Phytochemical Profile, Extraction and Characterization of Bioactive Compounds from Industrial Hemp (Cannabis sativa L.) Felina 32 Variety
Abstract
1. Introduction
2. Results and Discussion
2.1. Plant Source and Extraction Procedures
2.2. Profiling Cannabinoid Content in Plant Extracts
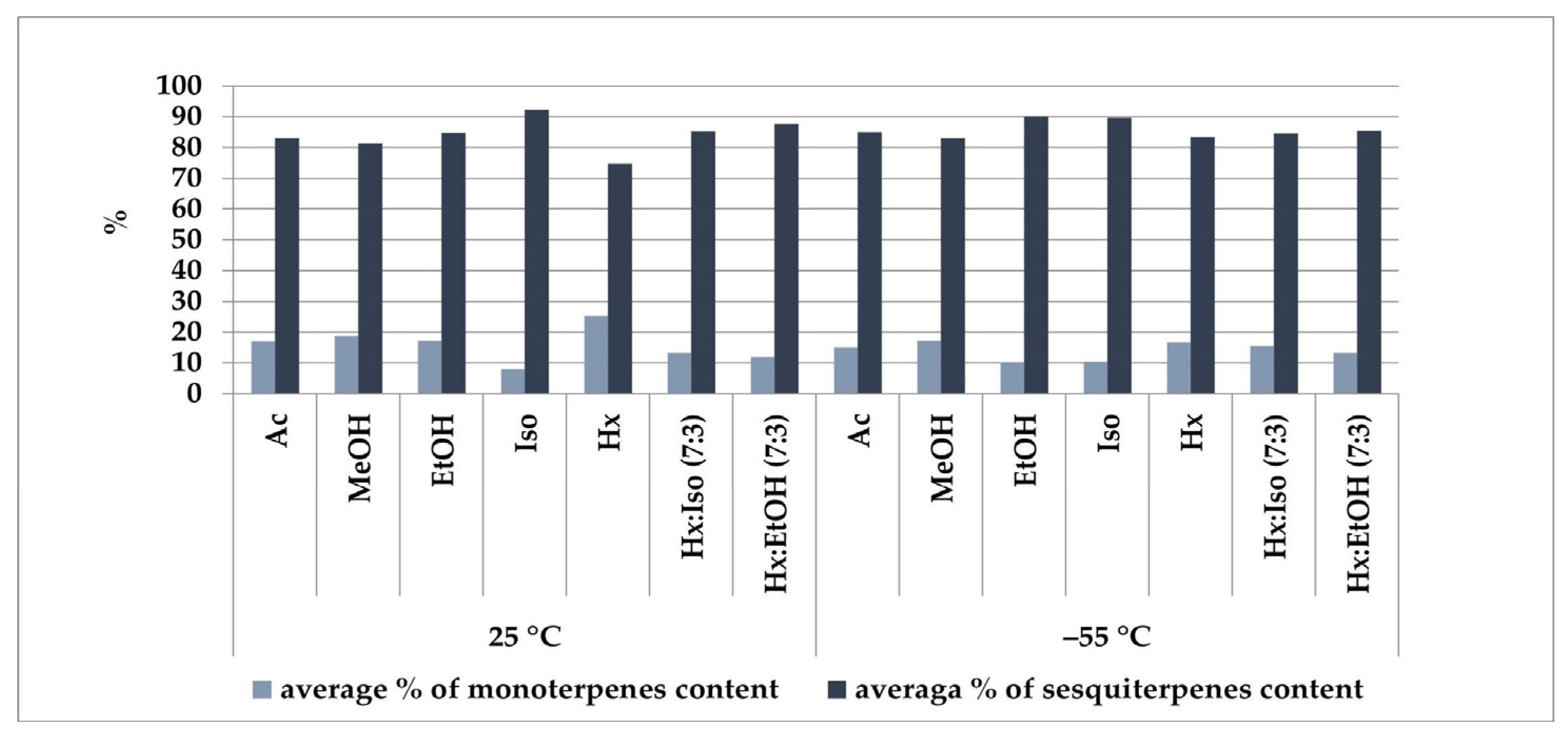
2.3. Comparative Analysis of Terpene Profiles in Plant Extracts
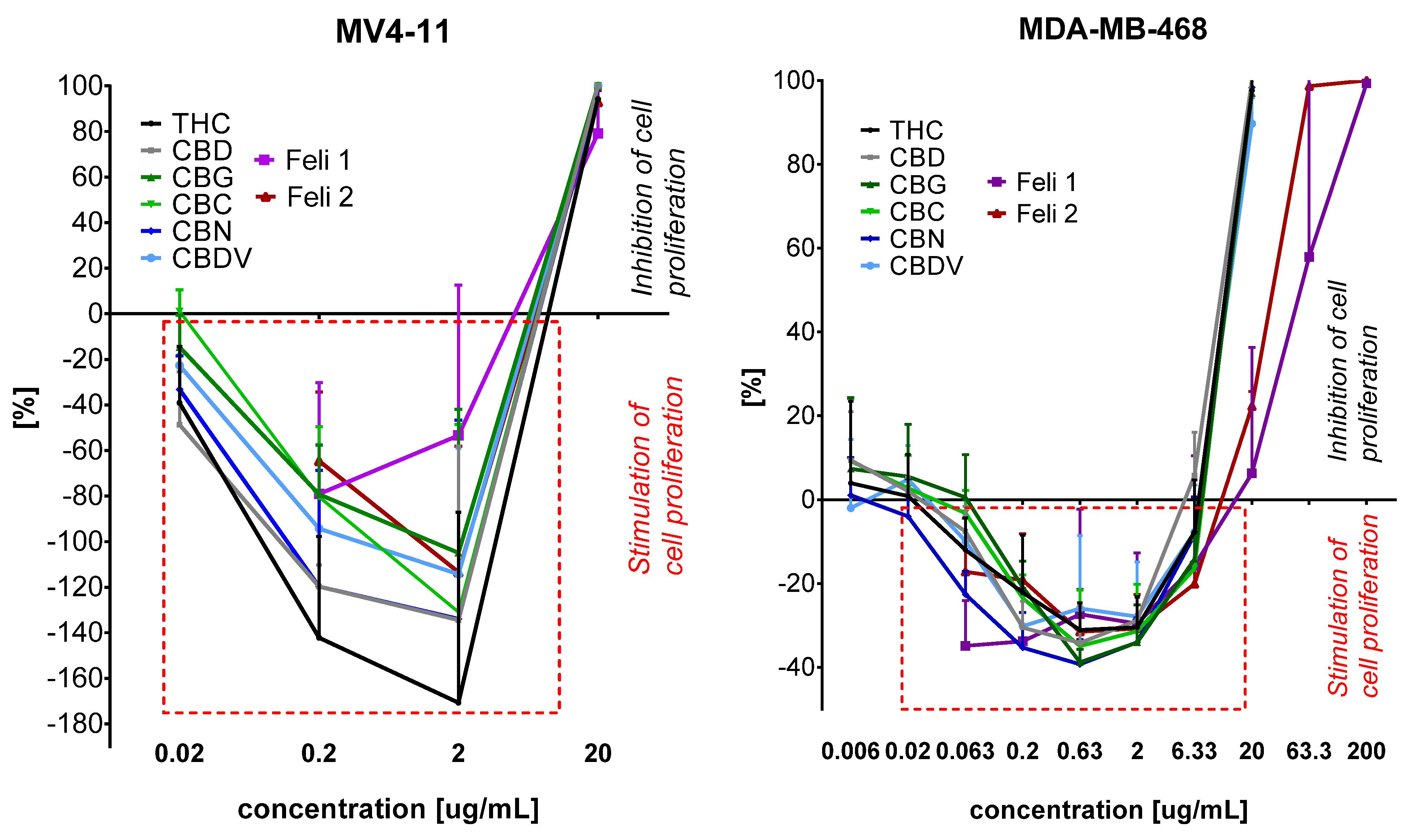
2.4. Antiproliferative Activity of Extracts Towards Cancer and Normal Cell Lines
3. Materials and Methods
3.1. Chemicals and Solvents
3.2. Plant Source and Sample Preparation
3.2.1. Plant Material
3.2.2. Low Pressure Extraction (LPE)
3.3. GC-MS Analysis of Cannabinoids in Prepared Extracts
3.4. Qualitative Analysis of Terpenoids in Prepared Extracts by HS-SPME
3.5. Biological Studies
3.5.1. Cell Lines and Cultured Mediums
3.5.2. The Antiproliferative Activity Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McPartland, J.M.; Small, E. A Classification of Endangered High-THC Cannabis (Cannabis Sativa Subsp. Indica) domesticates and their wild relatives. PhytoKeys 2020, 144, 81–112. [Google Scholar] [CrossRef] [PubMed]
- Kitdumrongthum, S.; Trachootham, D. An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules 2023, 28, 2791. [Google Scholar] [CrossRef] [PubMed]
- Hazekamp, A.; Tejkalová, K.; Papadimitriou, S. Cannabis: From Cultivar to Chemovar II—A Metabolomics Approach to Cannabis Classification. Cannabis Cannabinoid Res. 2016, 1, 202–215. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Borah, R.; Sharma, B.; Pandhi, S.; Tripathi, V.; Yadav, H.S.; Devi, S.; Patil, U.; et al. Pharmacological Properties, Therapeutic Potential, and Legal Status of Cannabis sativa L.: An Overview. Phytother. Res. 2021, 35, 6010–6029. [Google Scholar] [CrossRef]
- Salami, S.A.; Martinelli, F.; Giovino, A.; Bachari, A.; Arad, N.; Mantri, N. It Is Our Turn to Get Cannabis High: Put Cannabinoids in Food and Health Baskets. Molecules 2020, 25, 4036. [Google Scholar] [CrossRef]
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture 2021, 11, 384. [Google Scholar] [CrossRef]
- Hussain, T.; Jeena, G.; Pitakbut, T.; Vasilev, N.; Kayser, O. Cannabis Sativa Research Trends, Challenges, and New-Age Perspectives. iScience 2021, 24, 103391. [Google Scholar] [CrossRef]
- Koltai, H.; Namdar, D. Cannabis Phytomolecule “Entourage”: From Domestication to Medical Use. Trends Plant Sci. 2020, 25, 976–984. [Google Scholar] [CrossRef]
- Raz, N.; Eyal, A.M.; Zeitouni, D.B.; Hen-Shoval, D.; Davidson, E.M.; Danieli, A.; Tauber, M.; Ben-Chaim, Y. Selected Cannabis Terpenes Synergize with THC to Produce Increased CB1 Receptor Activation. Biochem. Pharmacol. 2023, 212, 115548. [Google Scholar] [CrossRef]
- Chacon, F.T.; Raup-Konsavage, W.M.; Vrana, K.E.; Kellogg, J.J. Secondary Terpenes in Cannabis sativa L.: Synthesis and Synergy. Biomedicines 2022, 10, 3142. [Google Scholar] [CrossRef]
- European Commission. Common Catalogue of Varieties of Agricultural Plant Species. 2025. Available online: https://food.ec.europa.eu/system/files/2023-08/plant-variety-catalogues_agricultural-plant-species.pdf (accessed on 22 January 2025).
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing Industrial Hemp (Cannabis sativa L.) by-Products: Cannabidiol Enrichment in the Inflorescence Essential Oil Optimizing Sample Pre-Treatment Prior to Distillation. Ind. Crops Prod. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Giannoulis, K.D.; Bartzialis, D.; Gintsioudis, I.; Danalatos, N.G. Cultivation Practices Affect Biomass Yield and Quality of “Felina 32”, an Industrial Hemp Variety. Agronomy 2024, 14, 2743. [Google Scholar] [CrossRef]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; Di Sotto, A.; Di Giacomo, S.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa L. Inflorescences from Monoecious Cultivars Grown in Central Italy: An Untargeted Chemical Characterization from Early Flowering to Ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Mariano, A.; Gullì, M.; Fraschetti, C.; Vitalone, A.; Filippi, A.; Mannina, L.; Scotto d’Abusco, A.; Di Sotto, A. Role of Caryophyllane Sesquiterpenes in the Entourage Effect of Felina 32 Hemp Inflorescence Phytocomplex in Triple Negative MDA-MB-468 Breast Cancer Cells. Molecules 2021, 26, 6688. [Google Scholar] [CrossRef]
- Haczkiewicz, M.; Świtalska, M.; Łyczko, J.; Pluta, M.; Wietrzyk, J.; Gliszczyńska, A. Extraction of Cannabinoids and Terpenes from Hemp Flowers and Leaves (Cannabis sativa L., Futura 75): Chemical Profiling and Evaluation of Anticancer Properties. Molecules 2025, 30, 1325. [Google Scholar] [CrossRef] [PubMed]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of Phenolic Compounds and Terpenes from Cannabis sativa L. By-Products: From Conventional to Intensified Processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef]
- Tzimas, P.S.; Petrakis, E.A.; Halabalaki, M.; Skaltsounis, L.A. Extraction Solvent Selection for Cannabis sativa L. by Efficient Exploration of Cannabinoid Selectivity and Phytochemical Diversity. Phytochem. Anal. 2024, 35, 163–183. [Google Scholar] [CrossRef]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and Extraction Methods of Medicinal Cannabis: A Narrative Review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [CrossRef]
- Selvaraj, S.; Nawfer, N.; Dharmawansa, K.V.S.; Ali Redha, A.; Rupasinghe, H.P.V. Recent Advances in Cannabidiol (CBD) Extraction: A Review of Potential Eco-Friendly Solvents and Advanced Technologies. Green Anal. Chem. 2025, 13, 100270. [Google Scholar] [CrossRef]
- López-Olmos, C.; García-Valverde, M.T.; Hidalgo, J.; Ferrerio-Vera, C.; Sánchez de Medina, V. Comprehensive Comparison of Industrial Cannabinoid Extraction Techniques: Evaluation of the Most Relevant Patents and Studies at Pilot Scale. Front. Nat. Prod. 2022, 1, 1043147. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential Oil of Cannabis sativa L.: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef]
- Dammann, I.; Keil, C.; Hardewig, I.; Skrzydlewska, E.; Biernacki, M.; Haase, H. Effects of Combined Cannabidiol (CBD) and Hops (Humulus Lupulus) Terpene Extract Treatment on RAW 264.7 Macrophage Viability and Inflammatory Markers. Nat. Prod. Bioprospect. 2023, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- LaVigne, J.; Hecksel, R.; Streicher, J. In Defense of the “Entourage Effect”: Terpenes Found in Cannabis sativa Activate the Cannabinoid Receptor 1 In Vivo. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Silva, B.I.M.; Nascimento, E.A.; Silva, C.J.; Silva, T.G.; Aguiar, J.S. Anticancer Activity of Monoterpenes: A Systematic Review. Mol. Biol. Rep. 2021, 48, 5775–5785. [Google Scholar] [CrossRef]
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004, 64, 1943–1950. [Google Scholar] [CrossRef]
- Parolaro, D.; Massi, P. Cannabinoids as potential new therapy for the treatment of gliomas. Expert Rev. Neurother. 2008, 8, 37–49. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, I.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vazquez, P.; Blazquez, C.; Torres, S.; Garcia, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Kappo, A.P. Anti-Cancer and Anti-Proliferative Potential of Cannabidiol: A Cellular and Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 5659. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, H.; Liu, C.; Liu, M.; Shangguan, F.; Liu, Y.; Yang, S.; Li, H.; An, J.; Song, S.; et al. A Novel Mechanism of Cannabidiol in Suppressing Ovarian Cancer through LAIR-1 Mediated Mitochondrial Dysfunction and Apoptosis. Environ. Toxicol. 2023, 38, 1118–1132. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska, A.; Świtalska, M.; Wietrzyk, J.; Wawrzeńczyk, C. Synthesis of a Natural γ-Butyrolactone from Nerylacetone by Acremonium roseum and Fusarium oxysporum Cultures. Nat. Prod. Commun. 2011, 6, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Nevozhay, D. Cheburator Software for Automatically Calculating Drug Inhibitory Concentrations from In Vitro Screening Assays. PLoS ONE 2014, 9, e106186. [Google Scholar] [CrossRef] [PubMed]
| Solvent | Temp. [°C] | Mass [g] ± SD | % Mass Loss After Winterization |
|---|---|---|---|
| Ac | 25 | 0.29 ± 0.01 | –27% |
| –55 | 0.26 ± 0.01 | –17% | |
| MeOH | 25 | 0.34 ± 0.02 | –37% |
| –55 | 0.30 ± 0.01 | –23% | |
| EtOH | 25 | 0.30 ± 0.02 | –32% |
| –55 | 0.27 ± 0.01 | –18% | |
| Iso | 25 | 0.26 ± 0.01 | –29% |
| –55 | 0.23 ± 0.00 | –19% | |
| Hx | 25 | 0.30 ± 0.01 | –49% |
| –55 | 0.25 ± 0.00 | –30% | |
| Hx:Iso (7:3) | 25 | 0.29 ± 0.01 | –29% |
| –55 | 0.26 ± 0.00 | –16% | |
| Hx:EtOH (7:3) | 25 | 0.31 ± 0.01 | –30% |
| –55 | 0.25 ± 0.01 | –17% |
| Solvent | Temp. [°C] | Δ9-THC % Mean ± SD | CBD % Mean ± SD | CBG % Mean ± SD | CBC % Mean ± SD | CBN % Mean ± SD | % Total Cannabinoids |
|---|---|---|---|---|---|---|---|
| Ac | 25 | 1.83 ± 0.00 | 49.02 ± 0.53 | 2.08 ± 0.08 | 1.98 ± 0.16 | 1.30 ± 0.04 | 56.20 |
| –55 | 1.66 ± 0.01 | 49.03 ± 0.08 | 2.11 ± 0.01 | 1.99 ± 0.07 | 1.30 ± 0.01 | 56.09 | |
| MeOH | 25 | 1.66 ± 0.02 | 48.67 ± 0.05 | 2.16 ± 0.21 | 2.12 ± 0.26 | 1.29 ± 0.12 | 55.90 |
| –55 | 1.66 ± 0.08 | 48.04 ± 0.57 | 2.04 ± 0.05 | 2.42 ± 0.18 | 1.28 ± 0.07 | 55.44 | |
| EtOH | 25 | 1.78 ± 0.16 | 46.86 ± 0.54 | 2.02 ± 0.10 | 1.88 ± 0.05 | 1.32 ± 0.02 | 53.86 |
| –55 | 1.86 ± 0.08 | 44.53 ± 0.25 | 1.98 ± 0.20 | 1.98 ± 0.20 | 1.28 ± 0.02 | 51.63 | |
| Iso | 25 | 1.64 ± 0.06 | 44.87 ± 0.19 | 2.05 ± 0.04 | 1.96 ± 0.02 | 1.33 ± 0.02 | 51.85 |
| –55 | 1.73 ± 0.02 | 46.94 ± 0.17 | 2.09 ± 0.02 | 1.94 ± 0.19 | 1.25 ± 0.08 | 53.95 | |
| Hx | 25 | 2.06 ± 0.09 | 53.98 ± 0.97 | 1.79 ± 0.12 | 2.10 ± 0.03 | 1.39 ± 0.02 | 61.32 |
| –55 | 1.84 ± 0.29 | 57.74 ± 0.93 | 1.74 ± 0.27 | 2.13 ± 0.65 | 1.31 ± 0.05 | 64.76 | |
| Hx:Iso 7:3 | 25 | 1.80 ± 0.03 | 50.67 ± 0.70 | 2.29 ± 0.06 | 2.04 ± 0.14 | 1.25 ± 0.08 | 58.05 |
| –55 | 1.64 ± 0.01 | 48.39 ± 0.07 | 2.13 ± 0.07 | 2.05 ± 0.16 | 1.23 ± 0.02 | 55.44 | |
| Hx:EtOH 7:3 | 25 | 1.78 ± 0.05 | 49.34 ± 0.074 | 2.22 ± 0.10 | 2.12 ± 0.03 | 1.28 ± 0.04 | 56.74 |
| –55 °C | 1.87 ± 0.04 | 48.47 ± 0.08 | 2.26 ± 0.12 | 2.26 ± 0.12 | 1.22 ± 0.01 | 56.08 |
| Compound | LRIexp1 | LRIlit2 | Content Range Min–Max [%] |
|---|---|---|---|
| α-Pinene | 933 | 933 | 0.01–1.16 |
| Myrcene | 991 | 991 | 0.64–5.54 |
| E-Caryophyllene | 1425 | 1424 | 18.37–30.07 |
| α-trans-Bergamotene | 1440 | 1432 | 3.52–7.30 |
| α-Humulene | 1458 | 1454 | 28.54–31.27 |
| Sesquisabinene | 1461 | 1455 | 3.54–9.21 |
| α-Selinene | 1499 | 1501 | 2.52–3.49 |
| Caryophyllene oxide | 1589 | 1587 | 2.41–6.39 |
| Compound | Cell Line/IC50 [μg/mL] | ||||||
|---|---|---|---|---|---|---|---|
| A549 | MCF-7 | AGS | HT-29 | MDA-MB-468 | MV4-11 | MCF-10A | |
| THC | 3.9 ± 0.4 * | 8.4 ± 1.3 * | 6.4 ± 1.4 * | 3.7 ± 0.6 * | 11.3 ± 0.4 | 6.7 ± 0.2 | 11.1 ± 0.7 * |
| CBD | 2.9 ± 0.2 * | 3.7 ± 0.6 * | 3.7 ± 0.3 * | 3.0 ± 0.5 * | 10.9 ± 0.8 | 6.33 ± 0.1 | 10.6 ± 0.6 * |
| CBG | 3.7 ± 0.5 * | 7.4 ± 1.4 * | 6.1 ± 1.3 * | 10.0 ± 1.1 * | 11.6 ± 0.4 | 6.28 ± 0.1 | 10.7 ± 0.7 * |
| CBC | 3.3 ± 0.2 * | 7.6 ± 1.5 * | 5.5 ± 0.9 * | 5.9 ± 1.9 * | 11.4 ± 0.1 | 6.33 ± 0.1 | 10.5 ± 0.5 * |
| CBN | 3.3 ± 0.2 * | 5.8 ± 1.8 * | 6.2 ± 0.98 * | 6.1 ± 2.1 * | 11.4 ± 0.2 | 6.38 ± 0.1 | 10.8 ± 0.7 * |
| CBDV | 6.2 ± 2.3 * | 8.4 ± 2.1 * | 8.4 ± 1.98 * | 3.6 ± 0.4 * | 12 ± 0.5 | 6.33 ± 0.1 | 11.2 ± 0.3 * |
| Feli 1 | 8.1 ± 2.6 | 10.2 ± 4.6 | 10.8 ± 1.9 | 8.1 ± 3.2 | 28.7 ± 7.7 | 6.8 ± 0.7 | 30.7 ± 4.2 |
| Feli 2 | 7.2 ± 1.0 | 8.6 ± 0.9 | 9.4 ± 1.8 | 6.5 ± 0.8 | 30.5 ± 0.9 | 6.9 ± 0.4 | 25.2 ± 8.6 |
| Compound | Cell Line/Calculated Selectivity Index SI | |||||
|---|---|---|---|---|---|---|
| A549 | MCF-7 | AGS | HT-29 | MDA-MB-468 | MV4-11 | |
| THC | 2.82 * | 1.33 * | 1.75 * | 2.98 * | 0.98 | 1.65 |
| CBD | 3.68 * | 2.87 * | 2.82 * | 3.47 * | 0.97 | 1.67 |
| CBG | 2.89 * | 1.45 * | 1.76 * | 1.07 * | 0.92 | 1.70 |
| CBC | 3.23 * | 1.39 * | 1.9 * | 1.77 * | 0.92 | 1.66 |
| CBN | 3.27 * | 1.87 * | 1.75 * | 1.78 * | 0.95 | 1.69 |
| CBDV | 1.81 * | 1.33 * | 1.34 * | 3.12 * | 0.93 | 1.77 |
| Feli 1 | 3.79 | 3.01 | 2.84 | 3.79 | 1.07 | 4.51 |
| Feli 2 | 3.5 | 2.93 | 2.68 | 3.88 | 0.83 | 3.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haczkiewicz, M.; Świtalska, M.; Łyczko, J.; Wietrzyk, J.; Gliszczyńska, A. Phytochemical Profile, Extraction and Characterization of Bioactive Compounds from Industrial Hemp (Cannabis sativa L.) Felina 32 Variety. Molecules 2025, 30, 4148. https://doi.org/10.3390/molecules30204148
Haczkiewicz M, Świtalska M, Łyczko J, Wietrzyk J, Gliszczyńska A. Phytochemical Profile, Extraction and Characterization of Bioactive Compounds from Industrial Hemp (Cannabis sativa L.) Felina 32 Variety. Molecules. 2025; 30(20):4148. https://doi.org/10.3390/molecules30204148
Chicago/Turabian StyleHaczkiewicz, Monika, Marta Świtalska, Jacek Łyczko, Joanna Wietrzyk, and Anna Gliszczyńska. 2025. "Phytochemical Profile, Extraction and Characterization of Bioactive Compounds from Industrial Hemp (Cannabis sativa L.) Felina 32 Variety" Molecules 30, no. 20: 4148. https://doi.org/10.3390/molecules30204148
APA StyleHaczkiewicz, M., Świtalska, M., Łyczko, J., Wietrzyk, J., & Gliszczyńska, A. (2025). Phytochemical Profile, Extraction and Characterization of Bioactive Compounds from Industrial Hemp (Cannabis sativa L.) Felina 32 Variety. Molecules, 30(20), 4148. https://doi.org/10.3390/molecules30204148








