The Action of Cannabidiol on Doxycycline Cytotoxicity in Human Cells—In Vitro Study
Abstract
1. Introduction
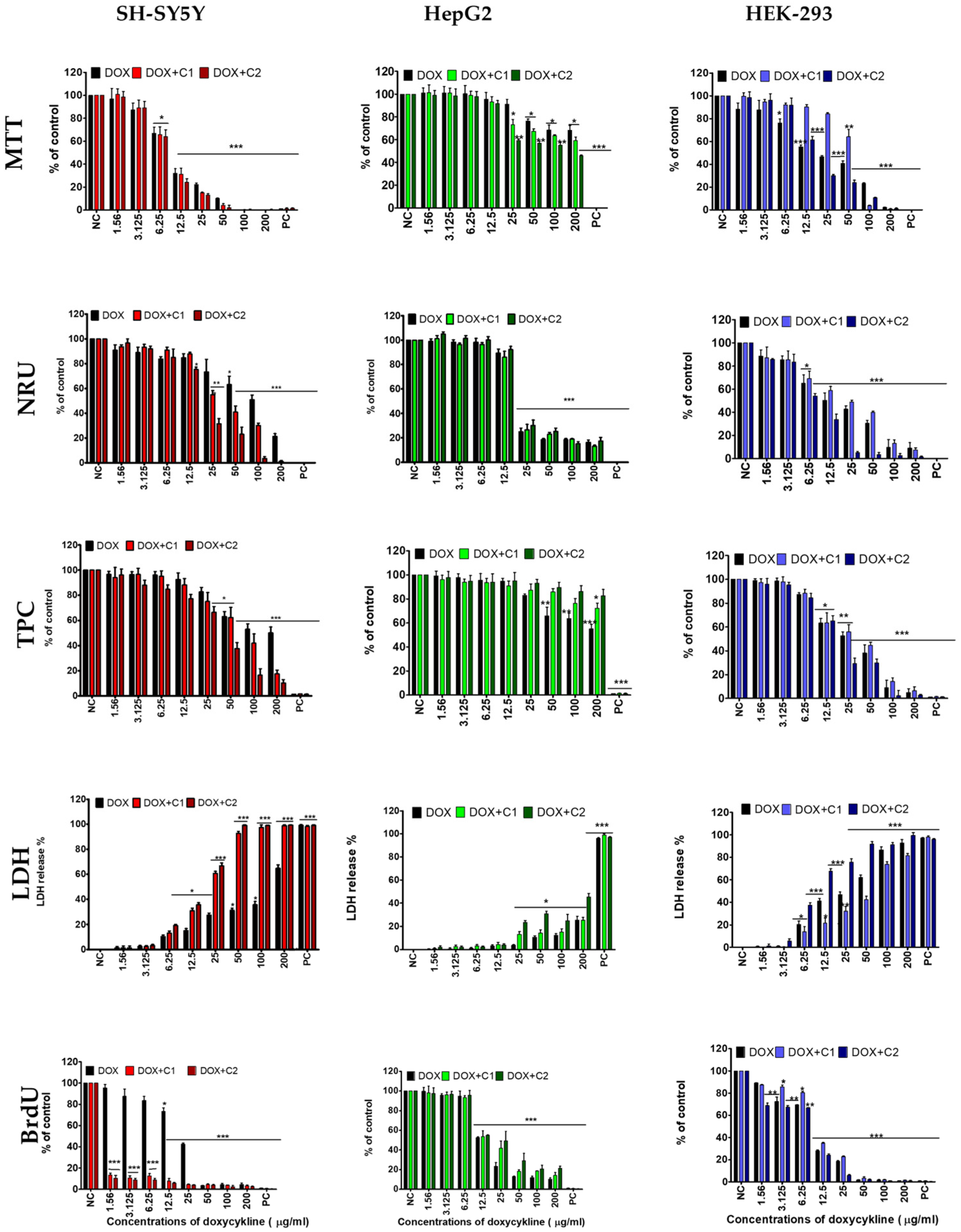
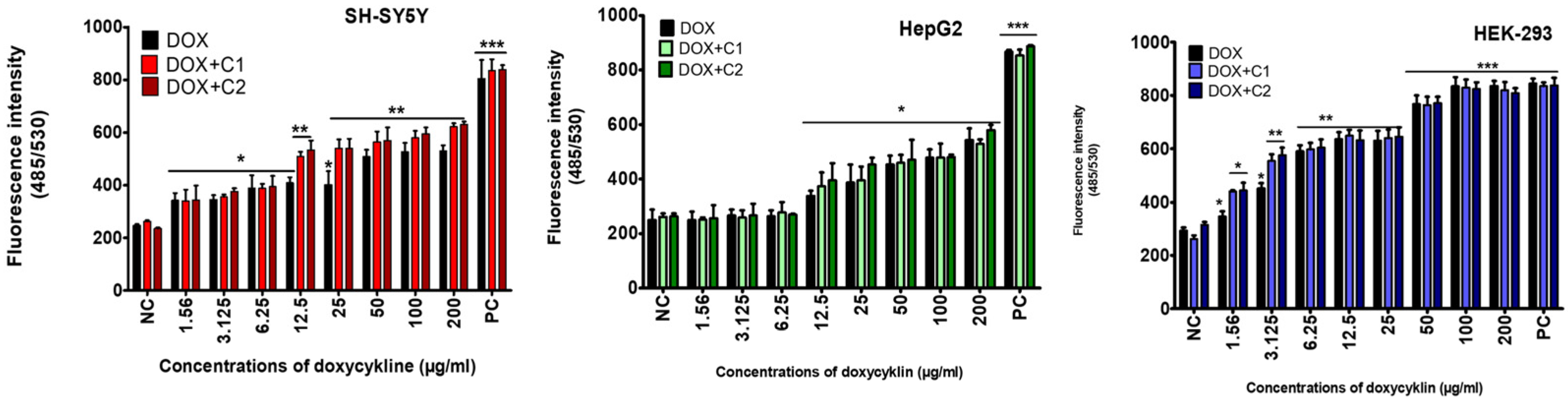
2. Results
2.1. The Effects of CBD on Doxycycline’s Activity
2.2. The Type of Interaction Between Cannabidiol and Doxycycline
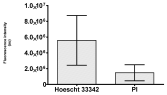
2.3. The Morphological Changes and Death of Cells
3. Discussion
3.1. Cytotoxicity of Doxycycline
3.2. Effect of Cannabidiol on the Drug’s Cytotoxicity
4. Materials and Methods
4.1. Materials
4.1.1. Cell Line and Culture Conditions
4.1.2. Metabolic Activity (MTT Assay)
4.1.3. Lysosomal Activity (NRU Assay)
4.1.4. Total Cellular Protein (TPC Assay)
4.1.5. Integrity of Cellular Membrane (LDH Leakage Assay)
4.1.6. DNA Synthesis (BrdU Assay)
4.1.7. Oxidative Stress (DCFH Assay)
4.1.8. Apoptosis and Necrosis Death Cells
4.1.9. Cellular Morphology Analysis, (May–Grünwald–Giemsa (MGG) Staining)
4.1.10. Assessment of Synergistic/Antagonistic Effects
4.1.11. Analysis of Statistical Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le, T.; Xu, R.; Yang, L.; Xie, Y. Development of a Highly Specific Fluoroimmuno-assay for the Detection of Doxycycline Residues in Water Environmental and Animal Tissue Samples. Micromachines 2022, 13, 1864. [Google Scholar] [CrossRef]
- Cárdenas Sierra, R.S.; Zúñiga-Benítez, H.; Peñuela, G.A. Elimination of cephalexin and doxycycline under low frequency ultrasound. Ultrason. Sonochem. 2021, 79, 105777. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Homenuik, K.; Nichol, K. The glycylcyclines: A comparative review with the tetracyclines. Drugs 2004, 64, 63–88. [Google Scholar] [CrossRef]
- Holmes, N.E.; Charles, P.G.P. Safety and Efficacy Review of Doxycycline. Clin. Med. Ther. 2009, 1, CMT-S2035. [Google Scholar] [CrossRef]
- Brihoum, M.; Amory, H.; Desmecht, D.; Rollin, F. Doxycycline poisoning in calves: 18 cases in Belgium. In Proceedings of the 23rd World Buiatrics Congress, Quebec City, QC, Canada, 11–16 July 2004; p. 102. [Google Scholar]
- Brihoum, M.; Amory, H.; Desmecht, D.; Cassart, D.; Deleuze, S.; Rollin, F. Descriptive study of 32 cases of doxycycline-overdosed calves. J. Vet. Intern. Med. 2010, 24, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Brihoum, M.; Rollin, F.; Desmecht, D.; Detilleux, J.; Amory, H. Clinical evaluation of cardiac effects of experimental doxycycline overdosing in healthy calves. BMC Vet. Res. 2011, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Deruham, I.; Perl, S.; Sharony, D.; Vishinisky, Y. Doxycycline toxicity in calves in two feedlots. J. Vet. Med. B Infect. Dis. Vet. Public Health 2002, 49, 406–408. [Google Scholar]
- El-Neweshy, M.S. Experimental doxycycline overdose in rats causes cardiomyopathy. Int. J. Exp. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef]
- Sani, A.A.; Rafiq, K.; Hossain, M.T.; Akter, F.; Haque, A.; Hasan, M.I.; Sachi, S.; Mustari, A.; Islam, M.Z.; Alam, M.M. Screening and quantification of antibiotic residues in poultry products and feed in selected areas of Bangladesh. Vet. World 2023, 16, 1747–1754. [Google Scholar] [CrossRef]
- Bartkiene, E.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; Zavistanaviciute, P.; Starkute, V.; Zokaityte, E.; Lele, V.; Dauksiene, A.; Grashorn, M.; et al. Study of the antibiotic residues in poultry meat in some of the EU countries and selection of the best compositions of lactic acid bacteria and essential oils against Salmonella enterica. Poult. Sci. 2020, 99, 4065–4076. [Google Scholar] [CrossRef]
- Okerman, L.; Croubels, S.; De Baere, S.; Van Hoof, J.; De Backer, P.; De Brabander, H. Inhibition tests for detection and presumptive identification of tetracyclines, beta-lactam antibiotics and quinolones in poultry meat. Food Addit. Contam. 2001, 18, 385–393. [Google Scholar] [CrossRef]
- Commission Regulation (EU). No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin; European Union: Brussels, Belgium, 2009. [Google Scholar]
- European Medicines Agency. European Surveillance of Veterinary Antimicrobial Consumption (ESVAC): Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2022. Available online: https://www.ema.europa.eu/en/documents/report/european-sales-use-antimicrobials-veterinary-medicine-annual-surveillance-report-2023_en.pdf (accessed on 3 July 2025).
- Gajda, A.; Posyniak, A. Doxycycline depletion and residues in eggs after oral admin-istration to laying hens. Food Addit. Contam. Part A 2015, 32, 1116–1123. [Google Scholar] [CrossRef]
- Gajda, A.; Nowacka–Kozak, E.; Gbylik–Sikorska, M.; Posyniak, A. Tetracycline antibiotics transfer from contaminated milk to dairy products and the effect of the skimming step and pasteurisation process on residue concentrations. Food Addit. Contam. Part A 2018, 35, 66–76. [Google Scholar] [CrossRef]
- Gajda, A.; Posyniak, A.; Tomczyk, G. LC-MS/MS analysis of doxycycline residues in chicken tissues after oral administration. Bull. Vet. Inst. Pulawy 2014, 58, 573–579. [Google Scholar] [CrossRef]
- Gajda, A.; Bladek, T.; Gbylik-Sikorska, M.; Posyniak, A. The influence of cooking procedures on doxycycline concentration in contaminated eggs. Food Chem. 2017, 15, 1666–1670. [Google Scholar] [CrossRef]
- Monir, H.H.; Fayez, Y.M.; Nessim, C.K.; Michael, A.M. When is it safe to eat different broiler chicken tissues after administration of doxycycline and tylosin mixture? J. Food Sci. 2021, 86, 1162–1171. [Google Scholar] [CrossRef]
- Gajda, A.; Szymanek-Bany, I.; Nowacka-Kozak, E.; Gbylik-Sikorska, M. Investigation of doxycycline residues in bones after oral administration to broiler chickens. J. Vet. Res. 2024, 68, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Gudda, F.; Odinga, E.S.; Tang, L.; Waigi, M.G.; Wang, J.; Abdalmegeed, D.; Gao, Y. Tetracyclines uptake from irrigation water by vegetables: Accumulation and antimicrobial resistance risks. Environ. Pollut. 2023, 1, 122696. [Google Scholar] [CrossRef] [PubMed]
- Borghi, A.A.; Silva, M.F.; Al Arni, S.; Converti, A.; Palma, M.S.A. Doxycycline Degradation by the Oxidative Fenton Process. J. Chem. 2015, 2015, 9. [Google Scholar] [CrossRef]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef]
- Eljaaly, K.; Alghamdi, H.; Al-mehmadi, H.; Aljawi, F.; Hassan, A.; Thabit, A.K. Long-term gastrointestinal adverse effects of doxycycline. J. Infect. Dev. Ctries. 2023, 28, 281–285. [Google Scholar] [CrossRef]
- Dağ, M.S.; Öztürk, Z.A.; Akın, I.; Tutar, E.; Çıkman, Ö.; Gülşen, M.T. Drug-induced esophageal ulcers: Case series and the review of the literature. Turk. J. Gastroenterol. 2014, 25, 180–184. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 15, 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 2, 2671. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-Term Antibiotic Treatment Has Differing Long-Term Impacts on the Human Throat and Gut Microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Toledano-Osorio, M.; Navarro-Hortal, M.D.; Varela-López, A.; Osorio, R.; Quiles, J.L. Novel Polymeric Nanocarriers Reduced Zinc and Doxycycline Toxicity in the Nematode Caenorhabditis elegans. Antioxidants 2019, 14, 550. [Google Scholar] [CrossRef]
- Wright, G.D. The antibiotic resistome: The nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 2007, 5, 175–186. [Google Scholar] [CrossRef]
- Chan, P.A.; Le Brazidec, D.L.; Becasen, J.S.; Martin, H.; Kapadia, J.; Reno, H.; Bachmann, L.; Bar-bee, L.A. Safety of Longer-Term Doxycycline Use: A Systematic Review and Meta-Analysis with Implications for Bacterial Sexually Transmitted Infection Chemoprophylaxis. Sex. Transm. Dis. 2023, 1, 701–712. [Google Scholar] [CrossRef]
- Grill, M.F.; Maganti, R.K. Neurotoxic effects associated with antibiotic use: Management considerations. Br. J. Clin. Pharmacol. 2011, 72, 381–393. [Google Scholar] [CrossRef]
- Rein, J.L. The nephrologist’s guide to cannabis and cannabinoids. Curr. Opin. Nephrol. Hypertens. 2020, 29, 248–257. [Google Scholar] [CrossRef]
- Soliman, N.A.; Dahmy, S.I.E.; Shalaby, A.A.; Mohammed, K.A. Prospective affirmative therapeutics of cannabidiol oil mitigates doxorubicin-induced abnormalities in kidney function, inflammation, and renal tissue changes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3897–3906. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, X.; Gao, X.; Li, Y.; Shen, X. Liver-Targeted Nanoparticles Loaded with Cannabidiol Based on Redox Response for Effective Alleviation of Acute Liver Injury. Foods 2024, 13, 2464. [Google Scholar] [CrossRef]
- Şahin, S.; Azarkan, S.Y.; Türksoy, V.A. Evaluation of the effect of cannabidiol on the THLE-2 liver cell line exposed to lead. Sci. Total Environ. 2024, 1, 170901. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.Y.; Seo, J.; Choi, I.S. Neuroprotective Effect of Cannabidiol Against Hydrogen Peroxide in Hippocampal Neuron Culture. Cannabis Cannabinoid Res. 2021, 12, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Pankowska, E.; Kończak, O.; Żakowicz, P.; Wojciechowicz, T.; Gogulski, M.; Radko, L. Protective Action of Cannabidiol on Tiamulin Toxicity in Humans—In Vitro Study. Int. J. Mol. Sci. 2024, 18, 13542. [Google Scholar] [CrossRef] [PubMed]
- Li, M.R.; Men, S.H.; Wang, Z.Y.; Liu, C.; Zhou, G.R.; Yan, Z.G. The application of human-derived cell lines in neurotoxicity studies of environmental pollutants. Sci. Total Environ. 2024, 20, 168839. [Google Scholar] [CrossRef]
- Jantas, D.; Leśkiewicz, M.; Regulska, M.; Procner, M.; Warszyński, P.; Lasón, W. Protective Effects of Cannabidiol (CBD) against Qxidative Stress, but Not Excitotoxic-Related Neuronal Cell Damage-An In Vitro Study. Biomolecules. 2024, 9, 564. [Google Scholar] [CrossRef]
- Radko, L.; Stypuła-Trębas, S.; Posyniak, A.; Żyro, D.; Ochocki, J. Silver(I) Complexes of the Pharmaceutical Agents Metronidazole and 4-Hydroxymethylpyridine: Comparison of Cytotoxic Profile for Potential Clinical Application. Molecules 2019, 21, 1949. [Google Scholar] [CrossRef]
- Radko, L.; Minta, M.; Jedziniak, P.; Stypuła-Tręba, S. Comparison of Albendazole Cytotoxicity in Terms of Metabolite Formation in Four Model Systems. J. Vet. Res. 2017, 61, 313–319. [Google Scholar] [CrossRef]
- Ahler, E.; Sullivan, W.J.; Cass, A.; Braas, D.; York, A.G.; Bensinger, S.J. Doxycycline Alters Metabolism and Proliferation of Human Cell Lines. PLoS ONE 2013, 8, e64561. [Google Scholar] [CrossRef]
- Majewski, M.A. Current opinion on the safety and efficacy of doxycycline including parenteral administration—A review. Pol. Ann. Med. 2014, 21, 57–62. [Google Scholar] [CrossRef]
- Kirse, D.J.; Stern, S.J.; Suen, J.Y.; Rudnicki, S.; Roberson, P.K.; Schaefer, R.F. Neurotic effects of doxycycline sclerotherapy. Otolaryngol. Head Neck Surg. 1998, 118, 356–362. [Google Scholar] [PubMed]
- Medina, L.; González-Lizárraga, F.; Dominguez-Meijide, A.; Ploper, D.; Parrales, V.; Sequeira, S.; Cima-Omori, M.S.; Zweckstetter, M.; Del Bel, E.; Michel, P.P.; et al. Doxycycline Interferes with Tau Aggregation and Reduces Its Neuronal Toxicity. Front. Aging Neurosci. 2021, 22, 635760. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, M.; Martin, S.; Mitkovski, M.; Vozari, R.R.; Stühmer, W.; Bel, E.D. Doxycycline restrains glia and confers neuroprotection in a 6-OHDA Parkinson model. Glia 2013, 61, 1084–1100. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.D.; Santos, N.A.G.D.; Sisti, F.M.; Del Bel, E.; Santos, A.C.D. The antibiotic doxycycline mimics the NGF signaling in PC12 cells: A relevant mechanism for neuroprotection. Chem. Biol. Interact. 2021, 25, 109454. [Google Scholar] [CrossRef]
- Dominguez-Meijide, A. Doxycycline inhibits α-synuclein-associated pathologies in vitro and in vivo. Neurobiol. Dis. 2021, 151, 105256. [Google Scholar] [CrossRef]
- Goranov, B.B. Overexpression of RARgamma increases death of SH-SY5Y neuroblastoma cells in response to retinoic acid but not fenretinide. Cell Death Differ. 2006, 13, 676–679. [Google Scholar] [CrossRef]
- Bo, C. Studies on antitumor activity spectrum of doxycycline. J. Solid Tumors 2016, 6, 103–106. [Google Scholar]
- Gardouh, A.R. Synthesis and Antitumor Activity of Doxycycline Polymeric Nanoparticles: Effect on Tumor Apoptosis in Solid Ehrlich Carcinoma. Molecules 2020, 25, 3230. [Google Scholar] [CrossRef]
- Heaton, P.C.; Fenwick, S.R.; Brewer, D.E. Association between tetracycline or doxycycline and hepatotoxicity: A population-based case-control study. J. Clin. Pharm. Ther. 2007, 32, 483–487. [Google Scholar] [CrossRef]
- Pan, J.J.; Promrat, K. Doxycycline-Induced Autoimmune Hepatitis. ACG Case Rep. J. 2020, 10, e00440. [Google Scholar] [CrossRef]
- Mohammed, A.F.E. Antitumor activity of doxycycline in HepG-2 cells. Int. J. Adv. Res. 2015, 3, 834–846. [Google Scholar]
- Donato, M.T. High-content imaging technology for the evaluation of drug-induced steatosis using a multiparametric cell-based assay. J. Biomol. Screen. 2012, 17, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Amrane, D. Synthesis and Antiplasmodial Evaluation of 4-Carboxamido- and 4-Alkoxy-2-Trichloromethyl Quinazolines. Molecules 2020, 25, 3929. [Google Scholar] [CrossRef] [PubMed]
- Peaslee, C. Doxycycline Significantly Enhances Induction of Induced Pluripotent Stem Cells to Endoderm by Enhancing Survival Through Protein Kinase B Phosphorylation. Hepatology 2021, 74, 2102–2117. [Google Scholar] [CrossRef]
- Ansong, S.; Doad, J.; Igweonu-Nwakile, E.O.; Okafor, C. Drug-Drug Interaction of Warfarin and Doxycycline Leading to a Large Rectus Sheath Hematoma. Cureus 2025, 27, e81312. [Google Scholar] [CrossRef]
- Abrahamian, A.; Burmeister, C.; Hejeebu, S. Suspected Doxycycline-Induced Acute Interstitial Nephritis. Transl. J. Med. Sci. 2023, 11, e1. [Google Scholar] [CrossRef]
- Campbell, R.E. Overview of Antibiotic-Induced Nephrotoxicity. Kidney Int. Rep. 2023, 8, 2211–2225. [Google Scholar] [CrossRef]
- Wang, B. Doxycycline sensitizes renal cell carcinoma to chemotherapy by preferentially inhibiting mitochondrial translation. J. Int. Med. Res. 2021, 49, 3000605211044368. [Google Scholar] [CrossRef]
- Brodaczewska, K.K.; Bielecka, Z.F.; Maliszewska-Olejniczak, K.; Szczylik, C.; Porta, C.; Bartnik, E.; Czarnecka, A.M. Metastatic renal cell carcinoma cells growing in 3D on poly D lysine or laminin present a stem like phenotype and drug resistance. Oncol. Rep. 2019, 42, 1878–1892. [Google Scholar] [CrossRef]
- Xu, X. The “steric-like” inhibitory effect and mechanism of doxycycline on florfenicol metabolism: Interaction risk. Food Chem. Toxicol. 2022, 169, 113431. [Google Scholar] [CrossRef]
- Melo, E.S.A.; Asevedo, E.A.; Duarte-Almeida, J.M.; Nurkolis, F.; Syahputra, R.A.; Park, M.N.; Kim, B.; Couto, R.O.d.; Ribeiro, R.I.M.d.A. Mechanisms of Cell Death Induced by Cannabidiol Against Tumor Cells: A Review of Preclinical Studies. Plants 2025, 14, 585. [Google Scholar] [CrossRef]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; Walker, E.A. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) re-ceptors without diminishing nervous system function or chemotherapy efficacy. Br. J. Pharmacol. 2014, 171, 636–645. [Google Scholar] [CrossRef]
- Marzęda, P.; Wróblewska-Łuczka, P.; Drozd, M.; Florek-Łuszczki, M.; Załuska-Ogryzek, K.; Łuszczki, J.J. Cannabidiol Interacts Antagonistically with Cisplatin and Additively with Mitoxantrone in Various Melanoma Cell Lines-An Isobolographic Anal-ysis. Int. J. Mol. Sci. 2022, 17, 6752. [Google Scholar] [CrossRef] [PubMed]
- Inkol, J.M.; Hocker, S.E.; Mutsaers, A.J. Combination therapy with cannabidiol and chemotherapeutics in canine urothelial carcinoma cells. PLoS ONE 2021, 16, e0255591. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Mukhopadhyay, P.; Rajesh, M.; Patel, V.; Mukhopadhyay, B.; Gao, B.; Haskó, G.; Pacher, P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 2009, 328, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Fleig, A.; Penner, R. CBGA ameliorates inflammation and fibrosis in nephropathy. Sci. Rep. 2023, 18, 6341. [Google Scholar] [CrossRef]
- Didik, S.; Palygin, O.; Chandy, M.; Staruschenko, A. The effects of cannabinoids on the kidney. Acta Physiol. 2024, 240, e14247. [Google Scholar] [CrossRef]
- Śledziński, P. In Vitro Evidence of Selective Pro-Apoptotic Action of the Pure Cannabidiol and Cannabidiol-Rich Extract. Molecules 2023, 1, 7887. [Google Scholar] [CrossRef]
- Fouad, A.A.; Al-Mulhim, A.S.; Gomaa, W. Protective effect of cannabidiol against cadmium hepatotoxicity in rats. J. Trace Elem. Med. Biol. 2013, 27, 355–363. [Google Scholar] [CrossRef]
- De Ternay, J.; Naassila, M.; Nourredine, M.; Louvet, A.; Bailly, F.; Sescousse, G.; Maurage, P.; Cottencin, O.; Carrieri, P.M.; Rolland, B. Therapeutic Prospects of Cannabidiol for Alcohol Use Disorder and Alcohol-Related Damages on the Liver and the Brain. Front. Pharmacol. 2019, 31, 627. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, G.; Zhang, X.; Zhang, H.; Liu, Y.; Wang, C.; Miao, L.; Li, Y.; Huang, Y.; Teng, H.; et al. Cannabidiol protects the liver from α-Amanitin-induced apoptosis and oxidative stress through the regulation of Nrf2. Food Chem. Toxicol. 2023, 182, 114196. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Behring, J.B.; Shao, D.; Sverdlov, A.L.; Whelan, S.A.; Elezaby, A.; Yin, X.; Siwik, D.A.; Seta, F.; Costello, C.E.; et al. Overexpression of Catalase Diminishes Oxidative Cysteine Modifications of Cardiac Proteins. PLoS ONE 2015, 7, e0144025. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Antiinflammatory Properties of Cannabidiol. Antioxidants 2019, 25, 21. [Google Scholar] [CrossRef]
- Böckmann, S.; Hinz, B. Cannabidiol Promotes Endothelial Cell Survival by Heme Oxygenase-1-Mediated Autophagy. Cells 2020, 16, 1703. [Google Scholar] [CrossRef]
- Vilela, L.R.; Gomides, L.F.; David, B.A.; Antunes, M.M.; Diniz, A.B.; Moreira Fde, A.; Menezes, G.B. Cannabidiol rescues acute hepatic toxicity and seizure induced by cocaine. Mediators Inflamm. 2015, 2015, 523418. [Google Scholar] [CrossRef]
- Yang, L.; Decas, T.; Zhang, Y.; Alassane-Kpembi, I. Cannabidiol Mitigates Deoxynivalenol-Induced Intestinal Toxicity by Regulating Inflammation, Oxidative Stress, and Barrier Integrity. Toxins 2025, 12, 241. [Google Scholar] [CrossRef]
- Yamaori, S.; Kinugasa, Y.; Jiang, R.; Takeda, S.; Yamamoto, I.; Watanabe, K. Cannabidiol induces expression of human cytochrome P450 1A1 that is possibly mediated through aryl hydrocarbon receptor signaling in HepG2 cells. Life Sci. 2015, 1, 87–93. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assay. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Radko, L.; Minta, M.; Stypuła-Trębas, S. Influence of fluoroquinolones on viability of Balb/c 3T3 and HepG2 cells. Bull. Vet. Inst. Pulawy 2013, 57, 599–606. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewski, C.; Calleawert, D.M. An enzyme-release assay for natural cytotoxicity. J. Immunol. Methods 1983, 64, 313–320. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Zając, A.; Sumorek-Wiadro, J.; Maciejczyk, A.; Langner, E.; Wertel, I.; Rzeski, W.; Jakubowicz-Gil, J. LY294002 and sorafenib as inhibitors of intracellular survival pathways in the elimination of human glioma cells by programmed cell death. Cell Tissue Res. 2021, 386, 17. [Google Scholar] [CrossRef]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]

| Cell Lines | Assay | DOX | DOX + C1 | CI | DOX + C2 | CI |
|---|---|---|---|---|---|---|
| SH-SY5Y | MTT | 9.8 ± 0.9 a | 9.4 ± 1.1 a | 0.87 | 10.4 ± 0.4 a | 0.85 |
| NRU | >200 | 41.0 ± 2.3 a | - | 17.7 ± 0.9 b | - | |
| TPC | >200 | 69.6 ± 4.3 a | - | 35.5 ± 1.4 b | - | |
| LDH | 149 ± 6.5 a | 37.6 ± 0.6 b | 0.47 | 18.3 ± 4.3 c | 0.09 | |
| BrdU | 15.6 ± 0.7 a | <1.56 | - | <1.56 | - | |
| HepG2 | MTT | >200 | >200 | - | 147.6 ± 3.9 a | |
| NRU | 13.4 ± 0.9 a | 17.4 ± 0.9 a | 0.94 | 16.5 ± 0.8 a | 0.93 | |
| TPC | >200 | >200 | - | >200 | - | |
| LDH | >200 | >200 | - | 116.4 ± 4.7 a | - | |
| BrdU | 24.0 ± 0.4 a | 43.5 ± 1.9 b | 1.97 | 35.5 ± 1.9 c | 1.43 | |
| HEK 293 | MTT | 17.4 ± 1.0 a | 88.7 ± 2.1 b | 4.35 | 20.5 ± 0.9 a | 0.97 |
| NRU | 12.7 ± 1.0 a | 13.6 ± 1.3 a | 0.93 | 11.2 ± 0.9 a | 0.90 | |
| TPC | 14.8 ± 1.3 a | 36.9 ± 2.0 b | 2.77 | 19.7 ± 1.1 c | 0.95 | |
| LDH | 30.4 ± 2.7 a | 62.1 ± 3.8 b | 2.09 | 8.9 ± 0.7 c | 0.35 | |
| BrdU | 8.9 ± 0.1 a | 9.1 ± 0.7 a | 0.91 | 9.3 ± 0.4 a | 0.95 |
| Control | Doxycycline (DOX) | DOX + C1 | DOX + C2 | |
|---|---|---|---|---|
| SH-SY5Y cells | ||||
| May–Grunwald staining | 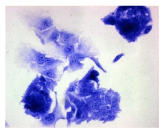 | 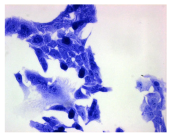 | 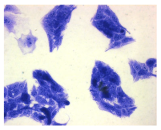 | 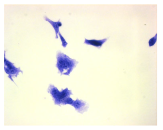 |
| Hoechst 33342 + propidium iodide |  |  | 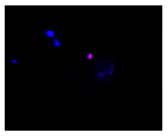 | 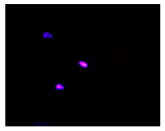 |
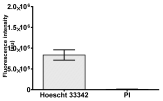 | 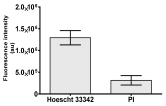 |  |  | |
| HepG2 cells | ||||
| May–Grunwald staining | 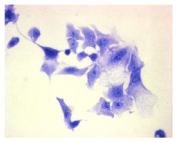 | 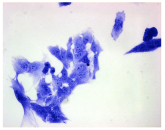 | 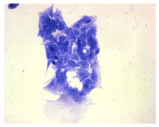 | 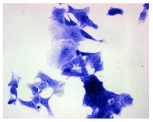 |
| Hoechst 33342 + propidium iodide |  | 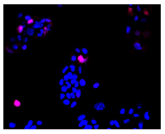 | 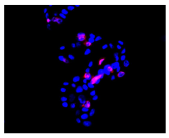 | 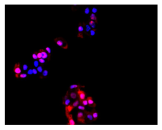 |
 |  |  |  | |
| HEK-293 cells | ||||
| May–Grunwald staining | 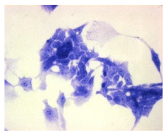 | 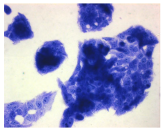 | 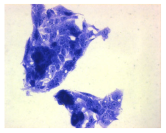 | 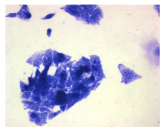 |
| Hoechst 33342 + propidium iodide | 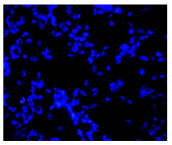 | 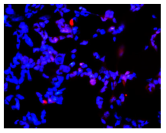 | 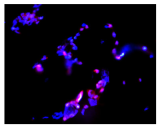 | 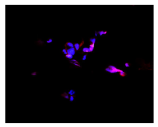 |
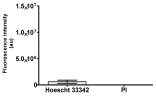 | 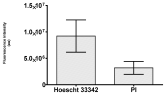 |  |  | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radko, L.; Wojciechowicz, T.; Kończak, O.; Żakowicz, P.; Łętowski, O.; Salmanowicz, J.; Skrzypczak, Z. The Action of Cannabidiol on Doxycycline Cytotoxicity in Human Cells—In Vitro Study. Molecules 2025, 30, 4319. https://doi.org/10.3390/molecules30214319
Radko L, Wojciechowicz T, Kończak O, Żakowicz P, Łętowski O, Salmanowicz J, Skrzypczak Z. The Action of Cannabidiol on Doxycycline Cytotoxicity in Human Cells—In Vitro Study. Molecules. 2025; 30(21):4319. https://doi.org/10.3390/molecules30214319
Chicago/Turabian StyleRadko, Lidia, Tatiana Wojciechowicz, Oliwia Kończak, Paula Żakowicz, Oskar Łętowski, Julia Salmanowicz, and Zuzanna Skrzypczak. 2025. "The Action of Cannabidiol on Doxycycline Cytotoxicity in Human Cells—In Vitro Study" Molecules 30, no. 21: 4319. https://doi.org/10.3390/molecules30214319
APA StyleRadko, L., Wojciechowicz, T., Kończak, O., Żakowicz, P., Łętowski, O., Salmanowicz, J., & Skrzypczak, Z. (2025). The Action of Cannabidiol on Doxycycline Cytotoxicity in Human Cells—In Vitro Study. Molecules, 30(21), 4319. https://doi.org/10.3390/molecules30214319









