Effect of Wheat Flour Substitution with Medicinal Mushroom Powder on Protein and Starch Digestibility and Functional Properties of Bread
Abstract
1. Introduction
2. Results and Discussion
2.1. Water Solubility Index (WSI) and Water Absorption Index (WAI)
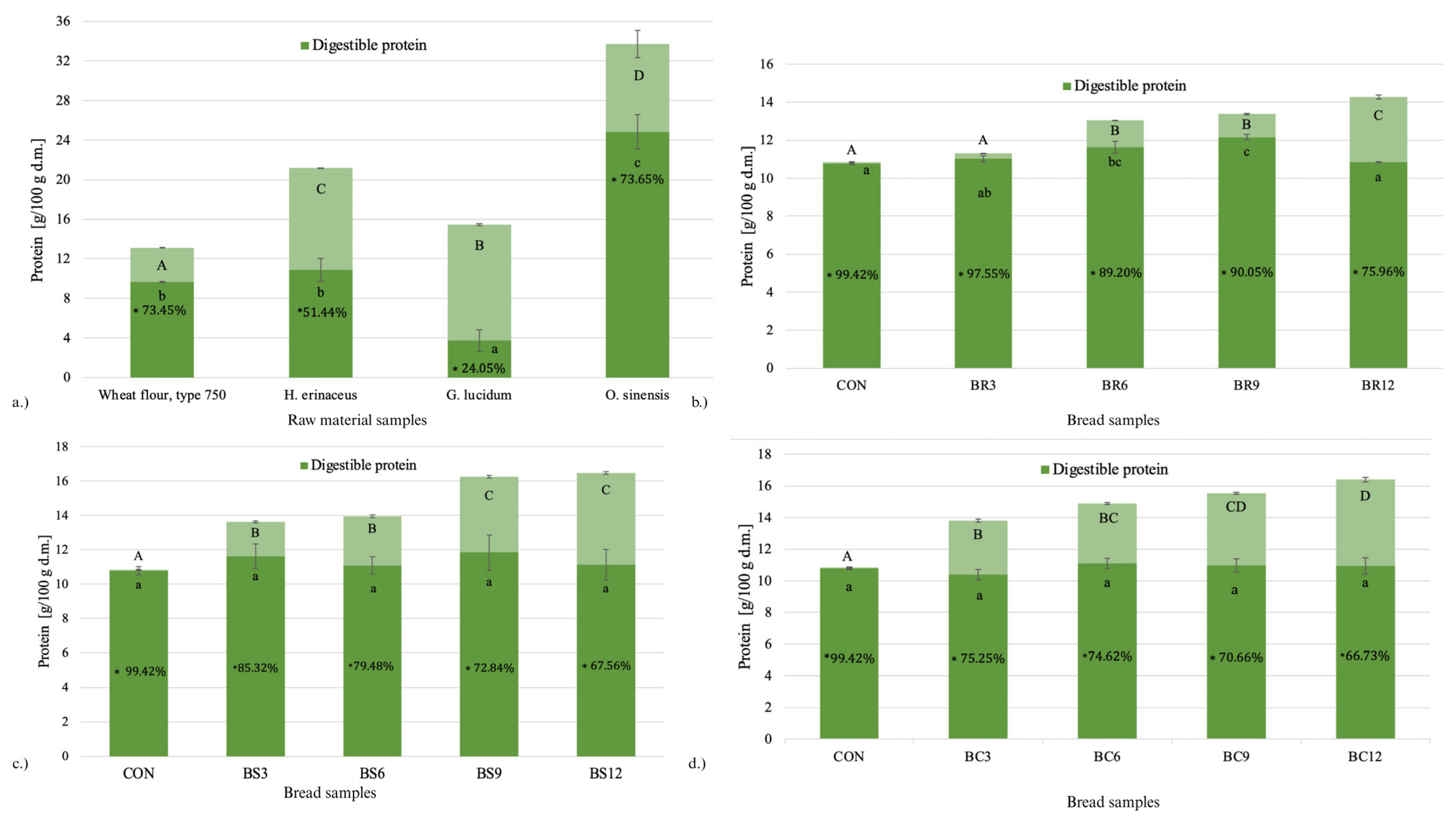
2.2. Protein Digestibility
2.3. Starch Digestibility
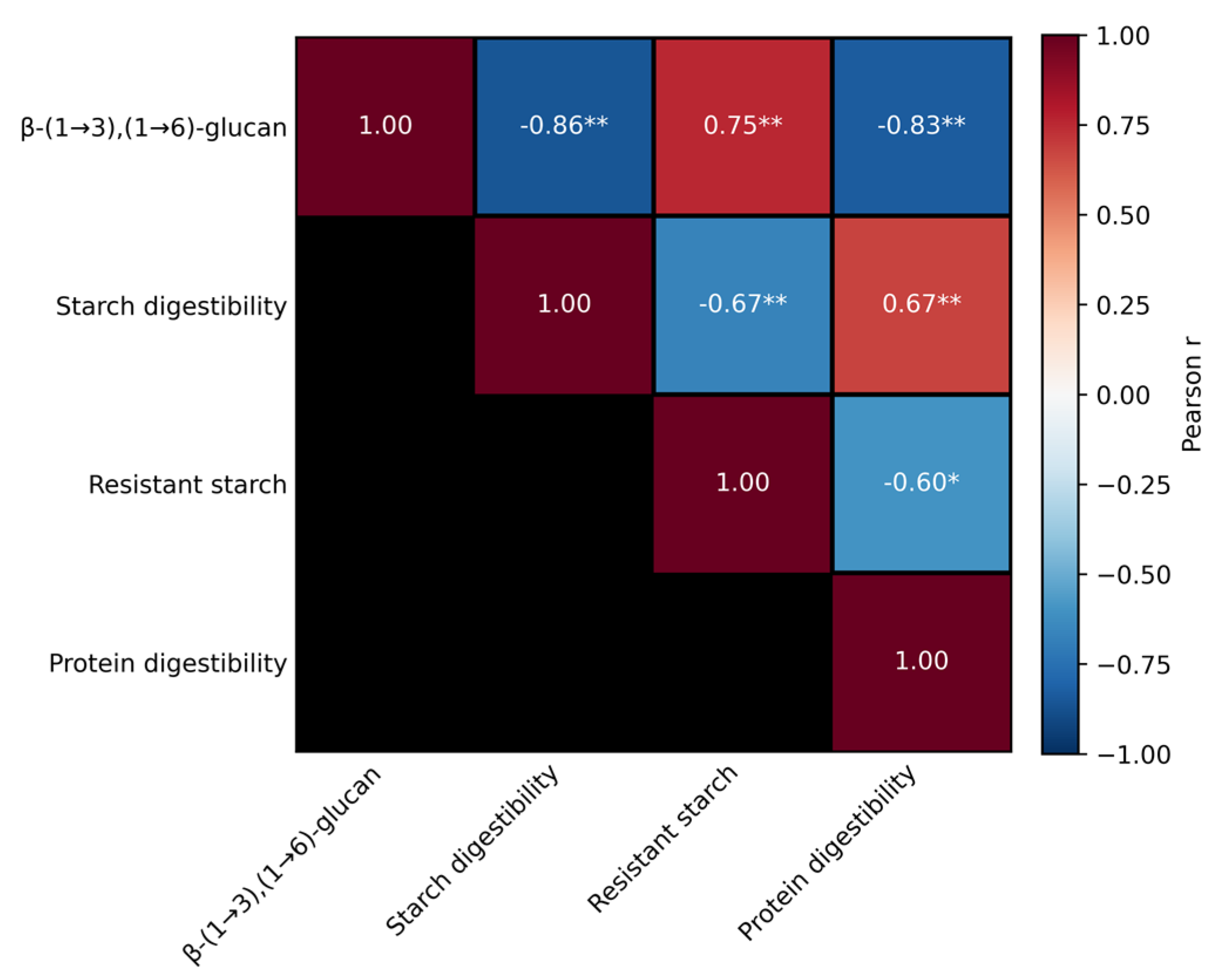
2.4. β-Glucan Content
3. Methodology
3.1. Plant Material
3.2. Chemicals
3.3. Bread Production
3.4. Water Solubility Index (WSI) and Water Absorption Index (WAI)
3.5. Determination of Protein Digestibility
3.6. Determination of Starch Digestibility
3.7. Determination of β-Glucan Content
3.8. Statistical Analysis
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masters, W.A.; Finaret, A.B.; Block, S.A. The economics of malnutrition: Dietary transition and food system transformation. In Handbook of Agricultural Economics; Elsevier: Amsterdam, The Netherlands, 2022; Volume 6, pp. 4997–5083. [Google Scholar] [CrossRef]
- Arrieta, E.M.; Aguiar, S. Healthy diets for sustainable food systems: A narrative review. Environ. Sci. Adv. 2023, 2, 684–694. [Google Scholar] [CrossRef]
- Meng, W.; Chao, W.; Kaiwei, Z.; Sijia, M.; Jiajia, S.; Shijie, X. Bioactive compounds from Chinese herbal plants for neurological health: Mechanisms, pathways and functional food applications. Front. Nutr. 2025, 12, 1537363. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Nutrition and immunity: Lessons from coronavirus disease-2019. Proc. Nutr. Soc. 2025, 84, 8–23. [Google Scholar] [CrossRef]
- Dicken, S.J.; Dahm, C.C.; Ibsen, D.B.; Olsen, A.; Tjønneland, A.; Louati-Hajji, M.; Cadeau, C.; Marques, C.; Schulze, M.B.; Jannasch, F.; et al. Food consumption by degree of food processing and risk of type 2 diabetes mellitus: A prospective cohort analysis of the European Prospective Investigation into Cancer and Nutrition (EPIC). Lancet Reg. Health Eur. 2024, 46, 101043. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.V.; Ulhas, R.S.; Malaviya, A. Bioactive compounds from Cordyceps and their therapeutic potential. Crit. Rev. Biotechnol. 2023, 44, 753–773. [Google Scholar] [CrossRef]
- Cha, S.; Bell, L.; Shukitt-Hale, B.; Williams, C.M. A review of the effects of mushrooms on mood and neurocognitive health across the lifespan. Neurosci. Biobehav. Rev. 2024, 158, 105548. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal mushrooms: Their bioactive components, nutritional value and application in functional food production—A review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- De MarcoCastro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-glucans and immunity: State of the art and future directions. Mol. Nutr. Food Res. 2020, 65, 1901071. [Google Scholar] [CrossRef]
- Seo, G.; Hyun, C.; Choi, S.; Kim, Y.M.; Cho, M. The wound healing effect of four types of beta-glucan. Appl. Biol. Chem. 2019, 62, 20. [Google Scholar] [CrossRef]
- Yang, F.; Cheung, P.C.K. Fungal β-Glucan-Based Nanotherapeutics: From Fabrication to Application. J. Fungi 2023, 9, 475. [Google Scholar] [CrossRef]
- Timm, T.G.; Costa, T.M.; Alberton, M.D.; Helm, C.V.; Tavares, L.B.B. Mushroom β-glucans: Application and innovation for food industry and immunotherapy. Appl. Microbiol. Biotechnol. 2023, 107, 5035–5049. [Google Scholar] [CrossRef]
- Khan, A.A.; Gani, A.; Khanday, F.A.; Masoodi, F.A. Biological and pharmaceutical activities of mushroom β-glucan discussed as a potential functional food ingredient. Bioact. Carbohydr. Diet. Fibre 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Steimbach, L.; Borgmann, A.V.; Gomar, G.G.; Hoffmann, L.V.; Rutckeviski, R.; de Andrade, D.P.; Smiderle, F.R. Fungal beta-glucans as adjuvants for treating cancer patients—A systematic review of clinical trials. Clin. Nutr. 2021, 40, 3104–3113. [Google Scholar] [CrossRef]
- Amoah, I.; Cairncross, C.; Osei, E.O.; Yeboah, J.A.; Cobbinah, J.C.; Rush, E. Bioactive properties of bread formulated with plant-based functional ingredients before consumption and possible links with health outcomes after consumption—A review. Plant Foods Hum. Nutr. 2022, 77, 329–339. [Google Scholar] [CrossRef]
- Alvarez-Ramirez, J.; Rodriguez-Huezo, E.; Meraz, M.; Garcia-Diaz, S.; Flores-Silva, P.C.; Mondragon-Reinoso, L. Spatial variation of in vitro starch and protein digestibility in white wheat bread. Starch Stärke 2018, 70, 1800025. [Google Scholar] [CrossRef]
- Ucar, E.M.; Kizil, M. A review on healthier bread making: Focusing on the underlying mechanisms for decreasing glycemic index. Food Rev. Int. 2025, 41, 1469–1508. [Google Scholar] [CrossRef]
- Zhou, D.; Ma, Z.; Hu, X. Isolated pea resistant starch substrates with different structural features modulate the production of short-chain fatty acids and the gut microbiota composition in vitro. J. Agric. Food Chem. 2021, 69, 5392–5404. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y.; Lee, Y.E.J.; Chan, A.; Lee, P.-R.; Yang, H. Effect of starch addition on the physicochemical properties, molecular interactions, structures, and in vitro digestibility of plant-based egg analogues. Food Chem. 2023, 403, 134390. [Google Scholar] [CrossRef]
- Tekin, T.; Dincer, E. Effect of resistant starch types as a prebiotic. Appl. Microbiol. Biotechnol. 2023, 107, 491–515. [Google Scholar] [CrossRef]
- Campos-Perez, W.; Martinez-Lopez, E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2021, 1866, 158900. [Google Scholar] [CrossRef] [PubMed]
- Makran, M.; Miedes, D.; Cilla, A.; Barberá, R.; Garcia-Llatas, G.; Alegría, A. Understanding the influence of simulated elderly gastrointestinal conditions on nutrient digestibility and functional properties. Trends Food Sci. Technol. 2022, 129, 283–295. [Google Scholar] [CrossRef]
- Araújo, D.; Rodrigues, T.; Alves, V.D.; Freitas, F. Chitin-glucan complex hydrogels: Optimization of gel formation and demonstration of drug loading and release ability. Polymers 2022, 14, 785. [Google Scholar] [CrossRef]
- Araújo, D.; Rodrigues, T.; Roma-Rodrigues, C.; Alves, V.D.; Fernandes, A.R.; Freitas, F. Chitin-glucan complex hydrogels: Physical-chemical characterization, stability, in vitro drug permeation, and biological assessment in primary cells. Polymers 2023, 15, 791. [Google Scholar] [CrossRef] [PubMed]
- Mandliya, A.; Kaur, M.; Mishra, P.; Riar, C.S. Modeling of vacuum drying of pressed mycelium (Pleurotus eryngii) and its application in bread. J. Food Process Eng. 2022, 45, e14030. [Google Scholar] [CrossRef]
- He, M.; Tang, C.-Y.; Wang, T.; Xiao, M.-J.; Li, Y.-L.; Li, X.-Z. Analysis of metabolic profiles and antioxidant activity of Chinese Cordyceps, Ophiocordyceps sinensis, and Paecilomyces hepiali based on untargeted metabolomics. Biology 2024, 13, 683. [Google Scholar] [CrossRef]
- Kong, X.-R.; Zhu, Z.-Y.; Zhang, X.-J.; Zhu, Y.-M. Effects of Cordyceps polysaccharides on pasting properties and in vitro starch digestibility of wheat starch. Food Hydrocoll. 2020, 102, 105604. [Google Scholar] [CrossRef]
- Hitayezu, E.; Kang, Y.-H. Effect of particle size on the physicochemical and morphological properties of Hypsizygus marmoreus mushroom powder and its hot-water extracts. Korean J. Food Preserv. 2021, 28, 504–549. [Google Scholar] [CrossRef]
- Uukule, E.N.; Embashu, W.; Kadhila, P.N.; Ueitele, I.S.E.; Nantanga, K.K.M. Climate smart, underutilised, healthful future cereal: Protein content, hydration properties, starch digestibility and consumer liking of pearl millet-based Oyster mushroom crackers. Food Chem. Adv. 2023, 3, 100467. [Google Scholar] [CrossRef]
- Ketnawa, S.; Ogawa, Y. In vitro protein digestibility and biochemical characteristics of soaked, boiled and fermented soybeans. Sci. Rep. 2021, 11, 14257. [Google Scholar] [CrossRef]
- Lavoignat, M.; Denis, S.; Faye, A.; Halupka, L.; Perrochon, S.; Rhazi, L.; Giraudeau, P.; Déjean, S.; Branlard, G.; Bancel, E.; et al. Differences in bread protein digestibility traced to wheat cultivar traits. J. Cereal Sci. 2022, 107, 103533. [Google Scholar] [CrossRef]
- Ali, S.A. Gelatinization and fermentation synergy: Functional and nutritional evaluation of breads fermented with Saccharomyces cerevisiae MK-157. Int. J. Food Prop. 2024, 27, e2350243. [Google Scholar] [CrossRef]
- Colunga, A.G.; Cruz-Hernández, M.; Losoya, C.; Nobre Gonçalves, C.; Treviño, A.; Rodriguez-Jasso, R.; Contreras-Esquivel, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef]
- Ayimbila, F.; Keawsompong, S. Nutritional quality and biological application of mushroom protein as a novel protein alternative. Curr. Nutr. Rep. 2023, 12, 281–298. [Google Scholar] [CrossRef]
- Tang, C.; Fan, Y.; Wang, T.; Wang, J.; Xiao, M.; He, M.; Chang, X.; Li, Y.; Li, X. Integrated Amino Acid Profiling and 4D-DIA Proteomics Reveal Protein Quality Divergence and Metabolic Adaptation in Cordyceps Species. J. Fungi 2025, 11, 365. [Google Scholar] [CrossRef]
- Chen, M.; Luo, J.; Jiang, W.; Chen, L.; Miao, L.; Han, C. Cordycepin: A review of strategies to improve the bioavailability and efficacy. Phytother. Res. 2023, 37, 3839–3858. [Google Scholar] [CrossRef]
- Wong, Y.Y.; Moon, A.; Duffin, R.; Barthet-Barateig, A.; Meijer, H.A.; Clemens, M.J.; De Moor, C.H. Cordycepin inhibits protein synthesis and cell adhesion through effects on signal transduction. J. Biol. Chem. 2010, 285, 2610–2621. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Russell, F.M.; Atrih, A.; Lamont, D.J.; Hardie, D.G. Mechanism of activation of AMPK by cordycepin. Cell Chem. Biol. 2020, 27, 214–222.e4. [Google Scholar] [CrossRef]
- Mansouri, M. Improving Extraction Procedure and a Comparative Analysis of Bioactive Molecules from Ganoderma lucidum, Hericium erinaceus and Cordyceps Mushrooms. Master’s Thesis, University of Windsor, Windsor, ON, Canada, 2024; p. 31295864, ProQuest Dissertations & Theses Global. [Google Scholar]
- Bonomini, M.G.; Prandi, B.; Caligiani, A. Black soldier fly (Hermetia illucens L.) whole and fractionated larvae: In vitro protein digestibility and effect of lipid and chitin removal. Food Res. Int. 2024, 196, 115102. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Wen, C.; Wang, N.; Yan, C.; Shen, C.; Song, S. Chitosan and chitosan oligosaccharide influence digestibility of whey protein isolate through electrostatic interaction. Int. J. Biol. Macromol. 2022, 222, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.-H.; Moon, C.-R.; Park, S.-W.; Choi, J.; Park, J.-D.; Sung, J.M.; Choi, E.-J.; Son, Y.-J. Quality and staling characteristics of white bread fortified with lysozyme-hydrolyzed mealworm powder (Tenebrio molitor L.). Curr. Res. Food Sci. 2024, 8, 100685. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Zarzycki, P. Evaluation of the physicochemical, antioxidant and sensory properties of wheat bread with partial substitution of wheat flour with Cordyceps sinensis powder. Pol. J. Food Nutr. Sci. 2025, 75, 170–183. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A.; Ivanišová, E. Lion’s Mane (Hericium erinaceus (Bull.) Pers.) as a functional component for wheat bread production: Influence on physicochemical, antioxidant, and sensory properties. Int. Agrophys. 2025, 39, 13–28. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A.; Zarzycki, P.; Blicharz-Kania, A. The impact of Ganoderma lucidum (Curtis) P. Karst. supplementation on the technological, chemical, and quality parameters of wheat bread. Foods 2024, 13, 3101. [Google Scholar] [CrossRef]
- Tańska, M.; Browarek, J.; Ruszkowska, M.; Purkiewicz, A. Comparative study on the incorporation of lesser mealworm (Alphitobius diaperinus) and house cricket (Acheta domesticus) powders into shortbread cookies: Effects on physical, chemical and sensory properties. Pol. J. Food Nutr. Sci. 2024, 74, 280–292. [Google Scholar] [CrossRef]
- Tu, J.; Brennan, M.; Brennan, C. An insight into the mechanism of interactions between mushroom polysaccharides and starch. Curr. Opin. Food Sci. 2021, 37, 17–25. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Brennan, M.; Brennan, C.; Qin, Y.; Cheng, G.; Liu, Y. Physical, chemical, sensorial properties and in vitro digestibility of wheat bread enriched with Yunnan commercial and wild edible mushrooms. LWT Food Sci. Technol. 2022, 169, 113923. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Sun, L.; Lei, J.; Yang, Y.-M.; Sun, W.-J.; Zan, X.-Y.; Meng, L.-J.; Cui, F.-J. Structural and catalytic characterization of β-1,3-glucan synthases of mushroom Ganoderma lucidum. Appl. Biochem. Biotechnol. 2025, 197, 4665–4684. [Google Scholar] [CrossRef]
- Feng, T.; Shui, M.; Chen, Z.; Zhuang, H.; Wang, W.; Yang, Y.; Zhang, J.; Ye, R. Hericium erinaceusβ-glucan modulates in vitro wheat starch digestibility. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Li, J.; Zheng, M.; Wang, Z.; Liu, Y.; Niu, Q.; Zhou, H.; Wang, D.; Song, J.; Bi, H.; Guo, B.; et al. Anti-tumor and anti-metastasis of water-soluble sulfated β-glucan derivatives from Saccharomyces cerevisiae. Carbohydr. Polym. 2024, 344, 122466. [Google Scholar] [CrossRef]
- Cui, Y.; Han, X.; Huang, X.; Xie, W.; Zhang, X.; Zhang, Z.; Yu, Q.; Tao, L.; Li, T.; Li, S. Effects of different sources of β-glucan on pasting, gelation, and digestive properties of pea starch. Food Hydrocoll. 2023, 135, 108172. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. Impact of the molecular weight, viscosity, and solubility of β-glucan on in vitro oat starch digestibility. J. Agric. Food Chem. 2013, 61, 3270–3277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Xia, Q.; Liu, L.; Wu, Z.; Pan, D. Recent advances of cereal β-glucan on immunity with gut microbiota regulation functions and its intelligent gelling application. Crit. Rev. Food Sci. Nutr. 2021, 63, 3895–3911. [Google Scholar] [CrossRef]
- Świeca, M.; Baraniak, B.; Gawlik-Dziki, U. In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 2013, 138, 1414–1420. [Google Scholar] [CrossRef]
- Wang, X.; Lao, X.; Bao, Y.; Guan, X.; Li, C. Effect of whole quinoa flour substitution on the texture and in vitro starch digestibility of wheat bread. Food Hydrocoll. 2021, 119, 106840. [Google Scholar] [CrossRef]
- Sobh, M.; Montroy, J.; Daham, Z.; Sibbald, S.; Lalu, M.; Stintzi, A.; Mack, D.; Fergusson, D.A. Tolerability and SCFA production after resistant starch supplementation in humans: A systematic review of randomized controlled studies. Am. J. Clin. Nutr. 2022, 115, 608–618. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, Y.; Tan, Y.; Zhang, Z.; McClements, D.J. Digestibility and gastrointestinal fate of meat versus plant-based meat analogs: An in vitro comparison. Food Chem. 2021, 364, 130439. [Google Scholar] [CrossRef]
- Corrado, M.; Zafeiriou, P.; Ahn-Jarvis, J.H.; Savva, G.M.; Edwards, C.H.; Hazard, B.A. Impact of storage on starch digestibility and texture of a high-amylose wheat bread. Food Hydrocoll. 2023, 135, 108139. [Google Scholar] [CrossRef]
- Qi, K.; Yi, X.; Li, C. Effects of endogenous macronutrients and processing conditions on starch digestibility in wheat bread. Carbohydr. Polym. 2022, 295, 119874. [Google Scholar] [CrossRef] [PubMed]
- Naseer, B.; Mousa, E.F. Production of nano beta-glucans from baking yeast Saccharomyces cerevisiae and its comparison with extracted beta-glucans. IOP Conf. Ser. Earth Environ. Sci. 2024, 1325, 012049. [Google Scholar] [CrossRef]
- Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Rubio-Moraga, Á. Food and human health applications of edible mushroom by-products. New Biotechnol. 2024, 81, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure–functional activity relationship of β-glucans from the perspective of immunomodulation: A mini-review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Liu, Y.; Tang, C. Recent advances in the preparation, structure, and biological activities of β-glucan from Ganoderma species: A review. Foods 2023, 12, 2975. [Google Scholar] [CrossRef]
- Case, S.; O’Brien, T.; Ledwith, A.E.; Chen, S.; Horneck Johnston, C.J.H.; Hackett, E.E.; O’Sullivan, M.; Charles-Messance, H.; Dempsey, E.; Yadav, S.; et al. β-glucans from Agaricus bisporus mushroom products drive trained immunity. Front. Nutr. 2024, 11, 1346706. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Q.; Zeng, L.; Liu, Y.; Zhang, Y.; Sun, Z.; Guo, Q.; Cui, S.W. Structural dynamics, gut microbiota modulation, and immunological impacts of shiitake mushroom β-glucan during in vitro intestinal fermentation. J. Agric. Food Chem. 2025, 73, 12049–12060. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, P.; Sobota, A.; Kuzawińska, E.; Wirkijowska, A.; Sykut-Domańska, E. Estimation of degree of starch gelatinisation in instant pasta using measurements of viscosity and water absorption of ground instant pasta dispersions. Acta Agrophys. 2017, 24, 625–632. [Google Scholar]
- Saunders, R.M.; Connor, M.A.; Booth, A.N.; Bickoff, E.M.; Kohler, G.O. Measurement of digestibility of alfalfa protein concentrates by in vivo and in vitro methods. J. Nutr. 1973, 103, 530–536. [Google Scholar] [CrossRef]
- ISO 20483:2013; Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2013.

| Sample | WAI [% d.m.] | WSI [% d.m.] |
|---|---|---|
| Lion’s Mane | 169.32 C ± 0.83 | 17.30 C ± 0.03 |
| Reishi | 213.08 D ± 1.23 | 13.23 B ± 0.14 |
| Cordyceps | 146.48 B ± 0.94 | 29.31 D ± 0.21 |
| Wheat Flour type 750 | 119.26 A ± 0.39 | 08.25 A ± 0.07 |
| CON | 134.69 a ± 1.31 | 13.08 a ± 0.04 |
| BS3 | 135.72 ab ± 0.24 | 13.23 ab ± 0.11 |
| BS6 | 137.80 bc ± 1.42 | 13.35 b ± 0.08 |
| BS9 | 140.92 cd ± 0.93 | 13.38 b ± 0.08 |
| BS12 | 145.08 e ± 0.31 | 13.53 bc ± 0.04 |
| BR3 | 137.04 bc ± 0.42 | 13.62 c ± 0.03 |
| BR6 | 140.43 cd ± 1.84 | 13.68 cd ± 0.14 |
| BR9 | 144.86 e ± 0.52 | 13.71 cd ± 0.04 |
| BR12 | 150.33 f ± 0.49 | 13.89 d ± 0.03 |
| BC3 | 135.04 ab ± 0.85 | 14.17 e ± 0.09 |
| BC6 | 137.57 bc ± 0.41 | 14.34 ef ± 0.09 |
| BC9 | 140.58 cd ± 0.94 | 14.98 f ± 0.04 |
| BC12 | 144.62 e ± 1.04 | 15.61 g ± 0.08 |
| Sample | Digestible Starch (%) | Resistant Starch (%) | Starch Digestibility (%) |
|---|---|---|---|
| Raw materials | |||
| Lion’s Mane | 9.36 B ± 0.12 | 3.26 C ± 0.08 | 74.17 B ± 0.42 |
| Reishi | 15.84 C ± 0.19 | 6.25 D ± 0.09 | 71.71 A ± 0.37 |
| Cordyceps | 2.26 A ± 0.06 | 0.91 A ± 0.03 | 71.29 A ± 0.45 |
| Wheat flour | 59.55 D ± 0.31 | 1.10 B ± 0.04 | 98.19 C ± 0.12 |
| Bread samples | |||
| CON | 72.71 f ± 0.71 | 0.80 a ± 0.06 | 98.91 g ± 0.07 |
| BS3 | 68.64 ef ± 0.76 | 1.12 ab ± 0.04 | 98.39 fg ± 0.04 |
| BS6 | 67.24 def ± 0.65 | 1.35 ab ± 0.05 | 98.03 ef ± 0.06 |
| BS9 | 66.11 cde ± 0.67 | 1.36 b ± 0.03 | 97.98 ef ± 0.02 |
| BS12 | 64.13 bcde ± 0.58 | 1.38 bc ± 0.03 | 97.89 ef ± 0.04 |
| BR3 | 65.43 bcde ± 0.73 | 1.93 cd ± 0.18 | 97.13 d ± 0.24 |
| BR6 | 62.08 bcd ± 0.43 | 2.46 d ± 0.11 | 96.19 c ± 0.14 |
| BR9 | 60.40 ab ± 0.73 | 3.66 e ± 0.24 | 94.29 b ± 0.29 |
| BR12 | 55.98 a ± 0.58 | 4.22 f ± 0.21 | 92.99 a ± 0.31 |
| BC3 | 71.83 f ± 0.69 | 1.21 ab ± 0.03 | 98.34 fg ± 0.02 |
| BC6 | 68.21 ef ± 0.47 | 1.23 ab ± 0.11 | 98.23 f ± 0.15 |
| BC9 | 65.83 bcde ± 0.58 | 1.37 b ± 0.10 | 97.96 ef ± 0.13 |
| BC12 | 61.42 abc ± 0.83 | 1.54 bc ± 0.13 | 97.55 de ± 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łysakowska, P.; Sobota, A.; Gumienna, M. Effect of Wheat Flour Substitution with Medicinal Mushroom Powder on Protein and Starch Digestibility and Functional Properties of Bread. Molecules 2025, 30, 4380. https://doi.org/10.3390/molecules30224380
Łysakowska P, Sobota A, Gumienna M. Effect of Wheat Flour Substitution with Medicinal Mushroom Powder on Protein and Starch Digestibility and Functional Properties of Bread. Molecules. 2025; 30(22):4380. https://doi.org/10.3390/molecules30224380
Chicago/Turabian StyleŁysakowska, Paulina, Aldona Sobota, and Małgorzata Gumienna. 2025. "Effect of Wheat Flour Substitution with Medicinal Mushroom Powder on Protein and Starch Digestibility and Functional Properties of Bread" Molecules 30, no. 22: 4380. https://doi.org/10.3390/molecules30224380
APA StyleŁysakowska, P., Sobota, A., & Gumienna, M. (2025). Effect of Wheat Flour Substitution with Medicinal Mushroom Powder on Protein and Starch Digestibility and Functional Properties of Bread. Molecules, 30(22), 4380. https://doi.org/10.3390/molecules30224380






