Electrodeposition of BiVO4 Nanoparticles on TiO2 Nanotubes: Characterization and Synergetic Photocatalytic Degradation Activity of Amido Black Dye
Abstract
1. Introduction
2. Results
2.1. Structural Analysis
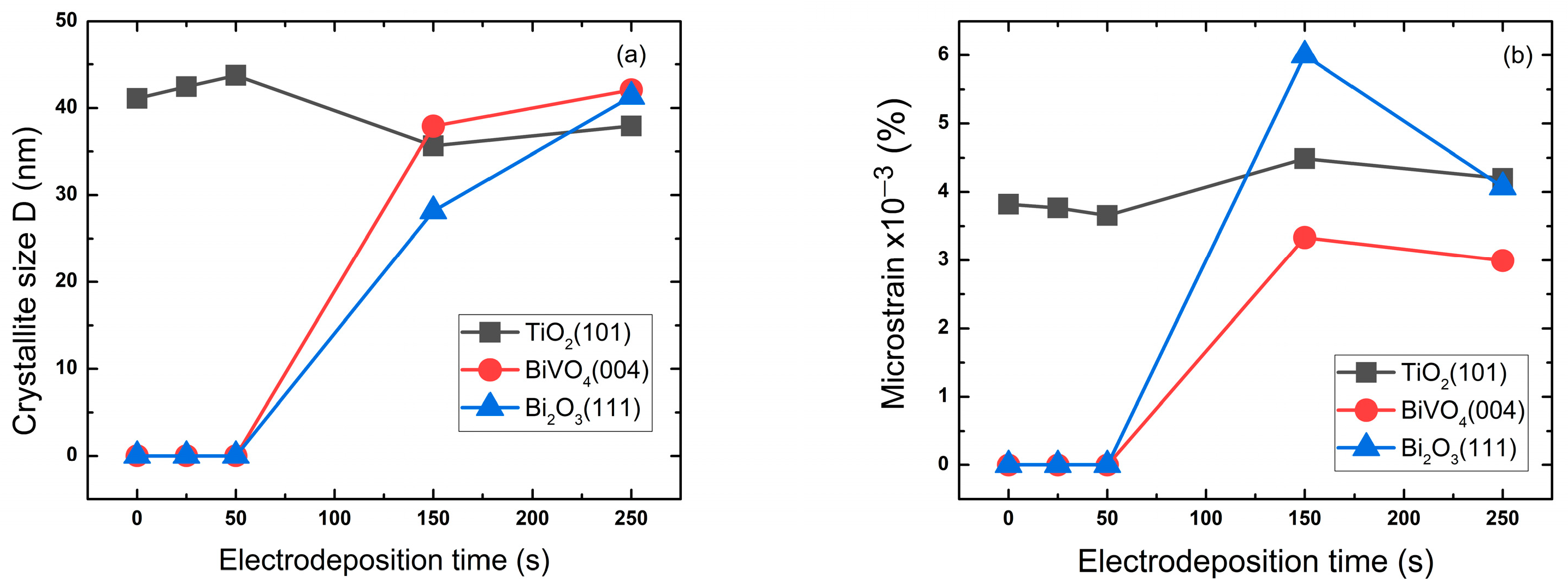
2.1.1. XRD Analysis
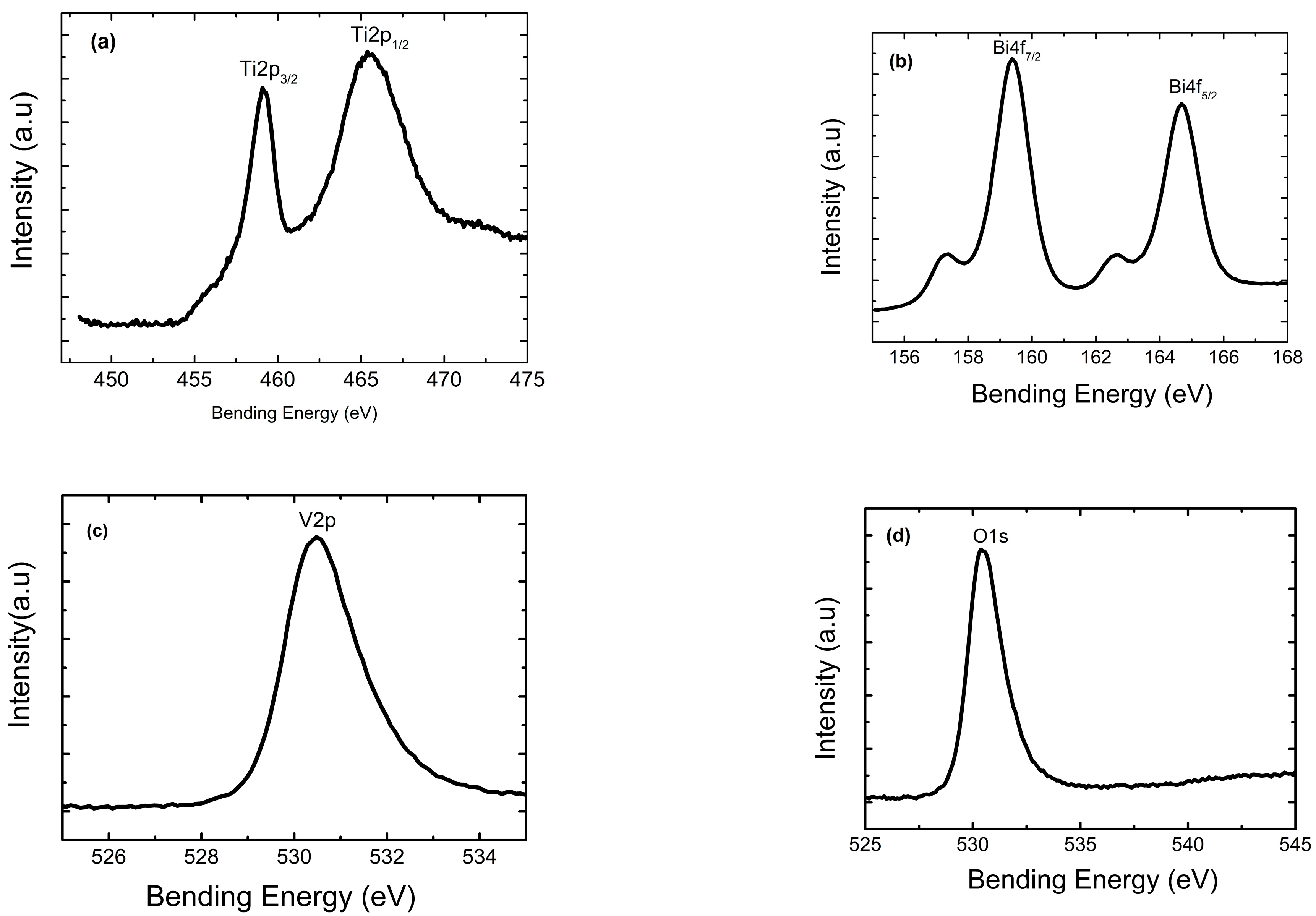
2.1.2. XPS Analysis
2.2. Morphological Analysis
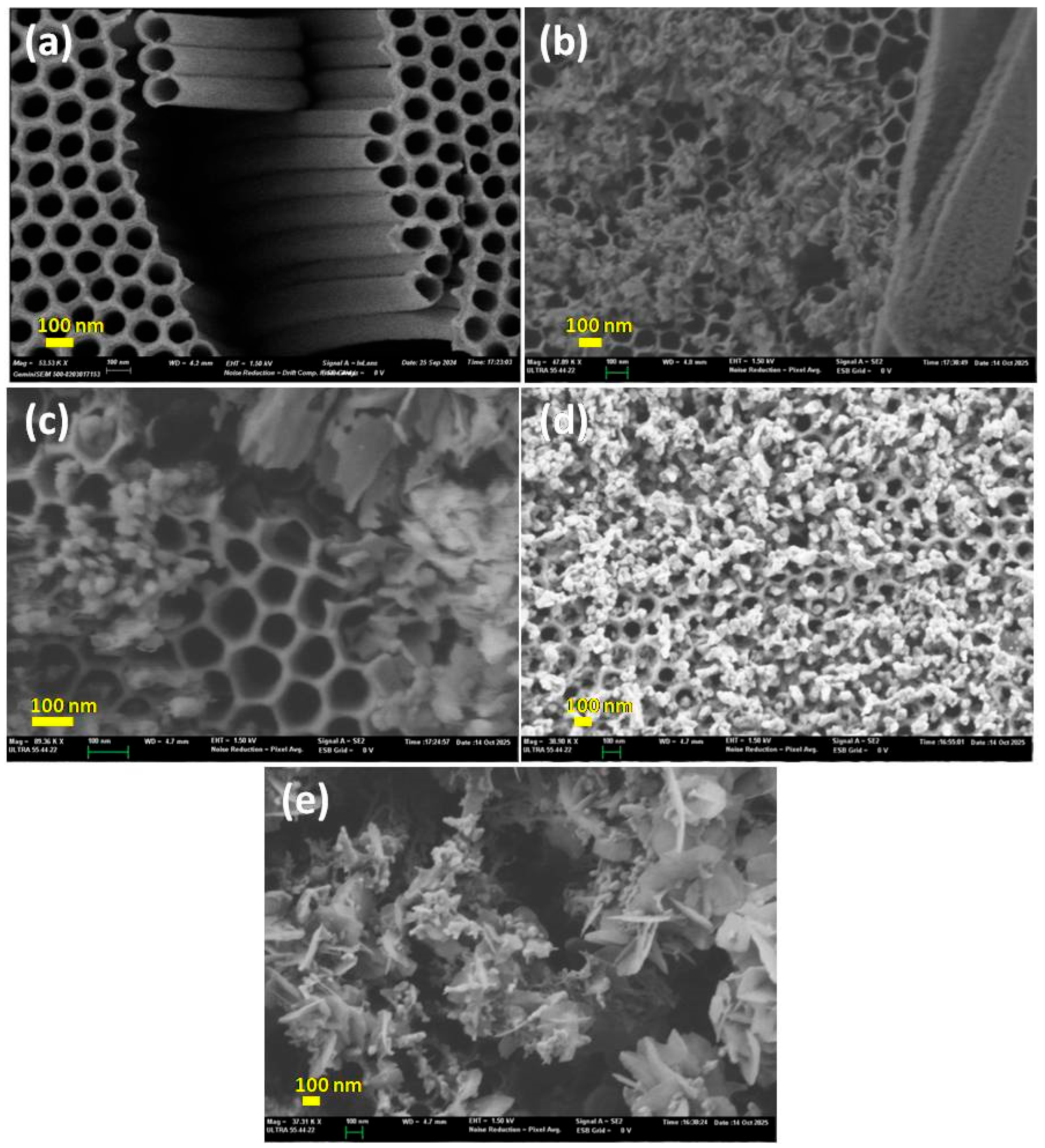
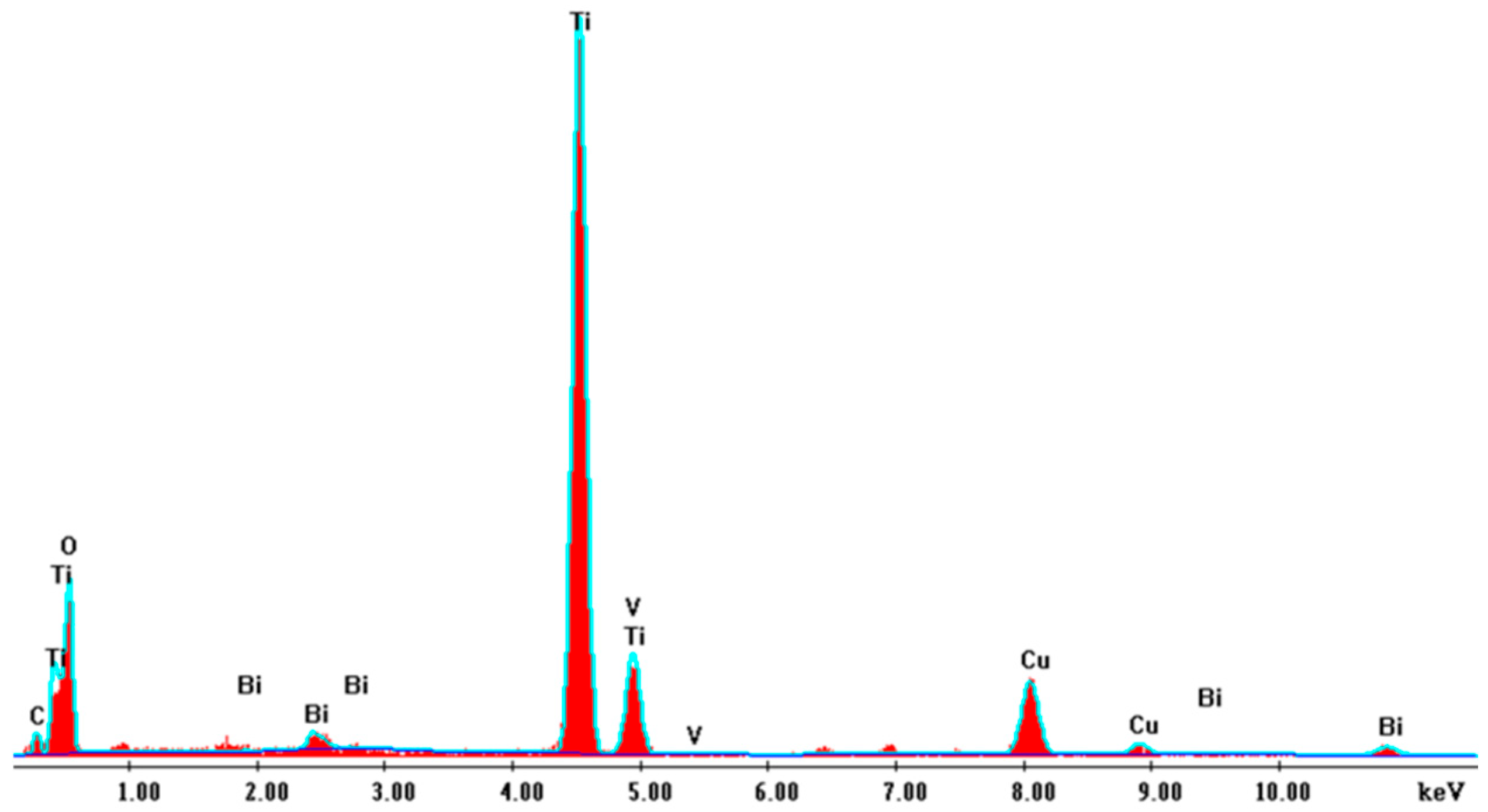
2.2.1. SEM Analysis
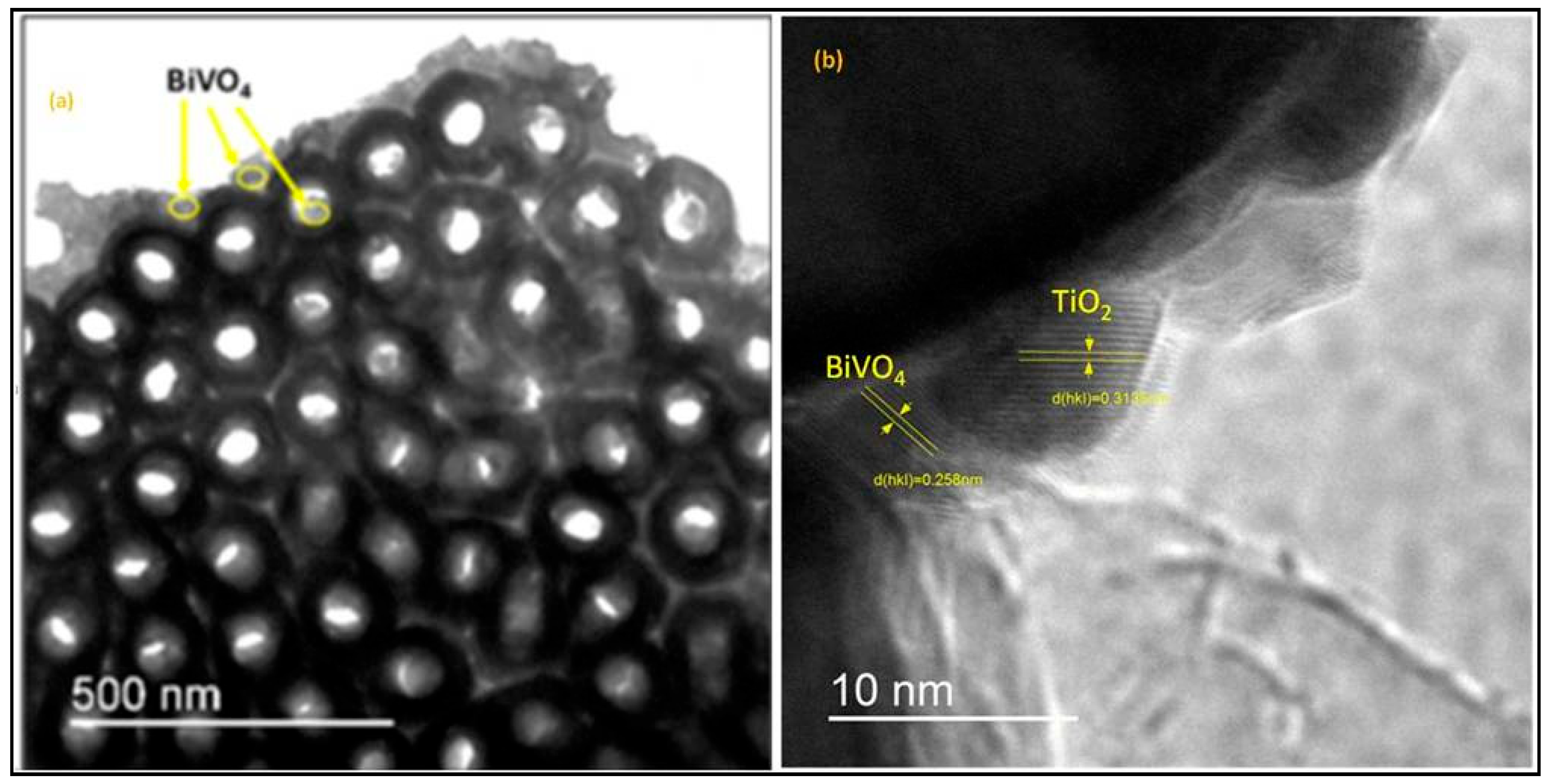
2.2.2. TEM Analysis
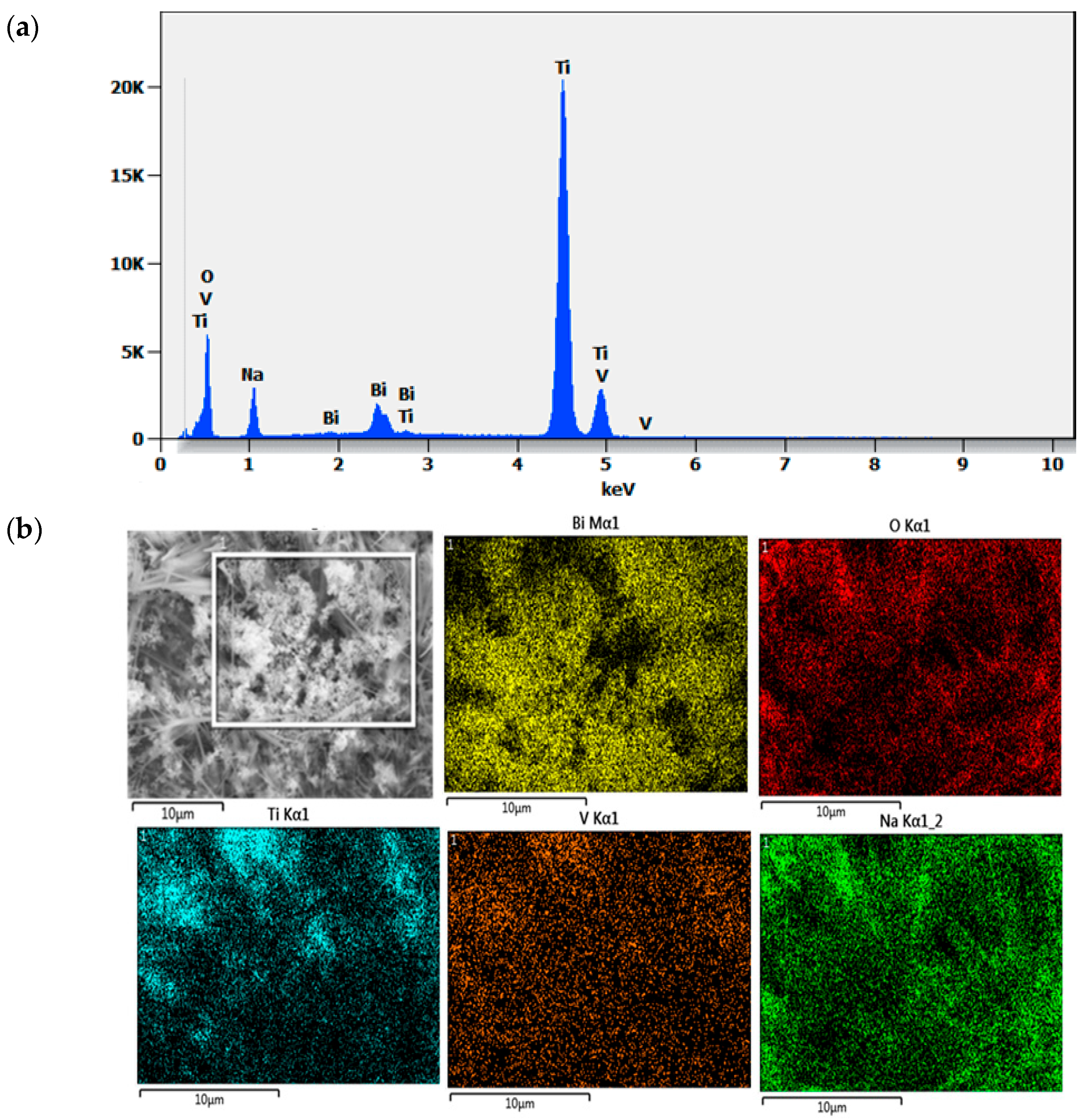
2.3. Optical Analysis
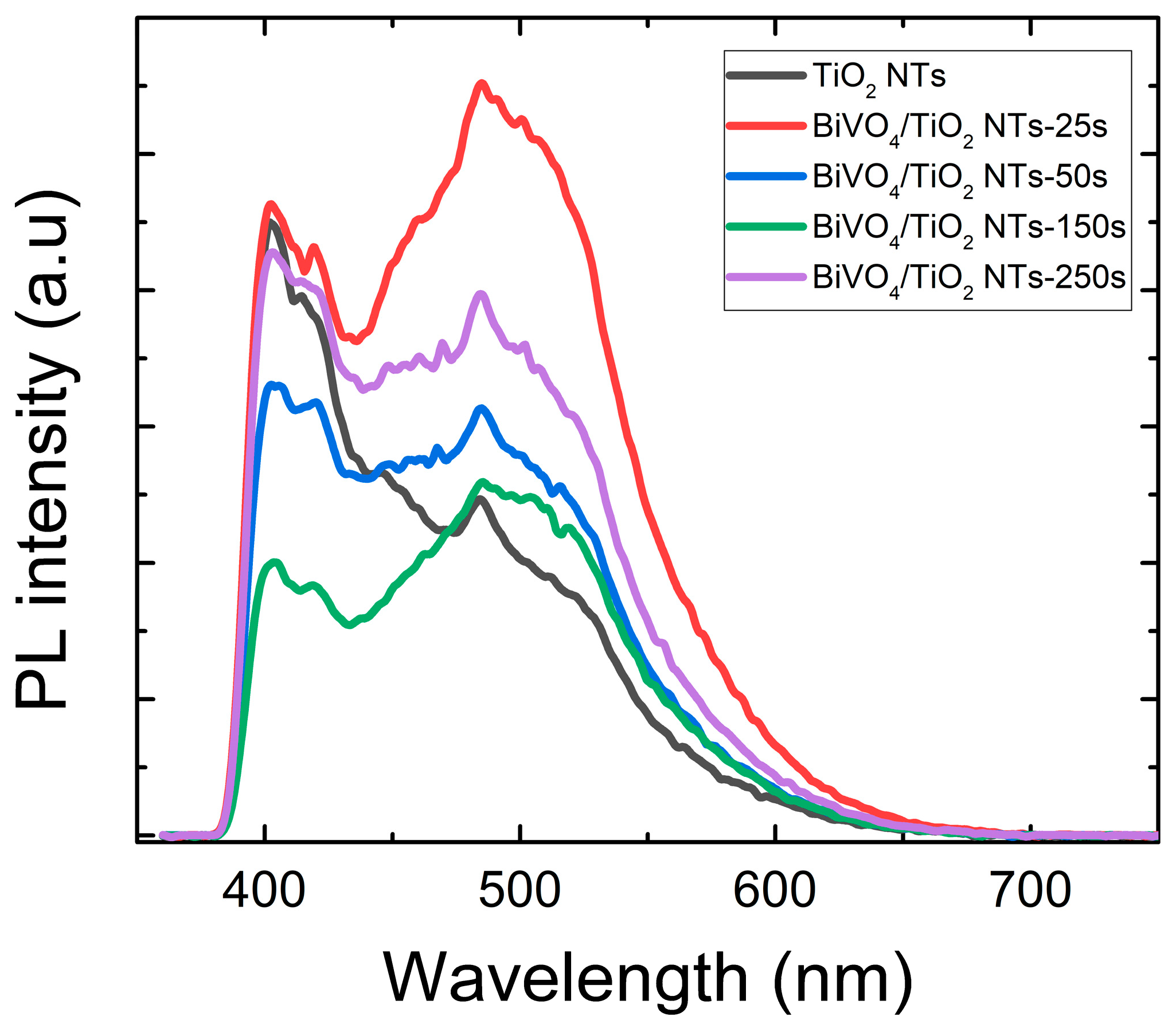
2.3.1. Photoluminescence
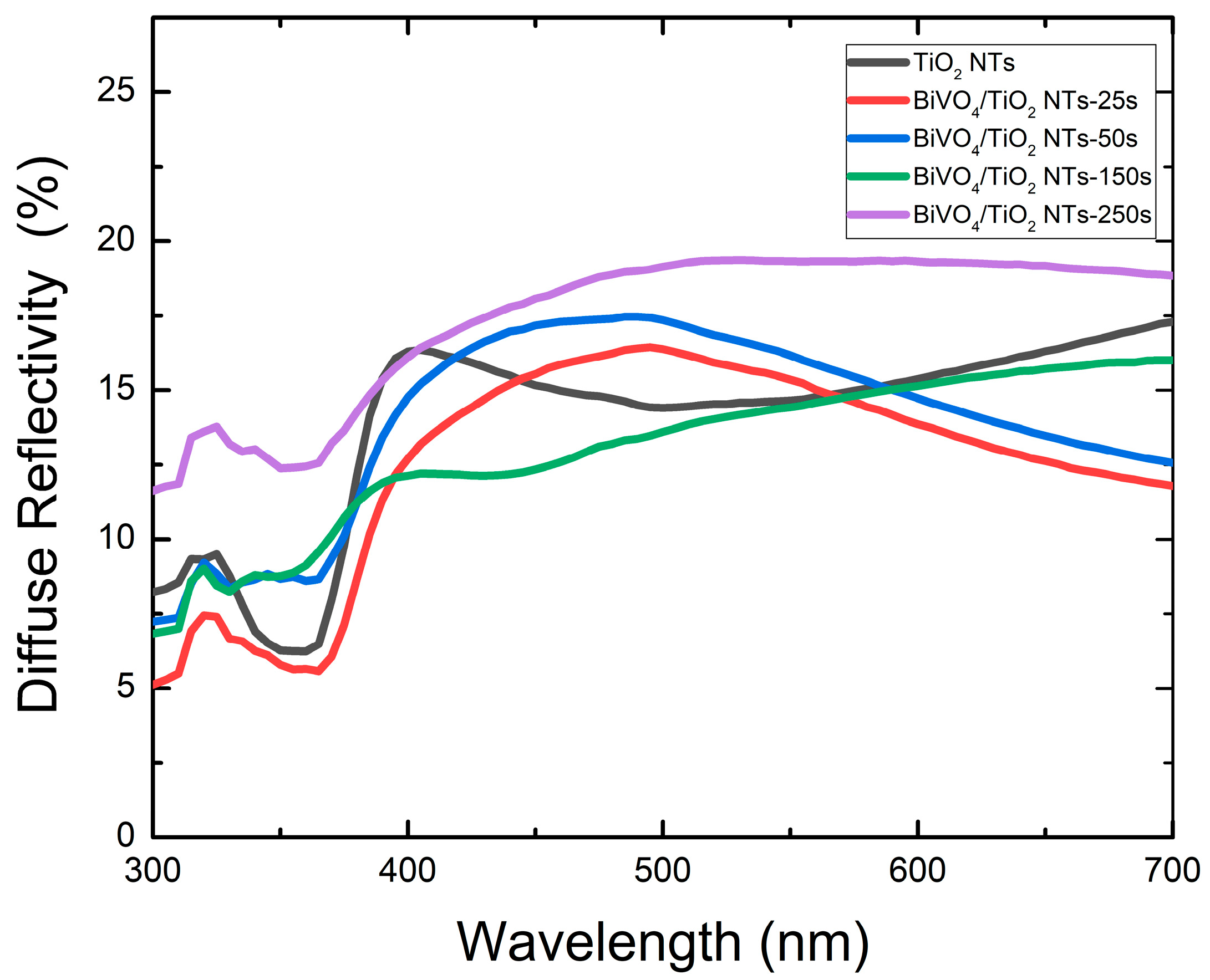
2.3.2. Diffuse Reflectivity
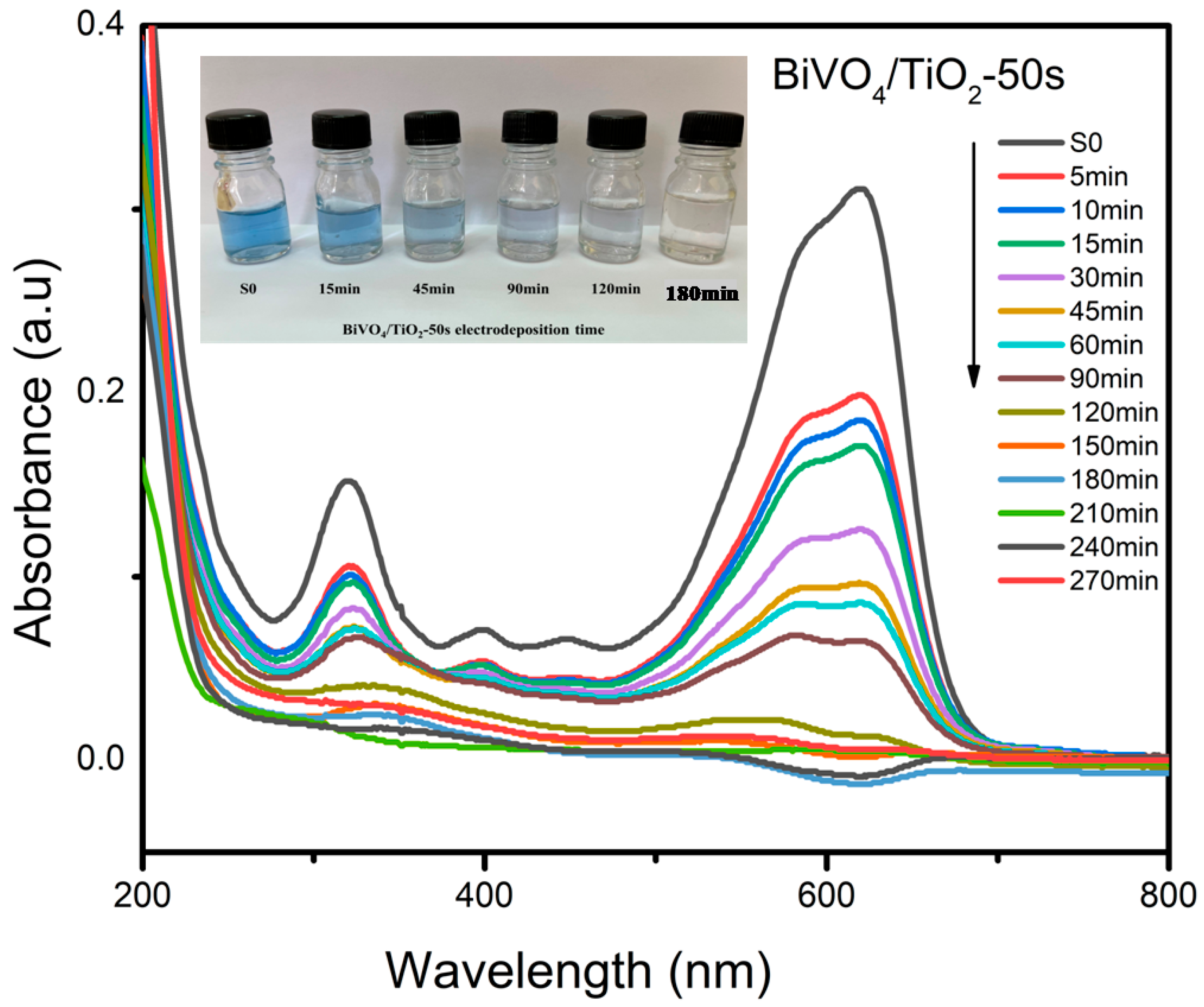
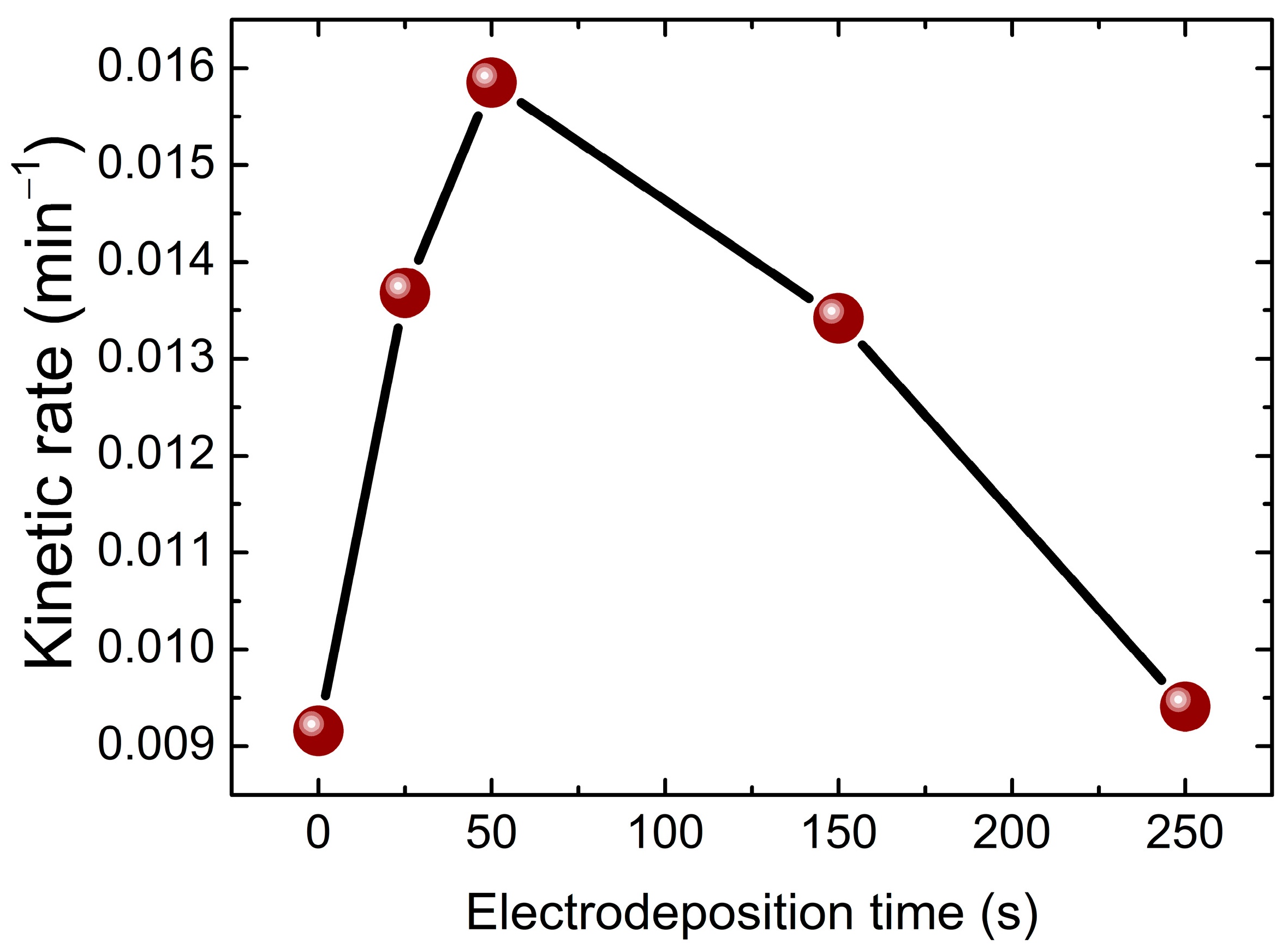
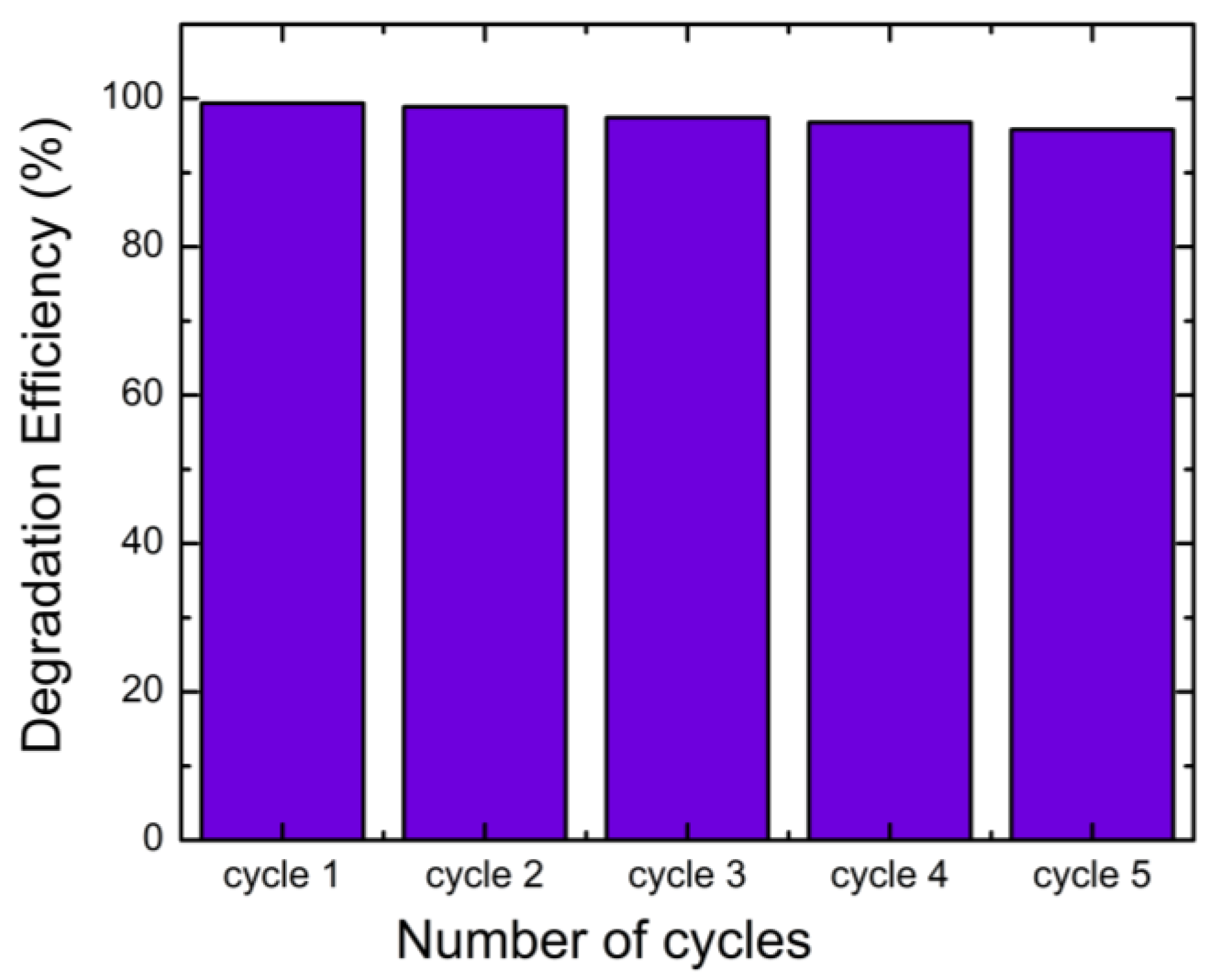
2.4. Photodegradation of Black Amido with BiVO4/TiO2
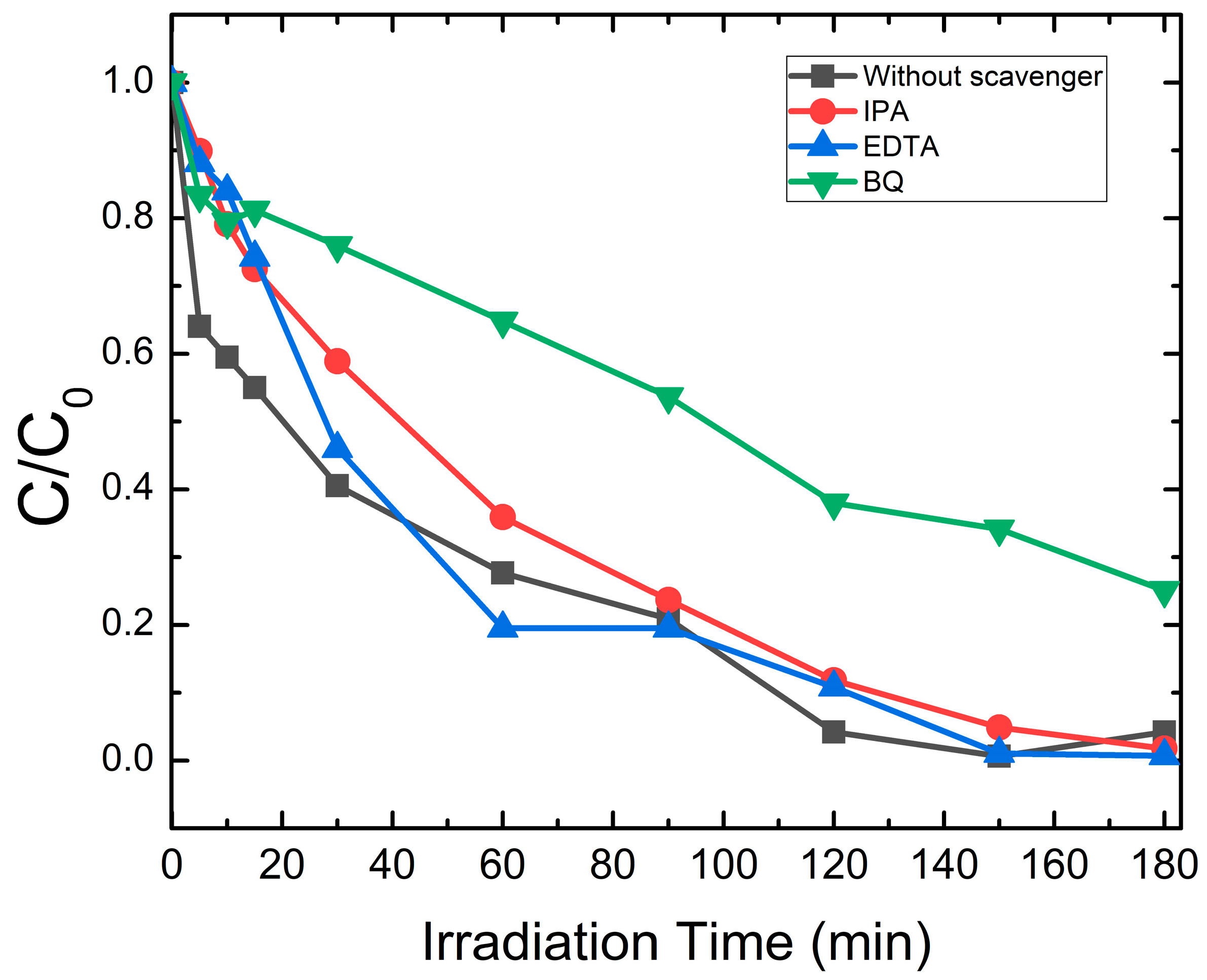
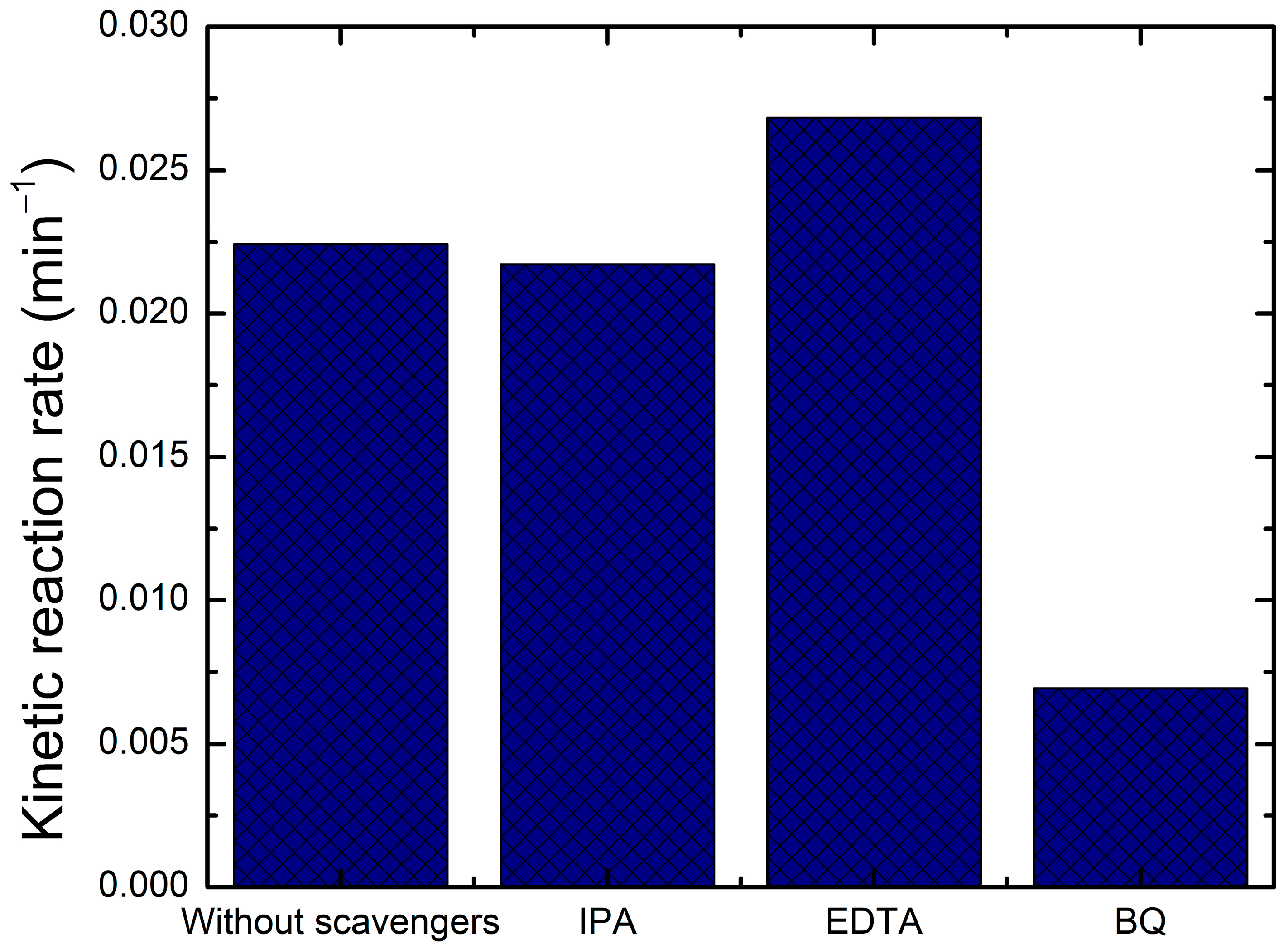
2.5. Quenching Test
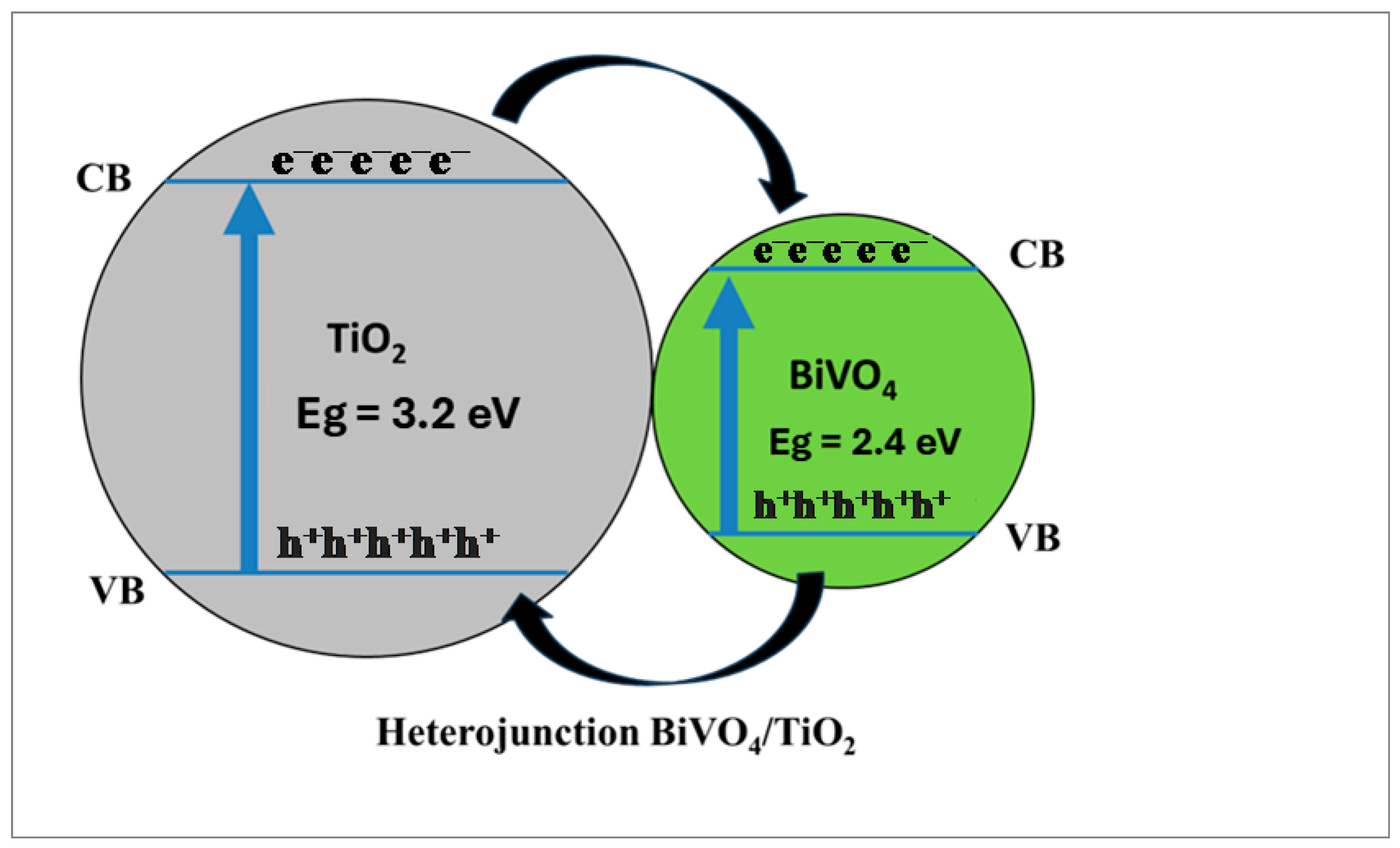
2.6. Mechanism of Photocatalysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of TiO2 Nanotubes (NTs)
4.3. Synthesis of BiVO4 Nanoparticles on TiO2-NTs
4.4. Samples Characterization
4.5. Photocatalytic Activity Measurements
4.5.1. Photodegradation of Black Amido
4.5.2. Trapping Test
- Quenching of Hydroxyl Radicals (OH•) by Isopropanol (IPA)Isopropanol reacts with hydroxyl radicals via hydrogen abstraction, leading to the formation of acetone and water.
- ▪
- Quenching of Superoxide Radical Anions (O2•−) by Benzoquinone (BQ)Benzoquinone (BQ) readily accepts an electron from the superoxide radical, forming a semiquinone radical and then hydroquinone after protonation.
- ▪
- Quenching of Photogenerated Holes (h+) by Ethylenediaminetetraacetic Acid (EDTA)EDTA acts as a sacrificial electron donor, capturing the photogenerated holes before they can generate OH• radicals or directly oxidize the dye.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaishree, G.; Divya, G.; Rao, T.S.; Chippada, M.L.V.P.; Raju, I.M. Biogenic surfactant mediated facile synthesis of visible light sensitized Zn/Mg co-doped TiO2 nanomaterials—A green approach: Evaluation of photocatalytic activity by degradation of Amido Black 10B. Sustain. Environ. Res. 2022, 32, 38. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Khan, S.; Noor, T.; Iqbal, N.; Yaqoob, L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega 2024, 9, 21751–21767. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Lemasle, M.; Wolbert, D. Removal of trimethylamine and isovaleric acid from gas streams in a continuous flow surface discharge plasma reactor. Chem. Eng. Res. Des. 2015, 93, 640–651. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Muddemann, T.; Haupt, D.; Sievers, M.; Kunz, U. Electrochemical reactors for wastewater treatment. ChemBioEng Rev. 2019, 6, 142–156. [Google Scholar] [CrossRef]
- Su, R.; Zhu, Y.; Gao, B.; Li, Q. Progress on mechanism and efficacy of heterogeneous photocatalysis coupled oxidant activation as an advanced oxidation process for water decontamination. Water Res. 2024, 251, 121119. [Google Scholar] [CrossRef]
- Feijoo, S.; Yu, X.; Kamali, M.; Appels, L.; Dewil, R. Generation of oxidative radicals by advanced oxidation processes (AOPs) in wastewater treatment: A mechanistic, environmental and economic review. Rev. Environ. Sci. Bio/Technol. 2023, 22, 205–248. [Google Scholar] [CrossRef]
- Terki, M.; Triaa, S.; Ali, F.K.; Youcef, R.; Brahim, I.O.; Trari, M. Sono-assisted degradation of rhodamine B using the Fe modified MgO nanostructures: Characterization and catalytic activity. React. Kinet. Catal. Lett. 2023, 136, 1143–1155. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Liu, J.; Yang, L.; Dai, C.; Ren, N.; Feng, Y. Construction of TiO2 nanotube clusters on Ti mesh for immobilizing Sb-SnO2 to boost electrocatalytic phenol degradation. J. Hazard. Mater. 2020, 393, 122329. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, W.; Li, H.; Yang, M.; Yang, Z. Separable magnetic Fe3O4@ MoS2 composite for adsorption and piezo-catalytic degradation of dye. Catalysts 2021, 11, 1403. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I. Recent development and futurs prospects of TiO2 photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Alkanad, K.; Hezam, A.; Al-Zaqri, N.; Bajiri, M.A.; Alnaggar, G.; Drmosh, Q.A.; Almukhlifi, H.A.; Krishnappagowda, L.N. One-step hydrothermal synthesis of anatase TiO2 nanotubes for efficient photocatalytic CO2 reduction. ACS Omega 2022, 7, 38686–38699. [Google Scholar] [CrossRef] [PubMed]
- Ikreedeegh, R.R.; Hossen, A.; Tahir, M.; Aziz, A.A. A comprehensive review on anodic TiO2 nanotube arrays (TNTAs) and their composite photocatalysts for environmental and energy applications: Fundamentals, recent advances and applications. Co-ord. Chem. Rev. 2024, 499, 215495. [Google Scholar] [CrossRef]
- Zakir, O.; Idouhli, R.; Elyaagoubi, M.; Khadiri, M.; Aityoub, A.; Koumya, Y.; Rafqah, S.; Abouelfida, A.; Outzourhit, A. Fabrication of TiO2 nanotube by electrochemical anodization: Toward photocatalytic application. J. Nanomater. 2020, 2020, 4745726. [Google Scholar] [CrossRef]
- David, T.M.; Dev, P.R.; Wilson, P.; Sagayaraj, P.; Mathews, T. A critical review on the variations in anodization parameters toward microstructural formation of TiO2 nanotubes. Electrochem. Sci. Adv. 2022, 2, e202100083. [Google Scholar] [CrossRef]
- Puga, M.; Venturini, J.; Caten, C.T.; Bergmann, C. Influencing parameters in the electrochemical anodization of TiO2 nanotubes: Systematic review and meta-analysis. Ceram. Int. 2022, 48, 19513–19526. [Google Scholar] [CrossRef]
- Hossen, A.; Aziz, A.A.; Ikreedeegh, R.R.; Muhammad, A.D.; Yaacof, N.; Leong, K.H.; Wu, L. Optimization of anodizing parameters for the morphological properties of TiO2 nanotubes based on response surface methodology. Next Mater. 2024, 2, 100061. [Google Scholar] [CrossRef]
- Beranek, R.; Tsuchiya, H.; Sugishima, T.; Macak, J.M.; Taveira, L.; Fujimoto, S.; Kisch, H.; Schmuki, P. Enhancement and limits of the photoelectrochemical response from anodic TiO2 nanotubes. Appl. Phys. Lett. 2005, 87, 243114. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Abidi, M.; Assadi, A.; Bouzaza, A.; Hajjaji, A.; Bessais, B.; Rtimi, S. Photocatalytic indoor/outdoor air treatment and bacterial inactivation on CuxO/TiO2 prepared by HiPIMS on polyester cloth under low intensity visible light. Appl. Catal. B Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Liu, D.; Chen, C.; Li, R.; Peng, T. Fabrication of PbS nanocrystal-sensitized ultrafine TiO2 nanotubes for efficient and unusual broadband-light-driven hydrogen production. Mater. Today Chem. 2020, 17, 100310. [Google Scholar] [CrossRef]
- Rezaei, M.; Nezamzadeh-Ejhieha, A. The ZnO-NiO nano-composite: A brief characterization, kinetic and thermodynamic study and study the Arrhenius model on the sulfasalazine photodegradation. Int. J. Hydrogen Energy 2020, 45, 24749–24764. [Google Scholar] [CrossRef]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Amato, L.S.; Maddalena, P. Charge carrier processes and optical properties in TiO2 and TiO2-based heterojunction photocatalysts: A Review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Hajjaji, A.; Jemai, S.; Rebhi, A.; Trabelsi, K.; Gaidi, M.; Alhazaa, A.; Al-Gawati, M.; El Khakani, M.; Bessais, B. Enhancement of photocatalytic and photoelectrochemical properties of TiO2 nanotubes sensitized by SILAR—Deposited PbS nanoparticles. J. Mater. 2020, 6, 62–69. [Google Scholar] [CrossRef]
- Hamdi, D.; Mansouri, L.; Srivastava, V.; Sillanpaa, M.; Bousselmi, L. Enhancement of Eu and Ce doped TiO2 thin films photoactivity: Application on amido black photodegradation. Inorg. Chem. Commun. 2021, 133, 108912. [Google Scholar] [CrossRef]
- Missaoui, K.; Ouertani, R.; Jbira, E.; Boukherroub, R.; Bessaïs, B. Morphological influence of BiVO4 nanostructures on peroxymonosulfate activation for highly efficient catalytic degradation of rhodamine B. Environ. Sci. Pollut. Res. 2021, 28, 52236–52246. [Google Scholar] [CrossRef]
- Shaban, M.; Ashraf, A.M.; Abukhadra, M.R. TiO2 nanoribbons/carbon nanotubes composite with enhanced photocatalytic activity; fabrication, characterization, and application. Sci. Rep. 2018, 8, 781. [Google Scholar] [CrossRef]
- Alias, S.S.; Harun, Z.; Azhar, F.H.; Ibrahim, S.A.; Johar, B. Comparison between commercial and synthesised nano flower-like rutile TiO2 immobilised on green super adsorbent towards dye wastewater treatment. J. Clean. Prod. 2020, 251, 119448. [Google Scholar] [CrossRef]
- Dong, G.; Wang, Y.; Lei, H.; Tian, G.; Qi, S.; Wu, D. Hierarchical mesoporous titania nanoshell encapsulated on polyimide nanofiber as flexible, highly reactive, energy saving and recyclable photocatalyst for water purification. J. Clean. Prod. 2020, 253, 120021. [Google Scholar] [CrossRef]
- Sahrin, N.T.; Nawaz, R.; Chong, F.K.; Lee, S.L.; Wirzal, M.D. Current perspectives of anodized TiO2 nanotubes towards photodegradation of formaldehyde: A short review. Environ. Technol. Innov. 2021, 22, 101418. [Google Scholar] [CrossRef]
- Feng, Y.; Rijnaarts, H.H.; Yntema, D.; Gong, Z.; Dionysiou, D.D.; Cao, Z.; Miao, S.; Chen, Y.; Ye, Y.; Wang, Y. Applications of anodized TiO2 nanotube arrays on the removal of aqueous contaminants of emerging concern: A review. Water Res. 2020, 186, 116327. [Google Scholar] [CrossRef] [PubMed]
- Palmas, S.; Mais, L.; Mascia, M.; Vacca, A. Trend in using TiO2 nanotubes as photoelectrodes in PEC processes for wastewater treatment. Curr. Opin. Electrochem. 2021, 28, 100699. [Google Scholar] [CrossRef]
- Niu, L.; Zhao, X.; Tang, Z.; Lv, H.; Wu, F.; Wang, X.; Zhao, T.; Wang, J.; Wu, A.; Giesy, J. Difference in performance and mechanism for methylene blue when TiO2 nanoparticles are converted to nanotubes. J. Clean. Prod. 2021, 297, 126498. [Google Scholar] [CrossRef]
- Hajjaji, M.A.; Missaoui, K.; Trabelsi, K.; Bouzaza, A.; Bessais, B.; Hajjaji, A.; Assadi, A.A. Electrodeposited Platinum Nanoparticles on Highly Ordered Titanium Dioxide Nanotubes for Photocatalytic Application: Enhancement of Photocatalytic Degradation of Amido Black Dye. Catal. Lett. 2024, 154, 1242–1254. [Google Scholar] [CrossRef]
- Le-Duy, N.; Hoang, L.-A.T.; Nguyen, T.D.; Lee, T. Pd nanoparticles decorated BiVO4 pine architectures for photocatalytic degradation of sulfamethoxazole. Chemosphere 2023, 321, 138118. [Google Scholar] [CrossRef]
- Rajkumar, S.; Perumalsamy, S.V.; Chidambaram, S.G.T.; Kulandaivel, J.; Paramasivam, T.; Ponnamma, D. Exploration of photocatalytic dye degradation and non-linear optical absorption of BiVO4–TiO2 nanocomposites. J. Mater. Sci. Mater. Electron. 2024, 35, 2209. [Google Scholar] [CrossRef]
- Kashyap, J.; Gautam, S.; Ashraf, S.M.; Riaz, U. Synergistic performance of sonolytically synthesized Poly (1-naphthylamine)/TiO2 nanohybrids: Degradation studies of amido black-10B dye. ChemistrySelect 2018, 3, 11926–11934. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Alothman, Z.A.; Badjah, A.Y. Kinetics, Thermodynamics, and modeling of amido black dye photodegradation in water using Co/TiO2 nanoparticles. Photochem. Photobiol. 2018, 94, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Sassi, S.; Bouich, A.; Hajjaji, A.; Khezami, L.; Bessais, B.; Soucase, B.M. Cu-Doped TiO2 Thin Films by Spin Coating: Investigation of Structural and Optical Properties. Inorganics 2024, 12, 188. [Google Scholar] [CrossRef]
- Goktas, A.; Modanlı, S.; Tumbul, A.; Kilic, A. Facile synthesis and characterization of ZnO, ZnO:Co, and ZnO/ZnO:Co nano rod-like homojunction thin films: Role of crystallite/grain size and microstrain in photocatalytic performance. J. Alloys Compd. 2022, 893, 162334. [Google Scholar] [CrossRef]
- Deb, A.K.; Chatterjee, P. Microstrain and lattice disorder in nanocrystalline titanium dioxide prepared by chemical route and its relation with phase transformation. J. Theor. Appl. Phys. 2020, 14, 285–293. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Zhao, Q.; Tadé, M.O.; Liu, S. Quantum-sized BiVO4 modified TiO2 microflower composite heterostructures: Efficient production of hydroxyl radicals towards visible light-driven degradation of gaseous toluene. J. Mater. Chem. A 2015, 3, 21655–21663. [Google Scholar] [CrossRef]
- Guan, Z.-C.; Wang, H.-P.; Wang, X.; Hu, J.; Du, R.-G. Fabrication of heterostructured β-Bi2O3-TiO2 nanotube array composite film for photoelectrochemical cathodic protection applications. Corros. Sci. 2018, 136, 60–69. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Sivakumar, J.; Reddy, M.R.; Sayanna, R. Photoluminescence and photocatalytic activity of spin coated Ag+ doped anatase TiO2 thin films. Opt. Mater. 2020, 108, 110401. [Google Scholar] [CrossRef]
- Sadeghzadeh-Attar, A. Boosting the photocatalytic ability of hybrid BiVO4-TiO2 heterostructure nanocomposites for H2 production by reduced graphene oxide (rGO). J. Taiwan Inst. Chem. Eng. 2020, 111, 325–336. [Google Scholar] [CrossRef]
- Khemissi, I.; Khezami, L.; Trabelsi, K.; Guesmi, A.; Kouki, A.; Kiwi, J.; Bessais, B.; Rtimi, S.; Hajjaji, A. Stable Ta2O5 nanotubes decorated by PbS by the SILAR method for photocatalytic dye degradation. J. Photochem. Photobiol. A Chem. 2023, 444, 114937. [Google Scholar] [CrossRef]
- Diwan, B.D.; Dubey, V.K. Influence of size on effective band gap of silicon nano-wire. Adv. Mater. Res. 2014, 938, 322–326. [Google Scholar] [CrossRef]
- Khammar, M.; Ynineb, F.; Guitouni, S.; Bouznit, Y.; Attaf, N. Crystallite size and intrinsic strain contribution in band gap energy redshift of ultrasonic-sprayed kesterite CZTS nanostructured thin films. Appl. Phys. A 2020, 126, 398. [Google Scholar] [CrossRef]
- Abidi, M.; Hajjaji, A.; Bouzaza, A.; Trablesi, K.; Makhlouf, H.; Rtimi, S.; Assadi, A.; Bessais, B. Simultaneous removal of bacteria and volatile organic compounds on Cu2O-NPs decorated TiO2 nanotubes: Competition effect and kinetic studies. J. Photochem. Photobiol. A Chem. 2020, 400, 112722. [Google Scholar] [CrossRef]
- Jemai, S.; Khezami, L.; Gueddana, K.; Trabelsi, K.; Hajjaji, A.; Amlouk, M.; Soucase, B.M.; Bessais, B.; Rtimi, S. Impact of annealing on ZrO2 nanotubes for photocatalytic application. Catalysts 2023, 13, 558. [Google Scholar] [CrossRef]
- Rebhi, A.; Amri, C.; Khemissi, I.; Zaghouani, R.B.; Gaidi, M.; Hajjaji, A.; Choubani, K.; Almeshaal, M.A.; Papathi, M.P.; Ben Rabha, M. Porous silicon layer decorated with PbS nanoparticles by SILAR method for enhanced photocatalytic degradation of amido black dye. J. Nanopart. Res. 2024, 26, 47. [Google Scholar] [CrossRef]
- Liaqat, M.; Kausar, S.; Iqbal, T.; Afsheen, S.; Younas, A.; Zubair, M.; Syed, A.; Elgorban, A.M.; Wong, L.S. Synergistic photocatalytic activity of TiO2/BiVO4 nanocomposites: Optimization, characterization, and recyclability for dye and antibiotic degradation. J. Inorg. Organomet. Polym. Mater. 2024, 34, 3246–3257. [Google Scholar] [CrossRef]
- Sassi, S.; Trabelsi, K.; El Jery, A.; Abidi, M.; Hajjaji, A.; Khezami, L.; Karrech, A.; Gaidi, M.; Soucase, B.; Bessais, B. Synergistic effect of CuxOy-NPs/TiO2-NTs heterostructure on the photodegradation of amido black staining. Optik 2023, 272, 170234. [Google Scholar] [CrossRef]
- Zalfani, M.; Van Der Schueren, B.; Hu, Z.Y.; Rooke, J.C.; Bourguiga, R.; Wu, M.; Li, Y.; Van Tendeloo, G.; Su, B.L. Novel 3DOM BiVO4/TiO2 nanocomposites for highly enhanced photocatalytic activity. J. Mater. Chem. A 2015, 3, 21244–21256. [Google Scholar] [CrossRef]
- Drisya, K.T.; Solís-López, M.; Ríos-Ramírez, J.J.; Durán-Álvarez, J.C.; Rousseau, A.; Velumani, S.; Asomoza, R.; Kassiba, A.; Jantrania, A.; Castaneda, H. Electronic and optical competence of TiO2/BiVO4 nanocomposites in the photocatalytic processes. Sci. Rep. 2020, 10, 13507. [Google Scholar] [CrossRef] [PubMed]
- Wetchakun, N.; Chainet, S.; Phanichphant, S.; Wetchakun, K. Efficient photocatalytic degradation of methylene blue over BiVO4/TiO2 nanocomposites. Ceram. Int. 2015, 41, 5999–6004. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, J.; Hu, Y.; Liu, Y.; Hu, S.; Zhu, C. Photochemical reactions between 1,4-benzoquinone and O2•−. Environ. Sci. Pollut. Res. 2020, 27, 31289–31299. [Google Scholar] [CrossRef]
- Mitra, S.P. Pharmacology and biochemistry behind the use of natural herbs to control arthritis—A review. Indian J. Nat. Prod. Resour. (IJNPR) [Former. Nat. Prod. Radiance (NPR)] 2018, 8, 204–223. [Google Scholar]


| Catalyst | Conditions | Removal Efficiency (%) | Reaction Time (min) | Ref. |
|---|---|---|---|---|
| Zn/Mg co-doped TiO2 (ZMT4) | [AB] = 10 mg.L−1 [Catalyst] = 100 mg.L−1 | 99% | 20 | [1] |
| PNA/TiO2 | [AB] = 100 mg.L−1 [Catalyst] = 200 mg | 90% | 60 | [42] |
| Co/TiO2 | [AB] = 100 μg.L−1 [Catalyst] = 1.0 g.L−1 | 90% | 60 | [43] |
| BiVO4/TiO2-NTs | [AB] = 5 mg.L−1 [Catalyst] = thin film | 99.4% | 150 | This work |
| Samples | TiO2 (101) | BiVO4 (004) | Bi2O3 (111) | |||
|---|---|---|---|---|---|---|
| Crystallite Size | Microstrain (×10−3%) | Crystallite Size | Microstrain (×10−3%) | Crystallite Size | Microstrain (×10−3%) | |
| 0 s | 41.09 | 3.82 | - | - | - | - |
| 25 s | 42.45 | 3.76 | - | - | - | - |
| 50 s | 43.74 | 3.65 | - | - | - | - |
| 150 s | 35.65 | 4.48 | 37.9 | 3.33 | 28.16 | 5.99 |
| 250 s | 37.92 | 4.19 | 42.1 | 2.99 | 41.31 | 4.07 |
| Element | Weight% | Atom% | Formula | Compound% |
|---|---|---|---|---|
| O | 40.69 | 64.94 | O | 40.69 |
| Na | 8.89 | 9.88 | Na | 8.89 |
| Ti | 45.56 | 24.29 | Ti | 45.56 |
| V | 0.79 | 0.39 | V | 0.79 |
| Bi | 4.08 | 0.50 | Bi | 4.08 |
| Total | 100.00 | 100.00 | 100.00 |
| Samples | Crystallite Size [TiO2 (101)] (nm) | Optical Band Energy (eV) |
|---|---|---|
| TiO2 | 41.09 | 3.3 |
| BiVO4/TiO2-25 s | 42.45 | 2.61 |
| BiVO4/TiO2-50 s | 43.74 | 2.6 |
| BiVO4/TiO2-150 s | 35.64 | 2.66 |
| BiVO4/TiO2-250 s | 37.92 | 2.7 |
| Catalyst | Conditions | Photodegradation Efficiency (%) | Degradation Time (min) | Ref. |
|---|---|---|---|---|
| 3DOM BiVO4/TiO2 | Concentration of RhB = 10−3 M Catalyst mass = 20 mg | 80 | 120 | [60] |
| BiVO4/TiO2 nanocomposites | Concentration of AB113 = 40 mg.L−1 Catalyst loading = 1 g.L−1 | 82 | 120 | [61] |
| BiVO4/TiO2 composite | Concentration of MB = 2 × 10−5 M Catalyst loading = 1 g.L−1 | 84 | 120 | [62] |
| BiVO4/TiO2-NTs | Concentration of AB = 5 mg.L−1 Catalyst = thin film | 99.4 | 150 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Mabrouk, K.; Sassi, S.; Khemissi, I.; Benabderrahmane Zaghouani, R.; Khezami, L.; Elfil, H.; Bouich, A.; Mari Soucase, B.; Hajjaji, A. Electrodeposition of BiVO4 Nanoparticles on TiO2 Nanotubes: Characterization and Synergetic Photocatalytic Degradation Activity of Amido Black Dye. Molecules 2025, 30, 4283. https://doi.org/10.3390/molecules30214283
Ben Mabrouk K, Sassi S, Khemissi I, Benabderrahmane Zaghouani R, Khezami L, Elfil H, Bouich A, Mari Soucase B, Hajjaji A. Electrodeposition of BiVO4 Nanoparticles on TiO2 Nanotubes: Characterization and Synergetic Photocatalytic Degradation Activity of Amido Black Dye. Molecules. 2025; 30(21):4283. https://doi.org/10.3390/molecules30214283
Chicago/Turabian StyleBen Mabrouk, Kawther, Syrine Sassi, Ines Khemissi, Rabia Benabderrahmane Zaghouani, Lotfi Khezami, Hamza Elfil, Amal Bouich, Bernabé Mari Soucase, and Anouar Hajjaji. 2025. "Electrodeposition of BiVO4 Nanoparticles on TiO2 Nanotubes: Characterization and Synergetic Photocatalytic Degradation Activity of Amido Black Dye" Molecules 30, no. 21: 4283. https://doi.org/10.3390/molecules30214283
APA StyleBen Mabrouk, K., Sassi, S., Khemissi, I., Benabderrahmane Zaghouani, R., Khezami, L., Elfil, H., Bouich, A., Mari Soucase, B., & Hajjaji, A. (2025). Electrodeposition of BiVO4 Nanoparticles on TiO2 Nanotubes: Characterization and Synergetic Photocatalytic Degradation Activity of Amido Black Dye. Molecules, 30(21), 4283. https://doi.org/10.3390/molecules30214283









