Advances in Naturally and Synthetically Derived Bioactive Sesquiterpenes and Their Derivatives: Applications in Targeting Cancer and Neurodegenerative Diseases
Abstract
1. Introduction
2. Antitumor Activity of Sesquiterpene Lactone Derivatives
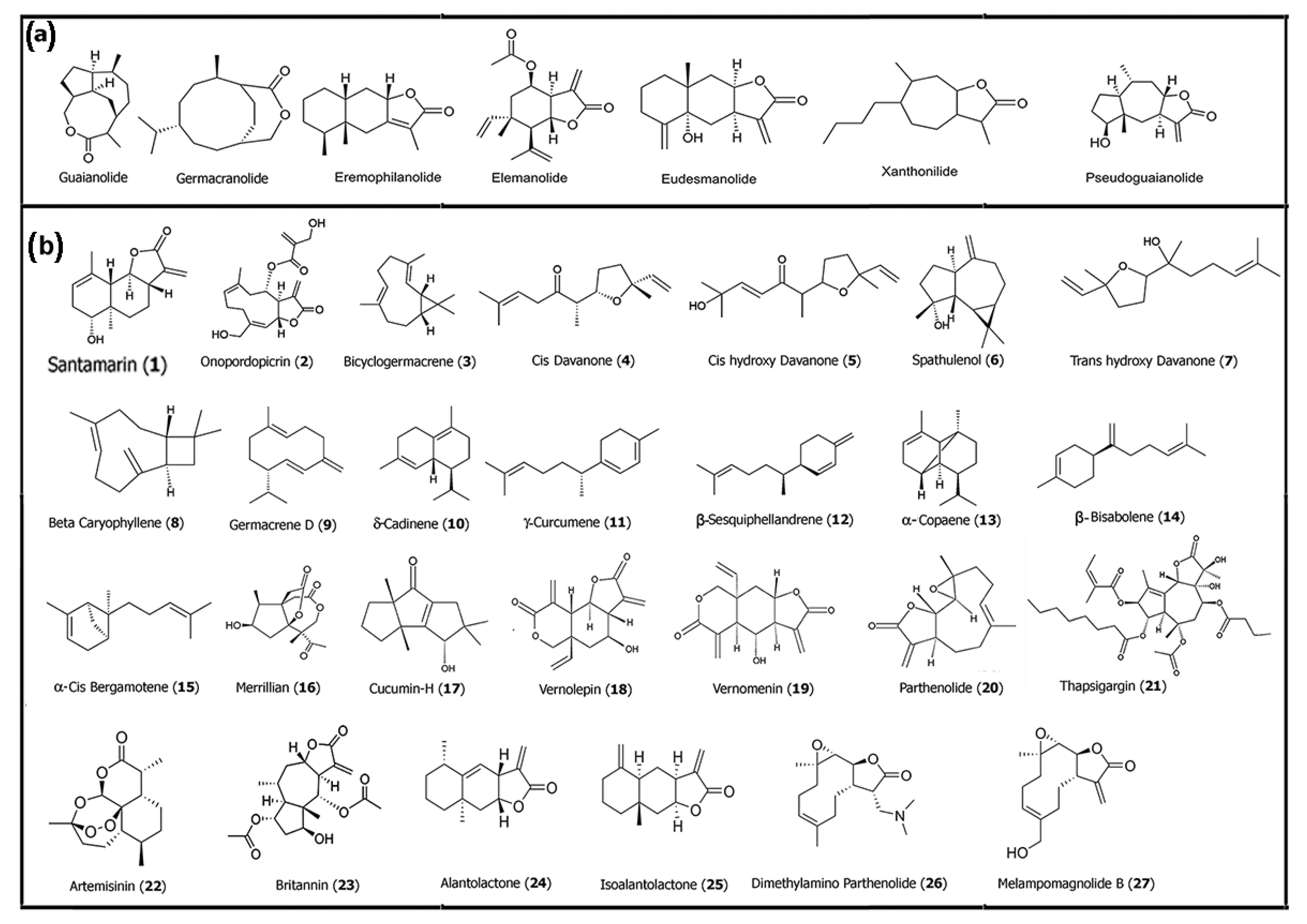
2.1. Naturally Derived Sesquiterpenes
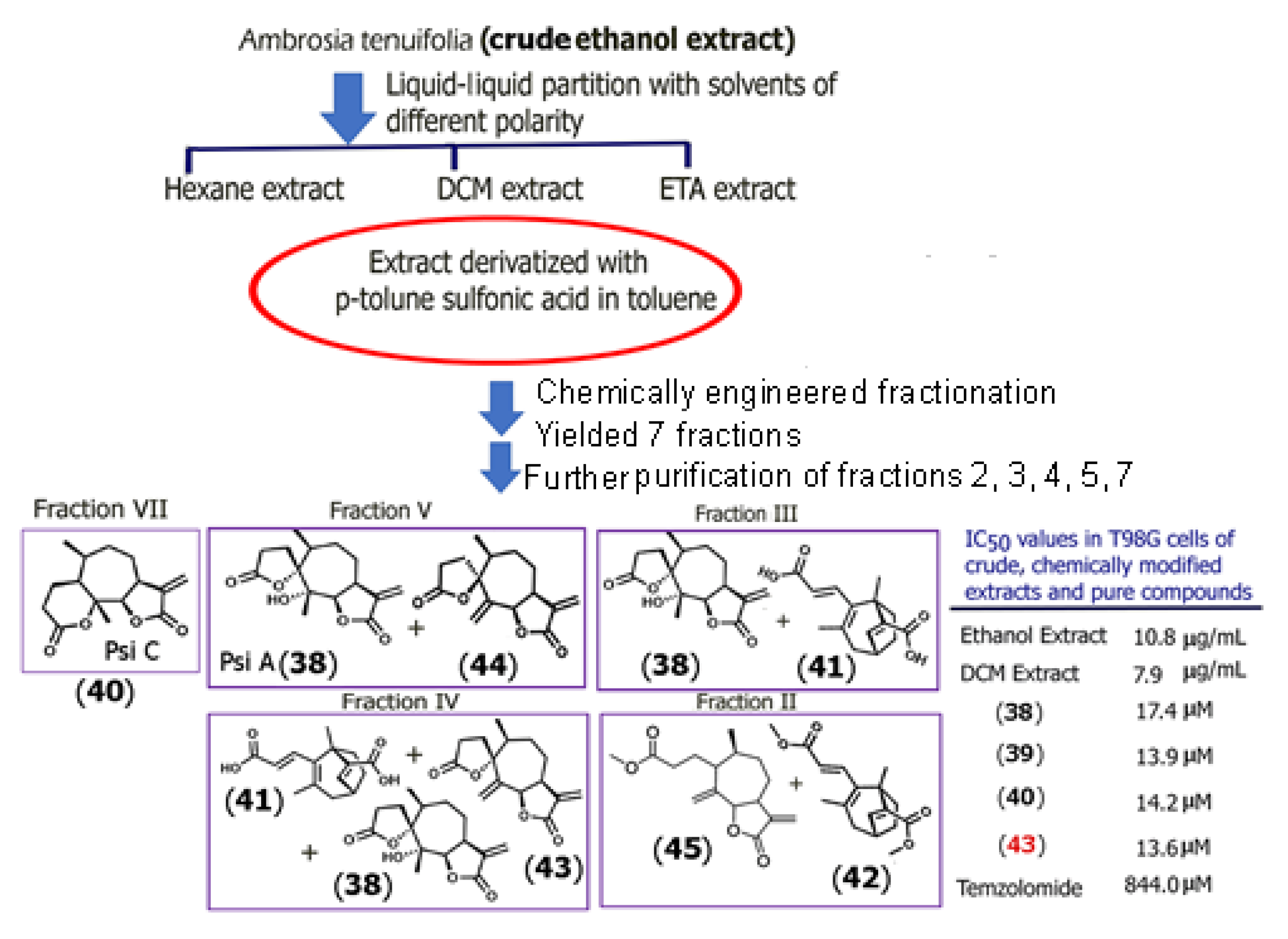
2.2. Remodeling of Sesquiterpene Extracts via In Situ Chemical Derivatization
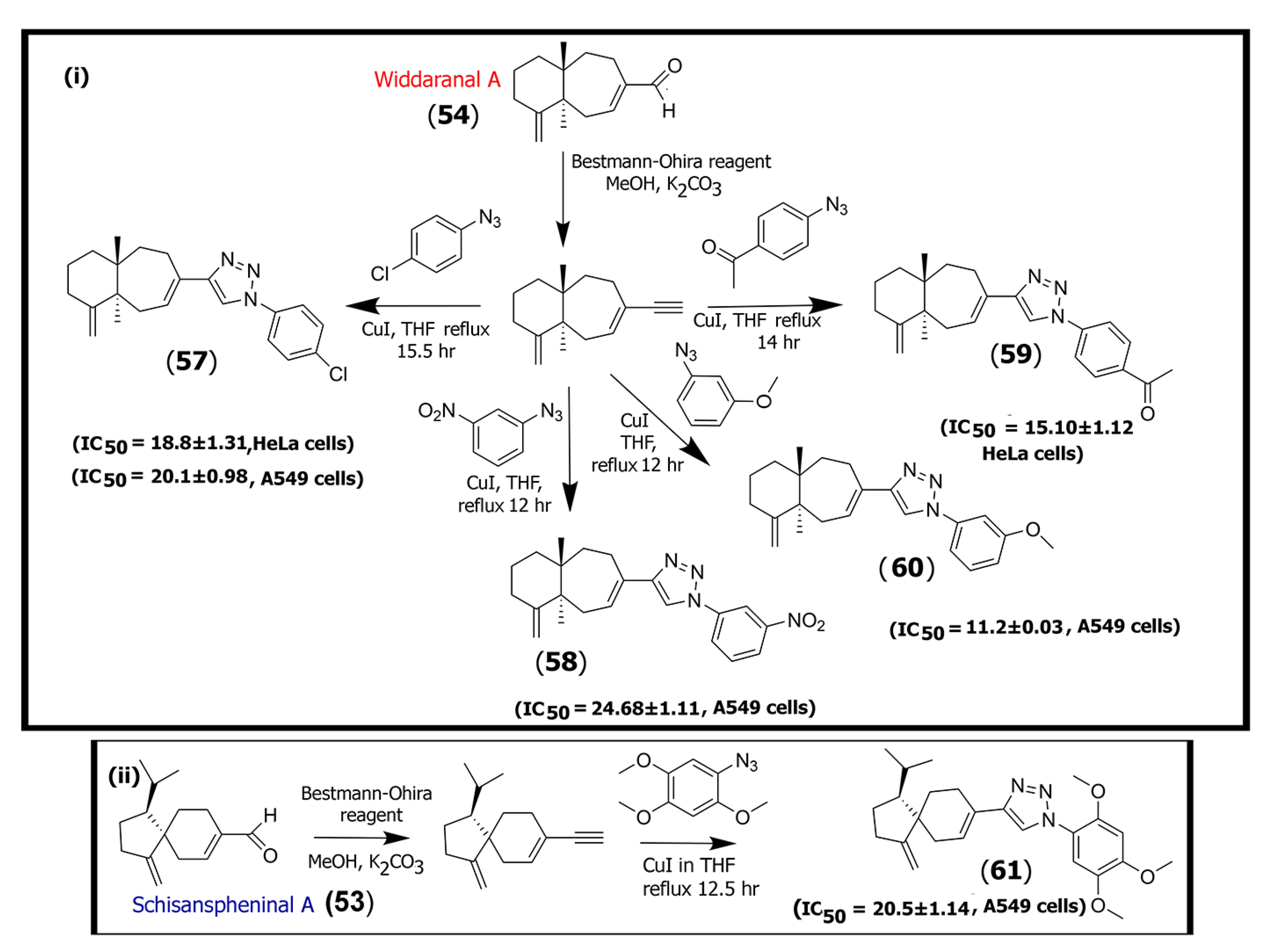
2.3. Synthetic Triazole Derivatives of Sesquiterpenes
| Synthesized SL-Derivatives | IC50 (μM) | Cell Line | In Vitro Assays | In Vivo Assays | Ref. # |
|---|---|---|---|---|---|
| (50) | 10.03 33.2 27.2 36.7 | HT-1080 A549 MCF-7 MDA-MB-231 | MTT assay, apoptosis assay (flow cytometry) Cell cycle analysis | - | [55] |
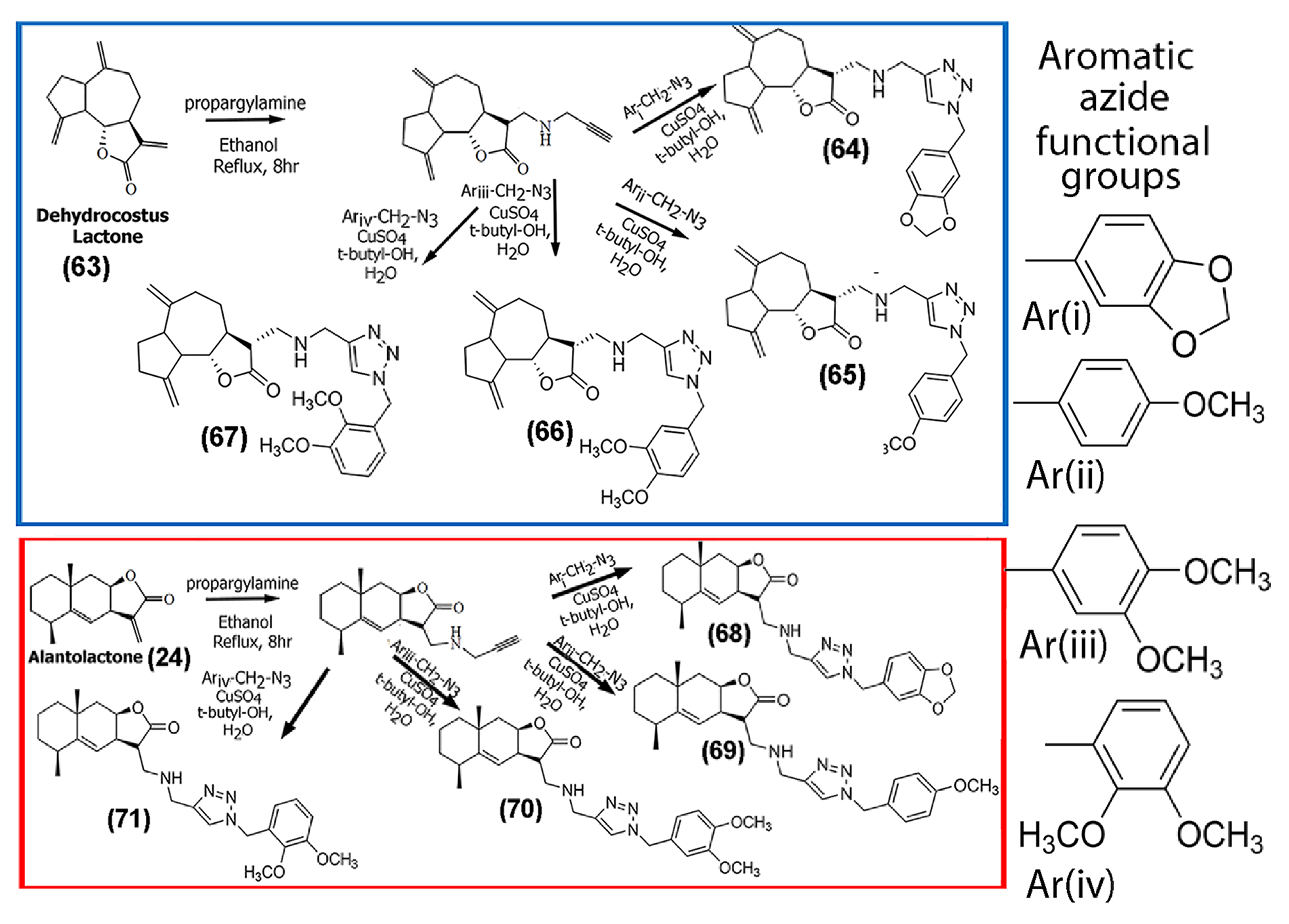
| (64) | 18.0 19.3 >100 >100 | HeLa SH-SY5Y Hep-2 A549 | MTT assay to test cytotoxicity | [59] | |
| (65) | 26.2 16.6 25.2 16.14 | Hep-2 HeLa A549 SH-SY5Y | Cytotoxicity—MTT assay “ “ “ | - | [59] |
| (66) | 82.6 26.4 >100 21.83 | Hep-2 HeLa A549 SH-SY5Y | Cytotoxicity—MTT assay “ “ “ | - | [59] |
| (67) | >100 79.6 >100 89.5 | Hep-2 HeLa A549 SH-SY5Y | Cytotoxicity—MTT assay “ “ “ | - | [59] |
| (68) through (71) | >100 >72 >70 >56 | Hep-2, HeLa, A549 SH-SY5Y | Cytotoxicity—MTT assay “ “ “ | - | [59] |
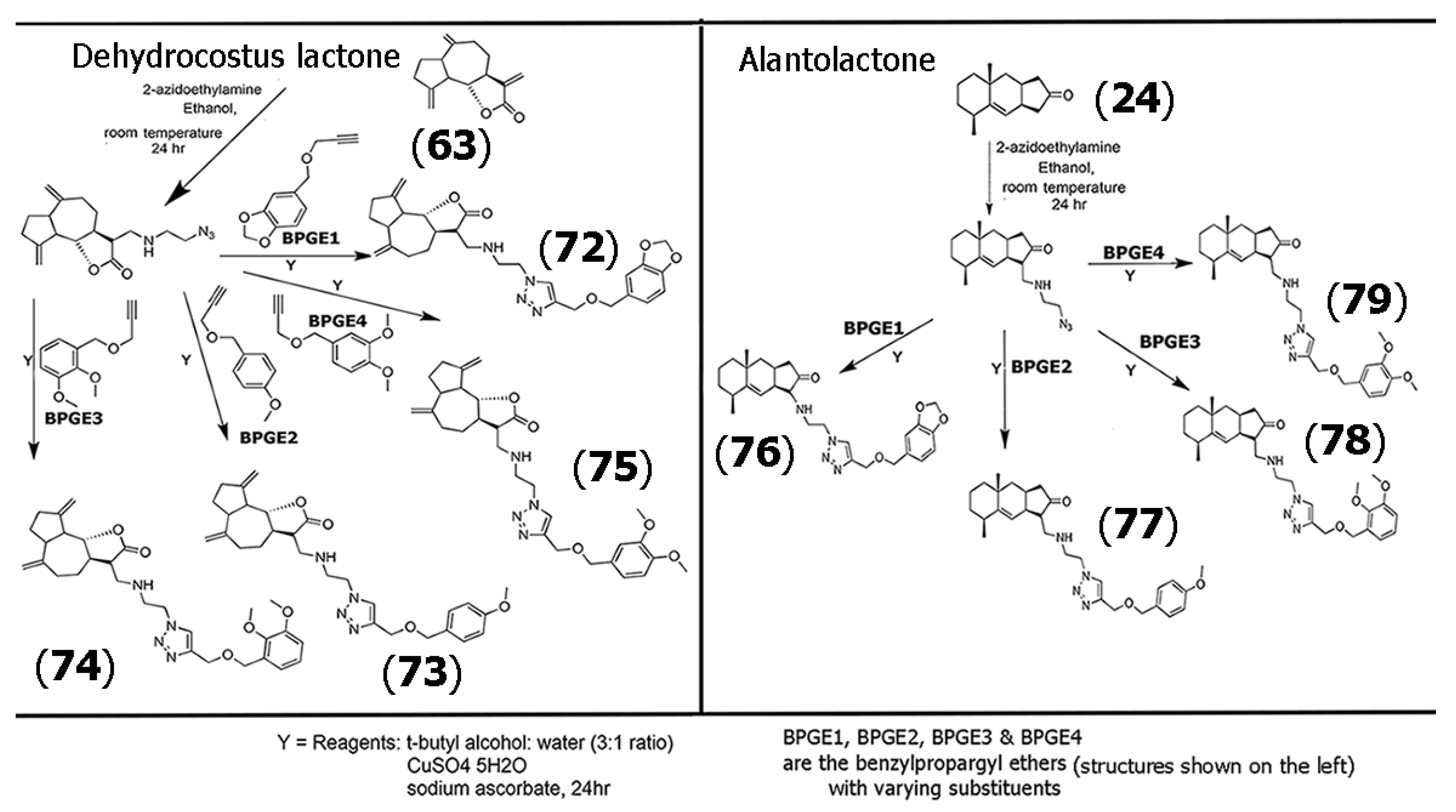
| (72) | 37.4 62.4 88.1 85.7 | SH-SY5Y Hela Hep-2 A549 | Cytotoxicity (MTT); mitochondria-dependent apoptosis and suppression of glycolysis process | Mitochondrial transmembrane potential from rat liver was tested | [63] |
| (73) | 36.3 61.5 >100 >100 | SH-SY5Y Hela Hep-2 A549 | Cytotoxicity (MTT); mitochondria-dependent apoptosis and suppression of glycolysis process | Mitochondrial transmembrane potential from rat liver was tested | [63] |
| (74) | 94.9 82.4 >100 >100 | SH-SY5Y Hela Hep-2 A549 | Cytotoxicity (MTT); mitochondria-dependent apoptosis and suppression of glycolysis process | Mitochondrial transmembrane potential from rat liver was tested | [63] |
| (75) | 57.8 >100 >100 >100 | SH-SY5Y Hela Hep-2 A549 | Cytotoxicity (MTT); mitochondria-dependent apoptosis and suppression of glycolysis process | Mitochondrial transmembrane potential from rat liver was tested | [63] |
| (76) | 33.6 38.9 55.7 29.3 | SH-SY5Y HeLa Hep2 A549 | Cytotoxicity (MTT); mitochondria-dependent apoptosis and suppression of glycolysis process | Mitochondrial transmembrane potential from rat liver was disrupted | [63] |
| (77) | 41.7 55.1 67.1 47.3 | SH-SY5Y HeLa Hep2 A549 | Cytotoxicity (MTT); mitochondria-dependent apoptosis and suppression of glycolysis | Determination of mitochondrial transmembrane potential from rat liver | [63] |
| (78) | 39.9 46.5 62.5 53.1 | SH-SY5Y HeLa Hep2 A549 | Cytotoxicity (MTT); mitochondria-dependent apoptosis and suppression of glycolysis | Determination of mitochondrial transmembrane potential from rat liver | [63] |
| (79) | 45.2 81.0 91.0 83.5 | SH-SY5Y HeLa Hep2 A549 | Cytotoxicity (MTT); mitochondria-dependent apoptosis and suppression of glycolysis process | Determination of mitochondrial transmembrane potential from rat liver | [63] |
| (80) | 8.6 10.01 9.97 16.41 | MCF-7 SH-SY5Y HeLa IMR-32 | MTT assay confirmed inhibition of cell proliferation. Glycolytic function in HeLa cells was assessed by a glycolysis stress test. Compounds (80) through (82) were identified as potential inhibitors of allosteric glycolytic enzyme and pyruvate kinase (PK) M2 oncoprotein. Docking studies revealed binding interactions. MTT assay confirmed inhibition of cell proliferation. Glycolytic function in HeLa cells was assessed by a glycolysis stress test. Some were identified as potential inhibitors of allosteric glycolytic enzyme and pyruvate kinase (PK) M2 oncoprotein. | - | [64] |
| (81) | 8.17 7.93 22.68 5.76 | MCF-7 SH-SY5Y HeLa IMR-32 | - | ||
| (82) | 18.6 19.01 21.31 17.38 | MCF-7 SH-SY5Y HeLa IMR-32 | - | ||
| (83) | 8.91 22.75 21.06 8.64 | MCF-7 SH-SY5Y HeLa IMR-32 | - | ||
| (84) | 8.07 7.41 6.58 6.07 | MCF-7 SH-SY5Y HeLa IMR-32 | - | [64] |
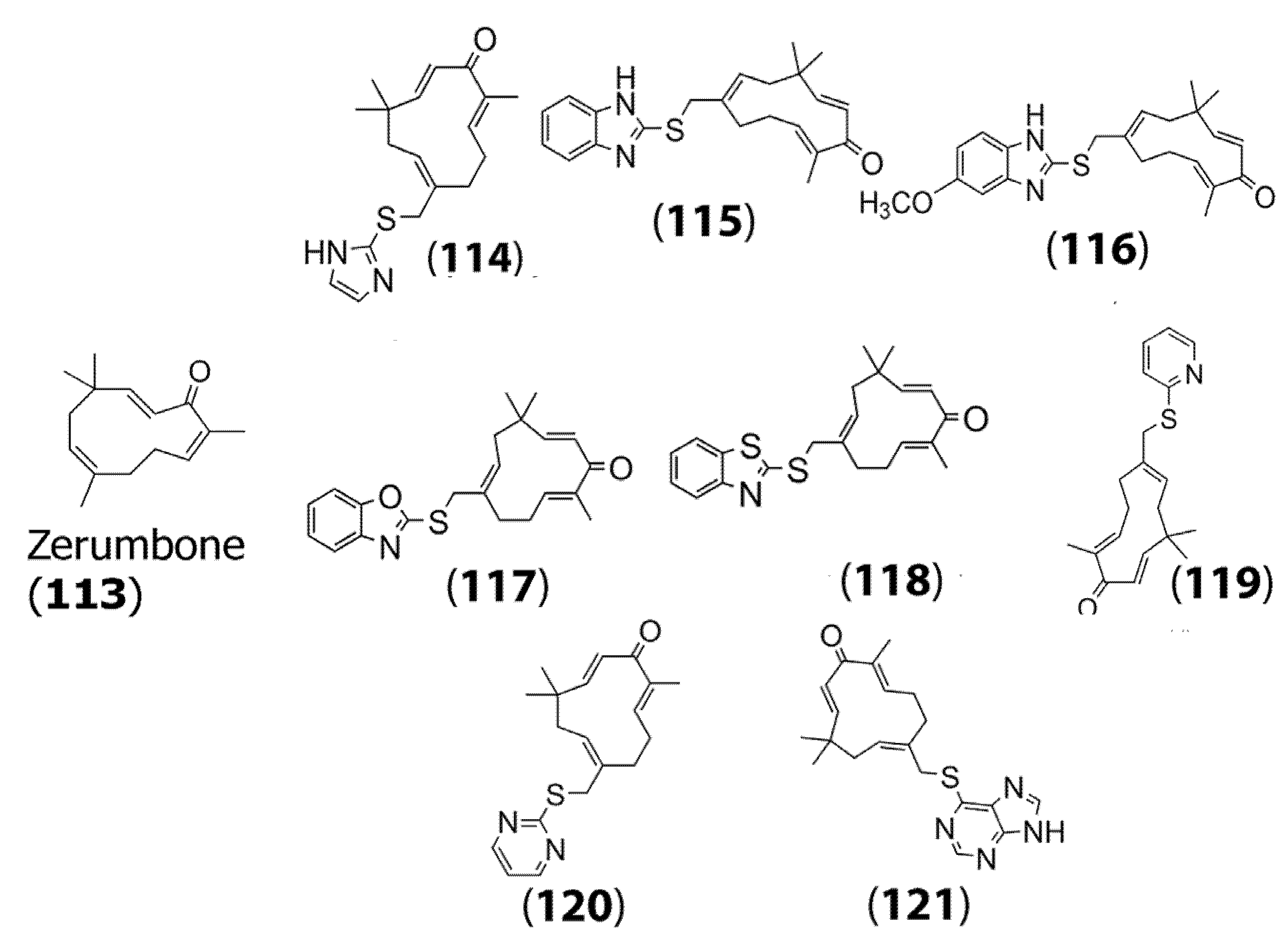
2.4. Mercapto Derivatives of Sesquiterpenes and Their Antitumor Activity
2.5. Conjugates of Sesquiterpene Lactones and Their Chemosensitization Ability
2.6. Anticancer Activity of Sesquiterpene Quinones and Their Derivatives
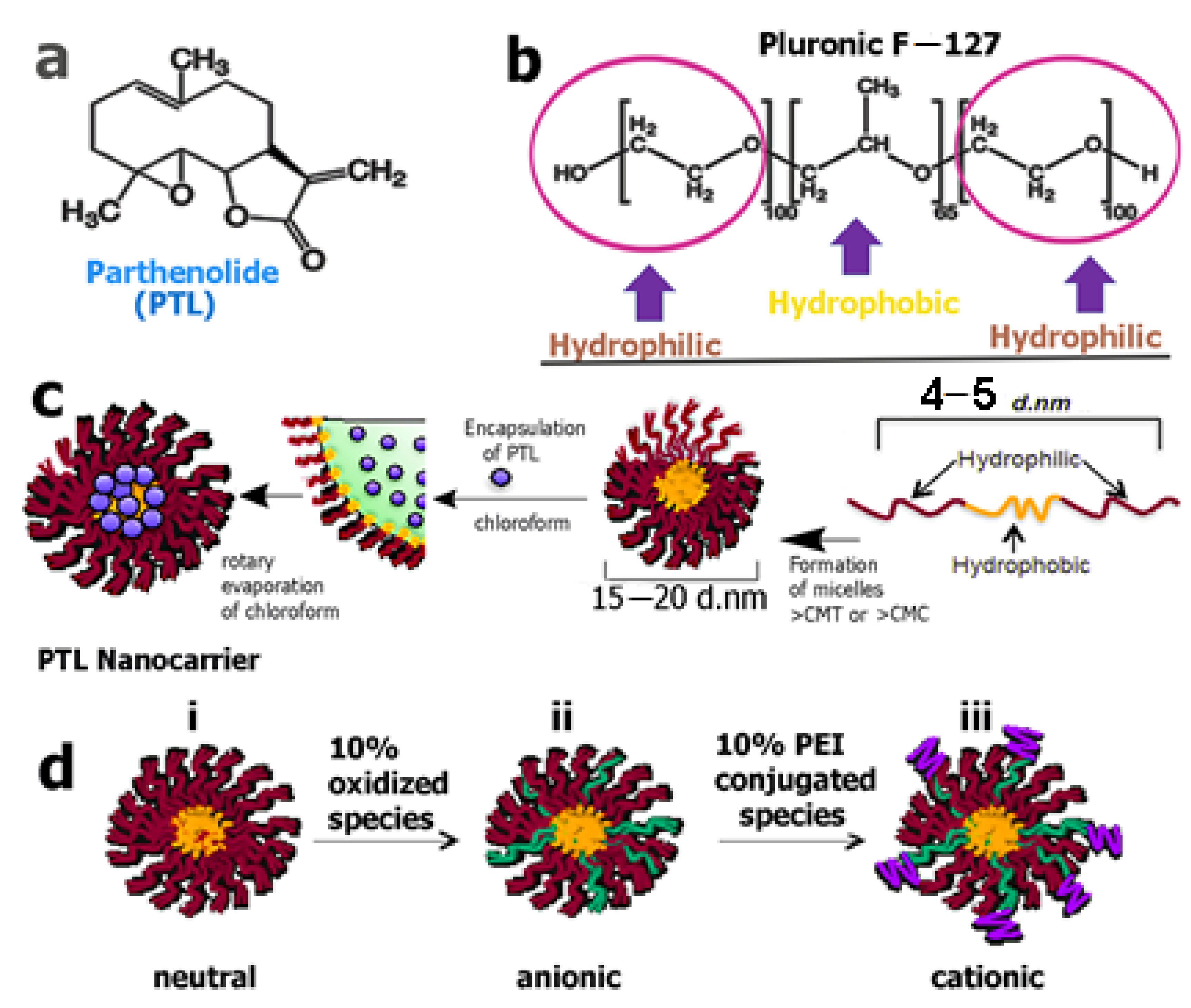
3. Nanoformulations of Sesquiterpenes
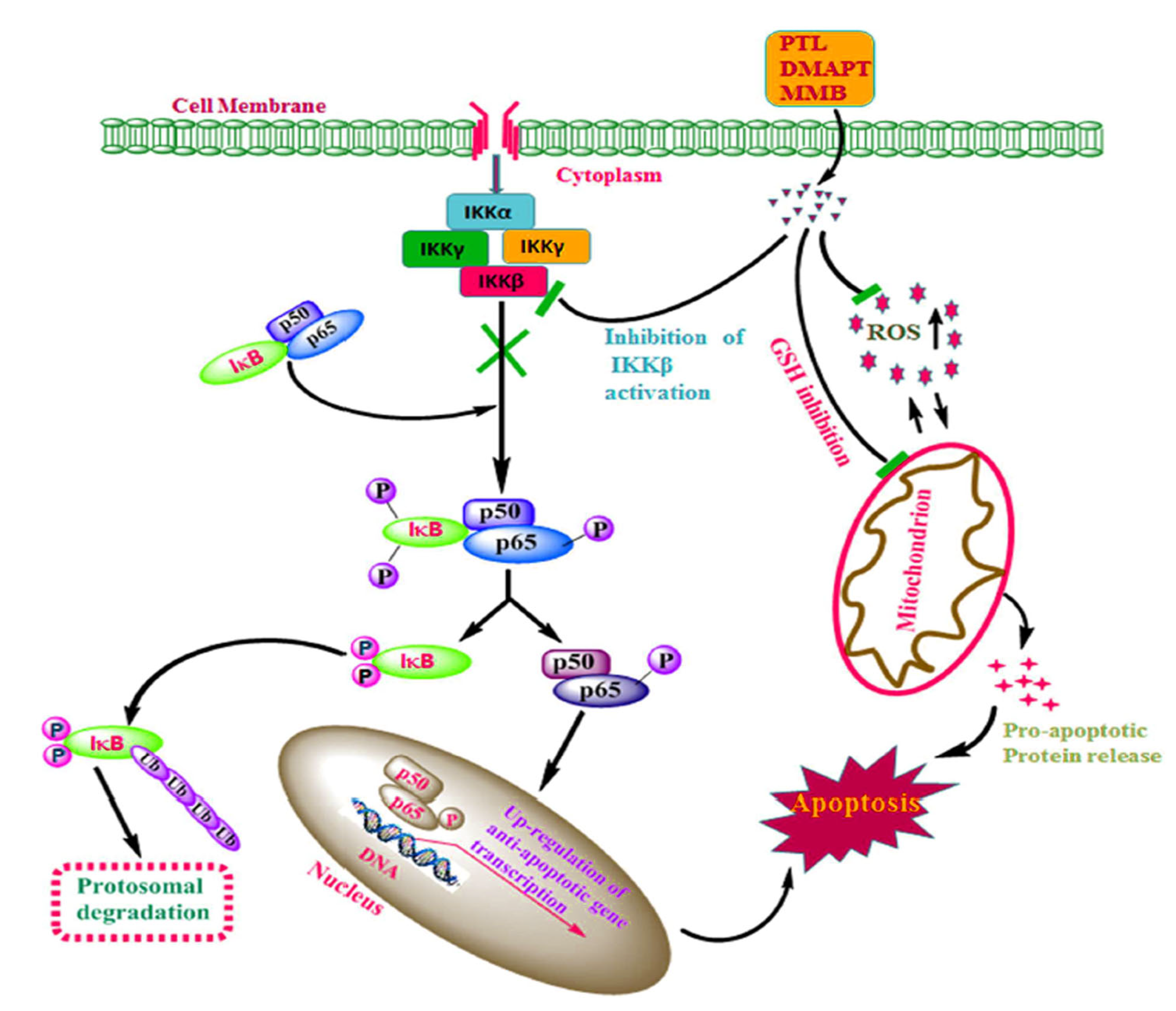
4. Clinical Trials
5. Sesquiterpenes as Potential Treatment for Neurodegenerative Diseases
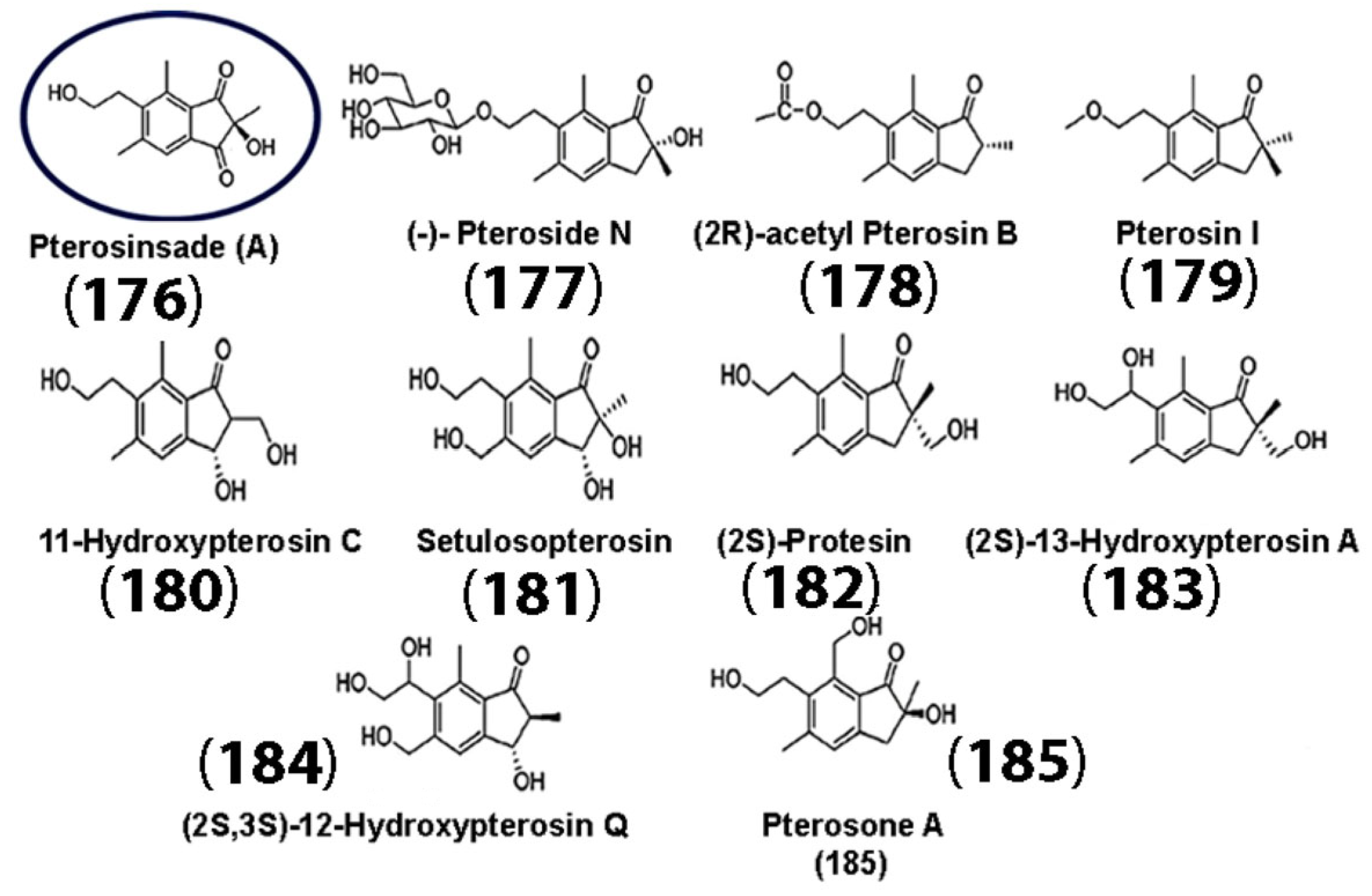
5.1. Pterosin Sesquiterpenes
5.2. Sesquiterpene Alcohols
5.3. Sesquiterpene Lactones in Neurodegenerative Diseases
6. Pharmacokinetics and Toxicity Studies of Sesquiterpenes and Their Derivatives
7. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, T.; Khan, M.U.; Sharma, V.; Gupta, K. Terpenoids in essential oils: Chemistry, classification, and potential impact on human health industry. Phytomedicine Plus 2024, 4, 100549. [Google Scholar] [CrossRef]
- Boncan, D.A.; Tsang, S.; Li, C.; Lee, I.; Lam, H.-M.; Chan, T.-F.; Hui, J. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene lactones from Artemisia genus: Biological activities and methods of analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef] [PubMed]
- Dumas, F.; Kousara, M.; Chen, L.; Wei, L.; Le Bideau, F. Non-halogenated heterotricyclic sesquiterpenes from marine origin I: Fused systems. In Studies in Natural Product Chemistry; Rahman, A.U., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2017; Volume 52, pp. 269–302. [Google Scholar]
- Miller, D.J.; Allemann, R.K. Sesquiterpene synthases: Passive catalysts or active players. Nat. Prod. Rep. 2012, 29, 60. [Google Scholar] [CrossRef]
- Fathy, N.; Morsy, S. Chemical structure, quality indices and bioactivity of essential oil constituents. In Active Ingredients from Aromatic Medicinal Plants; El-Shemy, H.A., Ed.; Intech Open: London, UK, 2017; Chapter 11. [Google Scholar]
- Sahoo, B.M.; Tiwari, V.; Tiwari, A.; Banik, B.K. Chapter 9, Sesquiterpenes from essential oils and their anticancer potential. J. Ind. Chem. Soc. 2023, 2023, 7841824. [Google Scholar] [CrossRef]
- Chizzola, R. Regular monoterpenes and sesquiterpenes (essential oils). In Natural Products; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–52. [Google Scholar]
- Khan, R.; Sultana, S. Farnesol attenuates 1,2-dimethylhydrazine induced oxidative stress, inflammation and apoptotic responses in the colon of wistar rats. Chem. Biol. Interact. 2011, 192, 193–200. [Google Scholar] [CrossRef]
- Choi, H.-G.; Lee, D.-S.; Li, B.; Choi, Y.-H.; Lee, S.-H.; Kim, Y.-C. Santamarin, a sesquiterpene lactone isolated from Sausseurea lappa, represses LPS-induced inflammatory responses via expression of heme oxygenase-1 in murine macrophage cells. Int. Immunopharmacol. 2012, 13, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Khatib, N.E.; Morel, S.; Hugon, G.; Rapior, S.; Carnac, G.; Saint, N. Identification of a sesquiterpene lactone from Arctium lappa leaves with antioxidant activity in primary human muscle cells. Molecules 2021, 26, 1328. [Google Scholar] [CrossRef]
- Singh, S.; Bhatt, D.; Singh, M.K.; Sundaresan, V.; Tandon, S.; Padalia, R.C.; Bawankule, D.U.; Verma, R.S. New insights into the chemical composition, pro-inflammatory cytokine inhibition profile of Davana (Artemisia pallens Wall. Ex DC.) essential oil and cis-davanone in primary macrophage cells. Chem. Biodivers. 2021, 18, e210053. [Google Scholar] [CrossRef]
- Ashmawy, A.M.; Ayoub, I.M.; Eldahshan, O.A. Chemical composition, cytotoxicity ad molecular profiling of Cordia africana Lam. On human breast cancer cell line. Nat. Prod. Res. 2021, 35, 4133–4138. [Google Scholar] [CrossRef]
- Calvopiña, K.; Malagon, O.; Capetti, F.; Sgorbini, B.; Verdugo, V.; Gilardoni, G. A new sesquiterpene essential oil from the native andean species Jungia rugosa Less (Asteraceae): Chemical analysis, enantiomeric evaluation and cholinergic activity. Plants 2021, 10, 2102. [Google Scholar] [CrossRef] [PubMed]
- Thalappil, M.A.; Singh, P.; de Prati, A.C.; Sahoo, S.K.; Mariotto, S.; Buttrini, E. Essential oils and their nanoformulations for breast cancer therapy. Phytother. Res. 2024, 38, 556–591. [Google Scholar] [CrossRef]
- Shiina, I.; Iizumi, T.; Taniguchi, S.; Sugimoto, M.; Shimazaki, T.; Yamai, Y.-S.; Ogawa, G.; Yamada, T.; Shinohara, S.; Kageyama, Y.; et al. Total synthesis of the sesquiterpene (-)-merrilianin. Org. Lett. 2023, 26, 3327–3331. [Google Scholar] [CrossRef]
- Srikrishna, A.H.; Dethe, D.H. Enantiospecific first total synthesis and assignment of absolute configuration of the sesquiterpene (-)-curcumin. Org. Lett. 2003, 5, 2295–2298. [Google Scholar] [CrossRef]
- Abraham, W.R. Bioactive sesquiterpenes produced by fungi are they useful for humans as well. Curr. Med. Chem. 2001, 8, 533–606. [Google Scholar] [CrossRef]
- Perassolo, M.; Cardillo, A.B.; Busto, V.D.; Giuliett, A.M.; Talou, J.R. Biosynthesis of sesquiterpene lactones in plants and metabolic engineering for their biotechnological production. In Sesquiterpene Lactones; Sulsen, V., Martion, V., Eds.; Springer Nature: Gewerbestrasse, Switzerland, 2018; Chapter 4; pp. 47–91. [Google Scholar]
- Grieco, P.A.; Nishizawa, M.; Oguri, T.; Burke, S.D.; Marinovic, N. Sesquiterpene lactones: Total synthesis of vernolepin and (±)-vernomenin. J. Am. Chem. Soc. 1977, 99, 5773–5780. [Google Scholar] [CrossRef]
- Saya, J.M.; Vos, K.; Kleinnijenhuis, R.; van Maarseveen, J.; Ingermann, S.; Hiemstra, H. Total synthesis of aquatolide. Org. Lett. 2015, 17, 3892–3894. [Google Scholar] [CrossRef]
- Dewanckele, J.M.; Zutterman, F.; Vadewalle, M. Sesquiterpene lactones: A total synthesis of (±) quadrone. Tetrahedron 1983, 39, 3235–3244. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Moharana, S.; Khatun, G.N. Recent advances in the syntheses of guaianolides. Org. Biomol. Chem. 2023, 21, 6652–6670. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.; Diaz, G. Studies on the synthesis of (±)-pathylactone A, a nor-sesquiterpene lactone isolated from marine sources. Tetrahedron 2002, 58, 1647–1656. [Google Scholar] [CrossRef]
- Jaskulska, A.; Janecka, A.; Gach-Janczak, K. Thapsigargin—From traditional medicine to anticancer drug. Int. J. Mol. Sci. 2020, 22, 4. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Beekman, A.; Wieranga, P.; Woerdenbag, H.; Van Uden, W.; Pras, N.; Konings, A.; El-Feraly, F.; Galal, A.; Wikstorm, H.V. Artemisinin-derived sesiquiterpene lactones as potential antitumor compounds: Cytotoxic action against bone marrow and tumor cells. Plant Med. 1998, 64, 615–619. [Google Scholar] [CrossRef]
- Dhyani, P.; Sati, P.; Sharma, E.; Attri, D.C.; Bahukhandi, A.; Tynybekov, B.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Suleria, H.; et al. Sesquiterpenoid lactones as potential anti-cancer agents: An update on molecular mechanism and recent studies. Cancer Cell Int. 2022, 22, 305. [Google Scholar] [CrossRef]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef]
- Kreuger, M.R.; Grootjans, S.; Biavatti, M.W.; Vandenabeele, P.; D’Herde, K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: Focus on parthenolide. AntiCancer Drugs 2012, 23, 883–1001. [Google Scholar] [CrossRef]
- Kim, J.-E.; You, D.-J.; Lee, C.; Ahn, C.; Seong, J.; Hwang, J. Suppression of NF-κB signaling by KEAP1 regulation of IKKβ activity through autophagic degradation and inhibition of phosphorylation. Cell. Signal. 2010, 22, 1645–1654. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer targets and signaling pathways activated by britannin and related pseudoguaianolide sesquiterpene lactones. Biomedicines 2021, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Vu, Q.V.; Sayama, S.; Ando, M.; Kataoka, T. Sesquiterpene lactones containing an α-methylene-γ-lactone moiety selectively downregulate the expression of tumor necrosis factor receptor 1 by promoting its ectodomain shedding in human lung adenocarcinoma A549 cells. Molecules 2024, 29, 1866. [Google Scholar] [CrossRef]
- Janganati, V.; Ponder, J.; Balasubramaniam, M.; Bhat-Nakshatri, P.; Bar, E.E.; Nakshatri, H.; Jordan, C.T.; Crooks, P.A. MMB triazole analogs are potent NF-κB inhibitors and anti-cancer agents against both hematological and solid tumor cells. Eur. J. Med. Chem. 2018, 157, 562–581. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, N.; Dhingra, S.; Jois, S.D.; Vicente, M. Molecular targeting of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR). Molecules 2021, 26, 1076. [Google Scholar] [CrossRef]
- Nerdy, N.; Lestari, P.; Fahdi, F.; Putra, E.D.L.; Amir, S.A.B.; Yusuf, F.; Bakri, T.K. In silico studies of sesquiterpene lactones from Veronica amygdalina Delile on the expression of EGFR and VEGFR as new anticancer potential. Pharmacogn. J. 2022, 14, 91–97. [Google Scholar]
- Moghadam, M.H.; Hajimehdipoor, H.; Saeidnia, S.; Atoofi, A.; Shahrestani, R.; Read, R.W.; Mosaddegh, M. Anti-proliferative activity and apoptotic potential of britannin, a sesquiterpene lactone from Inula aucheriana. Nat. Prod. Commun. 2012, 7, 979–980. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Pesic, M.; Pdolski-Renic, A.; Bankovic, J.; de Vos, R.; Peric, M.; Todorovic, S.; Tanic, N. Cytotoxic activity of sesquiterpene lactones from Inula britannica on human cancer cell lines. Phytochem. Lett. 2013, 6, 246–252. [Google Scholar] [CrossRef]
- Kabeer, F.A.; Rajalekshmi, D.S.; Nair, M.S.; Prathapan, R. Molecular mechanisms of anticancer activity of deoxyelephantopin in cancer cells. Integr. Med. Res. 2017, 6, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gao, H.; Zhang, Y.; Li, Y.; Vadev, N.; Gao, Y.; Chen, Y.; Zhang, Q. Alantolactone selectively ablates acute myeloid leukemia stem and progenitor cells. J. Hematol. Oncol. 2016, 9, 93. [Google Scholar] [CrossRef]
- Lin, M.; Bi, H.; Yan, Y.; Huang, W.; Zhang, G.; Tang, S.; Liu, Y.; Zhang, L.; Ma, J.; Zhang, J. Parthenolide suppresses non-small cell lung cancer GLC-82 cells growth via B-Raf/MAPK/Erk pathway. Oncotarget 2017, 8, 23436–23447. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-C.; Hui, X.-G.; Huo, L.; Sun, D.-X.; Peng, W.; Zhang, Y.; Li, X.-B.; Ma, T.; Li, W.-H.; Liang, J.; et al. Antiproliferative effects of isoalantolactone in human liver cancer cells are mediated through caspase dependent apoptosis, ROS generation, suppression of cell migration & invasion and targeting Ras/Raf/MEK signaling pathway. Acta Biochim. Polonica 2022, 69, 299–304. [Google Scholar]
- Hu, F.; Yang, P. Isoalantolactone exerts anticancer effects on human HEC-1-B endometrial cancer cells via induction of ROS mediated apoptosis and inhibition of MEK/ERK signaling pathway. Acta Biochim. Polonica 2022, 69, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Thongnesta, S.; Chawengrumb, P.; Keeratichamroenc, S.; Lirdprapamongkolc, K.; Eurtivongb, C.; Boonsombata, J.; Kittakoopb, P.; Svastic, J.; Ruchirawat, S.; Vernodalidimer, L. A sesquiterpene lactone dimer from Vernonia extensa and anti-tumor effects of vernodalin, vernolepin and vernolide on HepG2 liver cancer cells. Bioorg. Chem. 2019, 92, 103197. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, Y.-C.; Lim, J.S. Costunilide, a sesquiterpene lactone, suppresses skin cancer via induction of apoptosis and blockage of cell proliferation. Int. J. Mol. Sci. 2021, 22, 2075. [Google Scholar] [CrossRef]
- El-Far, A.; Godugu, K.; Salaheldin, T.; Darwish, N.; Saddiq, A.; Mousa, S.A. Nanonutraceuticals: Anti-cancer activity and improved safety of chemotherapy by costunolide and its nanoformulation against colon and breast cancer. Biomedicines 2021, 9, 990. [Google Scholar] [CrossRef]
- Aydogan, F.; Baykan, S.; Butnuner, B.D. Cytotoxic activity of sesquiterpene esters isolated from endemic Ferula tenuissima Hub. -Mor & Peşmen. Turkish J. Pharm. Sci. 2019, 16, 476–480. [Google Scholar]
- Adessi, T.; Wagner, P.M.; Bisogno, F.R.; Nicotra, V.; Guido, M.E.; Garcia, M.E. Enhancing structural diversity through chemical engineering of Ambrosia tenuifolia extract for novel anti-glioblastoma compounds. Sci. Rep. 2024, 14, 14229. [Google Scholar] [CrossRef]
- Safi, R.; Hamade, A.; Bteich, N.; El Shagir, J.; Assaf, M.; El-Sabban, M.; Najjar, F. A ferutinin analogue with enhanced potency and selectivity against ER positive breast cancer cells in vitro. Biomed. Pharmacother. 2018, 105, 267–273. [Google Scholar] [CrossRef]
- Lone, S.H.; Bhat, K.A.; Majeed, R.; Hamid, A.; Khuroo, M.A. Click chemistry inspired facile synthesis and bioevaluation of novel triazolyl analogues of ludartin. Bioorg. Med. Chem. Lett. 2014, 24, 1047. [Google Scholar] [CrossRef] [PubMed]
- Patrushev, S.; Kichkina, D.; Moralev, A.; Rybalova, T.; Krasnov, V.; Chernyak, E.; Zenkova, M.; Markov, A.; Shults, E. Synthesis and exploration of anticancer potential of spirocyclic 1,2,3-tirazoline and aziridine derivatives of natural eudesmanolide isoalantolactone. Bioorg. Chem. 2025, 155, 108124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, Y.; Ding, Y.; Zhai, J.; Ki, Q.; Ma, W.; Yang, M.; Fan, H.; Long, J.; Tong, Z.; et al. Guainolide sesquiterpene lactones, a source to discover agents that selectively inhibit acute myelogenous leukemia stem and progenitor cells. J. Med. Chem. 2012, 55, 8757–8769. [Google Scholar] [CrossRef]
- Zaki, M.; Oukhrib, A.; El Hakmaoui, A.; Hiebel, M.-A.; Berteina-Raboin, S.; Akssira, M. Synthesis of novel 1,2,3-triazole substituted tomentosins. Z. Naturforsch. 2019, 74, 273–281. [Google Scholar] [CrossRef]
- Patrushev, S.S.; Rybalova, T.V.; Shults, E.E. Synthetic transformations of sesquiterpene lactones. Controllable synthesis of 11,13-dihydroisoalantolactone azides and 13-(1,2,3-triazolyl) eudesmanolides based on sesquiterpene lactones. Chem. Heterocycl. Compd. 2021, 57, 1116–1129. [Google Scholar] [CrossRef]
- Faris, A.; Eddeer, Y.; Louchachha, I.; Lahcen, I.A.; Azzaoui, K.; Hammouti, B.; Merzouki, M.; Challioui, A.; Boulay, B.; Karim, A.; et al. From himachalenes to trans-himachalol: Unveiling bioactivity through hemisynthesis and molecular docking analysis. Sci. Rep. 2023, 13, 17653. [Google Scholar] [CrossRef]
- Bimoussa, A.; Oubella, A.; Laamari, Y.; Fawzi, M.; Hachim, M.; Itto, M.Y.; Morjani, H.; Ketatni, E.M.; Mentre, O.; Auhmani, A. Hybrid of the 1,2,3-triazole nucleus and sesquiterpene skeleton as a potential antitumor agent: Hemisynthesis, Molecular structure, Hirshfeld surface analysis, DFT, in vitro cytotoxic and apoptotic effects. J. Heterocycl. Chem. 2021, 58, 2334–2347. [Google Scholar] [CrossRef]
- Poornima, B.; Siva, B.; Shankaraiah, G.; Venkanna, A.; Nayak, V.L.; Ramakrishna, S.; Rao, C.V.; Babu, K.S. Novel sesquiterpenes from Schisandra grandiflora: Isolation, cytotoxicity and synthesis of their triazole derivatives using “click” reaction. Eur. J. Med. Chem. 2015, 92, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.-Y.; Zhang, Y.; Li, Y.-F.; Liu, Y.; Niu, W.-P.; Li, Y.; Zhang, C.-R.; Cao, H.; Hao, X.-J.; Yang, C. Discovery of natural sesquiterpene lactone 1-O-acetylbritannilactone analogues bearing oxadiazole, triazole or imidazole scaffolds for the development of new fungicidal candidates. J. Agric. Food Chem. 2023, 71, 11680–11691. [Google Scholar] [CrossRef] [PubMed]
- Artyushin, O.I.; Sharova, E.V.; Nikolaeva, N.S.; Alekasndrova, Y.; Semakov, A.; Ne-ganova, M.; Brel, V.K. Modification of sesquiterpene lactones—Dehydrocostus lactone and alantolactone—By click chemistry method. Cytotoxicity activity of the obtained conjugates. Russ. J. Gen. Chem. 2022, 92, 960–968. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, Z.; Chai, G.; Li, Y.; Xin, H.; Qian, X.; Tang, Z.; Wu, J.; Zhao, P. Dehydrocostus lactone suppresses LPS-induced acute lung injury and macrophage activation through NF-κB signaling pathway mediated by p38 MAPK and Akt. Molecules 2019, 24, 1510. [Google Scholar] [CrossRef]
- Sudevan, S.T.; Oh, J.M.; Abdelgawad, M.A.; Abourehab, M.A.S.; Rangarajan, T.M.; Kumar, S.; Ahmad, I.; Patel, H.; Kim, H.; Mathew, B. Introduction of benzyloxy pharmacophore into aryl/heteroaryl chalcone motifs as a new class of monoamine oxidase B inhibitors. Sci. Rep. 2022, 12, 22404. [Google Scholar] [CrossRef] [PubMed]
- Leveille, A.N.; Schwarzrock, T.; Brown, H.; True, B.; Plasencia, J.; Neudecker, P.; Üffing, A.; Weiergräber, O.H.; Willbold, D.; Kritzer, J.A. Exploring the arylidene-indolinone ligands for autophagy proteins LC3B and GABARAP. ACS Med. Chem. Lett. 2025, 16, 271–277. [Google Scholar] [CrossRef]
- Neganova, M.E.; Smirnova, E.V.; Sharova, E.V.; Artyushin, O.; Aleksandrova, Y.; Yandulova, E.; Nikolaeva, N.; Brel, V.K. Design of conjugates based on sesquiterpene lactones with polyalkoxybenzenes by “click” chemistry to create potential anticancer agents. Molecules 2022, 27, 8411. [Google Scholar] [CrossRef]
- Neganova, M.E.; Aleksandrova, Y.; Sharova, E.; Smirnova, E.; Artyushin, O.; Nikolaeva, N.; Semakov, A.; Schagina, I.; Akylbekov, N.; Kurmanbayev, R.; et al. Conjugates of 3,5-Bis(arylidene)-4-piperidone and sesquiterpene lactones have an anti-tumor effect via resetting the metabolic phenotype of cancer cells. Molecules 2024, 29, 2765. [Google Scholar] [CrossRef]
- Osman, H.; Idris, N.H.; Kamarulzaman, E.; Wahab, H.; Hassan, M. 3,5-bis(arylidene)-4-piperidones as potential dengue protease inhibitors. Acta Pharm. Sin. B 2017, 7, 479–484. [Google Scholar] [CrossRef]
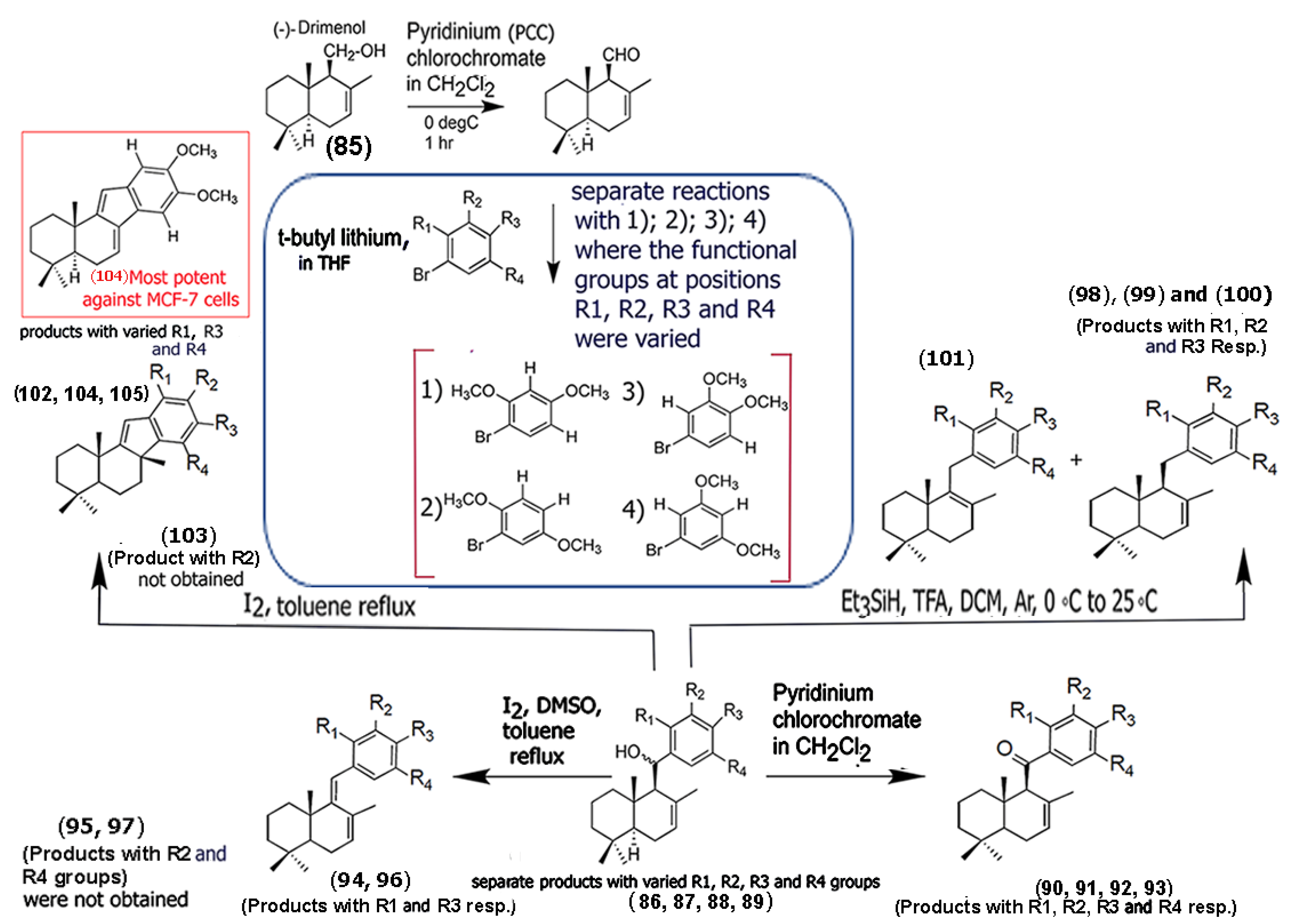
- Arque, I.; Vergara, R.; Mella, J.; Aránguiz, P.; Espinoza-Catalán, L.; Salas, C.; Barrero, A.; del Moral, J.; Villenga, J.; Cuellar, M. New dimethoxy aryl-sesquiterpene derivatives with cytotoxic activity against MCF-7 breast cancer cells: From synthesis to topoisomerase I/II inhibition and cell death mechanism studies. Int. J. Mol. Sci. 2025, 26, 4539. [Google Scholar] [CrossRef]
- Lü, J.-L.; Li, Z.; Guo, L.-M.; Zhang, L.-B. Sesquiterpene lactones with COX-2 inhibition activity with Artemisia lavandulaefolia. Chem. Biodivers. 2018, 15, e1700548. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.M.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cylcooxygenase-2 in cancer: A review. J. Cell Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Hashiba, K.; Yokoyama, K.; Hashimoto, K.; Kikuchi, H.; Nishakawa, H.; Kurihara, T.; Satoh, K.; Shioda, S.; Saito, S.; et al. Cytotoxic activity of azulenes against human oral tumor cell lines. Anticancer Res. 2003, 23, 4747–4756. [Google Scholar] [PubMed]
- Ma, Z.; Han, X.; Ren, J.; Liu, K.; Zhang, W.; Li, G. Design synthesis and biological activity of guaiazulene derivatives. Chem. Biodivers. 2023, 20, e202201174. [Google Scholar] [CrossRef]
- Imanari, K.; Hashimoto, M.; Wakabayashi, H.; Okudaira, N.; Bandow, K.; Nagai, J.; Tomomura, M.; Tomomura, A.; Uesawa, Y.; Sakagami, H. Quantitative structure-cytotoxicity relationship of azulene amide derivatives. Anticancer Res. 2019, 39, 3507–3518. [Google Scholar] [CrossRef]
- Murakami, A.; Takahashi, D.; Kinoshita, T.; Koshimizu, K.; Kim, H.W.; Yoshihiro, A.; Nakamura, Y.; Jiwajinda, S.; Terao, J.; Ohigashi, H. Zerumbone, a southeast asian ginger sesquiterpene markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: The α,β unsaturated carbonyl group is a prerequisite. Carcinogenesis 2002, 23, 795–802. [Google Scholar] [CrossRef]
- Utaka, Y.; Kashiwazaki, G.; Tajima, S.; Fujiwara, Y.; Sumi, K.; Itoh, T.; Kitayama, T. Antiproliferative effects of zerumbone-pendant derivatives on human T-cell lymphoid cell line jurkat cells. Tetrahedron 2019, 75, 1343–1350. [Google Scholar] [CrossRef]
- Hieu, L.X.; Ha, T.; Chi, H.; Vu, T.; Chung, P.; Hung, T.; Chinh, L. Novel derivatives based on zerumbone scaffold as potential anticancer inhibitors. Lett. Org. Chem. 2022, 19, 1062–1069. [Google Scholar] [CrossRef]
- Ren, H.; Wang, Y.-J.; Wang, X.-Y.; Li, X.; Han, Z.; Liwei, Z.; Gu, L.; Bai, M.; Yao, G.-D.; Liu, Q.; et al. Design of ROS-triggered sesquiterpene lactone SC prodrugs as TrXR1 covalent inhibitors for the treatment of non-small cell lung cancer. J. Med. Chem. 2025, 68, 3088–3122. [Google Scholar] [CrossRef] [PubMed]
- Cebula, M.; Schmidt, E.; Arner, E. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antiox. Redox Signal. 2015, 23, 823–853. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Luo, C.; Zhang, X.; Guo, M.; Sun, M.; Yu, H.; Chen, Q.; Yang, W.; Wang, M.; Zuo, S.; et al. Probing the impact of sulfur/selenium/carbon linkages on prodrug nanoassemblies for cancer therapy. Nat. Commun. 2019, 10, 3211. [Google Scholar] [CrossRef]
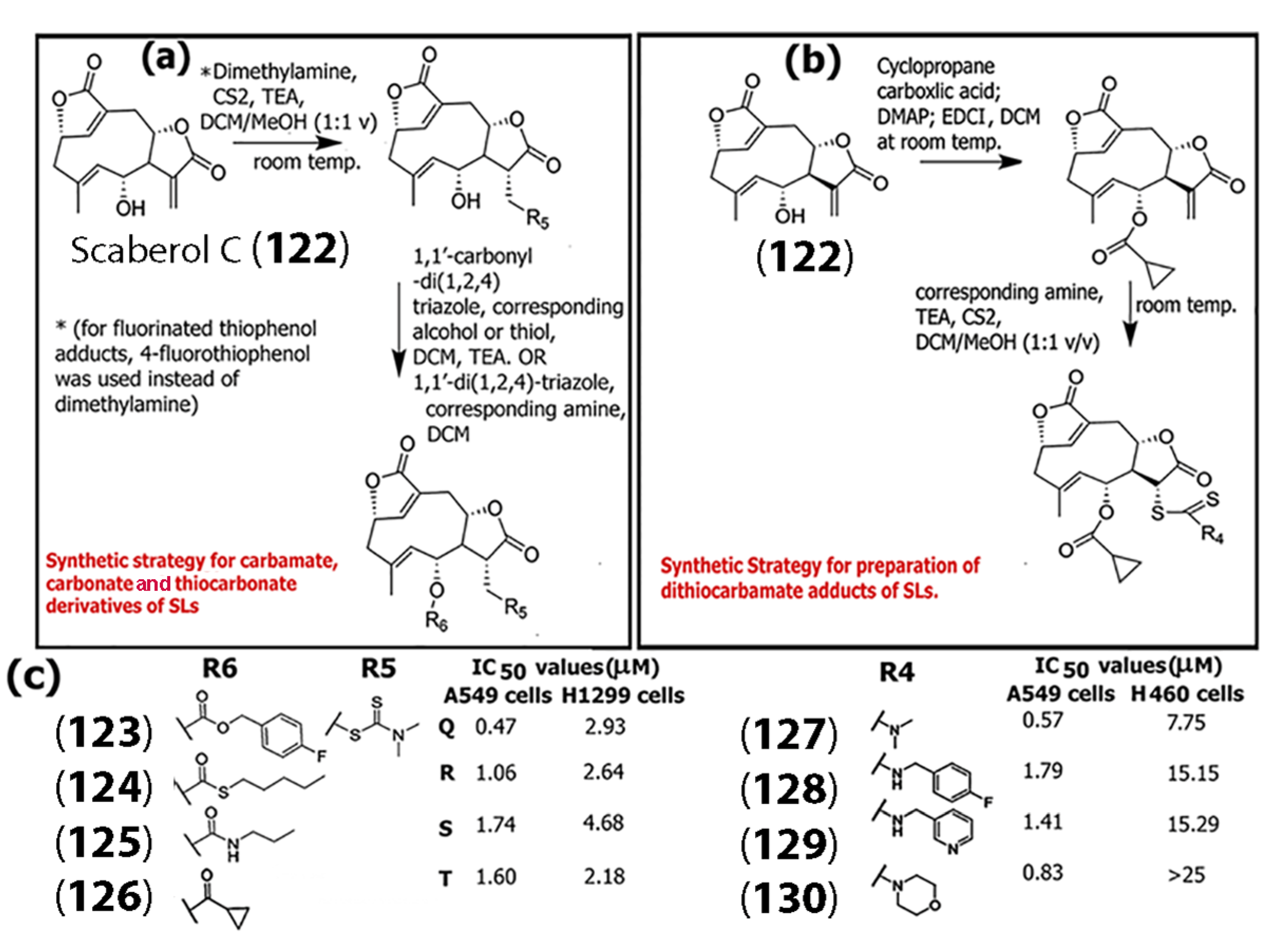
- Li, C.-L.; Han, Z.; Luo, D.-Y.; Ren, H.; Ye, L.; Yao, G.-D. Synthesis of scaberol C amino acid ester derivatives with anticancer activity. J. Asian Nat. Prod. 2025, 27, 189–206. [Google Scholar] [CrossRef]
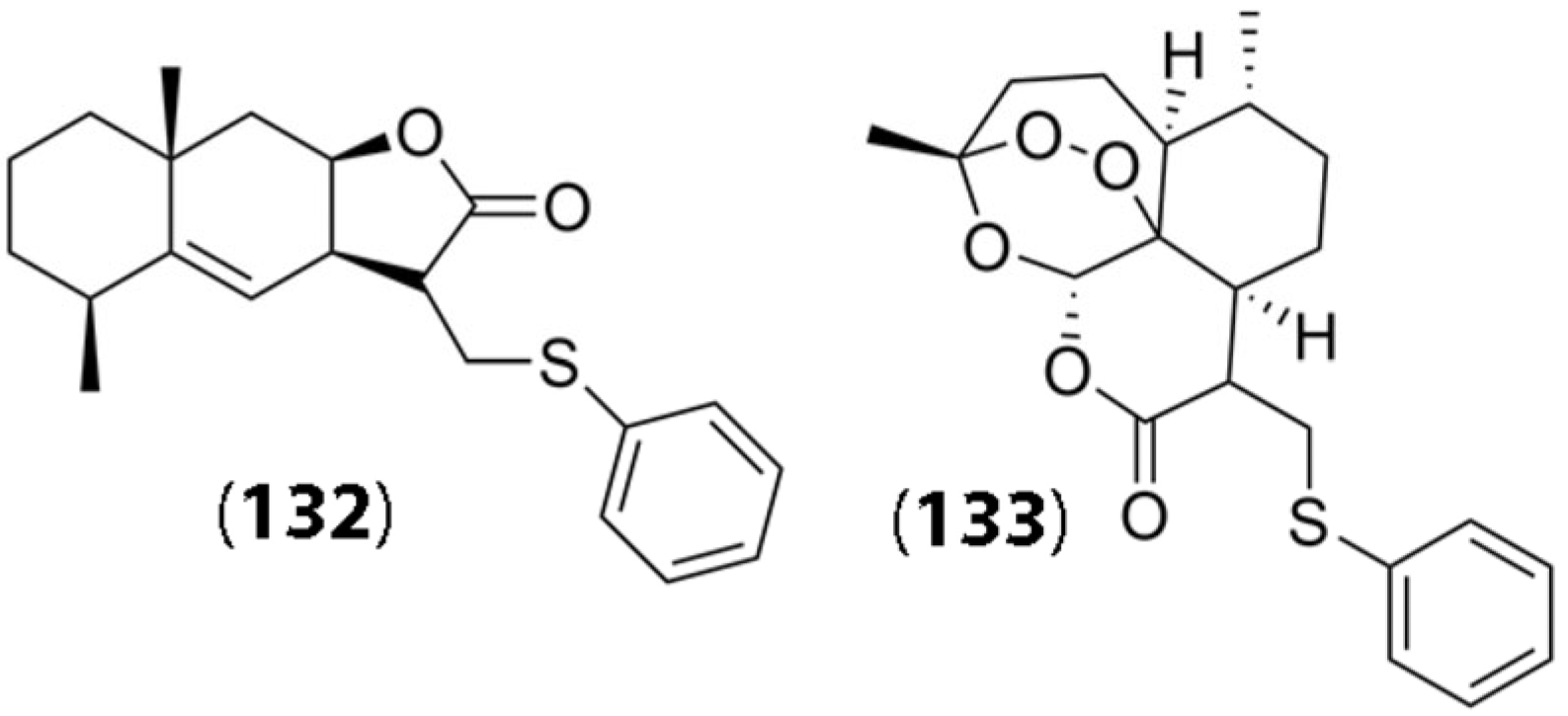
- Semakov, A.V.; Anikina, L.V.; Klochkov, S.G. Synthesis and cytotoxic activity of the products of addition of thiophenol to sesquiterpene lactones. Russian J. Bioorg. Chem. 2021, 47, 906–917. [Google Scholar] [CrossRef]
- Meng, X.; Huang, X.; Liu, Y.; Lv, H.; Ding, X.; Kian, T.; Niu, G.; Tong, B.; Ren, B.; Chen, J. Two amino acid-sesquiterpene lactone conjugates from chicory roots and their anti-inflammatory activity. Nat. Prod. Res. 2025, 1–8. [Google Scholar] [CrossRef]
- Weng, H.; He, L.; Zheng, J.; Li, Q.; Liu, X.; Wang, D. Low oral bioavailability and partial gut microbiotic and phase II metabolism of brussel/witloof chicory sesquiterpene lac-tones in healthy humans. Nutrients 2020, 12, 3675. [Google Scholar] [CrossRef]
- Amorim, M.H.R.; da Costa, R.; Lopes, C.; Bastos, M. Sesquiterpene lactones: Adverse health effects and toxicity mechanisms. Crit. Rev. Toxicol. 2013, 43, 559–579. [Google Scholar] [CrossRef]
- Neelakantan, S.; Nasim, S.; Guzman, M.L.; Jordan, C.T.; Crooks, P.A. Amino-parthenolides as novel anti-leukemic agents: Discovery of the NF-kappa B inhibitor, DMAPT (LC-1). Bioorg. Med. Chem. Lett. 2009, 19, 4346–4349. [Google Scholar] [CrossRef]
- Nakagawa-Goto, K.; Chen, J.-Y.; Cheng, Y.-T.; Lee, W.-L.; Takeya, M.; Saito, Y.; Lee, K.-H.; Shyur, L.-F. Novel sesquiterpene lactone analogues as potent anti-breast cancer agents. Mol. Oncol. 2016, 10, 921–937. [Google Scholar] [CrossRef] [PubMed]
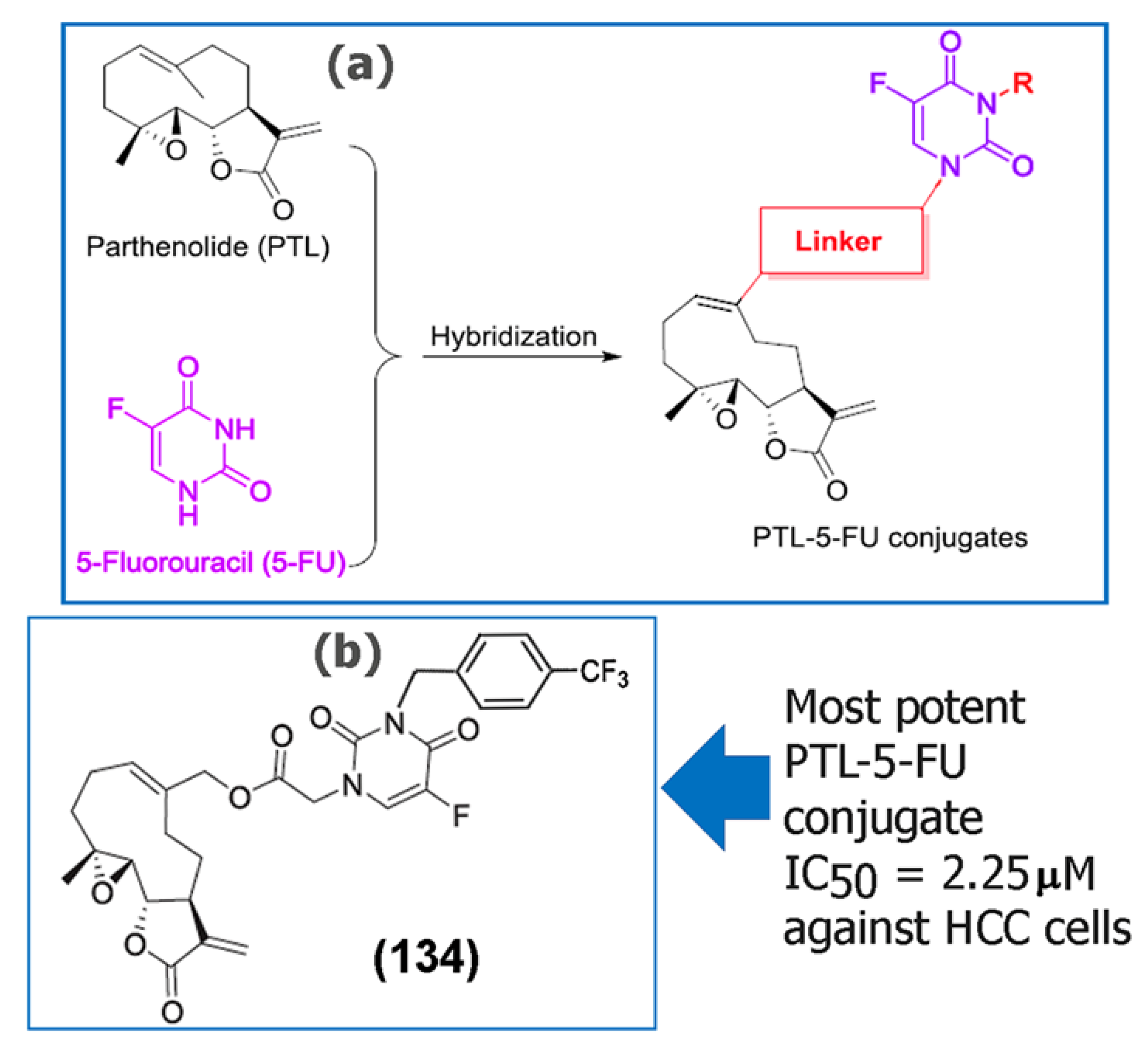
- Ding, Y.; Li, S.; Ge, W.; Liu, Z.; Zhang, X.; Wang, M.; Chen, T.; Chen, Y.; Zhang, Q. Design and synthesis of parthenolide and 5-fluorouracil conjugates as potential anticancer agents against drug resistant hepatocellular carcinoma. Eur. J. Med. Chem. 2019, 183, 111706. [Google Scholar] [CrossRef]
- Neganova, M.; Semakov, A.; Aleksandrova, Y.; Yandulova, E.; Pukhov, S.; Anikina, L.; Klochkov, S. N-Alkylation of anthracycline antibiotics by natural sesquiterpene lactones as a way to obtain antitumor agents with reduced side effects. Biomedicines 2021, 9, 547. [Google Scholar] [CrossRef]
- Anikina, L.V.; Semakov, A.V.; Afanas’eva, S.V.; Pukhov, S.A.; Klochkov, S.G. Synthesis and antiproliferative activity of daunorubicin conjugates with sesquiterpene lactones. Pharm. Chem. J. 2018, 52, 308–311. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Cheng, F.; Cao, W.; Geng, Y.; Chen, Z.; Wei, W.; Zhang, L. Xanthinin induce DDP-resistance lung cancer cells apoptosis through regulation of GLUT1 mediated ROS accumulation. Drug Devl. Res. 2023, 84, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, B.; Zou, Z.; Li, W.; Zhang, Y.; Xie, J.; Liu, C. Costunolide enhances doxorubicin-induced apoptosis in prostate cancer cells via activated mitogen-activated protein kinases and generation of reactive oxygen species. Oncotarget 2017, 8, 107701–107715. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Gulli, M.; Facchinetti, R.; Minacori, M.; Mancinelli, R.; Peraccio, E.; Scuderi, C.; Eufemi, M.; Di Sotto, A. Sorafenib chemosensitization by caryophyllene sesquiterpenes in liver biliary and pancreatic cancer cells. The role of STAT3/ABC transporter axis. Pharmaceutics 2022, 14, 1264. [Google Scholar] [CrossRef]
- Tian, X.-H.; Hong, L.-L.; Jiao, W.-H.; Lin, H.-W. Natural sesquiterpene quinone/quinols: Chemistry, biological activity and synthesis. Nat. Prod. Rep. 2023, 40, 718–749. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, B.-B.; Sun, F.; Xu, J.-R.; Jiao, W.-H.; Yu, H.-B.; Han, B.-N.; Yang, F.; Zhang, X.-C.; Lin, H.-W. Sesquiterpene quinones/hydroquinones from the marine sponge Spongia pertusa Esper. J. Nat. Prod. 2017, 80, 1436–1445. [Google Scholar] [CrossRef]
- Jiao, W.-H.; Xu, T.-T.; Gu, B.-B.; Shi, G.-H.; Zhu, Y.; Yang, F.; Han, B.-N.; Wang, S.-P.; Li, Y.-S.; Zhang, W.; et al. Bioactive sesquiterpene quinols and quinones from the marine sponge Dysidea avara. RSC Adv. 2015, 5, 87730–87738. [Google Scholar] [CrossRef]
- Ito, T.; Nguyen, H.M.; Win, N.N.; Vo, H.Q.; Nguyen, H.T.; Morita, H. Three new sesquiterpene aminoquinones from a Vietnamese spongia sp. and their biological activities. J. Nat. Med. 2018, 72, 298–303. [Google Scholar] [CrossRef]
- Jiao, W.-H.; Huang, X.-J.; Yang, J.-S.; Yang, F.; Piao, S.-J.; Gao, H.; Li, J.; Ye, W.-C.; Yao, X.-S.; Chen, W.-S.; et al. Dysidavarones A-D, New sesquiterpene quinones from the marine sponge Dysidea avara. Org. Lett. 2012, 14, 202–205. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, Y.; Wang, H.; Jiang, R.; Wei, J.; Zeng, H.; Sun, W.; Zhang, Y.; Hu, Z. Herqupenoid A, an unparalleled sesquiterpene-quinone hybrid featuring a multicyclic caged 2,7-dioxatetracyclo [5.4.0.04,11.05,9] hendecane fragment from Penicillium herquei. Org. Lett. 2024, 26, 10146–10151. [Google Scholar] [CrossRef]
- Hirata, A.; La Clair, J.; Jiminez, P.; Costa-Lotufo, L.; Fenical, W. Preclinical development of serinioquinones as selective dermcidin modulators for the treatment of melanoma. Mar. Drugs. 2022, 20, 301. [Google Scholar] [CrossRef]
- Novaković, I.; Annelković, U.; Zlatović, M.; Gašic, M.; Sladić, D. Bioconjugate of lysozyme and the antibacterial marine sesquiterpene quinone and its derivatives. Bioconj. Chem. 2012, 23, 57–65. [Google Scholar] [CrossRef]
- Barnaby, S.; Yu, S.; Fath, K.; Tsiola, A.; Khalpari, O.; Banerjee, I.A. Ellagic acid promoted biomimetic synthesis of shape-controlled silver nanochains. Nanotechnology 2011, 22, 225605. [Google Scholar] [CrossRef]
- Schwartz-Duval, A.; Wen, R.; Srivastava, I.; Moitra, P.; Pan, D. A simplistic single-step method for preparing biomimetic nanoparticles from endogenous biomaterials. ACS Appl. Mater. Interfaces 2023, 13, 46464–46477. [Google Scholar] [CrossRef]
- Rajendran, K.; Ahmed, U.; Meunier, A.C.; Shaikh, M.; Siddiq, U.R.; Anwar, A. Nanoparticle-terpene fusion: A game-changer in combating primary amoebic meningoencephalitis caused by Naegleria fowleri. ACS Omega 2024, 9, 11597–11607. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Babaei, G.; Aziz, S.; Abolhasani, S. Alantolactone and ZnO nanoparticles induce apoptosis activity of cisplatin in an ovarian cancer cell line (SKOV3). Res. Pharm. Sci. 2022, 17, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Ran, D.; Zhou, J.; Chai, Z.; Li, J.; Xie, C.; Mao, J.; Lu, L.; Zhang, Y.; Wu, S.; Zhan, C.; et al. All stage precisional glioma targeted therapy enabled by a well-designed D-peptide. Theranostics 2020, 10, 4073–4087. [Google Scholar] [CrossRef] [PubMed]
- Baranello, M.P.; Bauer, L.; Benoit, D.S.W. Poly(styrene-alt-maleic anhydride)-based diblock copolymer micelles exhibit versatile hydrophobic drug loading, drug dependent release and internalization by multidrug resistant ovarian cancer. Biomacromolecules 2014, 15, 2629–2641. [Google Scholar] [CrossRef]
- Deller, R.C.; Diamanti, P.; Morrison, G.; Reilly, J.; Ede, B.C.; Richardson, R.; Le Vay, K.; Collins, A.M.; Blair, A.; Perriman, A.W. Functionalized triblock copolymer vectors for the treatment of acute lymphoblastic leukemia. Mol. Pharm. 2017, 14, 722–732. [Google Scholar] [CrossRef]
- Mejias, F.; Fernandez, I.P.; Rial, C.; Varela, R.; Molinillo, M.; Calvino, J.; Trasobares, S.; Macias, F. Encapsulation of Cynara cardunculus guaiane-type lactones in fully organic nanotubes enhances phytotoxic properties. J. Agric. Food Chem. 2022, 70, 3644–3653. [Google Scholar] [CrossRef]
- Cheikh, I.A.; El-Baba, C.; Youssef, A.; Saliba, N.; Ghantous, A.; Darwiche, N. Lessons learned from the discovery and development of the sesquiterpene lactones in cancer therapy and prevention. Expert. Opin. Drug Del. 2022, 17, 1377–1405. [Google Scholar] [CrossRef]
- Krishna, S.; Ganapathi, S.; Ster, I.C.; Saeed, M.; Cowan, M.; Finlayson, C.; Kovacevics, H.; Jansen, H.; Kremsner, P.G.; Efferth, T.; et al. A randomized, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EbioMedicine 2014, 2, 82–90. [Google Scholar] [CrossRef] [PubMed]
- McDowell, A.; Hill, K.S.; McCorkle, J.; Gorski, J.; Zhang, Y.; Salahudeen, A.; Ueland, F.; Kolesar, J. Preclinical evaluation of artesnunate as an antineoplastic agent in ovarian cancer treatment. Diagonostics 2021, 11, 395. [Google Scholar] [CrossRef]
- Cao, D.; Chen, D.; Xia, J.-N.; Wang, W.-Y.; Zhu, G.-Y.; Chen, L.-W.; Zhang, C.; Tan, B.; Li, H.; Li, Y.-W. Artesnunate promoted anti-tumor immunity and overcame EGFR-TK resistance in non-small cell lung cancer by enhancing oncogenic TAZ degradation. Biomed. Pharmacother. 2022, 155, 113705. [Google Scholar] [CrossRef] [PubMed]
- Dings, R.; Van Laar, E.; Webber, J.; Zhang, Y.; Griffin, R.; Waters, S.; MacDonald, J.; Mayo, K. Ovarian tumor growth regression using a combination of vascular targeting anginex or topomimetic 0118 and chemotherapeutic irofulven. Cancer Lett. 2008, 265, 270–280. [Google Scholar] [CrossRef]
- Pietsch, K.; van Midwoud, P.; Villata, P.; Sturla, S. Quantification of acylfulvene- and illudin S-DNA adducts in cells with variable bioactivation capacities. Chem. Res. Toxicol. 2013, 26, 146–155. [Google Scholar] [CrossRef]
- Schilder, R.; Blessing, J.; Shahin, M.; Miller, D.; Tewari, K.S.; Muller, C.Y.; Warshal, D.; McMeekin, S.; Rotmensch, J. A phase II evaluation of Irofulven (IND#55804, NSC683863) as second-line treatment of recurrent or persistent intermediately platinum-sensitive ovarian or primary peritoneal cancer. A gynecologic oncology group trial. Int. J. Gynecol. Cancer 2010, 20, 1137–1141. [Google Scholar] [PubMed]
- Mahalingam, D.; Wilding, G.; Denmeade, S.; Saratopoulas, J.; Cosgrove, D.; Cetnar, J.; Azad, N.; Bruce, J.; Kurman, M.; Allgood, V.E.; et al. Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: Results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumors. Brit. J. Cancer. 2016, 114, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Peguero, J.; Cen, P.; Arora, S.P.; Sarantopolous, J.; Rowe, J.; Allgood, V.; Tubb, B.; Campos, L. A phase II, multicenter, single-arm study of mipsagargin (G-202) as a second-line therapy following Sorafenib for adult patients with progressive advanced hepatocellular carcinoma. Cancers 2019, 11, 833. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.; Radu, C.; Vladâcenco, O.; Roza, E.; Costach-escu, B.; Grumezescu, A.; Teleanu, R. An overview of oxidative stress, neuroinflammation and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Lin, B.; Wang, R.; Zhao, J.; Li, S.; Zhang, Y.; Zhang, X. Pteris Laeta Wall and its new phytochemical, pterosinsade A, promote hippocampal neurogenesis via activating the Wnt signaling pathway. J. Agric. Food Chem. 2023, 71, 4586–4598. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M. The role of oxidative stress in Parkinson’s disease. J. Parkinson Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and parkin mitochondrial quality control: A source of regional vulnerability in Parkinson’s disease. Mol. Neurodegenr. 2020, 15, 20. [Google Scholar] [CrossRef]
- Cramb, K.; Beccano-Kelly, D.; Cragg, S.; Wade-Martins, R. Impaired dopamine release in Parkinson’s disease. Brain 2023, 146, 3117–3132. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Hosseini, M.; Ahmadzadeh, A.M.; Pourbagher-Shahri, A.M. Neuroprotective effect of cedrol in a male rat model of Parkinson’s disease. Physiol. Rep. 2025, 13, e70309. [Google Scholar] [CrossRef]
- Farimani, F.D.; Hosseini, M.; Amirahmadi, S.; Akbarian, M.; Shirazinia, M.; Barabady, M.; Rajabian, A. Cedrol supplementation ameliorates memory deficits by regulating neuroinflammation and cholinergic function in lipopolysaccharide–induced cognitive impairment in rats. Heliyon 2024, 10, e30356. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, C.; Wang, H.; Jin, D.; Li, S.; Wang, M.; Ren, Q.; Xu, J.; Ohizumi, Y.; Guo, Y. Sesquiterpenes inhibiting the microglial activation from Laurus nobilis. J. Agric. Food Chem. 2014, 62, 4784–4788. [Google Scholar] [CrossRef] [PubMed]
- Amoah, S.; Vecchia, M.T.; Pedrini, B.; Carnhelutti, G.; Gonçalves, A.E.; Dos Santos, D. Inhibitory effect of sesquiterpene lactones and sesquiterpene alcohol aromadendrane-4β, 10α-diol on memory impairment in a mouse model Alzheimer. Eur. J. Pharmacol. 2015, 769, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Thiyagarajah, M.; Song, J.; Chen, C.; Herrmann, N.; Gallagher, D.; Rapoport, M.; Black, S.; Ramirez, J.; Andreazza, A.; et al. Altered central and blood glutathione in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Alzheimer’s Res. Ther. 2022, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Thau-Habermann, N.; Gschwendtberger, T.; Bodemer, C.; Petri, S. Parthenolide regulates microglial and astrocyte function in primary cultures from ALS mice and has neuroprotective effects on primary motor neurons. PLoS ONE 2025, 20, e0319866. [Google Scholar] [CrossRef]
- Ham, A.; Kim, B.; Koo, U.; Nam, K.-W.; Lee, S.-J.; Kim, K.-H.; Shin, J.; Mar, W. Spirafolide from bay leaf (Laurus nobilis) prevents dopamine induced apoptosis by decreasing re-active oxygen species production in human neuorblastoma SH-SY5Y cells. Arch. Pharm. Res. 2010, 33, 1953–1958. [Google Scholar] [CrossRef]
- Neganova, M.; Liu, J.; Aleksandrova, Y.; Vasilieva, N.; Semakov, A.; Yandulova, E.; Sukocheva, O.; Balakin, K.; Klochkov, S.; Fan, R. Development of neuroprotective agents for the treatment of Alzheimer’s disease using conjugates of serotonin with sesquiterpene lactones. Curr. Med. Chem. 2024, 31, 529–551. [Google Scholar] [CrossRef]
- Wu, X.; Gao, C.; Huang, Y.; Qin, L.; Zhang, Q.; Tan, D.; Zhao, Y.; Wu, J.; Yi, S.; Lu, Y.; et al. Pharmacokinetics and tissue distribution of key sesquiterpene glycosides in Dendrobium nobile analyzed by UHPLC-Q-Trap-MS/MS. J. Chromatogr. B 2025, 1250, 124386. [Google Scholar] [CrossRef]
- Tan, D.; Song, Y.; Wang, J.; Gao, C.; Qin, L.; Zhao, Y.; Lu, Y.; Yang, Z.; He, Y. Identification of sesquiterpepene glycosides from Dendrobium nobile and their α-glycosidase and α-amylase inhibitory activities. Food Sci. Technol. Camp. 2023, 43, e99722. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Lou, H.; Shan, L. Alpha-glucosidase inhibitors and risk of cancer in patients with diabetes mellitus: A systematic review and meta analysis. Oncotarget 2017, 8, 81027–81039. [Google Scholar] [CrossRef]
- ACT001 for the Treatment of Diffuse Intrinsic Pontine Gliomas and H3K27-Altered High Grade Gliomas. Clinical Trials.gov ID (NC06838675). Available online: https://www.clinicaltrials.gov/study/NCT06838676 (accessed on 24 February 2025).
- An, Y.; Guo, W.; Li, L.; Xu, C.; Yang, D.; Wang, S.; Lu, Y.; Zhang, Q.; Zhai, J.; Fan, H.; et al. Micheliolide derivative DMAMCL inhibits glioma cell growth in vitro and in vivo. PLoS ONE 2015, 10, e0116202. [Google Scholar] [CrossRef]
- Xi, X.-N.; Liu, N.; Wang, Q.-Q.; Wu, H.-T.; He, H.-B.; Wang, L.-L.; Zhang, T.-J.; Sun, L.; Yin, Z.; Chen, Y.; et al. Pharmacokinetics, tissue distribution and excretion of ACT001 in Sprague-Dawley rats and metabolism of ACT001. J. Chromatogr. B 2019, 1104, 29–39. [Google Scholar] [CrossRef]
- Xi, X.; Liu, N.; Wang, Q.; Chu, Y.; Yin, Z.; Ding, Y.; Lu, Y. ACT001, a novel PAI-1 inhibitor, exerts synergistic effects in combination with cisplatin by inhibiting PI3K/AKT pathway in glioma. Cell Death Dis. 2019, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Qin, T.; Huang, D. ACT001 inhibits the proliferation of non-small cell lung cancer cells by upregulating NKTR expression. Thorac. Cancer 2022, 13, 1772–1782. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Y.; Liu, B.; Li, J.; Hao, X.; Ge, W.; Zhang, X.; Bao, S.; Gong, J.; Jiang, Z.; et al. ACT001 modulates the NF-κB/MnSOD/ROS axis by targeting IKKβ to inhibit glioblastoma cell growth. J. Mol. Med. 2020, 98, 263–277. [Google Scholar] [CrossRef]
- Wang, M.; Xu, R.; Peng, Y.; Li, X. Metabolism and analysis of alantolactone and isoalantolactone in rats by oral administration. J. Chem. 2018, 2026357. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, G.; Chibber, P.; Saroch, D.; Kumar, C.; Ahmed, Z. Novel alantolactone derivative AL-04 exhibits potential anti-inflammatory activity via modulation of iNOS, COX-2 and NF-κB. Cytokine 2022, 158, 155978. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Kumar, A.; Nalli, Y.; Lone, W.; Satti, N.K.; Verma, M.; Ahmed, Z.; Ali, A. Design, synthesis and biological evaluation of alantolactone derivatives as potential anti-inflammatory agents. Med. Chem. Res. 2019, 28, 849–856. [Google Scholar] [CrossRef]
- Enciso-Roca, E.; Aguilar-Felices, E.; Tinco-Jayo, J.; Arroyo-Acevedo, J.; Herrera-Calderon, O.; Aguilar-Carranza, C.; Justil-Guerreo, H. Effects of acute and sub-acute oral toxicity studies of ethanol extract of Tancetum parthenium (L.) Sch. Bip. aerial parts in mice and rats. Ann. Res. Rev. Biol. 2017, 19, 1–10. [Google Scholar] [CrossRef]

| Sesquiterpene/SL/S-Alcohol/Esters | IC50 Values (μM) | Cancer Cell Lines Targeted | In Vitro Studies | In Vivo Studies | Ref. # |
|---|---|---|---|---|---|
| (20) | 6.07 ± 0.45 | non-small cell lung cancer GLC-82 cells | MTT assays proved cytotoxicity. Annexin-FITC apoptosis assays proved apoptosis. PTL targeted B-Raf and inhibited the MAPK-ERK pathway. Inhibition of STAT3 phosphorylation was observed using Western blot studies. Cell motility was reduced, as shown by scratch assay. | Human lung tumor tissues were examined. Immunohistochemistry was used to examine expression of B-Raf and c-Myc proteins in NSLC samples. | [41] |
| 15.38 ± 1.13 | A549 | MTT assays proved cytotoxicity | - | [41] | |
| 15.36 | PC-9 | “ ” | - | [41] | |
| 9.88 ± 0.09 | H1650 | “ ” | - | [41] | |
| 12.37 ± 1.21 | H1299 | “ ” | - | [41] | |
| (23) | 2.2 5.9 3.5 4.7 ± 0.1 3.3 ± 0.3 | HepG2 MCF-7 A549 NCI-H460 NCI-H460/R (MDR cancer cell lines with P-gp over-expression) | MTT assay was conducted to study cytotoxicity, Tunel assay was performed to confirm apoptosis. MTT assay was conducted to confirm cytotoxicity. | - | [37,38] |
| (24) | 0.15 ± 0.09/ 2.75 ± 0.64 | K562/A02 (Drug resistant cell line) K562 | Cytotoxicity was assayed by MTT to confirm the inhibition of cell proliferation. “ ” | - | [40] |
| 0.027 ± 0.02 3.26 ± 0.88 | HL60 Adriamycin Resistant cell line HL60 | Cytotoxicity was assayed by MTT. Induction of apoptosis and differentiation of AML stem and progenitor cells were observed. Cytotoxicity was attributed to apoptosis by the suppression of NF-kB and its downstream proteins. p65, XIAP, FLIP, Bcl-2, Bax, and the cleavage of PARP, caspase-3, and caspase-9 were detected by Western blots in primary CD34+ cells, HL60, and KG1a cells | (24) was converted to its prodrug, DMA-ATL (water soluble). Toxicity was first evaluated in normal Kunming mice. Then, tumors were induced in mice by injecting KG1 cells. Mice were treated with the pro-drug. Tumor sizes were significantly suppressed. | [40] | |
| 2.17 ± 0.72 | THP-1 | Cytotoxicity was confirmed through MTT assay. | - | [40] | |
| 2.75 ± 0.65 | KG1a | “ ” | - | [40] | |
| 35.99 ± 5.55 | GLC-82 | “ ” | - | [41] | |
| 32.89 ± 5.60 | A549 | “ ” | - | [41] | |
| (25) | 53.4 | HepG2 cells | Cytotoxicity was evaluated through MTT assays. Mechanistically, apoptosis was proven, and higher expression of Bax and caspases 3, 9, and 8 and lower expression of Bcl-2 proteins was observed. Ras/Raf/MEK signaling was suppressed. Flow cytometry showed induction of ROS, and cell migration and invasion assays showed inhibition. | - | [42] |
| 10 | HEC-1-B endometrial cancer cells | Cytotoxicity was evaluated through MTT assays. Induction of ROS mediated apoptosis, and inhibition of the MEK/ERK signaling pathway was proven through flow cytometry, fluorescence staining and Western blots, which showed reduction in the expression of ERK and MEK by inhibiting their phosphorylation. Cell migration was inhibited. | - | [43] | |
| 17.09 ± 1.68 | GLC-82 | Cytotoxicity was confirmed through MTT assay. | - | [41] | |
| 17.12 ± 1.62 | A549 | Cytotoxicity was confirmed through MTT assay. | - | [41] | |
| (28) | 81.45 ± 3.8 | A549 | MTT cytotoxicity assay and apoptosis assay. | - | [44] |
| 10.82 ± 0.5 | HeLa | “ ” | - | [44] | |
| 62.58 ± 5.1 | HepG2 | MTT assay and Annexin-V flow cytometry showed apoptosis. Cell cycle analysis showed G2/M phase cell cycle arrest. | - | [44] | |
| (29) | 13.61 ± 0.25 | A549 | Cytotoxicity was proven through MTT assay, and Annexin-FITC assays proved apoptosis. | - | [44] |
| 3.81 ± 0.36 | HeLa | “ ” | - | [44] | |
| 15.47 ± 1.69 | HepG2 | MTT assay and Annexin-V flow cytometry proved apoptosis. Cell cycle analysis showed G2/M phase cell cycle arrest. | - | [44] | |
| (32) | 19.7 | PC-3 | WST-1 cytotoxicity assay. | “ | [47] |
| (34) | 0.8 100.57 39.92 | A431 MDA-MB-231-Luc HCT116 | MTT assay confirmed cytotoxicity. Costunolide activated p38 and c-Jun N-terminal kinase pathways and suppressed ERK, STAT3, NFκ-B, and Akt pathways. MTT assay confirmed cytotoxicity. Apoptosis was confirmed through assessment of BAX and Bcl2 protein level measurements. | - Xenograft mice models were studied and showed decreased tumor growth. Significant necrotic and apoptotic cells were observed when given alongside the doxorubicin. | [45,46] |
| (35) | 3.8 ± 0.1/15.1 ± 0.4 | NCI-H460 NCI-H460R | MTT assay was conducted to study cytotoxicity. | - | [38] |
| (36) | 8.2 ± 0.1/ 4.5 ± 0.1 | NCI-H460 NCI-H460/R | MTT assay was conducted to confirm cytotoxicity. | - | [38] |
| (37) | 7.5 μg/mL 4.0 μg/mL 1.9 μg/mL 3.4 μg/mL | HCT 116 K562 T47D KB | MTT assay showed cytotoxicity. ROS was upregulated in tumor cells upon treatment. Both mitochondrial and receptor mediated apoptosis were observed. Autophagy and decrease in NF-κB and IκBα was observed. Downregulation of MMP-2 and MMP-9 was observed. | - | [39] |
| Compound | IC50 (μM) | Cell Line | In Vitro Studies | In Vivo Studies | Ref. # |
|---|---|---|---|---|---|
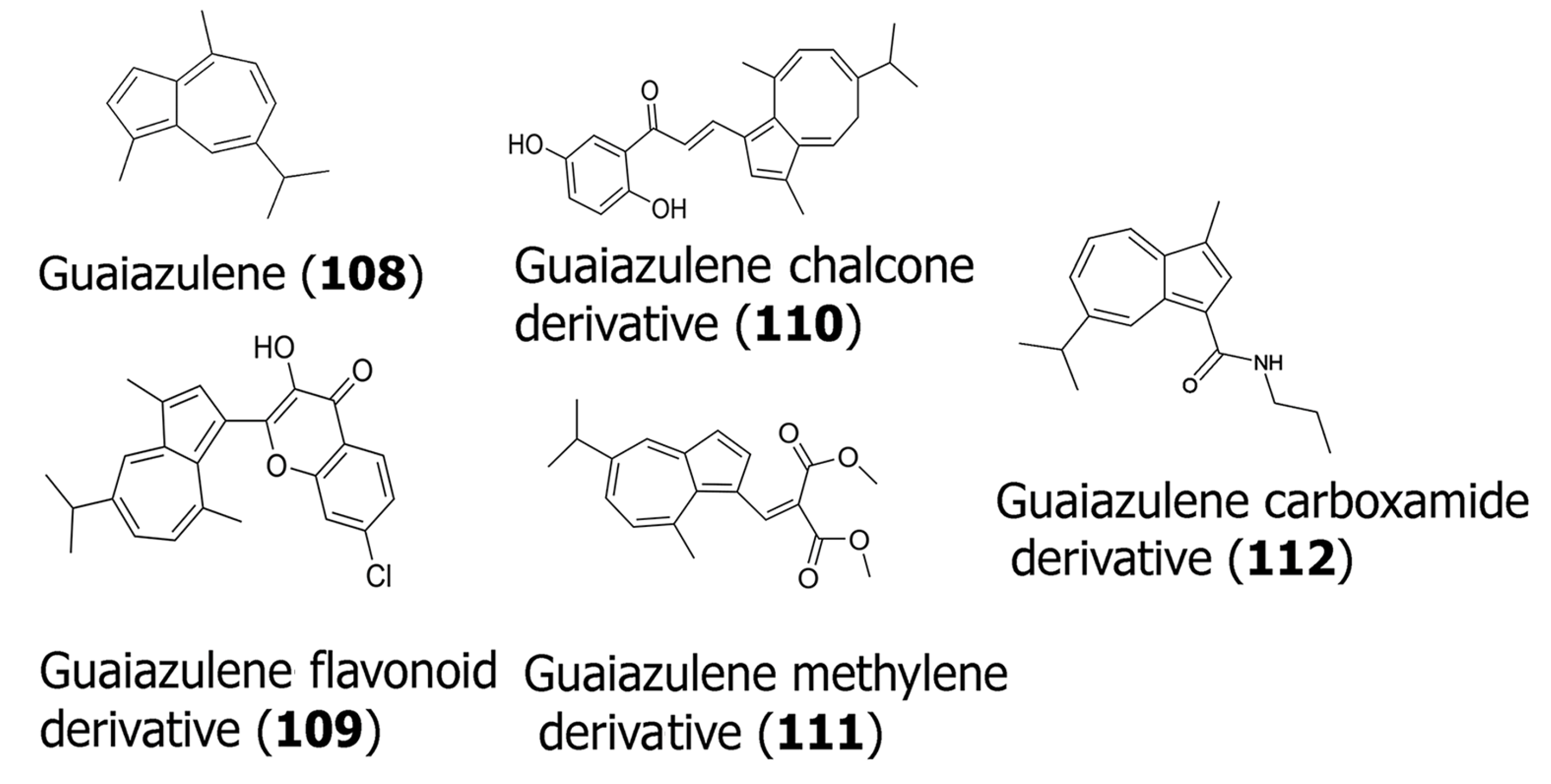
| (108) | 110 160 120 | HSG HSC-2 HSC-3 (Human oral cancer cell lines) | MTT assay was conducted to confirm cytotoxicity. DNA fragmentation analysis and induction of caspase 3 activity was studied. | - | [70] |
| (109) | 8.66 8.35 | K562 MDA-MB-231 | MTT assay for cytotoxicity; PPAR-γ signaling assay to determine the agonistic effects of the derivatives. | Anti-inflammatory activity was tested in zebrafish models. | [71] |
| (110) | 18.8 >30 | K562 MDA-MB-231 | MTT assay for cytotoxicity; PPAR-γ signaling assay to determine the agonistic effects of the derivatives. | Anti-inflammatory activity was tested in zebrafish models. | [71] |
| (111) | 5.21 30 | K562 MDA-MB-231 | MTT assay for cytotoxicity; PPAR-γ signaling assay to determine the agonistic effects of the derivatives. | Anti-inflammatory activity was tested in zebrafish models. | [71] |
| (112) | 35 48 47 38 | Ca9-22 HSC-2 HSC-3 HSC-4 | MTT assay for cytotoxicity studies were conducted. DNA fragmentation and caspase activation assays were conducted to determine apoptosis. Antioxidant assays were conducted to determine free-radical scavenging ability. | - | [72] |
| Chemotherapeutic Drug Conjugate with SL | IC50 (μM) | Cancer Cell Line | In Vitro Assay/Mechanism | In Vivo | Ref. # |
|---|---|---|---|---|---|
| 5-FU conjugates with PTL (135) | 2.67 3.24 | Bel-7402 (human hepatocellular carcinoma cells) Bel-7402/5-FU (5-FU resistant human hepatocellular carcinoma cells) | MTT assay proved inhibition of cell proliferation. Apoptosis assay confirmed its induction. Nuclear morphology changes were observed. | -- | [87] |
| (136) | 2.73 2.90 | Bel-7402 Bel-7402/5-FU | MTT assay proved inhibition of cell proliferation. Apoptosis assay confirmed its induction. Nuclear morphology changes were observed. | [86] | |
| (137) | 4.07 4.34 | Bel-7402 Bel-7402/5-FU 5-FU | MTT assay proved inhibition of cell proliferation. Apoptosis assay confirmed its induction. Nuclear morphology changes were observed. | [86] | |
| (138) | 3.98 4.27 | Bel-7402 (human hepatocellular carcinoma cells) Bel-7402/5-FU | MTT assay proved inhibition of cell proliferation. Apoptosis assay confirmed its induction. Nuclear morphology changes were observed. | [86] | |
| (144) | 0.75 0.04 0.73 2.48 | A549 HCT116 RD MCF7 | MTT assay proved inhibition of cell proliferation. | Cardiotoxicity lowered in rats | [88] |
| (145) | 1.38 0.41 1.16 4.13 | A549 HCT116 RD MCF7 | MTT assay proved inhibition of cell proliferation. | Cardiotoxicity lowered in rats | [88] |
| Compound | IC50 (μM) | Cell Line | Assays and Mechanism | In Vivo Studies | Ref. # |
|---|---|---|---|---|---|
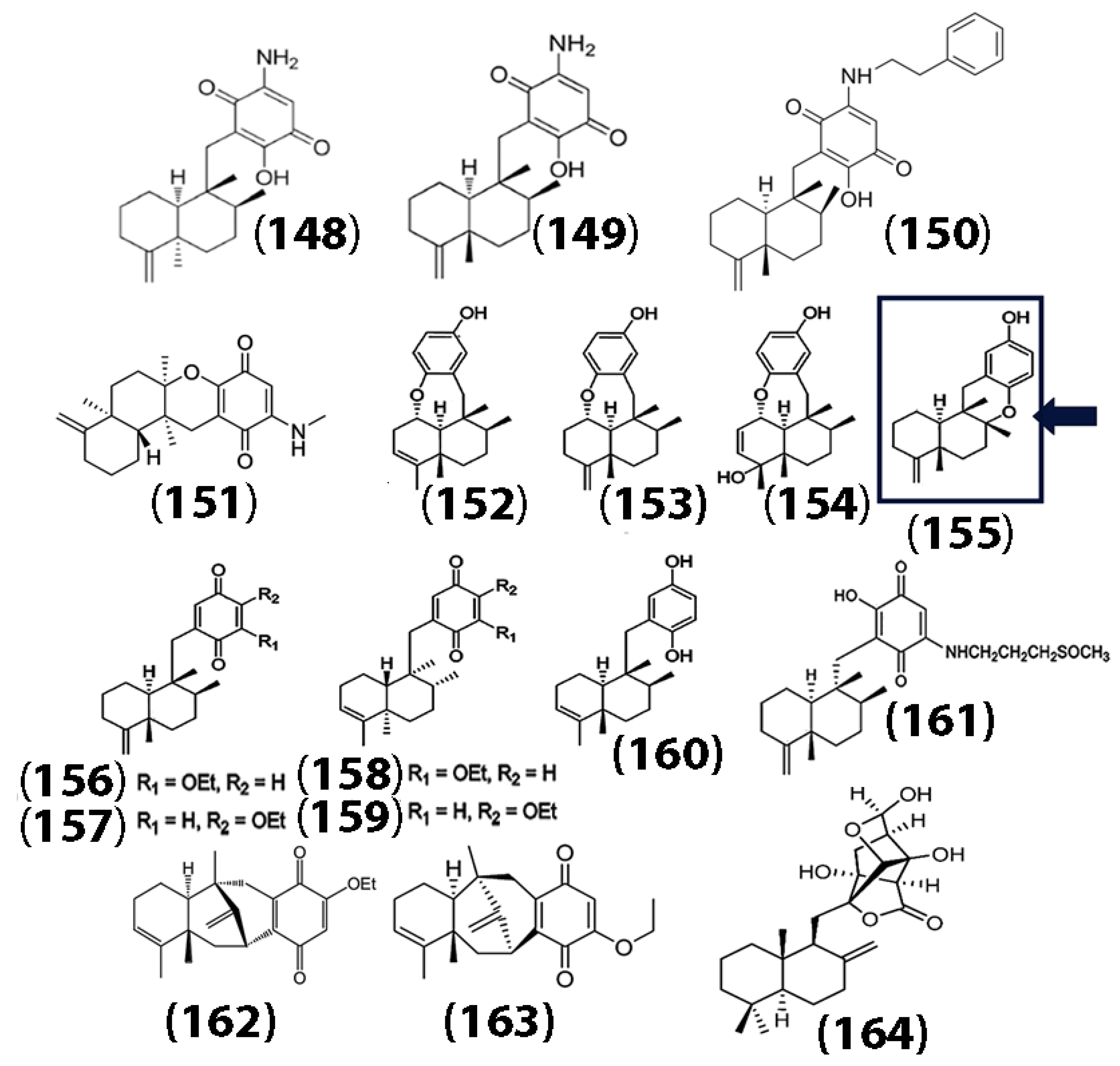
| (148) | 2.8 >10 >10 >10 | U937 A549 HeLa HepG2 | CCK-8 cytotoxicity assay proved inhibition of cell proliferation; cyclin-dependent kinase (CDK2) binding assays were also conducted using SPR. | - | [94] |
| (149) | 1.5 >10 8.6 6.7 | U937 A549 HeLa HepG2 | CCK-8 cytotoxicity assay proved inhibition of cell proliferation; CDK2 binding assays were also conducted using SPR. | - | [94] |
| (150) | 0.60 >10 5.4 3.5 | U937 A549 HeLa HepG2 | CCK-8 cytotoxicity assay proved inhibition of cell proliferation; CDK2 binding assays were also conducted using SPR. | - | [94] |
| (155) | 2.81 | NCI-H929 cells | MTT cytotoxicity assay proved inhibition of cell proliferation; NF-κB inhibition was proven by luciferase assays. | - | [95] |
| (156) | 5.45 | NCI-H929 cells | MTT cytotoxicity assay proved inhibition of cell proliferation; NF-κB inhibition was proven by luciferase assays. | - | [95] |
| (157) | 2.77 | NCI-H929 cells | MTT cytotoxicity assay proved inhibition of cell proliferation; NF-κB inhibition was proven by luciferase assays. | - | [95] |
| (160) | 3.2 | NCI-H929 cells | MTT cytotoxicity assay proved inhibition of cell proliferation; NF-κB inhibition was proven by luciferase assays. | - | [95] |
| (154) | 14.9 | NCI-H929 cells | MTT cytotoxicity assay proved inhibition of cell proliferation; NF-κB inhibition was proven by luciferase assays. | - | [95] |
| (161) | 8.9 5.9 8.6 | A549 MCF7 HeLa | WST-8 cytotoxicity assay was carried out to prove inhibition of cell proliferation. | - | [96] |
| (162) | 39.9 - - - | HeLa A549 MDA231 Hepatoma (QGY7703) | Cytotoxicity was confirmed by MTT assay. Protein tyrosine phosphatase B inhibition was also observed. | - | [97] |
| (163) | 28.8 21.4 11.6 28.1 | HeLa A549 MDA231 Hepatoma (QGY 7703) | Cytotoxicity was confirmed by MTT assay. Protein tyrosine phosphatase B inhibition was also observed. | - | [97] |
| (164) | 2.63 | LPS treated RAW 264.7 macrophage cells | Anti-inflammatory activity was exhibited through inhibition of the NF-κB-NLRP3 axis. NF-κB nuclear translocation was observed. | - | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutter, L.R.; Ren, A.R.; Banerjee, I.A. Advances in Naturally and Synthetically Derived Bioactive Sesquiterpenes and Their Derivatives: Applications in Targeting Cancer and Neurodegenerative Diseases. Molecules 2025, 30, 4302. https://doi.org/10.3390/molecules30214302
Cutter LR, Ren AR, Banerjee IA. Advances in Naturally and Synthetically Derived Bioactive Sesquiterpenes and Their Derivatives: Applications in Targeting Cancer and Neurodegenerative Diseases. Molecules. 2025; 30(21):4302. https://doi.org/10.3390/molecules30214302
Chicago/Turabian StyleCutter, Liana R., Alexandra R. Ren, and Ipsita A. Banerjee. 2025. "Advances in Naturally and Synthetically Derived Bioactive Sesquiterpenes and Their Derivatives: Applications in Targeting Cancer and Neurodegenerative Diseases" Molecules 30, no. 21: 4302. https://doi.org/10.3390/molecules30214302
APA StyleCutter, L. R., Ren, A. R., & Banerjee, I. A. (2025). Advances in Naturally and Synthetically Derived Bioactive Sesquiterpenes and Their Derivatives: Applications in Targeting Cancer and Neurodegenerative Diseases. Molecules, 30(21), 4302. https://doi.org/10.3390/molecules30214302







