Comprehensive Evaluation of OS Starch–Oleic Acid Mixtures: From Functional Properties to Their Application in Films with Improved Water Resistance
Abstract
1. Introduction
2. Results and Discussion
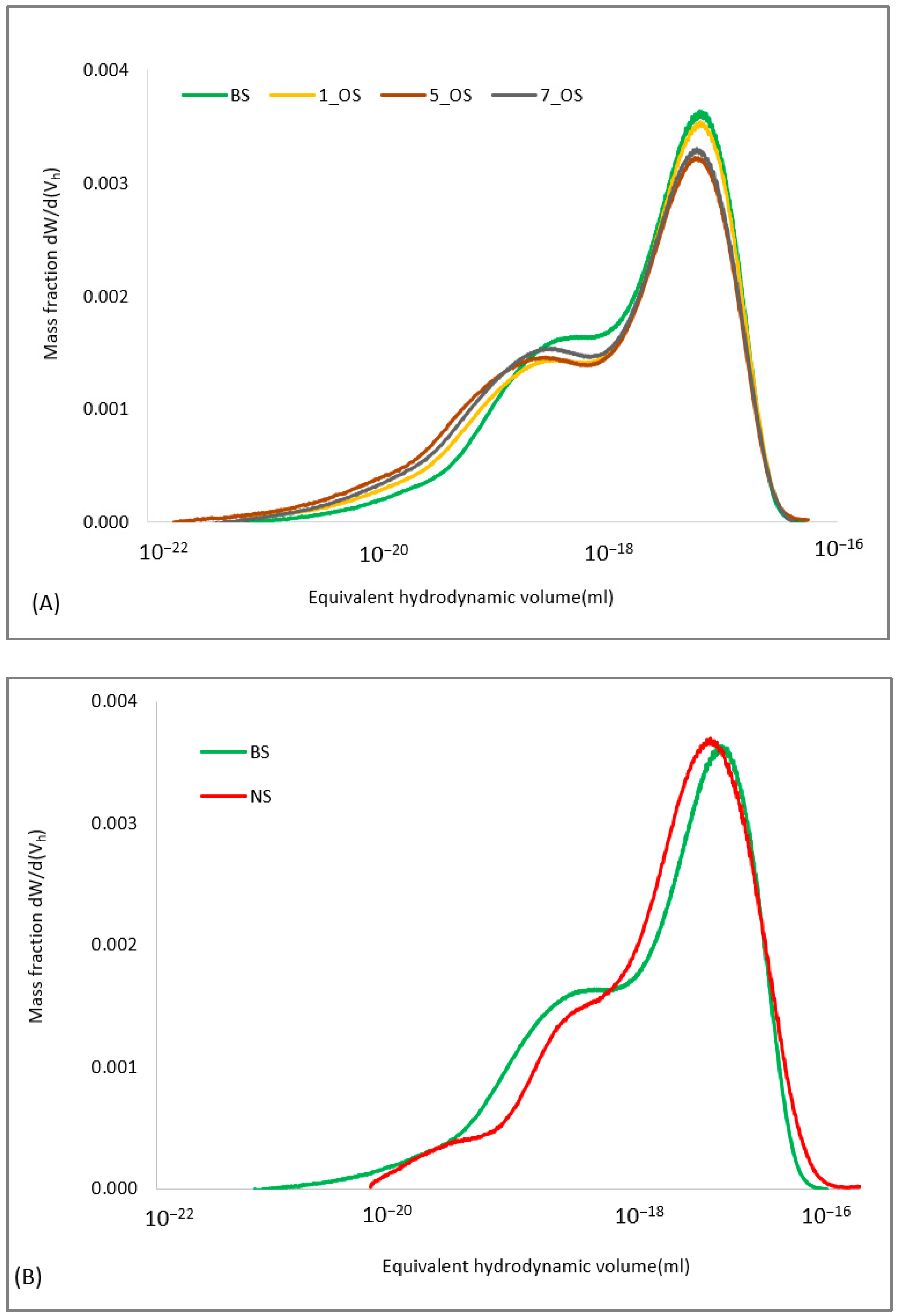
2.1. Evaluation of Oleic Acid-Octenyl Succinate Starch Mixture Properties
2.1.1. Effectiveness of Modification Procedures
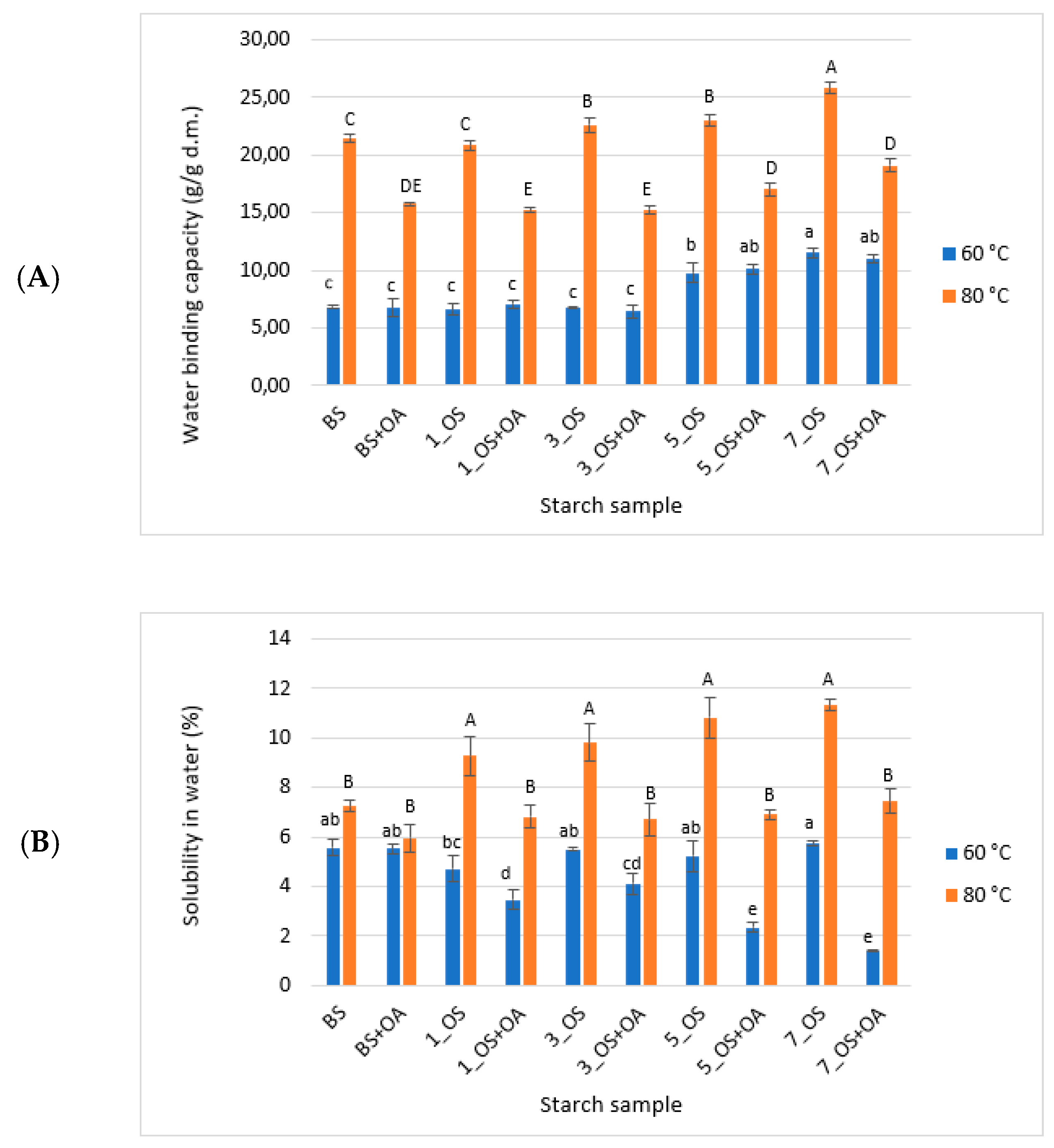
2.1.2. Water Binding Capacity and Solubility in Water
2.1.3. Thermodynamic Characteristics of Gelatinization
2.1.4. Pasting Characteristic
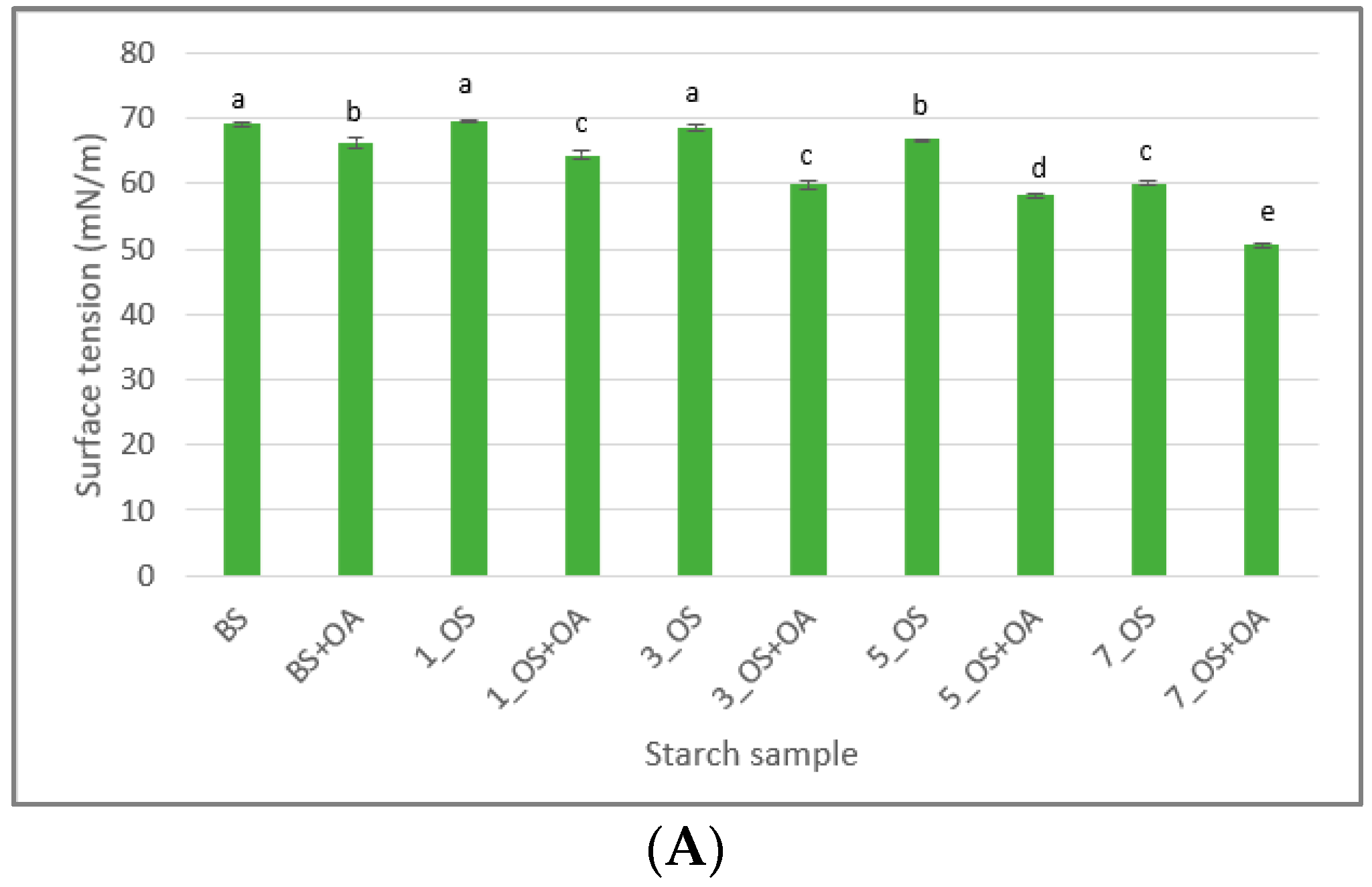
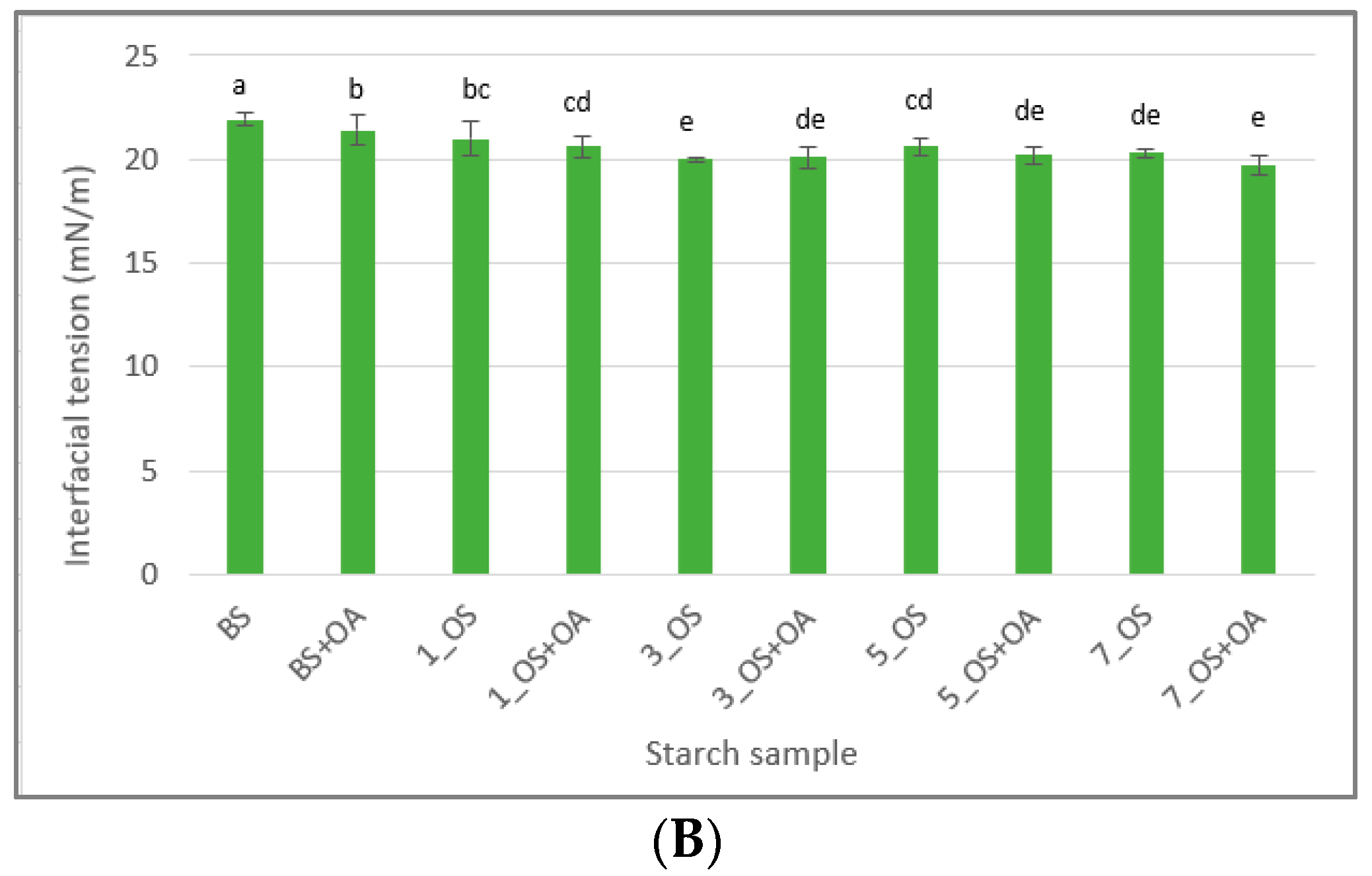
2.1.5. Surface and Interfacial Tension
2.2. Evaluation of Films Properties
2.2.1. Water Vapor Permeability of the Films (WVP)
2.2.2. Water Binding Capacity and Water Solubility
3. Materials
3.1. Preparation of Octenyl Succinate Starches
3.2. Production of OS Starch–Oleic Acid Mixtures
3.3. Formation of Edible Films Based on OS Starches and Their Mixtures with Oleic Acid
4. Methods
4.1. Effectiveness of Modification Procedures
4.1.1. Degree of Substitution
4.1.2. Determination of Complexing Index
4.1.3. Determination of Total Lipid Content
4.1.4. Determination of Equivalent Hydrodynamic Volume Distribution
4.2. Characterization of Octenyl Succinate Starches and Their Mixtures with Oleic Acid
4.2.1. Water Binding Capacity (WBC) and Solubility in Water (SW)
4.2.2. Thermodynamic Characteristics of Gelatinization
4.2.3. Pasting Characteristic
4.2.4. Surface and Interfacial Tensions of Starch and Mixture Pastes
4.3. Evaluation of the Functional Properties of the Films Prepared from Octenyl Succinate Starches or Their Mixtures with Oleic Acids
4.3.1. Microscopy Observation of Emulsions
4.3.2. Determination of Water Binding Capacity and Water Solubility
4.3.3. Water Vapor Permeability
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Królikowska, K.; Fortuna, T.; Pająk, P.; Witczak, M. Impact of the degree of octenyl succinylation on metal ions complexation and functional properties of maize starch. Food Chem. 2019, 278, 284–293. [Google Scholar] [CrossRef]
- Altuna, L.; Herrera, M.L.; Foresti, M.L. Synthesis and characterization of octenyl succinic anhydride modified starches for food applications. A review of recent literature. Food Hydrocoll. 2018, 80, 97–110. [Google Scholar] [CrossRef]
- Drusch, S.; Schwarz, K. Microencapsulation properties of two different types of n-octenylsuccinate-derivatised starch. Eur. Food Res. Technol. 2006, 222, 155–164. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, L.; Tong, J.; Ma, Y. Effect of surface esterification with octenyl succinic anhydride on hydrophilicity of corn starch films. J. Appl. Polym. Sci. 2009, 114, 940–947. [Google Scholar] [CrossRef]
- Commision Regulation (EU). No 231/2012 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. Off. J. Eur. Union 2012, L83, 1–295. [Google Scholar]
- Magnusson, E.; Nilsson, L. Interactions between hydrophobically modified starch and egg yolk proteins in solution and emulsions. Food Hydrocoll. 2011, 25, 764–772. [Google Scholar] [CrossRef]
- Wu, D.; Lin, Q.; Singh, H.; Ye, A. Complexation between whey protein and octenyl succinic anhydride (OSA)-modified starch: Formation and characteristics of soluble complexes. Food Res. Int. 2020, 136, 109350. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, M.; Yu, J.; Wang, S.; Copeland, L. Insights into the Formation and Structures of Starch-Protein-Lipid Complexes. J. Agric. Food Chem. 2017, 65, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hamaker, B.R. Starch-free fatty acid complexation in the presence of whey protein. Carbohydr. Polym. 2004, 55, 419–424. [Google Scholar] [CrossRef]
- Lu, X.; Shi, C.; Zhu, J.; Li, Y.; Huang, Q. Structure of starch-fatty acid complexes produced via hydrothermal treatment. Food Hydrocoll. 2019, 88, 58–67. [Google Scholar] [CrossRef]
- Marinopoulou, A.; Papastergiadis, E.; Raphaelides, S.N. An investigation into the structure, morphology and thermal properties of amylomaize starch-fatty acid complexes prepared at different temperatures. Food Res. Int. 2016, 90, 11–20. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Handarini, K.; Sauman Hamdani, J.; Cahyana, Y.; Siti Setiasih, I. Functional and pasting properties of a starch–lipid complex formed with gaseous ozone and palm oil. Int. J. Food Prop. 2020, 23, 1361–1372. [Google Scholar] [CrossRef]
- Kang, X.; Yu, B.; Zhang, H.; Sui, J.; Guo, L.; El-Aty, A.A.; Cui, B. The formation and in vitro enzymatic digestibility of starch-lipid complexes in steamed bread free from and supplemented with different fatty acids: Effect on textural and retrogradation properties during storage. Int. J. Biol. Macromol. 2021, 166, 1210–1219. [Google Scholar] [CrossRef]
- Lalush, I.; Bar, H.; Zakaria, I.; Eichler, S.; Shimoni, E. Utilization of amylose-lipid complexes as molecular nanocapsules for conjugated linoleic acid. Biomacromolecules 2005, 6, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Yu, J.; Wang, S. Effect of fatty acids on functional properties of normal wheat and waxy wheat starches: A structural basis. Food Chem. 2016, 190, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Królikowska, K.; Pietrzyk, S.; Łabanowska, M.; Kurdziel, M.; Pająk, P. The influence of acid hydrolysis on physicochemical properties of starch-oleic acid mixtures and generation of radicals. Food Hydrocoll. 2021, 118, 106780. [Google Scholar] [CrossRef]
- Wang, J.; Su, L.; Wang, S. Physicochemical properties of octenyl succinic anhydride-modified potato starch with different degrees of substitution. J. Sci. Food Agric. 2010, 90, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Choulis, N.H. Miscellaneous Drugs, Materials, Medical Devices, and Techniques, 1st ed.; Side Effects of Drugs Annual; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 33, pp. 1009–1029. [Google Scholar]
- Lopez-Huertas, E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef]
- Królikowska, K.; Pietrzyk, S.; Pustkowiak, H.; Wolak, K. The effect of cassava and wheat starches complexation with selected fatty acids on their functional properties. J. Food Sci. Technol. 2022, 59, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gallardo, A.; Bello-Pérez, L.A.; García-Almendárez, B.; Montejano-Gaitán, G.; Barbosa-Cánovas, G.; Regalado, C. Effect of structural characteristics of modified waxy corn starches on rheological properties, film-forming solutions, and on water vapor permeability, solubility, and opacity of films. Starch/Staerke 2012, 64, 27–36. [Google Scholar] [CrossRef]
- Pająk, P.; Gałkowska, D.; Juszczak, L.; Khachatryan, G. Octenyl succinylated potato starch-based film reinforced by honey-bee products: Structural and functional properties. Food Packag. Shelf Life 2022, 34, 100995. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Królikowska, K.; Grzyb, J.; Hetmańczyk, J.; Zachariasz, P. Characterization of octenyl succinylated potato-starch based films enriched with extracts from various honey-bee products. Int. J. Biol. Macromol. 2025, 285, 138293. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.; Ali, S.; Chatha, S.; Hussain, A.I.; Zia, K.M. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Ruan, H.; Chen, Q.; Fu, M.; Xu, Q.; He, G. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009, 114, 81–86. [Google Scholar] [CrossRef]
- Kurdziel, M.; Królikowska, K.; Łabanowska, M.; Pietrzyk, S.; Michalec, M. The effect of thermal and irradiation treatments on structural and physicochemical properties of octenyl succinate maize starches. Food Chem. 2020, 330, 127242. [Google Scholar] [CrossRef]
- Simsek, S.; Ovando-Martinez, M.; Marefati, A.; Sj, M.; Rayner, M. Chemical composition, digestibility and emulsification properties of octenyl succinic esters of various starches. Food Res. Int. 2015, 75, 41–49. [Google Scholar] [CrossRef]
- Wang, J.; Ren, F.; Yu, J.; Copeland, L.; Wang, S. Octenyl Succinate Modification of Starch Enhances the Formation of Starch-Lipid Complexes. J. Agric. Food Chem. 2021, 69, 14938–14950. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch–lipid and starch–lipid–protein complexes: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef]
- Eliasson, A.-C. Interactions between starch and lipids studied by DSC. Thermochim. Acta 1994, 246, 343–356. [Google Scholar] [CrossRef]
- Nie, H.; Li, C.; Liu, P.; Lei, C.; Li, J. Retrogradation, gel texture properties, intrinsic viscosity and degradation mechanism of potato starch paste under ultrasonic irradiation. Food Hydrocoll. 2019, 95, 590–600. [Google Scholar] [CrossRef]
- Koyakumaru, T.; Nakano, H. Thermal Characterization of the Gelatinization of Corn Starch Suspensions with Added Sodium Hydroxide or Urea as a Main Component of Corrugating Adhesives. J. Appl. Glycosci. 2016, 63, 87–98. [Google Scholar] [CrossRef]
- Gelders, G.G.; Goesaert, H.; Delcour, J.A. Amylose-lipid complexes as controlled lipid release agents during starch gelatinization and pasting. J. Agric. Food Chem. 2006, 54, 1493–1499. [Google Scholar] [CrossRef]
- Chumsri, P.; Panpipat, W.; Cheong, L.Z.; Chaijan, M. Formation of Intermediate Amylose Rice Starch–Lipid Complex Assisted by Ultrasonication. Foods 2022, 11, 2430. [Google Scholar] [CrossRef]
- Ulbrich, M.; Flöter, E. Modification of Starches with Different Amylose/Amylopectin-Ratios Using the Dual Approach with Hydroxypropylation and Subsequent Acid-Thinning—Impacts on Morphological and Molecular Characteristics. Starch/Staerke 2020, 72, 2000015. [Google Scholar] [CrossRef]
- Chung, H.J.; Lee, S.E.; Han, J.A.; Lim, S.T. Physical properties of dry-heated octenyl succinylated waxy corn starches and its application in fat-reduced muffin. J. Cereal Sci. 2010, 52, 496–501. [Google Scholar] [CrossRef]
- Liang, X.; King, J.M.; Shih, F.F. Pasting property differences of commercial and isolated rice starch with added lipids and β-cyclodextrin. Cereal Chem. 2002, 79, 812–818. [Google Scholar] [CrossRef]
- Zhao, S.; Tian, G.; Zhao, C.; Lu, C.; Bao, Y.; Liu, X.; Zheng, J. Emulsifying stability properties of octenyl succinic anhydride (OSA) modified waxy starches with different molecular structures. Food Hydrocoll. 2018, 85, 248–256. [Google Scholar] [CrossRef]
- Krstonošić, V.; Dokić, L.; Milanović, J. Micellar properties of OSA starch and interaction with xanthan gum in aqueous solution. Food Hydrocoll. 2011, 25, 361–367. [Google Scholar] [CrossRef]
- Królikowska, K.; Fortuna, T.; Pietrzyk, S.; Gryszkin, A. Effect of modification of octenyl succinate starch with mineral elements on the stability and rheological properties of oil-in-water emulsions. Food Hydrocoll. 2017, 66, 118–127. [Google Scholar] [CrossRef]
- Naseri, A.; Shekarchizadeh, H.; Kadivar, M. Octenylsuccination of sago starch and investigation of the effect of calcium chloride and ferulic acid on physicochemical and functional properties of the modified starch film. J. Food Process Preserv. 2019, 43, e13898. [Google Scholar] [CrossRef]
- Karnwal, A.; Rauf, A.; Jassim, A.Y.; Selvaraj, M.; Al-Tawaha, A.R.M.S.; Kashyap, P.; Kumar, D.; Malik, T. Advanced starch-based films for food packaging: Innovations in sustainability and functional properties. Food Chem X. 2025, 29, 102662. [Google Scholar] [CrossRef]
- Osés, J.; Fernández-Pan, I.; Mendoza, M.; Maté, J.I. Stability of the mechanical properties of edible films based on whey protein isolate during storage at different relative humidity. Food Hydrocoll. 2009, 23, 125–131. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Wang, Y.; Xiong, G.; Mei, X.; Wu, W.; Ding, A.; Li, X.; Qiao, Y.; Liao, L. Effects of fatty acid chain length on properties of potato starch–fatty acid complexes under partially gelatinization. Int. J. Food Prop. 2018, 21, 2121–2134. [Google Scholar] [CrossRef]
- PN-EN ISO 3947:2001; Starches, Native or Modified—Determination of Total Lipid Content. ISO: Geneva, Switzerland, 2001.
- Vilaplana, F.; Gilbert, R.G. Characterization of branched polysaccharides using multiple-detection size separation techniques. J. Sep. Sci. 2010, 33, 3537–3554. [Google Scholar] [CrossRef]
- Coseri, S.; Bercea, M.; Harabagiu, V.; Budtova, T. Oxidation vs. degradation in polysaccharides: Pullulan—A case study. Eur. Polym. J. 2016, 85, 82–91. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhu, F. Physicochemical properties of quinoa starch. Carbohydr. Polym. 2016, 137, 328–338. [Google Scholar] [CrossRef]
- Lima, E.R.A.; De Melo, B.M.; Baptista, L.T.; Paredes, M.L.L. Specific ion effects on the interfacial tension of water/hydrocarbon systems. Braz. J. Chem. Eng. 2013, 30, 55–62. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Rhim, J.W.; Roh, H.J.; Kim, S.S. Preparation and physical properties of zein-coated high-amylose corn starch film. Lwt 2002, 35, 680–686. [Google Scholar] [CrossRef]
- Šuput, D.; Lazić, V.; Pezo, L.; Markov, S.; Vaštag, Ž.; Popović, L.; Radulović, A.; Ostojic, S.; Zlatanovic, S.; Popović, S. Characterization of starch edible films with different essential oils addition. Pol. J. Food Nutr. Sci. 2016, 66, 277–285. [Google Scholar] [CrossRef]
- Kumaran, M.K. Interlaboratory Comparison of the ASTM Standard Test Methods for Water Vapor Transmission of Materials (E 96-95). J. Test. Eval. 1998, 26, 83–88. [Google Scholar] [CrossRef]

| Starch Derivative | Degree of Substitution (-) | Substitution (%) |
|---|---|---|
| 1_OS | 0.0043 ± 0.0005 d | 0.60 ± 0.06 d |
| 3_OS | 0.0092 ± 0.0007 c | 1.21 ± 0.08 c |
| 5_OS | 0.0156 ± 0.0003 b | 2.02 ± 0.03 b |
| 7_OS | 0.0217 ± 0.0003 a | 2.71 ± 0.05 a |
| Starch Derivative | Complexing Index (%) | Lipid Content (%) |
|---|---|---|
| BS+OA | 4.53 ± 0.40 e | 2.80 ± 0.10 a |
| 1_OS+OA | 5.69 ± 0.49 d | 2.87 ± 0.12 a |
| 3_OS+OA | 8.16 ± 0.14 c | 2.74 ± 0.09 a |
| 5_OS+OA | 15.57 ± 0.27 b | 2.98 ± 0.01 a |
| 7_OS+OA | 18.89 ± 0.40 a | 2.83 ± 0.16 a |
| Starch Derivative | To (°C) | Tp (°C) | Te (°C) | ΔH (J/g) |
|---|---|---|---|---|
| BS | 61.31 ± 0.03 cd | 65.79 ± 0.15 d | 72.67 ± 0.20 ab | 13.90 ± 0.36 a |
| BS+OA | 61.48 ± 0.06 bc | 66.08 ± 0.08 bc | 72.44 ± 0.12 bc | 12.35 ± 0.56 bcd |
| 1_OS | 61.23 ± 0.28 cd | 65.80 ± 0.18 cd | 72.48 ± 0.31 ab | 13.36 ± 0.48 a |
| 1_OS+OA | 61.03 ± 0.08 de | 65.81 ± 0.01 cd | 72.10 ± 0.19 cd | 12.61 ± 0.86 bcd |
| 3_OS | 62.01 ± 0.06 a | 66.52 ± 0.08 a | 72.51 ± 0.06 ab | 12.79 ± 0.11 bc |
| 3_OS+OA | 61.68 ± 0.14 b | 66.25 ± 0.17 ab | 72.42 ± 0.21 bc | 12.11 ± 0.91 cd |
| 5_OS | 61.25 ± 0.24 cd | 66.39 ± 0.24 a | 72.82 ± 0.27 a | 12.13 ± 0.35 cd |
| 5_OS+OA | 60.68 ± 0.11 f | 65.85 ± 0.11 cd | 71.88 ± 0.12 de | 11.79 ± 0.42 d |
| 7_OS | 57.18 ± 0.24 g | 64.24 ± 0.25 e | 71.05 ± 0.22 f | 12.05 ± 0.26 cd |
| 7_OS+OA | 60.80 ± 0.34 ef | 65.67 ± 0.24 d | 71.73 ± 0.21 e | 12.30 ± 0.11 cd |
| Starch Derivative | PV | HPV | BD | FV | SB | Pt |
|---|---|---|---|---|---|---|
| (mPa·s) | (mPa·s) | (mPa·s) | (mPa·s) | (mPa·s) | (°C) | |
| BS | 3969 ± 194 a | 1385 ± 28 c | 2584 ± 175 | 1693 ± 16 d | 308 ± 17 | 65.2 ± 0.1 c |
| BS+OA | 983 ± 4 d | 893 ± 22 f | 90 ± 19 | 1421 ± 15 e | 528 ± 13 | 67.2 ± 0.6 c |
| 1_OS | 2750 ± 70 b | 1600 ± 56 a | 1150 ± 81 | 2133 ± 16 b | 533 ± 47 | 64.4 ± 1.2 c |
| 1_OS+OA | 996 ± 7 d | 980 ± 17 e | 16 ± 11 | 1715 ± 49 d | 735 ± 32 | 73.0 ± 3.5 b |
| 3_OS | 1703 ± 33 c | 1305 ± 47 d | 398 ± 80 | 2150 ± 19 b | 845 ± 29 | 65.7 ± 0.1 c |
| 3_OS+OA | 637 ± 17 e | 618 ± 18 g | 18 ± 3 | 946 ± 26 f | 327 ± 16 | 74.6 ± 4.6 b |
| 5_OS | 1696 ± 20 c | 1633 ± 16 a | 63 ± 3 | 2204 ± 26 a | 571 ± 11 | 65.7 ± 0.1 c |
| 5_OS+OA | 568 ± 9 e | 541 ± 10 h | 27 ± 1 | 861 ± 13 g | 320 ± 4 | 81.2 ± 2.1 a |
| 7_OS | 1619 ± 29 c | 1488 ± 23 b | 131 ± 8 | 1926 ± 30 c | 438 ± 8 | 65.2 ± 0.5 c |
| 7_OS+OA | 644 ± 14 e | 618 ± 15 g | 25 ± 1 | 968 ± 14 f | 349 ± 1 | 75.9 ± 6.5 b |
| Film Component | Water Vapor Capacity | Water Binding Capacity (%) | Solubility in Water |
|---|---|---|---|
| (10−8 g/s·m·kPa) | (%) | ||
| BS | 6.97 ± 0.06 d | 9.02 ± 0.05 b | 38.3 ± 1.8 bc |
| BS+OA | 7.41 ± 0.23 b | 7.94 ± 1.12 e | 36.0 ± 2.4 cd |
| 1_OS | 7.82 ± 0.29 ac | 7.58 ± 0.49 e | 40.9 ± 1.9 ab |
| 1_OS+OA | 8.00 ± 0.06 a | 4.64 ± 0.60 d | 32.5 ± 0.3 e |
| 3_OS | 7.95 ± 0.30 a | 7.44 ± 0.62 e | 40.3 ± 1.2 ab |
| 3_OS+OA | 7.52 ± 0.26 bc | 6.95 ± 0.62 ce | 34.1 ± 2.0 de |
| 5_OS | 7.92 ± 0.24 ab | 11.39 ± 1.33 a | 39.4 ± 2.4 b |
| 5_OS+OA | 7.78 ± 0.10 ac | 4.67 ± 0.26 d | 28.9 ± 2.0 f |
| 7_OS | 7.64 ± 0.28 bc | Nd * | 42.7 ± 2.2 a |
| 7_OS+OA | 7.28 ± 0.26 d | 6.08 ± 0.22 c | 33.0 ± 2.4 de |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Królikowska, K.; Pająk, P.; Pietrzyk, S.; Czaplak, K.; Strządała, K. Comprehensive Evaluation of OS Starch–Oleic Acid Mixtures: From Functional Properties to Their Application in Films with Improved Water Resistance. Molecules 2025, 30, 4411. https://doi.org/10.3390/molecules30224411
Królikowska K, Pająk P, Pietrzyk S, Czaplak K, Strządała K. Comprehensive Evaluation of OS Starch–Oleic Acid Mixtures: From Functional Properties to Their Application in Films with Improved Water Resistance. Molecules. 2025; 30(22):4411. https://doi.org/10.3390/molecules30224411
Chicago/Turabian StyleKrólikowska, Karolina, Paulina Pająk, Sławomir Pietrzyk, Karolina Czaplak, and Katarzyna Strządała. 2025. "Comprehensive Evaluation of OS Starch–Oleic Acid Mixtures: From Functional Properties to Their Application in Films with Improved Water Resistance" Molecules 30, no. 22: 4411. https://doi.org/10.3390/molecules30224411
APA StyleKrólikowska, K., Pająk, P., Pietrzyk, S., Czaplak, K., & Strządała, K. (2025). Comprehensive Evaluation of OS Starch–Oleic Acid Mixtures: From Functional Properties to Their Application in Films with Improved Water Resistance. Molecules, 30(22), 4411. https://doi.org/10.3390/molecules30224411






