Chemical and Microscopic Characterization of the Yellow Passion Fruit Peel
Abstract
1. Introduction
2. Results and Discussion
2.1. Ultra-High-Performance Liquid Chromatography–Mass Spectrometry Electrospray (UHPLC-ESI) Analysis of Fractions 1 and 2
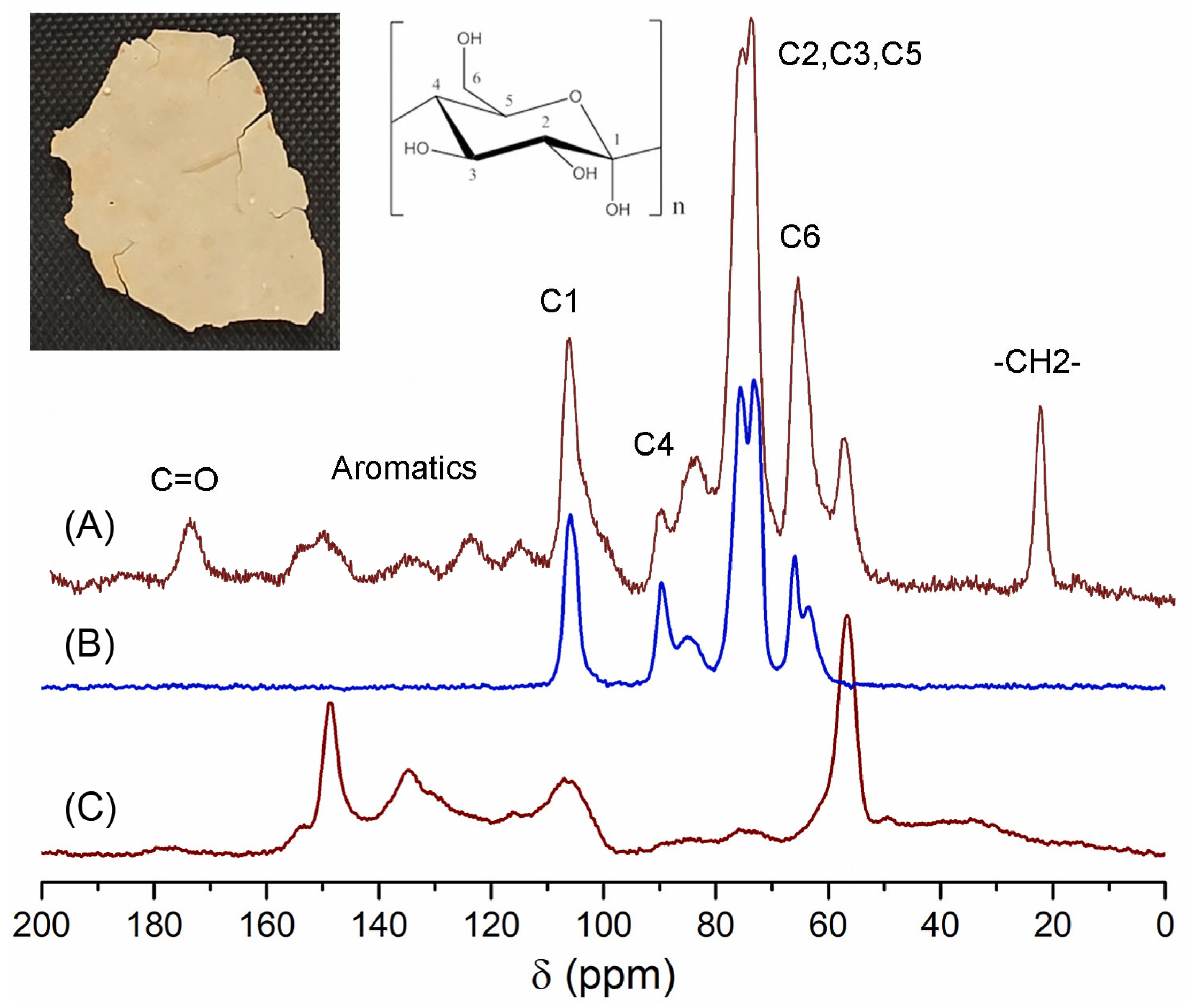
2.2. Solid State NMR (CPMAS 13C NMR) Analysis of the ICM of Passiflora edulis f. flavicarpa
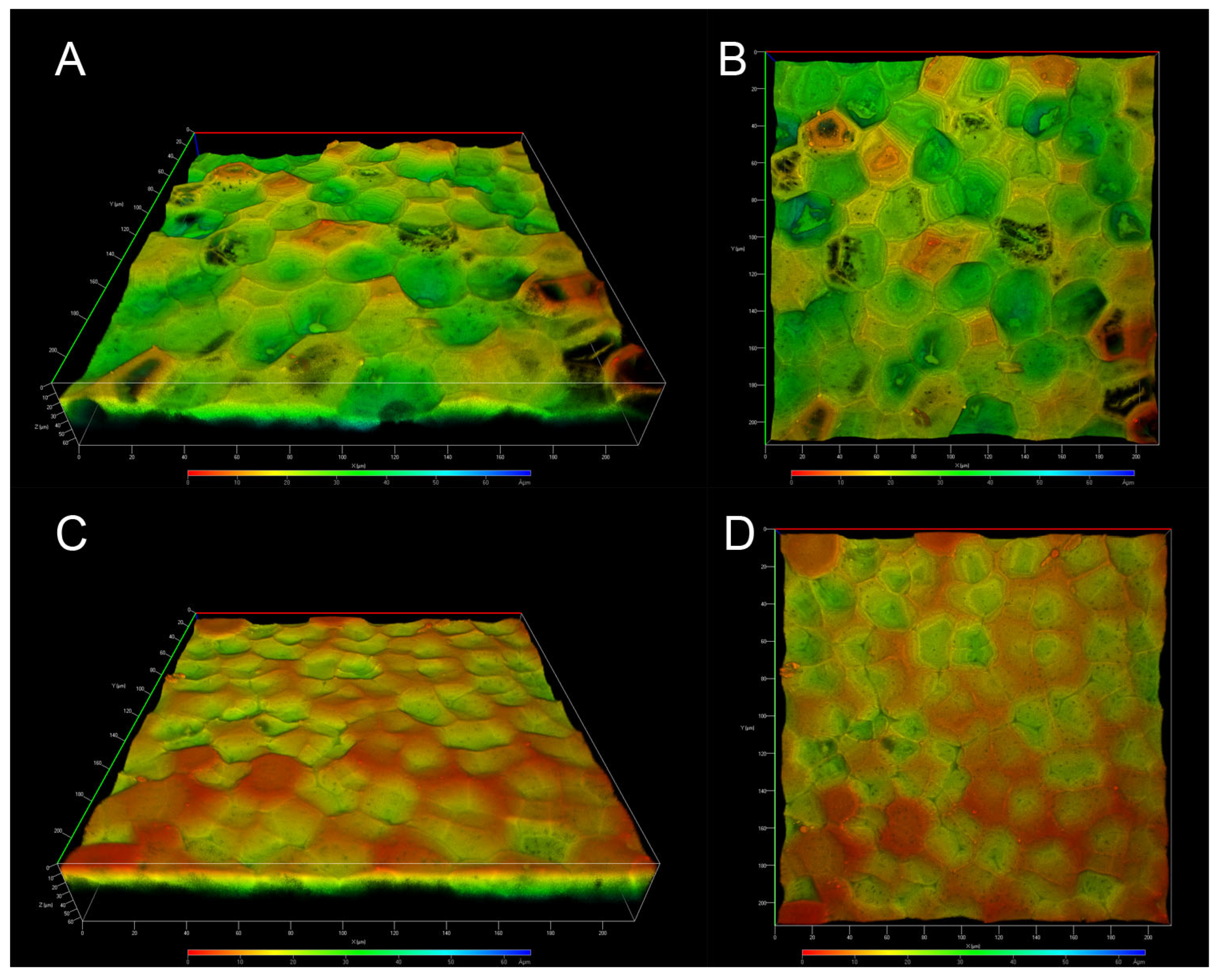
2.3. Confocal Laser Scanning Microscopy Analysis of the ICM of Passiflora edulis f. flavicarpa
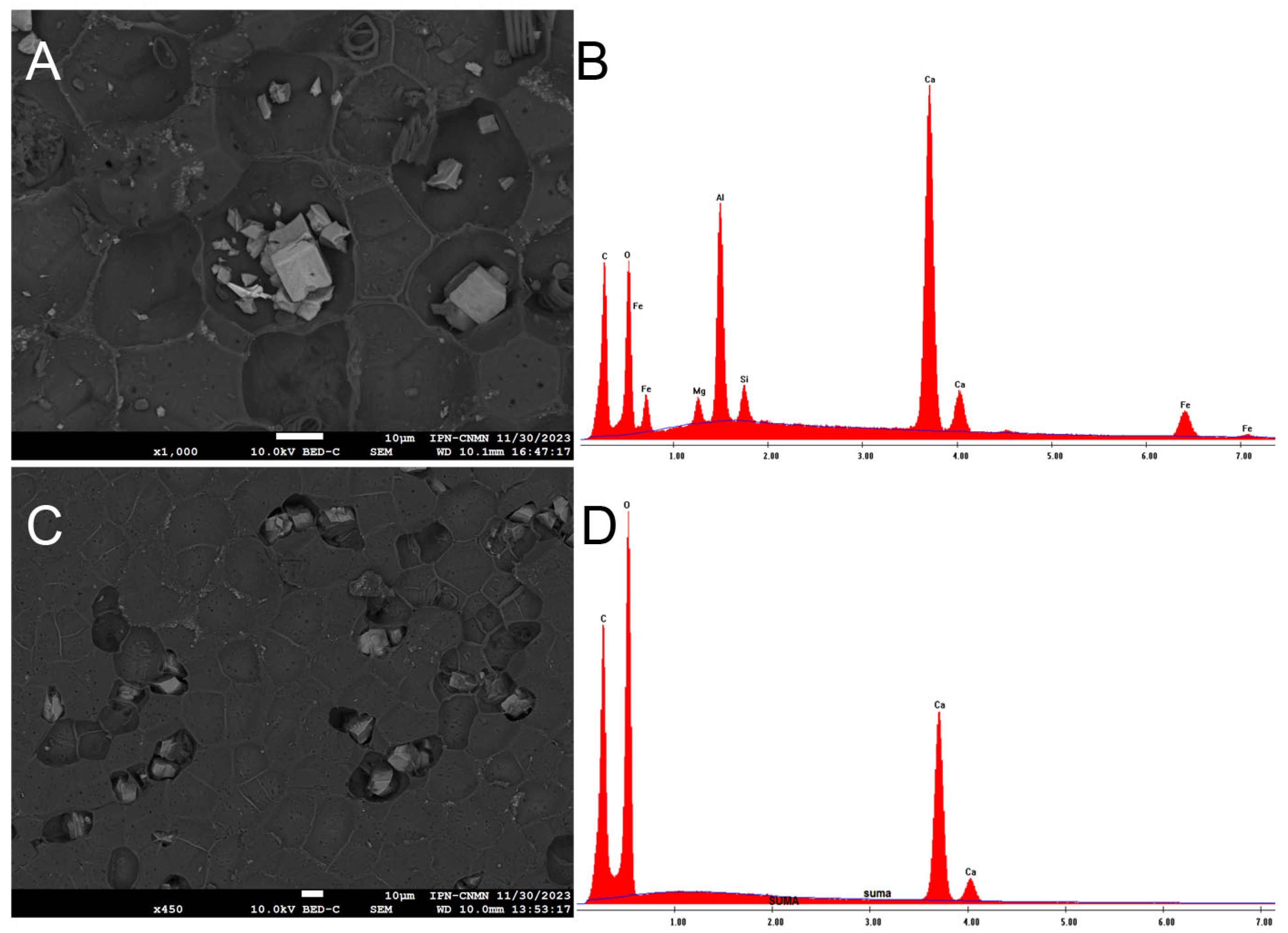
2.4. Scanning Electron Microscopy Analysis of ICM of Passiflora edulis f. flavicarpa
2.5. Direct Injection Electrospray Mass Spectrometry (DI-ESI-MS) Analysis of the KOH Hydrolyzed of ICM of Passiflora edulis f. flavicarpa
3. Materials and Methods
3.1. Isolation of Passiflora edulis f. flavicarpa ICM
3.2. Ultra-High-Performance Liquid Chromatography–Mass Spectrometry (UHPLC-MS) Analysis of Fractions 1 and 2
3.3. Solid State NMR Spectroscopy Analysis of Passiflora edulis f. flavicarpa ICM
3.4. Confocal Laser Scanning Microscopy Analysis of Passiflora edulis f. flavicarpa ICM
3.5. Scanning Electron Microscopy Analysis of Passiflora edulis f. flavicarpa ICM
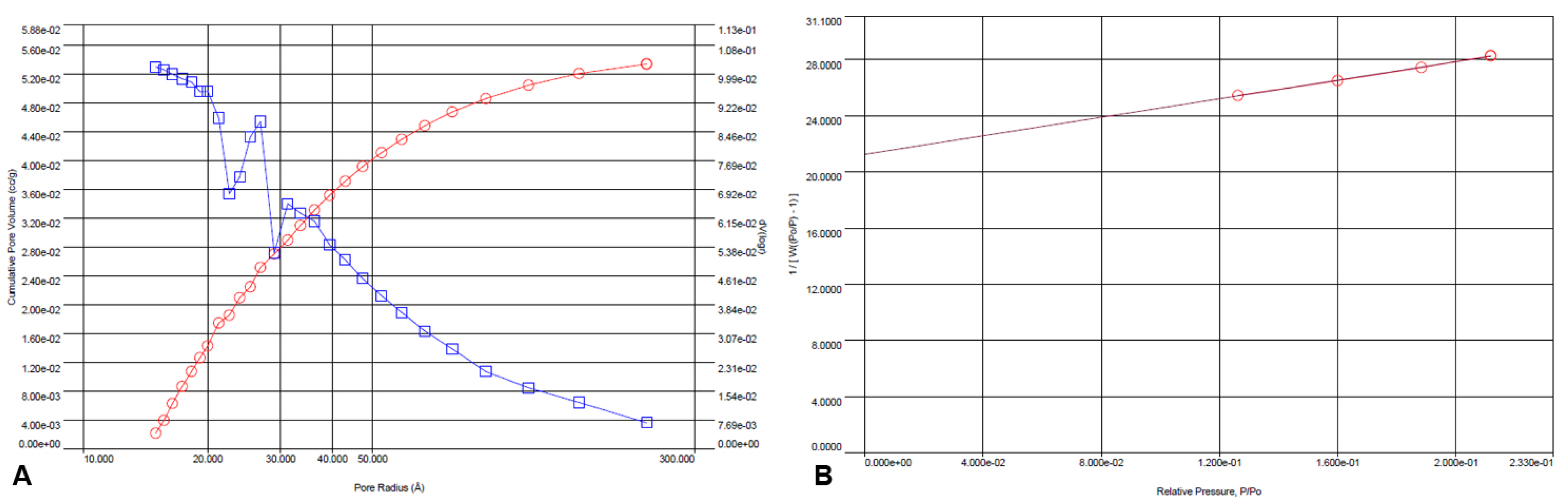
3.6. Brunauer–Emmett–Teller (BET) Analysis of Passiflora edulis f. flavicarpa ICM
3.7. Alkaline Hydrolysis Passiflora edulis f. flavicarpa ICM
3.8. Direct Injection Electrospray Mass Spectrometry (DI-ESI-MS) Analysis of the KOH Hydrolyzed of Passiflora edulis f. flavicarpa ICM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teles, G.; Costa dos Santos, E.; Barboza da Silva, G.; Lopes, D.; Maria da Silva, J.; de Barros, P.; Ribeiro, E. Full utilization of the yellow passion fruit peel: Chemical characterization and valorization to reduce biomass waste. Ind. Crops Prod. 2023, 206, 117593. [Google Scholar] [CrossRef]
- Pereira, Z.C.; Cruz, J.M.d.A.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; Bezerra, J.d.A. Passion fruit (Passiflora spp.) pulp: A review on bioactive properties, health benefits and technological potencial. Food Res. Int. 2023, 166, 112626. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. The past decade findings related with nutritional composition, bioactive molecules and biotechnological applications of Passiflora spp. (passion fruit). Trends Food Sci. Technol. 2016, 58, 79–95. [Google Scholar] [CrossRef]
- My-Thao Nguyen, T.; Anh-Thu Nguyen, T.; Tuong-Van Pham, N.; Ly, Q.-V.; Thuy-Quynh Tran, T.; Thach, T.-D.; Nguyen, C.-L.; Banh, K.-S.; Le, V.-D.; Nguyen, L.-P. Biosynthesis of metallic nanoparticles from waste Passiflora edulis peels for their antibacterial effect and catalytic activity. Arab. J. Chem. 2021, 14, 103096. [Google Scholar] [CrossRef]
- Fonseca, A.M.A.; Geraldi, M.V.; Junior, M.R.M.; Silvestre, A.J.D.; Rocha, S.M. Purple passion fruit (Passiflora edulis f. edulis): A comprehensive review on the nutritional value, phytochemical profile and associated health effects. Food Res. Int. 2022, 160, 111665. [Google Scholar] [CrossRef]
- Sahane, R.S.; Sanap, D.M. A review on passion fruit and it’s pharmacological benefits. IJNRD 2023, 8, 676–684. [Google Scholar]
- Aseervatham, G.S.B.; Abbirami, E.; Sivasudha, T.; Ruckmani, K. Passiflora caerulea L. fruit extract and its metabolites ameliorate epileptic seizure, cognitive deficit and oxidative stress in pilocarpine-induced epileptic mice. Metab. Brain Dis. 2020, 35, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Angonese, M.; Gomes, C.; Ferreira, R. Valorization of passion fruit (Passiflora edulis sp.) by-products: Sustainable recovery and biological activities. J. Supercrit. Fluids 2016, 111, 55–62. [Google Scholar] [CrossRef]
- Herrera, J.M.; Mora, J.T.; Argüello, G.E. El cultivo del maracuyá en Veracruz. Agroregion 2024, 20–21. [Google Scholar]
- Ciabotti, E.D. Alteraciones de características físico-químicas de pulpa de maracuyá amarillo sometido a diferentes técnicas de congelamiento inicial. Rev. Bra. Prod. Agroind. 2000, 51–60. [Google Scholar]
- Fischer, G.; Melgarejo, L.M.; Cutler, J. Preharvest factors that influence the quality of passion fruit: A review. Agron. Colomb. 2018, 36, 217–226. [Google Scholar] [CrossRef]
- Charan, S.M.S.; Gomez, K.B.; Sheela, P.B.; Pushpalatha; Suman, K.T. Effect of storage conditions and duration on quality of passion fruit (Passiflora edulis Sims.) nectar. Asian J. Dairy Food Res. 2017, 36, 161–165. [Google Scholar] [CrossRef]
- Shankar, K.; Singh, R.S.; Annu, T.; William, H.S.; Moyong, O.; Athokpam Gaitri, D.A.; Kavyashree, B.; Anush Sheikh, K.H.; Raju Debbarma, R. Shelf-life of Passiflora ligularis A. Juss, Passiflora quadrangularis L., Passiflora edulis Sim and Passiflora edulis f. flavicarpa at Ambient Room Temperature Found in North East Region of India. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3040–3043. [Google Scholar] [CrossRef]
- da Silva, W.A.O.; Aroucha, E.M.M.; de Araújo, N.O.; Santos, F.K.G.d.; de Medeiros, J.F.; de Sousa, A.L.V.; de Oliveira Queiroz, L.P.; de Lima Leite, R.H. The Shelf Life of Yellow Passion Fruit with an Edible Biocomposite Coating Based on Chitosan, Graphene Oxide Nanoparticles, and Beeswax. Horticulturae 2024, 10, 756. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, Y.; Lia, L.; Jiangb, K.; Gao, J.; Zhong, K.; Pan, M.; Yan, B. A multifunctional chitosan-derived conformal coating for the preservation of passion fruit. LWT 2022, 163, 113584. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhou, L.; Yu, K.; Jiang, F.; Xu, J.; Zou, L.; Du, L.; Liu, W. Effects of Microporous Packaging Combined with Chitosan Coating on the Quality and Physiological Metabolism of Passion fruit after Harvest. Food Bioprocess Technol. 2022, 15, 1836–1850. [Google Scholar] [CrossRef]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf Life Potential and the Fruit Cuticle: The Unexpected Player. Front. Plant Sci. 2019, 10, 770. [Google Scholar] [CrossRef]
- Huo, D.; Dai, J.; Yuan, S.; Cheng, X.; Pan, Y.; Wang, L.; Wang, R. Eco-friendly simultaneous extraction of pectins and phenolics from passion fruit (Passiflora edulis Sims) peel: Process optimization, physicochemical properties, and antioxidant activity. Int. J. Biol. Macromol. 2023, 243, 125229. [Google Scholar] [CrossRef]
- Santos, J.R.; Viana, G.C.C.; Barbosa, R.S.; de Borges, M.S.; Rambo, M.K.D.; Bertuol, D.A.; Scapin, E. Effect of different pretreatments of Passiflora edulis peel biomass on the conversion process into bioproducts for biorefineries. Sustain. Chem. Environ. 2023, 2, 100013. [Google Scholar] [CrossRef]
- Canteri, M.H.; Scheer, A.; Petkowicz, C.; Ginies, C.; Renard, C.; Wosiacki, G. Physicochemical composition of the yellow passion fruit pericarp fractions and respective pectic substances. Food Nutr. Res. 2010, 49, 113–122. [Google Scholar]
- Yu, Y.H.; Wu, L.B.; Liu, X.; Zhao, L.C.; Li, L.Q.; Jin, M.Y.; Yu, X.; Liu, F.; Li, Y.; Li, L.; et al. In vitro simulated digestion and fermentation characteristics of pectic polysaccharides from fresh passion fruit (Passiflora edulis f. flavicarpa L.) peel. Food Chem. 2024, 452, 139606. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.K.; Chen, Z.J.; Stark, R.E. Chemical Depolymerization studies of the Molecular Architecture of lime fruit cuticle. Phytochemistry 1998, 1, 65–70. [Google Scholar] [CrossRef]
- Arrieta-Baez, D.; Stark, R.E. Using Trifluoroacetic Acid to Augment Studies of Potato Suberin Molecular Structure. J. Agric. Food. Chem. 2006, 54, 9636–9641. [Google Scholar] [CrossRef]
- Arrieta-Baez, D.; Cruz-Carrillo, M.; Gómez-Patiño, M.B.; Zepeda-Vallejo, L.G. Derivatives of 10,16-dihydroxyhexadecanoic acid isolated from tomato (Solanum lycopersicum) as potential material for aliphatic polyesters. Molecules 2011, 16, 4923–4936. [Google Scholar] [CrossRef]
- Hernandez, V.B.L.; Arrieta-Baez, D.; Cortez, S.P.I.; Méndez-Méndez, J.V.; Berdeja, M.B.M.; Gómez-Patiño, M.B. Comparative studies of cutins from lime (Citrus aurantifolia) and grapefruit (Citrus paradisi) after TFA hydrolysis. Phytochemistry 2017, 144, 78–86. [Google Scholar] [CrossRef]
- Arrieta-Baez, D.; Quezada Huerta, C.; Rojas-Torres, G.S.; Perea-Flores, M.d.J.; Mendoza-León, H.F.; Gómez-Patiño, M.B. Structural Studies of Mexican Husk Tomato (Physalis ixocarpa) Fruit Cutin. Molecules 2024, 29, 184. [Google Scholar] [CrossRef] [PubMed]
- Bargel, H.; Neinhuis, C. Biomechanical properties of tomato (Lycopersicon esculentum Mill.) fruit skin and isolated cuticle during fruit growth and ripening. J. Exp. Bot. 2005, 56, 1049–1060. [Google Scholar] [CrossRef]
- Arrieta-Baez, D.; Perea Flores, M.d.J.; Méndez-Méndez, J.V.; Mendoza León, H.F.; Gómez-Patiño, M.B. Structural Studies of the Cutin from Two Apple Varieties: Golden Delicious and Red Delicious (Malus domestica). Molecules 2020, 25, 5955. [Google Scholar] [CrossRef]
- Araújo, R.; Arruda, A.; Lino Alves, J.; Soares, T.; Andrade, T.; Arruda, E. Biomimetic Application Potential of Agave sisalana Mechanical Properties, Lightness, Resistance Strategies, and Life Cycle for Digital Fabrication. In Developments in Design Research and Practice; Duarte, E., Rosa, C., Eds.; Springer: Lisbon, Portugal, 2022; Volume 17, pp. 67–79. [Google Scholar]
- Shanmugam, S.; Dutra, S.R.D.; Rajan, M.; Santos, L.N.M.T.; dos Santos, L.B.; de Melo, J.M.J.; Denadai, M.; Narain, N.; Thangaraj, P.; Russo, S.M.; et al. Volatile profiling and UHPLC-QqQ-MS/MS polyphenol analysis of Passiflora leschenaultii DC. fruits and its anti-radical and anti-diabetic properties. Food Res. Int. 2020, 133, 109202. [Google Scholar] [CrossRef]
- Ray, A.K.; Lin, Y.Y.; Gerard, H.; Chen, Z.; Osman, S.F.; Fett, W.F.; Moreau, R.A.; Stark, R.E. Separation and identification of lime cutin monomers by high-performance liquid chromatography and mass spectrometry. Phytochemistry 1995, 38, 1361–1369. [Google Scholar] [CrossRef]
- Deshmukh, A.P.; Simpson, A.J.; Hadad, C.M.; Hatcher, P.G. Insights into the structure of cutin and cutan from Agave americana leaf cuticle using HRMAS NMR spectroscopy. Org. Geochem. 2005, 36, 1072–1085. [Google Scholar] [CrossRef]



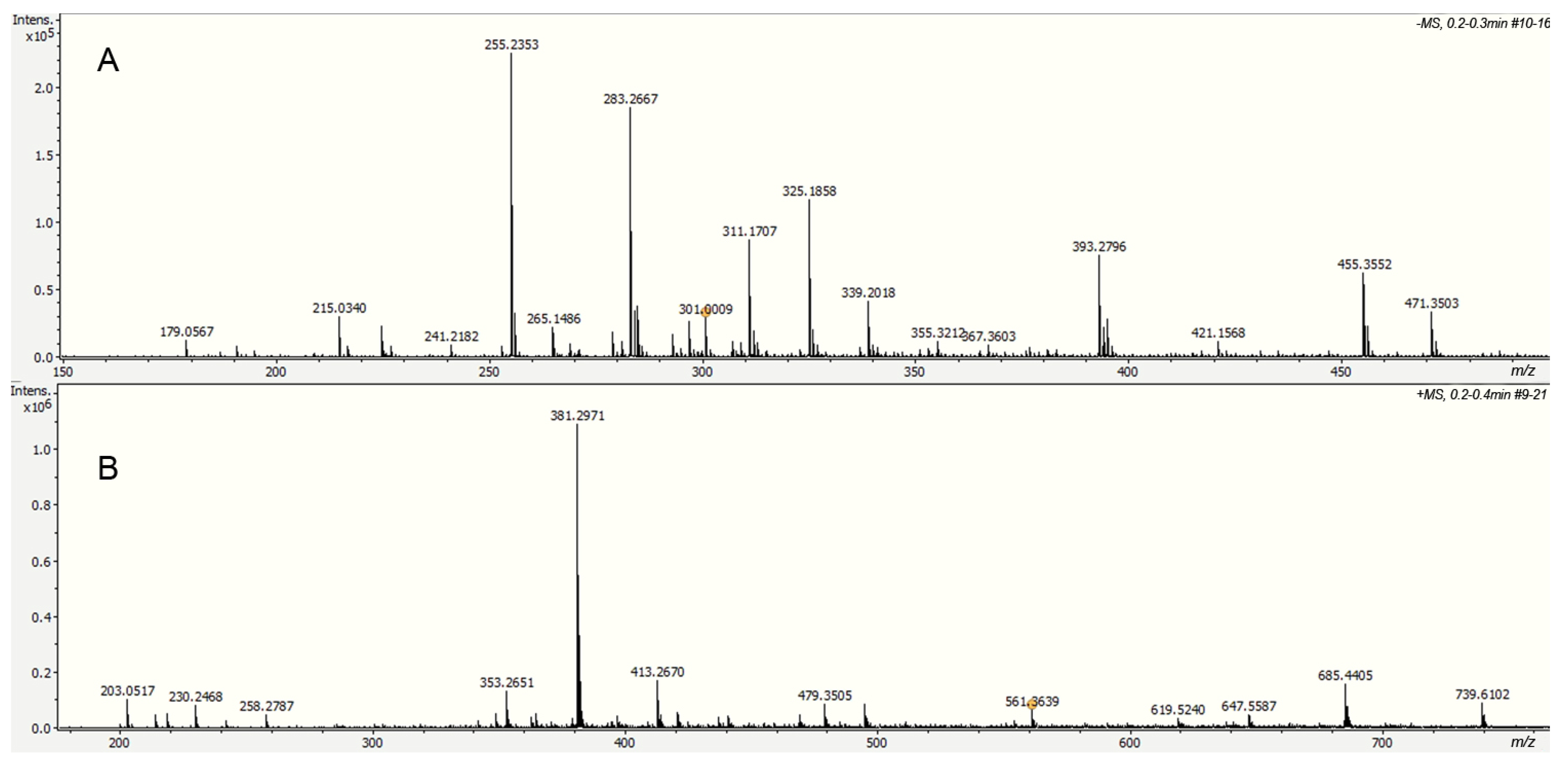
| Compound | Formula | [M − H]− Wexact | [M − H]− MWdetec | Error a | %Rel b |
|---|---|---|---|---|---|
| Hexose | C6H12O6 | 179.0561 | 179.0567 | −3.4 | 1.1 |
| (E)-3-(4-oxo-4H-chromen-3-yl) acrylic acid | C12H8O4 | 215.0350 | 215.0337 | 4.5 | 2.5 |
| Palmitic acid | C16H32O2 | 255.2330 | 255.2352 | 5.2 | 21.6 |
| Stearic acid | C18H36O2 | 283.2643 | 283.2668 | 3.1 | 19.6 |
| 4,9-Dioxopyrano[2,3-g]chromene-2,7-dicarboxylic acid | C14H6O8 | 300.9990 | 301.0008 | 3.9 | 0.4 |
| Acitretin | C21H26O3 | 325.1809 | 325.1862 | 1.5 | 15.1 |
| 2-[(E)-5-(4-methoxy-2,3,6-trimethylphenyl)- 3-methylpent-2-enyl] benzene-1,4-diol | C22H28O3 | 339.1966 | 339.2008 | 2.4 | 5.6 |
| Galloxanthin | C27H38O2 | 393.2858 | 393.2790 | 0.8 | 13.4 |
| Oleanolic Acid | C30H48O3 | 455.3531 | 455.3550 | 1.3 | 11.8 |
| Maslinic Acid | C30H48O4 | 471.9480 | 471.3509 | 3.5 | 6.80 |
| Compound | Formula | [M − H]+ MWexact | [M − H]+ MWdetec | Error a | %Rel b |
|---|---|---|---|---|---|
| Hexose (Na adduct) | C6H12O6Na+ | 203.0530 | 203.0515 | 0.9 | 0.35 |
| 5-(3-(dihydrocaffeoyl)oxy)- 2,3,4-trihydroxypentanoic acid | C14H18O9Na+ | 353.2643 | 353.2649 | 1.1 | 0.80 |
| methyl 2,3,4-trihydroxy-5-((dihydroferuloyl)oxy) pentanoate | C16H22O9Na+ | 381.2974 | 381.2970 | 3.9 | 4.30 |
| 2-(hydroxymethyl)-6-(4(octyloxy)phenetoxy) tetrahydro-2H-pyran-3,4,5-triol | C22H36O7 | 413.2540 | 413.2669 | 2.7 | 1.17 |
| Miquelianin | C21H18O13 | 479.0820 | 479.3503 | 3.3 | 0.83 |
| Cholest-5-en-3-yl 3,4,5-trihydroxybenzoate | C34H50O5 | 561.3630 | 561.3635 | 1.2 | 0.11 |
| Distearin | C39H76O5Na+ | 647.5530 | 647.5538 | 0.9 | 0.62 |
| [(2R,3R)-3-(acetyloxymethyl)-2-(1,3-benzodioxol -5-ylmethyl)-4-(3,4,5-trimethoxyphenyl)butyl] hexadecanoate | C40H60O9 | 685.3900 | 685.4406 | 1.5 | 1.74 |
| [(3S,8S,9S,10R,13R,14S,17R)-17-[(E,2R,5R)- 5-ethyl-6-methylhept-3-en-2-yl]-3-hydroxy-10,13- dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro- 1H-cyclopenta[a]phenanthren-1-yl] (2E,4E,6E,8E,10E,12E)- docosa-2,4,6,8,10,12-hexaenoate | C51H78O3 | 739.6081 | 739.6101 | 1.2 | 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrieta-Baez, D.; Díaz de la Torre, D.L.; Mendoza-León, H.F.; Perea-Flores, M.d.J.; Gómez-Patiño, M.B. Chemical and Microscopic Characterization of the Yellow Passion Fruit Peel. Molecules 2025, 30, 4293. https://doi.org/10.3390/molecules30214293
Arrieta-Baez D, Díaz de la Torre DL, Mendoza-León HF, Perea-Flores MdJ, Gómez-Patiño MB. Chemical and Microscopic Characterization of the Yellow Passion Fruit Peel. Molecules. 2025; 30(21):4293. https://doi.org/10.3390/molecules30214293
Chicago/Turabian StyleArrieta-Baez, Daniel, Denise Larissa Díaz de la Torre, Héctor Francisco Mendoza-León, María de Jesús Perea-Flores, and Mayra Beatriz Gómez-Patiño. 2025. "Chemical and Microscopic Characterization of the Yellow Passion Fruit Peel" Molecules 30, no. 21: 4293. https://doi.org/10.3390/molecules30214293
APA StyleArrieta-Baez, D., Díaz de la Torre, D. L., Mendoza-León, H. F., Perea-Flores, M. d. J., & Gómez-Patiño, M. B. (2025). Chemical and Microscopic Characterization of the Yellow Passion Fruit Peel. Molecules, 30(21), 4293. https://doi.org/10.3390/molecules30214293








