Oxidative and Glycation Stress Biomarkers: Advances in Detection Technologies and Point-of-Care Clinical Applications
Abstract
1. Introduction
2. Oxidative Stress–Related Biomarkers
2.1. ROS and RNS
2.2. Biomarkers of Oxidative Damage
2.3. Oxidized Albumin
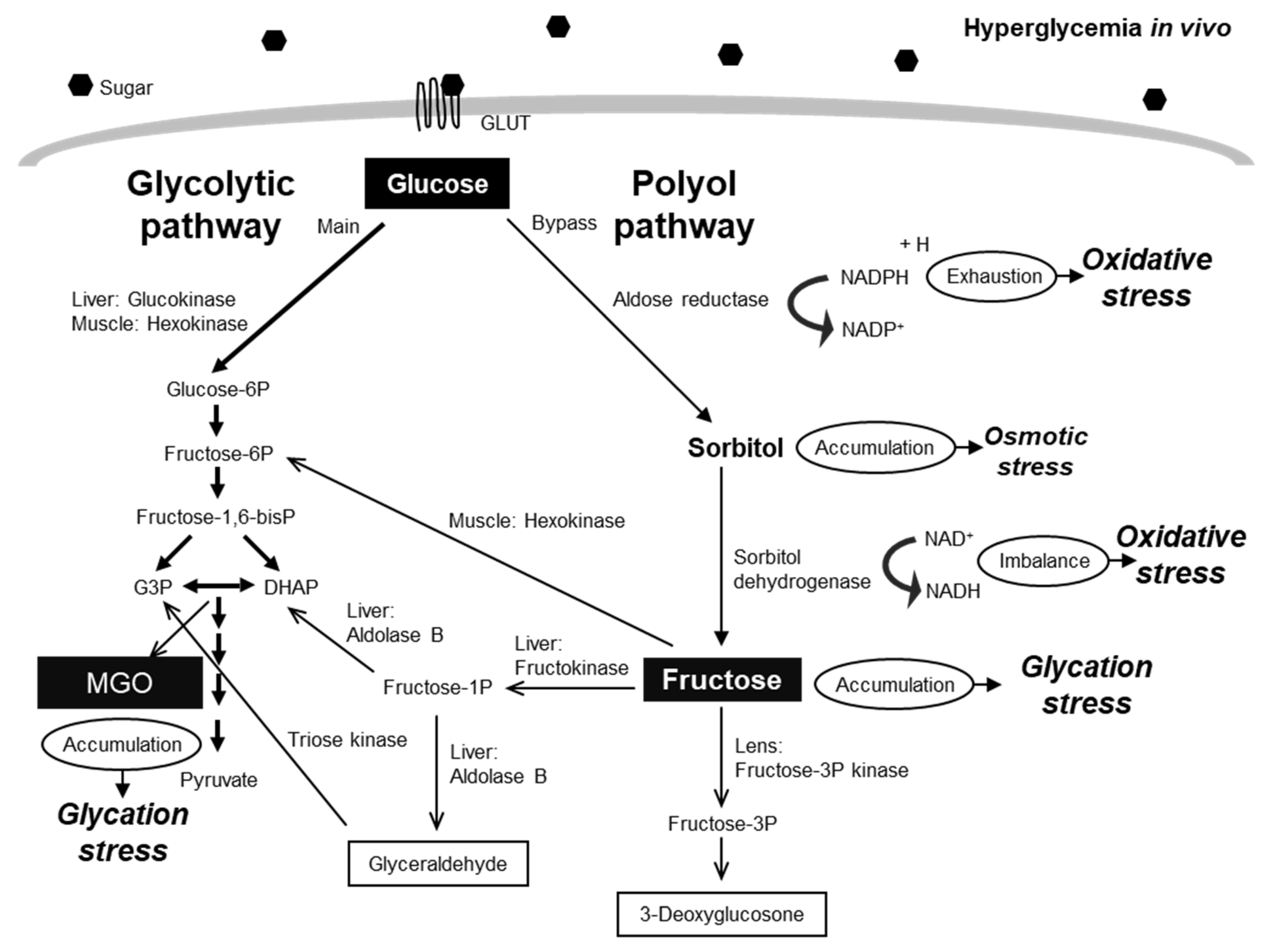
3. Glycation Stress-Related Biomarkers
3.1. Glucose and Glucose Metabolites
3.2. Glycated Proteins
3.3. AGEs
4. Clinical Applications and POC Testing
5. Technical Challenges and Future Perspectives
5.1. Limitations in Sensitivity and Specificity
5.2. Lack of Standardization
5.3. Non-Invasiveness and Real-Time Monitoring
5.4. Lack of Integrated Assessment
5.5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Takahashi, M.; Kizuka, Y.; Kitazume, S.; Shuvaev, V.V.; Ookawara, T.; Furuta, A. The Maillard Reaction in Aging and Diabetes: Chemistry, Biology, and Implications for Therapy. Clin. Chem. 2005, 51, 8–20. [Google Scholar]
- Thornalley, P.J. Advanced Glycation End Products in Renal Failure. J. Ren. Nutr. 2006, 16, 178–184. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced Glycation End-products: A Review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE, The Receptor for Advanced Glycation End Products. J. Mol. Med. 2005, 83, 876–886. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of Advanced Glycation End Products in Cellular Signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Kalapos, M.P. Methylglyoxal in Living Organisms: Chemistry, Biochemistry, Toxicology and Biological Implications. Toxicol. Lett. 1999, 110, 145–175. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, Glyoxalase 1 and the Dicarbonyl Proteome. Amino Acids 2012, 42, 1133–1142. [Google Scholar] [CrossRef]
- Baynes, J.W.; Thorpe, S.R. Role of Oxidative Stress in Diabetic Complications: A New Perspective on an Old Paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. The RAGE Axis and Endothelial Dysfunction: Maladaptive Roles in the Diabetic Vasculature and Beyond. Trends Cardiovasc. Med. 2005, 15, 237–243. [Google Scholar] [CrossRef]
- Wautier, J.L.; Guillausseau, P.J. Advanced Glycation End Products, their Receptors and Diabetic Angiopathy. Diabetes Metab. 2001, 27, 535–542. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Nagai, R. Insights from the Fructose-derived Product Glucoselysine: Revisiting the Polyol Pathway in Diabetic Complications. J. Diabetes Investig. 2025, 16, 569–577. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Pan, W.; Li, N.; Tang, B. Recent Progress of Oxidative Stress Associated Biomarker Detection. Chem. Commun. 2023, 59, 11345–11360. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Z.; Zhou, J.; Zhu, M.; Liu, J.; James, T.D. Recent Progress in the Development of Fluorescent Probes for Pathological Oxidative Stress. Chem. Soc. Rev. 2023, 52, 3873–3926. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Oxidized Albumin as a Potential Biomarker for Chronic Kidney Disease Progression. Biol. Pharm. Bull. 2022, 45, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, R.; Shi, Z.; Sui, Y.; Cheng, J.; Suda, M.; Niimi, M.; Gao, K.; Fan, J.; Yao, J. Oxidized Albumin Induces Renal Tubular Cell Death and Promotes the Progression of Renal Diseases Through Ferroptosis. Int. J. Mol. Sci. 2025, 26, 5924. [Google Scholar] [CrossRef]
- Nugnes, M.; Baldassarre, M.; Ribichini, D.; Tedesco, D.; Capelli, I.; Vetrano, D.; Marchignoli, F.; Brodosi, L.; Pompili, E.; Petroni, M.L.; et al. Association Between Albumin Alterations and Renal Function in Patients with Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 3168. [Google Scholar] [CrossRef]
- Tsinari, A.; Roumeliotis, S.; Neofytou, I.E.; Varouktsi, G.; Veljkovic, A.; Stamou, A.; Leivaditis, K.; Liakopoulos, V. The Clinical Utility and Plausibility of Oxidative and Antioxidant Variables in Chronic and End-Stage Kidney Disease: A Review of the Literature. Int. J. Mol. Sci. 2025, 26, 3376. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.A.; Neubaue, K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring Reactive Species and Oxidative Damage in Vivo and in Cell Culture: How Should You Do It and What Do the Results Mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence Probes Used for Detection of Reactive Oxygen Species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Ghaedamini, H.; Kim, D.S. Recent Advances in Electrochemical Detection of Reactive Oxygen Species: A Review. Analyst 2025, 150, 1490–1517. [Google Scholar] [CrossRef]
- Zhao, S.; Zang, G.; Zhang, Y.; Liu, H.; Wang, N.; Cai, S.; Durkan, C.; Xie, G.; Wang, G. Recent Advances of Electrochemical Sensors for Detecting and Monitoring ROS/RNS. Biosens. Bioelectron. 2021, 179, 113052. [Google Scholar] [CrossRef]
- Deshpande, A.S.; Muraoka, W.; Andreescu, S. Electrochemical Sensors for Oxidative Stress Monitoring. Curr. Opin. Electrochem. 2021, 29, 100809. [Google Scholar] [CrossRef]
- Giaretta, J.E.; Duan, H.; Oveissi, F.; Farajikhah, S.; Dehghani, F.; Naficy, S. Flexible Sensors for Hydrogen Peroxide Detection: A Critical Review. ACS Appl. Mater. Interfaces 2022, 14, 20491–20505. [Google Scholar] [CrossRef]
- Malinski, T.; Taha, Z. Nitric Oxide Release from a Single Cell Measured in Situ by a Porphyrinic-based Microsensor. Nature 1992, 358, 676–678. [Google Scholar] [CrossRef]
- Shibuki, K. An Electrochemical Microprobe for Detecting Nitric Oxide Release in Brain Tissue. Neurosci. Res. 1990, 9, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.C.; Mondal, S.P. Paper-based Electrochemical Sensor Integrated with Gold Nanoparticle-Decorated Carbon Cloth as a Working Electrode for Nitric Oxide Detection in Artificial Tears. ACS Appl Bio Mater. 2024, 7, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Faiza Bashir, F.; Kovács, S.; Ábrahám, Á.; Nagy, K.; Ayaydin, F.; Valkony-Kelemen, I.; Ferenc, G.; Galajda, P.; Tóth, S.Z.; Sass, L.; et al. Viable Protoplast Formation of the Coral Endosymbiont Alga Symbiodinium Spp. in a Microfluidics Platform. Lab Chip 2022, 22, 2986–2999. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Milne, G.L.; Dai, Q.; Roberts, L.J. The Isoprostanes—25 Years Later. Biochim. Biophys. Acta 2015, 1851, 433–445. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, Mutation, and Disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, L.; Wu, D.; Tu, S.; Chen, C.; Guo, H.; Xu, Y. Highly Sensitive Sandwich-type Immunosensor with Enhanced Electrocatalytic Durian-shaped MoS2/AuPtPd Nanoparticles for Human Growth Differentiation Factor-15 Detection. Anal. Chim. Acta 2022, 1223, 340194. [Google Scholar] [CrossRef]
- Cao, Q.; Ding, S.; Zheng, X.; Li, H.; Yu, L.; Zhu, Y.; Jiang, D.; Ruan, S. Luteolin Induces GPX4-dependent Ferroptosis and Enhances Immune Activation in Colon Cancer. Phytomedicine 2025, 146, 157117. [Google Scholar] [CrossRef]
- Jia, L.P.; Liu, J.F.; Wang, H.S. Electrochemical Performance and Detection of 8-Hydroxy-2′-deoxyguanosine at Single-stranded DNA Functionalized Graphene Modified Glassy Carbon Electrode. Biosens. Bioelectron. 2015, 67, 139–145. [Google Scholar] [CrossRef]
- Kusuma, S.A.F.; Harmonis, J.H.; Pratiwi, R.; Hasanah, A.N. Gold Nanoparticle-Based Colorimetric Sensors: Properties and Application in Detection of Heavy Metals and Biological Molecules. Sensors 2023, 23, 8172. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Stauber, R.E. Physiological and Pathological Changes in The Redox State of Human Serum Albumin. Redox Rep. 2007, 12, 236–242. [Google Scholar]
- Anraku, M.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Redox Properties of Serum Albumin. Biochim. Biophys. Acta 2013, 1830, 5465–5472. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Sohal, R.S. Analysis of Oxidative Modification of Proteins. In Current Protocols in Protein Science; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; Chapter 7, Unit 7.9. [Google Scholar]
- Shaikh, M.O.; Srikanth, B.; Zhu, P.Y.; Chuang, C.H. Impedimetric Immunosensor Utilizing Polyaniline/Gold Nanocomposite-Modified Screen-Printed Electrodes for Early Detection of Chronic Kidney Disease. Sensors 2019, 19, 3990. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Thornalley, P.J. Dicarbonyl Intermediates in the Maillard Reaction. Ann. N. Y. Acad. Sci. 2005, 1043, 111–117. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Dicarbonyls Linked to Damage in the Powerhouse: Glycation of Mitochondrial Proteins and Oxidative Stress. Biochem. Soc. Trans. 2008, 36, 1045–1050. [Google Scholar] [CrossRef]
- Rong, L.L.; Gooch, C.; Szabolcs, M.; Herold, K.C.; Lalla, E.; Hays, A.P.; Yan, S.F.; Yan, S.S.D.; Schmidt, A.M. RAGE: A Journey from the Complications of Diabetes to Disorders of the Nervous System—Striking a Fine Balance Between Injury and Repair. Restor. Neurol. Neurosci. 2005, 23, 355–365. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The Biology of the Receptor for Advanced Glycation End Products and its Ligands. Biochim. Biophys. Acta 2000, 1498, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.-X.; Wells-Knecht, K.J.; Blackledge, J.A.; Lyons, T.J.; Thorpe, S.R.; Baynes, J.W. Glycation, Glycoxidation, and Cross-linking of Collagen by Glucose. Kinetics, Mechanisms, and Inhibition of Late Stages of the Maillard Reaction. Diabetes 1994, 43, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.Y.; Cooper, M.E. Clinical Review: The Role of Advanced Glycation End Products in Progression and Complications of Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin Signalling and the Regulation of Glucose and Lipid Metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Rizza, R.A. Pathogenesis of Fasting and Postprandial Hyperglycemia in Type 2 Diabetes: Implications for Therapy. Diabetes 2010, 59, 2697–2707. [Google Scholar] [CrossRef]
- Thanopoulou, A.; Karamanos, B.; Archimandritis, A. Glycated Hemoglobin, Diabetes, and Cardiovascular Risk in Nondiabetic Adults. N. Engl. J. Med. 2010, 362, 2030–2031. [Google Scholar]
- Yoo, E.H.; Lee, S.Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based Microfluidic Point-of-care Diagnostic Devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent Developments in Paper-based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care Testing Based on Smartphone: The Current State-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.; Cobelli, C.; Dassau, E.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable Sensors: Modalities, Challenges, and Prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Chen, X.; Yu, H.; Wang, L.; Shen, D.; Li, C.; Zhou, W. Cross-linking-density-changeable Microneedle Patch Prepared from a Glucose-Responsive Hydrogel for Insulin Delivery. ACS Biomater. Sci. Eng. 2021, 7, 4870–4882. [Google Scholar] [CrossRef]
- Thornalley, P.J. Glyoxalase I—Structure, Function and a Critical Role in the Enzymatic Defence Against Glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef]
- Bhat, L.R.; Vedantham, S.; Krishnan, U.M.; Rayappan, J.B.B. A Non-enzymatic Two Step Catalytic Reduction of Methylglyoxal by Nanostructured V2O5 Modified Electrode. Biosens. Bioelectron. 2018, 103, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nesakumar, N.; Gopal, J.; Sivasubramanian, S.; Vedantham, S.; Rayappan, J.B.B. Clinical Validation of Electrochemical Biosensor for the Detection of Methylglyoxal in Subjects with Type-2 Diabetes Mellitus. Bioelectrochemistry 2024, 155, 108601. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.-R.; Govindhan, M.; Chen, A. Carbon Nanomaterials Based Electrochemical Sensors/Biosensors for the Sensitive Detection of Pharmaceutical and Biological Compounds. Sensors 2015, 15, 22490–22508. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Lilly Lecture 1993: Glycation and Diabetic Complications. Diabetes 1994, 43, 836–841. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced Glycation Endproducts—Role in Pathology of Diabetic Complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Singer, D.E.; Hurxthal, K.; Goodson, J.D. The Clinical Information Value of the Glycosylated Hemoglobin Assay. N. Engl. J. Med. 1984, 310, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Weykamp, C. HbA1c: A Review of Analytical and Clinical Aspects. Ann. Lab. Med. 2013, 33, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, T.; Jindal, A.; Kohli, P.; Thour, A.; Kaur, J.; Sachdev, A.; Gupta, Y. Utility and Limitations of Glycated Hemoglobin (HbA1c) in Patients with Liver Cirrhosis as Compared with Oral Glucose Tolerance Test for Diagnosis of Diabetes. Diabetes Ther. 2018, 9, 243–251. [Google Scholar] [CrossRef]
- Koga, M.; Kasayama, S. Clinical Impact of Glycated Albumin as Another Glycemic Control Marker. Endocr. J. 2010, 57, 751–762. [Google Scholar] [CrossRef]
- Peacock, T.P.; Shihabi, Z.K.; Bleyer, A.J.; Dolbare, E.L.; Byers, J.R.; Knovich, M.A.; Calles-Escandon, J.; Russell, G.B.; Freedman, B.I. Comparison of Glycated Albumin and Hemoglobin A1c Levels in Diabetic Subjects on Hemodialysis. Kidney Int. 2008, 73, 1062–1068. [Google Scholar] [CrossRef]
- Jeppsson, J.-O.; Kobold, U.; Barr, J.; Finke, A.; Hoelzel, W.; Hoshino, T.; Miedema, K.; Mosca, A.; Mauri, P.; Paroni, R.; et al. Approved IFCC Reference Method for The Measurement of HbA1c in Human Blood. Clin. Chem. Lab. Med. 2002, 40, 78–89. [Google Scholar] [CrossRef]
- Molazemhosseini, A.; Magagnin, L.; Vena, P.; Liu, C.C. Single-Use Disposable Electrochemical Label-Free Immunosensor for Detection of Glycated Hemoglobin (HbA1c) Using Differential Pulse Voltammetry (DPV). Sensors 2016, 16, 1024. [Google Scholar] [CrossRef]
- Rescalli, A.; Probst, D.; Hatada, M.; Nagata, M.; Cellesi, F.; Cerveri, P.; Sode, K. Electrochemical Aptamer-based Sensor for Single-step Quantification of Glycated Albumin in Point-of-care Diabetes and Pre-diabetes Management. Biosens. Bioelectron. 2025, 288, 117826. [Google Scholar] [CrossRef]
- Maraming, P.; Aye, N.N.S.; Boonsiri, P.; Daduang, S.; Buhome, O.; Daduang, J. Polydopamine Nanoparticles Functionalized Electrochemical DNA Aptasensor for Serum Glycated Albumin Detection. Int. J. Mol. Sci. 2022, 23, 13699. [Google Scholar] [CrossRef]
- Brownlee, M. Advanced Protein Glycosylation in Diabetes and Aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef]
- Chavakis, T.; Bierhaus, A.; Nawroth, P.P. RAGE (Receptor for Advanced Glycation End Products): A Central Player in the Inflammatory Response. Microbes Infect. 2004, 6, 1219–1225. [Google Scholar] [CrossRef]
- Sell, D.R.; Monnier, V.M. Structure Elucidation of a Senescence Cross-link from Human Extracellular Matrix. Implication of Pentoses in the Aging Process. J. Biol. Chem. 1989, 264, 21597–21602. [Google Scholar] [CrossRef]
- Schleicher, E.D.; Wagner, E.; Nerlich, A.G. Increased Accumulation of the Glycoxidation Product Nε-(Carboxymethyl)lysine in Human Tissues in Diabetes and Aging. J. Clin. Investig. 1997, 99, 457–468. [Google Scholar] [CrossRef]
- Ohno, R.I.; Ichimaru, K.; Tanaka, S.; Sugawa, H.; Katsuta, N.; Sakake, S.; Tominaga, Y.K.; Ban, I.; Shirakawa, J.I.; Yamaguchi, Y.; et al. Glucoselysine Is Derived from Fructose and Accumulates in the Eye Lens of Diabetic Rats. J. Biol. Chem. 2019, 294, 17326–17338. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Matsumura, T.; Sugawa, H.; Niimi, N.; Sango, K.; Nagai, R. Glucoselysine, a Unique Advanced Glycation End-product of the Polyol Pathway and its Association with Vascular Complications in Type 2 Diabetes. J. Biol. Chem. 2024, 300, 107479. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple Non-invasive Assessment of Advanced Glycation Endproduct Accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Radić, J.; Vučković, M.; Đogaš, H.; Gelemanović, A.; Belančić, A.; Radić, M. Are There Differences in Skin Autofluorescence-Measured Advanced Glycation End-Product Levels between Chronic Kidney Disease and Kidney Transplant Recipients? Diagnostics 2024, 14, 1383. [Google Scholar] [CrossRef]
- Kato, S.; Satoh, Y.; Okamoto, A.; Nagai, R. Non-invasive Evaluation of Advanced Glycation End Products in Hair as Early Markers of Diabetes and Aging. Sci. Rep. 2025, 15, 30232. [Google Scholar] [CrossRef]
- Ikeda, K.; Higashi, T.; Sano, H.; Jinnouchi, Y.; Yoshida, M.; Araki, T.; Ueda, S.; Horiuchi, S. Nε-(Carboxymethyl)lysine Protein Adduct Is a Major Immunological Epitope in Diabetic Nephropathy. Biochemistry 1996, 35, 8075–8083. [Google Scholar] [CrossRef]
- Teerlink, T.; Barto, R.; Hillebrand, J.J.G.; Schalkwijk, C.G. Measurement of Nε-(Carboxymethyl)lysine and Nε-(Carboxyethyl)lysine in Serum by Stable-isotope-dilution Tandem Mass Spectrometry. Clin. Chem. 2004, 50, 1222–1228. [Google Scholar] [CrossRef]
- Cheng, J.; Han, Y.; Deng, L.; Guo, S. Carbon Nanotube-bilirubin Oxidase Bioconjugate as a New Biofuel Cell Label for Self-powered Immunosensor. Anal. Chem. 2014, 86, 11782–11788. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Wang, F.; Xiong, W.; Shi, G.; Yuan, J.; Li, Y.; Zhang, H.; Xing, Y.; Jin, S.; Yang, K.; et al. Targeting RAGE with Nanobodies for Molecular Imaging of Cancers and Alzheimer’s Disease. Adv. Biol. 2025, 9, e00617. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of Oxidative Stress in Development of Complications in Diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Glycation in Diabetic Nephropathy: Characteristics, Consequences, Causes, and Therapeutic Options. Int. Rev. Neurobiol. 2002, 50, 37–57. [Google Scholar]
- Aronson, D. Hyperglycemia and the Pathobiology of Diabetic Complications. Adv. Cardiol. 2008, 45, 1–16. [Google Scholar]
- Goldstein, D.E.; Little, R.R.; Lorenz, R.A.; Malone, J.I.; Nathan, D.; Peterson, C.M.; Sacks, D.B. Tests of Glycemia in Diabetes. Diabetes Care 2004, 27, 1761–1773. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Ahmed, N.; Thornalley, P.J. Advanced Glycation Endproducts: What Is Their Relevance to Diabetic Complications? Diabetes Obes. Metab. 2007, 9, 233–245. [Google Scholar] [CrossRef]
- Lenters-Westra, E.; Slingerland, R.J. Six of Eight Hemoglobin A1c Point-of-care Instruments Do Not Meet the General Accepted Analytical Performance Criteria. Clin. Chem. 2010, 56, 44–52. [Google Scholar] [CrossRef]
- Chang, K.W.; Li, J.; Yang, C.H.; Shiesh, S.C.; Lee, G.B. An Integrated Microfluidic System for Measurement of Glycated Hemoglobin Levels by Using an Aptamer-antibody Assay on Magnetic Beads. Biosens. Bioelectron. 2015, 68, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, F.R.; Mattos, V.S.; Borghi-Silva, A.; Bagnato, V.S.; de Castro Neto, J.C. Advanced Glycation Endproducts as Biomarkers for Risk of Diabetes and Cardiovascular Diseases by Skin Autofluorescence: A Noninvasive Optical Screening. Photobiomodul. Photomed. Laser Surg. 2019, 37, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. Point-of-care Diagnostics: Recent Advances and Trends. Biosensors 2017, 7, 62. [Google Scholar] [CrossRef]
- Ghosh, G.; Sharma, R. Biomarkers in Wearables, Ingestible, and Implantable Sensors for Health Monitoring. Prog. Mol. Biol. Transl. Sci. 2025, 215, 35–62. [Google Scholar]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Varma, S. Electrochemical Studies on Reconstituted Horseradish Peroxidase Modified Carbon Paste Electrodes. Bioelectrochemistry 2002, 56, 107–111. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Montejo-Alvaro, F.; Peña-Castañeda, Y.A.; Matadamas-Ortiz, P.T.; Medina, D.I. Recent Developments in Graphene-Based Toxic Gas Sensors: A Theoretical Overview. Sensors 2021, 21, 1992. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Engelen, L.; Ferreira, I.; Scheijen, J.L.J.M.; van Greevenbroek, M.M.J.; van der Kallen, C.J.H.; Dekker, J.M.; Nijpels, G.; Stehouwer, C.D.A.; Schalkwijk, C.G. Plasma Advanced Glycation End Products Are Associated with Incident Cardiovascular Events in Individuals with Type 2 Diabetes: A Case–cohort Study with A Median Follow-up of 10 Years (EPIC-NL). Diabetes 2015, 64, 257–265. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Grune, T.; Höhn, A. Accumulation of Modified Proteins and Aggregate Formation in Aging. Exp. Gerontol. 2014, 57, 122–131. [Google Scholar] [CrossRef]
- Ge, A.; Xiang, W.; Li, Y.; Zhao, D.; Chen, J.; Daga, P.; Dai, C.C.; Yang, K.; Yan, Y.; Hao, M.; et al. Broadening Horizons: The Multifaceted Role of Ferroptosis in Breast Cancer. Front. Immunol. 2024, 15, 1455741. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Qiao, L.; Liu, B.; Gao, L.; Zhang, P. Development of A Microfluidic Wearable Electrochemical Sensor for the Non-invasive Monitoring of Oxidative Stress Biomarkers in Human Sweat. Biosens. Bioelectron. 2024, 261, 116502. [Google Scholar] [CrossRef] [PubMed]
- Pious, N.; Das, S.; Chakravorty, A.; Mini, A.A.; Raghavan, V. Gatifloxacin Detection in the Nanoscale: A Review Exploring Current Biosensing Technologies and Future Opportunities. RSC Adv. 2025, 15, 33018–33045. [Google Scholar] [CrossRef]
- Zelnick, L.R.; Trikudanathan, S.; Hall, Y.N.; Ayers, E.; Anderson, L.; Ashford, N.; Jones, E.; Hoofnagle, A.N.; de Boer, I.H.; Hirsch, I.B. Accuracy of Dexcom G6 Pro and G7 Continuous Glucose Monitors in Patients Treated with Maintenance Dialysis. Diabetes Technol. Ther. 2025. [Google Scholar] [CrossRef]
- Stewart, M.P.; Loera, L.J.; Onwukwe, B.; Reveles, K.R.; Lin, K.P. Assessing the Accessibility and Accuracy of Pharmacist counseling for Continuous Glucose Monitoring: A Secret Shopper Study of Community Pharmacies. J. Am. Pharm. Assoc. 2025, 65, 102493. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, Z.; Zhang, X.; Su, Z.; Yi, R.; Zou, D.; Xie, C.; Jin, N.; Long, W.; Liu, X. Generalized Machine Learning Based on Multi-omics Data to Profile the Effect of Ferroptosis Pathway on Prognosis and Immunotherapy Response in Patients with Bladder Cancer. Environ. Toxicol. 2024, 39, 680–694. [Google Scholar] [CrossRef]
- Rankin, D.; Black, M.; Flanagan, B.; Hughes, C.F.; Moore, A.; Hoey, L.; Wallace, J.; Gill, C.; Carlin, P.; Molloy, A.M.; et al. Identifying Key Predictors of Cognitive Dysfunction in Older People Using Supervised Machine Learning Techniques: Observational Study. JMIR Med. Inform. 2020, 8, e20995. [Google Scholar] [CrossRef]
| Category | Biomarker | Detection Methods | Clinical Relevance |
|---|---|---|---|
| Oxidative Stress | Lipid peroxidation product | TBARS, LC–MS/MS, electrochemical sensors | Atherosclerosis, progression of diabetes |
| Oxidized DNA modification | ELISA, LC–MS/MS, electrochemical sensors | Renal disease, cancer risk assessment | |
| Oxidized albumin | HPLC, biosensors | Indicator of CKD progression | |
| Glycation Stress | HbA1c | HPLC, immunoassays, POC devices | Diagnosis and management of diabetes |
| AGEs | ELISA, LC-MS/MS, HPLC, skin AF | Vascular complications, assessment of aging |
| Method | Principle | Target Analytes | Sensitivity/Specificity | Time Required | Cost | Suitability for POC |
|---|---|---|---|---|---|---|
| LC–MS/MS | Molecular identification and quantification via mass spectrometry | MDA, 8-OHdG, CML, Pentosidine | ◎ | Hours to 1 day | High | × |
| HPLC | Separation with UV/fluorescence detection | Pentosidine, MGO, 3-DG | ○ | Hours | Medium | × |
| ELISA | Antibody-based immunoassay | CML, Pentosidine, HbA1c | ○–◎ | Hours | Medium | △ |
| Electrochemical Sensors | Enzyme/antibody-modified nanomaterial electrodes | MDA, 8-OHdG, CML, HbA1c | ◎ | Minutes to tens of minutes | Low–Medium | ◎ |
| Optical Sensors | SPR, fluorescence, quantum dot–based probes | CML, Pentosidine, ROS | ◎ | Minutes to tens of minutes | Medium–High | ○ |
| Skin AF | Measurement of intrinsic skin fluorescence | Fluorescent AGEs (e.g., pentosidine) | △ | Immediate | Medium | ◎ |
| Challenge | Current Limitations | Future Directions |
|---|---|---|
| Sensitivity/Specificity | Short-lived ROS/RCS species lead to false positives | Enhancement of nanomaterial/enzyme-modified electrodes and molecular recognition elements |
| Standardization | Inconsistent LC–MS/MS and ELISA values; lack of reference ranges | Development of international standards and certified reference samples |
| Non-invasiveness | Skin autofluorescence lacks molecular specificity | Advancement of wearable sweat/saliva/tear sensors and hair analysis |
| Real-time Capability | Conventional methods provide only single time-point measurements | Adoption of continuous wearable monitoring systems |
| Integrated Evaluation | Oxidative and glycation markers measured separately | Multiplex detection platforms and AI-based integration |
| Clinical Implementation | High cost, reproducibility issues, limited multi-center data | Large-scale clinical validation and cost-reduction technologies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, H.; Yamaguchi, H. Oxidative and Glycation Stress Biomarkers: Advances in Detection Technologies and Point-of-Care Clinical Applications. Molecules 2025, 30, 4286. https://doi.org/10.3390/molecules30214286
Yamaguchi H, Yamaguchi H. Oxidative and Glycation Stress Biomarkers: Advances in Detection Technologies and Point-of-Care Clinical Applications. Molecules. 2025; 30(21):4286. https://doi.org/10.3390/molecules30214286
Chicago/Turabian StyleYamaguchi, Hiroko, and Hiroshi Yamaguchi. 2025. "Oxidative and Glycation Stress Biomarkers: Advances in Detection Technologies and Point-of-Care Clinical Applications" Molecules 30, no. 21: 4286. https://doi.org/10.3390/molecules30214286
APA StyleYamaguchi, H., & Yamaguchi, H. (2025). Oxidative and Glycation Stress Biomarkers: Advances in Detection Technologies and Point-of-Care Clinical Applications. Molecules, 30(21), 4286. https://doi.org/10.3390/molecules30214286





