2.1. Study of the Electronic Structure and Chemical Properties of the Reagents in the GS
2.1.1. Study of the Electronic Structure of the Reagents
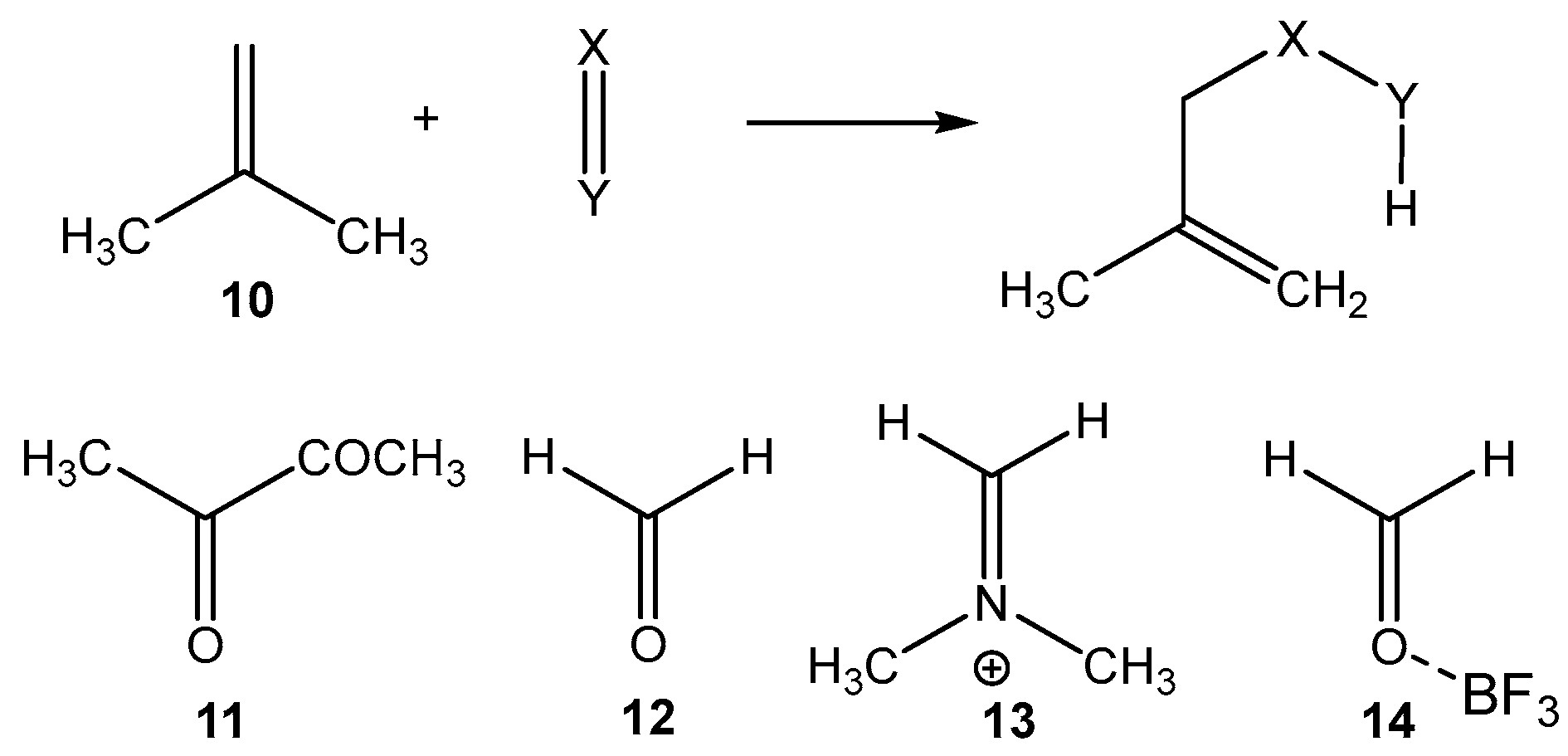
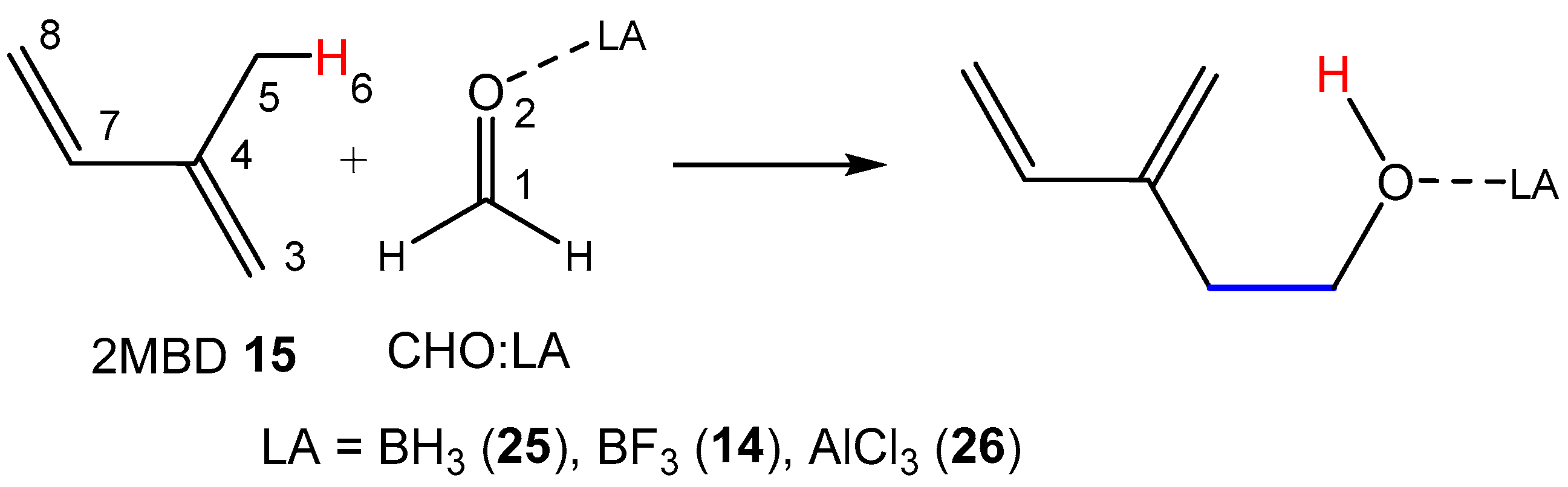
Before studying the LA-catalyzed AE reactions of 2MBD
15 with CHO
12, the electronic structures of reagents at the GS were investigated through topological analysis of the ELF [
17]. The ELF permits a quantitative characterization of the electron density distribution in a molecule [
36]. The attractor positions of the ELF basins and the natural atomic charges of 2MBD
15, CHO
12, and three representative CHO:LA complexes
14,
25, and
26 are shown in
Figure 2, while the populations of the more relevant valence basins are given in
Table 1.
ELF topological analysis of 2MBD
15 reveals the presence of two disynaptic basins, V(C3,C4) and V’(C3,C4), integrating a total of 3.42 e, associated with a depopulated C3-C4 double bond, one V(C4,C7) disynaptic basin integrating 2.22 e, associated with a populated C4-C7 single bond, and two disynaptic basins, V(C7,C8) and V’(C7,C8), integrating a total of 3.40 e, associated with a depopulated C7-C8 double bond. The methyl group at the C5 carbon does not substantially modify the ELF of
s-trans butadiene (see the
Figure S1 in the Supplementary Material). In 2MBD
15, the total electron density of the C=C-C=C framework is increased by 0.13 e due to the ER character of the C5 methyl group.
ELF topological analysis of CHO 12 shows the presence of two disynaptic basins, V(C1,O2) and V’(C1,O2), integrating a total of 2.46 e, associated with a highly depopulated C1-O2 double bond, and two monosynaptic basins, V(O2) and V’(O2), integrating 5.08 e, corresponding to the non-bonding electron density of the O2 oxygen atom. This electron density distribution results from the noticeable polarization of the electron density of the C1-O2 bonding region toward the strongly electronegative O2 oxygen atom.
Coordination of the three selected LAs; BH3, BF3, and AlCl3, to the carbonyl O2 oxygen of CHO causes slight changes in the electron density of the C-O bonding region of the three LA complexes 14, 25, and 26. Thus, while the C-O bonding region experiences variations in Δe = −0.04 (25, BH3), 0.00 (14, BF3), and 0.08 (26, AlCl3), the non-bonding O2 electron density varies by Δe = 0.12 (25, BH3), 0.03 (14, BF3), and 0.27 (26, AlCl3). Note that while LA complexes 14 and 25 display two V(C1,O2) and V’(C1,O2) disynaptic basins, LA complex 26 shows only one V(C1,O2) disynaptic basin due to the strong polarization of the carbonyl group upon AlCl3 coordination.
The natural atomic charges of the most relevant centers are presented in
Figure 2. The natural population analysis [
37,
38] (NPA) of 2MBD
15 shows that the four carbons of the butadiene system are negatively charged as a consequence of the greater electronegativity of the carbon atom than the hydrogen atom. Thus, the C4 carbon that is bonded to three carbons has a negligible charge −0.04 e. The NPA of CHO
12 shows that, while the carbonyl C1 carbon is markedly positively charged at +0.29 e, the O2 oxygen atom is strongly negatively charged at −0.48 e. This charge distribution results from the strong electronegative character of the O2 oxygen atom.
Coordination of the LAs to the oxygen atom of CHO 12 increases the positive charge of the carbonyl C1 carbon by between +0.07 and +0.11 e and decreases the negative charge of the oxygen by 0.06 e in CHO:BH3 25, while increasing it by 0.02 and 0.10 e in CHO:BF3 14 and CHO:AlCl3 26, respectively. The AlCl3 LA causes the strongest changes in the electron density distribution of CHO 12. Consequently, coordination of these LAs to the carbonyl O1 oxygen of CHO 12 does not substantially modify the electronic structure of the carbonyl C=O group at GS.
2.1.2. Analysis of the Chemical Properties of the Reagents
A useful method for comprehending reactivity in polar reactions is the analysis of DFT-based reactivity indices [
34,
35]. Subsequently, the DFT-based reactivity indices at the GS of the reagents were examined to study the chemical reactivity of the CHO and the corresponding CHO:LA complexes. The B3LYP/6-31G(d) computational level was used, as it was employed to establish electrophilicity and nucleophilicity scales [
35].
Table 2 summarizes the computed B3LYP/6-31G(d) global reactivity indices, including electronic chemical potential
μ, chemical hardness
η, electrophilicity ω and nucleophilicity
N indices. The
ωB97X-D/6-311G(d,p) global reactivity indices are given in
Table S1 in the Supplementary Material.
The electronic chemical potential [
39] μ of 2MBD
15, μ = −3.34 eV, is above that of CHO
12, μ = −4.23 eV, and those of the CHO:LA complexes
14,
25 and
26, which range from −5.29 to −6.09 eV. This suggests that in these polar AE reactions, the GEDT [
16] will occur from 2MBD
15 to CHO
12 and CHO:LA complexes
14,
25, and
26, in reactions classified as forward electron density flux [
40] (FEDF).
Ethylene
2 has electrophilicity [
41] ω and nucleophilicity [
42]
N indices of 0.73 and 1.86 eV, respectively, being classified as a marginal electrophile and a marginal nucleophile. Consequently, ethylene
2 will have no tendency to participate in polar reactions. This behavior accounts for the very high activation energy found in the AE reaction of isobutene
10 and ethylene
2, 34.7 kcal·mol
−1 [
14].
The electrophilicity and nucleophilicity of propene 1 and 1-butene 18, ca. 0.60 and 2.32 eV, respectively, classify these species as marginal electrophiles and moderate nucleophiles. These low values indicate that these alkenes will effectively participate in P-AE reactions only with superelectrophilic heteroethylenes with ω > 4.0 eV. The inclusion of a second ER CH3 methyl group in propene 1 increases the nucleophilicity of isobutene 10 to 2.60 eV.
2MBD
15 has electrophilicity ω and nucleophilicity
N indices of 0.99 and 2.97 eV, respectively. It is classified as a moderate electrophile and is located at the borderline of strong nucleophiles. Consequently, 2MBD
15 will participate in polar reactions with strong electrophilic species [
23].
CHO
12 has electrophilicity ω and nucleophilicity
N indices of 1.45 and 1.81 eV, respectively, and is, therefore, classified as a moderate electrophile and a marginal nucleophile. Consequently, CHO
12 will have a poor tendency to participate in polar reactions as an electrophile [
33].
Coordination of the LAs to the carbonyl O2 oxygen atom of CHO 12 markedly increases the electrophilicity ω index of the corresponding CHO:LA complexes 14, 25 and 26, between Δω = 1.28 (25, BH3) and 2.90 (26, AlCl3) eV. Thus, while CHO:BH3 25, ω = 2.87 eV, and CHO:BF3 14, ω = 2.73 eV, are categorized as strong electrophiles, the CHO:AlCl3 26, ω = 4.35 eV, with ω > 4.00 eV, is categorized as a superelectrophile.
The inclusion of an ER CH3 methyl group in the carbonyl group of CHO 12 decreases the electrophilic character of MeCHO 19, ω = 1.12 eV, and that of the corresponding MeCHO:AlCl3 complex 21, ω = 3.62 eV. These behaviors permit an explanation of the lower reactivity of these species compared to CHO 12 and CHO:AlCl3 26 when participating in AE reactions (see below).
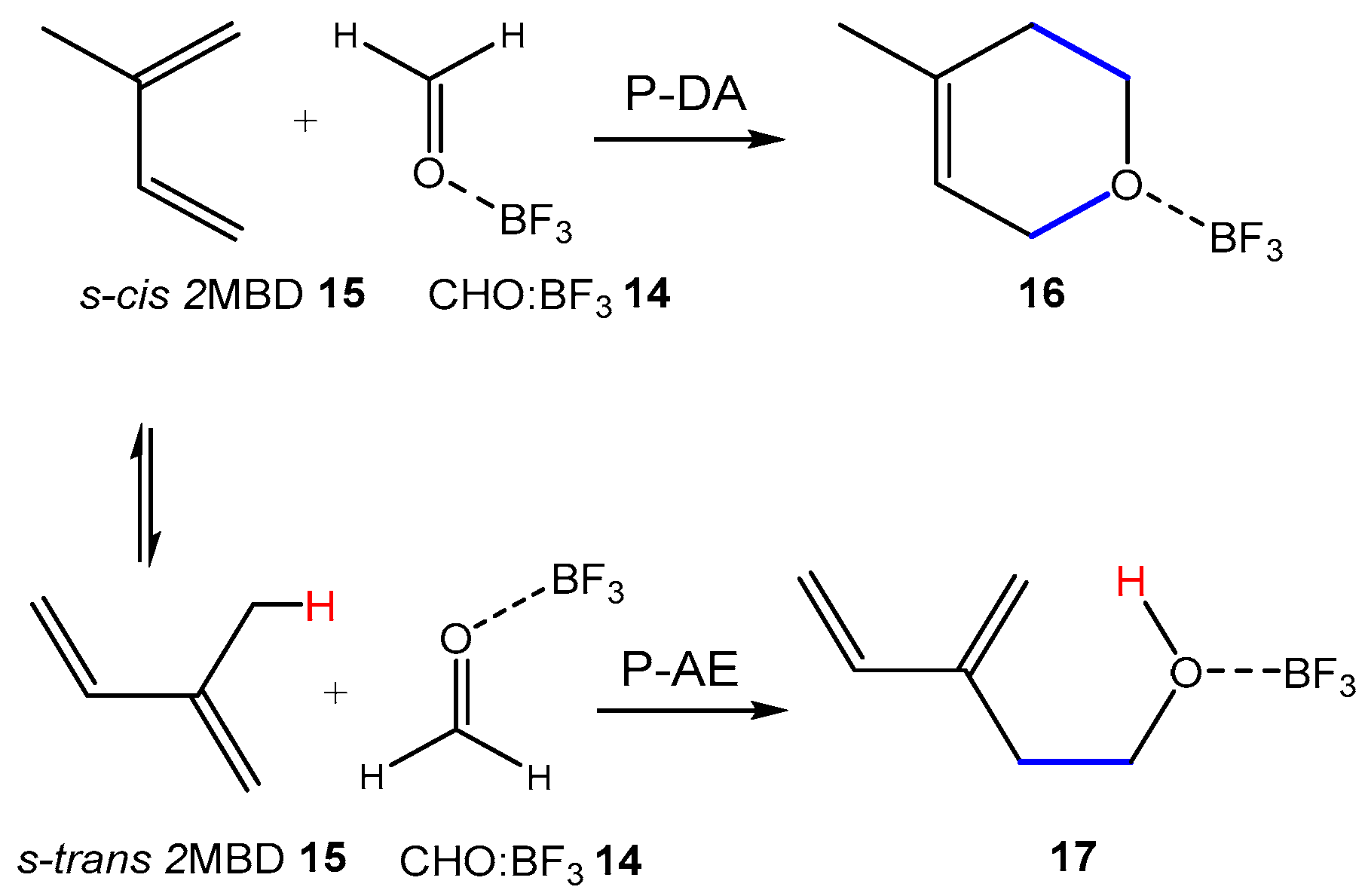
2.2. Study of the Non-Catalyzed AE Reaction of 2MBD 15 with CHO 12
Before studying the LA-catalyzed AE reactions of 2MBD
15 with CHO
12, the potential energy surface (PES) of the corresponding non-catalyzed AE reaction was investigated (see
Scheme 9). One molecular complex (MC),
MC-12, one TS,
TS-12, and the en-ol product
P-12 were located and characterized along the reaction path. Consequently, this AE reaction takes place through a one-step mechanism. The
ωB97X-D/6-311G(d,p) relative energies are given in
Scheme 9, and the total energies are provided in
Table S2 in the Supplementary Material.
Along the reaction path, an MC was found at the beginning of the reaction on the PES.
MC-12 is located 7.3 kcal·mol
−1 below the separated reagents.
TS-12 is located 21.3 kcal·mol
−1 above the separated reagent, the AE reaction being exothermic by 19.6 kcal·mol
−1. The activation energy for this AE reaction is 13.4 kcal·mol
−1 lower than that between isobutene
10 and ethylene
2, due to the low polar character of the latter [
14]. Despite this reduction, the activation energy remains high because of the moderate electrophilic nature of CHO
12 [
33]. Note that the AE reaction of 1-butene
18 with MeCHO
19, given in
Scheme 7 [
24], has an activation energy of 31.3 kcal·mol
−1 in dichloromethane as a consequence of the low nucleophilic character of
18 and the low electrophilic character of
19 (see
Table 2).
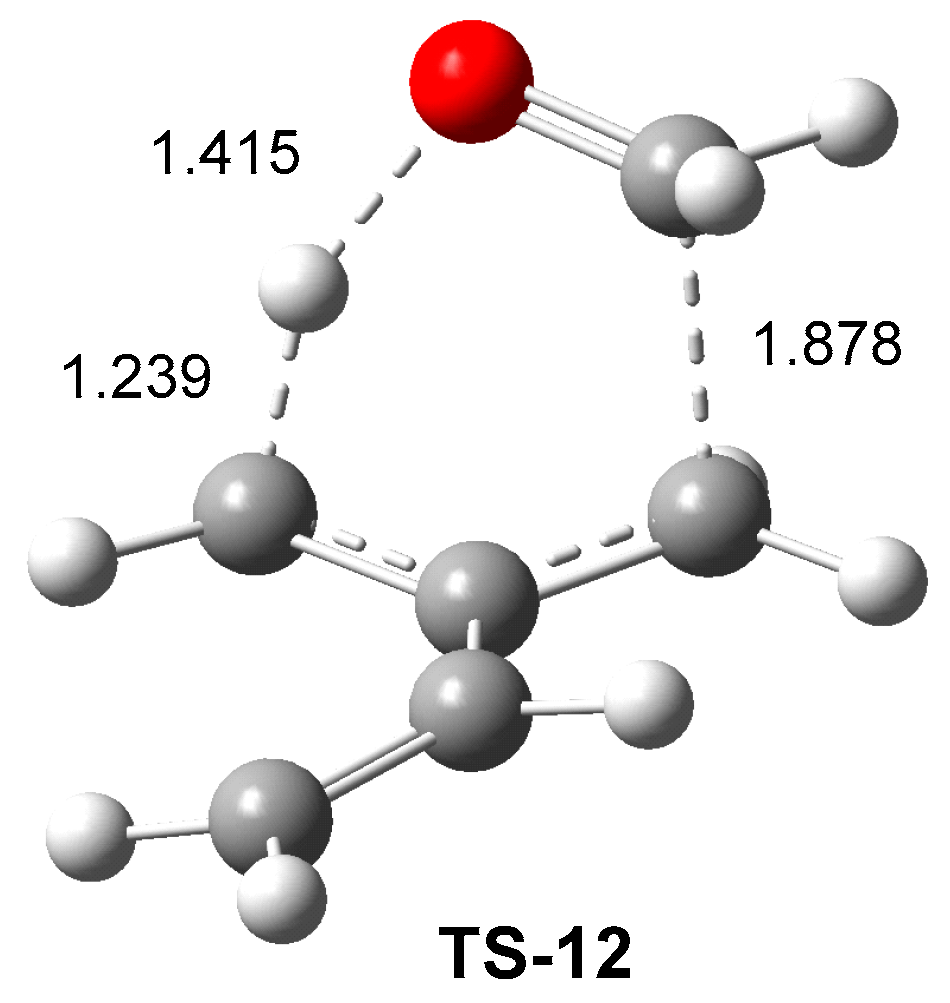
The optimized geometry of
TS-12 is shown in
Figure 3. The distance between the C3 carbon of 2MBD
15 and the carbonyl C1 carbon of CHO
12 is 1.878 Å, while the distances from the H6 hydrogen atom to the C5 carbon and O2 oxygen are 1.239 and 1.415 Å, respectively. These geometrical parameters indicate that the formation of the new C1-C3 single bond has already begun at this TS, while the hydrogen transfer process is still delayed (see below). Note that the C-C bond formation typically begins within the range of 1.90–2.0 Å [
16], while the O-H distance is shorter than the C-H one.
Analysis of the GEDT [
16] at
TS-12 involved in this AE reaction allows for quantifying its polar character [
14]. The sign of the GEDT computed at the TS unambiguously allows the classification of polar reactions as FEDF, with GEDT > +0.05 e, or REDF, with GEDT < −0.05 e [
40]. The sign of the GEDT values indicates that the propene framework, i.e., the hydrogen donor, is positively or negatively charged, meaning that propene acts as a nucleophile or electrophile at the corresponding TS. Non-polar reactions characterized by a negligible GEDT ≤ |0.05| e are classified as null electron density flux [
40] (NEDF). The GEDT at the 2MBD
15 framework of
TS-12 is 0.27 e. This high value indicates that the high polar character of this AE reaction is characterized by FEDF. Note that the GEDT at the AE reaction of isobutene
10 with ethylene
2 is 0.13 e [
14].
Both, the activation energy associated with the AE reaction of 2MBD
15 with CHO
12, and the GEDT taking place at
TS-12 classify the reaction as a P-AE reaction [
14].
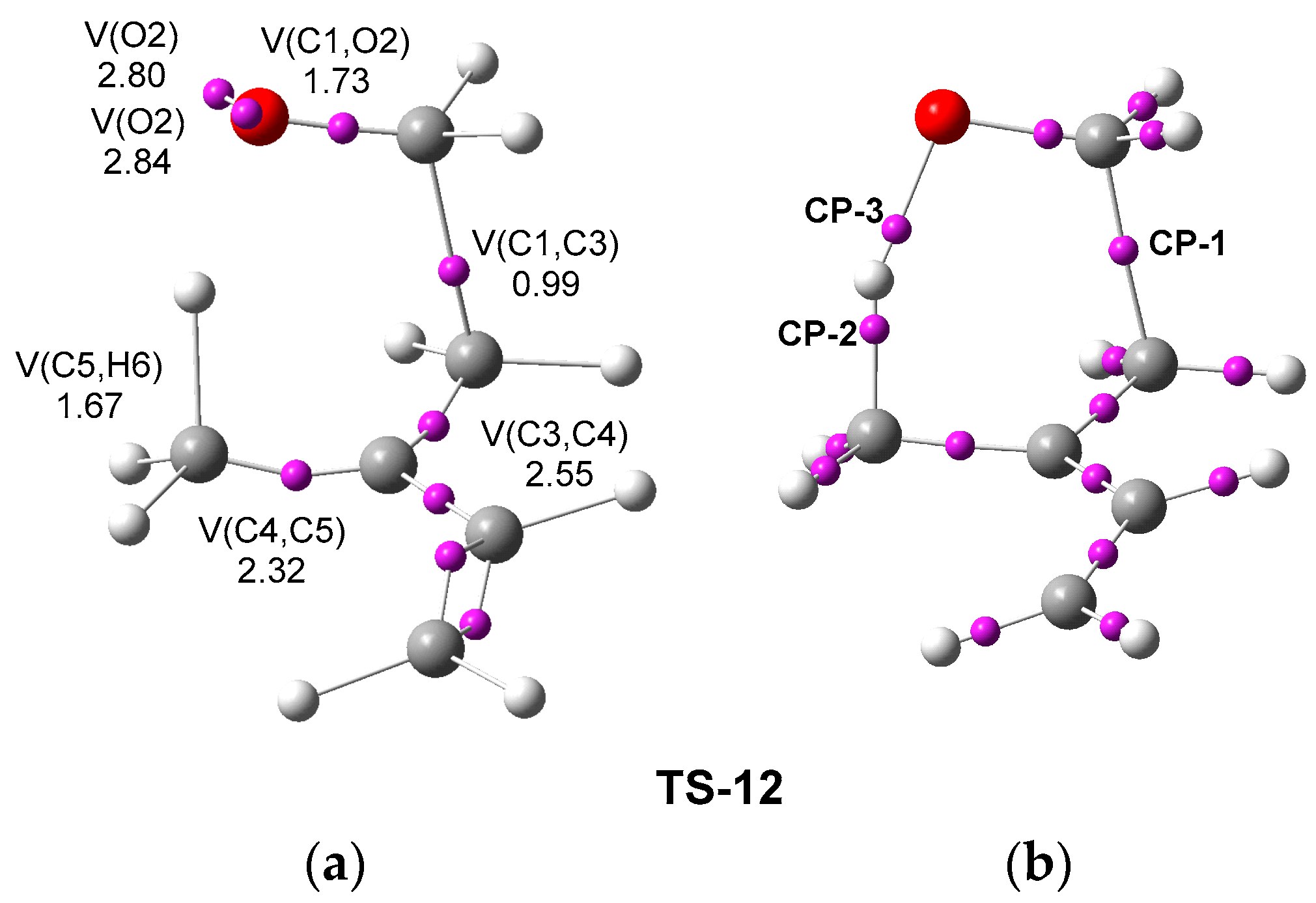
Next, an ELF [
17] and AIM [
28,
29] topological analysis of the electron density of
TS-12 was performed in order to understand the bonding changes taking place at this TS. The ELF basin attractor positions and the most relevant valence basin populations of
TS-12 are given in
Figure 4a, while the position of the AIM (3,−1) critical points (CPs) is given in
Figure 4b.
The ELF of
TS-12 shows the presence of a V(C1,C3) disynaptic basin integrating 0.99 e, indicating that the formation of the new C1-C3 single bond has already begun at this TS [
16]. On the other hand, the presence of the V(C5,H6) disynaptic basin integrating 1.67 e indicates that the C5-H6 single bond has not broken.
The AIM [
28,
29] topological analysis allows for comprehending the type of interatomic interactions at the C1-C3, C5-H6, and O2-H6 interacting regions of
TS-12. QTAIM parameters, namely the total electron density
ρ and Laplacian of electron density ∇
2ρ at the CPs associated with these interacting regions at the
TS-12 are listed in
Table 3, while the position of the CPs is shown in
Figure 4b.
The AIM of TS-12 shows the presence of a CP-1 in the C1-C3 interacting region with an electron density ρ = 0.2765 e, and a negative Laplacian, ∇2ρ, indicating a covalent interaction. On the other hand, CP-2, with ρ = 0.1880 e and a negative Laplacian, and CP-3, with a ρ = 0.1031 e, and a positive Laplacian, indicate that the C5-H6 single bond has not been broken, and the formation of the O2-H6 single bond has not yet begun.
Consequently, both ELF and AIM topological analyses of the electron density of TS-12 indicate that it corresponds to an asynchronous process, in which the formation of the C1-C3 single bond has already started, while the rupture of the C5-H6 single bond has not yet started.
2.3. Study of the LA-Catalyzed AE Reactions of 2MBD 15 with CHO 12
Next, the PESs of the LA-catalyzed AE reactions of 2MBD
15 with CHO
12 involving three common LAs of increasing acidic character: BH
3, BF
3, and AlCl
3, were studied. Analysis of the stationary points along the PES of these LA-catalyzed AE reactions indicated that, while the AE reaction involving the BH
3 takes place through a one-step mechanism, the other two AE reactions involving more acidic LAs take place through a two-step mechanism (see
Scheme 10). The relative energies of the TSs, intermediates, and products are given in
Table 4, while the total energies are provided in
Table S2 in the Supplementary Material.
Several conclusions can be obtained from the relative energies given in
Table 4: (i) along the three reaction paths, one MC is found in an early stage of the reaction. These MCs are found between 10.3 (
MC-25, BH
3) and 13.7 (
MC-26, AlCl
3) kcal·mol
−1 below the separated reagents; (ii) the three reactions are exothermic, with values ranging between 25.4 (
P-25, BH
3) and 30.4 (
P-26, AlCl
3) kcal·mol
−1; (iii) while
TS-25 is located 3.3 kcal·mol
−1 above the separated regents, the TSs associated with the first step of the stepwise mechanisms are located −4.3 (
TS1-14) and −10.3 (
TS1-26) kcal·mol
−1 below the separated reagents; (iv) if the formation of the MCs is considered, the activation energies become positive by 7.4 (
TS1-14) and 3.4 (
TS1-26) kcal·mol
−1; and finally, (v) the two intermediates
IN-X are stabilized by only 0.0 (
IN-14) and 0.5 (
IN-26) kcal·mol
−1 with respect the corresponding
TS1-X, while the activation energies associated with the proton transfer are less than 1.3 kcal·mol
−1. Thus, in the gas phase, the PESs around
IN-14 and
IN-26 are very flat.
The activation energy for the BH3-catalyzed AE reaction via TS-25 is 18.0 kcal·mol−1 lower than that for the non-catalyzed reaction via TS-12, indicating the strong effect of the BH3 catalyst. In the other two LA-catalyzed reactions, the relative energies of the TSs involved in the nucleophilic attack of 2MBD 15 on the carbonyl C1 of CHO:BF3 14 and CHO:AlCl3 26 are lowered by 7.6 (TS1-14) and 13.0 (TS1-26) kcal·mol−1 compared to TS-25. These highlights show that the nature of the LA catalyst influences the reaction rate of these AE reactions.
The geometries of the TSs and intermediates involved in the BH
3 and BF
3 LA-catalyzed AE reactions of 2MBD
15 with CHO
12 are shown in
Figure 5, while the main geometrical parameters of the TSs and intermediates involved in the three LA-catalyzed AE reactions are listed in
Table 5.
The C1-C3, C5-H6, and O2-H6 distances at the TSs associated with the nucleophilic attack of 2MBD 15 on the C1 carbon of CHO in the BH3 and BF3 LA-catalyzed AE reactions are found within the ranges 1.66–1.83 Å, 1.11–1.19 Å, and 1.58–2.10 Å, respectively. Consequently, the O2-H6 distance at TS-25, 1.579 Å, is less than the other parameters, which are very similar among the three TSs. The short C1-C3 distance at all three TSs, less than 1.827 Å, indicates that, similar to TS-12, the formation of the C1-C3 single bond has already begun. On the other hand, the C5-H6 and O2-H6 distances at TS1-14 and TS1-26 indicate that the transfer of hydrogen H6 to oxygen O2 has not yet begun.
The C1-C3, C5-H6, and O2-H6 distances at the two intermediates IN-14 and IN-25 are 1.68 and 1.74 Å, 1.12 Å for both, and 2.00 and 2.01 Å, respectively. These distances are close to those at TS1-14 and TS1-26 as a consequence of the highly advanced nature of the TSs and the very flat PES in these two-step reactions.
The C1-C3, C5-H6, and O2-H6 distances at TS2-14 and TS2-26 are 1.63 and 1.61 Å, 1.20 and 1.23 Å, and 1.57 and 1.52 Å, respectively. The shorter C5-H6 distances, approximately 1.21 Å, compared to the O2-H6 distance, roughly 1.54 Å, suggest that the proton transfer processes occur much later at the two TSs. The C5-H6 and O2-H6 distances at the two TSs are closer to those in TS-12.
The C5-H6 distances at TS1-14 and TS1-26, and IN-14 and IN-26, lesser than 1.12 Å, are only slightly longer than the other two allylic C5-H6 bonds, 1.09 Å. These observations, together with the similarly large O2-H6 distances at both TSs and intermediates, between 2.00 and 2.10 Å, suggest that the O2-H6 interactions in four species should be considered as a strong intramolecular O2…H6 hydrogen bond interaction rather than a hydrogen transfer process, as at TS-12.
Finally, a comparison of the geometrical parameters of
TS-25 and
TS2-14, as shown in
Figure 5, reveals a strong similarity between them, suggesting that the former is mainly associated with the hydrogen transfer process.
The GEDTs at
TS-25,
TS1-14, and
TS1-26 are 0.41, 0.48, and 0.63 e, respectively (see
Table 5). These high values indicate the strong polar character of these LA-catalyzed AE reactions characterized by FEDF. These values are markedly higher than those of the non-catalyzed AE reaction via
TS-12, 0.27 e, in agreement with the greater electrophilic character of the CHO:LA complexes (see
Table 2). The maximum GEDT value is obtained at intermediates
IN-14 and
IN-26, as GEDT depends on the electrophilic/nucleophilic character of the two interacting species, and their separation, i.e., the shorter the separation, the higher the GEDT.
The low activation energies associated with the LA-catalyzed AE reactions of CHO
12, which are lower than 20 kcal·mol
−1, and the high GEDT values (>0.40 e) found at the TSs, allow these reactions to be classified as H-AE reactions, which can take place at room temperature [
14].
The activation energy of the AE reaction of 1-butene
18 with MeCHO:AlCl
3 21 is 6.1 kcal·mol
−1 [
24]. This activation energy is 16.2 kcal·mol
−1 higher than that the relative energy for the AE reaction of 2MBD
15 with CHO:AlCl
3 26, −10.1 kcal·mol
−1 (see
Table S2 in Supplementary Material). This energy difference is a consequence of the moderate nucleophilic character of 1-butene
18, and the lower electrophilic character of MeCHO:AlCl
3 21 than CHO:AlCl
3 26 (see
Table 2). This finding reveals the importance of analyzing the DFT-based reactivity indices of the reagents to predict the polar character of an AE reaction, and consequently its feasibility (see
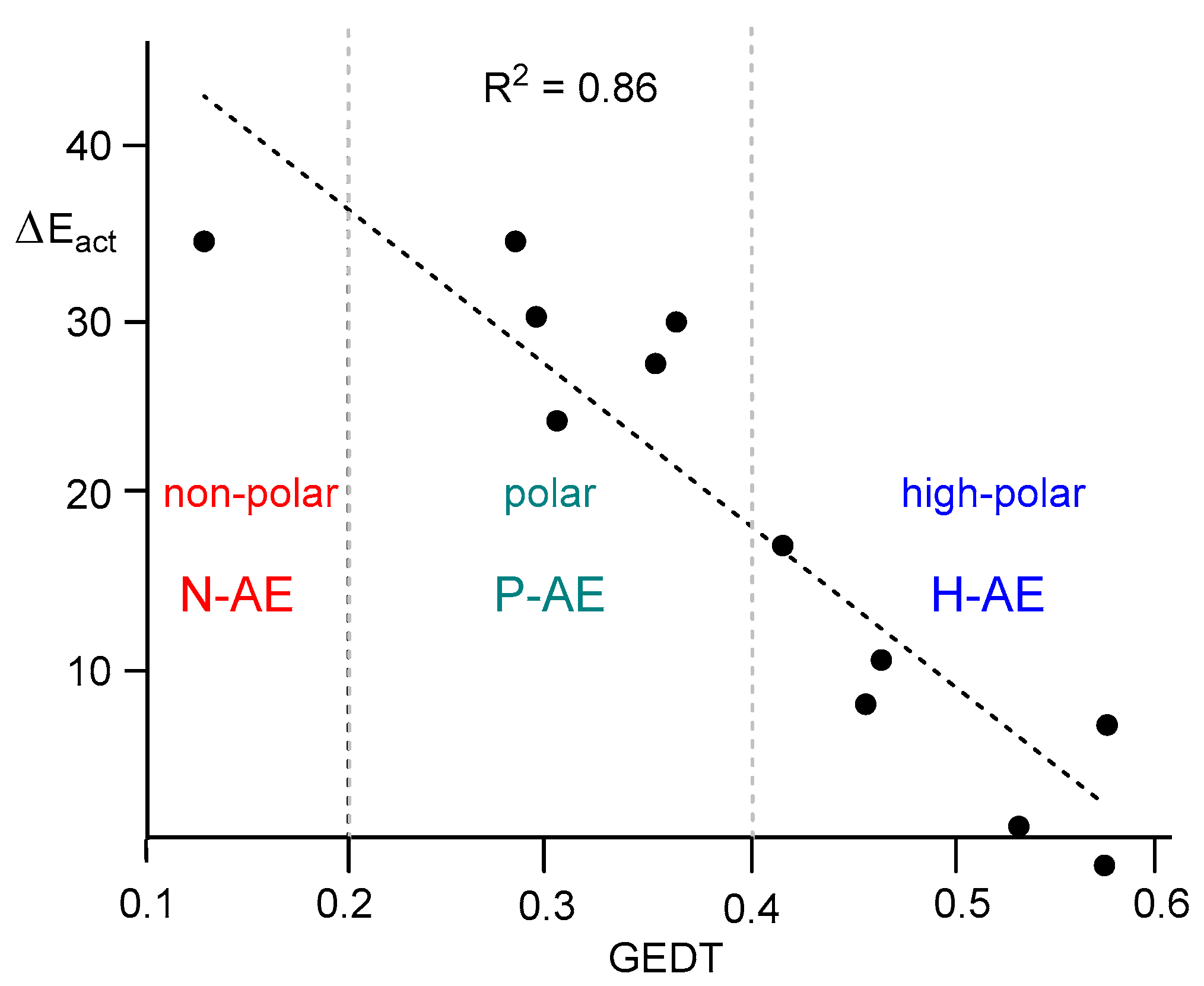
Figure 1).
Next, an ELF topological analysis of the TSs and intermediates involved in the BH
3 and BF
3 LA-catalyzed AE reactions of 2MBD
15 with CHO
12 was performed. The ELF basin attractor positions and the most relevant valence basin populations of
TS-25,
TS1-14,
IN-14, and
TS2-14 are shown in
Figure 6.
The four species show the same number and types of valence basins as those at
TS-12, but they present different populations. ELF of
TS-12 and
TS-25 shows a great similarity (see
Figure 4 and
Figure 6).
TS-25 shows a new V(C1,C3) disynaptic basin integrating 1.48 e. This population is higher than that at
TS-12, indicating the more advanced character of the former. The presence of this disynaptic basin shows that the formation of the new C1-C3 single bond has already begun. On the other hand, the presence of the V(C5,H6) disynaptic basin integrating 1.68 e indicates that the C5-H6 single bond has not broken.
The ELF topological analysis of
TS1-14,
IN-14, and
TS2-14 shows a great similarity between these species. Only some changes in the population are observed because of the evolution of the bonding changes along the reaction path. These behaviors agree with the closer relative energies and geometrical parameters of these species, in agreement with a very flat PES. Thus, the population of the V(C1,C3) disynaptic basins at the three species is 0.97, 1.19, and 1.55 e, respectively, showing the advancement in the formation of the C1-C3 single bond. Note that the population of the V(C1,C3) disynaptic basins at
P-14 is 1.99 e (see
Figure S2 in the Supplementary Material). Meanwhile, although the population of the V(C5,H6) disynaptic basin remains around 1.9 e at
TS1-26 and
IN-26, which is closer to the other two V(C5,H) disynaptic basins, at 2.0 e, it slightly decreases to 1.63 e at
TS2-26. In the three species, the population of the V(O2) monosynaptic basin remains at 4.05 e. These behaviors, along with the analysis of the geometric parameters, suggest that both
TS1-14 and
TS1-26 are solely related to the nucleophilic attack of 2MBD
15 on the C1 carbon of CHO:LA complexes
14 and
26. This attack is favored by the strong hydrogen bond formation between the carbonyl O2 oxygen and the H6 hydrogen present on the C5 methyl group, which will be transferred after passing
TS2-26.
Finally, the populations of the selected disynaptic basins of TS-25 are very close to those of TS2-14, indicating the similarity between the electronic structure of the two TSs. These results indicate that the more favorable TS1-14 is more delayed than TS-25.
2.4. Study of the Effects of the LAs on the Kinetics of the LA-Catalyzed AE Reactions
Next, the effects of the LAs on the kinetics of the LA-catalyzed AE reactions of 2MBD
15 with the CHO:LA complexes
14,
25, and
26 were examined through a comparative analysis of the relative reaction rate constants (RRRCs), k
r, computed using the Eyring-Polanyi equation [
43]. To this end, all gas phase stationary points involved in the AE reactions of 2MBD
15 with CHO
12, and with the CHO:LA complexes
14,
25, and
26 were first optimized in dioxane. Subsequently, the thermodynamic data were computed by frequency calculations performed in this solvent at 25 °C. The AE reaction of 2MBD
15 with the CHO
12 was used as the reference reaction.
Solvent effects stabilize the TSs and intermediates by 0.4 to 4.1 kcal·mol
−1 due to their polar character but destabilize MCs and products by 0.9–1.7 kcal·mol
−1. Despite these variations, solvent effects do not substantially modify the gas phase PESs. In addition, solvent effects do not produce remarkable changes in the gas-phase optimized geometries (see
Table S4 in the Supplementary Material).
Some valuable conclusions can be drawn from the thermodynamic parameters provided in
Table 6: (i) including thermal correction to the electronic energies only slightly modifies the relative enthalpies of TSs by between 0.0 and 2.2 kcal·mol
−1, and those of the intermediates by 2.8 kcal·mol
−1. As a result, the relative enthalpies follow the same trend as the relative electronic energies; (ii) including the entropies and temperature in the enthalpies increases the relative Gibbs free energies between 10.0 and 16.7 kcal·mol
−1, due to the unfavorable activation entropies associated with these bimolecular processes. (iii) although the formation of the MCs is exothermic ranging from −4.6 to −9.8 kcal·mol
−1, they are endergonic by between 1.0 and 3.4 kcal·mol
−1; (iv) the relative Gibbs free energies of the TSs involved in nucleophilic attack of 2MBD
15 on the C1 carbon of CHO in these LA-catalyzed AE reactions are endergonic by between 4.5 and 15.7 kcal·mol
−1, showing the three LA-catalyzed AE reactions positive activation Gibbs free energies; and finally, (v) for the AE reaction of 2MBD
15 with CHO:BF
3,
25 the second step becomes the rate-determining step (RDS) of this stepwise reaction, which is
TS2-14, 0.8 kcal·mol
−1 above
TS1-14 in Gibbs free energy.
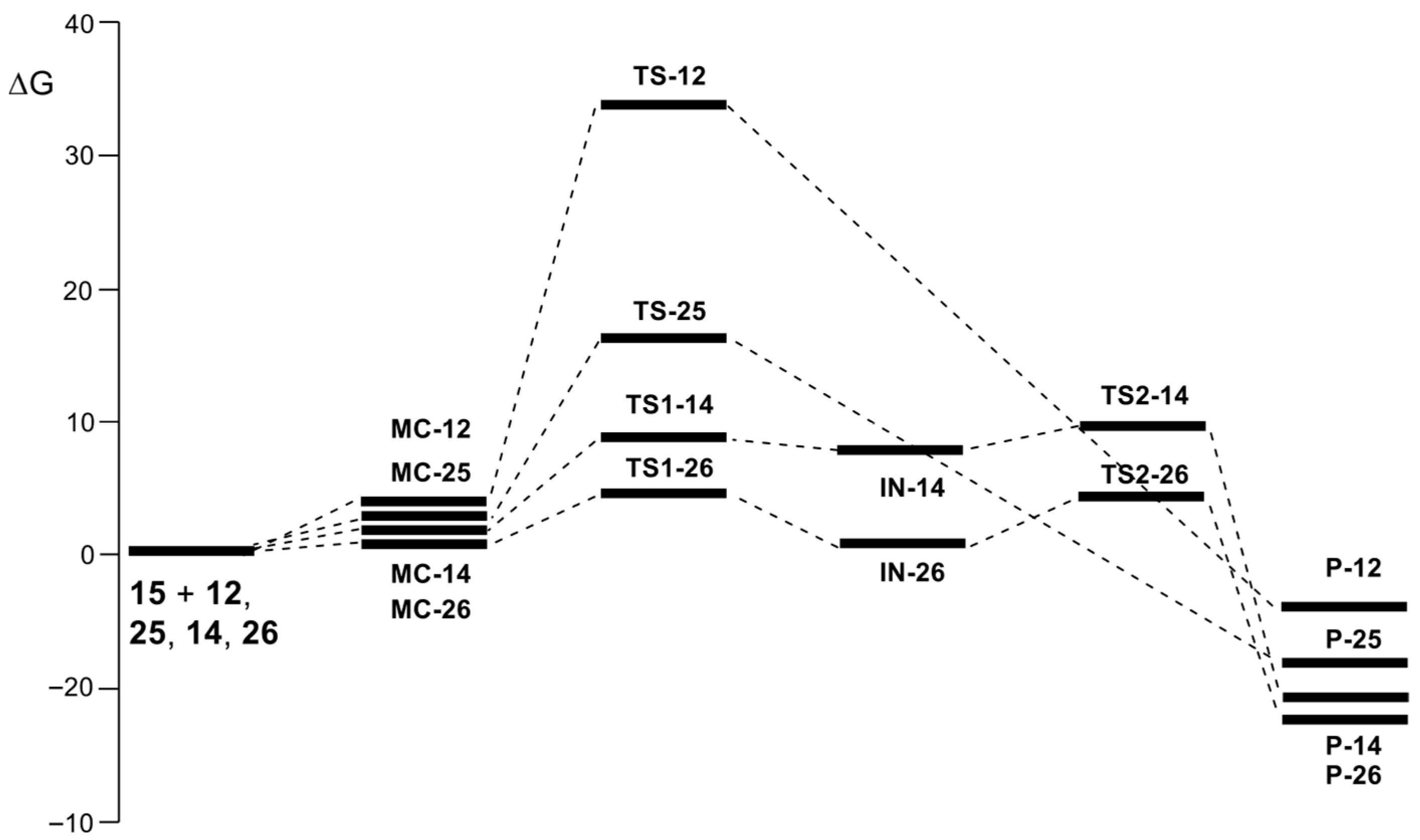
The Gibbs free energy profiles for the AE reactions of 2MBD
15 with CHO
12 and with the CHO:LA complexes
14,
25, and
26 are shown in
Figure 7. As observed, LAs have a significant impact on both the kinetic and thermodynamic aspects of these AE reactions. The activation Gibbs free energies of the LA-catalyzed AE reactions are significantly lowered by 18.3 to 29.5 kcal·mol
−1 compared to the non-catalyzed process, while the exergonic nature of these AE reactions is enhanced by 4.8 to 9.2 kcal·mol
−1. As can be seen, the formation of the four MC is endergonic, while all activation Gibbs free energies are positive. The most noticeable change in the analysis of the Gibbs free energy profiles is that for the LA-catalyzed AE reaction between 2MBD
15 and CHO:BF
3 14, the second step involving proton transfer becomes the RDS of this stepwise AE reaction (see
Figure 7).
Finally, the RRRCs, k
r, constants for the LA-catalyzed AE reactions of 2MBD
1 with the CHO:LA complexes
14,
25, and
26, relative to the non-catalyzed AE reaction of 2MBD
15 with CHO
12, were computed using the Eyring-Polanyi equation [
43]. The corresponding values are given in
Table 7. As can be seen, the coordination of an LA to the oxygen atom of CHO
12 causes a significant acceleration of the corresponding AE reactions, resulting in a k
r between 2.5 × 10
13 and 4.5 × 10
21 times higher compared to the non-catalyzed process. These high k
r values indicate the high efficiency of the LAs as catalysts of AE reactions involving carbonyl compounds. Note that the rate constants from the Eyring–Polanyi equation do not include possible compensation effects [
44]; thus, the acceleration factors in
Table 7 are qualitative, illustrating the enhancement of the reaction rate with the presence of the LAs.
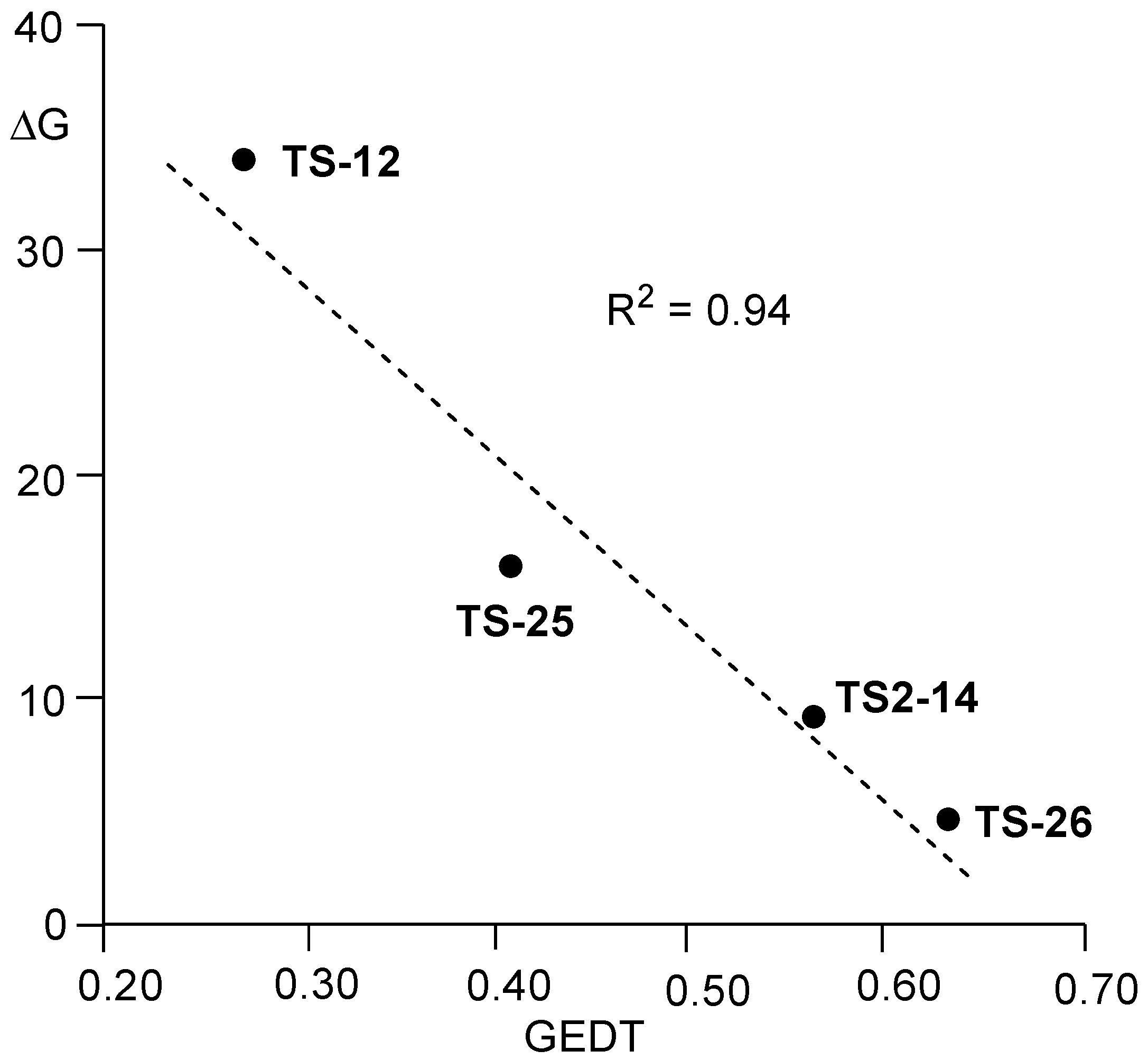
As shown in
Figure 8, a strong linear correlation between the activation Gibbs free energies associated with the RDS of the non-catalyzed and LA-catalyzed AE reactions and GEDT determined at the corresponding TS is found, R
2 = 0.94. Consequently, as in the LA-catalyzed DA reactions [
27], a strong correlation between the polar character of the reaction measured by the GEDT computed at the corresponding TSs, and the feasibility of the AE reaction can be established (see below).
2.5. RIAE Analysis of the Electronic Effects of LAs on the AE Reactions of 2MBD 15 with CHO 12
To gain deeper insight into the electronic factors governing the activation barriers, an EDA based on the RIAE approach [
27] was performed to evaluate the electronic effects of the LAs on the activation energies of the AE reactions of 2MBD
15 with CHO
12 and with the CHO:LA complexes
25,
14, and
26. This analysis was applied to the regents and TSs involved in the nucleophilic/electrophilic interaction step of these AE reactions, providing insight into the energy costs associated with electron density reorganization on going from the reagents to the TSs, on which MEDT is founded [
20]. The theoretical background of the RIAE method is outlined in the
Supplementary Material. As a reference, the AE reaction of 2MBD
15 with ethylene
2 via
TS-2 was also considered. The RIAE study was carried out at the M06-2X/6-311G(d,p) computational level in the gas phase, as required by the IQA [
26] calculations. The gas-phase ξ
intra-atomic, ξ
interatomic and ξ
total energies for the 2MBD and the CHO frameworks of the four TSs are presented in
Table 8. The sum of the ξ
values of both frameworks, denoted as ξ
, represents the RIAE relative energy.
RIAE analysis of TS-2, corresponding to the AE reaction of 2MBD 15 with ethylene 2, reveals that the main contributor to the RIAE relative energy is the destabilization of the 2MBD framework, ξ = 27.30 kcal·mol−1. Interestingly, the ethylene framework is only destabilized by 1.88 kcal·mol−1. Analysis of the unfavorable contribution to the ξ total energies shows that the unfavorable intra-atomic energies of the 2MBD framework, ξ 44.24 kcal·mol−1, are the primary factors responsible for the height of the high RIAE relative energy, 29.10 kcal·mol−1.
RIAE analysis of TS-12, corresponding to the non-catalyzed AE reaction of 2MBD 15 with CHO 12, shows a reduction in the RIAE relative energy by 8.40 kcal·mol−1 with respect to that of the AE reaction of 2MBD 15 with ethylene 2. Even though the 2MBD framework is more strongly destabilized, ξ = 48.88 kcal·mol−1, the CHO framework is stabilized by −28.18 kcal·mol−1. Note that while ethylene 2 is a marginal electrophile, CHO 12 is a moderate electrophile, thus favoring the GEDT from 2MBD to the CHO framework. Analysis of the factors responsible for the stabilization of ξ indicates that the strong intra-atomic stabilization of the CHO framework, ξ = −109.50 kcal·mol−1, is the main factor responsible for the reduction in the RIAE relative energy.
RIAE analysis of the LA-catalyzed AE reactions helps understand the electronic effects of the LAs in lowering the activation energies of these LA-catalyzed AE reactions. The RIAE relative energies of the three LA-catalyzed AE reactions decrease as the acidic character of the LA increases, with values of 1.54 (TS-25), −5.02 (TS1-14), and −10.05 (TS1-26) kcal·mol−1. In fact, the LA-catalyzed AE reactions involving BF3 and AlCl3 show negative RIAE relative energies, meaning that the TS associated with the first step is electronically more stabilized than the separated reactants.
Analysis of the factors responsible for this trend shows that while the nucleophilic 2MBD framework is destabilized by ξ = 44.94 (TS-25), 54.53 (TS1-14) and 71.04 (TS1-26) kcal·mol−1, the electrophilic CHO:LA framework is strongly stabilized by ξ = −43.40 (TS-25), −59.56 (TS1-14) and −81.09 (TS1-26) kcal·mol−1, with the increase in the acidic character of the LA. A detailed analysis of the factor responsible for these stabilizations reveals that, similar to the non-catalyzed process, the intra-atomic stabilization of the electrophilic CHO:LA framework, ξ = −76.27 (TS-25), −83.58 (TS1-14) and −121.99 (TS1-26) kcal·mol−1, is responsible for the decrease in the RIAE relative energies. A detailed analysis of the factor responsible for the increased stabilization of the CHO:AL framework indicates that the strong stabilization of the carbonyl C1 carbon, ξ = −68.12 (TS-25), −86.86 (TS1-14) and −98.98 (TS1-26) kcal·mol−1, is the main factor responsible.
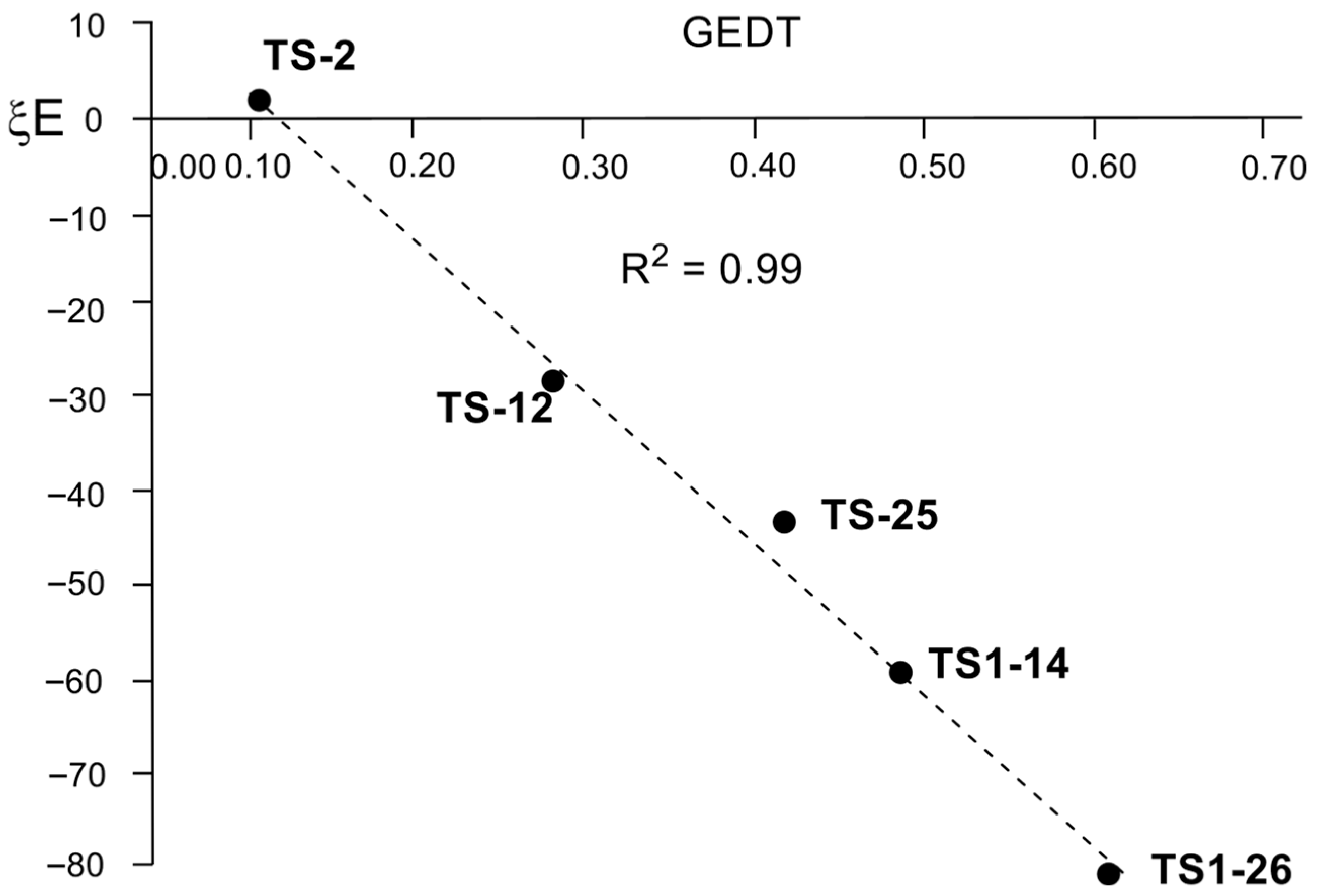
As shown in
Figure 9, a strong linear correlation between the ξ
total energies of the CHO framework and GEDT at the corresponding TSs are found, R
2 = 0.99. As in polar DA reactions [
27,
45], the present RIAE analysis demonstrates that the GEDT occurring at the TSs of these polar AE reactions, primarily at the carbonyl C1 atom, is the main factor responsible for lowering the RIAE relative energies in the LA-catalyzed AE reactions. In other words, increasing the acidic character of the LA enhances the electrophilic nature of the CHO:LA complex (see
Table 2), which increases the GEDT and thereby reduces the activation energies [
27]. Thus, similar to the LA-catalyzed DA reaction, coordination of the LAs to the oxygen atom of carbonyl compounds increases the electrophilicity of these species, promoting the GEDT at the TS, and consequently, lowering the activation barrier energies.
Finally,
Figure 10 shows a graphical representation of the ξ
energies of the 2MBD and CHO frameworks at the TSs, shown in red and blue, respectively. The ξ
energies, shown in black, represent the RIAE relative energies of these AE reactions.
Along this series of AE reactions, the ξ
energies associated with the electrophilic CHO framework become more negative, i.e., more favorable, while those associated with the nucleophilic 2MBD framework, ξ
, become more positive, i.e., more unfavorable. With the inclusion of the LA catalyst, the stabilization of the CHO:LA framework outweighs the destabilization of the 2MBD one, consequently decreasing the ξ
energies associated with these LA-catalyzed AE reactions, and even rendering these RIAE relative energies negative with the use of strongly acidic LAs (see
Figure 10). These behaviors result from the GEDT that takes place at the TSs (see
Figure 9); while the GEDT destabilizes the nucleophilic 2MBD framework due to the electron density loss, it stabilizes the electrophilic CHO:LA through electron density gain [
27,
45]. This behavior aligns with Parr’s electrophilicity ω index definition [
33,
41].
Many studies of P-DA reactions, including LA-catalyzed DA reactions, have shown that increasing the polar character of the reaction induces greater asynchronicity at the TSs, thereby increasing the distance between the two non-interacting centers [
15]. This behavior, caused by an increase in the nucleophilic/electrophilic two-center interactions at the polar TSs, could contribute to the apparent reduction in proposed ‘Pauli repulsions’ within the framework of the ASM [
46].