A Phthalocyanine Optical Probe Responding to Cationic Surfactants Containing Long Carbon Chains with High Selectivity in Total Water Phase and Its Applications
Abstract
1. Introduction
2. Results and Discussion
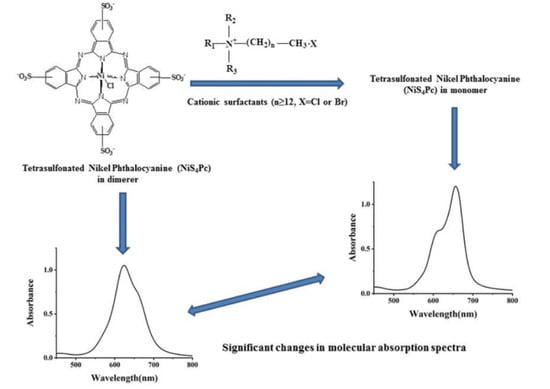
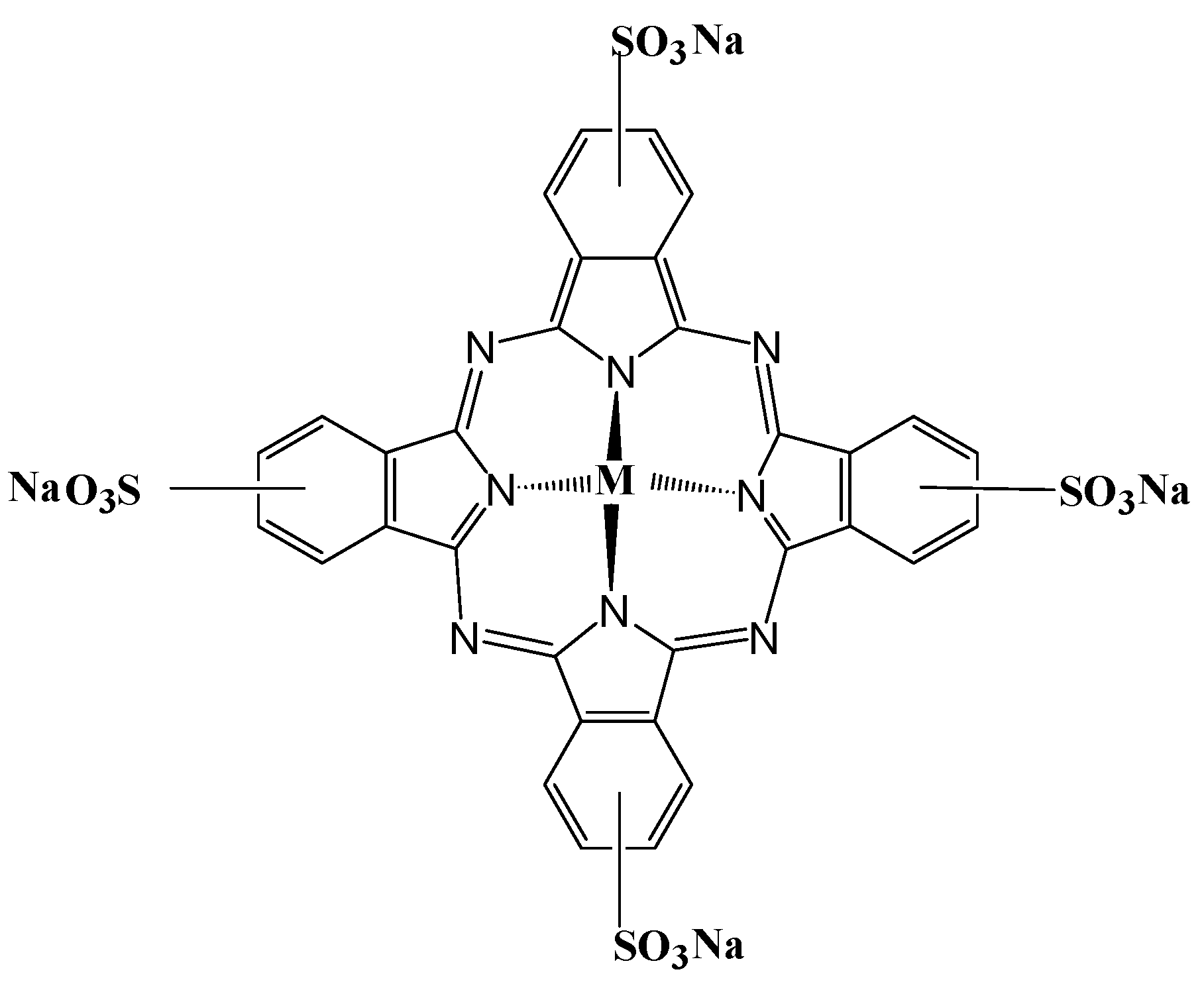
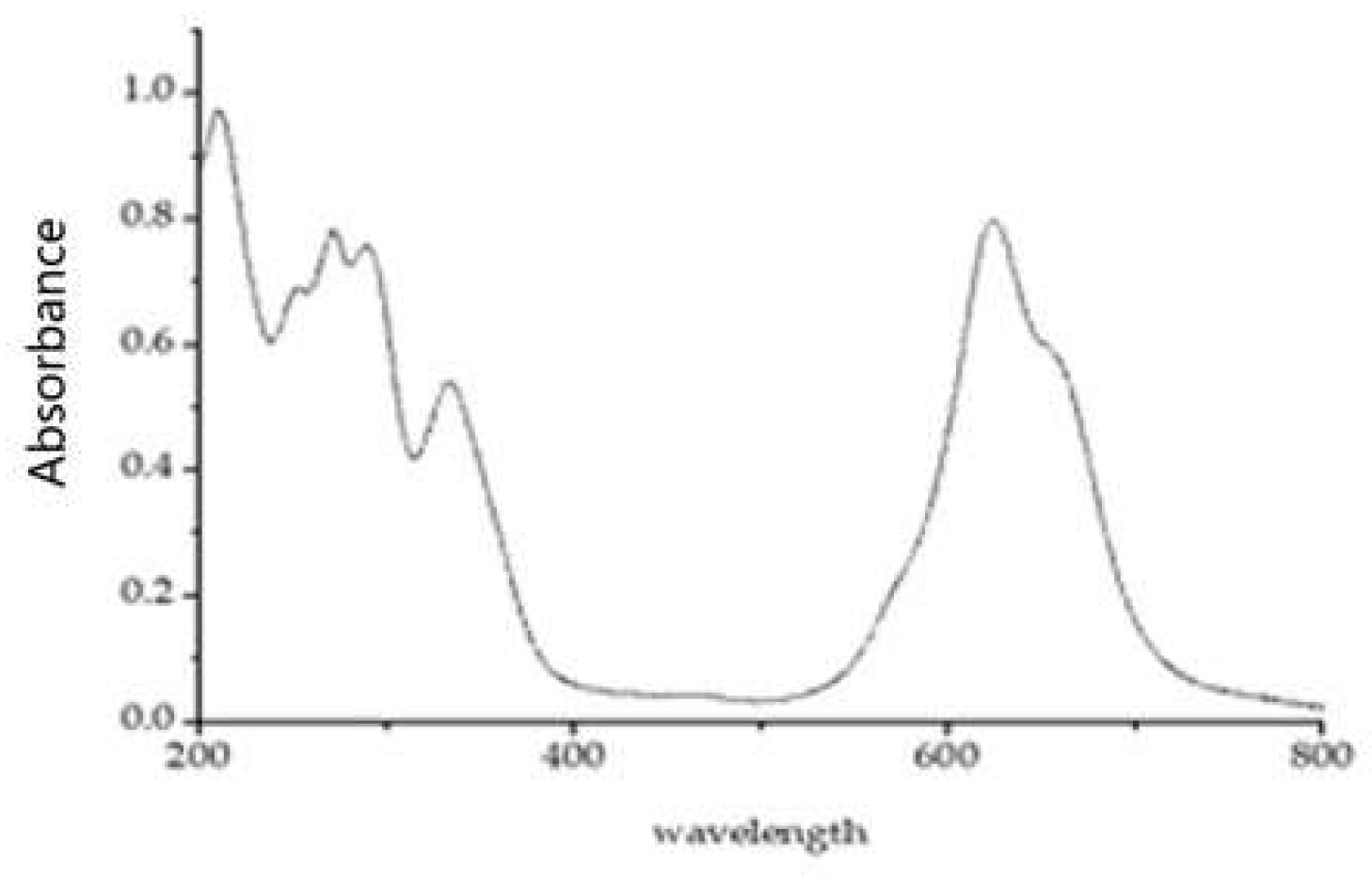
2.1. Molecular Structure and Spectral Characteristics of Tetra-Sulphonated Metal Phthalocyanines
2.2. Discussion on Reaction Mechanism
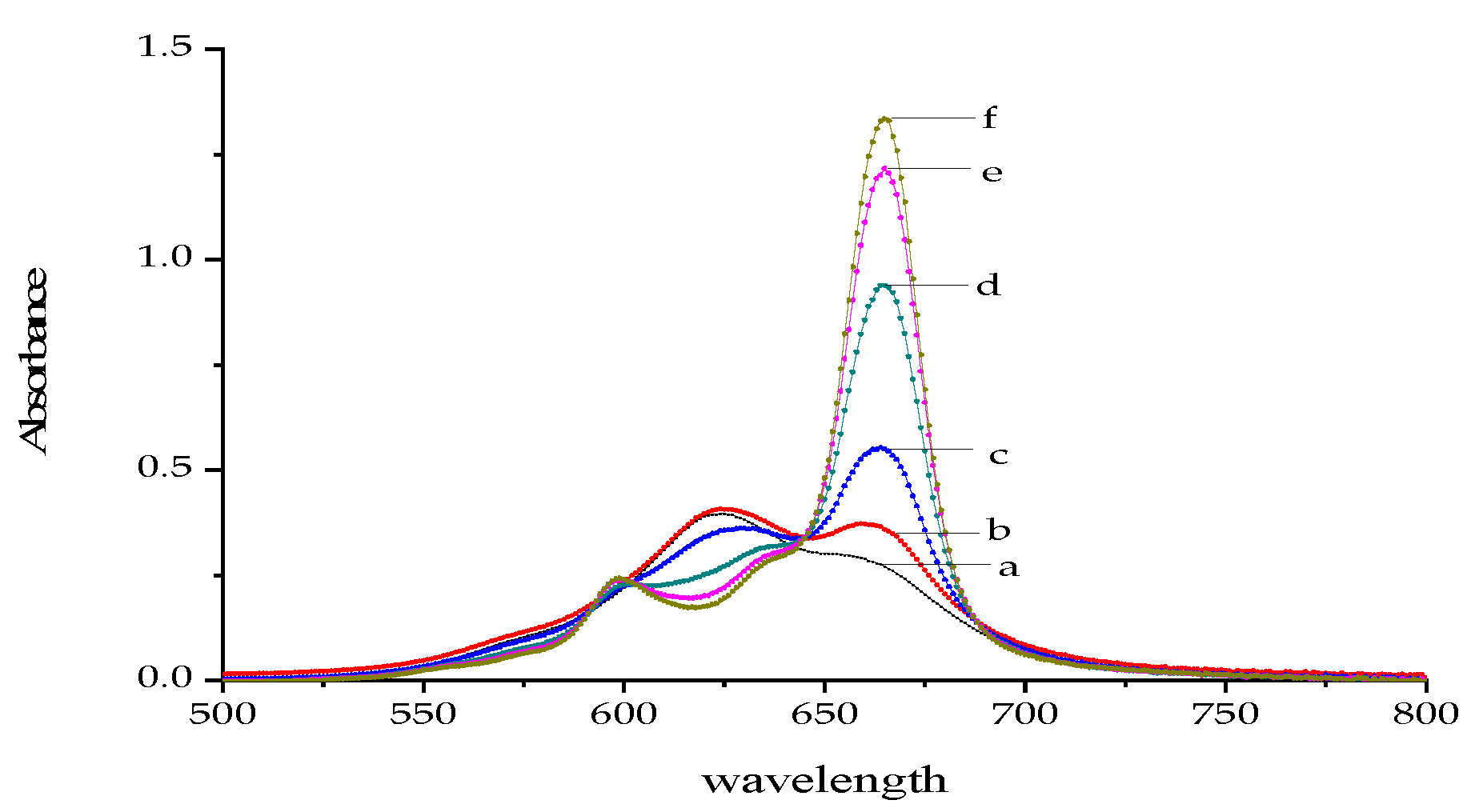
2.3. Optimization of the Experimental Conditions
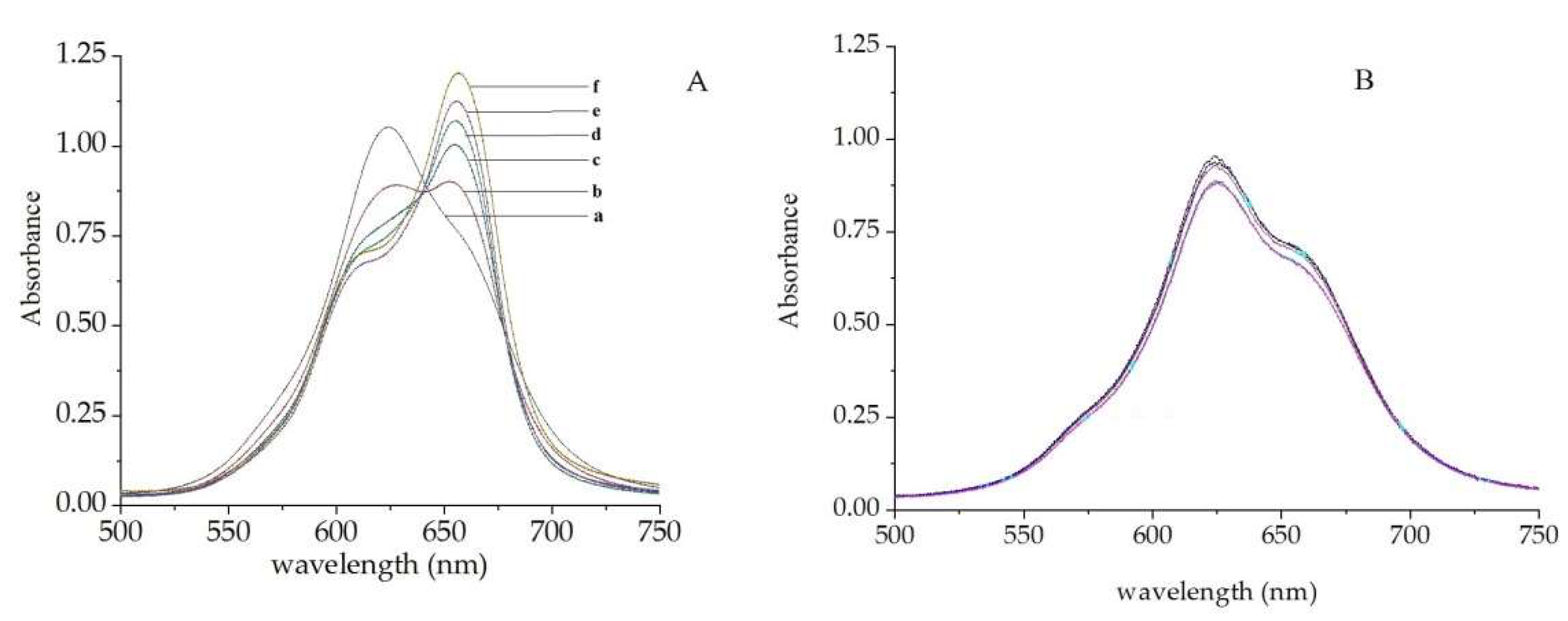
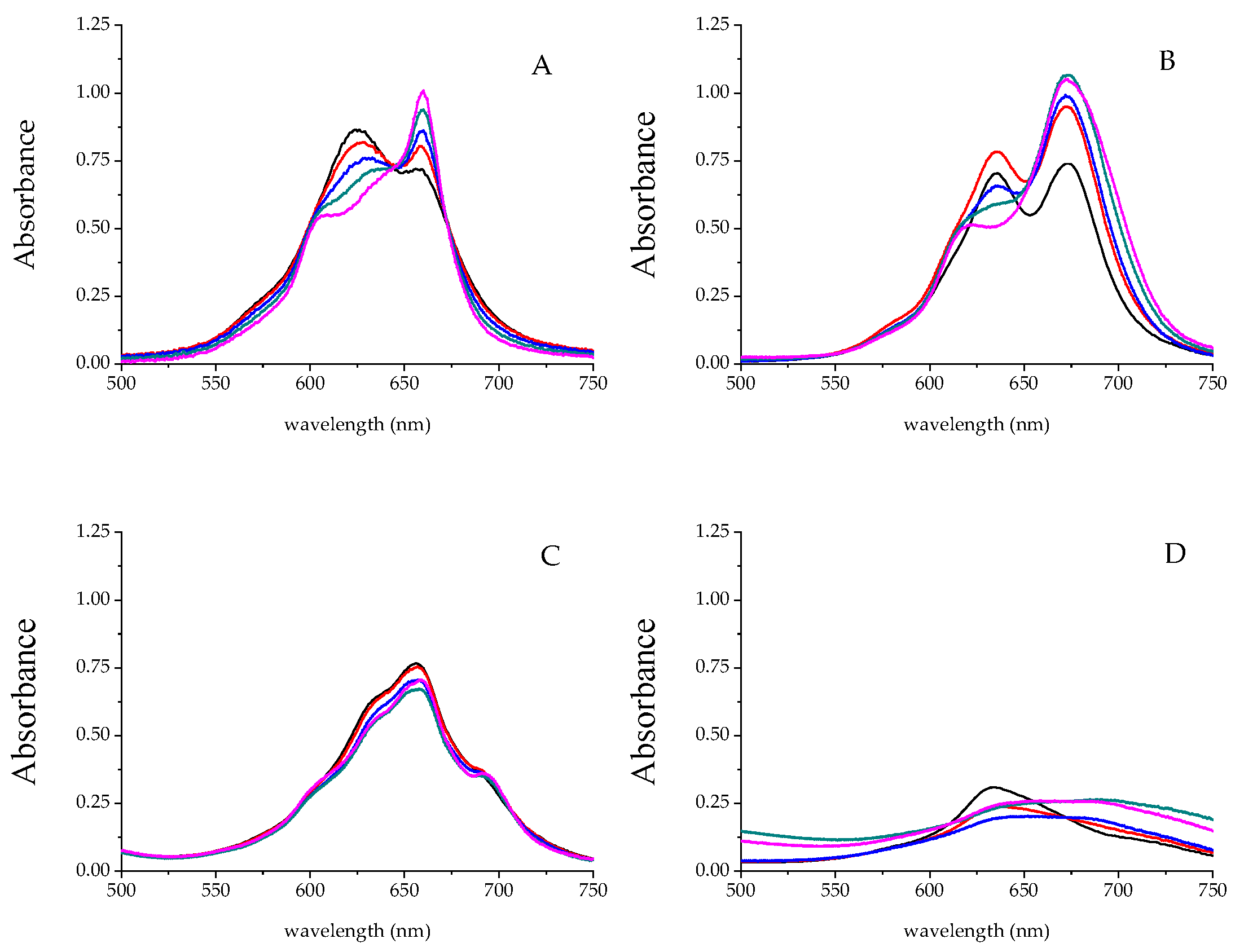
2.3.1. Selection of Metal Phthalocyanine Compounds
2.3.2. Effect of pH
2.3.3. Selection of Buffer Systems
2.3.4. Effect of Ion Strength
2.3.5. Selection of Wavelength Pair for Measurements
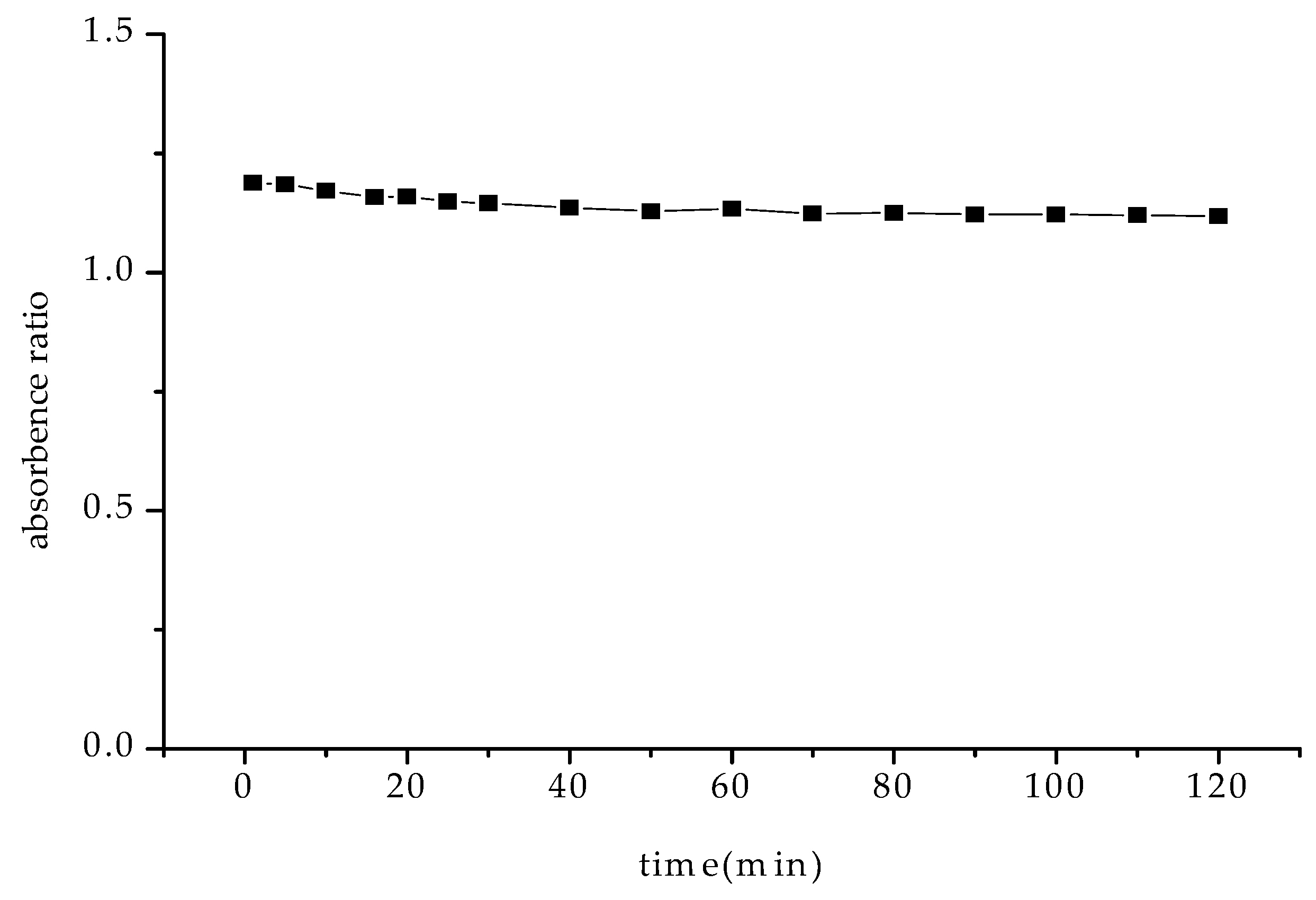
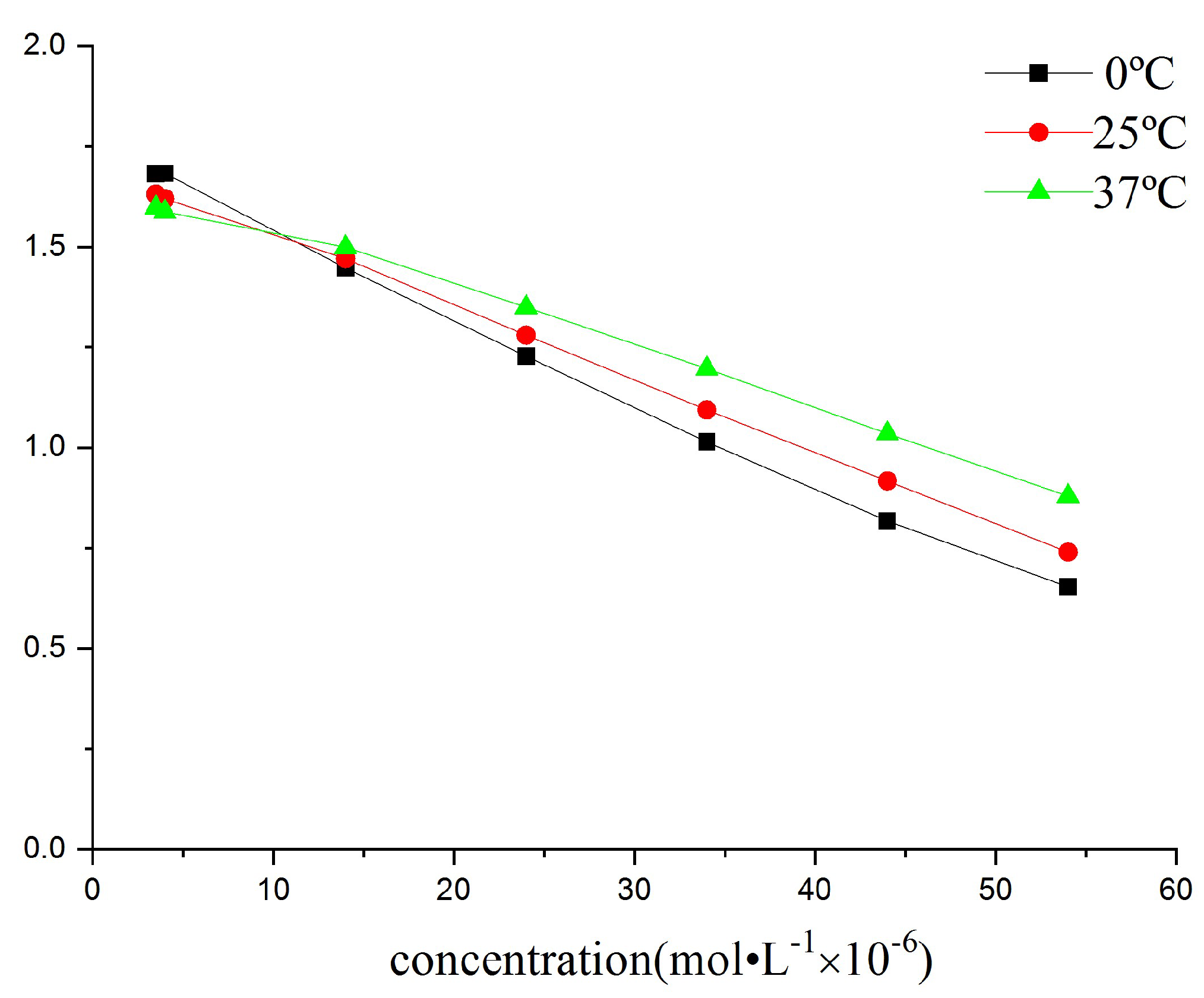
2.3.6. Effects of Reaction Time and Temperature
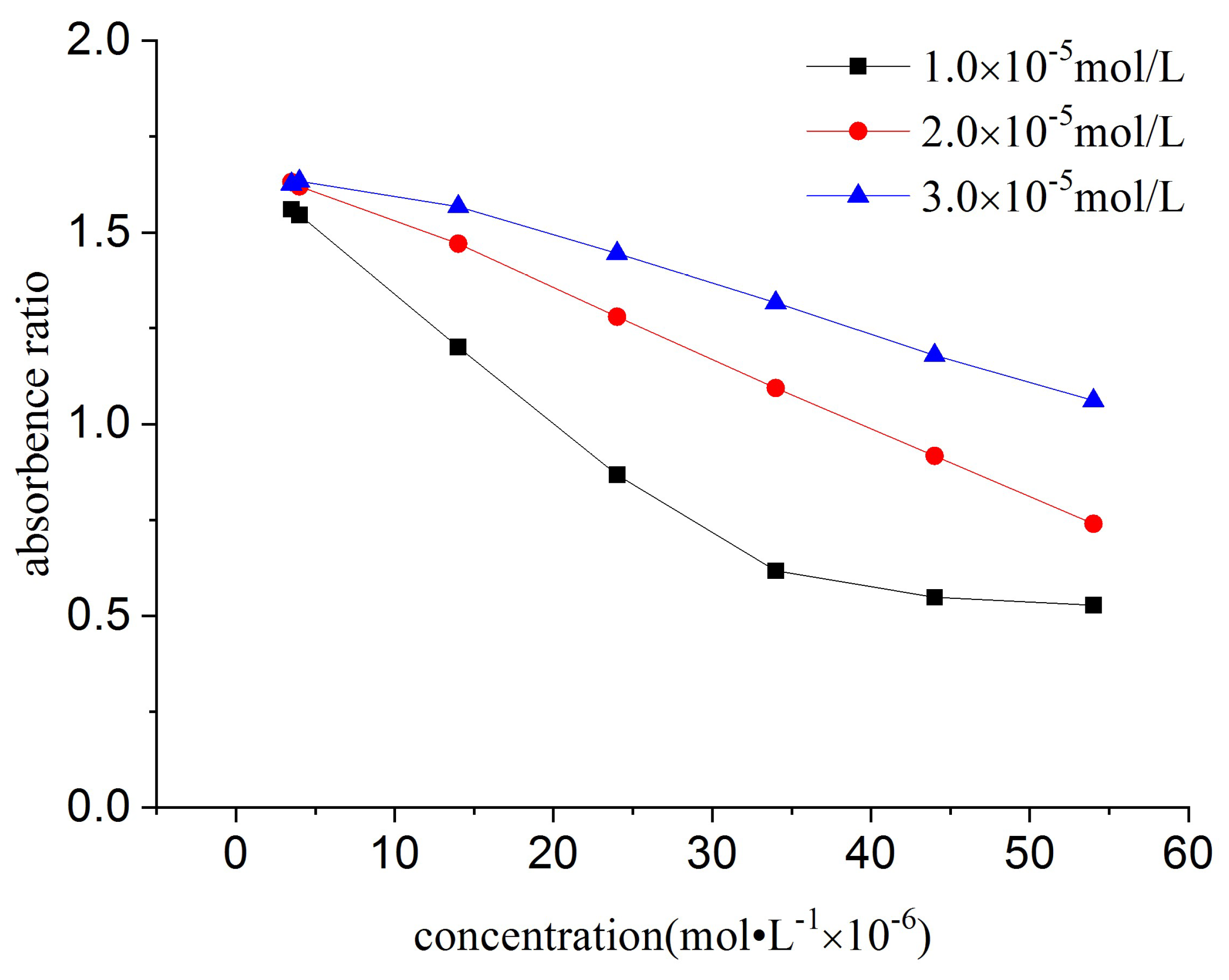
2.3.7. Effect of the Concentration of Tetra-Sulphonated Nickel Phthalocyanine
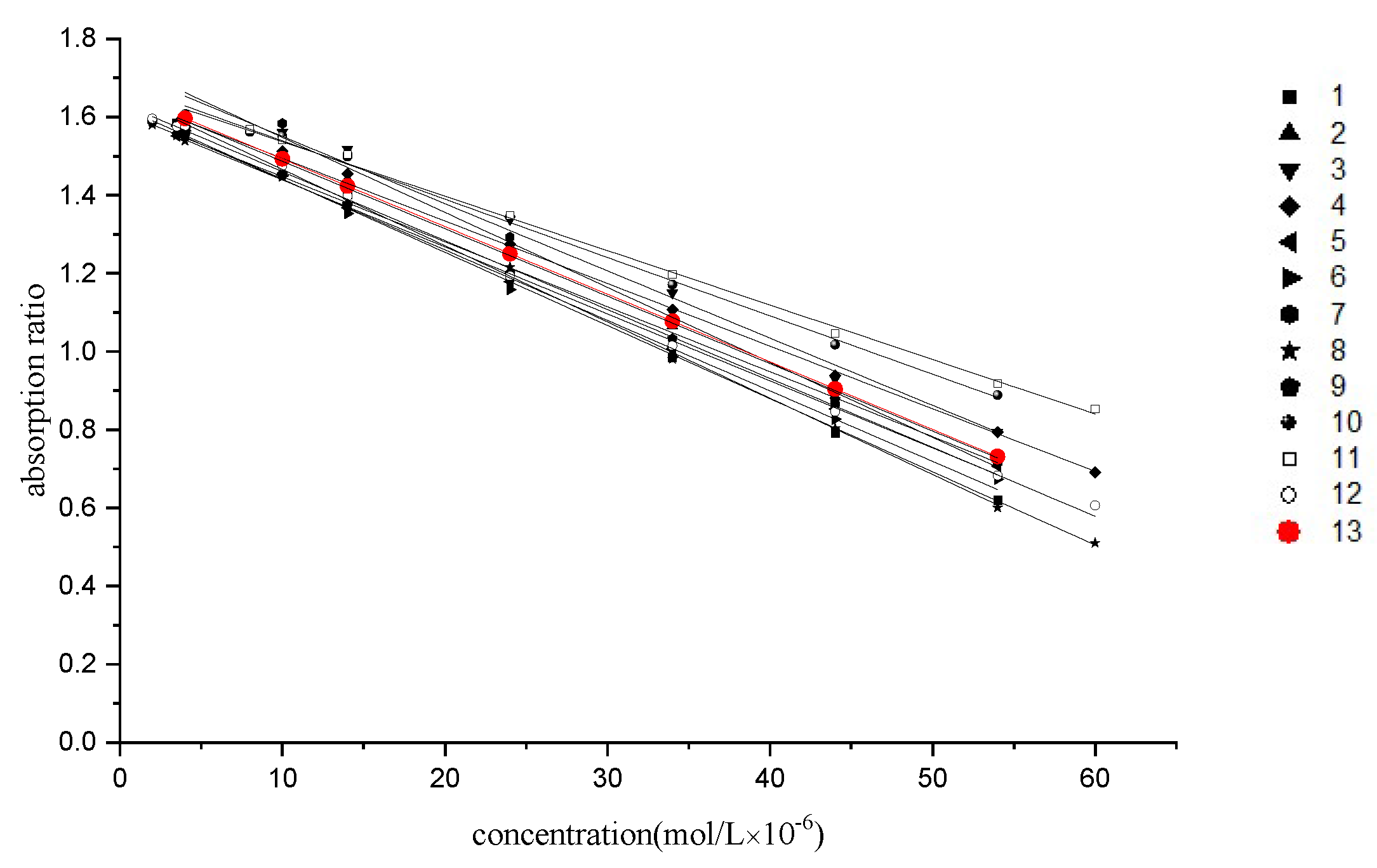
2.4. Averaging of Calibration Curves
2.5. Interference of Foreign Substances
2.6. Determination of Real Samples
3. Materials and Methods
3.1. Equipment and Reagents
3.2. Experimental Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Negm, N.A.; Elkholy, Y.M.; Ghuiba, F.M.; Zahran, M.K.; Mahmoud, S.A.; Tawfik, S.M. Benzothiazol-3-ium cationic schiff base surfactants: Synthesis, surface activity and antimicrobial applications against pathogenic and sulfur reducing bacteria in oil fields. J. Disper. Sci. Technol. 2011, 32, 512–518. [Google Scholar] [CrossRef]
- Gorbunova, T.I.; Bazhin, D.N.; Zapevalov, A.Y.; Saloutin, V.I. Synthesis and inhibiting capacity of new fluorine-containing quaternary ammonium salts. Russ. J. Appl. Chem. 2011, 84, 972–977. [Google Scholar] [CrossRef]
- Tanaka, M.; Rastogi, A.; Toepperwein, G.N.; Riggleman, R.A.; Felix, N.M.; de Pablo, J.J.; Ober, C.K. Fluorinated quaternary ammonium salts as dissolution aids for polar polymers in environmentally benign supercritical carbon dioxide. Chem. Mater. 2009, 21, 3125–3135. [Google Scholar] [CrossRef]
- Yildirim, G.; Acar, E.T. Determination of benzalkonium chloride in wet wipes by using a validated capillary electrophoresis method. J. Cosmet. Sci. 2017, 68, 1–10. [Google Scholar] [PubMed]
- Buffet-Bataillon, S.; Tattevin, P.; Maillard, J.Y.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Efflux pump induction by quaternary ammonium compounds and fluoroquinolone resistance in bacteria. Future Microbiol. 2016, 11, 81–92. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Suo, X.K.; Bai, X.Q.; Yuan, C.Q.; Li, H. Functionalizing aluminum substrata by quaternary ammonium for antifouling performances. Appl. Surf. Sci. 2018, 440, 300–307. [Google Scholar] [CrossRef]
- Yunhuan, X.; Cheng, Z.; Jing, L.; Taoyan, M.; Wuhuan, H. Research progress of synthesis and application of fluorinated quaternary ammonium salt surfactants. Chem. Ind. Eng. Progr. 2013, 32, 1641–1648. [Google Scholar]
- Zhang, C.; Cui, F.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Yu, Z.G.; Zhu, M.Y.; Shen, L.Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef]
- Cui, L.; Puerto, M.; Lopez-Salinas, J.L.; Biswal, S.L.; Hirasaki, G.J. Improved Methylene Blue Two-Phase Titration Method for Determining Cationic Surfactant Concentration in High-Salinity Brine. Anal. Chem. 2014, 86, 11055–11061. [Google Scholar] [CrossRef]
- Paun, I.; Iancu, V.I.; Cruceru, L.; Niculescu, M.; Chiriac, F.L. Simultaneous Determination of Anionic, Amphoteric and Cationic Surfactants Mixtures in Surface Water. Rev. Chim 2018, 69, 27–30. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zhan, H.W.; Ma, W.X. Spectrophotometric Determination of Cationic Surfactant with Titan Yellow. Asian J. Chem. 2013, 25, 2736–2738. [Google Scholar] [CrossRef]
- Shtykov, S.N.; Beloliptseva, G.M. Fluorescence enhancement and quenching effects in the magnesium-8-hydroxyquinoline-5-sulfonic acid-cationic surfactant system and their application to the determination of cetylpyridinium. J. Anal. Chem. 1998, 53, 264–268. [Google Scholar]
- Zhidong, M.; Zhong, G.; Wende, Z. Study on the oscilopolarographic determination of cationic surfactants of pyridinium salts. Phys. Test. Chem. Anal. (Part B Chem. Anal.) 2003, 39, 334–335. [Google Scholar]
- Mohammad, A.; Qasimullah; Khan, M.; Mobin, R. Thin-layer chromatography in the analysis of surfactants: At a glance. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 863–871. [Google Scholar] [CrossRef]
- Mack, J.; Stillman, M.J. Assignment of the optical spectra of metal phthalocyanines through spectral band deconvolution analysis and ZINDO calculations. Coordin. Chem. Rev. 2001, 219, 993–1032. [Google Scholar] [CrossRef]
- Jiang, J.Z. Functional Phthalocyanine Molecular Materials; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Xia, D.C. Synthesis and Optical Character Study of New Substituted Pthalocyanies and Phthalocyanine Crysta; Jilin University Press: Changchun, China, 2009. [Google Scholar]
- MeKeown, N.B. Phtalocyanine Materials—Synthesis, Structure, and Function; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Yu, F.; Guo, M.; Deng, Y.; Lu, Y.; Chen, L.; Huang, P.; Li, D. Structure-matched Phthalocyanine Ion Pair as a Red-emitting Fluorescent Optical Probe for the Analysis of Sodium Dodecylbenzenesulfonate with High Specificity and Sensitivity. Anal. Sci. 2016, 32, 201. [Google Scholar] [CrossRef]
- Duan, H.; Liu, Z.; Liu, S.; Yi, A. Resonance Rayleigh scattering, second-order scattering and frequency doubling scattering methods for the indirect determination of penicillin antibiotics based on the formation of Fe(3)[Fe(CN)6](2) nanoparticles. Talanta 2008, 75, 1253–1259. [Google Scholar] [CrossRef]
- Yongxin, L.; Dexing, Z.; Danhua, Z.; Shujuan, Z.; Changqing, Z.; Lun, W. Rayleigh Light-Scattering System of Manganese-Tetrasulfonatophthalocyanine-Proteins and Its Application. Chin. J. Anal. Chem. 2003, 31, 1372–1375. [Google Scholar]
- Okura, I. Photosensitization of Porphyrins and Phthalocayanies; Gordon and Breach Science Pubolishers: London, UK, 2000. [Google Scholar]
- Thomas, A.L. Phthalocyanie Research and Applications; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Moser, F.H.; Thomas, A.L. The Phthalocyanies (Volume I, Properties); CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Lu, L.; Wu, X.; Yuan, S.H. Dimerization of Aluminum, Zinc, Vanadium and Gallium Phthalocyanie-sulfonates in water, Aqueous Acolholic Solution and Micelles. Spectro. Spectra. Anal. 1999, 19, 750–754. [Google Scholar]
- Ball, D.J.; Wood, S.R.; Vernon, D.I.; Griffiths, J.; Dubbelman, T.M.; Brown, S.B. The characterisation of three substituted zinc phthalocyanines of differing charge for use in photodynamic therapy. A comparative study of their aggregation and photosensitising ability in relation to mTHPC and polyhaematoporphyrin. J. Photochem. Photobiol. B Biol. 1998, 45, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Zhao, Y.; Yao, G.Q. Dimerization of Metal-free Sulfonated Phthalocyanines in Aqueous Methanol Solution. Acta Phys.-Chim. Sin. 1996, 12, 163–168. [Google Scholar]
- Graham, R.C.; Yring, E.M.; Henderson, G.H. Molecular-Interactions in Non-Aqueous Solvents Part 3. Kinetics and Energetics of Dimerization of A Tetrasubstituted Copper(II) Phthalocyanine Dye. J. Chem. Soc. Perkin Trans. 2 1981, 763–769. [Google Scholar] [CrossRef]
- Yang, Y.C.; Ward, J.R.; Seiders, R.P. Dimerization of Cobalt (11) Tetrasulfonated Phthalocyanine in Water and Aqueous Alcoholic Solutions. J. Inorg. Chem. 1985, 24, 1765–1769. [Google Scholar] [CrossRef]
- Zhan, H.B.; Chen, W.Z.; Wang, M.Q. Dimerization of aluminum tetrasulfo-phthalocyanine chloride in sol-gel process. Mater. Lett. 2003, 57, 1108–1112. [Google Scholar] [CrossRef]
- Sahar, M.; Jahan, B.G.; Hamid, D. Chemometrics-spectroscopic study of the effect of temperature and pre-micellar to post-micellar forms of various surfactants on the dimerization of nickel and copper phthalocyanines. J. Mol. Liq. 2020, 300, 112350–112359. [Google Scholar]
- EI-Khouly, M.E.; ET-Said, W.A.; Yildirim, E.; EI-Shafei, A. Influence of cationic dimeric and trimeric-type surfactants on the aggregation behavior of water-soluble phthalocyanine: A combined experimental and computational study. J. Mol. Liq. 2024, 400, 124581–124592. [Google Scholar] [CrossRef]
- El-Nemma, E.M.; Badawi, N.M.; Hassan, S.S.M. Cobalt phthalocyanine as a novel molecular recognition reagent for batch and flow injection potentiometric and spectrophotometric determination of anionic surfactants. Talant 2009, 78, 723–729. [Google Scholar] [CrossRef]
- Huang, C.Z.; Zhang, Y.M.; Huang, X.H.; Li, Y.F.; Liu, S.P. Dual-Wavelength Spectrophotometric Determination of Cationic Surfactant by Using meso-Tetrakis(p-sulfpphenyl)porphyrin. Chin. J. Anal. Chem. 1998, 26, 823–826. [Google Scholar]
- Zhu, Z.Z.; Li, Z.J.; Liu, Y. A novel direct spectrophotometric determination of traces of polyoxyethylene non-ionic surfactant in environmental water using meso-tetra (3,5-dibromo-4-hydrooxylphenyl) porphyrin-Pb(II) complex. Int. J. Environ. Anal. Chem. 2004, 84, 267–275. [Google Scholar] [CrossRef]
- Isago, H. Optical Spectra of Phthalocyanies and Related Compounds; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
| pH | Selection of Wavelength Pairs | Linear Equation | Linear Range (μM) | r |
|---|---|---|---|---|
| 1.0 | 625/659 | y = −0.0183x + 1.6921 | 1.0–54.0 | 0.9987 |
| 2.0 | 625/659 | y = −0.0197x + 1.6150 | 0.2–44.0 | 0.9982 |
| 3.0 | 620/659 | y = −0.0215x + 1.6232 | 1.0–44.0 | 0.9973 |
| 4.0 | 620/659 | y = −0.0195x + 1.5785 | 4.0–54.0 | 0.9908 |
| 5.0 | 620/659 | y = −0.0199x + 1.5822 | 4.0–54.0 | 0.9884 |
| 6.0 | 621/660 | y = −0.0198x + 1.5974 | 4.0–54.0 | 0.9886 |
| 7.0 | 622/659 | y = −0.0176x + 1.5619 | 4.0–54.0 | 0.9850 |
| 8.0 | 620/659 | y = −0.0180x + 1.5832 | 4.0–54.0 | 0.9925 |
| 9.0 | 620/659 | y = −0.0182x + 1.5860 | 4.0–54.0 | 0.9898 |
| 10.0 | 620/658 | y = −0.0178x + 1.5729 | 4.0–54.0 | 0.9894 |
| 11.0 | 620/659 | y = −0.0177x + 1.5857 | 4.0–54.0 | 0.9923 |
| No. | Cationic Surfactant | Regression Equation | Linear Range (μM) | Correlation |
|---|---|---|---|---|
| 1 | octadecylpyridinium chloride | y = 1.66 − 0.019x | 3.5–54.0 | 0.9991 |
| 2 | cetylpyridinium chloride | y = 1.65 − 0.017x | 3.5–54.0 | 0.9991 |
| 3 | tetradecylpyridinium chloride | y = 1.75 − 0.018x | 10–54.0 | 0.9976 |
| 4 | hexadecylpyridinium bromide | y = 1.65 − 0.016x | 3.5–60.0 | 0.9990 |
| 5 | hexadecyltrimethylammonium chloride | y = 1.61 − 0.017x | 3.5–54.0 | 0.9988 |
| 6 | hexadecyltrimethylammonium bromide | y = 1.62 − 0.018x | 3.5–54.0 | 0.9982 |
| 7 | tetradecyltrimethylammonium bromide | y = 1.77 − 0.020x | 10–54.0 | 0.9984 |
| 8 | hexadecyldimethylbenzylammonium chloride | y = 1.62 − 0.019x | 2.0–60.0 | 0.9990 |
| 9 | tetradecyldimethylbenzylammonium chloride | y = 1.61 − 0.017x | 2.0–54.0 | 0.9992 |
| 10 | dodecyldimethylbenzylammonium chloride | y = 1.69 − 0.015x | 4.0–54.0 | 0.9982 |
| 11 | dodecydimethylbenzylammonium bromide | y = 1.67 − 0.014x | 4.0–60.0 | 0.9984 |
| 12 | dexadecyldimethylethylammonium bromide | y = 1.64 − 0.018x | 2.0–60.0 | 0.9990 |
| 13 | mean calibration curve | y = 1.67 − 0.017x | 4.0–54.0 | 0.9987 |
| Limit of Detection (LOD) | 3 × 10−6 μM | |||
| Substance | Concentration (mol/L) | Relative Error (%) | Substance | Concentration (mol/L) | Relative Error (%) |
|---|---|---|---|---|---|
| NaIO4 | 1.5 × 10−4 | −5.98 | KBr | 3 × 10−2 | −6.29 |
| Na2S2O3 | 3 × 10−4 | 0.36 | NH4Cl | 3 × 10−2 | −2.04 |
| CoCl2 | 3 × 10−4 | 4.92 | NaH2PO4 | 3 × 10−2 | −3.79 |
| Pb (NO3) 2 | 3 × 10−3 | 7.58 | Urea | 3 × 10−2 | 1.79 |
| BaCl2 | 3 × 10−3 | −4.37 | Boric acid | 3 × 10−2 | 0.86 |
| CaCl2 | 3 × 10−3 | 4.58 | Pyridine | 3 × 10−3 | −3.57 |
| CuSO4 | 3 × 10−3 | 8.62 | Decyltrimethylammonium Bromide | 3 × 10−5 | −2.70 |
| NaF | 3 × 10−3 | −1.19 | Benzyltributylammonium chloride | 3 × 10−4 | −0.18 |
| Na3PO4 | 3 × 10−3 | −0.07 | Tetrabutylammonium bromide | 3 × 10−3 | −0.52 |
| NaI | 3 × 10−3 | 1.94 | Tetramethylammonium bromide | 3 × 10−2 | −6.10 |
| EDTA | 3 × 10−3 | −3.71 | Tetraethylammonium bromide | 3 × 10−2 | −1.00 |
| Na2CO3 | 3 × 10−3 | −1.67 | Tetramethylammonium bromide | 3 × 10−2 | −1.08 |
| NaHCO3 | 3 × 10−3 | −3.39 | Dodecyltrimethylammonium bromide | 3 × 10−5 | −12.69 |
| NaClO4 | 3 × 10−3 | −0.81 | |||
| AgNO3 | 3 × 10−3 | −2.89 | |||
| 0 (without interference | 0 | 0.17 |
| Concentrations of CPC in Real Samples (μM) * | Absorbance Ratio (A625 nm/A659 nm) | Concentrations Measured by This Method (μM) | Relative Error (%) | RSD (%) |
|---|---|---|---|---|
| 4.65 | 1.58 | 4.68 | 0.64 | 2.84 |
| 18.60 | 1.35 | 18.42 | −0.97 | 1.07 |
| 27.90 | 1.15 | 29.86 | 7.02 | 0.26 |
| 37.20 | 1.02 | 37.53 | 0.90 | 0.46 |
| 46.50 | 0.81 | 49.31 | 6.03 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, T.; Deng, Y.; Zheng, X.; Guo, J.; Huang, P.; Li, D. A Phthalocyanine Optical Probe Responding to Cationic Surfactants Containing Long Carbon Chains with High Selectivity in Total Water Phase and Its Applications. Molecules 2025, 30, 4184. https://doi.org/10.3390/molecules30214184
Zhang Y, Zhou T, Deng Y, Zheng X, Guo J, Huang P, Li D. A Phthalocyanine Optical Probe Responding to Cationic Surfactants Containing Long Carbon Chains with High Selectivity in Total Water Phase and Its Applications. Molecules. 2025; 30(21):4184. https://doi.org/10.3390/molecules30214184
Chicago/Turabian StyleZhang, Yan, Tao Zhou, Yabin Deng, Xiao Zheng, Jiaqi Guo, Ping Huang, and Donghui Li. 2025. "A Phthalocyanine Optical Probe Responding to Cationic Surfactants Containing Long Carbon Chains with High Selectivity in Total Water Phase and Its Applications" Molecules 30, no. 21: 4184. https://doi.org/10.3390/molecules30214184
APA StyleZhang, Y., Zhou, T., Deng, Y., Zheng, X., Guo, J., Huang, P., & Li, D. (2025). A Phthalocyanine Optical Probe Responding to Cationic Surfactants Containing Long Carbon Chains with High Selectivity in Total Water Phase and Its Applications. Molecules, 30(21), 4184. https://doi.org/10.3390/molecules30214184