Vapor Pressure of Selected Aliphatic Hexanols by Static and Indirect Chromatographic Methods
Abstract
1. Introduction
2. Results
2.1. Vapor Pressures by the Static Method
2.2. Gas–Liquid Chromatographic Retention Times
2.3. Heat Capacities in the Ideal-Gas State
3. Discussion
3.1. Vapor Pressures
| Reference | Phase | N b | (Tmin–Tmax)/K | (pmin–pmax)/kPa | Method |
|---|---|---|---|---|---|
| 3H | |||||
| Hovorka et al. [33] | Liq | 13 | 298.15–411.15 | 0.960–109.391 | Isoteniscope c |
| Thomas et al. [31] | Liq | 9 | 253.95–294.75 | 0.006–0.306 | Ramsay–Young c |
| Sachek et al. [18] | Liq | 16 | 333.27–408.93 | 3.901–101.661 | Ebulliometry |
| N’Guimbi et al. [20] | Liq | 11 | 243.94–318.15 | 0.004–1.549 | Static |
| Kulikov et al. [32] | Liq | 11 | 278.3–311.5 | 0.067–1.020 | Transpiration |
| This work | Liq | 15 | 238.30–308.16 | 0.001–0.768 | Static |
| 2M2P | |||||
| Hovorka et al. [34] | Liq | 12 | 288.15–396.15 | 0.400–107.858 | Isoteniscope c |
| Markovnik et al. [26] | Liq | 15 | 331.07–396.39 | 6.37–103.76 | Ebulliometry |
| This work | Liq | 14 | 238.30–303.16 | 0.002–1.097 | Static |
| 2M3P | |||||
| Hovorka et al. [35] | Liq | 13 | 298.15–401.15 | 0.800–105.325 | Isoteniscope c |
| Loginova et al. [40] | Liq | 14 | 317.24–399.52 | 2.853–101.112 | Ebulliometry |
| Brazhnikov et al. [17] | Liq | 15 | 343.07–400.02 | 10.376–98.260 | Ebulliometry |
| Kulikov et al. [32] | Liq | 13 | 274.9–307.5 | 0.098–1.350 | Transpiration |
| This work | Liq | 16 | 233.29–308.15 | 0.001–1.322 | Static |
| 3M2P | |||||
| Hovorka et al. [36] | Liq | 13 | 298.15–408.15 | 0.667–103.325 | Isoteniscope c |
| Thomas et al. [31] | Liq | 11 | 255.05–294.45 | 0.006–0.293 | Ramsay–Young c |
| Kulikov et al. [32] | Liq | 13 | 275.1–310.3 | 0.058–1.076 | Transpiration |
| This work | Liq | 15 | 238.24–308.09 | 0.001–0.862 | Static |
| 3M3P | |||||
| Hovorka et al. [35] | Liq | 12 | 298.15–394.15 | 2.400–101.698 | Isoteniscope c |
| Kulikov et al. [32] | Liq | 11 | 275.2–301.5 | 0.137–1.212 | Transpiration |
| This work | Liq | 15 | 233.30–303.11 | 0.001–1.232 | Static |
| This work | Cr I | 4 | 233.31–248.37 | 0.001–0.008 | Static |
| 22M1B | |||||
| Hovorka et al. [38] | Liq | 14 | 298.15–415.95 | 0.400–121.590 | Isoteniscope c |
| This work | Liq | 12 | 253.26–308.15 | 0.005–0.784 | Static |
| This work | Cr I | 3 | 238.80–248.27 | 0.001–0.003 | Static |
| 23M2B | |||||
| Hovorka et al. [36] | Liq | 11 | 298.15–393.15 | 1.120–105.191 | Isoteniscope c |
| This work | Liq | 10 | 253.28–298.16 | 0.020–1.093 | Static |
| This work | Cr I | 5 | 238.30–258.26 | 0.002–0.032 | Static |
| 33M2B | |||||
| Kulikov et al. [32] | Liq | 13 | 279.9–315.3 | 0.248–3.254 | Transpiration |
| This work | Liq | 6 | 273.24–298.13 | 0.142–1.021 | Static |
| This work | Cr I | 7 | 248.33–278.22 | 0.009–0.218 | Static |
| This work | Cr II | 5 | 233.73–253.32 | 0.001–0.016 | Static |
 This work;
This work;  Belarus State University—BSU (3H: Sachek et al. [18]; 2M2P: Markovnik et al. [26]; 2M3P: Brazhnikov et al. [17]);
Belarus State University—BSU (3H: Sachek et al. [18]; 2M2P: Markovnik et al. [26]; 2M3P: Brazhnikov et al. [17]);  Hovorka et al. (3H: [33], 2M2P: [34], 2M3P and 3M3P [35], 3M2P and 23M2B [36], 22M1B [38]);
Hovorka et al. (3H: [33], 2M2P: [34], 2M3P and 3M3P [35], 3M2P and 23M2B [36], 22M1B [38]);  N’Guimbi et al. [20];
N’Guimbi et al. [20];  Thomas et al. [31];
Thomas et al. [31];  Kulikov et al. [32];
Kulikov et al. [32];  Loginova et al. [40]. Vapor pressures obtained by a combination of static and indirect chromatographic methods (GLC-ACRT (Activity Coefficients–Retention Times) method, Section 3.5 and Section 4.6): − reference 2M2P, − reference 2M3P, − reference 3H.
Loginova et al. [40]. Vapor pressures obtained by a combination of static and indirect chromatographic methods (GLC-ACRT (Activity Coefficients–Retention Times) method, Section 3.5 and Section 4.6): − reference 2M2P, − reference 2M3P, − reference 3H.  , data obtained by the SimCor procedure (Section 3.2 and Section 4.4). Data sets represented by filled symbols were used in the SimCor procedure.
, data obtained by the SimCor procedure (Section 3.2 and Section 4.4). Data sets represented by filled symbols were used in the SimCor procedure.
 This work;
This work;  Belarus State University—BSU (3H: Sachek et al. [18]; 2M2P: Markovnik et al. [26]; 2M3P: Brazhnikov et al. [17]);
Belarus State University—BSU (3H: Sachek et al. [18]; 2M2P: Markovnik et al. [26]; 2M3P: Brazhnikov et al. [17]);  Hovorka et al. (3H: [33], 2M2P: [34], 2M3P and 3M3P [35], 3M2P and 23M2B [36], 22M1B [38]);
Hovorka et al. (3H: [33], 2M2P: [34], 2M3P and 3M3P [35], 3M2P and 23M2B [36], 22M1B [38]);  N’Guimbi et al. [20];
N’Guimbi et al. [20];  Thomas et al. [31];
Thomas et al. [31];  Kulikov et al. [32];
Kulikov et al. [32];  Loginova et al. [40]. Vapor pressures obtained by a combination of static and indirect chromatographic methods (GLC-ACRT (Activity Coefficients–Retention Times) method, Section 3.5 and Section 4.6): − reference 2M2P, − reference 2M3P, − reference 3H.
Loginova et al. [40]. Vapor pressures obtained by a combination of static and indirect chromatographic methods (GLC-ACRT (Activity Coefficients–Retention Times) method, Section 3.5 and Section 4.6): − reference 2M2P, − reference 2M3P, − reference 3H.  , data obtained by the SimCor procedure (Section 3.2 and Section 4.4). Data sets represented by filled symbols were used in the SimCor procedure.
, data obtained by the SimCor procedure (Section 3.2 and Section 4.4). Data sets represented by filled symbols were used in the SimCor procedure.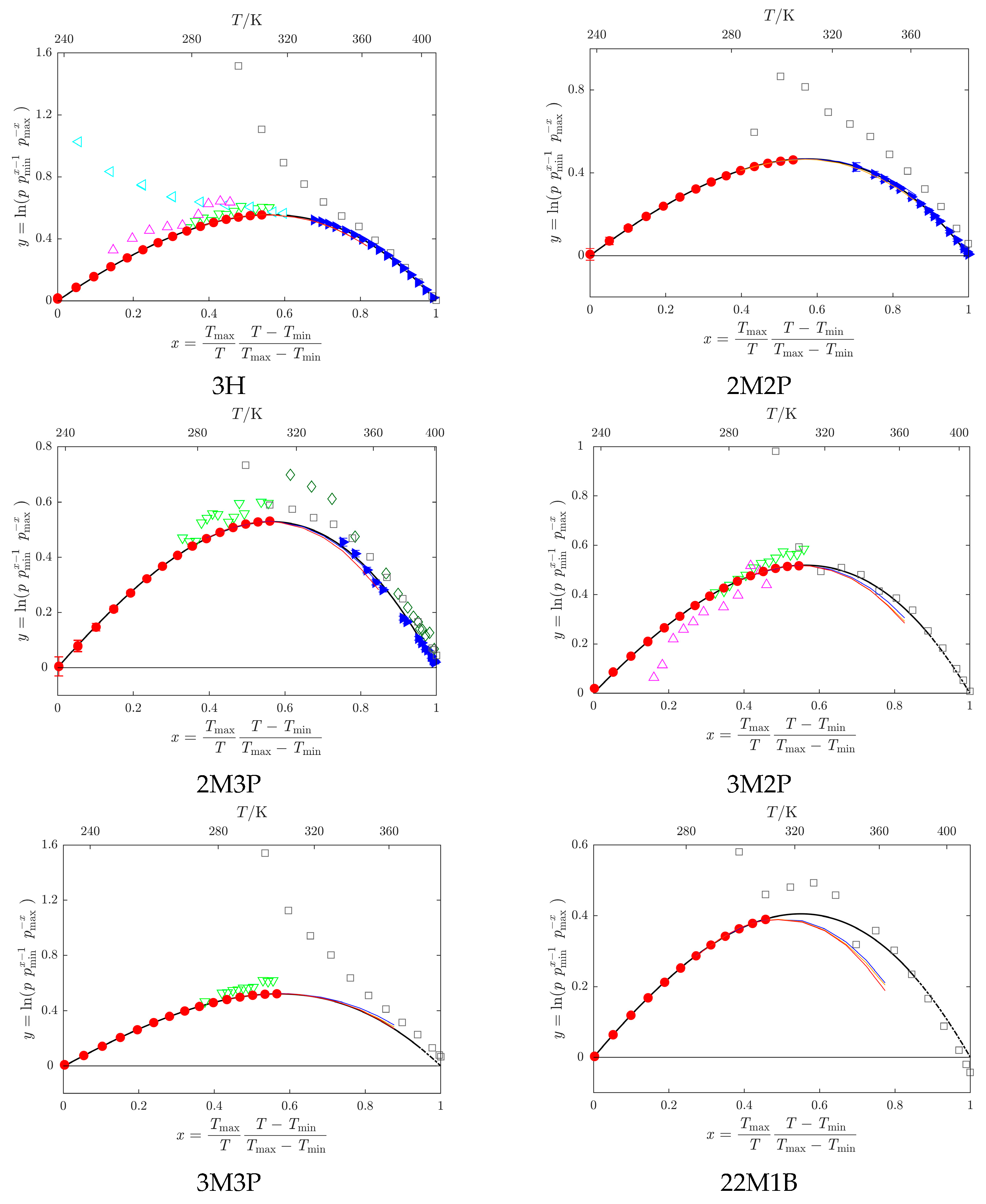

3.2. Simultaneous Correlation of Vapor Pressures and Related Thermal Data (SimCor)
| Compound | Phase | (Tmin−Tmax)/K a | T0/K | p0/Pa | A0 | A1·103 | A2·106 | A3·109 | σ, 100σr b |
|---|---|---|---|---|---|---|---|---|---|
| 3H | l | 238–409 | 408.52 | 100,000 | 2.7943507 ± 0.0059517 | 2.2516151 ± 0.0651354 | −12.252175 ± 0.23786466 | 12.970439 ± 0.2937654 | 59.9 Pa, 0.56 |
| 2M2P | l | 238–397 | 395.4686 | 100,000 | 2.7693308 ± 0.01399173 | 2.1093639 ± 0.15207155 | −10.943814 ± 0.55098098 | 10.504174 ± 0.6701256 | 174.3 Pa, 0.30 |
| 2M3P | l | 233–400 | 400.8863 | 100,000 | 2.6780467 ± 0.01883502 | 3.3340287 ± 0.20516587 | −16.41784 ± 0.7460045 | 17.757143 ± 0.9121549 | 269.6 Pa, 0.63 |
| 3M2P | 238–381 | 407.3302 | 100,000 | 2.8662138 ± 0.004850913 | 1.2889432 ± 0.05356992 | −8.9337753 ± 0.19629249 | 9.441982 ± 0.2440539 | 0.347 Pa, 0.48 | |
| 3M3P | l | 233–381 | 395.7459 | 100000 | 2.5391802 ± 0.00984653 | 4.7073126 ± 0.1078361 | −21.083236 ± 0.39316663 | 22.884878 ± 0.4827427 | 0.103 Pa 0.22 |
| crI | 215–250 | 346.0881 | 100,000 | 3.2357548 ± 0.00206600 | −0.2490463 ± 0.00813569 | 0 | 0 | 0.018 Pa, 0.50 | |
| 22M1B+ | l | 251–381 | 408.3389 | 100,000 | 2.7486585 ± 0.00918374 | 2.2959203 ± 0.1013443 | −11.991292 ± 0.37115292 | 13.142867 ± 0.4613495 | 0.337 Pa 0.07 |
| crI | 238–252 | 377.6666 | 100,000 | 2.9942036 ± 0.00088889 | 0 | 0 | 0 | 0.006 Pa 0.41 | |
| 23M2B | l | 253–381 | 390.6690 | 100,000 | 2.5801146 ± 0.01162609 | 4.1167729 ± 0.12632841 | −18.705154 ± 0.45832402 | 20.113871 ± 0.5584105 | 0.364 Pa 0.07 |
| crI | 215–262 | 352.6666 | 100,000 | 3.1640758 ± 0.00205365 | −0.29312593 ± 0.00933850 | 0 | 0 | 0.044 Pa 0.39 | |
| 33M2B | l | 265–381 | 394.1552 | 100,000 | 2.7453941 ± 0.01596937 | 2.2111073 ± 0.17130408 | −12.379211 ± 0.6142143 | 13.252975 ± 0.7411688 | 0.268 Pa 0.14 |
| crI | 248–279 | 366.4122 | 100,000 | 3.1057766 ± 0.00091999 | −0.51527251 ± 0.00412352 | 0 | 0 | 0.176 Pa 0.31 | |
| crII | 215–258 | 361.3012 | 100,000 | 3.1386075 ± 0.00229197 | −0.45595335 ± 0.01330643 | 0 | 0 | 0.058 Pa 0.82 |
 Cr II,
Cr II,  Cr I,
Cr I,  liquid;
liquid;  Belarus State University—BSU (3H: Sachek et al. [18]; 2M2P: Markovnik et al. [26]; 2M3P: Brazhnikov et al. [17]);
Belarus State University—BSU (3H: Sachek et al. [18]; 2M2P: Markovnik et al. [26]; 2M3P: Brazhnikov et al. [17]);  Hovorka et al. (3H: [33], 2M2P: [34], 2M3P and 3M3P [35], 3M2P and 23M2B [36], 22M1B [38]);
Hovorka et al. (3H: [33], 2M2P: [34], 2M3P and 3M3P [35], 3M2P and 23M2B [36], 22M1B [38]);  N’Guimbi et al. [20];
N’Guimbi et al. [20];  Thomas et al. [31];
Thomas et al. [31];  Kulikov et al. [32];
Kulikov et al. [32];  Loginova et al. [40]. Vapor pressures obtained by a combination of static and indirect chromatographic methods (GLC-ACRT (Activity Coefficients–Retention Times) method, Section 3.5 and Section 4.6): − reference 2M2P, − reference 2M3P, − reference 3H. Data sets represented by filled symbols were used in the SimCor procedure. For compounds for which ebulliometric data from BSU are available (3H, 2M2P, 2M3P), error bars are given to show data uncertainty.
Loginova et al. [40]. Vapor pressures obtained by a combination of static and indirect chromatographic methods (GLC-ACRT (Activity Coefficients–Retention Times) method, Section 3.5 and Section 4.6): − reference 2M2P, − reference 2M3P, − reference 3H. Data sets represented by filled symbols were used in the SimCor procedure. For compounds for which ebulliometric data from BSU are available (3H, 2M2P, 2M3P), error bars are given to show data uncertainty.
 Cr II,
Cr II,  Cr I,
Cr I,  liquid;
liquid;  Belarus State University—BSU (3H: Sachek et al. [18]; 2M2P: Markovnik et al. [26]; 2M3P: Brazhnikov et al. [17]);
Belarus State University—BSU (3H: Sachek et al. [18]; 2M2P: Markovnik et al. [26]; 2M3P: Brazhnikov et al. [17]);  Hovorka et al. (3H: [33], 2M2P: [34], 2M3P and 3M3P [35], 3M2P and 23M2B [36], 22M1B [38]);
Hovorka et al. (3H: [33], 2M2P: [34], 2M3P and 3M3P [35], 3M2P and 23M2B [36], 22M1B [38]);  N’Guimbi et al. [20];
N’Guimbi et al. [20];  Thomas et al. [31];
Thomas et al. [31];  Kulikov et al. [32];
Kulikov et al. [32];  Loginova et al. [40]. Vapor pressures obtained by a combination of static and indirect chromatographic methods (GLC-ACRT (Activity Coefficients–Retention Times) method, Section 3.5 and Section 4.6): − reference 2M2P, − reference 2M3P, − reference 3H. Data sets represented by filled symbols were used in the SimCor procedure. For compounds for which ebulliometric data from BSU are available (3H, 2M2P, 2M3P), error bars are given to show data uncertainty.
Loginova et al. [40]. Vapor pressures obtained by a combination of static and indirect chromatographic methods (GLC-ACRT (Activity Coefficients–Retention Times) method, Section 3.5 and Section 4.6): − reference 2M2P, − reference 2M3P, − reference 3H. Data sets represented by filled symbols were used in the SimCor procedure. For compounds for which ebulliometric data from BSU are available (3H, 2M2P, 2M3P), error bars are given to show data uncertainty.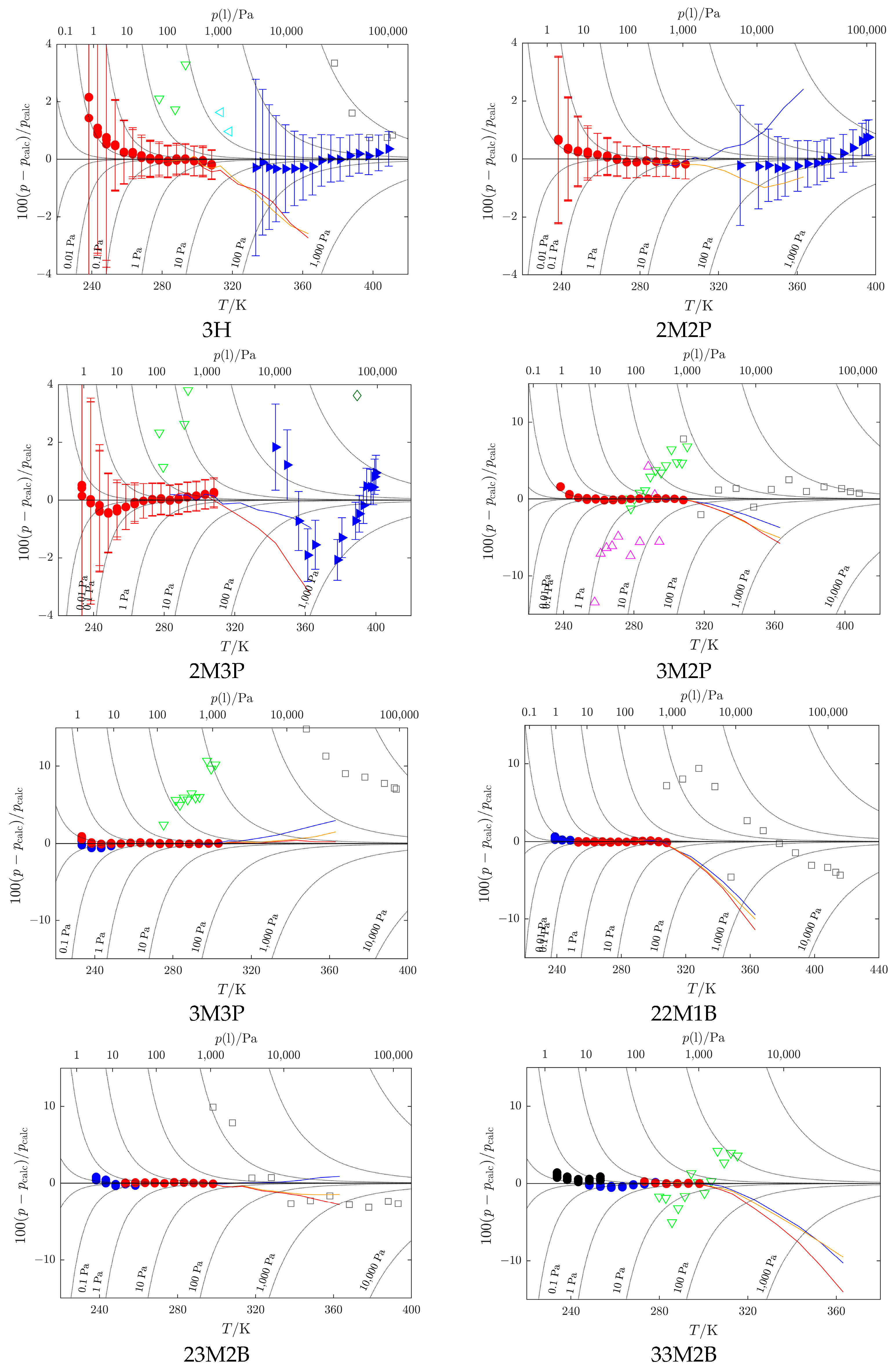
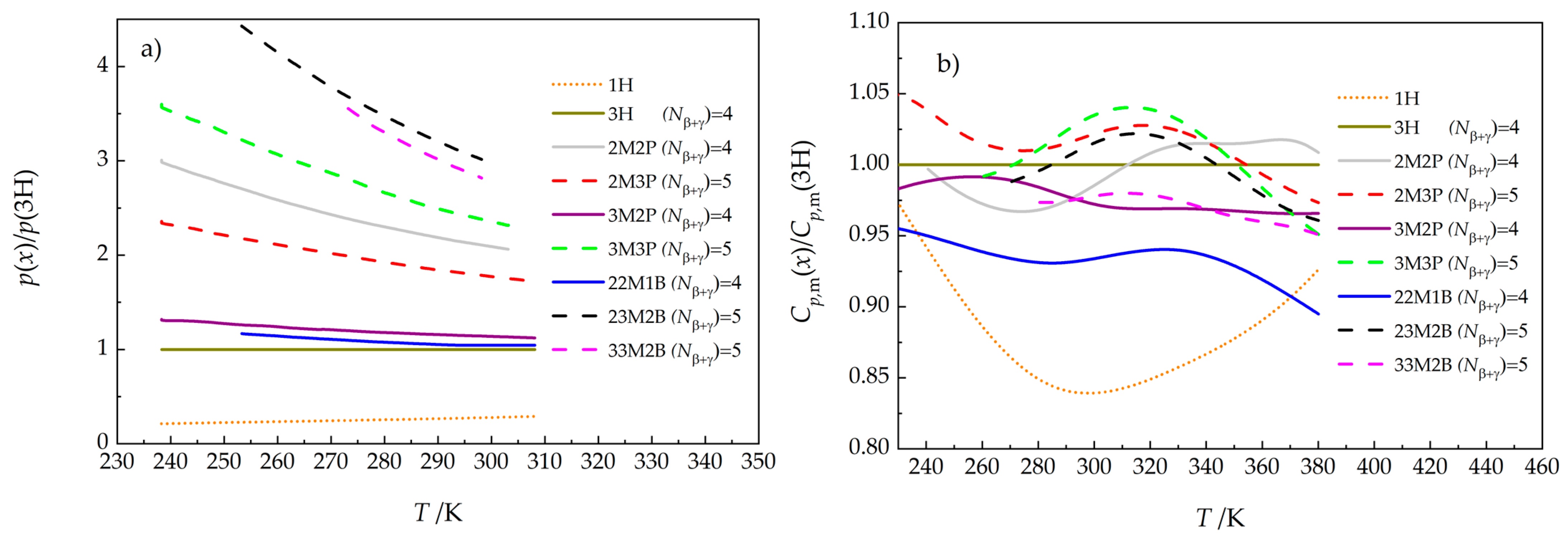
3.3. Sublimation Pressures
3.4. Enthalpies of Vaporization
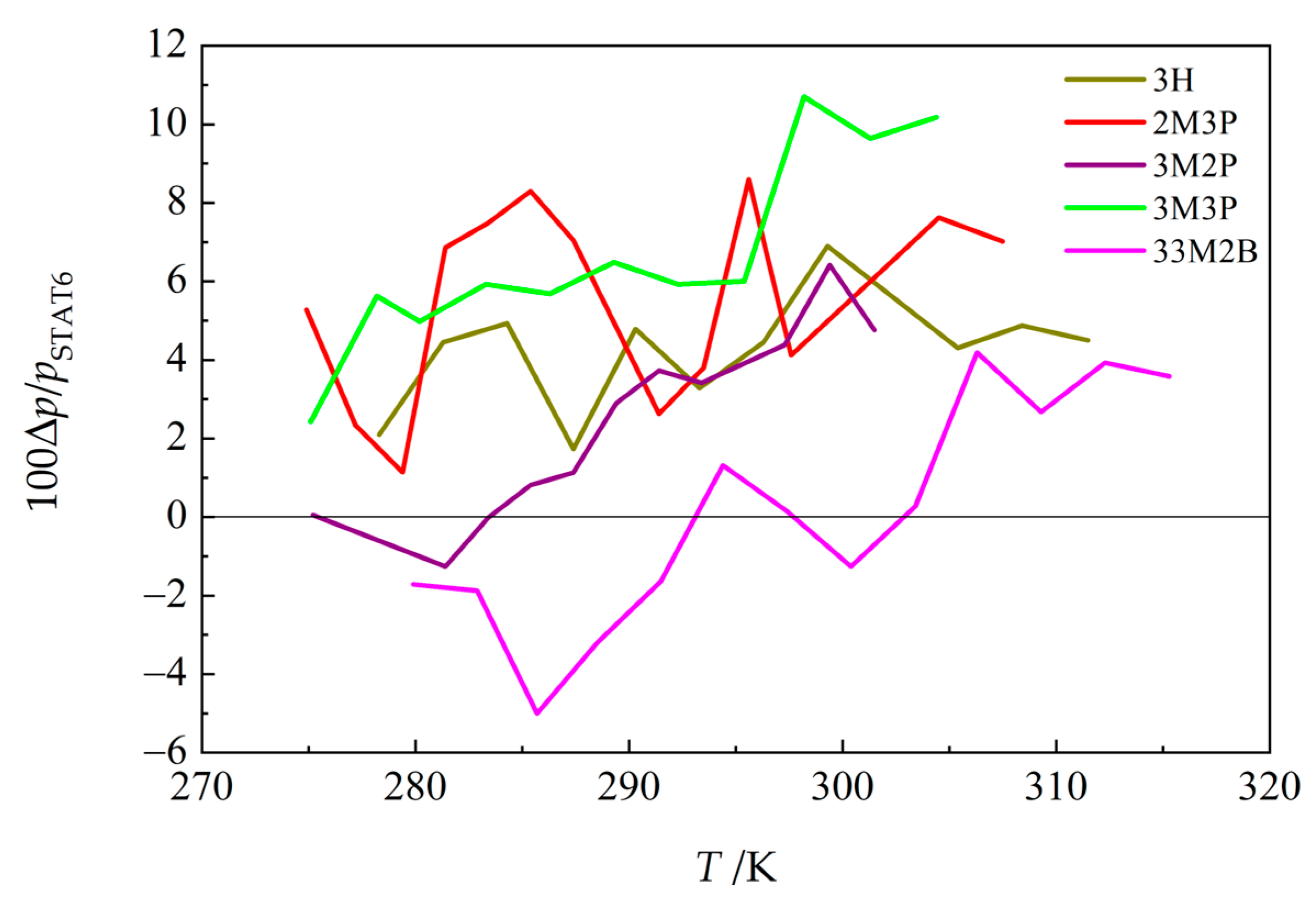
3.5. Vapor Pressures Obtained by Combining Static and Indirect Chromatographic Methods
3.6. Discussion of Trends in Thermodynamic Properties of the Studied Hexanols
4. Materials and Methods
4.1. Sample Description
| Compound | Abbreviation | CAS RN | Supplier | Original Molar Fraction a | Final Molar Fraction b | Water Mass Fraction wH2O (·10−6) c |
|---|---|---|---|---|---|---|
| (±)-3-hexanol | 3H | 623-37-0 | Aldrich (Burlington, MA, USA) | 0.994 | 0.9990 d | 28.8 |
| 2-methyl-2-pentanol | 2M2P | 590-36-3 | Aldrich | 0.997 | 0.9982 | 12.8 |
| (±)-2-methyl-3-pentanol | 2M3P | 565-67-3 | Aldrich | 0.994 | 0.9992 d | 26.0 |
| (±)-3-methyl-2-pentanol e | 3M2P | 565-60-6 | TCI (Tokyo, Japan) | 0.99 | 0.9998 | 14.8 |
| 3-methyl-3-pentanol | 3M3P | 77-74-7 | TCI | 0.996 | 0.9989 d | 20.9 |
| 2,2-dimethyl-1-butanol | 22M1B | 1185-33-7 | Synthesized f (London, UK) | - | 0.9993 d | 36.0 |
| 2,3-dimethyl-2-butanol | 23M2B | 594-60-5 | Aldrich | 0.993 | 0.9946 | 34.0 |
| (±)-3,3-dimethyl-2-butanol | 33M2B | 464-07-3 | Aldrich | 0.989 | 0.9967 | 16.6 |
4.2. Vapor Pressures
4.3. Thermodynamic Properties in the Ideal-Gas State
4.4. Simultaneous Treatment of Vapor Pressures and Related Thermal Data (SimCor Method)
4.5. Gas–Liquid Retention Time Determination
4.6. Indirect GLC-RT Method for Determination of Vapor Pressures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falbe, J.; Bahrmann, H.; Lipps, W.; Mayer, D.; Frey, G.D. Alcohols, Aliphatic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar] [CrossRef]
- Kenneally, C.J. Alcohols, Higher Aliphatic, Survey and Natural Alcohols Manufacture. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar] [CrossRef]
- Hua, Y. Research progress of higher alcohols as alternative fuels for compression ignition engines. Fuel 2024, 357, 129749. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W. Alcohols as Biofuel for a Diesel Engine with Blend Mode—A Review. Energies 2024, 17, 4516. [Google Scholar] [CrossRef]
- Gui, L.; Yang, J.; Yu, G.; Wu, J.; Meng, X. Thermodynamic properties of renewable n-butanol: Experimental and modeling investigations. Thermochim. Acta 2025, 751, 180080. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju Gautam, R.; Klein Roger, A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary David, C.; Crabtree Robert, H.; Dannenberg Joseph, J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Defining the hydrogen bond: An account (IUPAC Technical Report). Pur. Appl. Chem. 2011, 83, 1619–1636. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen Bond—Definitions, Criteria of Existence and Various Types. In Understanding Hydrogen Bonds; The Royal Society of Chemistry: Cambridge, UK, 2020; pp. 1–40. [Google Scholar] [CrossRef]
- Böhmer, R.; Gainaru, C.; Richert, R. Structure and dynamics of monohydroxy alcohols—Milestones towards their microscopic understanding, 100 years after Debye. Phys. Rep. 2014, 545, 125–195. [Google Scholar] [CrossRef]
- Wójcik, M.J.; Ozaki, Y. (Eds.) Spectroscopy and Computation of Hydrogen-Bonded Systems; Wiley-VCH: Weinheim, Germany, 2023. [Google Scholar] [CrossRef]
- Grabowski, S.J. Theoretical Approaches. In Understanding Hydrogen Bonds; The Royal Society of Chemistry: Cambridge, UK, 2020; pp. 22–224. [Google Scholar] [CrossRef]
- Jabłoński, M. Hydrogen Bonds. Molecules 2023, 28, 1616. [Google Scholar] [CrossRef]
- Wilhoit, R.C.; Zwolinski, B.J. Physical and Thermodynamic Properties of Aliphatic Alcohols; American Chemical Society: New York, NY, USA, 1973. Available online: https://srd.nist.gov/JPCRD/jpcrdS1Vol2.pdf (accessed on 13 October 2025).
- Cibulka, I. Saturated Liquid Densities of 1-Alkanols from C(1) to C(10) and n-Alkanes from C(5) to C(16)—A Critical-Evaluation of Experimental-Data. Fluid Phase Equilib. 1993, 89, 1–18. [Google Scholar] [CrossRef]
- Ambrose, D.; Ellender, J.H.; Sprake, C.H.S. Thermodynamic properties of organic oxygen compounds XXXV. Vapour pressures of aliphatic alcohols. J. Chem. Thermodyn. 1974, 6, 909–914. [Google Scholar] [CrossRef]
- van Miltenburg, J.C.; Oonk, H.A.J.; Ventola, L. Heat capacities and derived thermodynamic functions of 1-octadecanol, 1-nonadecanol, 1-eicosanol, and 1-docosanol between 10 K and 370 K. J. Chem. Eng. Data 2001, 46, 90–97. [Google Scholar] [CrossRef]
- Brazhnikov, M.M.; Andreevskii, D.N.; Sachek, A.I.; Peshchenko, A.D. Saturated vapor pressure of some secondary alcohols and calculations of heats of vaporization. Zh. Prikl. Khim. 1975, 48, 2181–2185. [Google Scholar]
- Sachek, A.I.; Markovnik, V.S.; Peshchenko, A.D.; Shvaro, A.V.; Andreevskii, D.N. Vapor pressure of normal C5–C8 secondary alcohols. Khimicheskaya Promyshlennost 1984, 337–340. [Google Scholar]
- Hales, J.L.; Ellender, J.H. Liquid densities from 293 to 490 K of nine aliphatic alcohols. J. Chem. Thermodyn. 1976, 8, 1177–1184. [Google Scholar] [CrossRef]
- N’Guimbi, J.; Berro, C.; Mokbel, I.; Rauzy, E.; Jose, J. Experimental vapour pressures of 13 secondary and tertiary alcohols—Correlation and prediction by a group contribution method. Fluid Phase Equilib. 1999, 162, 143–158. [Google Scholar] [CrossRef]
- Štejfa, V.; Lyshchuk, H.; Babková, K.; Krupička, M.; Ludík, J.; Fulem, M.; Červinka, C.; Růžička, K. Hierarchy of hydrogen bonding among constitutional isomers of hexanol. J. Mol. Liq. 2024, 394, 123804. [Google Scholar] [CrossRef]
- Pokorný, V.; Štejfa, V.; Klajmon, M.; Fulem, M.; Růžička, K. Vapor Pressures and Thermophysical Properties of 1-Heptanol, 1-Octanol, 1-Nonanol, and 1-Decanol: Data Reconciliation and PC-SAFT Modeling. J. Chem. Eng. Data 2021, 66, 805–821. [Google Scholar] [CrossRef]
- Koutek, B.; Mahnel, T.; Šimáček, P.; Fulem, M.; Růžička, K. Extracting Vapor Pressure Data from GLC Retention Times. Part 1: Analysis of Single Reference Approach. J. Chem. Eng. Data 2017, 62, 3542–3550. [Google Scholar] [CrossRef]
- Koutek, B.; Pokorný, V.; Mahnel, T.; Štejfa, V.; Řehák, K.; Fulem, M.; Růžička, K. Estimating Vapor Pressure Data from Gas–Liquid Chromatography Retention Times: Analysis of Multiple Reference Approaches, Review of Prior Applications, and Outlook. J. Chem. Eng. Data 2022, 67, 2017–2043. [Google Scholar] [CrossRef]
- Herington, E.F.G. Theoretical fundamentals of gas chromatography. In Vapour Phase Chromatography, Proceedings of the Symposium Sponsored by the Hydrocarbon Research Group of the Institute of Petroleum, London, UK, 30 May–1 June 1956; Desty, D.H., Harbourn, C.L.A., Eds.; Butterworth: London, UK, 1957; pp. 5–14. [Google Scholar]
- Markovnik, V.S.; Sachek, A.I.; Peshchenko, A.D.; Shvaro, O.V.; Andreevskii, D.N. Temperature dependence of the vapor pressure of some isostructural aliphatic alcohols. Termodinam. Organ. Soedin. (Gor’kii) 1978, 81–84. [Google Scholar]
- Ambrose, D.; Ghiassee, N.B. Vapour pressures, critical temperatures, and critical pressures of benzyl alcohol, octan-2-ol, and 2-ethylhexan-1-ol. J. Chem. Thermodyn. 1990, 22, 307–311. [Google Scholar] [CrossRef]
- Čenský, M.; Roháč, V.; Růžička, K.; Fulem, M.; Aim, K. Vapor pressure of selected aliphatic alcohols by ebulliometry. Part 1. Fluid Phase Equilib. 2010, 298, 192–198. [Google Scholar] [CrossRef]
- Čenský, M.; Vrbka, P.; Růžička, K.; Fulem, M. Vapor pressure of selected aliphatic alcohols by ebulliometry. Part 2. Fluid Phase Equilib. 2010, 298, 199–205. [Google Scholar] [CrossRef]
- Goldberg, R.N.; Weir, R.D. Conversion of temperatures and thermodynamic properties to the basis of the international temperature scale of 1990. Pure Appl. Chem. 1992, 64, 1545–1562. [Google Scholar] [CrossRef]
- Thomas, L.H.; Meatyard, R.; Smith, H.; Davies, G.H. Vapor pressures and molar entropies of vaporization of monohydric alcohols. J. Chem. Eng. Data 1979, 24, 159–161. [Google Scholar] [CrossRef]
- Kulikov, D.; Verevkin, S.P.; Heintz, A. Determination of vapor pressures and vaporization enthalpies of the aliphatic branched C-5 and C-6 alcohols. J. Chem. Eng. Data 2001, 46, 1593–1600. [Google Scholar] [CrossRef]
- Hovorka, F.; Lankelma, H.P.; Stanford, S.C. Thermodynamic Properties of the Hexyl Alcohols. II. Hexanols-1, -2, -3 and 2-Methylpentanol-1 and -4. J. Am. Chem. Soc. 1938, 60, 820–827. [Google Scholar] [CrossRef]
- Hovorka, F.; Lankelma, H.P.; Naujoks, C.K. Thermodynamic Properties of 2-Methylpentanol-2. J. Am. Chem. Soc. 1933, 55, 4820–4822. [Google Scholar] [CrossRef]
- Hovorka, F.; Lankelma, H.P.; Axelrod, A.E. Thermodynamic Properties of the Hexyl Alcohols. III. 2-Methylpentanol-3 and 3-Methylpentanol-3. J. Am. Chem. Soc. 1940, 62, 187–189. [Google Scholar] [CrossRef]
- Hovorka, F.; Lankelma, H.P.; Bishop, J.W. Thermodynamic Properties of the Hexyl Alcohols. VI. 2,3-Dimethylbutanol-2 and 3-Methylpentanol-21. J. Am. Chem. Soc. 1941, 63, 1097–1098. [Google Scholar] [CrossRef]
- Hovorka, F.; Lankelma, H.P.; Schneider, I. Thermodynamic Properties of the Hexyl Alcohols. IV. 3-Methylpentanol-1 and 2-Methylpentanol-5. J. Am. Chem. Soc. 1940, 62, 1096–1098. [Google Scholar] [CrossRef]
- Hovorka, F.; Lankelma, H.P.; Smith, W.R. Thermodynamic Properties of the Hexyl Alcohols. V. 2,2-Dimethylbutanol-1 and 2-Ethylbutanol-1. J. Am. Chem. Soc. 1940, 62, 2372–2374. [Google Scholar] [CrossRef]
- N’Guimbi, J.; Kasehgari, H.; Mokbel, I.; Jose, J. Tensions de vapeur d’alcools primaires dans le domaine 0.3 Pa à 1.5 kPa. Thermochim. Acta 1992, 196, 367–377. [Google Scholar] [CrossRef]
- Loginova, M.A.; Frolov, A.F.; Bolshako, L.; Simanov, N.A.; Obedkova, L.V. Liquid-Vapor-Equilibrium in Systems of Ethylisopropylketone Synthesis. Zh. Prikl. Khim. 1974, 47, 99–102. [Google Scholar]
- Ambrose, D. VaporPressures. In Experimental Thermodynamics Volume II; Neindre, B., Vodar, B., Eds.; Springer: New York, NY, USA, 1975; pp. 607–656. [Google Scholar]
- Zaitsau, D.H.; Pimerzin, A.A.; Verevkin, S.P. Fatty acids methyl esters: Complementary measurements and comprehensive analysis of vaporization thermodynamics. J. Chem. Thermodyn. 2019, 132, 322–340. [Google Scholar] [CrossRef]
- Majer, V.; Svoboda, V.; Uchytilová, V.; Finke, M. Enthalpies of vaporization of aliphatic C5 and C6 alcohols. Fluid Phase Equilib. 1985, 20, 111–118. [Google Scholar] [CrossRef]
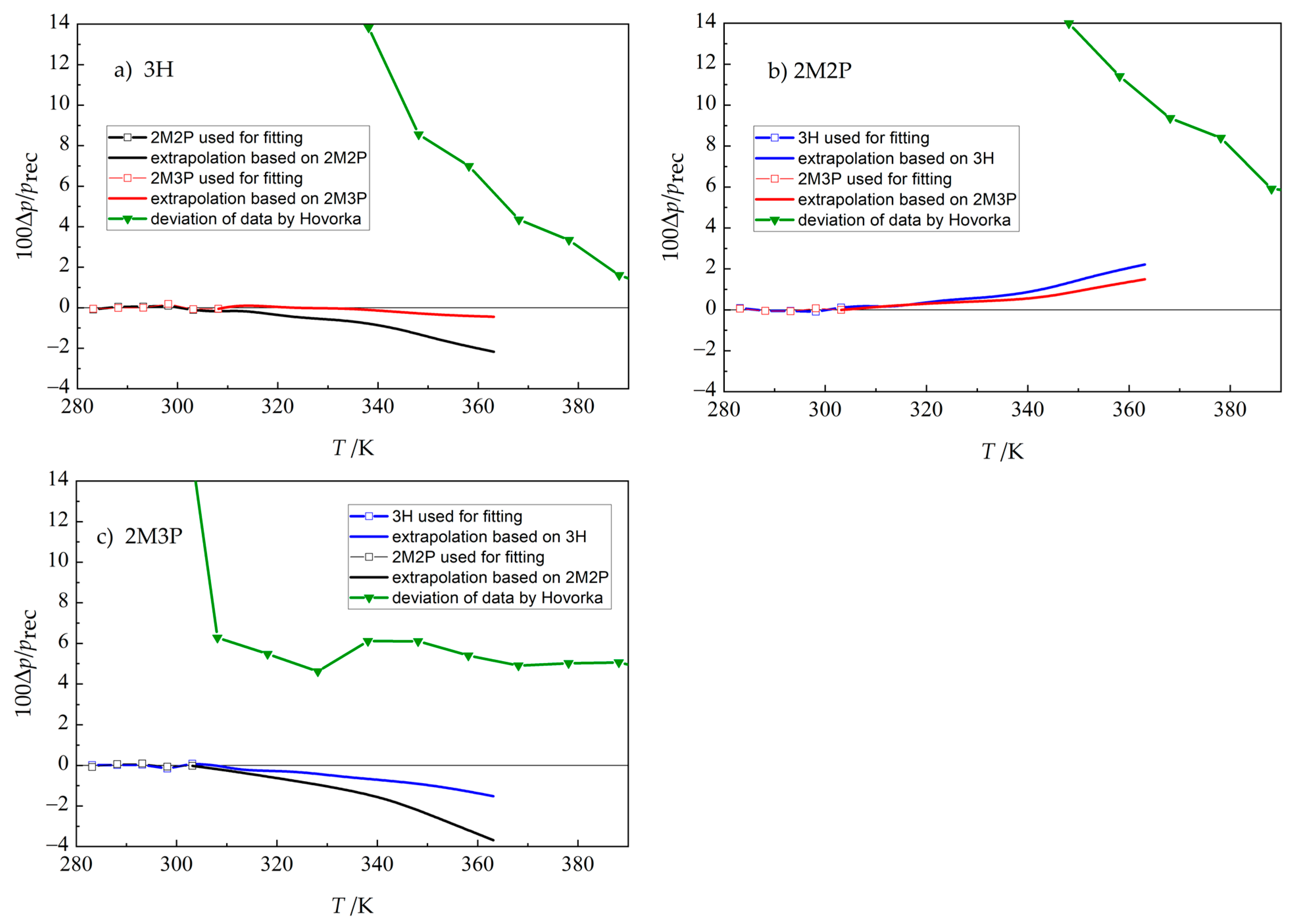
- Růžička, K.; Majer, V. Simple and controlled extrapolation of vapor pressures toward the triple point. AIChE J. 1996, 42, 1723–1740. [Google Scholar] [CrossRef]
- Růžička, K.; Majer, V. Simultaneous treatment of vapor pressures and related thermal data between the triple and normal boiling temperatures for n-alkanes C5–C20. J. Phys. Chem. Ref. Data 1994, 23, 1–39. [Google Scholar] [CrossRef]
- Tsonopoulos, C. Empirical correlation of second virial coefficients. AIChE J. 1974, 20, 263–272. [Google Scholar] [CrossRef]
- Gude, M.; Teja, A.S. Vapor-Liquid Critical Properties of Elements and Compounds. 4. Aliphatic Alkanols. J. Chem. Eng. Data 1995, 40, 1025–1036. [Google Scholar] [CrossRef]
- Nannoolal, Y.; Rarey, J.; Ramjugernath, D. Estimation of pure component properties: Part 2. Estimation of critical property data by group contribution. Fluid Phase Equilib. 2007, 252, 1–27. [Google Scholar] [CrossRef]
- Artist Software; DDBST Software and Separation Technology GmbH: Oldenburg, Germany, 2019; Volume p. 2019.2010.0151.
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Kolská, Z.; Růžička, V.; Gani, R. Estimation of the Enthalpy of Vaporization and the Entropy of Vaporization for Pure Organic Compounds at 298.15 K and at Normal Boiling Temperature by a Group Contribution Method. Ind. Eng. Chem. Res. 2005, 44, 8436–8454. [Google Scholar] [CrossRef]
- Majer, V.; Svoboda, V. International Union of Pure and Applied Chemistry Chemical Data Series, No. 32. Enthalpies of Vaporization of Organic Compounds: Critical Review and Data Compilation; Blackwell: Oxford, UK, 1985; p. 300. ISBN 300-632-01529-01522. [Google Scholar]
- Benson, S.W. Some Observations on the Structures of Liquid Alcohols and Their Heats of Vaporization. J. Am. Chem. Soc. 1996, 118, 10645–10649. [Google Scholar] [CrossRef]
- Serra, P.B.P.; Růžička, K.; Fulem, M.; Vlk, O.; Krakovský, I. Calorimetric and FTIR study of selected aliphatic heptanols. Fluid Phase Equilib. 2016, 423, 43–54. [Google Scholar] [CrossRef]
- Serra, P.B.P.; Krakovský, I.; Fulem, M.; Růžička, K. Calorimetric and FTIR study of selected aliphatic octanols. J. Therm. Anal. Calorim. 2018, 134, 2157–2170. [Google Scholar] [CrossRef]
- Zábranský, M.; Růžička, V. Estimation of the heat capacities of organic liquids as a function of temperature using group additivity: An amendment. J. Phys. Chem. Ref. Data 2004, 33, 1071–1081. [Google Scholar] [CrossRef]
- Kolská, Z.; Kukal, J.; Zábranský, M.; Růžička, V. Estimation of the Heat Capacity of Organic Liquids as a Function of Temperature by a Three-Level Group Contribution Method. Ind. Eng. Chem. Res. 2008, 47, 2075–2085. [Google Scholar] [CrossRef]
- Zábranský, M.; Růžička, V.; Majer, V.; Domalski, E.S. Heat Capacity of Liquids. Critical Review and Recommended Values; American Chemical Society: Washington, DC, USA, 1996; p. 1596. [Google Scholar]
- Zábranský, M.; Růžička, V.; Domalski, E.S. Heat Capacity of Liquids: Critical Review and Recommended Values. Supplement I. J. Phys. Chem. Ref. Data 2001, 30, 1199–1689. [Google Scholar] [CrossRef]
- Zábranský, M.; Kolská, Z.; Růžička, V.; Domalski, E.S. Heat Capacity of Liquids: Critical Review and Recommended Values. Supplement II. J. Phys. Chem. Ref. Data 2010, 39, 013103. [Google Scholar] [CrossRef]
- Hamilton, D.J. Gas chromatographic measurement of volatility of herbicide esters. J. Chromatogr. A 1980, 195, 75–83. [Google Scholar] [CrossRef]
- Bidleman, T.F. Estimation of Vapor-Pressures for Nonpolar Organic-Compounds by Capillary Gas-Chromatography. Anal. Chem. 1984, 56, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Ballschmiter, K. Relationship between experimentally determined vapour pressures and retention times of PCB on a 50% n-octyl-methylpolysiloxane stationary phase. Fresenius’ Z. Anal. Chem. 1989, 333, 731–732. [Google Scholar] [CrossRef]
- Hinckley, D.A.; Bidleman, T.F.; Foreman, W.T.; Tuschall, J.R. Determination of Vapor-Pressures for Nonpolar and Semipolar Organic-Compounds from Gas-Chromatographic Retention Data. J. Chem. Eng. Data 1990, 35, 232–237. [Google Scholar] [CrossRef]
- Letcher, T.M.; Naicker, P.K. Determination of vapor pressures using gas chromatography. J. Chromatogr. A 2004, 1037, 107–114. [Google Scholar] [CrossRef]
- Moller, B.; Rarey, J.; Ramjugernath, D. Estimation of the vapour pressure of non-electrolyte organic compounds via group contributions and group interactions. J. Mol. Liq. 2008, 143, 52–63. [Google Scholar] [CrossRef]
- Nannoolal, Y.; Rarey, J.; Ramjugernath, D.; Cordes, W. Estimation of pure component properties: Part 1. Estimation of the normal boiling point of non-electrolyte organic compounds via group contributions and group interactions. Fluid Phase Equilib. 2004, 226, 45–63. [Google Scholar] [CrossRef]
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; Bièvre, P.D.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Atomic weights of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 265–291. [Google Scholar] [CrossRef]
- Newell, D.B.; Cabiati, F.; Fischer, J.; Fujii, K.; Karshenboim, S.G.; Margolis, H.S.; de Mirandés, E.; Mohr, P.J.; Nez, F.; Pachucki, K.; et al. The CODATA 2017 values of h,e,k, and NA for the revision of the SI. Metrologia 2018, 55, L13–L16. [Google Scholar] [CrossRef]
- Fulem, M.; Růžička, K.; Morávek, P.; Pangrác, J.; Hulicius, E.; Kozyrkin, B.; Shatunov, V. Vapor Pressure of Selected Organic Iodides. J. Chem. Eng. Data 2010, 55, 4780–4784. [Google Scholar] [CrossRef]
- Pfaendtner, J.; Yu, X.; Broadbelt, L.J. The 1-D hindered rotor approximation. Theor. Chem. Acc. 2007, 118, 881–898. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
- Štejfa, V.; Fulem, M.; Růžička, K. First-principles calculation of ideal-gas thermodynamic properties of long-chain molecules by R1SM approach—Application to n-alkanes. J. Chem. Phys. 2019, 150, 224101. [Google Scholar] [CrossRef] [PubMed]
- Marston, C.C.; Balint-Kurti, G.G. The Fourier grid Hamiltonian method for bound state eigenvalues and eigenfunctions. J. Chem. Phys. 1989, 91, 3571–3576. [Google Scholar] [CrossRef]
- King, M.B.; Al-Najjar, H. Method for correlating and extending vapor pressure data to lower temperatures using thermal data. Vapor pressure equations for some n-alkanes at temperatures below the normal boiling point. Chem. Eng. Sci. 1974, 29, 1003–1011. [Google Scholar] [CrossRef]
- Cox, E.R. Hydrocarbon vapor pressures. Ind. Eng. Chem. 1936, 28, 613–616. [Google Scholar] [CrossRef]
- Rodgers, T.F.M.; Okeme, J.O.; Parnis, J.M.; Girdhari, K.; Bidleman, T.F.; Wan, Y.; Jantunen, L.M.; Diamond, M.L. Novel Bayesian Method to Derive Final Adjusted Values of Physicochemical Properties: Application to 74 Compounds. Environ. Sci. Tech. 2021, 55, 12302–12316. [Google Scholar] [CrossRef]
- Delle Site, A. The vapor pressure of environmentally significant organic chemicals: A review of methods and data at ambient temperature. J. Phys. Chem. Ref. Data 1997, 26, 157–193. [Google Scholar] [CrossRef]
- Orbey, H.; Sandler, S.I. Relative Measurements of Activity-Coefficients at Infinite Dilution by Gas-Chromatography. Ind. Eng. Chem. Res. 1991, 30, 2006–2011. [Google Scholar] [CrossRef]
 , from SETARAM Microcalvet calorimeter;
, from SETARAM Microcalvet calorimeter;  ,
,  from Perkin Elmer 8500 calorimeter;
from Perkin Elmer 8500 calorimeter;  , from the SimCor procedure;
, from the SimCor procedure;  , from the SimCor procedure (see Equation (S7)).
, from the SimCor procedure (see Equation (S7)).
 , from SETARAM Microcalvet calorimeter;
, from SETARAM Microcalvet calorimeter;  ,
,  from Perkin Elmer 8500 calorimeter;
from Perkin Elmer 8500 calorimeter;  , from the SimCor procedure;
, from the SimCor procedure;  , from the SimCor procedure (see Equation (S7)).
, from the SimCor procedure (see Equation (S7)).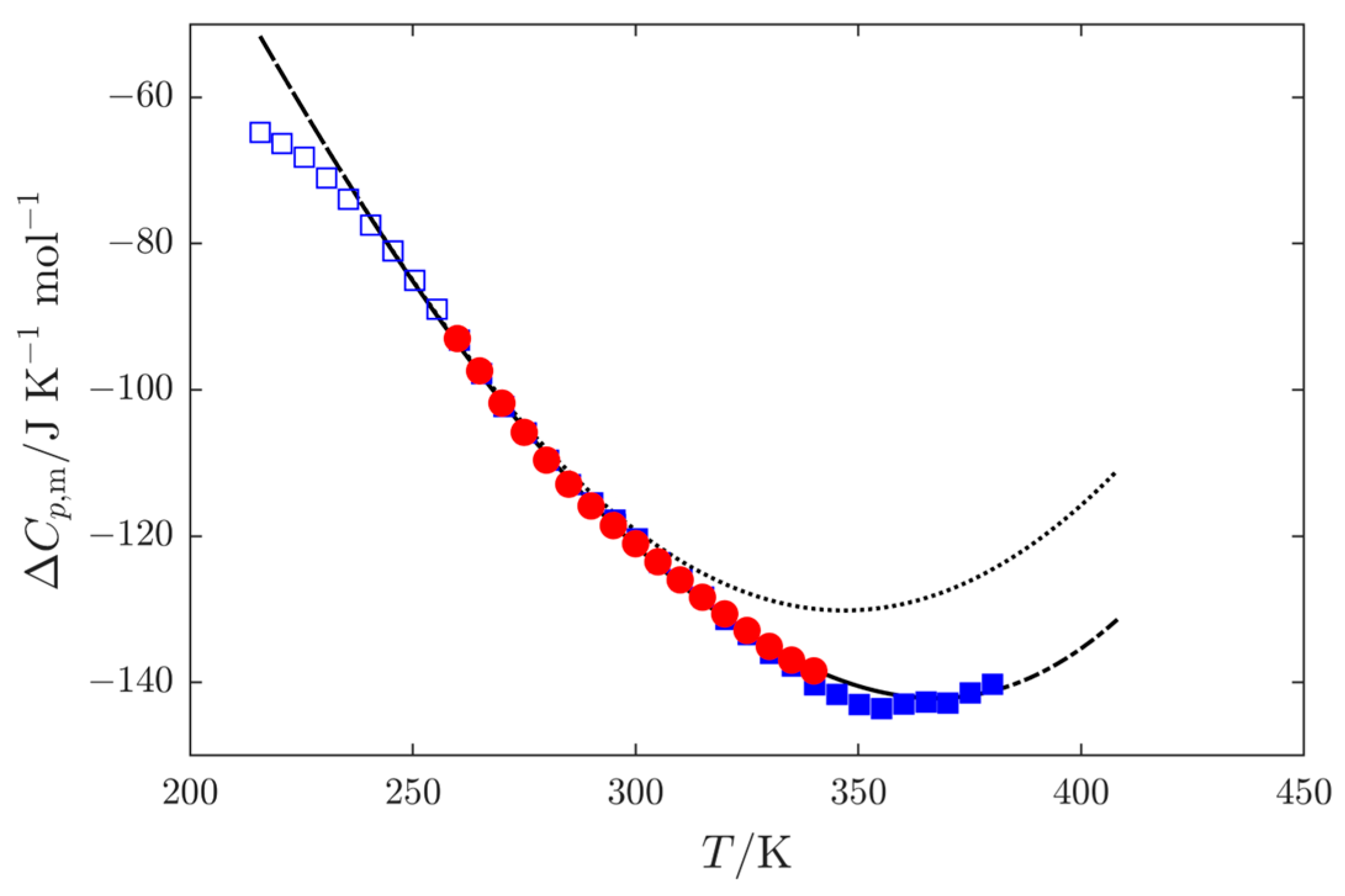
 Majer et al. [43], calorimetry) and estimated values (filled squares, group-contribution method by Kolská et al. [51]) from the results of this work, derived from the Cox equation (Equation (3)), with parameters from Table 3 (resulting vaporization enthalpies, including corrections for non-ideality applied in their calculation, are given in Table S7 in the Supplementary Materials). See Table 5 for compound abbreviations.
Majer et al. [43], calorimetry) and estimated values (filled squares, group-contribution method by Kolská et al. [51]) from the results of this work, derived from the Cox equation (Equation (3)), with parameters from Table 3 (resulting vaporization enthalpies, including corrections for non-ideality applied in their calculation, are given in Table S7 in the Supplementary Materials). See Table 5 for compound abbreviations.
 Majer et al. [43], calorimetry) and estimated values (filled squares, group-contribution method by Kolská et al. [51]) from the results of this work, derived from the Cox equation (Equation (3)), with parameters from Table 3 (resulting vaporization enthalpies, including corrections for non-ideality applied in their calculation, are given in Table S7 in the Supplementary Materials). See Table 5 for compound abbreviations.
Majer et al. [43], calorimetry) and estimated values (filled squares, group-contribution method by Kolská et al. [51]) from the results of this work, derived from the Cox equation (Equation (3)), with parameters from Table 3 (resulting vaporization enthalpies, including corrections for non-ideality applied in their calculation, are given in Table S7 in the Supplementary Materials). See Table 5 for compound abbreviations.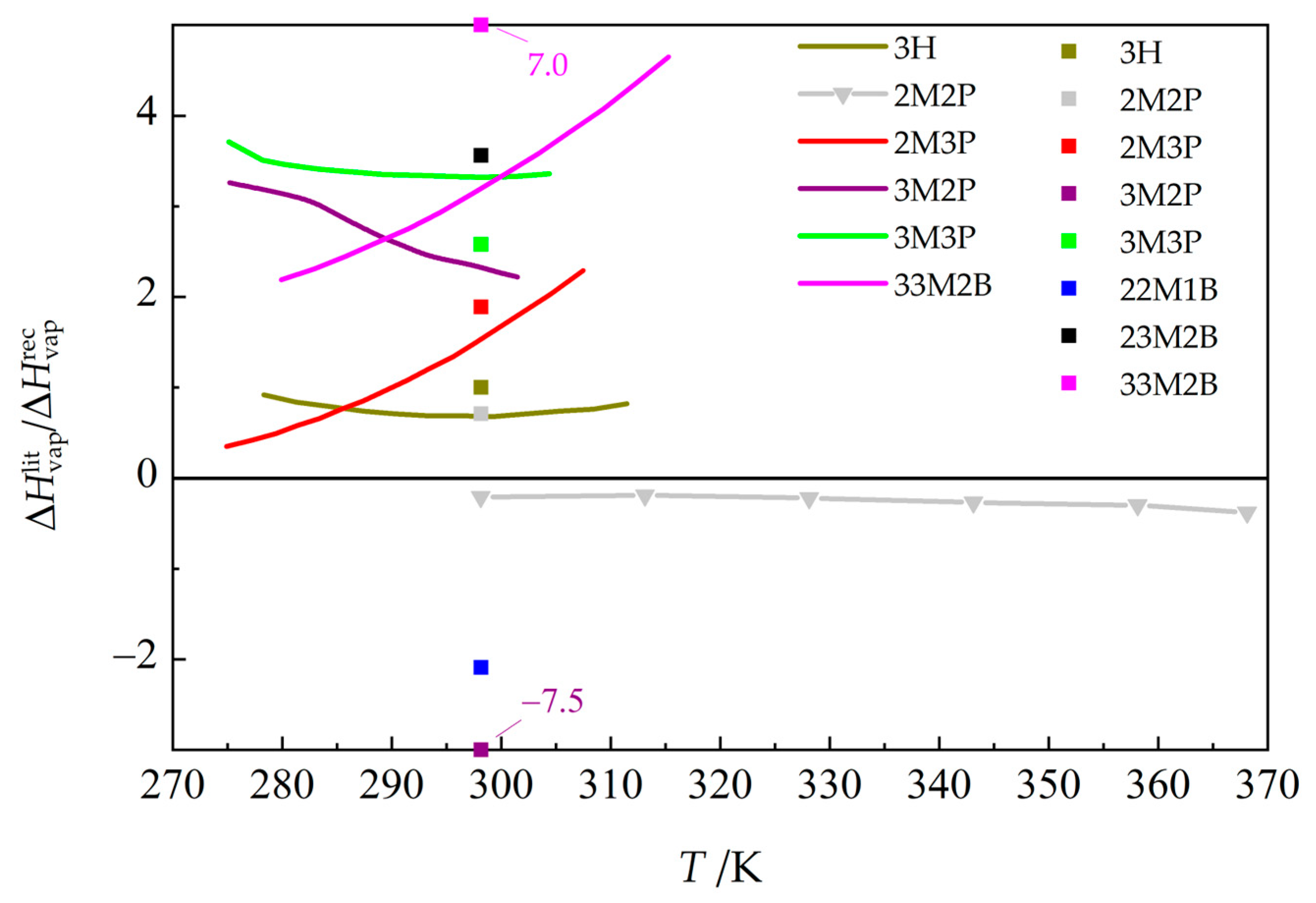

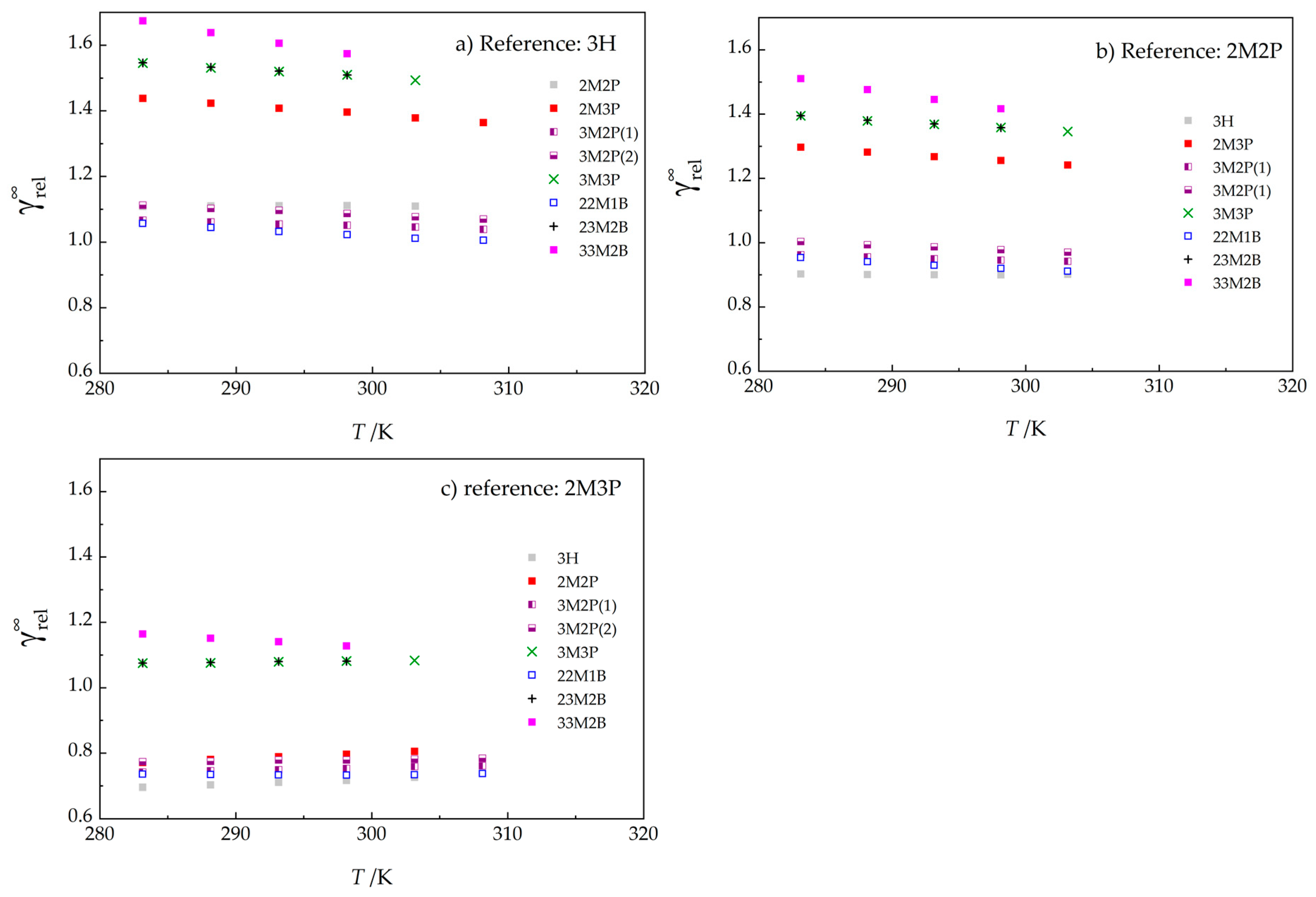
| HUT b | 3H c | 2M2P | 2M3P | 3M2P d | 3M2P d | 3M3P | 22M1B | 23M2B | 33M2B | |
|---|---|---|---|---|---|---|---|---|---|---|
| T/K | t/min | |||||||||
| 283.15 | 1.623 | 34.366 | 17.650 | 26.382 | 31.214 | 32.488 | 20.987 | 33.990 | 16.575 | 18.666 |
| 288.15 | 1.631 | 26.392 | 14.081 | 20.570 | 24.129 | 25.010 | 16.592 | 26.098 | 13.296 | 14.827 |
| 293.15 | 1.656 | 20.723 | 11.477 | 16.396 | 19.043 | 19.731 | 13.441 | 20.470 | 10.914 | 12.053 |
| 298.15 | 1.667 | 16.381 | 9.425 | 13.180 | 15.166 | 15.623 | 10.969 | 16.185 | 9.021 | 9.866 |
| 303.15 | 1.703 | 13.342 | 7.964 | 10.876 | 12.415 | 12.736 | 9.192 | 13.157 | 7.681 | 8.313 |
| 308.15 | 1.738 | 11.043 | 6.845 | 9.143 | 10.313 | 10.572 | 7.829 | 10.913 | 6.638 | 7.123 |
| 313.15 | 1.750 | 9.177 | 5.914 | 7.728 | 8.621 | 8.830 | 6.708 | 9.097 | 5.756 | 6.136 |
| 323.15 | 1.800 | 6.788 | 4.694 | 5.877 | 6.439 | 6.567 | 5.240 | 6.730 | 4.618 | 4.857 |
| 333.15 | 1.828 | 5.240 | 3.877 | 4.663 | 5.023 | 5.103 | 4.252 | 5.209 | 3.838 | 3.993 |
| 343.15 | 1.864 | 4.268 | 3.351 | 3.888 | 4.128 | 4.178 | 3.616 | 4.250 | 3.333 | 3.434 |
| 353.15 | 1.897 | 3.628 | 2.994 | 3.372 | 3.536 | 3.569 | 3.188 | 3.620 | 2.989 | 3.058 |
| 363.15 | 1.926 | 3.210 | 2.760 | 3.033 | 3.149 | 3.166 | 2.904 | 3.208 | 2.761 | 2.810 |
| Compound | Transition | Ti(lit) [21] a | Ti(SC) b | Ti(lit) − Ti(SC) | ΔiH(lit) [21] a | ΔiH(SC) b | ΔiH(lit) − ΔiH(SC) |
|---|---|---|---|---|---|---|---|
| 3M3P | crI-l | 250.1 ± 0.4 | 250.4 | −0.3 | 10.7 ± 0.3 | 10.81 | −0.11 |
| 22M1B | crI-l | 251.2 ± 0.4 | 251.2 | 0.0 | 1.6 ± 0.1 | 1.60 | 0.00 |
| 23M2B | crI-l | 261.6 ± 0.5 | 262.4 | −0.8 | 8.4 ± 0.5 | 8.71 | −0.31 |
| 33M2B | crI-l | 278.4 ± 0.4 | 278.9 | −0.5 | 6.4 ± 0.2 | 6.50 | −0.10 |
| crII-crI | 258.0 ± 0.5 | 257.6 | 0.4 | 1.96 ± 0.15 | 1.80 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štejfa, V.; Šimáček, P.; Koutek, B.; Fulem, M.; Růžička, K. Vapor Pressure of Selected Aliphatic Hexanols by Static and Indirect Chromatographic Methods. Molecules 2025, 30, 4287. https://doi.org/10.3390/molecules30214287
Štejfa V, Šimáček P, Koutek B, Fulem M, Růžička K. Vapor Pressure of Selected Aliphatic Hexanols by Static and Indirect Chromatographic Methods. Molecules. 2025; 30(21):4287. https://doi.org/10.3390/molecules30214287
Chicago/Turabian StyleŠtejfa, Vojtěch, Pavel Šimáček, Bohumír Koutek, Michal Fulem, and Květoslav Růžička. 2025. "Vapor Pressure of Selected Aliphatic Hexanols by Static and Indirect Chromatographic Methods" Molecules 30, no. 21: 4287. https://doi.org/10.3390/molecules30214287
APA StyleŠtejfa, V., Šimáček, P., Koutek, B., Fulem, M., & Růžička, K. (2025). Vapor Pressure of Selected Aliphatic Hexanols by Static and Indirect Chromatographic Methods. Molecules, 30(21), 4287. https://doi.org/10.3390/molecules30214287






