Green Synthesis of Gallium-Based Metal-Organic Frameworks with Antibacterial Properties
Abstract
1. Introduction
2. Results and Discussion
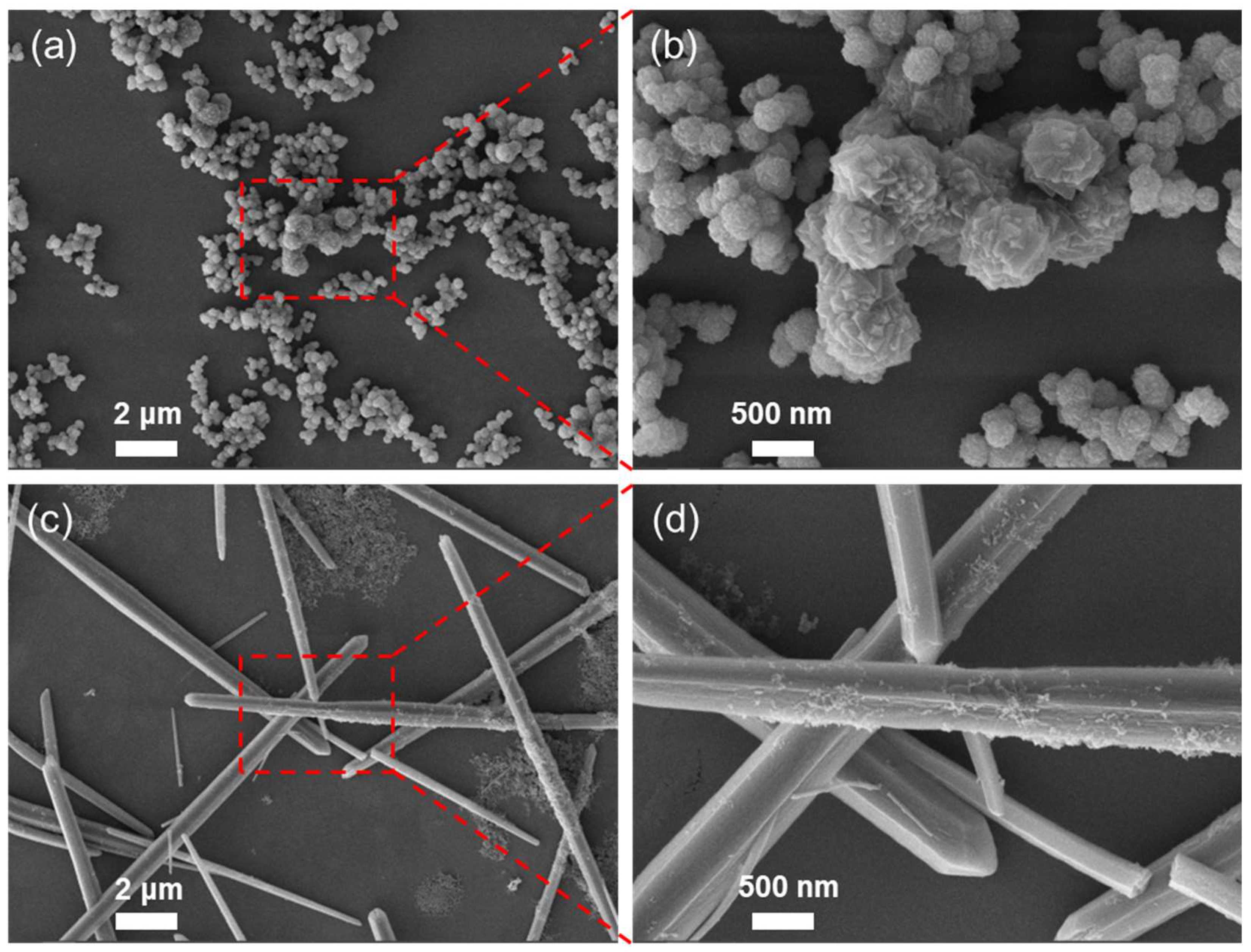
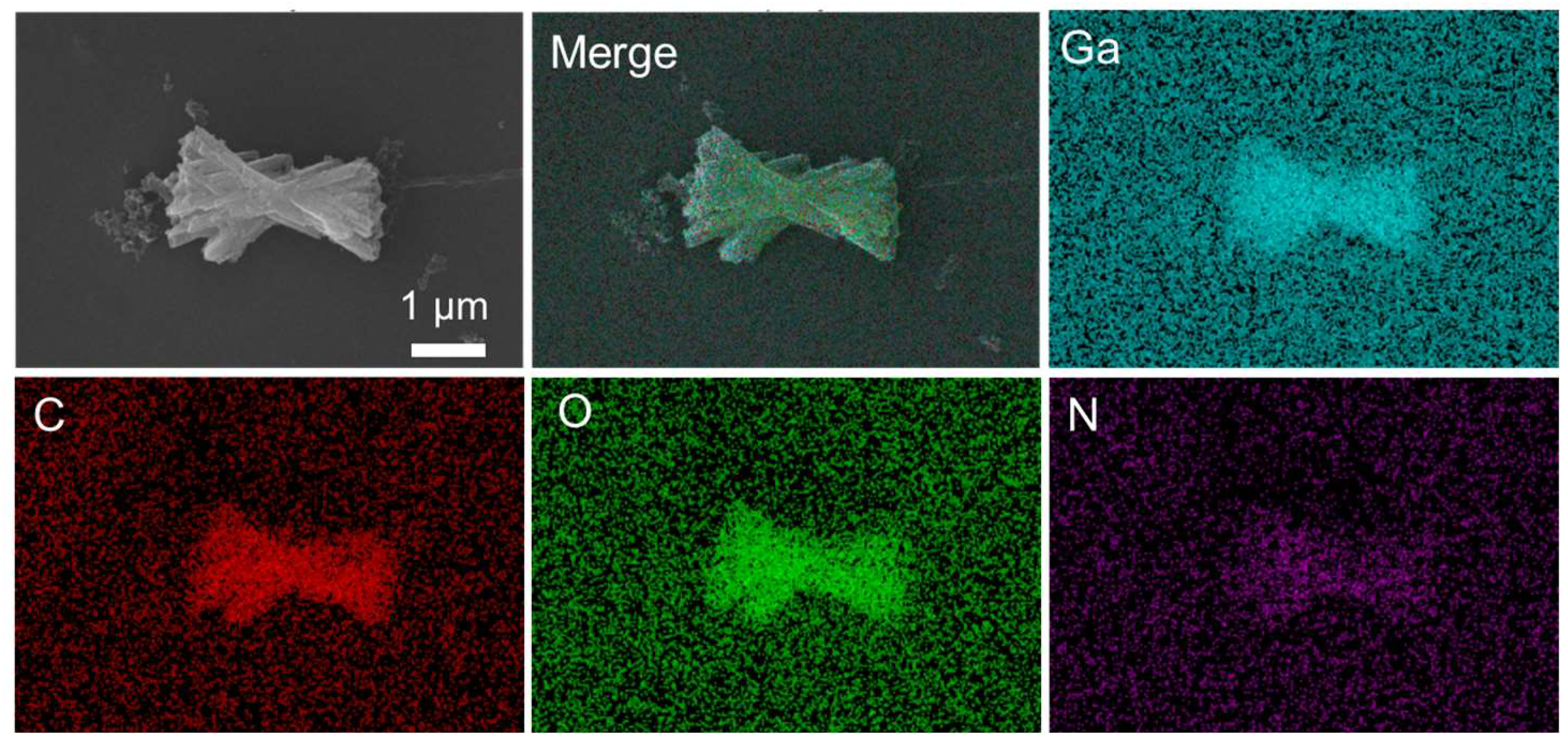
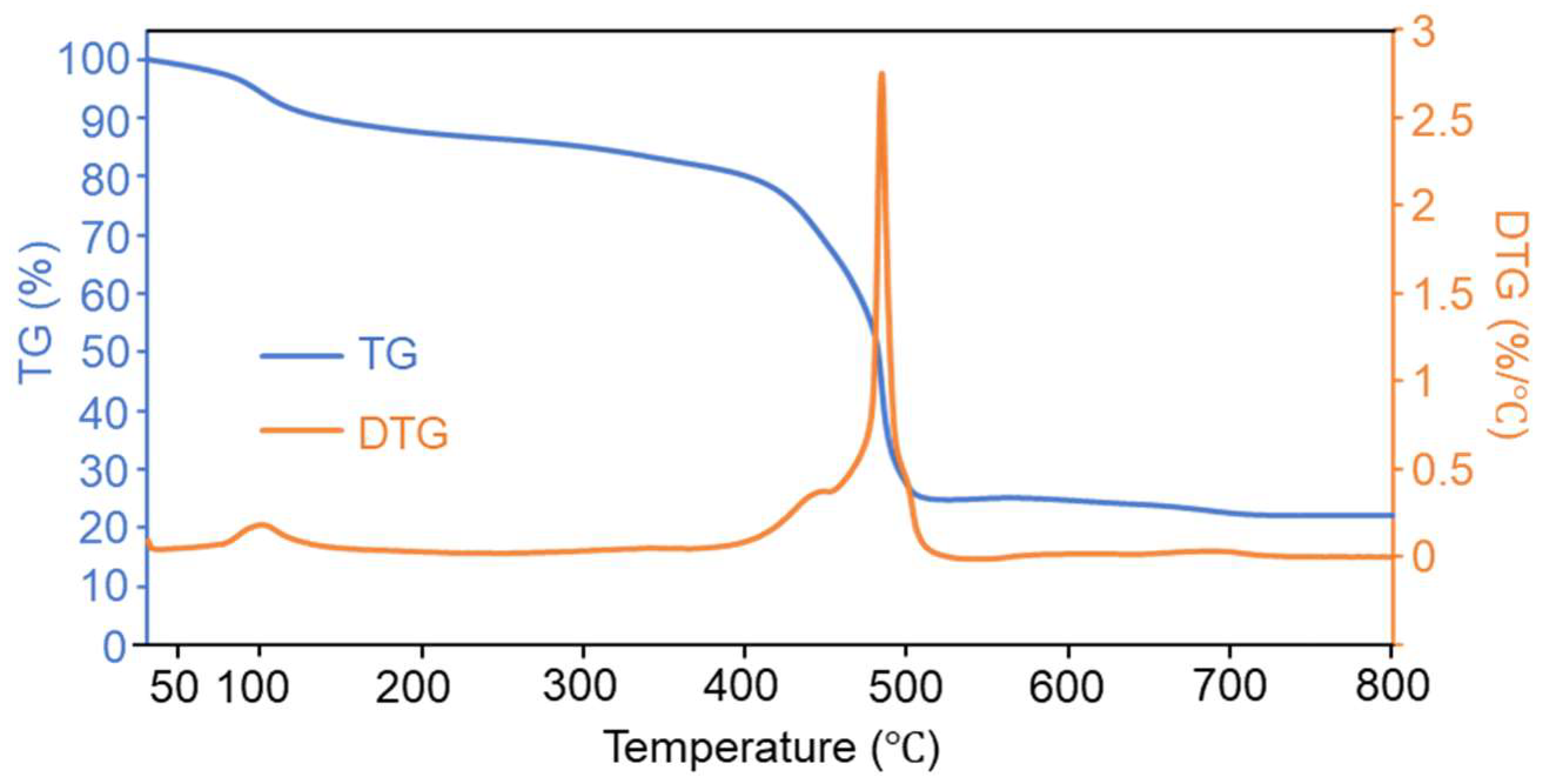
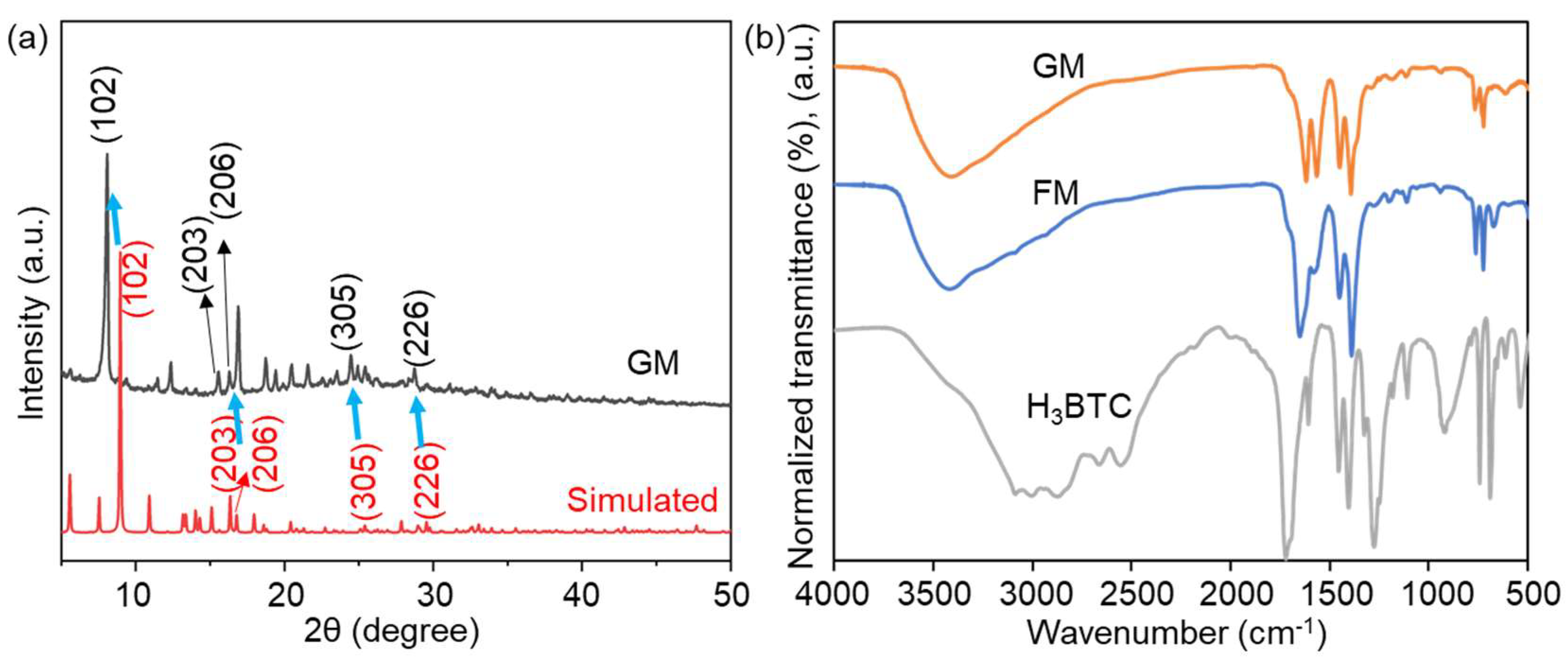
2.1. Preparation and Characterization of FM and GM
2.2. Antibacterial Activity of FM and GM
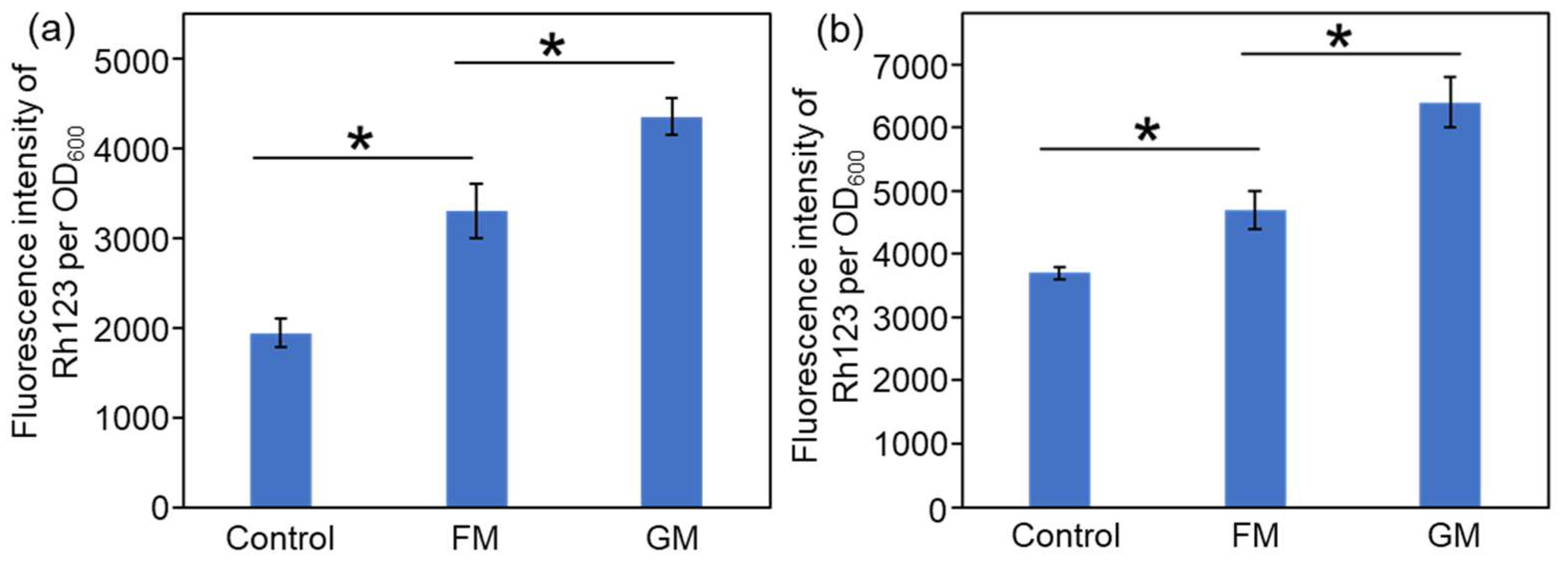
2.3. Antibacterial Mechanisms
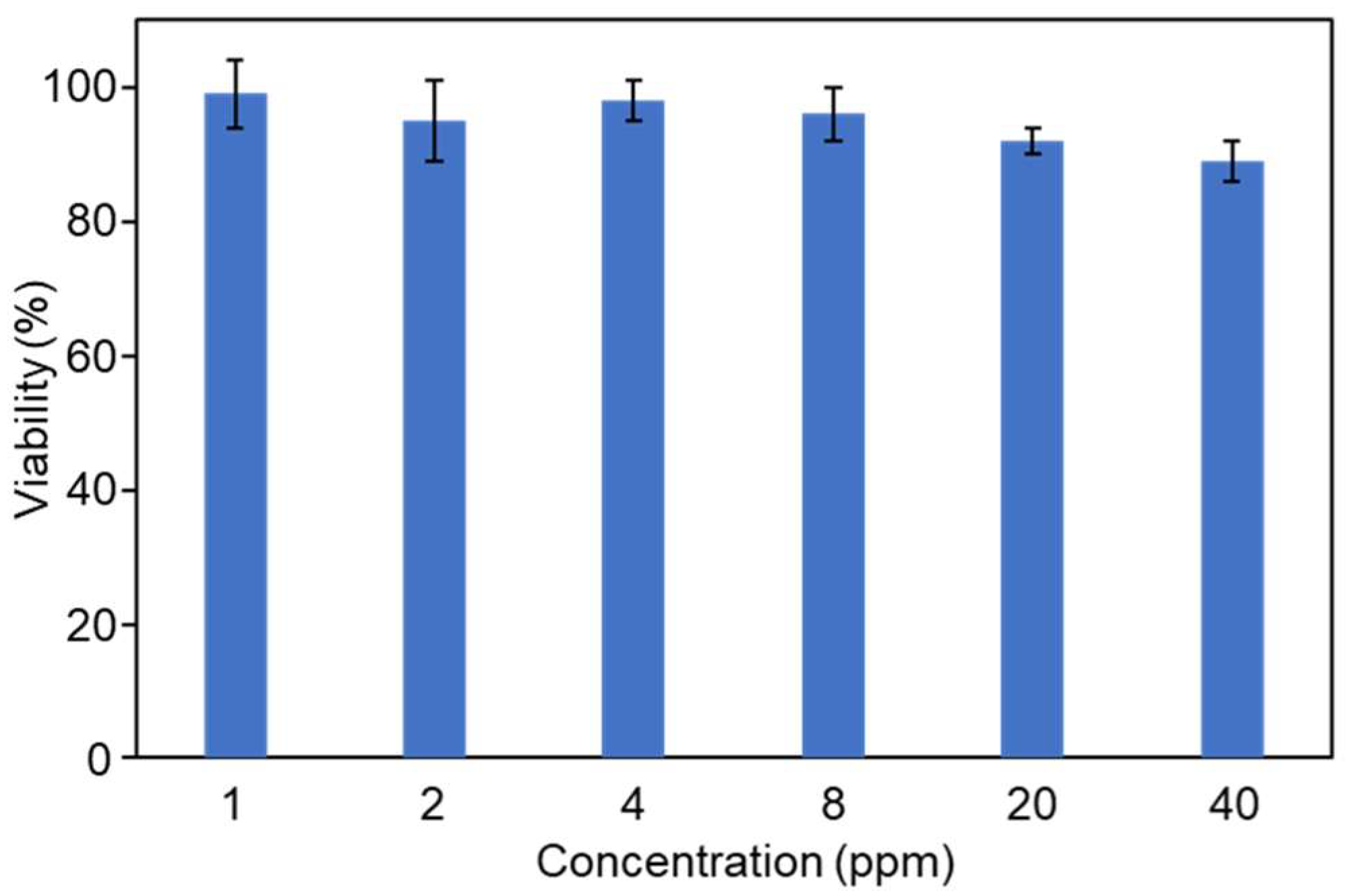
2.4. Cytotoxicity of GM
3. Materials and Methods
3.1. Preparation of FM and GM
3.2. Characterization of GM
3.3. Bacterial Culture
3.4. Growth Inhibition Assay
3.5. Mitochondrial Membrane Potential Detection
3.6. Evaluation of Reactive Oxygen Species–Scavenging Activities
3.7. Cell Viability Test
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galgano, M.; Pellegrini, F.; Catalano, E.; Capozzi, L.; Del Sambro, L.; Sposato, A.; Lucente, M.S.; Vasinioti, V.I.; Catella, C.; Odigie, A.E.; et al. Acquired bacterial resistance to antibiotics and resistance genes: From past to future. Antibiotics 2025, 14, 222. [Google Scholar] [CrossRef]
- Enshaie, E.; Nigam, S.; Patel, S.; Rai, V. Livestock antibiotics use and antimicrobial resistance. Antibiotics 2025, 14, 621. [Google Scholar] [CrossRef] [PubMed]
- Lajqi Berisha, N.; Poceva Panovska, A.; Hajrulai-Musliu, Z. Antibiotic resistance and aquatic systems: Importance in public health. Water 2024, 16, 2362. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M.; et al. Antibiotic action and resistance: Updated review of mechanisms, spread, influencing factors, and alternative approaches for combating resistance. Front. Pharmacol. 2024, 14, 1305294. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Badawy, N.K.; Ashraf, Y.; Zatioun, A.A.; Masriya, H.H.; Ammar, M.M.; Mohamed, N.A.; Mourad, S.; Assy, A.M. Combating antibiotic resistance: Mechanisms, multidrug-resistant pathogens, and novel therapeutic approaches: An updated review. Pharmaceuticals 2025, 18, 402. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Najafiyan, H.; Yousefi, M.H.; Khosravi, E.; Shabani, E.; Afkhami, H.; Aghaei, S.S. Beyond antibiotics: Exploring multifaceted approaches to combat bacterial resistance in the modern era: A comprehensive review. Front. Cell. Infect. Microbiol. 2025, 15, 1493915. [Google Scholar] [CrossRef]
- Solanki, R.; Makwana, N.; Kumar, R.; Joshi, M.; Patel, A.; Bhatia, D.; Sahoo, D.K. Nanomedicines as a cutting-edge solution to combat antimicrobial resistance. RSC Adv. 2024, 14, 33568–33586. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Nanomaterial-based strategies to combat antibiotic resistance: Mechanisms and applications. Antibiotics 2025, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.A.; Alyamani, H.; Alenaizat, A.; Tunc, T.; Aljabali, A.A.; Alsaadi, M.M. Nanomaterial-based drug delivery systems in overcoming bacterial resistance: Current review. Microb. Pathog. 2025, 203, 107455. [Google Scholar] [CrossRef]
- Bedair, H.M.; Hamed, M.; Mansour, F.R. New emerging materials with potential antibacterial activities. Appl. Microbiol. Biotechnol. 2024, 108, 515. [Google Scholar] [CrossRef]
- Fang, Q.; Pan, X. A systematic review of antibiotic resistance driven by metal-based nanoparticles: Mechanisms and a call for risk mitigation. Sci. Total Environ. 2024, 916, 170080. [Google Scholar] [CrossRef]
- Zarouki, M.A.; Hejji, L.; Azzouz, A.; Ali, Y.A.; Munoz, A.J.; Kailasa, S.K. Carbon nanostructures with antibacterial and wound healing activities: Recent progress and challenges. J. Mater. Chem. B 2025, 13, 9745–9803. [Google Scholar] [CrossRef] [PubMed]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Mendes, R.F.; Figueira, F.; Leite, J.P.; Gales, L.; Paz, F.A.A. Metal–organic frameworks: A future toolbox for biomedicine? Chem. Soc. Rev. 2020, 49, 9121–9153. [Google Scholar] [CrossRef]
- Oheix, E.; Daou, T.J.; Pieuchot, L. Antimicrobial zeolites and metal organic frameworks. Mater. Horiz. 2024, 11, 6222–6256. [Google Scholar] [CrossRef]
- Guo, L.; Kong, W.; Che, Y.; Liu, C.; Zhang, S.; Liu, H.; Tang, Y.; Yang, X.; Zhang, J.; Xu, C. Research progress on antibacterial applications of metal-organic frameworks and their biomacromolecule composites. Int. J. Biol. Macromol. 2024, 261, 129799. [Google Scholar] [CrossRef]
- Yan, X.; Lin, Z.; Shen, H.; Chen, Y.; Chen, L. Photo-responsive antibacterial metal organic frameworks. J. Mater. Chem. B 2025, 13, 6299–6328. [Google Scholar] [CrossRef]
- Hu, W.; Ouyang, Q.; Jiang, C.; Huang, S.; Alireza, N.E.; Guo, D.; Liu, J.; Peng, Y. Biomedical Metal–Organic framework materials on antimicrobial therapy: Perspectives and challenges. Mater. Today Chem. 2024, 41, 102300. [Google Scholar] [CrossRef]
- Doan, T.D.; Vu, N.N.; Hoang, T.L.G.; Nguyen-Tri, P. Metal-organic framework (MOF)-based materials for photocatalytic antibacterial applications. Coord. Chem. Rev. 2025, 523, 216298. [Google Scholar] [CrossRef]
- Shi, F.; Ma, S.S.; Liu, S.; Xin, R.; Chen, B.; Ye, W.; Sun, J. A novel antimicrobial strategy for bacterial infections: Gallium-based materials. Colloid Interface Sci. Commun. 2023, 56, 100735. [Google Scholar] [CrossRef]
- Li, F.; Liu, F.; Huang, K.; Yang, S. Advancement of gallium and gallium-based compounds as antimicrobial agents. Front. Bioeng. Biotechnol. 2022, 10, 827960. [Google Scholar] [CrossRef]
- Kircheva, N.; Dobrev, S.; Nikolova, V.; Yocheva, L.; Angelova, S.; Dudev, T. Implementation of three gallium-based complexes in the “Trojan Horse” antibacterial strategy against A. baumannii: A DFT approach. Inorg. Chem. 2024, 63, 15409–15420. [Google Scholar] [CrossRef] [PubMed]
- Kircheva, N.; Dobrev, S.; Petkova, V.; Yocheva, L.; Angelova, S.; Dudev, T. In silico analysis of the Ga3+/Fe3+ competition for binding the iron-scavenging siderophores of P. aeruginosa—Implementation of three gallium-based complexes in the “Trojan Horse” antibacterial strategy. Biomolecules 2024, 14, 487. [Google Scholar] [CrossRef]
- Yao, Z.; Yu, K.; Qian, C.; Zhou, B.; Lin, Y.; Zhang, X.; Zhang, Y.; Zhou, T.; Zeng, W.; Cao, J.; et al. Gallium nitrate inhibits multidrug-resistant Acinetobacter baumannii isolated from bloodstream infection by disrupting multiple iron-dependent metabolic processes. BMC Microbiol. 2025, 25, 216. [Google Scholar] [CrossRef]
- Liu, C.; Cui, C.; Tan, X.; Miao, J.; Wang, W.; Ren, H.; Wu, H.; Zheng, C.; Ren, H.; Kang, W. pH-mediated potentiation of gallium nitrate against Pseudomonas aeruginosa. Front. Microbiol. 2024, 15, 1464719. [Google Scholar] [CrossRef]
- Guo, M.; Tian, P.; Li, Q.; Meng, B.; Ding, Y.; Liu, Y.; Li, Y.; Yu, L.; Li, J. Gallium nitrate enhances antimicrobial activity of colistin against Klebsiella pneumoniae by inducing reactive oxygen species accumulation. Microbiol. Spectr. 2023, 11, e00334-23. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.D.C.; Alves, M.C.F.; Junior, S.A.; Medina, S.H. Bactericidal Metal–Organic Gallium Frameworks—Synthesis to Application. Mol. Pharm. 2025, 22, 638–646. [Google Scholar] [CrossRef]
- Song, G.; Li, M.; Zhou, B.; Qi, H.; Guo, J. Gallium-based metal–organic frameworks with antibacterial and anti-inflammatory properties for oral health protection. Heliyon 2024, 10, e31788. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, J.; Zhang, Q.; Yuan, K.; Yang, Y.; Li, F.; Sun, X.; Chang, H.; Liang, Y.; Zhao, J.; et al. Sub 150 nm nanoscale gallium based metal–organic frameworks armored antibiotics as super penetrating bombs for eradicating persistent bacteria. Adv. Funct. Mater. 2022, 32, 2204906. [Google Scholar] [CrossRef]
- Wang, M.; Li, R.; Sheng, S.; Yang, H.; Tang, X.; Wang, J.; Wang, F.; Zhang, Q.; Bai, L.; Chen, X.; et al. MOF nanozyme mediated bacterial metabolic regulation to intervene MRSA antibiotic tolerance for enhanced antimicrobial efficacy. Nano Today 2025, 63, 102753. [Google Scholar] [CrossRef]
- Liu, S.; Ji, Y.; Zhu, H.; Shi, Z.; Li, M.; Yu, Q. Gallium-based metal–organic frameworks loaded with antimicrobial peptides for synergistic killing of drug-resistant bacteria. J. Mater. Chem. B 2023, 11, 10446–10454. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Zhang, J.; Yu, Q.; Liu, S.; Li, M. Synthesis of the ternary nanocomposites composed of zinc 2-methylimidazolate frameworks, lactoferrin and melittin for antifungal therapy. J. Mater. Sci. 2022, 57, 16809–16819. [Google Scholar] [CrossRef]
- Sarkar, A.; Adhikary, A.; Mandal, A.; Chakraborty, T.; Das, D. Zn-BTC MOF as an adsorbent for iodine uptake and organic dye degradation. Cryst. Growth Des. 2020, 20, 7833–7839. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S.; Yildiz, M. Preparation and characterization of Bi-metallic and tri-metallic metal organic frameworks based on trimesic acid and Co(II), Ni(II), and Cu(II) ions. J. Electron. Mater. 2017, 46, 790–801. [Google Scholar] [CrossRef]
- Bhat, Z.U.H.; Hanif, S.; Rafi, Z.; Alam, M.J.; Ahmad, M.; Shakir, M. New mixed-ligand Zn(II)-based MOF as a nanocarrier platform for improved antibacterial activity of clinically approved drug levofloxacin. New J. Chem. 2023, 47, 7416–7424. [Google Scholar] [CrossRef]
- Xu, K.; Kong, L.; Ye, K.; Xu, B.; Deng, Y.; Wang, Y.; Wei, S. NiPc-Cu 2D MOF: Facile and scalable synthesis of an antimicrobial agent rivaling clinical antibiotics. Inorg. Chem. Commun. 2025, 177, 114343. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, W.; Zhao, M.; Ding, S.; Yu, L.; Hu, Q. Tuning d-band center of FeCu alloy aerogel nanozyme boosting biosensing and wound therapy. Adv. Funct. Mater. 2025, 35, 2424433. [Google Scholar] [CrossRef]
- Liu, J.; Wu, D.; Zhu, N.; Wu, Y.; Li, G. Antibacterial mechanisms and applications of metal-organic frameworks and their derived nanomaterials. Trends Food Sci. Technol. 2021, 109, 413–434. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, B.; Zhang, Z.; Kai, T.; Wu, P.; Ding, P. Recent advances in metal–organic frameworks for antibacterial applications: Mechanisms and emerging strategies. RSC Adv. 2025, 15, 26710–26727. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Teng, L.; Ping, J. Graphitic carbon nitride confers bacterial tolerance to antibiotics in wastewater relating to ATP depletion. Molecules 2024, 29, 5780. [Google Scholar] [CrossRef]
- Sharma, S.; Manhar, A.K.; Bora, P.J.; Dolui, S.K.; Mandal, M. Evaluation of antioxidant and antibacterial activity of various aspect ratio gold (Au) nanorods. Adv. Mater. Lett. 2015, 6, 235–241. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shi, Z.; Teng, L.; Nie, J.; Zhang, L. Biocompatible black phosphorus nanosheets-antimicrobial peptide nanocomposites for enhanced anti-infection therapy. Molecules 2025, 30, 872. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, L.; Yang, Y.; Shi, Z.; Jia, Y.; Lu, B.; Zou, Y.; Liu, S.; Zhang, L. Green Synthesis of Gallium-Based Metal-Organic Frameworks with Antibacterial Properties. Molecules 2025, 30, 4190. https://doi.org/10.3390/molecules30214190
Teng L, Yang Y, Shi Z, Jia Y, Lu B, Zou Y, Liu S, Zhang L. Green Synthesis of Gallium-Based Metal-Organic Frameworks with Antibacterial Properties. Molecules. 2025; 30(21):4190. https://doi.org/10.3390/molecules30214190
Chicago/Turabian StyleTeng, Lin, Yuxin Yang, Zhishang Shi, Yimeng Jia, Binbin Lu, Ying Zou, Shuo Liu, and Libing Zhang. 2025. "Green Synthesis of Gallium-Based Metal-Organic Frameworks with Antibacterial Properties" Molecules 30, no. 21: 4190. https://doi.org/10.3390/molecules30214190
APA StyleTeng, L., Yang, Y., Shi, Z., Jia, Y., Lu, B., Zou, Y., Liu, S., & Zhang, L. (2025). Green Synthesis of Gallium-Based Metal-Organic Frameworks with Antibacterial Properties. Molecules, 30(21), 4190. https://doi.org/10.3390/molecules30214190






