The Effect of Caffeic Acid on Zn Corrosion in NaCl: Electrochemical Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrochemical Studies
2.1.1. Corrosion Inhibition via CA Addition
2.1.2. Corrosion Inhibition by CA Coatings
2.2. Morphological Characterisation of Zn Surface
3. Materials and Methods
3.1. Materials Preparation
3.2. Electrochemical Measurements
3.3. Surface Characterisation of Zn Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, G. Cost of corrosion. In Trends in Oil and Gas Corrosion Research and Technologies Production and Transmission; El-Sherik, A.M., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2017; pp. 3–30. [Google Scholar] [CrossRef]
- Nady, C. Tricine [N-(Tri(hydroxymethyl)methyl)glycine]–A novel green inhibitor for the corrosion inhibition of zinc in neutral aerated sodium chloride solution. Egypt. J. Pet. 2017, 26, 905–913. [Google Scholar] [CrossRef]
- Pais, M.; Rao, P. Maltodextrin for corrosion mitigation of zinc in sulfamic acid: Electrochemical, surface and spectroscopic studies. Int. J. Biol. Macromol. 2020, 145, 575–585. [Google Scholar] [CrossRef]
- Deyab, M.A. Application of nonionic surfactant as a corrosion inhibitor for zinc in alkaline battery solution. J. Power Source 2015, 292, 66–71. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wei, Y.; Zhang, R.; Li, R.; Lin, Z.; Shi, S.; Qiu, R. Enhancing corrosion inhibition of zinc with biomimetic slippery liquid-infused porous surfaces (SLIPS): An on-site fabrication strategy. Colloids Surf. A 2024, 681, 132779. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.-Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zhen, Y.F. Comparative in vitro study on pure metals (Fe, Mn, Mg, Zn and W) as biodegradable metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2019, 24, 48. [Google Scholar] [CrossRef]
- Larios-Galvez, A.K.; Porcayo-Calderon, J.; Salinas-Bravo, V.M.; Chacon-Nava, J.G.; Gonzalez-Rodriguez, J.G.; Martinez-Gomez, L. Use of Salvia hispanica as an eco-friendly corrosion inhibitor for bronze in acid rain. Anti-Corros. Methods Mater. 2017, 64, 654–663. [Google Scholar] [CrossRef]
- Hlozek, M.; Komoroczy, B.; Trojek, T. X-ray fluorescence analysis of ancient and medieval brass artifacts from south Moravia. Appl. Radiat. Isot. 2012, 70, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Wu, Z.; Wang, J.; Huang, Y.; Liu, Q.; Zhou, S.-F. Corrosion inhibiting performance and mechanism of protic ionic liquids as green brass inhibitors in nitric acid. Green Energy Environ. 2020, 5, 214–222. [Google Scholar] [CrossRef]
- Dillmann, P.; Watkinson, D.; Angelini, E.; Adriaens, A. Corrosion and Conservation of Cultural Heritage Metallic Artefacts; Woodhead Publishing Limited: Philadelphia, PA, USA, 2013. [Google Scholar]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R. Plant extracts as sustainable and green corrosion inhibitors for protection of ferrous metals in corrosive media: A mini review. Corros. Commun. 2022, 5, 25–38. [Google Scholar] [CrossRef]
- Ouakki, M.; Galai, M.; Cherkaoui, M. Imidazole derivatives as efficient and potential class of corrosion inhibitors for metals and alloys in aqueous electrolytes: A review. J. Mol. Liq. 2022, 345, 117815. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Ebenso, E.E. Quinoline and its derivatives as corrosion inhibitors: A review. Surf. Interfaces 2020, 21, 100634. [Google Scholar] [CrossRef]
- Alotaibi, M.D.; Mckinley, A.J.; Patterson, B.M.; Reeder, A.Y. Benzotriazoles in the aquatic environment: A review of their occurrence, Toxicity, Degradation and analysis. Water Air Soil Pollut. 2015, 226, 226. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Wan Isahak, W.N.R.; Al-Azzawi, W.K. Sustainable corrosion Inhibitors: A key step towards environmentally responsible corrosion control. Ain Shams Eng. J. 2024, 15, 102672. [Google Scholar] [CrossRef]
- Maity, S.; Kinra, M.; Nampoothiri, M.; Arora, D.; Pai, K.S.R.; Mudgal, J. Caffeic acid, a dietary polyphenol, as a promising candidate for combination therapy. Chem. Pap. 2022, 76, 1271–1283. [Google Scholar] [CrossRef]
- Alam, M.; Ashraf, G.; Sheikh, K.; Khan, A.; Ali, S.; Ansari, M.; Adnan, M.; Pasupuleti, V.R.; Hassan, I. Potential therapeutic implications of caffeic acid in cancer signaling: Past, present, and future. Front. Pharmacol. 2022, 13, 845871. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Horna, A.; Adam, V.; Provaznik, I.; Kizek, R. Content of phenolic compounds and antioxidant capacity in fruits of apricot genotypes. Molecules 2010, 15, 6285–6305. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Simon, P.W.; Tanumihardjo, S.A. Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J. Agric. Food Chem. 2009, 57, 4142–4147. [Google Scholar] [CrossRef]
- Espindola, K.M.M.; Ferreira, R.G.; Naravez, L.E.M.; Rosario, A.C.R.S.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Fouda, A.S.; Mohamed, O.A.; Elabbasy, H.M. Ferula hermonis plant extract as safe corrosion inhibitor for zinc in hydrochloric acid solution. J. Bio. Tribo-Corr. 2021, 7, 135. [Google Scholar] [CrossRef]
- Ezeugo, J.N.O.; Onukwuli, O.D.; Omotioma, M. Optimization of corrosion inhibition of Picralima nitida leaves extract as green corrosion inhibitor for zinc in 1.0 M HCl. World News Nat. Sci. 2017, 15, 139–161. [Google Scholar]
- Singh, A.; Ansari, K.R.; Quraishi, M.A. Chondroitin sulfate as a green corrosion inhibitor for zinc in 26% ammonium chloride solution: Electrochemical and surface morphological analysis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125465. [Google Scholar] [CrossRef]
- Lebrini, M.; Suedile, F.; Salvin, P.; Roos, C.; Zarrouk, A.; Jama, C.; Bentiss, F. Bagassa guianensis ethanol extract used as sustainable eco-friendly inhibitor for zinc corrosion in 3% NaCl: Electrochemical and XPS studies. Surf. Interfaces 2020, 20, 100588. [Google Scholar] [CrossRef]
- Desai, P.S.; Parmar, B.B.; Desai, F.P.; Patel, A.M. Caesalpinia Crista (Kanchaki) as green corrosion inhibitor for zinc in hydrochloric acid solutions. Chem. Afr. 2024, 7, 2173–2187. [Google Scholar] [CrossRef]
- Fouda, A.S.; Shalabi, K.; Idress, A.A. Ceratonia siliqua extract as a green corrosion inhibitor for copper and brass in nitric acid solutions. Green Chem. Lett. Rev. 2015, 8, 17–29. [Google Scholar] [CrossRef]
- Fekri, M.H.; Omidali, F.; Alemnezhad, M.M.; Ghaffarinejad, A. Turnip peel extract as green corrosion bio-inhibitor for copper in 3.5% NaCl solution. Mater. Chem. Phys. 2022, 286, 126150. [Google Scholar] [CrossRef]
- Jmiai, A.; El Ibrahimi, B.; Tara, A.; Chadili, M.; El Issami, I.; Jbara, O.; Khallaayoun, A.; Bazzi, L. Application of Zizyphus lotuse—Pulp of Jujube extract as green and promising corrosion inhibitor for copper in acidic medium. J. Mol. Liq. 2018, 268, 102–113. [Google Scholar] [CrossRef]
- Ahmed, R.K.; Zhang, S. Alchemilla Vulgaris extract as green inhibitor of copper corrosion in hydrochloric acid. Int. J. Electrochem. Sci. 2019, 14, 10657–10669. [Google Scholar] [CrossRef]
- Martinovic, I.; Pilic, Z.; Zlatic, G.; Soldo, V.; Sego, M. N-Acetyl cysteine and d-penicillamine as green corrosion inhibitors for copper in 3% NaCl. Int. J. Electrochem. Sci. 2023, 18, 100238. [Google Scholar] [CrossRef]
- Damej, M.; Zouarhi, M.; Doubi, M.; Chellouli, M.; Benassaoui, H.; Erramli, H. Study of the protective effect of green inhibitor extracted from seeds oil of Cannabis sativa L. against corrosion of brass 60Cu–40Zn in seawater medium. Int. J. Corros. Scale Inhib. 2020, 9, 1564–1579. [Google Scholar] [CrossRef]
- Rivera-Bahena, G.B.; Ramirez-Arteaga, A.M.; Saldarriaga-Norena, H.A.; Larios-Galvez, A.K.; Gonzalez-Rodriguez, J.G.; Romero-Aguilar, M.; Sesenes, R.L. Hexane extract of Persea schiedeana Ness as green corrosion inhibitor for the brass immersed in 0.5 M HCl. Sci. Rep. 2024, 14, 6512. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Morillo, C.; Cisneros-Perez, P.; Llive, R.; Ricaurte, M.; Reinoso, C.; Meneses, M.A.; del Cisne Guaman, M.; Palma-Cando, A. Croton lechleri extracts as green corrosion inhibitors of admiralty brass in hydrochloric acid. Molecules 2021, 26, 7417. [Google Scholar] [CrossRef]
- Chraka, A.; Raissouni, I.; Seddik, N.B.; Khayar, S.; Mansour, A.I.; Tazi, S.; Chaouket, F.; Bouchta, D. Identification of potential green inhibitors extracted from Thymbra capitata (L.) Cav. for the corrosion of brass in 3% NaCl solution: Experimental. SEM–EDX Analysis. DFT computation and Monte Carlo simulation studies. J. Bio-Tribo-Corros. 2020, 6, 80. [Google Scholar] [CrossRef]
- Vazquez-Aguirre, I.D.; Torres-Islas, A.; Vazquez-Velez, E.; Martinez, H.; del Pozo-Mares, A.; Cotero-Villegas, A.M. Fatty imidazolines as a green corrosion inhibitor of bronze exposed to acid rain. Coatings 2024, 14, 1152. [Google Scholar] [CrossRef]
- Benzidia, B.; Barbouchi, M.; Hsissou, R.; Zouarhi, M.; Erramli, H.; Hajjaji, N. A combined experimental and theoretical study of green corrosion inhibition of bronze B66 in 3% NaCl solution by Aloe saponaria (syn. Aloe maculata) tannin extract. Curr. Res. Green Sustain. Chem. 2022, 5, 100299. [Google Scholar] [CrossRef]
- Elshahawi, A.; Rifai, M.; Hamid, Z.A. Corrosion inhibition of bronze alloy by Jatropha extract in neutral media for application on archaeological bronze artifacts. Egypt. J. Chem. 2022, 65, 869–878. [Google Scholar] [CrossRef]
- Varvara, S.; Bostan, R.; Bobis, O.; Gaina, L.; Popa, F.; Mena, V.; Souto, R.M. Propolis as a green corrosion inhibitor for bronze in weakly acidic solution. Appl. Surf. Sci. 2017, 426, 1100–1112. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, J.G.; Gutierrez-Granda, D.G.; Larios-Galvez, A.K.; Lopez-Sesenes, R. Use of Thymus vulgaris extract as green corrosion inhibitor for bronze in acid rain. J. Bio-Tribo-Corros. 2022, 8, 77. [Google Scholar] [CrossRef]
- Vrsalovic, L.; Gudic, S.; Kliskic, M.; Oguzie, E.E.; Carev, L. Inhibition of copper corrosion in NaCl solution by caffeic acid. Int. J. Electrochem. Sci. 2016, 11, 459–474. [Google Scholar] [CrossRef]
- de Souza, F.S.; Spinelli, A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Mei, D.; Tian, Y.; Mao, S.; Kong, T.; Liu, Z.; Liu, J.; Xie, H.; Xiao, Y.; Wang, L.; Zhu, S.; et al. The inhibitory effect of caffeic acid on localized corrosion of LAZ931 Mg alloy. Corros. Sci. 2025, 252, 112949. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, L.; Zhang, D.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L.-N. Initial formation of corrosion products on pure zinc in saline solution. Bioact. Mater. 2019, 4, 87–96. [Google Scholar] [CrossRef]
- Lodhi, Z.F.; Mol, J.M.C.; Hovestad, A.; Hoen-Velterop, L.; Terryn, H.; de Wit, J.H.W. Corrosion resistance of Zn-Co-Fe alloy coatings on high strength steel. Surf. Coat. Technol. 2009, 203, 1415–1422. [Google Scholar] [CrossRef]
- Teymouri, F.; Allahkaram, S.R.; Shekarchi, M.; Azamian, I.; Johari, M. A comprehensive study on the inhibition behaviour of four carboxylate based corrosion inhibitors focusing on efficiency drop after the optimum concentration for carbon steel in the simulated concrete pore solution. Constr. Build. Mater. 2021, 296, 123702. [Google Scholar] [CrossRef]
- Ferraa, N.; Ouakki, M.; El Harmouchi, H.; Cherkaoui, M.; Ziatni, M.B. Investigation of the inhibition behavior of an octacalcium phosphate as a green corrosion inhibitor against carbon steel in 3% NaCl medium. Inorg. Chem. Commun. 2023, 157, 111343. [Google Scholar] [CrossRef]
- Kumar, H.; Yadav, P.; Kumari, R.; Sharma, R.; Sharma, S.; Singh, D.; Dahiya, H.; Kumar, P.; Bhardwaj, S.; Kaur, P. Highly efficient green corrosion inhibitor for mild steel in sulfuric acid: Experimental and DFT approach. Colloid. Surf. A-Physicochem. Eng. Asp. 2023, 675, 132039. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Chakravarthy, M.P.; Mohana, K.N. Adsorption and corrosion inhibition characteristics of some nicotinamide derivatives on mild steel in hydrochloric acid solution. Int. Sch. Res. Not. 2014, 2014, 687276. [Google Scholar] [CrossRef]
- Miralrio, A.; Vazquez, A.E. Plant extracts as green corrosion inhibitors for different metal surfaces and corrosive media: A review. Processes 2020, 8, 942. [Google Scholar] [CrossRef]
- Ouakki, M.; Galai, M.; Rbaa, M.; Abousalem, A.S.; Lakhrissi, B.; Rifi, E.H.; Cherkaoui, M. Quantum chemical and experimental evaluation of the inhibitory action of two imidazole derivatives on mild steel corrosion in sulphuric acid medium. Heliyon 2019, 5, e02759. [Google Scholar] [CrossRef]
- Ouakki, M.; Rbaa, M.; Galai, M.; Lakhrissi, B.; Rifi, E.H.; Cherkaoui, M. Experimental and quantum chemical investigation of imidazole derivatives as corrosion inhibitors on mild steel in 1.0 M hydrochloric acid. J. Bio-Tribo Corros. 2018, 4, 35. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, S.; Fu, X.-Z.; Wang, X.-Z.; Luo, J.-L. Transition of self-passivation and semiconductor property of titanium in the simulated environments of proton exchange membrane fuel cells. Appl. Surf. Sci. 2023, 612, 155930. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Bastidas, D.M.; Baltazar, M.A.; Zambrano-Robledo, P.; Bastidas, J.M.; Almeraya-Calderon, F.M.; Gaona-Tiburcio, C. Corrosion behavior of Zn-TiO2 and Zn-ZnO electrodeposited coatings in 3.5% NaCl solution. Int. J. Electrochem. Sci. 2019, 14, 4226–4239. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of cyclic potentiodynamic polarization test results for study of corrosion behavior of metals: A Review. Prot. Met. Phys. Chem. Surf. 2018, 54, 976–989. [Google Scholar] [CrossRef]
- Maruthanila, V.L.; Elancheran, R.; Chandraboss, V.L.; Kabilan, S.; Miurnalini, S. Single crystal XRD analysis, molecular docking, DFT studies, Hirshfeld analysis, antioxidant and anticancer activities of three natural carboxylic acids identified in Carica papaya leaves. J. Mol. Struct. 2024, 1318, 139131. [Google Scholar] [CrossRef]
- Ou, G.; Liu, X.; Huang, X.; Wu, W. Influence of flow velocity on electrochemical corrosion resistance of carbon steel and 316 L stainless steels in 3.5% sodium chloride solution. Int. J. Electrochem. Sci. 2025, 20, 101130. [Google Scholar] [CrossRef]
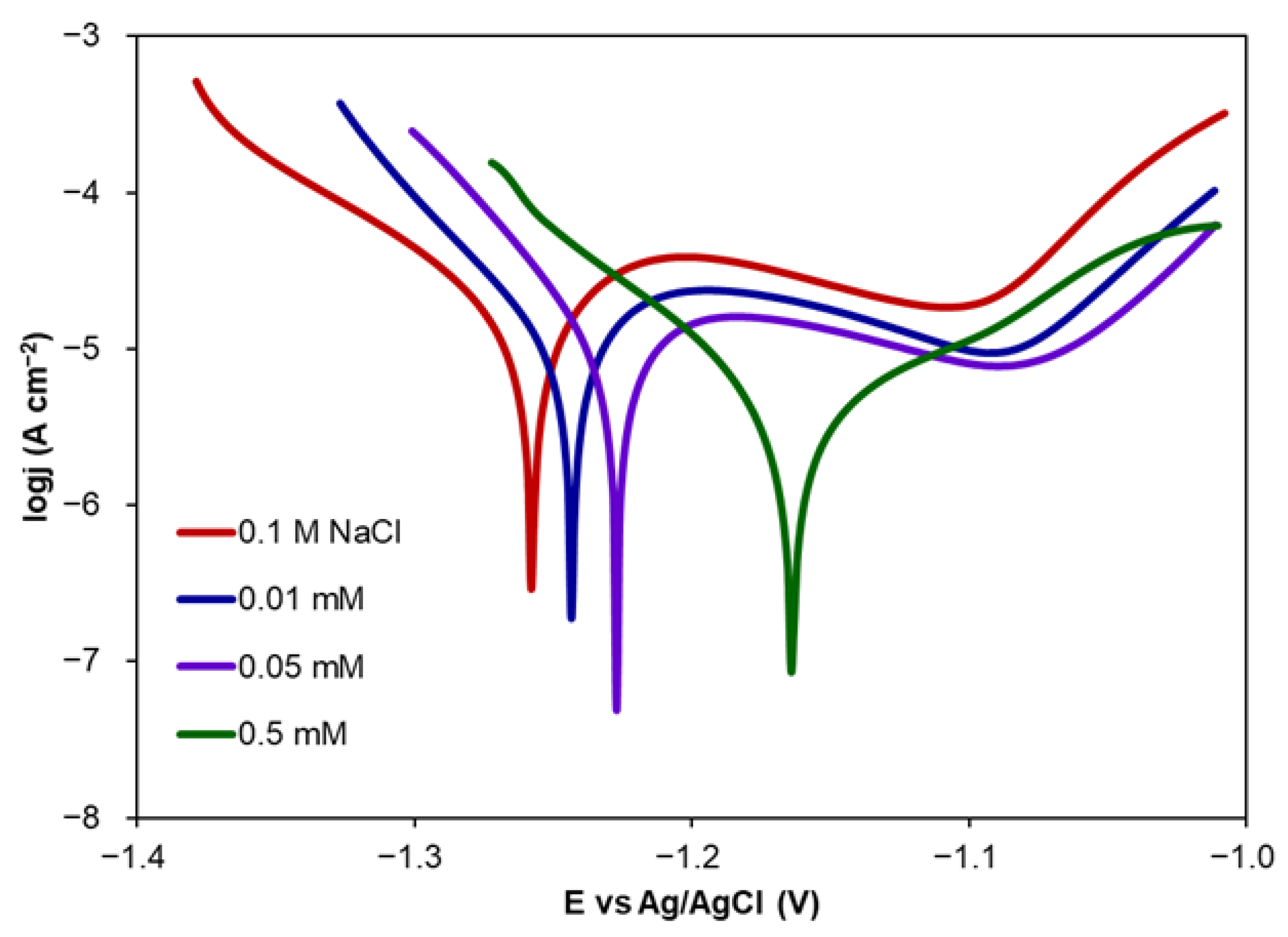
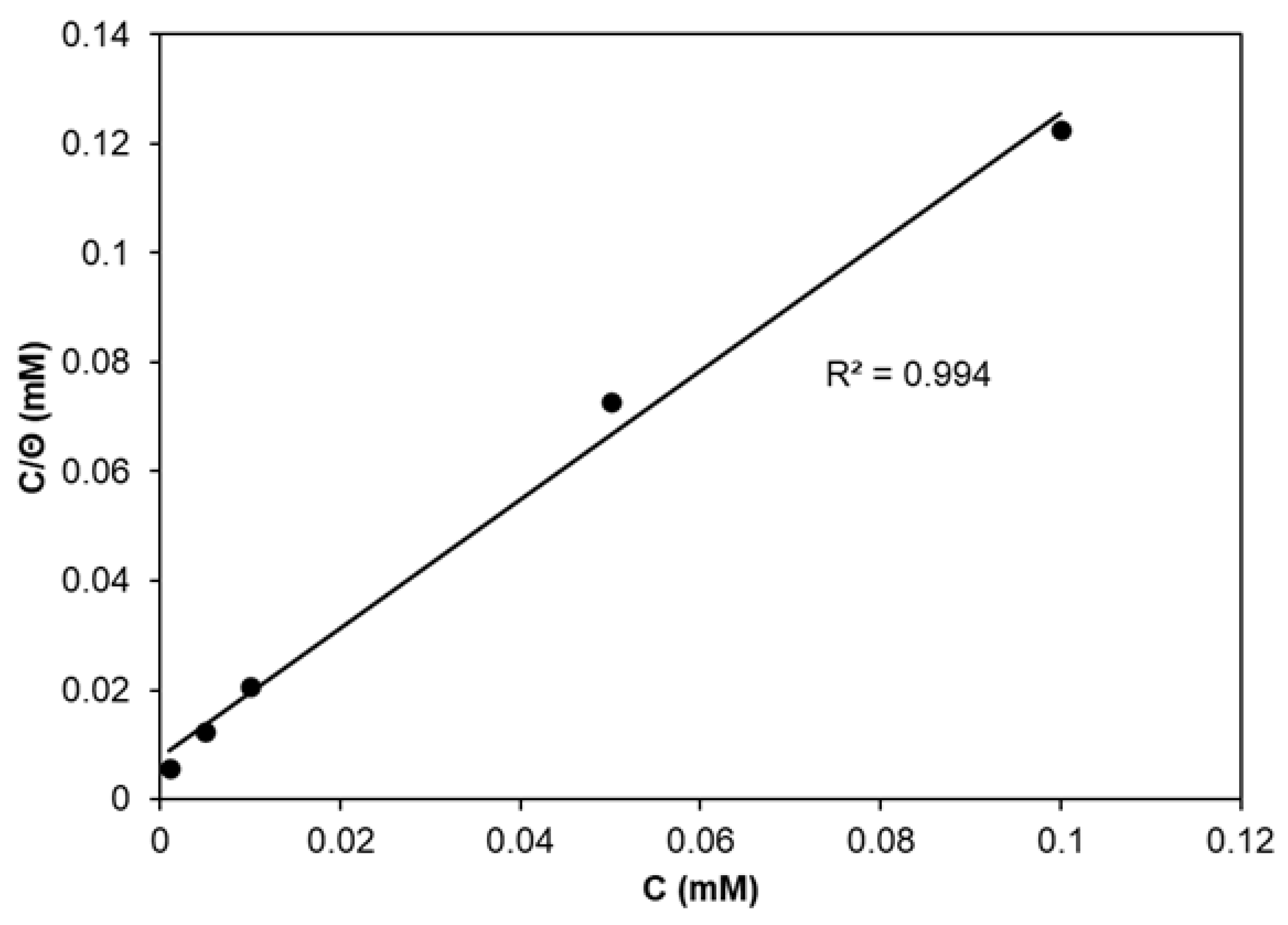

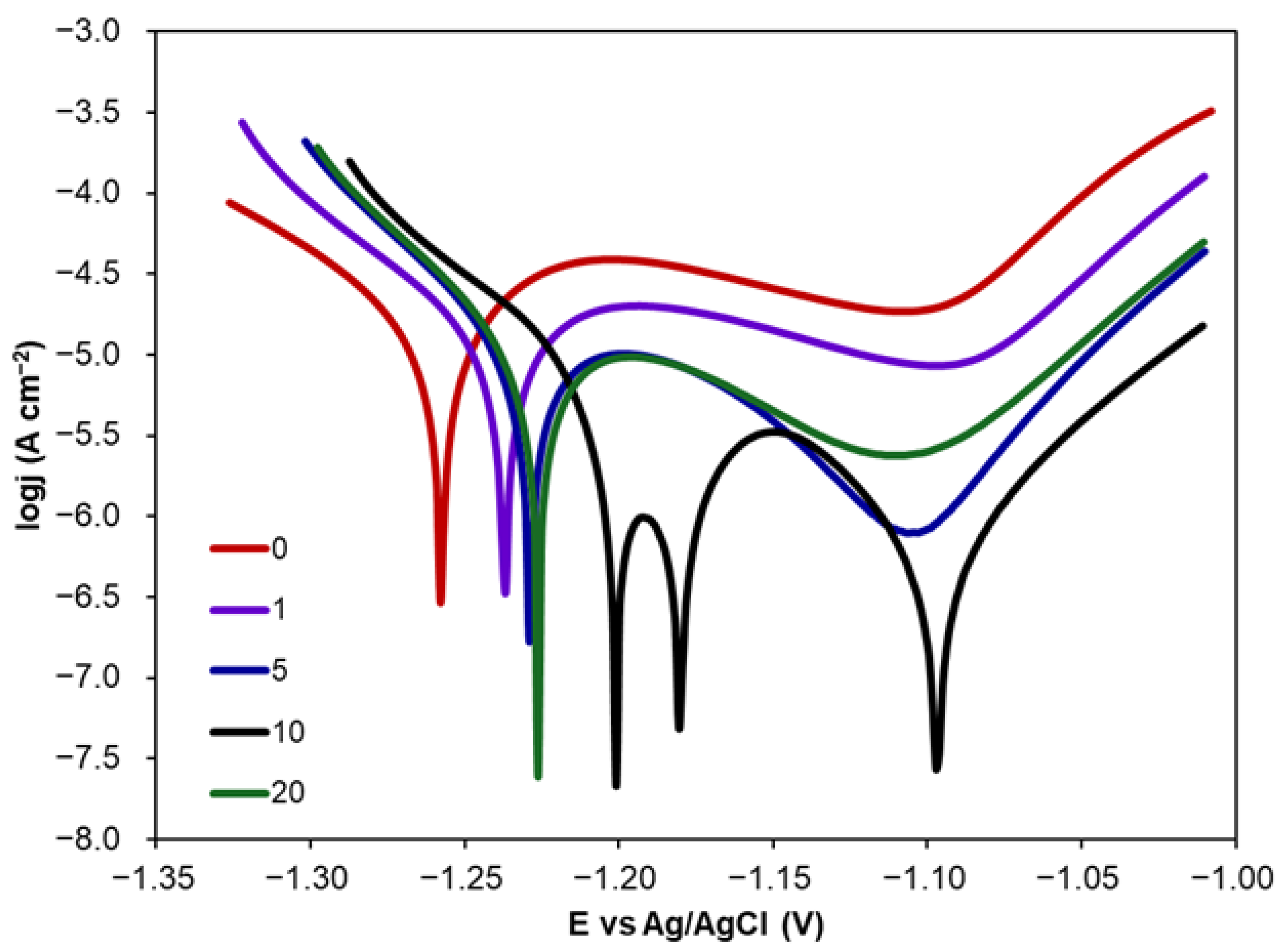
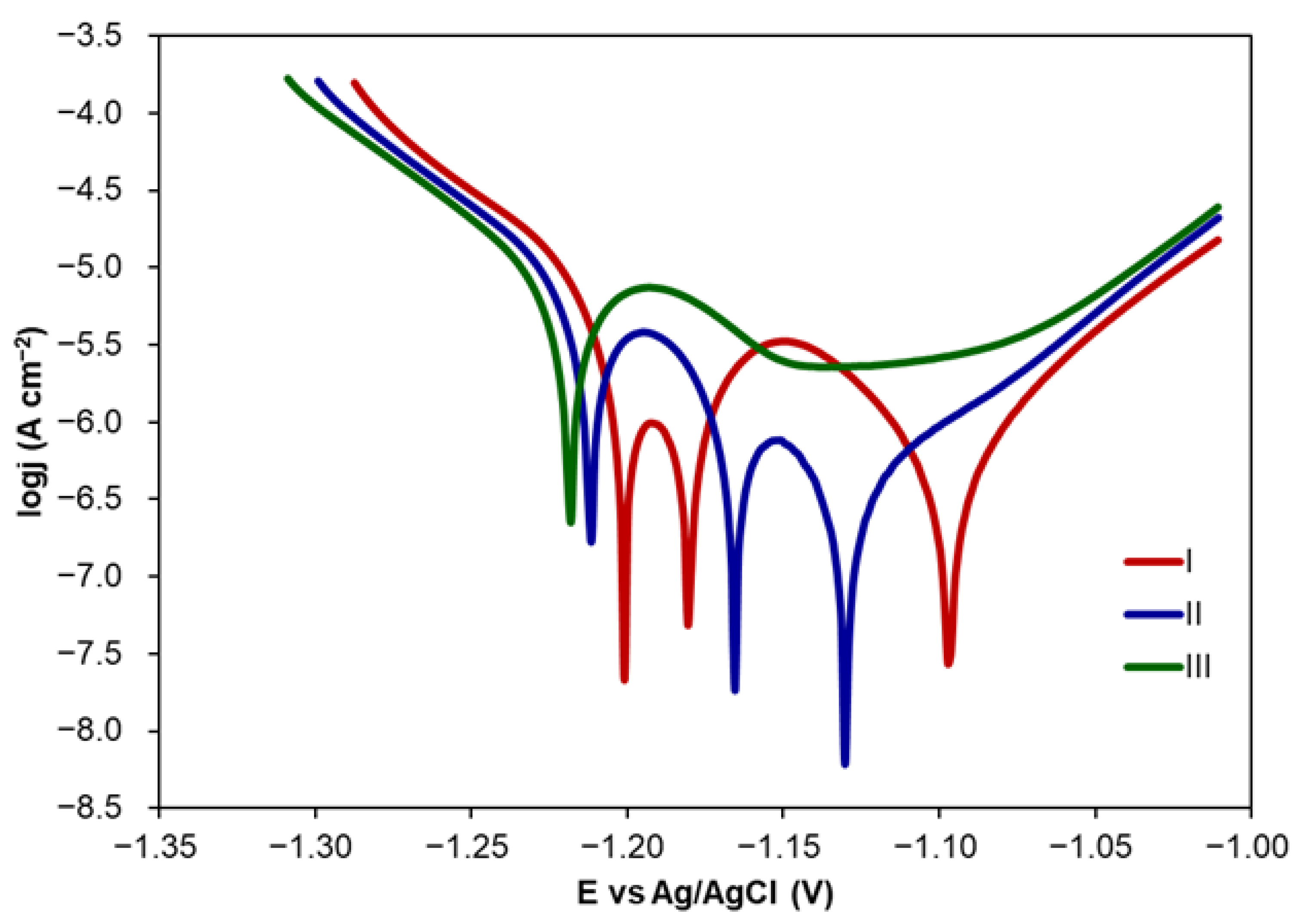

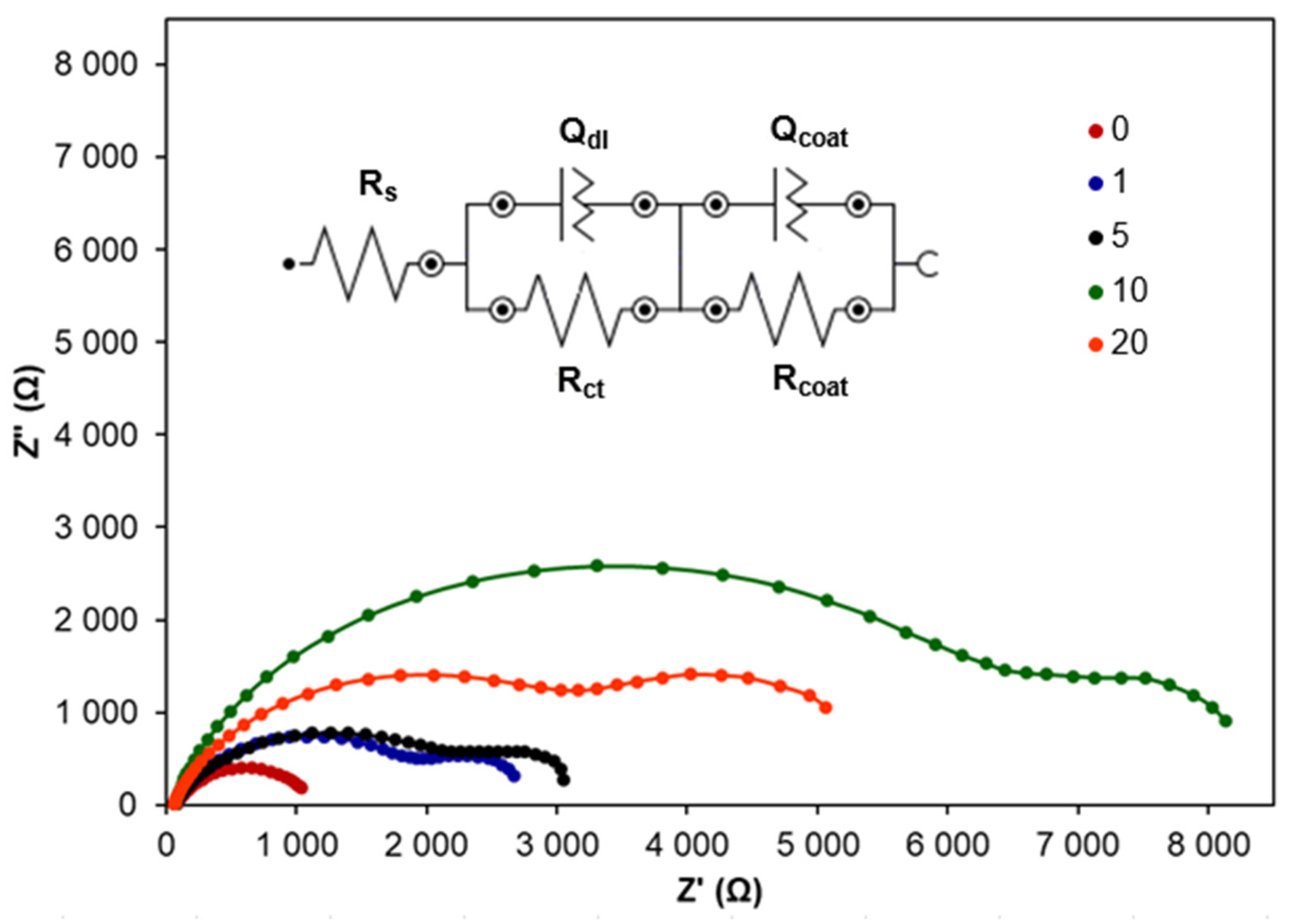
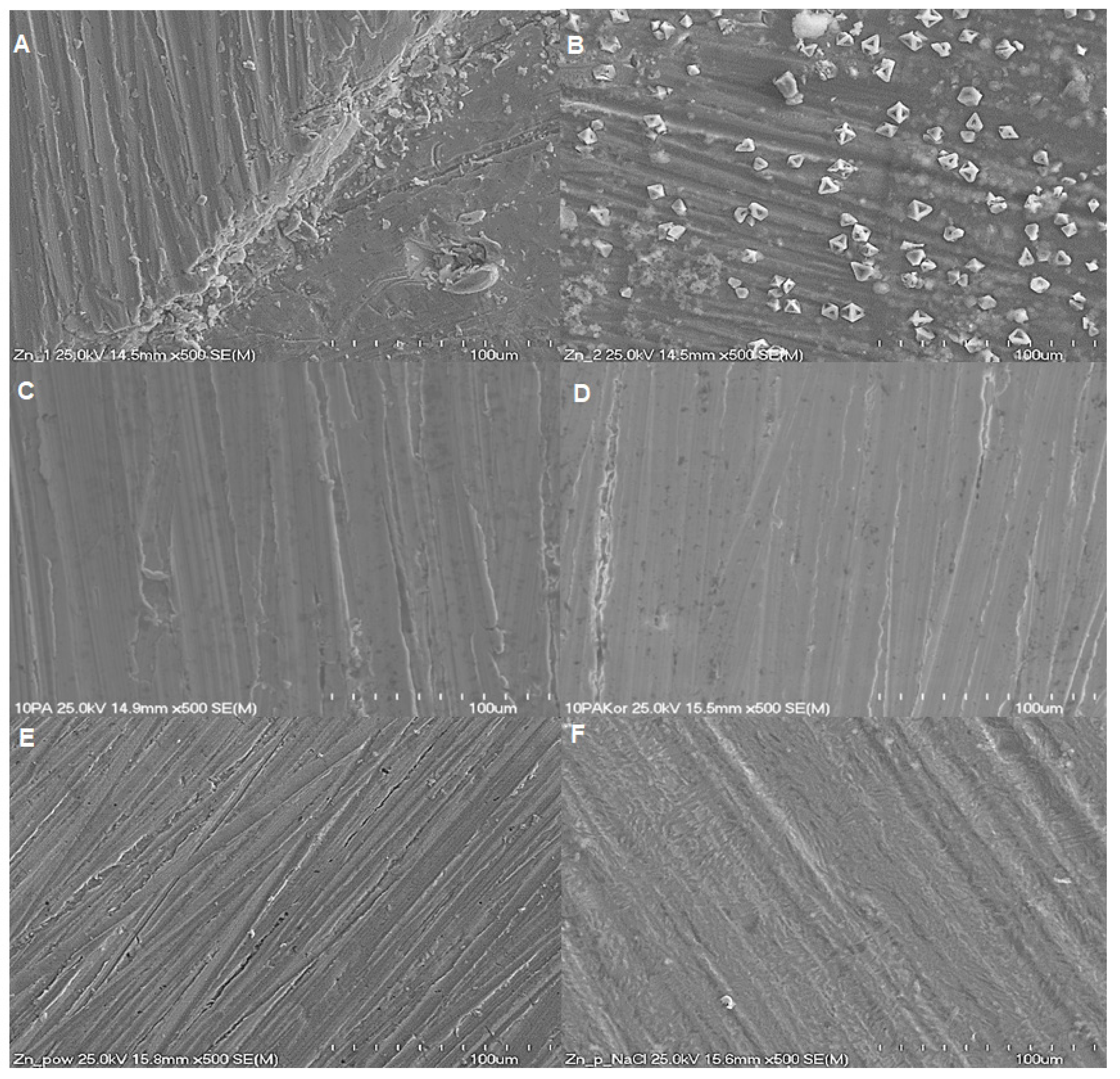


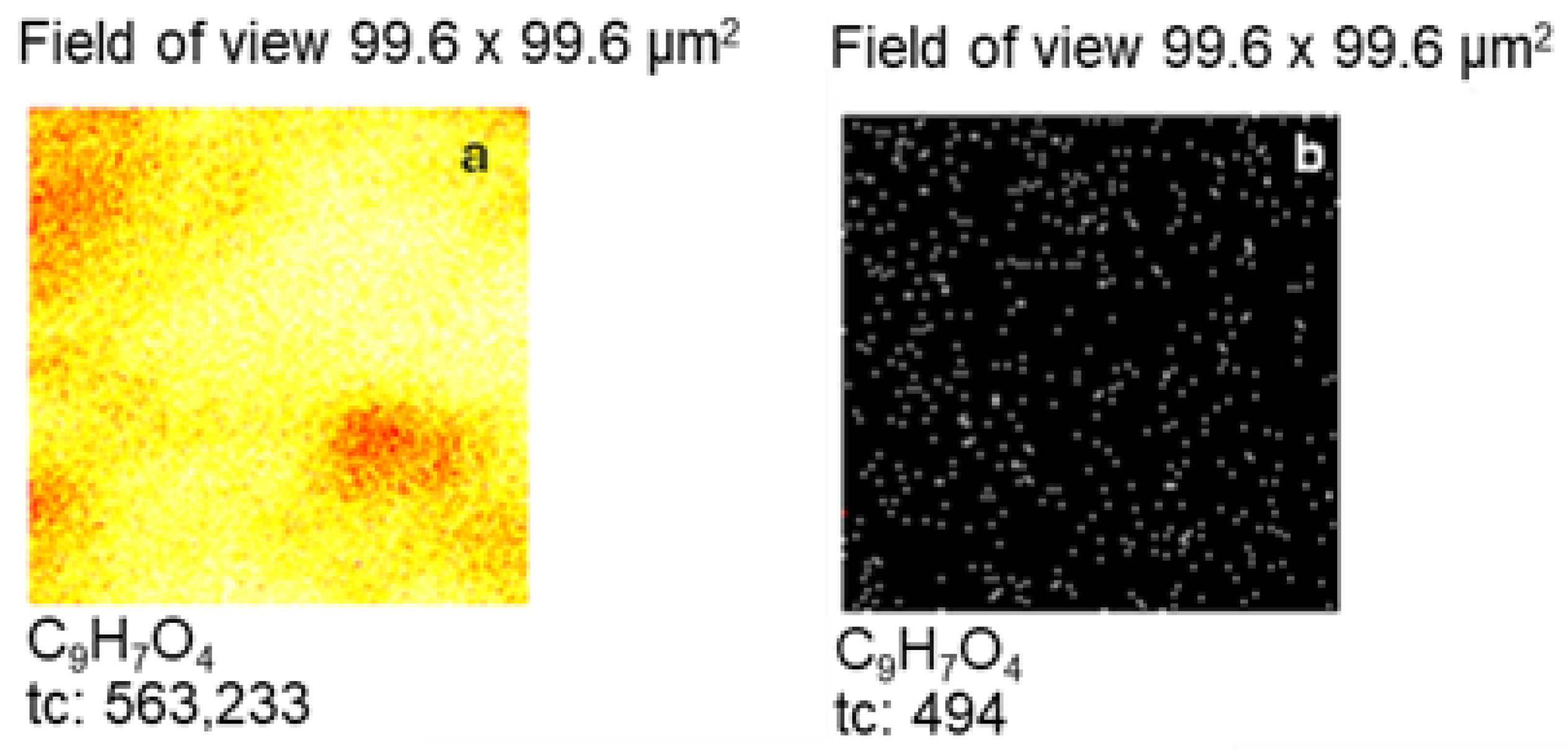
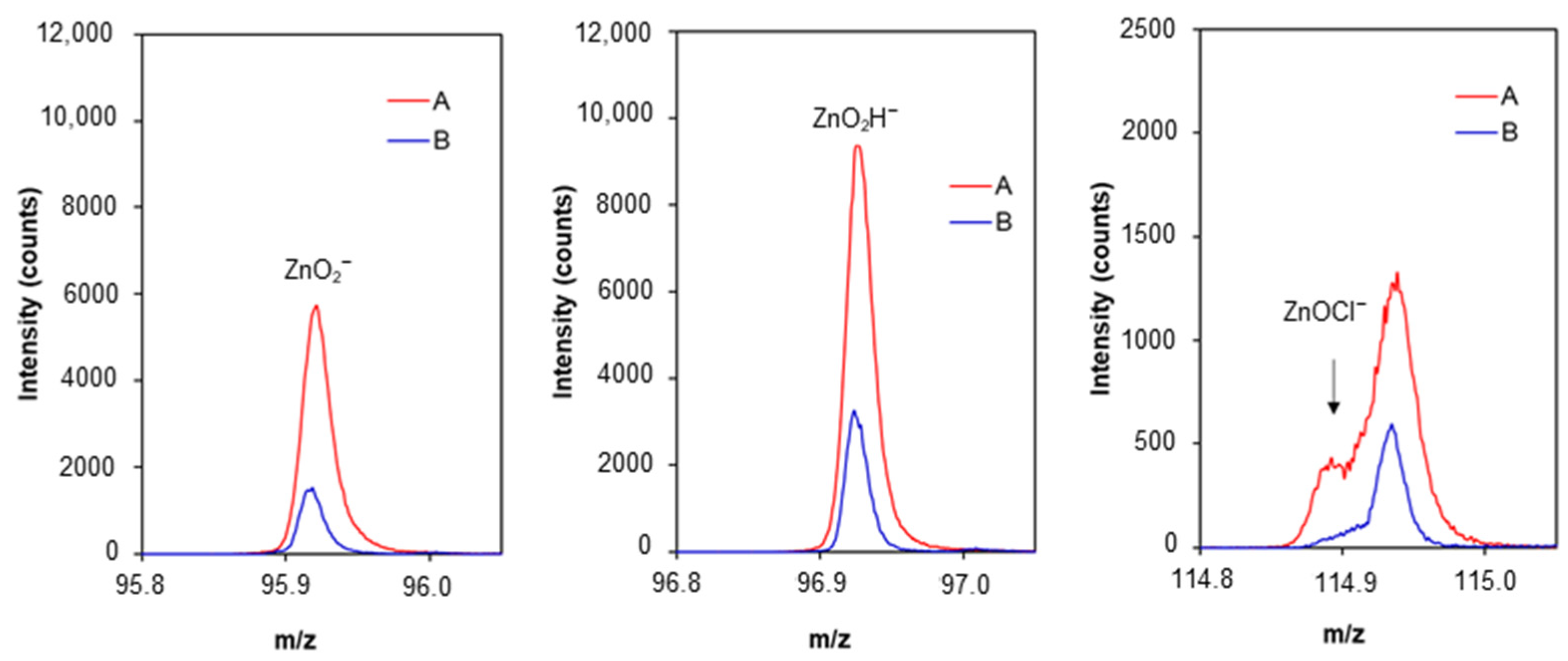
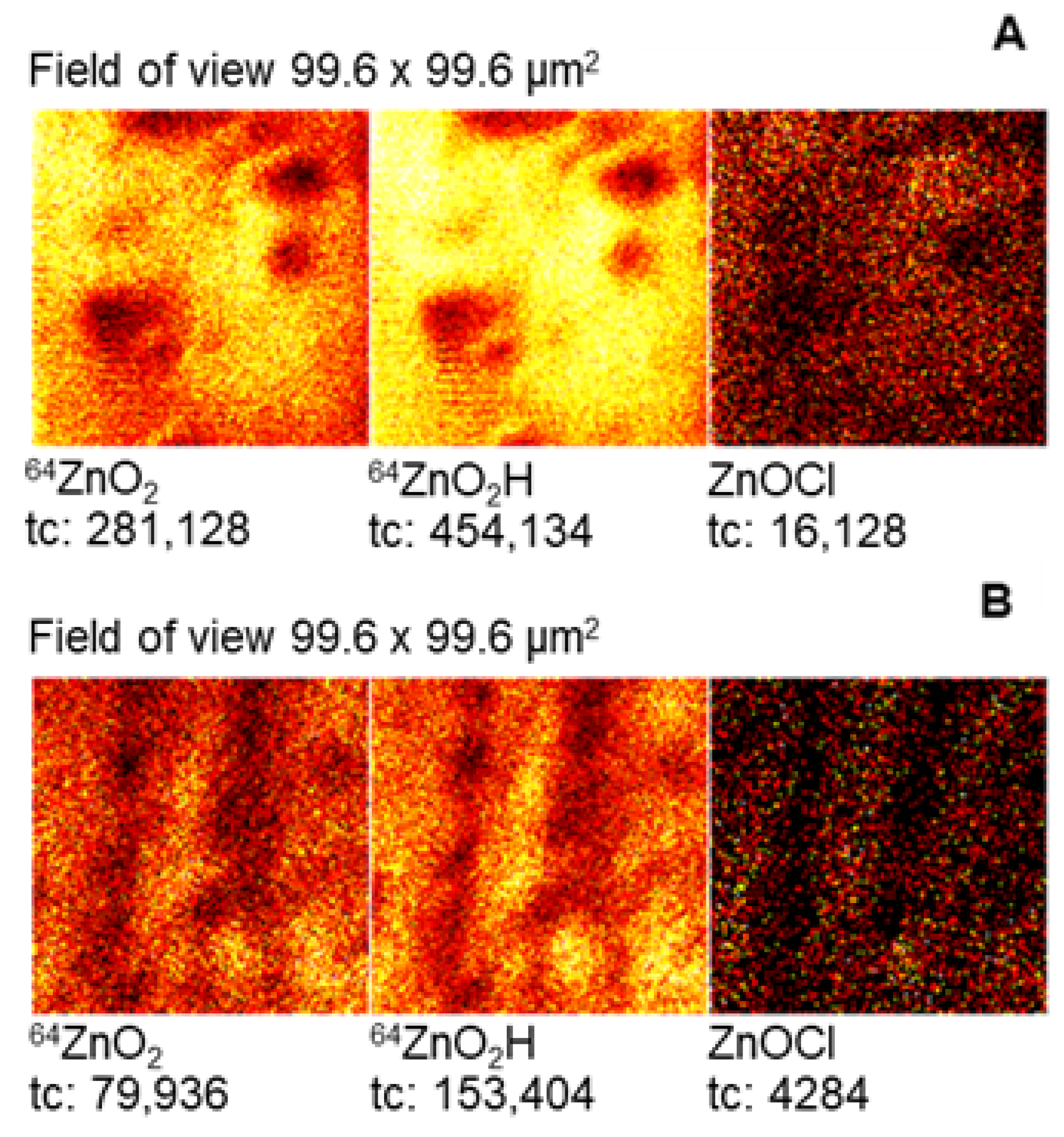

| CA Conc. (mM) | Ecorr (V) | ba (V dec−1) | bc (V dec−1) | jcorr (A cm−2) | CR (mm year−1) | IE (%) | Θ (−) |
|---|---|---|---|---|---|---|---|
| 0 | −1.258 | 0.069 | −0.067 | 1.09 × 10−5 | 0.1634 | − | − |
| 0.001 | −1.249 | 0.040 | −0.039 | 6.79 × 10−6 | 0.1018 | 37.7 | 0.38 |
| 0.005 | −1.256 | 0.041 | −0.047 | 6.48 × 10−6 | 0.0971 | 40.6 | 0.41 |
| 0.01 | −1.243 | 0.042 | −0.039 | 5.64 × 10−6 | 0.0845 | 48.3 | 0.48 |
| 0.05 | −1.227 | 0.034 | −0.024 | 3.40 × 10−6 | 0.0509 | 68.8 | 0.69 |
| 0.1 | −1.207 | 0.035 | −0.020 | 2.00 × 10−6 | 0.0301 | 81.6 | 0.82 |
| 0.5 | −1.164 | 0.069 | −0.058 | 2.96 × 10−6 | 0.0444 | 72.8 | 0.73 |
| Measur. No. | Ecorr (V) | ba (V dec−1) | bc (V dec−1) | jcorr (A cm−2) | CR (mm year−1) | IE (%) |
|---|---|---|---|---|---|---|
| 0.1 mM NaCl | ||||||
| 1 | −1.258 | 0.069 | −0.067 | 1.09 × 10−5 | 0.1634 | − |
| 2 | −1.250 | 0.103 | −0.072 | 1.00 × 10−5 | 0.1502 | − |
| 3 | −1.244 | 0.145 | −0.079 | 9.96 × 10−6 | 0.1493 | − |
| 0.1 mM NaCl + 0.1 mM CA | ||||||
| 1 | −1.207 | 0.030 | −0.020 | 2.00 × 10−6 | 0.0301 | 81.6 |
| 2 | −1.222 | 0.036 | −0.025 | 3.24 × 10−6 | 0.0486 | 67.6 |
| 3 | −1.225 | 0.030 | −0.023 | 3.00 × 10−6 | 0.0450 | 69.9 |
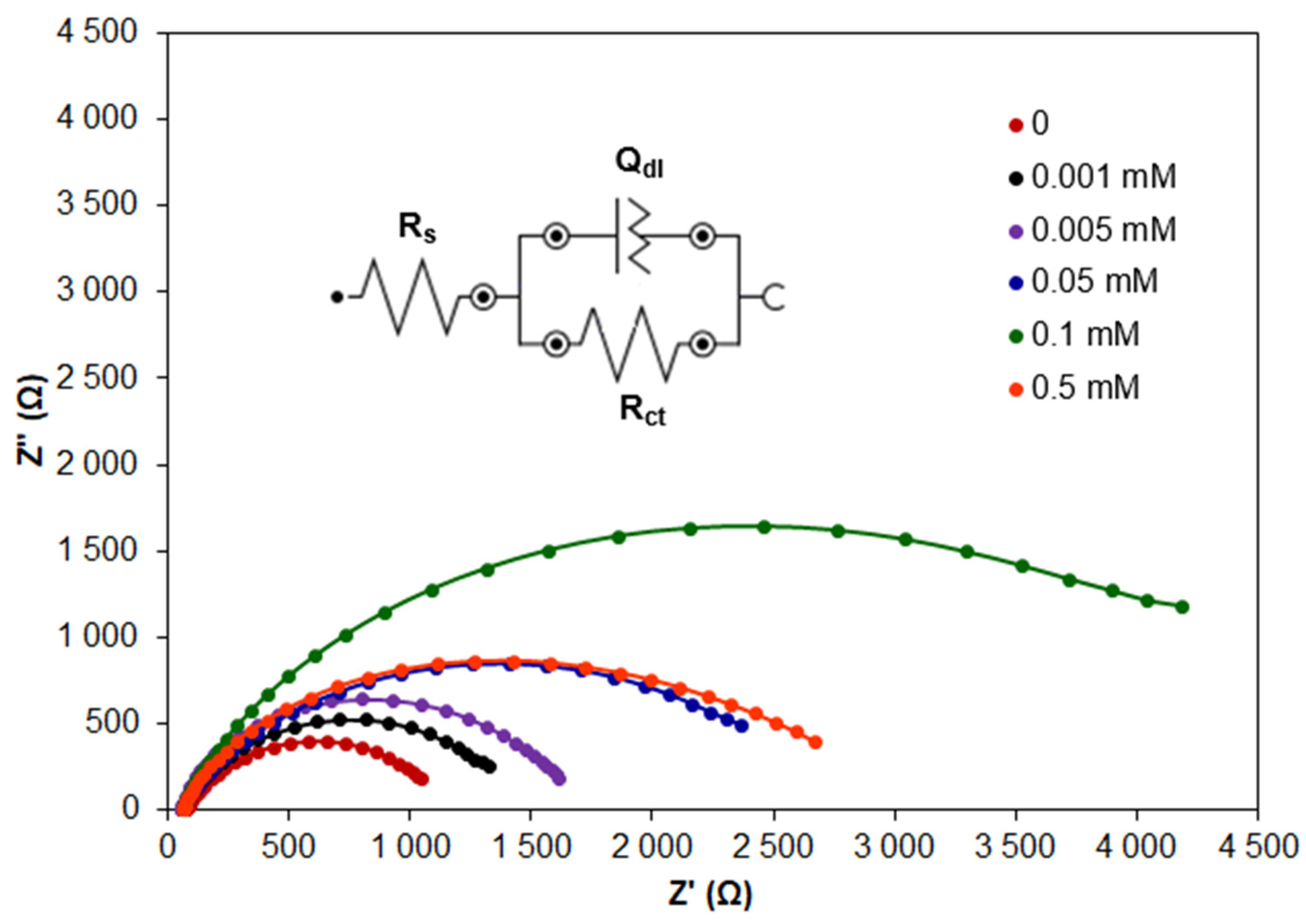
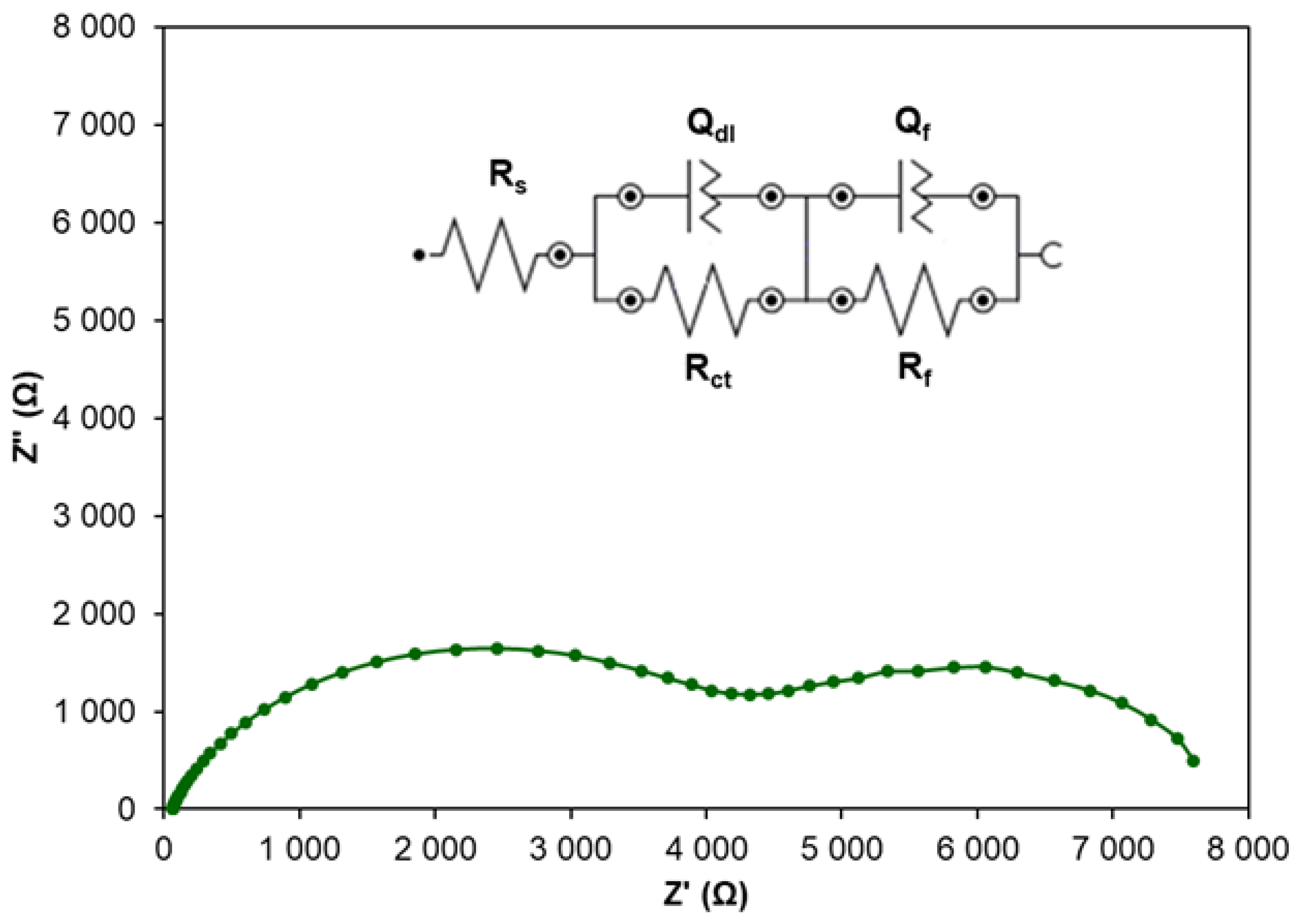
| CA (mM) | Rs (Ω) | Rct (Ω) | Qdl (S·s)n | n | Rf (Ω) | Qf (S·s)n | nf | IE (%) | Cdl (μF) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 75.3 | 1093.2 | 3.375 × 10−5 | 0.779 | - | - | - | - | 13.52 |
| 0.001 | 64.3 | 1422.5 | 2.610 × 10−5 | 0.782 | - | - | - | 23.1 | 11.13 |
| 0.005 | 63.2 | 1478.9 | 3.247 × 10−5 | 0.847 | - | - | - | 26.1 | 11.29 |
| 0.01 | 63.3 | 1580.8 | 4.314 × 10−5 | 0.864 | - | - | - | 30.8 | 11.84 |
| 0.05 | 57.0 | 2560.4 | 1.921 × 10−5 | 0.744 | - | - | - | 57.3 | 7.06 |
| 0.1 | 62.2 | 4071.0 | 1.758 × 10−5 | 0.812 | 3807.9 | 5.926 × 10−4 | 0.771 | 86.1 | 6.23 |
| 0.5 | 62.9 | 2586.6 | 3.323 × 10−5 | 0.784 | - | - | - | 57.7 | 8.26 |
| Number of Layers | Ecorr (V) | ba (V dec−1) | bc (V dec−1) | jcorr (A cm−2) | CR (mm year−1) | IE (%) |
|---|---|---|---|---|---|---|
| 5 mM CA | ||||||
| 1 | −1.233 | 0.048 | −0.037 | 4.69 × 10−6 | 0.0703 | 57.0 |
| 5 | −1.232 | 0.044 | −0.029 | 4.07 × 10−6 | 0.0610 | 62.7 |
| 10 | −1.234 | 0.030 | −0.025 | 3.52 × 10−6 | 0.0527 | 67.7 |
| 20 | −1.224 | 0.038 | −0.024 | 2.67 × 10−6 | 0.0400 | 75.5 |
| 10 mM CA | ||||||
| 1 | −1.237 | 0.042 | −0.034 | 5.26 × 10−6 | 0.0789 | 51.7 |
| 5 | −1.229 | 0.025 | −0.020 | 2.46 × 10−6 | 0.0369 | 77.4 |
| 10 | −1.201 | 0.035 | −0.012 | 5.95 × 10−7 | 0.0089 | 94.6 |
| 20 | −1.226 | 0.021 | −0.017 | 2.02 × 10−6 | 0.0303 | 81.5 |
| 20 mM CA | ||||||
| 1 | −1.224 | 0.040 | −0.023 | 2.72 × 10−6 | 0.0408 | 75.0 |
| 5 | −1.221 | 0.037 | −0.021 | 2.20 × 10−6 | 0.0330 | 79.8 |
| 10 | −1.219 | 0.035 | −0.021 | 2.12 × 10−6 | 0.0318 | 80.5 |
| 20 | −1.222 | 0.031 | −0.020 | 2.11 × 10−6 | 0.0317 | 80.6 |
| 30 mM CA | ||||||
| 1 | −1.228 | 0.068 | −0.039 | 5.65 × 10−6 | 0.0847 | 48.2 |
| 5 | −1.223 | 0.058 | −0.032 | 3.90 × 10−6 | 0.0584 | 64.3 |
| 10 | −1.218 | 0.044 | −0.022 | 2.12 × 10−6 | 0.0317 | 80.6 |
| 20 | −1.219 | 0.034 | −0.019 | 1.89 × 10−6 | 0.0283 | 82.7 |
| Measur. No. | Ecorr (V) | ba (V dec−1) | bc (V dec−1) | jcorr (A cm−2) | CR (mm year−1) | IE (%) |
|---|---|---|---|---|---|---|
| 1 | −1.201 | 0.035 | −0.012 | 5.95 × 10−7 | 0.0089 | 94.6 |
| 2 | −1.212 | 0.041 | −0.021 | 1.80 × 10−6 | 0.0270 | 82.0 |
| 3 | −1.218 | 0.020 | −0.023 | 2.11 × 10−6 | 0.0316 | 78.8 |
| Number of Layers | Rs (Ω) | Rct (Ω) | Qdl (S·s)n | n | Rcoat (Ω) | Qcoat (S·s)n | ncoat | IE (%) | Cdl (μF) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 75.3 | 1093.2 | 3.375 × 10−5 | 0.779 | - | - | - | - | 13.52 |
| 1 | 67.9 | 1974.3 | 2.864 × 10−5 | 0.777 | 942.8 | 1.292 × 10−3 | 0.868 | 62.5 | 13.27 |
| 5 | 63.9 | 1983.5 | 3.348 × 10−5 | 0.769 | 1417.9 | 9.707 × 10−4 | 0.696 | 67.9 | 13.42 |
| 10 | 69.0 | 5997.9 | 1.389 × 10−5 | 0.864 | 2921.7 | 6.387 × 10−4 | 0.789 | 87.7 | 8.97 |
| 20 | 62.5 | 3088.3 | 1.626 × 10−5 | 0.832 | 3035.7 | 1.071 × 10−4 | 0.797 | 82.1 | 10.63 |
| Element | wt. (%) | |
|---|---|---|
| Zn | Zn After Immersion | |
| C | 15.56 | 19.41 |
| O | 2.37 | 29.95 |
| Al | 1.13 | 1.85 |
| Cl | 0.00 | 0.28 |
| Zn | 80.94 | 48.51 |
| Zn 10 layers | Zn 10 layers after immersion | |
| C | 26.00 | 15.11 |
| O | 3.02 | 1.47 |
| Al | 3.25 | 3.33 |
| Cl | 0.00 | 0.02 |
| Zn | 67.73 | 80.07 |
| Zn 20 layers | Zn 20 layers after immersion | |
| C | 20.22 | 21.26 |
| O | 2.43 | 10.38 |
| Al | 1.87 | 1.93 |
| Cl | 0.00 | 0.11 |
| Zn | 75.48 | 66.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharek, A.; Kuśmierek, E.; Chrześcijańska, E.; Maniukiewicz, W.; Rogowski, J.; Bednarek, A.; Żarczyński, A. The Effect of Caffeic Acid on Zn Corrosion in NaCl: Electrochemical Studies. Molecules 2025, 30, 3648. https://doi.org/10.3390/molecules30173648
Kucharek A, Kuśmierek E, Chrześcijańska E, Maniukiewicz W, Rogowski J, Bednarek A, Żarczyński A. The Effect of Caffeic Acid on Zn Corrosion in NaCl: Electrochemical Studies. Molecules. 2025; 30(17):3648. https://doi.org/10.3390/molecules30173648
Chicago/Turabian StyleKucharek, Aleksander, Elżbieta Kuśmierek, Ewa Chrześcijańska, Waldemar Maniukiewicz, Jacek Rogowski, Aleksandra Bednarek, and Andrzej Żarczyński. 2025. "The Effect of Caffeic Acid on Zn Corrosion in NaCl: Electrochemical Studies" Molecules 30, no. 17: 3648. https://doi.org/10.3390/molecules30173648
APA StyleKucharek, A., Kuśmierek, E., Chrześcijańska, E., Maniukiewicz, W., Rogowski, J., Bednarek, A., & Żarczyński, A. (2025). The Effect of Caffeic Acid on Zn Corrosion in NaCl: Electrochemical Studies. Molecules, 30(17), 3648. https://doi.org/10.3390/molecules30173648







