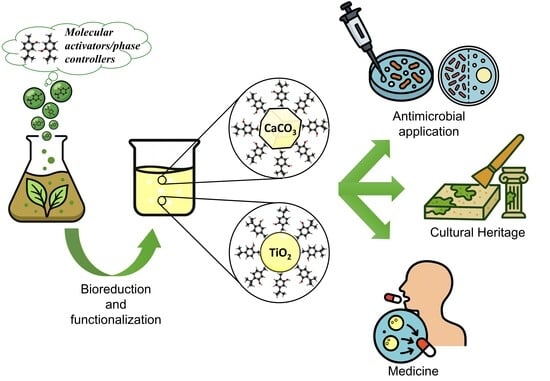
TiO2 and CaCO3 Microparticles Produced in Aqueous Extracts from Satureja montana: Synthesis, Characterization, and Preliminary Antimicrobial Test
Abstract
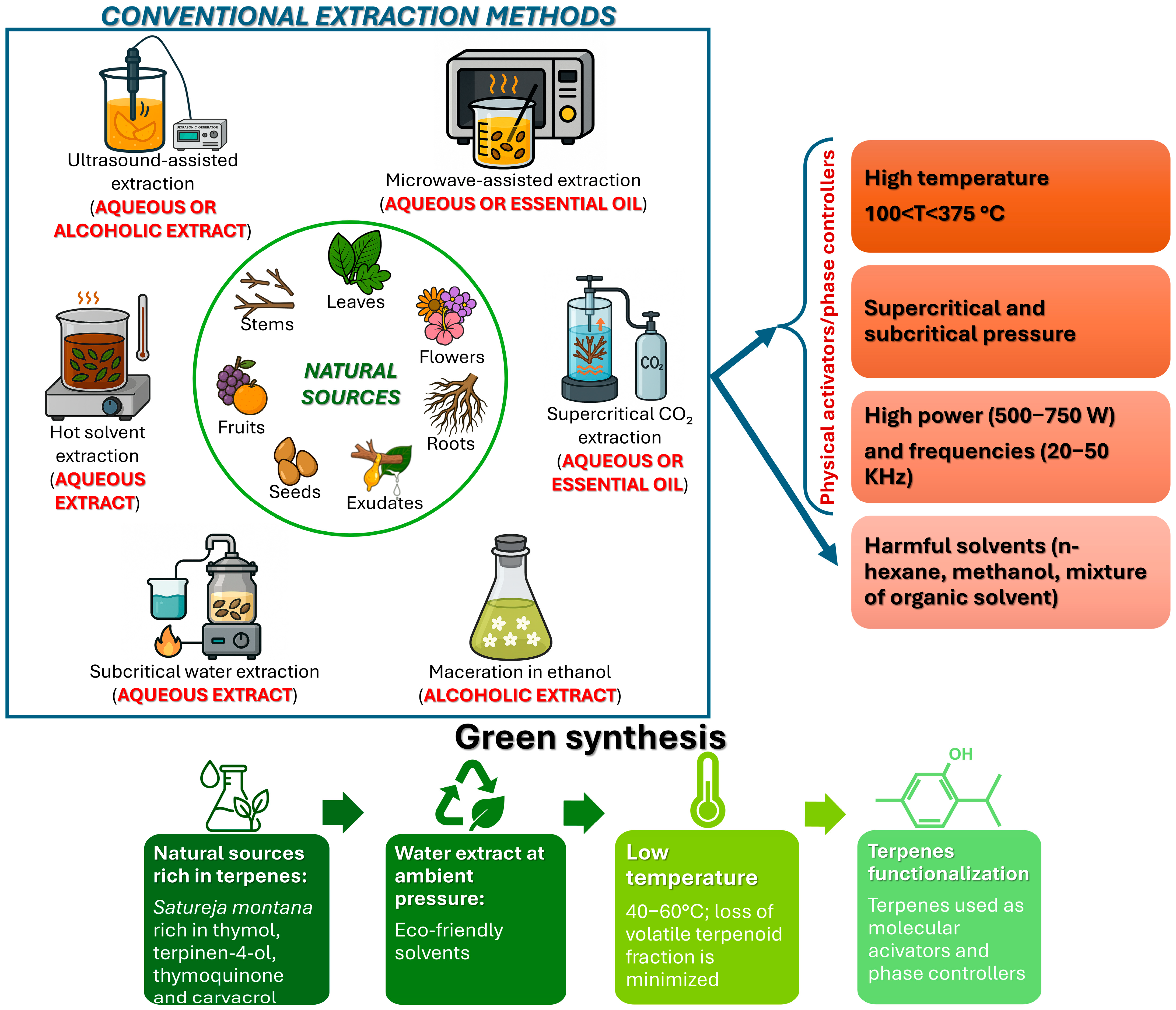
1. Introduction
2. Results and Discussion
2.1. Functionalized Microparticles and Their Characterization Study
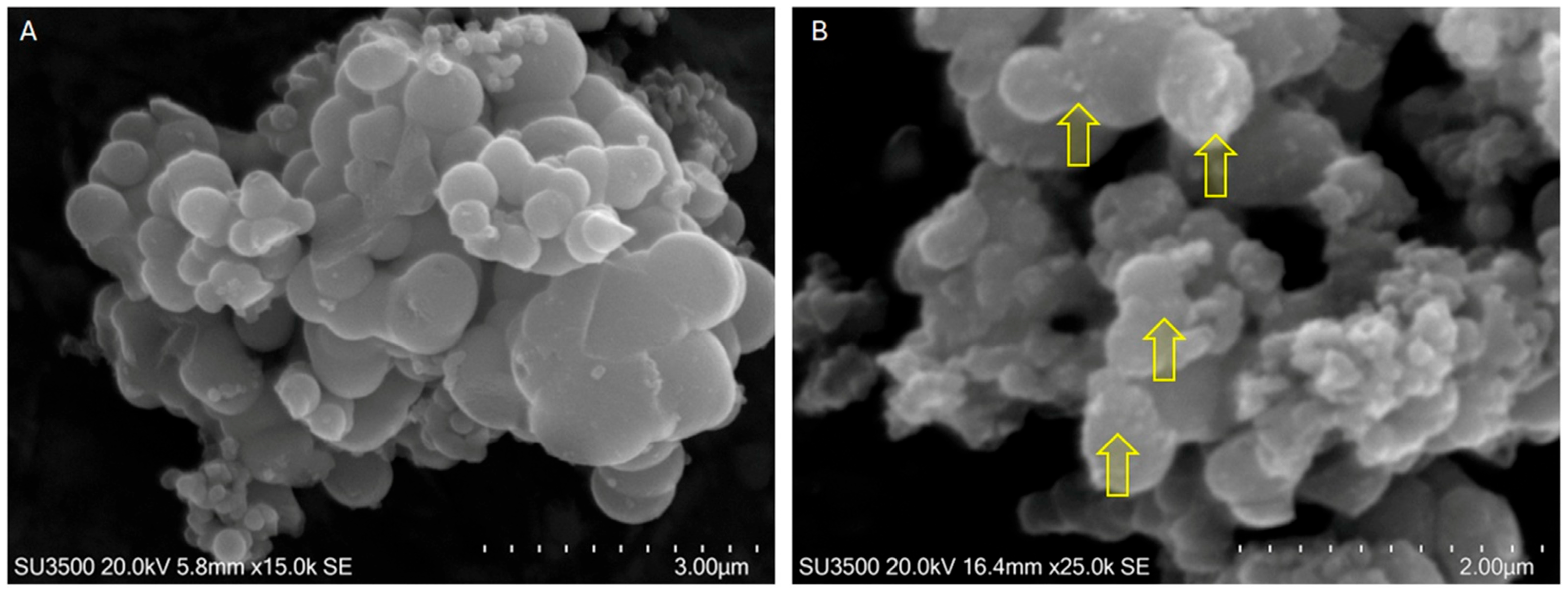
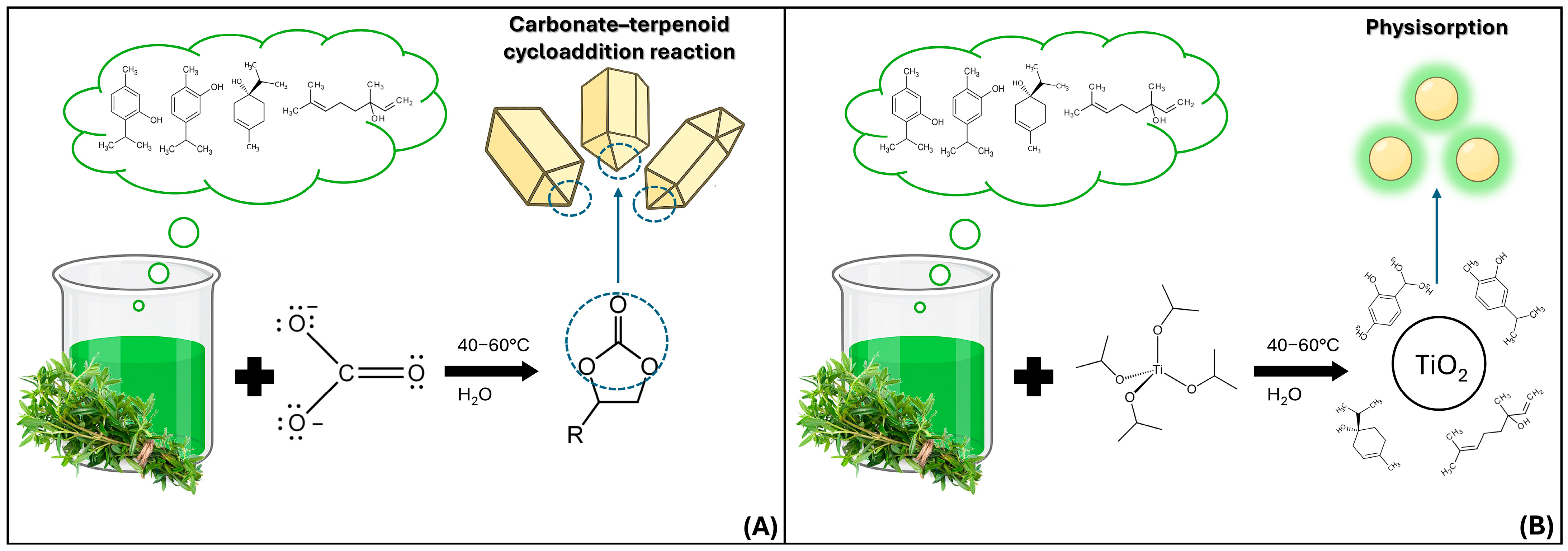
2.1.1. TiO2 MPs/SM
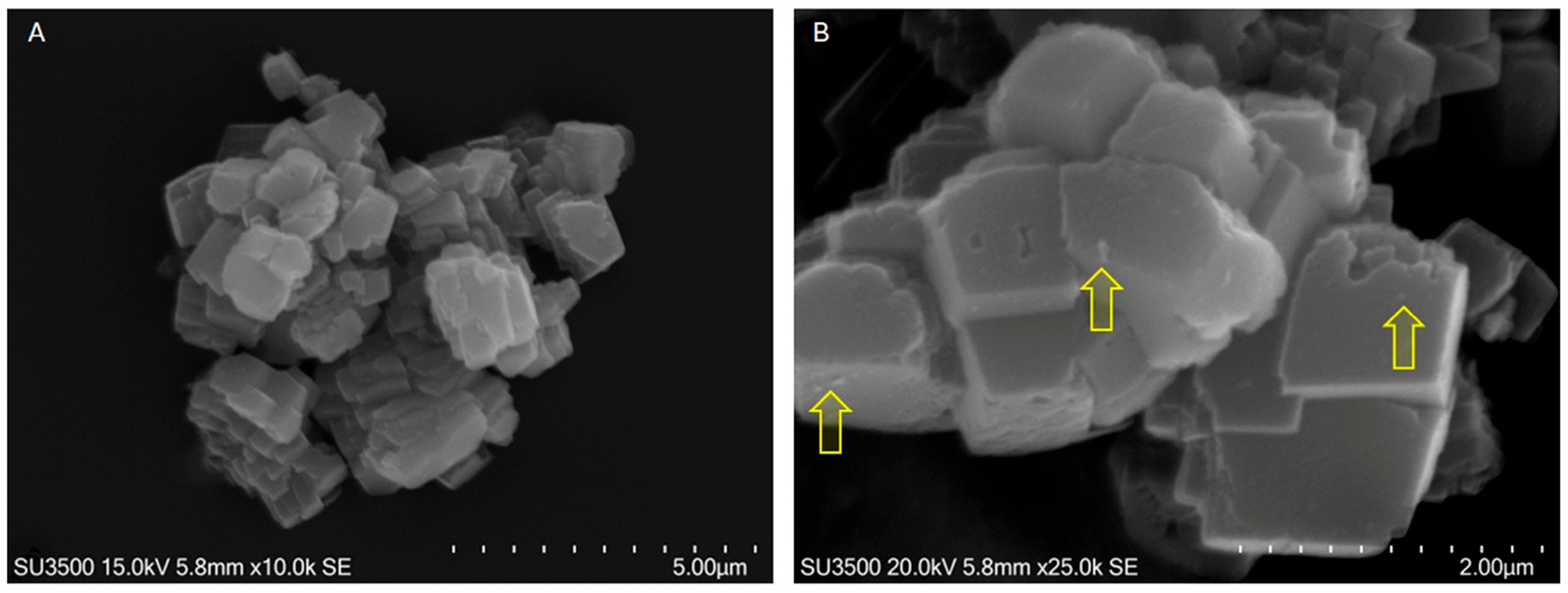
2.1.2. CaCO3 MPs/SM
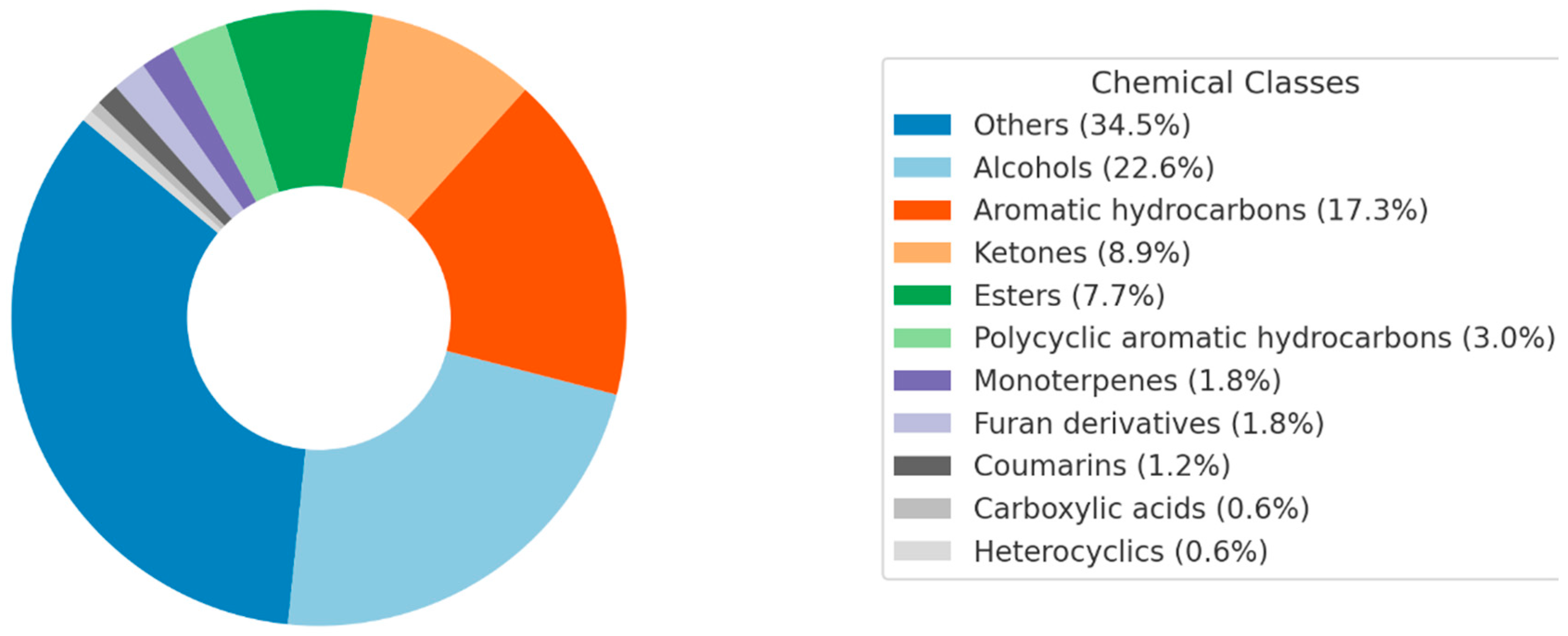
2.2. Chemical Composition of NPEs from SM by GC-MS
2.2.1. Targeted Analysis
2.2.2. Untargeted Analysis
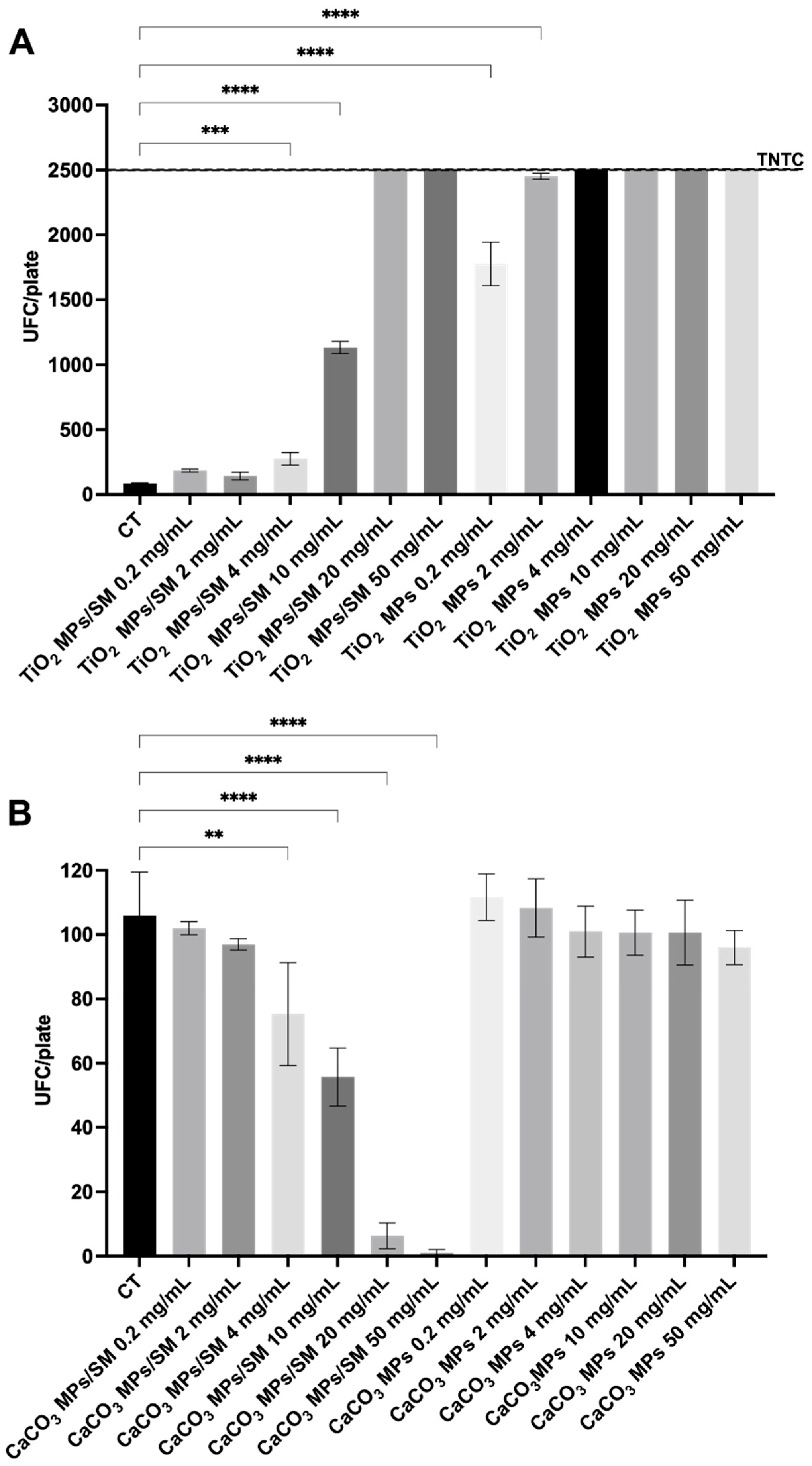
2.3. Antimicrobial Screening
3. Materials and Methods
3.1. Plant Materials and Chemicals/Reagents
3.2. Preparation of TiO2 Microparticles in SM Natural Extracts (TiO2 MPs/SM)
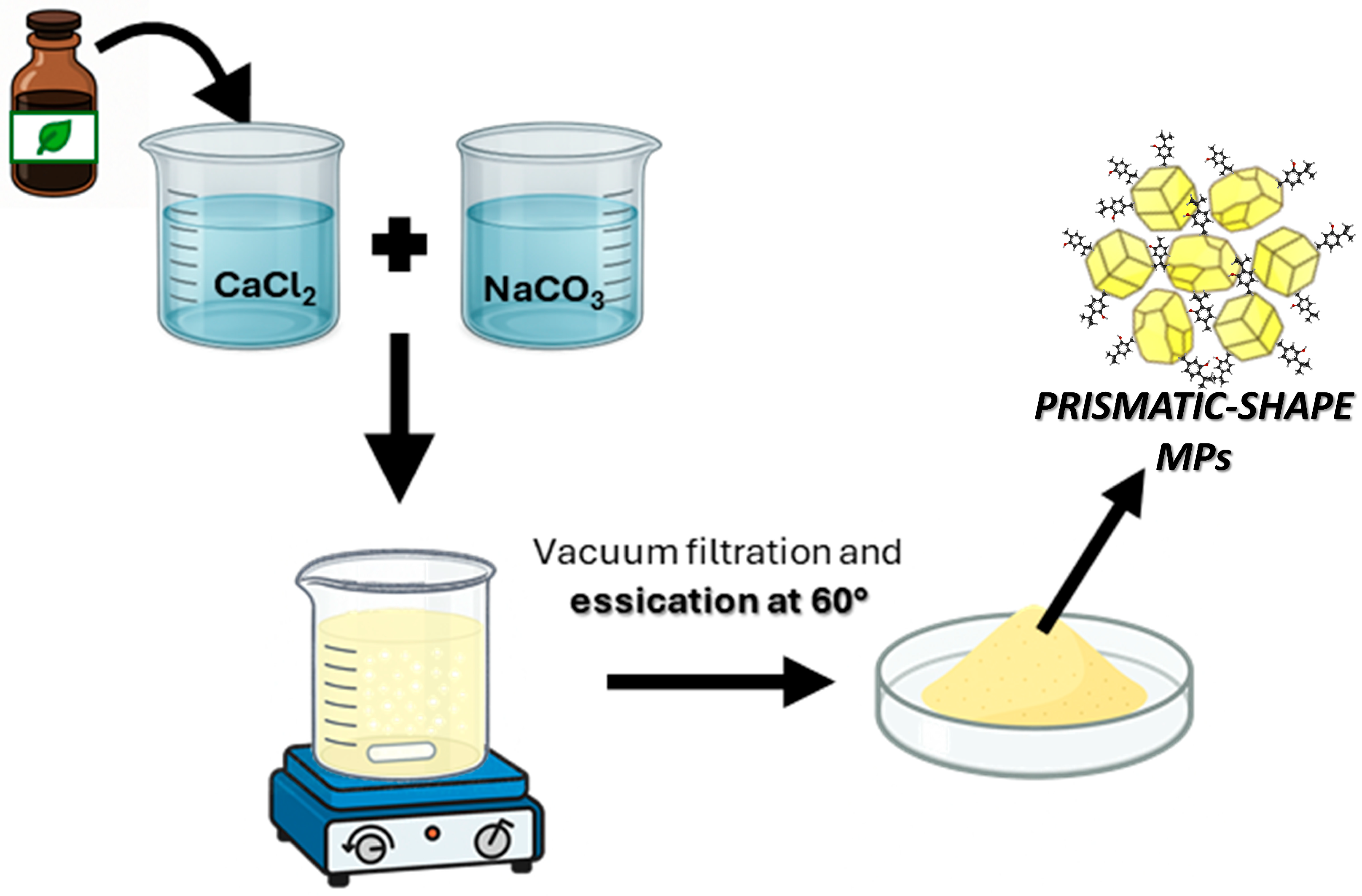
3.3. Preparation of CaCO3 Microparticles in SM Natural Extracts (CaCO3 MPs/SM)
3.4. TiO2 MPs/SM) and (CaCO3 MPs/SM) Characterization Study
3.4.1. SEM/EDX
3.4.2. XRD
3.4.3. Raman Spectroscopy
3.4.4. FTIR Spectroscopy
3.4.5. Zeta-Potential, DLS, and MALS
3.4.6. TGA
3.4.7. BET Analysis
3.4.8. GC-MS Analysis of SM Extract: Molecular Composition
3.5. Experimental Biological Testing and Sampling
Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patra, A.R.; Pattnaik, A.; Ghosh, P. The Latest Breakthroughs in Green and Hybrid Nanoparticle Synthesis for Multifaceted Environmental Applications. J. Taiwan Inst. Chem. Eng. 2025, 106157. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sahu, J.N.; Poonia, A.K.; Ghosh, P. Current Perspective of Nano-Engineered Metal Oxide Based Photocatalysts in Advanced Oxidation Processes for Degradation of Organic Pollutants in Wastewater. Chem. Eng. Res. Des. 2023, 190, 667–686. [Google Scholar] [CrossRef]
- Suman, T.Y.; Radhika Rajasree, S.R.; Ramkumar, R.; Rajthilak, C.; Perumal, P. The Green Synthesis of Gold Nanoparticles Using an Aqueous Root Extract of Morinda Citrifolia L. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Anbalagan, K.; Manosathyadevan, M.; Lee, K.J.; Cho, M.; Lee, S.M.; Park, J.H.; Oh, S.G.; Bang, K.S.; Oh, B.T. Green Synthesis of Silver and Gold Nanoparticles Using Zingiber Officinale Root Extract and Antibacterial Activity of Silver Nanoparticles against Food Pathogens. Bioprocess Biosyst. Eng. 2014, 37, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile Green Synthesis of Silver Nanoparticles Using Berberis Vulgaris Leaf and Root Aqueous Extract and Its Antibacterial Activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.Y.; Mazhar, K.; Raees, L.; Mukhtiar, A.; Khan, F.; Khan, M. Green Synthesis of Cobalt Oxide Nanoparticles Using Roots Extract of Ziziphus Oxyphylla Edgew Its Characterization and Antibacterial Activity. Mater. Res. Express 2022, 9, 105001. [Google Scholar] [CrossRef]
- Ganesh Kumar, V.; Dinesh Gokavarapu, S.; Rajeswari, A.; Stalin Dhas, T.; Karthick, V.; Kapadia, Z.; Shrestha, T.; Barathy, I.A.; Roy, A.; Sinha, S. Facile Green Synthesis of Gold Nanoparticles Using Leaf Extract of Antidiabetic Potent Cassia Auriculata. Colloids Surf. B Biointerfaces 2011, 87, 159–163. [Google Scholar] [CrossRef]
- Flexa-Ribeiro, B.; Garcia, M.D.N.; Silva, A.C.d.J.; Carvalho, J.C.T.; Rocha, L.; Faustino, S.M.M.; Fernandes, C.P.; da Silva, H.F.; Machado, F.P.; Hage-Melim, L.I.d.S.; et al. Essential Oil from Curcuma Longa Leaves: Using Nanotechnology to Make a Promising Eco-Friendly Bio-Based Pesticide from Medicinal Plant Waste. Molecules 2025, 30, 1023. [Google Scholar] [CrossRef]
- Savković, Ž.; Džamić, A.; Veselinović, J.; Grbić, M.L.; Stupar, M. Exploring the Potential of Essential Oils against Airborne Fungi from Cultural Heritage Conservation Premises. Sci. Nat. 2025, 112, 32. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Hernández, I.; Gómez-García, R.; Martínez-Ávila, G.C.G.; Medina-Herrera, N.; González-Hernández, M.D. Unlocking Essential Oils’ Potential as Sustainable Food Additives: Current State and Future Perspectives for Industrial Applications. Sustainability 2025, 17, 2053. [Google Scholar] [CrossRef]
- Omidian, H.; Cubeddu, L.X.; Gill, E.J. Harnessing Nanotechnology to Enhance Essential Oil Applications. Molecules 2025, 30, 520. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Sana; Tariq, A.; Khan, M.M.A. Conclusions and Future Prospects of Plant-Based Essential Oils. In Essential Oil-Bearing Plants: Agro-Techniques, Phytochemicals, and Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2025; pp. 381–384. ISBN 9780443248603. [Google Scholar]
- da Silva, E.F.; dos Santos, F.A.L.; Pires, H.M.; Bastos, L.M.; Ribeiro, L.N.d.M. Lipid Nanoparticles Carrying Essential Oils for Multiple Applications as Antimicrobials. Pharmaceutics 2025, 17, 178. [Google Scholar] [CrossRef] [PubMed]
- Hashempur, M.H.; Ghorat, F.; Karami, F.; Jahanbin, A.; Nouraei, H.; Abbasi, M.; Jafari, M.; Zare, A.; Barzegar, S.; Zareshahrabadi, Z. Topical Delivery Systems for Plant-Derived Antimicrobial Agents: A Review of Current Advances. Int. J. Biomater. 2025, 2025, 4251091. [Google Scholar] [CrossRef]
- Nayak, S.; Hegde, R.B.; Rao, A.S.; Poojary, R. Unlocking the Potential of Essential Oils in Aromatic Plants: A Guide to Recovery, Modern Innovations, Regulation and AI Integration. Planta 2025, 262, 6. [Google Scholar] [CrossRef]
- Mançano, R.R.; Matheus, L.R.; Castro, L.E.N.; Barroso, T.L.C.T.; da Rosa, R.G.; Ferreira, V.C.; Forster-Carneiro, T.; Colpini, L.M.S. Extraction Techniques for Propolis and Its Utilization in Silver Nanoparticle Synthesis: A Comprehensive Review. Eur. Food Res. Technol. 2025, 251, 1397–1433. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Rivas, S.; Rivas, J.; Domínguez, H. Subcritical Water Extraction of Essential Oils and Plant Oils. Sustain. Chem. Pharm. 2023, 36, 101332. [Google Scholar] [CrossRef]
- Matinfard, S.; Tavakolipour, H.; Sodeifian, G.; Sajadian, S.A.; Kalvandi, R. Supercritical CO2 Green Solvent Extraction of Nepeta Crispa: Evaluation of Process Optimization, Chemical Analysis, and Biological Activity. J. Supercrit. Fluids 2025, 216, 106451. [Google Scholar] [CrossRef]
- Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S.; et al. Satureja montana L. Essential Oils: Chemical Profiles/Phytochemical Screening, Antimicrobial Activity and o/w Nanoemulsion Formulations. Pharmaceutics 2020, 12, 7. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Macchia, A.; Valentini, F.; Colasanti, I.A.; Zaratti, C. Prediction of Green Solvent Applicability in Cultural Heritage Using Hansen Solubility Parameters, Cremonesi Method and Integrated Toxicity Index. Sustainability 2025, 17, 2944. [Google Scholar] [CrossRef]
- Bodea, I.M.; Garre Pérez, A.; Cătunescu, G.M.; Palop, A. A Review on Microwave and Ultrasound-Assisted Extractions of Essential Oil from Orange Peel Waste. Food Bioprocess Technol. 2025, 18, 7060–7082. [Google Scholar] [CrossRef]
- Jadhav, J.J.; Jadeja, G.C.; Desai, M.A. Ultrasound-Assisted Hydrodistillation for Extraction of Essential Oil from Clove Buds—A Step towards Process Improvement and Sustainable Outcome. Chem. Eng. Process.—Process Intensif. 2023, 189, 109404. [Google Scholar] [CrossRef]
- Dragomanova, S.; Tancheva, L.; Abarova, S.; Grigorova, V.B.; Gavazova, V.; Stanciu, D.; Tzonev, S.; Prandjev, V.; Kalfin, R. Protective Potential of Satureja montana-Derived Polyphenols in Stress-Related Central Nervous System Disorders, Including Dementia. Curr. Issues Mol. Biol. 2025, 47, 556. [Google Scholar] [CrossRef]
- Muhammad; Sofyan, N.; Yuwono, A.H.; Dhaneswara, D. A Review on Green Synthesis of TiO2 Nanoparticles: Enhancing DSSC Performance and Exploring Future Opportunities. Mater. Sci. Energy Technol. 2025, 8, 188–199. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, S.; Lim, S. Antimicrobial Properties of Thermally Processed Oyster Shell Powder for Use as Calcium Supplement. Foods 2025, 14, 2579. [Google Scholar] [CrossRef] [PubMed]
- Frassine, D.; Braglia, R.; Scuderi, F.; Redi, E.L.; Valentini, F.; Relucenti, M.; Colasanti, I.A.; Macchia, A.; Allegrini, I.; Gismondi, A.; et al. Enhancing Lettuce (Lactuca sativa) Productivity: Foliar Sprayed Fe-Alg-CaCO3 MPs as Fertilizers for Aquaponics Cultivation. Plants 2024, 13, 3416. [Google Scholar] [CrossRef] [PubMed]
- Frassine, D.; Braglia, R.; Scuderi, F.; Redi, E.L.; Valentini, F.; Relucenti, M.; Colasanti, I.A.; Zaratti, C.; Macchia, A.; Allegrini, I.; et al. Smart Foliar Fertilizer Based on Zn-Alg-CaCO3 Microparticles Improves Aquaponic Tomato Cultivation. Sci. Rep. 2025, 15, 18092. [Google Scholar] [CrossRef] [PubMed]
- Algadi, H.; Alhoot, M.A.; Yaaqoob, L.A. Systematic Review of Antibacterial Potential in Calcium Oxide and Silicon Oxide Nanoparticles for Clinical and Environmental Infection Control. J. Appl. Biomed. 2025, 23, 1–11. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Alluqmani, S.M.; Almarri, H.M.; AL-Zahrani, A.A. Physical, Chemical, and Biological Routes of Synthetic Titanium Dioxide Nanoparticles and Their Crucial Role in Temperature Stress Tolerance in Plants. Heliyon 2024, 10, e26537. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Chen, X.; Baesjou, P.J.; Imhof, A.; van Blaaderen, A. Synthesis and Characterization of Anatase TiO2 Nanorods: Insights from Nanorods’ Formation and Self-Assembly. Appl. Sci. 2022, 12, 1614. [Google Scholar] [CrossRef]
- Rezaee, M.; Mousavi Khoie, S.M.; Liu, K.H. The Role of Brookite in Mechanical Activation of Anatase-to-Rutile Transformation of Nanocrystalline TiO2: An XRD and Raman Spectroscopy Investigation. CrystEngComm 2011, 13, 5055–5061. [Google Scholar] [CrossRef]
- Yan, J.; Wu, G.; Guan, N.; Li, L.; Li, Z.; Cao, X. Understanding the Effect of Surface/Bulk Defects on the Photocatalytic Activity of TiO2: Anatase versus Rutile. Phys. Chem. Chem. Phys. 2013, 15, 10978–10988. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaima, A.; Bahnemann, D. Brookite versus Anatase TiO2 Photocatalysts: Phase Transformations and Photocatalytic Activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef]
- Hu, W.; Li, L.; Li, G.; Tang, C.; Sun, L. High-Quality Brookite TiO2 Flowers: Synthesis, Characterization, and Dielectric Performance. Cryst. Growth Des. 2009, 9, 3676–3682. [Google Scholar] [CrossRef]
- Anjos, O.; Santos, A.J.A.; Paixão, V.; Estevinho, L.M. Physicochemical Characterization of Lavandula Spp. Honey with FT-Raman Spectroscopy. Talanta 2018, 178, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Espina, A.; Sanchez-Cortes, S.; Jurašeková, Z. Vibrational Study (Raman, SERS, and IR) of Plant Gallnut Polyphenols Related to the Fabrication of Iron Gall Inks. Molecules 2022, 27, 279. [Google Scholar] [CrossRef]
- Minteguiaga, M.; Dellacassa, E.; Iramain, M.A.; Catalán, C.A.N.; Brandán, S.A. FT-IR, FT-Raman, UV–Vis, NMR and Structural Studies of Carquejyl Acetate, a Distinctive Component of the Essential Oil from Baccharis Trimera (Less.) DC. (Asteraceae). J. Mol. Struct. 2019, 1177, 499–510. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Sandoval Pauker, C.; Zárate Pozo, P.; Sinche Serra, M.; Jácome Camacho, G.; Rueda-Ayala, V.; Garrido, P.; Ramos Guerrero, L.; Ciobotă, V. Raman Spectroscopy in the Detection of Adulterated Essential Oils: The Case of Nonvolatile Adulterants. J. Raman Spectrosc. 2021, 52, 1055–1063. [Google Scholar] [CrossRef]
- Calheiros, R.; Machado, N.F.L.; Fiuza, S.M.; Gaspar, A.; Garrido, J.; Milhazes, N.; Borges, F.; Marques, M.P.M. Antioxidant Phenolic Esters with Potential Anticancer Activity: A Raman Spectroscopy Study. J. Raman Spectrosc. 2008, 39, 95–107. [Google Scholar] [CrossRef]
- Schulz, H.; Özkan, G.; Baranska, M.; Krüger, H.; Özcan, M. Characterisation of Essential Oil Plants from Turkey by IR and Raman Spectroscopy. Vib. Spectrosc. 2005, 39, 249–256. [Google Scholar] [CrossRef]
- Lafhal, S.; Vanloot, P.; Bombarda, I.; Valls, R.; Kister, J.; Dupuy, N. Raman Spectroscopy for Identification and Quantification Analysis of Essential Oil Varieties: A Multivariate Approach Applied to Lavender and Lavandin Essential Oils. J. Raman Spectrosc. 2015, 46, 577–585. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Daferera, D.J.; Mitsi, C.; Trigas, P.; Polissiou, M.; Tarantilis, P.A. Comparative Chemotype Determination of Lamiaceae Plants by Means of GC-MS, FT-IR, and Dispersive-Raman Spectroscopic Techniques and GC-FID Quantification. Ind. Crops Prod. 2014, 62, 22–33. [Google Scholar] [CrossRef]
- Ram Kumar, A.; Selvaraj, S.; Azam, M.; Sheeja Mol, G.; Kanagathara, N.; Alam, M.; Jayaprakash, P. Spectroscopic, Biological, and Topological Insights on Lemonol as a Potential Anticancer Agent. ACS Omega 2023, 8, 31548–31566. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological Activity of Vegetal Extracts Containing Phenols on Plant Metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Han, L.; Xu, Y.; Ke, H.; Zhou, N.; Dong, H.; Liu, S.; Qiao, G. Near Infrared Spectroscopic Study of Trioctahedral Chlorites and Its Remote Sensing Application. Open Geosci. 2019, 11, 815–828. [Google Scholar] [CrossRef]
- Ahmadi, O.; Jafarizadeh-Malmiri, H. Intensification Process in Thyme Essential Oil Nanoemulsion Preparation Based on Subcritical Water as Green Solvent and Six Different Emulsifiers. Green Process. Synth. 2021, 10, 430–439. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via Ft-Ir Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Abdallah, R.A.; El-Borady, O.M.; El-Sayed, A.F.; Fawzy, M. A Comparative Study of Chemo- and Phytosynthesized Silver Nanoparticles Using Ceratophyllum Demersum Extract: Characterization and Biological Activities. Biomass Convers. Biorefin. 2025, 15, 19013–19029. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Gibin, M.S.; Vollmann, A.; Pattaro, M.C.; Giacomelli, M.E.; Sato, F.; Nanni, M.R.; Antunes, W.C. Classification and Prediction by Pigment Content in Lettuce (Lactuca sativa L.) Varieties Using Machine Learning and ATR-FTIR Spectroscopy. Plants 2022, 11, 3413. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; Wiley: Hoboken, NJ, USA, 2005; ISBN 1118311655. [Google Scholar]
- Blessymol, B.; Yasotha, P.; Kalaiselvi, V.; Gopi, S. An Antioxidant Study of Titanium Dioxide (TiO2) Nanoparticles against Mace of Nutmeg in Myristica Fragrans Houtt, Rhizomes of Curcuma Longa Linn and Kaempferia Galanga Extracts. Results Chem. 2024, 7, 101291. [Google Scholar] [CrossRef]
- Pushpamalini, T.; Keerthana, M.; Sangavi, R.; Nagaraj, A.; Kamaraj, P. Comparative Analysis of Green Synthesis of TiO2 Nanoparticles Using Four Different Leaf Extract. Mater. Today Proc. 2021, 40, S180–S184. [Google Scholar] [CrossRef]
- Smith, B. The C=O Bond, Part VII: Aromatic Esters, Organic Carbonates, and More of the Rule of Three. Spectroscopy 2018, 33, 24–29. Available online: https://www.spectroscopyonline.com/view/co-bond-part-vii-aromatic-esters-organic-carbonates-and-more-rule-three (accessed on 30 September 2025).
- Kim, Y.; Caumon, M.C.; Barres, O.; Sall, A.; Cauzid, J. Identification and Composition of Carbonate Minerals of the Calcite Structure by Raman and Infrared Spectroscopies Using Portable Devices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 119980. [Google Scholar] [CrossRef]
- Alves, J.F.; Edwards, H.G.M.; Korsakov, A.; de Oliveira, L.F.C. Revisiting the Raman Spectra of Carbonate Minerals. Minerals 2023, 13, 1358. [Google Scholar] [CrossRef]
- Legodi, M.A.; De Waal, D.; Potgieter, J.H. Quantitative Determination of CaCO3 in Cement Blends by FT-IR. Appl. Spectrosc. 2001, 55, 361–365. [Google Scholar] [CrossRef]
- Vagenas, N.V.; Gatsouli, A.; Kontoyannis, C.G. Quantitative Analysis of Synthetic Calcium Carbonate Polymorphs Using FT-IR Spectroscopy. Talanta 2003, 59, 831–836. [Google Scholar] [CrossRef]
- Gismondi, A.; Di Marco, G.; Redi, E.L.; Ferrucci, L.; Cantonetti, M.; Canini, A. The Antimicrobial Activity of Lavandula angustifolia Mill. Essential Oil against Staphylococcus Species in a Hospital Environment. J. Herb. Med. 2021, 26, 100426. [Google Scholar] [CrossRef]
- Pino-Otín, M.R.; Gan, C.; Terrado, E.; Sanz, M.A.; Ballestero, D.; Langa, E. Antibiotic Properties of Satureja montana L. Hydrolate in Bacteria and Fungus of Clinical Interest and Its Impact in Non-Target Environmental Microorganisms. Sci. Rep. 2022, 12, 18460. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Šovljanski, O.; Pezo, L.; Travičić, V.; Tomić, A.; Zheljazkov, V.D.; Ćetković, G.; Švarc-Gajić, J.; Brezo-Borjan, T.; Sofrenić, I. Variability in Biological Activities of Satureja montana Subsp. Montana and Subsp. Variegata Based on Different Extraction Methods. Antibiotics 2022, 11, 1235. [Google Scholar] [CrossRef] [PubMed]
- Maravić-Vlahoviček, G.; Kindl, M.; Andričević, K.; Obranić, S.; Vladimir-Knežević, S. Modulatory Effects of Satureja montana L. Essential Oil on Biofilm Formation and Virulence Factors of Pseudomonas aeruginosa. Pharmaceuticals 2025, 18, 1269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhao, X.; Liu, Y. The Latest Research Progress on the Antibacterial Properties of TiO2 Nanocomposites. J. Text. Inst. 2025, 116, 634–660. [Google Scholar] [CrossRef]
- Khalaf Kenawe, L.; Abbas, R.S.; Kadhim, A.S. Comparative Study of Alcohol Clove Extract, TiO2 Nanoparticles, and Clove Extract-TiO2 Complex in Controlling Bacterial Growth in Burn Infections. Microbes Infect. Dis. 2025, 6, 680–688. [Google Scholar] [CrossRef]
- Fletes-Vargas, G.; Rodríguez-Rodríguez, R.; Pinto, N.V.; Kato, K.C.; Carneiro, G.; Rodrigues, A.P.; Rodrigues-Martins, H.; Espinosa-Andrews, H. TiO2 Nanoparticles Loaded with Polygonum Cuspidatum Extract for Wound Healing Applications: Exploring Their Hemolytic, Antioxidant, Cytotoxic, and Antimicrobial Properties. Nanomaterials 2025, 15, 926. [Google Scholar] [CrossRef]
- Al-darwesh, M.Y.; Babakr, K.A.; Qader, I.N.; Mohammed, M.A. Antimicrobial, Anti-Inflammatory, and Anticancer Potential of Green Synthesis TiO2 Nanoparticles Using Sophora Flavescens Root Extract. Chem. Pap. 2025, 79, 1207–1221. [Google Scholar] [CrossRef]
- Prakash, J.; Cho, J.; Ruzimuradov, O.; Fang, D. Antibacterial Applications of TiO2 Based Hybrid Semiconductor Nanomaterials: Recent Advances and Future Prospects. In Titanium Dioxide-Based Multifunctional Hybrid Nanomaterials; Engineering Materials; Springer: Cham, Switzerland, 2025; Part F212; pp. 313–337. [Google Scholar] [CrossRef]
- Sundaram, T.; Rajendran, S.; Natarajan, S.; Vinayagam, S.; Rajamohan, R.; Lackner, M. Environmental Fate and Transformation of TiO2 Nanoparticles: A Comprehensive Assessment. Alex. Eng. J. 2025, 115, 264–276. [Google Scholar] [CrossRef]
- Delgado, L.P.; Figueroa-Torres, M.Z.; Ceballos-Chuc, M.C.; García-Rodríguez, R.; Alvarado-Gil, J.J.; Oskam, G.; Rodriguez-Gattorno, G. Tailoring the TiO2 Phases through Microwave-Assisted Hydrothermal Synthesis: Comparative Assessment of Bactericidal Activity. Mater. Sci. Eng. C 2020, 117, 111290. [Google Scholar] [CrossRef]
- Shakeel, N.; Piwoński, I.; Iqbal, P.; Kisielewska, A. Green Synthesis of Titanium Dioxide Nanoparticles: Physicochemical Characterization and Applications: A Review. Int. J. Mol. Sci. 2025, 26, 5454. [Google Scholar] [CrossRef]
- Gu, C.C.; Long, X.E.; Chen, X.; Liu, J.; Li, C.C. Recent Natural Product Total Syntheses Involving Cycloadditions of Allenes. Chin. J. Chem. 2025, 43, 1059–1077. [Google Scholar] [CrossRef]
- Zang, Y.; Sun, R.; Feng, R.; Zhu, H.; Li, X. Recent Advances of Terpenoids with Intriguing Chemical Skeletons and Biological Activities. Chin. J. Chem. 2025, 43, 443–469. [Google Scholar] [CrossRef]
- Trapasso, G.; Aricò, F. Organic Carbonates as Green Media: From Laboratory Syntheses to Industrial Applications. Green Chem. 2025, 27, 6925–6966. [Google Scholar] [CrossRef]
- Wesolowska, A.; Grzeszczuk, M.; Jadczak, D. Influence of Harvest Term on the Content of Carvacrol, p-Cymene, Î3-Terpinene and Î2-Caryophyllene in the Essential Oil of Satureja montana. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 392–397. [Google Scholar] [CrossRef]
- Serrano, C.; Matos, O.; Teixeira, B.; Ramos, C.; Neng, N.; Nogueira, J.; Nunes, M.L.; Marques, A. Antioxidant and Antimicrobial Activity of Satureja montana L. Extracts. J. Sci. Food Agric. 2011, 91, 1554–1560. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M. Synthesis of TiO2 Nanoparticles by Chemical and Green Synthesis Methods and Their Multifaceted Properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Bahrom, H.; Goncharenko, A.A.; Fatkhutdinova, L.I.; Peltek, O.O.; Muslimov, A.R.; Koval, O.Y.; Eliseev, I.E.; Manchev, A.; Gorin, D.; Shishkin, I.I.; et al. Controllable Synthesis of Calcium Carbonate with Different Geometry: Comprehensive Analysis of Particle Formation, Cellular Uptake, and Biocompatibility. ACS Sustain. Chem. Eng. 2019, 7, 19142–19156. [Google Scholar] [CrossRef]
- Garg, R.; Kumari, M.; Kumar, M.; Dhiman, S.; Garg, R. Green Synthesis of Calcium Carbonate Nanoparticles Using Waste Fruit Peel Extract. Mater. Today Proc. 2021, 46, 6665–6668. [Google Scholar] [CrossRef]
- Correr, S.; Makabe, S.; Heyn, R.; Relucenti, M.; Naguro, T.; Familiari, G. Microplicae-like Structures of the Fallopian Tube in Postmenopausal Women as Shown by Electron Microscopy. Histol. Histopathol. 2006, 21, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Relucenti, M.; Heyn, R.; Correr, S.; Familiari, G. Cumulus Oophorus Extracellular Matrix in the Human Oocyte: A Role for Adhesive Proteins. Ital. J. Anat. Embryol. 2005, 110, 219–224. [Google Scholar]
- Relucenti, M.; Heyn, R.; Petruzziello, L.; Pugliese, G.; Taurino, M.; Familiari, G. Detecting Microcalcifications in Atherosclerotic Plaques by a Simple Trichromic Staining Method for Epoxy Embedded Carotid Endarterectomies. Eur. J. Histochem. 2010, 54, 143–147. [Google Scholar] [CrossRef]
- Di Giorgio, G.; Relucenti, M.; Iaculli, F.; Salucci, A.; Donfrancesco, O.; Polimeni, A.; Bossù, M. The Application of a Fluoride-and-Vitamin D Solution to Deciduous Teeth Promotes Formation of Persistent Mineral Crystals: A Morphological Ex-Vivo Study. Materials 2023, 16, 4049. [Google Scholar] [CrossRef]
- Bossù, M.; Matassa, R.; Relucenti, M.; Iaculli, F.; Salucci, A.; Di Giorgio, G.; Familiari, G.; Polimeni, A.; Di Carlo, S. Morpho-Chemical Observations of Human Deciduous Teeth Enamel in Response to Biomimetic Toothpastes Treatment. Materials 2020, 13, 1803. [Google Scholar] [CrossRef]
- Valentini, F.; Pallecchi, P.; Relucenti, M.; Donfrancesco, O.; Sottili, G.; Pettiti, I.; Mussi, V. Characterization of Calcium Carbonate Nanoparticles with Architectural Application for the Consolidation of Pietraforte. Anal. Lett. 2022, 55, 93–108. [Google Scholar] [CrossRef]
- Mathaes, R.; Winter, G.; Engert, J.; Besheer, A. Application of Different Analytical Methods for the Characterization of Non-Spherical Micro- and Nanoparticles. Int. J. Pharm. 2013, 453, 620–629. [Google Scholar] [CrossRef]
- Rübner, K.; Balköse, D.; Robens, E. Methods of Humidity Determination Part II: Determination of Material Humidity. J. Therm. Anal. Calorim. 2008, 94, 675–682. [Google Scholar] [CrossRef]
- Valentini, F.; Pallecchi, P.; Relucenti, M.; Donfrancesco, O.; Sottili, G.; Pettiti, I.; Mussi, V.; De Angelis, S.; Scatigno, C.; Festa, G. SiO2 Nanoparticles as New Repairing Treatments toward the Pietraforte Sandstone in Florence Renaissance Buildings. Crystals 2022, 12, 1182. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 2002, 73, 373–380. [Google Scholar] [CrossRef]
- Barsottelli, M.; Fratini, F.; Giorgetti, G.; Manganelli Del Fà, C. Microfabric and Alteration in Carrara Marble: A Preliminary Study. Sci. Technol. Cult. Herit. 1998, 7, 115–126. Available online: https://www.researchgate.net/publication/292312093_Microfabric_and_alteration_in_Carrara_marble_A_preliminary_study (accessed on 30 September 2025).
- Barsottelli, M.; Cellai, G.F.; Fratini, F.; Manganelli Del Fà, C. The Hygrometric Behaviour of Some Artificial Stone Materials Used as Elements of Masonry Walls. Mater. Struct./Mater. Et Constr. 2001, 34, 211–216. [Google Scholar] [CrossRef]
- Viani, I.; Colucci, M.E.; Pergreffi, M.; Rossi, D.; Veronesi, L.; Bizzarro, A.; Capobianco, E.; Affanni, P.; Zoni, R.; Saccani, E.; et al. Passive Air Sampling: The Use of the Index of Microbial Air Contamination. Acta Biomed. 2020, 91, 92–105. [Google Scholar] [CrossRef]
- Pasquarella, C.; Pitzurra, O.; Savino, A. The Index of Microbial Air Contamination. J. Hosp. Infect. 2000, 46, 241–256. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Agric. Nat. Resour. 2008, 42, 357–361. [Google Scholar]
- You, Y.F.; Xu, C.H.; Xu, S.S.; Cao, S.; Wang, J.P.; Huang, Y.B.; Shi, S.Q. Structural Characterization and Optical Property of TiO2 Powders Prepared by the Sol–Gel Method. Ceram. Int. 2014, 40, 8659–8666. [Google Scholar] [CrossRef]
- Bellardita, M.; Di Paola, A.; Megna, B.; Palmisano, L. Absolute Crystallinity and Photocatalytic Activity of Brookite TiO2 Samples. Appl. Catal. B 2017, 201, 150–158. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Khedr, T.M.; Zhang, G.; Vogiazi, V.; Ismail, A.A.; O’Shea, K.; Dionysiou, D.D. Tailored Synthesis of Anatase–Brookite Heterojunction Photocatalysts for Degradation of Cylindrospermopsin under UV–Vis Light. Chem. Eng. J. 2017, 310, 428–436. [Google Scholar] [CrossRef]
- Juhasz-Bortuzzo, J.A.; Myszka, B.; Silva, R.; Boccaccini, A.R. Sonosynthesis of Vaterite-Type Calcium Carbonate. Cryst. Growth Des. 2017, 17, 2351–2356. [Google Scholar] [CrossRef]
- Zhou, G.T.; Yu, J.C.; Wang, X.C.; Zhang, L.Z. Sonochemical Synthesis of Aragonite-Type Calcium Carbonate with Different Morphologies. New J. Chem. 2004, 28, 1027–1031. [Google Scholar] [CrossRef]
- Ramasamy, V.; Anand, P.; Suresh, G. Synthesis and Characterization of Polymer-Mediated CaCO3 Nanoparticles Using Limestone: A Novel Approach. Adv. Powder Technol. 2018, 29, 818–834. [Google Scholar] [CrossRef]
- Swain, S.K.; Pradhan, G.C.; Dash, S.; Mohanty, F.; Behera, L. Preparation and Characterization of Bionanocomposites Based on Soluble Starch/Nano CaCO3. Polym. Compos. 2018, 39, E82–E89. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Iqbal Faruque, M.R.; Din, I.U.; Alotaibi, M.A. Unmodified Titanium Dioxide Nanoparticles as a Potential Contrast Agent in Photon Emission Computed Tomography. Crystals 2021, 11, 171. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Wu, H.; Zhao, X.; Liu, Y. Green Synthesis of Titanium Dioxide Nanoparticles: Characterization, Mechanisms, and Antibacterial Properties of Brookite/Anatase Heterophase Systems. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; El-Sayyad, G.S.; El-Baz, F.K.; Ahmed, H.S. Recent Trends in Green Synthesis of Metal Nanoparticles Using Natural Resources and Their Biomedical Applications. Microb. Cell Fact. 2024, 23, 341. [Google Scholar] [CrossRef]
- Punz, B.; Christ, C.; Waldl, A.; Li, S.; Liu, Y.; Johnson, L.; Auer, V.; Cardozo, O.; Farias, P.M.A.; Andrade, A.C.D.S.; et al. Nano-Scaled Advanced Materials for Antimicrobial Applications—Mechanistic Insight, Functional Performance Measures, and Potential towards Sustainability and Circularity. Environ. Sci. Nano 2025, 12, 1710–1739. [Google Scholar] [CrossRef]




| Band (cm−1) | Assignment | References |
|---|---|---|
| RAMAN SPECTROSCOPY | ||
| 146 | Eg mode (anatase) | [32,33,34] |
| 219–279 | Eg (anatase) B1g + B2g mode (brookite) | [32,33,34,35] |
| 411 | B1g mode (anatase) | [32,33,34] |
| 517 | A1g + B1g mode (anatase) | [32,33,34] |
| 623 | Eg mode (anatase) | [32,33,34] |
| ~874 | νC–OH νC–C δCH/CH2 | [36,37,38,39,40,41,42,43,44,45] |
| ~1627 | νC=O νC=C δO–H | [36,37,38,39,40,41,42,43,44] |
| ~3352 | νO–H | [46] |
| FTIR SPECTROSCOPY | ||
| 3400 | νO–H | [47,48,49,50,51] |
| 1630 | δO–H νC=O νC=C | [47,48,49,50,51] |
| 2974 | νas–CH3 | [50,51] |
| 2893 | νs–CH2 | [50,51] |
| 1454, 1395 | δas/s–CH3 δas/s–CH2 | [50,51] |
| 1268 | νC–O νC–C | [50,51] |
| 1051 | νC–O νC–C ring vibration | [50,51] |
| 800–600 | ν + δ Ti–O–Ti | [52,53] |
| Band (cm−1) | Assignment | References |
|---|---|---|
| RAMAN SPECTROSCOPY | ||
| 248 | Eg lattice mode (calcite) τ + δ aromatic ring | [55,56] |
| 320 | τ aromatic ring | [36,37,38,39,40,44] |
| 412 | δop + τ aromatic ring | [36,37,38,39,40,44] |
| 507 | δop + τ aromatic ring | [36,37,38,39,40,44] |
| 626 | δop + τ aromatic ring | [36,37,38,39,40,44] |
| 718 | Eg mode (δipCO32−) | [55,56] |
| 746 | νC–C δC–O δ aromatic ring | [36,37,38,39,40,44] |
| 890 | νC–OH νC–C δCH/CH2 | [36,37,38,39,40,44] |
| 1009 | δipCH νC–O δ aromatic skeletal | [37,38,39,40,41,42,43,44] |
| 1092 | A1g mode (νsCO32−) | [55,56] |
| 1180 | δCH νC–O δ aromatic skeletal | [36,37,38,39,40,41,42,43,44,45] |
| 1339 | δCH3 (isopropyl) δCH2 | [36,37,38,39,40,41,42,43,44,45] |
| FTIR-ATR SPECTROSCOPY | ||
| 3600–3000 | νO–H | [48,49,50,51] |
| 2951 | νas–CH3 | [47,48,49,50,51] |
| 2922 | νas–CH2 | [47,48,49,50,51] |
| 2856 | νs–CH2 | [47,48,49,50,51] |
| 1710 | νC=O | [47,48,49,50,51] |
| 1637 | δO–H νC = O νC = C | [47,48,49,50,51] |
| 1404 | νasCO32− | [57,58] |
| 1231 | νC–O νC–C δ aromatic skeletal | [47,48,49,50,51] |
| 1070 | νC–O νC–C δ aromatic skeletal | [47,48,49,50,51] |
| 870 | δopCO32− | [57,58] |
| 705 | δipCO32− | [57,58] |
| Compound | Concentration [ng/mL] |
|---|---|
| α-thujone | 282 |
| α-pinene | n.d. |
| camphene | n.d. |
| sabinene | 1054 |
| β-pinene | n.d. |
| α-phellandrene | 264 |
| α-terpinene | 3814 |
| para-cymene | 157 |
| (R)-(+)-limonene | 535 |
| α-terpinolene | 284 |
| γ-terpinene | 882 |
| terpinen 4-ol | 10,305 |
| carvacrol methyl ether | 23.4 |
| L-carvone | n.d. |
| thymoquinone | 8271 |
| geraniol | n.d. |
| thymol | 63,906 |
| carvacrol | 5669 |
| β-caryophyllene | n.d. |
| α-humulene | 4.0 |
| caryophyllene oxide | 135 |
| cis-α-bisabolene (levomenol) | 4.7 |
| L-linalool | 1425 |
| aromadendrene oxide 2 | n.d. |
| Treatment | Concentration (mg/mL) | CFU/Plate | MPs Size _SEM (nm/µm) |
|---|---|---|---|
| Titanium Oxide | |||
| CT | - | 86.00 ± 3.60 | |
| TiO2 MPs/SM | 0.2 | 184.00 ± 10.58 | Diameters ranging from 160 nm to 1.3 µm, with a prevalence of elements with diameters around 400–600 nm |
| 2 | 142.40 ± 29.70 | ||
| 4 | 274.00 ± 47.62 *** | ||
| 10 | 1132.00 ± 46.13 **** | ||
| 20 | >2500 | ||
| 50 | >2500 | ||
| TiO2 MPs (pristine) | 0.2 | 1776.00 ± 166.40 **** | Diameters about 200 nm |
| 2 | 2452.00 ± 22.27 **** | ||
| 4 | >2500 | ||
| 10 | >2500 | ||
| 20 | >2500 | ||
| 50 | >2500 | ||
| Calcium Carbonate | |||
| CT | - | 106.00 ± 13.53 | |
| CaCO3 MPs/SM | 0.2 | 102.00 ± 2.00 | CaCO3 particles have prismatic shape, sharp edges, and variable size, with an average of 1 µm × 1 µm × 1 µm |
| 2 | 97.00 ± 1.73 | ||
| 4 | 75.33 ± 16.04 ** | ||
| 10 | 55.67 ± 9.02 **** | ||
| 20 | 6.33 ± 4.04 **** | ||
| 50 | 1.00 ± 1.00 **** | ||
| CaCO3 MPs (pristine) | 0.2 | 111.7 ± 7.23 | Diameters about 800 nm |
| 2 | 108.3 ± 9.07 | ||
| 4 | 101.00 ± 7.93 | ||
| 10 | 100.70 ± 7.02 | ||
| 20 | 100.70 ± 10.07 | ||
| 50 | 96.00 ± 5.29 | ||
| All Synthetized Samples | Z-Potential (ξ, mV) | MALS * Hydrodynamic Diameter (µm) | Acidic Sites (a) (nmol/mg) | Weight Loss % (TGA) |
|---|---|---|---|---|
| TiO2 MPs/SM (Anatase) | −8.92 | 1.57 (by DLS) spherical particles | 14.66 ± 0.97 (FTIR signal for C(=O) carbonyls) | 1.41 ± 0.40 |
| TiO2 MPs/SM (Brookite/Anatase) heterophase | −6.07 | 2.03 (by DLS) spherical particles | 9.98 ± 0.45 (FTIR signal for C(=O) carbonyls) | 0.95 ± 0.23 |
| TiO2 MPs (pristine) (b) | ------ | 0.31 (by DLS) spherical particles | ------ | ------ |
| CaCO3 MPs/SM (Calcite) | −25.14 | 2.83 (by MALS) prismatic particles | 41.31 ± 1.23 (FTIR signal for C(=O) O-H carboxyls) | 3.97 ± 0.34 |
| CaCO3 MPs (pristine) (b) | ------ | 0.72 nm (by MALS) prismatic particles | ------ | ------ |
| Samples | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Antimicrobial Activity (Figure 7 and Table 4) |
|---|---|---|---|---|
| TiO2 MPs/SM (Anatase) | 1.20 | 0.0023 | 560 | Not detected |
| CaCO3 MPs/SM (porous calcite) | 20.0 | 0.0383 | 1000 | Detected |
| TiO2 MPs/SM (Brookite/Anatase) heterophase | 0.84 | 0.0011 | 650 | Not detected |
| TiO2 (pristine) | 13.3 | 0.046 | 180 | Not detected |
| CaCO3 (pristine) | 100 | 0.581 | 650 | Not detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, F.; Colasanti, I.A.; Zaratti, C.; Filimon, D.; Macchia, A.; Neri, A.; Relucenti, M.; Reverberi, M.; Allegrini, I.; Guerriero, E.; et al. TiO2 and CaCO3 Microparticles Produced in Aqueous Extracts from Satureja montana: Synthesis, Characterization, and Preliminary Antimicrobial Test. Molecules 2025, 30, 4138. https://doi.org/10.3390/molecules30204138
Valentini F, Colasanti IA, Zaratti C, Filimon D, Macchia A, Neri A, Relucenti M, Reverberi M, Allegrini I, Guerriero E, et al. TiO2 and CaCO3 Microparticles Produced in Aqueous Extracts from Satureja montana: Synthesis, Characterization, and Preliminary Antimicrobial Test. Molecules. 2025; 30(20):4138. https://doi.org/10.3390/molecules30204138
Chicago/Turabian StyleValentini, Federica, Irene Angela Colasanti, Camilla Zaratti, Dumitrita Filimon, Andrea Macchia, Anna Neri, Michela Relucenti, Massimo Reverberi, Ivo Allegrini, Ettore Guerriero, and et al. 2025. "TiO2 and CaCO3 Microparticles Produced in Aqueous Extracts from Satureja montana: Synthesis, Characterization, and Preliminary Antimicrobial Test" Molecules 30, no. 20: 4138. https://doi.org/10.3390/molecules30204138
APA StyleValentini, F., Colasanti, I. A., Zaratti, C., Filimon, D., Macchia, A., Neri, A., Relucenti, M., Reverberi, M., Allegrini, I., Guerriero, E., Cerasa, M., De Luca, M., Santangeli, F., Braglia, R., Scuderi, F., Rugnini, L., Ranaldi, R., De Meis, R., & Canini, A. (2025). TiO2 and CaCO3 Microparticles Produced in Aqueous Extracts from Satureja montana: Synthesis, Characterization, and Preliminary Antimicrobial Test. Molecules, 30(20), 4138. https://doi.org/10.3390/molecules30204138