Phytochemicals as Epigenetic Modulators in Chronic Diseases: Molecular Mechanisms
Abstract
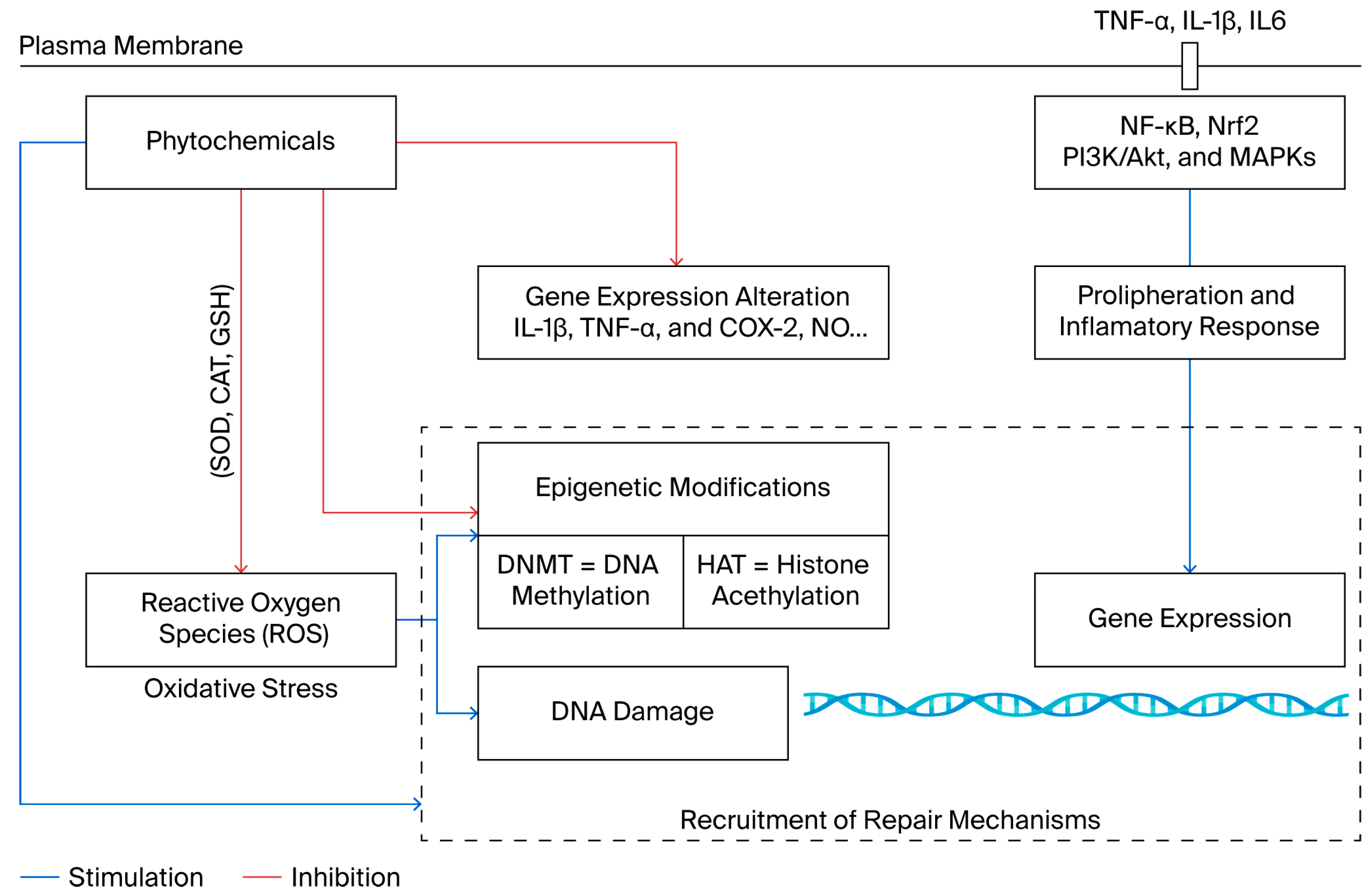
1. Introduction
2. Epigenetic Dysregulation in Chronic Diseases
2.1. Oxidative Stress Impacting Epigenetic Mechanisms
2.2. Epigenetic Dysregulation in the Inflammation Process
3. Phytochemicals as Antioxidant and Anti-Inflammatory Agents with Epigenetic Modulator Capacities
| Class of Compound | Phytochemicals | Study Design: Cell Culture Type; Phytochemicals Concentration | Key Outcomes | References | ||
|---|---|---|---|---|---|---|
| Antioxidant | Anti-Inflammatory | Epigenetic Modulator | ||||
| Flavonoids | Hesperidin | MCF-7 (50 µM) | Increases activities of SOD, CAT, GSH; reduces lipid peroxidation; activates Nrf2 | Reduces the expression of TNF-α and IL-6 | Inhibits DNMT1; hypo-methylation of p16 promoter | [54,55,56] |
| Phloretin | RAW 264.7 macrophages A549 cells (25 µM); | Inhibits ROS production and increases GSH activity | Suppresses NF-κB activation and reduces iNOS and COX-2 expression | Inhibits HDAC; increases histone acetylation, p21 expression | [139,140,141,142] | |
| Genistein | MCF-7, (10 µM) PC3 (25 µM–100 µM), HL-60 cells | Increases antioxidant enzymes; reduces lipid peroxidation | Reduces pro-inflammatory cytokines (IL-6, IL-1β) expression via NF-κB | Inhibits DNMT1, demethylates, and reactivates tumor suppressor genes | [143,144,145,146] | |
| Phenolic acids | Caffeic acid | Caco-2, HeLa cells (20 µM) | Neutralizes free radicals and boosts antioxidant enzymes | Inhibits pro-inflammatory cytokines and NF-κB activation | Inhibits DNMT; DNA hypomethylation; gene reactivation | [147,148,149] |
| Coumaric acid | LPS-stimulated RAW 264.7 cells; MDA-MB-231 cells (30 µM) | Free radical scavenger and increases SOD activity | Reduces NO production and COX-2 expression | Inhibits HDAC; increases histone acetylation | [150,151,152] | |
| Terpenoids | Lycopene | HepG2, PC3 cells (10 µM) | Reduces ROS and increases antioxidant enzyme activity | Inhibits COX-2 expression and lowers prostaglandin E2 levels | Inhibits DNMT1 and HDAC2 expression; modulates miR-let-7f-1/AKT2 axis; restores tumor suppressor genes (p21, RARB, SOCS3). | [136,153] |
| Silibinin | HepG2, DU145 cells (50 µM) | Increases SOD and CAT activity; reduces lipid peroxidation | Inhibits pro-inflammatory cytokines and NF-κB activation | Inhibits DNMT and HDAC; hypomethylation and histone acetylation | [154,155,156] | |
| Artemisinin | HeLa cells (50 µM) | Reduces oxidative stress by lowering ROS and activating Nrf2 | Inhibits IL-1β, TNF-α, and COX-2 expression | — | [157] | |
| Geraniol | RAW 264.7 macrophages (50 µM) | Reduces ROS production and increases GSH activity | Suppresses NO, IL-6, and TNF-α production by inhibiting NF-κB | — | [158] | |
| Organo-sulfur com-pounds | Allyl mercaptan | HUVEC, HT-29 cells (15 µM) | Enhances antioxidant enzyme activity and reduces ROS | Inhibits adhesion molecules and reduces vascular inflammation | Inhibits HDAC; increases histone acetylation; gene reactivation | [159,160] |
3.1. Flavonoids
3.2. Carotenoids
3.3. Phenolic Compounds
3.4. Organosulfur Compounds
3.5. Terpenoids
3.6. Synergistic Effects of Phytochemical Combinations
3.7. Challenges and Strategies for Clinical Translation
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Anjali; Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of Plant Secondary Metabolites in Defence and Transcriptional Regulation in Response to Biotic Stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Rabizadeh, F.; Mirian, M.S.; Doosti, R.; Kiani-Anbouhi, R.; Eftekhari, E. Phytochemical Classification of Medicinal Plants Used in the Treatment of Kidney Disease Based on Traditional Persian Medicine. Evid.-Based Complement. Altern. Med. 2022, 2022, 8022599. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of Plant Extracts upon Human Health: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Chihomvu, P.; Ganesan, A.; Gibbons, S.; Woollard, K.; Hayes, M.A. Phytochemicals in Drug Discovery—A Confluence of Tradition and Innovation. Int. J. Mol. Sci. 2024, 25, 8792. [Google Scholar] [CrossRef]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.; et al. Evolution of the Adaptogenic Concept from Traditional Use to Medical Systems: Pharmacology of Stress- and Aging-related Diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef]
- Bhat, R.A.; Hakeem, K.R.; Dervash, M.A. Phytomedicine: A Treasure of Pharmacologically Active Products from Plants; Academic Press: London, UK, 2021; ISBN 978-0-12-824109-7. [Google Scholar]
- Howes, M.R.; Perry, N.S.L.; Vásquez-Londoño, C.; Perry, E.K. Role of Phytochemicals as Nutraceuticals for Cognitive Functions Affected in Ageing. Br. J. Pharmacol. 2020, 177, 1294–1315. [Google Scholar] [CrossRef]
- Dar, R.A.; Shahnawaz, M.; Ahanger, M.A.; Majid, I.U. Exploring the Diverse Bioactive Compounds from Medicinal Plants: A Review. J. Phytopharm. 2023, 12, 189–195. [Google Scholar] [CrossRef]
- Albulescu, L.; Suciu, A.; Neagu, M.; Tanase, C.; Pop, S. Differential Biological Effects of Trifolium Pratense Extracts—In Vitro Studies on Breast Cancer Models. Antioxidants 2024, 13, 1435. [Google Scholar] [CrossRef]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Alarabei, A.A.; Abd Aziz, N.A.L.; Ab Razak, N.I.; Abas, R.; Bahari, H.; Abdullah, M.A.; Hussain, M.K.; Abdul Majid, A.M.S.; Basir, R. Immunomodulating Phytochemicals: An Insight into Their Potential Use in Cytokine Storm Situations. Adv. Pharm. Bull. 2023, 14, 105–119. [Google Scholar] [CrossRef]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Rajasingh, S.; Cao, T.; Dawn, B.; Rajasingh, J. Epigenetic Dysfunctional Diseases and Therapy for Infection and Inflammation. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 518–528. [Google Scholar] [CrossRef]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Kundu, T.K. Epigenetics: Development and Disease; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-4524-7. [Google Scholar]
- Bure, I.V.; Nemtsova, M.V.; Kuznetsova, E.B. Histone Modifications and Non-Coding RNAs: Mutual Epigenetic Regulation and Role in Pathogenesis. Int. J. Mol. Sci. 2022, 23, 5801. [Google Scholar] [CrossRef]
- Lorenzo, P.M.; Izquierdo, A.G.; Rodriguez-Carnero, G.; Fernández-Pombo, A.; Iglesias, A.; Carreira, M.C.; Tejera, C.; Bellido, D.; Martinez-Olmos, M.A.; Leis, R.; et al. Epigenetic Effects of Healthy Foods and Lifestyle Habits from the Southern European Atlantic Diet Pattern: A Narrative Review. Adv. Nutr. 2022, 13, 1725–1747. [Google Scholar] [CrossRef]
- Nakadate, K.; Ito, N.; Kawakami, K.; Yamazaki, N. Anti-Inflammatory Actions of Plant-Derived Compounds and Prevention of Chronic Diseases: From Molecular Mechanisms to Applications. Int. J. Mol. Sci. 2025, 26, 5206. [Google Scholar] [CrossRef]
- Dai, W.; Qiao, X.; Fang, Y.; Guo, R.; Bai, P.; Liu, S.; Li, T.; Jiang, Y.; Wei, S.; Na, Z.; et al. Epigenetics-Targeted Drugs: Current Paradigms and Future Challenges. Signal Transduct. Target. Ther. 2024, 9, 332. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Zhou, X.; Ding, L.; Liu, D.; Xu, H. DNMT1-Mediated PPARα Methylation Aggravates Damage of Retinal Tissues in Diabetic Retinopathy Mice. Biol. Res. 2021, 54, 25. [Google Scholar] [CrossRef] [PubMed]
- Gillette, T.G.; Hill, J.A. Readers, Writers, and Erasers: Chromatin as the Whiteboard of Heart Disease. Circ. Res. 2015, 116, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Pop, S.; Enciu, A.M.; Tarcomnicu, I.; Gille, E.; Tanase, C. Phytochemicals in Cancer Prevention: Modulating Epigenetic Alterations of DNA Methylation. Phytochem. Rev. 2019, 18, 1005–1024. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, C.; Wang, X. TET (Ten-Eleven Translocation) Family Proteins: Structure, Biological Functions and Applications. Signal Transduct. Target. Ther. 2023, 8, 297. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacol. Rev. 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Becker, P.B.; Workman, J.L. Nucleosome Remodeling and Epigenetics. Cold Spring Harb. Perspect. Biol. 2013, 5, a017905. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Lam, M.T.Y.; Glass, C.K. Non-Coding RNAs as Regulators of Gene Expression and Epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef]
- Pop, S.; Enciu, A.; Necula, L.G.; Tanase, C. Long Non-coding RNA s in Brain Tumours: Focus on Recent Epigenetic Findings in Glioma. J. Cell. Mol. Med. 2018, 22, 4597–4610. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic Regulation of Aging: Implications for Interventions of Aging and Diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-H.; Kuo, M.-Z.; Chen, I.-A.; Lin, C.-J.; Hsu, V.; HuangFu, W.-C.; Wu, T.-Y. Epigenetic Modifications as Novel Therapeutic Strategies of Cancer Chemoprevention by Phytochemicals. Pharm. Res. 2025, 42, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A.; Olhava, E.J.; Scott, M.P. Targeting Epigenetic Enzymes for Drug Discovery. Curr. Opin. Chem. Biol. 2010, 14, 505–510. [Google Scholar] [CrossRef]
- Casalino, L.; Verde, P. Multifaceted Roles of DNA Methylation in Neoplastic Transformation, from Tumor Suppressors to EMT and Metastasis. Genes 2020, 11, 922. [Google Scholar] [CrossRef]
- Lee, A.V.; Nestler, K.A.; Chiappinelli, K.B. Therapeutic Targeting of DNA Methylation Alterations in Cancer. Pharmacol. Ther. 2024, 258, 108640. [Google Scholar] [CrossRef] [PubMed]
- Brückmann, N.H.; Pedersen, C.B.; Ditzel, H.J.; Gjerstorff, M.F. Epigenetic Reprogramming of Pericentromeric Satellite DNA in Premalignant and Malignant Lesions. Mol. Cancer Res. 2018, 16, 417–427. [Google Scholar] [CrossRef]
- Sales, V.M.; Ferguson-Smith, A.C.; Patti, M.-E. Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations. Cell Metab. 2017, 25, 559–571. [Google Scholar] [CrossRef]
- Keleher, M.R.; Zaidi, R.; Hicks, L.; Shah, S.; Xing, X.; Li, D.; Wang, T.; Cheverud, J.M. A High-Fat Diet Alters Genome-Wide DNA Methylation and Gene Expression in SM/J Mice. BMC Genom. 2018, 19, 888. [Google Scholar] [CrossRef]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.; Salvador, C.; Skibola, C.; Tollefsbol, T.O. Influences of Diet and the Gut Microbiome on Epigenetic Modulation in Cancer and Other Diseases. Clin. Epigenet. 2015, 7, 112. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Aanniz, T.; Bouyahya, A.; Balahbib, A.; El Kadri, K.; Khalid, A.; Makeen, H.A.; Alhazmi, H.A.; El Omari, N.; Zaid, Y.; Wong, R.S.-Y.; et al. Natural Bioactive Compounds Targeting DNA Methyltransferase Enzymes in Cancer: Mechanisms Insights and Efficiencies. Chem.-Biol. Interact. 2024, 392, 110907. [Google Scholar] [CrossRef]
- Zheng, X.; Sawalha, A.H. The Role of Oxidative Stress in Epigenetic Changes Underlying Autoimmunity. Antioxid. Redox Signal. 2022, 36, 423–440. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Ray, B.K.; Roychoudhury, S. Handbook of Oxidative Stress in Cancer; Springer: Singapore, 2022; ISBN 978-981-15-9410-6. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Silva-Llanes, I.; Shin, C.H.; Jiménez-Villegas, J.; Gorospe, M.; Lastres-Becker, I. The Transcription Factor NRF2 Has Epigenetic Regulatory Functions Modulating HDACs, DNMTs, and miRNA Biogenesis. Antioxidants 2023, 12, 641. [Google Scholar] [CrossRef]
- Kietzmann, T.; Petry, A.; Shvetsova, A.; Gerhold, J.M.; Görlach, A. The Epigenetic Landscape Related to Reactive Oxygen Species Formation in the Cardiovascular System. Br. J. Pharmacol. 2017, 174, 1533–1554. [Google Scholar] [CrossRef]
- Madugundu, G.S.; Cadet, J.; Wagner, J.R. Hydroxyl-Radical-Induced Oxidation of 5-Methylcytosine in Isolated and Cellular DNA. Nucleic Acids Res. 2014, 42, 7450–7460. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Lü, J.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez De La Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Antioxidant Metabolism Pathways in Vitamins, Polyphenols, and Selenium: Parallels and Divergences. Int. J. Mol. Sci. 2024, 25, 2600. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Qin, S.; Chen, Z.; Wen, Y.; Yi, Y.; Lv, C.; Zeng, C.; Chen, L.; Shi, M. Phytochemical Activators of Nrf2: A Review of Therapeutic Strategy in Diabetes. Acta Biochim. Biophys. Sin. 2022, 55, 11. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; Astudillo-de La Vega, H.; Hernández De La Cruz, O.N.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Yasmeen, F.; Pirzada, R.H.; Ahmad, B.; Choi, B.; Choi, S. Understanding Autoimmunity: Mechanisms, Predisposing Factors, and Cytokine Therapies. Int. J. Mol. Sci. 2024, 25, 7666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Meng, Y.; Zhou, L.; Qiu, L.; Wang, H.; Su, D.; Zhang, B.; Chan, K.; Han, J. Targeting Epigenetic Regulators for Inflammation: Mechanisms and Intervention Therapy. MedComm 2022, 3, e173. [Google Scholar] [CrossRef]
- Rycyk-Bojarzyńska, A.; Kasztelan-Szczerbińska, B.; Cichoż-Lach, H.; Jargieło, A. Ganglioneuromatous Polyposis Associated with Type 2 B Multiple Endocrine Neoplasia (MEN 2B)—Case Report. Ann. Agric. Environ. Med. 2024, 31, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Kaszycki, J.; Kim, M. Epigenetic Regulation of Transcription Factors Involved in NLRP3 Inflammasome and NF-kB Signaling Pathways. Front. Immunol. 2025, 16, 1529756. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Z.; Shen, W.; Huang, G.; Sedivy, J.M.; Wang, H.; Ju, Z. Inflammation, Epigenetics, and Metabolism Converge to Cell Senescence and Ageing: The Regulation and Intervention. Signal Transduct. Target. Ther. 2021, 6, 245. [Google Scholar] [CrossRef]
- Wu, D.; Shi, Y.; Zhang, H.; Miao, C. Epigenetic Mechanisms of Immune Remodeling in Sepsis: Targeting Histone Modification. Cell Death Dis. 2023, 14, 112. [Google Scholar] [CrossRef]
- Maiolino, G.; Fernández-Pascual, E.; Ochoa Arvizo, M.A.; Vishwakarma, R.; Martínez-Salamanca, J.I. Male Infertility and the Risk of Developing Testicular Cancer: A Critical Contemporary Literature Review. Medicina 2023, 59, 1305. [Google Scholar] [CrossRef]
- Jurkowska, R.Z. Role of Epigenetic Mechanisms in the Pathogenesis of Chronic Respiratory Diseases and Response to Inhaled Exposures: From Basic Concepts to Clinical Applications. Pharmacol. Ther. 2024, 264, 108732. [Google Scholar] [CrossRef]
- Tan, S.Y.X.; Zhang, J.; Tee, W.-W. Epigenetic Regulation of Inflammatory Signaling and Inflammation-Induced Cancer. Front. Cell Dev. Biol. 2022, 10, 931493. [Google Scholar] [CrossRef]
- Vezzani, B.; Carinci, M.; Previati, M.; Giacovazzi, S.; Della Sala, M.; Gafà, R.; Lanza, G.; Wieckowski, M.R.; Pinton, P.; Giorgi, C. Epigenetic Regulation: A Link between Inflammation and Carcinogenesis. Cancers 2022, 14, 1221. [Google Scholar] [CrossRef]
- Rickey, L.M.; Mueller, E.R.; Newman, D.K.; Markland, A.D.; Falke, C.; Rudser, K.; Smith, A.L.; Mueller, M.G.; Lowder, J.L.; Lukacz, E.S.; et al. Reliability of Uroflowmetry Pattern Interpretation in Adult Women. Neurourol. Urodyn. 2024, 43, 2084–2092. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Dang, Y.-Q.; Ji, G. Role of Epigenetics in Transformation of Inflammation into Colorectal Cancer. World J. Gastroenterol. 2019, 25, 2863–2877. [Google Scholar] [CrossRef] [PubMed]
- Pandareesh, M.D.; Kameshwar, V.H.; Byrappa, K. Prostate Carcinogenesis: Insights in Relation to Epigenetics and Inflammation. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 253–267. [Google Scholar] [CrossRef]
- Deer, T.; Heros, R.; Tavel, E.; Wahezi, S.; Funk, R.; Buchanan, P.; Christopher, A.; Weisbein, J.; Gilligan, C.; Patterson, D.; et al. Comparing Conventional Medical Management to Spinal Cord Stimulation for the Treatment of Low Back Pain in a Cohort of DISTINCT RCT Patients. J. Pain Res. 2024, 17, 2741–2752. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; White, K.; Rostami, K. Pro and Anti-Inflammatory Diets as Strong Epigenetic Factors in Inflammatory Bowel Disease. World J. Gastroenterol. 2024, 30, 3284–3289. [Google Scholar] [CrossRef]
- Liu, L.; Ni, S.; Zhang, L.; Chen, Y.; Xie, M.; Huang, X. Molecular Insights and Clinical Implications of DNA Methylation in Sepsis-Associated Acute Kidney Injury: A Narrative Review. BMC Nephrol. 2025, 26, 253. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Karthik, N.; Taneja, R. Crosstalk Between Inflammatory Signaling and Methylation in Cancer. Front. Cell Dev. Biol. 2021, 9, 756458. [Google Scholar] [CrossRef]
- Bagni, G.; Biancalana, E.; Chiara, E.; Costanzo, I.; Malandrino, D.; Lastraioli, E.; Palmerini, M.; Silvestri, E.; Urban, M.L.; Emmi, G. Epigenetics in Autoimmune Diseases: Unraveling the Hidden Regulators of Immune Dysregulation. Autoimmun. Rev. 2025, 24, 103784. [Google Scholar] [CrossRef]
- Li, C.; Gu, S.; Zhang, Y.; Zhang, Z.; Wang, J.; Gao, T.; Zhong, K.; Shan, K.; Ye, G.; Ke, Y.; et al. Histone Deacetylase in Inflammatory Bowel Disease: Novel Insights. Therap. Adv. Gastroenterol. 2025, 18, 17562848251318833. [Google Scholar] [CrossRef]
- Bacher, S.; Meier-Soelch, J.; Kracht, M.; Schmitz, M.L. Regulation of Transcription Factor NF-κB in Its Natural Habitat: The Nucleus. Cells 2021, 10, 753. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Tak, P.P.; Reedquist, K.A. Targeting Histone Deacetylase Activity in Rheumatoid Arthritis and Asthma as Prototypes of Inflammatory Disease: Should We Keep Our HATs On? Arthritis Res. Ther. 2008, 10, 226. [Google Scholar] [CrossRef][Green Version]
- Susini, P.; Marcaccini, G.; Cuomo, R.; Grimaldi, L.; Nisi, G. Thighs Lift in the Post-Bariatric Patient—A Systematic Review. J. Plast. Reconstr. Aesthet. Surg. 2024, 98, 357–372. [Google Scholar] [CrossRef]
- Alivernini, S.; Gremese, E.; McSharry, C.; Tolusso, B.; Ferraccioli, G.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155—At the Critical Interface of Innate and Adaptive Immunity in Arthritis. Front. Immunol. 2018, 8, 1932. [Google Scholar] [CrossRef]
- Raisch, J. Role of microRNAs in the Immune System, Inflammation and Cancer. World J. Gastroenterol. 2013, 19, 2985. [Google Scholar] [CrossRef] [PubMed]
- Phimmachanh, M.; Han, J.Z.R.; O’Donnell, Y.E.I.; Latham, S.L.; Croucher, D.R. Histone Deacetylases and Histone Deacetylase Inhibitors in Neuroblastoma. Front. Cell Dev. Biol. 2020, 8, 578770. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.J.; Shanle, E.K.; Khan, A.; Chua, K.F.; Hong, T.; Boxer, L.D.; Allis, C.D.; Josefowicz, S.Z.; Garcia, B.A.; Rothbart, S.B.; et al. HDAC Inhibition Results in Widespread Alteration of the Histone Acetylation Landscape and BRD4 Targeting to Gene Bodies. Cell Rep. 2021, 34, 108638. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Attal, Z.G.; Solomon, A.; Shalata, S.; Abu Saleh, O.; Tourkey, L.; Abu Salamah, F.; Alatawneh, I.; Yakobson, A. Melanoma Management: Exploring Staging, Prognosis, and Treatment Innovations. Int. J. Mol. Sci. 2024, 25, 5794. [Google Scholar] [CrossRef] [PubMed]
- Al Bitar, S.; Gali-Muhtasib, H. The Role of the Cyclin Dependent Kinase Inhibitor P21cip1/Waf1 in Targeting Cancer: Molecular Mechanisms and Novel Therapeutics. Cancers 2019, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Sturmlechner, I.; Zhang, C.; Sine, C.C.; Van Deursen, E.-J.; Jeganathan, K.B.; Hamada, N.; Grasic, J.; Friedman, D.; Stutchman, J.T.; Can, I.; et al. P21 Produces a Bioactive Secretome That Places Stressed Cells under Immunosurveillance. Science 2021, 374, eabb3420. [Google Scholar] [CrossRef]
- Arora, I.; Sharma, M.; Sun, L.Y.; Tollefsbol, T.O. The Epigenetic Link between Polyphenols, Aging and Age-Related Diseases. Genes 2020, 11, 1094. [Google Scholar] [CrossRef]
- Manoukian, P.; Kuhnen, L.C.; van Laarhoven, H.W.M.; Bijlsma, M.F. Association of Epigenetic Landscapes with Heterogeneity and Plasticity in Pancreatic Cancer. Crit. Rev. Oncol. Hematol. 2025, 206, 104573. [Google Scholar] [CrossRef]
- Khan, M.I.; Rath, S.; Adhami, V.M.; Mukhtar, H. Targeting Epigenome with Dietary Nutrients in Cancer: Current Advances and Future Challenges. Pharmacol. Res. 2018, 129, 375–387. [Google Scholar] [CrossRef]
- Sood, U.; Garg, G.; Lal, R. Editorial: Thematic Issue on Modulating the Environment with Microbes. FEMS Microbes 2024, 5, xtae021. [Google Scholar] [CrossRef] [PubMed]
- He, W.-J.; Lv, C.-H.; Chen, Z.; Shi, M.; Zeng, C.-X.; Hou, D.-X.; Qin, S. The Regulatory Effect of Phytochemicals on Chronic Diseases by Targeting Nrf2-ARE Signaling Pathway. Antioxidants 2023, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, V.; Emanuelli, M.; Sartini, D.; Salvolini, E.; Pozzi, V.; Campagna, R. Curcumin and Its Analogues in Oral Squamous Cell Carcinoma: State-of-the-Art and Therapeutic Potential. Anticancer Agents Med. Chem. 2025, 25, 313–329. [Google Scholar] [CrossRef]
- Campagna, R.; Cecati, M.; Vignini, A. The Multifaceted Role of the Polyphenol Curcumin: A Focus on Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2025, 21, e15733998313402. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, V.; Emanuelli, M.; Milanese, G.; Galosi, A.B.; Pompei, V.; Salvolini, E.; Campagna, R. Nrf2 Signaling in Renal Cell Carcinoma: A Potential Candidate for the Development of Novel Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 13239. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Hou, L.; Luo, W.; Pan, L.-H.; Li, X.; Tan, H.-P.; Wu, R.-D.; Lu, H.; Yao, K.; Mu, M.-D.; et al. Myocardial Infarction Drives Trained Immunity of Monocytes, Accelerating Atherosclerosis. Eur. Heart J. 2024, 45, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Bergonzini, M.; Loreni, F.; Lio, A.; Russo, M.; Saitto, G.; Cammardella, A.; Irace, F.; Tramontin, C.; Chello, M.; Lusini, M.; et al. Panoramic on Epigenetics in Coronary Artery Disease and the Approach of Personalized Medicine. Biomedicines 2023, 11, 2864. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Marsit, C.J. Influence of Environmental Exposure on Human Epigenetic Regulation. J. Exp. Biol. 2015, 218, 71–79. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Li, Y.; Bai, G.; Pang, J.; Wu, M.; Li, J.; Zhao, X.; Xia, Y. Implications of Gut Microbiota-Mediated Epigenetic Modifications in Intestinal Diseases. Gut Microbes 2025, 17, 2508426. [Google Scholar] [CrossRef]
- Begum, N.; Mandhare, A.; Tryphena, K.P.; Srivastava, S.; Shaikh, M.F.; Singh, S.B.; Khatri, D.K. Epigenetics in Depression and Gut-Brain Axis: A Molecular Crosstalk. Front. Aging Neurosci. 2022, 14, 1048333. [Google Scholar] [CrossRef]
- Liu, X.; Shao, J.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.; Liu, Z.; He, D.; Li, C.; Zhang, X. Regulation of Short-Chain Fatty Acids in the Immune System. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Rubio, K.; Hernández-Cruz, E.Y.; Rogel-Ayala, D.G.; Sarvari, P.; Isidoro, C.; Barreto, G.; Pedraza-Chaverri, J. Nutriepigenomics in Environmental-Associated Oxidative Stress. Antioxidants 2023, 12, 771. [Google Scholar] [CrossRef]
- Ji, H.; Khurana Hershey, G.K. Genetic and Epigenetic Influence on the Response to Environmental Particulate Matter. J. Allergy Clin. Immunol. 2012, 129, 33–41. [Google Scholar] [CrossRef]
- Breton, C.V.; Landon, R.; Kahn, L.G.; Enlow, M.B.; Peterson, A.K.; Bastain, T.; Braun, J.; Comstock, S.S.; Duarte, C.S.; Hipwell, A.; et al. Exploring the Evidence for Epigenetic Regulation of Environmental Influences on Child Health across Generations. Commun. Biol. 2021, 4, 769. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-M.; Johnson, A.; Tarapore, P.; Janakiram, V.; Zhang, X.; Leung, Y.-K. Environmental Epigenetics and Its Implication on Disease Risk and Health Outcomes. ILAR J. 2012, 53, 289–305, Erratum in ILAR J. 2017, 58, 413. [Google Scholar] [CrossRef]
- Alegría-Torres, J.A.; Baccarelli, A.; Bollati, V. Epigenetics and Lifestyle. Epigenomics 2011, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Barouki, R.; Melén, E.; Herceg, Z.; Beckers, J.; Chen, J.; Karagas, M.; Puga, A.; Xia, Y.; Chadwick, L.; Yan, W.; et al. Epigenetics as a Mechanism Linking Developmental Exposures to Long-Term Toxicity. Environ. Int. 2018, 114, 77–86. [Google Scholar] [CrossRef]
- Wang, G.; Su, H.; Guo, Z.; Li, H.; Jiang, Z.; Cao, Y.; Li, C. Rubus Occidentalis and Its Bioactive Compounds against Cancer: From Molecular Mechanisms to Translational Advances. Phytomedicine 2024, 126, 155029. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Zhong, M.; Chen, Y.; Su, W.; Li, P. Epigenetic Regulation by Naringenin and Naringin: A Literature Review Focused on the Mechanisms Underlying Its Pharmacological Effects. Fitoterapia 2025, 181, 106353. [Google Scholar] [CrossRef]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Pricl, S.; Townley, H.; Thorat, N. Novel Epigenetic Therapeutic Strategies and Targets in Cancer. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2022, 1868, 166552. [Google Scholar] [CrossRef] [PubMed]
- Lagosz-Cwik, K.B.; Melnykova, M.; Nieboga, E.; Schuster, A.; Bysiek, A.; Dudek, S.; Lipska, W.; Kantorowicz, M.; Tyrakowski, M.; Darczuk, D.; et al. Mapping of DNA Methylation-Sensitive Cellular Processes in Gingival and Periodontal Ligament Fibroblasts in the Context of Periodontal Tissue Homeostasis. Front. Immunol. 2023, 14, 1078031. [Google Scholar] [CrossRef]
- Nisar, A.; Jagtap, S.; Vyavahare, S.; Deshpande, M.; Harsulkar, A.; Ranjekar, P.; Prakash, O. Phytochemicals in the Treatment of Inflammation-Associated Diseases: The Journey from Preclinical Trials to Clinical Practice. Front. Pharmacol. 2023, 14, 1177050. [Google Scholar] [CrossRef]
- Hossain, M.S.; Wazed, M.A.; Asha, S.; Amin, M.R.; Shimul, I.M. Dietary Phytochemicals in Health and Disease: Mechanisms, Clinical Evidence, and Applications—A Comprehensive Review. Food Sci. Amp. Nutr. 2025, 13, e70101. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.B.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Inflammatory Biomarkers and Immune Cell Populations: A Systematic Literature Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef]
- Caban, M.; Lewandowska, U. Polyphenols and the Potential Mechanisms of Their Therapeutic Benefits against Inflammatory Bowel Diseases. J. Funct. Foods 2022, 95, 105181. [Google Scholar] [CrossRef]
- Hasnat, H.; Shompa, S.A.; Islam, M.M.; Alam, S.; Richi, F.T.; Emon, N.U.; Ashrafi, S.; Ahmed, N.U.; Chowdhury, M.N.R.; Fatema, N.; et al. Flavonoids: A Treasure House of Prospective Pharmacological Potentials. Heliyon 2024, 10, e27533. [Google Scholar] [CrossRef]
- Fahmy, M.I.; Sadek, M.A.; Abdou, K.; El-Dessouki, A.M.; El-Shiekh, R.A.; Khalaf, S.S. Orientin: A Comprehensive Review of a Promising Bioactive Flavonoid. Inflammopharmacology 2025, 33, 1713–1728. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Scheidereit, C. The IκB Kinase Complex in NF-κB Regulation and Beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef]
- Roberti, A.; Chaffey, L.E.; Greaves, D.R. NF-κB Signaling and Inflammation-Drug Repurposing to Treat Inflammatory Disorders? Biology 2022, 11, 372. [Google Scholar] [CrossRef]
- Chauhan, A.; Islam, A.U.; Prakash, H.; Singh, S. Phytochemicals Targeting NF-κB Signaling: Potential Anti-Cancer Interventions. J. Pharm. Anal. 2022, 12, 394–405. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Romero-Durán, M.A.; Silva-García, O.; Perez-Aguilar, J.M.; Baizabal-Aguirre, V.M. Mechanisms of Keap1/Nrf2 Modulation in Bacterial Infections: Implications in Persistence and Clearance. Front. Immunol. 2024, 15, 1508787. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Bhattacharya, H.; Bhattacharyya, C.; Chakraborty, P.; Fleishman, J.; Alexiou, A.; Papadakis, M.; Jha, S.K. Nrf2/Keap1/ARE Regulation by Plant Secondary Metabolites: A New Horizon in Brain Tumor Management. Cell Commun. Signal. 2024, 22, 497. [Google Scholar] [CrossRef] [PubMed]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An Update of Nrf2 Activators and Inhibitors in Cancer Prevention/Promotion. Cell Commun. Signal. 2022, 20, 100. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.-Y.; Tang, F.; Liu, D.; Zhao, X.-L.; Zhang, J.-N.; Xia, J.; Wu, J.-J.; Yang, Y.; Peng, C.; et al. New Perspectives on the Therapeutic Potential of Quercetin in Non-Communicable Diseases: Targeting Nrf2 to Counteract Oxidative Stress and Inflammation. J. Pharm. Anal. 2024, 14, 100930. [Google Scholar] [CrossRef]
- Sharbafshaaer, M.; Pepe, R.; Notariale, R.; Canale, F.; Tedeschi, G.; Tessitore, A.; Bergamo, P.; Trojsi, F. Beyond Antioxidants: The Emerging Role of Nrf2 Activation in Amyotrophic Lateral Sclerosis (ALS). Int. J. Mol. Sci. 2025, 26, 9872. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Powell, K.; Giliberto, L.; LeDoux, C.; d’Abramo, C.; Sciubba, D.; Al Abed, Y. Non-Electrophilic Activation of NRF2 in Neurological Disorders: Therapeutic Promise of Non-Pharmacological Strategies. Antioxidants 2025, 14, 1047. [Google Scholar] [CrossRef]
- Schreiber, S.P.; Villalba, J.; Meyer-Ficca, M.L. Potential Epigenetic Impacts of Phytochemicals on Ruminant Health and Production: Connecting Lines of Evidence. Animals 2025, 15, 1787. [Google Scholar] [CrossRef]
- Thakur, V.S.; Deb, G.; Babcook, M.A.; Gupta, S. Plant Phytochemicals as Epigenetic Modulators: Role in Cancer Chemoprevention. AAPS J. 2014, 16, 151–163. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, H.; Li, H.; Wang, X.; Wei, P.; Yi, L.; Lin, S. Epigenetic Insights into Aging: Emerging Roles of Natural Products in Therapeutic Interventions. Phytother. Res. 2025, 39, 3300–3322. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Li, Y.; Lei, D.; Xiang, J.; Ouyang, L.; Wang, Y.; Yang, J. Recent Progress in DNA Methyltransferase Inhibitors as Anticancer Agents. Front. Pharmacol. 2022, 13, 1072651. [Google Scholar] [CrossRef]
- Akone, S.H.; Ntie-Kang, F.; Stuhldreier, F.; Ewonkem, M.B.; Noah, A.M.; Mouelle, S.E.M.; Müller, R. Natural Products Impacting DNA Methyltransferases and Histone Deacetylases. Front. Pharmacol. 2020, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Daskalos, A.; Oleksiewicz, U.; Filia, A.; Nikolaidis, G.; Xinarianos, G.; Gosney, J.R.; Malliri, A.; Field, J.K.; Liloglou, T. UHRF1-mediated Tumor Suppressor Gene Inactivation in Nonsmall Cell Lung Cancer. Cancer 2011, 117, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Cacan, E.; Ali, M.W.; Boyd, N.H.; Hooks, S.B.; Greer, S.F. Inhibition of HDAC1 and DNMT1 Modulate RGS10 Expression and Decrease Ovarian Cancer Chemoresistance. PLoS ONE 2014, 9, e87455. [Google Scholar] [CrossRef] [PubMed]
- Číž, M.; Dvořáková, A.; Skočková, V.; Kubala, L. The Role of Dietary Phenolic Compounds in Epigenetic Modulation Involved in Inflammatory Processes. Antioxidants 2020, 9, 691. [Google Scholar] [CrossRef]
- Boaru, D.L.; Fraile-Martinez, O.; De Leon-Oliva, D.; Garcia-Montero, C.; De Castro-Martinez, P.; Miranda-Gonzalez, A.; Saez, M.A.; Muñon-Zamarron, L.; Castillo-Ruiz, E.; Barrena-Blázquez, S.; et al. Harnessing the Anti-Inflammatory Properties of Polyphenols in the Treatment of Inflammatory Bowel Disease. Int. J. Biol. Sci. 2024, 20, 5608–5672. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez De Mejia, E. Phenolic Compounds from Coffee By-Products Modulate Adipogenesis-Related Inflammation, Mitochondrial Dysfunction, and Insulin Resistance in Adipocytes, via Insulin/PI3K/AKT Signaling Pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef]
- Dierckx, T.; Haidar, M.; Grajchen, E.; Wouters, E.; Vanherle, S.; Loix, M.; Boeykens, A.; Bylemans, D.; Hardonnière, K.; Kerdine-Römer, S.; et al. Phloretin suppresses neuroinflammation by autophagy-mediated Nrf2 activation in macrophages. J. Neuroinflammation 2021, 18, 148. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, M.; Hong, Y.; Wang, S.; Xu, Y.; Zhong, C.; Zhang, J.; Zhuang, Z.; Shan, S.; Ren, T. Isovalerylspiramycin I Suppresses Non-Small Cell Lung Carcinoma Growth through ROS-Mediated Inhibition of PI3K/AKT Signaling Pathway. Int. J. Biol. Sci. 2022, 18, 3714–3730. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Record, I.R.; Dreosti, I.E.; McInerney, J.K. The Antioxidant Activity of Genistein in Vitro. J. Nutr. Biochem. 1995, 6, 481–485. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, W.-S.; Kim, M.E.; Lee, H.-W.; Youn, H.-Y.; Seon, J.K.; Lee, H.-N.; Lee, J.S. Genistein Inhibits Pro-Inflammatory Cytokines in Human Mast Cell Activation through the Inhibition of the ERK Pathway. Int. J. Mol. Med. 2014, 34, 1669–1674. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhu, G.; Peng, Q.; Li, X.; Zou, X.; Zhang, W.; Zhao, L.; Li, X.; Wu, P.; Luo, A.; et al. Natural Products Combating EGFR-TKIs Resistance in Cancer. Eur. J. Med. Chem. Rep. 2025, 13, 100251. [Google Scholar] [CrossRef]
- Zhao, Z.; Shin, H.S.; Satsu, H.; Totsuka, M.; Shimizu, M. 5-Caffeoylquinic Acid and Caffeic Acid Down-Regulate the Oxidative Stress- and TNF-α-Induced Secretion of Interleukin-8 from Caco-2 Cells. J. Agric. Food Chem. 2008, 56, 3863–3868. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Bukowska-Strakova, K.; Kocemba-Pilarczyk, K.A.; Majka, M. Caffeic Acid Targets AMPK Signaling and Regulates Tricarboxylic Acid Cycle Anaplerosis While Metformin Downregulates HIF-1α-Induced Glycolytic Enzymes in Human Cervical Squamous Cell Carcinoma Lines. Nutrients 2018, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yue, P.; Dickinson, C.F.; Yang, J.K.; Datanagan, K.; Zhai, N.; Zhang, Y.; Miklossy, G.; Lopez-Tapia, F.; Tius, M.A.; et al. Natural Product Preferentially Targets Redox and Metabolic Adaptations and Aberrantly Active STAT3 to Inhibit Breast Tumor Growth in Vivo. Cell Death Dis. 2022, 13, 1022. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, J. Anti-Inflammatory Effects of p-Coumaric Acid in LPS-Stimulated RAW264.7 Cells: Involvement of NF-κB and MAPKs Pathways. Med. Chem. 2016, 6, 327–330. [Google Scholar] [CrossRef]
- Wu, C.; Sun, C.; Han, X.; Ye, Y.; Qin, Y.; Liu, S. Sanyin Formula Enhances the Therapeutic Efficacy of Paclitaxel in Triple-Negative Breast Cancer Metastases through the JAK/STAT3 Pathway in Mice. Pharmaceuticals 2022, 16, 9. [Google Scholar] [CrossRef]
- Casari, G.; Romaldi, B.; Scirè, A.; Minnelli, C.; Marzioni, D.; Ferretti, G.; Armeni, T. Epigenetic Properties of Compounds Contained in Functional Foods Against Cancer. Biomolecules 2024, 15, 15. [Google Scholar] [CrossRef]
- Surai, P. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Hachlafi, N.; Aanniz, T.; Bourais, I.; Mechchate, H.; Benali, T.; Shariati, M.A.; Burkov, P.; Lorenzo, J.M.; Wilairatana, P.; et al. Natural Bioactive Compounds Targeting Histone Deacetylases in Human Cancers: Recent Updates. Molecules 2022, 27, 2568. [Google Scholar] [CrossRef]
- Neelab; Zeb, A.; Jamil, M. Milk Thistle Protects against Non-Alcoholic Fatty Liver Disease Induced by Dietary Thermally Oxidized Tallow. Heliyon 2024, 10, e31445. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, A.; Nguyen, L.C.; Shamim, H.M.; Iida, T.; Nakase, M.; Takegawa, K.; Senda, M.; Jida, S.; Ueno, M. Mutation in Fission Yeast Phosphatidylinositol 4-Kinase Pik1 Is Synthetically Lethal with Defect in Telomere Protection Protein Pot1. Biochem. Biophys. Res. Commun. 2018, 496, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Baş, H.; Kalender, Y.; Pandir, D.; Kalender, S. Effects of Lead Nitrate and Sodium Selenite on DNA Damage and Oxidative Stress in Diabetic and Non-Diabetic Rat Erythrocytes and Leucocytes. Environ. Toxicol. Pharmacol. 2015, 39, 1019–1026. [Google Scholar] [CrossRef]
- Astrain-Redin, N.; Sanmartin, C.; Sharma, A.K.; Plano, D. From Natural Sources to Synthetic Derivatives: The Allyl Motif as a Powerful Tool for Fragment-Based Design in Cancer Treatment. J. Med. Chem. 2023, 66, 3703–3731. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, H.; Xu, Y.; Wang, X.; Qiu, Z.; Jiang, L. Allicin Decreases Lipopolysaccharide-Induced Oxidative Stress and Inflammation in Human Umbilical Vein Endothelial Cells through Suppression of Mitochondrial Dysfunction and Activation of Nrf2. Cell Physiol. Biochem. 2017, 41, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gu, H.; Ye, Y.; Lin, B.; Sun, L.; Deng, W.; Zhang, J.; Liu, J. Protective Effects of Hesperidin against Oxidative Stress of Tert-Butyl Hydroperoxide in Human Hepatocytes. Food Chem. Toxicol. 2010, 48, 2980–2987. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2021, 12, 29. [Google Scholar] [CrossRef]
- Li, C.; Schluesener, H. Health-Promoting Effects of the Citrus Flavanone Hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Martorell, M.; Capó, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential Anti-Inflammatory Effects of Hesperidin from the Genus Citrus. Curr. Med. Chem. 2019, 25, 4929–4945. [Google Scholar] [CrossRef]
- Fernández-Bedmar, Z.; Anter, J.; Alonso-Moraga, A.; Martín De Las Mulas, J.; Millán-Ruiz, Y.; Guil-Luna, S. Demethylating and Anti-hepatocarcinogenic Potential of Hesperidin, a Natural Polyphenol of Citrus Juices. Mol. Carcinog. 2017, 56, 1653–1662. [Google Scholar] [CrossRef]
- Jiang, W.; Xia, T.; Liu, C.; Li, J.; Zhang, W.; Sun, C. Remodeling the Epigenetic Landscape of Cancer—Application Potential of Flavonoids in the Prevention and Treatment of Cancer. Front. Oncol. 2021, 11, 705903. [Google Scholar] [CrossRef]
- Dixon, R. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A Review on Its Anti-Inflammatory Properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Cassidy, A. Dietary Isoflavones: Biological Effects and Relevance to Human Health. J. Nutr. 1999, 129, 758S–767S. [Google Scholar] [CrossRef]
- Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Rossi, A.; Sacerdote, P.; Colleoni, M. The Soy Isoflavone Genistein Reverses Oxidative and Inflammatory State, Neuropathic Pain, Neurotrophic and Vasculature Deficits in Diabetes Mouse Model. Eur. J. Pharmacol. 2011, 650, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yuan, L.; Zhao, X.; Hou, C.; Ma, W.; Yu, H.; Xiao, R. Genistein Antagonizes Inflammatory Damage Induced by β-Amyloid Peptide in Microglia through TLR4 and NF-κB. Nutrition 2014, 30, 90–95. [Google Scholar] [CrossRef]
- Smolińska, E.; Moskot, M.; Jakóbkiewicz-Banecka, J.; Węgrzyn, G.; Banecki, B.; Szczerkowska-Dobosz, A.; Purzycka-Bohdan, D.; Gabig-Cimińska, M. Molecular Action of Isoflavone Genistein in the Human Epithelial Cell Line HaCaT. PLoS ONE 2018, 13, e0192297. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Asmawi, M.; Vuorela, P.; Vapaatalo, H.; Moilanen, E. Effects of Flavonoids on Prostaglandin E2 Production and on COX-2 and mPGES-1 Expressions in Activated Macrophages. Planta Med. 2011, 77, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.-P.; Kim, S.-W.; Ma, S.H.; Park, B.; Ahn, Y.; Lee, J.W.; Lee, M.H.; Kang, E.; Kim, L.S.; Jung, Y.; et al. Dietary Intake and Breast Cancer among Carriers and Noncarriers of BRCA Mutations in the Korean Hereditary Breast Cancer Study. Am. J. Clin. Nutr. 2013, 98, 1493–1501. [Google Scholar] [CrossRef]
- Bilir, B.; Sharma, N.V.; Lee, J.; Hammarstrom, B.; Svindland, A.; Kucuk, O.; Moreno, C.S. Effects of Genistein Supplementation on Genome-Wide DNA Methylation and Gene Expression in Patients with Localized Prostate Cancer. Int. J. Oncol. 2017, 51, 223–234. [Google Scholar] [CrossRef]
- Lu, H.; Shi, J.-X.; Zhang, D.-M.; Wang, H.-D.; Hang, C.-H.; Chen, H.-L.; Yin, H.-X. Inhibition of Hemolysate-Induced iNOS and COX-2 Expression by Genistein through Suppression of NF-κB Activation in Primary Astrocytes. J. Neurol. Sci. 2009, 278, 91–95. [Google Scholar] [CrossRef]
- Habtemariam, S. The Molecular Pharmacology of Phloretin: Anti-Inflammatory Mechanisms of Action. Biomedicines 2023, 11, 143. [Google Scholar] [CrossRef]
- Hao, X.; Bai, Y.; Li, W.; Zhang, M.X. Phloretin Attenuates Inflammation Induced by Subarachnoid Hemorrhage through Regulation of the TLR2/MyD88/NF-kB Pathway. Sci. Rep. 2024, 14, 26583. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, G.; Jo, J.-H.; Yang, Y.; Ahn, J.-H. Biosynthesis of Phloretin and Its C-Glycosides through Stepwise Culture of Escherichia Coli. Appl. Biol. Chem. 2024, 67, 99. [Google Scholar] [CrossRef]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in Modulation of Cell Survival Signalling Pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Huang, W.-C.; Liou, C.-J. Evaluation of the Anti-Inflammatory Effects of Phloretin and Phlorizin in Lipopolysaccharide-Stimulated Mouse Macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N. Hepatoprotective Effect of Silymarin. World J. Hepatol. 2014, 6, 144. [Google Scholar] [CrossRef]
- Wadhwa, K.; Pahwa, R.; Kumar, M.; Kumar, S.; Sharma, P.C.; Singh, G.; Verma, R.; Mittal, V.; Singh, I.; Kaushik, D.; et al. Mechanistic Insights into the Pharmacological Significance of Silymarin. Molecules 2022, 27, 5327. [Google Scholar] [CrossRef]
- Lovelace, E.S.; Wagoner, J.; MacDonald, J.; Bammler, T.; Bruckner, J.; Brownell, J.; Beyer, R.P.; Zink, E.M.; Kim, Y.-M.; Kyle, J.E.; et al. Silymarin Suppresses Cellular Inflammation by Inducing Reparative Stress Signaling. J. Nat. Prod. 2015, 78, 1990–2000. [Google Scholar] [CrossRef]
- Chittezhath, M.; Deep, G.; Singh, R.P.; Agarwal, C.; Agarwal, R. Silibinin Inhibits Cytokine-Induced Signaling Cascades and down-Regulates Inducible Nitric Oxide Synthase in Human Lung Carcinoma A549 Cells. Mol. Cancer Ther. 2008, 7, 1817–1826. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Y.; Gong, T.; Liu, Z.; Yang, W.; Xiong, Y.; Xiao, D.; Cifuentes, A.; Ibáñez, E.; Lu, W. The Clinical Anti-Inflammatory Effects and Underlying Mechanisms of Silymarin. iScience 2024, 27, 111109. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Kulawik, A.; Cielecka-Piontek, J.; Czerny, B.; Kamiński, A.; Zalewski, P. The Relationship Between Lycopene and Metabolic Diseases. Nutrients 2024, 16, 3708. [Google Scholar] [CrossRef]
- Birková, A. Caffeic Acid: A Brief Overview of Its Presence, Metabolism, and Bioactivity. Bioact. Compd. Health Dis. 2020, 3, 74. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.-C.; Lee, S.-Y.; Yang, K.-C.; Kuo, Y.-H.; Su, M.-J. Modification of Caffeic Acid with Pyrrolidine Enhances Antioxidant Ability by Activating AKT/HO-1 Pathway in Heart. PLoS ONE 2016, 11, e0148545. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, N. Caffeic Acid and Diseases—Mechanisms of Action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Giménez-Bastida, J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Yao, J.; Liu, Y.; Lin, H.; Shao, C.; Jin, X.; Peng, T.; Liu, Y. Caffeic Acid Activates Nrf2 Enzymes, Providing Protection against Oxidative Damage Induced by Ionizing Radiation. Brain Res. Bull. 2025, 224, 111325. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.; Hu, Y.; Wang, J.; Wang, Y.; Lu, Q. Caffeic Acid Hinders the Proliferation and Migration through Inhibition of IL-6 Mediated JAK-STAT-3 Signaling Axis in Human Prostate Cancer. Oncol. Res. 2024, 32, 1881–1890. [Google Scholar] [CrossRef]
- Shin, K.-M.; Kim, I.-T.; Park, Y.-M.; Ha, J.; Choi, J.-W.; Park, H.-J.; Lee, Y.S.; Lee, K.-T. Anti-Inflammatory Effect of Caffeic Acid Methyl Ester and Its Mode of Action through the Inhibition of Prostaglandin E2, Nitric Oxide and Tumor Necrosis Factor-α Production. Biochem. Pharmacol. 2004, 68, 2327–2336. [Google Scholar] [CrossRef]
- Cortez, N.; Villegas, C.; Burgos, V.; Cabrera-Pardo, J.R.; Ortiz, L.; González-Chavarría, I.; Nchiozem-Ngnitedem, V.-A.; Paz, C. Adjuvant Properties of Caffeic Acid in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 7631. [Google Scholar] [CrossRef]
- Park, J.-Y.; Yasir, M.; Lee, H.; Han, E.-T.; Han, J.-H.; Park, W.; Kwon, Y.-S.; Chun, W. Caffeic Acid Methyl Ester Inhibits LPS-induced Inflammatory Response through Nrf2 Activation and NF-κB Inhibition in Human Umbilical Vein Endothelial Cells. Exp. Ther. Med. 2023, 26, 559. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Zhong, R.; Wang, M.; Zhou, Y.; Chen, Y.; Yi, B.; Hou, F.; Liu, L.; Zhao, Y.; Chen, L.; et al. Caffeic Acid Supplement Alleviates Colonic Inflammation and Oxidative Stress Potentially Through Improved Gut Microbiota Community in Mice. Front. Microbiol. 2021, 12, 784211. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, Y.-M.; Lau, A.T.Y. The Epigenetic Effects of Coffee. Molecules 2023, 28, 1770. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic Acids and Derivatives Formulations for Skin Damages and Disorders: A Review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and Bioactive Components from Vinegar: A Fermented and Functional Food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Yang, H.J.; Li, W.; Oh, Y.-C.; Choi, J.-G. Immune-Enhancing Effects of Gwakhyangjeonggi-San in RAW 264.7 Macrophage Cells through the MAPK/NF-κB Signaling Pathways. Int. J. Mol. Sci. 2024, 25, 9246. [Google Scholar] [CrossRef]
- Moustardas, P.; Aberdam, D.; Lagali, N. MAPK Pathways in Ocular Pathophysiology: Potential Therapeutic Drugs and Challenges. Cells 2023, 12, 617. [Google Scholar] [CrossRef]
- Moon, H.-R.; Yun, J.-M. P-Coumaric Acid Modulates Cholesterol Efflux and Lipid Accumulation and Inflammation in Foam Cells. Nutr. Res. Pract. 2024, 18, 774. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Linard, C.F.B.; Andrade-da-Costa, B.L.D.S.; Augusto, R.L.; Sereniki, A.; Trevisan, M.T.S.; Perreira, R.D.C.R.; De Souza, F.T.C.; Braz, G.R.F.; Lagranha, C.J.; De Souza, I.A.; et al. Anacardic Acids from Cashew Nuts Prevent Behavioral Changes and Oxidative Stress Induced by Rotenone in a Rat Model of Parkinson’s Disease. Neurotox. Res. 2018, 34, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Augusto, R.L.; Mendonça, I.P.; De Albuquerque Rego, G.N.; Pereira, D.D.; Da Penha Gonçalves, L.V.; Dos Santos, M.L.; De Souza, R.F.; Moreno, G.M.M.; Cardoso, P.R.G.; De Souza Andrade, D.; et al. Purified Anacardic Acids Exert Multiple Neuroprotective Effects in Pesticide Model of Parkinson’s Disease: In Vivo and in Silico Analysis. IUBMB Life 2020, 72, 1765–1779. [Google Scholar] [CrossRef]
- Tomasiak, P.; Janisiak, J.; Rogińska, D.; Perużyńska, M.; Machaliński, B.; Tarnowski, M. Garcinol and Anacardic Acid, Natural Inhibitors of Histone Acetyltransferases, Inhibit Rhabdomyosarcoma Growth and Proliferation. Molecules 2023, 28, 5292. [Google Scholar] [CrossRef]
- Rais, N.; Ved, A.; Ahmad, R.; Kumar, M.; Deepak Barbhai, M.; Radha; Chandran, D.; Dey, A.; Dhumal, S.; Senapathy, M.; et al. S-Allyl-L-Cysteine—A Garlic Bioactive: Physicochemical Nature, Mechanism, Pharmacokinetics, and Health Promoting Activities. J. Funct. Foods 2023, 107, 105657. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef] [PubMed]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A.; et al. Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Ansary, J.; Forbes-Hernández, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential Health Benefit of Garlic Based on Human Intervention Studies: A Brief Overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef]
- Nian, H.; Delage, B.; Pinto, J.T.; Dashwood, R.H. Allyl Mercaptan, a Garlic-Derived Organosulfur Compound, Inhibits Histone Deacetylase and Enhances Sp3 Binding on the P21WAF1 Promoter. Carcinogenesis 2008, 29, 1816–1824. [Google Scholar] [CrossRef]
- Taleghani, A.; Emami, S.A.; Tayarani-Najaran, Z. Artemisia: A Promising Plant for the Treatment of Cancer. Bioorganic Med. Chem. 2020, 28, 115180. [Google Scholar] [CrossRef]
- Shoaib, M.; Shah, I.; Ali, N.; Adhikari, A.; Tahir, M.N.; Shah, S.W.A.; Ishtiaq, S.; Khan, J.; Khan, S.; Umer, M.N. Sesquiterpene Lactone! A Promising Antioxidant, Anticancer and Moderate Antinociceptive Agent from Artemisia Macrocephala Jacquem. BMC Complement. Altern. Med. 2017, 17, 27. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Han, J.; Sun, Y.; Wang, S.; Yang, B.; Wang, Q.; Kuang, H. Artesunate Exerts Organ- and Tissue-Protective Effects by Regulating Oxidative Stress, Inflammation, Autophagy, Apoptosis, and Fibrosis: A Review of Evidence and Mechanisms. Antioxidants 2024, 13, 686. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, H.; Yao, H.; Lu, L.; He, G.; Wu, M.; Zheng, C.; Li, Y.; Chen, S.; Li, L.; et al. Artemisinin Derivatives Inhibit Non-Small Cell Lung Cancer Cells Through Induction of ROS-Dependent Apoptosis/Ferroptosis. J. Cancer 2021, 12, 4075–4085. [Google Scholar] [CrossRef]
- Efferth, T.; Oesch, F. The Immunosuppressive Activity of Artemisinin-type Drugs towards Inflammatory and Autoimmune Diseases. Med. Res. Rev. 2021, 41, 3023–3061. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Santos, A.R.D.O.D.; Carvalho, A.C.A.D.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.D.A.; Vargas Sinatora, R.; Araújo, A.C.; Barbalho, S.M. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites 2023, 13, 96. [Google Scholar] [CrossRef]
- Ma, Z.; Woon, C.Y.-N.; Liu, C.-G.; Cheng, J.-T.; You, M.; Sethi, G.; Wong, A.L.-A.; Ho, P.C.-L.; Zhang, D.; Ong, P.; et al. Repurposing Artemisinin and Its Derivatives as Anticancer Drugs: A Chance or Challenge? Front. Pharmacol. 2021, 12, 828856. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.M.; Yance, D.; Wong, R.K. Natural Health Products That Inhibit Angiogenesis: A Potential Source for Investigational New Agents to Treat Cancer—Part 1. Curr. Oncol. 2006, 13, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.T.B.; Fatima, H.; Naz, I.; Kanwal, N.; Haq, I. Pre-Clinical Studies Comparing the Anti-Inflammatory Potential of Artemisinic Compounds by Targeting NFκB/TNF-α/NLRP3 and Nrf2/TRX Pathways in Balb/C Mice. Front. Pharmacol. 2024, 15, 1352827. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.D.S.E.; Marques, J.N.D.J.; Linhares, E.P.M.; Bonora, C.M.; Costa, É.T.; Saraiva, M.F. Review of Anticancer Activity of Monoterpenoids: Geraniol, Nerol, Geranial and Neral. Chem.-Biol. Interact. 2022, 362, 109994. [Google Scholar] [CrossRef]
- Ben Ammar, R.; Mohamed, M.E.; Alfwuaires, M.; Abdulaziz Alamer, S.; Bani Ismail, M.; Veeraraghavan, V.P.; Sekar, A.K.; Ksouri, R.; Rajendran, P. Anti-Inflammatory Activity of Geraniol Isolated from Lemon Grass on Ox-LDL-Stimulated Endothelial Cells by Upregulation of Heme Oxygenase-1 via PI3K/Akt and Nrf-2 Signaling Pathways. Nutrients 2022, 14, 4817. [Google Scholar] [CrossRef]
- Kim, H.; Li, S.; Nilkhet, S.; Baek, S.J. Anti-Cancer Activity of Rose-Geranium Essential Oil and Its Bioactive Compound Geraniol in Colorectal Cancer Cells. Appl. Biol. Chem. 2025, 68, 30. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Dashwood, R.H. Epigenetic Regulation of NRF2/KEAP1 by Phytochemicals. Antioxidants 2020, 9, 865. [Google Scholar] [CrossRef]
- Nurkolis, F.; Taslim, N.A.; Syahputra, R.A.; d’Arqom, A.; Tjandrawinata, R.R.; Purba, A.K.R.; Mustika, A. Food Phytochemicals as Epigenetic Modulators in Diabetes: A Systematic Review. J. Agric. Food Res. 2025, 21, 101873. [Google Scholar] [CrossRef]
- Gowd, V.; Kanika; Jori, C.; Chaudhary, A.A.; Rudayni, H.A.; Rashid, S.; Khan, R. Resveratrol and Resveratrol Nano-Delivery Systems in the Treatment of Inflammatory Bowel Disease. J. Nutr. Biochem. 2022, 109, 109101. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Gao, L. Mechanism of Antioxidant Properties of Quercetin and Quercetin-DNA Complex. J. Mol. Model. 2020, 26, 133. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaokun, O.O. Apigenin and Inflammation in the Brain: Can Apigenin Inhibit Neuroinflammation in Preclinical Models? Inflammopharmacology 2024, 32, 3099–3108. [Google Scholar] [CrossRef]
- Pinto, C.; Cidade, H.; Pinto, M.; Tiritan, M.E. Chiral Flavonoids as Antitumor Agents. Pharmaceuticals 2021, 14, 1267. [Google Scholar] [CrossRef]
- Iordache, M.P.; Buliman, A.; Costea-Firan, C.; Gligore, T.C.I.; Cazacu, I.S.; Stoian, M.; Teoibaș-Şerban, D.; Blendea, C.-D.; Protosevici, M.G.-I.; Tanase, C.; et al. Immunological and Inflammatory Biomarkers in the Prognosis, Prevention, and Treatment of Ischemic Stroke: A Review of a Decade of Advancement. Int. J. Mol. Sci. 2025, 26, 7928. [Google Scholar] [CrossRef]
- Ding, K.; Jiang, W.; Jia, H.; Lei, M. Synergistically Anti-Multiple Myeloma Effects: Flavonoid, Non-Flavonoid Polyphenols, and Bortezomib. Biomolecules 2022, 12, 1647. [Google Scholar] [CrossRef]
- Fatima, Z.; Itrat, N.; Israr, B.; Ahmad, A.M.R. Therapeutic Efficacy of Soy-Derived Bioactives: A Systematic Review of Nutritional Potency, Bioactive Therapeutics, and Clinical Biomarker Modulation. Foods 2025, 14, 3447. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Bai, Q.; Zou, L.; Zhang, Q.; Zhou, Y.; Chang, H.; Yi, L.; Zhu, J.; Mi, M. Genistein Inhibits DNA Methylation and Increases Expression of Tumor Suppressor Genes in Human Breast Cancer Cells. Genes Chromosomes Cancer 2014, 53, 422–431. [Google Scholar] [CrossRef]
- Montgomery, M.; Srinivasan, A. Epigenetic Gene Regulation by Dietary Compounds in Cancer Prevention. Adv. Nutr. 2019, 10, 1012–1028. [Google Scholar] [CrossRef]
- Zahari, C.N.M.C.; Mohamad, N.V.; Akinsanya, M.A.; Gengatharan, A. The Crimson Gem: Unveiling the Vibrant Potential of Lycopene as a Functional Food Ingredient. Food Chem. Adv. 2023, 3, 100510. [Google Scholar] [CrossRef]
- Tufail, T.; Bader Ul Ain, H.; Noreen, S.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Nutritional Benefits of Lycopene and Beta-Carotene: A Comprehensive Overview. Food Sci. Nutr. 2024, 12, 8715–8741. [Google Scholar] [CrossRef]
- Maaz, M.; Sultan, M.T.; Khalid, M.U.; Raza, H.; Imran, M.; Hussain, M.; Al Abdulmonem, W.; Alsagaby, S.A.; Abdelgawad, M.A.; Ghoneim, M.M.; et al. A Comprehensive Review on the Molecular Mechanism of Lycopene in Cancer Therapy. Food Sci. Amp. Nutr. 2025, 13, e70608. [Google Scholar] [CrossRef]
- Mannino, F.; D’Angelo, T.; Pallio, G.; Ieni, A.; Pirrotta, I.; Giorgi, D.A.; Scarfone, A.; Mazziotti, S.; Booz, C.; Bitto, A.; et al. The Nutraceutical Genistein-Lycopene Combination Improves Bone Damage Induced by Glucocorticoids by Stimulating the Osteoblast Formation Process. Nutrients 2022, 14, 4296. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Xu, B. Critical Review on Molecular Mechanisms for Genistein’s Beneficial Effects on Health Through Oxidative Stress Reduction. Antioxidants 2025, 14, 904. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, S.; Zhang, X.; Du, Y.; Ni, T.; Hao, S. Crosstalk between Metabolic and Epigenetic Modifications during Cell Carcinogenesis. iScience 2024, 27, 111359. [Google Scholar] [CrossRef]
- Böhm, V.; Bitsch, R. Intestinal Absorption of Lycopene from Different Matrices and Interactions to Other Carotenoids, the Lipid Status, and the Antioxidant Capacity of Human Plasma. Eur. J. Nutr. 1999, 38, 118–125. [Google Scholar] [CrossRef]
- Naveen Kumar, P.; Elango, P.; Asmathulla, S.; Kavimani, S. A Systematic Review on Lycopene and Its Beneficial Effects. Biomed. Pharmacol. J. 2017, 10, 2113–2120. [Google Scholar] [CrossRef]
- Narra, F.; Piragine, E.; Benedetti, G.; Ceccanti, C.; Florio, M.; Spezzini, J.; Troisi, F.; Giovannoni, R.; Martelli, A.; Guidi, L. Impact of Thermal Processing on Polyphenols, Carotenoids, Glucosinolates, and Ascorbic Acid in Fruit and Vegetables and Their Cardiovascular Benefits. Comp. Rev. Food Sci. Food Safe 2024, 23, e13426. [Google Scholar] [CrossRef] [PubMed]
- Shanaida, M.; Mykhailenko, O.; Lysiuk, R.; Hudz, N.; Balwierz, R.; Shulhai, A.; Shapovalova, N.; Shanaida, V.; Bjørklund, G. Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications. Pharmaceuticals 2025, 18, 403. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Xu, X.; Li, Y.; Fang, H.; Ren, J. Lycopene as a Potential Anticancer Agent: Current Evidence on Synergism, Drug Delivery Systems and Epidemiology (Review). Oncol. Lett. 2025, 30, 462. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Wang, J.; Guan, R.; Sun, J.; Jin, P.; Shen, J. Role of Oxidative Stress in the Occurrence, Development, and Treatment of Breast Cancer. Antioxidants 2025, 14, 104. [Google Scholar] [CrossRef]
- Paschos, A.; Pandya, R.; Duivenvoorden, W.C.M.; Pinthus, J.H. Oxidative Stress in Prostate Cancer: Changing Research Concepts towards a Novel Paradigm for Prevention and Therapeutics. Prostate Cancer Prostatic Dis. 2013, 16, 217–225. [Google Scholar] [CrossRef]
- Selvaraj, N.R.; Nandan, D.; Nair, B.G.; Nair, V.A.; Venugopal, P.; Aradhya, R. Oxidative Stress and Redox Imbalance: Common Mechanisms in Cancer Stem Cells and Neurodegenerative Diseases. Cells 2025, 14, 511. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R. Cellular and Molecular Mechanisms in Oxidative Stress-Related Diseases. Int. J. Mol. Sci. 2022, 23, 8017. [Google Scholar] [CrossRef]
- López-Contreras, A.K.; Martínez-Ruiz, M.G.; Olvera-Montaño, C.; Robles-Rivera, R.R.; Arévalo-Simental, D.E.; Castellanos-González, J.A.; Hernández-Chávez, A.; Huerta-Olvera, S.G.; Cardona-Muñoz, E.G.; Rodríguez-Carrizalez, A.D. Importance of the Use of Oxidative Stress Biomarkers and Inflammatory Profile in Aqueous and Vitreous Humor in Diabetic Retinopathy. Antioxidants 2020, 9, 891. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; González-Sarrías, A.; Espín, J.C. New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study. Antioxidants 2021, 10, 435. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules 2025, 30, 1184. [Google Scholar] [CrossRef]
- Cord, D.; Rimbu, M.C.; Tanase, C.; Tablet, C.; Duca, G. Molecular Docking Study of Some Active Principles from Silybum Marianum, Chelidonium Majus, Ginkgo Biloba, Gelsemium Sempervirens, Artemisia Annua, and Taraxacum Officinale. Chem. J. Mold. 2025, 20, 100–105. [Google Scholar] [CrossRef]
- Rao, A.; Kumar, A.H.S. Computational Pharmacology Analysis of Lycopene to Identify Its Targets and Biological Effects in Humans. Appl. Sci. 2025, 15, 7815. [Google Scholar] [CrossRef]
- Liang, X.; Ma, C.; Yan, X.; Liu, X.; Liu, F. Advances in Research on Bioactivity, Metabolism, Stability and Delivery Systems of Lycopene. Trends Food Sci. Technol. 2019, 93, 185–196. [Google Scholar] [CrossRef]
- Yen, G.-C.; Cheng, H.-L.; Lin, L.-Y.; Chen, S.-C.; Hsu, C.L. The Potential Role of Phenolic Compounds on Modulating Gut Microbiota in Obesity. J. Food Drug Anal. 2020, 28, 195–205. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Korma, S.A.; Salem, H.M.; Abd El-Mageed, T.A.; Alkafaas, S.S.; Elsalahaty, M.I.; Elkafas, S.S.; Mosa, W.F.A.; Ahmed, A.E.; et al. Garlic Bioactive Substances and Their Therapeutic Applications for Improving Human Health: A Comprehensive Review. Front. Immunol. 2024, 15, 1277074. [Google Scholar] [CrossRef]
- Rîmbu, M.C.; Cord, D.; Savin, M.; Grigoroiu, A.; Mihăilă, M.A.; Gălățanu, M.L.; Ordeanu, V.; Panțuroiu, M.; Țucureanu, V.; Mihalache, I.; et al. Harnessing Plant-Based Nanoparticles for Targeted Therapy: A Green Approach to Cancer and Bacterial Infections. Int. J. Mol. Sci. 2025, 26, 7022. [Google Scholar] [CrossRef]
- Xia, Y.; Shi, C.; Lu, J.; Zhu, Z.; Li, M.; Pan, Y.; Huang, X.; Zhang, L.; Liu, A. Artemisinin and Its Derivatives from Molecular Mechanisms to Clinical Applications: New Horizons Beyond Antimalarials. Int. J. Mol. Sci. 2025, 26, 8409. [Google Scholar] [CrossRef]
- Pavan, B.; Dalpiaz, A.; Marani, L.; Beggiato, S.; Ferraro, L.; Canistro, D.; Paolini, M.; Vivarelli, F.; Valerii, M.C.; Comparone, A.; et al. Geraniol Pharmacokinetics, Bioavailability and Its Multiple Effects on the Liver Antioxidant and Xenobiotic-Metabolizing Enzymes. Front. Pharmacol. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, N.; Baldassarre, M.P.A.; Cichelli, A.; Pandolfi, A.; Formoso, G.; Pipino, C. Role of Polyphenols and Carotenoids in Endothelial Dysfunction: An Overview from Classic to Innovative Biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 6381380. [Google Scholar] [CrossRef] [PubMed]
- Rîmbu, M.C.; Popescu, L.; Mihăilă, M.; Sandulovici, R.C.; Cord, D.; Mihăilescu, C.-M.; Gălățanu, M.L.; Panțuroiu, M.; Manea, C.-E.; Boldeiu, A.; et al. Synergistic Effects of Green Nanoparticles on Antitumor Drug Efficacy in Hepatocellular Cancer. Biomedicines 2025, 13, 641. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cord, D.; Rîmbu, M.C.; Iordache, M.P.; Albulescu, R.; Pop, S.; Tanase, C.; Popa, M.-L. Phytochemicals as Epigenetic Modulators in Chronic Diseases: Molecular Mechanisms. Molecules 2025, 30, 4317. https://doi.org/10.3390/molecules30214317
Cord D, Rîmbu MC, Iordache MP, Albulescu R, Pop S, Tanase C, Popa M-L. Phytochemicals as Epigenetic Modulators in Chronic Diseases: Molecular Mechanisms. Molecules. 2025; 30(21):4317. https://doi.org/10.3390/molecules30214317
Chicago/Turabian StyleCord, Daniel, Mirela Claudia Rîmbu, Marius P. Iordache, Radu Albulescu, Sevinci Pop, Cristiana Tanase, and Maria-Linda Popa. 2025. "Phytochemicals as Epigenetic Modulators in Chronic Diseases: Molecular Mechanisms" Molecules 30, no. 21: 4317. https://doi.org/10.3390/molecules30214317
APA StyleCord, D., Rîmbu, M. C., Iordache, M. P., Albulescu, R., Pop, S., Tanase, C., & Popa, M.-L. (2025). Phytochemicals as Epigenetic Modulators in Chronic Diseases: Molecular Mechanisms. Molecules, 30(21), 4317. https://doi.org/10.3390/molecules30214317








