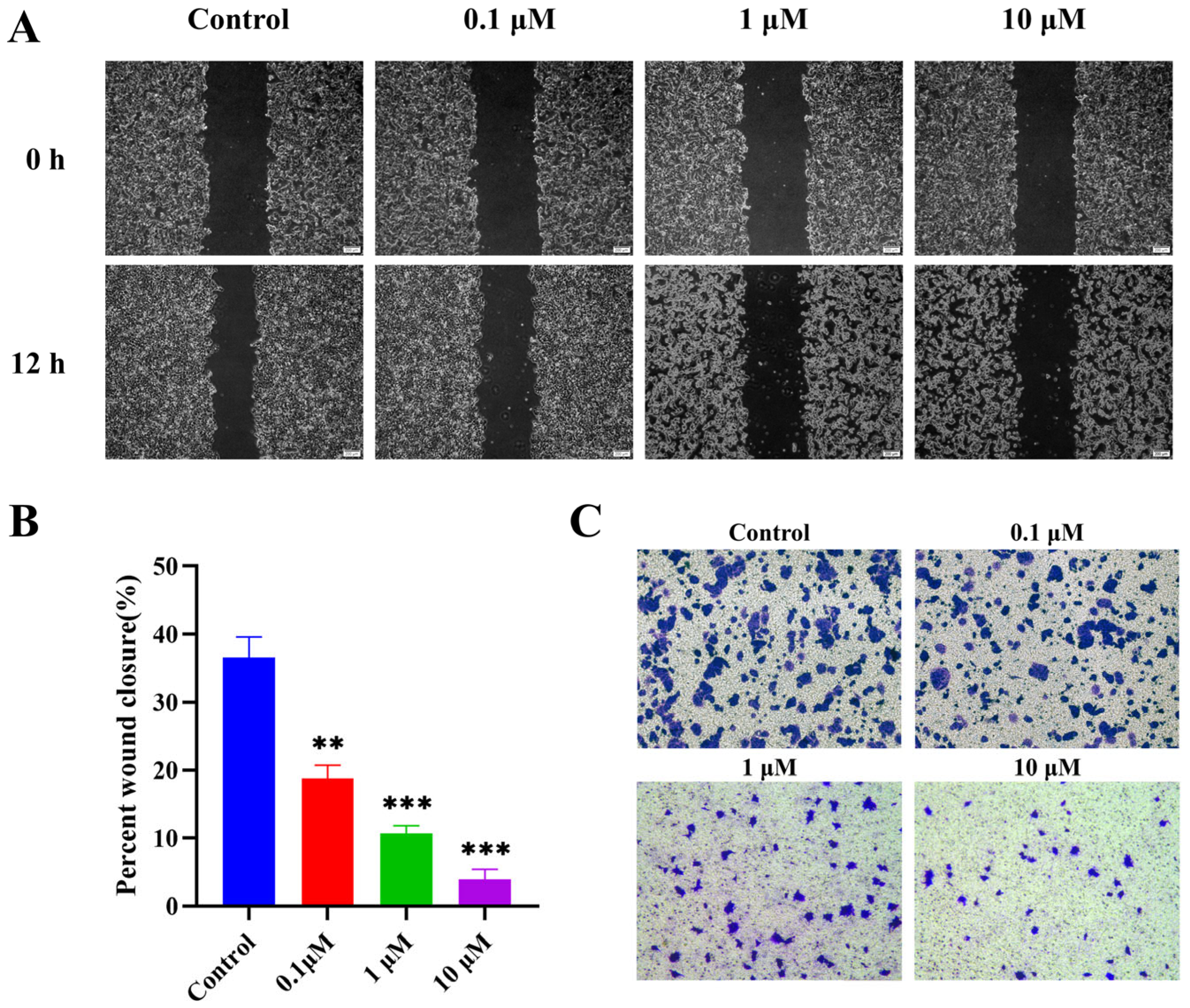
2.2.5. Suppression of MCF-7 Cell Migration
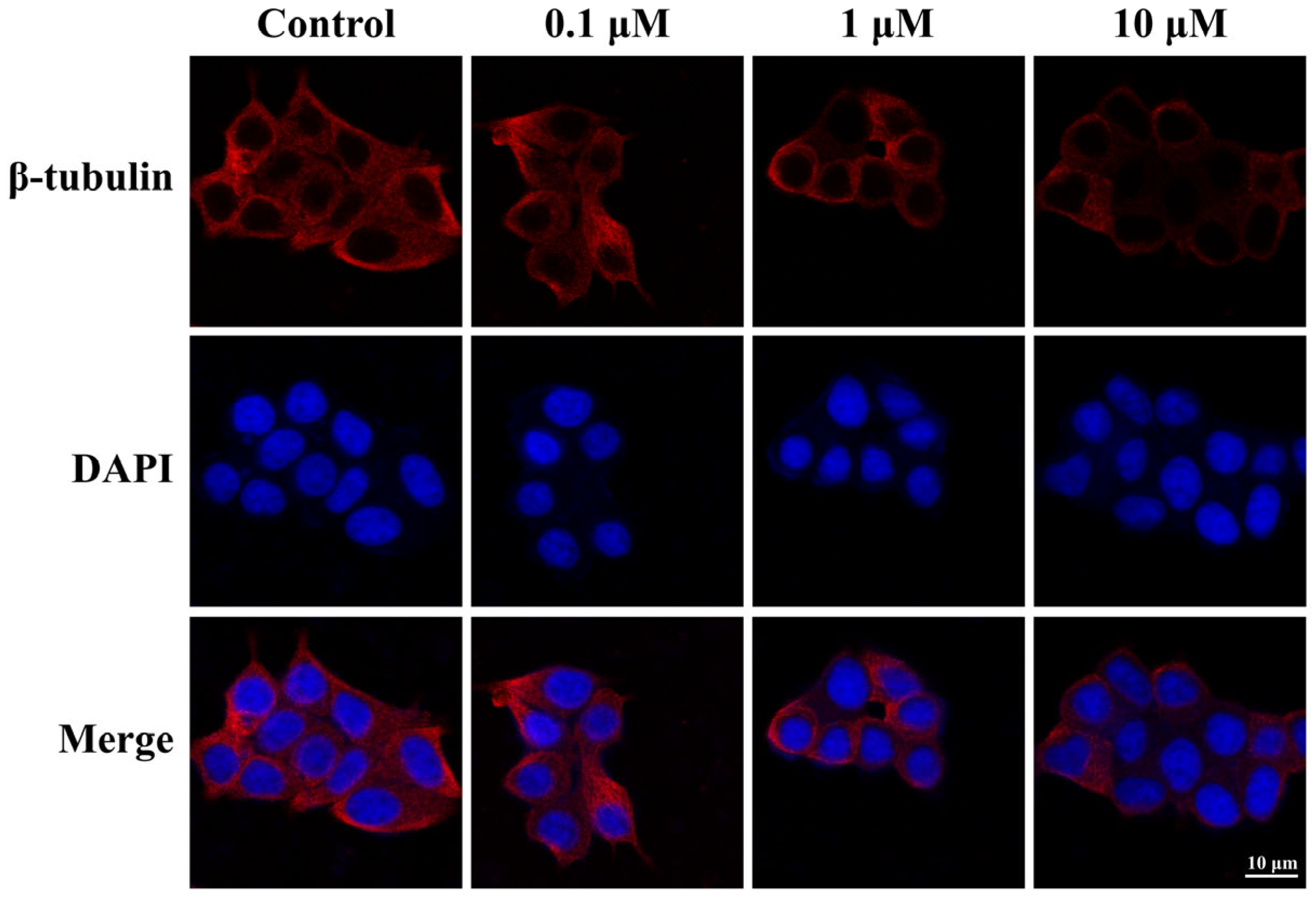
2.2.6. Antimicrotubule Activity in MCF-7 Cells
2.2.7. Analysis of Cell Cycle Analysis
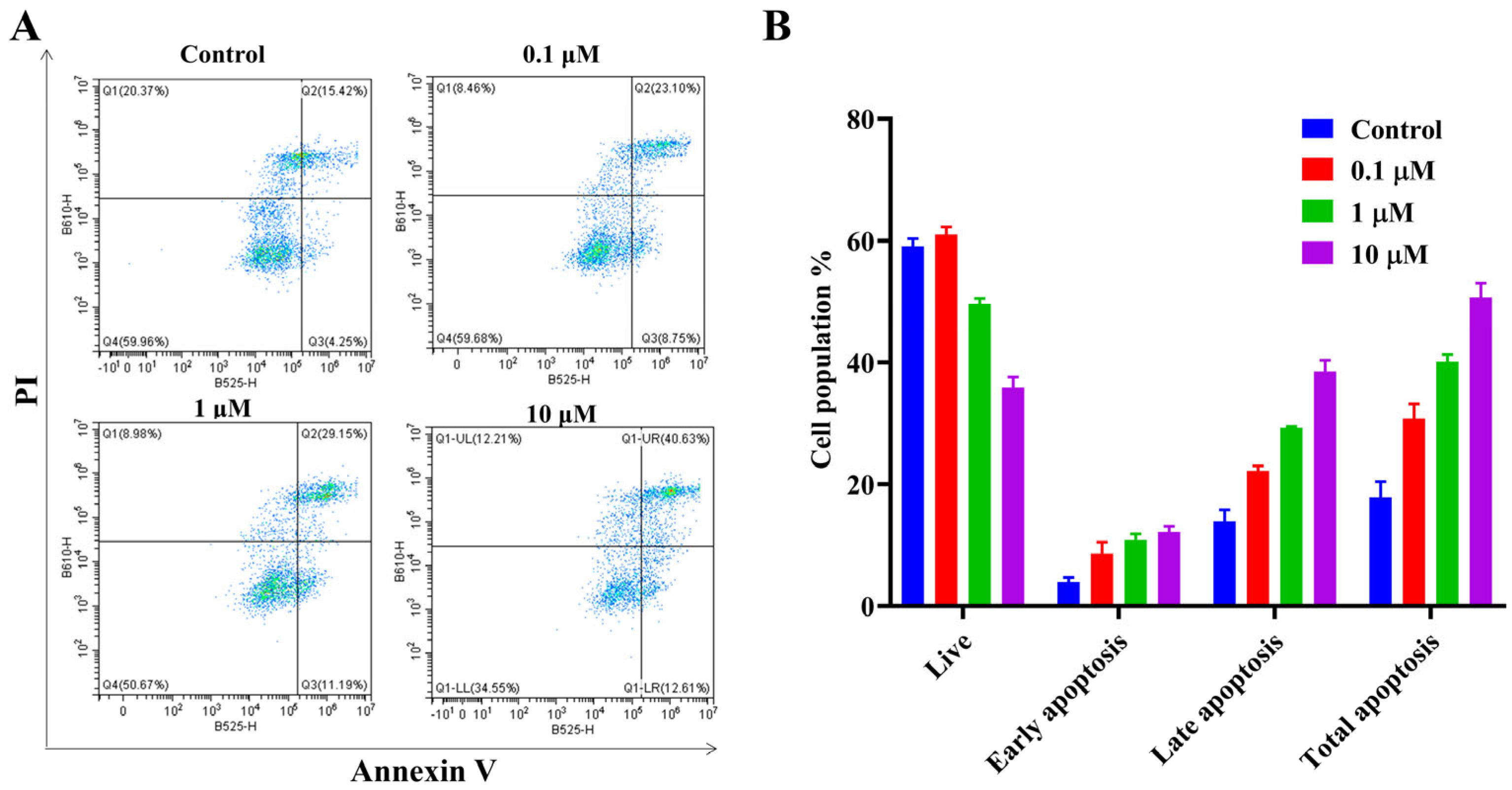
2.2.8. Analysis of Cancer Cell Apoptosis Analysis
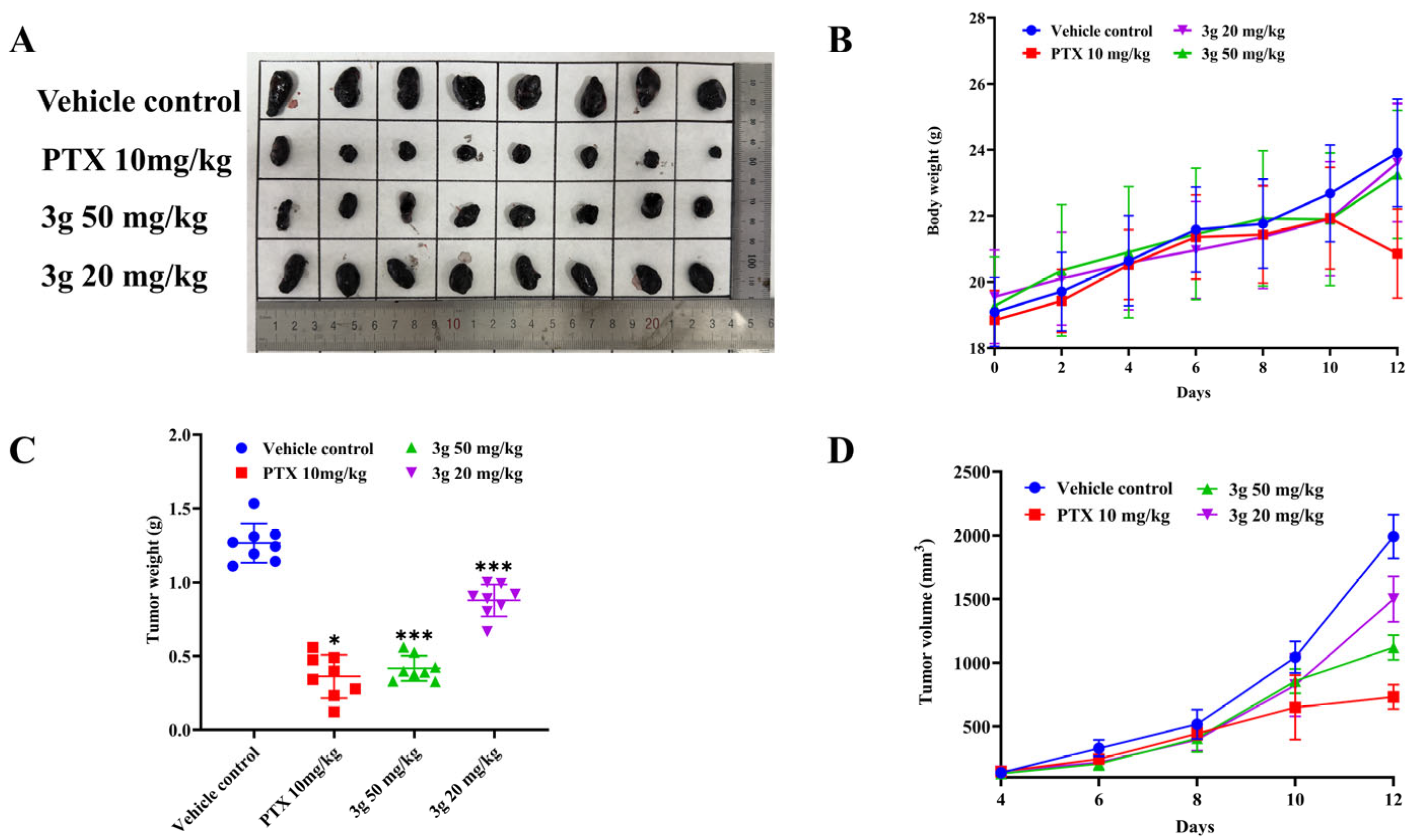
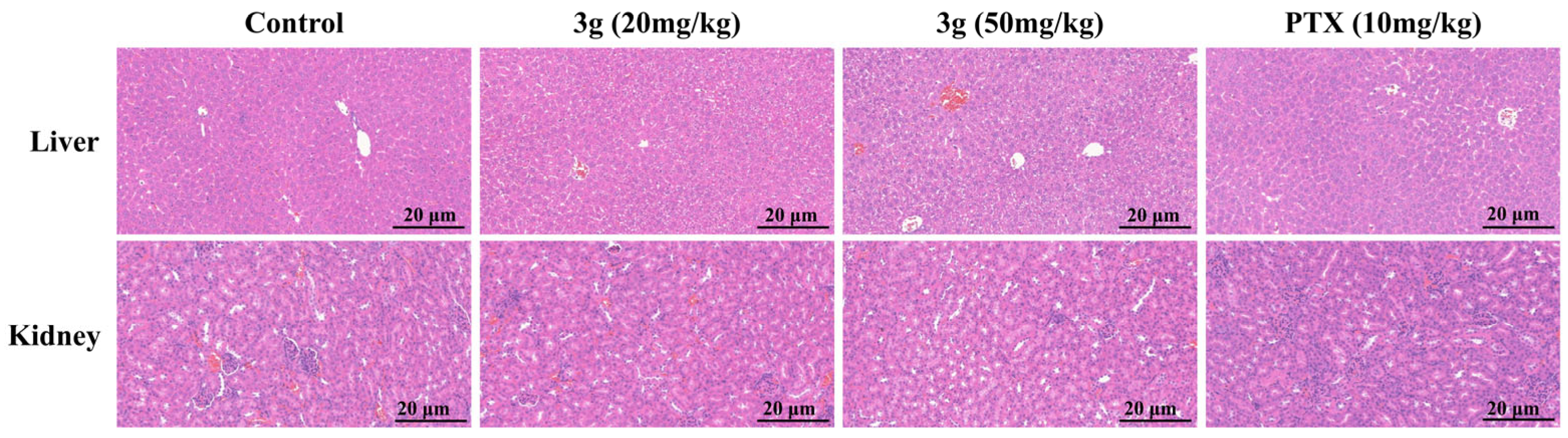
2.2.9. In Vivo Antitumor Efficacy
3. Materials and Methods
3.1. General Chemistry Methods
3.2. Docking Studies
3.3. Synthesis of 6-Phenyl-1H-indoles (Intermediate 1)
3.4. Synthesis of 6-Phenyl-1-(3,4,5-trimethoxyphenyl)-1H-indoles (Target Compound)
- 6-phenyl-1-(3,4,5-trimethoxyphenyl)-1H-indole (3a)
- 3-(1-(3,4,5-trimethoxyphenyl)-1H-indol-6-yl)benzonitrile (3b)
- 6-(m-tolyl)-1-(3,4,5-trimethoxyphenyl)-1H-indole (3c)
- 6-(2-ethylphenyl)-1-(3,4,5-trimethoxyphenyl)-1H-indole (3d)
- 6-(4-ethylphenyl)-1-(3,4,5-trimethoxyphenyl)-1H-indole (3e)
- 6-(2-isopropylphenyl)-1-(3,4,5-trimethoxyphenyl)-1H-indole (3f)
- 2-methyl-5-(1-(3,4,5-trimethoxyphenyl)-1H-indol-6-yl)benzonitrile (3g)
- 6-(3-(trifluoromethoxy)phenyl)-1-(3,4,5-trimethoxyphenyl)-1H-indole (3h)
- 6-(4-butylphenyl)-1-(3,4,5-trimethoxyphenyl)-1H-indole (3i)
- 6-(4-(methylthio)phenyl)-1-(3,4,5-trimethoxyphenyl)-1H-indole (3j)
- 6-(4-isopropylphenyl)-1-(3,4,5-trimethoxyphenyl)-1H-indole (3k)
- 2-(1-(3,4,5-trimethoxyphenyl)-1H-indol-6-yl)benzonitrile (3l)
- 4-(1-(3,4,5-trimethoxyphenyl)-1H-indol-6-yl)benzonitrile (3m)
- 3-fluoro-4-(1-(3,4,5-trimethoxyphenyl)-1H-indol-6-yl)benzonitrile (3n)
- 6-(4-Phenoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-1H-indole (3o)
- 2-(1-(3,4,5-trimethoxyphenyl)-1H-indol-6-yl)benzo[d]thiazole (3p)
3.5. Biological Assays
3.5.1. BLI Analysis
3.5.2. Cell Lines and Cell Culture
3.5.3. Cytotoxicity Assay
3.5.4. In Vitro Antiproliferative Assay
3.5.5. Colony Formation Assay
3.5.6. In Vitro Tubulin Polymerization Assay
3.5.7. Wound Healing Assay
3.5.8. Transwell Assay
3.5.9. Immunofluorescence Staining
3.5.10. Cell Cycle Analysis
3.5.11. Cancer Cell Apoptosis Analysis
3.5.12. In Vivo Antitumor Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binarová, P.; Tuszynski, J. Tubulin: Structure, Functions and Roles in Disease. Cells 2019, 8, 1294. [Google Scholar] [CrossRef]
- Gudimchuk, N.B.; McIntosh, J.R. Regulation of microtubule dynamics, mechanics and function through the growing tip. Nat. Rev. Mol. Cell Biol. 2021, 22, 777–795. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.R.; Grishchuk, E.L.; West, R.R. Chromosome-microtubule interactions during mitosis. Annu. Rev. Cell Dev. Biol. 2002, 18, 193–219. [Google Scholar] [CrossRef] [PubMed]
- Borys, F.; Joachimiak, E.; Krawczyk, H.; Fabczak, H. Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells. Molecules 2020, 25, 3705. [Google Scholar] [CrossRef] [PubMed]
- Halim, C.E.; Deng, S.; Crasta, K.C.; Yap, C.T. Interplay Between the Cytoskeleton and DNA Damage Response in Cancer Progression. Cancers 2025, 17, 1378. [Google Scholar] [CrossRef]
- Lopes, D.; Maiato, H. The Tubulin Code in Mitosis and Cancer. Cells 2020, 9, 2356. [Google Scholar] [CrossRef]
- Dominguez-Brauer, C.; Thu, K.L.; Mason, J.M.; Blaser, H.; Bray, M.R.; Mak, T.W. Targeting Mitosis in Cancer: Emerging Strategies. Mol. Cell 2015, 60, 524–536. [Google Scholar] [CrossRef]
- Parker, A.L.; Kavallaris, M.; McCarroll, J.A. Microtubules and their role in cellular stress in cancer. Front. Oncol. 2014, 4, 153. [Google Scholar] [CrossRef]
- Rieder, C.L.; Maiato, H. Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 2004, 7, 637–651. [Google Scholar] [CrossRef]
- Prota, A.E.; Bargsten, K.; Diaz, J.F.; Marsh, M.; Cuevas, C.; Liniger, M.; Neuhaus, C.; Andreu, J.M.; Altmann, K.H.; Steinmetz, M.O. A new tubulin-binding site and pharmacophore for microtubule-destabilizing anticancer drugs. Proc. Natl. Acad. Sci. USA 2014, 111, 13817–13821. [Google Scholar] [CrossRef]
- Abal, M.; Andreu, J.M.; Barasoain, I. Taxanes: Microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr. Cancer Drug Targets 2003, 3, 193–203. [Google Scholar] [CrossRef]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Karatoprak, G.; Küpeli Akkol, E.; Genç, Y.; Bardakci, H.; Yücel, Ç.; Sobarzo-Sánchez, E. Combretastatins: An Overview of Structure, Probable Mechanisms of Action and Potential Applications. Molecules 2020, 25, 2560. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.O.; Prota, A.E. Microtubule-Targeting Agents: Strategies To Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Hari, M.; Wang, Y.; Veeraraghavan, S.; Cabral, F. Mutations in alpha- and beta-tubulin that stabilize microtubules and confer resistance to colcemid and vinblastine. Mol. Cancer Ther. 2003, 2, 597–605. [Google Scholar]
- Hari, M.; Loganzo, F.; Annable, T.; Tan, X.; Musto, S.; Morilla, D.B.; Nettles, J.H.; Snyder, J.P.; Greenberger, L.M. Paclitaxel-resistant cells have a mutation in the paclitaxel-binding region of beta-tubulin (Asp26Glu) and less stable microtubules. Mol. Cancer Ther. 2006, 5, 270–278. [Google Scholar] [CrossRef]
- Ganguly, A.; Cabral, F. New insights into mechanisms of resistance to microtubule inhibitors. Biochim. Biophys. Acta 2011, 1816, 164–171. [Google Scholar] [CrossRef]
- Ning, N.; Yu, Y.; Wu, M.; Zhang, R.; Zhang, T.; Zhu, C.; Huang, L.; Yun, C.H.; Benes, C.H.; Zhang, J.; et al. A Novel Microtubule Inhibitor Overcomes Multidrug Resistance in Tumors. Cancer Res. 2018, 78, 5949–5957. [Google Scholar] [CrossRef]
- Weng, H.; Li, J.; Zhu, H.; Carver Wong, K.F.; Zhu, Z.; Xu, J. An update on the recent advances and discovery of novel tubulin colchicine binding inhibitors. Future Med. Chem. 2023, 15, 73–95. [Google Scholar] [CrossRef]
- Chan, B.; Lynch, N.B.; Tran, W.; Joyce, J.M.; Savage, G.P.; Meutermans, W.; Montgomery, A.P.; Kassiou, M. Fragment-based drug discovery for disorders of the central nervous system: Designing better drugs piece by piece. Front. Chem. 2024, 12, 1379518. [Google Scholar] [CrossRef] [PubMed]
- Mallakuntla, M.K.; Togre, N.S.; Santos, D.B.; Tiwari, S. Implications of Fragment-Based Drug Discovery in Tuberculosis and HIV. Pharmaceuticals 2022, 15, 1415. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.J.; Lopez-Fernandez, J.D.; Knight, L.E.; Al-Khawaldeh, I.; Gai, C.; Lin, S.; Martin, M.P.; Miller, D.C.; Cano, C.; Endicott, J.A.; et al. FragLites-Minimal, Halogenated Fragments Displaying Pharmacophore Doublets. An Efficient Approach to Druggability Assessment and Hit Generation. J. Med. Chem. 2019, 62, 3741–3752. [Google Scholar] [CrossRef]
- Lamoree, B.; Hubbard, R.E. Current perspectives in fragment-based lead discovery (FBLD). Essays Biochem. 2017, 61, 453–464. [Google Scholar] [CrossRef]
- Hope, I.; Martin, M.P.; Jiang, Z.; Waring, M.J.; Noble, M.E.M.; Endicott, J.A.; Tatum, N.J. Crystallographic fragment screening of CDK2-cyclin A: FragLites map sites of protein-protein interaction. Structure 2025, 33, 1971–1983. [Google Scholar] [CrossRef]
- Martin, M.P.; Endicott, J.A.; Noble, M.E.M.; Tatum, N.J. Crystallographic fragment screening in academic cancer drug discovery. Methods Enzymol. 2023, 690, 211–234. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, Z.; Jiang, Z.; Zhu, W.; Qiao, D. Indole-Based Tubulin Inhibitors: Binding Modes and SARs Investigations. Molecules 2022, 27, 1587. [Google Scholar] [CrossRef]
- Wang, Q.; Arnst, K.E.; Wang, Y.; Kumar, G.; Ma, D.; Chen, H.; Wu, Z.; Yang, J.; White, S.W.; Miller, D.D.; et al. Structural Modification of the 3,4,5-Trimethoxyphenyl Moiety in the Tubulin Inhibitor VERU-111 Leads to Improved Antiproliferative Activities. J. Med. Chem. 2018, 61, 7877–7891. [Google Scholar] [CrossRef]
- Li, L.; Jiang, S.; Li, X.; Liu, Y.; Su, J.; Chen, J. Recent advances in trimethoxyphenyl (TMP) based tubulin inhibitors targeting the colchicine binding site. Eur. J. Med. Chem. 2018, 151, 482–494. [Google Scholar] [CrossRef]
- Bates, T.A.; Gurmessa, S.K.; Weinstein, J.B.; Trank-Greene, M.; Wrynla, X.H.; Anastas, A.; Anley, T.W.; Hinchliff, A.; Shinde, U.; Burke, J.E.; et al. Biolayer interferometry for measuring the kinetics of protein-protein interactions and nanobody binding. Nat. Protoc. 2025, 20, 861–883. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, S.H.; Li, D.; Wang, S.Y.; Liu, X.; Song, J.; Wang, Y.T.; Zhang, S.Y. Progress of tubulin polymerization activity detection methods. Bioorg. Med. Chem. Lett. 2021, 37, 127698. [Google Scholar] [CrossRef]
- Jędrzejczyk, M.; Morabito, B.; Żyżyńska-Granica, B.; Struga, M.; Janczak, J.; Aminpour, M.; Tuszynski, J.A.; Huczyński, A. Novel Combretastatin A-4 Analogs—Design, Synthesis, and Antiproliferative and Anti-Tubulin Activity. Molecules 2024, 29, 2200. [Google Scholar] [CrossRef]
- Abdul Hussein, S.A.; Razzak Mahmood, A.A.; Tahtamouni, L.H.; Balakit, A.A.; Yaseen, Y.S.; Al-Hasani, R.A. New Combretastatin Analogs as Anticancer Agents: Design, Synthesis, Microtubules Polymerization Inhibition, and Molecular Docking Studies. Chem. Biodivers. 2023, 20, e202201206. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Oliva, P.; Salvador, M.K.; Manfredini, S.; Padroni, C.; Brancale, A.; Ferla, S.; Hamel, E.; Ronca, R.; Maccarinelli, F.; et al. A facile synthesis of diaryl pyrroles led to the discovery of potent colchicine site antimitotic agents. Eur. J. Med. Chem. 2021, 214, 113229. [Google Scholar] [CrossRef]

| ID | KD (μM) | ID | KD (μM) |
|---|---|---|---|
| 3a | 39 ± 2.60 | 3i | NB |
| 3b | 29 ± 4.10 | 3j | NB |
| 3c | 23 ± 2.12 | 3k | ND |
| 3d | 24 ± 2.13 | 3l | 26 ± 3.38 |
| 3e | 110 ± 14.46 | 3m | NB |
| 3f | 47 ± 5.62 | 3n | 220 ± 30.80 |
| 3g | 13 ± 1.17 | 3o | 31 ± 3.41 |
| 3h | ND | 3p | NB |
| Colchicine | 8.60 ± 1.20 |
| Compd. | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | A549 | Hela | A375 | B16-F10 | |
| 3a | 10.13 ± 0.68 | 78.92 ± 0.57 | 36.60 ± 1.78 | >100 | 1.16 ± 0.12 | 45.36 ± 1.11 |
| 3b | 3.33 ± 0.28 | 42.93 ± 3.13 | 41.20 ± 3.42 | 38.52 ± 2.25 | 27.53 ± 2.50 | 3.78 ± 0.14 |
| 3c | 9.15 ± 0.21 | 37.72 ± 0.89 | 92.44 ± 8.58 | 66.50 ± 6.21 | 53.16 ± 4.06 | 59.21 ± 5.86 |
| 3d | 34.22 ± 1.62 | 23.37 ± 0.44 | 60.24 ± 4.17 | 46.60 ± 3.65 | 24.84 ± 0.16 | 6.49 ± 0.40 |
| 3e | >100 | >100 | >100 | >100 | >100 | >100 |
| 3f | 17.39 ± 0.35 | 21.37 ± 0.12 | 87.43 ± 3.45 | 41.80 ± 1.11 | 21.07 ± 1.17 | 6.67 ± 0.29 |
| 3g | 2.94 ± 0.56 | 1.61 ± 0.004 | 6.30 ± 0.30 | 6.10 ± 0.31 | 0.57 ± 0.01 | 1.69 ± 0.41 |
| 3h | >100 | >100 | >100 | >100 | >100 | >100 |
| 3i | >100 | >100 | >100 | >100 | >100 | >100 |
| 3j | >100 | >100 | >100 | >100 | >100 | >100 |
| 3k | >100 | >100 | >100 | >100 | >100 | >100 |
| 3l | >100 | >100 | >100 | >100 | >100 | >100 |
| 3m | >100 | >100 | >100 | >100 | >100 | >100 |
| 3n | 14.17 ± 0.51 | 41.37 ± 1.21 | 48.01 ± 2.56 | 65.00 ± 3.20 | 16.10 ± 0.38 | 68.94 ± 3.17 |
| 3o | >100 | >100 | >100 | >100 | >100 | >100 |
| 3p | >100 | >100 | >100 | >100 | >100 | >100 |
| Colchicine | 0.26 ± 0. 08 | 0.48 ± 0.03 | 0.082 ± 0.006 | 0.027 ± 0.002 | 0.110 ± 0.02 | 0.013 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Gai, C.; Zou, S.; Song, Y.; Zhang, J.; Zhao, Q.; Chai, X.; Wang, P. Design, Synthesis and Anticancer Activity of 6-Substituted-1-(3,4,5-trimethoxyphenyl)-1H-indole Against Tubulin Polymerisation. Molecules 2025, 30, 4538. https://doi.org/10.3390/molecules30234538
Gu Y, Gai C, Zou S, Song Y, Zhang J, Zhao Q, Chai X, Wang P. Design, Synthesis and Anticancer Activity of 6-Substituted-1-(3,4,5-trimethoxyphenyl)-1H-indole Against Tubulin Polymerisation. Molecules. 2025; 30(23):4538. https://doi.org/10.3390/molecules30234538
Chicago/Turabian StyleGu, Yuanna, Conghao Gai, Sijie Zou, Yan Song, Juan Zhang, Qingjie Zhao, Xiaoyun Chai, and Peipei Wang. 2025. "Design, Synthesis and Anticancer Activity of 6-Substituted-1-(3,4,5-trimethoxyphenyl)-1H-indole Against Tubulin Polymerisation" Molecules 30, no. 23: 4538. https://doi.org/10.3390/molecules30234538
APA StyleGu, Y., Gai, C., Zou, S., Song, Y., Zhang, J., Zhao, Q., Chai, X., & Wang, P. (2025). Design, Synthesis and Anticancer Activity of 6-Substituted-1-(3,4,5-trimethoxyphenyl)-1H-indole Against Tubulin Polymerisation. Molecules, 30(23), 4538. https://doi.org/10.3390/molecules30234538







