Novel Halolactones Derived from Vanillin: Design, Synthesis, Structural Characterization, and Evaluation of Antiproliferative and Hemolytic Activities
Abstract
1. Introduction
2. Results and Discussion
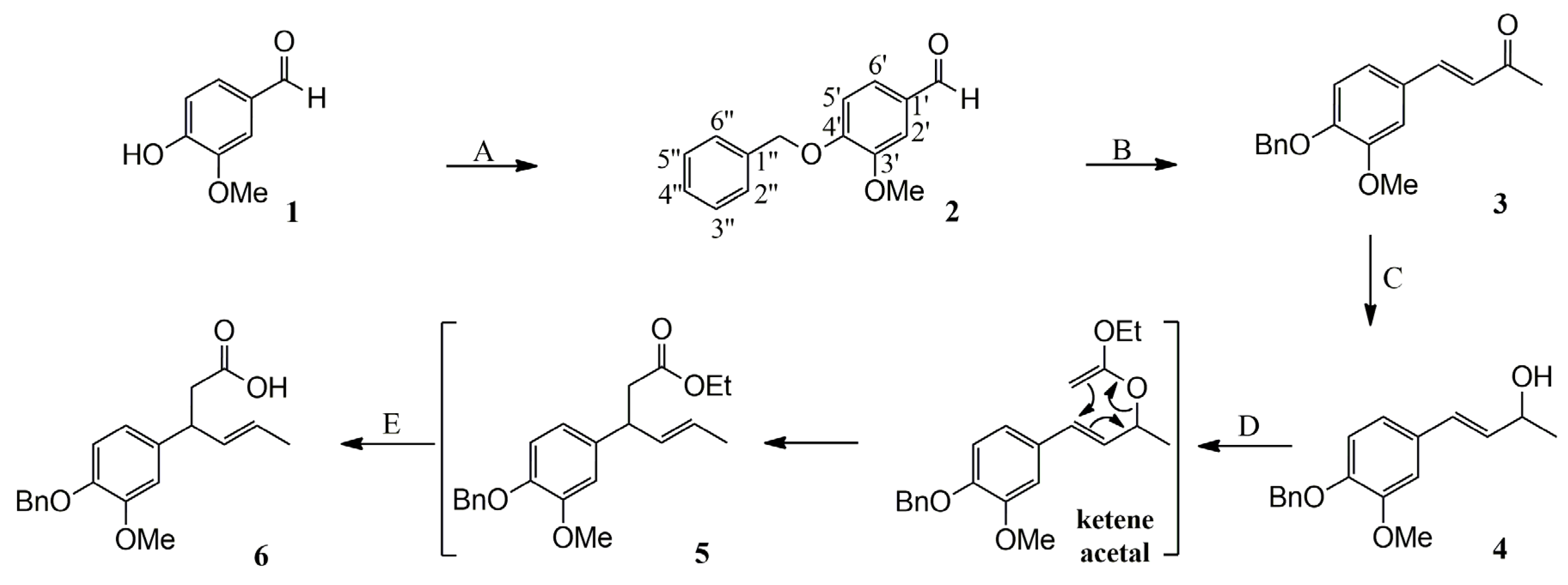
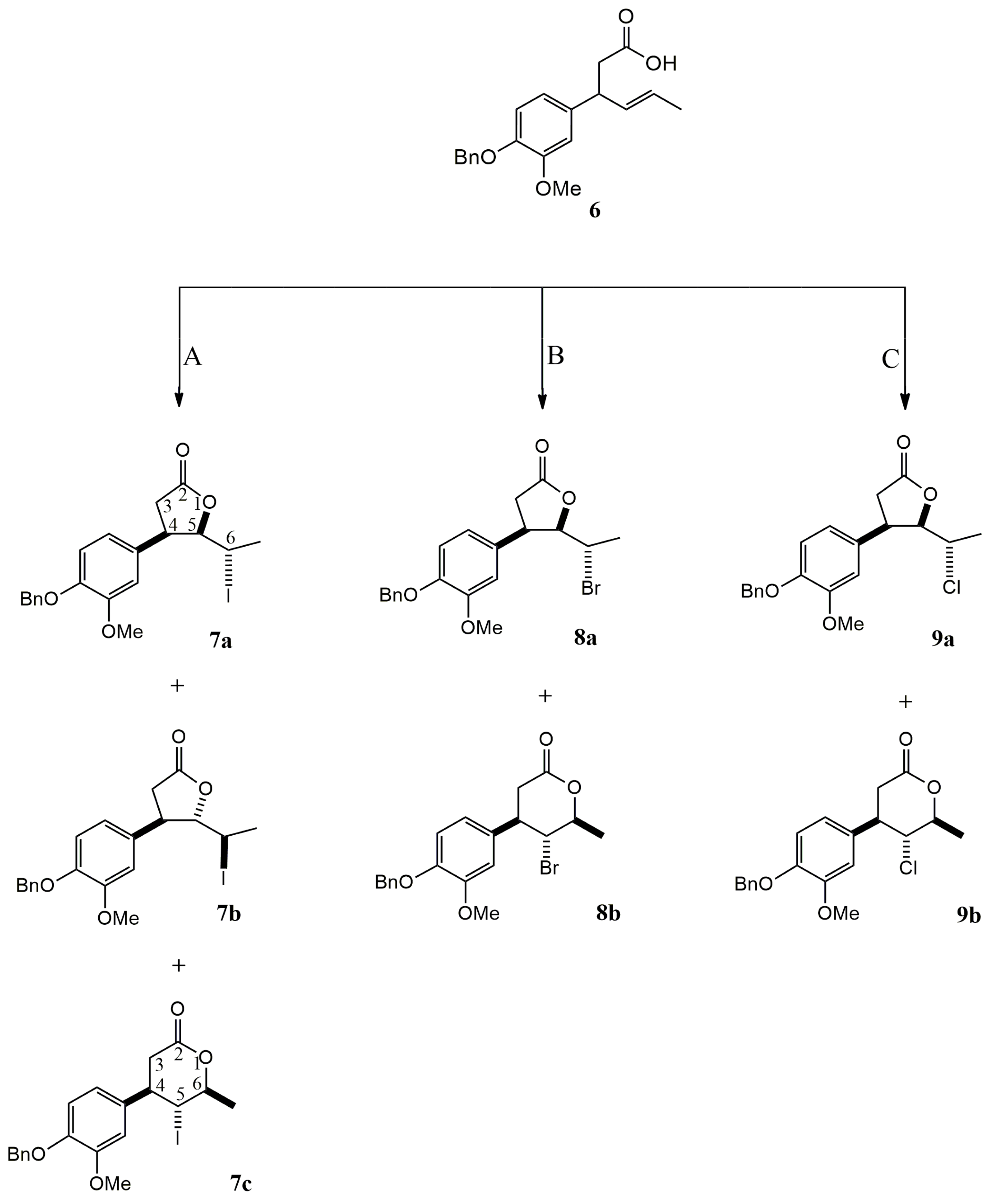
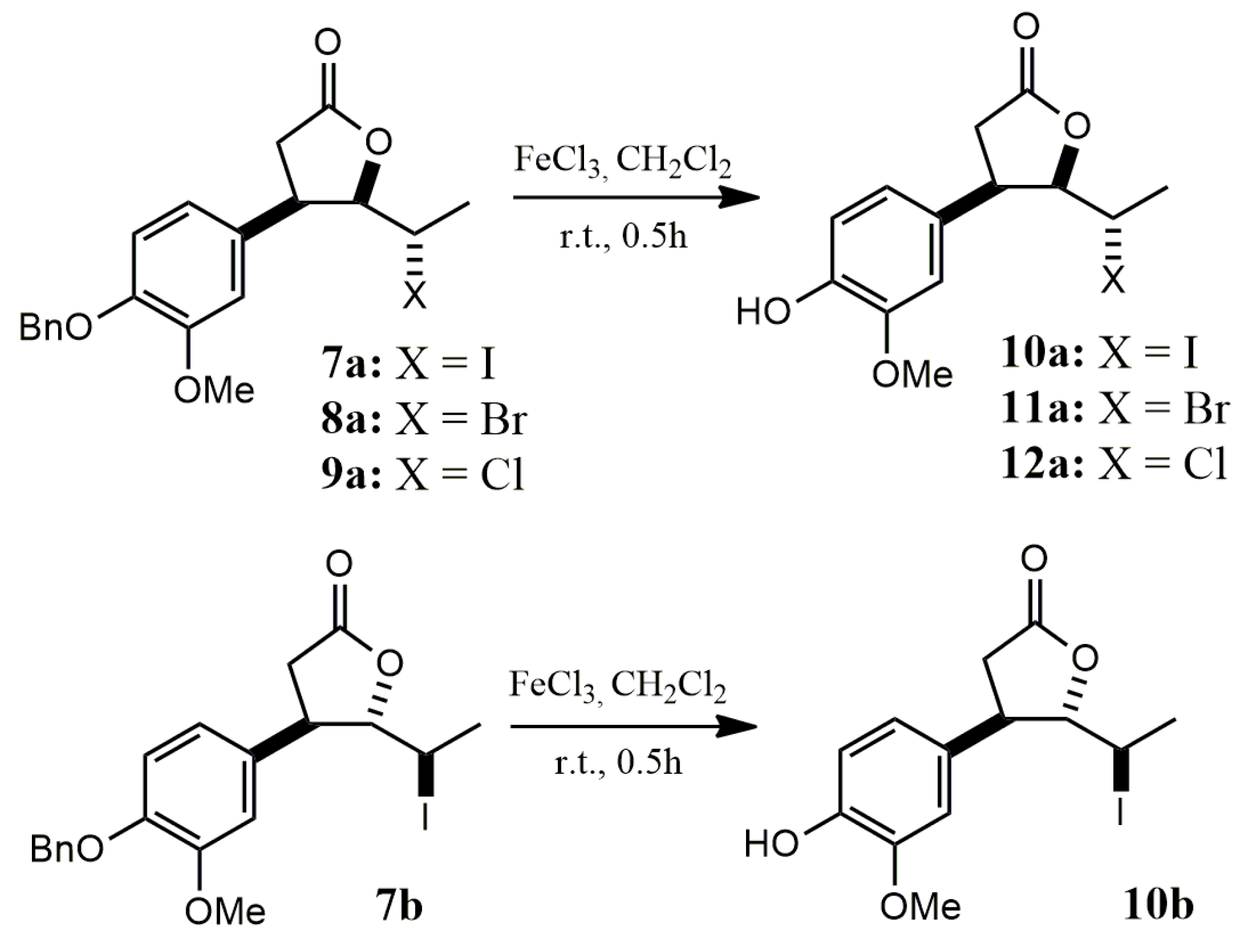
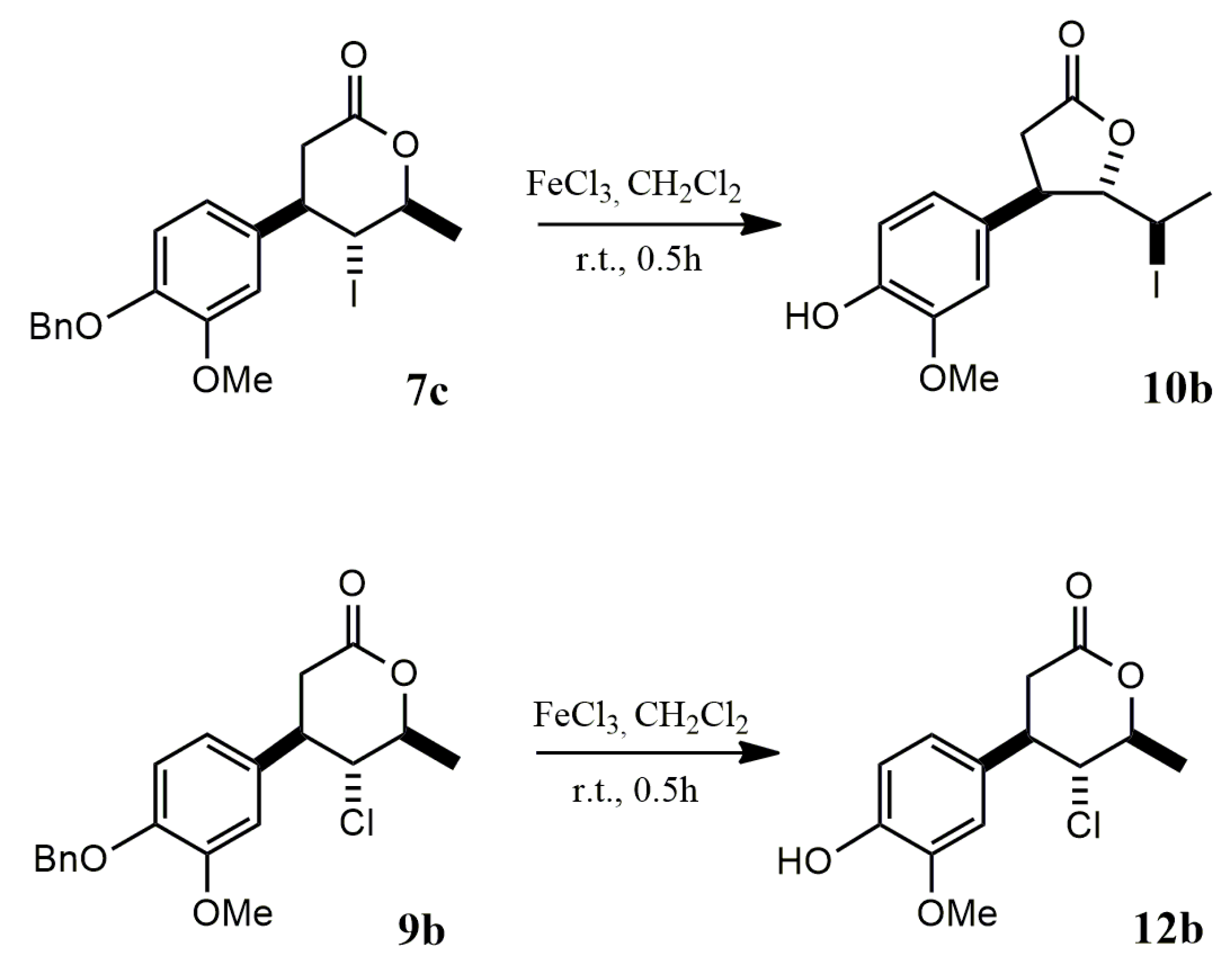
2.1. Synthesis of Vanillin-Derived Halolactones
2.1.1. Synthesis of α,β-Unsaturated Carboxylic Acid 6
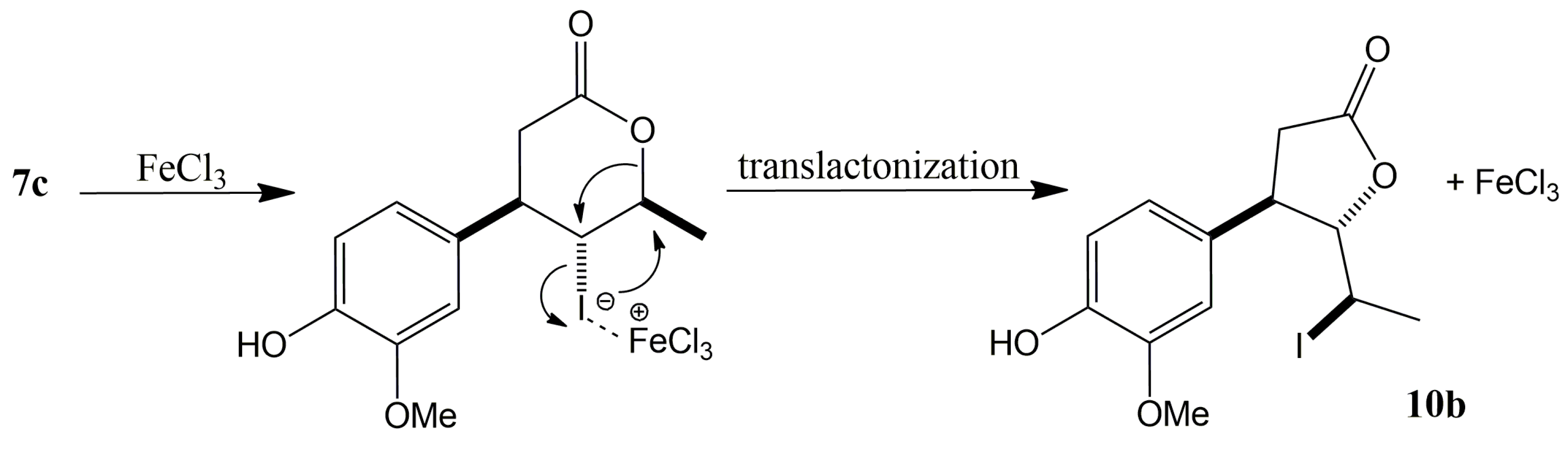
2.1.2. Halolactonization of γ,δ-Unsaturated Carboxylic Acid 6
2.1.3. Benzyl Deprotection of Halolactones 7a–c, 8a,b, 9a,b
2.2. Antiproliferative Activity
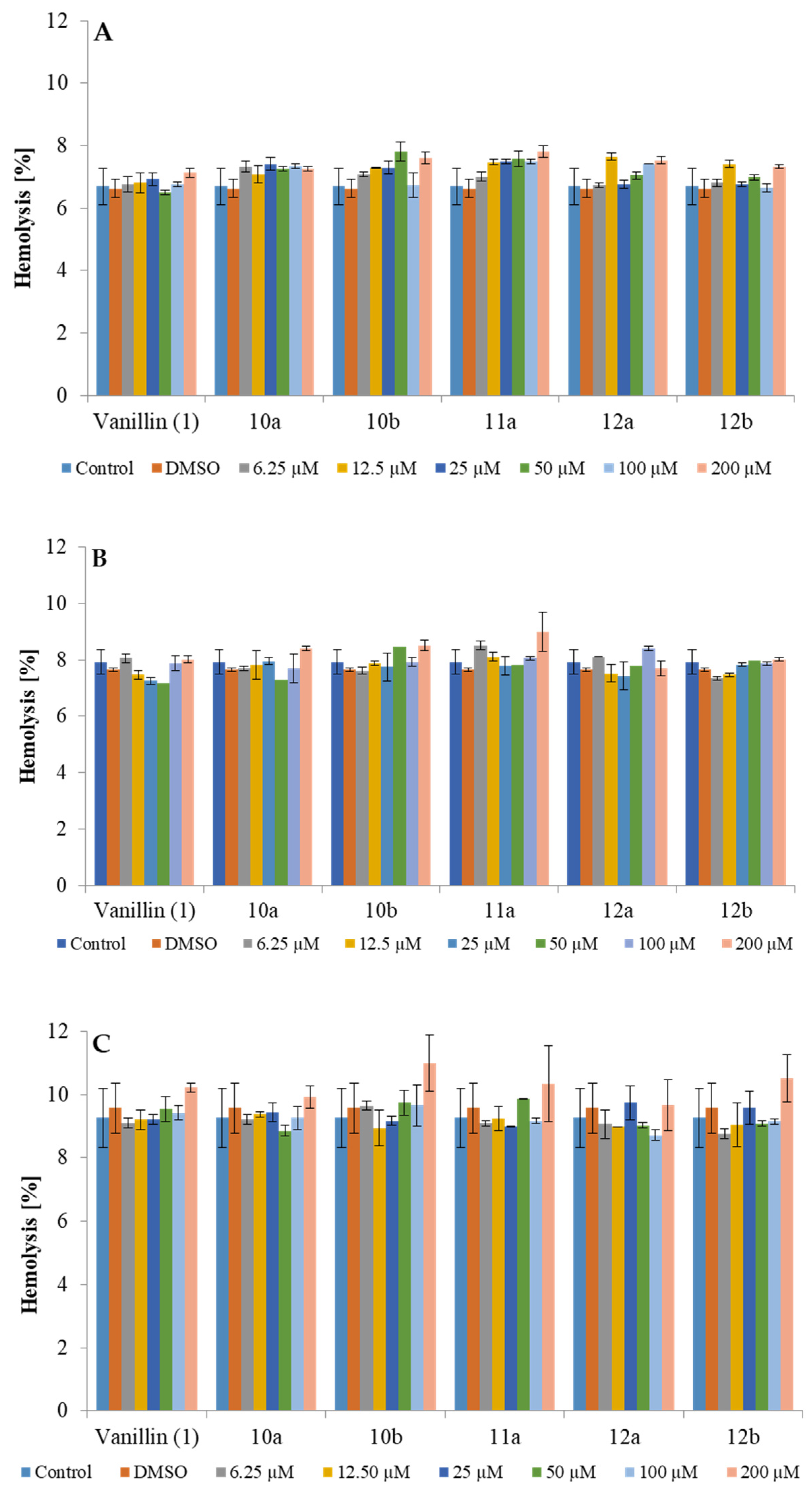
2.3. Cytotoxicity Against Red Blood Cells (RBCs)
3. Materials and Methods
3.1. Chemicals
3.2. Analysis and Purification
3.3. Preparation of Benzylvanillin (2)
3.4. Preparation of Ketone 3 via Claisen–Schmidt Condensation
3.5. Preparation of Allylic Alcohol 4
3.6. Preparation of Ester 5 by Johnson-Claisen Rearrangement
3.7. Preparation of Acid 6
3.8. Preparation of Iodolactones 7a–c
3.8.1. Cis-4-(4′-Benzyloxy-3′-methoxyphenyl)-5-(1-iodoethyl)dihydrofuran-2-One (7a)
3.8.2. Trans-4-(4′-Benzyloxy-3′-methoxyphenyl)-5-(1-iodoethyl)dihydrofuran-2-one (7b)
3.8.3. 4-r-(4′-Benzyloxy-3′-methoxyphenyl)-5-t-iodo-6-c-methyltetrahydropyran-2-one (7c)
3.9. Preparation of Bromolactones 8a,b
3.9.1. Cis-4-(4′-Benzyloxy-3′-methoxyphenyl)-5-(1-bromoethyl)dihydrofuran-2-one (8a)
3.9.2. 4-r-(4′-Benzyloxy-3′-methoxyphenyl)-5-t-bromo-6-c-methyltetrahydropyran-2-one (8b)
3.10. Preparation of Chlorolactones 9a,b
3.10.1. Cis-4-(4′-Benzyloxy-3′-methoxyphenyl)-5-(1-chloroethyl)dihydrofuran-2-one (9a)
3.10.2. 4-r-(4′-Benzyloxy-3′-methoxyphenyl)-5-t-chloro-6-c-methyltetrahydropyran-2-one (9b)
3.11. General Procedure for Benzyl Deprotection of Halolactones 7a–c, 8a,b and 9a,b
3.11.1. Cis-4-(4′-Hydroxy-3′-methoxyphenyl)-5-(1-iodoethyl)dihydrofuran-2-one (10a)
3.11.2. Trans-4-(4′-Hydroxy-3′-methoxyphenyl)-5-(1-iodoethyl)dihydrofuran-2-one (10b)
3.11.3. Cis-5-(1-Bromoethyl)-4-(4′-hydroxy-3′-methoxyphenyl)dihydrofuran-2-one (11a)
3.11.4. Cis-5-(1-Chloroethyl)-4-(4′-hydroxy-3′-methoxyphenyl)dihydrofuran-2-one (12a)
3.11.5. 5-t-Chloro-4-r-(4′-Hydroxy-3′-methoxyphenyl)-6-c-methyltetrahydropyran-2-one (12b)
3.12. Antiproliferative Activity
3.12.1. Chemicals for Biological Tests
3.12.2. Cell Lines and Cell Cultures
3.12.3. MTT Assay
3.13. Cytotoxicity Against Red Blood Cells (RBCs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sartori, S.K.; Diaz, M.A.N.; Diaz-Muñoz, G. Lactones: Classification, Synthesis, Biological Activities, and Industrial Applications. Tetrahedron 2021, 84, 132001. [Google Scholar] [CrossRef]
- Frey, M.; Vahabi, K.; Cankar, K.; Lackus, N.D.; Padilla-Gonzalez, F.; Ro, D.K.; Rieseberg, L.; Spring, O.; Tissier, A. Sesquiterpene Lactones–Insights into Biosynthesis, Regulation and Signalling Roles. CRC Crit. Rev. Plant Sci. 2024, 43, 131–157. [Google Scholar] [CrossRef]
- Sokovic, M.; Ciric, A.; Glamoclija, J.; Skaltsa, H. Biological Activities of Sesquiterpene Lactones Isolated from the Genus Centaurea L. (Asteraceae). Curr. Pharm. Des. 2017, 23, 2767–2786. [Google Scholar] [CrossRef]
- Kumar, P.; Wallis, M.; Zhou, X.; Li, F.; Holland, D.C.; Reddell, P.; Münch, G.; Raju, R. Triplinones A-H: Anti-Inflammatory Arylalkenyl α,β-Unsaturated-δ-Lactones Isolated from the Leaves of Australian Rainforest Plant Cryptocarya Triplinervis (Lauraceae). J. Nat. Prod. 2024, 87, 1817–1825. [Google Scholar] [CrossRef]
- López, S.; Acín, P.; Gómez-Zubiaur, A.; Corbella-Martorell, C.; Quero, C. A Shift in the Paradigm? A Male-Specific Lactone Increases the Response of Both Sexes of the Olive Fruit Fly Bactrocera Oleae to the Food Lure Ammonium Bicarbonate. J. Pest. Sci. 2024, 97, 965–978. [Google Scholar] [CrossRef]
- Da Silva Souza, I.H.; Nogueira, J.P.; Chaves, R.V.; Sandes, R.D.D.; Leite Neta, M.T.S.; Narain, N. Microbial Lactones: A Systematic Bibliometric Review of γ-Lactone Production by Biotechnological Processes and Technological Prospection with Focus on γ-Dodecalactone. Biocatal. Agric. Biotechnol. 2024, 60, 103318. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2024, 41, 151–324. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising Bioactive Compounds from the Marine Environment and Their Potential Effects on Various Diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Masłowiec, D. Antimicrobial Activity of Lactones. Antibiotics 2022, 11, 1327. [Google Scholar] [CrossRef] [PubMed]
- Kudumela, R.G.; Mazimba, O.; Masoko, P. Isolation and Characterisation of Sesquiterpene Lactones from Schkuhria Pinnata and Their Antibacterial and Anti-Inflammatory Activities. S. Afr. J. Bot. 2019, 126, 340–344. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Gawdzik, B.; Trzepizur, D.; Szymczak, M.; Skiba, G.; Raj, S.; Kramkowski, K.; Lizut, R.; Ostaszewski, R. δ-Lactones—A New Class of Compounds That Are Toxic to E. coli K12 and R2–R4 Strains. Materials 2021, 14, 2956. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Singh, T.; Fatima, H.; Maurya, A.C.; Dutta, T.; Khare, S.K. Lactones as Promising Biofilm Inhibitors: Disrupting Bacterial Communication for Next-Gen Therapies. Prep. Biochem. Biotechnol. 2025, 1532–2297. [Google Scholar] [CrossRef]
- Koszelewski, D.; Borys, F.; Brodzka, A.; Ostaszewski, R. Synthesis of Enantiomerically Pure 5,6-Dihydropyran-2-Ones via Chemoenzymatic Sequential DKR-RCM Reaction. Eur. J. Org. Chem. 2019, 7, 1653–1658. [Google Scholar] [CrossRef]
- Paço, A.; Brás, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef] [PubMed]
- Dancewicz, K.; Szumny, A.; Wawrzeńczyk, C.; Gabryś, B. Repellent and Antifeedant Activities of Citral-Derived Lactones against the Peach Potato Aphid. Int. J. Mol. Sci. 2020, 21, 8029. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M.; Díaz, C.E.; Bailén, M.; González-Coloma, A. Sesquiterpene Lactones from Artemisia Absinthium. Biotransformation and Rearrangement of the Insect Antifeedant 3α-Hydroxypelenolide. Plants 2021, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, M.; Dams, I.; Wawrzeńczyk, C. Feeding Deterrent Activity of Terpenoid Lactones with the p-Menthane System Against the Colorado Potato Beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 2005, 34, 1433–1440. [Google Scholar] [CrossRef]
- Silva, L.C.; Tauhata, S.B.F.; Baeza, L.C.; Oliveira, C.M.A.; Kato, L.; Borges, C.L.; Soares, C.M.A.; Pereira, M. Argentilactone Molecular Targets in Paracoccidioides brasiliensis Identified by Chemoproteomics. Antimicrob. Agents Chemother. 2018, 62, e00737-18. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, P.; Bhardwaj, U.; Kaur, R. Dehydrocostus Lactone: A Comprehensive Review on Its Isolation, Chemical Transformations, and Pharmacological Potential. Discov. Chem. 2025, 2, 131. [Google Scholar] [CrossRef]
- Zhi, X.Y.; Zhang, Y.; Li, Y.F.; Liu, Y.; Niu, W.P.; Li, Y.; Zhang, C.R.; Cao, H.; Hao, X.J.; Yang, C. Discovery of Natural Sesquiterpene Lactone 1-O-Acetylbritannilactone Analogues Bearing Oxadiazole, Triazole, or Imidazole Scaffolds for the Development of New Fungicidal Candidates. J. Agric. Food Chem. 2023, 71, 11680–11691. [Google Scholar] [CrossRef]
- Rasul, A.; Parveen, S.; Ma, T. Costunolide: A Novel Anti-Cancer Sesquiterpene Lactone. Bangladesh J. Pharmacol. 2012, 7, 6–13. [Google Scholar] [CrossRef]
- Kim, D.Y.; Choi, B.Y. Costunolide—A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential. Int. J. Mol. Sci. 2019, 20, 2926. [Google Scholar] [CrossRef] [PubMed]
- Wzorek, A.; Kwiatkowska, M.; Hodorowicz, M.; Kalwat, K.; Arabski, M.; Płoszaj, P.; Gonciarz, W.; Omelaniuk, A.; Gmiter, D.; Kaca, W.; et al. Syntheses, Structures and Biological Activities of New Pyridinyl Lactones. J. Mol. Struct. 2025, 1319, 139534. [Google Scholar] [CrossRef]
- Kim, Y.; Sengupta, S.; Sim, T. Natural and Synthetic Lactones Possessing Antitumor Activities. Int. J. Mol. Sci. 2021, 22, 1052. [Google Scholar] [CrossRef]
- Kamizela, A.; Gawdzik, B.; Urbaniak, M.; Lechowicz, Ł.; Białonska, A.; Kutniewska, S.E.; Gonciarz, W.; Chmiela, M. New γ-Halo-δ-Lactones and δ-Hydroxy-γ-Lactones with Strong Cytotoxic Activity. Molecules 2019, 24, 1875. [Google Scholar] [CrossRef]
- Kamizela, A.; Gawdzik, B.; Urbaniak, M.; Lechowicz, Ł.; Białońska, A.; Gonciarz, W.; Chmiela, M. Synthesis, Characterization, Cytotoxicity, and Antibacterial Properties of Trans-γ-Halo-δ-Lactones. ChemistryOpen 2018, 7, 543–550. [Google Scholar] [CrossRef]
- Fan, H.; Wei, X.; Si-Tu, M.X.; Lei, Y.H.; Zhou, F.G.; Zhang, C.X. γ-Aromatic Butenolides of Microbial Source—A Review of Their Structures, Biological Activities and Biosynthesis. Chem. Biodivers. 2022, 19, e202200208. [Google Scholar] [CrossRef]
- Albrecht, A.; Koszuk, J.F.; Modranka, J.; Różalski, M.; Krajewska, U.; Janecka, A.; Studzian, K.; Janecki, T. Synthesis and Cytotoxic Activity of γ-Aryl Substituted α-Alkylidene-γ-Lactones and α-Alkylidene-γ-Lactams. Bioorg. Med. Chem. 2008, 16, 4872–4882. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Shin, D. Arylnaphthalene Lactones: Structures and Pharmacological Potentials. Phytochem. Rev. 2021, 20, 1033–1054. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Thi Tran, L.T.; Truong Tan, T.; Pham, P.T.V.; Nguyen, L.T.K.; Nguyen, H.T.; Ho, D.V.; Tran, M.H. Isolation, Structural Elucidation, and Cytotoxic Activity Investigation of Novel Styryl-Lactone Derivatives from Goniothalamus elegans: In Vitro and in Silico Studies. RSC Adv. 2023, 13, 17587–17594. [Google Scholar] [CrossRef] [PubMed]
- Wzorek, A.; Gawdzik, B.; Gładkowski, W.; Urbaniak, M.; Barańska, A.; Malińska, M.; Woźniak, K.; Kempinska, K.; Wietrzyk, J. Synthesis, Characterization and Antiproliferative Activity of β-Aryl-ω-Iodo-γ-Lactones. J. Mol. Struct. 2013, 1047, 160–168. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Siepka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Białońska, A.; Poradowski, D.; Drynda, A.; Urbaniak, M. Synthesis and Anticancer Activity of Novel Halolactones with β-Aryl Substituents from Simple Aromatic Aldehydes. Tetrahedron 2013, 69, 10414–10423. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Gliszczyńska, A.; Czarnecka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Maciejewska, G.; Białońska, A. Chiral δ-Iodo-γ-Lactones Derived from Cuminaldehyde, 2,5-Dimethylbenzaldehyde and Piperonal: Chemoenzymatic Synthesis and Antiproliferative Activity. Tetrahedron Asymmetry 2016, 27, 227–237. [Google Scholar] [CrossRef]
- Gładkowski, W.; Włoch, A.; Pawlak, A.; Sysak, A.; Białońska, A.; Mazur, M.; Mituła, P.; Maciejewska, G.; Obmińska-Mrukowicz, B.; Kleszczyńska, H. Preparation of Enantiomeric β-(2′,5′-Dimethylphenyl) Bromolactones, Their Antiproliferative Activity and Effect on Biological Membranes. Molecules 2018, 23, 3035. [Google Scholar] [CrossRef]
- Pawlak, A.; Gładkowski, W.; Mazur, M.; Henklewska, M.; Obmińska-Mrukowicz, B.; Rapak, A. Optically Active Stereoisomers of 5-(1-Iodoethyl)-4-(4′-Isopropylphenyl)Dihydrofuran-2-One: The Effect of the Configuration of Stereocenters on Apoptosis Induction in Canine Cancer Cell Lines. Chem. Biol. Interact. 2017, 261, 18–26. [Google Scholar] [CrossRef]
- Pawlak, A.; Gładkowski, W.; Kutkowska, J.; Mazur, M.; Obmińska-Mrukowicz, B.; Rapak, A. Enantiomeric Trans β-Aryl-δ-Iodo-γ-Lactones Derived from 2,5-Dimethylbenzaldehyde Induce Apoptosis in Canine Lymphoma Cell Lines by Downregulation of Anti-Apoptotic Bcl-2 Family Members Bcl-XL and Bcl-2. Bioorg. Med. Chem. Lett. 2018, 28, 1171–1177. [Google Scholar] [CrossRef]
- Włoch, A.; Sengupta, P.; Szulc, N.; Kral, T.; Pawlak, A.; Henklewska, M.; Pruchnik, H.; Sykora, J.; Hof, M.; Gładkowski, W. Biophysical and Molecular Interactions of Enantiomeric Piperonal-Derived Trans β-Aryl-δ-Iodo-γ-Lactones with Cancer Cell Membranes, Protein and DNA: Implications for Anticancer Activity. Int. J. Biol. Macromol. 2025, 303, 140476. [Google Scholar] [CrossRef]
- Włoch, A.; Stygar, D.; Bahri, F.; Bażanów, B.; Kuropka, P.; Chełmecka, E.; Pruchnik, H.; Gładkowski, W. Antiproliferative, Antimicrobial and Antiviral Activity of β-Aryl-δ-Iodo-γ-Lactones, Their Effect on Cellular Oxidative Stress Markers and Biological Membranes. Biomolecules 2020, 10, 1594. [Google Scholar] [CrossRef]
- Olatunde, A.; Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Shuaibu, M.N. Vanillin: A Food Additive with Multiple Biological Activities. Eur. J. Med. Chem. Rep. 2022, 5, 100055. [Google Scholar] [CrossRef]
- Kafali, M.; Finos, M.A.; Tsoupras, A. Vanillin and Its Derivatives: A Critical Review of Their Anti-Inflammatory, Anti-Infective, Wound-Healing, Neuroprotective, and Anti-Cancer Health-Promoting Benefits. Nutraceuticals 2024, 4, 522–561. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A Review on the Therapeutic Prospects of a Popular Flavouring Molecule. Adv. Tradit. Med. 2021, 21, 415–431. [Google Scholar] [CrossRef]
- Yousuf, M.; Shamsi, A.; Queen, A.; Shahbaaz, M.; Khan, P.; Hussain, A.; Alajmi, M.F.; RizwanulHaque, Q.M.; Imtaiyaz Hassan, M. Targeting Cyclin-Dependent Kinase 6 by Vanillin Inhibits Proliferation of Breast and Lung Cancer Cells: Combined Computational and Biochemical Studies. J. Cell. Biochem. 2021, 122, 897–910. [Google Scholar] [CrossRef]
- Liang, J.A.; Wu, S.L.; Lo, H.Y.; Hsiang, C.Y.; Ho, T.Y. Vanillin Inhibits Matrix Metalloproteinase-9 Expression through down-Regulation of Nuclear Factor-ΚB Signaling Pathway in Human Hepatocellular Carcinoma Cells. Mol. Pharmacol. 2009, 75, 151–157. [Google Scholar] [CrossRef]
- Ho, K.L.; Yazan, L.S.; Ismail, N.; Ismail, M. Apoptosis and Cell Cycle Arrest of Human Colorectal Cancer Cell Line HT-29 Induced by Vanillin. Cancer Epidemiol. 2009, 33, 155–160. [Google Scholar] [CrossRef]
- Srinual, S.; Chanvorachote, P.; Pongrakhananon, V. Suppression of Cancer Stem-like Phenotypes in NCI-H460 Lung Cancer Cells by Vanillin through an Akt-Dependent Pathway. Int. J. Oncol. 2017, 50, 1341–1351. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, Y.M.; Oh, T.I.; Kim, B.M.; Lim, B.O.; Lim, J.H. Vanillin Suppresses Cell Motility by Inhibiting STAT3-Mediated HIF-1α MRNA Expression in Malignant Melanoma Cells. Int. J. Mol. Sci. 2017, 18, 532. [Google Scholar] [CrossRef]
- Gendron, D. Vanillin: A Promising Biosourced Building Block for the Preparation of Various Heterocycles. Front. Chem. 2022, 10, 949355. [Google Scholar] [CrossRef]
- Yıldırım, M.; Ünver, H.; Necip, A.; Çimentepe, M. Design, Synthesis, and Biological Evaluation of Novel Vanillin-Derived Hydrazone Compounds with Antimicrobial, Anticancer, and Enzyme Inhibition Activities, along with Molecular Structure and Drug-Likeness Assessment. Biochem. Biophys. Res. Commun. 2025, 775, 152173. [Google Scholar] [CrossRef]
- Scipioni, M.; Kay, G.; Megson, I.L.; Kong Thoo Lin, P. Synthesis of Novel Vanillin Derivatives: Novel Multi-Targeted Scaffold Ligands against Alzheimer’s Disease. MedChemComm 2019, 10, 764–777. [Google Scholar] [CrossRef]
- Li, Z.H.; Liu, H.M.; Fan, Z.Y.; Pang, W.; Cheng, L.P. Design, Synthesis and Evaluation of Vanillin Derivatives as Dual-Target Inhibitors for the Treatment of Alzheimer’s Disease. Bioorg. Med. Chem. 2025, 129, 118296. [Google Scholar] [CrossRef]
- Carrasco-Gomez, R.; Keppner-Witter, S.; Hieke, M.; Lange, L.; Schneider, G.; Schubert-Zsilavecz, M.; Proschak, E.; Spänkuch, B. Vanillin-Derived Antiproliferative Compounds Influence Plk1 Activity. Bioorg. Med. Chem. Lett. 2014, 24, 5063–5069. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Resch, V.; Wallner, S.; Lienhart, W.D.; Sattler, J.H.; Resch, J.; MacHeroux, P.; Kroutil, W. Biocatalytic Organic Synthesis of Optically Pure (S)-Scoulerine and Berbine and Benzylisoquinoline Alkaloids. J. Org. Chem. 2011, 76, 6703–6714. [Google Scholar] [CrossRef]
- Krishnamurty, H.G.; Ghosh, S. Synthesis of Dihydrocurcumin. Indian J. Chem. 1986, 25B, 411–412. [Google Scholar]
- Li, Y.; Manickam, G.; Ghoshal, A.; Subramaniam, P. More Efficient Palladium Catalyst for Hydrogenolysis of Benzyl Groups. Synth. Commun. 2006, 36, 925–928. [Google Scholar] [CrossRef]
- Lanthier, C.; Payan, H.; Liparulo, I.; Hatat, B.; Lecoutey, C.; Since, M.; Davis, A.; Bergamini, C.; Claeysen, S.; Dallemagne, P.; et al. Novel Multi Target-Directed Ligands Targeting 5-HT4 Receptors with in Cellulo Antioxidant Properties as Promising Leads in Alzheimer’s Disease. Eur. J. Med. Chem. 2019, 182, 111596. [Google Scholar] [CrossRef]
- Gadhiya, S.; Madapa, S.; Kurtzman, T.; Alberts, I.L.; Ramsey, S.; Pillarsetty, N.K.; Kalidindi, T.; Harding, W.W. Tetrahydroprotoberberine Alkaloids with Dopamine and σ Receptor Affinity. Bioorg. Med. Chem. 2016, 24, 2060–2071. [Google Scholar] [CrossRef]
- Adinolfi, M.; Barone, G.; Guariniello, L.; Iadonisi, A. Facile Cleavage of Carbohydrate Benzyl Ethers and Benzylidene Acetals Using the NaBrO3/Na2S2O4 Reagent under Two-Phase Conditions. Tetrahedron Lett. 1999, 40, 8439–8441. [Google Scholar] [CrossRef]
- Rodebaugh, R.; Debenham, J.S.; Fraser-Reid, B. Debenzylation of Complex Oligosaceharides Using Ferric Chloride. Tetrahedron Lett. 1996, 37, 5477–5478. [Google Scholar] [CrossRef]
- Giri, R.S.; Roy, S.; Dolai, G.; Manne, S.R.; Mandal, B. FeCl3-Mediated Boc Deprotection: Mild Facile Boc-Chemistry in Solution and on Resin. ChemistrySelect 2020, 5, 2050–2056. [Google Scholar] [CrossRef]
- Gładkowski, W.; Siepka, M.; Żarowska, B.; Białońska, A.; Gawdzik, B.; Urbaniak, M.; Wawrzeńczyk, C. Chalcone-Derived Lactones: Synthesis, Whole-Cell Biotransformation, and Evaluation of Their Antibacterial and Antifungal Activity. Molecules 2023, 28, 3800. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, S.; Zhao, L.; Zhang, T.; Zhao, J.; Wang, X.; Ma, T.; Sun, A.; Ye, L.; Li, G. The Mechanism by Which Cisplatin, Mitomycin, and Doxorubicin Inhibit Cell Growth in Bladder Cancer. Int. J. Clin. Exp. Med. 2022, 15, 208–214. [Google Scholar]
- Pawlak, A.; Ziolo, E.; Kutkowska, J.; Blazejczyk, A.; Wietrzyk, J.; Krupa, A.; Hildebrand, W.; Dziegiel, P.; Dzimira, S.; Obminska-Mrukowicz, B.; et al. A Novel Canine B-Cell Leukaemia Cell Line. Establishment, Characterisation and Sensitivity to Chemotherapeutics. Vet. Comp. Oncol. 2016, 15, 1218–1231. [Google Scholar] [CrossRef]
- Ben Toumia, I.; Sobeh, M.; Ponassi, M.; Banelli, B.; Dameriha, A.; Wink, M.; Ghedira, L.C.; Rosano, C. A Methanol Extract of Scabiosa Atropurpurea Enhances Doxorubicin Cytotoxicity against Resistant Colorectal Cancer Cells In Vitro. Molecules 2020, 25, 5265. [Google Scholar] [CrossRef]
- Pawlak, A.; Rapak, A.; Zbyryt, I.; Obmińska-Mrukowicz, B. The Effect of Common Antineoplastic Agents on Induction of Apoptosis in Canine Lymphoma and Leukemia Cell Lines. In Vivo 2014, 28, 843–850. [Google Scholar]
- Klimek, K.; Tyśkiewicz, K.; Miazga-Karska, M.; Dębczak, A.; Rój, E.; Ginalska, G. Bioactive Compounds Obtained from Polish “Marynka” Hop Variety Using Efficient Two-Step Supercritical Fluid Extraction and Comparison of Their Antibacterial, Cytotoxic, and Anti-Proliferative Activities in Vitro. Molecules 2021, 26, 2366. [Google Scholar] [CrossRef] [PubMed]
- Rütgen, B.C.; Hammer, S.E.; Gerner, W.; Christian, M.; de Arespacochaga, A.G.; Willmann, M.; Kleiter, M.; Schwendenwein, I.; Saalmüller, A. Establishment and Characterization of a Novel Canine B-Cell Line Derived from a Spontaneously Occurring Diffuse Large Cell Lymphoma. Leuk. Res. 2010, 34, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, A.; Duff, A.; Hu, T. DMSO Inhibits Human Cancer Cells and Downregulates the Expression of Cdk2 and Cyclin A. FASEB J. 2020, 34, S1. [Google Scholar] [CrossRef]
- Pruchnik, H.; Włoch, A.; Bonarska-Kujawa, D.; Kleszczyńska, H. An In Vitro Study of the Effect of Cytotoxic Triorganotin Dimethylaminophenylazobenzoate Complexes on Red Blood Cells. J. Membr. Biol. 2018, 251, 735–745. [Google Scholar] [CrossRef]

| Compound | Cell Line | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CLBL-1 | CLB70 | T-24 | CaCo-2 | NIH/3T3 | |||||
| IC50 [µM] | SI | IC50 [µM] | SI | IC50 [µM] | SI | IC50 [µM] | SI | IC50 [µM] | |
| 1 | 72.4 ± 9.4 1 | >1.4 2 | 72.8 ± 2.3 | >1.4 | 48.5 ± 13.2 | >2.1 | >100 | - | >100 |
| 10a | 73.6 ± 8.8 | >1.4 | 69.7 ± 13.7 | >1.4 | 98.1 ± 10.4 | >1.0 | >100 | - | >100 |
| 10b | 46.3 ± 4.1 | >2.2 | 71.5 ± 7.5 | >1.4 | 63.4 ± 6.2 | >1.6 | >100 | - | >100 |
| 11a | 63.2 ± 1.6 | >1.6 | 76.2 ± 5.3 | >1.3 | >100 | - | 76.4 ± 10.0 | >1.3 | >100 |
| 12a | 76.7 ± 11.5 | >1.3 | 75.2 ± 6.3 | >1.3 | >100 | - | 88.1 ± 4.4 | >1.1 | >100 |
| 12b | 85.7 ± 15.7 | >1.2 | 71.2 ± 11.6 | >1.4 | >100 | - | 72.5 ± 19.8 | >1.4 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunal, A.; Gładkowski, W.; Dejnaka, E.; Sulecka-Zadka, J.; Pawlak, A.; Włoch, A.; Pruchnik, H.; Maciejewska, G. Novel Halolactones Derived from Vanillin: Design, Synthesis, Structural Characterization, and Evaluation of Antiproliferative and Hemolytic Activities. Molecules 2025, 30, 4180. https://doi.org/10.3390/molecules30214180
Dunal A, Gładkowski W, Dejnaka E, Sulecka-Zadka J, Pawlak A, Włoch A, Pruchnik H, Maciejewska G. Novel Halolactones Derived from Vanillin: Design, Synthesis, Structural Characterization, and Evaluation of Antiproliferative and Hemolytic Activities. Molecules. 2025; 30(21):4180. https://doi.org/10.3390/molecules30214180
Chicago/Turabian StyleDunal, Anna, Witold Gładkowski, Ewa Dejnaka, Joanna Sulecka-Zadka, Aleksandra Pawlak, Aleksandra Włoch, Hanna Pruchnik, and Gabriela Maciejewska. 2025. "Novel Halolactones Derived from Vanillin: Design, Synthesis, Structural Characterization, and Evaluation of Antiproliferative and Hemolytic Activities" Molecules 30, no. 21: 4180. https://doi.org/10.3390/molecules30214180
APA StyleDunal, A., Gładkowski, W., Dejnaka, E., Sulecka-Zadka, J., Pawlak, A., Włoch, A., Pruchnik, H., & Maciejewska, G. (2025). Novel Halolactones Derived from Vanillin: Design, Synthesis, Structural Characterization, and Evaluation of Antiproliferative and Hemolytic Activities. Molecules, 30(21), 4180. https://doi.org/10.3390/molecules30214180







