Polyphenols and Bone Health: A Comprehensive Review of Their Role in Osteoporosis Prevention and Treatment
Abstract
1. Introduction
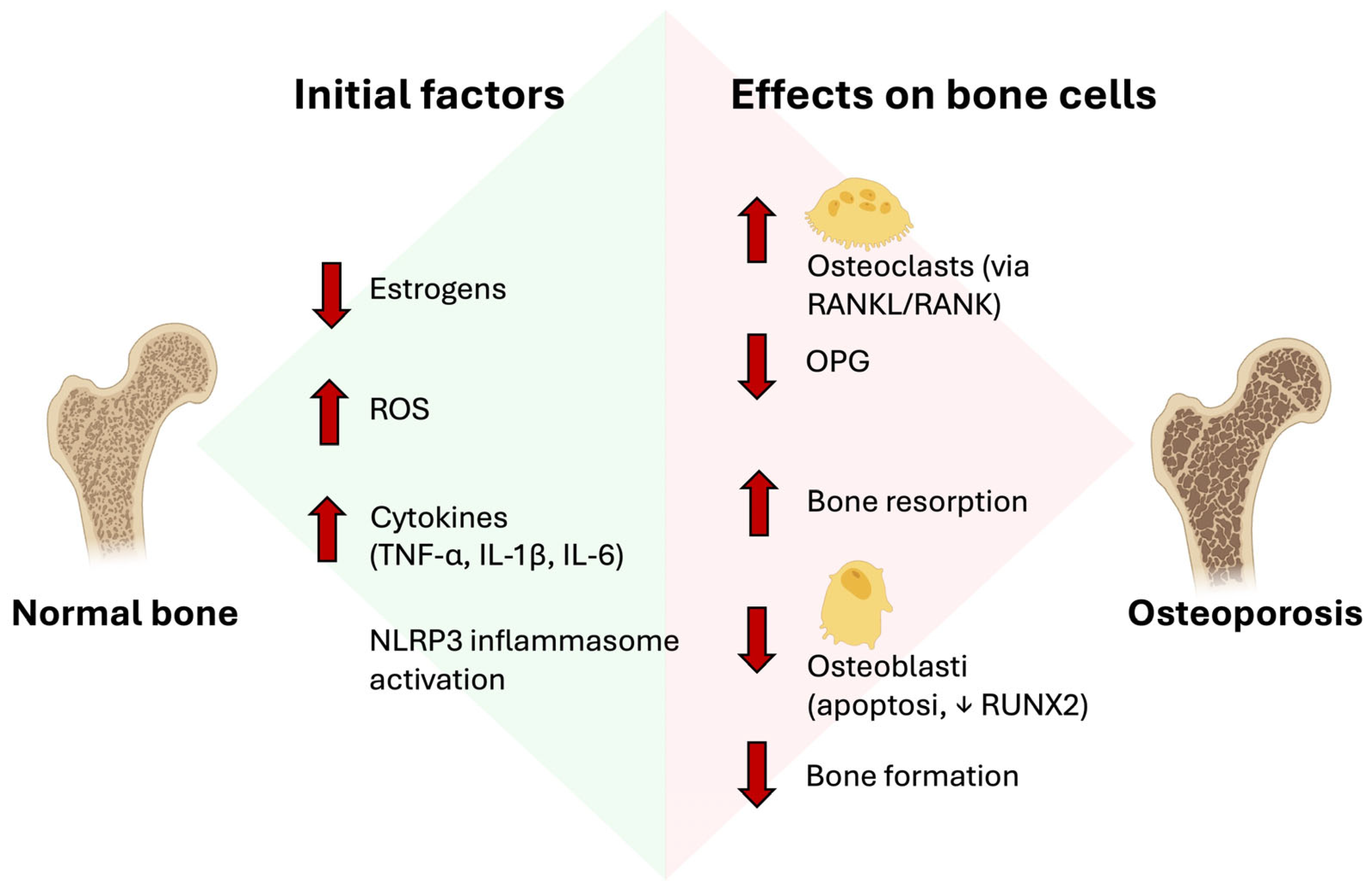
2. Pathophysiology of Osteoporosis
3. Polyphenols: Classification and Mechanisms of Action
Mechanisms of Action Relevant to Bone Health
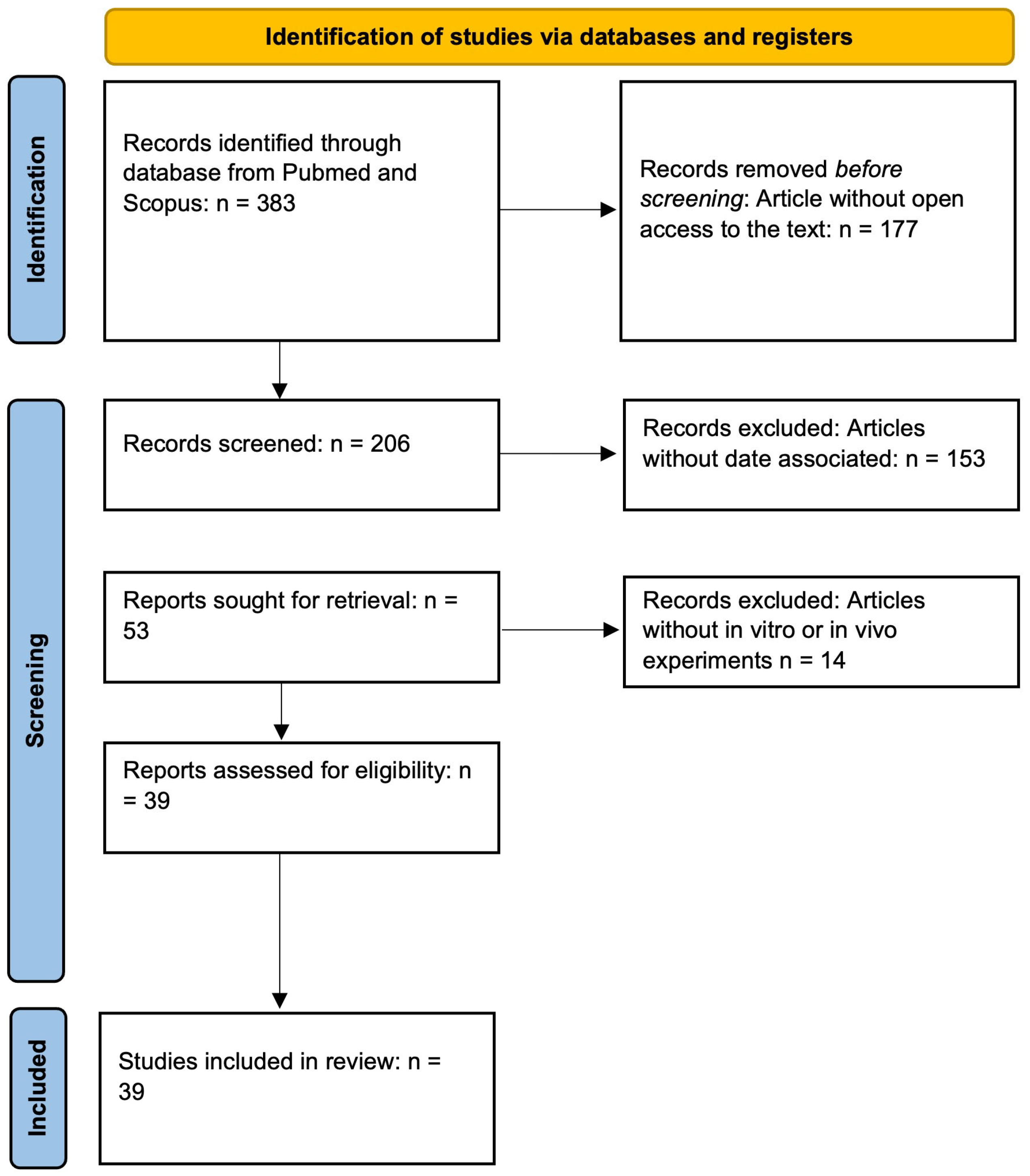
4. Methodology
5. Preclinical Evidence (In Vitro and In Vivo)
5.1. In Vitro Studies on Osteoblastic/Osteoclastic Cells
5.1.1. Quercetin
5.1.2. Resveratrol
5.1.3. Curcumin
5.1.4. Other Polyphenols
5.2. In Vivo Studies
5.2.1. Quercetin
5.2.2. Resveratrol
5.2.3. Fisetin
5.2.4. EGCG
5.2.5. Other Polyphenols
6. Clinical Studies
7. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HPA | 4-Hydroxyphenylacetic acid |
| AP-1 | Activator protein 1 |
| ALP | Alkaline phosphatase |
| BMD | Bone mineral density |
| BMP | Bone morphogenetic protein |
| CHH | Chronic high-altitude hypoxia |
| EGCG | Epigallocatechin gallate |
| MSC | Mesenchymal stem cells |
| MAPKs | Mitogen-Activated Protein Kinases |
| NF-κB | Nuclear factor kappa B |
| OPG | Osteoprotegerin |
| OSX | Osterix |
| OVX | Ovariectomy |
| ROS | Reactive oxygen species |
| RANKL | Receptor Activator of Nuclear Factor κB Ligand |
| RUNX2 | Runt-related transcription factor 2 |
| SCI | Spinal cord injury |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor-alpha |
References
- Aspray, T.J.; Hill, T.R. Osteoporosis and the Ageing Skeleton. Subcell. Biochem. 2019, 91, 453–476. [Google Scholar] [CrossRef]
- Xiao, P.-L.; Cui, A.-Y.; Hsu, C.-J.; Peng, R.; Jiang, N.; Xu, X.-H.; Ma, Y.-G.; Liu, D.; Lu, H.-D. Global, Regional Prevalence, and Risk Factors of Osteoporosis According to the World Health Organization Diagnostic Criteria: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- Li, M.; Ge, Z.; Zhang, B.; Sun, L.; Wang, Z.; Zou, T.; Chen, Q. Efficacy and Safety of Teriparatide vs. Bisphosphonates and Denosumab vs. Bisphosphonates in Osteoporosis Not Previously Treated with Bisphosphonates: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Arch. Osteoporos. 2024, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, L.; Wen, Z.; Wang, G. Romosozumab in Osteoporosis: Yesterday, Today and Tomorrow. J. Transl. Med. 2023, 21, 668. [Google Scholar] [CrossRef] [PubMed]
- Naik, A. The Role of Hormone Replacement Therapy in Osteoporosis: Benefits and Risks. J. Evid. Based Med. Healthc. 2024, 11, 1–2. [Google Scholar] [CrossRef]
- Gehrke, B.; Alves Coelho, M.C.; Brasil d’Alva, C.; Madeira, M. Long-Term Consequences of Osteoporosis Therapy with Bisphosphonates. Arch. Endocrinol. Metab. 2023, 68, e220334. [Google Scholar] [CrossRef]
- American Board of Family Medicine. Denosumab versus Bisphosphonates for Reducing Fractures in Postmenopausal Women with Osteoporosis: A Meta-Analysis. Available online: https://www.jabfm.org/content/early/2023/01/12/jabfm.2022.220099R1.abstract?utm_source=chatgpt.com (accessed on 17 September 2025).
- Bandeira, F.; de Oliveira, L.B.; Bilezikian, J.P. Long-Term Consequences of Osteoporosis Therapy with Denosumab. Arch. Endocrinol. Metab. 2022, 66, 717–723. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Chu, W.; Chou, W.-H.; Chu, W.-C.; Kang, Y.-N. Cardiovascular Safety of Romosozumab Compared to Commonly Used Anti-Osteoporosis Medications in Postmenopausal Osteoporosis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Drug Saf. 2025, 48, 7–23. [Google Scholar] [CrossRef]
- Lv, F.; Cai, X.; Yang, W.; Gao, L.; Chen, L.; Wu, J.; Ji, L. Denosumab or Romosozumab Therapy and Risk of Cardiovascular Events in Patients with Primary Osteoporosis: Systematic Review and Meta-Analysis. Bone 2020, 130, 115121. [Google Scholar] [CrossRef]
- Nannan, X.; Liyang, J.; Qiyan, L.I. Potential of Natural Medicines for Treatment of Osteoporosis: A Narrative Review. J. Tradit. Chin. Med. 2023, 43, 198–204. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Pompilio, C. Adherence to the Mediterranean Diet in Athletes. Sport Sci. 2020, 13, 58–63. [Google Scholar]
- Perrone, P.; D’Angelo, S. Original Article Extra Virgin Olive Oil as a Functional Food for Athletes: Recovery, Health, and Performance. J. Phys. Educ. Sport 2025, 25, 370–381. [Google Scholar] [CrossRef]
- Dernini, S.; Berry, E.M.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.X.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G.; et al. Med Diet 4.0: The Mediterranean Diet with Four Sustainable Benefits. Public Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef]
- D’Angelo, S. Current Evidence on the Effect of Dietary Polyphenols Intake on Brain Health. Curr. Nutr. Food Sci. 2020, 16, 1170–1182. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Cacciapuoti, G. Effects of Annurca Apple (Malus pumila cv Annurca) Polyphenols on Breast Cancer Cells. Curr. Nutr. Food Sci. 2019, 15, 745–751. [Google Scholar] [CrossRef]
- Notariale, R.; Moriello, C.; Alessio, N.; Del Vecchio, V.; Mele, L.; Perrone, P.; Manna, C. Protective Effect of Hydroxytyrosol against Hyperglycemia-Induced Phosphatidylserine Exposure in Human Erythrocytes: Focus on Dysregulation of Calcium Homeostasis and Redox Balance. Redox Biol. 2025, 85, 103783. [Google Scholar] [CrossRef]
- D’Angelo, S. Diet and Aging: The Role of Polyphenol-Rich Diets in Slow Down the Shortening of Telomeres: A Review. Antioxidants 2023, 12, 2086. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Lerner, U.H. Bone Remodeling in Post-Menopausal Osteoporosis. J. Dent. Res. 2006, 85, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Moon, R.J.; Dennison, E.M.; Harvey, N.C.; Cooper, C. Recent Advances in the Pathogenesis and Treatment of Osteoporosis. Clin. Med. 2016, 16, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Chen, L.-R.; Chen, K.-H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Kimball, J.S.; Johnson, J.P.; Carlson, D.A. Oxidative Stress and Osteoporosis. J. Bone Jt. Surg. 2021, 103, 1451–1461. [Google Scholar] [CrossRef]
- Edwards, C.J.; Williams, E. The Role of Interleukin-6 in Rheumatoid Arthritis-Associated Osteoporosis. Osteoporos. Int. 2010, 21, 1287–1293. [Google Scholar] [CrossRef]
- Ross, F.P. Interleukin 7 and Estrogen-Induced Bone Loss. Trends Endocrinol. Metab. 2003, 14, 147–149. [Google Scholar] [CrossRef]
- Jiang, N.; An, J.; Yang, K.; Liu, J.; Guan, C.; Ma, C.; Tang, X. NLRP3 Inflammasome: A New Target for Prevention and Control of Osteoporosis? Front. Endocrinol. 2021, 12, 752546. [Google Scholar] [CrossRef]
- Livshits, G.; Kalinkovich, A. Targeting Chronic Inflammation as a Potential Adjuvant Therapy for Osteoporosis. Life Sci. 2022, 306, 120847. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed. Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Chi, G.; Qiu, L.; Ma, J.; Wu, W.; Zhang, Y. The Association of Osteoprotegerin and RANKL with Osteoporosis: A Systematic Review with Meta-Analysis. J. Orthop. Surg. Res. 2023, 18, 839. [Google Scholar] [CrossRef]
- Deligiorgi, M.V.; Panayiotidis, M.I.; Siasos, G.; Trafalis, D.T. Osteoporosis Entwined with Cardiovascular Disease: The Implication of Osteoprotegerin and the Example of Statins. Curr. Med. Chem. 2021, 28, 1443–1467. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, R.; Rong, X.; Zhu, S.; Cui, Y.; Liu, H.; Li, M. Immunoporosis: Role of Immune System in the Pathophysiology of Different Types of Osteoporosis. Front. Endocrinol. 2022, 13, 965258. [Google Scholar] [CrossRef]
- Yan, C.; Shi, Y.; Yuan, L.; Lv, D.; Sun, B.; Wang, J.; Liu, X.; An, F. Mitochondrial Quality Control and Its Role in Osteoporosis. Front. Endocrinol. 2023, 14, 1077058. [Google Scholar] [CrossRef]
- Reid, I.R.; Bolland, M.J.; Grey, A. Effects of Vitamin D Supplements on Bone Mineral Density: A Systematic Review and Meta-Analysis. Lancet 2014, 383, 146–155. [Google Scholar] [CrossRef]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The Epidemiology of Osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Guzon-Illescas, O.; Perez Fernandez, E.; Crespí Villarias, N.; Quirós Donate, F.J.; Peña, M.; Alonso-Blas, C.; García-Vadillo, A.; Mazzucchelli, R. Mortality after Osteoporotic Hip Fracture: Incidence, Trends, and Associated Factors. J. Orthop. Surg. Res. 2019, 14, 203. [Google Scholar] [CrossRef]
- Bolat, E.; Sarıtaş, S.; Duman, H.; Eker, F.; Akdaşçi, E.; Karav, S.; Witkowska, A.M. Polyphenols: Secondary Metabolites with a Biological Impression. Nutrients 2024, 16, 2550. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids--Food Sources and Health Benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 79–85. [Google Scholar]
- Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. A Simple and Rapid Electrophoretic Method to Characterize Simple Phenols, Lignans, Complex Phenols, Phenolic Acids, and Flavonoids in Extra-Virgin Olive Oil. J. Sep. Sci. 2006, 29, 2221–2233. [Google Scholar] [CrossRef]
- Notariale, R.; Perrone, P.; Mele, L.; Lettieri, G.; Piscopo, M.; Manna, C. Olive Oil Phenols Prevent Mercury-Induced Phosphatidylserine Exposure and Morphological Changes in Human Erythrocytes Regardless of Their Different Scavenging Activity. Int. J. Mol. Sci. 2022, 23, 5693. [Google Scholar] [CrossRef]
- Perrone, P.; Spinelli, S.; Mantegna, G.; Notariale, R.; Straface, E.; Caruso, D.; Falliti, G.; Marino, A.; Manna, C.; Remigante, A.; et al. Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols. Cells 2023, 12, 424. [Google Scholar] [CrossRef]
- Perrone, P.; De Rosa, C.; D’Angelo, S. Mediterranean Diet and Agri-Food By-Products: A Possible Sustainable Approach for Breast Cancer Treatment. Antioxidants 2025, 14, 789. [Google Scholar] [CrossRef]
- Perrone, P.; Notariale, R.; Lettieri, G.; Mele, L.; La Pietra, V.; Piscopo, M.; Manna, C. Protective Effects of Olive Oil Antioxidant Phenols on Mercury-Induced Phosphatidylserine Externalization in Erythrocyte Membrane: Insights into Scramblase and Flippase Activity. Free Radic. Biol. Med. 2024, 227, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The Interplay between Cytokines, Inflammation, and Antioxidants: Mechanistic Insights and Therapeutic Potentials of Various Antioxidants and Anti-Cytokine Compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef] [PubMed]
- Notariale, R.; Längst, E.; Perrone, P.; Crettaz, D.; Prudent, M.; Manna, C. Effect of Mercury on Membrane Proteins, Anionic Transport and Cell Morphology in Human Erythrocytes. Cell Physiol. Biochem. 2022, 56, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Perrone, P.; Ortega-Luna, R.; Manna, C.; Álvarez-Ribelles, Á.; Collado-Diaz, V. Increased Adhesiveness of Blood Cells Induced by Mercury Chloride: Protective Effect of Hydroxytyrosol. Antioxidants 2024, 13, 1576. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health. Nutrients 2025, 17, 948. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Hormesis and Health: Molecular Mechanisms and the Key Role of Polyphenols. Food Chem. Adv. 2025, 7, 101030. [Google Scholar] [CrossRef]
- Perrone, P.; Palmieri, S.; Piscopo, M.; Lettieri, G.; Eugelio, F.; Fanti, F.; D’Angelo, S. Antioxidant Activity of Annurca Apple By-Products at Different Ripening Stages: A Sustainable Valorization Approach. Antioxidants 2025, 14, 941. [Google Scholar] [CrossRef]
- Vuoso, D.; Porcelli, M.; Cacciapuoti, G.; D’Angelo, S. Biological Activity of MelAnnurca Flesh Apple Biophenols. Curr. Nutr. Food Sci. 2020, 16, 1149–1162. [Google Scholar] [CrossRef]
- Vuoso, D.C.; D’Angelo, S.; Ferraro, R.; Caserta, S.; Guido, S.; Cammarota, M.; Porcelli, M.; Cacciapuoti, G. Annurca Apple Polyphenol Extract Promotes Mesenchymal-to-Epithelial Transition and Inhibits Migration in Triple-Negative Breast Cancer Cells through ROS/JNK Signaling. Sci. Rep. 2020, 10, 15921. [Google Scholar] [CrossRef]
- Ferrara, L.; Joksimovic, M.; D’Angelo, S. Could Polyphenolic Food Intake Help in the Control of Type 2 Diabetes? A Narrative Review of the Last Evidence. Curr. Nutr. Food Sci. 2022, 18, 785–798. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and Bioavailability: An Update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.A.; Lee, S.G.; Lee, S.-K.; Chun, O.K. Dietary Polyphenols, Berries, and Age-Related Bone Loss: A Review Based on Human, Animal, and Cell Studies. Antioxidants 2014, 3, 144–158. [Google Scholar] [CrossRef]
- Chisari, E.; Shivappa, N.; Vyas, S. Polyphenol-Rich Foods and Osteoporosis. Curr. Pharm. Des. 2019, 25, 2459–2466. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef]
- Su, Z.; Yao, B.; Liu, G.; Fang, J. Polyphenols as Potential Preventers of Osteoporosis: A Comprehensive Review on Antioxidant and Anti-Inflammatory Effects, Molecular Mechanisms, and Signal Pathways in Bone Metabolism. J. Nutr. Biochem. 2024, 123, 109488. [Google Scholar] [CrossRef]
- Wattel, A.; Kamel, S.; Prouillet, C.; Petit, J.-P.; Lorget, F.; Offord, E.; Brazier, M. Flavonoid Quercetin Decreases Osteoclastic Differentiation Induced by RANKL via a Mechanism Involving NF Kappa B and AP-1. J. Cell Biochem. 2004, 92, 285–295. [Google Scholar] [CrossRef]
- Hedbrant, A.; Persson, I.; Erlandsson, A.; Wijkander, J. Green, Black and Rooibos Tea Inhibit Prostaglandin E2 Formation in Human Monocytes by Inhibiting Expression of Enzymes in the Prostaglandin E2 Pathway. Molecules 2022, 27, 397. [Google Scholar] [CrossRef]
- Samiminemati, A.; Shahzaib, M.; Moriello, C.; Alessio, N.; Aprile, D.; Squillaro, T.; Di Bernardo, G.; Galderisi, U. Mesenchymal Stromal Cell (MSC) Isolation and Induction of Acute and Replicative Senescence. Methods Mol. Biol. 2025, 2960, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Samiminemati, A.; Shahzaib, M.; Moriello, C.; Alessio, N.; Aprile, D.; Squillaro, T.; Di Bernardo, G.; Galderisi, U. Methods to Detect and Compare Cellular and Mitochondrial Changes in Senescent and Healthy Mesenchymal Stem Cells. Methods Mol. Biol. 2025, 2960, 95–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-E.; Yang, Z.; Zhang, H.; Yao, G.; Liu, J.; Wei, Q.; Ma, B. Resveratrol Promotes Osteogenic Differentiation of Canine Bone Marrow Mesenchymal Stem Cells Through Wnt/Beta-Catenin Signaling Pathway. Cell. Reprogramm. 2018, 20, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-T.; Cheng, T.-L.; Lin, S.-Y.; Ho, C.-J.; Chyu, J.Y.; Yang, R.-S.; Chen, C.-H.; Shen, C.-L. Osteoprotective Roles of Green Tea Catechins. Antioxidants 2020, 9, 1136. [Google Scholar] [CrossRef]
- Bu, S.Y.; Hunt, T.S.; Smith, B.J. Dried Plum Polyphenols Attenuate the Detrimental Effects of TNF-Alpha on Osteoblast Function Coincident with up-Regulation of Runx2, Osterix and IGF-I. J. Nutr. Biochem. 2009, 20, 35–44. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Jie, Z. Effect of Polyphenols on Calcium Content and Alkaline Phosphatase Activity in Rat Femoral Tissues in Vitro. Biol. Pharm. Bull. 2001, 24, 1437–1439. [Google Scholar] [CrossRef]
- Jin, X.; Sun, J.; Yu, B.; Wang, Y.; Sun, W.J.; Yang, J.; Huang, S.H.; Xie, W.L. Daidzein Stimulates Osteogenesis Facilitating Proliferation, Differentiation, and Antiapoptosis in Human Osteoblast-like MG-63 Cells via Estrogen Receptor-Dependent MEK/ERK and PI3K/Akt Activation. Nutr. Res. 2017, 42, 20–30. [Google Scholar] [CrossRef]
- Chen, X.; Anderson, J.J.B. Isoflavones and Bone: Animal and Human Evidence of Efficacy. J. Musculoskelet. Neuronal Interact. 2002, 2, 352–359. [Google Scholar]
- Torre, E. Molecular Signaling Mechanisms behind Polyphenol-Induced Bone Anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef]
- Graef, J.L.; Rendina-Ruedy, E.; Crockett, E.K.; Ouyang, P.; King, J.B.; Cichewicz, R.H.; Lucas, E.A.; Smith, B.J. Select Polyphenolic Fractions from Dried Plum Enhance Osteoblast Activity through BMP-2 Signaling. J. Nutr. Biochem. 2018, 55, 59–67. [Google Scholar] [CrossRef]
- Prouillet, C.; Mazière, J.-C.; Mazière, C.; Wattel, A.; Brazier, M.; Kamel, S. Stimulatory Effect of Naturally Occurring Flavonols Quercetin and Kaempferol on Alkaline Phosphatase Activity in MG-63 Human Osteoblasts through ERK and Estrogen Receptor Pathway. Biochem. Pharmacol. 2004, 67, 1307–1313. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Chen, G.; Feng, S.; Wang, P.; Zhu, X.; Zhang, R. Quercetin Promotes the Osteogenic Differentiation of Rat Mesenchymal Stem Cells via Mitogen-Activated Protein Kinase Signaling. Exp. Ther. Med. 2015, 9, 2072–2080. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lezcano, V.; Morelli, S.; González-Pardo, V. Molecular and Cellular Outcomes of Quercetin Actions on Healthy and Tumor Osteoblasts. Biochimie 2022, 199, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.F.; Ehnert, S.; Freude, T.; Egaña, J.T.; Schenck, T.L.; Buchholz, A.; Schmitt, A.; Siebenlist, S.; Schyschka, L.; Neumaier, M.; et al. Quercetin Protects Primary Human Osteoblasts Exposed to Cigarette Smoke through Activation of the Antioxidative Enzymes HO-1 and SOD-1. Sci. World J. 2011, 11, 2348–2357. [Google Scholar] [CrossRef]
- Wang, X.-C.; Zhao, N.-J.; Guo, C.; Chen, J.-T.; Song, J.-L.; Gao, L. Quercetin Reversed Lipopolysaccharide-Induced Inhibition of Osteoblast Differentiation through the Mitogen-activated Protein Kinase Pathway in MC3T3-E1 Cells. Mol. Med. Rep. 2014, 10, 3320–3326. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Tripathy, N.; Lee, D.H.; Park, J.H.; Khang, G. Quercetin Inlaid Silk Fibroin/Hydroxyapatite Scaffold Promotes Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2018, 10, 32955–32964. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, G.; Chen, B.; He, Q.; Mai, J.; Chen, W.; Pan, Z.; Yang, J.; Li, J.; Ma, Y.; et al. Quercetin Protects against Iron Overload-Induced Osteoporosis through Activating the Nrf2/HO-1 Pathway. Life Sci. 2023, 322, 121326. [Google Scholar] [CrossRef]
- Pang, X.-G.; Cong, Y.; Bao, N.-R.; Li, Y.-G.; Zhao, J.-N. Quercetin Stimulates Bone Marrow Mesenchymal Stem Cell Differentiation through an Estrogen Receptor-Mediated Pathway. Biomed. Res. Int. 2018, 2018, 4178021. [Google Scholar] [CrossRef]
- Sharma, G.; Lee, Y.H.; Kim, J.-C.; Sharma, A.R.; Lee, S.-S. Bone Regeneration Enhanced by Quercetin-Capped Selenium Nanoparticles via miR206/Connexin43, WNT, and BMP Signaling Pathways. Aging Dis. 2025, 17, 2. [Google Scholar] [CrossRef]
- Messer, J.G.; Hopkins, R.G.; Kipp, D.E. Quercetin Metabolites Up-Regulate the Antioxidant Response in Osteoblasts Isolated from Fetal Rat Calvaria. J. Cell Biochem. 2015, 116, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Müller, J.; Schimke, I. Vascular Hypothesis Revisited: Role of Stimulating Antibodies against Angiotensin and Endothelin Receptors in the Pathogenesis of Systemic Sclerosis. Cabral-Marques, O, Riemekasten, G., Autoimmun Rev. 2016 Mar 10. Pii: S1568-9972(16)30053-2. doi: 10.1016/j.autrev.2016.03.005. [Epub Ahead of Print]. Autoimmun. Rev. 2016, 15, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.W.; Yoo, C.I.; Kim, H.T.; Kwon, C.H.; Park, J.Y.; Kim, Y.K. The Flavonoid Quercetin Induces Apoptosis and Inhibits Migration through a MAPK-Dependent Mechanism in Osteoblasts. J. Bone Miner. Metab. 2008, 26, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.; Posa, F.; De Maria, S.; Ravagnan, G.; Ballini, A.; Porro, C.; Trotta, T.; Grano, M.; Muzio, L.L.; Mori, G. Polydatin, Natural Precursor of Resveratrol, Promotes Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 944–952. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Z.; You, X.; Zhou, H.; He, W.; Li, B.; Xia, J.; Zhu, H.; Zhao, Y.; Yu, G.; et al. Resveratrol Promotes Osteogenesis and Alleviates Osteoporosis by Inhibiting P53. Aging 2020, 12, 10359–10369. [Google Scholar] [CrossRef]
- Chen, X.-H.; Shi, Z.-G.; Lin, H.-B.; Wu, F.; Zheng, F.; Wu, C.-F.; Huang, M.-W. Resveratrol Alleviates Osteoporosis through Improving the Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6352–6359. [Google Scholar] [CrossRef]
- Feng, Y.-L.; Jiang, X.-T.; Ma, F.-F.; Han, J.; Tang, X.-L. Resveratrol Prevents Osteoporosis by Upregulating FoxO1 Transcriptional Activity. Int. J. Mol. Med. 2018, 41, 202–212. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ding, S.; Chang, J.; Liu, G.; Hu, S. Curcumin Ameliorated Glucocorticoid-Induced Osteoporosis While Modulating the Gut Microbiota and Serum Metabolome. J. Agric. Food Chem. 2025, 73, 8254–8276. [Google Scholar] [CrossRef]
- Fan, D.; Lu, J.; Yu, N.; Xie, Y.; Zhen, L. Curcumin Prevents Diabetic Osteoporosis through Promoting Osteogenesis and Angiogenesis Coupling via NF-κB Signaling. Evid. Based Complement. Altern. Med. 2022, 2022, 4974343. [Google Scholar] [CrossRef]
- Sanjaya, S.S.; Park, J.; Choi, Y.H.; Park, H.S.; Sadanaga, T.; Jung, M.-J.; Kim, G.-Y. Polyphenol Extract from Tagetes erecta L. Flowers Stimulates Osteogenesis via β-Catenin Activation. Phytomedicine 2025, 136, 156313. [Google Scholar] [CrossRef]
- Dai, J.; Li, Y.; Zhou, H.; Chen, J.; Chen, M.; Xiao, Z. Genistein Promotion of Osteogenic Differentiation through BMP2/SMAD5/RUNX2 Signaling. Int. J. Biol. Sci. 2013, 9, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Jadai, R.; Venna, N.; Ajumeera, R.; Challa, S. Isoflavones Rich Cowpea and Vitamin D Induces the Proliferation and Differentiation of Human Osteoblasts via BMP-2/Smad Pathway Activation: Mechanistic Approach. IUBMB Life 2019, 71, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Min, J.; Zhao, Y.; Cheng, Q.; Wang, K.; Lin, S.; Luo, J.; Liu, H. Quercetin Rescued TNF-Alpha-Induced Impairments in Bone Marrow-Derived Mesenchymal Stem Cell Osteogenesis and Improved Osteoporosis in Rats. Am. J. Transl. Res. 2018, 10, 4313–4321. [Google Scholar] [PubMed]
- Feng, L.; Yang, Z.; Hou, N.; Wang, M.; Lu, X.; Li, Y.; Wang, H.; Wang, Y.; Bai, S.; Zhang, X.; et al. Long Non-Coding RNA Malat1 Increases the Rescuing Effect of Quercetin on TNFα-Impaired Bone Marrow Stem Cell Osteogenesis and Ovariectomy-Induced Osteoporosis. Int. J. Mol. Sci. 2023, 24, 5965. [Google Scholar] [CrossRef]
- Wang, S.; Zhai, J.; Heng, K.; Sha, L.; Song, X.; Zhai, H.; Dai, C.; Li, J.; Teng, F.; Huang, J.; et al. Senolytic Cocktail Dasatinib and Quercetin Attenuates Chronic High Altitude Hypoxia Associated Bone Loss in Mice. Sci. Rep. 2024, 14, 30417. [Google Scholar] [CrossRef]
- Sharan, K.; Mishra, J.S.; Swarnkar, G.; Siddiqui, J.A.; Khan, K.; Kumari, R.; Rawat, P.; Maurya, R.; Sanyal, S.; Chattopadhyay, N. A Novel Quercetin Analogue from a Medicinal Plant Promotes Peak Bone Mass Achievement and Bone Healing after Injury and Exerts an Anabolic Effect on Osteoporotic Bone: The Role of Aryl Hydrocarbon Receptor as a Mediator of Osteogenic Action. J. Bone Miner. Res. 2011, 26, 2096–2111. [Google Scholar] [CrossRef]
- Wei, L.; Chai, S.; Yue, C.; Zhang, H.; Li, J.; Qin, N. Resveratrol Protects Osteocytes against Oxidative Stress in Ovariectomized Rats through AMPK/JNK1-Dependent Pathway Leading to Promotion of Autophagy and Inhibition of Apoptosis. Cell Death Discov. 2023, 9, 16. [Google Scholar] [CrossRef]
- Song, C.-Y.; Guo, Y.; Chen, F.-Y.; Liu, W.-G. Resveratrol Promotes Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells Through miR-193a/SIRT7 Axis. Calcif. Tissue Int. 2022, 110, 117–130. [Google Scholar] [CrossRef]
- Lv, Y.-J.; Yang, Y.; Sui, B.-D.; Hu, C.-H.; Zhao, P.; Liao, L.; Chen, J.; Zhang, L.-Q.; Yang, T.-T.; Zhang, S.-F.; et al. Resveratrol Counteracts Bone Loss via Mitofilin-Mediated Osteogenic Improvement of Mesenchymal Stem Cells in Senescence-Accelerated Mice. Theranostics 2018, 8, 2387–2406. [Google Scholar] [CrossRef]
- Zhong, Q. Resveratrol Enhances the Protective Effects of Calcium Supplements on Spinal Cord Injury-Induced Osteoporosis by Targeting the SIRT1/FOXO3a Pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 2823–2832. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin Is a Senotherapeutic That Extends Health and Lifespan. eBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Léotoing, L.; Davicco, M.-J.; Lebecque, P.; Wittrant, Y.; Coxam, V. The Flavonoid Fisetin Promotes Osteoblasts Differentiation through Runx2 Transcriptional Activity. Mol. Nutr. Food Res. 2014, 58, 1239–1248. [Google Scholar] [CrossRef]
- Xi, J.; Li, Q.; Luo, X.; Li, J.; Guo, L.; Xue, H.; Wu, G. Epigallocatechin-3-gallate Protects against Secondary Osteoporosis in a Mouse Model via the Wnt/Β-catenin Signaling Pathway. Mol. Med. Rep. 2018, 18, 4555–4562. [Google Scholar] [CrossRef] [PubMed]
- Morinobu, A.; Biao, W.; Tanaka, S.; Horiuchi, M.; Jun, L.; Tsuji, G.; Sakai, Y.; Kurosaka, M.; Kumagai, S. (−)-Epigallocatechin-3-Gallate Suppresses Osteoclast Differentiation and Ameliorates Experimental Arthritis in Mice. Arthritis Rheum. 2008, 58, 2012–2018. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Li, Y.; Wang, Y.; Zheng, X.; Wang, F.; Tong, T.; Miao, D.; Li, W.; Chen, L.; et al. 4-Hydroxyphenylacetic Acid, a Microbial-Derived Metabolite of Polyphenols, Inhibits Osteoclastogenesis by Inhibiting ROS Production. Int. Immunopharmacol. 2024, 143, 113571. [Google Scholar] [CrossRef]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation with Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Corbi, G.; Nobile, V.; Conti, V.; Cannavo, A.; Sorrenti, V.; Medoro, A.; Scapagnini, G.; Davinelli, S. Equol and Resveratrol Improve Bone Turnover Biomarkers in Postmenopausal Women: A Clinical Trial. Int. J. Mol. Sci. 2023, 24, 12063. [Google Scholar] [CrossRef]
- Hodges, J.K.; Maiz, M.; Cao, S.; Lachcik, P.J.; Peacock, M.; McCabe, G.P.; McCabe, L.D.; Cladis, D.P.; Jackson, G.S.; Ferruzzi, M.G.; et al. Moderate Consumption of Freeze-Dried Blueberry Powder Increased Net Bone Calcium Retention Compared with No Treatment in Healthy Postmenopausal Women: A Randomized Crossover Trial. Am. J. Clin. Nutr. 2023, 118, 382–390. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Wallace, T.C. Dietary Flavonoid and Subclass Intakes Are Not Associated with Markers of Bone Health in U.S. Adults Age 50+ Years. J. Am. Nutr. Assoc. 2024, 43, 604–613. [Google Scholar] [CrossRef]
- Shen, C.-L.; Chyu, M.-C.; Yeh, J.K.; Felton, C.K.; Xu, K.T.; Pence, B.C.; Wang, J.-S. Green Tea Polyphenols and Tai Chi for Bone Health: Designing a Placebo-Controlled Randomized Trial. BMC Musculoskelet. Disord. 2009, 10, 110. [Google Scholar] [CrossRef]
- Al-Dashti, Y.A.; Holt, R.R.; Carson, J.G.; Keen, C.L.; Hackman, R.M. Effects of Short-Term Dried Plum (Prune) Intake on Markers of Bone Resorption and Vascular Function in Healthy Postmenopausal Women: A Randomized Crossover Trial. J. Med. Food 2019, 22, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.-K.; Chen, W.-F.; Lai, W.-P.; Leung, P.-C.; Wang, X.-L.; Yao, X.-S.; Wong, M.-S. Icariin Protects against Bone Loss Induced by Oestrogen Deficiency and Activates Oestrogen Receptor-Dependent Osteoblastic Functions in UMR 106 Cells. Br. J. Pharmacol. 2010, 159, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-Y. Therapeutic Potential of Ginsenosides on Bone Metabolism: A Review of Osteoporosis, Periodontal Disease and Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 5828. [Google Scholar] [CrossRef]
- Hu, J.-P.; Nishishita, K.; Sakai, E.; Yoshida, H.; Kato, Y.; Tsukuba, T.; Okamoto, K. Berberine Inhibits RANKL-Induced Osteoclast Formation and Survival through Suppressing the NF-kappaB and Akt Pathways. Eur. J. Pharmacol. 2008, 580, 70–79. [Google Scholar] [CrossRef]
- Zhou, L.; Song, F.; Liu, Q.; Yang, M.; Zhao, J.; Tan, R.; Xu, J.; Zhang, G.; Quinn, J.M.W.; Tickner, J.; et al. Berberine Sulfate Attenuates Osteoclast Differentiation through RANKL Induced NF-κB and NFAT Pathways. Int. J. Mol. Sci. 2015, 16, 27087–27096. [Google Scholar] [CrossRef]
- He, G.; Guo, W.; Lou, Z.; Zhang, H. Achyranthes Bidentata Saponins Promote Osteogenic Differentiation of Bone Marrow Stromal Cells Through the ERK MAPK Signaling Pathway. Cell Biochem. Biophys. 2014, 70, 467–473. [Google Scholar] [CrossRef]
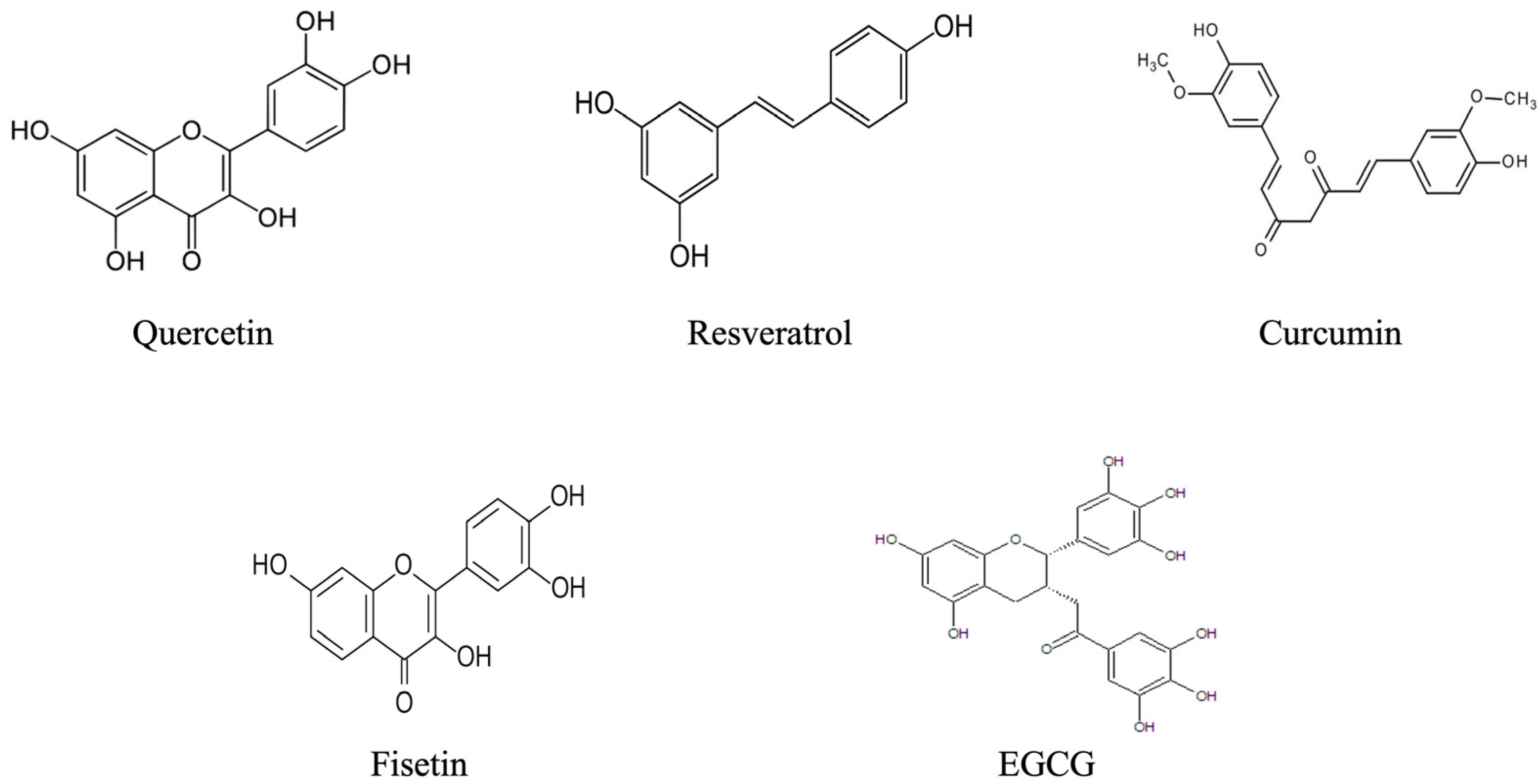
| Polyphenol Class | Subcategories | Examples | Main Dietary Sources |
|---|---|---|---|
| Flavonoids | Flavonols, Flavones, Isoflavones, Anthocyanins, etc. | Quercetin, Genistein | Green tea, red wine, soy, citrus, apple |
| Phenolic Acids | Derivatives of benzoic and cinnamic acids | Caffeic acid, Ferulic acid | Coffee, whole grains, fruit |
| Stilbenes | — | Resveratrol | Grapes, red wine, peanuts |
| Lignans | — | Secoisolariciresinol | Flaxseeds, cereals, legumes |
| Polyphenol | Key Molecular Actions | Pathways Involved | Effects on Bone Cells |
|---|---|---|---|
| Quercetin | ↑ ALP, ↑ RUNX2, ↓ ROS | MAPKs, ERK, NF-κB | Promotes osteoblasts, inhibits osteoclasts |
| Resveratrol | ↑ SIRT1, ↑ FoxO1, ↓ p53 | PI3K/AKT, MDM2/p53 | Anti-apoptotic, anti-inflammatory |
| Curcumin | ↑ Wnt/β-catenin, ↑ microbiota | NF-κB, BMP-2 | Enhances bone mineralization |
| EGCG | ↓ NF-ATc1, ↓ TRAP | Wnt/β-catenin, MAPKs | Reduces osteoclastogenesis |
| Isoflavones | ↑ ERβ binding, ↑ BMP-2 | ER-mediated, SMAD | Estrogen-like effects |
| Polyphenol/Extract | Experimental Model | Main Observed Effects | Molecular Mechanisms Involved |
|---|---|---|---|
| Quercetin | Osteoblasts in vitro | ↑ ALP (early osteogenesis marker) | ERK activation downstream of estrogen receptor |
| Osteoblasts in vitro | ↑ Osteoblast differentiation | ↑ TGF-β1, BMP-2, RUNX2; activation of MAPKs (ERK1/2, p38, JNK) | |
| Osteoblasts in vitro | ↑ Viability, adhesion, migration; protective effect | Safe on healthy cells; at high concentrations (20–100 μM) ↓ viability of osteoblastic tumor cells | |
| Osteoblasts exposed to cigarette smoke | ↑ Viability; protection against oxidative stress | ↑ HO-1, SOD; reduction in ROS-induced damage | |
| Osteoblasts exposed to LPS | Restores LPS-inhibited differentiation | MAPK pathway activation; protection from infection-related osteolysis | |
| MC3T3-E1 | ↑ ALP, mineralized nodules; ↓ apoptosis and ROS | ↑ Runx2, Osterix, Bcl-2; ↓ Caspase3, Bax | |
| Murine MSC | ↑ Proliferation and osteogenic differentiation | ↑ BMP pathways, OSX, RUNX2, OPN | |
| MSC | ↓ ALP, mineralization; ↑ adipogenesis | ↓ ALPL, Col1a1, OCN; dose-dependent and potentially adverse effects | |
| Osteoblasts from fetal rat calvaria | ↑ HO-1, GCLC; ↓ pERK1/2, NF-κB | Variable antioxidant effect; no synergy among metabolites | |
| Quercetin + biomaterials | Silk fibroin/HA scaffold with quercetin | ↑ Osteogenesis and bone regeneration | Creation of pro-osteoblastic biomimetic microenvironment |
| Quercetin (nanoformulations) | Hydrogel + quercetin | ↑ Cellular uptake, ↑ bone regeneration | ↑ Bioavailability and stability; promising for orthopedic applications |
| Resveratrol | MSC | ↑ Osteogenic differentiation | MDM2/p53, NF-κB/β-catenin pathways; protective effect |
| MSC | Protects differentiation under inflammation | ↓ NF-κB, β-catenin; ↑ Runx2, osteocalcin | |
| Oxidative stress models | ↓ RANKL, TRAP5b; ↑ OPG | ↑ FoxO1 via PI3K/AKT inhibition; ↓ osteoclastogenesis | |
| Curcumin | Glucocorticoid-induced osteoporosis | ↑ BMD, trabeculae, microbiota and beneficial metabolites | Gut–bone axis modulation; ↓ inflammatory cytokines |
| MSC under hyperglycemia | Restores osteogenesis–angiogenesis coupling | NF-κB inhibition under glucose-induced stress | |
| Tagetes erecta (extract) | MC3T3-E1 | ↑ ALP, mineralization, RUNX2, SP7 | β-catenin activation, GSK-3β inhibition; patuletin effective |
| Isoflavones | MC3T3-E1 | ↑ ALP, osteoblast proliferation | Estrogen receptor activation; ↑ RUNX2, BMP-2 |
| MG-63 | ↑ BMP-2, OSX, Smad1/5/8, ALP, OPN | Supports functional legumes as anti-osteoporotic agents |
| Polyphenol/Extract | Animal Model | Main Observed Effects | Molecular Mechanisms Involved |
|---|---|---|---|
| Quercetin | Rats, postmenopausal osteoporosis | ↑ BMD, improved bone biomechanical properties | Inhibits TNF-α-induced NF-κB activation, preserves β-catenin; promotes MSC proliferation and osteogenic differentiation |
| Murine RAW 264.7 cells + RANKL | ↓ Osteoclastogenesis, dose-dependent | IC50 ~1 µM; inhibits NF-κB and AP-1 activation, reduces osteoclast differentiation | |
| OVX mice | Restores OVX-induced bone loss and structural deterioration | Upregulates Wnt/β-catenin, inhibits NF-κB; effect mediated by lncRNA Malat1 | |
| Mice, chronic high-altitude hypoxia | Anti-senescent effects on MSC, improved bone marrow microenvironment | Potential therapeutic for CHH-associated osteoporosis | |
| Quercetin analogs (GTDF) | OVX and growing rats | ↑ Peak bone mass, trabecular/cortical bone strength, BFR | Stimulates osteoblast proliferation, survival, differentiation via AhR; non-estrogenic, selective anabolic effect |
| Resveratrol | OVX rats, H2O2-treated osteocytes | ↓ Osteocyte apoptosis, promotes autophagy | AMPK/JNK1 pathway; dissociation of Beclin-1/Bcl-2 complex |
| OVX rats, femoral trabecular tissue | ↑ Osteogenic differentiation | Suppresses miR-193a, upregulates SIRT7, modulates NF-κB signaling | |
| Senescence-accelerated mice | ↑ Bone formation, counters accelerated bone loss | Restores mitochondrial function, upregulates mitofilin, improves MSC osteogenesis | |
| SCI-induced osteoporosis in mice | Preserved bone mass, strength, microarchitecture | SIRT1/FOXO3a pathway; ↑ osteoblastic markers, ↓ osteoclastic markers | |
| Fisetin | Progeroid/aged mice | ↓ Senescence markers, extended health span & lifespan | Senolytic activity, tissue-specific, restores tissue homeostasis |
| LPS-induced inflammatory osteoporosis in mice | ↑ Osteoblastic markers (OCN, Col1a1) | Regulates RUNX2 to support osteoblast development and maturation | |
| EGCG | Secondary osteoporosis murine model | ↓ Serum & urinary calcium, ↑ leptin, improved trabecular structure | Activates Wnt/β-catenin signaling; inhibits bone resorption, ↑ cyclin D1 |
| Murine experimental arthritis | ↓ TRAP-positive osteoclasts, ↓ bone resorption | ↓ NF-ATc1 expression; modulates inflammation; protects against osteoclast differentiation | |
| 4-HPA | OVX murine osteoporosis model | Prevented bone loss, ↓ osteoclast differentiation | Regulates ROS, Nrf2 activation, inhibits NF-κB and MAPK pathways; gut microbiota-derived polyphenol metabolite |
| Polyphenol Studied | Population | Main Findings | Study Type |
|---|---|---|---|
| Resveratrol | Postmenopausal women | ↑ BMD, ↓ CTX | RCT, double-blind |
| Curcumin | Patients with GIOP | ↑ trabecular bone, ↑ beneficial microbiota | Controlled study |
| EGCG | Women with osteopenia | Modest increase in BMD | Intervention trial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, P.; De Rosa, C.; D’Angelo, S. Polyphenols and Bone Health: A Comprehensive Review of Their Role in Osteoporosis Prevention and Treatment. Molecules 2025, 30, 4154. https://doi.org/10.3390/molecules30214154
Perrone P, De Rosa C, D’Angelo S. Polyphenols and Bone Health: A Comprehensive Review of Their Role in Osteoporosis Prevention and Treatment. Molecules. 2025; 30(21):4154. https://doi.org/10.3390/molecules30214154
Chicago/Turabian StylePerrone, Pasquale, Chiara De Rosa, and Stefania D’Angelo. 2025. "Polyphenols and Bone Health: A Comprehensive Review of Their Role in Osteoporosis Prevention and Treatment" Molecules 30, no. 21: 4154. https://doi.org/10.3390/molecules30214154
APA StylePerrone, P., De Rosa, C., & D’Angelo, S. (2025). Polyphenols and Bone Health: A Comprehensive Review of Their Role in Osteoporosis Prevention and Treatment. Molecules, 30(21), 4154. https://doi.org/10.3390/molecules30214154








