Abstract
Silver nanoparticles (AgNPs) have drawn great attention, owing to their unique physico-chemical and biological properties and various applications, particularly in the biomedical field. In addition to conventional chemical and physical methods, materials scientists have been exploring the capabilities endowed by several bioresources, such as plants, bacteria, fungi and algae, in the cost-effective and eco-friendly production of AgNPs. This review article provides a comprehensive overview of the current state of research on the bioapplications of biogenic AgNPs (bio-AgNPs). The various bioresources used and methodologies followed to synthesize bio-AgNPs are briefly examined, along with some aspects of the underlying mechanisms. Then, the review surveys the toxicity of AgNPs, in general, and presents the unique biological properties of bio-AgNPs. Furthermore, the review details numerous applications of bio-AgNPs with paramount importance to human health, such as the control of infectious disease vectors, cancer therapy, antibiofilm activity and environmental remediation. Importantly, the review highlights the paradoxical effect of these nano-objects since they specifically seem to exert their action solely on targeted cells and (micro)organisms. By featuring the unique advantages of biogenic methods and their challenges, this article aims at serving as a valuable resource to attract research on bio-AgNPs and elicit further developments towards the scalable and sustainable production of AgNPs for large scale industrial and clinical use.
1. Introduction
Nanoparticles (NPs) are structures that have at least one dimension at the nanoscale, i.e., spanning from 1 to 100 nm [1,2,3,4]. They are 0-D (zero-dimensional) if all the dimensions are at the nanoscale [2,3,4]. Nanospheres, nanocubes and quantum dots are some examples of 0-D NPs. One-dimensional NPs have two dimensions at the nanoscale while the last one exceeds 100 nm [2,3,4]. This is the case, for instance, of nanorods and nanotubes. Two-dimensional NPs, such as nanoplates, nanofilms, nanolayers and nanocoatings, exhibit only one dimension at the nanoscale [2,3,4]. Lastly, 3-D NPs constitute a special category since they have no dimension at the nanoscale. However, their texture reveals the presence of features, such as spines or holes, at the nanoscale, which ensures them the classification as a nanomaterial [2,4]. Nano-urchins and nano-wells are examples of 3-D NPs.
Nanomaterials can be biological, organic, inorganic and hybrid [5,6]. Inorganic NPs have attracted significant attention across diverse fields owing to their unique properties that have given rise to an ever-growing body of research enabling their application in various fields and triggering vigorous advancements in multiple domains [7,8,9,10,11,12,13]. In the metallic category, silver NPs (AgNPs) have been extensively studied, owing to their remarkably unusual physical, chemical, and biological properties that are directly influenced by their size, shape, and surface chemistry. AgNPs are exploited for multiple applications, including the biomedical field, agriculture, the food industry and the environment [14,15,16,17]. In the biomedical field, AgNPs exhibit outstanding potential to control the growth of or eliminate various disease-causing organisms, such as pathogenic bacteria and a broad spectrum of viruses [15,18,19,20,21]. Additionally, AgNPs have witnessed exciting developments in wound healing, burn injuries, coatings for implants, drug delivery, biosensing and bioimaging in addition to cancer therapy [6,22,23,24,25].
In drug delivery systems, AgNPs offer unique advantages, such as adjustable size, large surface area, and tunable surface chemistry, allowing the efficient attachment of both drug molecules and recognition moieties, and subsequently the targeted and controlled delivery, and monitored release, of therapeutic agents [6,26,27,28]. AgNPs have also demonstrated promise in bioimaging due to their strong and tunable plasmonic properties, enabling enhanced contrast in various imaging modalities, such as optical imaging, computed tomography (CT), and photoacoustic imaging [29,30,31]. Furthermore, AgNPs have been extensively explored in biosensing applications due to their high sensitivity, selectivity, and stability, facilitating the detection of pathogens, biomarkers and other bioanalytes in very tiny concentrations, owing to their unique optical properties and outstanding surface enhanced Raman spectroscopy effect [29,32,33,34,35]. In the environment, AgNPs are used, for instance, in wastewater treatment, sensing of heavy metals, and photodegradation of dyes and colorants [36,37,38,39].
The present review surveys first the various methodologies developed to produce biosynthetically silver NPs (bio-AgNPs), summarizes some of the key mechanistic aspects that govern their toxicity, and extensively discusses their bioapplications in the biomedical and environmental fields. Importantly, it highlights, using specific examples, their paradoxical effects since these nano-objects may exhibit the desired activity against the target microorganisms/cell lines while they remain devoid of any unwanted side-effects towards the untargeted counterparts. This review also discusses the use of bio-AgNPs in the degradation of environmental pollutants like organic dyes. Finally, the findings are summarized, and some exciting emerging perspectives are provided.
2. Biosynthesis of AgNPs
To synthesize AgNPs, various chemical and physical methods are followed, such as sonochemistry, photochemistry, microwave chemistry, laser ablation and ball milling to name a few [30,40]. Some of these chemical routes may rely on costly and/or hazardous chemicals whereas their physical counterparts may require the use of sophisticated equipment in addition to issues encountered upon scaling up the NP production [41]. Chemically produced AgNPs offer many advantages, such as cost-effectiveness and scalability, shape and size control, adequate surface chemistry, and versatility to potentially find various applications in several fields [20,42,43,44]. However, some limitations restrict their integration into the market for many reasons, such as the use of toxic reagents and/or the generation of hazardous byproducts during the fabrication and/or the application stages, rendering them highly toxic or, at least, not sufficiently biocompatible [40,45].
To overcome these challenges, greener, sustainable and scalable methodologies have been devised to synthesize a very large variety of nanomaterials, among which AgNPs hold a special position [46,47,48]. These well-established yet fast-growing routes rely on living microorganisms, such as algae, bacteria, fungi, yeast, their extracts, plants’ extracts, and combinations of biomolecules to promote the fabrication of AgNPs (Figure 1). These processes fit within the bottom–up approach and, most often, meet several principles of Green Chemistry since they are easy to implement and are, usually, carried out in aqueous media at atmospheric pressure and room temperature or with mild heating, do not rely on any added chemicals except the precursors, exploit renewable biomolecules, and do not require sophisticated equipment [49,50,51]. When the organisms are directly used without any further processing, these routes are coined intracellular since the synthesis occurs inside the cells, as reported by Klaus et al. who carried out the synthesis of AgNPs in the shape of spheres, triangles, and hexagons, using the silver-resistant bacterium Pseudomonas stutzeri grown on AgNO3-containing agar substrate [52]. A similar procedure was implemented by other groups [53]. On the other hand, the process is termed extracellular when the synthesis occurs outside the cells thanks to the different metabolites released by the cells into the supernatant [54,55,56]. However, the epithet ‘extracellular’ is somehow flagrantly used in the literature to qualify the cell-free synthesis of NPs using processed microorganisms as extracts in reaction media devoid of any cells [57].
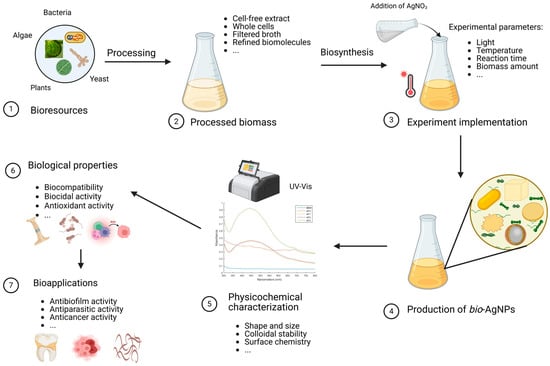
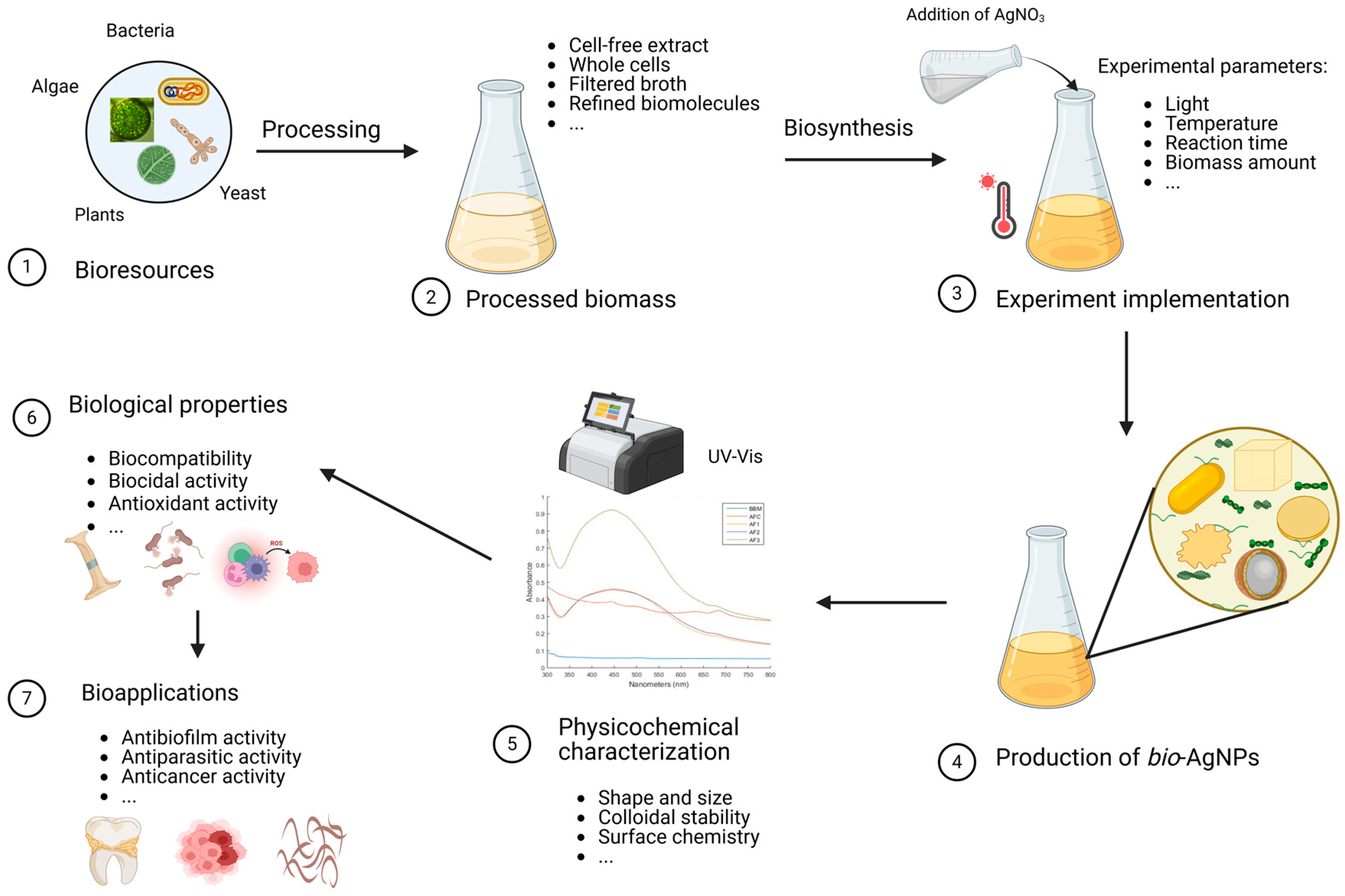
Figure 1.
Biosynthesis of silver nanoparticles. The first step consists of preparing the biomass. For instance, the relevant parts of the plants are directly harvested while the microorganisms (bacteria, fungi, microalgae) are cultured ①. Then, the biomass is processed according to the envisaged pathway ②. Subsequently, this bioresource, that can be made of the crude broth, refined biomolecules, disrupted cells, cell-free supernatant, or pristine cultures, is challenged by the precursor (aqueous solution of cationic silver in this case) under a set of selected experimental parameters (pH, temperature, reaction time, etc.) ③. This gives rise to bio-AgNPs, ④ whose features (size, shape, stability, etc.) are studied using a variety of characterization techniques ⑤. Then, the biological properties of these NPs are explored ⑥ from which some interesting bio-applications may emerge ⑦.
Several reviews have been fully or partially dedicated to the biosynthesis of AgNPs [58,59,60,61,62]. As one of the most popular methodologies, the cell-free pathway is highly advantageous since it is simple to implement and might be time- and cost-effective, especially when it utilizes extracts of plants, such as those derived from leaves, stems, or fruits, extracts of fungi, bacteria or algae and enables easy control over the environment in which the NPs are produced [58,63,64,65]. For instance, the polysaccharide-rich, cell-free supernatant of the green microalga Chlamydomonas reinhardtii, is used to produce bioAgNPs starting from aqueous solutions of cationic silver via a light-driven process [55]. However, aqueous suspensions of washed cells of the same microalga that are devoid of polysaccharides keep their bioreducing capabilities, although the obtained AgNPs lack any colloidal stability and display various shapes and forms [55]. Thus, this study highlights the double role that polysaccharides may fulfill during the biosynthesis of AgNPs: light-activated bioreducing agents and stabilizing moieties that protect the NP shape and size from any alteration. These findings regarding the stabilizing role played by the polysaccharides corroborate another study reporting the sedimentation of gold NPs made by Euglena gracilis, a microalga that does not produce polysaccharides [66].
Various experimental conditions have been screened for the cell-free synthesis of AgNPs, such as the pH, temperature, reaction time, and concentration of silver precursor and biomass content, resulting in desired morphologies and forming spherical, triangular, or hexagonal NPs [67,68,69,70,71,72,73]. For instance, AgNPs obtained using the cell-free supernatant of different strains of Bacillus sp. exhibit different shapes, as displayed in Figure 2. The underlying mechanistic aspects involve first the reduction of silver ions into their metallic counterparts by biomolecules present in the bioresource, such as sugars, polyphenols, flavonoids, proteins, or enzymes. Subsequently, these atoms assemble to form the AgNPs. Finally, the interaction of these nano-objects with biomolecules via weak or strong interactions determines the quality of their colloidal stability [74,75].
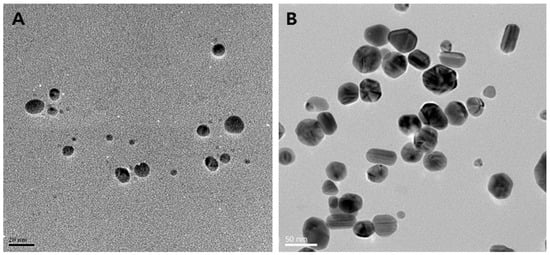
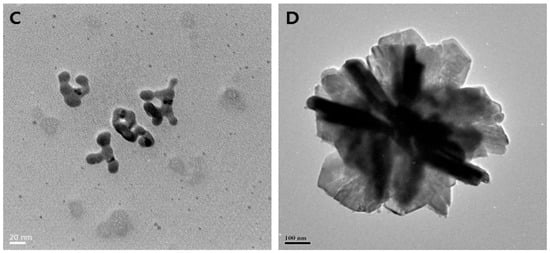
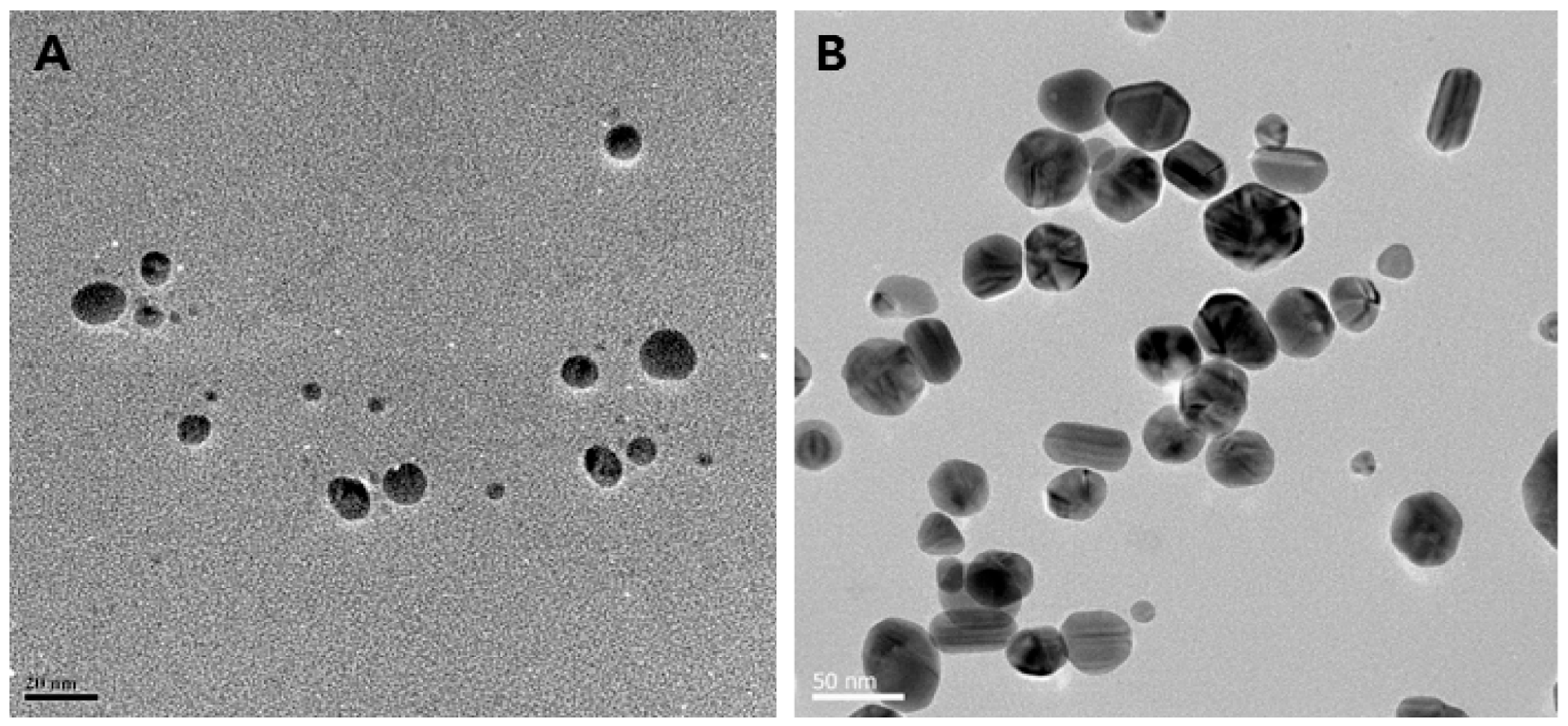
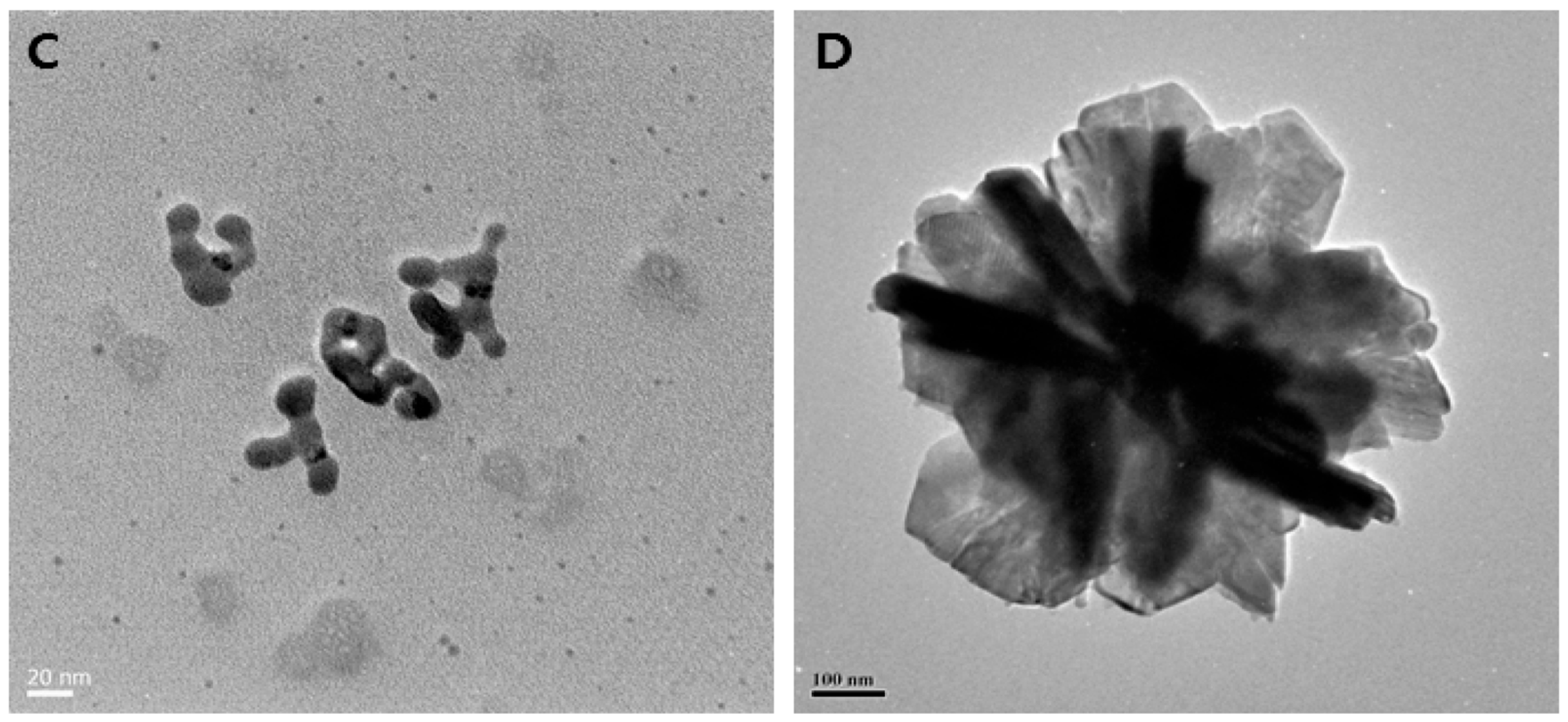
Figure 2.
Biosynthesis of different shapes of AgNPs using the supernatant of cultures of various Bacillus species. (A) Spherical (scale bar: 20 nm); (B) mixed populations (octagonal, rod, hexagonal, and icosahedral) (scale bar: 50 nm); (C) highly branched (scale bar: 20 nm); (D) flower-like in shape (scale bar: 100 nm). Reproduced from Ref. [76] with permission from MDPI under Creative Commons CC BY 4.0.
The extracellular pathway is another prominent approach used to synthesize AgNPs in the presence of living cells of bacteria, fungi, and microalgae, although the process itself occurs outside the cells in the culture medium [77]. In this approach, the experimental conditions play a crucial role, such as using different cell types under given growth conditions [53,78,79,80]. In a similar way to the cell-free pathway, the AgNP shape, size and colloidal stability are also influenced by several factors, such as the pH, temperature, and capping agents that are present [67,81,82]. The synthesis follows the different steps described for the extracellular pathway via a similar mechanism, although some specific nitrate-dependent reductases and shuttle quinones might be involved [83,84].
The intracellular synthesis of inorganic NPs constitutes the last methodology followed in the biosynthesis of nanomaterials, in general, and AgNPs in particular [47,85,86]. Compared to the two previous ones, this remains the least explored pathway. Although experimental conditions play a crucial role in this process, the physiology and viability of the living organisms restrict the extent to which these parameters, especially the pH, temperature, and precursor concentration, can be varied. Instead, the focus is usually placed on the employed microorganism, culture age, cell density, and reaction time [87]. Under certain conditions, the intracellular pathway allows the design of bioreactors for the continuous synthesis of NPs [88]. In addition, the very vast majority of studies report on the intracellular synthesis of noble metal, zero-valent NPs [47,86,89,90]. In a few instances, alloy metallic NPs are also obtained [91].
Several studies have explored the use of various bioresources for the intracellular synthesis of AgNPs, including microalgae, plants, mammalian cells and, exceptionally, animals [47]. Importantly, maintaining the cells in their growth media yields results that differ significantly from using either washed cells or cell-free supernatant, affecting many aspects, such as NP size, shape, production kinetics, and colloidal stability [55]. Several species of microalgae and cyanobacteria have demonstrated their ability to effectively synthesize AgNPs via an intracellular pathway, as evidenced by optical and electron microscopy [86,89,92]. On the other hand, whole cultures of the microalga C. reinhardtii promote the production of AgNPs simultaneously via extra- and intra-cellular pathways [55]. Although the contribution of each pathway is not quantified, this study clearly shows that the use of the cells in their original medium without any further processing gives the best results in terms of NP features (size, shape, stability, and yield). Other organisms, such as bacteria and fungi, have also potential in the intracellular synthesis of AgNPs [56,93].
3. Toxicity of Silver Nanoparticles
A great number of studies have been conducted to assess the toxicity of AgNPs [94,95,96]. Although NPs have several extraordinary features, not all of them are fit to achieve a proper function, thereby causing side effects in living organisms [97]. As NPs have been gaining increasing applications in several fields, such as electronics, energy and healthcare, to name a few, their toxicity has become a booming investigation field due to their impact on human health and the environment [98,99,100]. Several studies have found that the size, shape, surface charge, chemical composition, solubility, dose, exposure route, metabolism and excretion affect the toxicity and biokinetics of NPs [94,95,101,102,103,104,105,106,107,108]. The physical size-dependent characteristics of these materials contribute to hindering metabolism and excretion, provoking a long duration inside the host and consequently a prolonged harmful effect [109].
The oligodynamic effect of metals, especially heavy metals including silver, occurs even when their concentrations are low. The size of AgNPs controls their properties and, thus, their activity towards many species of bacteria [110,111]. The exact mechanism of action of silver NPs on the cell is yet to be fully unraveled [112]. However, a significant amount of data accumulated in this area indicates that AgNPs can physically interact with the cell surface of different microorganisms [82,113,114]. Several observations support this statement regarding NP adhesion to the cell wall and membrane of bacteria: penetration into the cell and disruption of intracellular organelles, induction of oxidative stress, and modulation of signal transduction pathways, among others [101,115,116]. In addition, AgNPs can putatively modulate cellular signaling by dephosphorylating tyrosine residues on key peptide substrates of bacteria, thus inhibiting their growth [30,117].
It is well-known that silver ions do not exert the same toxic effects as AgNPs, mainly because the former are more reactive [118]. The cytotoxicity of AgNPs begins with the significant release of toxic ions that follows their internalization [105]. Since silver ions play a key role in the toxicity of AgNP formulations due to carry-over, their amount should be frequently measured and reported [95]. However, recent findings using mammalian cells indicate that AgNP-induced toxicity might be an intrinsic effect of AgNPs that is independent of free Ag+ and the mode of action of AgNPs may differ from that of Ag+ since the latter increases H2O2 driving the oxidative stress and the apoptotic pathways while the former provoke lipid peroxidation causing proteotoxicity and necrotic pathway activation [119]. Furthermore, AgNPs capped with starch and bovine serum albumin (BSA) induce a dose-dependent toxicity in zebrafish embryos and prevent their normal development by exhibiting phenotypic defects and altered physiological functions, such as bradycardia, axial curvatures and degeneration of body parts [120].
The shape of AgNPs is another parameter that significantly affects their toxicity in environmental models [121]. Compared to quasi-spherical AgNPs and silver nanowires, Ag nanocubes exhibit a lower toxicity toward several environmental models, including the ryegrass Lolium multiflorum, the zebrafish Danio rerio, the nematode Caenorhabditis elegans, and bacterial species (Escherichia coli, Bacillus cereus, and P. aeruginosa) [122]. The surface functionalization and charge, temperature and nature of the immersion medium, including the presence of biomolecules and salts, are also major factors that affect the toxicity of AgNPs [123]. In this vein, the physical interactions between AgNPs and Bacillus sp. are mainly governed by the surface charge, which has a greater influence on the toxicity than the particle shape and size [124].
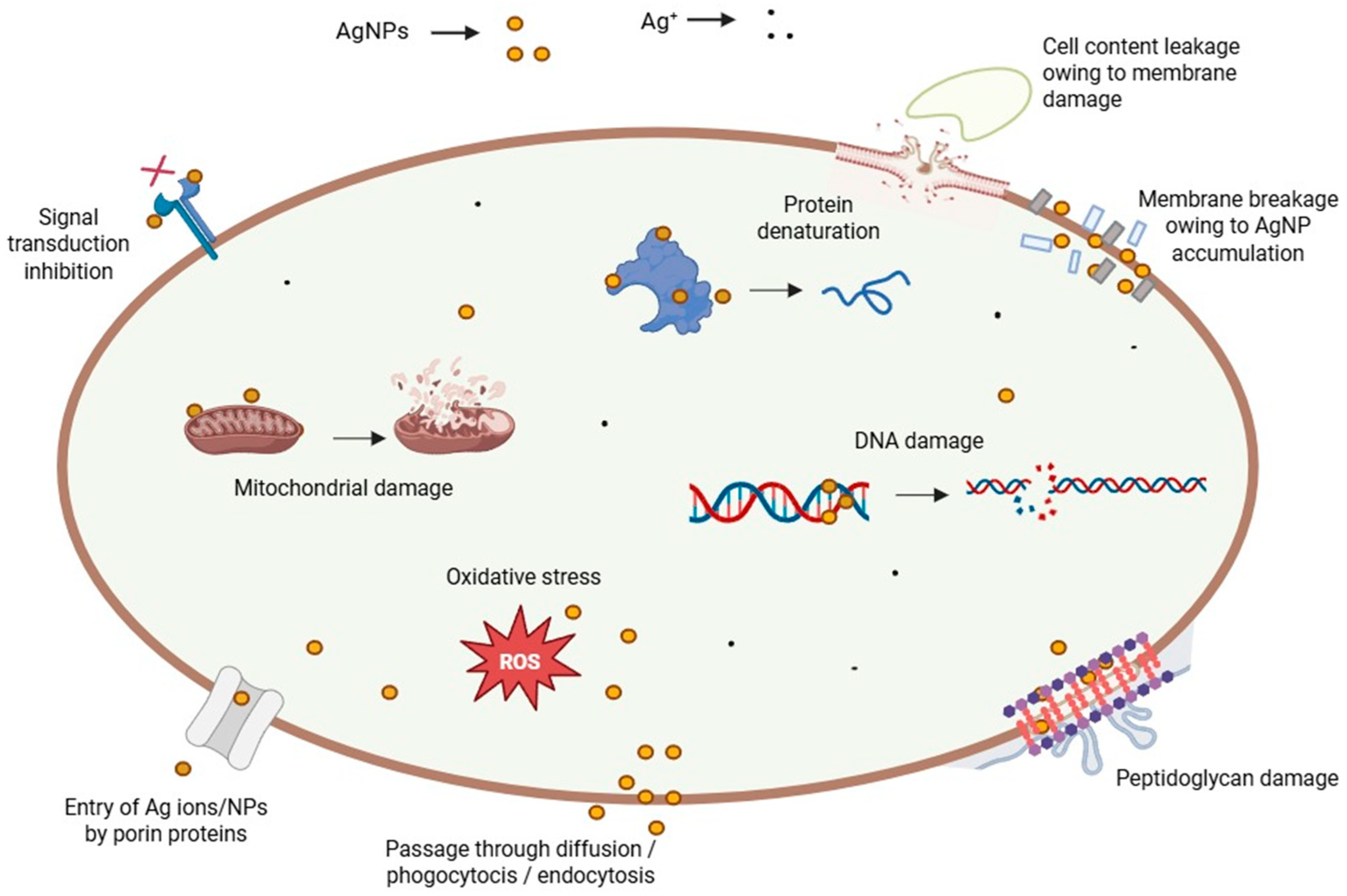
Some of the principal toxic effects of AgNPs in mammals are changes induced in the treated cells, including alteration of DNA, oxidative stress, and protein denaturation, among others (Figure 3) [96]. By affecting the dehydrogenase activity, reactive oxygen species (ROS) damage the mitochondria, resulting in diminished ATP production [105]. Consequently, the cell metabolism decreases, and vital functions dramatically plummet or even stop. DNA is also damaged by oxidative stress, which disturbs the correct process of the cell cycle during G2 and M phases [125]. Therefore, the cells undergo apoptosis or necrosis. In addition, the cells can present several chromosomal abnormalities. These toxic effects are concentration-dependent, thus an excessive NP dose during the exposure/treatment can increase the side effects and hurt the patient at a bigger scale [126]. Other related cytotoxic effects stem from the interference of NPs with specialized proteins, such as the membrane protein links and enzymes, like lactate dehydrogenase (LDH) [103]. In addition, peptidoglycans, which are an exclusive structural feature of bacteria, constitute the critical reason why AgNPs are highly toxic to these prokaryotes when compared to eukaryotic cells. AgNP interaction with bacteria leads to bacterial membrane damage, as observed by TEM and SEM. The membrane damage was confirmed by detecting the leakage of proteins and reducing sugars from treated bacterial cells [114,127]. Conversely, the protein corona that builds up on biogenic AgNPs appears to be responsible not only for making them more stable in time but also for masking and protecting eukaryotic cells against metal toxicity [128], thus explaining, at least in part, the term “paradoxical” in this review’s title.
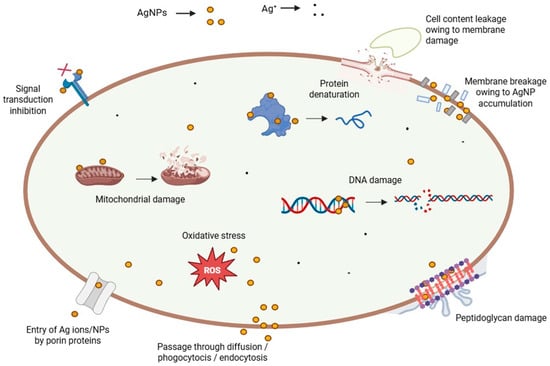
Figure 3.
Different toxicity pathways of AgNPs.
Several studies discuss the toxicological effects of bio-AgNPs. For instance, bio-AgNPs prepared with the aqueous leaf extract of Swertia chirata are equally toxic to Allium cepa cells as chemically synthesized AgNPs and silver ions [129]. Additionally, the induced chromosomal aberrations are similar both at mitotic and meiotic levels even at lower concentrations. Rheder et al. (2018) show that bio-AgNPs synthesized by the leaf extract of the plant Althaea officinalis (AgNP-L) are slightly more toxic towards the used mammalian cell lines than those synthesized with the dehydrated root infusion of the same plant (AgNP-R); this might be related most likely to a size effect since the former are smaller than the latter [130]. This trend is also observed when these two types of bio-AgNPs are tested on zebrafish. Whereas the two highest tested concentrations lead to fish death after 24 h exposure, the intermediate concentration causes death of fish exposed to AgNP-L and great damage to the gill cells in fish exposed to AgNP-R, while the lowest provokes DNA damage in blood cells, regardless of NP type. Furthermore, high concentrations of bio-AgNPs made using Rumex acetosa inhibit the proliferation of human umbilical vein endothelial cells (HUVECs) via a ROS-induced apoptotic pathway and cause morphological changes in the yolk sac and the tail of zebrafish [131].
4. Biological Properties of bio-AgNPs
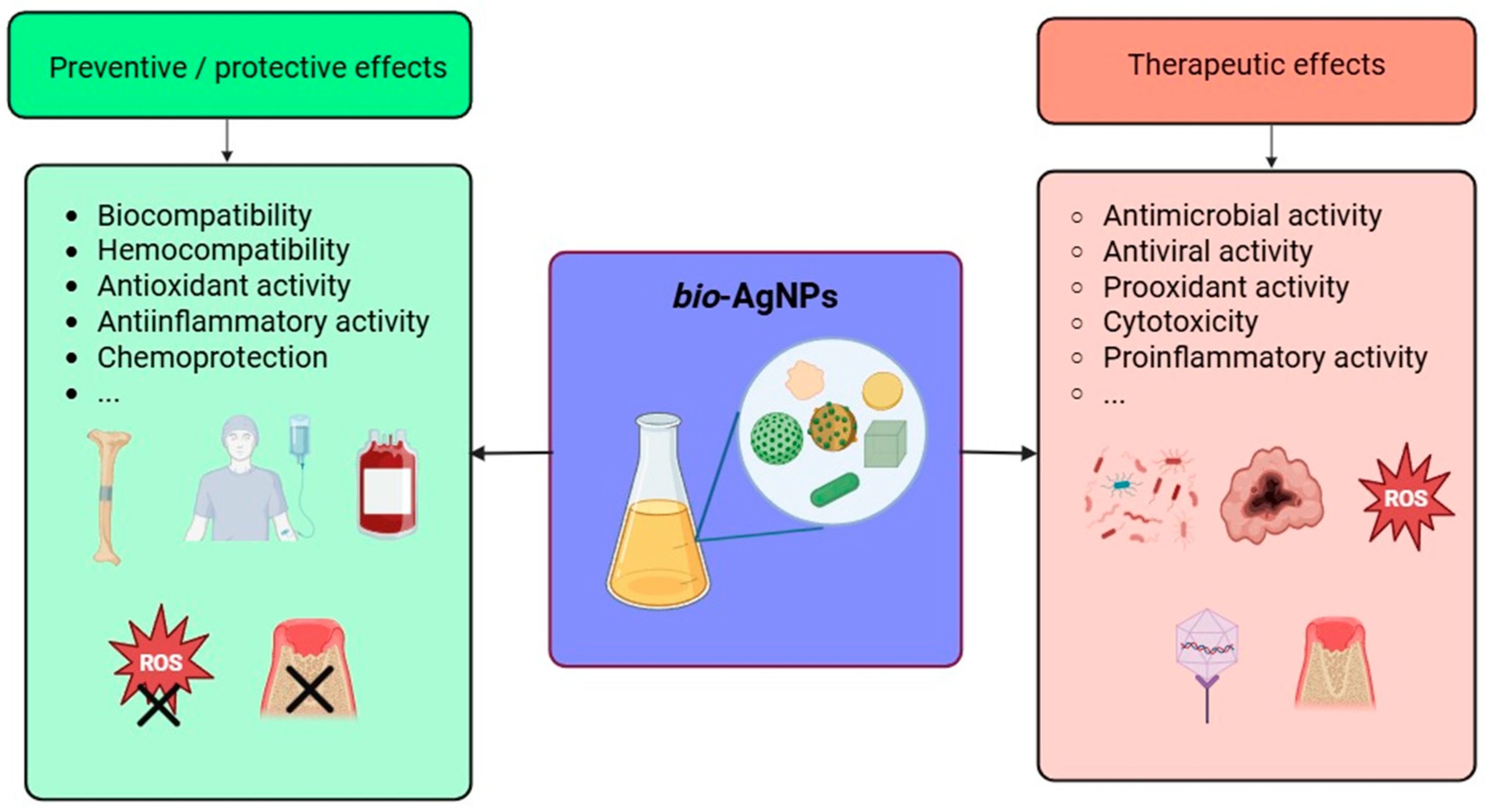
Inorganic NPs display a very diverse and rich set of biological properties that make them of paramount importance in various applications related to human health and well-being, such as the biomedical field, agriculture, and the environment (see above). As portrayed in Figure 4, these outstanding properties encompass but are not limited to anti- and pro-oxidant activity, anti- and pro-inflammatory activity, modulation of the immune system response, inhibition of enzymatic pathways, induction of apoptosis/necrosis, and, depending on the target, genotoxicity, cytotoxicity against pathogens and cancer cells, and biocompatibility for healthy cells and tissues, enabling their use in several biomedical applications (cf. next section). These properties, present among inorganic NPs that are obtained via a green approach, might even be enhanced due to the moieties of biological origin, i.e., biomolecules, phytochemicals, that coat their surface [132,133,134]. For instance, biogenic selenium NPs exhibit valuable characteristics in addition to the abovementioned properties since they also lessen the toxicity caused by drugs or heavy metal cations [135]. Similarly, bio-AgNPs are also known to possess unique biological properties and activities that are described in the following paragraphs.
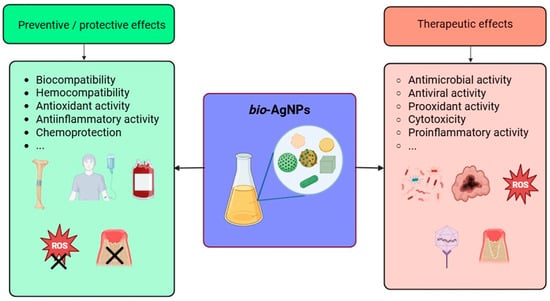
Figure 4.
Illustration of some of the most noteworthy biological properties of biogenic AgNPs.
The oxidative stress refers to the accumulation of reactive oxygen species (ROS) in cells and tissues because of an imbalance between their production and detoxification [136]. The excess and accumulation of ROS may lead to the onset of numerous diseases, including cancer, diabetes, Alzheimer’s, and atherosclerosis [137]. Oxidative stress is usually studied in vitro using several routine assays, such as 2′,7′-dichlorofluorescein diacetate (DCFH-DA) [138,139], 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH) [140,141], 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS) [142,143], ferric reduction antioxidant power (FRAP) [143,144], and phosphomolybdenum assays [145].
The ability of NPs to trigger the cellular production of ROS is known as the prooxidant activity. On the other hand, the scavenging of these radicals by the NPs defines the antioxidant activity. In this regard, bio-AgNPs are outstandingly versatile since they can either provoke a surge in the intracellular ROS levels, which make them toxic to pathogenic microbes, such as bacteria [146,147,148], fungi [149,150,151], and viruses [152], in addition to their cytotoxicity against countless cancer cell lines [153,154]. On the other hand, bio-AgNPs can lower the ROS levels, rendering them protective of healthy cells and organs [155,156,157]. These findings constitute a special feature of biologically synthesized NPs. However, bio-AgNPs hold a distinct position because of the ease and speed of their synthesis which is carried out, usually, under straightforward conditions: simple glassware, atmospheric pressure, room temperature, or very mild heating, either in the dark or under regular illumination, no pH adjustment, etc., (see above).
These oxidative radicals possess several modes of action against pathogens and cancer cells: they may (i) damage the cellular integrity by perforating the cytoplasmic membrane and cause the leakage of different components in bacteria [158,159,160,161]; (ii) induce DNA damage [158]; (iii) induce autophagy and apoptosis in cancer cells [138,140,150,162,163]; (iv) upregulate pro-apoptotic pathways and/or downregulate their anti-apoptotic analogs [164]. On the other hand, bio-AgNPs scavenge the ROS and, therefore, prevent their harmful effects against healthy cells [165]. This remarkable activity explains the surge in the exploration of the opportunities that bio-AgNPs might offer in combating infections and emerging threats to humans and their environment (see below).
Several studies underline the major role that bio-AgNPs may play in the modulation of inflammation in humans since they can favor the body’s defense mechanisms, yielding a pro-inflammatory response, or, on the contrary, fight against it via an anti-inflammatory pathway [166,167,168,169,170]. On a molecular level, bio-AgNPs decrease mRNA levels of inflammation-related enzymes and pro-inflammatory cytokines in lipopolysaccharide-stimulated RAW 264.7 cells [168], scavenge the activity of nitrite [143,171], attenuate the activity of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) [172], and prostaglandin E2 [171], lower the level of pro-inflammatory cytokines [167,172,173,174], promote the expression of anti-inflammatory cytokines [167], and inhibit the activity of proteinases [175]. Moreover, bio-AgNPs can reduce the heat-induced effect on bovine serum albumin (BSA) [176], greatly inhibit protein denaturation, and stabilize the membrane of human red blood cells [142,148,176,177,178,179]. In animal models, bio-AgNPs suppress pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [167,174,180], and promote the expression of the anti-inflammatory cytokine IL-10 on burn injury [167,174]. The modulation of the inflammatory response appears to be dose-dependent in xenograft-bearing mice [156]. In vivo experiments on mice bearing acute myeloid leukemia demonstrate clearly that bio-AgNPs lower the total count of the different leukocytes, favor body weight gain, and restore the lymphocyte, platelet, and RBC counts [174]. On a macroscopic level, bio-AgNPs have proven efficacious in reducing the volume of induced edema or preventing its onset in treated mice [180].
Other studies clearly demonstrate the ability of bio-AgNPs to inhibit the activity of several enzymes. For instance, the capacity to inhibit the activity of several metabolic enzymes, such as α-amylase, α-glycosidase, and dipeptidyl peptidase IV, confers these bio-NPs a valuable antidiabetic character [142,148,176,181]. bio-AgNPs can also inhibit the action of enzymes related to aging processes [182,183], in addition to others involved in the onset of Alzheimer’s disease [175,183] and in other biological processes [148,183,184].
Lastly, several in vitro and in vivo studies highlight the biocompatibility of bio-AgNPs. These findings are of crucial importance, especially from the point of view of developing bio-AgNP-based nanoformulations and devices for real-world applications related to human health and well-being. For instance, bio-AgNPs do not induce any hemolysis [164,185,186,187] and possess valuable thrombolytic activity [146,188,189]. bio-AgNPs seem not to interact with hemoglobin and human serum albumin [190], although another study reports that bio-AgNPs bind to the latter, resulting in a slight modification of its structure [191]. Other studies pinpoint the biocompatibility of bio-AgNPs towards, for instance, human peripheral blood lymphocytes [192] and fibroblasts [187,193,194]. These findings are corroborated by in vivo tests that reveal no noticeable toxicity of bio-AgNPs administered orally [195,196], intravenously [197], or intraperitoneally [198] in healthy mice. On the other hand, bio-AgNPs do not cause any major toxicity when used to treat tumor-bearing mice, and they might also protect these animal models against the toxicity induced by the conventional drugs used (see below).
5. Bioapplications of bio-AgNPs
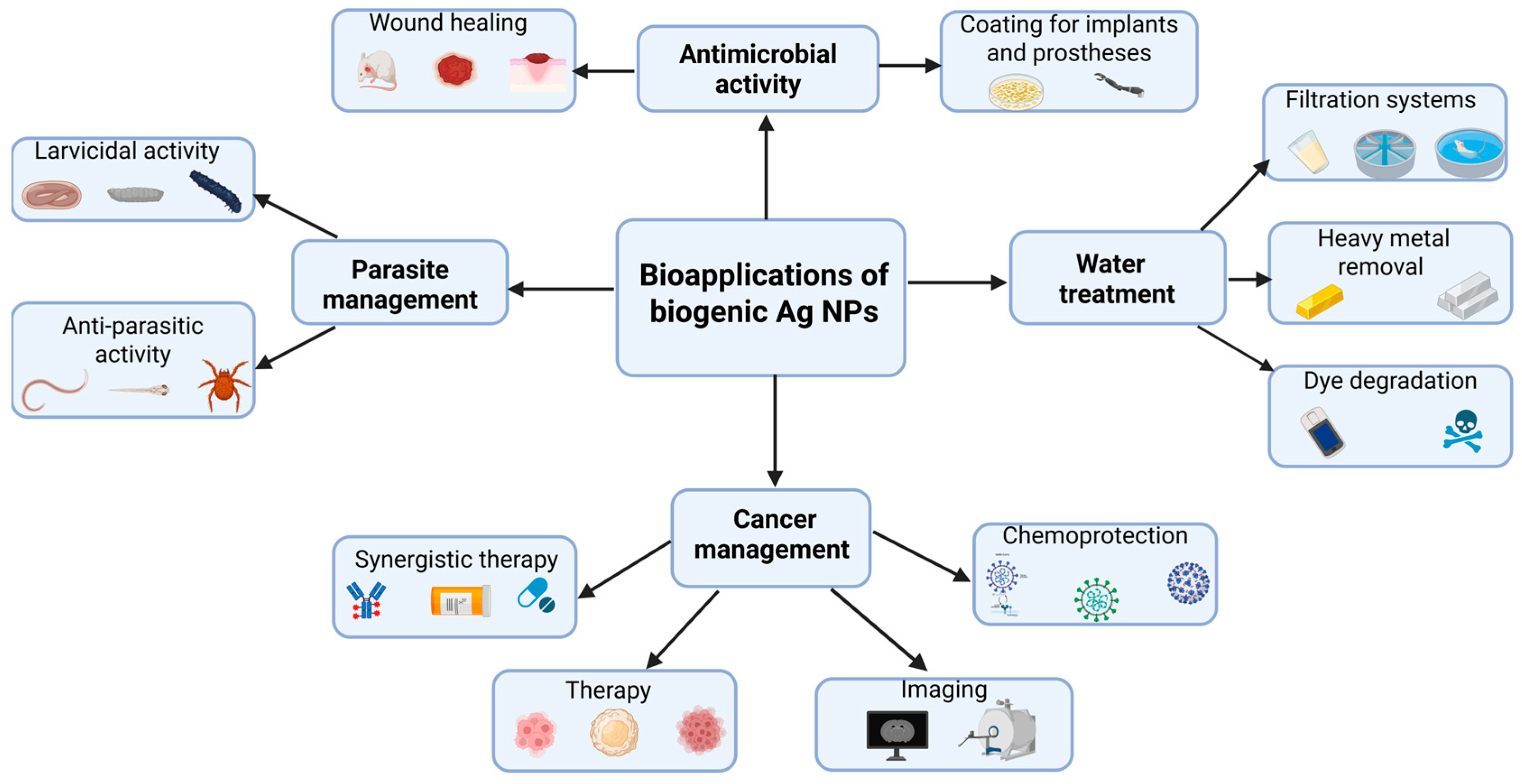
Owing to their outstanding biological properties, bio-AgNPs have been explored for numerous applications in the biomedical field and water treatment (Figure 5). The following paragraphs will provide insightful discussion on each of them with examples taken from recently published findings.
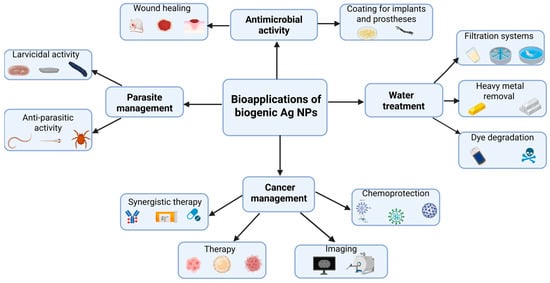
Figure 5.
Summary of the most widespread bioapplications of biogenic AgNPs.
5.1. Larvicidal Activity of bio-AgNPs
In the era of climate change and globalization, the control of vectors of dangerous diseases, such as mosquitoes, that transmit yellow fever, chikungunya fever, dengue hemorrhagic fever, malaria and filariasis, among others, is a major worldwide concern for health authorities [199,200]. As a green alternative to commercially available pesticides, much attention has been placed on eco-friendly yet cost-effective solutions for the control of proliferation of these insects thanks to the larvicidal efficacy of phytocompounds, plant extracts and plant-based metallic NPs [201,202,203]. Although still laboratory-based, these emerging approaches bring hope for field applications as they take advantage of the intrinsic toxicity of inorganic nanomaterials to destroy the colonies of mosquito eggs, larvae, and pupae [204,205]. Several studies describe the use of bio-AgNPs as a potent larvicidal agent [206,207,208]. For instance, bio-AgNPs produced using the leaf aqueous extract of Aegle marmelos exhibit a dose-dependent larvicidal activity against larvae of both Aedes aegypti and Culex quinquefasciatus, where the lethal concentration that kills 50% of the larvae, LC50, is much lower in the latter (132 ppm) than in the former (302 ppm) [209]. These findings corroborate other results that demonstrate the killing efficacy of bio-AgNPs, synthesized using other plant-based extracts, such as those originating from roots [210], fibers [211], stems, leaves [212], bark [213], fruits [214], latex [215], and shoots [216] against the larvae of several mosquitoes that are vectors of diseases. Likewise, bio-AgNPs of fungal or bacterial origin, are also a powerful weapon that destroys mosquito larvae in a dose-dependent manner [217,218,219,220,221,222].
Several experimental parameters controlling the larvicidal activity of bio-AgNPs have been explored, such as the applied dose, the exposure duration, development stage, and target species. Several studies have reported the dose-dependent action of bio-AgNPs against the larvae, regardless of the type of biomass used or the nature of the extract [219,220,221]. Usually, the higher the dose, the higher the larvicidal activity [217,223]. Sometimes, bio-AgNPs synthesized using a given biomass exhibit better larvicidal activity than the same dose of bio-AgNPs obtained through a different type of biomass. For instance, more than 33 ppm of bio-AgNPs, produced using an aqueous extract of B. amyloliquefaciens, are needed to reach the LC50 against the pupae of C. pipiens pallens, while less than 14 ppm are needed for the same goal when B. subtilis is used instead [222]. Exposure duration is another parameter that impacts the larvicidal activity [211,224]. For instance, a given dose of bio-AgNPs applied against A. aegypti larvae has a higher activity after 48 h of exposure when compared to an exposure of 24 h, although the difference tends to diminish when the applied dose increases [210]. Several studies shed light on the larvicidal activity of bio-AgNPs as a function of the development stage of the larvae [222,225,226]. Murugan et al. report that, for all screened doses of bio-AgNPs, the mortality of C. quinquefasciatus decreases as it progresses in its development, from instars I to IV as larvae to pupae [227]. A similar trend is reported regarding the mortality of larval instars of the same vector and of A. aegypti [221]. In other words, destroying larvae colonies requires higher doses of bio-AgNPs as the mosquitoes develop. However, instar II is reported to be more susceptible to bio-AgNPs when compared to the other stages since they show a 100% mortality within 1 h exposure [219]. Further, the same group demonstrated that bio-AgNPs are more efficient than their Au counterparts synthesized using the same Chrysosporium tropicum fungal supernatant against the same vector. Divergent trends have also been reported, i.e., increased % mortality for all screened concentrations of bio-AgNPs as the larvae develop [228], but with no adequate explanation.
Some studies compare the larvicidal activity inherent in the extract used to synthesize the bio-AgNPs with that of the resulting NPs [207]. For instance, it is reported that the concentration, in μg/mL, of the extract, prepared from the shoots of Echinochloa stagnina, should be multiplied by ~5–7 fold to reach the same larvicidal activity (LC50 and LC90) against Anopheles pharoensis and C. pipiens as that of the resulting bio-AgNPs [216]. To reach the same mortality effect on larvae at their II and IV instars, it has been shown that the dose in ppm of the aqueous crude latex should be multiplied by two orders of magnitude when compared to the effect of the resulting NPs [215]. As a general trend, the larvicidal activity of bio-AgNPs is always stronger than that of the biomass extract used for their formation [212,229,230,231]. However, the biomass itself seems to play a non-negligible role in the larvicidal activity. For instance, bio-AgNPs synthesized using clove aqueous extract exhibit a much higher and faster larvicidal activity than their analogs obtained using NaBH4 or glutathione; this effect might arise from the phytochemicals present in the extract [232]. This reinforces previous observations that the increase in polyphenols enhances the larvicidal activity of bio-AgNPs against Spodoptera littoralis larvae [233].
Several groups have attempted to unravel the mechanistic aspects underlying the larvicidal activity of bio-AgNPs and its specificity. Owing to their nanoscale size, AgNPs may cross the insect cuticle and penetrate the cells where they interfere with physiological processes, including molting [204]. When challenged by bio-AgNPs, IVth instars of C. pipiens pallens undergo morphological changes in their thorax and abdomen, resulting in significant damage in the anal region and cuticle layer [222]. On the other hand, the pupae of the same mosquito exhibit, after exposure to bio-AgNPs, severe distortions in the head, thorax and abdomen, and loss of breathing ability. Besides confirming the shrinkage of the internal cuticle, another study describes the pigmentation and swelling of apical cells of A. aegypti larvae after exposure to bio-AgNPs [234]. These findings highlight the disturbances induced by bio-AgNPs upon the normal development of the larvae. The uptake of bio-AgNPs might be facilitated by the biomolecules present in the synthesizing biomass extract since some of these moieties bind specifically to mosquito salivary proteins [235]. Histopathological images of bio-AgNP-treated larvae of several mosquito species display, among other things, altered structures of the midgut epithelial cells, apical enlargement in the gut lumen, and reduction in intercellular connections [220].
The above-mentioned studies underscore the strong larvicidal activity of bio-AgNPs when compared to their chemical analogs or to the extracts used in the synthesis process. However, there are concerns about the potential toxicity of these NPs should they be used on a large scale and translated from the lab bench to the field in the form of bio-AgNP-based commercial nanopesticides to combat the development of mosquito larvae responsible for spreading diseases to humans and livestock [207]. Further work is needed to address these issues by enabling the design of long-term safe bio-AgNP nanoformulations that are suitable for field use and by fully elucidating their mode of action. A handful of studies offer optimistic perspectives in this area since, paradoxically, bio-AgNPs do not show toxicity towards non-target species [230,236,237,238]. Other studies show that bio-AgNPs increase predation against the NP-treated larvae [226,239]. However, the long-term fate of released bio-AgNPs in the environment and their impact on the ecosystems should be meticulously monitored.
5.2. Antiparasitic Activity of bio-AgNPs
Parasite-borne illnesses affect humans and animals and cause mild-to-severe health problems that can lead to important economic and social consequences and, sometimes, to death [199]. For instance, leishmaniasis, the most widespread parasitic infection, is caused by the protozoans belonging to the Leishmania family, which are transmitted by the bite of infected female phlebotomine sandflies. Leishmaniasis affects between 700,000 and 1.2 million persons living in some of the poorest countries in the world; it has different clinical forms, the cutaneous, mucocutaneous, and visceral among which the latter is life-threatening if left untreated [240]. Despite the commercial availability of several antiparasitic drugs [241], some of them have disadvantages, such as their cost, given that these infections affect very poor populations, their toxicity, and the resistance some parasite carriers can acquire, rendering the therapeutic molecules progressively inefficient and useless [242]. In addition to conventional therapies, several groups have questioned the capabilities offered by nanotechnology in managing these infections [241,243,244,245]. Typically, several NPs of different provenance, such as those made of polymers, fibers, lipids, and metals, have been explored for their ability to inhibit the growth of these parasites and/or their activity, and induce lethal damage [241,246,247,248]. Several parasite-borne diseases have been investigated for potential treatment using bio-AgNPs, including malaria caused by Plasmodium spp. [249], leishmaniasis caused Leishmania spp. [250], trypanosomiasis (Chagas Disease and African Sleeping Sickness) caused by Trypanosoma cruzi [251] and T. brucei [252], schistosomiasis caused by Schistosoma spp. [253], toxoplasmosis caused by Toxoplasma gondii [254] and giardiasis caused by Giardia lamblia [255].
Using representative examples, the following paragraphs portray the activity of bio-AgNPs against different parasites causing infections that primarily impact humans, especially leishmaniasis which remains, by far, the most targeted infection of parasitic origin [244,256]. In vitro studies show that chemically synthesized NPs made of noble metals (Au, Ag and Pt) and their alloys restrict the growth of Leishmania tropica, Toxoplasma gondii and different Trypanosoma strains in a dose-dependent manner [252,257,258]. In the case of T. gondii, the best results, in terms of, for instance, parasite viability, parasite invasion rate, and parasite intracellular replication, are obtained with bio-AgNPs whereas the viability of host cells remains quite unaffected [258]. The action of these NPs against T. gondii might arise from the generation of ROS. It is also possible to enhance the antiparasitic activity of chem-AgNPs consequently to ultraviolet light irradiation [257].
bio-AgNPs exhibit a versatile and appreciable in vitro antiparasitic activity against several pathogens, such as L. amazonensis [259,260], L. donovani [261], L. major [262], L. tropica [263], Plasmodium falciparum [264,265], Giardia lamblia [255], Pythium insidiosum [266], Trypanosoma brucei gambiense [267,268], Schistosomiasis mansoni [253], T. gondii [254]. Interestingly, the same bio-AgNPs exert mild to negligible cytotoxic effects on mammalian cells showcasing, thus, their biocompatibility. In addition, bio-AgNPs inhibit the growth of L. major at both the promastigote and amastigote stages [269]. Moreover, bio-AgNPs possess an appreciable anthelmintic activity since those produced using the aqueous extract of Lansium parasiticum exhibit toxicity towards adult males and females, larvae, and eggs of Haemonchus contortus, a nematode causing infection in the gastrointestinal tract [270]. Furthermore, bio-AgNPs greatly enhance the antiparasitic activity of drugs, such as miltefosine [271]. However, in some cases bio-AgNPs are less (or equally) efficacious than a drug, e.g., pyrimethamine [272]. Lastly, bio-AgNPs obtained using the leaf extract of the medicinal plant Teucrium stocksianum possess a better antiparasitic activity against promastigotes of L. infantum than the ones made using the stem extract of the same plant although both show a dose-dependent activity [273].
The aqueous extract of the medicinal plant Sargentodoxa cuneata enables the production of bio-AgNPs that are lethal to L. tropica in a dose- and time-dependent manner since the viability of the parasite nearly collapses to zero while its growth is fully inhibited within 24 h of exposure [274]. At the same time, bio-AgNPs show the best results when compared to the extract alone or to bio-AuNPs obtained using the same extract. In vitro studies show that bio-AgNPs, obtained using the medicinal herb myrrh or Commiphora molmol, have the best sustained effect against L. major when compared to chemical analogs or the drug pentostam [275]. At the same time, the area of cutaneous lesion due to leishmaniasis in murine models recedes faster when bio-AgNPs are applied topically than when relying on chem-AgNPs or the drug pentostam [275]. Furthermore, bio-AgNPs appear to have no adverse effects on mice kidneys and livers. These findings corroborate the results reported earlier [259]. On the other hand, bio-AgNPs appear to enhance the therapeutic effect of amphotericin B against L. tropica since, in all tested concentrations, and the combined formulation yields a better effect enabling thus to lower the concentration of used silver; however, there is no mention of the sole effect of amphotericin B [276]. Likewise, ointments containing both bio-AgNPs and quercetin show a dramatic effect in healing leishmaniasis-induced ulcer in mice [277]. The same report also records an IC50 of 6.125 µg/mL for bio-AgNPs that should be compared to 100 µg/mL for the leaf extract of Artemisia aucheri used to synthesize the bio-AgNPs, and to 150 µg/mL for quercetin, thus demonstrating the antileishmanial efficacy of bio-AgNPs. Another in vivo study demonstrates the positive impact of bio-AgNPs in healing mice infected with schistosomiasis, especially when combined with the drug praziquantel [278]. From a mechanistic point of view, there is a lot to discover as various pathways might be involved [244]. A few studies clearly highlight the dependence of the results on the targeted parasite, infected cell lines and origin of bio-AgNPs [279]. Overall, bio-AgNPs hold great promise in managing parasitic infections, such as leishmaniasis, especially when used as part of topical formulations that might facilitate translational knowledge from animal models to humans [248,277].
5.3. Biogenic AgNPs as a Promising Tool in Cancer Imaging and Therapy
Owing to their unique properties, some of which are discussed above, bio-AgNPs exhibit a very large spectrum of activity against various cancer cell lines and experimental tumor xenografts [280,281]. Several articles extensively review the in vitro anticancer activity of bio-AgNPs against malignant cell lines, highlighting their quite universal cytotoxic (or antiproliferative) propensity as encountered among all tested NPs [282]. Therefore, the present section aims to sum up the most important, and updated findings regarding this aspect by portraying the richness and diversity of the potential of bio-AgNPs in cancer therapy. The biomass used for their fabrication may originate from different sources, such as plants [283,284], algae [285,286], bacteria [139,287,288,289], and fungi [290,291,292]. Both officinal [293,294,295,296] and non-officinal plants [142,297,298] are used to produce bio-AgNPs possessing an in vitro anticancer activity against, mostly, human cancer cell lines, and, in a few cases, murine cancer lines [149,174,299]. To reach the same goal, different parts of plants might be exploited, such as sea grass [300], leaves [301], stems [302], flowers [303], fruits [304,305], seeds [306], spices [307], roots [308], or specific biomolecules isolated from plants [164]. Although most studies detail the utilization of aqueous extracts in the biosynthesis of bio-AgNPs, a few others report the use of ethanolic extracts [309,310,311]. Whenever the extraction occurs using organic solvents, such as ethanol, the resulting solvent-free extract is transferred into water [312].
The in vitro anticancer activity of bio-AgNPs is dose-dependent since the cell viability decreases with increasing NP concentration [313,314,315]. It is also time-dependent [309,316]. Most often, bio-AgNPs exhibit a superior anticancer activity when compared to that of the extract itself [177,311,317,318,319]. However, in a few instances, no significant difference in the anticancer effect is observed when comparing bio-AgNPs with the aqueous extract itself [291,320]. So far, there is a lack of information regarding the impact of the size and shape of bio-AgNPs on their in vitro anticancer activity. When compared to other NPs synthesized via the same green process, i.e., same plant extract and protocol, such as bio-AuNPs, no clear trend emerges as contradictory data is reported; this might be explained by the variation in NP size and shape and the response of the tested cells [320,321,322]. However, bio-AgNPs seem to be more potent than cationic silver administered at the same concentration [323,324]. On the other hand, bio-AgNPs remain, by far, more powerful in inhibiting the in vitro growth of cancer cells, while at the same time remaining more biocompatible than their chemical analogs [193,313]. This extra anticancer effect might be attributable to the biopolymeric layer, rich in bioactive biomolecules, like terpenoids, saponins, flavonoids, and phenols, that surround the NPs [302,325,326]. The polymeric matrix may also foster a better binding between the bio-AgNPs and their target cells, thus enhancing the anticancer efficacy of the NPs [327].
When compared to conventional cancer drugs, there are no clear data that show any in vitro superiority of bio-AgNPs, since a few studies report contradictory results [328,329]. This might be attributed to the interplay of several parameters, including the NP features (size, shape, coating), the cell line, and the drug used. bio-AgNPs might also be used to enhance the efficacy of conventional chemotherapy drugs, e.g., cisplatin, while, at the same time, reducing their side-effects on healthy body tissues and organs [155,156,329]. Some studies pinpoint the fact that bio-AgNPs are toxic to cancer cell lines while they remain innocuous to healthy ones [330,331,332]. This is corroborated by other findings suggesting a discriminatory lethal action of bio-AgNPs against cancer cells while they remain biocompatible when tested on healthy cell lines [333]. This most likely originates from the NP coating made of the bioresource extract used. However, further investigations are needed to elucidate this fact.
It is possible to encapsulate the bio-AgNPs in another matrix made of chitosan, for instance; however, this does not always ensure the newly designed AgNP-based formulation any substantial advantages over the other AgNP-free formulations in terms of scavenging activity or cytotoxic effects on cancer cells [334]. In other instances, the post-functionalization greatly improves the biocompatibility of the bio-AgNPs without affecting their anticancer efficacy [299].
Several groups have investigated the mode of action of bio-AgNPs against various cancer cell lines [335,336,337,338,339,340]. This action may result in morphological alterations, DNA fragmentation, ROS generation, impairment of mitochondria function, gene and protein down-/up-regulation, and, ultimately, induction of apoptotic pathways [153,280,321,341,342,343,344]. For instance, bio-AgNPs, produced using the marine alga Chaetomorpha linum, act via an apoptotic pathway since they increase, on one hand, the expression of apoptotic proteins, such as caspase 3 and 9, and Bax, while, on the other hand, they decrease the expression of the anti-apoptotic proteins Bcl-2 and Bcl-xl, yielding, among other effects, mitochondrial dysfunction that results in the apoptosis of the bio-AgNP-treated HCT-116 cancer cell line [324]. Several studies corroborate these findings [303,344]. Besides clearly showing their modulation of gene expression, other studies reveal DNA fragmentation and nucleus condensation, thus, highlighting an apoptotic cell death as a consequence of bio-AgNP exposure [312,345]. Flow cytometry analyses indicate that the increase in bio-AgNP concentration impacts the viability of cancer cells as the proportion of dying cells increases with a clear shift towards late apoptosis and necrosis [346]. Moreover, bio-AgNPs downregulate the oncogenes PIK3Ca and KRAS [347]. The exposure to bio-AgNPs eventually leads to apoptosis as it triggers, in a dose-dependent manner, the impairment of cellular membranes and increased lactate dehydrogenase leakage; it also impairs the mitochondrial function indicated, for instance, by greater levels of ROS and malondialdehyde, when compared to untreated controls [314]. Interestingly, genes related to oxidation-reduction pathways are upregulated, especially the ones coding for cytochrome P450 monooxygenases; on the other hand, genes linked to aging are upregulated [314]. bio-AgNPs, obtained using the aqueous leaf extract of Eucalyptus globulus, are also able to inhibit the formation of cancer cell colonies [332].
Besides their intrinsic, rich biological activity, bio-AgNPs may also be used as a radiosensitizing agent that absorbs γ-rays [348]. Indeed, a 6-Gy dose of γ-rays reduced the viability of HepG-2 cells treated with bio-AgNPs synthesized using the leaf aqueous extract of Picrasma quassioides, by more than 90%. These findings may suggest the accumulation of these bio-AgNPs in the vicinity of the cells and/or their internalization owing to a passive accumulation process since no targeting functionalization is carried out. The in vivo anticancer activity is also found in biogenic NPs that are made of a mixture of silver and silver chloride [349,350]. On a cellular level, these NPs exert their toxicity by altering the expression of genes that eventually leads to apoptosis.
Several groups have taken a step forward by investigating the efficacy of bio-AgNPs in combating solid tumors in animal models. Bacterially synthesized bio-AgNPs were used at 500 nM via intraperitoneal (IP) injection for 15 days to monitor their effect on solid xenograft in female Swiss albino mice in addition to a thorough in vitro investigation [288]. First, it was confirmed that these purified, endotoxin-free bio-AgNPs had no adverse side effects on healthy mice, thus highlighting their biocompatibility. These findings were later corroborated by other studies [196,197,198,351]. Second, mice treated with bio-AgNPs had a longer survival time when compared to their control counterparts (32 vs. 18 days) and saw their tumor volume dramatically recede by two thirds (2.6 mL vs. 7.3 mL for the control) [288]. Third, only minor alterations in hematological parameters were observed among tumor-bearing mice that were treated with bio-AgNPs. This study suggests that bio-AgNPs naturally accumulate in tumors without the need for any further post-functionalization step to provide them with stealth and targeting properties. These early findings were corroborated by He al., who used the peel powder of the longan fruit to synthesize bio-AgNPs for IP injection in mice settling on 10 µg/g body weight as the working concentration [340]. bio-AgNPs slowed down the growth of the tumor and decreased its volume, when compared to the control group. On day 36, the tumor size had receded by more than a half in treated mice. Again, toxicity tests indicate that bio-AgNPs efficiently and preferentially target and accumulate in the tumor without any need for post-functionalization. This almost intrinsic and unique feature of bio-AgNPs is time-dependent since, for instance, the very weak Raman imaging signal, recorded 5 min after inoculation, becomes extremely strong when taken at 45 min [198].
bio-AgNPs can be administered via several modes: intraperitoneally (IP) [288], intratumorally [198], intravesically [352], intravenously [174], and orally [156,353]. Usually, bio-AgNPs are quite biocompatible [165,195,197,288,351] and seem to preferentially exert their major cytotoxic effects almost exclusively on cancer cells and tumors via oxidative stress [165,198,353] and/or inhibition of angiogenesis [171]. Therefore, several biochemical parameters, including the activity of specific enzymes, up- and down-regulation of genes, immunological markers, and hematological and cellular parameters, are screened by investigators [155,156,174,353]. Usually, the translation to in vivo studies confirms the up- and down-regulation of genes that are already implicated in in vitro studies [324,353,354]. The in vivo anticancer activity of bio-AgNPs is dose-dependent [355]; some biochemical and histological parameters of AgNP-treated tumor-bearing mice may, at first, undergo some changes before reverting to normal values [356].
Lab-extracted and -purified polysaccharides (PS) are utilized to synthesize AgNPs that exhibit unique anticancer efficacy in animal models [198,357]. For instance, galactoxyloglucan-coated bio-AgNPs (PS-AgNPs) do not induce any behavioral changes or noticeable toxicity in healthy male BALB/c mice after administration at a 1.1 µg/g body weight [198]. Biochemical and hematological analyses confirm the absence of any abnormalities for the tested concentrations. Moreover, histological analyses indicate that the vital organs, i.e., heart, kidney, liver, spleen, lungs, remain unaffected by PS-AgNP IP inoculation although some moderate alterations are observed at the highest tested concentration (222 µg/g body weight) [198]. All these findings highlight the biocompatibility of these bio-AgNPs that is ensured, most likely, by the coating polysaccharides. When tested on tumor-bearing mice, PS-AgNPs give the best results in terms of all screened parameters: reduction in tumor volume, maintenance of body weight, lowering tumor cell count, diminution of percentage of viable cells, and increase in survival time (Figure 6). In addition, no systemic toxicity is recorded. Owing to their optical properties, the biodistribution of these IP injected PS-AgNPs is monitored in the tumor, blood and vital organs of treated mice using a confocal Raman microscope and found to evolve with time. While the PS-AgNP concentration in blood decreases over time, it increases in the tumor to reach its maximum 4 h after inoculation, then decreases. A similar trend is recorded for the kidney. The amount of PS-AgNPs steadily increases for the screened 6 h period. For the other organs, only tiny amounts of PS-AgNPs are detected after 6 h. Except for kidney and lungs, these results are corroborated by fluorescence analyses [198].
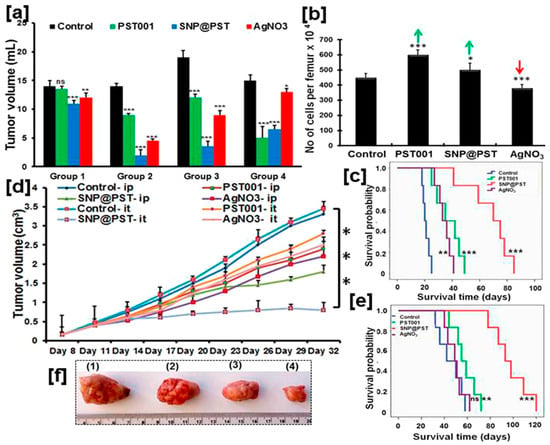
Figure 6.
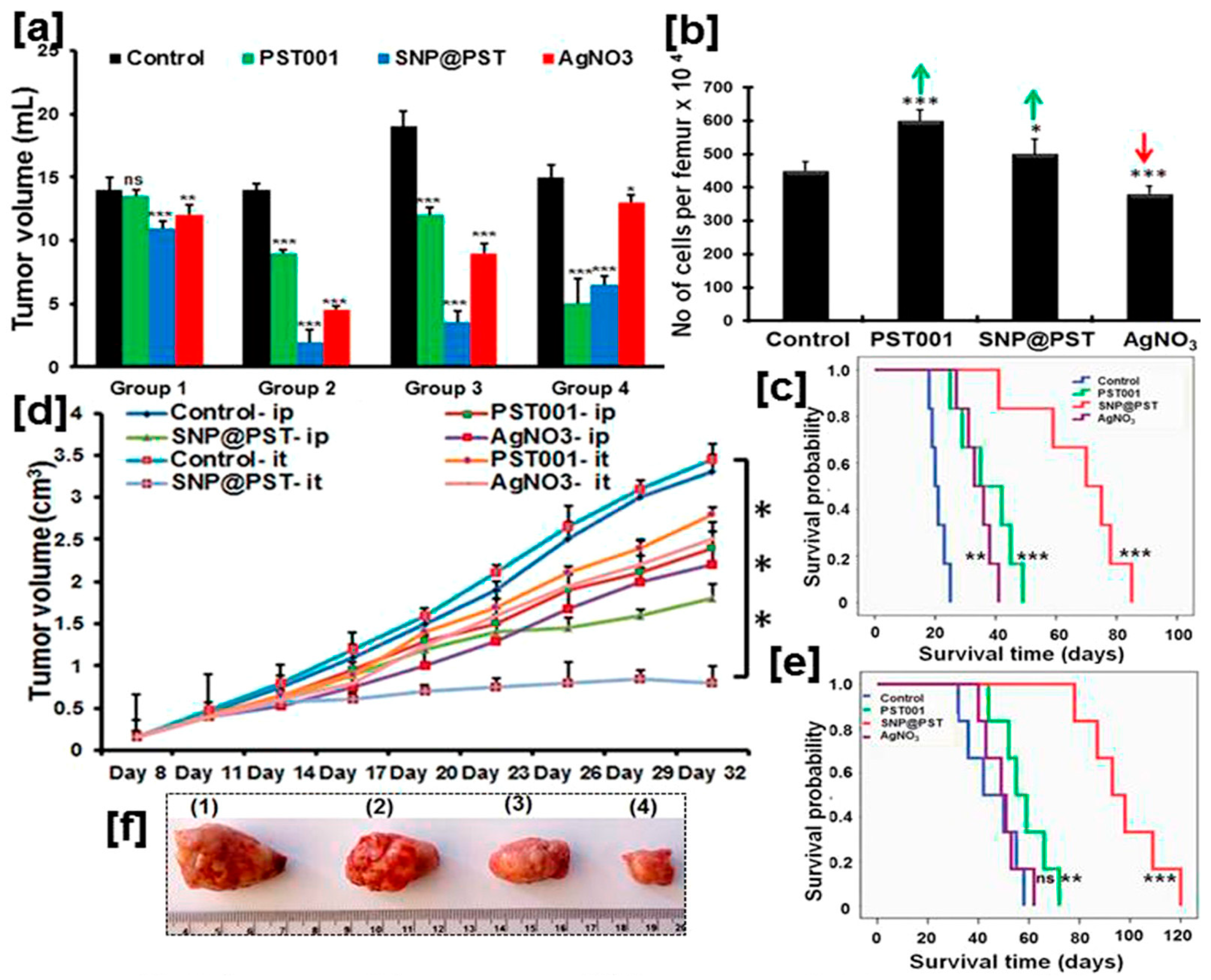
Antineoplastic activity of bio-AgNPs synthesized using the lab-purified polysaccharide PST001 galactoxyloglucan (SNP@PST). (a) Volume measurements in female BALB/c mice bearing Dalton’s Lymphoma ascites (DLA) xenograft. Groups 1 to 3 are inoculated with DLA on Day 1 while Group 4 is injected on Day 8. Regarding the treatment experiments, Group 1 is treated only once (Day 2), Group 2 is injected on Days 2 to 15, Group 3 is treated on Days 9 to 22, and Group 4 is injected on Days 1 to 7. (b) Quantification of bone marrow cellularity in Group 2. Green arrows indicate increase in cell number vs. the control; on the other hand, the red arrow indicates a decrease in cell number vs. the control. (c) Survival rate of Group 2 mice bearing DLA xenograft over time as a function of administered compounds. (d) Tumor volume measurements in mice bearing Ehrlich ascites carcinoma (EAC)-induced tumor syngraft as a function of received compound and mode of administration (ip = intraperitoneal administration; it = intratumoral administration). (e) Survival rate of Group 3 mice bearing EAC syngraft over time as a function of it-injected compounds. For (c–e), the results are expressed as the mean ± the standard deviation. Statistical significance is denoted as * p < 0.05, ** p < 0.01, *** p < 0.001, and ns (nonsignificant), all compared with the control group. (f) Images of resected solid tumors from mice that are it treated: (1) Control, (2) PST, (3) AgNO3, and (4) SNP@PST. Adapted from Ref. [198] with permission from the American Chemical Society.
Only a few papers have compared the in vivo anticancer efficacy of bio-AgNPs vs. conventional chemotherapy drugs, such as doxorubicin (DOX) [174,354], and cisplatin (cis-Pt) [329]. A few other papers carried out the same work using silymarin, a compound extracted from the plant milk thistle (Silybum marianum) that is sold as a dietary supplement and supposed to have an anticancer activity [155,156,327]. When silymarin was used at a 30 mg/g concentration, no statistically significant differences were observed for almost all the screened parameters when compared to doses of 20 or 30 mg/g of bio-AgNPs, obtained using the aqueous extract of leaves of the officinal plant Carissa caranda [155]. Similar trends were observed when the aqueous extracts of Madhuca longifolia [156] or of Ziziphus mauritiana were used [327]. Regarding DOX, minor to non-significant differences were reported for the studied hematological, cellular, immunological, and general patient parameters when compared to bio-AgNPs [174]. On the contrary, it was found that administered bio-AgNPs provide a better protection against the side effects of DOX, owing to the NP antioxidant activity [358]. Importantly, synergistic improvements were obtained by combining DOX and bio-AgNPs in terms of enhanced therapeutic effects and better protection against the side effects [354]. Moreover, comparable results were reported using cis-Pt instead of DOX [329].
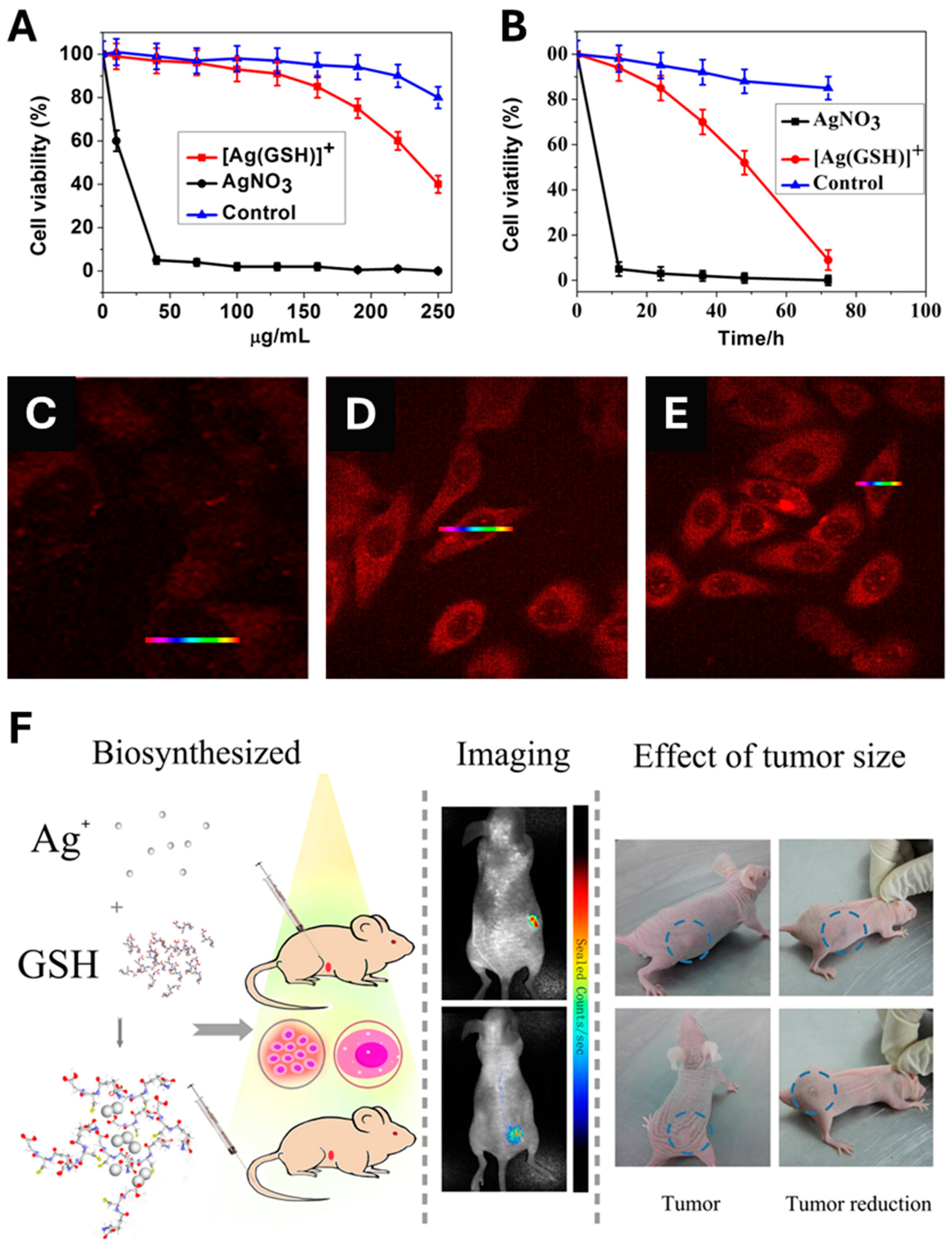
In contrast to the above-cited papers that typically, first, describe the biosynthesis of AgNPs; then, followed by their administration to finally monitor their in vivo anticancer activity, Gao et al. designed an original, single-step method for the in vivo synthesis of bio-AgNPs and their subsequent in vivo utilization [359]. Prior to the in vivo experiment, a comparative in vitro toxicity study of silver nitrate vs. a complex made by mixing silver nitrate and glutathione ([Ag(GSH)]+) was performed. As a result, [Ag(GSH)]+ shows no toxicity towards normal cells at all tested concentrations since the lowest cell viability exceeds 80% for the highest concentration tested (250 µg/mL) (Figure 7A). On the contrary, [Ag(GSH)]+ exerts a higher toxicity towards HeLa cancer cell line (Figure 7A). In addition, normal cells display a viability that exceeds 80% when challenged by 250 µg/mL of [Ag(GSH)]+ for 3 days while HeLa cells undergo a dramatic loss of viability (Figure 7B). Moreover, [Ag(GSH)]+ is found to penetrate cancer cells, thus offering the opportunity to implement near-infrared fluorescence imaging (NIR) whose intensity is dose-dependent (Figure 7C–E). Finally, the injection of [Ag(GSH)]+ into mice bearing xenograft tumors either via the tail vein or directly into the tumor yields the formation of silver nanoclusters (AgNCs), which exclusively occur within the tumor; this was confirmed by ex vivo analyses that found no presence of AgNCs in other parts of the body (Figure 7C–E). Owing to their NIR imaging features, these AgNCs allowed tumor imaging within a few hours after the injection of the complex (Figure 7F). Importantly, the tumors of mice treated with [Ag(GSH)]+ gradually receded until their total disappearance. Knowing their preferential accumulation in tumors, it is also possible to use bio-AgNPs, made using the ethanolic extract of Zinnia elegans, to implement NIR imaging and follow their biodistribution over time in tumors and vital organs, including the brain since the extract is known to accumulate in this organ [360].
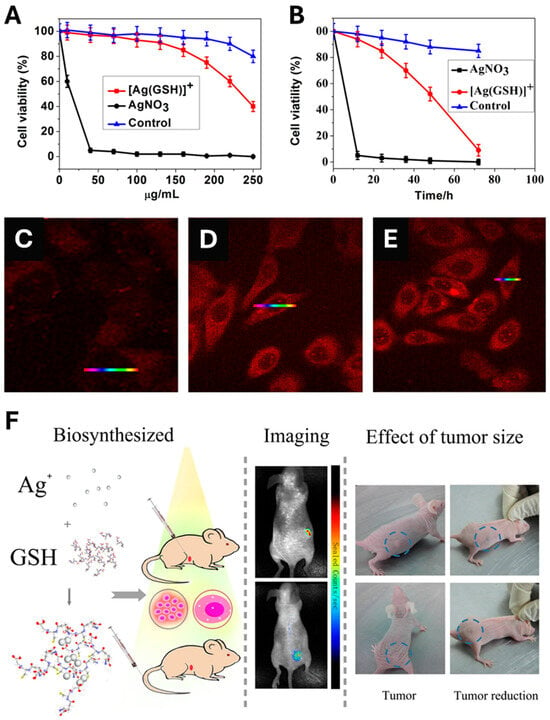
Figure 7.
(A) Cytotoxicity of AgNO3 vs. [Ag(GSH)]+ (a silver derivative made of cationic silver and glutathione) at various concentrations towards HeLa cancer cells. The control is made of L02 normal cell line challenged by [Ag(GSH)]+. (B) Time-dependent toxicity of AgNO3 at 40 μg/mL and [Ag(GSH)]+ at 130 μg/mL towards HeLa cancer cells. The control is made of L02 normal cell line challenged by [Ag(GSH)]+ at 130 μg/mL. (C–E) Intracellular formation and accumulation of silver nanoclusters (AgNCs) within HeLa cancer cells as a function of administered concentration of [Ag(GSH)]+: (C) 0 μg/mL, (D) 40 μg/mL, and (E) 100 μg/mL. (F) A schematic depicting the in vivo formation of AgNCs in mice bearing tumor xenograft to which [Ag(GSH)]+ is injected either directly to the tumor or in the tail vein. AgNCs accumulate in the tumor which allows the tumor in vivo bioimaging owing to AgNC fluorescence and monitor the tumor size reduction owing to the action of the same AgNCs. Reproduced from Ref. [359] with permission from Springer Nature under the Creative Commons CC-BY-NC-SA license.
In sum, bio-AgNPs are emerging as a potential anticancer therapeutic because of their preferential toxicity against tumors, in addition to their facile synthesis and low cost. Although most studies that report these significant anticancer effects of bio-AgNPs have been performed in vitro and much fewer in vivo animal models, there is hope that clinical therapeutic application of bio-AgNPs in the near future will position them as valuable agents in the fight against several human cancers [361], taking full advantage of the paradox of AgNP toxicity for cancerous and inertness for normal cells.
5.4. Exploitation of bio-AgNPs in Antibiofilm Action, Wound Healing and as Implant Coating
Antibiotics have gradually lost their activity due to their increasing and inappropriate use, leading to a major worldwide health concern as several strains of pathogenic bacteria do not respond to any available antibiotherapy following the evolutionary development of antibiotic resistance [362,363,364]. In addition to quorum sensing by which bacteria pass the information to their neighbors on circumventing the activity of antibiotics, microorganisms form biofilms in almost all environments which enable them to develop virulence factors and survive even in the presence of high concentrations of antibiotics [363,365,366]. Bacterial biofilms are structured clusters of cells or colonies embedded within a polymeric extracellular matrix and attached to a surface; this matrix acts as a barrier that hinders the action of host immune cells and the delivery of antibiotics should these bacteria be still active, therefore providing them protection by creating the suitable conditions for their survival, proliferation and spreading towards other parts of the body [367].
The presence of opportunistic pathogens in the form of variously structured biofilms is also a hallmark of most wounds, including chronic ones and others associated with burns or specific pathologies, such as diabetes. It is in these situations, research on the use of antimicrobial NPs, including AgNPs, has shown significant promise for combating infection and leading to wound healing. Choosing the right NP-based treatment depends on the wound’s specific requirements and biofilm type. Integrating NPs into wound dressings can improve therapeutic outcomes and reduce antibiotic use, thus addressing the serious challenge of antibiotic-tolerant infections. Ultimately, these strategies aim to replace or greatly lower antibiotic doses for managing biofilm infections [368].
Similarly, various commercially available accessories and devices improve the patients’ quality of life, such as prostheses and implants used to repair teeth, bones, and limbs, reestablish human mechanical functions, and improve mobility, comfort, and autonomy [369]. However, microbial infections and the subsequent biofilm formation on their surface constitute serious concerns as they potentially activate the patient’s immune response, induce implant rejection, and even cause septicemia in the most severe cases [370,371]. This can prove insurmountable with current antibiotics, necessitating the replacement of the implants/prostheses [372]. To prevent any biofouling, surface colonization and biofilm formation, the most widespread and relevant solution consists of the surface modification of implants and prostheses using coatings made of antibiotic-loaded polymers or inorganic NPs [372,373]. Among the latter, bio-AgNPs appear as a suitable and versatile solution thanks to the ease of their synthesis and casting on surfaces, and the large spectrum of their sustained biological activity against several microorganisms, including both gram+ and—bacteria, yeast, and fungi [294,374,375]. bio-AgNPs are also attracting interest to be incorporated into water filtering systems [376,377,378].
Given their well-documented and outstanding biocidal activity, several methodologies enable the exploitation of bio-AgNPs in the battle against the spread of pathogenic microorganisms and emerging health complications [294,379]. Achieving the same performance as their chemically synthesized analogs [380], bio-AgNPs could be directly cast on the surface of implants and prostheses since they prevent biofilm formation and inhibit microbial spreading [381,382,383,384]. They can also be incorporated into membranes, matrices, composites, and wound dressings [14,385]. Lastly, bio-AgNPs can also be topically applied to wounds and burns [386].
The antimicrobial activity of bio-AgNPs is size-dependent [351] and may stem from several factors, such as ROS generation, disruption of the cell membrane, protein denaturation and/or leakage, and cell lysis [159,387,388,389]. Spherical bio-AgNPs, prepared from Miscanthus khasiana plant extract and self-assembled within amine monolayers inhibit bacterial adhesion and biofilm formation of P. aeruginosa by reducing its viability by 67% [390]. bio-AgNPs prepared using the officinal plant Tinospora cordifolia reach 83% biofilm reduction in S. aureus, exhibiting a higher performance when compared to the antibiotic tetracycline or the extract used from the same plant [391]. Importantly, this effect lasts in time even after the removal of the NP treatment. Owing to ROS generation and impairment of cell membrane integrity, bio-AgNPs prepared using Anabaena variabilis cell extract disrupt Candida albicans biofilms in a dose-dependent manner [392]. In addition, a synergistic effect is observed by combining the same NPs with antibiotics [393]. Concretely, this drastically lowers the minimal inhibitory concentrations (MIC) of both the bio-AgNPs and antibiotics used to combat the tested bacterial and yeast strains, although its extent remains species- and strain-dependent. For instance, the MIC of bio-AgNPs and amphotericin B are, respectively, 12.5 and 3.12 µg/mL when used separately against C. albicans. It falls to 3.12 and 0.09 µg/mL, respectively, when combined. In the case of P. aeruginosa, the MIC plummets from 6.25 and 2.3 µg/mL of bio-AgNPs and streptomycin, respectively, when used separately, to 1.56 and 0.39 µg/mL of bio-AgNPs and streptomycin, respectively, when used in combination. Other studies confirm the observed synergistic effect [181,303,394,395,396]. A similar synergy is obtained by combining bio-AgNPs with peptides [397], metabolites [398] or a biosurfactant [399]. In all cited studies, the antimicrobial activity of bio-AgNPs is always and significantly much higher than that of the extract used [325,391,396,400]. Moreover, no clear trend is observed regarding the antibacterial effect of bio-AgNPs vs. antibiotics in general, since contradictory results have been reported [294,391,401]. However, a clear tendency advocates for bio-AgNPs in terms of antimicrobial efficacy when compared to their chemical/commercial counterparts [192,399,402].
bio-AgNPs may be topically applied as ingredients of formulations for different types of wounds aiming to accelerate the in vivo healing process by combating microbial infections and reducing inflammation [403,404,405,406]. Importantly, this healing efficacy may equal that of the conventional medication, BetadineTM [407]. The same healing effect is achieved when bio-AgNPs are part of hydrogels applied in rats bearing 6 mm diameter excision wounds [408]. A similar effect is obtained when bio-AgNPs enter the composition of an ointment used to treat induced burn wounds in rats by efficiently reducing the wound size, decreasing the epidermis layer, and lowering mast cell migration due to the proper regulation of inflammatory factors [167]. This healing effect of bio-AgNPs is corroborated in rats bearing not only an induced wound but also subjected to P. aeruginosa infection [405,409,410]. It is also possible to reach a synergistic effect by combining bio-AgNPs with the low-molecular-weight heparin enoxaparin (Enox) [411]. As displayed in Figure 8A, the best wound contraction was attained using bio-AgNPs + Enox (95%) when compared to the control (55%), bio-AgNPs alone (89%), and Enox alone (91%). In addition, the silver concentration in explored organs (liver, kidney, lung, and spleen) was lower in the group treated with bio-AgNPs + Enox than with bio-AgNPs (Figure 8B). An opposite trend was observed for skin until day 7 following the burn. On the other hand, silver concentration in blood was very low for both formulations, suggesting an elimination process that involves liver and spleen.
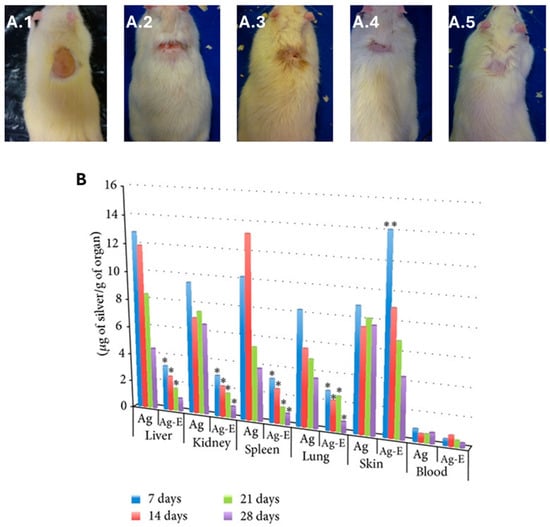
Figure 8.
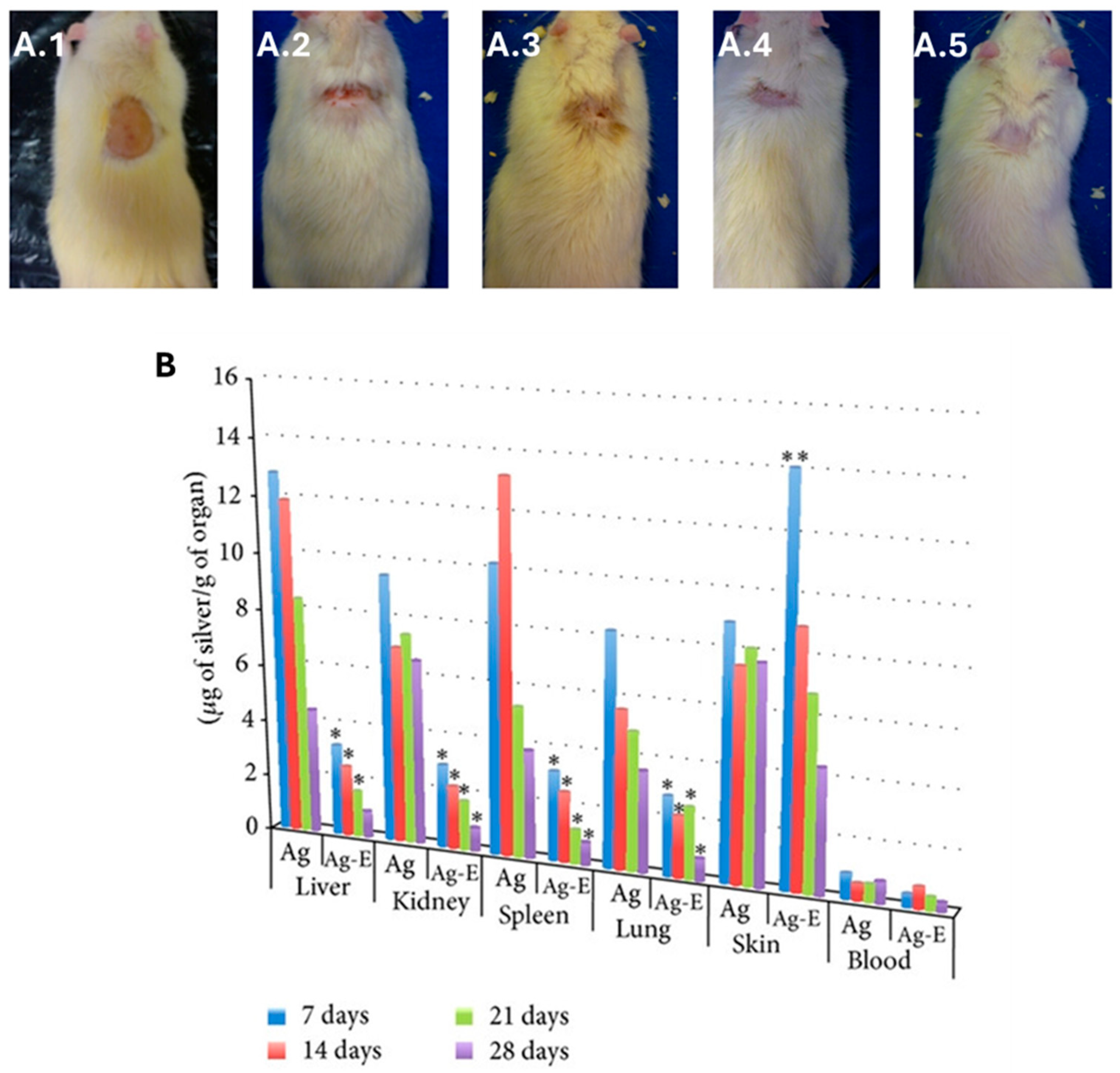
(A) Synergistic effect of bio-AgNPs, synthesized using the aqueous filtrate of Fusarium oxysporum, and Enoxaparin (Enox), an anticoagulant drug, on wound healing in male Wistar rats subjected to thermal injury: (A.1) provoked burn injury; (A.2) control rat, untreated after 28 days of burn injury; (A.3) bio-AgNP-treated rat after 28 days of burn injury; (A.4) Enox-treated rat after 28 days of burn injury; and (A.5) bio-AgNP + Enox-treated rat after 28 days of burn injury. (B) Silver concentration monitoring at different times in organs in rats treated with bio-AgNPs (Ag) and with bio-AgNP + Enox (Ag-E). Statistical significance is denoted as * p ≤ 0.001 for AgNP-Enox vs. AgNPs, ** p ≤ 0.01 for AgNP-Enox vs. AgNPs. Adapted from Ref. [411] with permission from Wiley under Creative Commons CC BY 3.0.
In addition to the previously mentioned antimicrobial effects of AgNPs via ROS generation and cell membrane disruption, the facilitation of Ag+ release by wound exudates may lead to the disruption of DNA replication and death of the microbial pathogens [412].
The effect of the specific AgNP formulation (e.g., in terms of polymeric matrix, colloidal status, etc.) on antibiofilm and wound-healing applications has been studied recently, indicating significant potential, as can be seen from the following examples. Chitosan-stabilized AgNPs tested against S. aureus and P. aeruginosa biofilms led to biofilm reduction by 48% and 78%, respectively [413]. Colloidal AgNPs tested in patients with infections by methicillin-sensitive and methicillin-resistant S. aureus (MSSA, MRSA), Enterococcus faecalis, and vancomycin-resistant Enterococci (VRE) were found to inhibit biofilm formation in the low, medium, and high biofilm producers by 91%, 83%, and 75%, respectively, when applied at the highest concentration of 52 ppm [414].
Casting or grafting bio-AgNPs onto surfaces constitutes an explored approach in combating biofilm formation and microbial colonization of surfaces, surgical sutures, catheters and wounds [415,416,417,418]. For instance, the efficacy of bio-AgNPs deposited on alumina disks in preventing colony formation is improved thanks to the coupling action of the extract used to produce them [381]. This resulted in a less wettable and very rough surface that reduced the number of colony formation units (CFUs) by 90–99.9% for the microbial isolates tested when compared to the bare alumina disks. On the other hand, the modified surface maintains a valuable biocompatibility towards human cell lines. These findings showcase the potential of bio-AgNPs in the design of antibiofouling yet biocompatible materials. Other findings show that coating a dental acrylic resin with bio-AgNPs yields a significant reduction in C. albicans biofilm formation [419]. It is also possible to modify cotton and polyester fabrics with bio-AgNPs to endow them with a large spectrum biocidal activity while maintaining, at the same time, a good biocompatibility towards moth larvae [420]. Interestingly, cotton fibers impregnated with bio-AgNPs maintain their biocidal activity against yeast and bacteria even after repeated wash cycles [421]. Other findings demonstrate that a firm anchoring of bio-AgNPs in a bandage gives better results regarding the wound healing activity [422]. In contrast to the above encouraging studies, there are also recent reports raising doubts about the presumed generality of AgNP antimicrobial and wound healing efficacy, depending on the context, with some works even suggesting the development of Ag resistance. For instance, an adverse effect of Ag in wound healing due to DNA damage of local skin and immune cells was recently reported [98]. The basis for potential tolerance of Ag by some pathogens goes back to the discovery in 2009 of Ag resistance genes in methicillin-resistant S. aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci (MR-CNS) isolated from wound and nasal sources [423]. Therefore, there is a real need for further context-focused research into AgNP-based antibiofilm and wound healing technologies.
6. Environmental Applications of bio-AgNPs
Environmental pollution is one of the biggest global issues due to the damage it causes to ecosystems and human health. Amongst its numerous sources, there are industries that discharge substantial amounts of contaminants like heavy metals, oils and dyes [424]. Industrial effluents, carrying heavy metals, such as Pb2+, Zn2+, Cu2+, Hg2+ and Ni2+, directly and indirectly commingle with both groundwater and surface water sources, endangering flora and fauna [425,426]. Different methods, such as chemical precipitation, adsorption, ion exchange, membrane filtration and electrolytic methods, have been developed for the removal of these contaminants [426,427]. Despite their efficiency in removing contaminants, most of these methods are neither eco-friendly nor can they remove all types of contaminants, not to mention the emerging ones, which are often harmful at very low concentrations (micropollutants). Hence, the use of metallic NPs appears as an alternative solution, thanks to their properties like high affinity, specificity, and a large surface area. NPs possess a high affinity to many types of contaminants, especially in water and wastewater [427]. Various NPs including AgNPs are currently being used in water and wastewater treatment technologies not only for their efficacy but also for their sustainability and eco-friendliness, especially when they are produced using benign biological sources. As pointed out above, the major advantages of the biosynthesized AgNPs are their high surface area for binding and specific affinity for metallic ions [424]. As a consequence of their superior qualities, bio-AgNPs are often used in combination with other agents, such as organic materials, membranes, fibers, and alloys to widen the spectrum of their environmental applications [36]. For instance, studies on hybrid composites made of bio-AgNPs and cellulose show that when used as a coated filter paper with the correct concentration, they can attain up to 100% removal of E. coli, thus proving to be an easy-to-use material in emergency antibacterial water filters [428].
Nowadays, the main problem with conventional water purification or disinfection using chemicals consists of the generation of potentially ecotoxic disinfection by-products, commonly referred to as DBPs [429]. Given their antimicrobial properties, bio-AgNPs appear to be effective water disinfectants, hence avoiding the generation of the harmful DBPs [430]. One of the novel uses of bio-AgNPs in water treatment lies in membrane technology. bio-AgNPs can significantly modify the membrane properties, leading to enhanced performance. For example, Wu et al. produced bio-AgNPs by reducing and capping cationic silver ions using bacteria [431]. Then, they embedded the resulting bio-AgNPs within polysulfone substrate to prepare a thin-film composite toward osmosis membranes, which resulted in refined porosity, greater water flux, enhanced surface hydrophilicity, and better antibacterial and antifouling properties compared to their pristine counterpart. Moreover, silver nanocomposite-activated carbons are currently being researched for the treatment of emerging drinking water contaminants, such as per- and poly-fluoroalkyl substances (PFAS) [432]. Known as “forever chemicals” for their persistence in the environment, these chemicals are major human health concerns owing to their toxicity and bioaccumulation potential [433]. Although specific studies pertaining to the use of biogenic nanomaterials including AgNPs for treating PFAS are still unavailable, the need for eco-friendly biogenic treatment approaches is critical as more of these chemicals emerge into the environment. In addition to their use in treatment technologies, bio-AgNPs have been widely used in sensor systems to monitor water pollution [434,435]. The straightforward and versatile surface functionalization of bio-AgNPs enables their selective use for specific analytes within sensor systems. Various sizes of bio-AgNPs with various morphologies and surface functionalities have been studied for water pollution monitoring and treatments (Table 1).

Table 1.
Removal of environmental pollutants using bio-AgNPs.
In addition to drinking water treatment, bio-AgNPs have found their way into wastewater treatment [450]. For example, bio-AgNPs are effective in removing major industrial contaminants, including toxic chemicals from textile wastewater [446,447]. Other applications rely on the catalytic properties of the bio-AgNPs to speed up the rate of reduction of selective textile-industry dyes and for the efficient removal of toxic non-biodegradable organic dyes, respectively [438,447]. Moreover, bio-AgNPs produced using two classes of fungi (Penicillium citreonigrum and Scopulariopsis brumptii) are used as an antimicrobial agent for the low-cost removal of pathogens from wastewater [451]. Since bio-AgNPs are produced using various natural bioresources, this eliminates the use of chemicals as reducing or stabilizing agents.
The rapid and precise detection of highly toxic heavy metals, such as As, Cd, Pb, Hg, Cr, and their derivatives, is strongly enabled using bio-AgNP-based sensors [38]. Furthermore, the simplicity, accuracy, and low cost of bio-AgNP-based colorimetric/plasmonic nanosensors are major assets in the detection of many other environmental pollutants in water [452]. Although bio-AgNPs have seen their widest use in water and wastewater treatment, it is obvious that their use will continue to expand in other areas of environmental application. A recent study clearly demonstrated that bio-AgNPs reduce the stress in plants that arises from environmental pollution [453]. Therefore, bio-AgNPs can play a vital role by mitigating both the cause and the effect of the ongoing environmental pollution. In sum, it is worth observing that the surface functionalities of bio-AgNPs can lead towards a sustainable solution to water pollution.
7. Conclusions and Perspectives
As amply documented from this extensive critical survey, the status of AgNPs is becoming consolidated in a range of biological (including health-related) and environmental applications thanks to their distinctive characteristics, such as their tiny size and commensurate high surface-to-volume ratio, surface-modifying capacity, stability with low chemical reactivity, biocompatibility, and biosafety. These attributes, together with appropriate conditioning from green biological synthesis, are placing bio-AgNPs at center stage for biosensing, drug delivery, antimicrobial and cancer therapy, and pollution control, justifying the welcome paradox of these nano-objects’ specificity (e.g., towards pathogens or malignancies) together with their inertness for non-target organisms and tissues.
The remarkable properties of bio-AgNPs need to be explored further since, in combination with synergistic formulations, the range of their applications is constantly extended. Because of increasing human exposure, the issue of their biosafety is bound to be scrutinized more thoroughly, with suggestions for complete and systematic genotoxicological characterization of AgNPs [454]. A recent massive evaluation of hundreds of studies over the last 20 years on metal nanoparticles including AgNPs found that bio-AgNPs exhibit lower genotoxicity compared to those synthesized by conventional methodologies [455]. In this connection, the combination of AgNPs with selected conventional chemotherapeutics aiming at synergistic antiproliferative effects promises to offer, as a bonus, lower genotoxicity as shown recently by extensive nanotoxicological assessment of AgNPs combined with tamoxifen on breast tumor cells [456].
In the area of combatting wound infections through the beneficial action of bio-AgNPs, efforts will increase in several complementary directions, where AgNPs become components of integrated solutions that include composite nanomaterials, flexible nanoplatforms, and minimally invasive nanodevices. The intelligent targeting of infections caused by methicillin-resistant S. aureus (MRSA) with acidic biofilms and alkaline wound microenvironments has recently been achieved by devising an acid−base responsive bionic claw microneedle (MN) loaded with Au@ZnO/Ag (AZA) core−shell nanoparticles [457]. Wound healing was promoted through the synergy of released Zn2+/Ag+. The beneficial role of Ag+ is emerging as an essential part of “silver nanomix”, a highly cost-effective new-generation nanomaterial for wound dressing developed from the systematic hybridization of metallic AgNPs with ionic Ag [458]. On the other hand, the MN component of the wound-healing solution can be loaded with sophisticated nano-entities like silk fibroin microspheres (SFMs) in combination with AgNPs and antibiotics for simultaneous delivery into biofilms to facilitate synergistic therapeutic effects and eventually eradicate bacterial biofilm infections [459]. Non-antibiotic approaches to fight infection are generating novel multifunctional nanoplatforms, as illustrated by the combined introduction of pH-dependent and ROS-scavenging antibacterial, anti-inflammatory, and proangiogenic properties to promote wound healing by integrating allicin (Ac) into an AgNP-loaded zeolite imidazole framework collectively termed Ac@ZIF-8/AgNPs [460].
The path to clinical and industrial translation for bio-AgNPs is fraught with several severe technical and regulatory bottlenecks. An important issue is the reliable attainment of reproducibility and control of batch-to-batch variation. Since biogenic synthesis uses complex, often non-standardized biological resources like plants, microbes or their extracts, variations in the source material, pH, or temperature at scale may result in inconsistent particle size, shape, and surface chemistry, which, in turn, could affect the nanoparticles’ efficacy and safety. This variability is incompatible with the stringent rules mandated by regulatory bodies like the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), which require strict adherence to Good Manufacturing Practice (GMP) standards to ensure a consistent, well-characterized product. An additional issue is the cost of downstream processing towards purification of the clinical- or industrial-grade product. The various separation and purification steps to remove residual biological components and unreacted precursors from the crude biogenic nanoparticulate formulation may even diminish or eliminate the initial cost advantage over conventional synthesis routes. Addressing these challenges necessitates the establishment of standardized protocols, the implementation of advanced Process Analytical Technology (PAT) for real-time quality control, and the development of more economical, high-throughput purification methods compatible with clinical production or commercial biomanufacturing.
Finally, a new front in the quest for ever more efficient antimicrobial nanoparticles, including AgNPs, for bioapplications is the necessity to address concerns about the emergence of microbial resistance, which could compromise their effectiveness as innovative solutions replacing antibiotics. The development of targeted interventions addressing the putative evolutionary mechanisms of nanoresistance development in bacteria could lead to its practical elimination. In one recent example, the resensitization of bacteria against AgNPs was achieved via physicochemical strategies blocking energy supply and neutralizing bacterial intracellular acidic pH [461].
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huynh, K.H.; Pham, X.H.; Kim, J.; Lee, S.H.; Chang, H.; Rho, W.Y.; Jun, B.H. Synthesis, Properties, and Biological Applications of Metallic Alloy Nanoparticles. Int. J. Mol. Sci. 2020, 21, 5174. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Gibi, C.; Liu, C.H.; Barton, S.C.; Wu, J.J. Recent Progress in Morphology-Tuned Nanomaterials for the Electrochemical Detection of Heavy Metals. Nanomaterials 2022, 12, 3930. [Google Scholar] [CrossRef]
- Yanchatuña Aguayo, O.P.; Mouheb, L.; Villota Revelo, K.; Vásquez-Ucho, P.A.; Pawar, P.P.; Rahman, A.; Jeffryes, C.; Terencio, T.; Dahoumane, S.A. Biogenic Sulfur-Based Chalcogenide Nanocrystals: Methods of Fabrication, Mechanistic Aspects, and Bio-Applications. Molecules 2022, 27, 458. [Google Scholar] [CrossRef]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and inorganic nanomaterials: Fabrication, properties and applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Dash, K.K.; Deka, P.; Bangar, S.P.; Chaudhary, V.; Trif, M.; Rusu, A. Applications of Inorganic Nanoparticles in Food Packaging: A Comprehensive Review. Polymers 2022, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhuang, T.; Dong, J.; Wang, L.; Xia, J.; Wang, H.; Cui, X.; Wang, Z. Sonochemical fabrication of inorganic nanoparticles for applications in catalysis. Ultrason. Sonochem. 2021, 71, 105384. [Google Scholar] [CrossRef]
- Vijayaram, S.; Tsigkou, K.; Zuorro, A.; Sun, Y.Z.; Rabetafika, H.; Razafindralambo, H. Inorganic nanoparticles for use in aquaculture. Rev. Aquac. 2023, 15, 1600–1617. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, L.; Anika, F.; Riya, A.A.; Kali, S.K.; Rauf, A.; Sharma, R. Bioinorganic Nanoparticles for the Remediation of Environmental Pollution: Critical Appraisal and Potential Avenues. Bioinorg. Chem. Appl. 2023, 2023, 2409642. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, Y.J.; Im, G.B.; Zhu, J.; Wu, Y.; Liu, Y.; Bhang, S.H. Inorganic Nanoparticles Applied as Functional Therapeutics. Adv. Funct. Mater. 2020, 31, 2008171. [Google Scholar] [CrossRef]
- Irvine, J.; Rupp, J.L.M.; Liu, G.; Xu, X.; Haile, S.; Qian, X.; Snyder, A.; Freer, R.; Ekren, D.; Skinner, S.; et al. Roadmap on inorganic perovskites for energy applications. J. Phys. Energy 2021, 3, 031502. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Feng Chong, K.; Ali, G.A.M. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Simon, S.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, S.; Josephs, J.; Onani, M.O.; Meyer, M.; Madiehe, A.M. Biomedical Applications of Plant Extract-Synthesized Silver Nanoparticles. Biomedicines 2022, 10, 2792. [Google Scholar] [CrossRef]
- Rakib-Uz-Zaman, S.M.; Hoque Apu, E.; Muntasir, M.N.; Mowna, S.A.; Khanom, M.G.; Jahan, S.S.; Akter, N.; Khan, M.A.R.; Shuborna, N.S.; Shams, S.M.; et al. Biosynthesis of Silver Nanoparticles from Cymbopogon citratus Leaf Extract and Evaluation of Their Antimicrobial Properties. Challenges 2022, 13, 18. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Hikal, W.M.; Bratovcic, A.; Baeshen, R.S.; Tkachenko, K.G.; Said-Al Ahl, H.A.H. Nanobiotechnology for the Detection and Control of Waterborne Parasites. Open J. Ecol. 2021, 11, 203–223. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Boddu, V.M.; Chadha, N.K.; Chakraborty, P.; Kumar, J.; Krishna, G.; Pathak, H. Metallic and non-metallic nanoparticles from plant, animal, and fisheries wastes: Potential and valorization for application in agriculture. Environ. Sci. Pollut. Res. Int. 2022, 29, 81130–81165. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.R.; Vindigni, V.; Azzena, B.; Barbante, C.; et al. Active silver nanoparticles for wound healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef]
- Haugen, H.J.; Makhtari, S.; Ahmadi, S.; Hussain, B. The Antibacterial and Cytotoxic Effects of Silver Nanoparticles Coated Titanium Implants: A Narrative Review. Materials 2022, 15, 5025. [Google Scholar] [CrossRef]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef]
- Liu, Y.; Miyoshi, H.; Nakamura, M. Nanomedicine for drug delivery and imaging: A promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int. J. Cancer 2007, 120, 2527–2537. [Google Scholar] [CrossRef]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The Future of Cancer Diagnosis and Therapy. CA Cancer J. Clin. 2018, 63, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Li, H.; Wang, J.; Gopinath, S.C.B. Silver nanoparticle in biosensor and bioimaging: Clinical perspectives. Biotechnol. Appl. Biochem. 2021, 68, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Kravets, V.; Almemar, Z.; Jiang, K.; Culhane, K.; Machado, R.; Hagen, G.; Kotko, A.; Dmytruk, I.; Spendier, K.; Pinchuk, A. Imaging of Biological Cells Using Luminescent Silver Nanoparticles. Nanoscale Res. Lett. 2016, 11, 30. [Google Scholar] [CrossRef]
- Shkilnyy, A.; Souce, M.; Dubois, P.; Warmont, F.; Saboungi, M.L.; Chourpa, I. Poly(ethylene glycol)-stabilized silver nanoparticles for bioanalytical applications of SERS spectroscopy. Analyst 2009, 134, 1868–1872. [Google Scholar] [CrossRef]
- Ibrahim, N.; Jamaluddin, N.D.; Tan, L.L.; Mohd Yusof, N.Y. A Review on the Development of Gold and Silver Nanoparticles-Based Biosensor as a Detection Strategy of Emerging and Pathogenic RNA Virus. Sensors 2021, 21, 5114. [Google Scholar] [CrossRef]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef]
- Ziai, Y.; Rinoldi, C.; Nakielski, P.; De Sio, L.; Pierini, F. Smart plasmonic hydrogels based on gold and silver nanoparticles for biosensing application. Curr. Opin. Biomed. Eng. 2022, 24, 100413. [Google Scholar] [CrossRef]
- Palani, G.; Trilaksana, H.; Sujatha, R.M.; Kannan, K.; Rajendran, S.; Korniejenko, K.; Nykiel, M.; Uthayakumar, M. Silver Nanoparticles for Waste Water Management. Molecules 2023, 28, 3520. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Z.; Li, P.; Gajaraj, S. Governing factors affecting the impacts of silver nanoparticles on wastewater treatment. Sci. Total Environ. 2016, 572, 852–873. [Google Scholar] [CrossRef] [PubMed]
- Sudarman, F.; Shiddiq, M.; Armynah, B.; Tahir, D. Silver nanoparticles (AgNPs) synthesis methods as heavy-metal sensors: A review. Int. J. Environ. Sci. Technol. 2023, 20, 9351–9368. [Google Scholar] [CrossRef]
- Eddy, N.O.; Garg, R.; Garg, R.; Ukpe, R.A.; Abugu, H. Adsorption and photodegradation of organic contaminants by silver nanoparticles: Isotherms, kinetics, and computational analysis. Environ. Monit. Assess. 2023, 196, 65. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Mussatto, A.; Ahad, I.U.I.; Mousavian, R.T.; Delaure, Y.; Brabazon, D. Advanced production routes for metal matrix composites. Eng. Rep. 2020, 3, e12330. [Google Scholar] [CrossRef]
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic Nanoparticles: Synthesis, Characterisation and Applications. Appl. Sci. 2021, 11, 2598. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Pereira, T.M.; Polez, V.L.P.; Sousa, M.H.; Silva, L.P. Modulating physical, chemical, and biological properties of silver nanoparticles obtained by green synthesis using different parts of the tree Handroanthus heptaphyllus (Vell.) Mattos. Colloid Interface Sci. Commun. 2020, 34, 100224. [Google Scholar] [CrossRef]
- Nqakala, Z.B.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Onani, M.O.; Madiehe, A.M. Advances in Nanotechnology towards Development of Silver Nanoparticle-Based Wound-Healing Agents. Int. J. Mol. Sci. 2021, 22, 11272. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Rahman, A.; Lin, J.; Jaramillo, F.E.; Bazylinski, D.A.; Jeffryes, C.; Dahoumane, S.A. In Vivo Biosynthesis of Inorganic Nanomaterials Using Eukaryotes—A Review. Molecules 2020, 25, 3246. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, L.M.; Zimmerman, J.B.; Plata, D.L.; Hutchison, J.E.; Anastas, P.T. Designing nanomaterials to maximize performance and minimize undesirable implications guided by the Principles of Green Chemistry. Chem. Soc. Rev. 2015, 44, 5758–5777. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, R.; Negi, B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Curr. Res. Green Sust. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Jeffryes, C.; Mechouet, M.; Agathos, S.N. Biosynthesis of Inorganic Nanoparticles: A Fresh Look at the Control of Shape, Size and Composition. Bioengineering 2017, 4, 14. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Biosynthetic Conversion of Ag+ to highly Stable Ag0 Nanoparticles by Wild Type and Cell Wall Deficient Strains of Chlamydomonas reinhardtii. Molecules 2018, 24, 198. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Lin, J.; Dahoumane, S.A.; Jeffryes, C. A Mechanistic View of the Light-Induced Synthesis of Silver Nanoparticles Using Extracellular Polymeric Substances of Chlamydomonas reinhardtii. Molecules 2019, 24, 3506. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Individual and Combined Effects of Extracellular Polymeric Substances and Whole Cell Components of Chlamydomonas reinhardtii on Silver Nanoparticle Synthesis and Stability. Molecules 2019, 24, 956. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Pineda, M.E.; Lizarazo Forero, L.M.; Sierra, Y.C.A. Mycosynthesis of silver nanoparticles: A review. Biometals 2023, 36, 745–776. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.G.; Ismail, G.A.; El-Gemizy, W.M.; Salem, M.A. Biosynthesis of silver nanoparticles by cell-free extracts from some bacteria species for dye removal from wastewater. Biotechnol. Lett. 2019, 41, 379–389. [Google Scholar] [CrossRef]
- Malik, M.; Aamir Iqbal, M.; Iqbal, Y.; Malik, M.; Bakhsh, S.; Irfan, S.; Ahmad, R.; Pham, P.V. Biosynthesis of silver nanoparticles for biomedical applications: A mini review. Inorg. Chem. Commun. 2022, 145, 109980. [Google Scholar] [CrossRef]
- Arif, R.; Uddin, R. A review on recent developments in the biosynthesis of silver nanoparticles and its biomedical applications. Med. Device Sens. 2020, 4, e10158. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Bamal, D.; Singh, A.; Chaudhary, G.; Kumar, M.; Singh, M.; Rani, N.; Mundlia, P.; Sehrawat, A.R. Silver Nanoparticles Biosynthesis, Characterization, Antimicrobial Activities, Applications, Cytotoxicity and Safety Issues: An Updated Review. Nanomaterials 2021, 11, 2086. [Google Scholar] [CrossRef]
- Kulkarni, N.; Muddapur, U. Biosynthesis of Metal Nanoparticles: A Review. J. Nanotechnol. 2014, 2014, 510246. [Google Scholar] [CrossRef]
- Moradi, F.; Sedaghat, S.; Moradi, O.; Arab Salmanabadi, S. Review on green nano-biosynthesis of silver nanoparticles and their biological activities: With an emphasis on medicinal plants. Inorg. Nano-Metal. Chem. 2020, 51, 133–142. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Ralte, V.; Zohmingliana, H.; Das, S.; Anal, J.M.H.; Lallianrawna, S.; Rokhum, S.L. A review of microbes mediated biosynthesis of silver nanoparticles and their enhanced antimicrobial activities. Heliyon 2024, 10, e32333. [Google Scholar] [CrossRef]
- Wahab, S.; Khan, T.; Adil, M.; Khan, A. Mechanistic aspects of plant-based silver nanoparticles against multi-drug resistant bacteria. Heliyon 2021, 7, e07448. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Improvement of kinetics, yield, and colloidal stability of biogenic gold nanoparticles using living cells of Euglena gracilis microalga. J. Nanopar Res. 2016, 18, 79. [Google Scholar] [CrossRef]
- Rajput, S.; Werezuk, R.; Lange, R.M.; McDermott, M.T. Fungal Isolate Optimized for Biogenesis of Silver Nanoparticles with Enhanced Colloidal Stability. Langmuir 2016, 32, 8688–8697. [Google Scholar] [CrossRef] [PubMed]
- AbdelRahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.; Husseiny, S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J. Biol. Sci. 2017, 24, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Biswas, S.; Mulaba-Bafubiandi, A.F. Optimization of process variables for the biosynthesis of silver nanoparticles by Aspergillus wentii using statistical experimental design. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045005. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Sun, D.; Lin, L.; Lin, W.; Li, J.; Jiang, X.; Wu, W.; Li, Q. Microfluidic biosynthesis of silver nanoparticles: Effect of process parameters on size distribution. Chem. Eng. J. 2012, 209, 568–576. [Google Scholar] [CrossRef]
- Manosalva, N.; Tortella, G.; Cristina Diez, M.; Schalchli, H.; Seabra, A.B.; Durán, N.; Rubilar, O. Green synthesis of silver nanoparticles: Effect of synthesis reaction parameters on antimicrobial activity. World J. Microbiol. Biotechnol. 2019, 35, 88. [Google Scholar] [CrossRef] [PubMed]
- Pourmortazavi, S.M.; Taghdiri, M.; Makari, V.; Rahimi-Nasrabadi, M. Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hong, Y.N.; Weyers, A.; Kim, Y.S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Sharma, A.; Sharma, B. Recent Advances in Biogenic Silver Nanoparticles for Their Biomedical Applications. Sustain. Chem. 2023, 4, 61–94. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Nain, R.; Patel, H.; Chahar, M.; Kumar, S.; Rohilla, D.; Pal, M. Biosynthesized metallic nanoparticles for sustainable environmental remediation: Mechanisms, applications, and future perspectives. Discov. Chem. 2025, 2, 124. [Google Scholar] [CrossRef]
- Pereira, L.; Mehboob, F.; Stams, A.J.; Mota, M.M.; Rijnaarts, H.H.; Alves, M.M. Metallic nanoparticles: Microbial synthesis and unique properties for biotechnological applications, bioavailability and biotransformation. Crit. Rev. Biotechnol. 2015, 35, 114–128. [Google Scholar] [CrossRef]
- Murillo-Rábago, E.I.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Garcia-Marin, L.E.; Quester, K.; Castro-Longoria, E. Optimized Synthesis of Small and Stable Silver Nanoparticles Using Intracellular and Extracellular Components of Fungi: An Alternative for Bacterial Inhibition. Antibiotics 2022, 11, 800. [Google Scholar] [CrossRef]
- Xie, J.; Lee, J.Y.; Wang, D.I.C.; Ting, Y.P. Silver Nanoplates: From Biological to Biomimetic Synthesis. ACS Nano 2007, 1, 429–439. [Google Scholar] [CrossRef]
- De Leersnyder, I.; De Gelder, L.; Van Driessche, I.; Vermeir, P. Revealing the Importance of Aging, Environment, Size and Stabilization Mechanisms on the Stability of Metal Nanoparticles: A Case Study for Silver Nanoparticles in a Minimally Defined and Complex Undefined Bacterial Growth Medium. Nanomaterials 2019, 9, 1684. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef]
- Parikh, R.Y.; Singh, S.; Prasad, B.L.; Patole, M.S.; Sastry, M.; Shouche, Y.S. Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella sp.: Towards understanding biochemical synthesis mechanism. Chembiochem 2008, 9, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Wujcik, E.K.; Jeffryes, C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzym. Microb. Technol. 2016, 95, 13–27. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Alvarez, F.J.; Agathos, S.N.; Jeffryes, C. Microalgae: An outstanding tool in nanotechnology. Bionatura 2016, 1, 196–201. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Djediat, C.; Yéprémian, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Species selection for the design of gold nanobioreactor by photosynthetic organisms. J. Nanopart Res. 2012, 14, 883. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Djediat, C.; Yepremian, C.; Coute, A.; Fievet, F.; Coradin, T.; Brayner, R. Recycling and adaptation of Klebsormidium flaccidum microalgae for the sustained production of gold nanoparticles. Biotechnol. Bioeng. 2012, 109, 284–288. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.M.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology—A review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. A global approach of the mechanism involved in the biosynthesis of gold colloids using micro-algae. J. Nanopart Res. 2014, 16, 2607. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Wijesekera, K.; Filipe, C.D.; Brennan, J.D. Stoichiometrically controlled production of bimetallic Gold-Silver alloy colloids using micro-alga cultures. J. Colloid Interface Sci. 2014, 416, 67–72. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An Overview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef]
- Javaid, A.; Oloketuyi, S.F.; Khan, M.M.; Khan, F. Diversity of Bacterial Synthesis of Silver Nanoparticles. BioNanoScience 2017, 8, 43–59. [Google Scholar] [CrossRef]
- Cunningham, B.; Engstrom, A.M.; Harper, B.J.; Harper, S.L.; Mackiewicz, M.R. Silver Nanoparticles Stable to Oxidation and Silver Ion Release Show Size-Dependent Toxicity In Vivo. Nanomaterials 2021, 11, 1516. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, S.; Han, J.; Wang, Z.; Zhang, S. On the developmental toxicity of silver nanoparticles. Mater. Des. 2021, 203, 109611. [Google Scholar] [CrossRef]
- Verkhovskii, R.; Kozlova, A.; Atkin, V.; Kamyshinsky, R.; Shulgina, T.; Nechaeva, O. Physical properties and cytotoxicity of silver nanoparticles under different polymeric stabilizers. Heliyon 2019, 5, e01305. [Google Scholar] [CrossRef] [PubMed]
- Nešporová, K.; Pavlík, V.; Šafránková, B.; Vágnerová, H.; Odráška, P.; Žídek, O.; Císařová, N.; Skoroplyas, S.; Kubala, L.; Velebný, V. Effects of wound dressings containing silver on skin and immune cells. Sci. Rep. 2020, 10, 15216. [Google Scholar] [CrossRef]
- Skalska, J.; Frontczak-Baniewicz, M.; Struzynska, L. Synaptic degeneration in rat brain after prolonged oral exposure to silver nanoparticles. Neurotoxicology 2015, 46, 145–154. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanopart Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Gibała, A.; Żeliszewska, P.; Gosiewski, T.; Krawczyk, A.; Duraczyńska, D.; Szaleniec, J.; Szaleniec, M.; Oćwieja, M. Antibacterial and Antifungal Properties of Silver Nanoparticles-Effect of a Surface-Stabilizing Agent. Biomolecules 2021, 11, 1481. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. Int. 2015, 22, 4122–4143. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Zhang, W.; Zhu, X.; Zhang, S. An updated overview of some factors that influence the biological effects of nanoparticles. Front. Bioeng. Biotechnol. 2023, 11, 1254861. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef]
- Kennedy, D.C.; Gies, V.; Jezierski, A.; Yang, L. Changes in the physical properties of silver nanoparticles in cell culture media mediate cellular toxicity and uptake. J. Nanopart Res. 2019, 21, 132. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, e1701503. [Google Scholar] [CrossRef]
- Khodashenas, B. The Influential Factors on Antibacterial Behaviour of Copper and Silver Nanoparticles. Indian Chem. Eng. 2015, 58, 224–239. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Durán, N.; Silveira, C.P.; Durán, M.; Martinez, D.S. Silver nanoparticle protein corona and toxicity: A mini-review. J. Nanobiotechnol. 2015, 13, 55. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organometal Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Rohde, M.M.; Snyder, C.M.; Sloop, J.; Solst, S.R.; Donati, G.L.; Spitz, D.R.; Furdui, C.M.; Singh, R. The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Part. Fibre Toxicol. 2021, 18, 37. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lian Wu, Y.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Li, V.; Shukla, R.; Bansal, V.; Nugegoda, D. The Toxicity of Silver Nanoparticles (AgNPs) to Three Freshwater Invertebrates with Different Life Strategies: Hydra vulgaris, Daphnia carinata, and Paratya australiensis. Front. Environ. Sci. 2018, 6, 152. [Google Scholar] [CrossRef]
- Gorka, D.E.; Osterberg, J.S.; Gwin, C.A.; Colman, B.P.; Meyer, J.N.; Bernhardt, E.S.; Gunsch, C.K.; DiGulio, R.T.; Liu, J. Reducing Environmental Toxicity of Silver Nanoparticles through Shape Control. Environ. Sci. Technol. 2015, 49, 10093–11098. [Google Scholar] [CrossRef]
- Wu, F.; Harper, B.J.; Harper, S.L. Differential dissolution and toxicity of surface functionalized silver nanoparticles in small-scale microcosms: Impacts of community complexity. Environ. Sci. Nano 2017, 4, 359–372. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.O.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gamboa, S.; Garcia-Garcia, M.R.; Piñon-Zarate, G.; Rojas-Lemus, M.; Jarquin-Yañez, K.; Angel Herrera-Enriquez, M.; Fortoul, T.I.; Toledano-Magaña, Y.; Garcia-Iglesias, T.; Pestryakov, A.; et al. Toxicity of silver nanoparticles in mouse bone marrow-derived dendritic cells: Implications for phenotype. J. Immunotoxicol. 2019, 16, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Oves, M.; Khan, A.U. Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS ONE 2017, 12, e0181363. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Kronberg, F.; Spedalieri, C.; Munarriz, E.; Giacometti, R. Protein corona on biogenic silver nanoparticles provides higher stability and protects cells from toxicity in comparison to chemical nanoparticles. J. Environ. Manag. 2021, 297, 113434. [Google Scholar] [CrossRef]
- Saha, N.; Dutta Gupta, S. Low-dose toxicity of biogenic silver nanoparticles fabricated by Swertia chirata on root tips and flower buds of Allium cepa. J. Hazard. Mater. 2017, 330, 18–28. [Google Scholar] [CrossRef]
- Rheder, D.T.; Guilger, M.; Bilesky-Jose, N.; Germano-Costa, T.; Pasquoto-Stigliani, T.; Gallep, T.B.B.; Grillo, R.; Carvalho, C.D.S.; Fraceto, L.F.; Lima, R. Synthesis of biogenic silver nanoparticles using Althaea officinalis as reducing agent: Evaluation of toxicity and ecotoxicity. Sci. Rep. 2018, 8, 12397. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Krishnan, M.; Shukla, S.; Lee, H.; Min, S.H.; Choi, D.K.; Cho, Y.; Bajpai, V.K.; Huh, Y.S.; et al. The effect of biogenic manufactured silver nanoparticles on human endothelial cells and zebrafish model. Sci. Total Environ. 2019, 679, 365–377. [Google Scholar] [CrossRef]
- Ghasempour, A.; Dehghan, H.; Ataee, M.; Chen, B.; Zhao, Z.; Sedighi, M.; Guo, X.; Shahbazi, M.A. Cadmium Sulfide Nanoparticles: Preparation, Characterization, and Biomedical Applications. Molecules 2023, 28, 3857. [Google Scholar] [CrossRef]
- Waris, A.; Din, M.; Ali, A.; Ali, M.; Afridi, S.; Baset, A.; Ullah Khan, A. A comprehensive review of green synthesis of copper oxide nanoparticles and their diverse biomedical applications. Inorg. Chem. Commun. 2021, 123, 108369. [Google Scholar] [CrossRef]
- Khairnar, B.A.; Dabhane, H.A.; Dashpute, R.S.; Girase, M.S.; Nalawade, P.M.; Gaikwad, V.B. Study of biogenic fabrication of zinc oxide nanoparticles and their applications: A review. Inorg. Chem. Commun. 2022, 146, 110155. [Google Scholar] [CrossRef]
- Zambonino, M.C.; Quizhpe, E.M.; Mouheb, L.; Rahman, A.; Agathos, S.N.; Dahoumane, S.A. Biogenic Selenium Nanoparticles in Biomedical Sciences: Properties, Current Trends, Novel Opportunities and Emerging Challenges in Theranostic Nanomedicine. Nanomaterials 2023, 13, 424. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.A.; Ali, M.A.; Chen, S.M.; Li, Y.; Al-Hemaid, F.M.; Abou-Tarboush, F.M.; Al-Anazi, K.M.; Lee, J. Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf. B Biointerfaces 2016, 141, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Nakkala, J.R.; Sadras, S.R. Biogenic silver nanoparticles from Abutilon indicum: Their antioxidant, antibacterial and cytotoxic effects in vitro. Colloids Surf. B Biointerfaces 2015, 128, 276–286. [Google Scholar] [CrossRef]
- Bagur, H.; Medidi, R.S.; Somu, P.; Choudhury, P.W.J.; Karua, C.S.; Guttula, P.K.; Melappa, G.; Poojari, C.C. Endophyte fungal isolate mediated biogenic synthesis and evaluation of biomedical applications of silver nanoparticles. Mater. Technol. 2020, 37, 167–178. [Google Scholar] [CrossRef]
- Kirubakaran, D.; Selvam, K.; Prakash, P.; Manimegalai, P.; Shivakumar, M.S.; SenthilNathan, S. Preparation and characterization of biogenic silver nanoparticles using Strobilanthes cordifolia (Vahl) J.R.I. Wood leaves and its biological applications. Biotechnol. Appl. Biochem. 2023, 70, 870–884. [Google Scholar] [CrossRef]
- Solaiman, M.A.; Ali, M.A.; Abdel-Moein, N.M.; Mahmoud, E.A. Synthesis of Ag-NPs developed by green-chemically method and evaluation of antioxidant activities and anti-inflammatory of synthesized nanoparticles against LPS-induced NO in RAW 264.7 macrophages. Biocatal. Agric. Biotechnol. 2020, 29, 101832. [Google Scholar] [CrossRef]
- Pangi, V.N.; Marukurti, A.; Reddy, A.M.; Medapalli, S.R. Synthesis of Biogenic Silver Nanoparticles (bAgNPs) Using Leaf Extract of Mirabilis jalapa and Evaluation of Anti-vibriocidal, Anti-oxidant properties and Cytotoxicity. BioNanoScience 2023, 13, 376–392. [Google Scholar] [CrossRef]
- Abida, A.; Almutairi, M.H.; Mushtaq, N.; Ahmed, M.; Sher, N.; Fozia, F.; Ahmad, I.; Almutairi, B.O.; Ullah, Z. Revolutionizing Nanotechnology with Filago desertorum Extracts: Biogenic Synthesis of Silver Nanoparticles Exhibiting Potent Antioxidant and Antibacterial Activities. ACS Omega 2023, 8, 35140–35151. [Google Scholar] [CrossRef]
- Kumar, D.G.; Achar, R.R.; Kumar, J.R.; Amala, G.; Gopalakrishnan, V.K.; Pradeep, S.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Silina, E.; et al. Assessment of antimicrobial and anthelmintic activity of silver nanoparticles bio-synthesized from Viscum orientale leaf extract. BMC Complement. Med. Ther. 2023, 23, 167. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elem. Res. 2020, 195, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.-K.; Ravishankar Rai, V.; Ramachandra, Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Result Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Netala, V.R.; Bukke, S.; Domdi, L.; Soneya, S.; Reddy, S.G.; Bethu, M.S.; Kotakdi, V.S.; Saritha, K.V.; Tartte, V. Biogenesis of silver nanoparticles using leaf extract of Indigofera hirsuta L. and their potential biomedical applications (3-in-1 system). Artif. Cells Nanomed. Biotechnol. 2018, 46, 1138–1148. [Google Scholar] [CrossRef]
- Gulbagca, F.; Ozdemir, S.; Gulcan, M.; Sen, F. Synthesis and characterization of Rosa canina-mediated biogenic silver nanoparticles for anti-oxidant, antibacterial, antifungal, and DNA cleavage activities. Heliyon 2019, 5, e02980. [Google Scholar] [CrossRef]
- Chandraker, S.K.; Lal, M.; Khanam, F.; Dhruve, P.; Singh, R.P.; Shukla, R. Therapeutic potential of biogenic and optimized silver nanoparticles using Rubia cordifolia L. leaf extract. Sci. Rep. 2022, 12, 8831. [Google Scholar] [CrossRef]
- Dhanasezhian, A.; Srivani, S.; Govindaraju, K.; Parija, P.; Sasikala, S.; Ramesh Kumar, M.R. Anti-Herpes Simplex Virus (HSV-1 and HSV-2) activity of biogenic gold and silver nanoparticles using seaweed Sargassum wightii. Ind. J. Geo-Mar. Sci. 2019, 48, 1252–1257. [Google Scholar]
- Li, J.; Zhang, B.; Chang, X.; Gan, J.; Li, W.; Niu, S.; Kong, L.; Wu, T.; Zhang, T.; Tang, M.; et al. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ. Pollut. 2020, 256, 113430. [Google Scholar] [CrossRef]
- Naveed, M.; Batool, H.; Rehman, S.u.; Javed, A.; Makhdoom, S.I.; Aziz, T.; Mohamed, A.A.; Sameeh, M.Y.; Alruways, M.W.; Dablool, A.S.; et al. Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton populneus Leaf Extract. Processes 2022, 10, 1521. [Google Scholar] [CrossRef]
- Singh, D.; Singh, M.; Yadav, E.; Falls, N.; Singh Dangi, D.; Kumar, V.; Ramteke, P.W.; Verma, A. Attenuation of diethylnitrosamine (DEN)—Induced hepatic cancer in experimental model of Wistar rats by Carissa carandas embedded silver nanoparticles. Biomed. Pharmacother. 2018, 108, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, M.; Yadav, E.; Falls, N.; Komal, U.; Dangi, D.S.; Kumar, V.; Verma, A. Amelioration of diethylnitrosamine (DEN)-induced hepatocellular carcinogenesis in animal models via knockdown oxidative stress and proinflammatory markers by Madhuca longifolia embedded silver nanoparticles. RSC Adv. 2018, 8, 6940–6953. [Google Scholar] [CrossRef]
- Roy, T.; Dey, S.K.; Pradhan, A.; Chaudhuri, A.D.; Dolai, M.; Mandal, S.M.; Choudhury, S.M. Facile and Green Fabrication of Highly Competent Surface-Modified Chlorogenic Acid Silver Nanoparticles: Characterization and Antioxidant and Cancer Chemopreventive Potential. ACS Omega 2022, 7, 48018–48033. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, M.; Ab Rahim, N.; Wan Omar, W.A.; Nik Mohamed Kamal, N.N.S. Cytotoxicity and Genotoxicity of Biogenic Silver Nanoparticles in A549 and BEAS-2B Cell Lines. Bioinorg. Chem. Appl. 2022, 2022, 8546079. [Google Scholar] [CrossRef]
- Senthil, B.; Devasena, T.; Prakash, B.; Rajasekar, A. Non-cytotoxic effect of green synthesized silver nanoparticles and its antibacterial activity. J. Photochem. Photobiol. B Biol. 2017, 177, 1–7. [Google Scholar] [CrossRef]
- Govindappa, M.; Tejashree, S.; Thanuja, V.; Hemashekhar, B.; Srinivas, C.; Nasif, O.; Pugazhendhi, A.; Raghavendra, V.B. Pomegranate fruit fleshy pericarp mediated silver nanoparticles possessing antimicrobial, antibiofilm formation, antioxidant, biocompatibility and anticancer activity. J. Drug Deliv. Sci. Technol. 2021, 61, 102289. [Google Scholar] [CrossRef]
- Rani, R.; Sharma, D.; Chaturvedi, M.; Yadav, J.P. Green synthesis of silver nanoparticles using Tridax procumbens: Their characterization, antioxidant and antibacterial activity against MDR and reference bacterial strains. Chem. Pap. 2019, 74, 1817–1830. [Google Scholar] [CrossRef]
- Ayromlou, A.; Masoudi, S.; Mirzaie, A. Scorzonera calyculata Aerial Part Extract Mediated Synthesis of Silver Nanoparticles: Evaluation of Their Antibacterial, Antioxidant and Anticancer Activities. J. Clust. Sci. 2019, 30, 1037–1050. [Google Scholar] [CrossRef]
- Netala, V.R.; Bethu, M.S.; Pushpalatha, B.; Baki, V.B.; Aishwarya, S.; Rao, J.V.; Tartte, V. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int. J. Nanomed. 2016, 11, 5683–5696. [Google Scholar] [CrossRef] [PubMed]
- Padinjarathil, H.; Joseph, M.M.; Unnikrishnan, B.S.; Preethi, G.U.; Shiji, R.; Archana, M.G.; Maya, S.; Syama, H.P.; Sreelekha, T.T. Galactomannan endowed biogenic silver nanoparticles exposed enhanced cancer cytotoxicity with excellent biocompatibility. Int. J. Biol. Macromol. 2018, 118, 1174–1182. [Google Scholar] [CrossRef]
- Kazaryan, S.; Farsiyan, L.; Tumoyan, J.; Kirakosyan, G.; Ayvazyan, N.; Gasparyan, H.; Buloyan, S.; Arshakyan, L.; Kirakosyan, A.; Hovhannisyan, A. Oxidative stress and histopathological changes in several organs of mice injected with biogenic silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2022, 50, 331–342. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Bolfa, P.; Clichici, S.; Filip, G.A. Modulatory effects of Cornus sanguinea L. mediated green synthesized silver nanoparticles on oxidative stress, COX-2/NOS2 and NFkB/pNFkB expressions in experimental inflammation in Wistar rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110709. [Google Scholar] [CrossRef]
- Bold, B.E.; Urnukhsaikhan, E.; Mishig-Ochir, T. Biosynthesis of silver nanoparticles with antibacterial, antioxidant, anti-inflammatory properties and their burn wound healing efficacy. Front. Chem. 2022, 10, 972534. [Google Scholar] [CrossRef]
- Wunnoo, S.; Bilhman, S.; Waen-ngoen, T.; Yawaraya, S.; Paosen, S.; Lethongkam, S.; Kaewnopparat, N.; Voravuthikunchai, S.P. Thermosensitive hydrogel loaded with biosynthesized silver nanoparticles using Eucalyptus camaldulensis leaf extract as an alternative treatment for microbial biofilms and persistent cells in tissue infections. J. Drug Deliv. Sci. Technol. 2022, 74, 103588. [Google Scholar] [CrossRef]
- Florkiewicz, W.; Malina, D.; Pluta, K.; Rudnicka, K.; Gajewski, A.; Olejnik, E.; Tyliszczak, B.; Sobczak-Kupiec, A. Assessment of cytotoxicity and immune compatibility of phytochemicals-mediated biosynthesised silver nanoparticles using Cynara scolymus. IET Nanobiotechnol 2019, 13, 726–735. [Google Scholar] [CrossRef]
- Sharma, A.; Sanjay; Jaiswal, V.; Park, M.; Lee, H.J. Biogenic silver NPs alleviate LPS-induced neuroinflammation in a human fetal brain-derived cell line: Molecular switch to the M2 phenotype, modulation of TLR4/MyD88 and Nrf2/HO-1 signaling pathways, and molecular docking analysis. Biomater. Adv. 2023, 148, 213363. [Google Scholar] [CrossRef]
- Antony, J.J.; Sithika, M.A.; Joseph, T.A.; Suriyakalaa, U.; Sankarganesh, A.; Siva, D.; Kalaiselvi, S.; Achiraman, S. In vivo antitumor activity of biosynthesized silver nanoparticles using Ficus religiosa as a nanofactory in DAL induced mice model. Colloids Surf. B Biointerfaces 2013, 108, 185–190. [Google Scholar] [CrossRef]
- Singh, P.; Ahn, S.; Kang, J.P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; El-Agamy Farh, M.; Aceituno, V.C.; Kim, Y.J.; et al. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: A green synthetic approach. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef]
- Zangeneh, M.M. Green synthesis and formulation a modern chemotherapeutic drug of Spinacia oleracea L. leaf aqueous extract conjugated silver nanoparticles; Chemical characterization and analysis of their cytotoxicity, antioxidant, and anti-acute myeloid leukemia properties in comparison to doxorubicin in a leukemic mouse model. Appl. Organometal Chem. 2019, 34, e5295. [Google Scholar] [CrossRef]
- Korkamaz, N.; Ceylan, Y.; Taslimi, P.; Kadarağ, A.; Savaş Bülbül, A.; Şen, F. Biogenic nano silver: Synthesis, characterization, antibacterial, antibiofilms, and enzymatic activity. Adv. Powder Technol. 2020, 31, 2942–2950. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.; Srivastava, B.; Bhadouria, R.; Singh, R. Green synthesis of silver nanoparticles using leaf extract of Holoptelea integrifolia and preliminary investigation of its antioxidant, anti-inflammatory, antidiabetic and antibacterial activities. J. Environ. Chem. Eng. 2019, 7, 103094. [Google Scholar] [CrossRef]
- Naik, J.R.; David, M. Green synthesis of silver nanoparticles using Caesalpinia bonducella leaf extract: Characterization and evaluation of in vitro anti-inflammatory and anti-cancer activities. Inorg. Nano-Metal. Chem. 2022, 54, 376–386. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F.; Álvarez-Suarez, J.M. Green Synthesis of Silver Nanoparticles Using Astragalus tribuloides Delile. Root Extract: Characterization, Antioxidant, Antibacterial, and Anti-Inflammatory Activities. Nanomaterials 2020, 10, 2383. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, M.S.; Nithya, S.; Vinoth Kumar, V.; Christena, L.R.; Vadivel, V.; Subramanian, N.S.; Anthony, S.P. Green synthesis of silver nanoparticles using Nardostachys jatamansi and evaluation of its anti-biofilm effect against classical colonizers. Microb. Pathog. 2019, 126, 1–5. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B Biointerfaces 2014, 122, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. Int. 2018, 25, 10392–10406. [Google Scholar] [CrossRef]
- Faisal, S.; Ullah, R.; Alotaibi, A.; Zafar, S.; Rizwan, M.; Tariq, M.H. Biofabrication of silver nanoparticles employing biomolecules of Paraclostridium benzoelyticum strain: Its characterization and their in-vitro antibacterial, anti-aging, anti-cancer and other biomedical applications. Microsc. Res. Tech. 2023, 86, 846–861. [Google Scholar] [CrossRef]
- Jan, H.; Zaman, G.; Usman, H.; Ansir, R.; Drouet, S.; Gigliolo-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Biogenically proficient synthesis and characterization of silver nanoparticles (Ag-NPs) employing aqueous extract of Aquilegia pubiflora along with their in vitro antimicrobial, anti-cancer and other biological applications. J. Mater. Res. Technol. 2021, 15, 950–968. [Google Scholar] [CrossRef]
- Rajakumar, G.; Gomathi, T.; Thiruvengadam, M.; Devi Rajeswari, V.; Kalpana, V.N.; Chung, I.M. Evaluation of anti-cholinesterase, antibacterial and cytotoxic activities of green synthesized silver nanoparticles using from Millettia pinnata flower extract. Microb. Pathog. 2017, 103, 123–128. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef] [PubMed]
- Balu, S.; Andra, S.; Kannan, S.; Vidyavathy, S.M.; Muthalagu, M. Facile synthesis of silver nanoparticles with medicinal grass and its biological assessment. Mater. Lett. 2020, 259, 126900. [Google Scholar] [CrossRef]
- Gholami, A.; Mousavi, S.M.; Shomali, A.; Hashemi, S.A.; Abootalebi, S.N.; Chiang, W.-H.; Barzegar, A.; Shokripoor, M.; Zadeh, A.M. One-Put Ferula-Mediated Synthesis of Biogenic Silver Nanoparticles with More Antimicrobial Effect and Promising Human Cell Biocompatibility. J. Nanomater. 2022, 2022, 5938952. [Google Scholar] [CrossRef]
- Hamedi, S.; Shojaosadati, S.A.; Mohammadi, A. Evaluation of the catalytic, antibacterial and anti-biofilm activities of the Convolvulus arvensis extract functionalized silver nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 167, 36–44. [Google Scholar] [CrossRef]
- Lateef, A.; Ojo, S.A.; Oladejo, S.M. Anti-candida, anti-coagulant and thrombolytic activities of biosynthesized silver nanoparticles using cell-free extract of Bacillus safensis LAU 13. Process Biochem. 2016, 51, 1406–1412. [Google Scholar] [CrossRef]
- Maji, A.; Beg, M.; Mandal, A.K.; Das, S.; Jha, P.K.; Kumar, A.; Sarwar, S.; Hossain, M.; Chakrabarti, P. Spectroscopic interaction study of human serum albumin and human hemoglobin with Mersilea quadrifolia leaves extract mediated silver nanoparticles having antibacterial and anticancer activity. J. Mol. Struct. 2017, 1141, 584–592. [Google Scholar] [CrossRef]
- Ali, M.S.; Altaf, M.; Al-Lohedan, H.A. Green synthesis of biogenic silver nanoparticles using Solanum tuberosum extract and their interaction with human serum albumin: Evidence of “corona” formation through a multi-spectroscopic and molecular docking analysis. J. Photochem. Photobiol. B Biol. 2017, 173, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Garibo, D.; Borbon-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 10805. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh-Taheri, R.; Ghasemi, A.; Meshkat, Z.; Sabouri, Z.; Mohtashami, M.; Darroudi, M. Green Synthesis of Silver Nanoparticles Using Salvadora persica and Caccinia macranthera Extracts: Cytotoxicity Analysis and Antimicrobial Activity Against Antibiotic-Resistant Bacteria. Appl. Biochem. Biotechnol. 2023, 195, 5120–5135. [Google Scholar] [CrossRef]
- Sankarganesh, P.; Ganesh Kumar, A.; Parthasarathy, V.; Joseph, B.; Priyadharsini, G.; Anbarasan, R. Synthesis of Murraya koenigii Mediated Silver Nanoparticles and Their In Vitro and In Vivo Biological Potential. J. Inorg. Organometal Polym. Mater. 2021, 31, 2971–2979. [Google Scholar] [CrossRef]
- Jacob, S.J.P.; Prasad, V.L.S.; Sivasankar, S.; Muralidharan, P. Biosynthesis of silver nanoparticles using dried fruit extract of Ficus carica—Screening for its anticancer activity and toxicity in animal models. Food Chem. Toxicol. 2017, 109, 951–956. [Google Scholar] [CrossRef]
- Niloy, M.S.; Hossain, M.M.; Takikawa, M.; Shakil, M.S.; Polash, S.A.; Mahmud, K.M.; Uddin, M.F.; Alam, M.; Shubhra, R.D.; Shawan, M.; et al. Synthesis of Biogenic Silver Nanoparticles Using Caesalpinia digyna and Investigation of Their Antimicrobial Activity and In Vivo Biocompatibility. ACS Appl. Bio Mater. 2020, 3, 7722–7733. [Google Scholar] [CrossRef]
- Polash, S.A.; Hamza, A.; Hossain, M.M.; Tushar, M.H.; Takikawa, M.; Shubhra, R.D.; Saiara, N.; Saha, T.; Takeoka, S.; Sarker, S.R. Diospyros malabarica Fruit Extract Derived Silver Nanoparticles: A Biocompatible Antibacterial Agent. Front. Nanotechnol. 2022, 4, 888444. [Google Scholar] [CrossRef]
- Joseph, M.M.; Nair, J.B.; Adukkadan, R.N.; Hari, N.; Pillai, R.K.; Nair, A.J.; Maiti, K.K.; Therakathinal, T.S. Exploration of Biogenic Nano-chemobiotics Fabricated by Silver Nanoparticle and Galactoxyloglucan with an Efficient Biodistribution in Solid Tumor Investigated by SERS Fingerprinting. ACS Appl. Mater. Interface 2017, 9, 19578–19590. [Google Scholar] [CrossRef]
- Dahmana, H.; Mediannikov, O. Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens 2020, 9, 310. [Google Scholar] [CrossRef]
- Anoopkumar, A.N.; Aneesh, E.M. A critical assessment of mosquito control and the influence of climate change on mosquito-borne disease epidemics. Environ. Dev. Sustain. 2021, 24, 8900–8929. [Google Scholar] [CrossRef]
- Shaalan, E.A.; Canyon, D.; Younes, M.W.; Abdel-Wahab, H.; Mansour, A.H. A review of botanical phytochemicals with mosquitocidal potential. Environ. Int. 2005, 31, 1149–1166. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Ganesan, P.; Samuel, R.; Mutheeswaran, S.; Pandikumar, P.; Reegan, A.D.; Aremu, A.O.; Ignacimuthu, S. Phytocompounds for mosquito larvicidal activity and their modes of action: A review. S. Afr. J. Bot. 2023, 152, 19–49. [Google Scholar] [CrossRef]
- Benelli, G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: A review. Parasitol. Res. 2016, 115, 23–34. [Google Scholar] [CrossRef]
- Benelli, G.; Jeffries, C.L.; Walker, T. Biological Control of Mosquito Vectors: Past, Present, and Future. Insects 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, C.; Rahuman, A.A. Acaricidal efficacy of synthesized silver nanoparticles using aqueous leaf extract of Ocimum canum against Hyalomma anatolicum anatolicum and Hyalomma marginatum isaaci (Acari: Ixodidae). Parasitol. Res. 2012, 111, 1369–1378. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, P.; Singh, H.; Agrawal, V. Biocontrol of mosquito vectors through herbal-derived silver nanoparticles: Prospects and challenges. Environ. Sci. Pollut. Res. Int. 2020, 27, 25987–26024. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Kirthi, A.V.; Jayaseelan, C.; Bagavan, A.; Zahir, A.A.; Elango, G.; Kamaraj, C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res. 2011, 108, 1541–1549. [Google Scholar] [CrossRef]
- Sampath, G.; Govarthanan, M.; Rameshkumar, N.; Vo, D.-V.N.; Krishnan, M.; Sivasankar, P.; Kayalvizhi, N. Eco-friendly biosynthesis metallic silver nanoparticles using Aegle marmelos (Indian bael) and its clinical and environmental applications. Appl. Nanosci. 2021, 13, 663–674. [Google Scholar] [CrossRef]
- Suresh, G.; Gunasekar, P.H.; Kokila, D.; Prabhu, D.; Dinesh, D.; Ravichandran, N.; Ramesh, B.; Koodalingam, A.; Vijaiyan Siva, G. Green synthesis of silver nanoparticles using Delphinium denudatum root extract exhibits antibacterial and mosquito larvicidal activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 127, 61–66. [Google Scholar] [CrossRef]
- Roopan, S.M.; Rohit; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crops Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. One-pot green synthesis of silver nanocrystals using Hymenodictyon orixense: A cheap and effective tool against malaria, chikungunya and Japanese encephalitis mosquito vectors? RSC Adv. 2016, 6, 59021–59029. [Google Scholar] [CrossRef]
- Karthiga, P.; Rajeshkumar, S.; Annadurai, G. Mechanism of Larvicidal Activity of Antimicrobial Silver Nanoparticles Synthesized Using Garcinia mangostana Bark Extract. J. Clust. Sci. 2018, 29, 1233–1241. [Google Scholar] [CrossRef]
- Vimala, R.T.; Sathishkumar, G.; Sivaramakrishnan, S. Optimization of reaction conditions to fabricate nano-silver using Couroupita guianensis Aubl. (leaf & fruit) and its enhanced larvicidal effect. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 110–115. [Google Scholar] [CrossRef]
- Patil, C.D.; Patil, S.V.; Borase, H.P.; Salunke, B.K.; Salunkhe, R.B. Larvicidal activity of silver nanoparticles synthesized using Plumeria rubra plant latex against Aedes aegypti and Anopheles stephensi. Parasitol. Res. 2012, 110, 1815–1822. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Elbahnasawy, M.A.; Hasaballah, A.I. Green Phytosynthesis of Silver Nanoparticles Using Echinochloa stagnina Extract with Reference to Their Antibacterial, Cytotoxic, and Larvicidal Activities. BioNanoScience 2021, 11, 526–538. [Google Scholar] [CrossRef]
- Shukla, G.; Gaurav, S.S.; Singh, A.; Rani, P. Synthesis of mycogenic silver nanoparticles by Fusarium pallidoroseum and evaluation of its larvicidal effect against white grubs (Holotrichia sp.). Mater. Today Proc. 2022, 49, 3517–3527. [Google Scholar] [CrossRef]
- Seetharaman, P.K.; Chandrasekaran, R.; Gnanasekar, S.; Chandrakasan, G.; Gupta, M.; Manikandan, D.B.; Sivaperumal, S. Antimicrobial and larvicidal activity of eco-friendly silver nanoparticles synthesized from endophytic fungi Phomopsis liquidambaris. Biocatal. Agric. Biotechnol. 2018, 16, 22–30. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae. Parasitol. Res. 2012, 110, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.J.; Harimuralikrishnaa, T.; Sivakumar, T.; Mahendran, S.; Ponmanickam, P.; Thangaraj, R.; Sevarkodiyone, S.; Alharbi, N.S.; Kadaikunnan, S.; Venkidasamy, B.; et al. Biogenic Synthesis of Silver Nanoparticles Using Pantoea stewartii and Priestia aryabhattai and Their Antimicrobial, Larvicidal, Histopathological, and Biotoxicity Potential. Bioengineering 2023, 10, 248. [Google Scholar] [CrossRef]
- Banu, A.N.; Balasubramanian, C. Extracellular synthesis of silver nanoparticles using Bacillus megaterium against malarial and dengue vector (Diptera: Culicidae). Parasitol. Res. 2015, 114, 4069–4079. [Google Scholar] [CrossRef]
- Fouad, H.; Hongjie, L.; Yanmei, D.; Baoting, Y.; El-Shakh, A.; Abbas, G.; Jianchu, M. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Srinivasan, M.; Mohanraj, J. Biosynthesis of silver nanoparticles from mangrove plant (Avicennia marina) extract and their potential mosquito larvicidal property. J. Parasit. Dis. 2016, 40, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Xavier, R.J.; Arumugam, M. Larvicidal property of green synthesized silver nanoparticles against vector mosquitoes (Anopheles stephensi and Aedes aegypti). J. King Saud Univ. Sci. 2016, 28, 318–323. [Google Scholar] [CrossRef]
- Ga’al, H.; Fouad, H.; Mao, G.; Tian, J.; Jianchu, M. Larvicidal and pupicidal evaluation of silver nanoparticles synthesized using Aquilaria sinensis and Pogostemon cablin essential oils against dengue and zika viruses vector Aedes albopictus mosquito and its histopathological analysis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Labeeba, M.A.; Panneerselvam, C.; Dinesh, D.; Suresh, U.; Subramaniam, J.; Madhiyazhagan, P.; Hwang, J.S.; Wang, L.; Nicoletti, M.; et al. Aristolochia indica green-synthesized silver nanoparticles: A sustainable control tool against the malaria vector Anopheles stephensi? Res. Vet. Sci. 2015, 102, 127–135. [Google Scholar] [CrossRef]
- Murugan, K.; Benelli, G.; Ayyappan, S.; Dinesh, D.; Panneerselvam, C.; Nicoletti, M.; Hwang, J.S.; Kumar, P.M.; Subramaniam, J.; Suresh, U. Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasitol. Res. 2015, 114, 2243–2253. [Google Scholar] [CrossRef]
- Sundaravadivelan, C.; Nalini Padmanabhan, M.; Sivaprasath, P.; Kishmu, L. Biosynthesized silver nanoparticles from Pedilanthus tithymaloides leaf extract with anti-developmental activity against larval instars of Aedes aegypti L. (Diptera; Culicidae). Parasitol. Res. 2013, 112, 303–311. [Google Scholar] [CrossRef]
- Rajakumar, G.; Abdul Rahuman, A. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop. 2011, 118, 196–203. [Google Scholar] [CrossRef]
- Govindarajan, M.; Vijayan, P.; Kadaikunnan, S.; Alharbi, N.S.; Benelli, G. One-pot biogenic fabrication of silver nanocrystals using Quisqualis indica: Effectiveness on malaria and Zika virus mosquito vectors, and impact on non-target aquatic organisms. J. Photochem. Photobiol. B Biol. 2016, 162, 646–655. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Veerakumar, K.; Muthukumaran, U.; Hoti, S.L.; Benelli, G. Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: Mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp. Parasitol. 2016, 161, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Preet, S. Comparative assessment of green and chemically synthesized glutathione capped silver nanoparticles for antioxidant, mosquito larvicidal and eco-toxicological activities. Sci. Rep. 2023, 13, 8152. [Google Scholar] [CrossRef]
- Saad, A.M.; El-Saadony, M.T.; El-Tahan, A.M.; Sayed, S.; Moustafa, M.A.M.; Taha, A.E.; Taha, T.F.; Ramadan, M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021, 28, 5674–5683. [Google Scholar] [CrossRef]
- Mahyoub, J.A.; Aziz, A.T.; Panneerselvam, C.; Murugan, K.; Roni, M.; Trivedi, S.; Nicoletti, M.; Hawas, U.W.; Shaher, F.M.; Bamakhrama, M.A.; et al. Seagrasses as Sources of Mosquito Nano-Larvicides? Toxicity and Uptake of Halodule uninervis-Biofabricated Silver Nanoparticles in Dengue and Zika Virus Vector Aedes aegypti. J. Clust. Sci. 2016, 28, 565–580. [Google Scholar] [CrossRef]
- Govindan, L.; Anbazhagan, S.; Altemimi, A.B.; Lakshminarayanan, K.; Kuppan, S.; Pratap-Singh, A.; Kandasamy, M. Efficacy of Antimicrobial and Larvicidal Activities of Green Synthesized Silver Nanoparticles Using Leaf Extract of Plumbago auriculata Lam. Plants 2020, 9, 1577. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Caselli, A.; Canale, A. Nanoparticles for mosquito control: Challenges and constraints. J. King Saud Univ. Sci. 2017, 29, 424–435. [Google Scholar] [CrossRef]
- Elumalai, K.; Kavipriya, M.R.; Lakshmi Prabha, A.; Krishnappa, K.; Pandiyan, J.; Nicoletti, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Govindarajan, M. Green synthesis of silver nanoparticles using Atalantia monophylla: A potential eco-friendly agent for controlling blood-sucking vectors. Green Process. Synth. 2022, 11, 915–930. [Google Scholar] [CrossRef]
- Rawani, A.; Ghosh, A.; Chandra, G. Mosquito larvicidal and antimicrobial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of Solanum nigrum L. (Solanaceae: Solanales). Acta Trop. 2013, 128, 613–622. [Google Scholar] [CrossRef]
- Subramaniam, J.; Murugan, K.; Jebanesan, A.; Pontheckan, P.; Dinesh, D.; Nicoletti, M.; Wei, H.; Higuchi, A.; Kumar, S.; Canale, A.; et al. Do Chenopodium ambrosioides-Synthesized Silver Nanoparticles Impact Oryzias melastigma Predation Against Aedes albopictus Larvae? J. Clust. Sci. 2016, 28, 413–436. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martinez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Santana, É.S.d.; Belmiro, V.B.d.S.; de Siqueira, L.B.d.O.; do Nascimento, T.; Santos-Oliveira, R.; dos Santos Matos, A.P.; Ricci-Junior, E. Nanotechnology as an alternative to improve the treatment of cutaneous leishmaniasis: A systematic review of the literature. J. Drug Deliv. Sci. Technol. 2022, 75, 103622. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, D.; Pan, Y.; Qu, W.; Hao, H.; Wang, X.; Liu, Z.; Xie, S. Nanoparticles for antiparasitic drug delivery. Drug Deliv. 2019, 26, 1206–1221. [Google Scholar] [CrossRef] [PubMed]
- Nafari, A.; Cheraghipour, K.; Sepahvand, M.; Shahrokhi, G.; Gabal, E.; Mahmoudvand, H. Nanoparticles: New agents toward treatment of leishmaniasis. Parasite Epidemiol. Control 2020, 10, e00156. [Google Scholar] [CrossRef]
- Aftab, A.; Ullah, S.; Syed, F.; Tahir, K.; Khan, A.U.; Yuan, Q. Biogenic metal nanoparticles as a potential class of antileishmanial agents: Mechanisms and molecular targets. Nanomedicine 2020, 15, 809–828. [Google Scholar] [CrossRef]
- AlGabbani, Q. Nanotechnology: A promising strategy for the control of parasitic infections. Exp. Parasitol. 2023, 250, 108548. [Google Scholar] [CrossRef]
- Akbari, M.; Oryan, A.; Hatam, G. Application of nanotechnology in treatment of leishmaniasis: A Review. Acta Trop. 2017, 172, 86–90. [Google Scholar] [CrossRef]
- Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Machado, L.F.; Bortoleti, B.; Sahd, C.S.; Chagas, A.F.; Assolini, J.P.; Oliveira, F.J.A.; Pavanelli, W.R.; Conchon-Costa, I.; et al. Nanotechnology as a potential therapeutic alternative for schistosomiasis. Acta Trop. 2017, 174, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gong, J.; Jiang, Y.; Long, Y.; Lei, W.; Gao, X.; Guo, D. Application of Silver Nanoparticles in Parasite Treatment. Pharmaceutics 2023, 15, 1783. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Kumar, R.; Devi, S.; Sharma, P.; Chaudhary, N.R.; Negi, S.; Tandel, N.; Marepally, S.; Pied, S.; Tyagi, R.K. Biogenically synthesized green silver nanoparticles exhibit antimalarial activity. Discov. Nano 2024, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Isaac-Márquez, A.P.; Talamás-Rohana, P.; Galindo-Sevilla, N.; Gaitan-Puch, S.E.; Díaz-Díaz, N.A.; Hernández-Ballina, G.A.; Lezama-Dávila, C.M. Decanethiol functionalized silver nanoparticles are new powerful leishmanicidals in vitro. World J. Microbiol. Biotechnol. 2018, 34, 38. [Google Scholar] [CrossRef]
- Souza, A.O.; Oliveira, J.W.F.; Moreno, C.J.G.; de Medeiros, M.J.C.; Fernandes-Negreiros, M.M.; Souza, F.R.M.; Pontes, D.L.; Silva, M.S.; Rocha, H.A.O. Silver Nanoparticles Containing Fucoidan Synthesized by Green Method Have Anti-Trypanosoma cruzi Activity. Nanomaterials 2022, 12, 2059. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Molefe, N.I.; Awakan, O.J.; Nwonuma, C.O.; Alejolowo, O.O.; Olaolu, T.; Maimako, R.F.; Suganuma, K.; Han, Y.; Kato, K. Metal nanoparticles restrict the growth of protozoan parasites. Artif. Cells Nanomed. Biotechnol. 2018, 46, S86–S94. [Google Scholar] [CrossRef]
- Detoni, M.B.; Bortoleti, B.; Tomiotto-Pellissier, F.; Concato, V.M.; Goncalves, M.D.; Silva, T.F.; Ortiz, L.S.F.; Gomilde, A.C.; Rodrigues, A.C.J.; de Matos, R.L.N.; et al. Biogenic silver nanoparticle exhibits schistosomicidal activity in vitro and reduces the parasitic burden in experimental Schistosomiasis mansoni. Microbes Infect. 2023, 25, 105145. [Google Scholar] [CrossRef]
- Sanfelice, R.; Bortoleti, B.; Tomiotto-Pellissier, F.; Silva, T.F.; Bosqui, L.R.; Nakazato, G.; Castilho, P.M.; de Barros, L.D.; Garcia, J.L.; Lazarin-Bidóia, D.; et al. Biogenic silver nanoparticles (AgNp-Bio) reduce Toxoplasma gondii infection and proliferation in HeLa cells, and induce autophagy and death of tachyzoites by apoptosis-like mechanism. Acta Trop. 2021, 222, 106070. [Google Scholar] [CrossRef]
- Said, D.E.; Elsamad, L.M.; Gohar, Y.M. Validity of silver, chitosan, and curcumin nanoparticles as anti-Giardia agents. Parasitol. Res. 2012, 111, 545–554. [Google Scholar] [CrossRef]
- Ovais, M.; Nadhman, A.; Khalil, A.T.; Raza, A.; Khuda, F.; Sohail, M.F.; Shinwari, Z.K. Biosynthesized Colloidal Silver and Gold Nanoparticles As Emerging Leishmanicidal Agents: An Insight. Nanomedicine 2017, 12, 2807–2819. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Ustundag, C.B.; Kaya, C.; Kaya, F.; Rafailovich, M. Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. Int. J. Nanomed. 2011, 6, 2705–2714. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Kato, K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int. J. Nanomed. 2017, 12, 1647–1661. [Google Scholar] [CrossRef]
- Rossi-Bergmann, B.; Pacienza-Lima, W.; Marcato, P.D.; de Conti, R.; Durán, N. Therapeutic Potential of Biogenic Silver Nanoparticles in Murine Cutaneous Leishmaniasis. J. Nano Res. 2012, 20, 89–97. [Google Scholar] [CrossRef]
- Fanti, J.R.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Cataneo, A.H.D.; Andrade, C.; Panis, C.; Rodrigues, J.; Wowk, P.F.; Kuczera, D.; Costa, I.N.; et al. Biogenic silver nanoparticles inducing Leishmania amazonensis promastigote and amastigote death in vitro. Acta Trop. 2018, 178, 46–54. [Google Scholar] [CrossRef]
- Baranwal, A.; Chiranjivi, A.K.; Kumar, A.; Dubey, V.K.; Chandra, P. Design of commercially comparable nanotherapeutic agent against human disease-causing parasite, Leishmania. Sci. Rep. 2018, 8, 8814. [Google Scholar] [CrossRef]
- Bagirova, M.; Dinparvar, S.; Allahverdiyev, A.M.; Unal, K.; Abamor, E.S.; Novruzova, M. Investigation of antileshmanial activities of Cuminum cyminum based green silver nanoparticles on L. tropica promastigotes and amastigotes in vitro. Acta Trop. 2020, 208, 105498. [Google Scholar] [CrossRef]
- Javed, B.; Mashwani, Z.U.; Sarwer, A.; Raja, N.I.; Nadhman, A. Synergistic response of physicochemical reaction parameters on biogenesis of silver nanoparticles and their action against colon cancer and leishmanial cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1340–1353. [Google Scholar] [CrossRef]
- Ghotekar, S.; Pansambal, S.; Pawar, S.P.; Pagar, T.; Oza, R.; Bangale, S. Biological activities of biogenically synthesized fluorescent silver nanoparticles using Acanthospermum hispidum leaves extract. SN Appl. Sci. 2019, 1, 1342. [Google Scholar] [CrossRef]
- Tripathy, S.; Rademan, S.; Matsabisa, M.G. Effects of Silver Nanoparticle from Dicoma anomala Sond. Root Extract on MCF-7 Cancer Cell Line and NF54 Parasite Strain: An In Vitro Study. Biol. Trace Elem. Res. 2020, 195, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.S.S.; Brasil, C.L.; Braga, C.Q.; Zamboni, R.; Sallis, E.S.V.; Albano, A.P.N.; Zambrano, C.G.; Franz, H.C.; Pötter, L.; Panagio, L.A.; et al. Biogenic silver nanoparticles in the treatment of experimental pythiosis Bio-AgNP in pythiosis therapy. Med. Mycol. 2020, 58, 913–918. [Google Scholar] [CrossRef]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Bipinchandra, S.; Mouray, E.; Grellier, P.; Satish, P. In vitro antiparasitic activity of microbial pigments and their combination with phytosynthesized metal nanoparticles. Parasitol. Int. 2015, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Larayetan, R.; Ojemaye, M.O.; Okoh, O.O.; Okoh, A.I. Silver nanoparticles mediated by Callistemon citrinus extracts and their antimalaria, antitrypanosoma and antibacterial efficacy. J. Mol. Liq. 2019, 273, 615–625. [Google Scholar] [CrossRef]
- Hashemi, Z.; Mohammadyan, M.; Naderi, S.; Fakhar, M.; Biparva, P.; Akhtari, J.; Ebrahimzadeh, M.A. Green synthesis of silver nanoparticles using Ferula persica extract (Fp-NPs): Characterization, antibacterial, antileishmanial, and in vitro anticancer activities. Mater. Today Commun. 2021, 27, 102264. [Google Scholar] [CrossRef]
- Goel, V.; Kaur, P.; Singla, L.D.; Choudhury, D. Biomedical Evaluation of Lansium parasiticum Extract-Protected Silver Nanoparticles Against Haemonchus contortus, a Parasitic Worm. Front. Mol. Biosci. 2020, 7, 595646. [Google Scholar] [CrossRef]
- Kalangi, S.K.; Dayakar, A.; Gangappa, D.; Sathyavathi, R.; Maurya, R.S.; Narayana Rao, D. Biocompatible silver nanoparticles reduced from Anethum graveolens leaf extract augments the antileishmanial efficacy of miltefosine. Exp. Parasitol. 2016, 170, 184–192. [Google Scholar] [CrossRef]
- Hematizadeh, A.; Ebrahimzadeh, M.A.; Sarvi, S.; Sadeghi, M.; Daryani, A.; Gholami, S.; Nayeri, T.; Hosseini, S.A. In Vitro and In Vivo Anti-parasitic Activity of Sambucus ebulus and Feijoa sellowiana Extracts Silver Nanoparticles on Toxoplasma gondii Tachyzoites. Acta Parasitol. 2023, 68, 557–565. [Google Scholar] [CrossRef]
- Ullah, I.; Cosar, G.; Abamor, E.S.; Bagirova, M.; Shinwari, Z.K.; Allahverdiyev, A.M. Comparative study on the antileishmanial activities of chemically and biologically synthesized silver nanoparticles (AgNPs). 3 Biotech. 2018, 8, 98. [Google Scholar] [CrossRef]
- Ahmad, A.; Syed, F.; Shah, A.; Khan, Z.; Tahir, K.; Khan, A.U.; Yuan, Q. Silver and gold nanoparticles from Sargentodoxa cuneata: Synthesis, characterization and antileishmanial activity. RSC Adv. 2015, 5, 73793–73806. [Google Scholar] [CrossRef]
- Awad, M.A.; Al Olayan, E.M.; Siddiqui, M.I.; Merghani, N.M.; Alsaif, S.S.A.; Aloufi, A.S. Antileishmanial effect of silver nanoparticles: Green synthesis, characterization, in vivo and in vitro assessment. Biomed. Pharmacother. 2021, 137, 111294. [Google Scholar] [CrossRef]
- Ahmad, A.; Wei, Y.; Syed, F.; Khan, S.; Khan, G.M.; Tahir, K.; Khan, A.U.; Raza, M.; Khan, F.U.; Yuan, Q. Isatis tinctoria mediated synthesis of amphotericin B-bound silver nanoparticles with enhanced photoinduced antileishmanial activity: A novel green approach. J. Photochem. Photobiol. B Biol. 2016, 161, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, E.; Karamian, M.; Abedi, F.; Hanafi-Bojd, M.Y. Topical treatment of cutaneous leishmaniasis lesions using quercetin/Artemisia-capped silver nanoparticles ointment: Modulation of inflammatory response. Acta Trop. 2022, 228, 106325. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, Z.K.; Soliman, M.I.; Taha, H.A.; Khalil, M.M.H.; Nigm, A.H. Antischistosomal effects of green and chemically synthesized silver nanoparticles: In vitro and in vivo murine model. Acta Trop. 2023, 244, 106952. [Google Scholar] [CrossRef]
- Machado, L.F.; Sanfelice, R.A.; Bosqui, L.R.; Assolini, J.P.; Scandorieiro, S.; Navarro, I.T.; Depieri Cataneo, A.H.; Wowk, P.F.; Nakazato, G.; Bordignon, J.; et al. Biogenic silver nanoparticles reduce adherence, infection, and proliferation of Toxoplasma gondii RH strain in HeLa cells without inflammatory mediators induction. Exp. Parasitol. 2020, 211, 107853. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Vahidi, H.; Damavandi Kamali, K.; Rashedi, M.; Saravanan, M. Antineoplastic Biogenic Silver Nanomaterials to Combat Cervical Cancer: A Novel Approach in Cancer Therapeutics. J. Clust. Sci. 2020, 31, 659–672. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Shinwari, Z.K. Green Synthesis of Silver Nanoparticles Via Plant Extracts: Beginning a New Era in Cancer Theranostics. Nanomedecine 2016, 11, 3157–3177. [Google Scholar]
- Kanagamani, K.; Muthukrishnan, P.; Shankar, K.; Kathiresan, A.; Barabadi, H.; Saravanan, M. Antimicrobial, Cytotoxicity and Photocatalytic Degradation of Norfloxacin Using Kleinia grandiflora Mediated Silver Nanoparticles. J. Clust. Sci. 2019, 30, 1415–1424. [Google Scholar] [CrossRef]
- Vinay, S.P.; Udayabhanu; Nagaraju, G.; Chandrappa, C.P.; Chandrasekhar, N. Rauvolfia tetraphylla (Devil Pepper)-Mediated Green Synthesis of Ag Nanoparticles: Applications to Anticancer, Antioxidant and Antimitotic. J. Clust. Sci. 2019, 30, 1545–1564. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Pandurangan, M.; Kim, D.H.; Lee, Y.R. Green Synthesis: In-vitro Anticancer Activity of Silver Nanoparticles on Human Cervical Cancer Cells. J. Clust. Sci. 2016, 27, 671–681. [Google Scholar] [CrossRef]
- Ulagesan, S.; Nam, T.J.; Choi, Y.H. Cytotoxicity against human breast carcinoma cells of silver nanoparticles biosynthesized using Capsosiphon fulvescens extract. Bioprocess. Biosyst. Eng. 2021, 44, 901–911. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Vanaja, M.; Annadurai, G. Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. J. Mol. Struct. 2016, 1116, 165–173. [Google Scholar] [CrossRef]
- Sriram, M.I.; Kanth, S.B.; Kalishwaralal, K.; Gurunathan, S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int. J. Nanomed. 2010, 5, 753–762. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Ostrowski, M.; Trzcińska, J.; Rai, M.; Golińska, P. Biogenic Silver Nanoparticles: Assessment of Their Cytotoxicity, Genotoxicity and Study of Capping Proteins. Molecules 2020, 25, 3022. [Google Scholar] [CrossRef]
- Mistry, H.; Thakor, R.; Bariya, H. Biogenesis and characterization of proficient silver nanoparticles employing marine procured fungi Hamigera pallida and assessment of their antioxidative, antimicrobial and anticancer potency. Biotechnol. Lett. 2022, 44, 1097–1107. [Google Scholar] [CrossRef]
- Tavan, M.; Hanachi, P.; Mirjalili, M.H.; Dashtbani-Roozbehani, A. Comparative assessment of the biological activity of the green synthesized silver nanoparticles and aqueous leaf extract of Perilla frutescens (L.). Sci. Rep. 2023, 13, 6391. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, S.; Divya, K.; Jisha, M.S. In vitro anticancer evaluation of chitosan/biogenic silver nanoparticle conjugate on Si Ha and MDA MB cell lines. Appl. Nanosci. 2019, 10, 715–728. [Google Scholar] [CrossRef]
- Dutt, Y.; Pandey, R.P.; Dutt, M.; Gupta, A.; Vibhuti, A.; Raj, V.S.; Chang, C.M.; Priyadarshini, A. Silver Nanoparticles Phytofabricated through Azadirachta indica: Anticancer, Apoptotic, and Wound-Healing Properties. Antibiotics 2023, 12, 121. [Google Scholar] [CrossRef]
- Aabed, K.; Mohammed, A.E. Synergistic and Antagonistic Effects of Biogenic Silver Nanoparticles in Combination with Antibiotics Against Some Pathogenic Microbes. Front. Bioeng. Biotechnol. 2021, 9, 652362. [Google Scholar] [CrossRef]
- Khalili, H.; Sadat Shandiz, S.A.; Baghbani-Arani, F. Anticancer Properties of Phyto-Synthesized Silver Nanoparticles from Medicinal Plant Artemisia tschernieviana Besser Aerial Parts Extract Toward HT29 Human Colon Adenocarcinoma Cells. J. Clust. Sci. 2017, 28, 1617–1636. [Google Scholar] [CrossRef]
- Khan, M.S.; Alomari, A.; Tabrez, S.; Hassan, I.; Wahab, R.; Bhat, S.A.; Alafaleq, N.O.; Altwaijry, N.; Shaik, G.M.; Zaidi, S.K.; et al. Anticancer Potential of Biogenic Silver Nanoparticles: A Mechanistic Study. Pharmaceutics 2021, 13, 707. [Google Scholar] [CrossRef]
- Oves, M.; Ahmar Rauf, M.; Aslam, M.; Qari, H.A.; Sonbol, H.; Ahmad, I.; Sarwar Zaman, G.; Saeed, M. Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci. 2022, 29, 460–471. [Google Scholar] [CrossRef]
- Oves, M.; Rauf, M.A.; Qari, H.A. Therapeutic Applications of Biogenic Silver Nanomaterial Synthesized from the Paper Flower of Bougainvillea glabra (Miami, Pink). Nanomaterials 2023, 13, 615. [Google Scholar] [CrossRef]
- Gul, A.R.; Shaheen, F.; Rafique, R.; Bal, J.; Waseem, S.; Park, T.J. Grass-mediated biogenic synthesis of silver nanoparticles and their drug delivery evaluation: A biocompatible anti-cancer therapy. Chem. Eng. J. 2021, 407, 127202. [Google Scholar] [CrossRef]
- Palaniappan, P.; Sathishkumar, G.; Sankar, R. Fabrication of nano-silver particles using Cymodocea serrulata and its cytotoxicity effect against human lung cancer A549 cells line. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Datkhile, K.D.; Durgavale, P.P.; Patil, M.N. Biogenic Silver Nanoparticles from Nothapodytes foetida Kills Human Cancer Cells In Vitro Through Inhibition of Cell Proliferation and Induction of Apoptosis. J. Bionanosci. 2017, 11, 416–427. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Bhat, R.S.; Al-Onazi, M.A.; Alwhibi, M.S.; Soliman, D.A.; Aljebrin, N.A.; Al-Suhaibani, L.S.; Al Daihan, S. Anticancer potential of biogenic silver nanoparticles using the stem extract of Commiphora gileadensis against human colon cancer cells. Green Process. Synth. 2022, 11, 435–444. [Google Scholar] [CrossRef]
- Halawani, E.M.; Hassan, A.M.; Gad El-Rab, S.M.F. Nanoformulation of Biogenic Cefotaxime-Conjugated-Silver Nanoparticles for Enhanced Antibacterial Efficacy Against Multidrug-Resistant Bacteria and Anticancer Studies. Int. J. Nanomed. 2020, 15, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Alanazi, A.M.; Alsaif, N.; Wani, T.A.; Bhat, M.A. Pomegranate peel induced biogenic synthesis of silver nanoparticles and their multifaceted potential against intracellular pathogen and cancer. Saudi J. Biol. Sci. 2021, 28, 4191–4200. [Google Scholar] [CrossRef]
- Korkmaz, N.; Ceylan, Y.; Hamid, A.; Karadağ, A.; Bülbül, A.S.; Aftab, M.N.; Çevik, Ö.; Şen, F. Biogenic silver nanoparticles synthesized via Mimusops elengi fruit extract, a study on antibiofilm, antibacterial, and anticancer activities. J. Drug Deliv. Sci. Technol. 2020, 59, 101864. [Google Scholar] [CrossRef]
- Poor, M.H.S.; Khatami, M.; Azizi, H.; Abazari, Y. Cytotoxic activity of biosynthesized Ag Nanoparticles by Plantago major towards a human breast cancer cell line. Rend. Lincei 2017, 28, 693–699. [Google Scholar] [CrossRef]
- Satsangi, N. Synthesis and Characterization of Biocompatible Silver Nanoparticles for Anticancer Application. J. Inorg. Organometal Polym. Mater. 2019, 30, 1907–1914. [Google Scholar] [CrossRef]
- Shaniba, V.S.; Aziz, A.A.; Joseph, J.; Jayasree, P.R.; Manish Kumar, P.R. Synthesis, Characterization and Evaluation of Antioxidant and Cytotoxic Potential of Annona muricata Root Extract-derived Biogenic Silver Nanoparticles. J. Clust. Sci. 2021, 33, 467–483. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Zarkesh Esfahani, S.H.; Khosropour, A.R.; Razmjou, A. Green synthesis of anisotropic silver nanoparticles with potent anticancer activity using Taxus baccata extract. RSC Adv. 2014, 4, 61394–61403. [Google Scholar] [CrossRef]
- Delalat, R.; Sadat Shandiz, S.A.; Pakpour, B. Antineoplastic effectiveness of silver nanoparticles synthesized from Onopordum acanthium L. extract (AgNPs-OAL) toward MDA-MB231 breast cancer cells. Mol. Biol. Rep. 2022, 49, 1113–1120. [Google Scholar] [CrossRef]
- Manikandan, R.; Manikandan, B.; Raman, T.; Arunagirinathan, K.; Prabhu, N.M.; Jothi Basu, M.; Perumal, M.; Palanisamy, S.; Munusamy, A. Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti-inflammatory activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 120–129. [Google Scholar] [CrossRef]
- Acharya, D.; Satapathy, S.; Yadav, K.K.; Somu, P.; Mishra, G. Systemic Evaluation of Mechanism of Cytotoxicity in Human Colon Cancer HCT-116 Cells of Silver Nanoparticles Synthesized Using Marine Algae Ulva lactuca Extract. J. Inorg. Organometal Polym. Mater. 2022, 32, 596–605. [Google Scholar] [CrossRef]
- Kummara, S.; Patil, M.B.; Uriah, T. Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles—A comparative study. Biomed. Pharmacother. 2016, 84, 10–21. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Park, C.; Yoo, H.; Kim, J.H.; Hong, K. Cytotoxic Potential and Molecular Pathway Analysis of Silver Nanoparticles in Human Colon Cancer Cells HCT116. Int. J. Mol. Sci. 2018, 19, 2269. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Wang, C.; Yang, R.; Ahmed, M.; Kumaran, S.; Velu, P.; Li, B. Pseudomonas aeruginosa synthesized silver nanoparticles inhibit cell proliferation and induce ROS mediated apoptosis in thyroid cancer cell line (TPC1). Artif. Cells Nanomed. Biotechnol. 2020, 48, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Lokina, S.; Stephen, A.; Kaviyarasan, V.; Arulvasu, C.; Narayanan, V. Cytotoxicity and antimicrobial activities of green synthesized silver nanoparticles. Eur. J. Med. Chem. 2014, 76, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Sriranjani, R.; Srinithya, B.; Vellingiri, V.; Brindha, P.; Anthony, S.P.; Sivasubramanian, A.; Muthuraman, M.S. Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J. Mol. Liq. 2016, 220, 926–930. [Google Scholar] [CrossRef]
- Sargazi, A.; Barani, A.; Heidari Majd, M. Synthesis and Apoptotic Efficacy of Biosynthesized Silver Nanoparticles Using Acacia luciana Flower Extract in MCF-7 Breast Cancer Cells: Activation of Bak1 and Bclx for Cancer Therapy. BioNanoScience 2020, 10, 683–689. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H.; Yi, D.k. Synthesis of Biogenic Silver Nanoparticles with Eco-Friendly Processes Using Ganoderma lucidum Extract and Evaluation of Their Theranostic Applications. J. Nanomater. 2021, 2021, 6135920. [Google Scholar] [CrossRef]
- Mukundan, D.; Mohankumar, R.; Vasanthakumari, R. Comparative study of synthesized silver and gold nanoparticles using leaves extract of Bauhinia tomentosa Linn and their anticancer efficacy. Bull. Mater. Sci. 2017, 40, 335–344. [Google Scholar] [CrossRef]
- Banu, H.; Renuka, N.; Faheem, S.M.; Ismail, R.; Singh, V.; Saadatmand, Z.; Khan, S.S.; Narayanan, K.; Raheem, A.; Premkumar, K.; et al. Gold and Silver Nanoparticles Biomimetically Synthesized Using Date Palm Pollen Extract-Induce Apoptosis and Regulate p53 and Bcl-2 Expression in Human Breast Adenocarcinoma Cells. Biol. Trace Elem. Res. 2018, 186, 122–134. [Google Scholar] [CrossRef]
- Botha, T.L.; Elemike, E.E.; Horn, S.; Onwudiwe, D.C.; Giesy, J.P.; Wepener, V. Cytotoxicity of Ag, Au and Ag-Au bimetallic nanoparticles prepared using golden rod (Solidago canadensis) plant extract. Sci. Rep. 2019, 9, 4169. [Google Scholar] [CrossRef] [PubMed]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of silver nanoparticles using the extract of Alternanthera sessilis-antiproliferative effect against prostate cancer cells. Cancer Nanotechnol. 2013, 4, 137–143. [Google Scholar] [CrossRef]
- Acharya, D.; Satapathy, S.; Somu, P.; Parida, U.K.; Mishra, G. Apoptotic Effect and Anticancer Activity of Biosynthesized Silver Nanoparticles from Marine Algae Chaetomorpha linum Extract Against Human Colon Cancer Cell HCT-116. Biol. Trace Elem. Res. 2021, 199, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, F.; Chahardoli, A.; Sadrjavadi, K.; Fattahi, A.; Shokoohinia, Y. Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: Characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. 2020, 31, 1323–1332. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Rashid, U.; Javed, Z.; Hamid, Z.; Imran, K.; Kabeer, A.; Raza, S.; Almarhoon, Z.M.; Reiner, Ž.; Bagiu, I.-C.; et al. Biosynthesized silver nanoparticles and miR34a mimics mediated activation of death receptor in MCF-7 human breast cancer cell lines. Cancer Nanotechnol. 2022, 13, 31. [Google Scholar] [CrossRef]
- Sameem, S.; Neupane, N.P.; Saleh Ansari, S.M.; Uzzaman Khan, M.M.; Kumar, V.; Pathak, P.; Grishina, M.; Verma, A. Phyto-fabrication of silver nanoparticles from Ziziphus mauritiana against hepatic carcinoma via modulation of Rho family-alpha serine/threonine protein kinase. J. Drug Deliv. Sci. Technol. 2022, 70, 103227. [Google Scholar] [CrossRef]
- Noorbazargan, H.; Amintehrani, S.; Dolatabadi, A.; Mashayekhi, A.; Khayam, N.; Moulavi, P.; Naghizadeh, M.; Mirzaie, A.; Mirzaei Rad, F.; Kavousi, M. Anti-cancer & anti-metastasis properties of bioorganic-capped silver nanoparticles fabricated from Juniperus chinensis extract against lung cancer cells. AMB Express 2021, 11, 61. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.A.; Edrees, N.; El-Sayed, H.; Khamis, T.; Arisha, A.H.; Metwally, M.M.M.; Eleiwa, N.Z.; Galal, A.A.A. Could Cisplatin Loading on Biosynthesized Silver Nanoparticles Improve Its Therapeutic Efficacy on Human Prostate Cancer Cell Line and Reduce Its In Vivo Nephrotoxic Effects? Biol. Trace Elem. Res. 2022, 200, 582–590. [Google Scholar] [CrossRef]
- Kumari, R.; Saini, A.K.; Kumar, A.; Saini, R.V. Apoptosis induction in lung and prostate cancer cells through silver nanoparticles synthesized from Pinus roxburghii bioactive fraction. J. Biol. Inorg. Chem. 2020, 25, 23–37. [Google Scholar] [CrossRef]
- Maity, P.; Bepari, M.; Pradhan, A.; Baral, R.; Roy, S.; Maiti Choudhury, S. Synthesis and characterization of biogenic metal nanoparticles and its cytotoxicity and anti-neoplasticity through the induction of oxidative stress, mitochondrial dysfunction and apoptosis. Colloids Surf. B Biointerface 2018, 161, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Narasimha, V.R.; Latha, T.S.; Pallu, R.; Panati, K.; Narala, V.R. Anticancer Activities of Biogenic Silver Nanoparticles Targeting Apoptosis and Inflammatory Pathways in Colon Cancer Cells. J. Clust. Sci. 2021, 33, 2215–2231. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chelliah, R.; MubarakAli, D.; Oh, D.H.; Kathiresan, K.; Wang, M.H. Unveiling the potentials of biocompatible silver nanoparticles on human lung carcinoma A549 cells and Helicobacter pylori. Sci. Rep. 2019, 9, 5787. [Google Scholar] [CrossRef]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef]
- Barabadi, H.; Mahjoub, M.A.; Tajani, B.; Ahmadi, A.; Junejo, Y.; Saravanan, M. Emerging Theranostic Biogenic Silver Nanomaterials for Breast Cancer: A Systematic Review. J. Clust. Sci. 2019, 30, 259–279. [Google Scholar] [CrossRef]
- Atmaca, H.; Camli Pulat, C.; Ilhan, S. Synthesis of silver nanoparticles using Alpinia officinarum rhizome extract induces apoptosis through down-regulating Bcl-2 in human cancer cells. Biol. Futur. 2022, 73, 327–334. [Google Scholar] [CrossRef]
- Behboodi, S.; Baghbani-Arani, F.; Abdalan, S.; Sadat Shandiz, S.A. Green Engineered Biomolecule-Capped Silver Nanoparticles Fabricated from Cichorium intybus Extract: In Vitro Assessment on Apoptosis Properties Toward Human Breast Cancer (MCF-7) Cells. Biol. Trace Elem. Res. 2019, 187, 392–402. [Google Scholar] [CrossRef]
- Datkhile, K.D.; Patil, S.R.; Durgawale, P.P.; Patil, M.N.; Jagdale, N.J.; Deshmukh, V.N.; More, A.L. Biogenic Silver Nanoparticles Synthesized Using Mexican Poppy Plant Inhibits Cell Growth in Cancer Cells through Activation of Intrinsic Apoptosis Pathway. Nano Biomed. Eng. 2020, 12, 241–252. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Ma, S.; Cheng, S.; Jiang, S.; Liu, Y.; Li, D.; Huang, H.; Zhang, K.; Zheng, X. Biosynthesis, Antibacterial Activity and Anticancer Effects Against Prostate Cancer (PC-3) Cells of Silver Nanoparticles Using Dimocarpus Longan Lour. Peel Extract. Nanoscale Res. Lett. 2016, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Du, Z.; Ma, S.; Liu, Y.; Li, D.; Huang, H.; Jiang, S.; Cheng, S.; Wu, W.; Zhang, K.; et al. Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int. J. Nanomed. 2016, 11, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Roy, B.; Shaw, P.; Mondal, P.; Mondal, M.K.; Chowdhury, P.; Bhattacharya, S.; Chattopadhyay, A. Cytotoxic effect of green synthesized silver nanoparticles in MCF7 and MDA-MB-231 human breast cancer cells in vitro. Nucleus 2019, 63, 191–202. [Google Scholar] [CrossRef]
- Bethu, M.S.; Netala, V.R.; Domdi, L.; Tartte, V.; Janapala, V.R. Potential anticancer activity of biogenic silver nanoparticles using leaf extract of Rhynchosia suaveolens: An insight into the mechanism. Artif. Cells Nanomed. Biotechnol. 2018, 46, 104–114. [Google Scholar] [CrossRef]
- Shiripoure Ganjineh Ketab, R.; Tafvizi, F.; Khodarahmi, P. Biosynthesis and Chemical Characterization of Silver Nanoparticles Using Satureja Rechingeri Jamzad and Their Apoptotic Effects on AGS Gastric Cancer Cells. J. Clust. Sci. 2020, 32, 1389–1399. [Google Scholar] [CrossRef]
- Plackal Adimuriyil George, B.; Kumar, N.; Abrahamse, H.; Ray, S.S. Apoptotic efficacy of multifaceted biosynthesized silver nanoparticles on human adenocarcinoma cells. Sci. Rep. 2018, 8, 14368. [Google Scholar] [CrossRef]
- Acharya, D.; Satapathy, S.; Thathapudi, J.J.; Somu, P.; Mishra, G. Biogenic synthesis of silver nanoparticles using marine algae Cladophora glomerata and evaluation of apoptotic effects in human colon cancer cells. Mater. Technol. 2022, 37, 569–580. [Google Scholar] [CrossRef]
- Alfuraydi, A.A.; Devanesan, S.; Al-Ansari, M.; AlSalhi, M.S.; Ranjitsingh, A.J. Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J. Photochem. Photobiol. B Biol. 2019, 192, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bin Saeed, H.A.; Daghestani, M.H.; Ambreen, K.; Daghestani, M.H.; Al-Zahrani, S.A.; Alobaid, H.; Al-Malahi, N.M. Low Dose of Green Synthesized Silver Nanoparticles is Sufficient to Cause Strong Cytotoxicity via its Cytotoxic Efficiency and Modulatory Effects on the Expression of PIK3CA and KRAS Oncogenes, in Lung and Cervical Cancer Cells. J. Clust. Sci. 2022, 34, 2471–2485. [Google Scholar] [CrossRef]
- Jyoti, K.; Singh, A.; Fekete, G.; Singh, T. Cytotoxic and radiosensitizing potential of silver nanoparticles against HepG-2 cells prepared by biosynthetic route using Picrasma quassioides leaf extract. J. Drug Deliv. Sci. Technol. 2020, 55, 101479. [Google Scholar] [CrossRef]
- Kabir, S.R.; Dai, Z.; Nurujjaman, M.; Cui, X.; Asaduzzaman, A.K.M.; Sun, B.; Zhang, X.; Dai, H.; Zhao, X. Biogenic silver/silver chloride nanoparticles inhibit human glioblastoma stem cells growth in vitro and Ehrlich ascites carcinoma cell growth in vivo. J. Cell. Mol. Med. 2020, 24, 13223–13234. [Google Scholar] [CrossRef]
- Kabir, S.R.; Islam, F.; Asaduzzaman, A.K.M. Biogenic silver/silver chloride nanoparticles inhibit human cancer cells proliferation in vitro and Ehrlich ascites carcinoma cells growth in vivo. Sci. Rep. 2022, 12, 8909. [Google Scholar] [CrossRef]
- Mahmud, K.M.; Hossain, M.M.; Polash, S.A.; Takikawa, M.; Shakil, M.S.; Uddin, M.F.; Alam, M.; Ali Khan Shawan, M.M.; Saha, T.; Takeoka, S.; et al. Investigation of Antimicrobial Activity and Biocompatibility of Biogenic Silver Nanoparticles Synthesized using Syzigyum cymosum Extract. ACS Omega 2022, 7, 27216–27229. [Google Scholar] [CrossRef]
- Ferreira, L.A.B.; Garcia-Fossa, F.; Radaic, A.; Durán, N.; Fávaro, W.J.; de Jesus, M.B. Biogenic silver nanoparticles: In vitro and in vivo antitumor activity in bladder cancer. Eur. J. Pharm. Biopharm. 2020, 151, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Albasher, G.; Bin-Meferij, M.M.; Cherukula, K. Apoptotic Responses Mediated by Nostoc-Synthesized Silver Nanoparticles against Ehrlich Ascites Carcinoma Tumor-Bearing Mice. J. Nanomater. 2023, 2023, 4648571. [Google Scholar] [CrossRef]
- Abosharaf, H.A.; Salah, M.; Diab, T.; Tsubaki, M.; Mohamed, T.M. Biogenic silver nanoparticles induce apoptosis in Ehrlich ascites carcinoma. Biomed. Res. Ther. 2020, 7, 4100–4113. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; Khattab, S.M.; Abu-Youssef, M.A.; Badr, A.M.A. Green synthesis of novel stable biogenic gold nanoparticles for breast cancer therapeutics via the induction of extrinsic and intrinsic pathways. Sci. Rep. 2022, 12, 11518. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Raja, K.; Khub Chandra, V.; Sadras, S.R. Green synthesized silver nanoparticles: Catalytic dye degradation, in vitro anticancer activity and in vivo toxicity in rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Syama, H.P.; Unnikrishnan, B.S.; Sreekutty, J.; Archana, M.G.; Joseph, M.M.; Preethi, G.U.; Anusree, K.S.; Reshma, P.L.; Shiji, R.; Sreelekha, T.T. Bio fabrication of galactomannan capped silver nanoparticles to apprehend Ehrlich ascites carcinoma solid tumor in mice. J. Drug Deliv. Sci. Technol. 2022, 76, 103649. [Google Scholar] [CrossRef]
- Lalsangpuii, F.; Rokhum, S.L.; Nghakliana, F.; Fakawmi, L.; Ruatpuia, J.V.L.; Laltlanmawii, E.; Lalfakzuala, R.; Siama, Z. Green Synthesis of Silver Nanoparticles Using Spilanthes acmella Leaf Extract and its Antioxidant-Mediated Ameliorative Activity against Doxorubicin-Induced Toxicity in Dalton’s Lymphoma Ascites (DLA)-Bearing Mice. ACS Omega 2022, 7, 44346–44359. [Google Scholar] [CrossRef]
- Gao, S.; Chen, D.; Li, Q.; Ye, J.; Jiang, H.; Amatore, C.; Wang, X. Near-infrared fluorescence imaging of cancer cells and tumors through specific biosynthesis of silver nanoclusters. Sci. Rep. 2014, 4, 4384. [Google Scholar] [CrossRef]
- Haque, S.; Norbert, C.C.; Acharyya, R.; Mukherjee, S.; Kathirvel, M.; Patra, C.R. Biosynthesized Silver Nanoparticles for Cancer Therapy and In Vivo Bioimaging. Cancers 2021, 13, 6114. [Google Scholar] [CrossRef]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer Therapy by Silver Nanoparticles: Fiction or Reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Singh, A.; Amod, A.; Pandey, P.; Bose, P.; Pingali, M.S.; Shivalkar, S.; Varadwaj, P.K.; Sahoo, A.K.; Samanta, S.K. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed. Mater. 2022, 17, 022003. [Google Scholar] [CrossRef]
- Flemming, H.C.; Baveye, P.; Neu, T.R.; Stoodley, P.; Szewzyk, U.; Wingender, J.; Wuertz, S. Who put the film in biofilm? The migration of a term from wastewater engineering to medicine and beyond. npj Biofilms Microbiomes 2021, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, O.; Bednarke, B.; Sherriff, H.; Doiron, A.L. Nanoparticle-Based Strategies for Managing Biofilm Infections in Wounds: A Comprehensive Review. ACS Omega 2024, 9, 27853–27871. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K. Nanoengineered Antibacterial Coatings and Materials: A Perspective. Coatings 2019, 9, 654. [Google Scholar] [CrossRef]
- Hajji, S.; Khedir, S.B.; Hamza-Mnif, I.; Hamdi, M.; Jedidi, I.; Kallel, R.; Boufi, S.; Nasri, M. Biomedical potential of chitosan-silver nanoparticles with special reference to antioxidant, antibacterial, hemolytic and in vivo cutaneous wound healing effects. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 241–254. [Google Scholar] [CrossRef]
- Ye, H.; Cheng, J.; Yu, K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol. 2019, 121, 633–642. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. H 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Bohara, S.; Suthakorn, J. Surface coating of orthopedic implant to enhance the osseointegration and reduction of bacterial colonization: A review. Biomater. Res. 2022, 26, 26. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- De Gusseme, B.; Sintubin, L.; Baert, L.; Thibo, E.; Hennebel, T.; Vermeulen, G.; Uyttendaele, M.; Verstraete, W.; Boon, N. Biogenic silver for disinfection of water contaminated with viruses. Appl. Environ. Microbiol. 2010, 76, 1082–1087. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, J.; Yu, R.; Yan, H.; Liang, R. Silver Nanoparticle-Incorporated Porous Renewable Film as Low-Cost Bactericidal and Antifouling Filter for Point-of-Use Water Disinfection. Ind. Eng. Chem. Res. 2020, 59, 10857–10867. [Google Scholar] [CrossRef]
- Fernández, J.G.; Almeida, C.A.; Fernández-Baldo, M.A.; Felici, E.; Raba, J.; Sanz, M.I. Development of nitrocellulose membrane filters impregnated with different biosynthesized silver nanoparticles applied to water purification. Talanta 2016, 146, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Boegli, L.; Agostinho, A.; Sánchez, E.M.; Bach, H.; Ruiz, F.; James, G. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 2013, 29, 651–660. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Singh, S.; Wunnoo, S.; Lerwittayanon, K.; Voravuthikunchai, S.P. Facile deposition of biogenic silver nanoparticles on porous alumina discs, an efficient antimicrobial, antibiofilm, and antifouling strategy for functional contact surfaces. Biofouling 2021, 37, 538–554. [Google Scholar] [CrossRef]
- Poon, T.K.C.; Iyengar, K.P.; Jain, V.K. Silver Nanoparticle (AgNP) Technology applications in trauma and orthopaedics. J. Clin. Orthop. Trauma. 2021, 21, 101536. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Al-Dahmash, N.D.; Ranjitsingh, A.J.A. Synthesis of silver nanoparticles using gum Arabic: Evaluation of its inhibitory action on Streptococcus mutans causing dental caries and endocarditis. J. Infect. Public Health 2021, 14, 324–330. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnol. 2015, 13, 91. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Pandey, M.; Lim, Y.Q.; Low, C.Y.; Lee, C.T.; Marilyn, T.C.L.; Loh, H.S.; Lim, Y.P.; Lee, C.F.; Bhattamishra, S.K.; et al. Silver nanoparticles: Advanced and promising technology in diabetic wound therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110925. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.B.; Raffaelli, S.; Mitchell, S.G.; Faccio, R.; Alborés, S. Biofilm Eradication Using Biogenic Silver Nanoparticles. Molecules 2020, 25, 2023. [Google Scholar] [CrossRef] [PubMed]
- Yadi, M.; Azizi, M.; Dianat-Moghadam, H.; Akbarzadeh, A.; Abyadeh, M.; Milani, M. Antibacterial activity of green gold and silver nanoparticles using ginger root extract. Bioprocess. Biosyst. Eng. 2022, 45, 1905–1917. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Jers, C.; Joshi, A.S.; Garnaes, J.; Mijakovic, I. Silver nanoparticles produced from Cedecea sp. exhibit antibiofilm activity and remarkable stability. Sci. Rep. 2021, 11, 12619. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Pandey, L.M. Design of antibiofilm surfaces by immobilization of biogenic silver nanoparticles on amine self-assembled monolayers. Mater. Lett. 2022, 311, 131574. [Google Scholar] [CrossRef]
- Ghosh, S.; Mondol, S.; Lahiri, D.; Nag, M.; Sarkar, T.; Pati, S.; Pandit, S.; Alarfaj, A.A.; Mohd Amin, M.F.; Edinur, H.A.; et al. Biogenic silver nanoparticles (AgNPs) from Tinosporacordifolia leaves: An effective antibiofilm agent against Staphylococcus aureus ATCC 23235. Front. Chem. 2023, 11, 1118454. [Google Scholar] [CrossRef]
- Ahamad, I.; Bano, F.; Anwer, R.; Srivastava, P.; Kumar, R.; Fatma, T. Antibiofilm Activities of Biogenic Silver Nanoparticles Against Candida albicans. Front. Microbiol. 2021, 12, 741493. [Google Scholar] [CrossRef]
- Ahamad, I.; Aziz, N.; Zaki, A.; Fatma, T. Synthesis and characterization of silver nanoparticles using Anabaena variabilis as a potential antimicrobial agent. J. Appl. Phycol. 2021, 33, 829–841. [Google Scholar] [CrossRef]
- Thomas, R.; Nair, A.P.; Kr, S.; Mathew, J.; Ek, R. Antibacterial activity and synergistic effect of biosynthesized AgNPs with antibiotics against multidrug-resistant biofilm-forming coagulase-negative staphylococci isolated from clinical samples. Appl. Biochem. Biotechnol. 2014, 173, 449–460. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Synergistic Antifungal Efficiency of Biogenic Silver Nanoparticles with Itraconazole against Multidrug-Resistant Candidal Strains. Crystals 2022, 12, 816. [Google Scholar] [CrossRef]
- Mohanty, S.; Jena, P.; Mehta, R.; Pati, R.; Banerjee, B.; Patil, S.; Sonawane, A. Cationic antimicrobial peptides and biogenic silver nanoparticles kill mycobacteria without eliciting DNA damage and cytotoxicity in mouse macrophages. Antimicrob. Agents Chemother. 2013, 57, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Spoladori, L.F.A.; Andriani, G.M.; Castro, I.M.; Suzukawa, H.T.; Gimenes, A.C.R.; Bartolomeu-Gonçalves, G.; Ishida, K.; Nakazato, G.; Pinge-Filho, P.; Machado, R.R.B.; et al. Synergistic Antifungal Interaction between Pseudomonas aeruginosa LV Strain Metabolites and Biogenic Silver Nanoparticles against Candida auris. Antibiotics 2023, 12, 861. [Google Scholar] [CrossRef]
- Joanna, C.; Marcin, L.; Ewa, K.; Grażyna, P. A nonspecific synergistic effect of biogenic silver nanoparticles and biosurfactant towards environmental bacteria and fungi. Ecotoxicology 2018, 27, 352–359. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Vo, T.N.N.; Nguyen, N.T.; Ching, Y.C.; Hoang Thi, T.T. Comparison of biogenic silver nanoparticles formed by Momordica charantia and Psidium guajava leaf extract and antifungal evaluation. PLoS ONE 2020, 15, e0239360. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel Biogenic Silver Nanoparticle-Induced Reactive Oxygen Species Inhibit the Biofilm Formation and Virulence Activities of Methicillin-Resistant Staphylococcus aureus (MRSA) Strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Barabadi, H.; Mojab, F.; Vahidi, H.; Marashi, B.; Talank, N.; Hosseini, O.; Saravanan, M. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg. Chem. Commun. 2021, 129, 108647. [Google Scholar] [CrossRef]
- Al-saggaf, M.S.; Saravanan, R. Formulation of Insect Chitosan Stabilized Silver Nanoparticles with Propolis Extract as Potent Antimicrobial and Wound Healing Composites. Int. J. Polym. Sci. 2021, 2021, 5578032. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, X.; Li, M.; Yan, L.; Lu, Y.; Jiang, C.; Liu, Y.; Pan, Z.; Shi, J. Antibacterial and wound healing-promoting effect of sponge-like chitosan-loaded silver nanoparticles biosynthesized by iturin. Int. J. Biol. Macromol. 2021, 181, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Velramar, B.; Pandiyan, R.; Velu, R.K. Anti-pseudomonal and anti-endotoxic effects of surfactin-stabilized biogenic silver nanocubes ameliorated wound repair in streptozotocin-induced diabetic mice. Artif. Cells Nanomed. Biotechnol. 2018, 46, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Velramar, B.; Ramatchandirin, B.; Abraham, G.C.; Duraisamy, N.; Pandiyan, R.; Velu, R.K. Effect of biogenic silver nanocubes on matrix metalloproteinases 2 and 9 expressions in hyperglycemic skin injury and its impact in early wound healing in streptozotocin-induced diabetic mice. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 146–152. [Google Scholar] [CrossRef]
- Attallah, N.G.M.; Elekhnawy, E.; Negm, W.A.; Hussein, I.A.; Mokhtar, F.A.; Al-Fakhrany, O.M. In Vivo and In Vitro Antimicrobial Activity of Biogenic Silver Nanoparticles against Staphylococcus aureus Clinical Isolates. Pharmaceuticals 2022, 15, 194. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Koh, T.W.; Bhatnagar, S. Synthesis, Characterization, Antibacterial and Wound Healing Efficacy of Silver Nanoparticles From Azadirachta indica. Front. Microbiol. 2021, 12, 611560. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, N.M.; Abo-Eleneen, M.A.; Al-Madboly, L.A.; Sharaf, M.M.; Othman, S.S.; Ibrahim, O.M.; Mubarak, M.S. Biogenically Synthesized Polysaccharides-Capped Silver Nanoparticles: Immunomodulatory and Antibacterial Potentialities Against Resistant Pseudomonas aeruginosa. Front. Bioeng. Biotechnol. 2020, 8, 643. [Google Scholar] [CrossRef]
- Lakkim, V.; Reddy, M.C.; Pallavali, R.R.; Reddy, K.R.; Reddy, C.V.; Inamuddin; Bilgrami, A.L.; Lomada, D. Green Synthesis of Silver Nanoparticles and Evaluation of Their Antibacterial Activity against Multidrug-Resistant Bacteria and Wound Healing Efficacy Using a Murine Model. Antibiotics 2020, 9, 902. [Google Scholar] [CrossRef]
- Marcato, P.D.; De Paula, L.B.; Melo, P.S.; Ferreira, I.R.; Almeida, A.B.A.; Torsoni, A.S.; Alves, O.L.; Choy, J.-H. In Vivo Evaluation of Complex Biogenic Silver Nanoparticle and Enoxaparin in Wound Healing. J. Nanomater. 2015, 2015, 439820. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional Silver Nanoparticles Based on Chitosan: Antibacterial, Antibiofilm, Antifungal, Antioxidant, and Wound-Healing Activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Banerjee, T.; Pratap, A.; Shukla, V.K. Significant in-Vitro and in-Vivo Antimicrobial and Antibiofilm Activity of Colloidal Silver Nanoparticles (cAgNPs) in Chronic Diabetic Foot Ulcers. Int. J. Low. Extrem. Wounds 2025, 24, 303–311. [Google Scholar] [CrossRef]
- Babu, S.M.; Ittiachen, L. Biogenic silver nanoparticle-coated low density polyethylene film with antibacterial and antioxidant properties. J. Coat. Technol. Res. 2024, 21, 1409–1420. [Google Scholar] [CrossRef]
- Syukri, D.M.; Singh, S.; Nwabor, O.F.; Ontong, J.C.; Dejyong, K.; Sunghan, J.; Dejyong, K.; Lethongkam, S.; Voravuthikunchai, S.P. Prevention of Post-operative Bacterial Colonization on Mice Buccal Mucosa Using Biogenic Silver Nanoparticles-Coated Nylon Sutures. Regen. Eng. Transl. Med. 2024, 10, 294–308. [Google Scholar] [CrossRef]
- De Simone, S.; Gallo, A.L.; Paladini, F.; Sannino, A.; Pollini, M. Development of silver nano-coatings on silk sutures as a novel approach against surgical infections. J. Mater. Sci. Mater. Med. 2014, 25, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Divya, M.; Kiran, G.S.; Hassan, S.; Selvin, J. Biogenic synthesis and effect of silver nanoparticles (AgNPs) to combat catheter-related urinary tract infections. Biocatal. Agric. Biotechnol. 2019, 18, 101037. [Google Scholar] [CrossRef]
- Sato, T.P.; Conjo, C.I.; Rossoni, R.D.; Junqueira, J.C.; Melo, R.M.d.; Durán, N.; Borges, A.L.S. Antimicrobial and mechanical acrylic resin properties with silver particles obtained from Fusarium oxysporum. Braz. Dent. Sci. 2018, 21, 96–103. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, A.G.; Romano de Oliveira Gonçalves, P.J.; Ottoni, C.A.; de Cassia Ruiz, R.; Morgano, M.A.; de Araújo, W.L.; de Melo, I.S.; De Souza, A.O. Functional textiles impregnated with biogenic silver nanoparticles from Bionectria ochroleuca and its antimicrobial activity. Biomed. Microdevice 2019, 21, 56. [Google Scholar] [CrossRef]
- Ballottin, D.; Fulaz, S.; Cabrini, F.; Tsukamoto, J.; Durán, N.; Alves, O.L.; Tasic, L. Antimicrobial textiles: Biogenic silver nanoparticles against Candida and Xanthomonas. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 582–589. [Google Scholar] [CrossRef]
- Ahmad, F.; Ashraf, N.; Elahi, M.I.; Zhou, Y.; Lu, Y.; Yin, D.-C. Fabrication of biogenic-silver nanoparticles functionalized electrospun membranes counteracting bacteria and enhance wound healing. Mater. Today Commun. 2022, 31, 103493. [Google Scholar] [CrossRef]
- Loh, J.V.; Percival, S.L.; Woods, E.J.; Williams, N.J.; Cochrane, C.A. Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int. Wound J. 2009, 6, 32–38. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Awwad, N.S.; Nabil, S.; Salama, A.; Youssef, M.A. Oxidized alginate/gelatin decorated silver nanoparticles as new nanocomposite for dye adsorption. Int. J. Biol. Macromol. 2019, 141, 1280–1286. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Chandra, R. Environmental pollutants of paper industry wastewater and their toxic effects on human health and ecosystem. Bioresour. Technol. Rep. 2022, 20, 101250. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Z.; Cao, H.; Xu, M.; Xu, L. Concentrations, speciation, and ecological risk of heavy metals in the sediment of the Songhua River in an urban area with petrochemical industries. Chemosphere 2019, 219, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Uma, V. Rapid extraction of Cu(II) heavy metal from industrial waste water by using silver nanoparticles anchored with novel Schiff base. Sep. Sci. Technol. 2018, 54, 1182–1193. [Google Scholar] [CrossRef]
- Praveena, S.M.; Han, L.S.; Than, L.T.L.; Aris, A.Z. Preparation and characterisation of silver nanoparticle coated on cellulose paper: Evaluation of their potential as antibacterial water filter. J. Exp. Nanosci. 2016, 11, 1307–1319. [Google Scholar] [CrossRef]
- Nowak, A.; Pacek, G.; Mrozik, A. Transformation and ecotoxicological effects of iodinated X-ray contrast media. Rev. Environ. Sci. Biotechnol. 2020, 19, 337–354. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fang, F.; Zhang, B.; Wu, J.J.; Zhang, K. Biogenic silver nanoparticles-modified forward osmosis membranes with mitigated internal concentration polarization and enhanced antibacterial properties. npj Clean Water 2022, 5, 41. [Google Scholar] [CrossRef]
- Omo-Okoro, P.N.; Curtis, C.J.; Marco, A.M.; Melymuk, L.; Okonkwo, J.O. Removal of per- and polyfluoroalkyl substances from aqueous media using synthesized silver nanocomposite-activated carbons. J. Environ. Health Sci. Eng. 2021, 19, 217–236. [Google Scholar] [CrossRef]
- Smalling, K.L.; Romanok, K.M.; Bradley, P.M.; Morriss, M.C.; Gray, J.L.; Kanagy, L.K.; Gordon, S.E.; Williams, B.M.; Breitmeyer, S.E.; Jones, D.K.; et al. Per- and polyfluoroalkyl substances (PFAS) in United States tapwater: Comparison of underserved private-well and public-supply exposures and associated health implications. Environ. Int. 2023, 178, 108033. [Google Scholar] [CrossRef]
- Fiorati, A.; Bellingeri, A.; Punta, C.; Corsi, I.; Venditti, I. Silver Nanoparticles for Water Pollution Monitoring and Treatments: Ecosafety Challenge and Cellulose-Based Hybrids Solution. Polymers 2020, 12, 1635. [Google Scholar] [CrossRef]
- Heinemann, M.G.; Rosa, C.H.; Rosa, G.R.; Dias, D. Biogenic synthesis of gold and silver nanoparticles used in environmental applications: A review. Trend Environ. Anal. Chem. 2021, 30, e00129. [Google Scholar] [CrossRef]
- Arya, G.; Mankamna Kumari, R.; Sharma, N.; Chatterjee, S.; Gupta, N.; Kumar, A.; Nimesh, S. Evaluation of antibiofilm and catalytic activity of biogenic silver nanoparticles synthesized from Acacia nilotica leaf extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 045003. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Kiran Kumar, H.A.; Mandal, B.K. Biofabricated silver nanoparticles as green catalyst in the degradation of different textile dyes. J. Environ. Chem. Eng. 2016, 4, 56–64. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for Efficient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202. [Google Scholar] [CrossRef]
- González-Pedroza, M.G.; Benítez, A.R.T.; Navarro-Marchal, S.A.; Martínez-Martínez, E.; Marchal, J.A.; Boulaiz, H.; Morales-Luckie, R.A. Biogeneration of silver nanoparticles from Cuphea procumbens for biomedical and environmental applications. Sci. Rep. 2023, 13, 790. [Google Scholar] [CrossRef]
- Hamedi, S.; Shojaosadati, S.A. Rapid and green synthesis of silver nanoparticles using Diospyros lotus extract: Evaluation of their biological and catalytic activities. Polyhedron 2019, 171, 172–180. [Google Scholar] [CrossRef]
- Rajan, A.; Vilas, V.; Philip, D. Catalytic and antioxidant properties of biogenic silver nanoparticles synthesized using Areca catechu nut. J. Mol. Liq. 2015, 207, 231–236. [Google Scholar] [CrossRef]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Photocatalytic activity of biogenic silver nanoparticles synthesized using potato (Solanum tuberosum) infusion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 146, 286–291. [Google Scholar] [CrossRef]
- Santhosh, A.S.; Sandeep, S.; Kumara Swamy, N. Green synthesis of nano silver from Euphorbia geniculata leaf extract: Investigations on catalytic degradation of methyl orange dye and optical sensing of Hg2+. Surf. Interface 2019, 14, 50–54. [Google Scholar] [CrossRef]
- Sharbaf Moghadas, M.R.; Motamedi, E.; Nasiri, J.; Naghavi, M.R.; Sabokdast, M. Proficient dye removal from water using biogenic silver nanoparticles prepared through solid-state synthetic route. Heliyon 2020, 6, e04730. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Darroudi, M.; Amiri, M.S.; Zarrinfar, H.; Hosseini, H.A.; Mashreghi, M.; Mozafarri, H.; Ghorbani, A.; Mousavi, S.H. Antimycobacterial, Anticancer, Antioxidant and Photocatalytic Activity of Biosynthesized Silver Nanoparticles Using Berberis Integerrima. Iran. J. Sci. Technol. Trans. A Sci. 2021, 46, 1–11. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Shahid, M.; Niazi, M.B.K.; Hussain, S.; Manzoor, N.; Wang, X.; Li, B. Green synthesis of silver nanoparticles transformed synthetic textile dye into less toxic intermediate molecules through LC-MS analysis and treated the actual wastewater. Environ. Res. 2020, 191, 110142. [Google Scholar] [CrossRef]
- Naseem, K.; Zia Ur Rehman, M.; Ahmad, A.; Dubal, D.; AlGarni, T. Plant Extract Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for Degradation of Toxic Dyes. Coatings 2020, 10, 1235. [Google Scholar] [CrossRef]
- Vélez, E.; Campillo, G.; Morales, G.; Hincapié, C.; Osorio, J.; Arnache, O. Silver Nanoparticles Obtained by Aqueous or Ethanolic Aloe vera Extracts: An Assessment of the Antibacterial Activity and Mercury Removal Capability. J. Nanomater. 2018, 2018, 7215210. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, K.; De Gusseme, B.; Verstraete, W. Biogenic silver nanoparticles (bio-Ag0) decrease biofouling of bio-Ag0/PES nanocomposite membranes. Water Res. 2012, 46, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.T. Removal of pathogenic bacteria from wastewater using silver nanoparticles synthesized by two fungal species. Water Sci. 2019, 31, 164–176. [Google Scholar] [CrossRef]
- De, A.; Kalita, D. Bio-Fabricated Gold and Silver Nanoparticle Based Plasmonic Sensors for Detection of Environmental Pollutants: An Overview. Crit. Rev. Anal. Chem. 2023, 53, 672–688. [Google Scholar] [CrossRef]
- Abasi, F.; Raja, N.I.; Mashwani, Z.U.R.; Amjad, M.S.; Ehsan, M.; Mustafa, N.; Haroon, M.; Proćków, J. Biogenic Silver Nanoparticles as a Stress Alleviator in Plants: A Mechanistic Overview. Molecules 2022, 27, 3378. [Google Scholar] [CrossRef]
- Rodriguez-Garraus, A.; Azqueta, A.; Vettorazzi, A.; Lopez de Cerain, A. Genotoxicity of Silver Nanoparticles. Nanomaterials 2020, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Okus, F.; Yuzbasioglu, D.; Unal, F. Green synthesized metal nanoparticles appear to meet expectations of low ecotoxicity: What about genotoxicity? Toxicol. Mech. Methods 2025, 35, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.D.; Vazquez-Duhalt, R.; Castro-Longoria, E.; Juarez-Moreno, K. Synergistic anticancer effects and reduced genotoxicity of silver nanoparticles and tamoxifen in breast cancer cells. J. Biochem. Mol. Toxicol. 2024, 38, e23823. [Google Scholar] [CrossRef]
- Zheng, W.; Shen, H.; Cheng, Y.; Liu, L.; Sun, J.; Chu, Z.; Wang, W.; Qian, H. Au Nanorods Activate Zn2+/Ag+ Mediated Anti-inflammatory for Enhanced Methicillin-Resistant Wound Repair via Bionic Claw Microneedles. ACS Mater. Lett. 2024, 6, 4791–4800. [Google Scholar] [CrossRef]
- Polaka, S.; Tekade, R.K. Development and Evaluation of Silver Nanomix as a Next-Generation Tool for Wound Healing and Dressing Applications. ACS Appl. Bio Mater. 2023, 6, 1832–1848. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.Y.; Wang, P.; Fu, H.; Yousef, F.; Xie, R.; Wang, W.; Liu, Z.; Pan, D.W.; Ju, X.J.; et al. Dissolving microneedle system containing Ag nanoparticle-decorated silk fibroin microspheres and antibiotics for synergistic therapy of bacterial biofilm infection. J. Colloid Interface Sci. 2024, 661, 123–138. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, M.; Xie, Y.; Wang, Y.; You, Y.; Ke, Y.; Zhang, C.; Chen, X.; Yang, Y.; Zhang, C.; et al. Multifunctional Ac@ZIF-8/AgNPs nanoplatform with pH-responsive and ROS scavenging antibacterial properties promotes infected wound healing. Chem. Eng. J. 2024, 489, 151485. [Google Scholar] [CrossRef]
- Sun, R.; Cui, Y.; Wu, Y.; Gao, M.; Xue, S.; Li, R.; Zboril, R.; Zhang, C. Overcoming Nanosilver Resistance: Resensitizing Bacteria and Targeting Evolutionary Mechanisms. ACS Nano 2025, 19, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).