Fungal Polysaccharides as Modulators of Molecular Pathways in Liver Health
Abstract
1. Introduction
2. Methodology
3. General Overview of Fungal Polysaccharides
3.1. Homoglycans
3.2. Protein-Bound Polysaccharides and Proteoglycans
3.3. Heteroglycans
4. Effect of the Fungal Polysaccharide for Hepatoprotection, Antioxidant Activity, and Anti-Inflammation Processes Through Regulation of TLR4/NF-κB Pathway
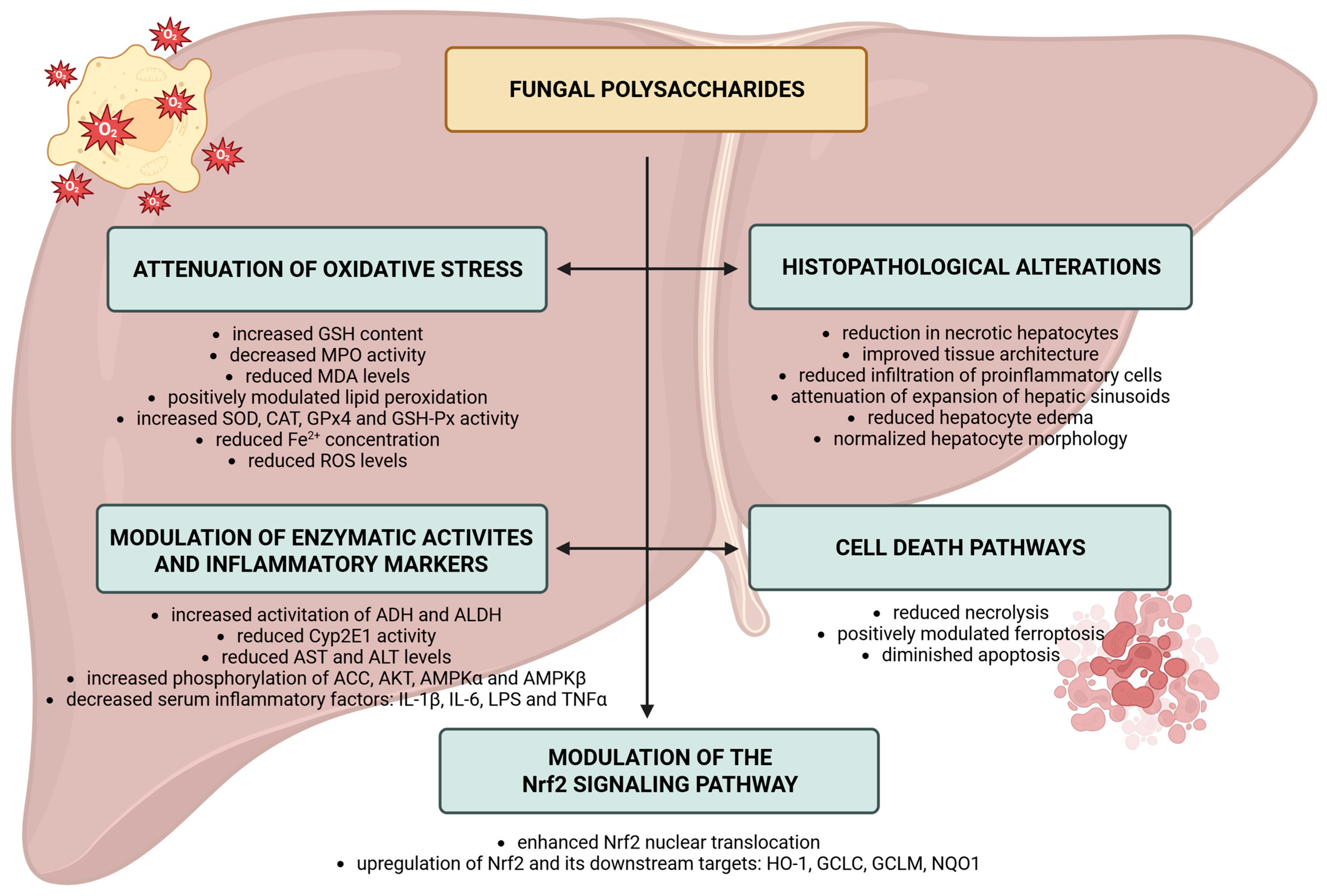
5. Effect of the Fungal Polysaccharide for Hepatoprotection, Antioxidant Activity, and Anti-Inflammation Processes Through Regulation of Nrf2 Pathway
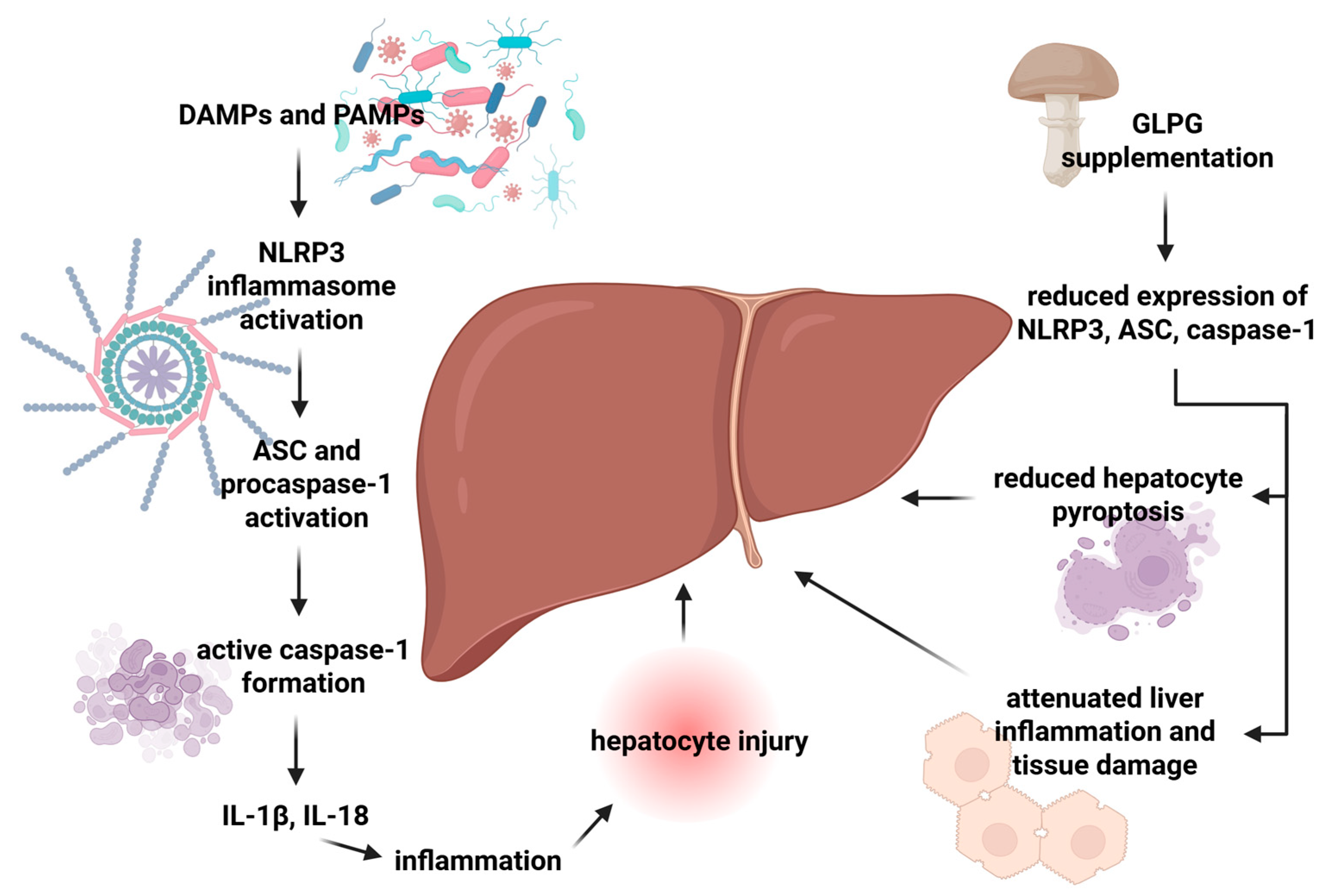
6. Effect of Fungal Polysaccharides on the NLRP3 Inflammasome
7. Challenges and Future Perspectives
7.1. Limitations
7.2. Prospects
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAP | Auricularia auricula polysaccharide |
| Acc | Acetyl-CoA Carboxylase |
| ACP | Antrodia cinnamomea polysaccharide |
| ADH | alcohol dehydrogenase |
| AKT | Protein kinase B |
| ALD | alcohol-related liver disease |
| ALDH | acetal dehydrogenase |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| APAP | Acetaminophen |
| AREs | antioxidant response elements |
| ASC | apoptosis-associated speck-like protein containing a caspase recruitment domain CARD |
| AST | aspartate aminotransferase |
| BAX | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| Cat | catalase |
| CCL4 | C-C Motif Chemokine Ligand 4 |
| Cd14 | Cluster of Differentiation 14 |
| ChNPs | chitosan-based nanoparticles |
| Cox2 | Cyclooxygenase-2 |
| CVP | Coriolus versicolor polysaccharide |
| Cyp | cytochrome P450 |
| DAMPs | damage-associated molecular patterns |
| DIP | Dictyophora indusiata polysaccharide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EnPs | endopolysaccharides |
| EPCM | Enzymatic-extractable polysaccharides from Cordyceps militaris |
| FPMPS | Fomitopsis pinicola mycelial polysaccharides |
| GCLC | Glutamate–Cysteine Ligase Catalytic Subunit |
| GCLM | Glutamate–Cysteine Ligase Modifier Subunit |
| GFP | Grifola frondosa polysaccharide |
| GGT | Gamma-Glutamyl Transferase |
| GLPG | Ganoderma lucidum proteoglycan |
| GPx4 | glutathione peroxidase 4 |
| GSH-Px | glutathione peroxidase |
| GSK3β | cascade glycogen synthase kinase 3 beta |
| HCC | hepatocellular carcinoma |
| HFD | high-fat diet |
| HO-1 | Heme Oxygenase-1 |
| HSCs | hepatic stellate cells |
| HSP | Hirsutella sinensis polysaccharide |
| IFNα | interferon α |
| IL | interleukin |
| IMPP | intracellular mycelium polysaccharides from Pleurotus geesteranus |
| iNos | Inducible Nitric Oxide Synthase |
| Ifnγ | Interferon gamma |
| IPS | intracellular polysaccharide |
| JNK | c-Jun N-terminal kinase |
| KCs | Kupffer cells |
| Lbp | Lipopolysaccharide Binding Protein |
| LDH | lactate dehydrogenase |
| LDL-C | low-density lipoprotein cholesterol |
| LPO | lipid peroxidation |
| LPS | lipopolysaccharide |
| LSECs | liver sinusoidal endothelial cells |
| MAFLD | metabolic dysfunction-associated fatty liver disease |
| MCP | Morchella esculenta polysaccharide |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MDA | malondialdehyde |
| MEP2 | Morchella esculenta polysaccharide 2 |
| MPO | myeloperoxidase |
| MyD88 | Myeloid differentiation primary response 88 |
| NASH | Non-Alcoholic Steatohepatitis |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-like receptor protein 3 |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| PAMPs | pathogen-associated molecular patterns |
| PCP | Poria cocos polysaccharides |
| Phps | Phellinus linteus polysaccharides |
| PINK1 | PTEN-indiced kinase 1 |
| PI3K | Phosphoinositide 3-Kinase |
| p-NF-κB p65 | phosphorylated NF-κB, p65 subunit |
| p-IκBα | phosphorylated inhibitor of kappa B alpha |
| Pparα | Peroxisome Proliferator-Activated Receptor Alpha |
| PRRs | pattern recognition receptors |
| PSK | polysaccharide-K |
| PSP | polysaccharopeptide |
| Ptp1b | Protein Tyrosine Phosphatase 1B |
| PUFAs | polyunsaturated fatty acids |
| RGD | Arginylglycylaspartic acid |
| ROS | reactive oxygen species |
| RR | response rates |
| Sirt1 | sirtuin 1 |
| Socs2 | Suppressor of Cytokine Signaling 2 |
| SOD | superoxide dismutase |
| T-AOC | Total Antioxidant Capacity |
| TLR4 | Toll-like receptor 4 |
| TNFα | tumor necrosis factor α |
| WEGL | polysaccharides from the water extract of Ganoderma lucidum |
References
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Schulze, R.J.; Schott, M.B.; Casey, C.A.; Tuma, P.L.; McNiven, M.A. The cell biology of the hepatocyte: A membrane trafficking machine. J. Cell Biol. 2019, 218, 2096–2112. [Google Scholar] [CrossRef] [PubMed]
- Gibert-Ramos, A.; Sanfeliu-Redondo, D.; Aristu-Zabalza, P.; Martínez-Alcocer, A.; Gracia-Sancho, J.; Guixé-Muntet, S.; Fernández-Iglesias, A. The Hepatic Sinusoid in Chronic Liver Disease: The Optimal Milieu for Cancer. Cancers 2021, 13, 5719. [Google Scholar] [CrossRef]
- Shah, V.; Haddad, F.G.; Garcia-Cardena, G.; Frangos, J.A.; Mennone, A.; Groszmann, R.J.; Sessa, W.C. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J. Clin. Investig. 1997, 100, 2923–2930. [Google Scholar] [CrossRef]
- Deleve, L.D.; Wang, X.; Guo, Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 2008, 48, 920–930. [Google Scholar] [CrossRef]
- Malik, R.; Selden, C.; Hodgson, H. The role of non-parenchymal cells in liver growth. Semin. Cell Dev. Biol. 2002, 13, 425–431. [Google Scholar] [CrossRef]
- Kostallari, E.; Shah, V.H. Pericytes in the Liver. Adv. Exp. Med. Biol. 2019, 1122, 153–167. [Google Scholar] [CrossRef]
- Winau, F.; Quack, C.; Darmoise, A.; Kaufmann, S.H. Starring stellate cells in liver immunology. Curr. Opin. Immunol. 2008, 20, 68–74. [Google Scholar] [CrossRef]
- Heymann, F.; Peusquens, J.; Ludwig-Portugall, I.; Kohlhepp, M.; Ergen, C.; Niemietz, P.; Martin, C.; van Rooijen, N.; Ochando, J.C.; Randolph, G.J.; et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015, 62, 279–291. [Google Scholar] [CrossRef]
- Løvdal, T.; Andersen, E.; Brech, A.; Berg, T. Fc receptor mediated endocytosis of small soluble immunoglobulin G immune complexes in Kupffer and endothelial cells from rat liver. J. Cell Sci. 2000, 113 Pt 18, 3255–3266. [Google Scholar] [CrossRef]
- Gregory, S.H.; Cousens, L.P.; van Rooijen, N.; Döpp, E.A.; Carlos, T.M.; Wing, E.J. Complementary adhesion molecules promote neutrophil-Kupffer cell interaction and the elimination of bacteria taken up by the liver. J. Immunol. 2002, 168, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Bilzer, M.; Roggel, F.; Gerbes, A.L. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006, 26, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; Masyuk, A.I.; Masyuk, T.V.; O’Hara, S.P.; LaRusso, N.F. Physiology of cholangiocytes. Compr. Physiol. 2013, 3, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Syal, G.; Fausther, M.; Dranoff, J.A. Advances in cholangiocyte immunobiology. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1077–G1086. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Flores, G.A.; Cusumano, G.; Venanzoni, R.; Angelini, P. The Glucans Mushrooms: Molecules of Significant Biological and Medicinal Value. Polysaccharides 2024, 5, 212–224. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Singh, A.; Saini, R.K.; Kumar, A.; Chawla, P.; Kaushik, R. Mushrooms as Nutritional Powerhouses: A Review of Their Bioactive Compounds, Health Benefits, and Value-Added Products. Foods 2025, 14, 741. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, W.; Zhang, Y. Fungal polysaccharides. Adv. Pharmacol. 2020, 87, 277–299. [Google Scholar] [CrossRef]
- Barcan, A.S.; Barcan, R.A.; Vamanu, E. Therapeutic Potential of Fungal Polysaccharides in Gut Microbiota Regulation: Implications for Diabetes, Neurodegeneration, and Oncology. J. Fungi 2024, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Song, X.; Li, X.; Jia, L.; Zhang, C. Structural characterization of Hericium erinaceus polysaccharides and the mechanism of anti-T2DM by modulating the gut microbiota and metabolites. Int. J. Biol. Macromol. 2023, 242, 125165. [Google Scholar] [CrossRef] [PubMed]
- Minemura, M.; Shimizu, Y. Gut microbiota and liver diseases. World J. Gastroenterol. 2015, 21, 1691–1702. [Google Scholar] [CrossRef]
- Schwenger, K.J.; Clermont-Dejean, N.; Allard, J.P. The role of the gut microbiome in chronic liver disease: The clinical evidence revised. JHEP Rep. 2019, 1, 214–226. [Google Scholar] [CrossRef]
- Kumla, J.; Thangrongthong, S.; Kaewnunta, A.; Suwannarach, N. Research advances in fungal polysaccharides: Production, extraction, characterization, properties, and their multifaceted applications. Front. Cell. Infect. Microbiol. 2025, 15, 1604184. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.-M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.X.; Liu, Y.H.; Chen, Y.; Chen, J.; Lu, C.H. Auricularia auriculaPolysaccharides Exert Anti-inflammatory Effects in Hepatic Fibrosis by the Gut-Liver Axis and Enhancing SCFA Metabolism. J. Agric. Food Chem. 2025, 73, 4617–4629. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, C.; Fan, Q.; Lin, X.; Wang, Y.; Azzam, M.; Alhotan, R.; Alqhtani, A.; Jiang, S. Antrodia cinnamomea polysaccharide improves liver antioxidant, anti-inflammatory capacity, and cecal flora structure of slow-growing broiler breeds challenged with lipopolysaccharide. Front. Vet. Sci. 2022, 9, 994782. [Google Scholar] [CrossRef]
- Izadi, H.; Asadi, H.; Bemani, M. Chitin: A comparison between its main sources. Front. Mater. 2025, 12, 1537067. [Google Scholar] [CrossRef]
- Tang, H.; Zha, Z.; Tan, Y.; Li, Y.; Jiao, Y.; Yang, B.; Xiong, Q.; Yin, H.; Wang, H. Extraction and characterization of polysaccharide from fermented mycelia of Coriolus versicolor and its efficacy for treating nonalcoholic fatty liver disease. Int. J. Biol. Macromol. 2023, 248, 125951. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, L.; Zeng, Z.; Zhang, Z.; Zheng, B.; Zhang, Y. Chemical structure and prebiotic activity of a Dictyophora indusiata polysaccharide fraction. Food Chem. 2025, 463, 141086. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure characterization of a novel polysaccharide from Dictyophora indusiata and its macrophage immunomodulatory activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, B.; Li, D.; Jia, L.; Yang, F. Enzymatic-extractable polysaccharides from Cordyceps militaris alleviate carbon tetrachloride-induced liver injury via Nrf2/ROS/NF-κB signaling pathway. J. Funct. Foods 2022, 95, 105152. [Google Scholar] [CrossRef]
- Karunarathna, S.C.; Patabendige, N.M.; Kumla, J.; Hapuarachchi, K.K.; Suwannarach, N. The bioactive compounds, beneficial medicinal properties, and biotechnological prospects of Fomitopsis: A comprehensive overview. Front. Cell. Infect. Microbiol. 2025, 15, 1534617. [Google Scholar] [CrossRef]
- Cheng, J.-J.; Lin, C.-Y.; Lur, H.-S.; Chen, H.-P.; Lu, M.-K. Properties and biological functions of polysaccharides and ethanolic extracts isolated from medicinal fungus, Fomitopsis pinicola. Process Biochem. 2008, 43, 829–834. [Google Scholar] [CrossRef]
- Li, X.; Zeng, F.; Huang, Y.; Liu, B. The Positive Effects of Grifola frondosa Heteropolysaccharide on NAFLD and Regulation of the Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5302. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Y.; Zhu, H.; Liu, X.; Xue, M.; Yang, G.; Chen, Y.; Chen, S.; Wen, Z. In Vitro Gastrointestinal Digestion of Grifola frondosa Polysaccharides and Their Enhancement of GABA Production via Gut Microbiota Modulation. Nutrients 2025, 17, 3332. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Lan, J.; Wang, Y.; Pan, Y.; Song, T.; Liu, S.; Gu, Z.; Ge, Y. Structure characterization of Grifola frondosa polysaccharide and its effect on insulin resistance in HFD-fed mice. NPJ Sci. Food 2025, 9, 3. [Google Scholar] [CrossRef]
- Yang, X.J.; Liu, J.; Ye, L.B.; Yang, F.; Ye, L.; Gao, J.R.; Wu, Z.H. In vitro and in vivo protective effects of proteoglycan isolated from mycelia of Ganoderma lucidum on carbon tetrachloride-induced liver injury. World J. Gastroenterol. 2006, 12, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Zhao, Y. Possible mechanism underlying the antiherpetic activity of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. J. Biochem. Mol. Biol. 2005, 38, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Rong, L.; Zhang, X.; Li, G.; Wang, Q.; Li, C.; Xiao, Y.; Wei, L.; Bi, H. Hirsutella sinensis mycelium polysaccharides attenuate the TGF-β1-induced epithelial-mesenchymal transition in human intrahepatic bile duct epithelial cells. Int. J. Biol. Macromol. 2024, 254, 127834. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Liu, X.C.; Fang, X.N.; Sun, H.Q.; Yang, X.Y.; Zhang, Y.M. Structural characterization and anti-tumor activity of polysaccharide produced by Hirsutella sinensis. Int. J. Biol. Macromol. 2016, 82, 959–966. [Google Scholar] [CrossRef]
- Song, X.; Shen, Q.; Liu, M.; Zhang, C.; Zhang, L.; Ren, Z.; Wang, W.; Dong, Y.; Wang, X.; Zhang, J.; et al. Antioxidant and hepatoprotective effects of intracellular mycelium polysaccharides from Pleurotus geesteranus against alcoholic liver diseases. Int. J. Biol. Macromol. 2018, 114, 979–988. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L. Physicochemical properties and antitumor activities for sulfated derivatives of lentinan. Carbohydr. Res. 2009, 344, 2209–2216. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Zhang, Y.; Yin, Z.; Zhang, S.; Zhao, Z.; Duan, Y. Hepatoprotective effects of polysaccharide from Morchella esculenta are associated with activation of the AMPK/Sirt1 signaling pathway in mice with NAFLD. Int. J. Biol. Macromol. 2025, 301, 140444. [Google Scholar] [CrossRef]
- Teng, S.; Zhang, Y.; Jin, X.; Zhu, Y.; Li, L.; Huang, X.; Wang, D.; Lin, Z. Structure and hepatoprotective activity of Usp10/NF-κB/Nrf2 pathway-related Morchella esculenta polysaccharide. Carbohydr. Polym. 2023, 303, 120453. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef]
- Song, X.; Sun, W.; Cui, W.; Jia, L.; Zhang, J. A polysaccharide of PFP-1 from Pleurotus geesteranus attenuates alcoholic liver diseases via Nrf2 and NF-κB signaling pathways. Food Funct. 2021, 12, 4591–4605. [Google Scholar] [CrossRef] [PubMed]
- Kou, F.; Mei, Y.; Wang, W.; Wei, X.; Xiao, H.; Wu, X. Phellinus linteus polysaccharides: A review on their preparation, structure-activity relationships, and drug delivery systems. Int. J. Biol. Macromol. 2024, 258, 128702. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Liaw, C.-C.; Pan, S.-Z.; Yang, H.-C.; Ng, L.-T. Phellinus linteus polysaccharides and their immunomodulatory properties in human monocytic cells. J. Funct. Foods 2013, 5, 679–688. [Google Scholar] [CrossRef]
- Karácsonyi, Š.; Kuniak, Ľ. Polysaccharides of Pleurotus ostreatus: Isolation and structure of pleuran, an alkali-insoluble β-d-glucan. Carbohydr. Polym. 1994, 24, 107–111. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Kujawowicz, K.; Witkowska, A.M. Beta-Glucans from Fungi: Biological and Health-Promoting Potential in the COVID-19 Pandemic Era. Nutrients 2021, 13, 3960. [Google Scholar] [CrossRef]
- Sun, C.; Rosendahl, A.H.; Wang, X.D.; Wu, D.Q.; Andersson, R. Polysaccharide-K (PSK) in cancer—Old story, new possibilities? Curr. Med. Chem. 2012, 19, 757–762. [Google Scholar] [CrossRef]
- Ng, T.B. A review of research on the protein-bound polysaccharide (polysaccharopeptide, PSP) from the mushroom Coriolus versicolor (Basidiomycetes: Polyporaceae). Gen. Pharmacol. 1998, 30, 1–4. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef]
- Camilli, G.; Tabouret, G.; Quintin, J. The Complexity of Fungal β-Glucan in Health and Disease: Effects on the Mononuclear Phagocyte System. Front. Immunol. 2018, 9, 673. [Google Scholar] [CrossRef]
- Meng, Y.; Lyu, F.; Xu, X.; Zhang, L. Recent Advances in Chain Conformation and Bioactivities of Triple-Helix Polysaccharides. Biomacromolecules 2020, 21, 1653–1677. [Google Scholar] [CrossRef]
- Roszczyk, A.; Turło, J.; Zagożdżon, R.; Kaleta, B. Immunomodulatory Properties of Polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qu, Y.; Li, H.; Tang, S.; Chen, C.; Wang, Y.; Wang, H. Improved Extraction Yield, Water Solubility, and Antioxidant Activity of Lentinan from Lentinula edodes via Bacillus subtilis natto Fermentation. Fermentation 2023, 9, 333. [Google Scholar] [CrossRef]
- Muszyńska, B.; Pazdur, P.; Lazur, J.; Sułkowska-Ziaja, K. Lentinula edodes (Shiitake)—Biological activity. Med. Int. Rev. 2017, 28, 189–195. [Google Scholar]
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B. Lentinus edodes: A macrofungus with pharmacological activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef]
- Ahmad, I.; Arif, M.; Xu, M.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]
- Bugajewski, M.; Angerhoefer, N.; Pączek, L.; Kaleta, B. Lentinula edodes as a Source of Bioactive Compounds with Therapeutical Potential in Intestinal Inflammation and Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 3320. [Google Scholar] [CrossRef]
- Zhang, L.N.; Zhang, X.; Zhou, Q.; Zhang, P.Y.; Zhang, M.; Li, X.L. Triple Helix of β-D-Glucan from Lentinus edodes in 0.5 M NaCl Aqueous Solution Characterized by Light Scattering. Polym. J. 2001, 33, 317–321. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Zhang, L. Thermally induced conformation transition of triple-helical lentinan in NaCl aqueous solution. J. Phys. Chem. B 2008, 112, 10343–10351. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhou, Q.; Zhang, X.; Chen, R. Transition from Triple Helix to Coil of Lentinan in Solution Measured by SEC, Viscometry, and 13C NMR. Polym. J. 2002, 34, 443–449. [Google Scholar] [CrossRef]
- Chihara, G.; Maeda, Y.; Hamuro, J.; Sasaki, T.; Fukuoka, F. Inhibition of Mouse Sarcoma 180 by Polysaccharides from Lentinus edodes (Berk.) Sing. Nature 1969, 222, 687–688. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Zhang, L.; Tian, Q. Chapter Thirteen—Mushroom Polysaccharide Lentinan for Treating Different Types of Cancers: A Review of 12 Years Clinical Studies in China. In Progress in Molecular Biology and Translational Science; Zhang, L., Ed.; Academic Press: Oxford, UK, 2019; Volume 163, pp. 297–328. [Google Scholar]
- Zhang, Y.; Li, Q.; Wang, J.; Cheng, F.; Huang, X.; Cheng, Y.; Wang, K. Polysaccharide from Lentinus edodes combined with oxaliplatin possesses the synergy and attenuation effect in hepatocellular carcinoma. Cancer Lett. 2016, 377, 117–125. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Li, Y.D.; Zhao, S.Y.; Zhang, D.J.; Zhao, Z.H.; Wang, Y.B. Effects of tumor-specific antigen induced by lentinan on murine H22 hepatocellular carcinoma immunoprophylaxis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4516–4524. [Google Scholar]
- You, J.; Wu, Q.; Li, Y.; Li, X.; Lin, Z.; Huang, J.; Xue, Y.; Gulimiran, A.; Pan, Y. Lentinan induces apoptosis of mouse hepatocellular carcinoma cells through the EGR1/PTEN/AKT signaling axis. Oncol. Rep. 2023, 50, 142. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, W.; Xie, Q.; Zhan, Y.; Wu, B. Lentinan reduces tumor progression by enhancing gemcitabine chemotherapy in urothelial bladder cancer. Surg. Oncol. 2015, 24, 28–34. [Google Scholar] [CrossRef]
- Harada, K.; Itashiki, Y.; Takenawa, T.; Ueyama, Y. Effects of lentinan alone and in combination with fluoropyrimidine anticancer agent on growth of human oral squamous cell carcinoma in vitro and in vivo. Int. J. Oncol. 2010, 37, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jia, Y.; Sun, X.; Li, H.; Yin, M.; Dai, L.; Han, L.; Wang, L.; Qian, M.; Du, J.; et al. The Dectin-1 Receptor Signaling Pathway Mediates the Remyelination Effect of Lentinan through Suppression of Neuroinflammation and Conversion of Microglia. J. Immunol. Res. 2022, 2022, 3002304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Zhao, Y.; Zong, S.; Tian, Y.; Chen, S.; Li, M.; Liu, H.; Zhang, Q.; Jing, X.; et al. Therapeutic effects of lentinan on inflammatory bowel disease and colitis-associated cancer. J. Cell. Mol. Med. 2019, 23, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Zhang, C.; Cai, Z.; Chen, L.; Chen, Z.; Zhao, K.; Qiao, S.; Wang, Y.; Meng, L.; et al. Anti-Influenza Effect and Mechanisms of Lentinan in an ICR Mouse Model. Front. Cell. Infect. Microbiol. 2022, 12, 892864. [Google Scholar] [CrossRef]
- Melanouri, E.-M.; Diamantis, I.; Dedousi, M.; Dalaka, E.; Antonopoulou, P.; Papanikolaou, S.; Politis, I.; Theodorou, G.; Diamantopoulou, P. Pleurotus ostreatus: Nutritional Enhancement and Antioxidant Activity Improvement Through Cultivation on Spent Mushroom Substrate and Roots of Leafy Vegetables. Fermentation 2025, 11, 20. [Google Scholar] [CrossRef]
- Jesenak, M.; Majtan, J.; Rennerova, Z.; Kyselovic, J.; Banovcin, P.; Hrubisko, M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 2013, 15, 395–399. [Google Scholar] [CrossRef]
- Bergendiova, K.; Tibenska, E.; Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 2011, 111, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Urbancikova, I.; Hudackova, D.; Majtan, J.; Rennerova, Z.; Banovcin, P.; Jesenak, M. Efficacy of Pleuran (β-Glucan from Pleurotus ostreatus) in the Management of Herpes Simplex Virus Type 1 Infection. Evid. Based Complement. Alternat Med. 2020, 2020, 8562309. [Google Scholar] [CrossRef] [PubMed]
- Bobek, P.; Ginter, E.; Jurcovicová, M.; Ozdín, L.; Mekinová, D. Effect of oyster fungus (Pleurotus ostreatus) on serum and liver lipids of Syrian hamsters with a chronic alcohol intake. Physiol. Res. 1991, 40, 327–332. [Google Scholar] [PubMed]
- Llauradó, G.; Beltrán, Y.; Morris-Quevedo, H.; Marcos, E.; Díaz, U.; Marcos, J.; García Díaz, J.; Disotuar, D.; Cos, P. Restoration of liver function in malnourished mice orally administered with Pleurotus ostreatus fruiting bodies extract. J. Pharm. Pharmacogn. Res. 2020, 8, 32–42. [Google Scholar] [CrossRef]
- Wijesekara, T.; Xu, B. New Insights into Sources, Bioavailability, Health-Promoting Effects, and Applications of Chitin and Chitosan. J. Agric. Food Chem. 2024, 72, 17138–17152. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Chitin and chitinase: Role in pathogenicity, allergenicity and health. Int. J. Biol. Macromol. 2017, 97, 331–338. [Google Scholar] [CrossRef]
- Kandile, N.G.; Mohamed, H.M.; Nasr, A.S. Novel hydrazinocurcumin derivative loaded chitosan, ZnO, and au nanoparticles formulations for drug release and cell cytotoxicity. Int. J. Biol. Macromol. 2020, 158, 1216–1226. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Karimi, K.; Mojtabavi, S.; Tehrany, P.M.; Nejad, M.M.; Rezaee, A.; Mohtashamian, S.; Hamedi, E.; Yousefi, F.; Salmani, F.; Zandieh, M.A.; et al. Chitosan-based nanoscale delivery systems in hepatocellular carcinoma: Versatile bio-platform with theranostic application. Int. J. Biol. Macromol. 2023, 242, 124935. [Google Scholar] [CrossRef]
- Chen, J.; Sun, T.; You, Y.; Wu, B.; Wang, X.; Wu, J. Proteoglycans and Glycosaminoglycans in Stem Cell Homeostasis and Bone Tissue Regeneration. Front. Cell Dev. Biol. 2021, 9, 760532. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Chang, Y.; Zhang, L. Coriolus versicolor polysaccharopeptide as an immunotherapeutic in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Kleinschek, K.S.; Kargl, R. Polysaccharide peptide conjugates: Chemistry, properties and applications. Carbohydr. Polym. 2022, 280, 118875. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar]
- Yunoki, S.; Tanaka, N.; Hizuta, A.; Orita, K. Enhancement of antitumor cytotoxicity of hepatic lymphocytes by oral administration of PSK. Int. J. Immunopharmacol. 1994, 16, 123–130. [Google Scholar] [CrossRef]
- Hu, T.; Shen, L.; Huang, Q.; Wu, C.; Zhang, H.; Zeng, Q.; Wang, G.; Wei, S.; Zhang, S.; Zhang, J.; et al. Protective Effect of Dictyophora Polysaccharides on Sodium Arsenite-Induced Hepatotoxicity: A Proteomics Study. Front Pharmacol 2021, 12, 749035. [Google Scholar] [CrossRef]
- Hu, T.; Lu, J.; Wu, C.; Duan, T.; Luo, P. Dictyophora Polysaccharide Attenuates As-Mediated PINK1/Parkin Pathway-Induced Mitophagy in L-02 Cell through Scavenging ROS. Molecules 2022, 27, 2806. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, K.; Pan, D.; Pan, X.; Yang, H.; Xiao, J.; Shen, X.; Luo, P. Inhibition Effect of Dictyophora Polysaccharides on Human Hepatocellular Carcinoma Cell Line HCC-LM3. Med. Sci. Monit. 2020, 26, e918870. [Google Scholar] [CrossRef]
- Yan, X.; Chen, X.; Zhang, X.; Qureshi, A.; Wang, Y.; Tang, X.; Hu, T.; Zhuang, H.; Ran, X.; Ma, G.; et al. Proteomic analysis of the effects of Dictyophora polysaccharide on arsenic-induced hepatotoxicity in rats. Exp. Mol. Pathol. 2024, 138, 104910. [Google Scholar] [CrossRef]
- Soares, A.A.; de Sá-Nakanishi, A.B.; Bracht, A.; da Costa, S.M.; Koehnlein, E.A.; de Souza, C.G.; Peralta, R.M. Hepatoprotective effects of mushrooms. Molecules 2013, 18, 7609–7630. [Google Scholar] [CrossRef]
- Fei, N.; Bruneau, A.; Zhang, X.; Wang, R.; Wang, J.; Rabot, S.; Gérard, P.; Zhao, L. Endotoxin Producers Overgrowing in Human Gut Microbiota as the Causative Agents for Nonalcoholic Fatty Liver Disease. mBio 2020, 11, e03263-19. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tan, W.; Huang, J.; Li, Q.; Wang, J.; Su, H.; Guo, C.; Liu, H. Small intestinal bacterial overgrowth and metabolic dysfunction-associated steatotic liver disease. Front. Nutr. 2024, 11, 1502151. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, Y.; Dai, X.; Wang, B.; Zhang, J.; Cao, H. Polysaccharides: The Potential Prebiotics for Metabolic Associated Fatty Liver Disease (MAFLD). Nutrients 2023, 15, 3722. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.X.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.F.; Ni, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef]
- Zou, P.; Li, X.; Wang, L.; She, Y.; Xiao, C.; Peng, Y.; Qian, X.; Luo, P.; Wei, S. Grifola frondosa Polysaccharide Ameliorates Inflammation by Regulating Macrophage Polarization of Liver in Type 2 Diabetes Mellitus Rats. Mol. Nutr. Food Res. 2024, 68, e2400392. [Google Scholar] [CrossRef]
- Jiang, T.; Shen, S.; Wang, L.; Zhao, M.; Li, Y.; Huang, S. Grifola frondosa Polysaccharide Ameliorates Early Diabetic Nephropathy by Suppressing the TLR4/NF-κB Pathway. Appl. Biochem. Biotechnol. 2022, 194, 4093–4104. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chen, Q.Z.; Wang, Z.J.; Hua, C. Anti-Inflammatory and Hepatoprotective Effects of Ganoderma lucidum Polysaccharides against Carbon Tetrachloride-Induced Liver Injury in Kunming Mice. Pharmacology 2019, 103, 143–150. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Wang, Y.; Fang, L.; Guo, C.; Sang, T.; Peng, H.; Zhao, Q.; Chen, S.; Lin, X.; et al. Ganoderma lucidum polysaccharide inhibits HSC activation and liver fibrosis via targeting inflammation, apoptosis, cell cycle, and ECM-receptor interaction mediated by TGF-β/Smad signaling. Phytomedicine 2023, 110, 154626. [Google Scholar] [CrossRef]
- Zhang, N.; Han, Z.; Zhang, R.; Liu, L.; Gao, Y.; Li, J.; Yan, M. Ganoderma lucidum Polysaccharides Ameliorate Acetaminophen-Induced Acute Liver Injury by Inhibiting Oxidative Stress and Apoptosis along the Nrf2 Pathway. Nutrients 2024, 16, 1859. [Google Scholar] [CrossRef]
- Yang, Y.; Song, S.; Nie, Y.; Chen, R.; Chen, P. Lentinan alleviates arsenic-induced hepatotoxicity in mice via downregulation of OX40/IL-17A and activation of Nrf2 signaling. BMC Pharmacol. Toxicol. 2022, 23, 16. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, M.; Zhou, M.; Zhou, L.; Ge, X.; Pang, N.; Li, H.; Li, X.; Li, M.; Zhang, J.; et al. Lentinan Supplementation Protects the Gut-Liver Axis and Prevents Steatohepatitis: The Role of Gut Microbiota Involved. Front. Nutr. 2021, 8, 803691. [Google Scholar] [CrossRef]
- Wang, K.L.; Lu, Z.M.; Mao, X.; Chen, L.; Gong, J.S.; Ren, Y.; Geng, Y.; Li, H.; Xu, H.Y.; Xu, G.H.; et al. Structural characterization and anti-alcoholic liver injury activity of a polysaccharide from Coriolus versicolor mycelia. Int. J. Biol. Macromol. 2019, 137, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Geng, Y.; Chen, H.; Lu, Z.-M.; Shi, J.-S.; Xu, Z. Polysaccharide peptides from Coriolus versicolor: A multi-targeted approach for the protection or prevention of alcoholic liver disease. J. Funct. Foods 2018, 40, 769–777. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Zhou, S. Poria cocos polysaccharides improve alcoholic liver disease by interfering with ferroptosis through NRF2 regulation. Aging 2024, 16, 6147–6162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Wang, X.Z. Interleukin-10 and chronic liver disease. World J. Gastroenterol. 2006, 12, 1681–1685. [Google Scholar] [CrossRef]
- Durum, S.K.; Mazzucchelli, R.I. Live from the liver: Hepatocyte IL-7. Immunity 2009, 30, 320–321. [Google Scholar] [CrossRef]
- Pan, C.X.; Tang, J.; Wang, X.Y.; Wu, F.R.; Ge, J.F.; Chen, F.H. Role of interleukin-22 in liver diseases. Inflamm. Res. 2014, 63, 519–525. [Google Scholar] [CrossRef]
- Kanwal, S.; Aliya, S.; Xin, Y. Anti-Obesity Effect of Dictyophora indusiata Mushroom Polysaccharide (DIP) in High Fat Diet-Induced Obesity via Regulating Inflammatory Cascades and Intestinal Microbiome. Front. Endocrinol. 2020, 11, 558874. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, L.; Li, Z.; Jin, M.; Wang, Q.; Cheng, J.; Li, J.; Feng, H. Phellinus linteus polysaccharides mediates acetaminophen-induced hepatotoxicity via activating AMPK/Nrf2 signaling pathways. Aging 2022, 14, 6993–7002. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Park, J.S.; Rustamov, N.; Roh, Y.S. The Roles of NFR2-Regulated Oxidative Stress and Mitochondrial Quality Control in Chronic Liver Diseases. Antioxidants 2023, 12, 1928. [Google Scholar] [CrossRef] [PubMed]
- Petsouki, E.; Cabrera, S.N.S.; Heiss, E.H. AMPK and NRF2: Interactive players in the same team for cellular homeostasis? Free Radic. Biol. Med. 2022, 190, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Peng, H.; Dong, L.; Chen, L.; Ma, X.; Peng, Y.; Dai, S.; Liu, Q. Activation of the NRF2-ARE signalling pathway by the Lentinula edodes polysaccharose LNT alleviates ROS-mediated cisplatin nephrotoxicity. Int. Immunopharmacol. 2016, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Huo, R.; Wang, Y.; Ma, N.; Shi, X.; Shen, X.; Chang, G. Lentinan inhibits oxidative stress and alleviates LPS-induced inflammation and apoptosis of BMECs by activating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 2022, 222, 2375–2391. [Google Scholar] [CrossRef]
- Charan, H.V.; Dwivedi, D.K.; Khan, S.; Jena, G. Mechanisms of NLRP3 inflammasome-mediated hepatic stellate cell activation: Therapeutic potential for liver fibrosis. Genes Dis. 2023, 10, 480–494. [Google Scholar] [CrossRef]
- Huang, T.T.; Ojcius, D.M.; Young, J.D.; Wu, Y.H.; Ko, Y.F.; Wong, T.Y.; Wu, C.Y.; Lu, C.C.; Lai, H.C. The anti-tumorigenic mushroom Agaricus blazei Murill enhances IL-1β production and activates the NLRP3 inflammasome in human macrophages. PLoS ONE 2012, 7, e41383. [Google Scholar] [CrossRef]
- Li, H.; Feng, J.; Liu, C.; Hou, S.; Meng, J.; Liu, J.Y.; Zilong, S.; Chang, M.C. Polysaccharides from an edible mushroom, Hericium erinaceus, alleviate ulcerative colitis in mice by inhibiting the NLRP3 inflammasomes and reestablish intestinal homeostasis. Int. J. Biol. Macromol. 2024, 167, 131251. [Google Scholar] [CrossRef]
- Li, J.; Qu, C.; Li, F.; Chen, Y.; Zheng, J.; Xiao, Y.; Jin, Q.; Jin, G.; Huang, X.; Jin, D. Inonotus obliquus Polysaccharide Ameliorates Azoxymethane/Dextran Sulfate Sodium-Induced Colitis-Associated Cancer in Mice via Activation of the NLRP3 Inflammasome. Front. Pharmacol. 2021, 11, 621835. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, H.; Ren, C.; Chen, K.; Luo, S.; Wang, Q.; Guo, M.; Xu, Y.; Hu, M.; Fang, J.; et al. Inflammation unleashed: The role of pyroptosis in chronic liver diseases. Int. Immunopharmacol. 2024, 141, 113006. [Google Scholar] [CrossRef]
- Yang, B.; Xu, Y.; Zhang, W.; Zhu, D.; Huang, B.; Yang, Y.; Jia, X.; Feng, L. Oral absorption mechanisms of polysaccharides and potential as carriers for the construction of nano-delivery systems: A review. Int. J. Biol. Macromol. 2025, 310, 143184. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, H.; Wen, A.C.A.; Wang, S.; Zhang, L.; Yang, H.; Mao, Y.; Jia, J.; Wang, D.; Wang, J.; et al. Combined Mulberry Leaf Polysaccharide-Caged Liposomes for Effective Oral Drug Delivery in Rat Model. Int. J. Nanomed. 2025, 20, 5377–5391. [Google Scholar] [CrossRef]
- Sun, H.; Nai, J.; Deng, B.; Zheng, Z.; Chen, X.; Zhang, C.; Sheng, H.; Zhu, L. Angelica sinensis Polysaccharide-Based Nanoparticles for Liver-Targeted Delivery of Oridonin. Molecules 2024, 29, 731. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Allela, O.Q.B.; Hussein, A.M.; Mustafa, M.A.; Kaur, M.; Alaraj, M.; Al-Hussainy, A.F.; Radi, U.K.; Ubaid, M.; Idan, A.H.; et al. Recent advances in polysaccharide-based drug delivery systems for cancer therapy: A comprehensive review. Artif. Cells Nanomed. Biotechnol. 2024, 52, 564–586. [Google Scholar] [CrossRef]
- Liu, G.; Ling, J.; He, L.; Xu, Y.; Chen, T.; Shi, C.; Luo, L. Theranostic Cancer Treatment Using Lentinan-Coated Selenium Nanoparticles and Label-Free CEST MRI. Pharmaceutics 2022, 15, 120. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, W.; Song, H.; Hu, Y.; Li, W. Beyond biologics: Fungal polysaccharides as dual-function materials for nanomedicine. Nanomedicine 2025, 20, 2275–2289. [Google Scholar] [CrossRef] [PubMed]

| Polysaccharide | Family | Fungi | Structure/Composition | Molecular Weight | References |
|---|---|---|---|---|---|
| Auricularia auricula polysaccharide (AAP) | heteropolysaccharide | Auricularia auricula | monosaccharides: glucose, arabinose, fucose, mannose, rhamnose, galactose, xylose | 1.63 × 106 Da | [29] |
| Antrodia cinnamomea polysaccharide (ACP) | heteropolysaccharide | Antrodia cinnamomea | D-glucan (76.3%) | - | [30] |
| Chitin | homoglycan | Mucor rouxii Aspergillus niger Lentinus edodes | N-acetyl-D-glucosamine and D-glucosamine units | 50–300 kDa | [31] |
| Coriolus versicolor polysaccharide (CVP) | heteropolysaccharide | Coriolus versicolor | monosaccharides: mannose, glucose, galactose, xylose, fucose; glucuronic acid (→1)-β-D-Man-(6,4→1)-α-D-Gal-(3→1)-α-D-Man-(4→1)-α-D-Gal-(6→) backbone [(→1)-α-D-Glc-(6→1)-α-D-Man-(4,3→1)-β-D-Xyl-(2→1)-β-D-Glc] (O-6 position) and [(→1)-α-D-Fuc-(4→1)-α-D-Man] (O-4 position) branches | 17,478 Da | [32] |
| Dictyophora indusiata polysaccharide (DIP) | heteropolysaccharide | Dictyophora indusiata | monosaccharides: glucose, galactose, mannose, xylose; (→3)-Glcp-(1→, →4)-Glcp-(1→, →3,4)-Glcp-(→1→, →3,4)-Galp-(1→); branches at (→6)-Manp-(1→) | 1132 Da | [33,34] |
| Cordyceps militaris polysaccharides (EPCM) | heteropolysaccharide | Cordyceps militaris | monosaccharides: mannose, ribose, rhamnose, glucuronic acid, galacturonic acid, N-acetyl-glucosamine, N-acetyl-galactosamine, glucose, galactose, xylose, arabinose, fucose (→3)-α-L-Fucp(1→, →4)-α-D-Glcp-(1→, →2,6)-α-D-Galp-(1→, →3)-α-Glcp-(1→, →6)-β-D-Galp-(1→) and β-D-Manp-(1→) | 20,792 Da | [35] |
| Fomitopsis pinicola mycelial polysaccharides (FPMPS) | heteropolysaccharide | Fomitopsis pinicola | myo-inositol, fucose, galactose, glucose, mannose, fructose | - | [36,37] |
| Grifola frondosa polysaccharides (GFPs) | heteropolysaccharide | Grifola frondosa | varies with fraction monosaccharides: predominantly mannose, glucosamine, glucose, galactose, fucose GFP-N1:1 →3, 1→4, and 1→6 glycosidic bonds GFP-N2:1→2, 1→3, 1→4, and 1→6 glycosidic bonds | varies with fraction GFP-N1: 3.323 × 103 kDa GFP-N2: 10.8 kDa | [38,39,40] |
| Ganoderma lucidum proteoglycan (GLPG) | proteoglycan | Ganoderma lucidum | carbohydrate: protein ratio of 10.4:1 | - | [41,42] |
| Hirsutella sinensis polysaccharides (HSP) | heteropolysaccharide | Hirsutella sinensis | varies with fraction HSWP-1a: α-(1,4)-D-glucan HSWP-1b: mainly mannoglucans with a 1,4-Glc/1,4,6-Man backbone and 1-linked Glc side chains at O-6 of 1,4-Glc HSWP-1c: mainly galactomannoglucans HSWP-1d: mainly mannoglucans with a 1,4-Glc/1,4,6-Man backbone and 1-linked Glc side chains at O-6 of 1,4-Glc HSP-III: mannose, galactose, rhamnose, arabinose, xylose, glucose; majorly composed of (1→3) glucose | varies with fraction fraction H1: >300 kDa fraction HSP-III: 513.90 kDa | [43,44,45] |
| Intracellular mycelium polysaccharides from Pleurotus geesteranus (IMPP) | heteropolysaccharide | Pleurotus geesteranus | monosaccharides: fucose, arabinose, xylose, mannose, galactose, glucose | - | [46] |
| Lentinan | β-glucan | Lentinula edodes | β(1→3) backbone of D-glucose units with two β(1→6) D-glucosyl residues | 146–504 kDa | [47] |
| Morchella exculenta polysaccharide (MCP) | heteropolysaccharide | Morchella exculenta | glucose, mannose, galactose | 1.69 × 105 Da | [48] |
| Morchella esculenta polysaccharide 2 (MEP2) | heteropolysaccharide | Morchella esculenta | monosaccharides: glucose, galactose, mannose, glucuronic acid (→4)-α-D-Glcp-(1→) glucan backbone with α-D-Glcp-(1→4)-α-D-Glcp-(1→) residue and an α-D-Glcp-(1→) residue at H-6 position | 959 kDa | [49] |
| Poria cocos polysaccharides (PCP) | β-glucan | Poria cocos | monosaccharides: glucose, fucose, arabinose, xylose, mannose, galactose β-(1→3)-linked glucose backbone with β-(1→6)-linked glucose side chains | 4.1 × 104 to 5 × 106 Da | [50] |
| Polysaccharide isolated from Pleuroteus geestranus (PFP-1) | heteropolysaccharide | Pleuroteus geestranus | monosaccharides: fucose, arabinose, galactose, glucose, xylose, mannose, ribose pyranose-polysaccharide in a triple-helical conformation linked by t-β-Glcp, 1,6-α-Glcp and 1,2,6-α-Galp | 15.5 kDa | [51] |
| Phellinus linteus polysaccharides (Phps) | heteropolysaccharide | Phellinus linteus | monosaccharides: glucose, mannose, galactose, N-acetylglucosamine β-(1→3) glycosidic bonds in backbone with (1→6) branches | 22–1700 kDa | [52,53] |
| Pleuran | β-glucan | Pleurotus ostreatus | β-(1→3)-linked D-glucopyranosyl units branched at the O-6 position every fourth glucose residue | 600–700 kDa | [54,55] |
| Polysaccharide-K (PSK) | Protein-bound polysaccharide | Trametes versicolor | mainly glucose with minor amounts of mannose, fucose, xylose, and galactose peptide fraction: glutamic acid, aspartic acid, leucine, valine, threonine, serine, glycine β-glucan backbone with (1→4) linkages, branched at the 3- and 6-positions, and incorporates (1→3) and (1→6) glucopyranosidic bonds | 94 kDa | [56] |
| Polysaccharopeptide (PSP) | Protein-bound polysaccharide | Trametes versicolor (syn. Coriolus versicolor) | glucose with predominant α-1,4 and β-1,3 glucosidic linkages; arabinose and rhamnose peptide fraction: rich in aspartic acid and glutamic acid | 100 kDa | [57] |
| Polysaccharides extracted from the water extract of Ganoderma lucidum (WEGL) | heteropolysaccharide | Ganoderma lucidum | G1: mannose, glucose, galactose, glucoctosamine, arabinose, galactosamine, rhamnose, fucose | varies with fraction G1: high molecular weight polysaccharides (>300 kDa) G2: 190,399 kDa G3: <10 kDa | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szelenberger, R.; Więckowska, M. Fungal Polysaccharides as Modulators of Molecular Pathways in Liver Health. Molecules 2025, 30, 4384. https://doi.org/10.3390/molecules30224384
Szelenberger R, Więckowska M. Fungal Polysaccharides as Modulators of Molecular Pathways in Liver Health. Molecules. 2025; 30(22):4384. https://doi.org/10.3390/molecules30224384
Chicago/Turabian StyleSzelenberger, Rafał, and Magdalena Więckowska. 2025. "Fungal Polysaccharides as Modulators of Molecular Pathways in Liver Health" Molecules 30, no. 22: 4384. https://doi.org/10.3390/molecules30224384
APA StyleSzelenberger, R., & Więckowska, M. (2025). Fungal Polysaccharides as Modulators of Molecular Pathways in Liver Health. Molecules, 30(22), 4384. https://doi.org/10.3390/molecules30224384






