Spatial Distribution of Minerals and Selected Bioactive Compounds in White Mold-Ripened and Blue-Veined Cheeses
Abstract
1. Introduction
2. Results and Discussion
2.1. Minerals and Selected Bioactive Compounds in White Mold-Ripened and Blue-Veined Cheeses
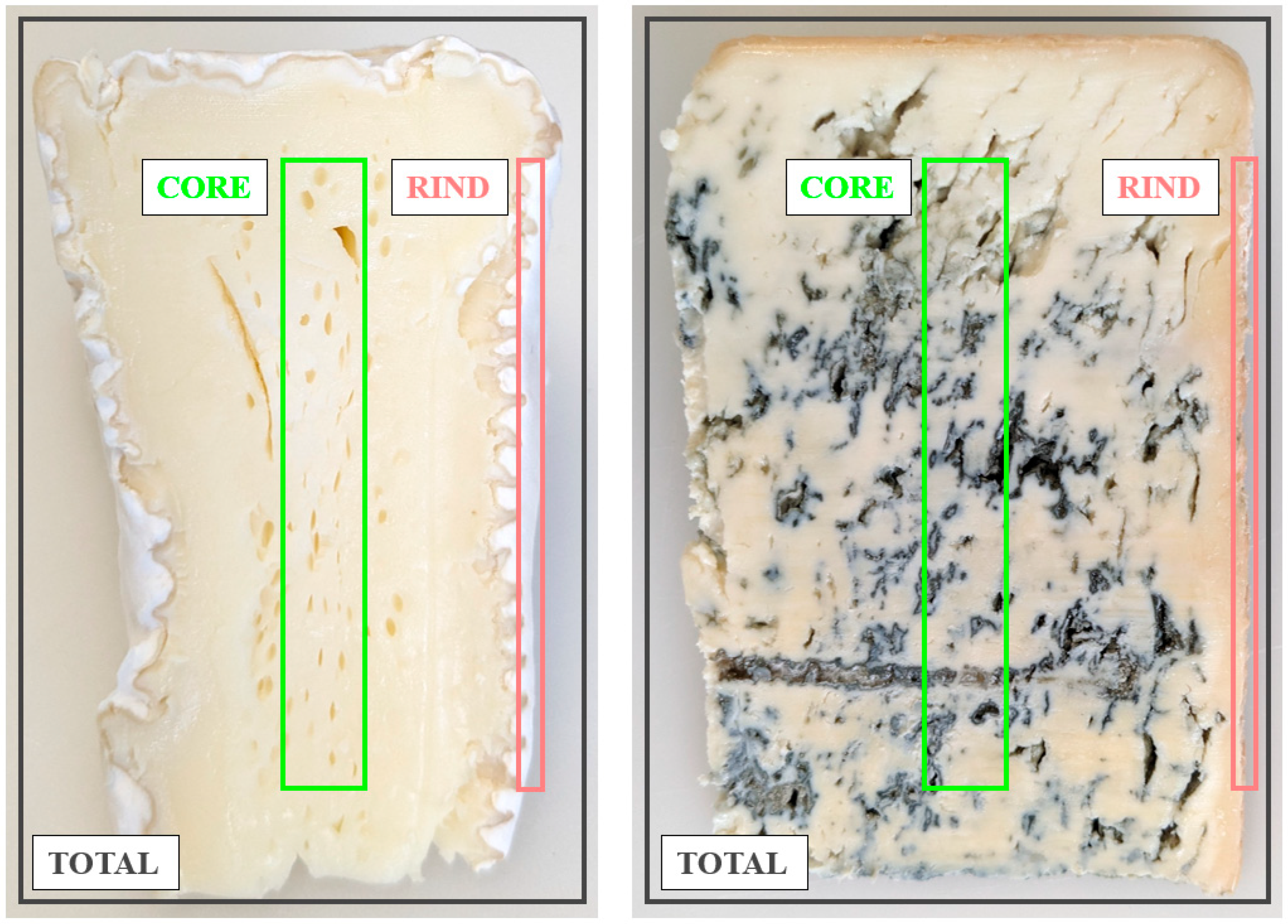
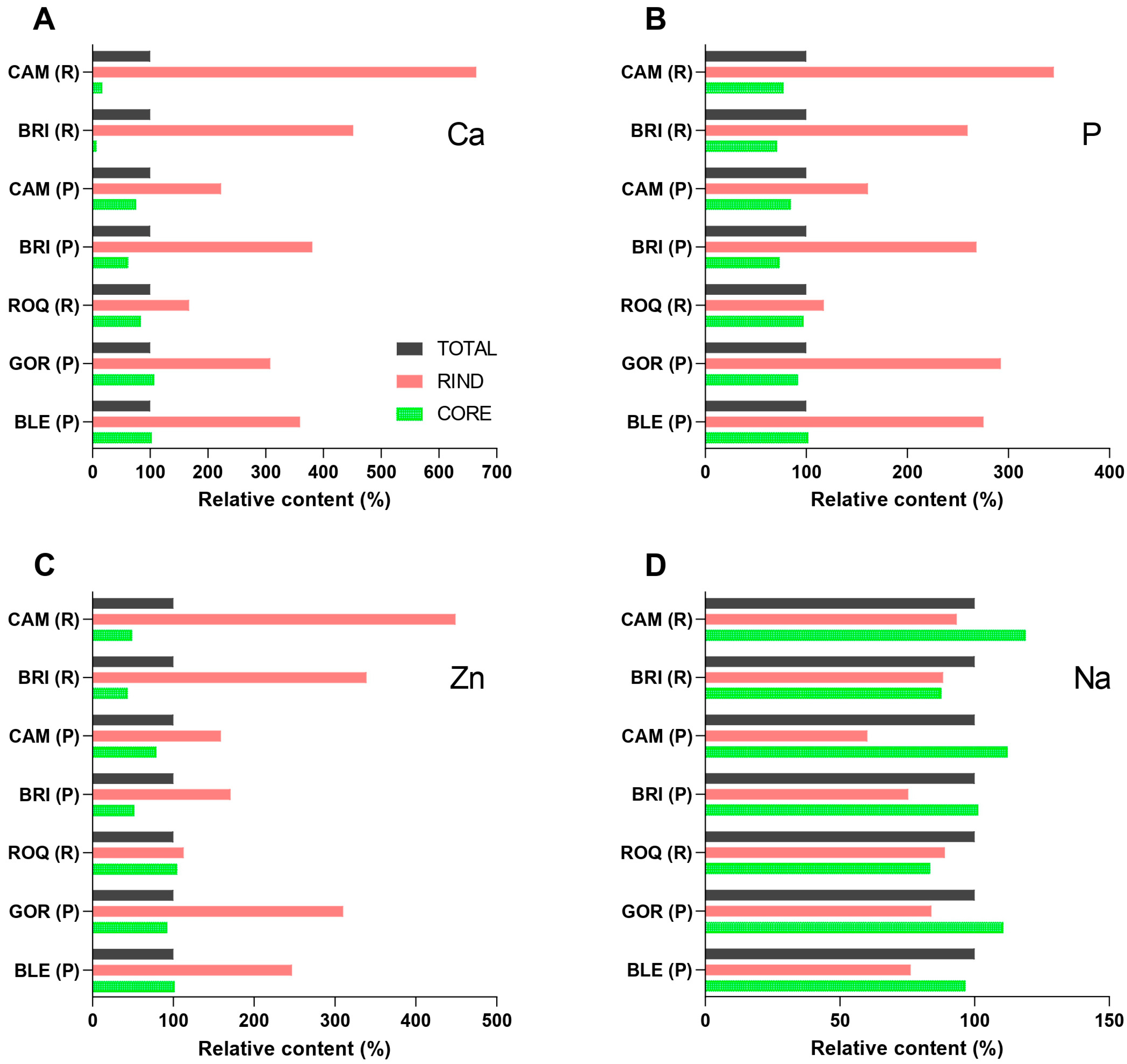
2.2. Spatial Distribution of Minerals in White Mold-Ripened and Blue-Veined Cheeses
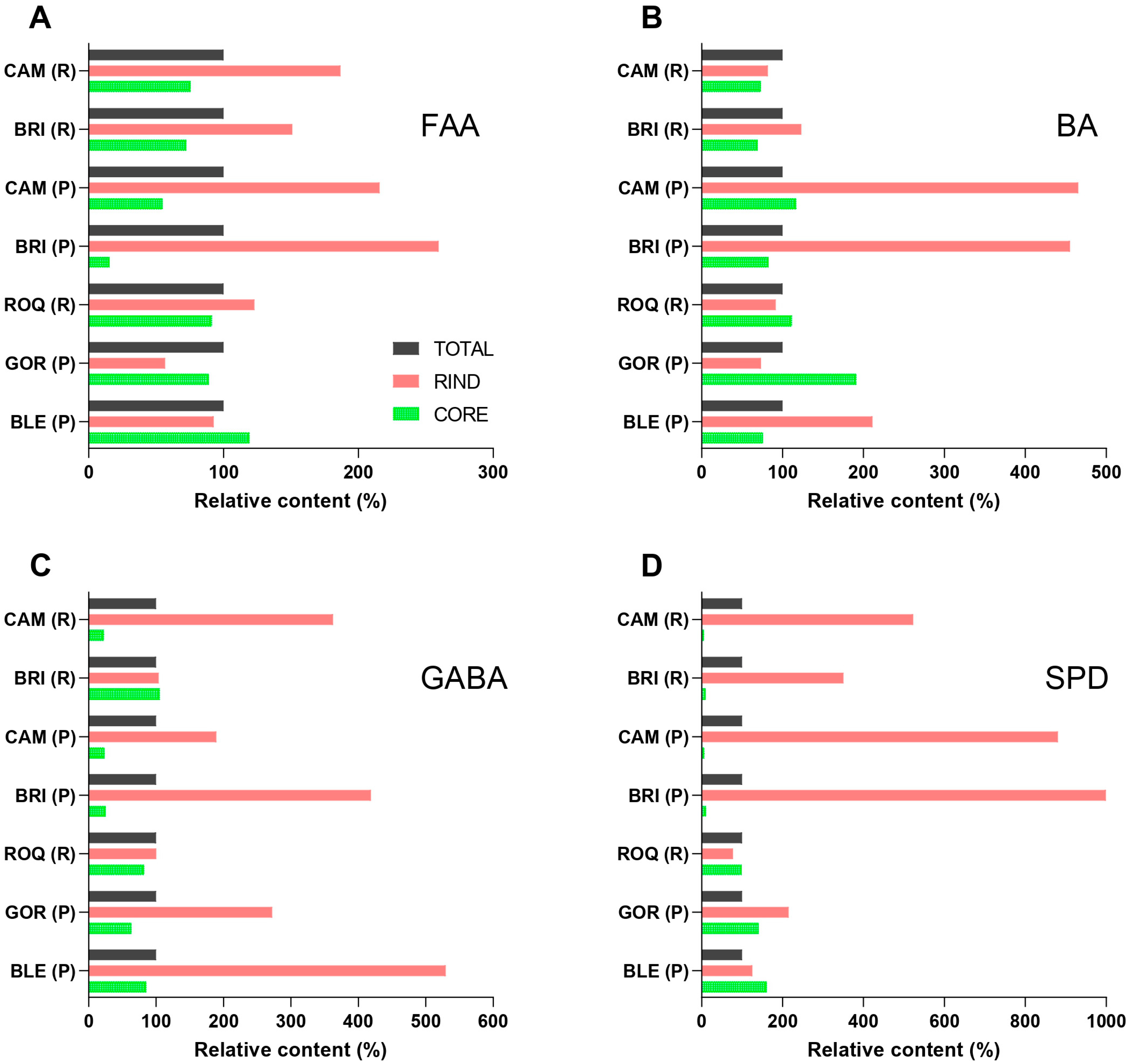
2.3. Spatial Distribution of Free Amino Acids and Their Metabolites in White Mold-Ripened and Blue-Veined Cheeses
3. Materials and Methods
3.1. Materials
3.1.1. Cheese
3.1.2. Chemicals
3.2. Methods
3.2.1. Preparation of Cheese Samples
3.2.2. Determination of pH Value
3.2.3. Elemental Analysis
3.2.4. Determination of Free Proteinogenic Amino Acids and GABA
3.2.5. Determination of Biogenic Amines and Polyamines
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAB | Lactic acid bacteria |
| FAAs | Free amino acids |
| BAs | Biogenic amines |
| PUT | Putrescine |
| CAD | Cadaverine |
| TYM | Tyramine |
| HIM | Histamine |
| GABA | γ-aminobutyric acid |
| PAs | Polyamines |
| SPD | Spermidine |
| TPM | Tryptamine |
| PEA | Phenylethylamine |
| SPM | Spermine |
| CAM | Camembert |
| BRI | Brie |
| ROQ | Roquefort |
| GOR | Gorgonzola |
| BLE | Bleu de Laqueuille |
| AAs | Amino acids |
| R | Produced from raw milk |
| P | Produced from pasteurized milk |
| PDO | Protected designation of origin |
| MQ | Milli-Q |
| ICP-OES | Inductively coupled plasma optical emission spectrometer |
| IS | Internal standard 1,7-diaminoheptane |
| HPLC-MS/MS | High-performance liquid chromatography coupled with tandem mass spectrometry |
| ESI | Electrospray ionization |
References
- Ferroukhi, I.; Chassard, C.; Mardon, J. A Comprehensive Overview of Blue-Veined Cheeses. Int. Dairy J. 2024, 154, 105926. [Google Scholar] [CrossRef]
- Guizani, N.; Kasapis, S.; Al Attabi, Z.; Al-Rizeiqi, M. Microbiological, Physicochemical, and Biochemical Changes during Ripening of Camembert Cheese Made of Pasteurized Cow’s Milk. Int. J. Food Prop. 2002, 5, 483–494. [Google Scholar] [CrossRef]
- Ferroukhi, I.; Bord, C.; Alvarez, S.; Fayolle, K.; Theil, S.; Lavigne, R.; Chassard, C.; Mardon, J. Functional Changes in Bleu d’Auvergne Cheese during Ripening. Food Chem. 2022, 397, 133850. [Google Scholar] [CrossRef]
- McSweeney, P.L.H. Biochemistry of Cheese Ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Mane, A.; Ciocia, F.; Beck, T.K.; Lillevang, S.K.; McSweeney, P. Proteolysis in Danish Blue Cheese during Ripening. Int. Dairy J. 2019, 97, 191–200. [Google Scholar] [CrossRef]
- Collins, Y.; McSweeney, P.; Wilkinson, M. Lipolysis and Free Fatty Acid Catabolism in Cheese: A Review of Current Knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Khattab, A.; Guirguis, H.; Tawfik, S.; Farag, M. Cheese Ripening: A Review on Modern Technologies towards Flavor Enhancement, Process Acceleration and Improved Quality Assessment. Trends Food Sci. Technol. 2019, 88, 343–360. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Gómez-Mascaraque, L.G.; Fenelon, M.; Huppertz, T. Determination of Minerals in Soft and Hard Cheese Varieties by ICP-OES: A Comparison of Digestion Methods. Molecules 2023, 28, 3988. [Google Scholar] [CrossRef]
- Gore, E.; Mardon, J.; Cécile, B.; Lebecque, A. Calcium Lactate as an Attractive Compound to Partly Replace Salt in Blue-Veined Cheese. J. Dairy Sci. 2019, 102, 1–13. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Fox, P.F.; Ciocia, F. Chapter 16—Metabolism of Residual Lactose and of Lactate and Citrate. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 411–421. ISBN 978-0-12-417012-4. [Google Scholar]
- Natrella, G.; Vacca, M.; Minervini, F.; Faccia, M.; De Angelis, M. A Comprehensive Review on the Biogenic Amines in Cheeses: Their Origin, Chemical Characteristics, Hazard and Reduction Strategies. Foods 2024, 13, 2583. [Google Scholar] [CrossRef]
- Garbowska, M.; Pluta, A.; Berthold-Pluta, A. Contents of Functionally Bioactive Peptides, Free Amino Acids, and Biogenic Amines in Dutch-Type Cheese Models Produced with Different Lactobacilli. Molecules 2020, 25, 5465. [Google Scholar] [CrossRef] [PubMed]
- Galgano, F.; Caruso, M.; Condelli, N.; Favati, F. Focused Review: Agmatine in Fermented Foods. Front. Microbiol. 2012, 3, 199. [Google Scholar] [CrossRef]
- Diezhandino, I.; Fernández, D.; Combarros-Fuertes, P.; Renes, E.; Fresno, J.M.; Tornadijo, M.E. Characteristics and Proteolysis of a Spanish Blue Cheese Made with Raw or Pasteurised Milk. Int. J. Dairy Technol. 2022, 75, 630–642. [Google Scholar] [CrossRef]
- Saha Turna, N.; Chung, R.; McIntyre, L. A Review of Biogenic Amines in Fermented Foods: Occurrence and Health Effects. Heliyon 2024, 10, e24501. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Battistelli, N.; Pepe, A.; Ianni, A.; Martino, G.; Suzzi, G. Accumulation γ-Aminobutyric Acid and Biogenic Amines in a Traditional Raw Milk Ewe’s Cheese. Foods 2019, 8, 401. [Google Scholar] [CrossRef]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-Aminobutyric Acid as a Bioactive Compound in Foods: A Review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Chávez, R.; Vaca, I.; García-Estrada, C. Secondary Metabolites Produced by the Blue-Cheese Ripening Mold Penicillium Roqueforti; Biosynthesis and Regulation Mechanisms. J. Fungi 2023, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Casquete, R.; Benito, M.J.; Córdoba, M.d.G.; Ruiz-Moyano, S.; Galván, A.I.; Martín, A. Physicochemical Factors Affecting the Growth and Mycotoxin Production of Penicillium Strains in a Synthetic Cheese Medium. LWT 2018, 89, 179–185. [Google Scholar] [CrossRef]
- Boisard, P. The Future of a Tradition: Two Ways of Making Camembert, the Foremost Cheese of France. Food Foodways 1991, 4, 173–207. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and Lifespan Extension by the Natural Polyamine Spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher Spermidine Intake Is Linked to Lower Mortality: A Prospective Population-Based Study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef]
- Ali, M.A.; Poortvliet, E.; Strömberg, R.; Yngve, A. Polyamines in Foods: Development of a Food Database. Food Nutr. Res. 2011, 55, 5572. [Google Scholar] [CrossRef]
- Löser, C. Polyamines in Human and Animal Milk. Br. J. Nutr. 2000, 84 (Suppl. S1), 55–58. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Dong, L.; Li, Y.; Zhang, Q.; Ding, Y.; Wu, J.; Wang, Z. Identification of Polyamines in Human Milk, Formula Milk, and Other Dairy Products: A Special Method Based on Precolumn Derivatization, Double-Florisil Purification, and Gas Chromatography–Triple Quadrupole Mass Spectrometry. J. Agric. Food Chem. 2025, 73, 8621–8631. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.-T.; Arias-Roth, E.; Jakob, E. Cheese Yeasts. Yeast 2019, 36, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Ehmer, A.; Chaize, D.; Rimbach, G. Quantitative Determination of Spermidine in 50 German Cheese Samples on a Core-Shell Column by High-Performance Liquid Chromatography with a Photodiode Array Detector Using a Fully Validated Method. J. Agric. Food Chem. 2016, 64, 2105–2111. [Google Scholar] [CrossRef]
- Novella-Rodríguez, S.; Veciana-Nogués, M.T.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. Distribution of Biogenic Amines and Polyamines in Cheese. J. Food Sci. 2003, 68, 750–756. [Google Scholar] [CrossRef]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in Polyamines with Aging and Their Ingestion from Food and Drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Zdolec, N.; Bogdanović, T.; Severin, K.; Dobranić, V.; Kazazić, S.; Grbavac, J.; Pleadin, J.; Petričević, S.; Kiš, M. Biogenic Amine Content in Retailed Cheese Varieties Produced with Commercial Bacterial or Mold Cultures. Processes 2022, 10, 10. [Google Scholar] [CrossRef]
- Engel, E.; Tournier, C.; Salles, C.; Le Quéré, J.L. Evolution of the Composition of a Selected Bitter Camembert Cheese during Ripening: Release and Migration of Taste-Active Compounds. J. Agric. Food Chem. 2001, 49, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Johnson, M.E.; Lucey, J.A. Changes in the Proportions of Soluble and Insoluble Calcium during the Ripening of Cheddar Cheese. J. Dairy Sci. 2004, 87, 854–862. [Google Scholar] [CrossRef]
- Upreti, P.; Metzger, L.E. Influence of Calcium and Phosphorus, Lactose, and Salt-to-Moisture Ratio on Cheddar Cheese Quality: pH Changes during Ripening. J. Dairy Sci. 2007, 90, 1–12. [Google Scholar] [CrossRef]
- Manzi, P.; Di Costanzo, M.G.; Ritota, M. Content and Nutritional Evaluation of Zinc in PDO and Traditional Italian Cheeses. Molecules 2021, 26, 6300. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Bajda, T.; Rogowska, M.; Pawłowska, A. Synthesis and Solubility of Hopeite Zn3(PO4)2·4H2O. Mineralogia 2023, 54, 78–81. [Google Scholar] [CrossRef]
- Pomastowski, P.; Sprynskyy, M.; Buszewski, B. The Study of Zinc Ions Binding to Casein. Colloids Surf. B Biointerfaces 2014, 120, 21–27. [Google Scholar] [CrossRef]
- Nogalska, A.; Momot, M.; Nogalski, Z. The Mineral Composition of Milk from High-Yielding Dairy Cows Depending on the Month of Lactation and Udder Health. Appl. Sci. 2020, 10, 4803. [Google Scholar] [CrossRef]
- López González, N.; Abarquero, D.; Combarros-Fuertes, P.; Prieto, B.; Fresno, J.M.; Tornadijo, M.E. Influence of Salting on Physicochemical and Sensory Parameters of Blue-Veined Cheeses. Dairy 2024, 5, 93–105. [Google Scholar] [CrossRef]
- Moreira, G.M.M.; Costa, R.G.B.; Teodoro, V.A.M.; Paula, J.C.J.; Sobral, D.; Fernandes, C.; Gloria, M.B.A. Effect of Ripening Time on Proteolysis, Free Amino Acids, Bioactive Amines and Texture Profile of Gorgonzola-Type Cheese. LWT 2018, 98, 583–590. [Google Scholar] [CrossRef]
- Mane, A.; McSweeney, P.L.H. Proteolysis in Irish Farmhouse Camembert Cheese during Ripening. J. Food Biochem. 2020, 44, e13101. [Google Scholar] [CrossRef]
- Chen, X.; Gu, Z.; Peng, Y.; Quek, S.Y. What Happens to Commercial Camembert Cheese under Packaging? Unveiling Biochemical Changes by Untargeted and Targeted Metabolomic Approaches. Food Chem. 2022, 383, 132437. [Google Scholar] [CrossRef]
- Kumar, D.; Chatli, M.K.; Singh, R.; Mehta, N.; Kumar, P. Enzymatic Hydrolysis of Camel Milk Casein and Its Antioxidant Properties. Dairy Sci. Technol. 2016, 96, 391–404. [Google Scholar] [CrossRef]
- Rafiq, S.; Huma, N.; Pasha, I.; Sameen, A.; Mukhtar, O.; Khan, M.I. Chemical Composition, Nitrogen Fractions and Amino Acids Profile of Milk from Different Animal Species. Asian Australas. J. Anim. Sci. 2016, 29, 1022–1028. [Google Scholar] [CrossRef]
- Chan, J.C.M.; Chaimovitz, C.; Ma, R.S.W. Acidity, Osmolality, Electrolyte and Amino Acid Concentration of Casein-Hydrolysate and Synthetic Amino Acid Solutions. Clin. Biochem. 1973, 6, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Carafa, I.; Stocco, G.; Nardin, T.; Larcher, R.; Bittante, G.; Tuohy, K.; Franciosi, E. Production of Naturally γ-Aminobutyric Acid-Enriched Cheese Using the Dairy Strains Streptococcus Thermophilus 84C and Lactobacillus Brevis DSM 32386. Front. Microbiol. 2019, 10, 93. [Google Scholar] [CrossRef]
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited Review: Bioactive Compounds Produced during Cheese Ripening and Health Effects Associated with Aged Cheese Consumption. J. Dairy Sci. 2018, 101, 3742–3757. [Google Scholar] [CrossRef] [PubMed]
- Redruello, B.; Szwengiel, A.; Ladero, V.; del Rio, B.; Alvarez, M.A. Identification of Technological/Metabolic/Environmental Profiles of Cheeses with High GABA Contents. LWT 2020, 130, 109603. [Google Scholar] [CrossRef]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, A.; Guo, A.C.; Vazquez-Fresno, R.; Lipfert, M.; Zhang, L.; Zheng, J.; Badran, H.; Budinski, Z.; Mandal, R.; Ametaj, B.N.; et al. Chemical Composition of Commercial Cow’s Milk. J. Agric. Food Chem. 2019, 67, 4897–4914. [Google Scholar] [CrossRef]
- Benkerroum, N. Biogenic Amines in Dairy Products: Origin, Incidence, and Control Means. Compr. Rev. Food Sci. Food Saf. 2016, 15, 801–826. [Google Scholar] [CrossRef]
- Abraham, S.; Cachon, R.; Colas, B.; Feron, G.; De Coninck, J. Eh and pH Gradients in Camembert Cheese during Ripening: Measurements Using Microelectrodes and Correlations with Texture. Int. Dairy J. 2007, 17, 954–960. [Google Scholar] [CrossRef]
- Graet, Y.L.; Brulé, G. Migration des macro et oligo-éléments dans un fromage à pâte molle de type Camembert. Le Lait 1988, 68, 219–234. [Google Scholar] [CrossRef]
- Gagnaire, V.; Pierre, A.; Molle, D.; Leonil, J. Phosphopeptides Interacting with Colloidal Calcium Phosphate Isolated by Tryptic Hydrolysis of Bovine Casein Micelles. J. Dairy Res. 1996, 63, 405–422. [Google Scholar] [CrossRef]
- Runthala, A.; Mbye, M.; Ayyash, M.; Xu, Y.; Kamal-Eldin, A. Caseins: Versatility of Their Micellar Organization in Relation to the Functional and Nutritional Properties of Milk. Molecules 2023, 28, 2023. [Google Scholar] [CrossRef]
- Amrane, A.; Prigent, Y. Diffusion of Calcium and Inorganic Phosphate at the Surface of a Solid Model Medium in Relation with Growth of Geotrichum Candidum and Penicillium Camembertii. J. Food Biochem. 2008, 32, 813–825. [Google Scholar] [CrossRef]
- Mai, W.; Wang, F.; He, S.; Wen, Y.; Yu, G.; Zhang, L.; Dong, H. Zinc Contents in Foods and Estimates of Dietary Intakes in Guangzhou, Guangdong Province, China. Front. Nutr. 2024, 11, 1364033. [Google Scholar] [CrossRef]
- Gibson, R.S. A Historical Review of Progress in the Assessment of Dietary Zinc Intake as an Indicator of Population Zinc Status. Adv. Nutr. 2012, 3, 772–782. [Google Scholar] [CrossRef]
- Namuswe, F.; Berg, J.M. Secondary Interactions Involving Zinc-Bound Ligands: Roles in Structural Stabilization and Macromolecular Interactions. J. Inorg. Biochem. 2012, 111, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Cerning, J.; Gripon, J.C.; Lamberet, G.; Lenoir, J. Les activités biochimiques des Penicillium utilisés en fromagerie. Le Lait 1987, 67, 3–39. [Google Scholar] [CrossRef]
- Su, A.; Yu, Q.; Luo, Y.; Yang, J.; Wang, E.; Yuan, H. Metabolic Engineering of Microorganisms for the Production of Multifunctional Non-Protein Amino Acids: γ-Aminobutyric Acid and δ-Aminolevulinic Acid. Microb. Biotechnol. 2021, 14, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Paventi, G.; Di Martino, C. Biosynthesis of Gamma-Aminobutyric Acid (GABA) by Lactiplantibacillus Plantarum in Fermented Food Production. Curr. Issues Mol. Biol. 2024, 46, 200–220. [Google Scholar] [CrossRef]
- Bardócz, S.; Grant, G.; Brown, D.S.; Ralph, A.; Pusztai, A. Polyamines in Food—Implications for Growth and Health. J. Nutr. Biochem. 1993, 4, 66–71. [Google Scholar] [CrossRef]
- Hymery, N.; Vasseur, V.; Coton, M.; Mounier, J.; Jany, J.-L.; Barbier, G.; Coton, E. Filamentous Fungi and Mycotoxins in Cheese: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Uutela, P.; Ketola, R.A.; Piepponen, P.; Kostiainen, R. Comparison of Different Amino Acid Derivatives and Analysis of Rat Brain Microdialysates by Liquid Chromatography Tandem Mass Spectrometry. Anal. Chim. Acta 2009, 633, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kralj Cigić, I.; Rupnik, S.; Rijavec, T.; Poklar Ulrih, N.; Cigić, B. Accumulation of Agmatine, Spermidine, and Spermine in Sprouts and Microgreens of Alfalfa, Fenugreek, Lentil, and Daikon Radish. Foods 2020, 9, 547. [Google Scholar] [CrossRef]

| White Mold-Ripened | Blue-Veined | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Raw | Pasteurized | Average | Raw | Pasteurized | Average | ||||
| CAM | BRI | CAM | BRI | ROQ | GOR | BLE | |||
| Ca (g/kg) | 2.7 | 3.7 | 5.5 | 4.5 | 4 ± 1 * | 6.9 | 6.2 | 6.1 | 6.4 ± 0.7 * |
| P (g/kg) | 3.1 | 3.7 | 4.2 | 3.5 | 3.6 ± 0.5 * | 4.6 | 4.4 | 4.3 | 4.4 ± 0.2 * |
| Zn (mg/kg) | 23.9 | 31.2 | 30.5 | 22.5 | 27 ± 5 | 21.5 | 36.5 | 29.6 | 29 ± 7 |
| Na (g/kg) | 5.9 | 6.6 | 6.4 | 6.1 | 6.2 ± 0.5 * | 11.5 | 8.5 | 9.5 | 9.8 ± 1.4 * |
| FAAs (mg/kg) | 404.4 | 305.3 | 221.0 | 189.5 | 300 ± 100 * | 1896.9 | 2865.4 | 1518.5 | 2100 ± 700 * |
| BAs (mg/kg) | 1494.6 | 1884.1 | 11.0 | 8.0 | 900 ± 900 | 24.6 | 18.6 | 10.0 | 18 ± 7 |
| GABA (mg/kg) | 25.0 | 180.8 | 13.1 | 51.7 | 70 ± 70 | 13.3 | 126.2 | 41.1 | 60 ± 60 |
| SPD (mg/kg) | 6.5 | 3.8 | 5.6 | 4.8 | 5 ± 2 * | 19.7 | 26.6 | 31.9 | 26 ± 6 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drašler, V.; Kralj Cigić, I.; Polak, T.; Marolt, G.; Imperl, J.; Čanžek Majhenič, A.; Cigić, B. Spatial Distribution of Minerals and Selected Bioactive Compounds in White Mold-Ripened and Blue-Veined Cheeses. Molecules 2025, 30, 3819. https://doi.org/10.3390/molecules30183819
Drašler V, Kralj Cigić I, Polak T, Marolt G, Imperl J, Čanžek Majhenič A, Cigić B. Spatial Distribution of Minerals and Selected Bioactive Compounds in White Mold-Ripened and Blue-Veined Cheeses. Molecules. 2025; 30(18):3819. https://doi.org/10.3390/molecules30183819
Chicago/Turabian StyleDrašler, Varineja, Irena Kralj Cigić, Tomaž Polak, Gregor Marolt, Jernej Imperl, Andreja Čanžek Majhenič, and Blaž Cigić. 2025. "Spatial Distribution of Minerals and Selected Bioactive Compounds in White Mold-Ripened and Blue-Veined Cheeses" Molecules 30, no. 18: 3819. https://doi.org/10.3390/molecules30183819
APA StyleDrašler, V., Kralj Cigić, I., Polak, T., Marolt, G., Imperl, J., Čanžek Majhenič, A., & Cigić, B. (2025). Spatial Distribution of Minerals and Selected Bioactive Compounds in White Mold-Ripened and Blue-Veined Cheeses. Molecules, 30(18), 3819. https://doi.org/10.3390/molecules30183819









