Discovering the Bioactive and Antibacterial Potential of Essential Oils from Aromatic Plants of Northeastern Peru
Abstract
1. Introduction
2. Results and Discussion
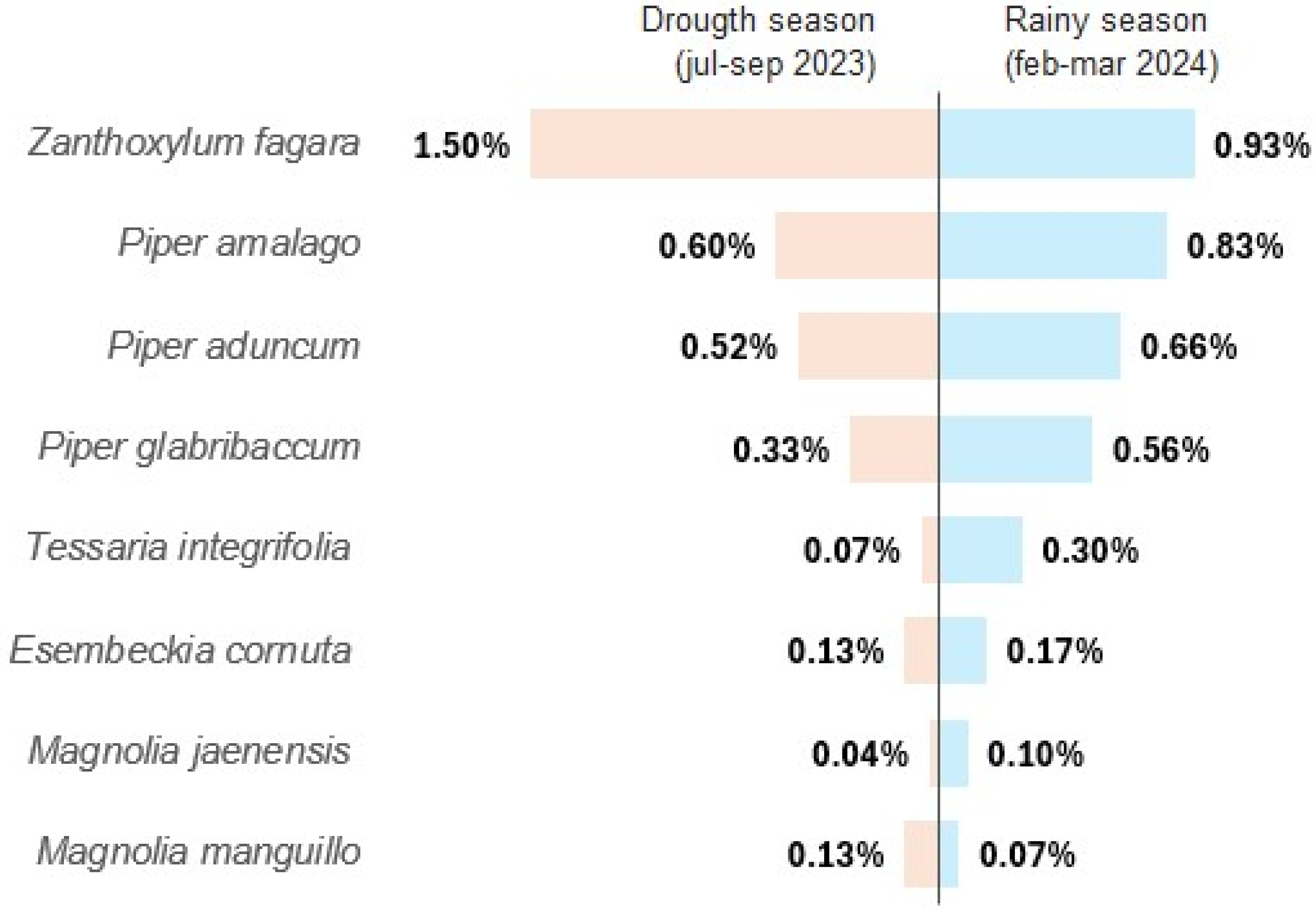
2.1. Extraction Yield of Essential Oils
2.2. Volatile Profile of Essential Oils
2.3. Evaluation of the Antibacterial Activity of Essential Oils
2.4. Evaluation of Antioxidant Capacity and Total Content of Phenolic Compounds of Essential Oils
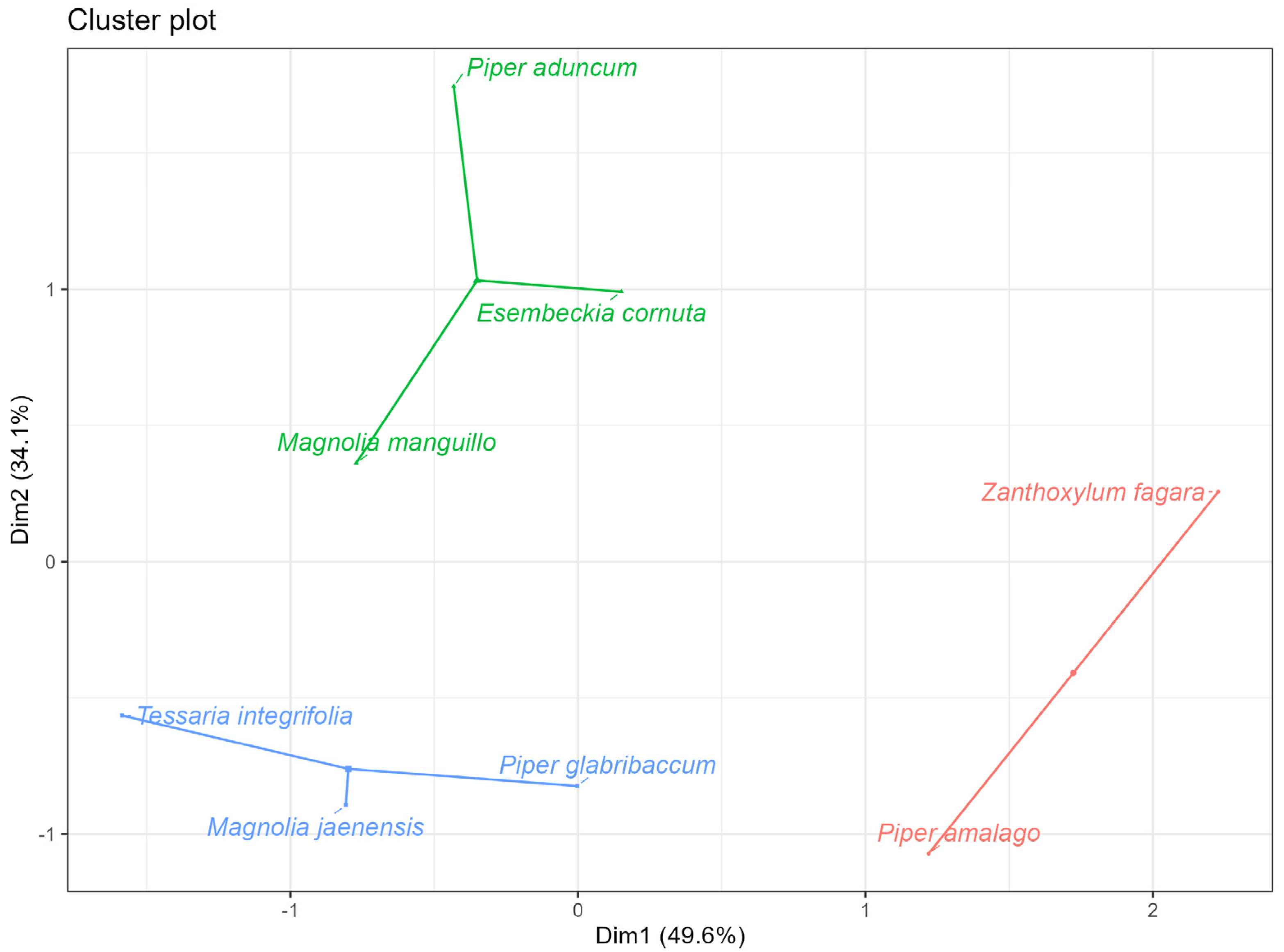
2.5. Grouping of the Best Performing Essential Oils in Terms of Antibacterial Activity and Antioxidant Activity
2.6. Chemical Composition of Essential Oils
3. Materials and Methods
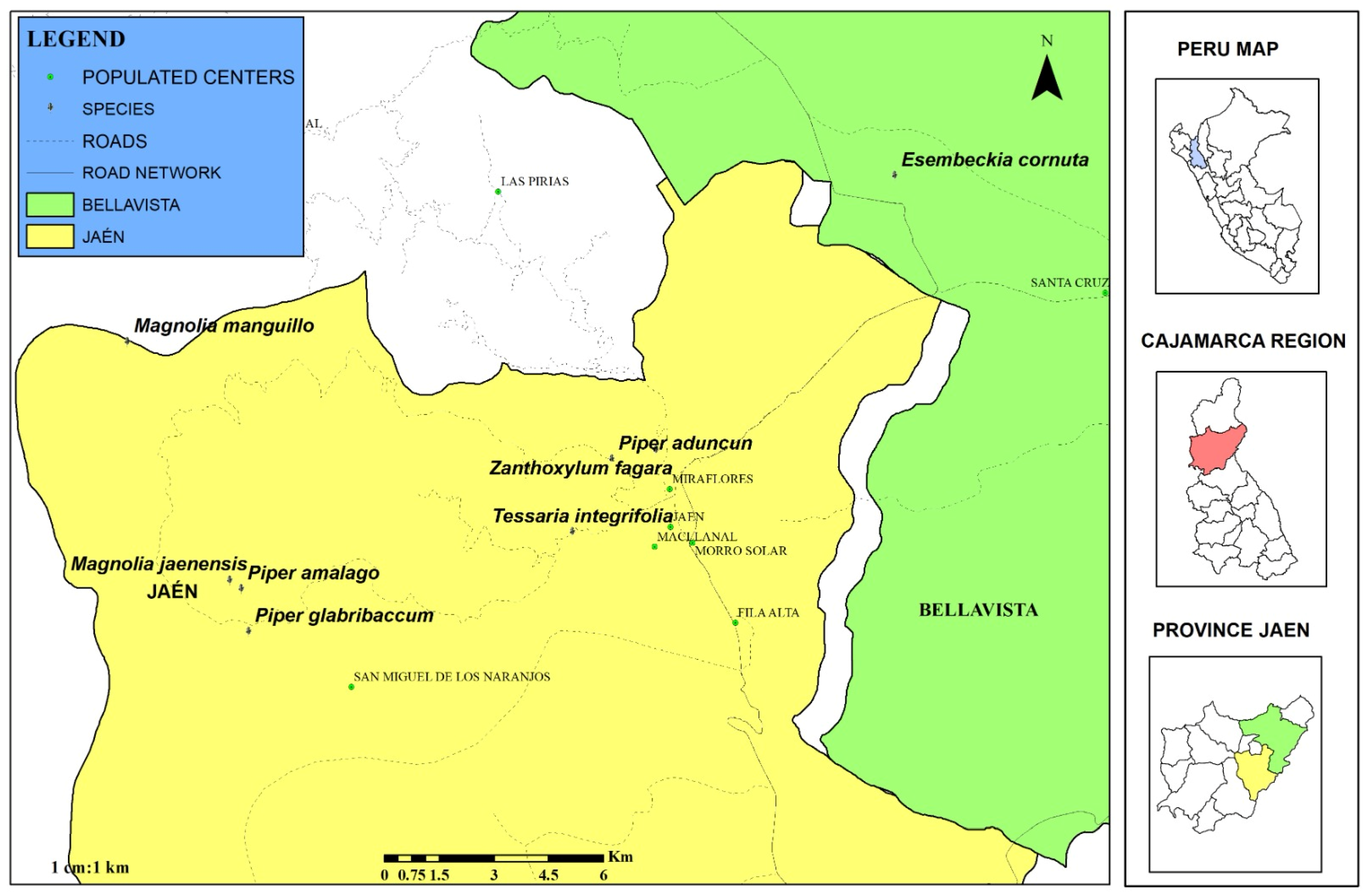
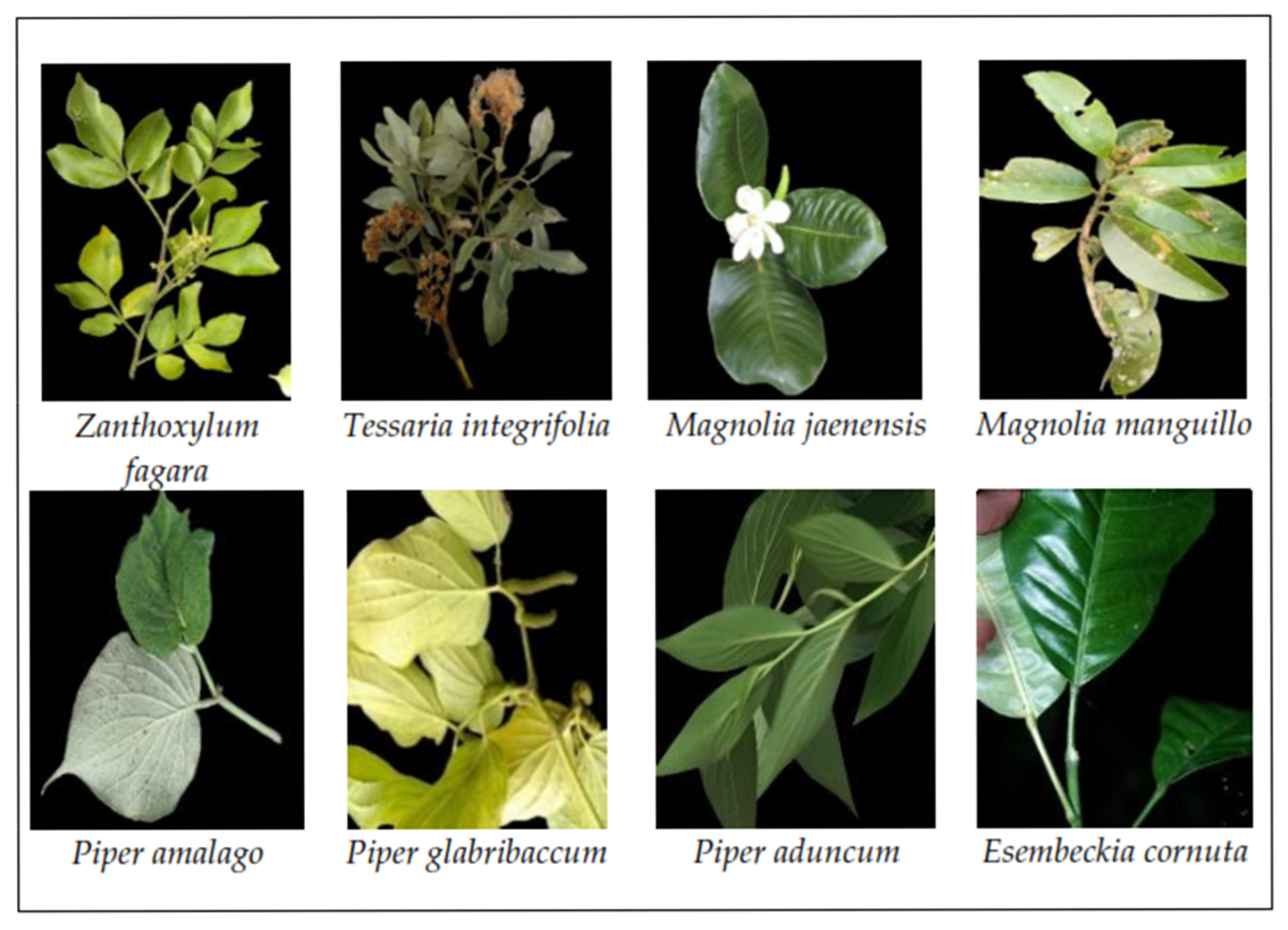
3.1. Georeferencing and Collection of Biological Samples
3.2. Essential Oil Extraction
3.3. Chemical Characterization of Essential Oils
3.4. Antibacterial Activity
3.5. Antioxidant Capacity
3.6. Total Content of Phenolic Compounds
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kloucek, P.; Polesny, Z.; Svobodova, B.; Vlkova, E.; Kokoska, L. Antibacterial Screening of Some Peruvian Medicinal Plants Used in Callería District. J. Ethnopharmacol. 2005, 99, 309–312. [Google Scholar] [CrossRef]
- Quinteros-Gómez, Y.; Macedo-Bedoya, J.; Santos-Linares, V.; Angeles-Alvarez, F.; Gómez-Ticerán, D.; Campos-De la Cruz, J.; Solis Sarmiento, J.; Salinas-Inga, A.; Valencia-Saavedra, Z. Floristic Diversity and Distribution Pattern along an Altitudinal Gradient in the Central Andes: A Case Study of Cajatambo, Peru. Plants 2024, 13, 3328. [Google Scholar] [CrossRef] [PubMed]
- Bohórquez-Medina, S.L.; Bohórquez-Medina, A.L.; de Lukacs Pereny, S.G.; Cárdenas-Jarama, M. Traditional Culinary Uses, Food Applications, and Potential Health Benefits of Peruvian Mesquite (Prosopis juliflora, Prosopis pallida), Research Advances and Challenges: A Review. J. Ethn. Food 2025, 12, 10. [Google Scholar] [CrossRef]
- Castro–Alayo, E.M.; Chávez–Quintana, S.G.; Auquiñivín-Silva, E.A.; Fernández-Jeri, A.B.; la Cruz, O.A.-D.; Rodríguez-Hamamura, N.; Olivas-Orozco, G. Aceites esenciales de plantas nativas del Perú: Efecto del lugar de cultivo en las características fisicoquímicas y actividad antioxidante. Sci. Agropecu. 2019, 10, 479–487. [Google Scholar] [CrossRef]
- Andes Amazon Fund. New Regional Conservation Area Protects the Largest Extension of Páramo in Cajamarca and over 250 Species of Birds; Andes Amazon Fund: Washington, DC, USA, 2021. [Google Scholar]
- Marcelo-Peña, J.L. Vegetación Leñosa, Endemismos y Estado de Conservación En Los Bosques Estacionalmente Secos de Jaén, Perú. Rev. Peru. Biol. 2008, 15, 43–52. [Google Scholar] [CrossRef]
- Funk, V.A.; Bayer, R.J.; Keeley, S.; Chan, R.; Watson, L.; Gemeinholzer, B.; Schilling, E.; Panero, J.L.; Baldwin, B.G.; Garcia-Jacas, N. Everywhere but Antarctica: Using a Supertree to Understand the Diversity and Distribution of the Compositae. Biol. Skr. 2005, 55, 343–373. [Google Scholar]
- Roeble, L.; van Benthem, K.J.; Weigelt, P.; Kreft, H.; Knope, M.L.; Mandel, J.R.; Vargas, P.; Etienne, R.S.; Valente, L. Island Biogeography of the Megadiverse Plant Family Asteraceae. Nat. Commun. 2024, 15, 7276. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, C. Compositae: Introduction with Key to Tribes. Fam. Genera Vasc. Plants 2007, 8, 61–87. [Google Scholar]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Stevens, P.F. Angiosperm Phylogeny Website; Version 12; Missouri Botanical Garden (MBG): St. Louis, MO, USA, 2001. [Google Scholar]
- Perigo, C.V.; Torres, R.B.; Bernacci, L.C.; Guimarães, E.F.; Haber, L.L.; Facanali, R.; Vieira, M.A.; Quecini, V.; Marques, M.O.M. The Chemical Composition and Antibacterial Activity of Eleven Piper Species from Distinct Rainforest Areas in Southeastern Brazil. Ind. Crops Prod. 2016, 94, 528–539. [Google Scholar] [CrossRef]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E.; Tognolini, M.; Maldonado, M.E.; Bruni, R. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) Essential Oils from Eastern Ecuador. Environ. Toxicol. Pharmacol. 2009, 27, 39–48. [Google Scholar] [CrossRef]
- Samain, M.-S.; Mathieu, G.; Wanke, S.; Neinhuis, C.; Goetghebeur, P. Verhuellia Revisited-Unravelling Its Intricate Taxonomic History and a New Subfamilial Classification of Piperaceae. Taxon 2008, 57, 583–587. [Google Scholar]
- Saleem, M.; Ali, M.; Gulshan, A.B. Nutritional Uses of the Family Rutaceae. In Phytochemical and Pharmacological Investigation of the Family Rutaceae; Apple Academic Press: Oakville, ON, Canada, 2024. [Google Scholar]
- Marcelo-Peña, J.L.; Santini, L.; Tomazello Filho, M. Wood Anatomy and Growth Rate of Seasonally Dry Tropical Forest Trees in the Marañón River Valley, Northern Peru. Dendrochronologia 2019, 55, 135–145. [Google Scholar] [CrossRef]
- Figlar, R.B.; Nooteboom, H.P. Notes on Magnoliaceae IV. Blumea-Biodivers. Evol. Biogeogr. Plants 2004, 49, 87–100. [Google Scholar] [CrossRef]
- Hernández-Vera, G.; Navarrete-Heredia, J.L.; Vázquez-García, J.A. Beetles as Floral Visitors in the Magnoliaceae: An Evolutionary Perspective. Arthropod-Plant Interact. 2021, 15, 273–283. [Google Scholar] [CrossRef]
- Saha, P.; Saha, S.; Semwal, A.; Prinsa, P.; Parashar, T.; Jakhmola, V. Geographical Distribution, Chemical Constituents, and Activity Profile of Magnolia. Maj. Obat Tradis. 2023, 28, 122–131. [Google Scholar] [CrossRef]
- Mabberley, D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses; Cambridge University Press: New York, NY, USA, 2017. [Google Scholar]
- Marcelo-Peña, J.L.; Arroyo, F. Magnolia Jaenensis y M. Manguillo, Nuevas Especies de Magnoliaceae Del Norte de Perú. Brittonia 2013, 65, 106–112. [Google Scholar] [CrossRef]
- Bimpizas-Pinis, M.; Santagata, R.; Kaiser, S.; Liu, Y.; Lyu, Y. Additives in the Food Supply Chain: Environmental Assessment and Circular Economy Implications. Environ. Sustain. Indic. 2022, 14, 100172. [Google Scholar] [CrossRef]
- Hernández, T.; García-Bores, A.M.; Serrano, R.; Ávila, G.; Dávila, P.; Cervantes, H.; Peñalosa, I.; Flores-Ortiz, C.M.; Lira, R. Fitoquímica y Actividades Biológicas de Plantas de Importancia En La Medicina Tradicional Del Valle de Tehuacán-Cuicatlán. TIP. Rev. Espec. Cienc. Quím.-Biol. 2015, 18, 116–121. [Google Scholar] [CrossRef][Green Version]
- Martínez Álvarez, Ó.; Iriondo-DeHond, A.; Gómez Estaca, J.; Castillo, M. Nuevas Tendencias En La Producción y Consumo Alimentario. Distrib. Consumo 2021, 1, 51–62. [Google Scholar]
- Rodilla, J.M.; Rosado, T.; Gallardo, E. Essential Oils: Chemistry and Food Applications. Foods 2024, 13, 1074. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Malik, T.; Sarkar, O.; Pant, S. Synergistic Antibacterial Effects of Trachyspermum Ammi L. Essential Oil and Sodium Nitrite in Combination on Artificially Inoculated Food Models. Grasas Aceites 2024, 75, e544. [Google Scholar] [CrossRef]
- Ben Akacha, B.; Švarc-Gajić, J.; Elhadef, K.; Ben Saad, R.; Brini, F.; Mnif, W.; Smaoui, S.; Ben Hsouna, A. The Essential Oil of Tunisian Halophyte Lobularia Maritima: A Natural Food Preservative Agent of Ground Beef Meat. Life 2022, 12, 1571. [Google Scholar] [CrossRef]
- Cesca, R.S.; Fonseca, G.G.; da Paz, M.F.; Cortez-Vega, W.R. Advances and Perspectives on the Application of Essential Oils in Food Packaging Films, Coatings, and Nanoencapsulated Materials. Bragantia 2024, 83, e20230132. [Google Scholar] [CrossRef]
- Enayatifard, R.; Akbari, J.; Babaei, A.; Rostamkalaei, S.S.; Hashemi, S.M.H.; Habibi, E. Anti-Microbial Potential of Nano-Emulsion Form of Essential Oil Obtained from Aerial Parts of Origanum Vulgare L. as Food Additive. Adv. Pharm. Bull. 2021, 11, 327–334. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Filho, L.E.P.T.; de Andrade, M.F.; de Freitas, L.F.; Palha, M.D.L.A.P.F.; Vinhas, G.M. Development and Characterization of Poly(Butylene Adipate-Co-Terephthalate) (PBAT) Antimicrobial Films with Clove and Cinnamon Essential Oils. J. Food Process. Preserv. 2022, 46, e16489. [Google Scholar] [CrossRef]
- Carsono, N.; Tumilaar, S.G.; Kurnia, D.; Latipudin, D.; Satari, M.H. A Review of Bioactive Compounds and Antioxidant Activity Properties of Piper Species. Molecules 2022, 27, 6774. [Google Scholar] [CrossRef]
- Miguel, M.G.; Gago, C.; Antunes, M.D.; Lagoas, S.; Faleiro, M.L.; Megías, C.; Cortés-Giraldo, I.; Vioque, J.; Figueiredo, A.C. Antibacterial, Antioxidant, and Antiproliferative Activities of Corymbia Citriodora and the Essential Oils of Eight Eucalyptus Species. Medicines 2018, 5, 61. [Google Scholar] [CrossRef]
- Setzer, W.N.; Schmidt, J.M.; Eiter, L.C.; Haber, W.A. The Leaf Oil Composition of Zanthoxylum fagara (L.) Sarg. from Monteverde, Costa Rica, and Its Biological Activities. J. Essent. Oil Res. 2005, 17, 333–335. [Google Scholar] [CrossRef]
- dos Santos, A.L.; Novaes, A.d.S.; Polidoro, A.d.S.; de Barros, M.E.; Mota, J.S.; Lima, D.B.; Krause, L.C.; Cardoso, C.A.; Jacques, R.A.; Caramão, E.B. Chemical Characterisation of (Piperaceae) Essential Oil by Comprehensive Two-Dimensional Gas Chromatography Coupled with Rapid-Scanning Quadrupole Mass Spectrometry (GC×GC/qMS) and Their Antilithiasic Activity and Acute Toxicity. Phytochem. Anal. 2018, 29, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Bergo, C.L.; Amaral, W.D.; Biasi, L.A.; Deschamps, C.; Junior, C.C.; da Silva, L.E.; Côcco, L.C. Essential Oil Yield and Composition of Piper Species in Parana. Rev. Bras. Plantas Med. 2017, 19, 177–183. [Google Scholar] [CrossRef]
- Ahrar, A.E. Variación Estacional del Aceite Esencial Obtenido de Diferentes Quimiotipos de Mentha longifolia L. Bachelor’s Thesis, Universitat Politècnica de València, Valencia, Spain, 2016. [Google Scholar]
- Smitha, G.R.; Tripathy, V. Seasonal Variation in the Essential Oils Extracted from Leaves and Inflorescence of Different Ocimum Species Grown in Western Plains of India. Ind. Crops Prod. 2016, 94, 52–64. [Google Scholar] [CrossRef]
- Liao, Z.; Huang, Q.; Cheng, Q.; Khan, S.; Yu, X. Seasonal Variation in Chemical Compositions of Essential Oils Extracted from Lavandin Flowers in the Yun-Gui Plateau of China. Molecules 2021, 26, 5639. [Google Scholar] [CrossRef]
- Pinheiro, C.G.; Machado, C.M.; Amaral, L.P.; Silva, D.T.; Almeida, C.A.A.; Longhi, S.J.; Mallmann, C.A.; Heinzmann, B.M. Seasonal Variability of the Essential Oil of Hesperozygis ringens (Benth.) Epling. Braz. J. Biol. 2016, 76, 176–184. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Blank, A.F.; Fontes, S.M.; Carvalho Filho, J.L.S.; Alves, P.B.; Silva-Mann, R.; Mendonça, M.C.; Arrigoni-Blank, M.F.; Rodrigues, M.O. Influência Do Horário de Colheita e Secagem de Folhas No Óleo Essencial de Melissa (Melissa officinalis L.) Cultivada Em Dois Ambientes. Rev. Bras. Plantas Med. 2005, 8, 73–78. [Google Scholar]
- Gobbo-Neto, L.; Lopes, N.P. Medicinal Plants: Factors of Influence on the Content of Secondary Metabolites. Quím. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Lakušić, B.; Ristić, M.; Slavkovska, V.; Milenković, M.; Lakušić, D. Environmental and Seasonal Impacts on the Chemical Composition of Satureja horvatii Šilić (Lamiaceae) Essential Oils. Chem. Biodivers. 2011, 8, 483–493. [Google Scholar] [CrossRef]
- Mossi, A.J.; Pauletti, G.F.; Rota, L.; Echeverrigaray, S.; Barros, I.B.I.; Oliveira, J.V.; Paroul, N.; Cansian, R.L. Efeito de Diferentes Níveis de Calagem Na Produção de Biomassa e No Rendimento de Extração de Óleo Essencial de Cunila Galioides Benth. Braz. J. Biol. 2012, 72, 787–793. [Google Scholar] [CrossRef]
- Venskutonis, P.R. Effect of Drying on the Volatile Constituents of Thyme (Thymus vulgaris L.) and Sage (Salvia officinalis L.). Food Chem. 1997, 59, 219–227. [Google Scholar] [CrossRef]
- Solorzano-Santos, F.; Miranda-Novales, M.G. Essential Oils from Aromatic Herbs as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Cossolin, J.F.S.; Pereira, M.J.B.; Martínez, L.C.; Turchen, L.M.; Fiaz, M.; Bozdoğan, H.; Serrão, J.E. Cytotoxicity of Piper Aduncum (Piperaceae) Essential Oil in Brown Stink Bug Euschistus Heros (Heteroptera: Pentatomidae). Ecotoxicology 2019, 28, 763–770. [Google Scholar] [CrossRef]
- Silva, L.S.; Mar, J.M.; Azevedo, S.G.; Rabelo, M.S.; Bezerra, J.A.; Campelo, P.H.; Machado, M.B.; Trovati, G.; Dos Santos, A.L.; Da Fonseca Filho, H.D.; et al. Encapsulation of Piper aduncum and Piper hispidinervum Essential Oils in Gelatin Nanoparticles: A Possible Sustainable Control Tool of Aedes Aegypti, Tetranychus urticae and Cerataphis lataniae. J. Sci. Food Agric. 2019, 99, 685–695. [Google Scholar] [CrossRef]
- Oliveira, G.L.; Cardoso, S.K.; Lara Junior, C.R.; Vieira, T.M.; GUIMARãES, E.F.; Figueiredo, L.S.; Martins, E.R.; Moreira, D.L.; Kaplan, M.A.C. Chemical Study and Larvicidal Activity against Aedes Aegypti of Essential Oil of Piper aduncum L. (Piperaceae). An. Acad. Bras. Ciênc. 2013, 85, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Santana, H.T.; Trindade, F.T.T.; Stabeli, R.G.; Silva, A.A.E.; Militão, J.; Facundo, V.A. Essential Oils of Leaves of Piper Species Display Larvicidal Activity against the Dengue Vector, Aedes Aegypti (Diptera: Culicidae). Rev. Bras. Plantas Med. 2015, 17, 105–111. [Google Scholar] [CrossRef][Green Version]
- Jaramillo-Colorado, B.E.; Duarte-Restrepo, E.; Pino-Benítez, N. Evaluación de La Actividad Repelente de Aceites Esenciales de Plantas Piperáceas Del Departamento de Chocó, Colombia. Rev. Toxicol. 2015, 32, 112–116. [Google Scholar]
- Mamood, S.N.H.; Hidayatulfathi, O.; Budin, S.B.; Rohi, G.A.; Zulfakar, M.H. The Formulation of the Essential Oil of Piper Aduncum Linnaeus (Piperales: Piperaceae) Increases Its Efficacy as an Insect Repellent. Bull. Entomol. Res. 2017, 107, 49–57. [Google Scholar] [CrossRef]
- Durofil, A.; Radice, M.; Blanco-Salas, J.; Ruiz-Téllez, T. Piper Aduncum Essential Oil: A Promising Insecticide, Acaricide and Antiparasitic. A Review. Parasite 2021, 28, 42. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.G.S.; Zohhbi, M.D.G.B.; Andrade, E.H.A.; Santos, A.S.; Da Silva, M.H.L.; Luz, A.I.R.; Bastos, C.N. Constituents of the Essential Oil ofPiper Aduncum L. Growing Wild in the Amazon Region. Flavour Fragr. J. 1998, 13, 269–272. [Google Scholar] [CrossRef]
- Monzote, L.; Scull, R.; Cos, P.; Setzer, W.N. Essential Oil from Piper Aduncum: Chemical Analysis, Antimicrobial Assessment, and Literature Review. Medicines 2017, 4, 49. [Google Scholar] [CrossRef]
- da Silva Mota, J.; de Souza, D.S.; Boone, C.V.; Lima Cardoso, C.A.; Bastos Caramão, E. Identification of the Volatile Compounds of Leaf, Flower, Root and Stem Oils of Piper amalago (Piperaceae). J. Essent. Oil Bear. Plants 2013, 16, 11–16. [Google Scholar] [CrossRef]
- Potzernheim, M.; Bizzo, H.R.; Agostini-Costa, T.S.; Vieira, R.F.; Carvalho-Cilva, M.; Gracindo, L. Chemical Characterization of Seven Piper Species (Piperaceae) from Federal District, Brazil, Based on Volatile Oil Constituents. Rev. Bras. Plantas Med. 2006, 8, 10–12. [Google Scholar]
- Mesquita, J.M.O.; Cavaleiro, C.; Cunha, A.P.; Lombardi, J.A.; Oliveira, A.B. Estudo Comparativo Dos Óleos Voláteis de Algumas Espécies de Piperaceae. Rev. Bras. Farmacogn. 2005, 15, 6–12. [Google Scholar] [CrossRef]
- Morandim-Giannetti, A.d.A.; Pin, A.R.; Santo Pietro, N.A.; de Oliveira, H.C.; Mendes-Giannini, M.J.S.; Alecio, A.C.; Kato, M.J.; de Oliveira, J.E.; Furlan, M. Composition and Antifungal Activity against Candida Albicans, Candida Parapsilosis, Candida Krusei and Cryptococcus Neoformans of Essential Oils from Leaves of Piper and Peperomia Species. J. Med. Plants Res. 2010, 4, 1810–1814. [Google Scholar]
- Ferraz, A.; Balbino, J.M.; Zini, C.A.; Ribeiro, V.L.S.; Bordignon, S.A.L.; Von Poser, G. Acaricidal Activity and Chemical Composition of the Essential Oil from Three Piper Species. Parasitol. Res. 2010, 107, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Feo, V.; de D’Agostino, M.; Simone, F.; de Pizza, C. Constituents of Tessaria Integrifolia; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Peluso, G.; De Feo, V.; De Simone, F.; Bresciano, E.; Vuotto, M.L. Studies on the Inhibitory Effects of Caffeoylquinic Acids on Monocyte Migration and Superoxide Ion Production. J. Nat. Prod. 1995, 58, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Silva-Correa, C.R.; Cruzado-Razco, J.L.; González-Blas, M.V.; García-Armas, J.M.; Ruiz-Reyes, S.G.; Villarreal-La Torre, V.E.; Gamarra-Sánchez, C.D. Identificación y determinación estructural de un sesquiterpeno de las hojas de Tessaria integrifolia Ruiz & Pav. y evaluación de su actividad leishmanicida. Rev. Peru. Med. Exp. Salud Pública 2018, 35, 221–227. [Google Scholar] [CrossRef]
- Ono, M.; Masuoka, C.; Odake, Y.; Ito, Y.; Nohara, T. Eudesmane Derivatives from Tessaria Integrifolia. Phytochemistry 2000, 53, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, L.A.; de la Torre, Y.C.; Cirio, A.T.; de Torres, N.W.; Flores Suárez, A.E.; Aranda, R.S. Essential Oils from Zanthoxylum fagara Wild Lime, Ruta chalepensis L. and Thymus vulgaris L.: Composition and Activity against Aedes Aegypti larvae. Pak. J. Pharm. Sci. 2015, 28, 1911–1915. [Google Scholar]
- Pino, J.A.; Agüero, J.; Marbot, R.; Fernandes, P. Composition of the Essential Oil of Zanthoxylum fagara (L.) Sargent. from Cuba. J. Essent. Oil Res. 2005, 17, 413–414. [Google Scholar] [CrossRef]
- Prieto, J.A.; Patiño, O.J.; Delgado, W.A.; Moreno, J.P.; Cuca, L.E. Chemical composition, insecticidal, and antifungal activities of fruit essential oils of three Colombian Zanthoxylum species. Chil. J. Agric. Res. 2011, 71, 73–82. [Google Scholar] [CrossRef]
- Braga Carneiro, S.; Kreutz, T.; Limberger, R.P.; da Veiga Júnior, V.F.; Koester, L.S. Development, Validation and Application of a Gas Chromatography Method for the Determination of Dillapiole from Piper aduncum Essential Oil in Skin Permeation Samples. Biomed. Chromatogr. 2023, 37, e5544. [Google Scholar] [CrossRef]
- Yasunaka, K.; Abe, F.; Nagayama, A.; Okabe, H.; Lozada-Pérez, L.; López-Villafranco, E.; Muñiz, E.E.; Aguilar, A.; Reyes-Chilpa, R. Antibacterial Activity of Crude Extracts from Mexican Medicinal Plants and Purified Coumarins and Xanthones. J. Ethnopharmacol. 2005, 97, 293–299. [Google Scholar] [CrossRef]
- Araujo Baptista, L.M.; Rondón Rivas, M.E.; Cruz Tenempaguay, R.E.; Guayanlema Chávez, J.D.; Vargas Córdova, C.A.; Morocho Zaragocin, S.V.; Cornejo Sotomayor, S.X. Antimicrobial Activity of the Essential Oil of Piper amalago L. (Piperaceae) Collected in Coastal Ecuador. CABI Databases 2019, 3, 15–27. [Google Scholar]
- Setzer, W.N.; Park, G.; Agius, B.R.; Stokes, S.L.; Walker, T.M.; Haber, W.A. Chemical Compositions and Biological Activities of Leaf Essential Oils of Twelve Species of Piper from Monteverde, Costa Rica. Nat. Prod. Commun. 2008, 3, 1934578X0800300. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K.; on behalf of the CLSI Methods Development and Standardization Working Group of the Subcommittee on Antimicrobial Susceptibility Testing. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, 10–1128. [Google Scholar] [CrossRef]
- Lemos, M.F.; Lemos, M.F.; Pacheco, H.P.; Guimarães, A.C.; Fronza, M.; Endringer, D.C.; Scherer, R. Seasonal Variation Affects the Composition and Antibacterial and Antioxidant Activities of Thymus vulgaris. Ind. Crops Prod. 2017, 95, 543–548. [Google Scholar] [CrossRef]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Yu, C.-W.; Wu, S.-C.; Yih, K.-H. DPPH Free-Radical Scavenging Activity, Total Phenolic Contents and Chemical Composition Analysis of Forty-Two Kinds of Essential Oils. J. Food Drug Anal. 2020, 17, 9. [Google Scholar] [CrossRef]
- Vásquez-Ocmín, P.G.; Cojean, S.; Roumy, V.; Marti, G.; Pomel, S.; Gadea, A.; Leblanc, K.; Dennemont, I.; Ruiz-Vásquez, L.; Ricopa Cotrina, H.; et al. Deciphering Anti-Infectious Compounds from Peruvian Medicinal Cordoncillos Extract Library through Multiplexed Assays and Chemical Profiling. Front. Pharmacol. 2023, 14, 1100542. [Google Scholar] [CrossRef]
- Sánchez-Velásquez, L.R.; Pineda-López, M.D.R.; Vásquez-Morales, S.G.; Avendaño-Yáñez, M.D.L.L. Ecology and Conservation of Endangered Species: The Case of Magnolias. Endangered Species; Nova Sciences Publishers, Inc.: New York, NY, USA, 2016; pp. 63–84. [Google Scholar]
- Rao, B.R.; Kaul, P.; Syamasundar, K.; Ramesh, S. Chemical Profiles of Primary and Secondary Essential Oils of Palmarosa (Cymbopogon martinii (Roxb.) Wats Var. Motia Burk.). Ind. Crops Prod. 2005, 21, 121–127. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Cockerill, F.R.; Wikler, M.; Bush, K.; Dudley, M.; Eliopoulos, G.; Hardy, D. Clinical and Laboratory Standards Institute. In Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Second Informational Supplement; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012. [Google Scholar]
- Sinche Ambrosio, C.M. Unravelling the Potential of Citrus Essential Oils Derived from Citrus Processing as an Alternative Antimicrobial Feed Additive in Pigs. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Spain, 2020. [Google Scholar]
- Martínez, M.J.; Betancourt Badell, J.; Alonso González, N. Ausencia de Actividad Antimicrobiana de Un Extracto Acuoso Liofilizado de Áloe Vera (Sábila). Rev. Cuba. Plantas Med. 1996, 1, 18–20. [Google Scholar]
- Ramirez, L.S.; Diaz, H.E. Actividad Antibacteriana de Extractos y Fracciones Del Ruibarbo (Rumex conglomeratus). Sci. Tech. 2007, 13, 397–400. [Google Scholar]
- Scherer, R.; Godoy, H.T. Antioxidant Activity Index (AAI) by the 2, 2-Diphenyl-1-Picrylhydrazyl Method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Ikeda, N.Y.; Ambrosio, C.M.S.; Miano, A.C.; Rosalen, P.L.; Gloria, E.M.; Alencar, S.M. Essential Oils Extracted from Organic Propolis Residues: An Exploratory Analysis of Their Antibacterial and Antioxidant Properties and Volatile Profile. Molecules 2021, 26, 4694. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- de Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the Bioactive Compounds, Antioxidant Activity and Chemical Composition of Brazilian Blackberry, Red Raspberry, Strawberry, Blueberry and Sweet Cherry Fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Ayed, A.; Caputo, L.; De Feo, V.; Elshafie, H.S.; Fratianni, F.; Nazzaro, F.; Hamrouni, L.; Amri, I.; Mabrouk, Y.; Camele, I.; et al. Antimicrobial, Anti-Enzymatic and Antioxidant Activities of Essential Oils from Some Tunisian Eucalyptus Species. Heliyon 2024, 10, e34518. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]

| Group | Subclass | Compound Name | Relative Abundance (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zanthoxylum fagara | Piper amalago | Piper aduncum L. | Piper glabribaccum | Esembeckia cornuta | Magnolia manguillo | Magnolia jaenensis | Tessaria integrifolia | |||
| Monoterpenoids | Acyclic Monoterpene | β-myrcene | - | 0.29 | - | - | 0.19 | - | - | - |
| citral | - | 0.06 | - | 0.09 | 0.12 | 0.09 | 0.46 | - | ||
| neral | - | 0.04 | - | - | 0.07 | 0.05 | 0.28 | - | ||
| geranial | - | - | - | - | - | - | - | - | ||
| trans-2-decenal | - | - | - | - | - | - | 0.02 | - | ||
| Monocyclic Monoterpene | D-limonene | 0.08 | 0.71 | - | - | - | 0.25 | - | - | |
| α-terpinene | - | 0.08 | - | - | - | - | - | - | ||
| γ-terpinene | - | 0.15 | - | - | - | - | - | - | ||
| α-terpinolene | - | 0.08 | - | - | - | - | - | - | ||
| p-cymene | 0.05 | 0.28 | - | - | 0.06 | 0.16 | - | - | ||
| α-phellandrene | 0.07 | - | - | - | 0.07 | - | - | - | ||
| β-pinene | - | 9.96 | - | - | - | - | 0.25 | - | ||
| sabinene | 0.07 | 0.15 | - | - | - | - | - | - | ||
| camphene | - | 0.08 | - | - | - | - | - | - | ||
| Bicyclic Monoterpene | eucalyptol (1,8-cineole) | - | 4.5 | - | - | 0.11 | - | 0.14 | - | |
| L-α-terpineol | - | 1.73 | - | - | 0.17 | - | 0.11 | - | ||
| (−)-terpinen-4-ol | - | 0.4 | - | - | - | - | - | - | ||
| α-terpineol | - | - | - | - | - | 0.07 | - | - | ||
| 4-terpineol | - | - | - | - | - | 0.54 | - | - | ||
| (−)-borneol | - | 0.16 | - | - | - | - | - | |||
| (−)-myrtenol | - | 0.1 | - | - | - | 0.11 | - | 0.09 | ||
| myrtenal | - | - | - | - | - | 0.19 | - | - | ||
| pinocarvone | - | - | - | - | - | - | - | 0.11 | ||
| isobornyl acetate | - | - | - | - | - | - | 0.03 | |||
| α-terpinyl acetate | - | 0.22 | - | - | - | - | - | - | ||
| myrtenyl acetate | - | 0.03 | - | - | - | - | - | - | ||
| Sesquiterpenoids | Acyclic Sesquiterpene | trans-nerolidol | - | 0.42 | 0.73 | 11.46 | - | 6.46 | 1.21 | 0.35 |
| farnesol | - | - | - | - | - | 1.17 | - | - | ||
| Monocyclic Sesquiterpene | β-bisabolene | - | - | - | - | - | 0.24 | - | ||
| Bicyclic Sesquiterpene | β-caryophyllene | 3.23 | 3.23 | 11.1 | - | 16.79 | 11.59 | 15.26 | 9.0 | |
| caryophyllene oxide | - | - | - | - | 2.4 | 0.65 | 1.07 | 2.09 | ||
| guaiol | 4.48 | - | - | 8.19 | - | - | - | - | ||
| bulnesol | 1.73 | - | - | - | - | - | - | - | ||
| δ-cadinene | - | 13.63 | 2.01 | - | - | - | - | - | ||
| τ-cadinol | 0.18 | 0.41 | 1.68 | 1.35 | 1.06 | 2.38 | 0.85 | |||
| α-cadinol | 1.0 | 0.76 | - | - | 1.13 | 4.94 | 0.29 | |||
| γ-eudesmol | 1.84 | - | - | - | - | - | - | 0.4 | ||
| α-Epi-7-epi-5-eudesmol | 3.1 | 1.4 | - | - | 2.03 | - | - | - | ||
| globulol | - | - | - | 1.06 | - | - | - | - | ||
| ledol | 0.87 | - | - | - | - | - | - | - | ||
| β-copaene | - | 4.21 | 3.12 | - | 1.18 | - | 0.74 | - | ||
| α-copaene | 3.26 | 3.16 | 0.74 | 5.25 | 3.94 | 5.89 | - | 1.53 | ||
| β-elemene | - | 0.78 | - | - | - | - | - | - | ||
| γ-elemene | 0.96 | - | - | 0.13 | 0.57 | - | 0.11 | - | ||
| δ-elemene | 0.99 | 0.12 | - | - | - | - | - | - | ||
| (−)-cis-β-elemene | 9.08 | - | 2.30 | 9.63 | 6.58 | - | 23.59 | 1.81 | ||
| α-cubebene | 0.74 | 1.73 | - | 14.3 | - | 1.21 | - | - | ||
| (−)-α-gurjunene | 0.09 | 0.84 | - | 5.22 | 0.29 | 0.15 | - | - | ||
| γ-gurjunene | 0.72 | - | - | 0.8 | 0.33 | - | - | |||
| alloaromadendrene | - | - | - | 2.85 | - | - | - | - | ||
| isoaromadendrene epoxide | - | - | 8.4 | 0.18 | - | - | - | - | ||
| humulene epoxide II | - | 1.47 | 1.62 | 1.14 | - | 2.19 | 0.13 | - | ||
| spathulenol | - | - | - | - | - | - | - | - | ||
| isospatulenol | - | - | - | - | - | 3.89 | - | - | ||
| γ-muurolene | 1.2 | 8.26 | - | - | 0.73 | 2.31 | 2.24 | 0.41 | ||
| α-muurolene | 2.17 | 0.83 | - | - | 1.08 | 0.8 | 1.09 | - | ||
| δ-amorphene | 3.77 | 0.37 | - | - | 14.58 | 4.44 | 10.15 | 2.11 | ||
| α-selinene | - | - | - | 4.51 | - | - | - | - | ||
| β-selinene | 0.41 | - | - | - | - | - | 1.88 | - | ||
| γ-selinene | - | - | - | 1.60 | - | - | 2.43 | - | ||
| β-calacorene | - | 0.71 | - | 1.1 | 0.45 | 0.38 | 0.17 | - | ||
| trans-calamenene | 0.17 | 1.31 | - | 1.46 | 0.31 | 0.89 | - | 0.47 | ||
| cadalene | - | - | - | 2.08 | - | - | - | - | ||
| Tricyclic Sesquiterpene | α-cedrene | - | - | - | - | - | 0.21 | - | - | |
| ylangene | - | 0.55 | - | - | - | - | - | - | ||
| sesquiterpene alcohols (hedycaryol) | 10.86 | - | - | - | 0.96 | - | - | 0.34 | ||
| (1aR,4aR,7S,7aR,7bR)-1,1,7-trimethyl-4-methylenedecahydro-1H-cyclopropa[e]azulen-7-ol | 1.63 | 3.73 | 10.04 | 3.62 | 6.13 | 15.0 | 0.82 | 1.67 | ||
| (3R,3aR,3bR,4S,7R,7aR)-4-isopropyl-3,7-dimethyloctahydro-1H-cyclopenta[1,3]cyclopropa[1,2]benzen-3-ol | 0.49 | 2.34 | 6.63 | 2.17 | 0.66 | 0.72 | 0.19 | 2.03 | ||
| 7R,8R-8-hydroxy-4-isopropylidene-7-methylbicyclo[5.3.1]undec-1-ene | - | 0.16 | - | 0.59 | - | 2.76 | - | - | ||
| eudesm-7(11)-en-4-ol | - | - | - | 1.67 | - | - | - | - | ||
| selin-6-en-4α-ol | - | - | - | - | - | - | 0.27 | - | ||
| neointermedeol | - | - | - | - | - | - | 0.61 | - | ||
| (+)-isovalencenol | - | - | - | - | - | - | - | 0.25 | ||
| Sesquiterpene Ketones | dehydrofukinone | - | - | - | - | 0.35 | - | - | 6.82 | |
| salvial-4(14)-en-1-one | - | - | - | - | - | 0.85 | - | - | ||
| β-vatirenone | - | - | - | - | - | - | - | 0.56 | ||
| Diterpenoids | Acyclic Diterpene | phytol | - | - | - | 0.19 | 3.54 | 0.97 | - | - |
| hexahydrofarnesyl acetone | - | - | - | 0.06 | - | - | - | - | ||
| phytone | - | - | - | - | - | - | - | 0.15 | ||
| Phenylpropanoids | Allylbenzenes | myristicin | - | 0.15 | 38.26 | - | - | - | 0.29 | - |
| apiol | - | 7.21 | 3.08 | - | 2.19 | 0.49 | 14.08 | 1.01 | ||
| Fatty Acids and Derivatives | Fatty Acids | palmitic acid | - | - | - | - | 0.56 | 0.4 | - | - |
| Fatty Alcohols | 1-octadecanol | - | - | - | 0.09 | - | - | - | - | |
| Fatty Aldehydes | pentadecanal | - | - | - | - | - | - | 0.08 | - | |
| Fatty Esters | methyl palmitate | - | - | - | - | 0.13 | - | - | - | |
| homosalate | - | - | - | - | 0.19 | - | - | - | ||
| Benzenoids | Phenols | phenol, 2,4-bis(1,1-dimethylethyl)-6-methyl- | - | - | - | - | - | - | - | 10.01 |
| thymol | - | - | - | - | - | - | 0.08 | - | ||
| Naphthalenes | 1,2,9,10-tetradehydroaristolane | - | - | - | 0.97 | - | - | - | - | |
| agarospirole | - | - | - | - | - | - | - | 2.14 | ||
| α-agarofuran | - | - | - | - | - | - | - | 0.23 | ||
| dihydroagarofuran | - | - | - | - | - | - | - | 8.08 | ||
| 2-tert-butylquinoline | - | - | - | - | 1.01 | - | - | - | ||
| Other Compounds | Alkanes | pentadecane | - | - | 5.86 | - | - | - | - | - |
| 1,4-diisopropylbenzene | - | - | - | - | 0.73 | - | - | - | ||
| Alkenes | 2-methyl-1-pentene | - | - | - | - | - | - | 16.7 | - | |
| 1,4-dimethyl-4-vinylcyclohexene | - | - | - | - | - | 0.15 | - | - | ||
| Ketones | 6-methyl-5-hepten-2-one | - | - | - | 0.49 | - | - | - | - | |
| 6-methyl-3,5-heptadien-2-one | - | - | - | 0.01 | - | - | - | - | ||
| Aldehydes | benzaldehyde | - | - | - | 0.01 | - | - | - | - | |
| Alcohols | cis-3-hexen-1-ol | - | - | - | - | - | 0.12 | - | - | |
| linalool | - | - | - | - | 0.14 | 0.13 | 0.12 | - | ||
| Esters | 3,8-dimethyl-5-α-hydroxy-δ^9-octa-hydroazulene acetate | - | 0.93 | - | - | - | - | - | 8.74 | |
| 5-azulenemethanol | 0.23 | - | - | - | 0.67 | - | - | - | ||
| (1S,3S,5S)-1-isopropyl-4-methylenebicyclo[3.1.0]hexan-3-yl acetate | - | 0.02 | - | - | - | - | - | - | ||
| Ethers | liguloxide | 1.16 | ||||||||
| Miscellaneous | oplopenone | - | - | - | - | - | - | - | 0.97 | |
| oxo-tremorine | - | - | - | - | 0.64 | - | 0.21 | - | ||
| teaspirane | - | 0.06 | - | - | - | - | - | - | ||
| peruviol | 0.07 | - | - | - | 6.23 | - | - | - | ||
| 2-propenoic acid, 3-[4-[(3-methyl-1-butenyl)oxy]phenyl]-, methyl ester | - | - | - | - | 1.95 | - | - | - | ||
| Essential Oils | Microorganism | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. enteritidis | S. aureus | ||||||||||
| I.C.A. (mm) | I.E.O. (mm) | RI (%) | Act | I.C.A. (mm) | I.E.O. (mm) | RI (%) | Act | I.C.A. (mm) | I.E.O. (mm) | RI (%) | Act | |
| E. cornuta | 19.43 ± 4.93 | 6.48 ± 0.47 | 33.51 | L | 24.00 ± 8.19 | 10.92 ± 4.98 | 45.51 | L | 19.33 ± 2.89 | 6.33 ± 0.58 | 32.76 | L |
| M. jaenensis | 17.00 ± 2.65 | 7.11 ± 1.06 | 41.83 | L | 16.67 ± 0.58 | 6.56 ± 0.96 | 39.33 | L | 35.00 ± 0.00 | 35.00 ± 0.00 | 100 | H |
| M. manguillo | 14.67 ± 1.53 | 6.24 ± 0.37 | 42.58 | L | 15.00 ± 6.08 | 7.33 ± 2.03 | 48.89 | L | 10.67 ± 1.15 | 6.49 ± 0.73 | 60.83 | I |
| P. aduncum | 22.00 ± 4.36 | 6.11 ± 0.19 | 27.78 | L | 18.67 ± 2.52 | 6.33 ± 0.58 | 33.93 | L | 23.00 ± 7.55 | 6.00 ± 0.00 | 26.09 | L |
| P. amalago | 20.00 ± 3.61 | 7.04 ± 3.02 | 35.22 | L | 20.33 ± 0.58 | 6.60 ± 0.93 | 32.46 | L | 33.33 ± 2.89 | 33.56 ± 2.50 | 100.67 | H |
| P. glabribaccum | 19.67 ± 9.61 | 6.06 ± 0.10 | 30.79 | L | 20.00 ± 3.46 | 6.92 ± 1.51 | 34.61 | L | 16.00 ± 16.5 | 15.89 ± 16.6 | 99.31 | H |
| T. integrifolia | 14.00 ± 1.00 | 6.40 ± 0.32 | 45.71 | L | 17.00 ± 5.57 | 7.47 ± 1.62 | 43.92 | L | 35.00 ± 0.00 | 35.00 ± 0.00 | 100 | H |
| Z. fagara | 20.00 ± 4.58 | 6.46 ± 0.34 | 32.28 | L | 20.33 ± 9.07 | 6.89 ± 1.54 | 33.88 | L | 16.00 ± 0.00 | 10.04 ± 3.98 | 62.78 | I |
| Essential Oils | Antioxidant Activity | TPC (mg GAE/g) | ||
|---|---|---|---|---|
| DPPH (µmol TE/g) | FRAP (µmol Fe2+/g) | ABTS (µmol TE/g) | ||
| E. cornuta | 7.72 ± 0.08 e | 39.39 ± 2.03 f | 9.91 ± 0.19 e | 67.74 ± 1.08 g |
| M. jaenensis | 8.61 ± 0.21 d | 73.69 ± 2.80 c | 16.43 ± 0.13 b | 96.09 ± 1.65 d |
| M. manguillo | 7.88 ± 0.14 e | 71.19 ± 1.97 c | 8.44 ± 0.10 f | 113.65 ± 0.13 c |
| P. aduncum | 19.28 ± 0.09 a | 111.79 ± 0.78 a | 19.02 ± 0.09 a | 132.64 ± 0.23 b |
| P. amalago | 7.61 ± 0.08 e | 22.59 ± 1.55 h | 8.86 ± 0.16 f | 50.11 ± 0.77 h |
| P. glabribaccum | 7.20 ± 0.07 f | 64.99 ± 0.81 d | 7.86 ± 0.10 g | 86.81 ± 1.68 e |
| T. integrifolia | 11.12 ± 0.10 c | 104.64 ± 1.29 b | 15.20 ± 0.10 c | 159.34 ± 0.19 a |
| Z. fagara | 7.16 ± 0.09 e | 32.86 ± 1.15 g | 7.86 ± 0.41 g | 52.49 ± 0.06 h |
| Essential Oils | Extraction Yield Cumulative Var: 94% | Antioxidant Activity Cumulative Var: 97% | Antimicrobial Activity Cumulative Var: 94% |
|---|---|---|---|
| E. cornuta | 20.18 | 78.07 | 34.49 |
| M. jaenensis | 8.82 | 122.10 | 102.39 |
| M. manguillo | 14.71 | 133.42 | 63.21 |
| P. aduncum | 79.64 | 175.14 | 27.65 |
| P. amalago | 95.64 | 53.79 | 102.61 |
| P. glabribaccum | 58.27 | 108.82 | 100.73 |
| T. integrifolia | 22.28 | 190.35 | 102.64 |
| Z. fagara | 176.44 | 62.06 | 64.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Rosillo, F.; Aguirre, E.; Quiñones Huatangari, L.; Chavez, S.G.; Caetano, A.C.; Iliquin-Chavez, A.F.; Silva-Zuta, M.Z.; Castro-Alayo, E.M.; Balcázar-Zumaeta, C.R. Discovering the Bioactive and Antibacterial Potential of Essential Oils from Aromatic Plants of Northeastern Peru. Molecules 2025, 30, 4236. https://doi.org/10.3390/molecules30214236
Fernandez-Rosillo F, Aguirre E, Quiñones Huatangari L, Chavez SG, Caetano AC, Iliquin-Chavez AF, Silva-Zuta MZ, Castro-Alayo EM, Balcázar-Zumaeta CR. Discovering the Bioactive and Antibacterial Potential of Essential Oils from Aromatic Plants of Northeastern Peru. Molecules. 2025; 30(21):4236. https://doi.org/10.3390/molecules30214236
Chicago/Turabian StyleFernandez-Rosillo, Frank, Elza Aguirre, Lenin Quiñones Huatangari, Segundo G. Chavez, Aline C. Caetano, Angel F. Iliquin-Chavez, Miguelina Z. Silva-Zuta, Efraín M. Castro-Alayo, and César R. Balcázar-Zumaeta. 2025. "Discovering the Bioactive and Antibacterial Potential of Essential Oils from Aromatic Plants of Northeastern Peru" Molecules 30, no. 21: 4236. https://doi.org/10.3390/molecules30214236
APA StyleFernandez-Rosillo, F., Aguirre, E., Quiñones Huatangari, L., Chavez, S. G., Caetano, A. C., Iliquin-Chavez, A. F., Silva-Zuta, M. Z., Castro-Alayo, E. M., & Balcázar-Zumaeta, C. R. (2025). Discovering the Bioactive and Antibacterial Potential of Essential Oils from Aromatic Plants of Northeastern Peru. Molecules, 30(21), 4236. https://doi.org/10.3390/molecules30214236











