Black Chokeberry Extracts (Aronia melanocarpa) as an Ingredient of Functional Food—Potential, Challenges and Directions of Development
Abstract
1. Introduction
2. Functional Food—Definitions and Market Significance
3. Black Chokeberry—Health-Promoting Properties and Bioactive Composition
4. Modern Techniques for Obtaining Plant Extracts
4.1. Ultrasound-Assisted Extraction
4.2. Microwave-Assisted Extraction
4.3. Supercritical Fluid Extraction
4.4. Accelerated Solvent Extraction
4.5. Extraction with Natural Eutectic Liquids
4.6. Enzyme-Assisted Extraction
4.7. Pulsed Electric Field
4.8. Hybrid Extractions and an Integrated Approach
5. Stabilization of Extracts Using Microencapsulation
6. Aronia Extracts—Methods of Obtaining
7. Application and Stability of Extracts in Processing
7.1. Functional Enrichment and Polyphenol Stability in Aronia-Enriched Foods
7.2. Antioxidant and Antimicrobial Roles of Aronia Extracts in Food Preservation
7.3. Encapsulation and Functional Stabilization Strategies for Aronia Bioactives
7.4. Sensory Impact of Chokeberry Extracts in Food Products
8. Future Challenges and Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef]
- Rutkowska, J.; Pasqualone, A. Plant Extracts as Functional Food Ingredients. Foods 2025, 14, 374. [Google Scholar] [CrossRef]
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Sreedharan, S.; Nair, V.; Bhargava, P.; Cisneros-Zevallos, L. Protective Role of Polyphenols from Aronia Berry (Aronia melanocarpa) Against LPS-Induced Inflammation in Colon Cells and Macrophages. Nutrients 2025, 17, 1652. [Google Scholar] [CrossRef] [PubMed]
- Chamberlin, M.L.; Peach, J.T.; Wilson, S.M.G.; Miller, Z.T.; Bothner, B.; Walk, S.T.; Yeoman, C.J.; Miles, M.P. Polyphenol-Rich Aronia melanocarpa Fruit Beneficially Impact Cholesterol, Glucose, and Serum and Gut Metabolites: A Randomized Clinical Trial. Foods 2024, 13, 2768. [Google Scholar] [CrossRef]
- Zapolska-Downar, D.; Bryk, D.; Małecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur. J. Nutr. 2011, 51, 563–572. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.; Zheng, M.; Sheng, L.; Shi, D.; Song, K. A Comprehensive Review of the Functional Potential and Sustainable Applications of Aronia melanocarpa in the Food Industry. Plants 2024, 13, 3557. [Google Scholar] [CrossRef]
- Raczkowska, E.; Nowicka, P.; Wojdyło, A.; Styczyńska, M.; Lazar, Z. Chokeberry Pomace as a Component Shaping the Content of Bioactive Compounds and Nutritional, Health-Promoting (Anti-Diabetic and Antioxidant) and Sensory Properties of Shortcrust Pastries Sweetened with Sucrose and Erythritol. Antioxidants 2022, 11, 190. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.; Kapoor, A.; Vo, D.-N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef] [PubMed]
- Intrasook, J.; Tsusaka, T.W.; Anal, A.K. Trends and current food safety regulations and policies for functional foods and beverages containing botanicals. J. Food Drug Anal. 2024, 32, 112–139. [Google Scholar] [CrossRef]
- Diplock, A.T.; Aggett, P.J.; Ashwell, M.; Bornet, F.; Fern, E.B.; Roberfroid, M.B. Scientific Concepts of Functional Foods in Europe—Consensus Document. Br. J. Nutr. 1999, 81 (Suppl. S1), S1–S27. [Google Scholar] [CrossRef]
- Iwatani, S.; Yamamoto, N. Functional food products in Japan: A review. Food Sci. Hum. Wellness 2019, 8, 96–101. [Google Scholar] [CrossRef]
- Sadohara, R.; Martirosyan, D. Functional Food Center’s Vision on Functional Food Definition and Science in Comparison to FDA’s Health Claim Authorization and Japan’s Foods for Specified Health Uses. Funct. Foods Health Dis. 2020, 10, 465–481. [Google Scholar] [CrossRef]
- Obayomi, O.V.; Olaniran, A.F.; Owa, S.O. Unveiling the Role of Functional Foods with Emphasis on Prebiotics and Probiotics in Human Health: A Review. J. Funct. Foods 2024, 119, 106337. [Google Scholar] [CrossRef]
- Martirosyan, D.; Kanya, H.; Nadalet, C. Can Functional Food Reduce the Risk of Disease? Advancement of Functional Food Definition and Steps to Create Functional Food Products. Funct. Foods Health Dis. 2021, 11, 213–221. [Google Scholar] [CrossRef]
- Egbuna, C.; Tupas, G.D. (Eds.) Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Global Market Insights. Functional Foods Market Size, Share & Growth Trend 2025–2034. 2025. Available online: https://www.gminsights.com/industry-analysis/functional-foods-market (accessed on 7 July 2025).
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Benson, T.; Lavelle, F.; Bucher, T.; McCloat, A.; Mooney, E.; Egan, B.; Collins, C.E.; Dean, M. The Impact of Nutrition and Health Claims on Consumer Perceptions and Portion Size Selection: Results from a Nationally Representative Survey. Nutrients 2018, 10, 656. [Google Scholar] [CrossRef]
- Fernqvist, F.; Spendrup, S.; Tellström, R. Understanding food choice: A systematic review of reviews. Heliyon 2024, 10, e32492. [Google Scholar] [CrossRef]
- Hajzer, Z.E.; Alibrahem, W.; Kharrat Helu, N.; Oláh, C.; Prokisch, J. Functional Foods in Clinical Trials and Future Research Directions. Foods 2025, 14, 2675. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Ageing and Health 2020; WHO Press: Geneve, Switzerland, 2020. [Google Scholar]
- Shi, D.; Xu, J.; Sheng, L.; Song, K. Comprehensive Utilization Technology of Aronia melanocarpa. Molecules 2024, 29, 1388. [Google Scholar] [CrossRef]
- Piasecka, I.; Wiktor, A.; Górska, A. Alternative Methods of Bioactive Compounds and Oils Extraction from Berry Fruit By-Products—A Review. Appl. Sci. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Do Thi, N.; Hwang, E. Effects of Drying Methods on Contents of Bioactive Compounds and Antioxidant Activities of Black Chokeberries (Aronia melanocarpa). Food Sci. Biotechnol. 2016, 25, 55–61. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Chrubasik, C.; Li, G.; Chrubasik, S. The Clinical Effectiveness of Chokeberry: A Systematic Review. Phytother. Res. 2010, 24, 1107–1114. [Google Scholar] [CrossRef]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, Anthocyanins and Procyanidins from Aronia melanocarpa as Radical Scavengers and Enzyme Inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef]
- Francik, R.; Krośniak, M.; Sanocka, I.; Bartoń, H.; Hebda, T.; Francik, S. Aronia melanocarpa Treatment and Antioxidant Status in Selected Tissues in Wistar Rats. BioMed Res. Int. 2014, 2014, 457085. [Google Scholar] [CrossRef]
- Dufour, C.; Villa-Rodriguez, J.A.; Furger, C.; Lessard-Lord, J.; Gironde, C.; Rigal, M.; Badr, A.; Desjardins, Y.; Guyonnet, D. Cellular Antioxidant Effect of an Aronia Extract and Its Polyphenolic Fractions Enriched in Proanthocyanidins, Phenolic Acids, and Anthocyanins. Antioxidants 2022, 11, 1561. [Google Scholar] [CrossRef]
- Sasmaz, H.K.; Kilic-Buyukkurt, Ö.; Selli, S.; Bouaziz, M.; Kelebek, H. Antioxidant Capacity, Sugar Content, and Tandem HPLC–DAD–ESI/MS Profiling of Phenolic Compounds from Aronia melanocarpa Fruits and Leaves (Nero and Viking Cultivars). ACS Omega 2024, 9, 14963–14976. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Xu, H.; Zhu, F.; Li, Z.; Lu, H.; Zhang, J.; Yang, Z.; Liu, Y. The Protective Effect of Anthocyanins Extracted from Aronia melanocarpa Berry in Renal Ischemia-Reperfusion Injury in Mice. Mediat. Inflamm. 2021, 2021, 7372893. [Google Scholar] [CrossRef]
- Kaloudi, T.; Tsimogiannis, D.; Oreopoulou, V. Aronia melanocarpa: Identification and Exploitation of Its Phenolic Components. Molecules 2022, 27, 4375. [Google Scholar] [CrossRef]
- Bushmeleva, K.; Vyshtakalyuk, A.; Terenzhev, D.; Belov, T.; Nikitin, E.; Zobov, V. Aronia melanocarpa Flavonol Extract—Antiradical and Cytoprotective Effects. Antioxidants 2023, 12, 2976. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- Dobros, N.; Zielińska, A.; Siudem, P.; Zawada, K.D.; Paradowska, K. Profile of Bioactive Components and Antioxidant Activity of Aronia melanocarpa Fruits at Various Stages of Their Growth, Using Chemometric Methods. Antioxidants 2024, 13, 462. [Google Scholar] [CrossRef]
- Mania, S.; Staszczyk, K.; Banach-Kopeć, A.; Krawcewicz, R.; Mielewczyk-Gryń, A.; Tylingo, R. From waste to value: Functionalized chokeberry-filled PLA filaments for 3D printing. J. Environ. Chem. Eng. 2025, 13, 118832. [Google Scholar] [CrossRef]
- Kaczmarczyk, S.; Dziewiecka, H.; Pasek, M.; Ostapiuk-Karolczuk, J.; Kasparska, A.; Skarpińska-Stejnborn, A. Effects of Black Chokeberry (Aronia melanocarpa) Supplementation on Oxidative Stress, Inflammation and Gut Microbiota: A Systematic Review of Human and Animal Studies. Br. J. Nutr. 2025, 133, 58–81. [Google Scholar] [CrossRef]
- Sarıkaya, B.; Kolay, E.; Guney-Coskun, M.; Yiğit-Ziolkowski, A. The Effect of Black Chokeberry (Aronia melanocarpa) on Human Inflammation Biomarkers and Antioxidant Enzymes: A Systematic Review of Randomized Controlled Trials. Nutr. Rev. 2024, 83, 1083–1098. [Google Scholar] [CrossRef]
- Mesárošová, A.; Bobko, M.; Jurčaga, L.; Bobková, A.; Poláková, K.; Demianová, A.; Lidiková, J.; Bučko, O.; Tóth, T.; Mendelová, A. Chokeberry (Aronia melanocarpa) as a Natural Antioxidant for the Meat Industry. Czech J. Food Sci. 2024, 42, 69–76. [Google Scholar] [CrossRef]
- Skąpska, S.; Marszałek, K.; Woźniak, Ł.; Zawada, K.; Wawer, I. Aronia Dietary Drinks Fortified with Selected Herbal Extracts Preserved by Thermal Pasteurization and High Pressure Carbon Dioxide. LWT Food Sci. Technol. 2017, 85, 423–426. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Liu, W.-Y.; Shi, Y.-C.; Wu, S.-C. Unveiling the Therapeutic Benefits of Black Chokeberry (Aronia melanocarpa) in Alleviating Hyperuricemia in Mice. Front. Nutr. 2025, 12, 1556527. [Google Scholar] [CrossRef]
- Siyahli, O.; Tırpancı Sivri, G.; Demirci, A.Ş. Probiotic stability and bioactive enhancement in black chokeberry (Aronia melanocarpa) juice: A functional beverage study. J. Sci. Food Agric. 2025, 105, 1–10. [Google Scholar] [CrossRef]
- Lupu, M.I.; Canja, C.M.; Maier, A.; Padureanu, V.; Branescu, G.R.; Manolica, A.-M. The Impact of the Infusion Method of Chokeberry Powder in White Tea. Carpathian J. Food Sci. Technol. 2024, 16, 28–39. [Google Scholar] [CrossRef]
- Broncel, M.; Koziróg-Kołacińska, M.; Andryskowski, G.; Duchnowicz, P.; Koter-Michalak, M.; Owczarczyk, A.; Chojnowska-Jezierska, J. Effect of anthocyanins from Aronia melanocarpa on blood pressure, concentration of endothelin-1 and lipids in patients with metabolic syndrome. Pol. Merkur. Lek. 2007, 23, 116–119. [Google Scholar]
- Wen, H.; Cui, H.; Tian, H.; Zhang, X.; Ma, L.; Ramassamy, C.; Li, J. Isolation of Neuroprotective Anthocyanins from Black Chokeberry (Aronia melanocarpa) against Amyloid-β-Induced Cognitive Impairment. Foods 2021, 10, 63. [Google Scholar] [CrossRef]
- Young Go, M.; Kim, J.; Young Jeon, C.; Wook Shin, D. Functional Activities and Mechanisms of Aronia melanocarpa in Our Health. Curr. Issues Mol. Biol. 2024, 46, 8071–8087. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Doma, A.O.; Cristina, R.T.; Dumitrescu, E.; Degi, D.; Moruzi, R.F.; Brezovan, D.; Petroman, I.; Muselin, F. The antioxidant effect of Aronia melanocarpa extract in rats oxidative stress induced by cisplatin administration. J. Trace Elem. Med. Biol. 2023, 79, 127205. [Google Scholar] [CrossRef]
- Trusov, N.V.; Shipelin, V.A.; Mzhelskaya, K.V.; Shumakova, A.A.; Timonin, A.N.; Riger, N.A.; Apryatin, S.A.; Gmoshinski, I.V. Effect of resveratrol on behavioral, biochemical, and immunological parameters of DBA/2J and tetrahybrid DBCB mice receiving diet with excess fat and fructose. J. Nutr. Biochem. 2021, 88, 108527. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A Review on the Characteristic Components and Potential Health Effects. Planta Hum. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Soja, J.; Gancarz, M.; Wojtunik-Kulesza, K.; Markut-Miotła, E.; Oniszczuk, A. The Efficacy of Black Chokeberry Fruits against Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 6541. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Mocan, A.; Rohn, S.; Gavrilaș, L. Impact of a Chokeberry (Aronia melanocarpa (Michx.) Elliott) Supplementation on Cardiometabolic Outcomes: A Critical Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2025, 17, 1488. [Google Scholar] [CrossRef]
- Shah, K.; Shah, P. Effect of Anthocyanin Supplementations on Lipid Profile and Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cholesterol 2018, 2018, 8450793. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Jin, H.; Qian, S.; Chen, P.; Wang, M.; Chen, N.; Ding, L. Comparison of Antioxidant Capacity and Muscle Amino Acid and Fatty Acid Composition of Nervous and Calm Hu Sheep. Antioxidants. 2023, 12, 459. [Google Scholar] [CrossRef]
- Palai, S.; Shekhawat, N. Novel Extraction Techniques for Phytochemicals: A Comprehensive Review. Int. J. Pharm. Res. 2022, 14, 21–30. [Google Scholar]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Bitwell, C.; Sen, I.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Lazović, M.Č.; Jović, M.D.; Petrović, M.; Dimkić, I.Z.; Gašić, U.M.; Milojković Opsenica, D.M.; Ristivojević, P.M.; Trifković, J.Đ. Potential Application of Green Extracts Rich in Phenolics for Innovative Functional Foods: Natural Deep Eutectic Solvents as Media for Isolation of Biocompounds from Berries. Food Funct. 2024, 15, 4122–4139. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Kordowska-Wiater, M.; Sosnowska, B.; Pytka, M. Effects of Plant Extracts on Microorganisms; Lublin University of Life Sciences Press: Lublin, Poland, 2020; p. 27. [Google Scholar]
- Kuzmanović Nedelijković, S.; Radan, M.; Ćujić Nikolić, N.; Mutavski, Z.; Krgović, N.; Marković, S.; Stević, T.; Živković, J.; Šavkin, K. Microencapsulated Bilberry and Chokeberry Leaf Extracts with Potential Health Benefits. Plants 2023, 12, 3979. [Google Scholar] [CrossRef]
- Zhu, R.; Shen, J.; Law, C.L.; Ma, X.; Li, D.; Han, Y.; Kiani, H.; Manickam, S.; Tao, Y. Combined calcium pretreatment and ultrasonic/microwave drying to dehydrate black chokeberry: Novel mass transfer modeling and metabolic pathways of polyphenols. Innov. Food Sci. Emerg. Technol. 2023, 83, 103215. [Google Scholar] [CrossRef]
- Zlabur, J.; Dobricevic, N.; Pliestić, S.; Galić, A.; Bilić, D.; Voca, S. Antioxidant Potential of Fruit Juice with Added Chokeberry Powder (Aronia melanocarpa). Molecules 2017, 22, 2158. [Google Scholar] [CrossRef]
- Gheorghita, R.E.; Lupaescu, A.V.; Gâtlan, A.M.; Dabija, D.; Lobiuc, A.; Iatcu, O.C.; Buculei, A.; Andriesi, A.; Dabija, A. Biopolymers-Based Macrogels with Applications in the Food Industry: Capsules with Berry Juice for Functional Food Products. Gels 2024, 10, 71. [Google Scholar] [CrossRef]
- Galván D’Alessandro, L.; Vauchel, P.; Przybylski, R.; Chataigné, G.; Nikov, I.; Dimitrov, K. Integrated Process Extraction–Adsorption for Selective Recovery of Antioxidant Phenolics from Aronia melanocarpa Berries. Molecules 2013, 120, 92–101. [Google Scholar] [CrossRef]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a Noble Technique for the Application of Bioactive Compounds in the Food Industry: A Comprehensive Review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Tzatsi, P.; Goula, A. Encapsulation of Extract from Unused Chokeberries by Spray Drying, Co-Crystallization, and Ionic Gelation. Waste Biomass Valorization 2021, 12, 4567–4585. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Bleive, U.; Kaldmäe, H.; Aluvee, A.; Ratsep, R.; Sats, A.; Pap, N.; Jarvenpaa, E.; Rinken, T. Characterization of Plant-Based Spray-Dried Powders Using Oilseed Proteins and Chokeberry Extract from Wine By-Product. Sci. Rep. 2024, 14, 27429. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Kudra, T.; Mujumdar, A.S.; Kudra, T. Advanced Drying Technologies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Chezanoglou, E.; Goula, C.M. Co-crystallization in sucrose: A promising method for encapsulation of food bioactive components. Trends Food Sci. Technol. 2021, 114, 262–274. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic Hydrocolloids for the Intestinal Delivery of Protein Drugs: Alginate and Chitosan—A Review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Ferrarese, I.; Carmo Dos Santos, N.; Rizzo, F.; Gargari, G.; Bertoli, N.; Gobbi, E.; Perosa, A.; Selva, M.; Dall’Acqua, S. Sustainable Extraction of Bioactive Compounds and Nutrients from Agri-Food Wastes: Potential Reutilization of Berry, Honey, and Chicory Byproducts. Appl. Sci. 2024, 14, 10785. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and Characterization of Phenolic Compounds and Their Potential Antioxidant Activities. Environ. Sci. Pollut. Res. Int. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Ivanova, M.; Petkova, N.; Balabanova, T.; Ognyanov, M.; Vlaseva, R. Food Design of Dairy Desserts with Encapsulated Cornelian Cherry, Chokeberry and Blackberry Juices. Ann. Univ. Dunarea De Jos Galati Fasc. VI Food Technol. 2018, 42, 137–146. [Google Scholar]
- Wang, J.; Wang, J.; Hao, J.; Jiang, M.; Zhao, C.; Fan, Z. Antioxidant Activity and Structural Characterization of Anthocyanin–Polysaccharide Complexes from Aronia melanocarpa. Int. J. Mol. Sci. 2024, 25, 13347. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Smeriglio, A.; Trombetta, D.; Nieddu, M.; Piras, F.; Sogos, V.; Rosa, A. Chemical Composition, Nutritional, and Biological Properties of Extracts Obtained with Different Techniques from Aronia melanocarpa Berries. Molecules 2024, 29, 2577. [Google Scholar] [CrossRef]
- Sönmez Gürer, E.; Karadağ, A.E.; Altıntaş, A. Comparison of Chemical Profiles of Aronia melanocarpa Fruit Extracts. Turk. J. Agric. Food Sci. Technol. 2023, 11, 323–328. [Google Scholar] [CrossRef]
- Park, Y.; Puligundla, P.; Mok, C. Decontamination of Chokeberries (Aronia melanocarpa L.) by Cold Plasma Treatment and Its Effects on Biochemical Composition and Storage Quality of Their Corresponding Juices. Food Sci. Biotechnol. 2021, 30, 405–411. [Google Scholar] [CrossRef]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern methods of pre-treatment of plant material for the extraction of bioactive compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef]
- Martins, R.F.; Advinha, B.; Barbosa, A.; Sales, H.J.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit. Rev. Food Sci. Nutr. 2010, 50, 146–161. [Google Scholar] [CrossRef]
- Kowalska, J.; Marzec, A.; Domian, E.; Galus, S.; Ciurzyńska, A.; Lenart, A.; Kowalska, H. The Use of Antioxidant Potential of Chokeberry Juice in Creating Pro-Healthy Dried Apples by Hybrid (Convection-Microwave-Vacuum) Method. Molecules 2020, 25, 5680. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Popkowicz, P.; Galus, S.; Janowicz, M. Innovative Freeze-Dried Snacks with Sodium Alginate and Fruit Pomace (Only Apple or Only Chokeberry) Obtained within the Framework of Sustainable Production. Molecules 2022, 27, 3095. [Google Scholar] [CrossRef]
- Masztalerz, K.; Łyczko, J.; Lech, K.; Szumny, A.; Figiel, A. The Effect of Filtrated Osmotic Solutions Based on Chokeberry Juice Enriched with Mint Extract on Volatile Compounds in Dried Apples. J. Food Process Eng. 2021, 44, e13803. [Google Scholar] [CrossRef]
- Sadowska, A.; Świderski, F.; Hallmann, E.; Świąder, K. Assessment of Chokeberry Powders Quality Obtained Using an Innovative Fluidized-Bed Jet Milling and Drying Method with Pre-Drying Compared with Convection Drying. Foods 2021, 10, 292. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Brzozowska, A.; Gramza-Michałowska, A. The Effect of Plant Additives on the Stability of Polyphenols in Dried Black Chokeberry. Foods 2021, 10, 44. [Google Scholar] [CrossRef]
- Repajić, M.; Zorić, M.; Magnabosca, I.; Pedisić, S.; Dragović-Uzelac, V.; Elez Garofulić, I. Bioactive Power of Black Chokeberry Pomace as Affected by Advanced Extraction Techniques and Cryogrinding. Molecules 2025, 30, 3383. [Google Scholar] [CrossRef]
- Nemetz, N.; Schieber, A.; Weber, F. Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff. Molecules 2021, 26, 2689. [Google Scholar] [CrossRef]
- Żbikowska, A.; Łukasiak, P.; Kowalska, M.; Łukasiak, A.; Kozłowska, M.; Marciniak-Łukasiak, K. Incorporation of Chokeberry Pomace into Baked Products: Influence on the Quality of the Dough and the Muffins. Appl. Sci. 2024, 14, 9675. [Google Scholar] [CrossRef]
- Karwacka, M.; Ciurzyńska, A.; Galus, S.; Janowicz, M. The Effect of Storage Time and Temperature on Quality Changes in Freeze-Dried Snacks Obtained with Fruit Pomace and Pectin Powders as a Sustainable Approach for New Product Development. Sustainability 2024, 16, 4736. [Google Scholar] [CrossRef]
- Drożdż, W.; Boruczkowska, H.; Boruczkowski, T.; Tomaszewska-Ciosk, E.; Zdybel, E. Use of Blackcurrant and Chokeberry Press Residue in Snack Products. Pol. J. Chem. Technol. 2019, 21, 13–19. [Google Scholar] [CrossRef]
- Babaoglu, A.; Unal, K.; Dilek, N.; Pocan, H.; Karakaya, M. Antioxidant and Antimicrobial Effects of Blackberry, Black Chokeberry, Blueberry, and Red Currant Pomace Extracts on Beef Patties Subject to Refrigerated Storage. Meat Sci. 2022, 187, 108794. [Google Scholar] [CrossRef] [PubMed]
- Strugała-Danak, P.; Gładkowski, W.; Kucharska, A.; Sokół-Łętkowska, A.; Gabrielska, J. Antioxidant Activity and Anti-Inflammatory Effect of Fruit Extracts from Blackcurrant, Chokeberry, Hawthorn, and Rosehip, and Their Mixture with Linseed Oil on a Model Lipid Membrane. Eur. J. Lipid Sci. Technol. 2016, 118, 461–474. [Google Scholar] [CrossRef]
- Eisinaitė, V.; Kazernavičiūtė, R.; Kaniauskienė, I.; Venskutonis, P.; Leskauskaitė, D. Effect of Black Chokeberry Pomace Extract Incorporation on Physical and Oxidative Stability of Water-Inioil-in-Water Emulsion. J. Sci. Food Agric. 2021, 101, 4570–4577. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cristea, E.; Sturza, R.; Niculaua, M.; Patras, A. Synthetic Dye Substitution with Chokeberry Extract in Jelly Candies. J. Food Sci. Technol. 2020, 57, 4383–4394. [Google Scholar] [CrossRef]
- Wathon, M.H.; Beaumont, N.; Benohoud, M.; Blackburn, R.S.; Rayner, C. Extraction of anthocyanins from Aronia melanocarpa skin waste as a sustainable source of natural colorants. Color. Technol. 2019, 135, 5–16. [Google Scholar] [CrossRef]
- Koç, S.T.; Kök, S.; Atalay, S.; Ersoy, O. Effect of Microencapsulated and Nonencapsulated Aronia Extract on Paraoxonase 1 Gene Expression and Aortic Histopathology in High-Fat Diet-Fed Rats. J. Med. Food 2025, 28, 885–896. [Google Scholar] [CrossRef]
- Szajnar, K.; Pawlos, M.; Znamirowska, A. The Effect of the Addition of Chokeberry Fiber on the Quality of Sheep’s Milk Fermented by Lactobacillus rhamnosus and Lactobacillus acidophilus. Int. J. Food Sci. 2021, 2021, 7928745. [Google Scholar] [CrossRef]
- Repajić, M.; Elez Garofulić, I.; Cegledi, E.; Dobroslavić, E.; Peisić, S.; Durgo, K.; Huđek Turković, A.; Mrvčić, J.; Hanousek Čiča, K.; Dragović-Uzelac, V. Bioactive and Biological Potential of Black Chokeberry Leaves Under the Influence of Pressurized Liquid Extraction and Microwave-Assisted Extraction. Antioxidants 2024, 13, 1582. [Google Scholar] [CrossRef]
- Tolić, M.-T.; Landeka Jurčević, I.; Panjkota Krbavčić, I.; Marković, K.; Vahčić, N. Phenolic content, antioxidant capacity and quality of chokeberry (Aronia melanocarpa) products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Cichowska, J.; Samborska, K.; Kowalska, H. Influence of Chokeberry Juice Concentrate Used as Osmotic Solution on the Quality of Differently Dried Apples During Storage. Eur. Food Res. Technol. 2018, 244, 1773–1782. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Gałązka-Czarnecka, I.; Otlewska, A.; Czyżowska, A.; Nowak, A. Aronia melanocarpa (Michx.) Elliot, Chaenomeles superba Lindl. and Cornus mas L. leaf extracts as natural preservatives for pork meat products. Molecules 2021, 26, 3009. [Google Scholar] [CrossRef]
- Olas, B.; Wachowicz, B.; Nowak, P.; Kędzierska, M.; Tomczak, A.; Stochmal, A.; Oleszek, W.; Jeziorski, A.; Piekarski, J. Studies on antioxidant properties of polyphenol-rich extract from berries of Aronia melanocarpa in blood platelets. J. Physiol. Pharmacol. 2008, 59, 823–835. [Google Scholar]
- Li, S.; Chen, J.; Sarengaowa; Chen, C.; Hu, W. Application of Procyanidins from Aronia melanocarpa (Michx.) Elliott in Fresh-Cut Apple Preservation. Horticulturae 2024, 10, 556. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Domaradzki, P.; Materska, M.; Florek, M.; Kaliniak-Dziura, A.; Skałecki, P.; Żółkiewski, P.; Grenda, T.; Pabich, M. Effect of the Addition of Chokeberry Leaf Extract on the Physicochemical and Sensory Properties of Burgers from Dark Cutting Veal. Food Chem. 2023, 399, 133937. [Google Scholar] [CrossRef]
- Saglam, A.; Asan-Ozusaglam, M. Development of edible films containing Aronia (Aronia melanocarpa) and probiotic. J. Microbiol. Biotechnol. Food Sci. 2025, 14, e11152. [Google Scholar] [CrossRef]
- Aydogdu Emir, A.; Yıldız, E.; Öz, E.; Amarowicz, R.; Proestos, C.; Khan, M.R.; Elobeid, T.; Öz, F. Development of simultaneous antioxidant and visual pH-sensing films based on guar gum loaded with Aronia melanocarpa extract. Int. J. Food Sci. Technol. 2023, 58, 58–4376. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, S.; Shang, D.; Hamid, N.; Ma, Q.; Xiao, Y.; Ren, L.; Liu, G.; Sun, A. Enhancing stability and physiological activity of Aronia melanocarpa L. anthocyanin by polysaccharides. J. Sci. Food Agric. 2025, 105, 3052–3063. [Google Scholar] [CrossRef] [PubMed]
- Samborska, K.; Eliasson, L.; Marzec, A.; Kowalska, J.; Piotrowski, D.; Lenart, A.; Kowalska, H. The Effect of Adding Berry Fruit Juice Concentrates and By-Product Extract to Sugar Solution on Osmotic Dehydration and Sensory Properties of Apples. J. Food Sci. Technol. 2019, 56, 1927–1938. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Petković, M.; Filipović, V.; Lončar, B.; Filipović, J.; Miletić, N.; Malasević, Z.; Jevremović, D. A Comparative Analysis of Thin-Layer Microwave and Microwave/Convective Dehydration of Chokeberry. Foods 2023, 12, 1651. [Google Scholar] [CrossRef]
- Habschied, K.; Nisević, J.; Krstanović, V.; Lončarić, A.; Lendić, K.; Mastanjević, K. Formulation of a Wort-Based Beverage with the Addition of Chokeberry (Aronia melanocarpa) Juice and Mint Essential Oil. Appl. Sci. 2023, 13, 2334. [Google Scholar] [CrossRef]
- Wawer, I.; Gralec, M.; Zawada, K. Aronia melanocarpa berries: Phenolics composition and antioxidant properties changes during fruit development and ripening. Emir. J. Food Agric. 2019, 31, 214–221. [Google Scholar] [CrossRef]
- Arshad, Z.; Shahid, S.; Hasnain, A.; Yaseen, E.; Rahimi, M. Functional Foods Enriched with Bioactive Compounds: Therapeutic Potential and Technological Innovations. Food Sci. Nutr. 2025, 13, e71024. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, X.; Zheng, H.; Li, J.; Wu, X.; Xu, J.; Zhen, Z.; Du, C. The Application of Encapsulation Technology in the Food Industry: Classifications, Recent Advances, and Perspectives. Food Chem. X 2024, 21, 101240. [Google Scholar] [CrossRef] [PubMed]
- Pedisić, S.; Zorić, Z.; Repajić, M.; Levaj, B.; Dobrinčić, A.; Balbino, S.; Čošić, Z.; Dragović-Uzelac, V.; Elez Garofulić, I. Valorization of Berry Fruit By-Products: Bioactive Compounds, Extraction, Health Benefits, Encapsulation and Food Applications. Foods 2025, 14, 1354. [Google Scholar] [CrossRef]
- Marino, M.; Venturi, S.; Gargari, G.; Del Bo’, C.; Martini, D.; Porrini, M.; Guglielmetti, S.; Riso, P. Berries-Gut Microbiota Interaction and Impact on Human Health: A Systematic Review of Randomized Controlled Trials. Food Rev. Int. 2024, 40, 2618–2640. [Google Scholar] [CrossRef]
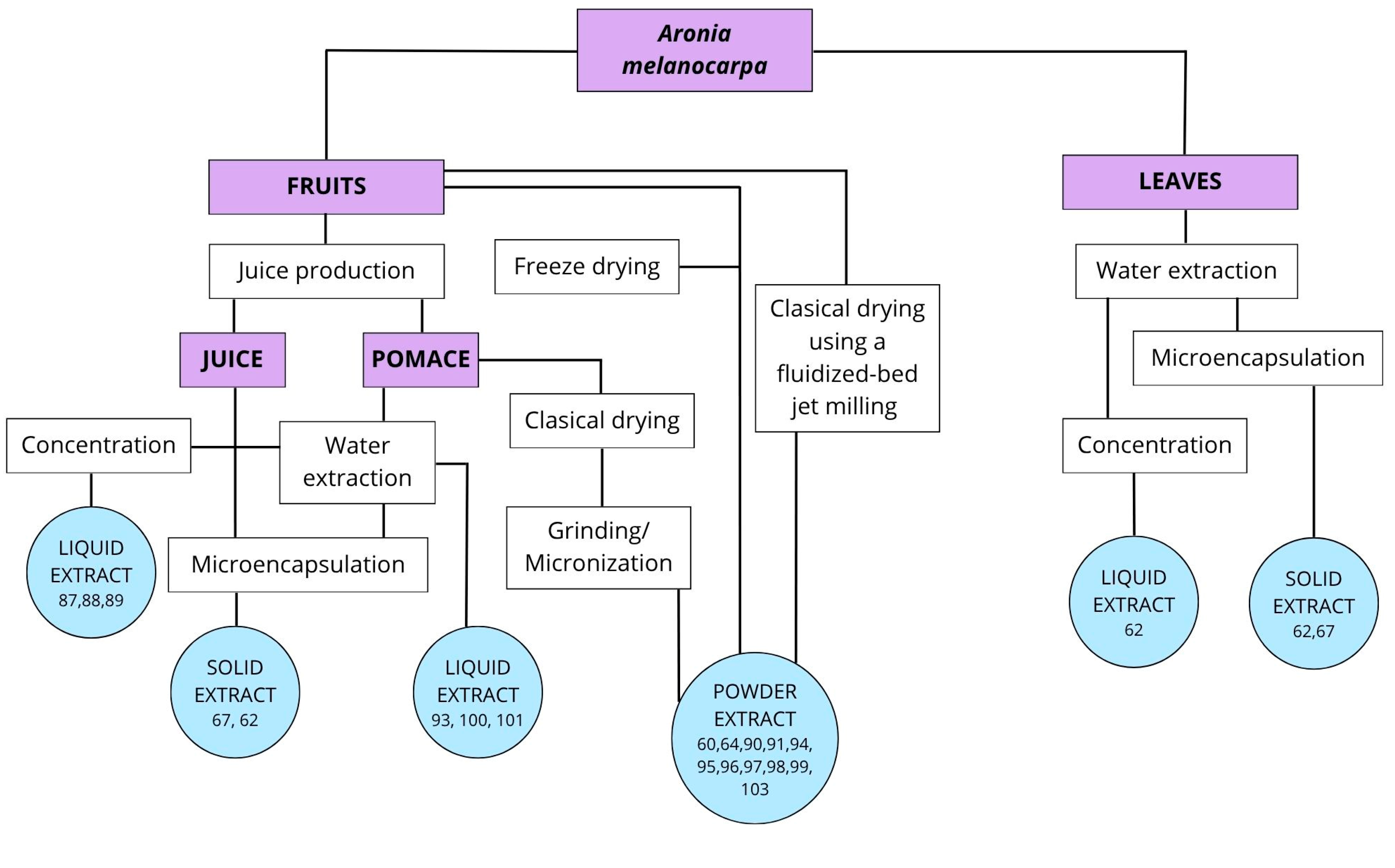
| Bioactive Compounds | Approximate Amount in Fruit | Health Benefits and Functions | |
|---|---|---|---|
| Anthocyanins [32,33] | 600–1000 mg/100 g fresh weight | Cyanidin 3-O-galactoside, Cyanidin 3-O-arabinoside, Cyanidin 3-O-glucoside, Cyanidin 3-O-xyloside | Strong antioxidants; anti-inflammatory; anti-cancer; cardiovascular protection by lowering cholesterol and improving vessel elasticity; improve glucose metabolism; reduce inflammatory markers |
| Phenolic acids [34] | 200–490 mg/100 g fresh weight | Chlorogenic acid (5-O-caffeoylquinic acid), Neochlorogenic acid (3-O-caffeoylquinic acid), dicaffeoylquinic acids | Anti-inflammatory; antioxidant; antibacterial; antidiabetic; DNA protection and chemopreventive effects against cancer; reduce heavy metal toxicity |
| Flavonols [35] | 35–77 mg/100 g fresh weight | Quercetin 3-O-rutinoside, Quercetin 3-O-glucoside, Quercetin 3-O-vicianoside, Isorhamnetin glycosides, Kaempferol derivatives | Potent antioxidants and anti-inflammatory agents; support immune system; promote vascular health |
| Flavanols [36] | 2–3.5 mg/100 g fresh weight | (-)-Epicatechin | Antioxidant; improve glucose metabolism; cardiovascular support |
| Polyol [32] | ~1–2 g/100 g fresh weight | Sorbitol | Acts as a prebiotic; safe for diabetics; supports oral health; mild laxative effect; contributes to sweetness with low glycemic index |
| Dietary fibre [37,38] | 3–8 g/100 g fresh weight | Cellulose (~35%), hemicellulose (~34%), lignins (~24%) | Supports digestive health by promoting beneficial gut bacteria; improves bowel movement; enhances satiety; aids detoxification |
| Ascorbic acid [36] | 8–30 mg/100 g fresh weight | Vitamin C (ascorbic acid) | Strong antioxidant; boosts immune function; supports collagen production; protects cells from oxidative stress |
| Extract Type | Technique | Application | The Added Value of the Enriched Product | References |
|---|---|---|---|---|
| Aronia juice concentrate/juice | Cold pressing, filtration, pasteurization, concentration (up to 65 Brix) | Osmotic impregnation before drying fruit, | Improved colour (ΔE > 6.0), increased antioxidant capacity (DPPH + 45%); inhibition of anthocyanin degradation by 30% during storage | [87,88,89] |
| Aronia powder (freeze-dried) | Freeze-drying (−40 °C, 0.1 mbar for 48 h); convection drying (60 °C for 24 h); Pressurized Liquid Extraction, PLE, Microwave-Assisted Extraction, MAE, Supercritical CO2 Extraction, scCO2. | Sweet confectionery, drinks, dairy desserts, supplements, food additive | Higher polyphenol content (820–900 mg GAE/100 g for freeze-dried vs. 470 mg GAE/100 g for hot air drying); Anthocyanin behaviour above 85%; PLE yielded the highest total phenolics but was less effective for heat-sensitive anthocyanins. MAE achieved a balanced extraction of phenolics and anthocyanins with strong antioxidant activity. scCO2 extraction was more selective and eco-friendly but generally yielded lower phenolics than PLE and MAE. | [64,90,91,92] |
| Aronia pomace | Drying with hot air (50–60 °C); grinding | Bread, snacks, pectin substitutes dairy products | Improvement of fibre content (up to 22% d.m.), reduction in polyphenol losses by up to 10% during baking, increase in moisture retention in baked goods by 15–18% | [93,94,95,96] |
| Conventional ethanol extract rich in phenolic/polyphenolic compounds | Extraction with 50% ethanol (1:10 m/v, 60 °C, 30 min); ultrasound-assisted (20 kHz, 30 min) | Oil emulsions, meat, supplements | Total polyphenols (TPC) up to 2400 mg GAE/100 g; reduction in TBARS in meat by 40–60% during storage (14 days, 4 °C) | [60,97,98,99] |
| Innovative ethanol extract rich in anthocyanin/procyanide | SPE (Solid Phase Extraction) from ethanol and water (50:50), purification on C18 columns. Extraction-adsorption method. | Jelly beans, natural colourants. | Maintaining colour stability (up to 85%) at pH 3–4; inhibition of ascorbic acid oxidation by 52%; colour fastness 28 days at 4 °C. Increased yield and purity of anthocyanin extract produced from chokeberry pomace using a new method compared to the traditional SPE method. | [100,101] |
| Aronia leaf extract | Hydroalcoholic extraction (60% ethanol, 1:15 m/v, 40 °C, 2 h), microencapsulation | Meat products | Reduction in lipid oxidation (TBARS) by 42% in beef burgers, increase in sensory acceptability (panel 8/9 pts.) | [62] |
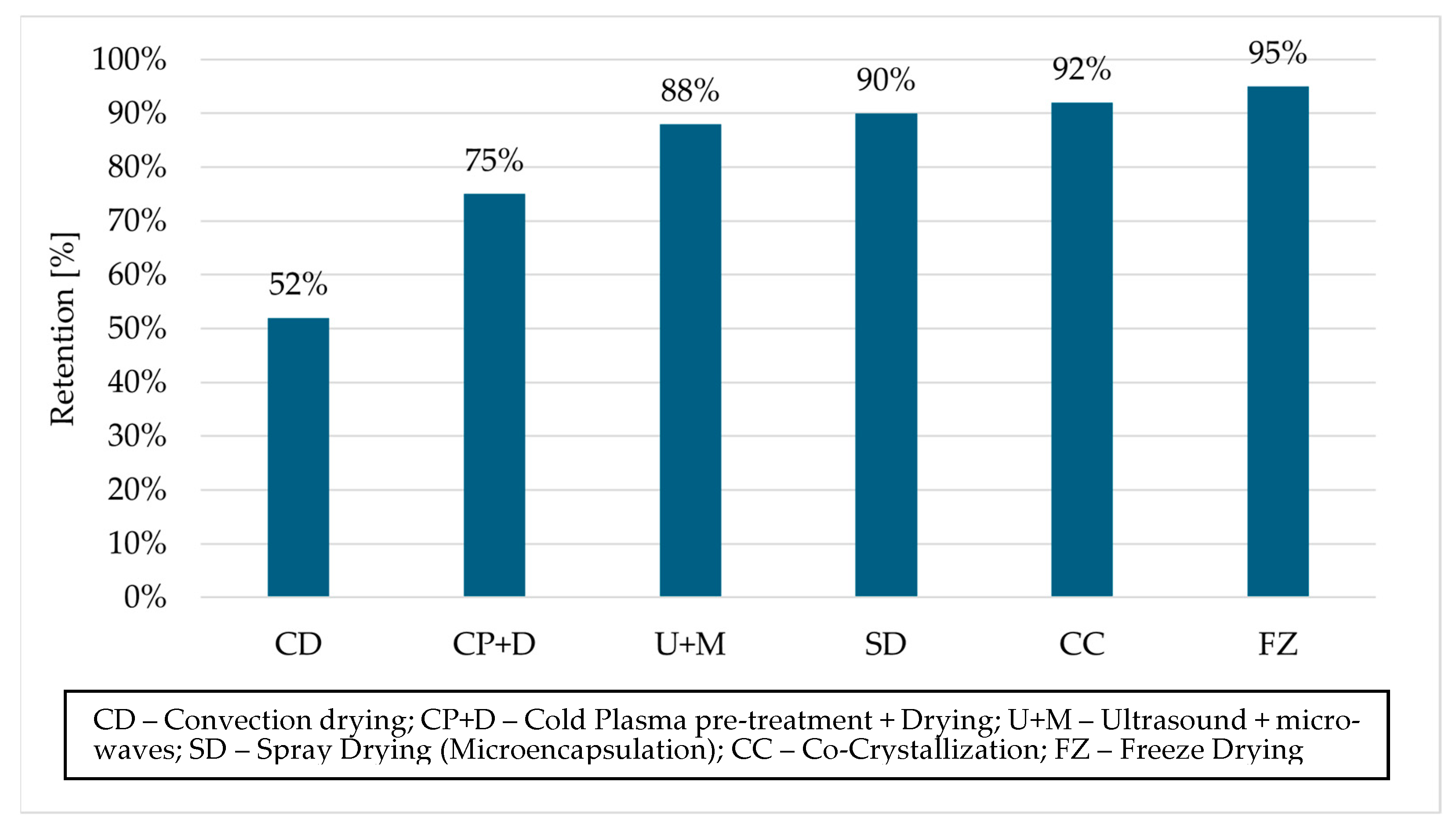
| Microencapsulated Aronia extracts/Nonencapsulated extract | Spray drying (inlet temperature 170 °C, output temperature 80 °C); co-crystallization with maltodextrin or alginate gelation | Yoghurts, dairy desserts, dietary supplements | Retention of 90–95% of polyphenols after 6 weeks of storage, reduction in Maillard reaction, greater stability at pH 4–5 | [67,102] |
| Aronia powder rich in dietary fibre | Pomace drying (55 °C, 24 h), mechanical separation | Fermented products (e.g., sheep’s milk) | Increase in the number of LAB by 1.5 log CFU/mL; improved texture, increase in overall sensory acceptance | [103] |
| Macrogels with aronia juice | Gelation of biopolymers (e.g., carboxymethylcellulose, pea protein) | Functional gummies, gelled products | Anthocyanin retention at 80%, improving antioxidant stability, masking astringent taste | [65] |
| Aronia natura dye extract for replacing synthetic bye E-131, E-162 | l Water-ethanol extraction, filtration | Jelly beans, pastries, drinks | Colour fastness for 4 weeks (4 °C, pH 3.0); 65% increase in ORAC of gummies, compliance with “clean label” standards | [93,100] |
| Fixed oil from berries | Supercritical Fluid Extraction (SFE–CO2) | Oil fraction from whole dried A. melanocarpa berries | Rich in essential fatty acids (≈70% PUFA: linoleic + α-linolenic), high β-carotene and α-tocopherol—nutritional and cosmetic potential | [79] |
| Polyphenol-rich extract from pomace | Pressurized Liquid Extraction (PLE) Microwave-Assisted Extraction (MAE) + cryogrinding preprocessing | Recovery of polyphenols and anthocyanins from chokeberry pomace | Higher initial polyphenol concentration; efficient waste recycling; high functional value product Improved behaviour of heat-sensitive compounds; rapid achievement of high concentrations | [92] |
| Extract from leaves | PLE and MAE | Leaves of A. melanocarpa (rather than fruit) | Allows use of leaves as raw material; varies polyphenol profile according to technique—greater flexibility in functional production | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieloch, D.; Konopacka, D. Black Chokeberry Extracts (Aronia melanocarpa) as an Ingredient of Functional Food—Potential, Challenges and Directions of Development. Molecules 2025, 30, 4237. https://doi.org/10.3390/molecules30214237
Wieloch D, Konopacka D. Black Chokeberry Extracts (Aronia melanocarpa) as an Ingredient of Functional Food—Potential, Challenges and Directions of Development. Molecules. 2025; 30(21):4237. https://doi.org/10.3390/molecules30214237
Chicago/Turabian StyleWieloch, Dawid, and Dorota Konopacka. 2025. "Black Chokeberry Extracts (Aronia melanocarpa) as an Ingredient of Functional Food—Potential, Challenges and Directions of Development" Molecules 30, no. 21: 4237. https://doi.org/10.3390/molecules30214237
APA StyleWieloch, D., & Konopacka, D. (2025). Black Chokeberry Extracts (Aronia melanocarpa) as an Ingredient of Functional Food—Potential, Challenges and Directions of Development. Molecules, 30(21), 4237. https://doi.org/10.3390/molecules30214237








